- 1Department of Genitourinary Medicine and Infectious Diseases, St James’s Hospital, Dublin, Ireland
- 2Department of Intensive Care Medicine, St James’s Hospital, Dublin, Ireland
- 3Department of Neurology, St James’s Hospital, Dublin, Ireland
- 4School of Medicine, Trinity College Dublin, Dublin, Ireland
- 5Department of Microbiology, St James’s Hospital, Dublin, Ireland
- 6Department of Parasitology, The Hospital for Tropical Diseases, University College London Hospitals National Health Service (NHS) Foundations Trust, London, United Kingdom
- 7Diagnostic Parasitology Laboratory, London School of Hygiene and Tropical Medicine, London, United Kingdom
- 8Department of Neurosurgery, Beaumont Hospital, Dublin, Ireland
- 9Department of Neurohistopathology, Beaumont Hospital, Dublin, Ireland
Chagas disease is a neglected tropical disease caused by the protozoan parasite Trypanosoma cruzi. Chagas disease reactivation is an opportunistic infection in HIV-positive patients that commonly presents as meningoencephalitis and/or central nervous system abscesses. We describe the first known Irish case of reactivation disease in a young patient from a Chagas-endemic region with previously undiagnosed HIV and profound immunocompromise. More than 15 years after migrating, he presented to the hospital with a headache, fever, and reduced consciousness. A diagnosis of Chagas disease reactivation was made with neuroimaging, epidemiological history, Chagas serology, and a brain biopsy. Despite the commencement of anti-parasitic therapy followed by anti-retroviral therapy (ART), he deteriorated and died 1 month after admission. There is no international consensus on the dosing of antiparasitic treatment or the timing of ART initiation in reactivation disease with T. cruzi in people living with HIV. This case highlights the need for further research into the management of this complex and highly morbid illness.
Introduction
Chagas disease is a neglected tropical disease caused by the protozoan parasite Trypanosoma cruzi (1). Approximately 6-7 million people worldwide are estimated to be infected with T. cruzi, resulting in around 12,000 deaths per year (1). Chagas disease is endemic in Latin America, with Bolivia reporting the highest prevalence in the world, at 6% (2). Cases in non-endemic settings have been reported, attributable to international travel and migration.
The T. cruzi parasite is primarily transmitted via triatomine insects. In endemic areas, these insects are typically found in the walls or roofing of homes, chicken coops, and warehouses, particularly in structures made of mud brick. Triatomine insects feed on animal and human blood. They are active at night and tend to bite exposed skin areas such as the face. The parasite enters the body when a person scratches the bite area, inadvertently smearing an infected insect’s faeces or urine into the wound. More rarely, transmission can occur through contaminated food or drink, congenitally, via blood transfusion, or through organ transplantation from an infected donor (3).
T. cruzi infection is characterised clinically by two phases, acute and chronic. The acute phase typically begins after an incubation period of 1 or 2 weeks and lasts 4–8 weeks (4, 5). There is microscopically detectable parasitaemia, with non-specific, generally mild symptoms such as malaise, fever, and anorexia (5). There may be inflammation and swelling at the inoculation site, known as a chagoma. Inoculation via the conjunctiva may produce unilateral upper and lower eyelid swelling, classically known as Romaña’s sign (6). In the chronic phase, which may occur many years after infection, gastrointestinal and cardiac symptoms predominate (7).
Both acute and chronic Chagas disease have been described in people living with HIV (PLWH). The reported prevalence of T. cruzi infection in PLWH in endemic countries varies widely from 1% to 28% (8). Individuals with profound immunocompromise due to HIV are at additional risk of a severe and often fatal T. cruzi-mediated reactivation disease (8, 9), which typically presents as central nervous system disease, such as meningoencephalitis or an intracerebral mass (10). Clinically and radiographically, it may therefore resemble cerebral toxoplasmosis.
Given the high mortality of this syndrome, some have called for T. cruzi screening in all PLWH from endemic countries (7). There is no international consensus on the dosing of antiparasitic treatment or the timing of anti-retroviral therapy (ART) initiation in reactivation disease with T. cruzi in PLWH.
We describe the first known Irish case of reactivation disease in a young patient from a Chagas-endemic region with profound HIV-related immunocompromise.
Case presentation
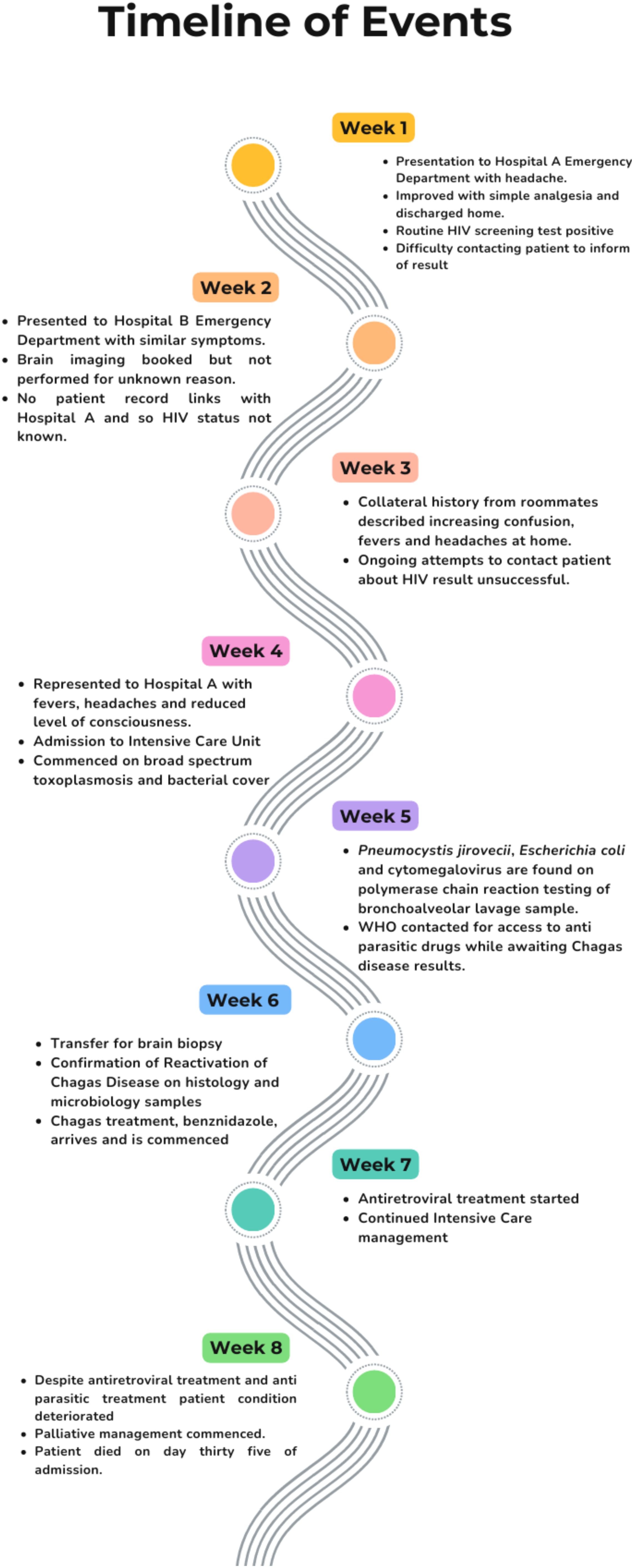
A 29-year-old presented to the emergency department (ED) of hospital A with a 4-week history of headache and fevers, followed by an acute deterioration in consciousness. He had no known medical comorbidities or allergies. He was originally from Bolivia but had lived in Europe for the last 15 years (Ireland for the past year). The patient worked as a mechanic, had no pets, no recent travel history and no unusual contacts or hobbies. In the preceding 4 weeks and prior to his deterioration, he had presented on two separate occasions to a hospital emergency department – once to hospital A and once to hospital B, a different hospital in the city.
At the first visit, which was to hospital A, the patient reported a mild headache, which responded to analgesia with no red flag symptoms. Blood tests were taken, including a routine opt-out bloodborne viral (BBV) screen. The patient was discharged from the ED. The BBV screen was subsequently reported as reactive for HIV antigen and antibody. Multiple unsuccessful attempts were made by staff to contact the patient by telephone and post.
Two weeks later, he presented to the emergency department in hospital B with a more severe headache. Neuroimaging [computed tomography (CT)] was requested; however, it was not performed, and the patient was discharged home with analgesia. Hospital B did not have access to the results from Hospital A. Further, hospital B does not operate an opt-out ED BBV testing programme.
Two weeks later, the patient was brought back to the emergency department of hospital A. He presented with reduced consciousness. The patient’s housemates, who accompanied him to the hospital, reported worsening drowsiness over the preceding days. On initial examination, Glasgow Coma Scale (GCS) was 12/15, with unequal pupils. His temperature was 40.0°C and his heart rate was 120 beats per minute. Initial oxygen saturation was 99% in room air. One hour after presentation, GCS deteriorated from 12 to 6.
Due to worsening level of consciousness and onset of respiratory failure, he was intubated and transferred to the intensive care unit (ICU). Initial blood tests showed lymphopaenia of 0.6 x 109/L (1.5-3.5 109/L), Hb 10.5g/dL (13.5 – 18g/dL), and CRP 59 mg/L (0.00 – 5.00mg/L). A chest radiograph showed diffuse, patchy opacification bilaterally. A brain CT showed multiple supratentorial ring-enhancing lesions bilaterally, with significant associated cerebral oedema and rightward midline shift. The patient was commenced on empiric therapy for meningitis (ceftriaxone, amoxicillin, and vancomycin) and cerebral toxoplasmosis (sulfadiazine and pyrimethamine, with folinic acid). It was deemed unsafe to perform a lumbar puncture at this stage. The case was discussed with neurosurgical colleagues in the national neurosurgical unit, as the admitting hospital does not have a neurosurgery service. The neurosurgical team recommended dexamethasone and levetiracetam therapy for seizure prophylaxis. The patient was not accepted for transfer for biopsy at this stage to give time to assess his response to current therapies. Thoracic CT was also performed, which showed findings suggestive of Pneumocystis jirovecii pneumonia (PJP); cotrimoxazole was commenced.
Admission and diagnosis
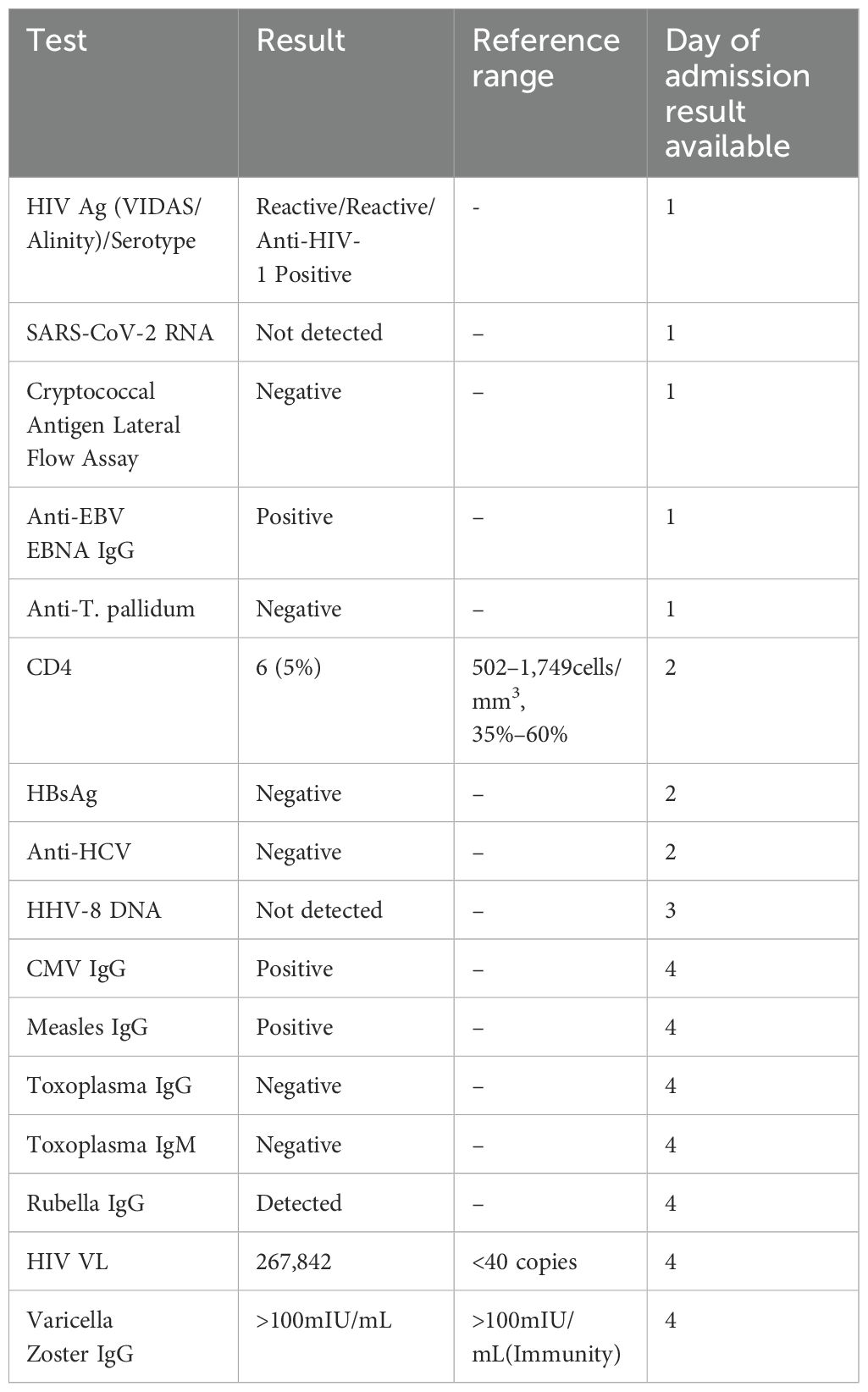
The patient’s inpatient care was coordinated by the intensive care and infectious diseases teams, with ongoing discussions with neurosurgical colleagues. CD4 count was 6 cells/mm3, (5%) (502–1,749 cells/mm3, 35%–60%). The results of further tests conducted are shown in Table 1. Magnetic resonance imaging (MRI) of the brain on day 1 showed multiple ring-enhancing supratentorial (Figure 1) and infratentorial (Figure 2) lesions with extensive mass effect and a 7mm midline shift. The patient’s clinical status and neuroimaging were re-discussed with neurosurgical colleagues, who advised against brain biopsy at that stage. The differential diagnosis included neuro-toxoplasmosis and other infectious and non-infectious causes of multiple brain lesions in an immunosuppressed host. Multiple broad-spectrum anti-infective therapies were commenced and continued.
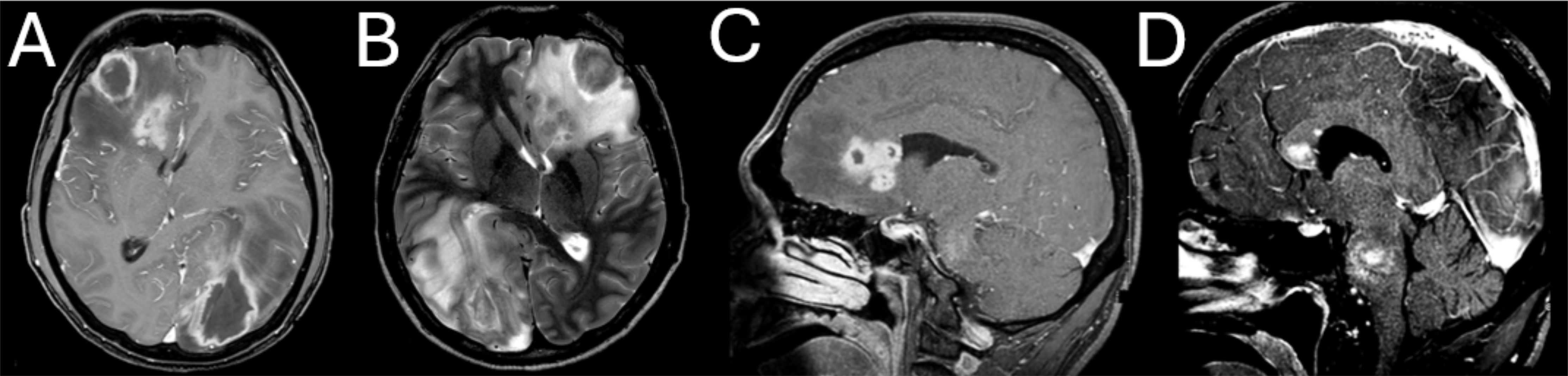
Figure 1. Large, multi-lobar enhancing supratentorial lesions with surrounding vasogenic oedema and resulting midline shift. (A) Axial T1-weighted (T1W) post gadolinium (GD). (B) Axial T2-weighted (T2W). (C) Sagittal T1 high-resolution isotropic volume excitation (THRIVE). (D) THRIVE Sagittal.
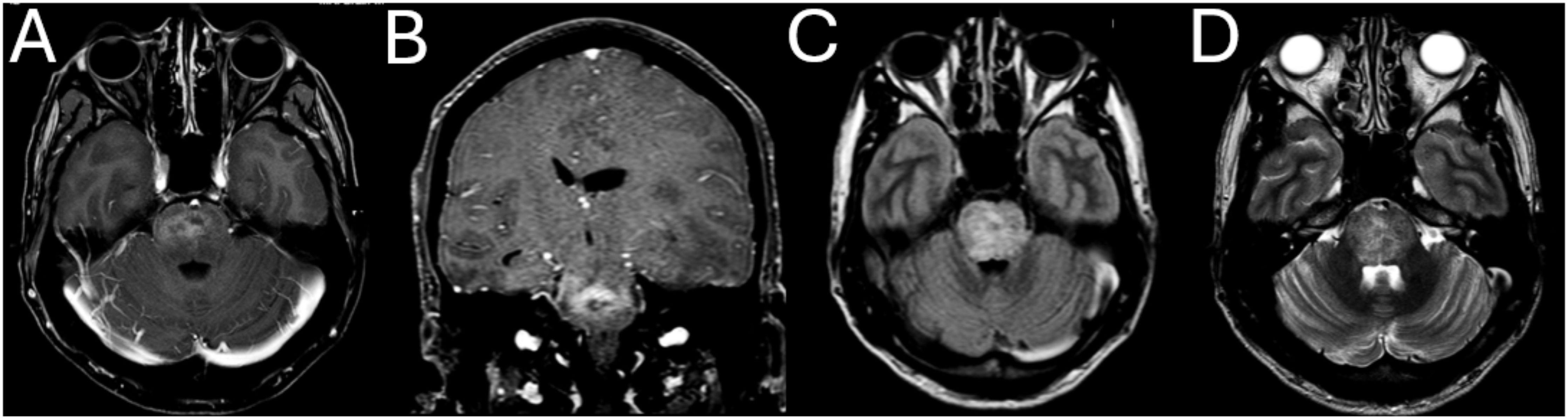
Figure 2. Large, enhancing necrotic pontine lesions with vasogenic oedema. (A) Axial T1W GD. (B) Coronal MPR GD. (C) Axial fluid attenuated inversion recovery (FLAIR). (D) Axial T2W.
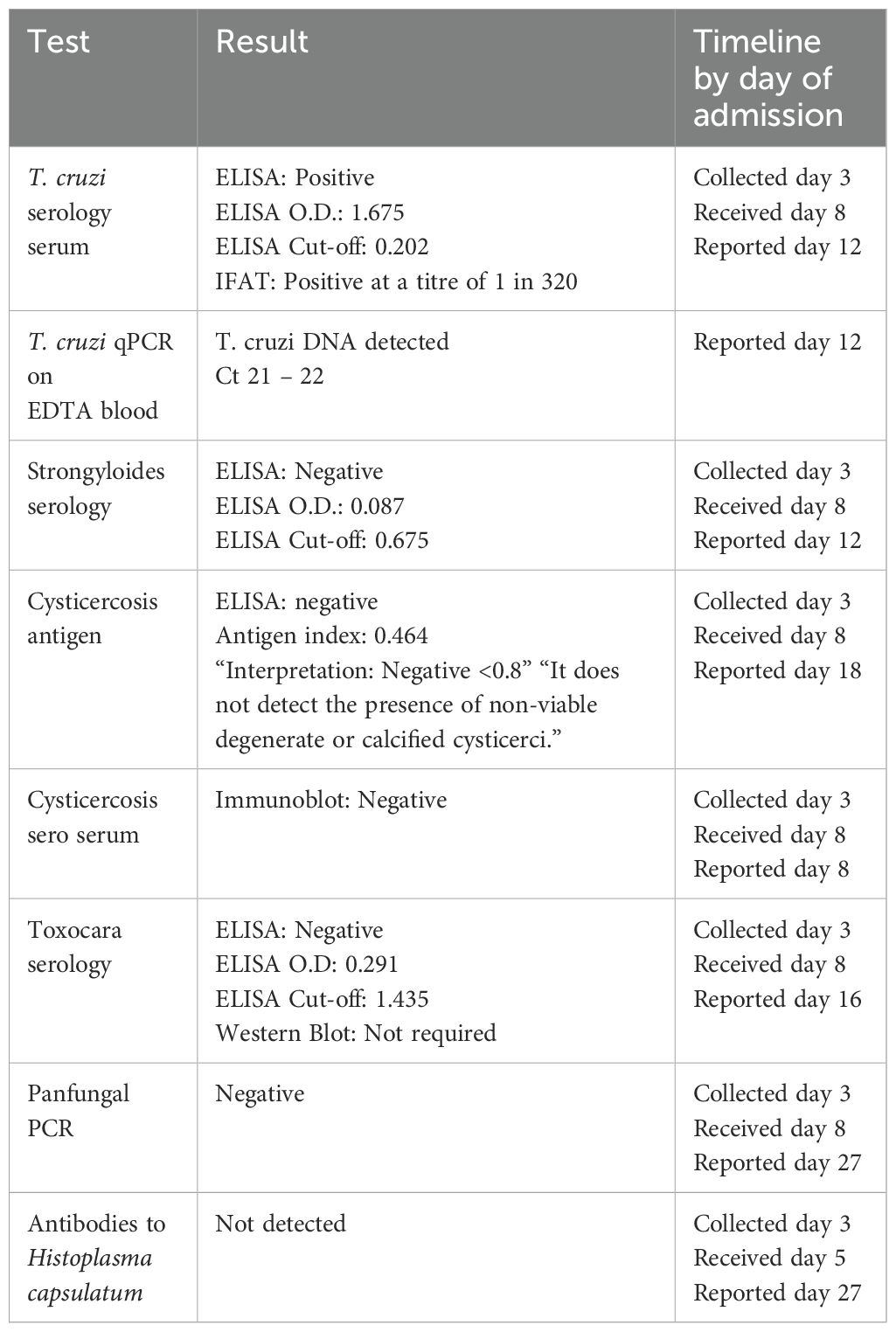
Three days into admission, a family history of Chagas disease was disclosed. This increased the consideration of T. cruzi as casaul pathogen, though trypanosoma serology was already sent and pending. The case was discussed with parasitology colleagues in the United Kingdom. Peripheral blood films from this time did not show detectable parasitaemia. The results of further tests requested are shown (Table 2).
Given the patient’s epidemiological risk profile and lack of improvement on toxoplasmosis therapy, and while Chagas disease test results remained pending, access to benznidazole therapy was arranged. This required communication with World Health Organisation (WHO) officials and transport of the drug from the WHO offices in Geneva, Switzerland. Prior departmental experience with delays in access to this drug led us to seek its delivery before we had confirmation of the diagnosis. Toxoplasmosis serology results returned negative on day 4 of admission.
Bronchoalveolar lavage (BAL) sample demonstrated Pneumocystis jirovecii, Escherichia coli, and cytomegalovirus (CMV) on polymerase chain reaction (PCR) testing. CT of the abdomen and pelvis showed evidence of large bowel loop dilatation, but was otherwise unremarkable. Electroencephalography did not detect evidence of seizure activity.
Transfer for brain biopsy
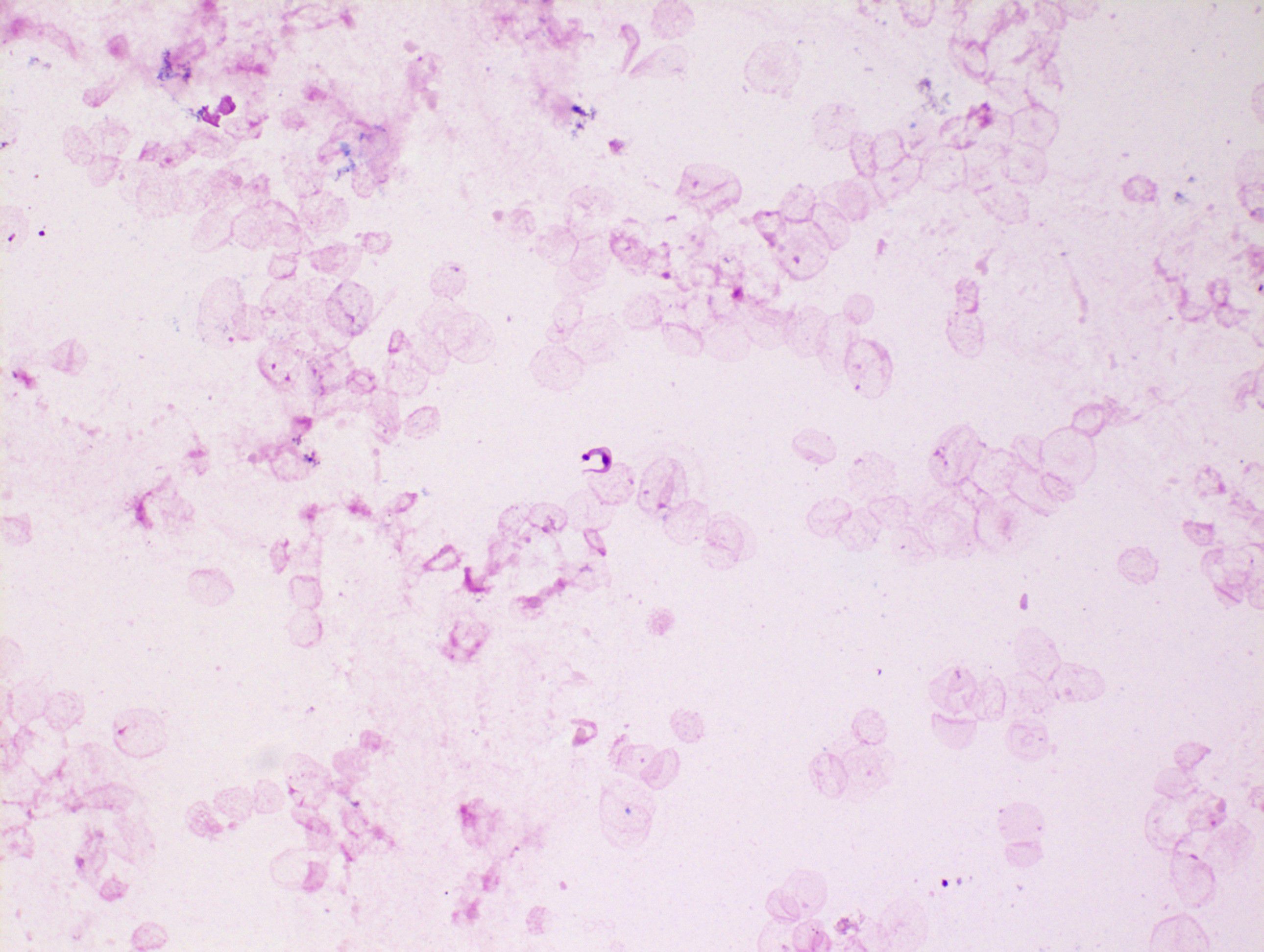
Given the ongoing requirement for sedation and limited radiographic change in neuroimaging (improvement in surrounding oedema only), the patient was transferred to the national neurosurgical unit for tissue biopsy on day 11 of admission. Samples were analysed in Dublin and in the United Kingdom. T. cruzi trypomastigotes were evident microscopically in both the ventricular cerebrospinal fluid (CSF), tissue biopsy, and biopsy fluid. Amastigote forms were abundant both inside macrophages and free in the tissue fluid and tissue preparations. Microscopic images from the specimens are shown (Figures 3–5). Unsurprisingly, the cycle threshold (Ct) values in the T. cruzi PCR were under 10 for all samples, with a Ct of ~4 for the biopsy fluid. Immunostaining for Toxoplasma was negative. Culture of CSF and dural tissue exhibited no growth.
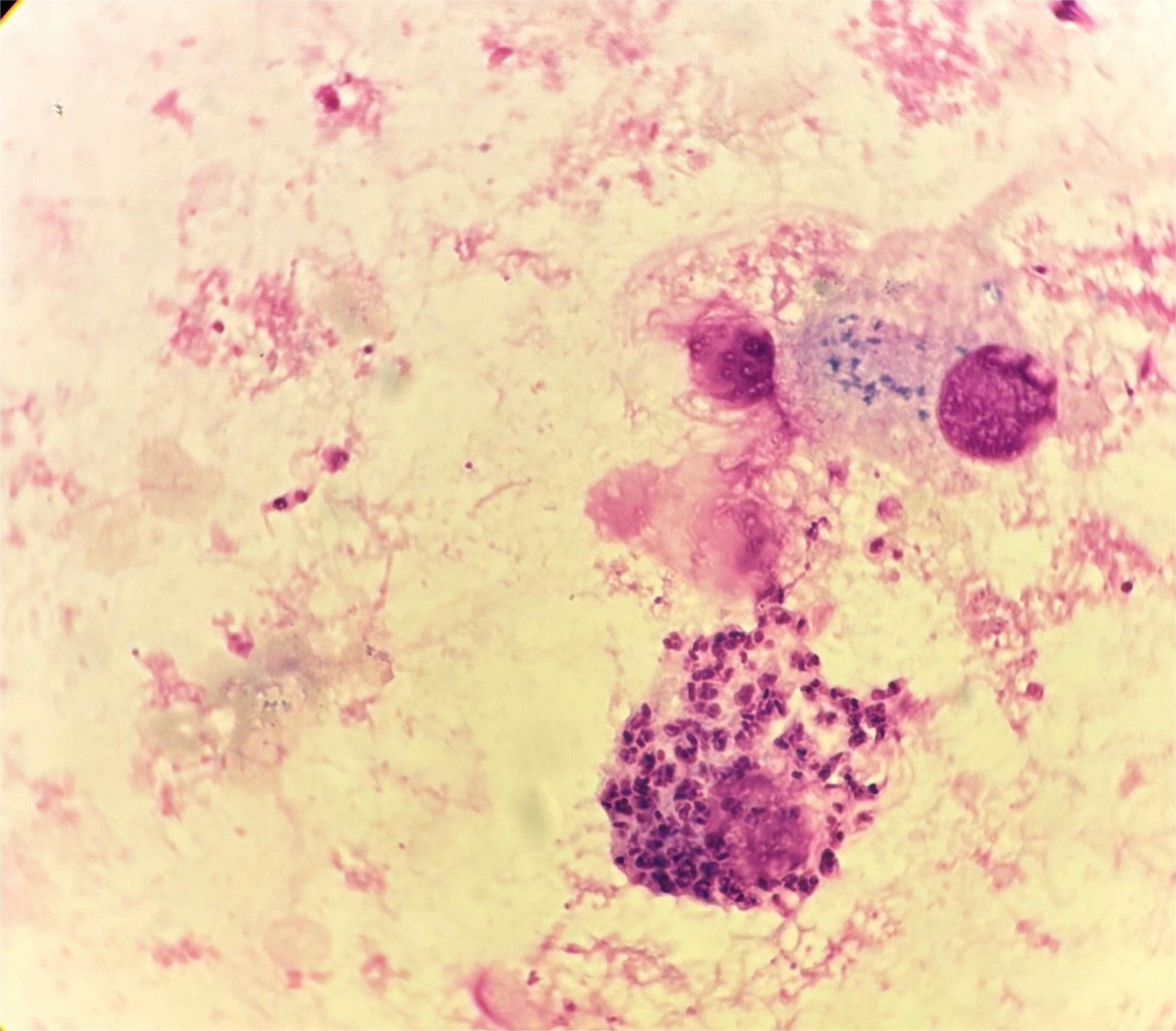
Figure 4. Macrophage packed with T. cruzi amastigotes in biopsy fluid. Trypomastigotes and extracellular amastigotes can also be seen.
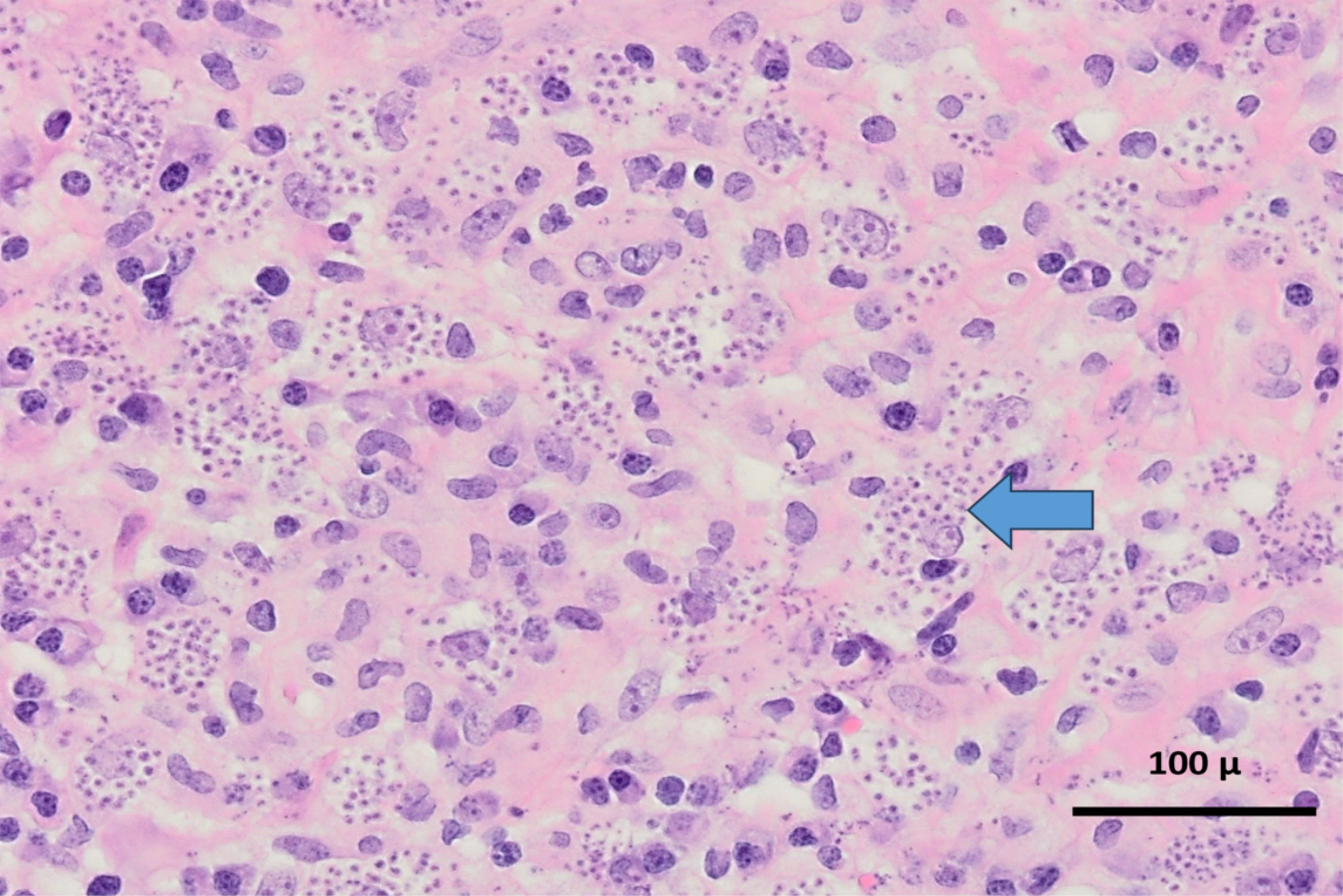
Figure 5. Haematoxylin and eosin (H&E) x 400 mag. Glial tissue within which there is a diffuse mixed inflammatory infiltrate composed of lymphocytes, microglial cells, macrophages and plasma cells, associated with innumerable parasites (arrow). The parasites are located both extracellularly and intracellularly and are morphologically consistent with the amastigote form/stage of Trypanosoma spp.
On day 15 of admission, benznidazole was obtained by the hospital and the patient was started on oral therapy via nasogastric tube comprising benznidazole 350mg once daily.
Advice was sought from colleagues in other centres with clinical experience of intracranial Chagas infection in PLWH. In the absence of standardised protocols, treatment options included benznidazole or nifurtimox (8, 11). Standard and high-dose benznidazole regimens have been employed (12–14). In addition, consideration was given as to the optimal point at which to commence ART.
Benznidazole was administered without any issues, and the dose was increased on day 19 to 300mg three times daily. The high dose was also well-tolerated with no adverse effects noted. Repeat T. cruzi PCR testing on day twenty-six of admission showed an increase in ct value from 21 to 32, suggesting treatment response.
On day 25 of admission ART, with tenofovir alafenamide fumarate (TAF), emtricitabine (FTC), and dolutegravir (DTG), was started. His HIV viral load fell from over two hundred thousand copies on admission to one hundred copies on day 31 of admission.
In spite of appropriate antimicrobial therapies and ICU supportive care, there was no objective clinical improvement in neurologic status over time. Repeat MRI neuroimaging showed a new, large haemorrhage in the pontine lesions. A decision was made with his family to redirect goals of care to palliative measures on day 32 of admission. The patient died on day 35 of admission. A summary of the timeline is shown in Figure 6.
Discussion
This complex case of fatal Chagas disease in a man presenting with advanced HIV infection raises several salient points of discussion.
First, our case identifies that many patients do not know their HIV status and, if undiagnosed, are at risk of developing opportunistic infections. An opportunity was lost to notify the patient of this HIV diagnosis several weeks earlier, due to difficulty communicating the result. This case highlighted the importance of considering patients’ epidemiological risk profile, making use of translation services where language barriers exist, and involving next of kin in instances where patients cannot provide a history.
Second, cerebral Chagas disease associated with profound HIV-related immunocompromise is an important yet rare diagnosis to consider in persons with epidemiological risk factors. Existing literature suggests a very poor prognosis (15, 16). A mortality rate of 79% was described in a small case series in Argentina (15).
Third, standardised protocols are lacking for Chagas disease treatment in PLWH. Varying combinations and dosages of benznidazole and nifurtimox were discussed for our patient with international experts. Benznidazole was commenced and then escalated from standard to high dosing. However, benznidazole has well-established side effects that limit treatment (12). These include skin reactions in up to 20% of patients, ranging from mild dermatitis to severe Stevens–Johnson syndrome; gastrointestinal upset; bone marrow suppression; and peripheral neuropathy (12). Knowledge is lacking on central nervous system (CNS) penetration by benznidazole and on the requisite duration of treatment or role for secondary prophylaxis for patients who respond clinically. In this case, treatment decisions were made by balancing the risks and benefits for the patient. High-dose treatment was tolerated without any adverse effects.
Fourth, international consensus does not exist on the optimal timing of ART in patients with Chagas reactivation disease. In this case, consideration was given to the potential for immune reconstitution syndrome following ART commencement, as is well-described in cryptococcal or tuberculous meningitis (18, 19). Cerebral Chagas-related Immune reconstitution inflammatory syndrome (IRIS) has not been commonly described to date. In this case, ART was started, and no IRIS symptoms were seen. Further research is needed to arrive at a consensus on how best to manage this clinical scenario.
Conclusion
Chagas disease should be considered in individuals living in or migrating from endemic countries in Latin America. This report describes a case of rare and fatal Chagas disease reactivation in a person who migrated from an endemic area 15 years previously presenting with a newly diagnosed, advanced HIV infection. This case is unusual compared to other reports of CNS Chagas in that we have brain biopsy confirmation with histopathological images and magnetic resonance images of the brain. This case report highlights the need for international guidelines for managing Chagas disease reactivation. Further, in the United States, some have argued for T. cruzi screening in all individuals newly diagnosed with HIV (17). In Ireland, the emergence of a growing Latin American population may warrant consideration of such a policy. We hope that this case will add to the literature on the management of this under researched and highly morbid disease.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the patient(s)' next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
LO: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. AW: Data curation, Writing – original draft, Writing – review & editing. AA: Writing – review & editing. AF: Data curation, Writing – review & editing. AH: Writing – review & editing. PB: Data curation, Writing – review & editing. BC: Data curation, Investigation, Writing – review & editing. LN: Data curation, Investigation, Writing – review & editing. DN: Data curation, Investigation, Writing – review & editing. CR: Data curation, Investigation, Writing – review & editing. CM: Investigation, Writing – review & editing. AB: Data curation, Investigation, Writing – review & editing. SK: Investigation, Writing – review & editing. MC: Data curation, Writing – review & editing. ED: Data curation, Investigation, Supervision, Writing – review & editing. CB: Data curation, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chagas disease(2025). Available online at: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (Accessed January 6, 2025).
2. World Health Organisation. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. (2015) 90:33–43. Available at: https://iris.who.int/handle/10665/242316.
3. Coura JR. Chagas disease: control, elimination and eradication. Is it possible? Mem Inst Oswaldo Cruz. (2013) 108:962–7. doi: 10.1590/0074-0276130565
5. Bern C, Martin DL, and Gilman RH. Acute and congenital Chagas disease. Adv Parasitol. (2011) 75:19–47. doi: 10.1016/B978-0-12-385863-4.00002-2
6. Romaña C. Acerca de um síntoma inicial de valor para o diagnóstico de forma aguda de la enfermedad de Chagas. La conjuntivitis esquizotripanósica unilateral: hipótesis sobre la puerta de entrada conjuntiva de la enfermedad. Publicaciones Mepra. (1935) 22:16–28.
7. Forsyth CJ, Manne-Goehler J, Bern C, Whitman J, Hochberg NS, Edwards M, et al. Recommendations for screening and diagnosis of Chagas disease in the United States. J Infect Dis. (2022) 225:1601–10. doi: 10.1093/infdis/jiab513
8. Clark EH and Bern C. Chagas disease in people with HIV: A narrative review. Trop Med Infect Disease. (2021) 6:198. doi: 10.3390/tropicalmed6040198
9. Del Castillo M, Mendoza G, Oviedo J, Perez Bianco RP, Anselmo AE, and Silva M. AIDS and Chagas’ disease with central nervous system tumor-like lesion. Am J Med. (1990) 88:693–4. doi: 10.1016/0002-9343(90)90544-N
10. Gluckstein D, Ciferri F, and Ruskin J. Chagas’ disease: another cause of cerebral mass in the acquired immunodeficiency syndrome. Am J Med. (1992) 92:429–32. doi: 10.1016/0002-9343(92)90275-G
11. Chagas Disease: Adult and Adolescent OIs | NIH (2023). Available online at: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/chagas-disease (Accessed January 6, 2025).
12. Pinazo MJ, Muñoz J, Posada E, López-Chejade P, Gállego M, Ayala E, et al. Tolerance of benznidazole in treatment of Chagas’ disease in adults. Antimicrob Agents Chemother. (2010) 54:4896–9. doi: 10.1128/AAC.00537-10
13. Altcheh J, Moscatelli G, Mastrantonio G, Moroni S, Giglio N, Marson ME, et al. Population pharmacokinetic study of benznidazole in pediatric Chagas disease suggests efficacy despite lower plasma concentrations than in adults. PLoS Negl Trop Dis. (2014) 8:e2907. doi: 10.1371/journal.pntd.0002907
14. Torrico F, Gascón J, Barreira F, Blum B, Almeida IC, Alonso-Vega C, et al. New regimens of benznidazole monotherapy and in combination with fosravuconazole for treatment of Chagas disease (BENDITA): a phase 2, double-blind, randomised trial. Lancet Infect Dis. (2021) 21:1129–40. doi: 10.1016/S1473-3099(20)30844-6
15. Cordova E, Boschi A, Ambrosioni J, Cudos C, and Corti M. Reactivation of Chagas disease with central nervous system involvement in HIV-infected patients in Argentina, 1992-2007. Int J Infect Dis. (2008) 12:587–92. doi: 10.1016/j.ijid.2007.12.007
16. Shelton WJ and Gonzalez JM. Outcomes of patients in Chagas disease of the central nervous system: a systematic review. Parasitology. (2024) 151:15–23. doi: 10.1017/S0031182023001117
17. Clark EH, Marquez C, Whitman JD, and Bern C. Screening for Chagas disease should be included in entry-to-care testing for at-risk people with human immunodeficiency virus (HIV) living in the United States. Clin Infect Dis. (2022) 75:901–6. doi: 10.1093/cid/ciac154
18. DeSimone JA, Pomerantz RJ, and Babinchak TJ. Inflammatory reactions in HIV-1-infected persons after initiation of highly active antiretroviral therapy. Ann Intern Med. (2000) 133:447–54. doi: 10.7326/0003-4819-133-6-200009190-00013
Keywords: Chagas disease, human immunodeficiency virus (HIV), immunocompromise, Trypanosomiasis cruzi, chagoma
Citation: O’Doherty L, White A, Almajrafi A, Fennessy A, Heekin A, Bede P, Crowley B, Nabarro L, Nolder D, Rogers C, Moran C, Beausang A, Keane S, Cruz M, Devitt E and Bergin C (2025) Case Report: Reactivation of Chagas disease in a patient with advanced HIV 15 Years after migration. Front. Trop. Dis. 6:1588423. doi: 10.3389/fitd.2025.1588423
Received: 05 March 2025; Accepted: 23 April 2025;
Published: 22 May 2025.
Edited by:
Manuela Berto Pucca, Sao Paulo State University, BrazilReviewed by:
Jader Oliveira, University of São Paulo, BrazilLeticia Gomes De Pontes, Universidade de Minas Gerais, Brazil
Copyright © 2025 O’Doherty, White, Almajrafi, Fennessy, Heekin, Bede, Crowley, Nabarro, Nolder, Rogers, Moran, Beausang, Keane, Cruz, Devitt and Bergin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura O’Doherty, b2RvaGVybEB0Y2QuaWU=
 Laura O’Doherty
Laura O’Doherty Annmarie White
Annmarie White Ali Almajrafi1
Ali Almajrafi1 Colm Bergin
Colm Bergin