- 1Virology Department, Noguchi Memorial Institute for Medical Research, College of Health Sciences, University of Ghana, Legon, Ghana
- 2Disease Surveillance Department, Ghana Health Service, Ministry of Health, Accra, Ghana
- 3World Health Organization, Country Office, Accra, Ghana
Introduction: Ghana joined the Global Polio Eradication Initiative in 1996 to interrupt wild poliovirus transmission in the country. This was a collaborative effort by the Ghana Health Service (Disease Surveillance Department and the Expanded Program on Immunization) and the Polio Laboratory in the Noguchi Memorial Institute for Medical Research, University of Ghana.
Methods: The polio surveillance started from the southern regions and was extended to the northern regions over time. Surveillance officers were sensitized to improve case detection. The most important surveillance indicators—annualized non-polio AFP rate and stool adequacy—continued to improve, and the WHO targets for laboratory indicators of timeliness were met. The introduction of the oral polio vaccine in 1978 by the Expanded Program on Immunization led to a significant reduction in polio cases. The routine immunization coverage increased from 72% in 1999 to 94% in 2007, with an improvement in supplementary immunization activities.
Results: Molecular characterization of wild poliovirus from Ghana between 1995 and 2008 and vaccine-derived poliovirus from 2019 to 2022 revealed that the transmission of wild poliovirus and vaccine-derived poliovirus can be interrupted with active acute flaccid paralysis surveillance and adequate and efficient implementation of immunization activities. The country attained a polio- free status in 2015 after successfully submitting documentation to the Regional Certification Committee. Analysis of vaccine-derived polioviruses contributed to a better understanding of the poliovirus transmission, showing that the VDPV is indistinguishable from wild poliovirus and therefore poses a risk as a source of paralytic polio in a polio-free world.
Discussion: Ghana will sustain efforts to maintain polio- free status; intensify routine immunization to improve equity and OPV3 coverage; improve vaccine management and logistics; and enhance surveillance and outbreak preparedness, community engagement, and mobilization to eliminate the circulating vaccine-derived poliovirus. Furthermore, the country will strengthen partnerships with the WHO, UNICEF, CDC, Rotary International, and other stakeholders and secure dedicated funding to ensure consistent support for immunization and surveillance activities.
1 Introduction
Ghana is situated on West Africa’s Gulf of Guinea, with a latitude of 5°36′ north of the Equator and a longitude of 0°10′ east of the Greenwich Meridian. The total surface area of the country is 238,450 km2 and shares borders with the Republics of Togo to the East, Burkina Faso to the North, and Ivory Coast to the West. The South of the country is bounded by the Gulf of Guinea. Ghana has two distinct rainy seasons in May–June and August–September with a tropical climate. The population of Ghana as projected in the 2021 National Population and Housing Census (PHC) was 31,742,357. Of this, ~40% (13,009,835) were children under 15 years of age. Ghana has 16 administrative regions and 276 decentralized administrative districts involving a total of 770 health subdistricts. The geographical distribution of the population is shown in Figure 1.
Ofosu-Amaah and colleagues in 1976 conducted a search for poliomyelitis cases among school-aged children in Ghana using a postal survey, and they projected the prevalence of paralysis due to poliomyelitis to be 5–8 per 100,000 with a mean annual incidence of paralytic poliomyelitis of 23 per 100,000 population (1). The recent school children survey of lameness in schools by Opare et al. estimated 1.0/1,000 (2). Ghana introduced the Expanded Program on Immunization (EPI) in 1978 with six antigens [Bacillus Calmette–Guérin (BCG), diphtheria-pertussis-tetanus, measles, and polio] (3), and the program has been functioning in all regions since 1985 (4). Due to the early success of polio vaccines in reducing the incidence of poliomyelitis, the World Health Assembly passed a resolution in 1988 to eradicate poliomyelitis globally by the year 2000 (5). The African Ministers of Health adopted the resolution in 1995, urging member states to implement the Global Polio Eradication Initiative (GPEI). Ghana assumed the global target of polio eradication in 1996 and soon made significant progress (6, 7). As of September 2006, all 110 districts in the country had been polio-free for at least 3 years. This was made possible by the successful implementation of GPEI strategies. They included intensifying routine polio immunization; implementing supplementary immunization activities (SIAs) such as national, subnational, synchronized, and mop-up immunization campaigns; and introducing active surveillance for poliovirus. Active surveillance for cases of acute flaccid paralysis (AFP) syndrome, the most common clinical manifestation of paralytic poliomyelitis, with full laboratory support to detect poliovirus was formally established in Ghana in 1996 (6). A few performance indicators have been used to monitor the quality of AFP surveillance, and laboratory work is also subjected to a full accreditation process to assess the efficiency of isolation and characterization of poliovirus from AFP stool and lately from environmental samples.
Thirty-eight (38) wild-type 1 polioviruses (WPV-1) from clinical cases were confirmed in Ghana from 1995 to 1999, when it was thought that the last wild-type poliovirus had been seen. However, eight more WPV-1 poliovirus isolates were each identified in the country in 2003 and 2008 from AFP samples. In addition, 15 WPV-1 isolates were also isolated from a survey in the northern part of the country that involved 100 children in an area known to be of poor sanitation and low vaccination coverage. Additional national immunization days and mop-up activities followed, and no wild poliovirus has been detected in Ghana to date.
This paper offers a comprehensive overview of polio eradication efforts in Ghana, effectively highlighting the successes achieved, the challenges faced, and recommendations for further action. It showcases the polio immunization and surveillance in the country and the molecular analysis of wild-type 1 and circulating vaccine-derived poliovirus type 2 (cVDPV2) from Ghana during this period, exploring the relationships between themselves and other WPV-1 and cVDPV2 polioviruses isolated in the region of Africa and other parts of the world. The results shown here represent the first comprehensive polio story that covers the period between the establishment of AFP surveillance in a country endemic for polio and the complete interruption of WPV-1 and cVDPV2 poliovirus circulation. The information was used to catalog possible geographical transmission routes and detect poliovirus introduction events and the effect of vaccination and surveillance on poliovirus circulation.
2 Methodology
We collected historic and current data from the Disease Surveillance Department and the Expanded Program on Immunization of the Ghana Health Service, the Polio Laboratory, and publications from the public database. The data were systematically reviewed and validated through consultations with past and present program directors. The online literature review focused on published works on polio eradication activities of Ghana.
Verbal approval was granted by the Disease Surveillance Department and the Expanded Program on Immunization of the Ghana Health Service. We protected the confidentiality of patients by using codes.
2.1 Polio vaccination activity
2.1.1 Polio immunization
Ghana used the Sabin trivalent live-attenuated oral polio vaccine (tOPV) for all polio immunizations from 1974 to 2016, when it was changed to the bivalent oral polio vaccine (bOPV) following the global switch (8). Then, in 2018, the inactivated polio vaccine (IPV) was added to provide protection for type 2, which was lacking in the bOPV. The vaccines were supplied by the United Nations Children’s Fund (UNICEF) and the WHO. Since global polio eradication strategies were officially introduced in Ghana, improved routine immunization rates were soon achieved in the more developed southern regions and gradually reached acceptable levels in the northern districts with less well-established health systems. In the routine polio immunization program, four doses of live attenuated oral polio vaccine (OPV) are administered. The first polio vaccination (OPV0) is given at birth. The remaining three doses (OPV1, OPV2, and OPV3) are respectively given at 6 weeks, 10 weeks, and 14 weeks after birth. The IPV was introduced in June 2018 and has been administered at 14 weeks since its introduction. In 1995, the vaccine coverage reported for Ghana (based on a nationwide survey) was 85% for BCG (the anti-tuberculosis vaccine based on the Bacillus Calmette–Guerin), 71% for DPT3 (three doses of the triple vaccine against diphtheria, tetanus, and pertussis), 68% for measles, and 61% for OPV3 (9). Due to the low coverage, a minimum target of 75% DPT3/OPV3 coverage by 2001 was set as part of the medium-term health strategy of the Ghana Ministry of Health. By the year 2000, the health system had improved across the country. Routine OPV3 coverage rose from 61% in 1997 to 80% in 2000 and has since remained stable at around that rate. Supplementary polio immunization activities were instituted in Ghana in 1996 and included national and subnational immunization days (NIDs and sub-NIDs) during which large numbers of children less than 5 years of age were vaccinated with OPV on the same day. Between 1996 and mid-2000, the strategy used fixed vaccination posts that the children attended with their parents. However, from mid-2000 and 2005, NIDs have been carried out every year mostly on a house-to-house basis by vaccination teams trying to reach all targeted children.
In 2001, the WHO introduced synchronized NIDs, which allowed countries sharing borders to conduct NIDs on the same day. This ensures that children crossing borders for any reason on those days are identified and immunized. Coordinated planning also allows health teams to cross borders themselves to immunize children on an island that may be less accessible from the other side or those in pockets of territory otherwise isolated by rivers, mountains, or other impassable terrain. Most NIDs conducted in Ghana from 2001 were in the form of synchronized activities involving neighboring countries. On 23 February 2004, for example, 10 countries across West and Central Africa held simultaneous immunization campaigns, targeting 63 million children in Benin, Burkina Faso, Cameroon, Central African Republic, Ghana, Niger, Nigeria, Ivory Coast, Togo, and Chad. Political, religious, and local leaders teamed up to launch the activities, and tens of thousands of vaccinators went from house to house over 3 days to administer the vaccine directly to every child (10).
Table 1 summarizes the NID and sub-NID campaigns and routine polio immunization carried out in Ghana from 1996 to 2008, including the proportion of children immunized in each of the two rounds. The second rounds always covered more children than the first round, either because mothers who were not willing or able to have their children immunized did so because of the awareness created by the first round or because of overreporting. Although the national coverage has always been high, the overall immunization rate may hide the reality on the ground where the poorer and less privileged sections of many communities, as well as hard to reach areas, may have much lower coverage than those reported and may not be reached by the immunization teams at all (11). Bonsu and colleagues attributed poor coverage in these communities to poor knowledge about immunization, lack of suitable venues and furniture at outreach clinics, financial difficulties, poorly motivated service providers, and weak intersectoral collaboration (12). Supplementary immunization campaigns were significantly reduced in Ghana during 2002 and early 2003 due to limited financial resources for GPEI activities, which concentrated on immunization campaigns in the seven endemic areas and on maintaining high-quality surveillance in all countries. Consequently, only one sub-NID was carried out in Ghana in October 2002 and another in March 2003, resulting in reduced numbers of immunized children (Table 1). Following the isolation of wild poliovirus from eight AFP cases between February and September 2003, high-quality NIDs were resumed from October 2003 to December 2005. In 2006 and 2007, only one NID was done each year until there was another wild poliovirus outbreak in 2008 from eight AFP cases before the country conducted one round of NID in November followed by a sub-NID in December 2008 when NID activities in Ghana virtually stopped.
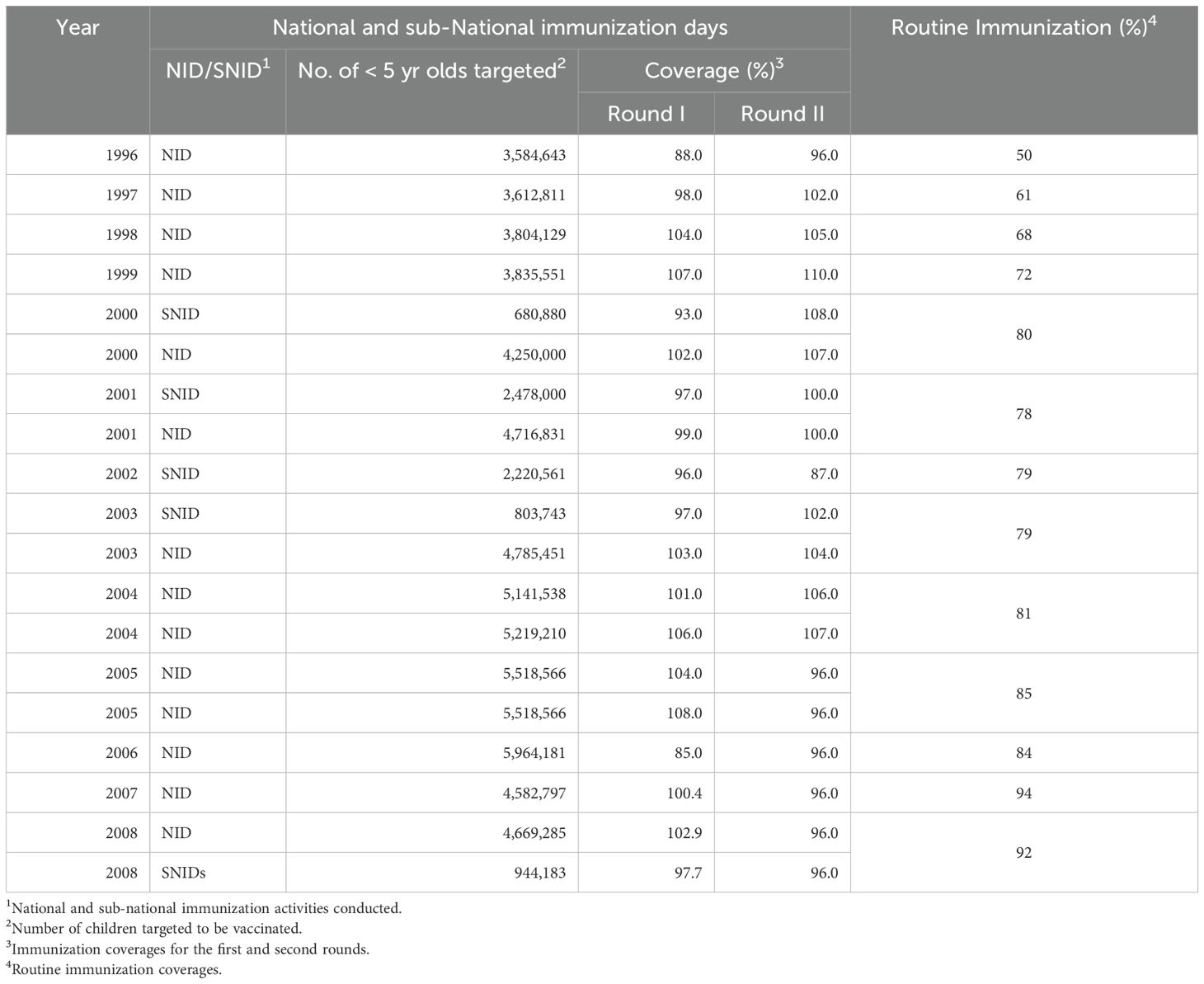
Table 1. Summary of polio immunisation activities in Ghana. 1996-2008 when wild poliovirus circulated in the country.
2.1.2 Switch from tOPV to bOPV
Following the certification of the eradication of WPV-type 2 by the Global Commission for the Certification of the eradication of poliomyelitis in 2015, the type 2 component in the tOPV remained redundant and has been responsible for over 90% of cVDPV2. To stop this, the eradication program decided to withdraw tOPV and replace it with bOPV and at the same time introduce the IPV (13, 14). The largest withdrawal of one vaccine and associated rollout of another vaccine in history was the switch from tOPV to bOPV in 2016 that affected over 150 countries.
Ghana switched from the use of tOPV to bOPV following the global switch in April 2016 (15). The implementation of the switch was done by first constituting a National Switch Planning Committee (NSPC) to work with the Ghana Health Service (GHS), the WHO, and UNICEF to plan the switch. To ensure a successful switch, 22 switch process monitors were also trained at the national level and deployed to all regions to assess the preparedness of the regions, districts, and health facilities and to train and build capacity. They were also trained to check documentation of the switch process at lower levels and observe vaccination sites post-switch. During the switch, public health nurses were identified to monitor the process together with coordinators. The initial global switch plan in 2013 was to introduce IPV (16) to mitigate the gap in immunity to poliovirus type 2 during the switch, but this was not met because of the global shortage of IPV (17, 18). This notwithstanding, the switch was carried out, and children born from that day received bOPV without any protection for poliovirus type 2. After the switch, the National Polio Certification Committee (NCC), the committee mandated to monitor the countrywide progress of polio eradication activities, was tasked to serve as the National Switch Validation Committee (NSVC). The role of the NSVC was to validate the switch by reviewing reports by independent monitors as well as other monitoring reports. The Committee assessed all monitoring and supervisory reports, as well as reports from independent monitors. The Committee also undertook field visits to selected regions, districts, and health facilities. Findings from the monitors indicated that there was no tOPV present in any of the facilities visited.
In June 2018, Ghana introduced the single dose of IPV (19) into the routine, making the new polio immunization schedule: OPV0 at birth, OPV1 at 6 weeks after birth, OPV2 at 10 weeks after birth, and OPV3 and IPV1 at 14 weeks after birth. At the time of the IPV introduction, approximately 2.7 million children across the country had received bOPV with immunity gap to poliovirus type 2. This buildup of susceptibility played a pivotal role in the first cVDPV2 outbreak in the country in 2019.
3 AFP surveillance in Ghana
Stopping poliovirus transmission has been pursued through a combination of routine immunization and supplementary immunization campaigns, which are guided by high-quality surveillance. Hence, the World Health Organization recommends that countries conduct AFP surveillance. In order not to miss any case of AFP, a broad case definition that includes Guillain–Barré syndrome, transverse myelitis, and transient paralysis associated with non-polio infections among children below 15 years and all cases of suspected poliomyelitis among persons of any age is used. The surveillance strategies also provide reliable information to monitor the quality of surveillance in a large population or an individual country.
AFP surveillance was established in Ghana in 1996 to detect wild-type poliovirus transmission and improve immunization strategies to eradicate poliomyelitis. The country currently has 261 districts with each implementing AFP surveillance to detect and report all cases of AFP and collect appropriate samples for laboratory investigation and follow-up. The Disease Surveillance Department of the Ghana Health Service manages AFP surveillance data. When poliomyelitis is suspected, clinicians collect data for any case of AFP in a child aged <15 years.
Like what occurred during the implementation of polio vaccination described above, AFP surveillance was initially started in the southern regions and was gradually introduced in the northern areas of the country. During the early years of AFP surveillance, the four most northerly regions, namely, Brong Ahafo, Northern, Upper West, and Upper East, accounting for ~28% of the total population in Ghana, only produced 13% and 5.2% of the total AFP cases in 1996 and 1997, respectively. However, from 1998, the situation improved with uniform numbers of AFP cases distributed nationwide.
When a patient is suspected of polio, much data including clinical and demographic are collected with every AFP case. Demographic data include name, age, sex, location, and names of parents as well as clinical data such as clinical symptoms and immunization history and technical aspects that affect the quality of stool sampling and laboratory analysis. The quality of AFP surveillance is evaluated with the data. The evolution of the more representative AFP and laboratory performance indicators in Ghana from 1995 to 2008 during the period when wild poliovirus circulated and the performance target for each indicator established by the WHO are shown in Figures 2, 3. The number of specimens received in the lab and the levels of performance were low during the initial stages of the program (Figure 2) due to the lack of inadequate trained personnel with sufficient knowledge. The infrastructure in the north was also not as good as it was in the south where relatively better human resources and a better health system existed. However, adequate levels were reached for most AFP performance indicators by the year 2000. Between 1996 and the year 2000, the WHO target for non-polio AFP rate was 1, but the country could not achieve it until 1999.
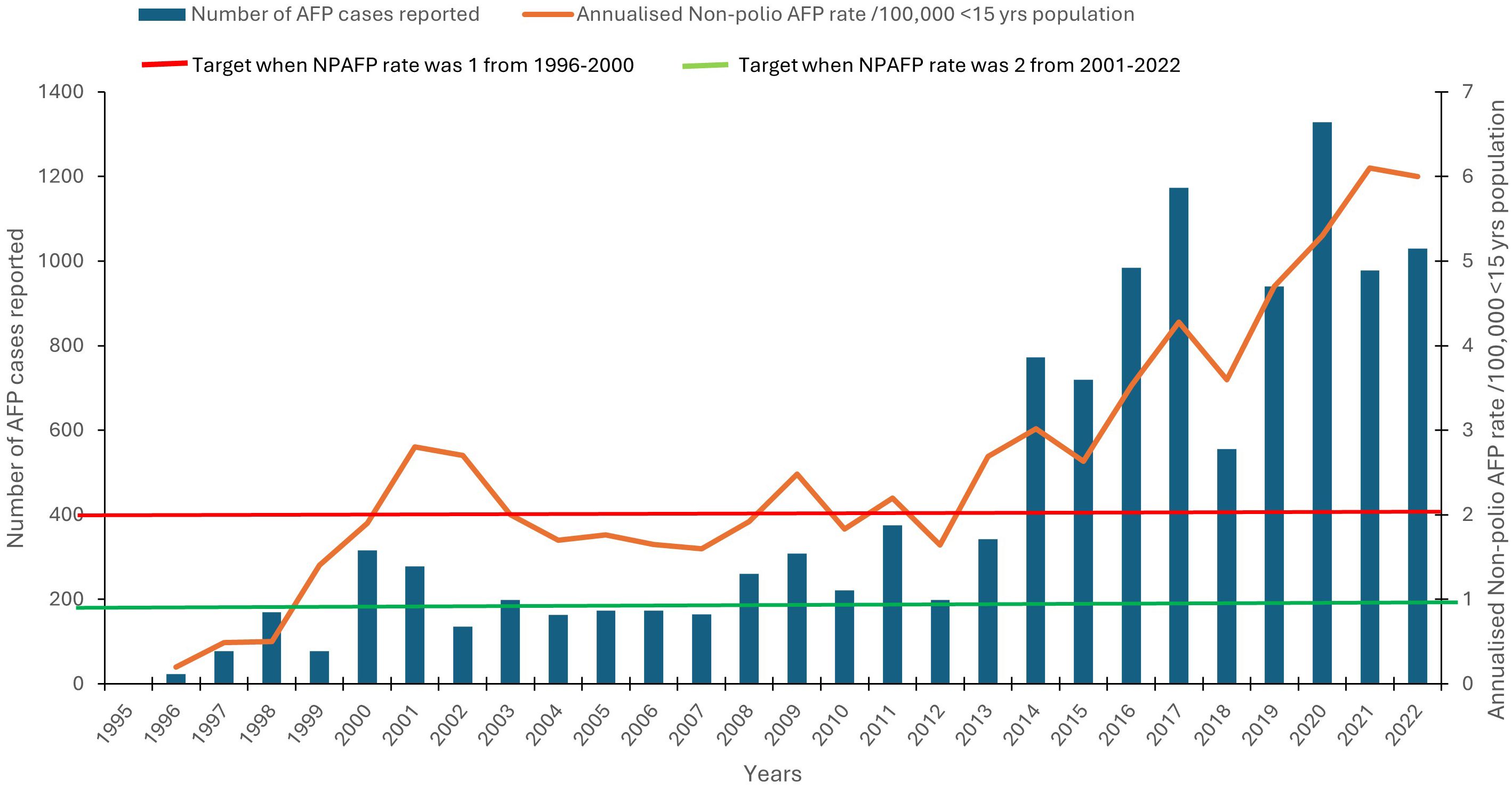
Figure 2. Annual specimens processed with the non-polio AFP rate from 1996 to 2022. The number of AFP cases (left axis) is indicated by dark bars, and the annualized non-polio AFP rate (right axis) is indicated by an orange line. The green bar represents the non-polio AFP rate of 1 between the period 1996 and 1999, and the red bar represents the target of 2 between the period of 2000 and 2008.
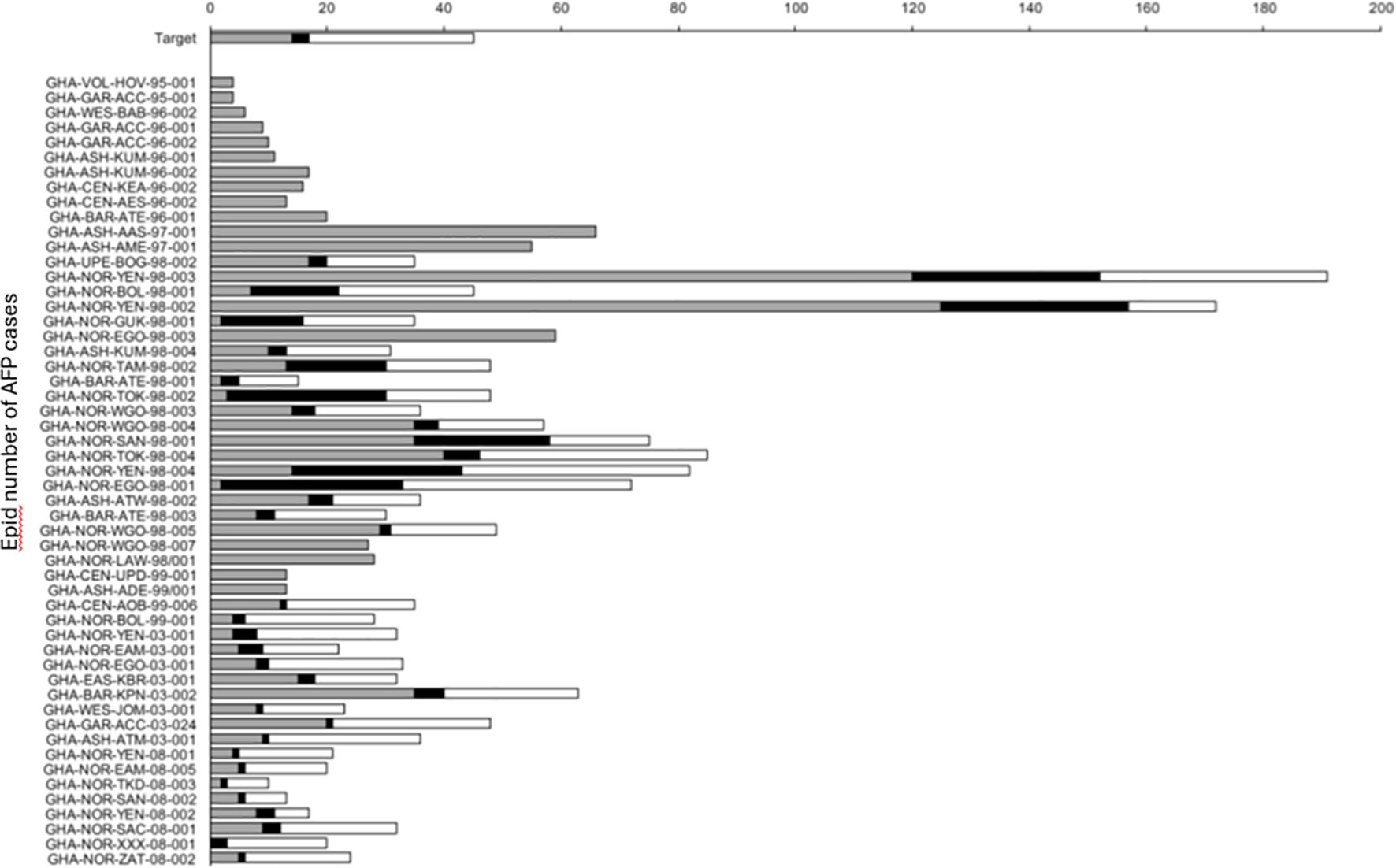
Figure 3. Quality indicators for poliovirus isolations in Ghana between 1995 and 2008. Gray bars indicate the time between onset of paralysis and collection of stool samples, black bars show time transport of stool samples to the laboratory and white bars indicate time for virus characterization. Satisfactory target times are 14, 3, and 28 days, respectively.
In addition, Ghana struggled to achieve the timely collection of two adequate stool specimens from each AFP case, one of the two most important quality markers for AFP surveillance. The target of 80% of AFP cases with two specimens collected within 14 days of onset of paralysis was achieved only from 2002 (Figure 4). The second most important AFP indicator, non-polio AFP rate, reached acceptable values from 1999 when the rate of AFP cases due to causes other than poliomyelitis was well above the WHO target of 1 per 100,000 children under 15 years of age (Figure 2). This indicator was later changed to 2 per 100,000 children under 15 years. Despite the occasional drop in one of the AFP indicators, Ghana has been able to maintain high levels of AFP surveillance performance during the last few years, ensuring an efficient surveillance system for poliovirus. In 2001, Ghana shifted from the clinical to the more advanced and specific virological classification scheme of AFP cases. The virological scheme, which relies on virological criteria for the final classification of AFP cases, is used at the advanced stages of polio eradication in those countries that meet and sustain a high level of AFP surveillance performance: non-polio AFP rate of at least 1/100,000 children under 15 years of age, adequate stool collection for at least 80% of AFP cases, and all specimens processed in a WHO-accredited laboratory.
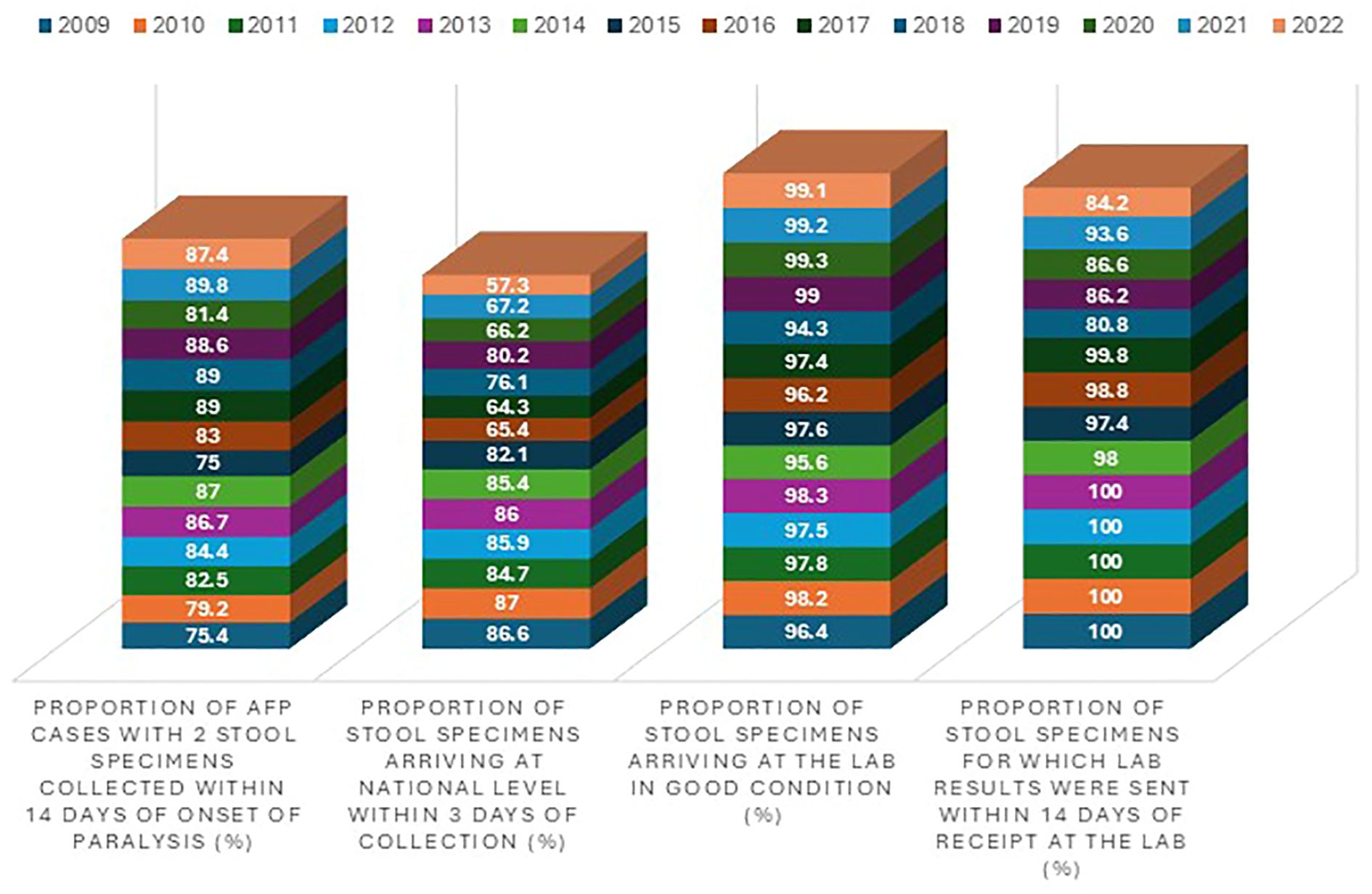
Figure 4. AFP surveillance quality indicators in Ghana from 2009 and 2022. The four blocks (from left to right) represent the stool adequacy, timeliness of stools arriving in the lab, stool condition, and timeliness of reporting results, respectively. The different colors (from bottom up) indicate the years from 2009 to 2022.
AFP surveillance has continued to improve in recent years in terms of the number of samples reaching the polio lab annually with the performance indicators as shown in Figures 2-4. Apart from the years 2010 and 2012 when the non-polio AFP indicator was not achieved, the remaining years recorded a range of 2.2 to 6.0. The turnaround time of 14 days exceeded the 80% target. No wild poliovirus was detected from 2009 to 2022; however, cVDPV2 was found in 2019, 2020, and 2022. Figure 5 shows the distribution of wild poliovirus isolated in the country from 1995 when the virus was first seen in the country to 2008 when it was last seen.
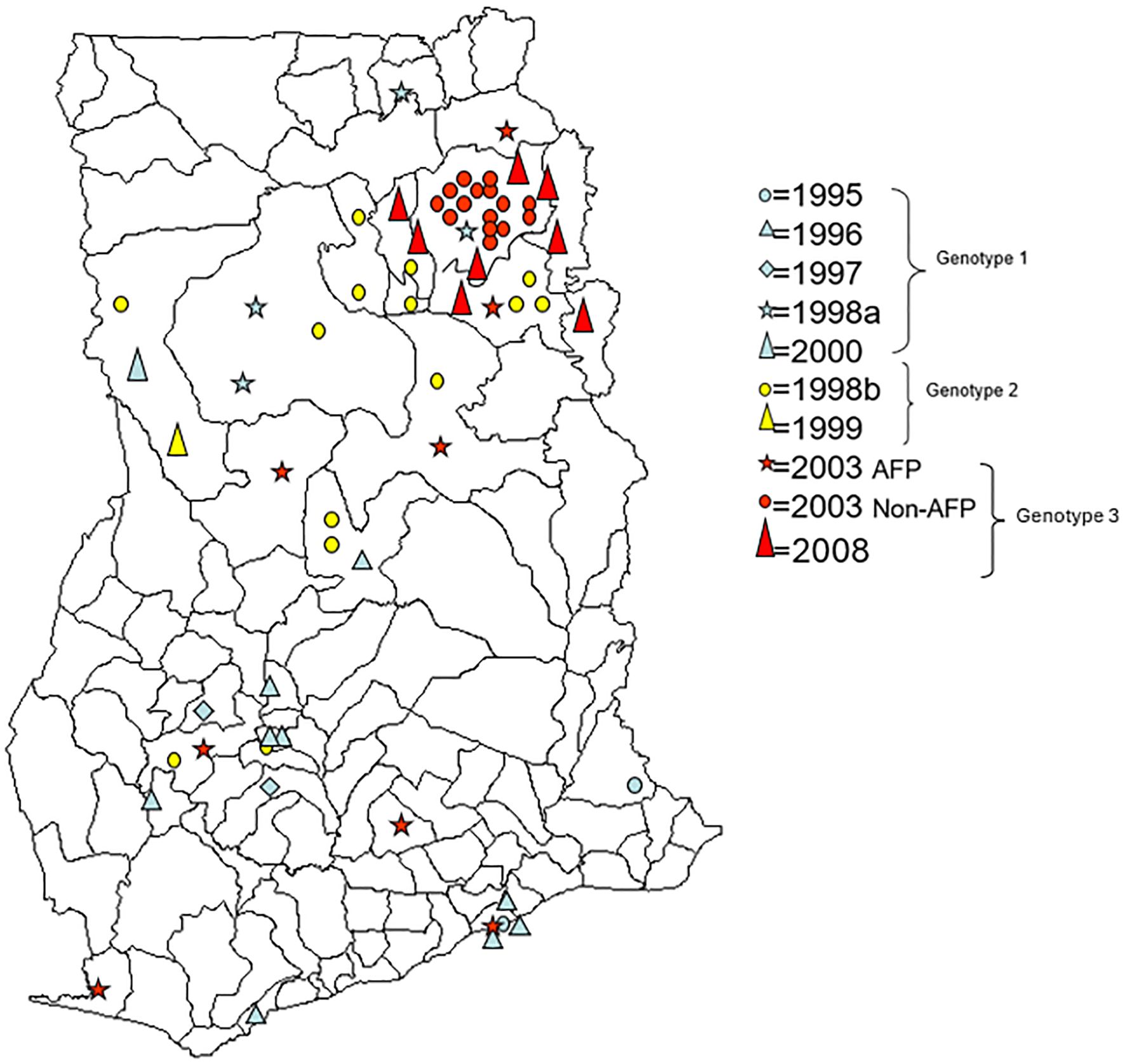
Figure 5. District map of Ghana showing the distribution of wild-type 1 strains isolated from 1995 to 2008. Isolates in red were Ghanaian samples belonging to West Africa genotype A, isolates in blue were Ghanaian samples belonging to West Africa genotype B, and isolates in green belong to West Africa genotype C.
4 Ghana attained a polio-free status
Ghana submitted its first documentation to the Regional Certification Committee (RCC) in 2007, 4 years after the successful interruption of wild poliovirus due to importation in 2003. As the process was ongoing in 2008, another wild poliovirus outbreak due to importation occurred in 2008 annulling the efforts made toward the polio-free status. With active surveillance and intensified immunization, the outbreak was interrupted in 2008. Since then, Ghana has maintained an active surveillance coupled with efficient and effective immunization with high coverage to sustain the gains. Subsequently, Ghana submitted another documentation to the RCC in 2014 and achieved a significant milestone by attaining a polio-free status in 2015.
5 Environmental surveillance for polio
Apart from AFP surveillance that links poliovirus to specific individuals and allows for a focused investigation of that individual and the immediate community at risk, environmental surveillance (ES), in the form of supplementary surveillance, has also become more crucial especially toward attaining eradication. The examination of composite human fecal samples through environmental surveillance links poliovirus isolates from unknown individuals to a population served by the wastewater system (WHO, 2003). Piloting of environmental surveillance for polio to test the country’s capacity to implement environmental surveillance to complement AFP surveillance was done at the polio reference laboratory in 2016 (20). In early 2016, the polio lab constituted a technical team with members from the Disease Surveillance Department, Polio Laboratory, and the Environmental Health to scout for environmental sites for polio surveillance. The selection criteria used by a technical assessment team to select the sewer lines and wastewater sites in respect of the two regions included the following: 1) whether based on existing data, the region is classified as high risk for poliovirus transmission (i.e., population density, high-risk population, sanitation, living conditions, routine immunization, and SIA coverage); 2) the presence or absence of sewer lines in the catchment area; 3) no evidence of industrial waste in the proposed site; and 4) suboptimal AFP performance indicators. The report of the Technical Assessment Team showed four high-risk districts in the Greater Accra region to be suitable for environmental surveillance. These were 1) Osu in the Osu-Klottey submetro with a population of 121,723, 2) Shiabu in Ablekuma submetro that had a population of approximately 213,914, 3) Agbogbloshie in Okaikoi submetro with a population of approximately 228,271, and 4) Legon in the Ayawaso West submetro with a population of 70,667. The Technical Assessment Team in consultation with the Local Government Regional Environment Health Services Department and Sanitary Offices obtained information on the sanitation for Greater Accra and sewer maps of the selected sites (Figures 6A, B). The team also found that the Accra Sewerage Improvement Project (ASIP) of the Accra Metropolitan Assembly (AMA) was renovating some non-functional sewage-pumping stations at the time.
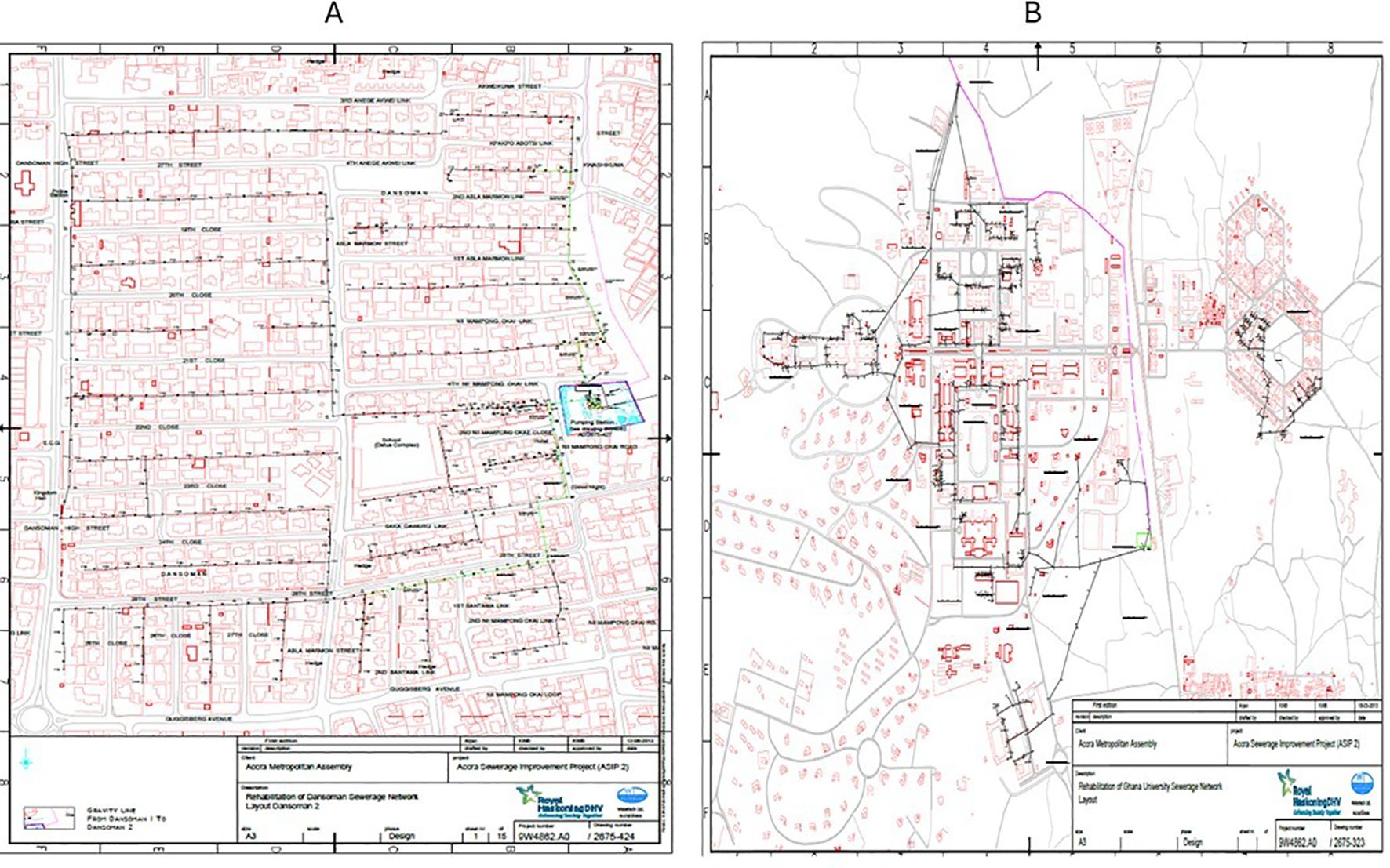
Figure 6. Sewer maps of the environmental surveillance site in Accra, Ghana. (A) The ES sewer map at Dansoman with a population of more than 210,000 contributing to the sewage. (B) The ES sewer map of the University of Ghana with a population of more than 100,000 draining into the sewage.
The feasibility assessment of the Eastern region revealed only one suitable site at Akosombo in the Asuogyaman District. It was a closed sewage channel system that served a population of approximately 17,000 inhabitants. Sewage from the residential areas drains into the channel through three smaller and medium-sized sewage channels. The second site in the Eastern region was a drainage that runs through many communities and receives wastewater from large areas within Koforidua township (Figures 7A, B).
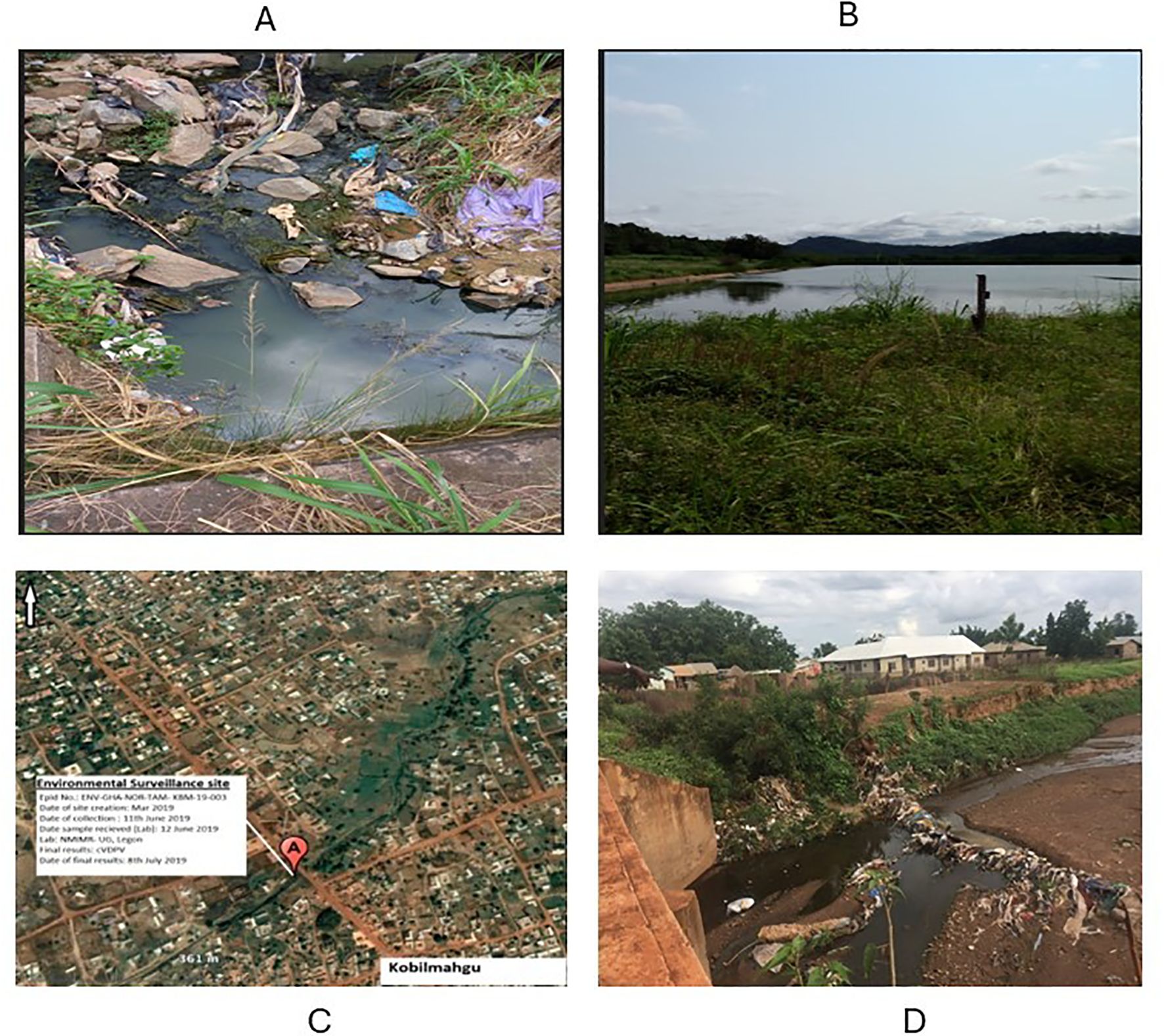
Figure 7. Environmental surveillance sites in the Eastern and Northern regions. (A) ES collection site (drainage) at the Nkubem, Koforidua in the New Juaben District with a population of 80,000. (B) The Akosombo ES site (sewage) in the Asuogyaman District with a population of over 30,000. (C) The settlement of Koblimahgu where the first cVDPV2 was detected in the country in 2019. (D) The Koblimahgu ES site where the first cVDPV2 was detected.
Ghana conducted the pilot study in 2016 with the support from the WHO Country Office and found environmental surveillance to be feasible. The pilot was done with six ES sites: four in Greater Accra and two in the Eastern region (20). The WHO reviewed the report and recommended that Ghana should process ES samples for Mali in 2017. With the support of the Ghana PolioPlus Committee, Ghana rolled out ES in October 2018 with 10 sites in Greater Accra (4), Eastern (2), Volta (2), and Northern (2). During the middle of 2019, the first ever cVDPV2 was detected in one of the two ES sites in the northern region (Figures 7C, D). Later, the virus was detected in AFP samples as well as other ES sites in Greater Accra and Northern and Eastern regions (21, 22). In 2020, the ES sites were increased from 10 to 14 by the addition of Ashanti (2), Bono (1), and Bono East (1) regions.
Furthermore, a second cVDPV2 outbreak occurred in the country in 2022 with the first detection from the two ES sites in the northern region. The virus was later found in two sites in Greater Accra, one site in Ashanti, and the site in Bone East as well as three AFP cases. The detection of cVDPV2 in the ES sites always serves as an early warning for surveillance and EPI to adequately prepare to respond to the outbreak. The ES sites are evaluated annually with the support of the WHO-AFRO. During the evaluation, coordinators from the local and WHO-AFRO conduct desk review, visit the sites and interact with the sample collectors and supervisors, review the sites based on the performance, and have a review meeting with the site teams. The site characteristics are also reviewed.
6 Laboratory
The WHO established a strong global polio laboratory network in over 150 laboratories in 1989 that work hand in hand with the field surveillance for polio to enhance the eradication of the polio program. The laboratory network has three arms: national, regional, and specialized laboratories. The laboratories at all levels have well-defined responsibilities that are assessed annually by a comprehensive accreditation process to ensure that the laboratories are fully accredited to meet WHO requirements. The Polio Laboratory in the Noguchi Memorial Institute for Medical Research (NMIMR) was started by Dr. Osei-Kwasi Mubarak in the mid-1980s when he brought polio cell lines and seed viruses from Germany to commence tissue culture and virus isolation activities. There were initial challenges to characterize poliovirus isolates as wild or Sabin as a result of a lack of appropriate reagents (23). However, techniques improved in the following years with the availability of reagents, and the polio laboratory was able to successfully identify polioviruses in a panel of unknowns sent to the lab by the WHO. Following this performance, the WHO in 1992 designated the polio laboratory of the Institute as the first World Health Organization Regional Reference Polio Laboratory (WHO-RRPL) in the African Region. The polio laboratory was chosen as a center to help build the Polio Laboratory Network in the Africa region from 1992 to 2000 and subsequently became a Centre of Excellence for training programs on the laboratory diagnosis of polio, measles, and yellow fever. In 1995, the Institute isolated and well-characterized the first two documented poliovirus isolates in Ghana from the Greater Accra and Volta regions (22). When AFP surveillance started in Ghana in 1996, the polio laboratory was strengthened to support the polio surveillance activities. The laboratory was set by the WHO to act as the National Laboratory for Benin, Ghana, and Togo as well as the Regional Reference Laboratory for countries such as Benin, Ghana, and Togo; however, currently, it supports Liberia, Mali, Niger, and Sierra Leone. The polio lab has also supported Nigeria, Uganda, Guinea, the Democratic Republic of Congo, and Zambia in the past. The main role of the laboratory is the isolation and intratypic characterization of polioviruses from AFP samples to determine their wild or vaccine origin. Since 1996, the polio lab has investigated thousands of AFP cases from all these countries and has remained fully accredited except for the years 2000 and 2004 when it was provisionally accredited.
7 Wild poliovirus outbreak in Ghana
Following the introduction of active surveillance and strengthening of the polio laboratory in 1996, eight epidemic cases associated with wild-type 1 poliovirus were seen in 1996. Three of the eight cases clustered in Greater Accra with patients having asymmetrical paralysis, but their OPV status was unknown. In 1997, two cases occurred in the Ashanti region. The routine immunization was intensified, and a national immunization day was conducted that year to interrupt poliovirus transmission. This notwithstanding, there was a polio outbreak in 1998 between June and November leading to 21 cases. Two of the cases occurred in Kumasi and Atwiman both in the Ashanti region, 2 cases occurred in Atebubu in the Brong Ahafo region, 1 in Lawra in the Upper West Region, and 16 cases clustered in the Northern region presumably because of increased contact and poor hygienic conditions. The last indigenous case has the date of onset in October 1999 from a 13-year-old boy in the Bole District in the Northern region. No cases associated with wild poliovirus were seen for almost 3 years after that.
Over 3 years of being polio-free, when the country was preparing the documentation for the Regional Certification Committee to be given the polio-free status, another outbreak occurred. Between February and September 2003, eight wild-type 1 polioviruses were isolated from AFP cases scattered throughout the country. In addition, in April 2003, the polio laboratory isolated 15 wild-type 1 polioviruses from 100 pairs of samples collected from Gushegu District in the Northern region as part of their annual enterovirus survey. Additional NIDs and mop-up activities followed and transmission was interrupted. Although a definite cause for the 2003 outbreak was not found, it was possible that reduced immunization campaigns might have contributed to a decrease in population immunity against polio. Due to limited financial resources for GPEI activities, only two sub-NIDs were carried out in Ghana in October and November 2002 and when approximately 2 million children each, representing 44% of the entire children population, were immunized, respectively. Routine immunization remained stable at approximately 80% since 1996, and although it may have limited the extent of the 2003 outbreak, it was insufficient to prevent poliovirus circulation. The age distribution of the 2003 polio cases showed an increase in the 0–1-year-old age group compared with 1995–2000 when the age distribution of polio cases was closer to the actual age distribution in the population. This would suggest that the circulation of wild-type 1 poliovirus in 2003 in Ghana was limited and mainly affected unvaccinated or poorly vaccinated children, the proportion of which had increased due to a reduction in immunization campaigns.
The last wild poliovirus outbreak occurred in 2008 with eight cases confined to the Northern region. Two rounds of effective and efficient NIDs were conducted, and since then, no wild poliovirus has been seen in Ghana.
In all, 63 wild-type 1 polioviruses representing 91.3% (63 of 69) of the total number of wild-type 1 viruses isolated in Ghana during the period 1995–2008 have been characterized. No VDPV, wild-type 2 or 3 polioviruses were isolated in Ghana during the period. As shown in Figure 8, three different genotypes of wild-type 1 poliovirus circulated in Ghana from 1995 to 2008. The sequence analysis also showed the possibility that frequent recombination events occurred during the evolution of wild-type polioviruses in Ghana (Figure 8).
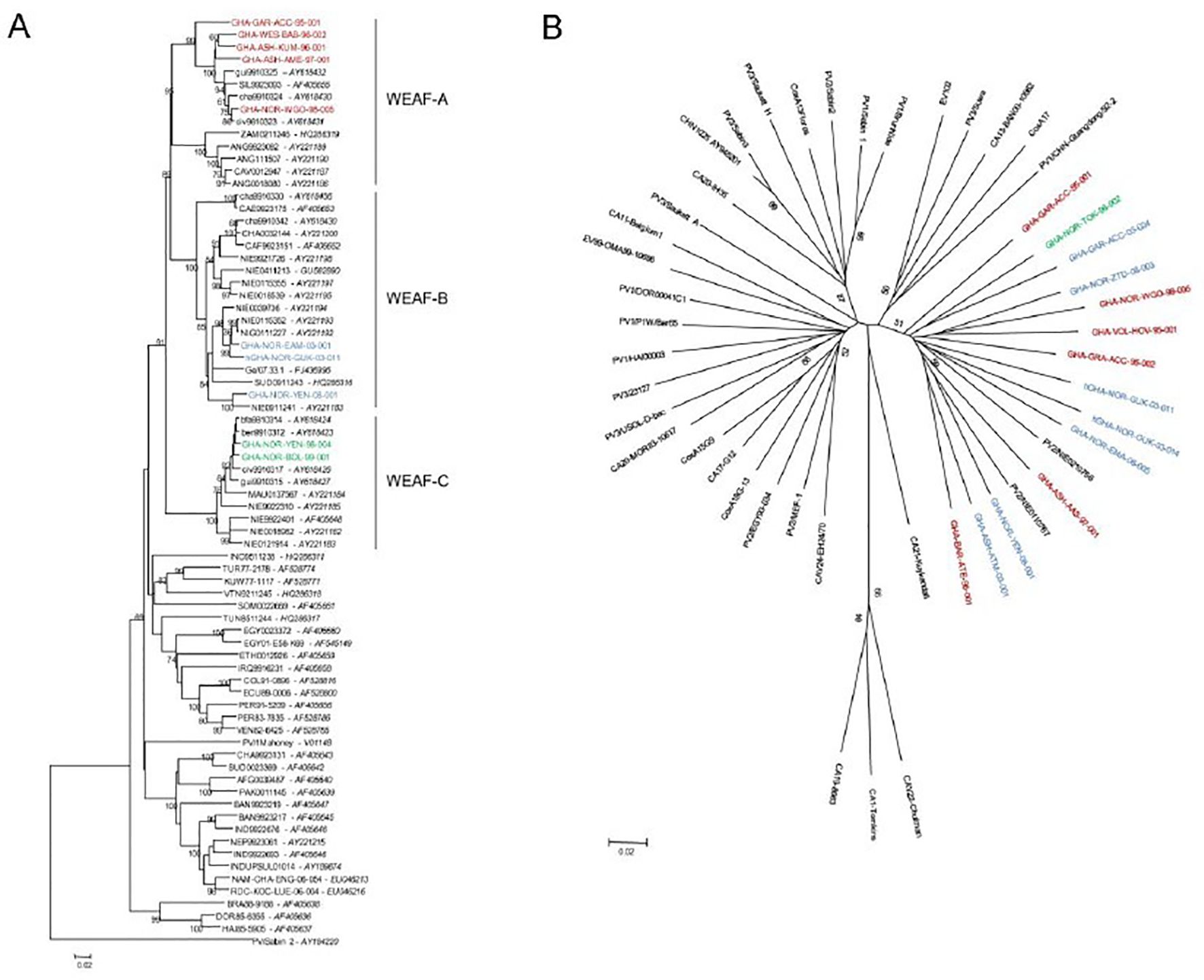
Figure 8. Neighbor-joining trees representing phylogenetic relationships between selected wild-type 1 poliovirus strains isolated in Ghana between 1995 and 2008 and other human enterovirus C strains isolated around the world. (A) VP1 coding region (nucleotides 2,480–3,385) of wild-type 1 polioviruses. (B) Partial 3CD coding region (nucleotides 5,846–6,478) of polio and non-polio human enterovirus C strains.
8 VDPVs
8.1 Circulating vaccine-derived poliovirus outbreak in Ghana
The first cVDPV2 was detected on 8 July 2019 following a collection on 11 June 2019 at the Kublimahgu ES site in the Vittin subdistrict of the Tamale Metropolis in the Northern region. Approximately 45% of the population was children under 15 (126,283 out of 280,629). The site where the sample was isolated is a large drain stretching for approximately 5 km (Figure 7D). Children defecate around the drain, and the rainy season stretches from May to September (peak in July/August). The cVDPV2 had the closest match of 99.23% similar to the Nigerian AFP strain NIE-KWS-KSB-18-006 that circulated in 2018.
While the investigation was ongoing, another cVDPV2 was detected from a case of AFP from Chereponi. The polio lab received the AFP samples on 8 August 2019 and processed them for virus isolation. The isolate obtained was molecularly characterized by real-time reverse transcription polymerase chain reaction as poliovirus type 2 (PV2). Further characterization by genetic sequencing of the VP1 region revealed vaccine-derived poliovirus type 2 (VDPV2) with 25 nucleotide difference from the reference Sabin 2 strain and 6 nucleotide difference from the cVDPV strain isolated from the environment. The closest match determination was linked to the 2018 Nigerian cVDPV2 strain NIE-KWS-KSB-18-006HC29 isolated from an AFP case with 6 nucleotide difference (99.34% similarity). The virus later spread to seven environmental surveillance sites from the north to the south of the country and in several AFP cases and their contacts.
8.2 cVDPV2 outbreak response
With the declaration of the cVDPV2 outbreaks, the Director General (DG) of the Ghana Health Service (GHS), with the support of polio partners, formed a multidisciplinary, multisectorial team from the national level drawn from the Ghana Health Service, Disease Surveillance Department, Expanded Program on Immunization, Polio Laboratory, Ghana Field Epidemiology Laboratory Training Program, World Health Organization, Centers for Disease Control and Prevention, and UNICEF to support the region and affected district. The terms of reference (TORs) for the team were a) to assess the potential for a polio outbreak by conducting a desk review of vaccination coverage, reviewing the immunization system, appraising local surveillance system, conducting case search for AFP, determining at risk groups, assessing the risk of spread of the virus, and assessing regional response preparedness; b) to recommend appropriate immediate response strategies to prevent future outbreaks; c) to collect additional sewage samples for testing; and d) to review the environmental surveillance system including assessment for additional sites. While on the field, the teams visited health institutions and facilities for record review, interview, and discussions. A community survey was conducted by visiting households and the drain site to collect stool samples from healthy individuals under 5 years of age and sewage samples from different points of the drain. Other activities implemented included social mobilization; case management; infection prevention and control, water sanitation and hygiene (WASH); immunization survey; AFP surveillance; and assessment of community knowledge, attitude, and practices on polio using assessment tools. Key findings and recommendations are presented to the National Coordinating Committee, chaired by the DG of GHS, and together with the polio partners, they take the appropriate steps.
Based on the decisions of the Coordinating Committee, polio partners, and the National Immunization Technical Advisory Group (NITAG) on the cVDPV2 outbreak from 2019 to 2020, Ghana conducted three phases of monovalent oral polio vaccine type 2 (mOPV2) campaigns to cover the entire country from 2019 to 2020. The phase 1 mOPV2 campaign covered 73 districts within the Upper East, Greater Accra, and the Northern regions. The second phase campaign was from December 2019 to February 2020, and it covered 38 districts. Three rounds of mOPV2 campaigns were conducted during the phase 3 response in 179 districts covering 8 of the 16 regions to interrupt the transmission of cVDPV2 in 2020. The regions included Greater Accra, Ashanti, Volta, Eastern, Western, Western North, Central, and Upper West regions. This notwithstanding, the country experienced another cVDPV2 outbreak in 2022 which was interrupted by two rounds of NID with the novel oral polio vaccine type 2 (nOPV2) campaigns to cover the entire country. This completely interrupted the transmission, and the virus has since not been seen. The routine OPV3 and IPV coverage in 2022 was 90% and 101%, respectively.
8.3 Limitations
We were unable to interact with past and current regional directors for their input, which could lead to loss of some information.
9 Outlook for polio eradication in Ghana
9.1 Improving immunization coverage
Ghana has made significant progress in polio immunization since its introduction. However, gaps remain, including regional disparities, rural–urban inequalities, and declining immunization coverage, which need to be addressed to improve routine and supplemental immunization efforts (24, 25). Immunization vulnerabilities like low immunity in some areas and the risk of cVDPV2 are key areas that need attention. The vaccine cold chain management must be strengthened to ensure proper storage and transportation. This will ensure potent vaccine availability to improve distribution and reduce stockouts. Enhanced health facility infrastructure must be ensured to upgrade facilities, equipment, and staffing as well as train healthcare workers to improve vaccination skills and knowledge. Another strategy will be to improve public awareness campaigns by educating communities on vaccine benefits and engaging the community through traditional leaders, community groups, and influencers, as well as addressing vaccine hesitancy through counter misinformation, managing concerns, and offering rewards for vaccinated children. Strategies involving outreach services, such as conducting vaccination in hard-to-reach areas, schools, and markets, and mobile vaccination teams that target underserved populations must be implemented. Integrated services that offer vaccination with other health services and appointment reminders to improve follow-up vaccination rates must be encouraged.
Ghana should consider signing on to the Global Electronic Vaccination Registry (EVR) Initiatives, which are international efforts to support the development, implementation, and scalability of electronic vaccination registries. These initiatives aim to improve vaccination data management, enhance immunization coverage, and promote global health security. This will allow the Expanded Immunization Program to develop EVR to capture patient registry, manage vaccination schedule, and send automated reminders. The vaccination records will be tracked electronically at the facility level to improve registry accuracy, accessibility, and efficiency and reduce paperwork.
9.2 Improving surveillance
Ghana has made significant accomplishments in polio surveillance. Lessons from the outbreak of the circulating vaccine-derived polio virus type 2 indicate the risks posed to Ghana’s polio eradication efforts when other aspects of polio eradication efforts fail. Therefore, Ghana’s polio eradication efforts must be extended beyond the health sector with polio and other water-borne diseases given prominence in addressing access to potable water. Given the overwhelming evidence of the presence of the virus in the environment from the detection of the virus in multiple environmental polio surveillance sites, there is a need to strengthen non-health sector-related issues. These include improving access to potable water to reduce the risk of ingestion of the virus that may be shed into the environment by some persons who may have been exposed. Where the opportunity for ingesting contaminated water is eliminated, the risk of polio transmission can be significantly reduced. Beyond the provision of potable water, sanitation and hygiene services must be spread to cover all communities and households in Ghana. Improved human waste management and proper disposal of human excreta with no risk of such waste entering the water consumption system is a sure way to eliminate any risk of exposure to the virus and hence reduce any risk of polio virus infection. Within the health sector, active efforts at risk communication and community engagement with clearly articulated and context-specific messaging to all communities and individuals can alter behavior and reduce the risk of disease transmission and contribute to the eradication of polio.
9.3 Surveillance monitoring
Though Ghana has made significant progress in the polio eradication initiative, some gaps and vulnerabilities still exist that need to be addressed. These include subnational surveillance performance, case detection and investigation, specimen collection and transportation, and environmental surveillance. These gaps can affect the quality of surveillance across the different regions and compromise the quality of surveillance data as well and the stool adequacy and non-polio AFP quality indicators. As a result, the polio surveillance system in the country must be further strengthened by enhancing the reporting and investigation of suspected cases (26). This can be achieved by ensuring timely and complete reporting of AFP cases; expansion of surveillance sites to include private hospitals, clinics, and traditional healing centers; and training of health workers to enhance AFP detection and reporting skills. The community-based surveillance must also be strengthened to engage community volunteers to report AFP cases and collaborate with traditional leaders to promote surveillance awareness. Cross-border surveillance must also be strengthened by collaborating with neighboring countries to share intelligence and best practices to enhance border surveillance to monitor travelers and migrants. There should be regular supervisory visits to ensure quality surveillance and the evaluation of key performance indicators to identify areas of improvement. The ES for polio must also be enhanced to monitor wastewater for poliovirus. Additional ES sites must be identified in high-risk areas, focusing on densely populated districts, areas with low vaccination coverage, and locations with previous polio outbreaks. Community engagement must be increased to educate the public on the importance of wastewater surveillance. A regular review meeting to assess AFP and ES surveillance performance must be instituted.
Regular training at all levels must be organized to update immunization and surveillance officers’ skills. Mentorship programs should be instituted to afford new participants in the surveillance and immunization programs the opportunity to obtain knowledge and skills. Dedicated funding to ensure consistent support for immunization and surveillance activities and resource allocation must be efficiently distributed, prioritizing high-risk areas.
The current surveillance data management system for Ghana, the Surveillance Outbreak Response Management Analysis System (SORMAS), is a well-developed robust health information system from the Helmholtz Centre for Infection Research in Germany for data-driven decision-making (23). The system provides real-time electronic surveillance of outbreaks, especially in resource-limited settings. Given that it is deployed nationwide, it has allowed data managers in the field to key in patient information, the laboratory to add on with laboratory data and results, and district directors to log in to obtain their results. There is a need to establish data governance policies and procedures to guide the usage to ensure data quality, integrity, and security for surveillance data in Ghana including that for polio.
In sustaining the gains and making more strides in the polio eradication effort, the Government of Ghana should continue to work with donor partners, civil society organizations (CSOs) and non-governmental organizations (NGOs), private sector engagement, and national and international collaborations. Ghana Health Service’s (GHS) Immunization Program, EPI, must come out with a National Vaccine Deployment Plan to continue working with the Global Alliance for Vaccines and Immunization (GAVI) for support. They should also work with the WHO, UNICEF, and CDC for technical assistance. These, in addition to strengthening immunization activities and surveillance (both human and environmental), are critical in Ghana’s polio eradication efforts.
10 Conclusion
With the strong routine immunization and effective surveillance in place and the rapid response to wild poliovirus and cVDPV2 outbreaks, Ghana has performed well in its effort to eradicate polio. However, adequate steps are needed to sustain efforts to maintain polio-free status by closing immunization and surveillance gaps to enhance surveillance and outbreak preparedness. Furthermore, the country needs to strengthen partnerships with the WHO, UNICEF, CDC, Rotary International, and other stakeholders and secure dedicated funding to ensure consistent support for immunization and surveillance activities.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The requirement of ethical approval was waived by Noguchi Memorial Institute for Medical Research IRB for the studies involving humans. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board also waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin.
Author contributions
JO: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Formal Analysis, Software. DL: Data curation, Investigation, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing, Visualization. NN: Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Resources. KA: Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. MA: Data curation, Investigation, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing, Formal Analysis. ED: Formal Analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CA: Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. EG: Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. IB-N: Data curation, Investigation, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. PA: Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. AD: Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. JB: Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. JM: Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. CO: Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. SB: Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. DO: Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. GA: Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. NO: Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. FA-B: Investigation, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. EO: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We are most grateful for the technical support provided by the polio team. Special thanks to the National EPI, Disease Surveillance Department, and the Department of Virology, Noguchi Memorial Institute for Medical Research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ofosu-Amaah S, Kratzer JH, and Nicholas DD. Is poliomyelitis a serious problem in developing countries? lameness in Ghanaian schools. Br Med J. (1977) 1:1012–4. doi: 10.1136/bmj.1.6067.1012
2. Opare JKL, Odoom JK, Akweongo P, Afari EA, and Pappoe M. Poliovirus antibody levels and lameness among individuals in three regions of Ghana. Hum Vaccin Immunother. (2019) 15:2050–9. doi: 10.1080/21645515.2019.1637235
3. W.H.O. Immunization program comprehensive multi-year plan (2010-2014). In: In line with Global Immunization Vision Strategies. EPI in Ghana. (2011) Accra, Ghana: Ministry of Health, Ghana. p. 11.
4. Kim-Farley R. Global immunization. The expanded program on immunization team. Annu Rev Public Health. (1992) 13:223–37. doi: 10.1146/annurev.pu.13.050192.001255
5. W.H.O. Global eradication of poliomyelitis by the year 2000. In: The 41st World Health Assembly. World Health Organization, Geneva, Switzerland.
6. Centers for Disease Control and Prevention. Tracking progress toward global polio eradication-worldwide, 2009–2010. Morbidity Mortality Weekly Rep. (2011) 60:441–5.
7. W.H.O. Polio laboratory manual. 4th edition. Geneva, Switzerland: World Health Organization (2004).
8. Tevi-Benissan C, Okeibunor J, du Chatellier GM, Assefa A, Biey JN, Cheikh D, et al. Introduction of inactivated poliovirus vaccine and trivalent oral polio vaccine/bivalent oral polio vaccine switch in the African Region. J Infect Dis. (2017) 216:S66–75. doi: 10.1093/infdis/jiw616
9. Global Technical Consultative Group on Global Eradication of Poliomyelitis. Meeting (2nd: 1998: Geneva SWEPoI. Global eradication of poliomyelitis: report of the second meeting of the Global Technical Consultative Group (TCG). Geneva: World Health Organization (1998).
10. West Africa prepares for the final battle against polio Vol. 82. Geneva, Switzerland: Bulletin of the World Health Organization (2004). p. 237.
11. Atkinson SJ and Cheyne J. Immunization in urban areas: issues and strategies. Bull World Health Organ. (1994) 72:183–94.
12. Bosu WK, Ahelegbe D, Edum-Fotwe E, Bainson KA, and Turkson PK. Factors influencing attendance to immunization sessions for children in a rural district of Ghana. Acta Trop. (1997) 68:259–67. doi: 10.1016/S0001-706X(97)00094-6
13. Garon J, Seib K, Orenstein WA, Ramirez Gonzalez A, Chang Blanc D, Zaffran M, et al. Polio endgame: the global switch from tOPV to bOPV. Expert Rev Vaccines. (2016) 15:693–708. doi: 10.1586/14760584.2016.1140041
14. Patel M, Zipursky S, Orenstein W, Garon J, and Zaffran M. Polio endgame: the global introduction of inactivated polio vaccine. Expert Rev Vaccines. (2015) 14:749–62. doi: 10.1586/14760584.2015.1001750
15. Odoom JK, Dzotse EK, Nii-Trebi NI, Opare D, Akyereko E, Attiku K, et al. Outbreak response to circulating vaccine-derived poliovirus in three northern regions of Ghana, 2019. BioMed Res Int. (2024) 2024:5515777. doi: 10.1155/2024/5515777
16. Bassey BE, Braka F, Vaz RG, Komakech W, Maleghemi ST, Koko R, et al. The global switch from trivalent oral polio vaccine (tOPV) to bivalent oral polio vaccine (bOPV): facts, experiences and lessons learned from the south-south zone; Nigeria, April 2016. BMC Infect Dis. (2018) 18:57.
17. Sutter RW and Cochi SL. Inactivated poliovirus vaccine supply shortage: is there light at the end of the tunnel? J Infect Dis. (2019) 220:1545–6. doi: 10.1186/s12879-018-2963-60
18. W.H.O. Polio Eradication & Endgame Strategic Plan 2013-2018. Geneva, Switzerland: World Health Organization (2013). Available at: https://polioeradication.org/wp-content/uploads/2016/07/PEESP_EN_A4.Pdf (Accessed June 4, 2024).
19. W.H.O. Ghana adopts use of Inactivated Polio Vaccine Ghana News Agency. Accra, Ghana: Business Ghana (2020). Available at: https://www.businessGhana.com/ (Accessed July 9, 2024).
20. Odoom JK, Obodai E, Diamenu S, Ahove V, Addo J, Banahene B, et al. Environmental surveillance for poliovirus in greater accra and eastern regions of Ghana-2016. Virol Curr Res. (2017) 1:101.
21. Odoom JK, Obodai E, Boateng G, Diamenu S, Attiku K, Avevor P, et al. Detection of vaccine-derived poliovirus circulation by environmental surveillance in the absence of clinical cases. Hum Vaccin Immunother. (2021) 17:2117–24. doi: 10.1080/21645515.2020.1852009
22. Odoom JK, Forrest L, Dunn G, Osei-Kwasi M, Obodai E, Arthur-Quarm J, et al. Interruption of poliovirus transmission in Ghana: molecular epidemiology of wild-type 1 poliovirus isolated from 1995 to 2008. J Infect Dis. (2012) 206:1111–20. doi: 10.1093/infdis/jis474
23. Yeboah-Manu D, Odoom JK, Osei-Wusu S, Adoma-Boakye A, Osae-Amoako G, Asante-Poku A, et al. From research to health policy: The Noguchi story in the past, present and next 25 years. Front Trop Dis. (2023) 4. doi: 10.3389/fitd.2023.1135354
24. Yawson AE, Bonsu G, Senaya LK, Yawson AO, Eleeza JB, Awoonor-Williams JK, et al. Regional disparities in immunization services in Ghana through a bottleneck analysis approach: implications for sustaining national gains in immunization. Arch Public Health. (2017) 75:10. doi: 10.1186/s13690-017-0179-7
25. Asuman D, Ackah CG, and Enemark U. Inequalities in child immunization coverage in Ghana: evidence from a decomposition analysis. Health Economics Rev. (2018) 8:9. doi: 10.1186/s13561-018-0193-7
26. Available online at: https://www.who.int/about/accountability/results/who-results-report-2020-mtr/country-story/2020/strengthening-Ghana-s-health-system-in-response-to-a-poliovirus-outbreak (Accessed April 29, 2025).
Keywords: polio eradication, immunization, surveillance, laboratory, Ghana
Citation: Odoom JK, Laryea DO, Ntim NAA, Attiku K, Adjabeng M, Duker EO, Antwi CN, Gberbi E, Baffoe-Nyarko I, Adams PL, Dickson AE, Boakye JD, Mensah JY, Odoom C, Bimpong SA, Odame D, Agboste GD, Odoom N, Asiedu-Bekoe F and Obodai E (2025) Polio eradication in Ghana: past, present, and future. Front. Trop. Dis. 6:1577945. doi: 10.3389/fitd.2025.1577945
Received: 17 February 2025; Accepted: 19 May 2025;
Published: 17 June 2025.
Edited by:
Yenddy Carrero, Technical University of Ambato, EcuadorReviewed by:
Nazish Bostan, COMSATS University, PakistanGuillaume Ngoie Mwamba, University of Kamina, Democratic Republic of Congo
Copyright © 2025 Odoom, Laryea, Ntim, Attiku, Adjabeng, Duker, Antwi, Gberbi, Baffoe-Nyarko, Adams, Dickson, Boakye, Mensah, Odoom, Bimpong, Odame, Agboste, Odoom, Asiedu-Bekoe and Obodai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Evangeline Obodai, ZW9ib2RhaUBub2d1Y2hpLnVnLmVkdS5naA==
 John Kofi Odoom
John Kofi Odoom Dennis Odai Laryea2
Dennis Odai Laryea2 Ewurabena Oduma Duker
Ewurabena Oduma Duker Comfort Nuamah Antwi
Comfort Nuamah Antwi Emmanuel Gberbi
Emmanuel Gberbi Jessica Dufie Boakye
Jessica Dufie Boakye Jude Yayra Mensah
Jude Yayra Mensah Christabel Odoom
Christabel Odoom Sharon Ansong Bimpong
Sharon Ansong Bimpong Nancy Odoom
Nancy Odoom Evangeline Obodai
Evangeline Obodai