- 1Family and Community Medicine, College of Medicine, King Khalid University, Abha, Saudi Arabia
- 2Tropical Health Department, High Institute of Public Health, Alexandria University, Alexandria, Egypt
- 3Family Medicine, Jeddah Second Health Cluster, Jeddah, Saudi Arabia
- 4Family Medicine, Aseer Health Cluster, Abha, Saudi Arabia
- 5Pediatric Department, King Abdulaziz Hospital & Oncology Center, Jeddah, Saudi Arabia
- 6Family Medicine, Makkah Health Cluster, Al Qunfudah, Saudi Arabia
- 7Department of Medical Parasitology, Faculty of Medicine, Kafrelsheikh University, Kafrelsheikh, Egypt
Background: Tropical infectious diseases continue to impose a significant public health burden, particularly in regions with poor sanitation, limited healthcare access, and favorable environmental conditions for pathogen transmission. Gastrointestinal (GI) involvement is a key clinical feature across many of these diseases, contributing significantly to global morbidity and mortality. Despite increasing awareness, few existing reviews comprehensively integrate the pathophysiology of GI involvement, diagnostic challenges, and multidisciplinary public health strategies, including the One Health approach.
Methods: This narrative review synthesizes current evidence on tropical diseases with GI manifestations. A structured literature search was conducted using major databases. Key themes were identified through thematic synthesis, including disease mechanisms, diagnostic limitations, treatment options, and prevention strategies.
Results: A significant number of tropical diseases spread through the fecal-oral route, primarily due to contaminated food or water, particularly in areas with poor sanitation. These include helminthic, protozoal, bacterial, and viral infections. Vector-borne diseases and zoonotic infections also present substantial GI involvement. Chronic parasitic presence triggers inflammation, fibrosis, and oxidative stress, leading to tissue damage and long-term complications, including cancers. Misdiagnosis of GI symptoms as more common conditions like irritable bowel syndrome delays appropriate care and worsens outcomes. The globalization of travel and commerce has widened the spread of these diseases, with travelers, immigrants, and refugees frequently exhibiting unfamiliar GI symptoms in non-endemic regions.
Conclusion: A multifaceted strategy is essential for effective management, including improved sanitation, enhanced diagnostic tools, mass drug administration, and vector control. The One Health framework provides a sustainable model by integrating human, animal, and environmental health perspectives. Aligning interventions with global targets such as the Sustainable Development Goals (SDGs) and the WHO’s 2030 roadmap for neglected tropical diseases (NTDs) can reduce health disparities, improve nutrition, and strengthen resilience against emerging threats.
Introduction
Tropical areas are situated between the Tropic of Cancer and the Tropic of Capricorn, known for their consistently warm temperatures and abundant rainfall throughout the year. Subtropical regions are located just outside the tropics, extending to approximately 35° latitude. Tropical diseases are illnesses commonly found in, or specific to, tropical and subtropical regions. Tropical diseases encompass communicable and non-communicable diseases, genetic disorders, and conditions resulting from nutritional deficiencies or environmental factors, such as heat, humidity, and altitude (1).
In 2022, 1.62 billion people required interventions against neglected tropical diseases (NTDs), marking a 26% decrease from 2010. However, this progress falls short of the trajectory needed to achieve the global target of a 90% reduction by 2030, as outlined in the road map for NTDs. The data underscores the urgent need for accelerated efforts, increased funding, and more effective strategies to address the persistent burden of these diseases and meet the ambitious 2030 goals (2). The primary portals of entry for the organisms responsible for most infections, both in tropical countries and globally, are the skin, respiratory tract, and gastrointestinal tract (GIT). In tropical climates, a significant proportion of infections arise from the ingestion of contaminated water and food (3).
This review emphasizes the crucial importance of recognizing and addressing gastrointestinal (GI) symptoms in tropical diseases. By integrating current evidence on pathophysiology, diagnosis, treatment, and prevention, we aim to enhance clinical management, strengthen public health strategies, and align with global health objectives, such as the Sustainable Development Goals (SDGs) 3.3 and the World Health Organisation’s (WHO) 2030 roadmap for NTDs. According to the World Health Organization’s 2021–2030 NTD Road Map, the global goals include eliminating at least one NTD in 100 countries and achieving a 90% reduction in the number of people requiring NTDs-related interventions (4). Ultimately, this effort will help reduce health disparities, improve nutritional outcomes, and build resilience to emerging health threats.
Despite a growing global awareness of tropical diseases, there is still a considerable lack of literature addressing the integration of GI pathophysiology, diagnostic hurdles, and multidisciplinary public health strategies like the One Health approach. While various reviews investigate specific pathogens or clinical manifestations, very few provide a comprehensive synthesis of the mechanisms of GI involvement, the current diagnostic challenges, and the broader implications for human, animal, and environmental health, all within a unified framework. This holistic perspective is vital for guiding clinicians in both endemic and non-endemic areas, developing public health interventions, and aligning with global objectives such as the SDGs and the World Health Organization’s 2030 roadmap for NTDs.
In this review, we focused on digestive disorders for several reasons. First, digestive disorders, including diarrheal diseases, rank among the leading causes of illness and death globally, with their most significant impact observed in tropical and subtropical regions (5). Second, the undiagnosed and improper treatment of GI problems in tropical diseases poses considerable obstacles to both healthcare and public health. GI symptoms, such as chronic diarrhea caused by parasitic infections, are often mistakenly attributed to more common conditions like irritable bowel syndrome (IBS) or inflammatory bowel disease (IBD), especially in non-endemic areas. Another instance is that conditions such as schistosomiasis or strongyloidiasis may exhibit symptoms that are similar to those of celiac disease or other malabsorption syndromes (6). Healthcare professionals may not be familiar with tropical pathogens. Additionally, these infections can cause health issues in tropical regions (7). Third, the diagnostic disparity not only postpones appropriate care but also worsens the long-term effects of these diseases on nutritional health and overall well-being. Fourth, ongoing GI involvement can result in malabsorption, malnutrition, and hindered growth, particularly in children, thus perpetuating cycles of poverty and health issues (8). Fifth, the globalization of travel and commerce has widened the spread of tropical diseases, with travelers, immigrants, and refugees frequently exhibiting unfamiliar GI symptoms in temperate regions (9). Finally, health issues such as travelers’ diarrhea, caused by pathogens like enterotoxigenic Escherichia coli (ETEC) or Campylobacter jejuni, underscore the necessity for increased awareness and enhanced diagnostic procedures to prevent outbreaks and guarantee effective management of these important yet often neglected health conditions (10).
Therefore, it is important to recognize GI symptoms in tropical diseases, as this is significant for both clinical management and public health. By bringing attention to these symptoms, we can enhance diagnostic precision, leading to prompt treatment for conditions such as amebic dysentery and typhoid fever, which in turn minimizes complications and reduces mortality rates (11). This awareness also reinforces focused efforts, such as improving access to clean water, adopting mass drug distribution (MDA) for helminths (12), implementing the One Health approach for infectious diseases management (13, 14), and bolstering disease surveillance to identify outbreaks of diseases like cholera or shigellosis (15). Moreover, tracking GI symptoms contributes to diminishing health inequalities, educating healthcare professionals in endemic areas, and aligning with global health objectives like the SDGs, ultimately promoting fair healthcare practices and resilience to health threats (16).
Methods
This paper presents a narrative review aimed at providing a comprehensive overview of tropical diseases that have GI manifestations. Unlike systematic reviews, which adhere to strict methodological protocols, narrative reviews offer broader interpretations and the integration of diverse evidence. This approach enables a more holistic understanding of complex, interdisciplinary topics. We chose the narrative format because of the wide-ranging nature of the subject and the variability of available evidence across different pathogens and geographic regions. This method supports thematic synthesis, enhancing coherence and offering practical insights for both clinicians and public health professionals. Our goal goes beyond simply summarizing existing knowledge; we strive to integrate findings across disciplines and produce actionable recommendations for the diagnosis, management, and prevention of GI-related manifestations in tropical diseases.
Search strategy
A structured yet flexible search strategy was implemented to identify relevant studies and documents. Keywords such as “tropical diseases,” “gastrointestinal symptoms,” “diarrhea,” “diagnosis,” “global health,” and related terms were combined using Boolean operators (AND/OR) to formulate search queries. Searches were conducted in major databases, including PubMed, Web of Science, Scopus, and Google Scholar, as well as in gray literature from organizations such as the WHO and the Centers for Disease Control and Prevention (CDC).
Inclusion and exclusion criteria
The inclusion criteria targeted studies, reports, and reviews that addressed tropical diseases with GI involvement. Sources discussing diagnosis, treatment, and public health impact were prioritized. No date restrictions were applied to ensure a historical perspective on the topic. Exclusion criteria eliminated irrelevant or non-peer-reviewed studies that did not fit within the scope of this review. However, seminal papers, landmark studies, and key guidelines were included regardless of their publication date to provide foundational insights.
The synthesis of data was conducted thematically rather than through quantitative meta-analysis. Themes were identified based on recurring patterns in the literature, which include: Pathophysiology (mechanisms of GI involvement in tropical diseases), clinical manifestations (common and unique GI symptoms associated with specific pathogens) diagnosis and treatment (challenges in diagnosing tropical diseases in both endemic and non-endemic regions, along with current therapeutic options, and public health strategies (prevention measures, vector control, vaccination, and the impact of social determinants of health).
Study findings
Figure 1 provides a summary of the main tropical diseases affecting the GIT in tropical regions. The figure categorizes these diseases into four major groups: protozoal infections, helminthic infections, bacterial infections, and viral infections.
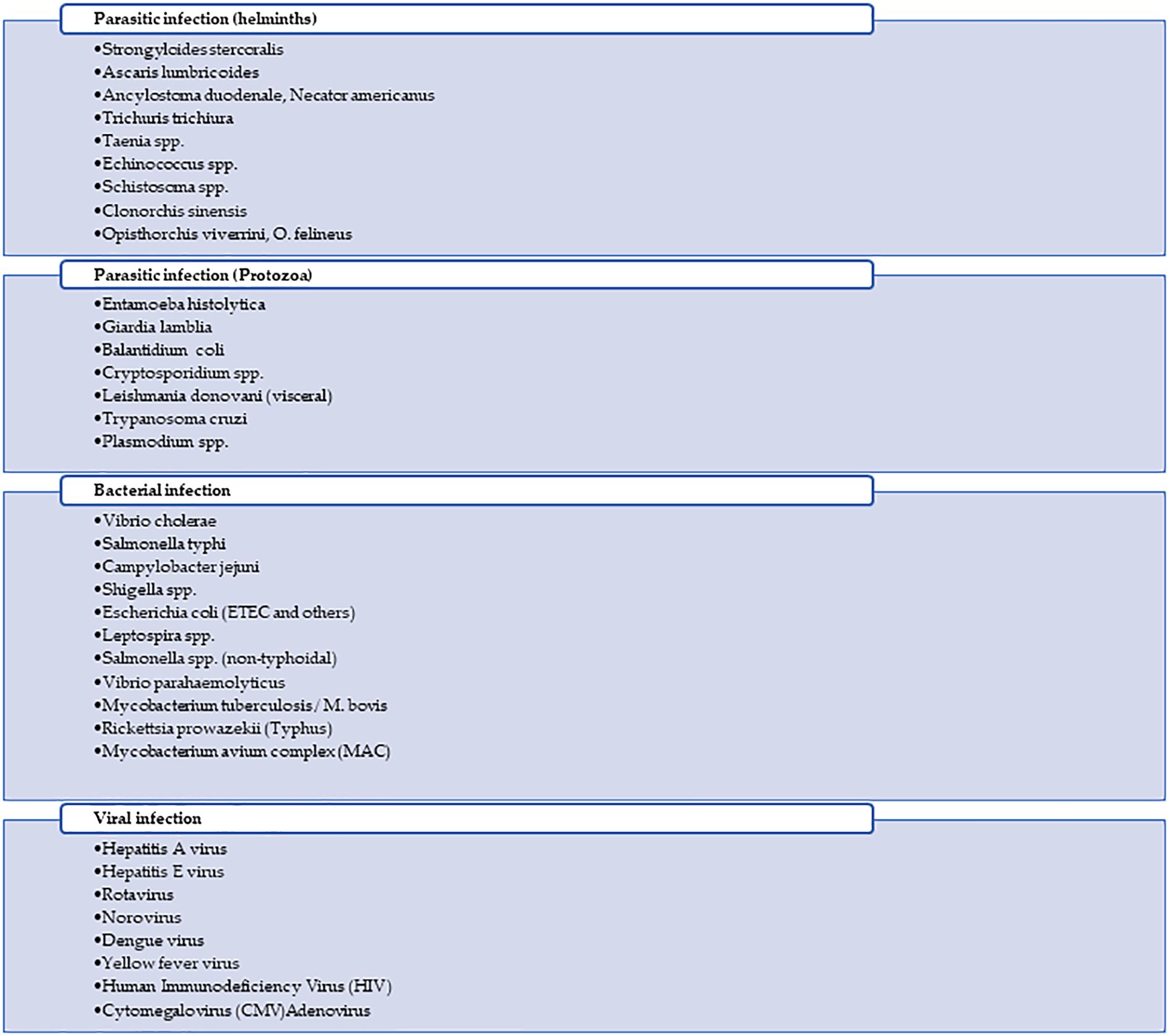
Figure 1. Summary of infectious diseases affecting the gastrointestinal tract (GIT) in tropical regions, classified into helminthic, protozoal, bacterial, and viral infections.
Mode of infection and GIT involvement
A significant number of diseases spread through the fecal-oral route, primarily due to contaminated food or water, particularly in areas with poor sanitation. These include helminthic diseases like ascariasis, hookworm, and strongyloidiasis, acquired via soil, food, or water contaminated with parasite eggs or larvae. Protozoal infections such as amebiasis, giardiasis, balantidiasis, and cryptosporidiosis are transmitted by ingesting cysts, oocysts, other contaminated materials, and vector-borne. Bacterial infections like cholera, typhoid fever, campylobacteriosis, shigellosis, salmonellosis, and ETEC also spread this way, often through contaminated food or water, with flies and cross-contamination acting as mechanical vectors (17, 18). Viral infections, including hepatitis A, hepatitis E, rotavirus, norovirus, and adenovirus, similarly spread via the fecal–oral route. Another group of diseases is vector-borne; for example, leishmaniasis is transmitted by sandflies, Chagas disease by triatomine bugs, dengue and yellow fever by Aedes mosquitoes, and typhus by lice or fleas. Zoonotic and environmentally acquired infections include schistosomiasis, which occurs through contact with freshwater containing parasite larvae from infected snails, and leptospirosis, contracted through exposure of wounds, abraded skin, or mucous membranes to urine or blood of infected animals or water or soil contaminated by Leptospira (19). Intact skin can also be the portal of entry for Strongyloides stercoralis and hookworms. Additionally, Fasciola hepatica, Opisthorchis, and Clonorchiasis are acquired through the ingestion of raw or undercooked aquatic plants or fish containing parasite larvae. Opportunistic infections such as cytomegalovirus (CMV) and Mycobacterium avium complex (MAC) are significant causes of GIT disease in immunocompromised individuals, often acquired from bodily fluids or environmental sources. Lastly, while human immunodeficiency virus (HIV) is not primarily a Gl infection, it can lead to HIV enteropathy and is transmitted through sexual contact, blood transfusions, and from mother to child. Mycobacterium tuberculosis, though primarily airborne, can also cause Gl tuberculosis, particularly in advanced cases (Table 1).
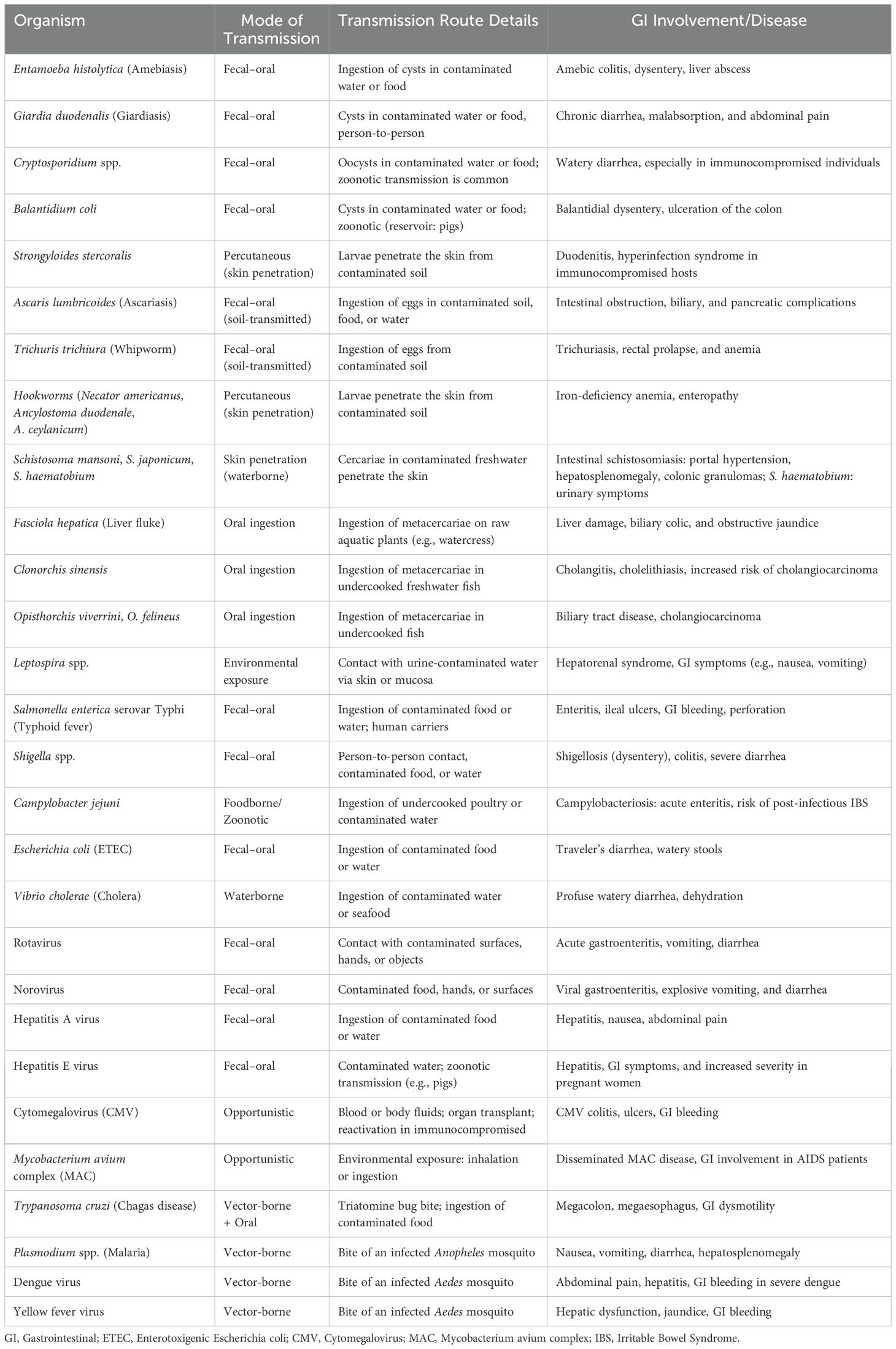
Table 1. Common tropical diseases affecting the gastrointestinal tract: pathogens, transmission, and clinical features.
Pathophysiology of GIT involvement and management
Parasitic diseases
Parasitic protozoans and helminths cause tissue damage through migration, feeding, and reproduction inside the host. They release enzymes that disrupt the GIT mucosa and immune responses. Chronic parasitemia triggers inflammation, fibrosis, and oxidative stress, leading to tissue damage. Organ dysfunction may persist even after parasite clearance, causing long-term complications such as lymphadenopathy and lipo-lymphedema, with significant morbidity (20).
Entamoeba histolytica (E. histolytica): Global estimates suggest that around 500 million people are infected with E. histolytica, with about 10% developing invasive forms of amebiasis, highlighting its significant public health impact (21). Pathogenesis involves adhesion, cytolysis, and immune evasion through lectin, amebapores, and cysteine proteases. Molecules like lipophosphopeptidoglycan, peroxiredoxin, and arginase enhance invasiveness. Virulent strains overexpress oxidative stress genes, aiding survival and suggesting therapeutic targets (22). Noninvasive intestinal amebiasis infections are often asymptomatic, with cysts passing in the stool. Invasive intestinal amebiasis occurs when trophozoites breach the intestinal lining, leading to symptoms like acute diarrhea, bloody stools, and abdominal tenderness (23). Severe cases may cause fulminant colitis, perforation, or amebomas, with right lower quadrant pain and bowel obstruction. Amebic colitis typically resolves within a month, but chronic forms may persist with intermittent diarrhea and weight loss. Extraintestinal complications include liver abscesses, cerebral and pleuropulmonary involvement (24). Diagnosis of amebiasis involves stool microscopy, antigen detection, Polymerase Chain Reaction (PCR), and serology. PCR is the gold standard, while Enzyme-Linked Immunosorbent Assay (ELISA) aids in rapid detection. Serology is useful for extraintestinal cases. Imaging supports liver abscess diagnosis. Colonoscopy may reveal flask-shaped ulcers. Combining methods improves accuracy and helps distinguish pathogenic from nonpathogenic Entamoeba species (23). Genetic analysis of E. histolytica isolates from different endemic regions helps link parasite genotypes to infection outcomes, aiding in improved diagnosis and treatment (25). All E. histolytica infections require treatment to prevent transmission and recurrence. Effective management involves a tissue amoebicidal (e.g., metronidazole) followed by a luminal agent (e.g., paromomycin). Combination therapy is superior to monotherapy. Follow-up stool PCR confirms clearance. Prevention includes hygiene, safe food practices, and treating contacts if positive (26). Corticosteroids and antimotility drugs are contraindicated. Follow-up stool testing is advised post-treatment (24).
Giardia lamblia: Giardiasis is the most common enteric protozoal infection worldwide, affecting about 2% of adults and 8% of children in developed nations (27). It exerts its pathogenic effects in the upper small intestine by adhering to the epithelial brush border using its ventral disk, leading to disruption of brush border enzymes, tight junctions, and epithelial barrier function (28). These changes result in malabsorption, increased intestinal permeability, and apoptosis, often mediated by caspase-3 (29). Histopathological findings may include villous atrophy and crypt hyperplasia, particularly in symptomatic cases (27). Giardia consumes host arginine, limiting nitric oxide synthesis and epithelial cell proliferation, which may aid parasite persistence. Although inflammation is often minimal, the functional impact is significant (30). The disease severity and pathology vary depending on parasite strain, host nutrition, and microbiota composition. Giardia alters the gut microbiome, enhancing the virulence of certain commensals and prolonging GI symptoms post-infection. These multifactorial interactions underline Giardia’s ability to cause both acute and chronic intestinal dysfunction (30). Giardiasis causes symptoms like diarrhea, bloating, cramps, malabsorption, and weight loss. If it is left untreated, it can become chronic and leads to growth delay, anemia, and cognitive issues in children. Chronic infection is linked to conditions like IBS, food allergies, and fatigue (31). Though it often does not cause severe diarrhea, it may increase the risk of persistent diarrhea in children. It has been incidentally found in patients with liver and pancreatic diseases, suggesting possible associations with certain cancers (32). Zinc sulfate flotation is a low-cost, effective test for the diagnosis of Giardiasis, especially with serial stool samples. While immunochromatographic tests are sensitive and specific, they are costly and limited to Giardia detection. PCR is useful for epidemiology but may miss light infections in asymptomatic carriers or animals with low parasite levels (33). Tinidazole and nitazoxanide are FDA-approved treatments for giardiasis, both offering high efficacy and good safety profiles. Tinidazole is preferred for its single-dose regimen, while nitazoxanide is available in suspension and causes few side effects. Metronidazole, though not FDA-approved, is also effective and less expensive, but has more side effects and requires multiple doses over several days (34).
Balantidium coli is the largest pathogenic protozoan in humans and the only ciliate known to cause disease. Infection begins with the ingestion of cysts, which excyst in the small intestine. Trophozoites colonize the large intestine and invade the mucosa using enzymes like hyaluronidase and cysteine proteases, forming flask-shaped ulcers similar to those in amebiasis (35). Clinically, balantidiasis often mimics amoebic dysentery, presenting with bloody diarrhea, cramps, nausea, and fever. Severe cases may involve extraintestinal spread to the liver, lungs, or bladder, particularly in immunocompromised individuals. Host immunity, malnutrition, and comorbidities are critical in disease progression and dissemination (34, 36). Balantidiasis is often asymptomatic but can cause dysentery, abdominal pain, and tenesmus, especially in immunocompromised individuals. Severe cases may involve bloody diarrhea, intestinal ulcers, and rare complications like liver abscesses or spinal infections. The parasite can spread to other organs, including the lungs (34). A case report highlighted balantidiasis infection during pregnancy, likely due to immune suppression and contact with animals or raw vegetables (36, 37). For treating intestinal balantidiasis, tetracycline is the preferred option, but it is contraindicated in pregnancy and for children below 8 years. Other investigational drugs include iodoquinol, metronidazole, and nitazoxanide, with variable success from paromomycin and chloroquine (35).
Cryptosporidium spp.: This infection is commonly acquired from contaminated water, particularly in developed regions, where it can persist in water treatment facilities and swimming pools (38). It is the second most frequent cause of diarrheal disease and related mortality in children worldwide, after rotavirus (39). More than a million deaths from gastroenteritis linked to Cryptosporidium spp. have been recorded in the late 20th century and early 21st century (40). In individuals with HIV/AIDS, the infection can become chronic and difficult to treat. In healthy individuals, infection often causes self-limiting diarrhea, which may be accompanied by nausea, vomiting, abdominal pain, and low-grade fever (41). Immunocompromised patients may experience chronic or prolonged diarrhea, with potential extraintestinal spread to the biliary tree and pancreas (42). While stool microscopy is the gold standard for diagnosing Cryptosporidium spp., newer methods like multiplex PCR and antigen-based rapid diagnostic tests (RDTs) offer higher sensitivity and quicker results, with RDTs being particularly useful for rapid, point-of-care testing (43). Nitazoxanide is FDA-approved for treating cryptosporidiosis in immunocompetent individuals but is less effective in HIV patients, transplant recipients, and malnourished children (44). Emerging evidence suggests antiparasitic combinations, such as nitazoxanide with azithromycin and, in some cases, rifaximin, may be effective for treating cryptosporidiosis in transplant recipients. For HIV-infected individuals, restoring immune function with antiretroviral drugs (ARVs) is key to resolving the infection (44, 45).
Plasmodium spp. (Malaria): Despite being preventable and treatable, malaria continues to have a devastating impact on human health. It is endemic in 83 countries, most of which are located in tropical and subtropical regions, causing approximately 263 million infections and 597,000 deaths annually worldwide (46, 47). Species that commonly infect humans include Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, Plasmodium ovale curtisi, Plasmodium ovale wallikeri, and Plasmodium knowlesi. Among these, P. falciparum and P. vivax are the most important due to their widespread prevalence and clinical significance (48). Malaria causes GIT pathophysiology by sequestration of infected red blood cells (iRBCs) in the GIT, including the stomach, colon, jejunum, and ileum, resulting in epithelial detachment, villous shortening, and increased intestinal permeability. As a result, ruptures and leakage of iRBCs occur, contributing to intestinal bleeding and dysbiosis (49). Moreover, malaria is a well-known risk factor for invasive bacterial diseases, which are predominantly caused by invasive enteropathogens. Among these, non-typhoidal salmonella (NTS) is particularly significant (50). GIT symptoms of malaria include abdominal pain, anorexia, heartburn, nausea, vomiting, diarrhea, and hepatic dysfunction (51–53). Artemisinin-based combination therapies (ACTs) are the main treatment for falciparum malaria, but emerging resistance is a concern, particularly in young children and those in low transmission areas (54). Side effects of antimalarial drugs are generally mild, with some risk of hematologic issues like hemolysis. Severe malaria may require blood transfusion, with guidelines recommending 30 mL/kg for afebrile children and 20 mL/kg for febrile children. Exchange blood transfusion may be considered for severe cases, but there is no strong evidence supporting its use. The high number of affected children and limited treatment options make this a significant public health concern (55).
Trypanosoma cruzi: More than 7 million people around the world, primarily in Latin America, are believed to be infected with Trypanosoma cruzi, the parasite responsible for causing Chagas disease (56). The chronic infection primarily affects the cardiac tissues, esophagus, and colon, leading to severe GI complications. Pathophysiology involves the destruction of enteric nervous system neurons and a reduction in interstitial cells of Cajal, causing megavisceral presentations like abdominal pain, nausea, vomiting, megacolon (severe chronic constipation, abdominal bloating, abdominal pain, fecaloma, risk of volvulus or bowel obstruction) and megaoesophagus (Dysphagia, odynophagia, regurgitation, chest pain, weight loss, aspiration pneumonia), and hepatosplenomegaly. Patients may experience malnutrition and weight loss over time (57, 58). Mixed mechanisms, including parasitic invasion, autoimmune responses, microvascular changes, and autonomic denervation, contribute to damage. Parasites persist in the GIT, especially in chronically infected individuals, with evidence of parasite DNA found in 20-50% of megaesophagus cases. Animal models show the colon and stomach as major reservoirs for the parasite, highlighting ongoing infection in the digestive tract (59). During the acute phase, high parasitemia allows direct detection through microscopy or PCR. In the chronic phase, low parasite levels require indirect methods like serologic tests (IgG detection) and molecular amplification techniques (60). Diagnostic strategies for Trypanosoma cruzi depend on disease stage. During the acute or reactivated phase, direct parasitological tests are preferred. Microscopy methods like fresh drop, thick drop, and buffy coat are low-cost and simple, but may miss low parasitemia and require trained staff. Microhematocrit and Strout techniques offer better sensitivity. Xenodiagnosis, though sensitive, is slow and impractical. Blood culture allows parasite isolation and typing, but is time-consuming and unsuitable for routine or urgent use (61). Treatment includes antiparasitic drugs—benznidazole or nifurtimox—which are most effective during the acute or early phases. However, these drugs often cause side effects such as nausea, vomiting, neuropathies, and central nervous system disturbances, leading to therapy discontinuation in 10–30% of cases. Nifurtimox is better tolerated in children (62).
Schistosoma spp.: The WHO seeks to eliminate schistosomiasis as a public health concern by 2030 (63). Species of the genus Schistosoma are digenetic trematodes responsible for the NTD, schistosomiasis. This parasitic disease ranks second only to malaria in terms of socioeconomic impact. Over 220 million people are currently infected worldwide, with 90% of cases occurring in sub-Saharan Africa (64). Katayama syndrome, an acute phase of schistosomiasis, typically occurs 2–6 weeks post-exposure. It presents with fever, fatigue, myalgia, cough, urticaria, and eosinophilia. Severe cases may involve neurological, cardiac, or pulmonary complications (65, 66). Chronic intestinal schistosomiasis, mainly caused by S. mansoni and S. japonicum, leads to inflammation, granulomas, ulcers, and fibrosis in the colon and rectum (67). Symptoms include abdominal pain, diarrhea, weight loss, and anemia. Polyps and, rarely, intestinal obstruction may occur. Hepatosplenic schistosomiasis causes portal hypertension, splenomegaly, anemia, and fibrosis with preserved liver function, often complicating diagnosis and monitoring (68). However, current morbidity indicators show weak correlation with infection intensity, posing challenges for effective disease surveillance and assessment. Even light-intensity infections contribute to both short-term and long-term schistosomiasis-related disability, affecting health-related quality of life (69). This raises concerns about the current WHO elimination strategy, which primarily targets the reduction of heavy infections, potentially overlooking the broader burden of disease (69, 70). Diagnosis requires high suspicion, especially in endemic-area patients. Despite its low sensitivity, microscopy remains the gold standard for diagnosing schistosomiasis. While several diagnostic approaches have been explored—based on circulating parasite proteins, genetic markers, egg morphology, and paramagnetic properties—none have yet proven robust enough to fully replace microscopy (71). However, protein-based biomarkers such as circulating cathodic antigen (CCA), circulating anodic antigen (CAA), and antibodies are widely used targets for detecting Schistosoma infection. Among these, the point-of-care CCA (POC-CCA) test is recommended by the WHO for surveillance of S. mansoni, offering a practical alternative, particularly in endemic regions (72). Antibody-based diagnostics for schistosomiasis face limitations in specificity and in distinguishing past from current infections. Nanobody technology, derived from the heavy chain of IgG antibodies, offers a promising alternative (73). Schistosomiasis treatment relies heavily on praziquantel, but its short-term effect and inability to prevent reinfection limit its impact. Drug repurposing and combination therapies targeting multiple parasite stages are essential to improve outcomes and reduce resistance. Integrated, multifaceted strategies are needed to effectively combat this NTD (74).
Fasciola spp.: It is estimated that between 2.6 million and 17 million people are infected with Fasciola spp. Globally. It primarily affects the hepatobiliary system, but it also has indirect effects on the GIT (75). The pathogenesis of acute fascioliasis is mainly caused by mechanical damage from the parasite’s tegument causes abrasion, tissue digestion, and damage from its suckers as it moves within the liver (76). The parasites’ suckers cause significant tissue damage, attaching to bile duct walls and puncturing blood vessels for feeding. These actions create hemorrhagic migratory lesions in the liver parenchyma (77). The new infective juveniles manipulate the host’s immune system, preventing a Th1-mediated response and promoting a Th2 immune response, aiding parasite survival (76). After 3 to 4 months, the parasites mature in the bile ducts and begin egg production. Chronic infections may be asymptomatic but can lead to biliary obstruction, abdominal pain, and other complications. Over time, adult parasites cause bile duct damage, leading to chronic inflammation, cholangitis, and cholecystitis (78). The pathological signs include fibrotic lesions, micro-abscesses, and necrotic tracts in the liver, with rare ectopic migration to other tissues like the lungs or brain. Early Fasciola spp. infection presents as white or gray liver nodules, progressing as the parasite invades the bile ducts. Its movement and spiny surface damage the biliary epithelium, causing fibrosis, necrosis, abscesses, and cholangiohepatitis. Severe cases can impair nutrition, lead to cholangiocarcinoma, or result in death (67, 75). Fascioliasis detection is essential for disease control, with direct and indirect methods used for diagnosis. Direct methods, such as fecal egg count, are effective in chronic infections but have limited sensitivity in the early stages. Improved flotation-sedimentation-based techniques like Flukefinder® and Mini-FLOTAC® enhance sensitivity for low egg burdens (79). Indirect methods, including ELISA, detect antibodies or antigens and offer early detection, though they can be costly and prone to false results. Molecular techniques like PCR provide high sensitivity and species-specific identification but require specialized equipment, limiting their field application (80). Triclabendazole is highly effective against both adult and immature flukes, but its overuse has led to resistance in some regions (81). Other drugs like albendazole, rafoxanide, and clorsulon also show varying effectiveness depending on the host species and infection stage.
Clonorchis sinensis and Opisthorchis spp.: Clonorchis sinensis infection causes biliary epithelial irritation, leading to hyperplasia, metaplasia, and mucinous bile production. This results in bile stasis, recurrent cholangiohepatitis, fibrosis, and possible stone formation. Though biliary cirrhosis is rare, chronic infection increases the risk of cholangiocarcinoma due to prolonged epithelial changes and carcinogen exposure (82). Chronic Opisthorchis spp. infection increases the risk of cholangiocarcinoma, recognized as a category I carcinogen. Persistent infection leads to bile duct dilation, fibrosis, metaplasia, and hyperplasia. Carcinogenesis may involve mechanical irritation, parasite metabolites, and carcinogenic nitrosamines, with host cytokine gene polymorphisms further enhancing susceptibility to malignancy (83).
Ascaris lumbricoides: Human ascariasis is a major NTD globally and remains a significant public health concern, particularly in low-resource settings, with 10.73% prevalence rate for studies published between 2018 and 2021 (84). In the GIT, Ascaris lumbricoides causes mechanical damage through its migration. Mature worms can obstruct the small intestine, leading to symptoms like abdominal pain, distention, nausea, and vomiting. Severe cases result in intestinal obstruction, necrosis, intussusception, or volvulus. Migration into the bile or pancreatic ducts can lead to biliary colic, obstructive jaundice, or pancreatitis (85). The most common method for diagnosing ascaris infection is the microscopic identification of eggs in stool samples (86). Although counting adult worms expelled after treatment is considered the “gold standard” for assessing infection intensity, this method is rarely used and typically limited to research studies rather than routine parasite surveillance or mass deworming program monitoring (84). A 2020 Cochrane review found that a single dose of albendazole, mebendazole, or ivermectin is a safe and effective treatment for ascariasis in both children and adults, achieving a 93% cure rate with minimal differences in effectiveness or side effects among the drugs (87).
Trichuris trichiura is a widespread intestinal parasite, commonly co-infecting with Ascaris. It often causes no symptoms, but children are more likely to develop symptoms such as growth delay, anemia, clubbing, colitis, and rectal prolapse (88). Adults with heavy infections may also suffer from inflammatory colitis, bloody diarrhea, and tenesmus. Severe cases are linked to increased tumor necrosis factor (TNF)-α production in the colon, which can cause appetite loss and wasting (89). Diagnosis is typically done through standard stool microscopy due to the high number of eggs shed, which appear barrel-shaped with distinctive plugs at both ends. Worms may also be seen during colonoscopy or in prolapse cases. PCR offers better species identification but with varied sensitivity. Eosinophilia may be present (89). A single dose of albendazole or mebendazole results in a cure rate of 25% to 33%. However, prolonged treatment with albendazole 400 mg for 3 to 7 days or mebendazole 500 mg daily or 100 mg twice a day for 3 to 7 days may be more effective in treating heavy infections with at least 1000 eggs per gram of stool (90).
Hookworm: Human hookworm infections are primarily caused by Necator americanus, A. ceylanicum and Ancylostoma duodenale. A. ceylanicum is a soil-transmitted helminth. A. ceylanicum is the second most common hookworm infecting humans in the Asia-Pacific region and is zoonotic, with dogs as the main reservoir. Unlike human-specific hookworms, it infects both animals and humans. The parasite has a direct life cycle, with infection occurring through ingestion or skin penetration of larvae from the environment. After entering the body, larvae migrate through the bloodstream and trachea before maturing in the small intestine (88, 91). Hookworm infections impose a substantial burden on endemic populations, with an estimated loss of 800,000–4 million disability-adjusted life years (92). Hookworm infections cause chronic intestinal blood loss through the feeding of adult worms, leading to anemia. The parasites disrupt the intestinal epithelium, affecting nutrient and iron absorption. Iron metabolism is altered, reducing iron availability and stimulating erythropoiesis. These effects contribute to anemia, especially in iron-deficient hosts (93). Hookworm-related blood loss occurs through capillary bleeding from feeding larvae and adults, and leakage at their intestinal attachment sites. The severity depends on the species and parasite load. Chronic bleeding, combined with poor iron intake and low iron stores, often leads to anemia, particularly in low-resource areas (94). Hepcidin (HAMP), a liver-derived peptide, plays a central role in this regulation by controlling the hepcidin-ferroportin axis, which is necessary for stabilizing serum iron levels. Factors like inflammation, low oxygen levels, and existing iron concentrations affect HAMP expression (95). During hookworm infection, HAMP is often downregulated, disrupting iron flow and influencing red blood cell production. This leads to increased erythropoietin release, which promotes red blood cell formation to compensate for oxygen loss (96). For uncomplicated cases, a 3-day regimen of mebendazole, with 100 mg taken twice daily 12 hours apart, is effective. Additionally, pyrantel pamoate, dosed at 11 mg/kg (up to a maximum of 1 g) daily for three days, is also effective in treating hookworm infections (97).
Strongyloides stercoralis: is a common parasitic roundworm that infects over 600 million people worldwide, especially in tropical climates. Despite its widespread presence, it remains largely ignored and is classified as a NTD due to insufficient awareness and limited research (98). This intestinal parasite can infect both humans and domestic animals such as cats and dogs, with potential for transmission between species (99). A particularly concerning feature of Strongyloides stercoralis is its capacity for autoinfection, where it reproduces inside the host without needing to exit, potentially leading to a dangerous condition called Strongyloides hyperinfection syndrome (100). This syndrome is especially deadly, with mortality rates surpassing 90 % in severe cases (101). Additionally, the parasite can weaken the host’s immune response, posing a serious risk to those with weakened immune systems, including individuals with HIV, those undergoing immunosuppressive treatment, people with alcoholism, and the malnourished (100). GI symptoms of Strongyloides hyperinfection syndrome range from mild discomfort to severe issues like hematemesis, distension, and shock. Diarrhea is common due to mucosal damage, enteritis, villous atrophy, and ulceration, potentially leading to sepsis. Though rare, liver and biliary complications include jaundice, papillary stenosis, and enzyme elevation, which can mimic graft-versus-host disease (102). Diagnosing this parasite is challenging because the number of larvae in the stool is often low and inconsistent, resulting in up to 70 % of cases being missed through routine fecal exams. Charcoal culture and agar plate culture also enhance sensitivity significantly. To increase diagnostic accuracy, a combination of methods such as duodenal fluid examination and serological testing is often needed (103). However, diagnostic techniques for strongyloidiasis lack standardization across methods (104). For uncomplicated strongyloides infection, ivermectin is preferred as it is effective against both larvae and adult worms. For patients who are not able to tolerate ivermectin, an alternative successful therapy consists of albendazole (400 mg for adults or 15 μg/kg for children twice a day for 3 to 7 days), which targets only the adult worms (105).
Bacterial infection
Vibrio cholerae (V. cholerae) is the causative agent of Cholera, which is an acute, watery diarrheal illness of a short incubation period (0.5–5 days) (106). Vibrio is a curved, rod-shaped, motile, Gram-negative bacterium that inhabits aquatic environments (107). It is classified into over 200 serogroups based on the structure of the O-antigen in its lipopolysaccharide (LPS). Among these, only strains from the O1 (three serotypes Ogawa, Hikojima, and Inaba) and O139 serogroups are capable of causing cholera and epidemics, as they produce cholera toxin (108). After ingestion, V. cholerae withstands the acidic environment of the stomach through an acid tolerance response. In the small intestine, it uses its flagellum to navigate the mucus layer and reach the epithelial cells, while evading host immune defenses and gut microbiota resistance. Successful colonization relies on the expression of key virulence factors, including toxin-coregulated pilus (TCP) and cholera toxin (CTX). Cholera is marked by profuse watery diarrhea and vomiting, which can quickly result in hypovolemic shock, metabolic acidosis, and death. Without treatment, the case-fatality rate may reach up to 50% (109). The infection can be confirmed using reliable and accessible diagnostic methods, such as PCR and RDTs. If not treated promptly, cholera can lead to severe dehydration and death. Management depends on the severity of the condition and may include oral rehydration salts (ORS), intravenous fluids, and antibiotics (108).
Salmonella enterica: Typhoid fever presents as a severe systemic illness characterized by fever and abdominal pain. It is marked by potentially life-threatening fever with a multitude of clinical signs and symptoms. The primary causative agent is a gram-negative bacterium, Salmonella enterica serotype Typhi (formerly S. typhi), although other serotypes, such as Paratyphi A, B, and C, can produce a similar clinical picture. After an incubation period of 6–30 days, the primary symptom of enteric fever is high fever, often accompanied by nausea, abdominal pain, and altered bowel habits (110). Increasing fever begins with secondary bacteremia in established typhoid infection. The gallbladder is colonized via hematogenous or local spread, especially with gallstones or structural pathology. Lymphoid tissue in Peyer’s patches is involved in primary, reinfection, and chronic infection, leading to fecal excretion and transmission. Proliferation of lymphoid tissue can cause constipation, while endotoxin-induced necrosis may lead to intestinal bleeding, perforation, or tertiary bacteremia (111). Blood culture remains the gold standard for diagnosing typhoid fever, though its sensitivity is limited. While bone marrow culture offers higher sensitivity, it is invasive, difficult to perform, and not practical for routine use. The Widal test is a simple, low-cost serological method used to detect antibodies against Salmonella O and H antigens. However, it has low sensitivity, is highly operator dependent, and its accuracy varies by region (112). In response to multidrug resistance (MDR) S. Typhi, fluoroquinolones (ciprofloxacin, ofloxacin, fleroxacin, pefloxacin) became common treatments. However, reports of reduced fluoroquinolone effectiveness emerged in endemic regions and among travelers. In areas with high MDR and fluoroquinolone resistance, azithromycin or ceftriaxone are preferred treatments (113).
Enterotoxigenic Escherichia coli (ETEC) is a leading cause of diarrheal disease, especially in children under five in endemic regions, contributing to around 100 million cases and 60,000 deaths in 2015. It is also a major cause of traveler’s diarrhea, with about one-third of affected travelers seeking medical care for GI symptoms (114–116). ETEC causes disease by producing heat-labile (LT) and/or heat-stable (ST) enterotoxins. A key step in ETEC infection is its attachment to the intestinal epithelium via colonization factors (CFs). After adherence, the enterotoxins trigger secretion of water and electrolytes into the intestinal lumen, leading to diarrhea (117–119). Diarrhea can lead to rapid dehydration and weakness, resembling cholera. Other symptoms may include vomiting, stomach cramps, headache, and occasionally mild fever (120). Beyond immediate illness, ETEC has been linked to long-term effects such as childhood stunting, weakened immunity, increased susceptibility to other infections, and impaired cognitive development (117). Traveler’s diarrhea caused by ETEC is also associated with post-infectious IBS (115). Management relies on adequate fluid intake, oral rehydration (or intravenous fluids if needed), avoiding sugary drinks, zinc supplementation, and antibiotics in severe cases. Recommended antibiotics include fluoroquinolones, macrolides, or rifaximin.
Leptospira: Leptospirosis, caused by Leptospira, is a worldwide zoonotic disease that is estimated to result in approximately 1 million cases and 60,000 deaths each year (121). Certain occupations, such as farming, ranching, veterinary work, rice farming, and military service, which involve exposure to animals or water, are linked to a higher risk of leptospirosis. In developed countries, travel-related infections and recreational activities have also emerged as significant sources of Leptospira infection. The clinical presentation of leptospirosis is often nonspecific, with symptoms ranging from asymptomatic cases to severe manifestations, including fatal multi-system organ failure (122). The incidence of leptospirosis in tropical regions is up to ten times higher than in other areas. This disparity is likely due to a combination of environmental and socioeconomic factors. Environmental factors, such as higher temperatures, humidity, and rainfall, create conditions that favor the survival of the causative organism. Socioeconomic factors, including poor sanitation and increased human contact with rodents and domestic animals, further contribute to the higher incidence in these regions (122, 123). The incubation period for leptospirosis typically ranges from 7 to 12 days. Leptospirosis typically begins with sudden fever, chills, muscle pain, and a throbbing frontal headache, resembling many other febrile illnesses. A hallmark sign is conjunctival suffusion (redness without discharge). GI symptoms like nausea, vomiting, diarrhea, and anorexia are common, and about half of the patients develop a dry cough. Aseptic meningitis occurs in up to 80% of cases. Less commonly, patients may experience hepatosplenomegaly, lymphadenopathy, or pharyngitis. Fulminant disease in leptospirosis can manifest as Weil’s disease, a severe form of the illness characterized by the triad of jaundice, renal failure, and hemorrhagic manifestations (124, 125). In many cases, clinical suspicion alone may justify the initiation of empiric antibiotic treatment. Definitive diagnosis of leptospirosis can generally be achieved through traditional microbiological methods, such as direct detection or culture, or through serological testing. Serological methods are the most widely used approach for confirming a diagnosis of leptospirosis. Among these, the microscopic agglutination test (MAT) is considered the “gold standard. For mild cases, oral antibiotics (doxycycline, azithromycin, ampicillin, or amoxicillin) are options. While in severe cases, intravenous antibiotics are preferred (126).
Shigella: Shigellosis caused by Shigella species (Shigella flexneri [commonest], Shigella sonnei, Shigella dysenteriae [most severe], and Shigella boydii) infect humans using virulence factors such as Shiga toxin (Stx), which is cytotoxic, neurotoxic, and enterotoxin, and a type III secretion system (T3SS) with effector proteins that cause the severe symptoms of shigellosis (127). The incubation period is 1–4 days, with an infectious dose of as few as 200 living organisms. Key challenges in controlling shigellosis include the high transmissibility of Shigella between individuals and its swift development of antimicrobial resistance (AMR). The most common symptoms of shigellosis include acute diarrhea (which may be watery, mucoid, or bloody), tenesmus, abdominal discomfort, nausea, and vomiting (128). Shigellosis-associated diarrheal diseases account for an estimated 125 million cases annually, leading to approximately 160,000 deaths worldwide (129). Besides travelers’ diarrhea, shigellosis has been associated with complications such as post-infectious IBS in travelers (130) and acalculous cholecystitis (131). Although most patients recover without complications within seven to ten days, serious outcomes can occur, including metabolic disturbances, sepsis, convulsions, rectal prolapse, toxic megacolon, intestinal perforation, and hemolytic-uremic syndrome (HUS) (128). Microscopy of fresh stool is a quick, inexpensive test for invasive bacterial diarrhea, with polymorphonuclear leukocytes (PMNs) suggesting a bacterial cause but not specific to Shigella. Definitive diagnosis requires stool culture and serotyping, as well as antimicrobial sensitivity testing. PCR methods are sensitive but not routine or useful for resistance testing (128, 132). Treatment primarily involves supportive care (hydration and electrolyte management). Antimotility drugs are contraindicated. Antimicrobial therapy is reserved for severe cases or high-risk patients, with fluoroquinolones, azithromycin, or third-generation cephalosporins as options. Increasing AMR, particularly to macrolides, penicillin, and cephalosporins, complicates treatment. In high-risk populations, carbapenems or colistin may be necessary. For pediatric cases, azithromycin is the empiric therapy, with cefixime as alternative (132).
Campylobacter: Campylobacteriosis is caused by Campylobacter which is a gram-negative, spiral-shaped, microaerophilic bacterium frequently linked to foodborne diseases. Over 95% of human infections are caused by C. jejuni and C. coli (133). The infectious dose of Campylobacter is relatively low, requiring only 500–800 bacteria to cause infection. Children below 4 years of age and elderly individuals aged 75 or older are more susceptible to the infection. Infection may lead to long-term complications such as IBS, arthritis, and Guillain–Barré Syndrome (GBS), septic thrombophlebitis, endocarditis, neonatal sepsis, pneumonia, sepsis, acute colitis associated with IBD, colorectal cancer, Barrett’s esophagus, carcinoid syndrome, and acute appendicitis (134–136). The isolation and identification of Campylobacter species depend on culture-based methods that use selective media with antibiotics, such as Skirrow’s medium, mCCDA, and Bolton broth. Antibiotics are not routinely recommended for healthy patients with Campylobacter infections, but may be considered for high-risk groups, such as immunocompromised or elderly individuals, and those with severe symptoms like fever, bloody stools, or significant abdominal pain. Immunocompromised patients may need multiple antibiotic courses. Antibiotics usually are not required; however, it is indicated if there is high fever, excessive bowel movements (>8 stools per day), bloody diarrhea, persistence of symptoms for longer than 1 week, pregnancy, or immunocompromised states. Macrolides (Azithromycin with a typical regimen of 500 mg/d for 3 days) are the preferred treatment due to rising AMR patterns (137).
Viral infection
Rotaviruses: Rotaviruses are non-enveloped, double-stranded RNA viruses that represent a leading cause of severe and potentially fatal diarrhea in children under five years old worldwide. Nearly every child globally is infected by the age of 3 to 5 years (138). Along with non-bloody diarrhea, which is more than average in severity, rotavirus infection often leads to vomiting, malaise, and fever. Notably, vomiting is a distinctive and characteristic feature of rotavirus disease (139). Rotavirus causes osmotic diarrhea due to malabsorption, which results from damage or death of enterocytes, as well as reduced epithelial absorptive function. Additionally, it induces secretory diarrhea through the action of the viral protein nonstructural protein 4 (NSP4) and the activation of the enteric nervous system (ENS) (140). Rotavirus antigens can be detected in stool samples using ELISA or immunochromatography. Reverse Transcription Polymerase Chain Reaction (RT-PCR) offers higher sensitivity and specificity, enabling the identification of rotavirus genotypes and providing detailed insights into viral circulation, strain diversity, and vaccine effectiveness (141).
HIV: Gastrointestinal complications in HIV include primary small bowel lymphoma, which predominantly affects the terminal ileum and presents with bowel wall thickening and masses (142). Cytomegalovirus (CMV) colitis, common in immunocompromised patients with low CD4 counts, shows bowel thickening and ulceration, often mistaken for IBD. Anal squamous cell carcinoma, linked to persistent Human papilloma virus (HPV) infection, is more prevalent in HIV patients and appears as enhancing masses on imaging (143). Gl tuberculosis, typically affecting the terminal ileum, presents with thickening and lymphadenopathy (144), while Kaposi sarcoma, an AIDS-related cancer, often involves the duodenum, displaying polypoid masses and lymphadenopathy (145).
Diagnostic approach
Diagnosing GI manifestations of tropical diseases is challenging due to symptom overlap with common GI conditions. A thorough exposure history—including recent travel to endemic areas, occupational risks, animal contact, and consumption of untreated water or raw foods—is essential. Understanding the patient’s immune status, especially in immunocompromised individuals, is also crucial. Symptom characterization helps narrow the differential diagnosis: watery diarrhea may indicate Vibrio cholerae, ETEC, or rotavirus; bloody diarrhea suggests Shigella, E. histolytica, or Campylobacter; while chronic diarrhea should prompt evaluation for Giardia, Cryptosporidium, or Strongyloides stercoralis. Additional systemic symptoms, such as fever or weight loss, further refine the clinical picture.
Laboratory investigations play a key role in diagnosis. First-line tests include microscopy for parasites, stool antigen detection, bacterial cultures, and serological tests for various infections. However, many pathogens are difficult to detect due to intermittent shedding, which may require repeat stool samples and advanced techniques like multiplex PCR. In resource-limited settings, cost-effective methods remain valuable, particularly for parasitic infections. Emerging technologies, such as point-of-care diagnostics and artificial intelligence (AI) assisted microscopy, offer potential improvements in diagnostic speed and sensitivity (Figure 2).
Prevention and public health strategies
Improvement of social determinants of health: Addressing infectious diseases through the lens of social determinants of health requires integrating public health measures, personal hygiene practices, vector control, food safety, therapeutics, and education. Access to clean water, sanitation, and healthcare infrastructure reduces exposure to infectious agents, while education and hygiene resources empower preventive behaviors (146). Improved housing and environmental management minimize vector exposure, such as mosquitoes and ticks. Food safety relies on pasteurization, proper cooking, regulatory enforcement, and consumer knowledge (146, 147). Equitable access to affordable medications and timely healthcare ensures effective treatment, and tailored education campaigns enhance disease prevention awareness. Programs like Water, Sanitation, and Hygiene (WASH) and Health Education Learning Package (HELP), along with measures like wearing shoes and routine deworming, help prevent hookworm infections (147). However, reinfection is common in endemic areas, and current mass MDA efforts often fall short in reducing anemia or long-term prevalence, with drug resistance emerging as a concern (97).
Surveillance: Surveillance involves the regular collection, analysis, interpretation, and dissemination of data. It is crucial for the effective control of tropical diseases. Prompt detection and reporting of bloody diarrhea cases are crucial for monitoring endemic shigellosis, controlling epidemic shigellosis, and managing dysentery outbreaks caused by enterohemorrhagic E. coli. For HIV and bloodborne infections, safe sex practices, needle exchange programs, and blood donation screening are vital, supported by health education, and surveillance to prevent outbreaks and reduce transmission.
Chemotherapy: The two primary intervention strategies for NTDs are preventive chemotherapy (PCT) and transmission control, which encompass MDA and innovative and intensified disease management (148). However, the WHO has found that less than two-thirds of the global population in need of treatment for NTDs currently receive it (149). PCT-based MDA programs have been considered effective; however, the potential development of drug resistance due to prolonged and continuous use remains a significant challenge (150). In recent years, AMR has become an escalating concern due to the widespread use of antimicrobials, leading to a faster rate of resistance development and spread to many infections, including Salmonella (151), Vibrio cholerae (152), ETEC (119), and shigellosis (153). Unfortunately, the geographic spread of emerging AMR has been driven by increased global travel, impacting both individuals in endemic regions and international travelers, while the development of new antimicrobials has not kept pace with this growing threat (154). Addressing AMR requires coordinated, multidisciplinary action across local, national, and global levels. Strong political will is essential for developing and enforcing evidence-based policies, regulating antibiotic use in both humans and animals, and ensuring ongoing educational initiatives. Controlling unethical antibiotic promotion and curbing misuse are critical steps. Emerging strategies aimed at improving antibiotic effectiveness—such as targeting, inactivating, or editing resistance genes—show promise, especially since many of these innovative approaches do not contribute to further resistance (155).
Vaccination: This measure is not restricted to humans; it extends to animal vaccination as well. Animal vaccination can indirectly protect human health. For instance, Campylobacter poses no threat to poultry health, vaccinating chickens could significantly reduce human campylobacteriosis by lowering bird-to-human transmission and decreasing the need for costly post-harvest interventions. However, since there is no direct economic benefit to farmers, government involvement is likely needed to support vaccine adoption (156). Regarding human vaccination: several vaccines are currently available for this tropical disease, with additional candidates in the development pipeline (17, 119). Oral cholera vaccines (OCVs) are effective in preventing cholera and should be integrated into a comprehensive cholera control strategy, particularly in endemic regions and during outbreaks. In addition, there are two widely available typhoid vaccines: Vivotif®, an enteric-coated capsule containing the live attenuated Ty21a strain, and TYPHIM Vi®, a liquid formulation of the unconjugated Vi polysaccharide (ViPS) vaccine (110). However, it should be used in conjunction with other interventions, such as WASH measures (152). As of December 2023, rotavirus vaccines have been introduced in 123 countries, achieving an estimated global coverage rate of 55% (157). This widespread implementation has led to significant declines in disease burden: hospitalizations due to all-cause diarrhea in children under five decreased by a median of 38% (ranging from 5–63%), hospitalizations specifically linked to rotavirus disease dropped by a median of 67% (ranging from 18–84%), and deaths from all-cause diarrhea fell by 42% (ranging from 3–64%) (158).
One Health approach: This approach aims to achieve improved health outcomes for humans, animals, and the environment through collaborative and interdisciplinary efforts (159). The One Health approach recognizes that the health of humans, domestic and wild animals, plants, and the wider environment (including ecosystems) are closely interconnected and interdependent. This framework is increasingly being utilized in the management, control, and even elimination of tropical diseases (160). The One Health approach continuously aims to interrupt ongoing transmission, diagnose and treat current cases in both humans and animals, and prevent future transmission scenarios from re-emerging (161). To achieve the goals of the One Health approach, key strategies such as epidemiological surveys, identification and diagnosis of NTDs, effective disease control measures, and vector and pest management must be comprehensively addressed (162). The WHO has developed a road map to support a range of stakeholders, including countries where endemic, international organizations, and non-state actors. This road map emphasizes the importance of a transdisciplinary, cross-cutting One Health approach to achieve effective control and, ultimately, the elimination of these diseases (159). Due to the nature of zoonotic NTDs, eradication is rarely achievable. Persistent animal reservoirs continually pose a risk of re-infection, making local elimination in human populations the most realistic public health objective. Achieving this goal requires a One Health approach that integrates coordination across various sectors and disciplines. This includes interrupting active transmission, diagnosing and treating existing cases in both humans and animals, and preventing future outbreaks by addressing human behaviors that increase contact with animals or disease vectors (160). One major barrier to unlocking the full potential of multisectoral approaches is the absence of targeted advocacy, sustainable funding models, and shared indicators that are meaningful and applicable across all involved sectors (163).
Climate change and tropical disease: Climatic factors play a significant role in influencing the growth rate and expression of virulence in pathogens. Most Vibrio species, including Vibrio cholerae, exhibit accelerated growth at elevated temperatures, which is reflected in their higher environmental isolation rates during warmer months (164). Many NTDs are often influenced by changing environmental conditions such as temperature, rainfall, humidity, and extreme weather events (165). Climatic factors play a critical role in shaping disease epidemiology. For instance, temperature affects vector reproduction, metabolism, survival, pathogen replication, and the geographic distribution of both vectors and hosts. Rainfall, on the other hand, influences the availability of breeding sites, thereby determining the suitability of habitats for vectors and hosts (166). Diseases like dengue and chikungunya, transmitted by Aedes mosquitoes, are spreading to new regions due to warmer temperatures, which accelerate mosquito breeding and expand their range. This has led to surges in dengue cases in areas such as Nepal, North America, and central Europe. Similarly, climatic changes favoring insect survival and mobility are likely to increase the spread of sand flies that transmit leishmaniasis (167). Furthermore, extreme meteorological events, such as floods and heat waves, can disrupt ecosystems by destroying habitats and increasing mortality rates among vectors and hosts (168).
Conclusions
Tropical diseases with GI manifestations pose significant challenges to global public health, particularly in low-resource settings. Caused by a diverse array of pathogens—including helminths, protozoa, bacteria, and viruses—these diseases contribute substantially to morbidity and mortality worldwide. This review highlights the critical need for interdisciplinary collaboration to enhance understanding of disease mechanisms, improve diagnostic accuracy, and develop effective therapeutic and preventive strategies. Despite advancements in detection technologies and treatment options, numerous barriers persist, including limited healthcare access, AMR, and inadequate surveillance systems. Addressing these challenges requires a coordinated, multisectoral approach aligned with global initiatives such SDG 3.3 and the WHO’s 2030 roadmap for NTDs.
The One Health framework emerges as a vital strategy for tackling zoonotic and environmentally driven infections. By integrating human, animal, and environmental health perspectives, this approach offers a sustainable model for disease control that also addresses underlying socioeconomic factors contributing to health disparities.
To achieve global health objectives and reduce the burden of tropical diseases, intensified efforts, increased funding, and innovative strategies are essential. These include improving water and sanitation infrastructure, strengthening vector control, expanding vaccination programs, and enhancing education to prevent misdiagnosis and ensure timely treatment. Such measures will not only mitigate disease impact but also promote equitable healthcare, improve nutritional outcomes, and build resilience to emerging health threats.
Recommendation for future research
Future research should prioritize the development of standardized diagnostic protocols to improve accuracy and consistency in identifying GI manifestations of tropical diseases. Expanding vaccine coverage, particularly for preventable infections such as cholera and typhoid fever, and implementing targeted interventions tailored to endemic populations are also essential steps in reducing disease burden.
In addition, longitudinal studies are needed to assess the long-term health outcomes of GI tropical diseases, especially among vulnerable groups such as children and immunocompromised individuals. These studies can help identify chronic sequelae such as post-infectious Irritable bowel syndrome (PI-IBS), malnutrition, and developmental delays, which contribute significantly to morbidity and perpetuate cycles of poverty. Emerging technologies offer promising tools for advancing diagnosis and treatment. AI, for instance, has the potential to enhance the detection of rare or asymptomatic cases through improved image analysis, pattern recognition, and predictive modeling. Furthermore, investigating the interaction between tropical pathogens and the gut microbiome may uncover novel disease mechanisms and inform the development of microbiota-based therapeutics.
An interdisciplinary approach that integrates environmental science, sociology, economics, and public health will be crucial for understanding the root causes behind the persistence of tropical diseases in certain regions. Such insights can guide more effective, context-specific interventions. Finally, comparative effectiveness research is warranted to evaluate key public health strategies such as WASH programs, MDA, and vaccination campaigns. This evidence will support data-driven policy decisions and optimize resource allocation to achieve sustainable reductions in disease prevalence.
Author contributions
RG: Writing – original draft, Visualization, Conceptualization, Writing – review & editing, Methodology, Supervision. SMA: Writing – original draft, Visualization, Conceptualization, Writing – review & editing, Methodology, Supervision. SAA: Data curation, Formal analysis, Investigation, Writing – review & editing. HA: Investigation, Validation, Writing – review & editing. AAA: Methodology, Software, Visualization, Writing – review & editing. AFA: Formal analysis, Writing – review & editing. HE: Data curation, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. It was used to check grammar mistakes and linguistic improvement. Artificial intelligence was also used to generate the diagnostic approach figure. We provided the data, and MindMap generated the illustration.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
NTDs: Neglected Tropical Diseases
GI: Gastrointestinal
GIT: Gastrointestinal Tract
WHO: World Health Organization
CDC: Centers for Disease Control and Prevention
ETEC: Enterotoxigenic Escherichia coli
IBS: Irritable Bowel Syndrome
IBD: Inflammatory Bowel Disease
SDGs: Sustainable Development Goals
iRBCs: Infected Red Blood Cells
LPPG: Lipophosphopeptidoglycan
PPGs: Proteophosphoglycans
ALA: Amebic Liver Abscess
SBO: Small Bowel Obstruction
STHs: Soil-Transmitted Helminths
OCVs: Oral Cholera Vaccines
ViPS: Vi Polysaccharide
WASH: Water, Sanitation, and Hygiene
AMR: Antimicrobial Resistance
MDA: Mass Drug Administration
PCT: Preventive Chemotherapy and Transmission Control
LT: Heat-Labile Toxin (of ETEC)
ST: Heat-Stable Toxin (of ETEC)
CFs: Colonization Factors (of ETEC)
TCP: Toxin-Coregulated Pilus (of Vibrio cholerae)
CTX: Cholera Toxin (of Vibrio cholerae)
MAT: Microscopic Agglutination Test
HUS: Hemolytic-Uremic Syndrome
GBS: Guillain–Barré Syndrome
PI-IBS: Post-Infectious Irritable Bowel Syndrome
ORS: Oral Rehydration Salts
PCR: Polymerase Chain Reaction
RDTs: Rapid Diagnostic Tests
ELISA: Enzyme-Linked Immunosorbent Assay
RT-PCR: Reverse Transcription Polymerase Chain Reaction
NSP4: Nonstructural Protein 4 (of Rotavirus)
CMV: Cytomegalovirus
HPV: Human Papillomavirus
MAC: Mycobacterium Avium Complex
HAMP: Hepcidin
PMNs: Polymorphonuclear Leukocytes
T3SS: Type III Secretion System
Stx: Shiga Toxin
ENS: Enteric Nervous System
References
1. Zumla A and Ustianowski A. Tropical diseases: definition, geographic distribution, transmission, and classification. Infect Dis Clin North Am. (2012) 26:195–205. doi: 10.1016/j.idc.2012.02.007
2. World Health Organization. Global report on neglected tropical diseases 2023. Geneva, Switzerland: World Health Organization (2023).
3. Cook GC. Tropical gastroenterological problems. Manson’s Trop Dis. (2009), 107–41. doi: 10.1016/B978-1-4160-4470-3.50014-8
4. Organization, W.H. Ending the neglect to attain the sustainable development goals: a rationale for continued investment in tackling neglected tropical diseases 2021–2030. Geneva, Switzerland: World Health Organization (2022).
5. Becker SL, Vogt J, Knopp S, Panning M, Warhurst DC, Polman K, et al. Persistent digestive disorders in the tropics: causative infectious pathogens and reference diagnostic tests. BMC Infect Dis. (2013) 13:37. doi: 10.1186/1471-2334-13-37
6. Vanhooren M, Stoefs A, Van Den Broucke S, Van Esbroeck M, Demuyser T, and Kindt S. Intestinal helminthic infections: a narrative review to guide the hepatogastro-enterologist. Europe. (2023) 3:4.doi: 10.51821/86.3.11895
7. Anderson MS, Mahugu EW, Ashbaugh HR, Wellbrock AG, Nozadze M, Shrestha SK, et al. Etiology and epidemiology of travelers’ Diarrhea among US military and adult travelers, 2018–2023. Emerging Infect Dis. (2024) 30:S19. doi: 10.3201/eid3014.240308
8. Fauziah N, Aviani JK, Agrianfanny YN, and Fatimah SN. Intestinal parasitic infection and nutritional status in children under five years old: A systematic review. Trop Med Infect Dis. (2022) 7:1–26. doi: 10.3390/tropicalmed7110371
9. Findlater A and Bogoch II. Human mobility and the global spread of infectious diseases: A focus on air travel. Trends Parasitol. (2018) 34:772–83. doi: 10.1016/j.pt.2018.07.004
10. Leung AKC, Leung AAM, Wong AHC, and Hon KL. Travelers’ Diarrhea: A clinical review. Recent Pat Inflammation Allergy Drug Discov. (2019) 13:38–48. doi: 10.2174/1872213X13666190514105054
11. Polman K, Becker SL, Alirol E, Bhatta NK, Bhattarai NR, Bottieau E, et al. Diagnosis of neglected tropical diseases among patients with persistent digestive disorders (diarrhoea and/or abdominal pain ≥14 days): Pierrea multi-country, prospective, non-experimental case-control study. BMC Infect Dis. (2015) 15:338. doi: 10.1186/s12879-015-1074-x
12. Ghazy RM, Tahoun MM, Abdo SM, El-Badry AA, and Hamdy NA. Evaluation of praziquantel effectivenss after decades of prolonged use in an endemic area in Egypt. Acta Parasitologica. (2021) 66:81–90. doi: 10.1007/s11686-020-00242-x
13. Lapat JJ, Opee J, Apio MC, Akello S, Ojul CL, Onekalit R, et al. A One Health approach toward the control and elimination of soil-transmitted helminthic infections in endemic areas. IJID One Health. (2024) 2:100021. doi: 10.1016/j.ijidoh.2024.100021
14. Ghazy RM, Saidouni A, and Taha SHN. Promoting child health through a comprehensive One Health perspective: a narrative review. Egyptian Pediatr Assoc Gazette. (2024) 72:3. doi: 10.1186/s43054-023-00243-1
15. Khan AI, Islam MT, Tanvir NA, Khan ZH, Amin MA, Firoj MG, et al. Diarrhea and cholera surveillance for early warning and preparedness to prevent epidemics among Rohingya Myanmar nationals in Cox’s Bazar, Bangladesh. Heliyon. (2024) 10:e37562. doi: 10.1016/j.heliyon.2024.e37562
16. Bangert M, Molyneux DH, Lindsay SW, Fitzpatrick C, and Engels D. The cross-cutting contribution of the end of neglected tropical diseases to the sustainable development goals. Infect Dis Poverty. (2017) 6:73. doi: 10.1186/s40249-017-0288-0
17. Williams PC and Berkley JA. Guidelines for the treatment of dysentery (shigellosis): a systematic review of the evidence. Paediatrics Int Child Health. (2018) 38:S50–65. doi: 10.1080/20469047.2017.1409454
18. Luangtongkum T, Morishita TY, Ison AJ, Huang S, McDermott PF, and Zhang Q. Effect of conventional and organic production practices on the prevalence and antimicrobial resistance of Campylobacter spp. in poultry. Appl Environ Microbiol. (2006) 72:3600–7. doi: 10.1128/AEM.72.5.3600-3607.2006
19. Faisal SM, McDonough SP, and Chang Y-F. Leptospira: invasion, pathogenesis and persistence. In: Embers ME, editor. The Pathogenic Spirochetes: strategies for evasion of host immunity and persistence. Springer US, Boston, MA (2012). p. 143–72.
20. Mustafa HM. Pathophysiology of medical parasites: mechanisms of disease and immune evasion. Eur J Ecology Biol Agric. (2024) 1:49–64. doi: 10.59324/ejeba.2024.1(5).04
21. Van Den Broucke S, Van Den Broucke S, Verschueren J, Van Esbroeck M, Bottieau E, and Van den Ende J. Clinical and microscopic predictors of Entamoeba histolytica intestinal infection in travelers and migrants diagnosed with Entamoeba histolytica/dispar infection. PLoS Negl Trop Dis. (2018) 12:e0006892. doi: 10.1371/journal.pntd.0006892
23. Garcia LS. Amebiasis. In: Sexually Transmitted Diseases. Boca Raton, FL, USA: CRC Press (2023). p. 167–88.
24. Gupta S, Smith L, and Diakiw A. Amebiasis and amebic liver abscess in children. Pediatr Clin North Am. (2022) 69:79–97. doi: 10.1016/j.pcl.2021.08.003
25. Yanagawa Y, Izumiyama S, Saito-Nakano Y, Nakada-Tsukui K, Kobayashi S, Yoshida N, et al. Gene expression of axenically-isolated clinical Entamoeba histolytica strains and its impact on disease severity of amebiasis. PLoS Pathog. (2022) 18:e1010880. doi: 10.1371/journal.ppat.1010880
26. Kaufman AR and Tu EY. Advances in the management of Acanthamoeba keratitis: A review of the literature and synthesized algorithmic approach. Ocul Surf. (2022) 25:26–36. doi: 10.1016/j.jtos.2022.04.003
27. Dunn N and Juergens AL. Giardiasis. In: StatPearls. StatPearls Publishing Copyright © 2025, StatPearls Publishing LLC, Treasure Island (FL (2025).
28. Allain T, Amat CB, Motta JP, Manko A, and Buret AG. Interactions of Giardia sp. with the intestinal barrier: Epithelium, mucus, and microbiota. Tissue Barriers. (2017) 5:e1274354.doi: 10.1080/21688370.2016.1274354
29. Yang Y, Zhao Y, Liu L, Zhu W, Jia S, Li X, et al. The anti-apoptotic role of COX-2 during in vitro infection of human intestinal cell line by Giardia duodenalis and the potential regulators. Infection Immun. (2022) 90:e00672–21. doi: 10.1128/iai.00672-21
30. Solaymani-Mohammadi S. Mucosal defense against giardia at the intestinal epithelial cell interface. Front Immunol. (2022) 13:817468. doi: 10.3389/fimmu.2022.817468
32. Hurník P, Žiak D, Dluhošová J, Židlík V, Šustíková J, Uvírová M, et al. Another case of coincidental Giardia infection and pancreatic cancer. Parasitol Int. (2019) 71:160–2. doi: 10.1016/j.parint.2019.04.013
33. Uchôa FFM, Sudré AP, Campos SDE, and Almosny NRP. Assessment of the diagnostic performance of four methods for the detection of Giardia duodenalis in fecal samples from human, canine and feline carriers. J Microbiol Methods. (2018) 145:73–8. doi: 10.1016/j.mimet.2018.01.001
34. Rossi F, Amadoro C, Santonicola S, Marino L, and Colavita G. Protozoan Infections Acquired from Food or Drinking Water: An Update. Basel, Switzerland: Preprints. org (2024).
35. da Silva RKM, Dib LV, Amendoeira MR, Class CC, Pinheiro JL, Fonseca ABM, et al. Balantidiasis in humans: A systematic review and meta-analysis. Acta Trop. (2021) 223:106069. doi: 10.1016/j.actatropica.2021.106069
36. Almaw A, Berhan A, Solomon Y, Malkamu B, Eyayu T, Workineh L, et al. Balantidium coli; rare and accidental finding in the urine of pregnant woman: case report. Int Med Case Rep J. (2022) 15:105–9. doi: 10.2147/IMCRJ.S355536
37. de Oliveira AS, Gómez-Hernández C, and Rezende-Oliveira K. Balantidium coli infection, immune status and comorbidities: literature review. Rev Patologia Tropical/Journal Trop Pathol. (2021) 50:265–84. doi: 10.22201/facmed.24486757e.2021.50.4.3270
38. Kombo Mpindou GOM, Escuder Bueno I, and Chordà Ramón E. Review on emerging waterborne pathogens in Africa: the case of Cryptosporidium. Water. (2021) 13:2966. doi: 10.3390/w13212966
39. Helmy YA and Hafez HM. Cryptosporidiosis: from prevention to treatment, a narrative review. Microorganisms. (2022) 10:2456. doi: 10.3390/microorganisms10122456
40. O’Leary JK, Blake L, Corcoran GD, Sleator RD, and Lucey B. A novel genotyping method for Cryptosporidium hominis. Exp Parasitol. (2021) 225:108113. doi: 10.1016/j.exppara.2021.108113
41. Khalil IA, Troeger C, Rao PC, Blacker BF, Brown A, Brewer TG, et al. Morbidity, mortality, and long-term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: a meta-analyses study. Lancet Global Health. (2018) 6:e758–68. doi: 10.1016/S2214-109X(18)30283-3
42. Balendran T, Iddawela D, and Lenadora S. Cryptosporidiosis in a zoonotic gastrointestinal disorder perspective: present status, risk factors, pathophysiology, and treatment, particularly in immunocompromised patients. J Trop Med. (2024) 2024:6439375. doi: 10.1155/2024/6439375
43. Goudal A, Laude A, Valot S, Desoubeaux G, Argy N, Nourrisson C, et al. Rapid diagnostic tests relying on antigen detection from stool as an efficient point of care testing strategy for giardiasis and cryptosporidiosis? Evaluation of a new immunochromatographic duplex assay. Diagn Microbiol Infect Dis. (2019) 93:33–6. doi: 10.1016/j.diagmicrobio.2018.07.012
44. Caravedo MA and White AC Jr. Treatment of cryptosporidiosis: nitazoxanide yes, but we can do better. Expert Rev Anti Infect Ther. (2023) 21:167–73. doi: 10.1080/14787210.2023.2160704
45. Khan SM and Witola WH. Past, current, and potential treatments for cryptosporidiosis in humans and farm animals: A comprehensive review. Front Cell infection Microbiol. (2023) 13:1115522. doi: 10.3389/fcimb.2023.1115522
46. Organization, W.H. World malaria report 2023. Geneva, Switzerland: World Health Organization (2023).
47. Venkatesan P. WHO world malaria report 2024. Lancet Microbe. (2025) p:101073. doi: 10.1016/j.lanmic.2025.101073
48. Poespoprodjo JR, Douglas NM, Ansong D, Kho S, Anstey NM, et al. Malaria. Lancet. (2023) 402:2328–45. doi: 10.1016/S0140-6736(23)01249-7
49. Coban C, Lee MSJ, and Ishii KJ. Tissue-specific immunopathology during malaria infection. Nat Rev Immunol. (2018) 18:266–78. doi: 10.1038/nri.2017.138
50. Mabey D, Brown A, and Greenwood B. Plasmodium falciparum malaria and Salmonella infections in Gambian children. J Infect Dis. (1987) 155:1319–21. doi: 10.1093/infdis/155.6.1319
51. Sey IC, Ehimiyein AM, Bottomley C, Riley EM, and Mooney JP. Does malaria cause diarrhoea? A systematic review. Front Med. (2020) 7:589379. doi: 10.3389/fmed.2020.589379
52. Mutinda L, Gichuru D, Siria E, Lumarai M, Lee G, and Maina S. New onset heartburn as a gastrointestinal symptom of severe malaria: 244. Off J Am Coll Gastroenterol | ACG. (2011) 106:S244. doi: 10.14309/00000434-201110002-00244
53. Mirsky IA, Brecht RV, and Williams LD. Hepatic dysfunction in malaria. Science. (1944) 99:20–1. doi: 10.1126/science.99.2558.20
54. Kokori E, Akinoso A, Egbunu E, Aremu SA, et al. Triple artemisinin-based combination therapy (TACT): advancing malaria control and eradication efforts. Malar J. (2024) 23:25. doi: 10.1186/s12936-024-04844-y
55. Ippolito MM, Kamavu LK, Banda PM, Yanek LR, et al. Whole blood transfusion for severe malarial anemia in a high plasmodium falciparum transmission setting. Clin Infect Dis. (2022) 75:1893–902. doi: 10.1093/cid/ciac304
56. World Health Organization. Chagas disease (also known as American trypanosomiasis) (2025). Available online at: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (Accessed April 1, 2025).
57. Iantorno G, Lumi CM, Cabanne AM, Fisogni S, et al. The enteric nervous system in chagasic and idiopathic megacolon. Am J Surg Pathol. (2007) 31:460–8. doi: 10.1097/01.pas.0000213371.79300.a8
58. Orozco VHG, Ocampo CMI, Sandoval CID, Gallegos CAO, et al. Digestive disorders in chagas disease: megaesophagus and chagasic megacolon. In: Chagas Disease-From Cellular and Molecular Aspects of Trypanosoma cruzi-Host Interactions to the Clinical Intervention. London, UK: IntechOpen (2022).
59. Carvalho LM, de Souza Marques F, Roatt BM, da Silva Fonseca K, et al. Histopathological changes in the gastrointestinal tract and systemic alterations triggered by experimental oral infection with Trypanosoma cruzi. Exp Parasitol. (2020) 218:108012. doi: 10.1016/j.exppara.2020.108012
60. Mendicino D, Colussi C, and Moretti E. Simultaneous use of two rapid diagnostic tests for the diagnosis of Chagas disease. Trop Doctor. (2019) 49:23–6. doi: 10.1177/0049475518813792
61. Lopez-Albizu C, Rivero R, Ballering G, Freilij H, Santini MS, Bisio MMC, et al. Laboratory diagnosis of Trypanosoma cruzi infection: a narrative review. Front Parasitol. (2023) 2:1138375. doi: 10.3389/fpara.2023.1138375
62. García-Huertas P and Cardona-Castro N. Advances in the treatment of Chagas disease: Promising new drugs, plants and targets. Biomedicine Pharmacotherapy. (2021) 142:112020. doi: 10.1016/j.biopha.2021.112020
63. Organization, W.H. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030. In: Ending the neglect to attain the sustainable development goals: a road map for neglected tropical diseases 2021–2030. Geneva, Switzerland: World Health Organization (2020).
64. Mawa PA, Kincaid-Smith J, Tukahebwa EM, Webster JP, and Wilson S. Schistosomiasis morbidity hotspots: roles of the human host, the parasite and their interface in the development of severe morbidity. Front Immunol. (2021) 12:635869. doi: 10.3389/fimmu.2021.635869
65. Leshem E, Maor Y, Meltzer E, Assous M, and Schwartz E. Acute schistosomiasis outbreak: clinical features and economic impact. Clin Infect Dis. (2008) 47:1499–506. doi: 10.1086/593191
66. Bottieau E, Clerinx J, De Vega MR, Van den Enden E, Colebunders R, Van Esbroeck M, et al. Imported Katayama fever: clinical and biological features at presentation and during treatment. J Infection. (2006) 52:339–45. doi: 10.1016/j.jinf.2005.07.022
67. ElShewy K. Liver parasites. In: Medical Parasitology: A Body System Approach. Cham, Switzerland: Springer (2024). p. 99–121.
68. Salas-Coronas J, Vázquez-Villegas J, Lozano-Serrano AB, Soriano-Pérez MJ, Cabeza-Barrera I, Cabezas-Fernández MT, et al. Severe complications of imported schistosomiasis, Spain: A retrospective observational study. Travel Med Infect Dis. (2020) 35:101508. doi: 10.1016/j.tmaid.2019.101508
69. Alonso S, Arinaitwe M, Atuhaire A, Nankasi AB, Prada JM, McIntosh E, et al. The short-term impact of Schistosoma mansoni infection on health-related quality of life: implications for current elimination policies. Proc Biol Sci. (2024) 291:20240449. doi: 10.1098/rspb.2024.0449
70. King CH, Dickman K, and Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. (2005) 365:1561–9. doi: 10.1016/S0140-6736(05)66457-4
71. Vaillant MT, Philippy F, Neven A, Barré J, Bulaev D, Olliaro PL, et al. Diagnostic tests for human Schistosoma mansoni and Schistosoma haematobium infection: a systematic review and meta-analysis. Lancet Microbe. (2024) 5:e366–78. doi: 10.1016/S2666-5247(23)00377-4
72. Feleke DG, Alemu Y, Bisetegn H, Debash H, et al. Accuracy of Diagnostic Tests for Detecting Schistosoma mansoni and S. haematobium in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. BioMed Res Int. (2023) 2023:3769931. doi: 10.1155/2023/3769931
73. Hu Y, Lin J, Wang Y, Wu S, Wu J, Lv H, et al. Identification of serum ferritin-specific nanobodies and development towards a diagnostic immunoassay. Biomolecules. (2022) 12:1–15. doi: 10.3390/biom12081080
74. Villamizar-Monsalve MA, López-Abán J, Vicente B, Peláez R, Muro A, et al. Current drug strategies for the treatment and control of schistosomiasis. Expert Opin Pharmacother. (2024) 25:409–20. doi: 10.1080/14656566.2024.2333372
75. Lalor R, Cwiklinski K, Calvani NED, Dorey A, Hamon S, Corrales JL, et al. Pathogenicity and virulence of the liver flukes Fasciola hepatica and Fasciola gigantica that cause the zoonosis Fasciolosis. Virulence. (2021) 12:2839–67. doi: 10.1080/21505594.2021.1996520
76. Sharma R, Godara R, and Thilagar M. Epizootiology, pathogenesis and immunoprophylactic trends to control tropical bubaline fasciolosis: an overview. J Parasitic Dis. (2011) 35:1–9. doi: 10.1007/s12639-011-0025-8
77. Mohamed YA. Pathological Status Of Fascioliasis Of Bovine Liver Collected From Slaughter House Of Dinajpur District Of Bangladesh. Dinajpur: Hajee Mohammod Danesh Science And Technology University (2020).
78. Hawash Y. Parasitic liver diseases. In: Liver Diseases: A Multidisciplinary Textbook. Cham, Switzerland: Springer (2020). p. 161–72.
79. Rufino-Moya PJ, Zafra Leva R, Martínez-Moreno Á, Buffoni L, Valderas García E, Pérez Arévalo J, et al. Advancement in Diagnosis, Treatment, and Vaccines against Fasciola hepatica: A Comprehensive Review. Pathogens. (2024) 13:1–19. doi: 10.3390/pathogens13080669
80. Nur Hafizah S, Noor Izani NJ, Ahmad Najib M, and Wan-Nor-Amilah WAW. Immunodiagnosis of fascioliasis in ruminants by ELISA method: A mini-review. Malays J Med Sci. (2023) 30:25–32. doi: 10.21315/mjms2023.30.4.3
81. Choi YJ, Rosa BA, Fernandez-Baca MV, Ore RA, Martin J, Ortiz P, et al. Independent origins and non-parallel selection signatures of triclabendazole resistance in Fasciola hepatica. Nat Commun. (2025) 16:2996. doi: 10.1038/s41467-025-57796-5
82. Zhang X-H, Huang D, Li YL, and Chang B. Novel mechanism of hepatobiliary system damage and immunoglobulin G4 elevation caused by Clonorchis sinensis infection. World J Clin cases. (2021) 9:6639. doi: 10.12998/wjcc.v9.i23.6639
83. Rather SA, Wani ZA, Mustafa RA, Bharti P, Kousar R, Ashraf MV, et al. Carcinogenic parasites: Insights into the epidemiology and possible mechanisms of cancer. Mutagenesis. (2025), geaf007. doi: 10.1093/mutage/geaf007
84. Holland C, Sepidarkish M, Deslyper G, Abdollahi A, Valizadeh S, Mollalo A, et al. Global prevalence of Ascaris infection in humans (2010-2021): a systematic review and meta-analysis. Infect Dis Poverty. (2022) 11:113. doi: 10.1186/s40249-022-01038-z
85. Kadem JO and Al-Jashamy K. Incidence and pathophysiology of Ascaris lumbricoides infection among hospital patients. In: AIP Conference Proceedings. Melville, NY, USA: AIP Publishing (2023).
86. Walker M, Hall A, and Basáñez M-G. Ascaris lumbricoides: new epidemiological insights and mathematical approaches. In: Ascaris: the neglected parasite. Amsterdam, Netherlands: Elsevier (2013). p. 155–201.
87. Narayan KG, Sinha DK, and Singh DK. Ascariasis. In: Handbook of Management of Zoonoses. Cham, Switzerland: Springer (2024). p. 1021–4.
88. Schlosser-Brandenburg J, Midha A, Mugo RM, Ndombi EM, Gachara G, Njomo D, et al. Infection with soil-transmitted helminths and their impact on coinfections. Front Parasitol. (2023) 2:1197956. doi: 10.3389/fpara.2023.1197956
89. Darlan DM, Rozi MF, and Yulfi H. Overview of immunological responses and immunomodulation properties of Trichuris sp.: Prospects for better understanding human trichuriasis. Life. (2021) 11:188. doi: 10.3390/life11030188
90. Gebreyesus TD, Makonnen E, Tadele T, Mekete K, Gashaw H, Gerba H, et al. Reduced efficacy of single-dose albendazole against Ascaris lumbricoides, and Trichuris trichiura, and high reinfection rate after cure among school children in southern Ethiopia: a prospective cohort study. Infect Dis Poverty. (2024) 13:8. doi: 10.1186/s40249-024-01176-6
91. Colella V, Bradbury R, and Traub R. Ancylostoma ceylanicum. Trends Parasitol. (2021) 37:844–5. doi: 10.1016/j.pt.2021.04.013
92. Kyu HH, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1859–922. doi: 10.1016/S0140-6736(18)32335-3
93. Furtado LFV, Alves WP, da Silva VJ, and Rabelo ÉML. Hookworm infection as a model for deepen knowledge of iron metabolism and erythropoiesis in anemia. Cytokine. (2024) 177:156559. doi: 10.1016/j.cyto.2024.156559
94. Hoogerwerf MA, Janse JJ, Kuiper VP, van Schuijlenburg R, Kruize YC, Sijtsma JC, et al. Protective efficacy of short-term infection with Necator americanus hookworm larvae in healthy volunteers in the Netherlands: a single-centre, placebo-controlled, randomised, controlled, phase 1 trial. Lancet Microbe. (2023) 4:e1024–34. doi: 10.1016/S2666-5247(23)00218-5
95. Schwartz A. Hepcidin/Ferroportin/HIF-2a Regulation of Iron Metabolism at the Systemic and Cellular Level. (2019).
96. Sebastiani G, Wilkinson N, and Pantopoulos K. Pharmacological targeting of the hepcidin/ferroportin axis. Front Pharmacol. (2016) 7:160. doi: 10.3389/fphar.2016.00160
97. Mrimi EC, Welsche S, Ali SM, Hattendorf J, and Keiser J. Emodepside for Trichuris trichiura and hookworm infection. N Engl J Med. (2023) 388:1863–75. doi: 10.1056/NEJMoa2212825
98. Liu Y. Population Genomics of Strongyloides stercoralis from People and Dogs in Asia. Liverpool, UK: University of Liverpool (2024).
99. Weinstock JV and Leung J. Parasitic diseases: helminths. In: Yamada’s Textbook of Gastroenterology (2022). p. 3039–78.
100. Gordon CA, Utzinger J, Muhi S, Becker SL, Keiser J, Khieu V, et al. Strongyloidiasis. Nat Rev Dis Primers. (2024) 10:6. doi: 10.1038/s41572-023-00490-x
101. Pillai RK, Pillai RK, Illankovan VR, Kumarasamy V, Reddy S, Gowtham K, et al. Understanding Strongyloides Stercoralis infection and its relationship to chronic alcohol abuse: Understanding pathogenesis and therapeutic strategies. Toxicol Rep. (2024) 13:101754. doi: 10.1016/j.toxrep.2024.101754
102. Vasquez-Rios G, Pineda-Reyes R, Pineda-Reyes J, Marin R, Ruiz EF, and Terashima A. Strongyloides stercoralis hyperinfection syndrome: a deeper understanding of a neglected disease. J Parasit Dis. (2019) 43:167–75. doi: 10.1007/s12639-019-01090-x
103. Ming DK, Armstrong M, Lowe P, Chiodini PL, Doherty JF, Whitty CJM, et al. Clinical and diagnostic features of 413 patients treated for imported strongyloidiasis at the hospital for tropical diseases, London. Am J Trop Med Hyg. (2019) 101:428–31. doi: 10.4269/ajtmh.19-0087
104. Gebretsadik HG. Estimation of ivermectin treatment demand for the management of strongyloidiasis cases worldwide: A systematic review. J Orthopaedics Sports Med. (2023) 5:214–21. doi: 10.26502/josm.511500103
105. Ndao M, Perera DJ, and Kadkhoda K. Strongyloides: a minireview and update. Clin Microbiol Newslett. (2022) 44:161–7. doi: 10.1016/j.clinmicnews.2022.09.001
106. Zeitoun A, Ibrahim A, Reda El Sayed S, Hobeika E, and Karam R. Descriptive analysis of adverse events following immunization with oral cholera vaccine in Lebanon. Front Public Health. (2024) 12:1480744. doi: 10.3389/fpubh.2024.1480744
107. Ganesan D, Gupta SS, and Legros D. Cholera surveillance and estimation of burden of cholera. Vaccine. (2020) 38 Suppl 1:A13–a17. doi: 10.1016/j.vaccine.2019.07.036
108. Montero DA, Vidal RM, Velasco J, George S, Lucero Y, Gómez LA, et al. Vibrio cholerae, classification, pathogenesis, immune response, and trends in vaccine development. Front Med (Lausanne). (2023) 10:1155751. doi: 10.3389/fmed.2023.1155751
109. Hsiao A and Zhu J. Pathogenicity and virulence regulation of Vibrio cholerae at the interface of host-gut microbiome interactions. Virulence. (2020) 11:1582–99. doi: 10.1080/21505594.2020.1845039
110. Masuet-Aumatell C and Atouguia J. Typhoid fever infection–Antibiotic resistance and vaccination strategies: A narrative review. Travel Med Infect Dis. (2021) 40:101946. doi: 10.1016/j.tmaid.2020.101946
111. Khan M and Shamim S. Understanding the mechanism of antimicrobial resistance and pathogenesis of salmonella enterica serovar typhi. Microorganisms. (2022) 10:2006–20. doi: 10.3390/microorganisms10102006
112. Wijedoru L, Mallett S, and Parry CM. Rapid diagnostic tests for typhoid and paratyphoid (enteric) fever. Cochrane Database Systematic Rev. (2017) 5. doi: 10.1002/14651858.CD008892.pub2
113. Crump John A, Sjölund-Karlsson M, Gordon AM, and Parry MC. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive salmonella infections. Clin Microbiol Rev. (2015) 28:901–37. doi: 10.1128/CMR.00002-15
114. Bagamian KH, Anderson JDt, Muhib F, Cumming O, Laytner LA, Wierzba TF, et al. Heterogeneity in enterotoxigenic Escherichia coli and shigella infections in children under 5 years of age from 11 African countries: a subnational approach quantifying risk, mortality, morbidity, and stunting. Lancet Glob Health. (2020) 8:e101–12. doi: 10.1016/S2214-109X(19)30456-5
115. Steffen R, Hill DR, and DuPont HL. Traveler’s diarrhea: a clinical review. JAMA. (2015) 313:71–80. doi: 10.1001/jama.2014.17006
116. Anderson J, Bagamian KH, Muhib F, Amaya MP, Laytner LA, Wierzba T, et al. Burden of enterotoxigenic Escherichia coli and shigella non-fatal diarrhoeal infections in 79 low-income and lower middle-income countries: a modelling analysis. Lancet Glob Health. (2019) 7:e321–30. doi: 10.1016/S2214-109X(18)30483-2
117. Qadri F, Saha A, Ahmed T, Al Tarique A, Begum YA, and Svennerholm AM. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infect Immun. (2007) 75:3961–8. doi: 10.1128/IAI.00459-07
118. Qadri F, Svennerholm AM, Faruque AS, and Sack RB. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev. (2005) 18:465–83. doi: 10.1128/CMR.18.3.465-483.2005
119. Zhang Y, Tan P, Zhao Y, and Ma X. Enterotoxigenic Escherichia coli: intestinal pathogenesis mechanisms and colonization resistance by gut microbiota. Gut Microbes. (2022) 14:2055943. doi: 10.1080/19490976.2022.2055943
120. Youmans BP, Ajami NJ, Jiang ZD, Campbell F, Wadsworth WD, Petrosino JF, et al. Characterization of the human gut microbiome during travelers’ diarrhea. Gut Microbes. (2015) 6:110–9. doi: 10.1080/19490976.2015.1019693
121. Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, et al. Global morbidity and mortality of leptospirosis: A systematic review. PLoS Negl Trop Dis. (2015) 9:e0003898. doi: 10.1371/journal.pntd.0003898
122. Hartskeerl RA, Collares-Pereira M, and Ellis WA. Emergence, control and re-emerging leptospirosis: dynamics of infection in the changing world. Clin Microbiol Infect. (2011) 17:494–501. doi: 10.1111/j.1469-0691.2011.03474.x
123. Lane AB and Dore MM. Leptospirosis: a clinical review of evidence based diagnosis, treatment and prevention. World J Clin Infect Dis. (2016) 6:61–6. doi: 10.5495/wjcid.v6.i4.61
125. Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. (2003) 3:757–71. doi: 10.1016/S1473-3099(03)00830-2
126. Petakh P, Behzadi P, Oksenych V, and Kamyshnyi O. Current treatment options for leptospirosis: a mini-review. Front Microbiol. (2024) 15:1403765. doi: 10.3389/fmicb.2024.1403765
127. Schroeder GN and Hilbi H. Molecular pathogenesis of Shigella spp.: Controlling host cell signaling, invasion, and death by type III secretion. Clin Microbiol Rev. (2008) 21:134–56. doi: 10.1128/CMR.00032-07
128. Muzembo BA, Kitahara K, Mitra D, Ohno A, Khatiwada J, Dutta S, et al. Burden of Shigella in South Asia: a systematic review and meta-analysis. J Travel Med. (2022) 30. doi: 10.1093/jtm/taac132
129. Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, et al. World health organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: A data synthesis. PLoS Med. (2015) 12:e1001921. doi: 10.1371/journal.pmed.1001921
130. Adler AV, Ciccotti HR, Trivitt SJH, Watson RCJ, and Riddle MS. What’s new in travellers’ diarrhoea: updates on epidemiology, diagnostics, treatment and long-term consequences. J Travel Med. (2021) 29:taab099. doi: 10.1093/jtm/taab099
131. Williams E, Lew TE, Fuller A, Spelman DW, and Jenney AW. A case of multi-drug resistant ESBL-producing Shigella sonnei acute acalculous cholecystitis and gastroenteritis in a returned traveller. J Travel Med. (2018) 25. doi: 10.1093/jtm/tay029
133. Young KT, Davis LM, and Dirita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol. (2007) 5:665–79. doi: 10.1038/nrmicro1718
134. Je HJ, Singh S, Kim DW, Hur HS, Kim AL, Seo EJ, et al. Systematic review and meta-analysis of campylobacter species contamination in poultry, meat, and processing environments in South Korea. Microorganisms. (2023) 11. doi: 10.3390/microorganisms11112722
135. Alnimr AM. A case of bacteremia caused by Campylobacter fetus: An unusual presentation in an infant. Infection Drug Resistance. (2014) 7:37–40. doi: 10.2147/IDR.S58645
136. Lagler H, Kiesewetter B, and Raderer M. Infection with multidrug-resistant Campylobacter coli mimicking recurrence of carcinoid syndrome: A case report of a neuroendocrine tumor patient with repeated diarrhea. BMC Infect Dis. (2016) 16. doi: 10.1186/s12879-016-1743-4
137. Fischer GH, Hashmi MF, and Paterek E. Campylobacter infection. In: StatPearls. StatPearls Publishing (2024).
138. Crawford SE, Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M, et al. Rotavirus infection. Nat Rev Dis Primers. (2017) 3:17083. doi: 10.1038/nrdp.2017.83
139. Hagbom M, Istrate C, Engblom D, Karlsson T, Rodriguez-Diaz J, Buesa J, et al. Rotavirus stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting. PLoS Pathog. (2011) 7:e1002115. doi: 10.1371/journal.ppat.1002115
140. Ball JM, Tian P, Zeng CQY, Morris AP, and Estes MK. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. (1996) 272:101–4. doi: 10.1126/science.272.5258.101
141. Pickering LK, Bartlett AVIII, Reves RR, and Morrow A. Asymptomatic excretion of rotavirus before and after rotavirus diarrhea in children in day care centers. J Pediatr. (1988) 112:361–5. doi: 10.1016/S0022-3476(88)80313-5
142. Ghai S, Pattison J, Ghai S, OMalley ME, Khalili K, and Stephens M. Primary gastrointestinal lymphoma: spectrum of imaging findings with pathologic correlation. RadioGraphics. (2007) 27:1371–88. doi: 10.1148/rg.275065151
143. Dandapani SV, Eaton M, Thomas CRJ, and Pagnini PG. HIV- positive anal cancer: an update for the clinician. J Gastrointest Oncol. (2010) 1:34–44. doi: 10.3978/j.issn.2078-6891.2010.005
144. Jaryal A, Raina R, Sarkar M, and Sharma A. Manifestations of tuberculosis in HIV/AIDS patients and its relationship with CD4 count. Lung India. (2011) 28.
145. Restrepo CS, Martínez S, Lemos JA, Carrillo JA, Lemos DF, Ojeda P, et al. Imaging manifestations of Kaposi sarcoma. RadioGraphics. (2006) 26:1169–85. doi: 10.1148/rg.264055129
146. Ali S, Li Z, Moqueet N, Moghadas SM, Galvani AP, Cooper LA, et al. Incorporating social determinants of health in infectious disease models: A systematic review of guidelines. Med Decis Making. (2024) 44:742–55. doi: 10.1177/0272989X241280611
147. Al-Delaimy AK, Al-Mekhlafi HM, Lim YA, Nasr NA, Sady H, Atroosh WM, et al. Developing and evaluating health education learning package (HELP) to control soil-transmitted helminth infections among Orang Asli children in Malaysia. Parasit Vectors. (2014) 7:416. doi: 10.1186/1756-3305-7-416
148. Akinsolu FT, DePaiva VN, Souza SS, and Varga O. Patent landscape of neglected tropical diseases: an analysis of worldwide patent families. Globalization Health. (2017) 13:1–13. doi: 10.1186/s12992-017-0306-9
149. Yajima A, Mikhailov A, Mbabazi PS, Gabrielli AF, Minchiotti S, Montresor A, et al. Preventive Chemotherapy and Transmission Control (PCT) databank: a tool for planning, implementation and monitoring of integrated preventive chemotherapy for control of neglected tropical diseases. Trans R Soc Trop Med Hygiene. (2012) 106:215–22. doi: 10.1016/j.trstmh.2012.01.003
150. Smits HL. Prospects for the control of neglected tropical diseases by mass drug administration. Expert Rev Anti-infective Ther. (2009) 7:37–56. doi: 10.1586/14787210.7.1.37
151. Andrews JR, Qamar FN, Charles RC, and Ryan ET. Extensively drug-resistant typhoid—are conjugate vaccines arriving just in time? New Engl J Med. (2018) 379:1493–5.
152. Pezzoli L. Global oral cholera vaccine use, 2013–2018. Vaccine. (2020) 38:A132–40. doi: 10.1016/j.vaccine.2019.08.086
153. Baker KS, Dallman TJ, Behar A, Weill FX, Gouali M, Sobel J, et al. Travel- and community-based transmission of multidrug-resistant Shigella sonnei lineage among international Orthodox Jewish communities. Emerging Infect Dis. (2016) 22:1545–53. doi: 10.3201/eid2209.151953
154. O’neill J. Review on antimicrobial resistance: tackling drug-resistant infections globally. Wellcome Trust and the Department of Health of UK Government (2016).
155. Murugaiyan J, Kumar PA, Rao GS, Iskandar K, Hawser S, Hays JP, et al. Progress in alternative strategies to combat antimicrobial resistance: focus on antibiotics. Antibiotics (Basel). (2022) 11. doi: 10.3390/antibiotics11020200
156. Johnson TJ, Shank JM, and Johnson JG. Current and potential treatments for reducing Campylobacter colonization in animal hosts and disease in humans. Front Microbiol. (2017) 8:487. doi: 10.3389/fmicb.2017.00487
157. Prunas O, Asare EO, Sajewski E, Li Y, Pithawala Z, Weinberger DM, et al. Global estimates of rotavirus vaccine efficacy and effectiveness: a rapid review and meta-regression analysis. eClinicalMedicine. (2025) 81. doi: 10.1016/j.eclinm.2025.103122
158. Parashar UD, Hummelman EG, Bresee JS, Miller MA, and Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerging Infect Dis. (2003) 9:565. doi: 10.3201/eid0905.020562
159. Samhouri D, Mahrous H, Saidouni A, ElKholy A, Ghazy RM, Sadek M, et al. Review on progress, challenges, and recommendations for implementing the One Health approach in the Eastern Mediterranean Region. One Health. (2025) 20:101057. doi: 10.1016/j.onehlt.2025.101057
160. Standley CJ, Bakuza J, and Peterson JK. One Health and Neglected Tropical Diseases. MDPI-Multidisciplinary Digital Publishing Institute (2021).
161. Peterson JK, Bakuza J, and Standley CJ. One health and neglected tropical diseases-multisectoral solutions to endemic challenges. Trop Med Infect Dis. (2020) 6.
162. Hendawy SH and Alzan HF. One Health in action: neglected tropical diseases, vector and pest management. Front Trop Dis. (2024) 5:1462918. doi: 10.3389/fitd.2024.1462918
163. Laing G, Vigilato MAN, Cleaveland S, Thumbi SM, Blumberg L, Salahuddin N, et al. One Health for neglected tropical diseases. Trans R Soc Trop Med Hyg. (2021) 115:182–4. doi: 10.1093/trstmh/traa117
164. Calistri P, Iannetti S, Danzetta ML, Narcisi V, Cito F, DiSabatino D, et al. The components of ‘one world–one health’approach. Transboundary emerging Dis. (2013) 60:4–13. doi: 10.1111/tbed.2013.60.issue-s2
165. Bryson JM, Bishop-Williams KE, Berrang-Ford L, Nunez EC, Lwasa S, Namanya DB, et al. Neglected tropical diseases in the context of climate change in East Africa: a systematic scoping review. Am J Trop Med hygiene. (2020) 102:1443. doi: 10.4269/ajtmh.19-0380
166. Tidman R, Abela-Ridder B, and de Castañeda RR. The impact of climate change on neglected tropical diseases: a systematic review. Trans R Soc Trop Med Hygiene. (2021) 115:147–68. doi: 10.1093/trstmh/traa192
167. The Lancet Global H. Climate change and NTDs: a perfect storm. Lancet Global Health. (2025) 13:e172. doi: 10.1016/S2214-109X(25)00014-2
168. Esser HJ, Mögling R, Cleton NB, VanDerJeugd H, Sprong H, Stroo A, et al. Risk factors associated with sustained circulation of six zoonotic arboviruses: a systematic review for selection of surveillance sites in non-endemic areas. Parasites Vectors. (2019) 12:1–17. doi: 10.1186/s13071-019-3515-7
Keywords: tropical diseases, gastrointestinal symptoms, neglected tropical diseases (NTDs), fecal–oral route, vector-borne diseases, One Health approach
Citation: Ghazy RM, Alshaikhi SA, Assiri HAH, Almozaini AA, Alhazmi AF, Elhasaneen HEM and Abdo SM (2025) Tropical diseases and the gastrointestinal tract: an overlooked connection. Front. Trop. Dis. 6:1612952. doi: 10.3389/fitd.2025.1612952
Received: 16 April 2025; Accepted: 22 May 2025;
Published: 26 June 2025.
Edited by:
Abdulqadir J. Nashwan, Hamad Medical Corporation, QatarReviewed by:
Hilal Bedir, Kafkas University, TürkiyeIsra Alsaady, King Abdulaziz University, Saudi Arabia
Copyright © 2025 Ghazy, Alshaikhi, Assiri, Almozaini, Alhazmi, Elhasaneen and Abdo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ramy Mohamed Ghazy, cmFteV9naGF6eUBhbGV4dS5lZHUuZWc=
†ORCID: Ramy Mohamed Ghazy, orcid.org/0000-0001-7611-706X
 Ramy Mohamed Ghazy
Ramy Mohamed Ghazy Saleh Ahmed Alshaikhi3
Saleh Ahmed Alshaikhi3 Sarah M. Abdo
Sarah M. Abdo