- 1Department of Community Health, Mbarara University of Science and Technology, Mbarara, Uganda
- 2Department of Research, Eye Health Africa, Kampala, Uganda
- 3Department of Community Medicine, Jos University Teaching Hospital, Plateau State, Nigeria
- 4Global Health and Infectious Diseases Research Group, Kumasi Centre for Collaborative Research in Tropical Medicine, Kumasi, Ghana
- 5Malaria Research and Training Centre, Bamako, Mali
- 6Department of Research, Uganda UK Health Alliance, Kampala, Uganda
- 7Moroto Regional Referral Hospital, Ministry of Health, Moroto, Uganda
- 8Department of Research, Ubora Foundation Africa, Kampala, Uganda
- 9Department of Community Medicine and Primary Health Care, Bingham University, Karu Nasarawa State, Nigeria
- 10Department of Community Medicine, David Umahi Federal University Teaching Hospital, Uburu, Nigeria
- 11Department of Research, Global Health Partnerships, Kampala, Uganda
Over time, numerous health issues have challenged Africa’s health systems, including reemerging and emerging pandemics and epidemics. International health bodies, such as the World Health Organisation (WHO), have developed various frameworks to help health systems maintain service delivery to their respective communities and individuals. The WHO’s health system framework is a six-pronged strategy to enhance healthcare service delivery. However, emerging epidemics, such as mpox, have hindered the integration of these components. This review explored the health preparedness of African countries to mitigate emerging and re-emerging epidemics using the WHO health system framework with a focus on mpox. The review found most African countries lack adequate health products, such as vaccines against mpox, and have limited human resources available to care for affected individuals. For instance, Africa’s health worker staffing is estimated at 1.55 per 1000 people compared to the 4.45 per 1000 WHO threshold. Many African countries, like Somalia, Uganda, Eritriea lack efficient health preparedness plans to enhance their readiness to address the epidemic. Nevertheless, these plans provide detailed information regarding mpox risks and how to mitigate them based on risk factors, such as reducing zoonotic spillover. Healthcare financing in is still challenged in many African countries like Uganda, Tanzania, and Ghana due to limited budgetary allocations, which affects the purchase and distribution of necessary resources for mpox prevention, control, and management. Cuts in funding from major donors, including United States Agency for International Development (USAID) and UK Aid (formerly known as Department of International Development, DFID), worsen the situation. However, African countries can leverage on innovation and risk factor mitigation, to fully equip their healthcare systems based on available frameworks for other re-emerging epidemics. Additionally, they must strategise avenues of self-sustenance, such as political commitment and depending on other resources to fund their health programs.
Introduction to health system preparedness in SSA
The WHO defines health systems as “all organisations, people, and actions whose primary intent is to promote, restore, or maintain health” (1). The WHO Health System Framework outlines six interconnected building blocks that form the foundation of a well-functioning health system. These are the health workforce, service delivery, health governance, financial management, health information, health products, for example, drugs, and health infrastructure, as demonstrated in Figure 1. These components are designed to strengthen health systems and improve health outcomes. The framework emphasises how these building blocks work together to ensure equitable access, quality care, and system resilience. SSA Countries have diverse health systems shaped by multiple factors. Most SSA countries rely on a mixed system of public, private, and nongovernmental organisation (NGO)-led services with an emphasis on primary health care (PHC) (3). However, these systems are challenged by multiple factors. A nation’s socioeconomic, historical, and political factors determine its capacity for health promotion. Also, factors such as infrastructure deficits, limited healthcare financing, funding cuts, and health worker shortages have shaped most SSA countries’ health systems. For example, SSA is affected by a shortage of health workers, estimated at 1.55 per 1000 people, compared to the WHO-recommended threshold of 4.45 per 1000 people (4).
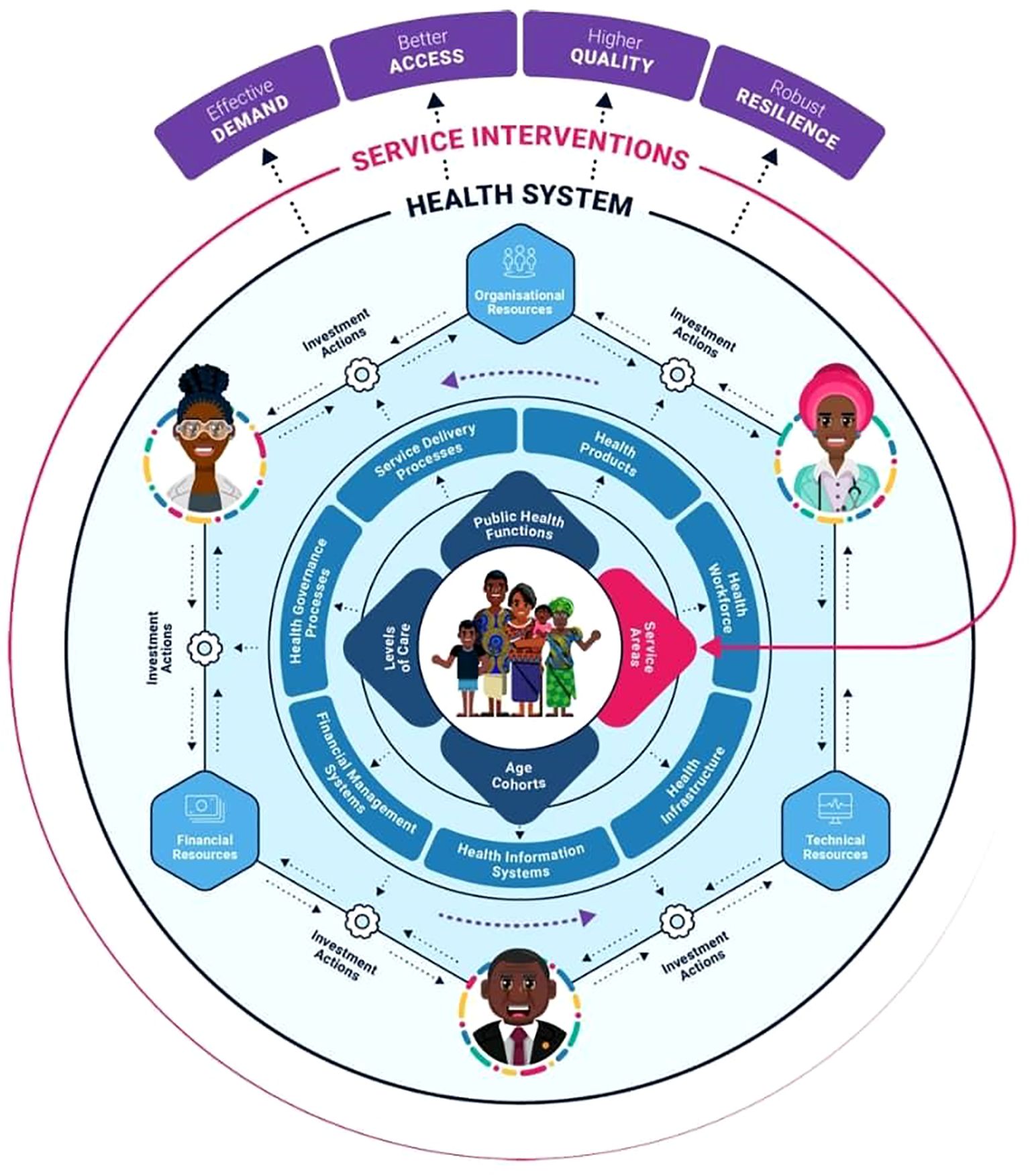
Figure 1. An illustration of the complex interplay of the entities within a health system framework (2). The complex interplay of the health system framework.
SSA has been prone to different epidemics and pandemics, for example, the coronavirus disease, mpox, and Viral Haemorrhagic fever (VHF) infections, such as Ebola and Crimean Congo. These epidemics and pandemics have significantly affected the healthcare infrastructure, producing undesirable outcomes, such as increased mortalities, morbidities, and reduced productivity. Across the pre- and post-colonial eras, the continent has faced multiple infectious disease outbreaks, such as influenza in the early 1910s, Malaria in the early 1950s and 1960s, HIV/AIDS and Ebola in the late 1970s, and the most recent coronavirus disease (COVID-19) in 2019/2020 (5). Notably, the region’s tropical climate, diverse ecosystems, and human settlement patterns contribute to the emergence and persistence of many infectious diseases throughout its history. These outbreaks have significantly crippled and challenged the different healthcare systems in different African Countries. However, these African countries have also made significant efforts concerning readiness and preparedness to mitigate emerging pandemics and epidemics. For example, countries like Kenya, Tanzania, and Ghana have introduced digital assessment tools to explore health system preparedness during outbreaks (6). Other countries, such as Rwanda, Ethiopia, and Egypt, have strategic health reforms, such as increasing healthcare funding to enhance their capacity to manage healthcare emergencies effectively (47). These initiatives have significantly improved SSA’s capacity to tackle the next epidemics. However, multiple issues still exist, some embedded in these initiatives that demonstrate a deficit concerning pandemic preparedness among African countries.
Mpox is among the zoonotic diseases of viral origin that have affected the African population and communities. The disease was discovered in early 1958 among the different monkey colonies, with the first human case reported in the Democratic Republic of Congo (DRC) around 1970 (5). Mpox remains endemic in different parts of western and central Africa and has been a global concern following the rise in cases and geographical spread since August 2024 (7). The Africa Centres for Disease Control (Africa CDC) has confirmed over 48,800 mpox cases through laboratory diagnosis among 31 African countries, with over 200 mortalities among 28 countries by 27th July 2025 (8). Phylogenetic analysis of the mpox virus has identified two distinct clades, I and II, with clade Ib being the most virulent and carrying the highest risk of community transmission. The Clade Ib subclass has mostly affected non-endemic areas, especially the Democratic Republic of Congo (DRC), with a sustained human-to-human transmission. Over 27800 and 7648 clade Ib cases had been confirmed in DRC and Uganda, respectively (8). Other African countries are reported in Table 1 below. However, the transmission of clade Ia has been endemic in some African countries such as Cameroon, Congo, DRC, Sudan, and the Central African Republic, yet, these countries have recorded a sustained community transmission of clade Ib.
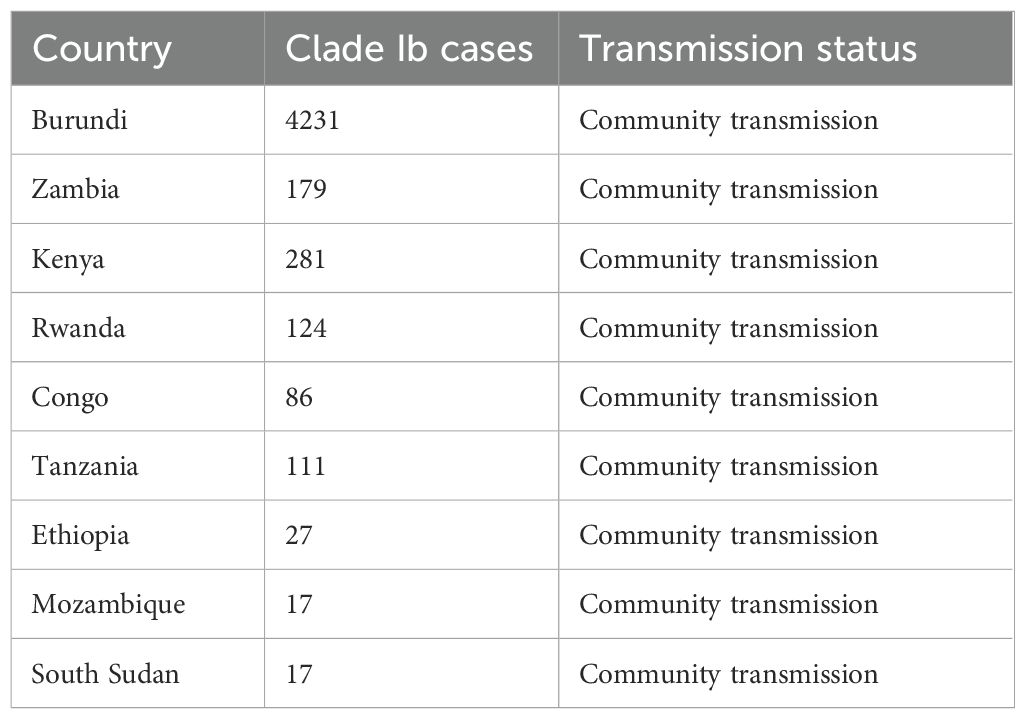
Table 1. A table demonstrating the different clade I b cases identified among African countries as per 27th July 2025 (8).
Among the 31 countries identified, the DRC, Uganda, and Burundi have reported the highest number of cases, which are estimated at 21439, 7645, and 4967, respectively (8). These are significant numbers and impact the healthcare system in multiple aspects. This evaluation is warranted due to the limited evaluation of health systems and their readiness to deal with reemerging epidemics and pandemics within SSA, especially using the WHO framework (9). We aim to answer the following question: What is the capacity and level of health preparedness of SSA’s healthcare systems to combat emerging and reemerging epidemics like mpox using the WHO health system framework?
Mpox risk assessment and preparedness planning across SSA
Multiple factors, such as human-to-human transmission, zoonotic spillover, ecological changes, and weak healthcare systems, drive mpox transmission across SSA (10). Some primary risk factors for transmission include contact with infected animals, particularly rodents and primates, which serve as natural reservoirs. Often, humans can encounter some infected rodents and primates through poorly regulated bushmeat trade, deforestation, and high-risk exposure to the virus reserves, especially in the Central African Republic (CAR) (11). Evidence has highlighted the absence of vaccination against smallpox as a predisposing risk factor for mpox transmission. Both mpox and smallpox are caused by orthopoxviruses with different structural similarities. Variola virus vaccines against smallpox, such as Dryvax and the ACAM2000, offers cross-protection by inducing an immune response that recognises and neutralises the mpox Virus (12). However, vaccination against smallpox did not confer protection against mpox among some cases identified in DRC, necessitating further evaluation (10, 13).
Other factors, such as sharing rooms with an infected person, sharing beds, sharing utensils like plates and cups, and communal sleeping areas, were also identified as potential factors for transmission (13). Additionally, unprotected sex has also been linked to the spread of mpox during the human-to-human transmission patterns. Evidence from the Democratic Republic of Congo (DRC) has found mpox viral DNA on multiple oral, penile, and vaginal swabs (14). Further, evidence from Nigeria demonstrated higher odds of contracting mpox after sexually engaging with an mpox-positive patient (15). Multiple factors have also been identified affecting the disease severity in Nigeria, such as having advanced HIV disease and preexisting co-infections like Varicella zoster virus infection (16). These risk factors require extensive national and regional disease prevention and control preparedness plans.
However, most SSA countries have limited national preparedness plans focusing on the risk factors to prevent the spread of mpox. For example, a few SSA countries have enforced plans or strategies to mitigate the zoonotic spillover, which leads to frequent contact between primates and humans, as illustrated in the zoonotic spillover cycle in Figure 2. Yet, multiple human activities that exploit natural resources accelerate most zoonotic spillovers, predisposing to reemerging epidemics and pandemics (18). Besides, most SSA countries have predominantly focused on the curative arm of managing different diseases, which includes diagnosing and treating affected individuals, rather than the preventative arm, which focuses on risk factor mitigation (18). For example, the Africa CDC has established regional mpox testing laboratories in countries like Burundi, Malawi, Uganda, and the DRC and issued recommendations for vaccine stockpiling and deployment strategies (19). Upstream strategies have been under-exploited, such as risk reduction activities, understanding infection dynamics, and addressing anthropogenic drivers of disease spread, which minimise spillover and spread. Thus, these strategies must be enhanced for better outcomes concerning mpox reemergence prevention and control. Further, scenarios-based planning, simulation, and modeling must be prioritised in SSA to enhance anticipation of outbreaks, optimise resource allocation, and identify potential response gaps within the healthcare systems. For example, the modeling studies have estimated that early intervention could significantly reduce mpox transmission. Notably, a stochastic model on a hypothetical cohort of 6500 students demonstrated that the detection and isolation of 50% and 80% of the students reduced the population cases to 37 and 8, respectively (20). These interventions can enhance the preparedness of SSA countries to mitigate future outbreaks like mpox effectively.
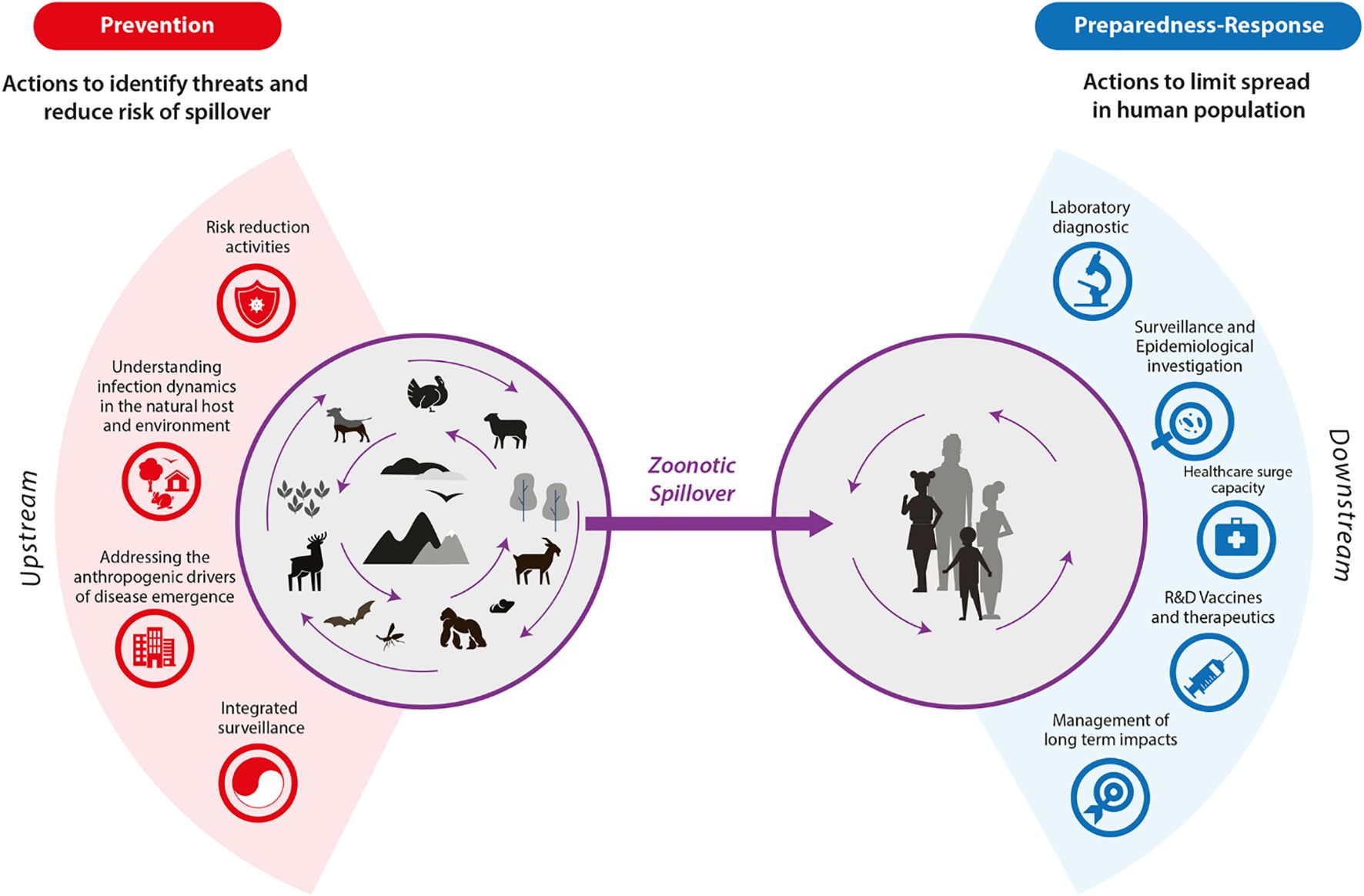
Figure 2. An illustration of the zoonotic spillover cycle illustrating both the preventive and preparedness response (17). The zoonotic spillover cycle.
Governance and leadership of mpox response in SSA
Leadership and governance are critical in health system preparedness as per the WHO health systems framework, even during the mpox epidemic. National governments develop policy frameworks, mobilise resources, and implement outbreak response strategies. Ministries of Health under these governments can collaborate with the national public health institutes and lead case surveillance, vaccine distribution, risk communication, and treatment strategies. Some SSA countries have made significant strides; for example, Nigeria, Senegal, and Benin have already developed mpox-related training material, especially concerning case-based management, contributing to approximately 60% readiness to handle the disease (21). Additionally, Nigeria and Ghana have gazetted mpox referral centres with sufficient intensive care unit capacity for critical cases. The Ugandan and Kenyan Ministries of Health have also developed training material about mpox case-based management. These countries have also translated into the local language and distributed information, education, and communication material (IEC) to aid accurate mpox information dissemination (21). Additionally, they have developed emergency supply and distribution mechanisms to ensure that mpox resources are available in the country. These efforts collectively contribute to strengthening the health systems in the respective countries, enhancing their fight against the disease. Strong governance propels response efforts that are well-coordinated across different sectors, given the zoonotic nature of mpox, including animal, environmental, and human health.
International organisations, such as the Africa CDC, the WHO, the Coalition for Epidemic Preparedness Innovations (CEPI), and the Vaccine Alliance (GAVI), are critical in the fight against mpox across SSA (22). Through the Access and Allocation Mechanism (AMM), these bodies provided vaccine doses to SSA countries affected by the disease by November 2024 (2). Most of these vaccine doses were outsourced from entities and countries outside Africa, signifying the strategic discussions and engagements from the regional bodies in the mpox fight. Also, the Africa CDC has established regional hubs, strengthened laboratory work, and provided sufficient guidance on mpox case management, vaccination, and infection prevention. Another key action by the Africa CDC has been vaccine allocation and distribution to heavily affected regions and high-risk populations, such as the DRC (23).
Other regional bodies like the African Union have negotiated with global vaccine manufacturers and donors to secure funding and vaccine procurement deals for SSA countries. The union has also donated over $10.4 million to over 8 African countries affected by mpox (23). These donations have supplemented other donations from former agencies, such as USAID, which donated over $10 million to the affected countries. Through these donations, multiple regional preparedness and response plans for mpox have been developed targeting affected countries, such as Uganda, Rwanda, the DRC, Kenya, and Cameroon (23). However, national preparedness and action plans addressing mpox are still lacking in most countries affected by the disease.
The role of these regional bodies extends beyond donations and the development of action plans to policy harmonisation and ensuring that countries adhere to standardised public health protocols and successful data-sharing mechanisms. Through strategic leadership, the entities can coordinate with the presidential task forces and public health authorities to ensure their participation in the fight against the outbreak within their countries. These can also extend to the leadership at the local level to ensure that community leaders, district health officials, and village healthcare workers are actively involved and on the frontline against mpox. Therefore, strengthening the existence of these entities is critical in the fight against mpox and in enhancing the healthcare systems in the SSA countries to combat different outbreaks.
Case management, clinical care, and infection prevention and control measures for mpox
The WHO health system framework prioritises service delivery to manage outbreaks and diseases effectively and may involve case-by-case management and therapeutic support. Mpox management involves supportive and symptomatic treatment since no specific antiviral therapy is universally approved for the disease. Management aims to alleviate symptoms, manage complications, and prevent long-term sequelae. The World Health Organisation (WHO) and Centres for Disease Control and Prevention (CDC) have outlined guidelines emphasising the importance of early detection, isolation of suspected cases, and supportive care to mitigate symptoms and prevent complications (24, 25). However, implementing these guidelines within some of the countries affected is questionable. Most patients’ primary intervention is supportive care, especially when hospitalisation is not required. This includes analgesics like acetaminophen or non-steroidal anti inflammatory drugs (NSAIDs) for pain relief in some complications, especially those involving the mucosa, like proctitis, pharyngitis, or vaginal lesions, antipyretics for fever management, and maintaining proper hydration (26). Skincare is key in preventing secondary bacterial infections of the lesions. In severe cases, such as those involving immunocompromised individuals or those with complications like sepsis, pneumonia, and encephalitis, more intensive care, including antiviral agents like Tecovirimat, may be considered under compassionate use protocols (27). Other antivirals like cidofovir and brincidofovir, primarily developed for cytomegalovirus infections, have also been considered for mpox treatment in cases where tecovirimat is unavailable. Other management forms for severe cases include intravenous fluids, oxygen therapy, or mechanical ventilation; others may need supportive neurological care (25).
However, resource-limited settings like countries within Sub-Saharan Africa continue facing several challenges in managing mpox outbreaks. These include limited access to diagnostic tools, a shortage of trained healthcare professionals, and inadequate healthcare infrastructure (7). Yet, mpox outbreaks require dedicated care facilities and isolation centres to control outbreaks. These centres are designed to reduce transmission, particularly in hospital settings, and to provide specialised care. However, establishing and maintaining such facilities is often challenging, particularly in low-resource settings (28, 29). Moreover, the lack of mpox-specific isolation centres in these regions exacerbates the spread of the disease, as isolation is critical to controlling outbreaks. Financial constraints through these outbreaks further limit the ability to procure essential medical supplies and protective equipment. In countries with robust healthcare systems like the United States of America or the United Kingdom, isolation centres with appropriate infection control measures are more readily available. In contrast, resource-limited settings may struggle with inadequate infrastructure, hindering effective isolation and treatment (30). Therefore, comprehensive measures to enhance service delivery are critical in mitigating the epidemic and future outbreaks.
Further, service delivery in managing emerging and reemerging epidemics and infectious diseases such as mpox entails promoting Infection Prevention and Control (IPC) measures. These measures include providing health workers with personal protective equipment (PPE). Suitable PPE, including gloves, gowns, eye protection, and N95 respirators, must be available when managing suspected or confirmed mpox cases. However, a recent scoping review demonstrated that more than 70% of healthcare workers were exposed to mpox due to a lack of PPE within SSA (31). The review emphasised different aspects, such as routine training and education concerning PPE use and implementation of hygiene measures in fighting against mpox. Also, disinfectants should be used to clean surfaces in the patient’s room. It is essential to promote other good hygiene practices, including regular handwashing, use of hand sanitisers, and proper waste disposal. Appropriate medical waste disposal and contaminated laundry handling are crucial prevention strategies. Most SSA countries affected by mpox, like Nigeria, Ghana, and the CAR, have achieved and implemented some of these practices (21). High-risk populations such as healthcare workers and close contacts of confirmed cases should be vaccinated where available (32). There should be animal surveillance and active case search. Confirmed animal cases are to be isolated and quarantined with associated restrictions on movement for suspected infected animals. Biosafety and biosecurity should also be observed in animal holding facilities. Together, these efforts can strengthen IPC measures against mpox within the region.
Also, the supply chain of IPC materials requires a strategic approach to ensure availability, cost-effectiveness, and quality. Procurement strategies should include diversification of Suppliers, centralised procurement, and long-term contracts with suppliers. Effective inventory management should ensure that IPC materials such as medical and nonmedical consumables, PPE, pharmaceuticals, or vaccines are available when needed while minimising wastage and maintaining strategic reserves of IPC materials. The logistics and distribution should ensure that supplies are delivered to the right place and at the right time through efficient transportation networks, cold chain management, and distribution prioritisation. Continuous monitoring and evaluation of the supply chain process are essential to identify bottlenecks, assess the effectiveness of strategies, and make data-driven decisions for future improvements.
Mpox vaccine preparedness and deployment in SSA
The WHO health systems framework prioritises the availability of different medical products, vaccines, and technologies to promote disease prevention and control. Yet, the availability of the mpox vaccine across SSA countries is limited due to various factors, such as the inability to manufacture the vaccines locally, inequalities in the distribution, and other global constraints (2). Unlike high-income countries that secured large vaccine stockpiles during the 2022 mpox outbreak, SSA has struggled to secure sufficient doses to protect its population. Two primary vaccines, JYNNEOS (MVA-BN) and ACAM2000, have been utilised for mpox prevention. By November 2024, approximately 900,000 vaccine doses had been donated by the “Access and Allocation Mechanism (AMM) for mpox” to nine countries affected by mpox (2). These countries included the DRC, Uganda, Rwanda, South Africa, Liberia, Nigeria, Kenya, the Central African Republic, and Côte d’Ivoire.
It was anticipated that over 5 million doses would be donated by the end of 2024, with 1.85 million doses from the European Union, Canada, and the United States, 3 million doses from LC16 Japan, 500,000 doses from GAVI, the vaccine alliance, and approximately 500,000 doses from UNICEF (2). Some of these organisations honoured their commitment and proceeded with their donation efforts to affected African countries, as demonstrated in the Table 2 below. Additionally, GAVI has supported vaccine administration with logistics and other financial-related capacity to an estimated US$6.21 million, with the DRC receiving around US$2.7 million (33).However, these doses are still insufficient to cover and protect the entire African population. Therefore, this has led to most countries relying on ring vaccination strategies rather than widespread immunisation for disease mitigation. Thus, responsible stakeholders must prioritise strategies to enhance the availability of these vaccine products across the countries affected, primarily through vaccine manufacture.
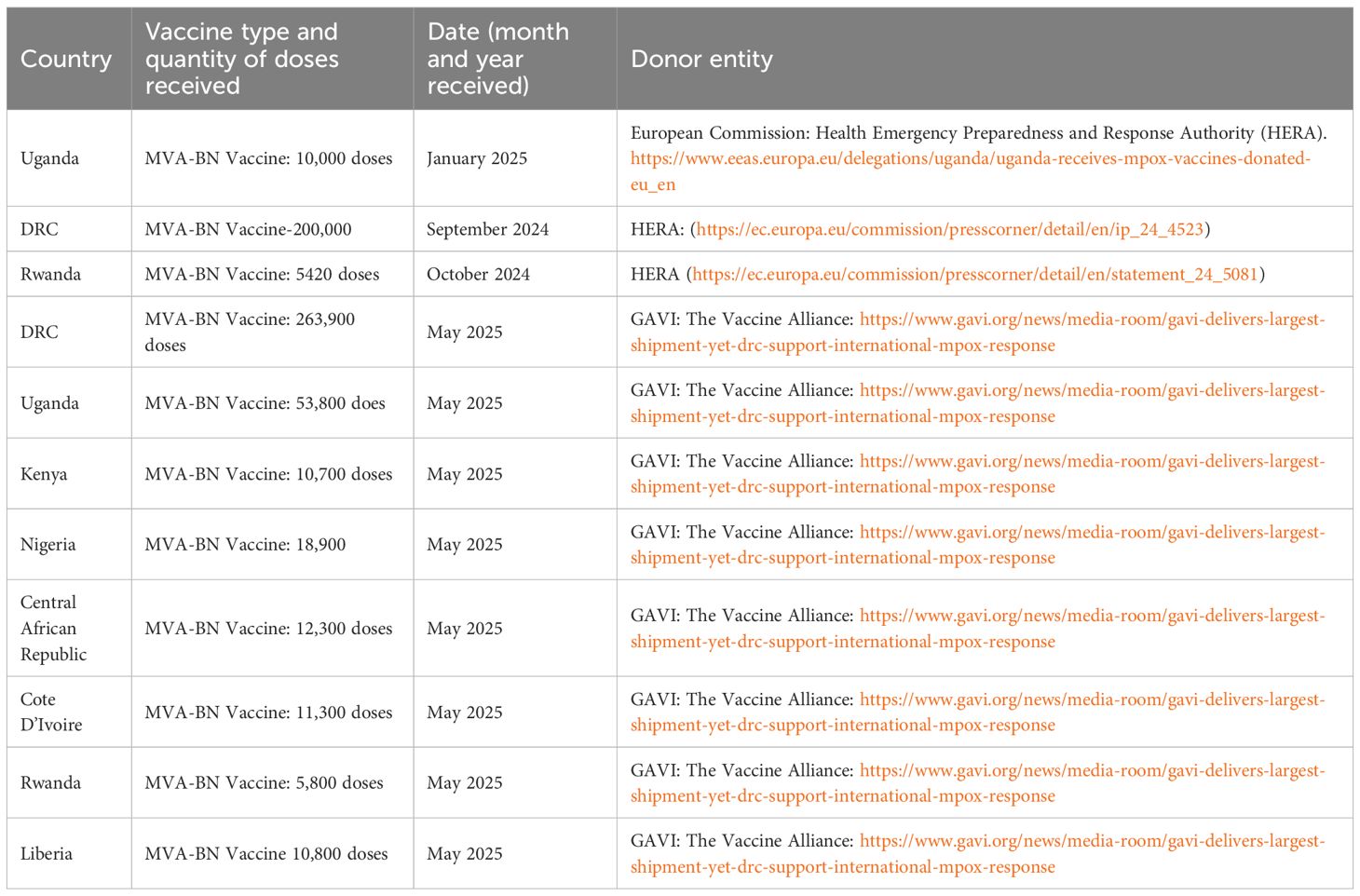
Table 2. A table showing some of the vaccine doses received by the African countries and the different donors.
Prioritisation has been critical to ensure maximum public health impact, even for the available doses. The Africa CDC, as one of the donors and regional entities, has prioritised and encouraged high-risk population vaccination, for example, the health workers like laboratory personnel handling the samples, contacts of the affected individuals, and immunocompromised individuals (34). However, this does not guarantee sufficient protection to the population against the outbreak. Often, this prioritisation is attributed to multiple factors such as limited vaccine doses, logistical challenges to distribute the available doses, slow vaccine rollout, and other regulatory barriers. Additionally, effective deployment of mpox vaccines depends on cold chain logistics, as vaccines like JYNNEOS require storage between -15 °C and -20 °C, while ACAM2000 requires refrigeration between 2 °C and 8 °C (35). Maintaining these temperatures is challenging, particularly in SSA’s remote and resource-limited settings where the electricity supply is unreliable. The freeze-dried and liquid-frozen formulations of Jeynneos have been recently approved in Europe, the United States, and Canada, and these developments could minimize dependence on cold chains (36). A multifactorial approach against these challenges is necessary for better outbreak management and prevention. Addressing vaccine access inequities and cold chain limitations through innovation and utilising solar-powered cold storage units is critical to ensuring effective mpox preparedness in SSA. Regional production capacity, supported through initiatives like the WHO’s mRNA hubs and local biotech partnerships, reduces dependence on global supply chains and strengthens local vaccine production. Also, implementing tiered distribution models based on risk and vulnerability ensures that both urban and rural populations have fair access.
Challenges to health system preparedness and response in SSA
Pandemics and reemerging epidemics have caused global and local economic depressions, which are reflected in healthcare financing and expenditure. For example, following the COVID-19 pandemic, SSA healthcare financing fell by 9.9% of the gross domestic product (GDP) in 2021 (37). However, governmental and out-of-pocket healthcare expenditures generally increased within SSA following the COVID-19 pandemic, with a slight depression after 2019, as demonstrated in Figure 3 (38). The increase in local spending was most likely due to the urgent need to respond to the pandemic with strategies such as scaling up testing, treatment, and vaccination efforts. However, reversing this trend by 2019 could reflect the region’s economic vulnerabilities and dependency on external funding. Yet, despite this depression, healthcare spending across SSA averaged 5.2% of the GDP by 2021, compared to the global average of 9.9% (37). Significantly, this depression in healthcare spending post-pandemic aligns with the decreased donor contributions and a constrained fiscal environment across multiple SSA countries. The reliance on out-of-pocket payments also demonstrates the health system weaknesses in SSA, such as the limited public health insurance systems to tackle these re-emerging epidemics, such as mpox. These deficits exacerbate the financial healthcare burden within SSA. The current global donor aid cuts, for example, from USAID and UKAID, further aggravate the issue. Therefore, SSA countries must strengthen healthcare financing mechanisms by diversifying funding sources, improving efficiency, and advocating for increased domestic support. SSA countries can prioritise strategies to avail funds for healthcare budgets, such as spending reviews, evidence-based saving, tax-based funding, performance-based budgeting, and program budgeting (39). Other strategies include macro-fiscal analyses, earmarking, social healthcare insurance programs, and automatic stabilisers. Some of these programs, such as automatic stabilisers, have been implemented in SSA countries like Kenya, enabling them to identify potential reserves to finance their healthcare budget (39). Budgeting strategies like performance-based and program budgeting depend solely on the program indicators, not the economic, administrative, or functional categories. Investments in universal health coverage and health system resilience are necessary to mitigate future pandemics and financial and health impacts.
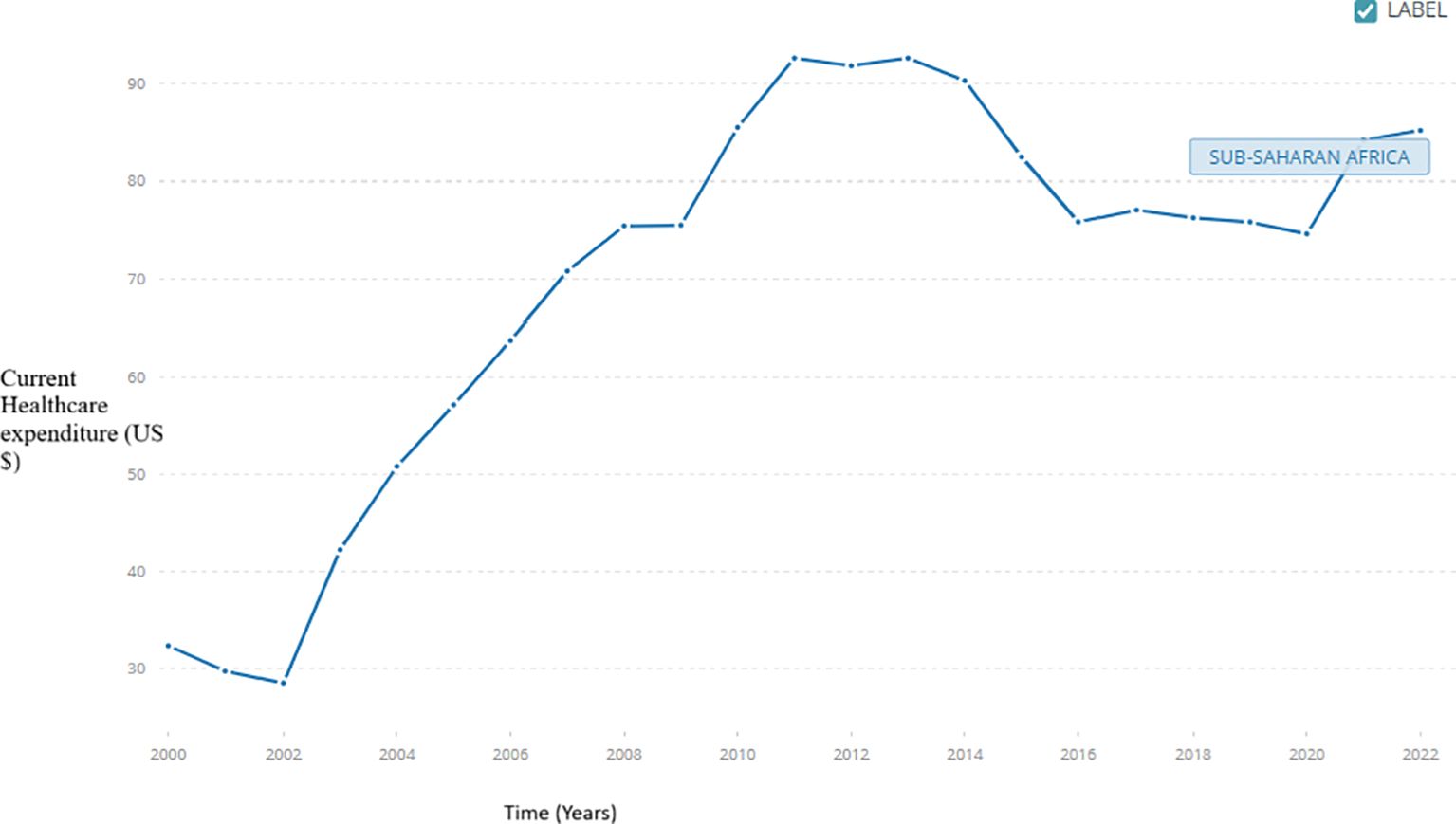
Figure 3. An illustration of the domestic government expenditure per capita for SSA. SSA government expenditure per capita.
Countries severely affected by mpox, such as the DRC, still lack the necessary resources for effective mpox surveillance and contact tracing programs (7). These programs are critical for the timely identification and isolation of cases. Yet, they are often underdeveloped due to the region’s long-standing struggles with underfunded and overstretched health systems, which are ill-equipped to handle emerging infectious diseases like mpox. The DRC’s healthcare system is characterised by significant weaknesses, including poor infrastructure, shortages of medical supplies, and barriers to accessing care, particularly in remote areas (40). Healthcare facilities in the DRC lack essential amenities such as consistent electricity, running water, and reliable sanitation systems. These deficiencies make it difficult to provide quality care, especially for infectious diseases like mpox, which require strict infection control measures (40). Political instability contributes to weak governance, which negatively impacts the design and implementation of health policies (41). For example, the health policy environment is often marked by inadequate coordination and inconsistent implementation, particularly in areas under the control of armed groups, such as the M23 in Goma or where the central government’s authority is weak (41). This fragile environment hinders mounting a coherent and effective response to mpox health emergencies (42).
Policy recommendations for strengthening preparedness and response in SSA
Multiple policy frameworks have been proposed to enhance the health system readiness of SSA countries to manage emerging and reemerging pandemics. However, incorporating strategies such as zoonotic spillover prevention activities is essential in minimising these outbreaks. With both an upstream and downstream focus, these activities can limit the zoonotic spread among humans and identify potential threats before occurrence (17). Upstream actions, such as reducing risk activities, for example, limiting the number of people hunting in the forests and game reserves, tracking infection dynamics within the vector and host environments using mathematical modeling tools and artificial intelligence, and integrating surveillance, must be incorporated into the national action plans. Downstream actions include enhancing laboratory diagnostics through the development of rapid diagnostic tests, improving the healthcare capacity at the different healthcare centres through integrating training courses and continuous professional development sessions, and enhancing surveillance (17). Other actions include increasing access to vaccines through boosting local manufacturing and enhancing the government’s political will, as well as managing the long-term effects of the disease, as demonstrated in Figure 1. These downstream actions align with the WHO health system framework and entail primary prevention strategies. Primary preventative strategies, for example, improved sanitation, safe handling of wild animals, and safe sex practices, like using a condom and being faithful to one partner, must be prioritised among SSA countries. These strategies predominantly focus on hindering the pandemic from happening in the first place. However, in the presence of cases, secondary prevention, entailing actions such as vaccination, early detection, health promotion, improving health systems, and behaviour change, can be prioritised (17). For example, financing existing cases must be averted, and attention must be directed to preventing these cases among different populations, especially in SSA (18). These options could minimise the spending required to counteract the next pandemic if it were prevented in the first place. Sadly, minimal information concerning the estimated costs of mpox primary prevention across the SSA countries is available, indicating another significant gap.
Long-term mpox prevention and control strategies must focus on improving vaccine access, public education, and health system financing, especially among countries with lower incidence rates in Africa. These countries could borrow from strategies such as those implemented in Rwanda to better combat future outbreaks (43). Surveillance and response mechanisms for mpox should also be linked with existing programs for HIV/AIDS, tuberculosis, and other infectious diseases. Adopting a One Health approach that connects human, animal, and environmental health will help address the zoonotic nature of mpox and prevent future outbreaks. For example, different stakeholders, like public health specialists, zoologists, epidemiologists, and virologists, can work together to identify, map, and corroborate information concerning a given mpox strain (44). Countries should negotiate vaccine procurement deals and establish stockpiles of vaccines and essential medical supplies, as noted by other developed countries (33). Besides, integrating mpox vaccination into routine immunisation schedules for high-risk populations, including healthcare workers and immunocompromised individuals, will help reduce future outbreaks. Also, increasing the health expenditure in SSA has been recommended to ensure that critical healthcare services are met and to produce a resilient health system. For example, Garcia-Escribano et al. (45) recommended an increase in healthcare spending by 4.6% by 2030 of the GDP. Also, capacity-building activities, such as Continued webinars prioritising aspects such as mpox case identification, clinical management, sample collection, and infection prevention and control, must be prioritised and enhanced among SSA countries. The capacity-building training must also enhance risk communication and community engagement to handle mpox-related stigma and misinformation. Vulnerable groups, including men who have sex with men, may be underserved due to social stigma, despite being at higher risk. Failure to address stigma compromises equitable access and violates human rights. Thus, community engagements have also been relevant, especially in addressing related stigma. Efforts, such as enhanced community engagement with the Key populations, as noted in Uganda, and the education sessions for the individuals affected, have been critical in minimizing the stigma related to the disease (46).
Conclusion and recommendations
Practical strategies to combat emerging and reemerging epidemics and pandemics in SSA, such as mpox, require strong and resilient healthcare systems. Resilient healthcare systems need a multifaceted investment from key players, such as global, regional, national, and local entities and the community. These investments build the different entities within the healthcare system structure, which is vital for responding to community healthcare needs. SSA countries must sustainably invest in their health workforce and health systems infrastructure to fight mpox and other emerging infectious diseases, especially with the turbulent global health atmosphere characterised by funding cuts. SSA countries also must prioritise self-reliance approaches, such as national budget re-evaluations and generating local revenue directed to healthcare, to ensure their health systems are sufficiently upheld. Sustainable and well-built health systems enhance early detection, response, and recovery from different outbreaks while maintaining other essential healthcare services. Integrating mpox preparedness into broader national health policies is necessary and aligns perfectly with the WHO health systems framework. Mpox preparedness plans should be embedded within universal health coverage (UHC) programs, ensuring equitable access to diagnostics, vaccines, and treatment.
Author contributions
IA: Conceptualization, Writing – original draft, Writing – review & editing. PM: Conceptualization, Writing – original draft, Writing – review & editing. AO: Writing – original draft, Writing – review & editing. JG: Writing – original draft, Writing – review & editing. PA: Writing – original draft, Writing – review & editing. EI: Writing – original draft, Writing – review & editing. HM: Writing – original draft, Writing – review & editing. TE: Writing – original draft, Writing – review & editing. LA: Writing – original draft. JD: Writing – original draft, Writing – review & editing. LO: Writing – original draft, Writing – review & editing. EM: Writing – original draft, Writing – review & editing. SN: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AMM, Access and Allocation Mechanism; SSA, Subsaharan Africa; UHC, Universal Health Coverage; IPC, Infection Prevention and Control; Mpox, MonkeyPox; DRC, Democratic Republic of Congo; CDC, Centres for Disease Control and Prevention.
References
1. WHO. Health systems resilience toolkit: a WHO global public health good to support building and strengthening of sustainable health systems resilience in countries with various contexts. In: Health systems resilience toolkit: a WHO global public health good to support building and strengthening of sustainable health systems resilience in countries with various contexts Geneva, Switzerland (2022). Available online at: https://iris.who.int/bitstream/handle/10665/354177/9789240048751-eng.pdf.
2. WHO. Vaccine doses allocated to 9 African countries hardest hit by mpox surge. Geneva, Switzerland: Joint press release Africa CDC, CEPI, Gavi, UNICEF, WHO (2024). Available online at: https://www.who.int/news/item/06-11-2024-vaccine-doses-allocated-to-9-african-countries-hardest-hit-by-mpox-surge (Accessed April 20, 2025).
3. Hanson K, Brikci N, Erlangga D, Alebachew A, De Allegri M, Balabanova D, et al. The Lancet Global Health Commission on financing primary health care: putting people at the centre. Lancet Global Health. (2022) 10:e715–72. doi: 10.1016/S2214-109X(22)00005-5
4. Ahmat A, Okoroafor SC, Kazanga I, Asamani JA, Millogo JJS, Illou MMA, et al. The health workforce status in the WHO African Region: findings of a cross-sectional study. BMJ Global Health. (2022) 7:e008317. doi: 10.1136/bmjgh-2021-008317
5. Gashema P, Musafiri T, Ndahimana F, Iradukunda H, Saramba E, Nyakatswau ST, et al. mpox in East Africa: learning from COVID-19 and Ebola to strengthen Public Health responses. Viruses. (2024) 16:1578. doi: 10.3390/v16101578
6. Gómez-Pérez GP, de Graaff AE, Dekker JT, Agyei BB, Dada I, Milimo E, et al. Preparing healthcare facilities in sub-Saharan Africa for future outbreaks: insights from a multi-country digital self-assessment of COVID-19 preparedness. BMC Health Serv Res. (2024) 24:254. doi: 10.1186/s12913-024-10761-2
7. Ndembi N, Folayan MO, Komakech A, Mercy K, Tessema S, Mbala-Kingebeni P, et al. Evolving epidemiology of mpox in Africa in 2024. New Engl J Med. (2025) 392(7):666–76. doi: 10.1056/NEJMoa2411368
8. WHO. Global mpox Trends(2025). Available online at: https://worldhealthorg.shinyapps.io/mpx_global/sec-clades (Accessed August 6 2025).
9. Ayanore MA, Amuna N, Aviisah M, Awolu A, Kipo-Sunyehzi DD, Mogre V, et al. Towards resilient health systems in sub-Saharan Africa: a systematic review of the English language literature on health workforce, surveillance, and health governance issues for health systems strengthening. Ann Global Health. (2019) 85:113. doi: 10.5334/aogh.2514
10. Enitan SS, Akele RY, Omoare AA, Dogonyaro BB, Iduh MU, Digban KA, et al. mpox resurgence: preventing a potential pandemic through lessons learned from COVID-19 experience and future research directions. Avicenna J Clin Microbiol Infection. (2024) 11:191–203. doi: 10.34172/ajcmi.3564
11. Besombes C, Gonofio E, Konamna X, Selekon B, Gessain A, Berthet N, et al. Intrafamily transmission of monkeypox virus, Central African Republi. Emerging Infect Dis. (2019) 25:1602. doi: 10.3201/eid2508.190112
12. Kalthan E, Tenguere J, Ndjapou SG, Koyazengbe TA, Mbomba J, Marada RM, et al. Investigation of an outbreak of monkeypox in an area occupied by armed groups, Central African Republic. Medecine maladies infectieuses. (2018) 48:263–8. doi: 10.1016/j.medmal.2018.02.010
13. Musuka G, Moyo E, Tungwarara N, Mhango M, Pierre G, Saramba E, et al. A critical review of mpox outbreaks, risk factors, and prevention efforts in Africa: lessons learned and evolving practices. IJID regions. (2024) 12:100402. doi: 10.1016/j.ijregi.2024.100402
14. Masirika LM, Udahemuka JC, Ndishimye P, Martinez GS, Kelvin P, Nadine MB, et al. Epidemiology, clinical characteristics, and transmission patterns of a novel mpox (Monkeypox) outbreak in eastern Democratic Republic of the Congo (DRC): an observational, cross-sectional cohort study. medRxiv. (2024), 2024–03. doi: 10.1101/2024.03.05.24303395
15. Ogoina D, Dalhat MM, Denue BA, Okowa M, Chika-Igwenyi NM, Oiwoh SO, et al. Mpox epidemiology and risk factors, Nigeri. Emerging Infect Dis. (2024) 30:1799. doi: 10.3201/eid3009.240135
16. Ogoina D, Dalhat MM, Denue BA, Okowa M, Chika-Igwenyi NM, Yusuff HA, et al. Clinical characteristics and predictors of human mpox outcome during the 2022 outbreak in Nigeria: a cohort study. Lancet Infect Dis. (2023) 23:1418–28. doi: 10.1016/S1473-3099(23)00427-9
17. Authored by the members of the One Health High-Level Expert Panel (OHHLEP), Markotter W, Mettenleiter TC, Adisasmito WB, Almuhairi S, Barton Behravesh C, et al. Prevention of zoonotic spillover: From relying on response to reducing the risk at source. PloS Pathog. (2023) 19:e1011504. doi: 10.1371/journal.ppat.1011504
18. Bernstein AS, Ando AW, Loch-Temzelides T, Vale MM, Li BV, Li H, et al. The costs and benefits of primary prevention of zoonotic pandemics. Sci Adv. (2022) 8:eabl4183. doi: 10.1126/sciadv.abl4183
19. Africa CDC. Africa CDC Strengthens Laboratory Capacity for mpox and Other Outbreaks in Burundi(2025). Available online at: https://africacdc.org/news-item/africa-cdc-strengthens-laboratory-capacity-for-mpox-and-other-outbreaks-in-Burundi/ (Accessed March 27 2025).
20. Savinkina A, Chitwood M, Kwon J, Pitzer VE, Gonsalves G, and Paltiel AD. Planning for mpox on a college campus: A model-based decision-support tool. Ann Internal Med. (2023) 176:340–7. doi: 10.7326/M22-2734
21. Lokossou VK, Awori AS, Fatimehin V, Usman AB, Sogbossi L, Agbla F, et al. Assessing mpox epidemic readiness status in ECOWAS region: strengths, gaps, and recommendations for an improved response. Pan Afr Med J. (2025) 50(1):2. doi: 10.11604/pamj.supp.2025.50.1.46077
22. Nishtar S. The mpox emergency and the role of Gavi. Lancet. (2024) 404:729–31. doi: 10.1016/S0140-6736(24)01706-9
23. Rahim FO, Fallah M, Jain U, Richardson ET, Ndembi N, Ngongo N, et al. Challenges and ongoing actions to address the mpox emergency in Africa. Ann Global Health. (2024) 90:68. doi: 10.5334/aogh.4580
24. Centres for Disease Control. Mpox Treatment Information for Healthcare Professionals | mpox | Poxvirus | CDC(2024). Available online at: https://www.cdc.gov/poxvirus/mpox/clinicians/treatment.html (Accessed January 27,2025).
25. Moore MJ, Rathish B, and Zahra F. Mpox (monkeypox). In: StatPearls. Treasure Island (FL): StatPearls Publishing (2023).
26. Adler H, Gould S, Hine P, Snell LB, Wong W, Houlihan CF, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. (2022) 22:1153–62. doi: 10.1016/S1473-3099(22)00228-6
27. McLean JR, Zucker JE, Vail RM, Shah SS, Fine SM, McGowan JP, et al. Prevention and treatment of mpox. (2024). https://www.ncbi.nlm.nih.gov/books/NBK603707/.
28. Wolf T. Maintenance of preparedness. In: Bioemergency Planning: A Guide for Healthcare Facilities (2018). p. 157–68. https://link.springer.com/chapter/10.1007/978-3-319-77032-1_13.
29. Mwine P, Atuhaire I, Ahirirwe SR, Nansikombi HT, Senyange S, Elayeete S, et al. Readiness of health facilities to manage individuals infected with COVID-19, Uganda, June 2021. BMC Health Serv Res. (2023) 23:441. doi: 10.1186/s12913-023-09380-0
30. Alum S, Asiimwe M, Kanyomozi G, Nalikka J, Okwaro P, Migisha I, et al. Optimising highly infectious disease isolation unit management: experiences from the infectious diseases isolation and research unit, Fort Portal, Uganda. Disaster Med Public Health preparedness. (2023) 17:e72. doi: 10.1017/dmp.2021.339
31. Lulli LG, Baldassarre A, Mucci N, and Arcangeli G. Prevention, risk exposure, and knowledge of monkeypox in occupational settings: a scoping review. Trop Med Infect Dis. (2022) 7:276. doi: 10.3390/tropicalmed7100276
32. Rallapalli S, Razai MS, Majeed A, and Drysdale SB. Diagnosis and management of monkeypox in primary care. J R Soc Med. (2022) 115:384–9. doi: 10.1177/01410768221131914
33. GAVI. Gavi delivers largest shipment yet to DRC in support of international mpox response(2025). Available online at: https://www.gavi.org/news/media-room/gavi-delivers-largest-shipment-yet-drc-support-international-mpox-response (Accessed August 6 2025).
34. Lee SS, Traore T, and Zumla A. The WHO mpox public health emergency of international concern declaration: need for reprioritisation of global public health responses to combat the MPXV Clade I epidemic. Int J Infect Dis. (2024) 147. doi: 10.1016/j.ijid.2024.107227
35. Rizk Y, Lippi G, Henry BM, Notarte KI, and Rizk JG. Update on mpox management: epidemiology, vaccines and therapeutics, and regulatory changes. Drugs. (2025) 85:1–9. doi: 10.1007/s40265-024-02117-1
36. Greenberg RN, Schmidt D, Reichhardt D, Roesch S, Vidojkovic S, Maclennan J, et al. Equivalence of freeze-dried and liquid-frozen formulations of MVA-BN as smallpox and mpox vaccine. Hum Vaccines Immunotherapeutics. (2024) 20:2384189. doi: 10.1080/21645515.2024.2384189
37. World Bank Group. Current health expenditure (% of GDP) - Sub-Saharan Africa(2024). Available online at: https://data.worldbank.org/indicator/SH.XPD.CHEX.GD.ZS?end=2021&locations=ZG&name_desc=false&start=2021&view=map (Accessed January 27 2025).
38. World Bank Group. Current health expenditure per capita (current US$) - Sub-Saharan Africa(2024). Available online at: https://data.worldbank.org/indicator/SH.XPD.CHEX.PC.CD?end=2022&locations=ZG&name_desc=false&start=2000&view=chart&year=2021 (Accessed January 27 2025).
39. Kurowski C, Evans DB, Tandon A, Eozenou PHV, Schmidt M, Irwin A, et al. From double shock to double recovery. (2021). doi: 10.1596/35298
40. Abubakar I, Lutwama J, Kyobutungi C, and Sankoh O. Mpox global emergency: strengthening African leadership. Lancet. (2024) 404:1286–8. doi: 10.1016/S0140-6736(24)02068-3
41. Ndi HN. Violent conflict, diplomacy, and health in Africa. In: Health Diplomacy in Africa: Trends, Challenges, and Perspectives. Springer International Publishing, Cham (2023). p. 121–42.
42. Ratevosian J, Heisler M, Carpino T, McHale T, Mushekuru J, and Beyrer C. Addressing transnational exploitation and armed conflict in the response to mpox. Lancet. (2024) 404:2137–40. doi: 10.1016/S0140-6736(24)02418-8
43. Rwibasira G, Dzinamarira T, NGabonziza JC, Tuyishime A, Ahmed A, and Muvunyi CM. The mpox Response Among Key Populations at High Risk of or Living with HIV in Rwanda: Leveraging the Successful National HIV Control Program for More Impactful Interventions. Vaccines. (2025) 13:307. doi: 10.3390/vaccines13030307
44. Najimudeen M, Chen HW, Jamaluddin NA, Myint MH, and Marzo RR. Monkeypox in pregnancy: susceptibility, maternal and fetal outcomes, and one health concept. Int J Maternal Child Health AIDS. (2022) 11:e594. doi: 10.21106/ijma.594
45. Garcia-Escribano MM, Juarros P, and Mogues MT. Patterns and drivers of health spending efficiency. Int Monetary Fund. (2022). doi: 10.5089/9798400204388.001
46. WHO. Leveraging collaboration to combat mpox among Uganda’s most vulnerable communities(2025). Available online at: https://www.afro.who.int/countries/Uganda/news/leveraging-collaboration-combat-mpox-among-Ugandas-most-vulnerable-communities (Accessed August 6 2025).
Keywords: health systems; health systems preparedness, mpox, health system framework, Sub-Saharan Africa (SSA), zoonotic disease
Citation: Ayesiga I, Magala P, Ovye A, Gmanyami JM, Atwau P, Ismaila E, Muwonge H, Ediamu TD, Atimango L, Dogo JM, Okoro LN, Mulogo EM and Nakacubo SG (2025) Health system preparedness among African countries for disease outbreaks using the World Health Organisation Health systems framework: an awakening from the recent mpox outbreak. Front. Trop. Dis. 6:1618205. doi: 10.3389/fitd.2025.1618205
Received: 25 April 2025; Accepted: 25 August 2025;
Published: 30 September 2025.
Edited by:
Basavaraj S. Mathapati, Indian Council of Medical Research (ICMR), IndiaReviewed by:
Hana Chen, Curtin University Sarawak, MalaysiaJeremiah Oghuan, University of Texas Health Science Centre at Houston, United States
Copyright © 2025 Ayesiga, Magala, Ovye, Gmanyami, Atwau, Ismaila, Muwonge, Ediamu, Atimango, Dogo, Okoro, Mulogo and Nakacubo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Innocent Ayesiga, YXllc2lnYTQ5QGdtYWlsLmNvbQ==
 Innocent Ayesiga
Innocent Ayesiga Primrose Magala2
Primrose Magala2