- 1Department of Public Health Pharmacy and Management, School of Pharmacy, Sefako Makgatho Health Sciences University, Ga-Rankuwa, South Africa
- 2Saselamani Pharmacy, Polokwane, South Africa
- 3School of Health Sciences, University of Manchester, Manchester, United Kingdom
- 4Department of Pharmacology, Faculty of Health Sciences, University of Pretoria, Pretoria, South Africa
- 5Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow, United Kingdom
- 6Centre for Neonatal and Paediatric Infection, Institute for Infection and Immunity, City St. George’s, University of London, London, United Kingdom
- 7South African Vaccination and Immunisation Centre, Sefako Makgatho Health Sciences University, Ga-Rankuwa, South Africa
Background: Antimicrobial resistance (AMR) poses a global health threat, particularly in low- and middle-income countries (LMICs) including South Africa where limited resources and knowledge gaps exacerbate inappropriate antimicrobial use. To address this, the community antimicrobial use scale (CAMUS) was developed to assess patients’ knowledge, attitudes and behaviors regarding antimicrobial use in South African primary healthcare (PHC) settings, with the aim of informing antimicrobial stewardship (AMS) strategies.
Methods: Development of the CAMUS was informed by a scoping review and theoretical constructs from the Health Belief Model, Social Cognitive Theory, and Theory of Planned Behavior. A pilot study was subsequently conducted in two South African districts, an urban and a rural district, with 30 adult participants to provide insights into patients’ understanding of the items. Data collection involved administering CAMUS alongside a health literacy test followed by cognitive interviews to refine clarity and ensure understanding. A feasibility assessment was also conducted to evaluate the practical use of CAMUS in PHC settings.
Results: Participants demonstrated varied knowledge of antimicrobial use. While 60% correctly identified antibiotics as effective for bacterial infections, 93.33% incorrectly believed antibiotics could treat viral illnesses such as colds. Marginal health literacy was prevalent (86.67%). The CAMUS demonstrated feasibility, with an average completion time of 10 minutes. Questions were iteratively revised to improve future clarity and relevance based on the results of the cognitive interviews. Key findings highlighted misconceptions about antibiotics and the influence of social norms and systemic barriers on antimicrobial use behaviors.
Conclusion: The CAMUS effectively captures the knowledge, attitudes and behaviors of antimicrobial use in South African PHC settings. Pilot testing demonstrated its feasibility to use it as a tool to assess patient knowledge, attitudes and behaviors related to antimicrobial use in a larger population, to subsequently guide AMS initiatives by addressing knowledge gaps and related barriers to improve future antimicrobial use. Future research will include development of a shorter version of the CAMUS, followed by validation in larger, more diverse populations and in local languages to enhance its usability when investigating antimicrobial use and AMR across LMICs.
1 Introduction
Antimicrobial resistance (AMR) poses a global threat, undermining the effectiveness of treatments for bacterial infections and placing immense pressure on healthcare systems worldwide (1). AMR contributes to millions of deaths annually, with its impact particularly severe in sub-Saharan Africa, where infectious disease burdens and limited healthcare resources exacerbate the problem (2–7). Misuse and overuse of antimicrobials, including non-prescription access and self-medication with leftover antibiotics from friends and family members, are significant drivers of AMR in low- and middle-income countries (LMICs) including South Africa (1, 8–10).
South Africa has a two-tiered health system with a public sector serving most of the population and a private sector for those with private health insurance or the ability to pay for healthcare. Primary healthcare (PHC) facilities are the first point of contact for most patients and provide essential services such as diagnosis, treatment, and health promotion (10, 11). Given the high burden of infectious diseases and AMR, PHC facilities should play a key role in antimicrobial stewardship (AMS) and patient education (12).
In South Africa, inappropriate antimicrobial use is particularly problematic in PHC settings, where economic constraints, self-purchasing of antibiotics without a prescription, and critical knowledge gaps among patients contribute to widespread misuse (10, 12–14). A pilot study by Sono et al. (9) found that 60% of patients who obtained antibiotics in independent pharmacies (privately owned and operated pharmacies that function separately from large retail chains) did so without a prescription, often driven by convenience and the inability to afford medical consultations. These practices, coupled with patient misconceptions including believing antibiotics are effective against viral infections, highlight a need for targeted education and intervention strategies in South Africa to improve future antibiotic use (9, 10, 15–18). Similar issues are seen in other LMICs, where patient expectations for antibiotics, even for self-limiting conditions, influence inappropriate prescribing patterns by healthcare providers (19–24).
Efforts to combat AMR including South Africa’s Antimicrobial Resistance Strategy Framework, emphasize antimicrobial stewardship (AMS) through regulatory measures, public education, and healthcare interventions (25, 26). However, AMS programs face significant challenges in PHC settings across LMICs, including inadequate diagnostic capabilities, resource constraints, and limited public awareness; although, this is beginning to change across Africa (27–30). A critical gap in these initiatives is the lack of robust tools to assess patients’ knowledge, attitudes, and practices (KAP) regarding antimicrobial use, which has been found to be a prominent theme regarding antimicrobial use among community members in PHC settings (23).
Existing scales have provided valuable insights into antibiotic use behaviors; however, they do exhibit significant limitations when applied to LMICs. For instance, Byrne et al. (31) developed a questionnaire based on the Theory of Planned Behavior (TpB) to assess social and behavioral predictors of antibiotic misuse, identifying perceived behavioral control and social norms as key factors. However, this tool’s sample size, weak internal consistency for some constructs, and exclusive testing in Australia limit its applicability to diverse socio-economic contexts including among African countries. Hill and Watkins (32) introduced the Appropriate Antibiotic Use Self-Efficacy Scale (AAUSES), which measures confidence in avoiding inappropriate antibiotic use. While valid and internally consistent, the tool relies on data from Amazon Mechanical Turk participants, raising concerns about its generalizability as well as its lack of inclusion of healthcare providers’ perspectives.
Community-based surveys, including those in Zambia and China (33, 34), and studies in South-East Asia (35–37) underscore the importance of capturing local behaviors and access patterns. However, these tools often rely on cross-sectional designs, small sample sizes, and self-reported data, which introduce bias and limit their ability to monitor behavioral change over time.
To address these gaps, the community antimicrobial use scale (CAMUS) was developed to assess antimicrobial use behaviors in South Africa’s PHC settings. Grounded in established behavioral theories, including the Health Belief Model (HBM), Social Cognitive Theory (SCT), and the TpB, the CAMUS captures the complex cognitive, social, and systemic drivers of antimicrobial use. By incorporating context-specific indicators and behavioral insights, the CAMUS aims to provide actionable data to inform AMS interventions. It also seeks to address gaps in existing scales by ensuring cultural and contextual relevance, robust theoretical grounding, and practical feasibility for use in South African communities.
As a result, CAMUS builds on existing antimicrobial use assessment tools by integrating multiple theoretical perspectives to provide a more comprehensive measure of the cognitive, social, and structural influences on antimicrobial use. Unlike previous scales, which often emphasize knowledge or awareness, CAMUS encompasses attitudes, perceived risks and benefits, social norms, and healthcare system barriers that shape antimicrobial-seeking behavior. By incorporating constructs from HBM, SCT, and TpB, CAMUS ensures a more holistic assessment. Consequently, capturing individual perceptions of antimicrobial necessity and risk (HBM), the role of social influence and self-efficacy (SCT), and how perceived behavioral control and norms impact decision-making (TpB). This multidimensional approach ensures that CAMUS is not only theoretically robust but also practically useful to inform the design of targeted AMS interventions or to use as a tool to measure the effectiveness of any intervention to improve the appropriateness of future antimicrobial use.
Consequently, this study aims to: i) Develop the CAMUS using theoretical and contextual insights to assess knowledge, attitudes, and behaviors related to antimicrobial use in South African PHC settings, and ii) Pilot test the CAMUS and evaluate its feasibility and face validity in capturing actionable data to guide AMS interventions prior to full implementation in a larger population.
2 Materials and methods
2.1 Development of the CAMUS
The CAMUS, designed to capture the drivers of antimicrobial use, was developed to investigate and measure AMS related knowledge, attitudes and behaviors in South African PHC settings. The development process began with a scoping review to identify the key factors influencing antimicrobial use especially among patients (23). This review synthesized evidence from diverse contexts, highlighting themes such as perceptions of disease threat, social norms surrounding antibiotic use, and barriers to accessing appropriate healthcare (23). These findings informed the design of the CAMUS and emphasized the importance of grounding its constructs in established behavioral theories to ensure a comprehensive approach.
2.2 Theoretical framework
To comprehensively capture the cognitive, social, and contextual factors influencing antimicrobial use, the CAMUS was designed using constructs from three well-established health behavior theories:
● The HBM explains health behavior as a rational evaluation of perceived risks and benefits. It focuses on constructs such as perceived susceptibility, severity, benefits, barriers, and self-efficacy (38–41). In the CAMUS, the HBM informs items assessing patients’ perceptions of risks associated with antimicrobial misuse, including beliefs about the efficacy of antibiotics for viral infections and the consequences of inappropriate use.
● The SCT emphasizes reciprocal determinism, the interaction between individual, environmental, and behavioral factors, and highlights self-efficacy, outcome expectations, and perceived facilitators and barriers (39). SCT constructs in the CAMUS assess behaviors such as purchasing antibiotics without prescriptions, prematurely discontinuing treatment, and the impact of external barriers like access to healthcare.
● The TpB links behavior to intentions shaped by attitudes, subjective norms, and perceived behavioral control (39–42). The CAMUS incorporates TpB constructs to explore patients’ attitudes toward antibiotic use, the influence of societal norms and expectations, and their perceived ease or difficulty in adhering to appropriate practices.
2.3 Structure and dimensions of the CAMUS
The CAMUS was structured to assess four primary dimensions namely patients’ knowledge, attitudes, motivations, and expectations related to antimicrobial treatments. These dimensions were derived from the scoping review themes and the theoretical framework:
● Knowledge: Assesses patients’ understanding of antimicrobial use, including awareness of AMR and the appropriate indications for antimicrobials.
● Attitudes: Explores patients’ perceptions and beliefs about antimicrobials, including their trust in healthcare providers and their views on self-medication.
● Motivations: Examines the factors driving decisions to use antimicrobials, such as past experiences, convenience, and societal or cultural influences.
● Expectations: Investigates patients’ expectations regarding the effectiveness of antibiotics across a range of infectious diseases, particularly their beliefs about the necessity of antibiotics for treating self-limiting infections including colds, coughs and influenza.
By integrating these dimensions, the CAMUS captures the multifaceted drivers of antimicrobial use, guided by the constructs of the HBM, SCT, and TpB. For example, questions informed by the HBM address perceived risks and benefits of antibiotic use, while TpB shapes items on social norms and attitudes. SCT contributes insights into self-efficacy and barriers influencing behaviors such as adherence to prescribed treatments. By integrating theoretically grounded constructs and context-specific indicators, the CAMUS comprehensively captures key drivers of antimicrobial use. Its design ensures it is well-suited for evaluating knowledge, attitudes, motivations, and expectations related to antimicrobial use in South African PHC settings, providing a robust foundation for targeted AMS interventions in South Africa and wider across LMICs.
2.4 Methodology
2.4.1 Study design
This cross-sectional pilot study aimed to evaluate the feasibility and face validity of the CAMUS, which was designed to assess patients’ knowledge, attitudes, motivations, and expectations regarding antimicrobial use. Cognitive interviews were conducted alongside the administration of the CAMUS to refine its design. Additionally, a health literacy test was administered to all participants prior to instigating the CAMUS to assess their ability to understand health-related information, which could influence their responses.
2.4.2 Study population
The study population consisted of adult patients attending public PHC facilities in two districts of South Africa.
2.4.2.1 Inclusion criteria
Participants were eligible for inclusion if they met the following criteria:
● Aged 18 years or older.
● Able to provide informed consent.
● Willing and able to participate in the interviewer-administered CAMUS and cognitive interview.
● Able to understand and communicate in English.
2.4.2.2 Exclusion criteria
Participants were excluded if they met any of the following criteria:
● Patients with severe illnesses requiring urgent medical attention or hospitalization.
● Individuals unable to understand or communicate in English.
● Patients unwilling to provide consent or participate in the study
2.4.3 Study site
The study was conducted in two phases, with the first phase conducted in a district in one province representing an urban population. For this phase of the study, urban areas included city, suburban, and township settings to capture a diverse range of healthcare access and living environments. The second phase was conducted in one district in another province representing a rural population.
2.4.4 Sample size and sampling technique
A total of 30 patients participated in this pilot study, with 30 patients chosen for this initial study building on our experiences with previous pilot studies undertaken in South Africa among patients and looking to add to this given the various strands of the questionnaire (17, 18).
● Phase 1: 15 patients were recruited from one urban district.
● Phase 2: 15 patients were recruited from one rural district in a different Province.
Participants were recruited using convenience sampling in the waiting areas of healthcare facilities. Patients sitting in the waiting area were approached and invited to participate in the study. Patients who met the inclusion criteria and provided informed consent were included in the study.
2.4.5 Data collection procedures
Phase 1 data collection in the urban district:
● A health literacy test was administered to all 15 participants before the CAMUS. Cognitive interviews were conducted alongside the administration of the CAMUS to evaluate clarity, comprehension, and relevance.
● Responses were analyzed to identify unclear or misinterpreted questions. Questions were refined based on the feedback to improve the CAMUS.
Phase 2 data collection in the rural district:
● The revised CAMUS was subsequently administered to another 15 participants, preceded by the health literacy test. Cognitive interviews were conducted to gather additional feedback, and further adjustments to the CAMUS were made based on these insights.
● Additionally, a practical feasibility assessment was conducted on 5 participants in the rural district. These participants completed only the CAMUS to evaluate the time required for completion, ensuring its suitability for real-world PHC settings where time constraints are a significant consideration.
Data collection was carried out in a structured and systematic manner to ensure the accuracy and reliability of the information gathered. The following procedures were followed:
2.4.5.1 Participant recruitment and consent
In both districts, patients were approached in the waiting areas of PHC facilities. They were informed about the study’s purpose, objectives, and procedures and invited to participate. Written informed consent was obtained from all participants before commencing any data collection activities. Participants were assured of confidentiality and anonymity throughout the study.
2.4.5.2 Health literacy assessment
Before administering the CAMUS, all participants completed a health literacy test adapted from the Health Literacy Test for Limited Literacy Populations (HELT-LL) (43). The test, used without modifications, assessed participants’ ability to understand common medical instructions, prescription labels, and basic healthcare terminology. Based on their test scores, participants were categorized into three health literacy levels: inadequate (0–10), marginal (11–20), and adequate (21–24). These categorizations were used to contextualize participants’ responses to the CAMUS.
2.4.5.3 Administration of the CAMUS and cognitive interviews
The CAMUS was administered to participants immediately following the health literacy test. It was conducted as an interviewer-administered survey to accommodate varying literacy levels and ensure accurate comprehension. Cognitive interviews were conducted concurrently with the CAMUS to evaluate participants’ understanding and interpretation of the CAMUS items. Participants were asked follow-up questions to clarify their thought processes, identify any confusion or misinterpretation, and suggest improvements to the wording or structure of the questions.
All cognitive interviews and CAMUS responses were audiorecorded with participant consent to ensure accurate data capture and enable transcription and analysis.
In Phase 1, initial responses and cognitive interview feedback highlighted areas of ambiguity, leading to a revision of the CAMUS. In Phase 2, the refined CAMUS was administered to an additional cohort of participants, and further adjustments were made based on their feedback (see Section 2.5.2).
2.4.5.4 Feasibility assessment
In the rural district, a separate group of five participants completed the CAMUS without cognitive interviews to assess the time required for completion. This step evaluated the practical feasibility of implementing the CAMUS in real-world PHC settings, considering time constraints and patient engagement.
2.5 Data management and analysis
Data from the health literacy test, CAMUS responses, and cognitive interviews were captured into a Microsoft Excel spreadsheet for organization and analysis.
2.5.1 Health literacy test
Results were analyzed to understand participants’ health literacy levels and their potential impact on the CAMUS responses. Descriptive statistics were used to summarize literacy levels across the sample.
2.5.2 Cognitive interviews
Recordings were transcribed to identify issues with question clarity and interpretation. This informed iterative refinements to the CAMUS after each phase.
2.5.3 CAMUS data
Quantitative data from the CAMUS were analyzed to assess knowledge, attitudes, motivations, and expectations regarding antimicrobial use. Descriptive statistics were used to summarize participant responses.
The average time to complete the CAMUS was calculated based on the feasibility assessment.
2.6 Ethical considerations
Ethical approval for this study was obtained from the Sefako Makgatho University Research Ethics Committee (SMUREC/P/220/2023). In addition, approval to conduct the study in the two provinces was granted by the respective provincial and district research committees. All patient responses were treated with strict confidentiality, and data will be securely stored in a password-protected database for a period of five years to ensure compliance with ethical standards and data protection protocols. Access to the data will be restricted to authorized research personnel only. In addition, no personal identifiable information will be included in reports or publications to safeguard participant confidentiality.
3 Results
30 participants took part in the pilot study, completing the health literacy assessment, the CAMUS and the cognitive interviews. An additional 5 participants completed the CAMUS only, in order to determine the duration taken to complete the interview. These patients' responses are not included in the results presented.
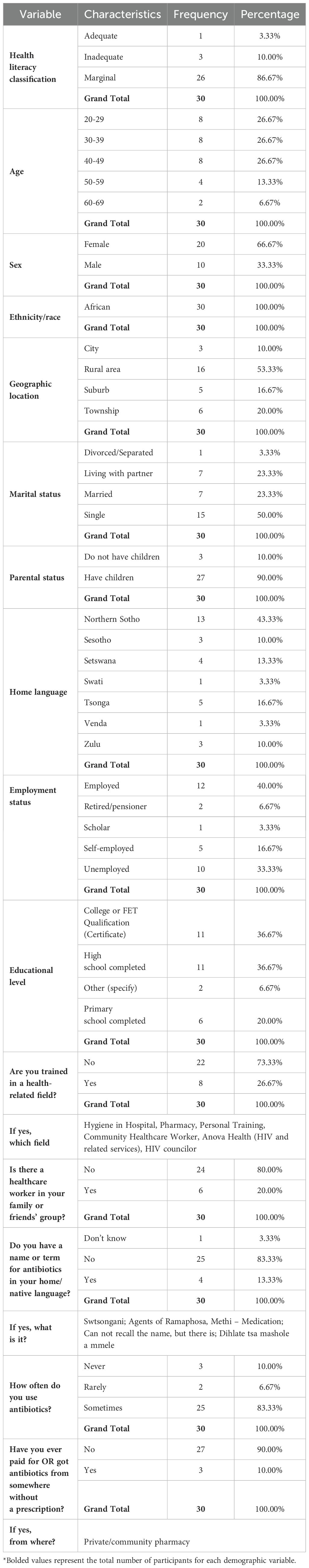
Participant demographics are summarized in Table 1. Most participants had marginal health literacy (85.67%, n = 26), while only one participant had adequate health literacy. The majority of participants were female (66.67%, n = 20), with participants aged between 20 and 49 years, accounting for 80.0% (n = 24) of the study participants.
Just more than half (53.33%, n = 16) of participants resided in and were living in rural areas. All participants were African (100%, n = 30). In terms of marital status, 50% (n = 15) were single, and 90% (n = 27) had children.
The employed group was the largest (40%, n = 12) and 33.33% (n= 10) unemployed. With regards to educational level, 36.67% (n = 11) had completed high school and had a college or Further Education and Training (FET) qualification. Only 26.67% (n = 8) were trained in a health-related field, though 20.0% (n = 6) had family or friends that were healthcare workers.
When asked if participants had a name or term for antibiotics in their home or native language, 83.33% (n = 25) indicated that they did not have a term. The majority of participants (83.33%, n=25) reported to have used antibiotics at some point while 10% (n=3) claimed to have never used antibiotics. The majority (90.0%; n=27) of participants had never accessed antibiotics without a prescription, while 3 (10%) indicated that they accessed antibiotics without a prescription through a private or community pharmacy. These are pharmacies that provide medication and pharmaceutical services to local populations, including both independent and chain pharmacies (17).
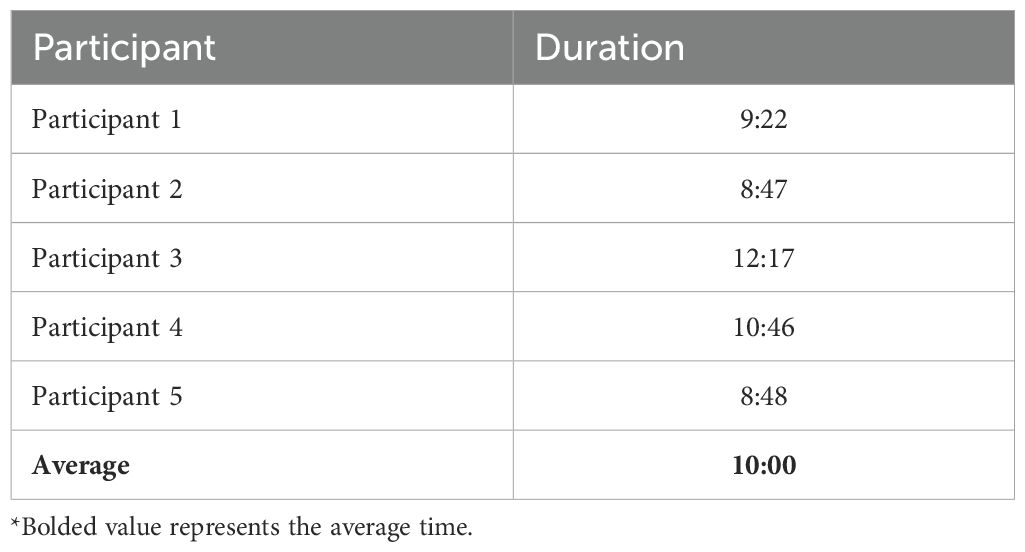
3.1 Average time to complete the CAMUS
Five participants completed the CAMUS only to gauge the time it took to complete. On average it took 10 minutes to complete the CAMUS, see Table 2.
3.2 Cognitive interviews
In the first phase of cognitive interviews, feedback from the initial 15 participants led to revisions of 28 of the 30 questions and statements, ensuring they were clear and easy to understand. These adjustments, outlined in Table 3, focused on simplifying language and enhancing clarity. The revised items were then used in Phase 2 with 15 participants to confirm their usability. Only two questions/statements were revised after Phase 2 of data collection.
3.3 Antimicrobial knowledge
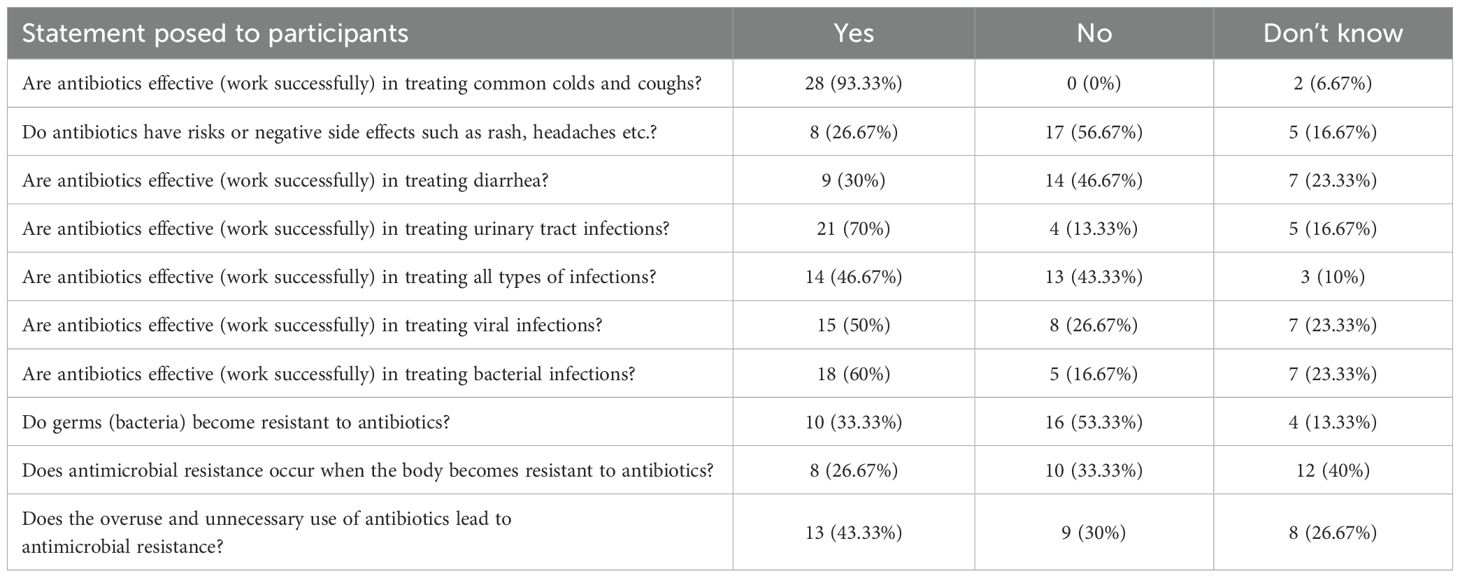
The results from the antimicrobial knowledge section (Table 4) highlights a mixed understanding of antibiotic use among participants. The majority of participants (60.0%, n=18) correctly recognized that antibiotics are effective against bacterial infections; however, there was confusion and misconceptions about their use for viral infections. Notably, 93.33% (n=28) incorrectly believed antibiotics treat colds and coughs, which are typically viral illnesses. While most coughs are viral in origin, certain bacterial infections, such as pertussis, do require antibiotic treatment. When asked explicitly, 50.0% of patients (n=15) believed that antibiotics treat viral infections and a further 23.33% (n=7) were uncertain.
Additionally, 56.67% (n=17) did not associate antibiotics with potential side-effects, indicating a gap in awareness about the possible risks of antibiotics. Understanding of specific conditions varied; while 70.0% (n=21) identified antibiotics as effective for urinary tract infections (UTIs), there was some misunderstanding regarding their use for other conditions. Notably, 30.0% (n=9) believed antibiotics were effective for treating diarrhea. While most cases of diarrhea are viral or self-limiting and require fluid replacement and supportive care, certain bacterial infections, such as Shigella or Clostridium difficile, may require antibiotic therapy. Additionally, 23.33% (n=7) expressed uncertainty regarding the appropriate treatment for diarrhea.
Regarding AMR, many participants showed an understanding of its causes, with 43.33% (n=13) recognizing that the overuse of antibiotics leads to resistance. However, some confusion persisted as 13.33% (n=4) were unsure about bacteria becoming resistant to antibiotics, 53.33% (n=16) indicated that bacteria do not become resistant to antibiotics and as mentioned, 93.33% (n=28) incorrectly believed antibiotics can treat viral infections such as colds and coughs. Overall, the findings suggest a need for targeted education to improve patients’ and the public’s knowledge about when antibiotics are appropriate, and the risks associated with their misuse.
3.4 Perceptions, attitudes, family and community behaviors and self-medication regarding antibiotics
The data in Table 5 shows a range of patient perceptions, attitudes, and behaviors regarding antibiotic use and healthcare provider roles in prescribing them. Of concern is that an appreciable portion of participants, 46.67% (n= 14), strongly agreed that they have the right to request antibiotics from their healthcare providers, with 10% (n= 3) moderately agreeing and 6.67% (n= 2) slightly agreeing. However, 16.67% (n= 5) strongly disagreed, indicating diverse opinions on patient agency in requesting antibiotics. This finding suggests that while some patients view antibiotic prescriptions as a right and expect healthcare providers to comply with their requests, others acknowledge the prescriber’s authority in determining the necessity of antibiotics. This variation highlights the potential influence of patient expectations on prescribing practices and the need for patient education on appropriate antibiotic use. When it comes to symptom relief, half of the participants (50.0%, n= 15) strongly expect antibiotics to quickly alleviate their symptoms with only 20.0% (n= 6) strongly disagree with this expectation, reflecting some awareness that antibiotics may not always lead to immediate relief.
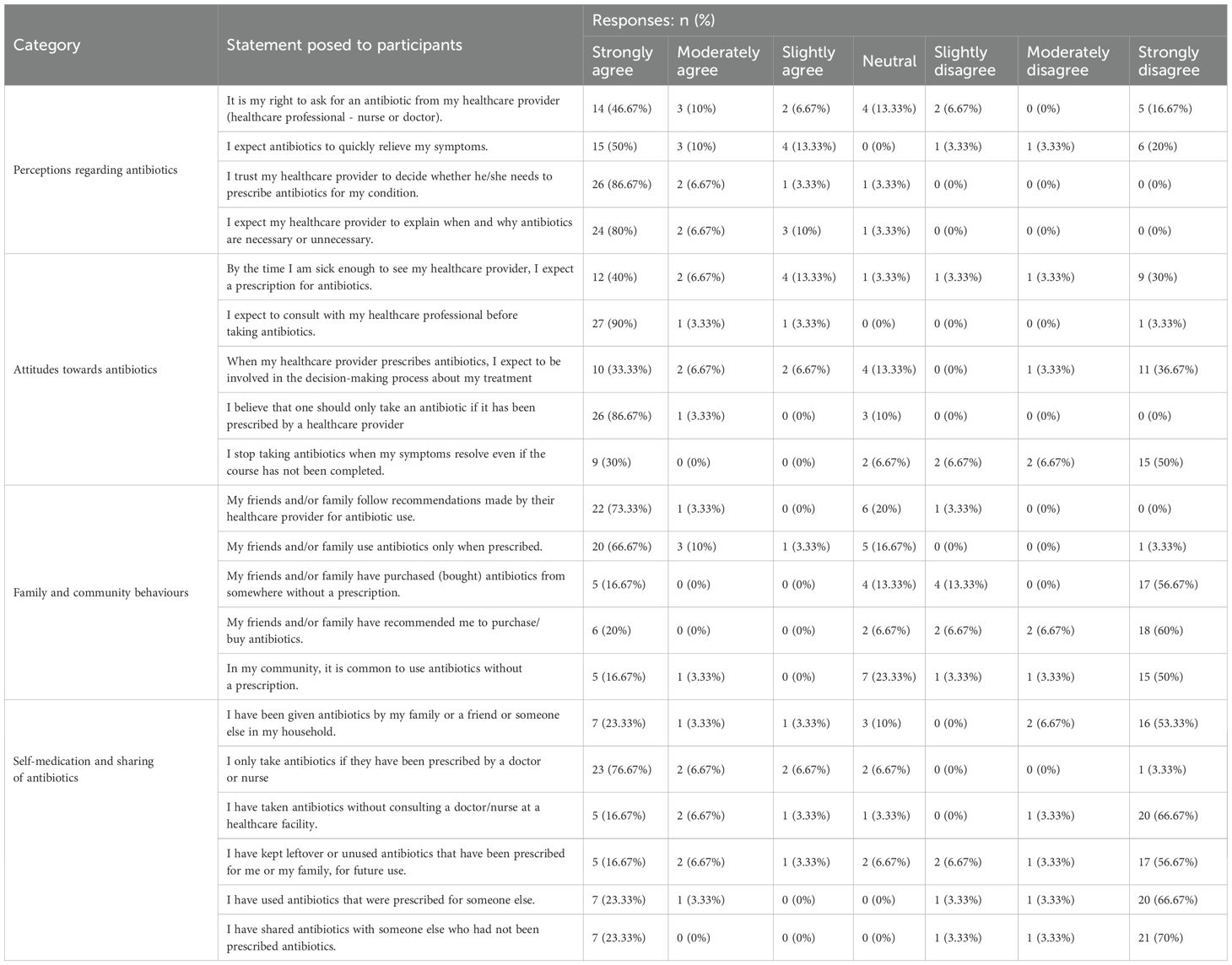
Table 5. Perceptions, attitudes, family and community behaviors and self-medication regarding antibiotics.
Trust in healthcare providers’ judgment was notably high, with 86.67% (n= 26) strongly agreeing that their providers should decide when antibiotics are necessary, underscoring the participants’ reliance on professional guidance. Furthermore, 80.0% (n= 24) strongly agree that they expect their providers to explain the necessity or lack thereof for antibiotic prescriptions, highlighting a strong desire for communication and understanding in the decision-making process. Having said this, a notable number of participants, 40.0% (n= 12) strongly agreed that by the time they seek medical attention, they expect a prescription for antibiotics, though 30% (n= 9) strongly disagreed showing divided expectations about receiving antibiotics when ill.
A majority (90.0%, n= 27) strongly agreed that they should consult their healthcare professional before taking antibiotics, underscoring a preference for professional guidance. Similarly, 86.67% (n= 26) strongly believe that antibiotics should only be taken if prescribed by a healthcare provider, reinforcing strong adherence to prescription-based use.
However, attitudes about treatment decisions and adherence to antibiotic courses varied. While 33.33% (n= 10) strongly agreed that they expect to be involved in treatment decisions, 36.67% (n= 11) strongly disagreed, indicating mixed preferences regarding patient-provider decision-making involvement. Additionally, 50.0% (n= 15) strongly disagreed with stopping antibiotics once symptoms resolve, suggesting that there is a keen understanding the importance of completing the full course. Having said this, 30.0% (n= 9) admitted they would stop antibiotics early when feeling better, highlighting an area for educational reinforcement.
The majority of participants (73.33%, n=22) strongly agreed that their friends and family follow healthcare provider recommendations for antibiotic use, and 66.67% (n=20) strongly agreed that antibiotics are only taken when prescribed. This suggests that adherence to prescription-based use is commonly valued within these social circles. However, certain behaviors show deviations. While 56.67% (n=17) strongly disagreed that their friends or family have bought antibiotics without a prescription, 16.67% (n=5) strongly agreed that this has occurred, and 20.0% (n=6) also report being encouraged by friends or family to purchase antibiotics without a prescription, although 60.0% (n=18) strongly disagreed with these practices. Perceptions of community norms further reinforce these findings. Half, 50.0% (n=15), of participants strongly disagreed that non-prescription antibiotic use is common in their community, though 16.67% (n=5) strongly agreed. These results highlight both adherence to healthcare provider guidance and variability in behaviors around prescription adherence, influenced by family or community practices.
With regards to self-medication and the sharing of antibiotics, most participants (66.67%, n=20) strongly disagreed with taking antibiotics without consulting a healthcare provider, indicating a strong preference for professional guidance. Similarly, a significant majority (76.67%, n=23) strongly agreed that they only take antibiotics if prescribed by a doctor or nurse, showing adherence to prescription-based use of antibiotics. Despite this adherence, certain behaviors show deviation. For instance, 16.67% (n=5) strongly agreed that they have kept leftover antibiotics for future use, while 56.67% (n=17) strongly disagreed, indicating mixed practices regarding leftover medication. Additionally, 23.33% (n=7) strongly agreed that they have used antibiotics prescribed for someone else, although the majority (66.67%, n=20) strongly disagreed. Antibiotic sharing appears to be limited. A large majority, 70.0% (n=21), strongly disagreed with sharing antibiotics with others who were not prescribed antibiotics. Likewise, 53.33% (n=16) strongly disagreed with receiving antibiotics from friends or family. However, a notable 23.33% (n=7) strongly agreed, suggesting a potential source of antibiotics within households.
4 Discussion
This study successfully developed, and pilot tested the CAMUS, designed to assess patients’ knowledge, attitudes, motivations, and behaviors related to antimicrobial use in South African PHC settings. The results demonstrate the potential of the CAMUS to capture the nuanced factors influencing antimicrobial use, providing actionable insights to guide AMS initiatives, which is important for South Africa as well as other African countries given concerns with rising AMR rates in this sub-continent (4, 5, 10, 17).
A notable finding was the prevalence of marginal health literacy among participants (86%), which aligns with previous studies linking limited health literacy to poorer understanding of antibiotic use and AMR in LMICs (44, 45). This underscores the importance of ensuring that health education and AMS interventions are accessible to patients with varying literacy levels. The CAMUS, with its iterative refinements based on cognitive interviews, addresses this challenge by simplifying language and enhancing clarity.
Misconceptions about antibiotic use were evident, with 93.33% (n=28) of participants incorrectly believing that antibiotics can treat common colds and coughs, which are typically viral illnesses. Additionally, 50.0% (n=15) believed that antibiotics could treat viral infections, while 23.33% (n=7) were uncertain. Regarding antibiotic adherence, 30.0% (n=9) of participants indicated that they would stop taking antibiotics once symptoms improved, which reflects a significant misconception about appropriate antibiotic use. This finding is consistent with other studies in South Africa and LMICs that report widespread misunderstanding of antibiotic efficacy and use (45–50). However, the recognition of AMR causes, including the overuse of antibiotics, among 43.33% of participants suggests a partial understanding of the issue. These combined findings highlight the importance of targeted education to bridge knowledge gaps and promote appropriate antibiotic use through AMS (12, 50–52). However, it is important to consider potential language barriers, particularly in cases where there are no specific terms for words such as antibiotics and AMR in certain populations and languages in South Africa and beyond (17, 18, 36, 53). We will be exploring the implications further for all key stakeholder groups in South Africa building on the suggestions from our previous work (9, 10, 17).
The study findings suggest that CAMUS can serve as a valuable tool in AMS initiatives by identifying key knowledge gaps and behavioral patterns related to antimicrobial use. Implementing CAMUS within PHC settings could enable targeted patient education, and guiding tailored interventions to correct misconceptions. Additionally, integrating CAMUS findings into provider training programs may help healthcare professionals address patient expectations regarding antibiotic prescriptions more effectively. Future studies should explore how CAMUS-based interventions impact antimicrobial use behaviors over time.
The demographic diversity of the sample, including participants from both urban and rural settings, provided insights into regional variations in antimicrobial use behaviors. However, the small sample size of this pilot study limits the generalizability of these findings, warranting further research with larger and more representative populations.
The CAMUS theoretical foundation, drawing on the HBM, SCT, and the TpB, ensured that it captured cognitive, social, and systemic drivers of antimicrobial use. For example, constructs addressing perceived risks, social norms, and self-efficacy were effectively incorporated, enabling a comprehensive assessment of key behavioral determinants. The CAMUS also demonstrated its reliability and feasibility in PHC settings, with participants completing the interview in an average of 10 minutes. The integration of cognitive interviews further enhanced its usability by addressing potential ambiguities and tailoring items to the local context, which is important going forward.
A limitation of this study in particular is that CAMUS was tested only in English, which may restrict its applicability in South Africa’s multilingual context. Many South African populations may not have precise terminology for terms such as ‘antibiotics’ or ‘antimicrobial resistance’ in their native languages, potentially impacting comprehension and response accuracy. However, because CAMUS is a new tool, validation in English should precede translation to other languages with subsequent validation. As CAMUS relies on self-reported data, there is also potential for recall and social desirability biases. Future studies should focus on firstly using CAMUS in a much larger and more diverse population to develop a shorter version of CAMUS (possibly 10 items) which will best predict AMS-related use behaviors. Thereafter, translating the shorter version into multiple local languages, and refining its applicability to different healthcare settings. It is envisaged that the shorter version will be quick and easy to administer.
Bearing in mind that this was only a pilot study, another potential limitation of the study is the presence of bias due to the fact that nearly one-third of participants had received health-related education. Participants with prior health-related training may have had greater baseline knowledge of antimicrobial use, which could have influenced their responses and potentially overestimated the level of understanding in the broader population. This may limit the generalizability of the findings to individuals without a healthcare background. Future studies should aim to balance participant demographics by including a more representative sample of the general population to minimize this potential bias and ensure the CAMUS is tested across a diverse range of knowledge levels.
If validated in larger populations, CAMUS could serve as a valuable tool to support AMS initiatives by providing data on patient knowledge, attitudes and behaviors. This information could help shape targeted educational interventions, guide healthcare provider communication strategies, and inform regulatory policies aimed at reducing inappropriate antimicrobial use in PHC settings.
Future studies with larger and more diverse samples will now take place to validate the CAMUS findings and identify the items that will best predict AMS-related behavior. As mentioned, we are aware that the CAMUS was only tested in English, potentially limiting its applicability in South Africa’s multilingual context (18, 24). Consequently, validation in local languages will also take place to ensure its broader relevance and utility. Hence, future research will involve translating and adapting the CAMUS into multiple local languages. This process will incorporate a rigorous translation and back-translation methodology, cognitive testing, and cultural adjustments to ensure broader relevance. This is important across Africa given the many languages that can exist in a number of African countries. While the data should be interpreted with caution as this was a pilot study with the aim of testing the questionnaire, we believe the findings from this pilot study are robust, providing direction for further studies with larger populations in South Africa and wider.
5 Conclusion
The CAMUS demonstrated its relevance and usefulness in capturing key constructs related to antimicrobial use behaviors in South African PHC settings. Pilot testing confirmed its feasibility, and face validity, with iterative refinements improving clarity and comprehension. The tool provides a structured method for assessing patient knowledge, attitudes, and behaviors, which can contribute to improving AMS efforts.
A key strength of this pilot was the use of cognitive interviews, which enhanced the clarity and relevance of the questionnaire. While this study was limited in scope, the findings support further validation of CAMUS in larger and more diverse populations. Future research should focus on shortening, refining and validating the tool across multiple settings to ensure its applicability and effectiveness in guiding AMS initiatives.
Next steps will include scaling up the validation process, incorporating a possibly shorter scale in diverse linguistic and cultural contexts, and evaluating CAMUS in broader healthcare environments. Ensuring its usability across different healthcare settings will be essential for optimizing its role in addressing inappropriate antimicrobial use and supporting AMS strategies which are very necessary to meet the new United Nations Global Assembly targets for AMR going forward.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Sefako Makgatho University Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
NR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. TB: Data curation, Formal analysis, Investigation, Validation, Writing – review & editing. MT: Data curation, Formal analysis, Investigation, Validation, Writing – review & editing. MS: Conceptualization, Formal analysis, Methodology, Resources, Validation, Writing – review & editing. TS: Conceptualization, Methodology, Validation, Writing – review & editing. SC: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing. NS: Conceptualization, Methodology, Supervision, Visualization, Writing – review & editing. BG: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. JM: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Research Foundation of South Africa, grant numbers 129365 and 138721.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Laxminarayan R. The overlooked pandemic of antimicrobial resistance. Lancet. (2022) 399:606–7. doi: 10.1016/S0140-6736(22)00087-3
2. Nkengasong JN, Tessema SK. Africa needs a new public health order to tackle infectious disease threats. Cell. (2020) 183:296–300. doi: 10.1016/j.cell.2020.09.041
3. Bell D, Schultz Hansen K. Relative burdens of the COVID-19, malaria, tuberculosis, and HIV/AIDS epidemics in sub-saharan africa. Am J Trop Med Hyg. (2021) 105:1510–5. doi: 10.4269/ajtmh.21-0899
4. Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. (2022) 399:629–55. doi: 10.1016/S0140-6736(21)02724-0
5. Antimicrobial Resistance Collaborators. The burden of bacterial antimicrobial resistance in the WHO African region in 2019: a cross-country systematic analysis. Lancet Glob Health. (2024) 12:e201–e16. doi: 10.1016/S2214-109X(23)00539-9
6. Saleem Z, Godman B, Cook A, Khan MA, Campbell SM, Seaton RA, et al. Ongoing efforts to improve antimicrobial utilization in hospitals among african countries and implications for the future. Antibiotics. (2022) 11:1824. doi: 10.3390/antibiotics11121824
7. Lewnard JA, Charani E, Gleason A, Hsu LY, Khan WA, Karkey A, et al. Burden of bacterial antimicrobial resistance in low-income and middle-income countries avertible by existing interventions: an evidence review and modelling analysis. Lancet. (2024) 403:2439–54. doi: 10.1016/S0140-6736(24)00862-6
8. Godman B, Egwuenu A, Haque M, Malande OO, Schellack N, Kumar S, et al. Strategies to improve antimicrobial utilization with a special focus on developing countries. Life. (2021) 11:(6). doi: 10.3390/life11060528
9. Sono TM, Yeika E, Cook A, Kalungia A, Opanga SA, Acolatse JEE, et al. Current rates of purchasing of antibiotics without a prescription across sub-Saharan Africa; rationale and potential programmes to reduce inappropriate dispensing and resistance. Expert Rev Anti Infect Ther. (2023) 21:1025–55. doi: 10.1080/14787210.2023.2259106
10. Chigome A, Ramdas N, Skosana P, Cook A, Schellack N, Campbell S, et al. A narrative review of antibiotic prescribing practices in primary care settings in South Africa and potential ways forward to reduce antimicrobial resistance. Antibiotics. (2023) 12:1540. doi: 10.3390/antibiotics12101540
11. Meyer JC, Schellack N, Stokes J, Lancaster R, Zeeman H, Defty D, et al. Ongoing initiatives to improve the quality and efficiency of medicine use within the public healthcare system in South Africa; A preliminary study. Front Pharmacol. (2017) 8:751. doi: 10.3389/fphar.2017.00751
12. Farley E, Stewart A, Davies MA, Govind M, Van den Bergh D, Boyles TH. Antibiotic use and resistance: Knowledge, attitudes and perceptions among primary care prescribers in South Africa. S Afr Med J. (2018) 108:763–71. doi: 10.7196/SAMJ.2018.v108i9.12933
13. Gasson J, Blockman M, Willems B. Antibiotic prescribing practice and adherence to guidelines in primary care in the Cape Town Metro District, South Africa. S Afr Med J. (2018) 108:304–10. doi: 10.7196/SAMJ.2018.v108i4.12564
14. Guma SP, Godman B, Campbell SM, Mahomed O. Determinants of the empiric use of antibiotics by general practitioners in South Africa: observational, analytic, cross-sectional study. Antibiotics. (2022) 11:1423. doi: 10.3390/antibiotics11101423
15. Farley E, van den Bergh D, Coetzee R, Stewart A, Boyles T. Knowledge, attitudes and perceptions of antibiotic use and resistance among patients in South Africa: A cross-sectional study. S Afr J Infect Dis. (2019) 34:118. doi: 10.4102/sajid.v34i1.118
16. Mokoena TTW, Schellack N, Brink AJ. Driving antibiotic stewardship awareness through the minibus-taxi community across the Tshwane District, South Africa-a baseline evaluation. JAC Antimicrob Resist. (2021) 3(3):dlab106. doi: 10.1093/jacamr/dlab106
17. Sono TM, Maluleke MT, Ramdas N, Jelic AG, Campbell S, Markovic-Pekovic V, et al. Pilot study to evaluate the feasibility of a patient questionnaire for the purpose of investigating the extent of purchasing antibiotics without a prescription in a rural province in South Africa: rationale and implications. Adv Hum Biol. (2024) 14:138–47. doi: 10.4103/aihb.aihb_140_23
18. Sono TM, Mboweni V, Jelić AG, Campbell SM, Marković-Peković V, Ramdas N, et al. Pilot study to evaluate patients’ Understanding of key terms and aspects of antimicrobial use in a rural province in South Africa findings and implications. Adv Hum Biol. (2025) 15:108–12. doi: 10.4103/aihb.aihb_119_24
19. Mboya EA, Davies ML, Horumpende PG, Ngocho JS. Inadequate knowledge on appropriate antibiotics use among clients in the Moshi municipality Northern Tanzania. PloS One. (2020) 15:e0239388. doi: 10.1371/journal.pone.0239388
20. Nyeko R, Otim F, Obiya EM, Abala C. Pre-hospital exposures to antibiotics among children presenting with fever in northern Uganda: a facility-based cross-sectional study. BMC Pediatr. (2022) 22:322. doi: 10.1186/s12887-022-03375-2
21. Massele A, Rogers AM, Gabriel D, Mayanda A, Magoma S, Cook A, et al. A narrative review of recent antibiotic prescribing practices in ambulatory care in Tanzania: findings and implications. Medicina. (2023) 59:2195. doi: 10.3390/medicina59122195
22. Otieku E, Fenny AP, Labi AK, Owusu-Ofori AK, Kurtzhals J, Enemark U. Knowledge, attitudes and practices regarding antimicrobial use and resistance among healthcare seekers in two tertiary hospitals in Ghana: a quasi-experimental study. BMJ Open. (2023) 13:e065233. doi: 10.1136/bmjopen-2022-065233
23. Ramdas N, Meyer JC, Schellack N, Godman B, Turawa E, Campbell SM. Knowledge, attitudes, motivations, expectations, and systemic factors regarding antimicrobial use amongst community members seeking care at the primary healthcare level: A scoping review. Antibiotics. (2025) 14:78. doi: 10.3390/antibiotics14010078
24. Sono TM, Schellack N, Godman B. The role of patients with addressing inappropriate dispensing of antibiotics without a prescription especially in developing countries. . Adv Hum Biol. (2025) 15:1–4. doi: 10.4103/aihb.aihb_124_24
25. Department of Health Republic of South Africa. South african antimicrobial resistance national strategy framework; A one health approach - 2017 – 2024 (2017). Available online at: https://www.knowledgehub.org.za/system/files/elibdownloads/2020-03/AMR%20National%20Action%20Plan%202018%20-%202024.pdf (Accessed February 27, 2025).
26. Godman B, Egwuenu A, Wesangula E, Schellack N, Kalungia AC, Tiroyakgosi C, et al. Tackling antimicrobial resistance across sub-Saharan Africa: current challenges and implications for the future. Expert Opin Drug Saf. (2022) 21:1089–111. doi: 10.1080/14740338.2022.2106368
27. Cox JA, Vlieghe E, Mendelson M, Wertheim H, Ndegwa L, Villegas MV, et al. Antibiotic stewardship in low- and middle-income countries: the same but different? Clin Microbiol Infect. (2017) 23:812–8. doi: 10.1016/j.cmi.2017.07.010
28. Akpan MR, Isemin NU, Udoh AE, Ashiru-Oredope D. Implementation of antimicrobial stewardship programmes in African countries: a systematic literature review. J Glob Antimicrob Resist. (2020) 22:317–24. doi: 10.1016/j.jgar.2020.03.009
29. Siachalinga L, Godman B, Mwita JC, Sefah IA, Ogunleye OO, Massele A, et al. Current antibiotic use among hospitals in the sub-saharan africa region; findings and implications. Infect Drug Resist. (2023) 16:2179–90. doi: 10.2147/IDR.S398223
30. Cohn J, Mendelson M, Kanj SS, Shafiq N, Boszczowski I, Laxminarayan R. Accelerating antibiotic access and stewardship: a new model to safeguard public health. Lancet Infect Dis. (2024) 24:e584–e90. doi: 10.1016/S1473-3099(24)00070-7
31. Byrne MK, Miellet S, McGlinn A, Fish J, Meedya S, Reynolds N. The drivers of antibiotic use and misuse: the development and investigation of a theory driven community measure. BMC Public Health. (2019) 19:(1). doi: 10.1186/s12889-019-7796-8
32. Hill EM, Watkins K. Development and initial validation of the appropriate antibiotic use self-efficacy scale. Patient Educ Couns. (2018) 101:1838–45. doi: 10.1016/j.pec.2018.05.020
33. Ngoma MT, Sitali D, Mudenda S, Mukuma M, Bumbangi FN, Bunuma E, et al. Community antibiotic consumption and associated factors in Lusaka district of Zambia: findings and implications for antimicrobial resistance and stewardship. JAC-Antimicrobial Resistance. (2024) 6:dlae034. doi: 10.1093/jacamr/dlae034
34. Lin R, Duan L, Liu C, Wang D, Zhang X, Wang X, et al. The public’s antibiotic use behavioural patterns and their determinants for upper respiratory tract infections: a latent class analysis based on consumer behaviour model in China. Front Public Health. (2023) 11:1231370. doi: 10.3389/fpubh.2023.1231370
35. Nepal G, Bhatta S. Self-medication with antibiotics in WHO southeast asian region: A systematic review. Cureus. (2018) 10:e2428. doi: 10.7759/cureus.2428
36. Haenssgen MJ, Charoenboon N, Zanello G, Mayxay M, Reed-Tsochas F, Lubell Y, et al. Antibiotic knowledge, attitudes and practices: new insights from cross-sectional rural health behaviour surveys in low-income and middle-income South-East Asia. BMJ Open. (2019) 9:e028224. doi: 10.1136/bmjopen-2018-028224
37. Pham-Duc P, Sriparamananthan K. Exploring gender differences in knowledge and practices related to antibiotic use in Southeast Asia: A scoping review. PloS One. (2021) 16:e0259069. doi: 10.1371/journal.pone.0259069
38. Jones CL, Jensen JD, Scherr CL, Brown NR, Christy K, Weaver J. The Health Belief Model as an explanatory framework in communication research: exploring parallel, serial, and moderated mediation. Health Commun. (2015) 30:566–76. doi: 10.1080/10410236.2013.873363
39. Munro S, Lewin S, Swart T, Volmink J. A review of health behaviour theories: how useful are these for developing interventions to promote long-term medication adherence for TB and HIV/AIDS? BMC Public Health. (2007) 7:104. doi: 10.1186/1471-2458-7-104
40. Huang X, Dai S, Xu H. Predicting tourists’ health risk preventative behaviour and travelling satisfaction in Tibet: Combining the theory of planned behaviour and health belief model. Tour Manag Perspect. (2020) 33:100589. doi: 10.1016/j.tmp.2019.100589
41. Miellet S, Byrne MK, Reynolds N. A confirmation of the predictive utility of the Antibiotic Use Questionnaire. BMC Public Health. (2024) 24:1925. doi: 10.1186/s12889-024-18901-3
42. Bosnjak M, Ajzen I, Schmidt P. The theory of planned behavior: Selected recent advances and applications. Eur J Psychol. (2020) 16:352–6. doi: 10.5964/ejop.v16i3.3107
43. Marimwe C, Dowse R. Health literacy test for limited literacy populations (HELT-LL): Validation in South Africa. Cogent Medicine. (2019) 6(1). doi: 10.1080/2331205x.2019.1650417
44. Castro-Sánchez E, Chang PWS, Vila-Candel R, Escobedo AA, Holmes AH. Health literacy and infectious diseases: why does it matter? Int J Infect Dis. (2016) 43:103–10. doi: 10.1016/j.ijid.2015.12.019
45. Godman B, Haque M, McKimm J, Abu Bakar M, Sneddon J, Wale J, et al. Ongoing strategies to improve the management of upper respiratory tract infections and reduce inappropriate antibiotic use particularly among lower and middle-income countries: findings and implications for the future. Curr Med Res Opin. (2020) 36:301–27. doi: 10.1080/03007995.2019.1700947
46. Antwi AN, Stewart A, Crosbie M. Fighting antibiotic resistance: a narrative review of public knowledge, attitudes, and perceptions of antibiotics use. Perspect Public Health. (2020) 140:338–50. doi: 10.1177/1757913920921209
47. Khan FU, Khan FU, Hayat K, Chang J, Saeed A, Khan Z, et al. Knowledge, attitude and practices among consumers toward antibiotics use and antibiotic resistance in Swat, Khyber-Pakhtunkhwa, Pakistan. Expert Rev Anti Infect Ther. (2020) 18:937–46. doi: 10.1080/14787210.2020.1769477
48. Sachdev C, Anjankar A, Agrawal J. Self-medication with antibiotics: an element increasing resistance. Cureus (2022) 14:e30844. doi: 10.7759/cureus.30844
49. Saleem Z, Moore CE, Kalungia AC, Schellack N, Ogunleye O, Chigome A, et al. Status and implications of the knowledge, attitudes and practices towards AWaRe antibiotic use, resistance, and stewardship among low- and middle-income countries. JAC-Antimicrobial Resistance (2025) 7(2):1–16. doi: 10.1093/jacamr/dlaf033
50. Haenssgen MJ, Xayavong T, Charoenboon N, Warapikuptanun P, Khine Zaw Y. The consequences of AMR education and awareness raising: outputs, outcomes, and behavioural impacts of an antibiotic-related educational activity in lao PDR. Antibiotics. (2018) 7:(4). doi: 10.3390/antibiotics7040095
51. Maarouf L, Amin M, Evans BA, Abouelfetouh A. Knowledge, attitudes and behaviour of Egyptians towards antibiotic use in the community: can we do better? Antimicrob Resist Infect Control. (2023) 12:50. doi: 10.1186/s13756-023-01249-5
52. Nayiga S, MacPherson EE, Mankhomwa J, Nasuwa F, Pongolani R, Kabuleta R, et al. “Arming half-baked people with weapons!” Information enclaving among professionals and the need for a care-centred model for antibiotic use information in Uganda, Tanzania and Malawi. Global Health Action. (2024) 17(1). doi: 10.1080/16549716.2024.2322839
53. Anstey Watkins J, Wagner F, Xavier Gómez-Olivé F, Wertheim H, Sankoh O, Kinsman J. Rural South African community perceptions of antibiotic access and use: qualitative evidence from a health and demographic surveillance system site. Am J Trop Med Hyg. (2019) 100:1378–90. doi: 10.4269/ajtmh.18-0171
Keywords: antimicrobial resistance, antimicrobial stewardship, primary healthcare, patient knowledge, attitudes, behaviors, South Africa
Citation: Ramdas N, Biyela T, Thema M, Sibanda M, Sono TM, Campbell SM, Schellack N, Godman B and Meyer JC (2025) Patient knowledge, attitudes and behaviors related to antimicrobial use in South African primary healthcare settings: development and testing of the CAMUS and its implications. Front. Trop. Dis. 6:1569076. doi: 10.3389/fitd.2025.1569076
Received: 31 January 2025; Accepted: 21 March 2025;
Published: 14 May 2025.
Edited by:
Annick Lenglet, University of KwaZulu-Natal, South AfricaReviewed by:
Celine Nguefeu Nkenfou, University of Yaounde I, CameroonGayathri Govindaraju, Rutgers, The State University of New Jersey, United States
Copyright © 2025 Ramdas, Biyela, Thema, Sibanda, Sono, Campbell, Schellack, Godman and Meyer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nishana Ramdas, bmlzaGFuYXJhbWRhc0BnbWFpbC5jb20=; Brian Godman, QnJpYW4uR29kbWFuQHN0cmF0aC5hYy51aw==
 Nishana Ramdas
Nishana Ramdas Thobani Biyela1
Thobani Biyela1 Mncengeli Sibanda
Mncengeli Sibanda Stephen M. Campbell
Stephen M. Campbell Natalie Schellack
Natalie Schellack Brian Godman
Brian Godman Johanna C. Meyer
Johanna C. Meyer