Abstract
Climate change is reshaping the epidemiology of vector-borne diseases, with zoonotic cutaneous leishmaniasis (ZCL) caused by Leishmania major emerging as a growing public health concern in Morocco. This study employs ecological niche modeling (ENM) to assess the current distribution and project future impacts of climate change on L. major, its primary vector (Phlebotomus papatasi), and reservoir host (Meriones shawi) under four Representative Concentration Pathway (RCP) scenarios (2.6, 4.5, 6.0 and 8.5). Under present climate conditions, our models reveal distinct distribution patterns: L. major is concentrated in southeastern Morocco, P. papatasi is widespread across central regions, and M. shawi occupies nearly nationwide distribution except Western Sahara. Projections indicate L. major will extend its range into eastern, High Atlas, and Rif regions (1.5–1.6% habitat gain), while P. papatasi and M. shawi will expand across central and southern Morocco (3.5–5.9% gain), with minimal habitat loss (<0.6%). These findings demonstrate a possible climate-driven shift in ZCL transmission geography, with current endemic areas expanding and new risk zones emerging in previously unaffected regions. The projections underscore the urgent need for integrated surveillance and climate-adaptive control strategies to mitigate outbreaks in vulnerable regions. By linking observed distributions to future environmental shifts, this work provides a framework for proactive public health interventions in Morocco and similar endemic areas facing climate change impacts.
1 Introduction
Climate change, through altered temperature, precipitation, and extreme weather, is reshaping the distribution and dynamics of vectors and vector-borne diseases globally (1). Among these, zoonotic cutaneous leishmaniasis (ZCL), caused by Leishmania major and transmitted by infected female Phlebotomus sandflies, has shown notable shifts in incidence and geographic range (2). In Morocco, ZCL continues to pose a public health challenge, particularly in arid and semi-arid regions, where ecological conditions strongly influence both vector and reservoir host populations (3). Climatic factors such as increased temperature and reduced rainfall have been correlated with higher sandfly densities and extended transmission seasons (4). Changes in land use, agricultural practices, and urbanization are further contributing to sandfly habitat expansion and increased human-vector contact (5). Moreover, human migration and mobility, coupled with animal trade and environmental disruption, facilitate the introduction and spread of infected vectors and hosts to new areas (6). The One Health approach is increasingly recognized as critical to understanding and managing ZCL, highlighting the interconnectedness of human, animal, and environmental health (7).
The potential distribution of L. major vectors and reservoirs is influenced by various climatic factors, including temperature, humidity, and precipitation, which affect the life cycles and habitats of these organisms (8). As climate change continues to alter these environmental parameters, the geographical range of ZCL may expand or contract, leading to changes in the epidemiology of the disease (9). This necessitates a thorough understanding of how climate change may impact the distribution of the main vectors and reservoirs of ZCL in Morocco.
Previous studies have demonstrated that climate change can significantly impact the distribution of sandflies and reservoirs. For instance, Paz, 2024 found that climate change scenarios projected an increase in suitable habitats for sandflies in Europe, while other studies have highlighted the role of climate variables in influencing the distribution of sandfly species in the Mediterranean region (10–12). Similarly, Elith et al., 2010 and Peterson, 2006 have discussed the utility of Ecological niche modeling (ENM) in predicting shifts in species distributions under changing climatic conditions (13, 14).
ENM has emerged as a critical tool in predicting the spatial and temporal dynamics of vector-borne diseases in response to environmental and anthropogenic changes. As global mobility increases and climate change continues to reshape ecological boundaries, the distribution of vector species and associated pathogens is shifting at unprecedented rates (15, 16). This is particularly evident in North Africa, where zoonotic cutaneous leishmaniasis (ZCL), primarily caused by L. major and transmitted by P. papatasi, is becoming increasingly prevalent. Morocco has reported a significant rise in ZCL cases over recent decades, with outbreaks often occurring in newly affected areas due to environmental changes, urban expansion, and movement of people and animals (11, 17).
ENM allows researchers and policymakers to map areas of potential risk by integrating climatic variables (e.g., temperature, humidity, precipitation), land use, host distribution, and vector biology (14, 18). In Morocco, ENM has been successfully applied to anticipate the spread of P. papatasi and its reservoir hosts, thereby identifying vulnerable regions before outbreaks occur (19). These predictive tools are vital, as it help in anticipating new foci due to human migration, conflict, and ecological disturbance (20, 21).
Moreover, the psychosocial consequences of ZCL particularly the disfiguring scars it leaves on affected individuals, many of whom are women and children are profound in Moroccan society and often overlooked in public health discourse (22, 23).
This study aims to model the potential distribution of the primary vectors and reservoirs of zoonotic cutaneous leishmaniasis in Morocco under different climate change scenarios. By utilizing ENM and climate projection data, we seek to predict future changes in the habitats suitable for sandflies and reservoir hosts. These predictions are crucial for public health planning and for the implementation of effective control measures to mitigate the impact of ZCL in a changing climate.
2 Materials and methods
2.1 Occurrence data
Comprehensive occurrence records for P. papatasi and M. shawi were compiled through multiple complementary approaches to ensure robust spatial representation across Morocco. Field collections conducted between 2003 and 2020 targeted known ZCL foci in northern and central regions, as identified in Moroccan Ministry of Health (MMH) surveillance reports (MMH, 2017). These field efforts employed standardized trapping protocols for sandflies and rodent surveys in endemic areas. To augment field data, we systematically reviewed literature from 1991–2020 through searches of PubMed and Scopus using the terms “P. paptasi” and “Morocco,” yielding additional georeferenced occurrence points. For L. major case locations, we extracted epidemiological data from MMH reports (2010-2017) supplemented by published case studies to ensure coverage of emerging transmission zones. All occurrence data underwent rigorous quality control, including removal of duplicate records and verification of coordinate precision to <1 km resolution. Spatial filtering was applied to minimize sampling bias, particularly around health facilities and easily accessible sites. The final curated dataset comprised 143 P. papatasi, 111 M. shawi, and 69 L. major occurrences (Figure 1), which we partitioned using a stratified random approach - allocating 70% for model calibration and 30% for evaluation. The full data set is available at Supplementary Material S1.
Figure 1
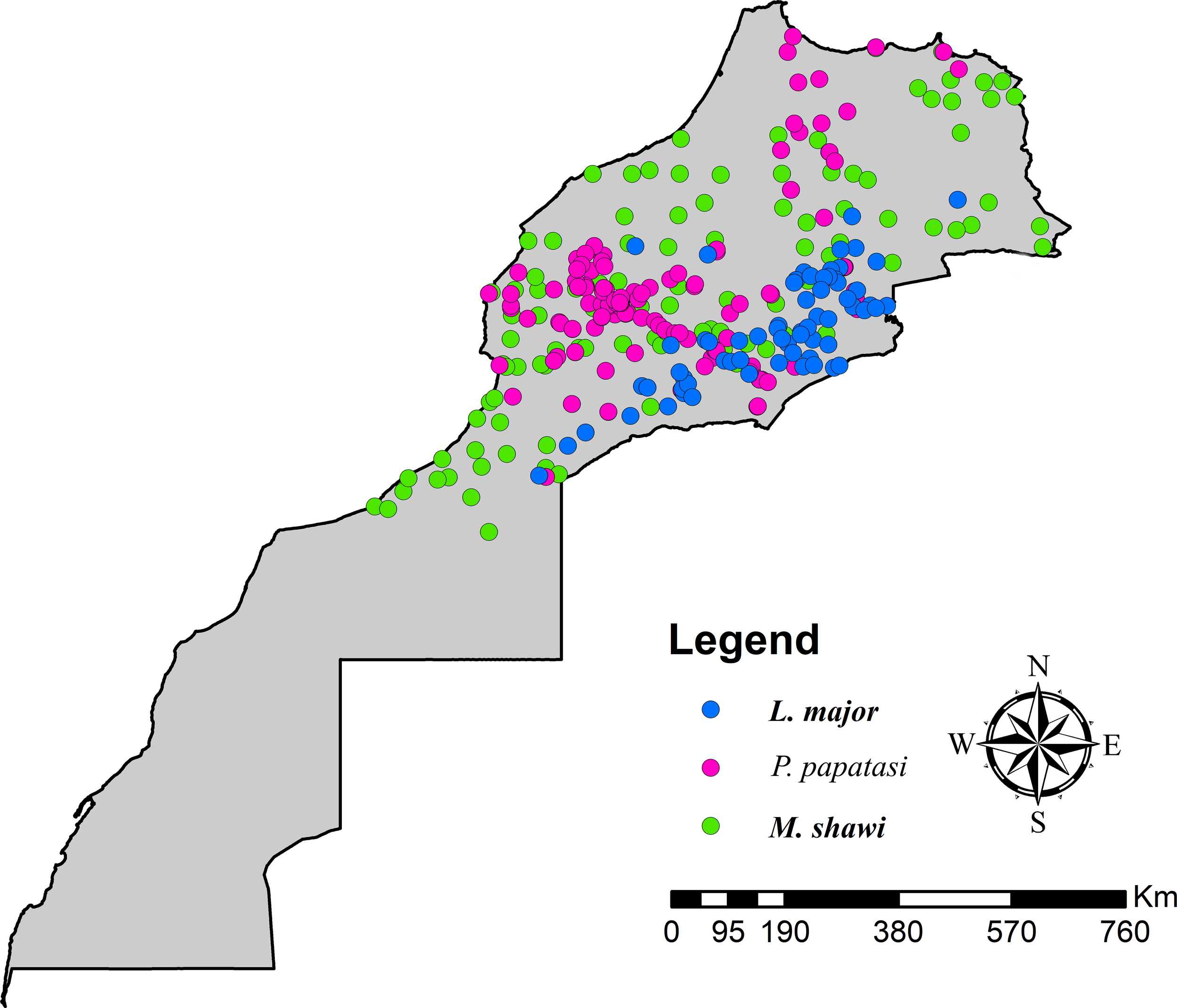
Species occurrences used for predictions.
2.2 Climatic data
Data from WorldClim (www.worldclim.org, version 1.4) were used to characterize current global climates, including 19 bioclimatic variables originally derived from monthly temperature and rainfall values collected from weather stations in 1950–2000 (Hijmans et al., 2005). We selected data coarsest at the higher spatial resolutions (i.e., 30 arcsecond). To characterize influences of climate change on the distribution of each species modeled, we selected parallel data sets for four representative concentration pathways (RCPs; RCP 2.6 and RCP 8.5) accounting for different future emission scenarios from the Coupled Model Intercomparison Project Phase 5 (CMIP5) available in WorldClim archive (version 1.4, 30 arcsecond). Each RCP leading to specific radiative forcing characteristics (https://www.ipcc-data.org); RCP 2.6 is lowest, RCP 4.5 and RCP 6.0 intermediate, RCP 8.5 high greenhouse gas emissions and higher resulting radiative forcing. For each RCP, we included 9 General Circulation Models (GCMs): BCC-CSM1-1, CCSM4, GISS-E2-R,HadGEM2-AO, HadGEM2-ES, IPSL-CM5A-LR, MIROC-ESM-CHEM, MIROC5, and MRICGCM3, for a total of 18 combinations (9 x 2 RCPs). Bioclimatic variables 8–9 and 18–19 was omitted from analysis, considering known spatial artifacts in those variables. The remaining of 15 variables was submitted to a principal component analysis (PCA) to reduce the dimensionality and avoid multicollinearity between variables (S2a) (Supplementary Material S2) (24). The component loadings in the present-day data were used to transform future-climate data using the PCA Projection function in Niche Analyst software version 3.0 (25, 26).
2.3 Ecological niche modeling
Modeling was carried out using Maxent version 3.4.1, which uses an optimization procedure that compares records of species in presence only to a “background” sample of environments across the region of concern, using the maximum entropy principle (27). Maxent typically outperforms other methods based on predictive accuracy, even for species with scarce occurrence records (28, 29). For each modeled species, we used a combination of different features (linear, quadratic, product, threshold, and hinge), and regularizations multiplier values; we used cross-validation to select optimal settings. The models were calibrated based on the first eight components of the PCA analyses described above, summarizing cumulatively 99.99% of total climate variance (S2b). The extrapolation and clamping options were deactivated to avoid any over-prediction risk in heterogeneous environments (24). To overcome the lack of occurrence records in some areas and the non-availability of absence data, a bias file was used to fine-tune background point selection in Maxent to a maximum radial distance of 50 km from observation points, using SDMtoolbox (30). We ran 50 bootstrap replicates in MaxEnt, and the median output was used in analyses. The median of medians across all GCMs for each RCP was used as an estimation of conditions under that RCP, and final models were threshold based on a maximum allowable omission error rate of 5% (29), assuming that up to 5% of occurrence data may include errors that misrepresented environmental values. Model performance was evaluated using two different metrics: area under the curve (AUC) and partial receiver operating characteristic (pROC) approach. Occurrence datasets and obtained binary maps were subjected to over 1000 bootstrap iterations analyses, each based on 50% random points resampling with replacement and with an omission error threshold of 1% (p < 0.01). The pROC statistic test was calculated using the pROC function available in package NicheToolBox under R.
2.4 Niche visualization
The overlapping niches of the three considered species were visualized in a 3D environmental space, using NicheA software version 3.0 (25). We used the first three principal components, out of the 8 PC generated in the previous section, to draw the environmental background cloud under current and future-climate conditions separately (26). Then we created a virtual niche from occurrence data sets of each species in that environmental background cloud.
3 Results
Models have demonstrated proficiency in accurately depicting the geographical range of observed occurrence records under current climate conditions, surpassing mere representation. The potential distribution of the three species considered exhibited a high level of suitability across a significant portion of the center and the northern region of the country (Figure 2). The areas with the greatest risk of L. major potential distribution are limited in the center-estern side (Figure 2A). Currently, suitable habitats for P. papatasi were predominantly situated to the central and north part of the country (Figure 2B). As for M. shawi, the environmental conditions in Morocco, which encompass up to half of the country’s land area, appear to align with its potential distribution requirements (Figure 2C). The areas classified as highly suitable were primarily concentrated in the central and northern parts, as well the southern part, inhabiting the dry and hot deserts.
Figure 2
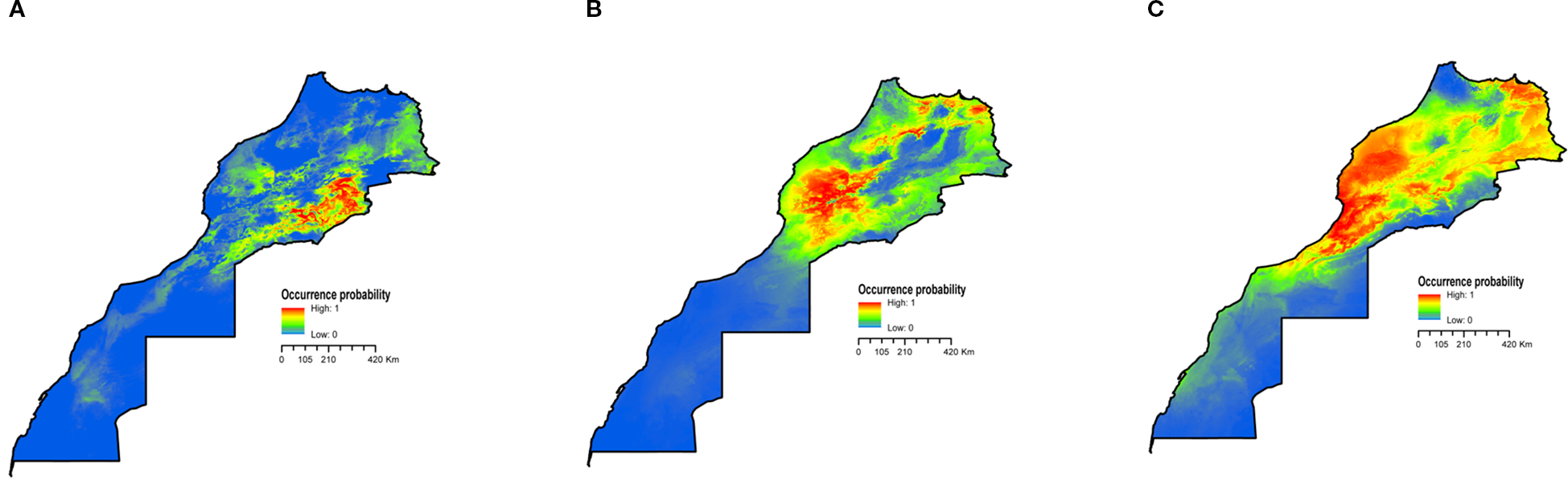
Predicted potential distribution for L. major(A), P. papatasi(B), and M. shawi(C) under current climat conditions.
The anticipated range changes for the three species under various climatic assumptions are depicted in Figure 3. By 2050, it is expected that L. major will exhibit a considerably broader potential distribution across the central-eastern side of the country, regardless of the RCP scenarios (Figure 3A). Moreover, there is a likelihood of the species expanding its habitat range to new areas in the central and south-eastern parts. Transitioning from RCP 2.6 to RCP 8.5, the three species are projected to persist and further increase its range in many similar areas as predicted under RCP 2.6, with same level of certainty under RCP 8.5, except for M. shawi were expanded under RCP 2.6 slightly more than under RCP 8.5 (Figure 3B). The model indicates an extension of the suitable habitat for P. papatasi towards central areas under RCP 8.5 (Figure 3C). The species has also expanded its range to encompass newly discovered areas on the northern and southern side. It is anticipated that the areas with suitable habitat will continue to expand under all the considered RCPs. Looking ahead, the climatic conditions most favorable for M. shawi are projected to prevail across the entire country, except for the extreme eastern central part of Morocco. The expansion of suitable habitats is predicted to persist under RCP 2.6 and RCP 8.5. Figure 4 illustrate the changes in distribution between current conditions and future projections. These figures also demonstrate the consensus among all GCMs in predicting areas where no changes are expected. The maps display varying degrees of expansion and contraction of habitat for each species under consideration. Detailed calculations regarding the changes in distribution can be found in Supplementary File 3. In the case of L. major, the potential distributional areas are projected to increase from current conditions to RCP 2.6, RCP 4.5, RCP 6.0, and RCP 8.5 by 1.548%, 1.556%, 1.572%, and 1.637%, respectively (Figure 4A). Among these scenarios, RCP 8.5 results in the most expansion. In general, the areas potentially suitable for P. papatasi are projected to increase from the present-day to 2050. Specifically, under RCP 2.6, RCP 4.5, RCP 6.0, and RCP 8.5, the increase is estimated to be 3.489%, 3.721%, 3.807%, and 3.807%, respectively (Figure 4B). However, the species is expected to experience a contraction of only 0.590%, 0.465%, 0.506%, and 0.506% under the respective RCPs mentioned above. Among the four modeled RCPs, RCP 8.5 and RCP 6.0 present the most favorable scenarios for habitat expansion, while RCP 2.6 results in a significant loss of predicted habitat suitability. Regarding M. shawi, the potential distribution areas are anticipated to increase under all RCPs (Figure 4C). Specifically, the increases are projected to be 5.711% (RCP 2.6), 5.802% (RCP 4.5), 5.562% (RCP 6.0), and 5.872% (RCP 8.5). However, there is also an expected reduction of 0.067% (RCP 2.6), 0.067% (RCP 4.5), 0.084% (RCP 6.0), and 0.124% (RCP 8.5). Notably, RCP 8.5 exhibits both the largest gained area and the largest reduction area.
Figure 3
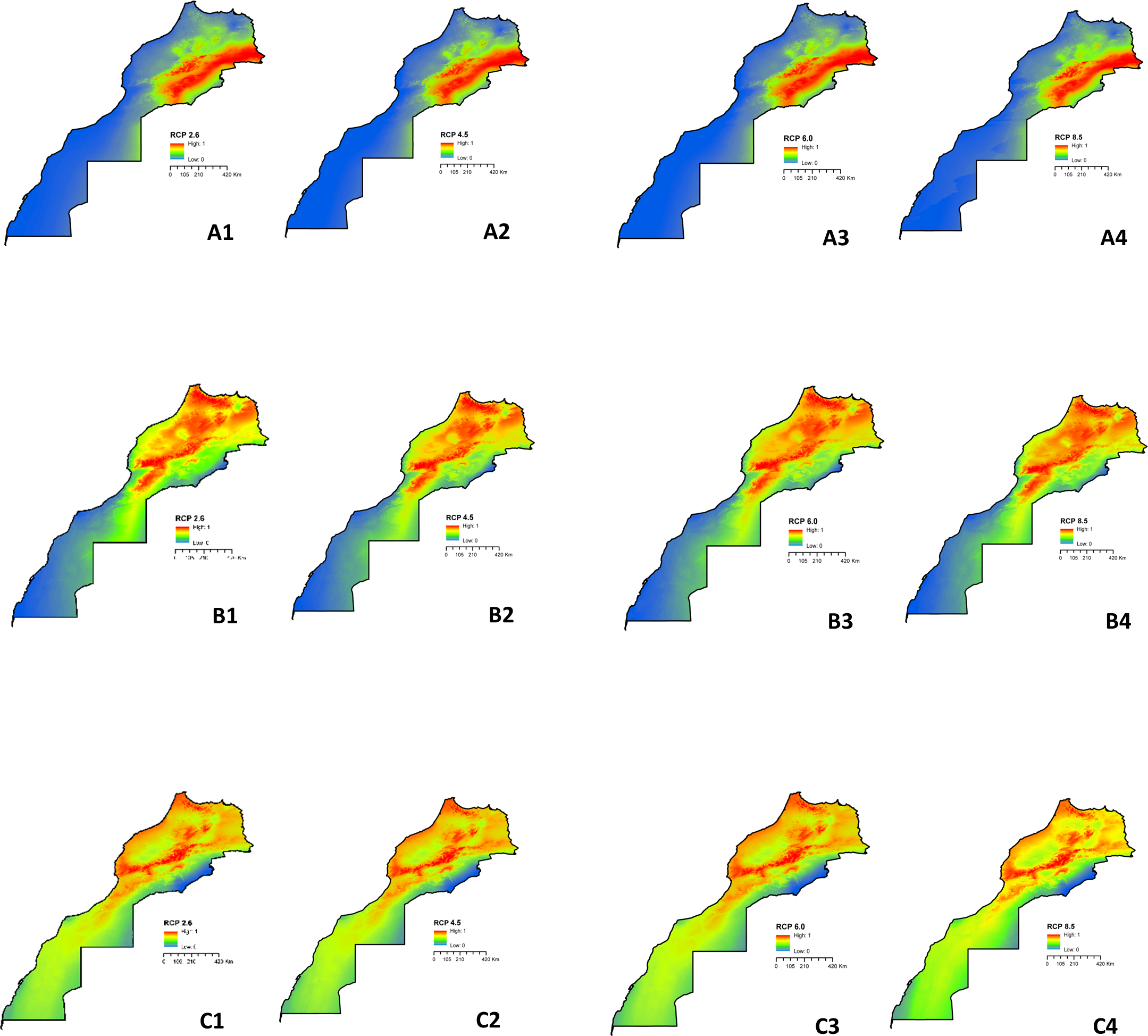
Predicted potential distribution for L. major(A), P. papatasi(B), and M. shawi(C) under RCP 2.6 (1), RCP 4.5 (2), RCP 6.0 (3) and RCP 8.5 (4).
Figure 4
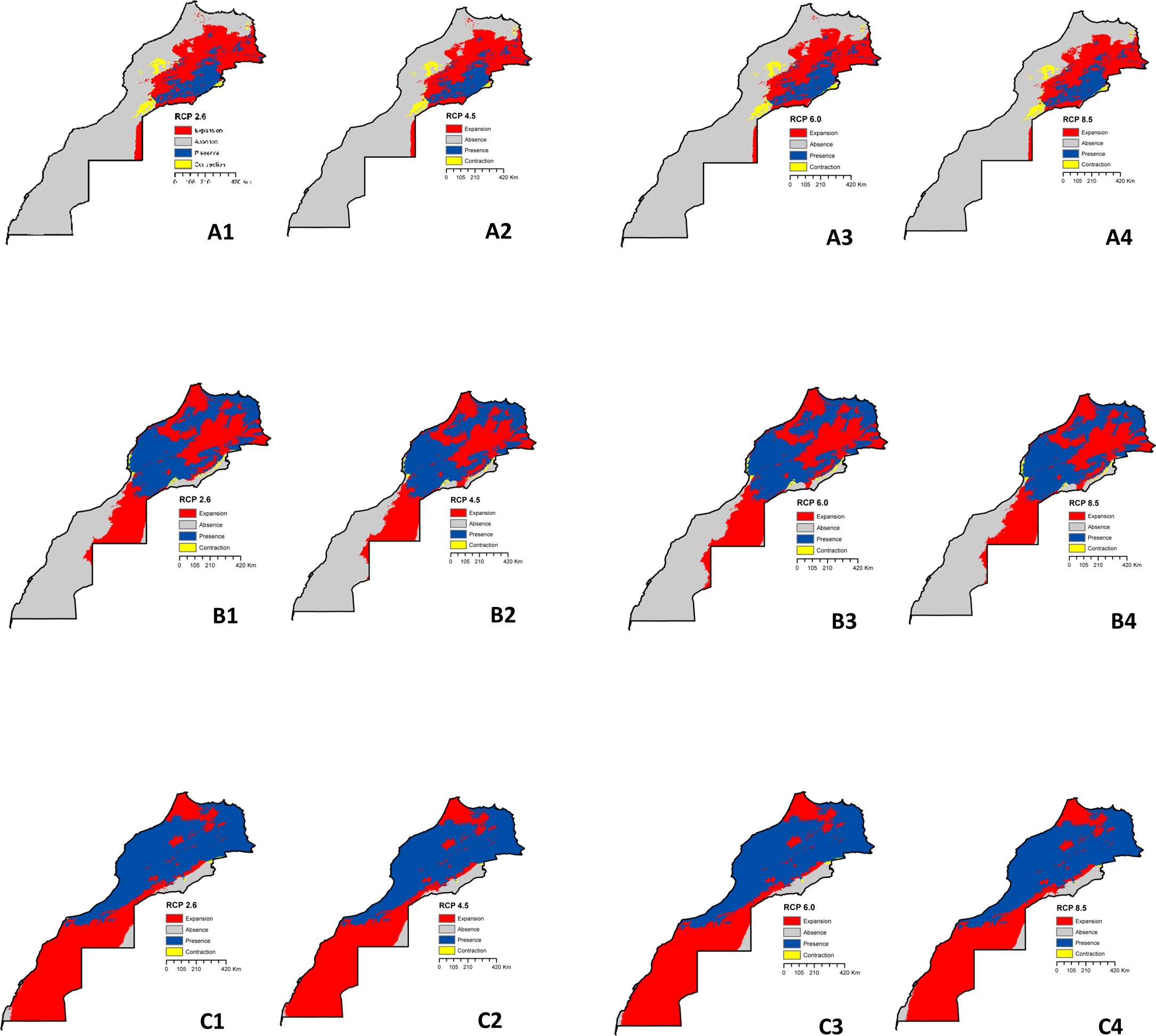
Distribution changes between present-day conditions and future projections for L. major(A), P. papatasi(B), and M. shawi(C). 1= RCP 2.6; 2= RCP 4.5; 3= RCP 6.0; 4= RCP 8.5. Bleu = suitble areas indicating model stability under both current and future conditions. Gray = unsuitable areas indicating model stability under both current and future conditions. Red= Areas of expansion in future. Yellow= Areas of contraction in future.
According to partial ROC tests (Table 1), all models made predictions with statistically significant results (p < .05). Uncertainty estimates associated with different GCMs in each RCP overall showed few differences between the four modeled scenarios for all three specie.
Table 1
| Species | Mean AUC* | Bootstrap iterations | pROC ratio | P < 0.01 | ||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean | Median | |||||
| L. major | Current | 0.973 ± 0.013 | 1000 | 1.97 | 1.98 | 1.98 | 1.98 | 0 *** |
| RCP 2.6 | 0,876 ± 0,007 | 1000 | 1.70 | 1.74 | 1.72 | 1.72 | 0 *** | |
| RCP 4.5 | 0,879 ± 0,004 | 1000 | 1.70 | 1.74 | 1.72 | 1.72 | 0 *** | |
| RCP 6.0 | 0,800 ± 0,006 | 1000 | 1.49 | 1.52 | 1.50 | 1.50 | 0 *** | |
| RCP 8.5 | 0,875 ± 0,019 | 1000 | 1.49 | 1.53 | 1.51 | 1.51 | 0 *** | |
| P. papatasi | Current | 0.956 ± 0.006 | 1000 | 1.93 | 1.94 | 1.93 | 1.93 | 0 *** |
| RCP 2.6 | 0,880 ± 0,005 | 1000 | 1.79 | 1.83 | 1.81 | 1.81 | 0 *** | |
| RCP 4.5 | 0,878 ± 0,004 | 1000 | 1.79 | 1.83 | 1.81 | 1.81 | 0 *** | |
| RCP 6.0 | 0,878 ± 0,007 | 1000 | 1.79 | 1.83 | 1.81 | 1.81 | 0 *** | |
| RCP 8.5 | 0,874 ± 0,008 | 1000 | 1.79 | 1.83 | 1.81 | 1.81 | 0 *** | |
| M. shawi | Current | 0.932 ± 0.011 | 1000 | 1.85 | 1.89 | 1.87 | 1.87 | 0 *** |
| RCP 2.6 | 0,870 ± 0,005 | 1000 | 1.71 | 1.78 | 1.75 | 1.75 | 0 *** | |
| RCP 4.5 | 0,870 ± 0,005 | 1000 | 1.72 | 1.77 | 1.75 | 1.75 | 0 *** | |
| RCP 6.0 | 0,868 ± 0,012 | 1000 | 1.70 | 1.78 | 1.74 | 1.74 | 0 *** | |
| RCP 8.5 | 0,863 ± 0,012 | 1000 | 1.72 | 1.78 | 1.75 | 1.75 | 0 *** | |
Area under the curve (AUC) measures and partial receiver operating characteristic (pROC) ratios summarizing the performance of ecological niche models (average over 50 runs).
*0.5 (random) < AUC < 1 (perfect).
***Highly significant.
The 3D ecological niche visualization of L. major, its primary vector P. papatasi, and reservoir host M. shawi (Figure 5) demonstrated significant overlap in their environmental tolerances under both current and projected climate scenarios. This convergence suggests shared habitat suitability across Morocco’s arid and semi-arid regions, where temperature and precipitation range simultaneously support parasite development, vector survival, and reservoir activity. Notably, the niche overlap was most pronounced in existing endemic zones (e.g., southeastern Morocco), but expanded under RCP scenarios toward the High Atlas and Rif Mountains regions where climatic conditions may become concurrently favorable for all three transmission cycle components by 2050.
Figure 5
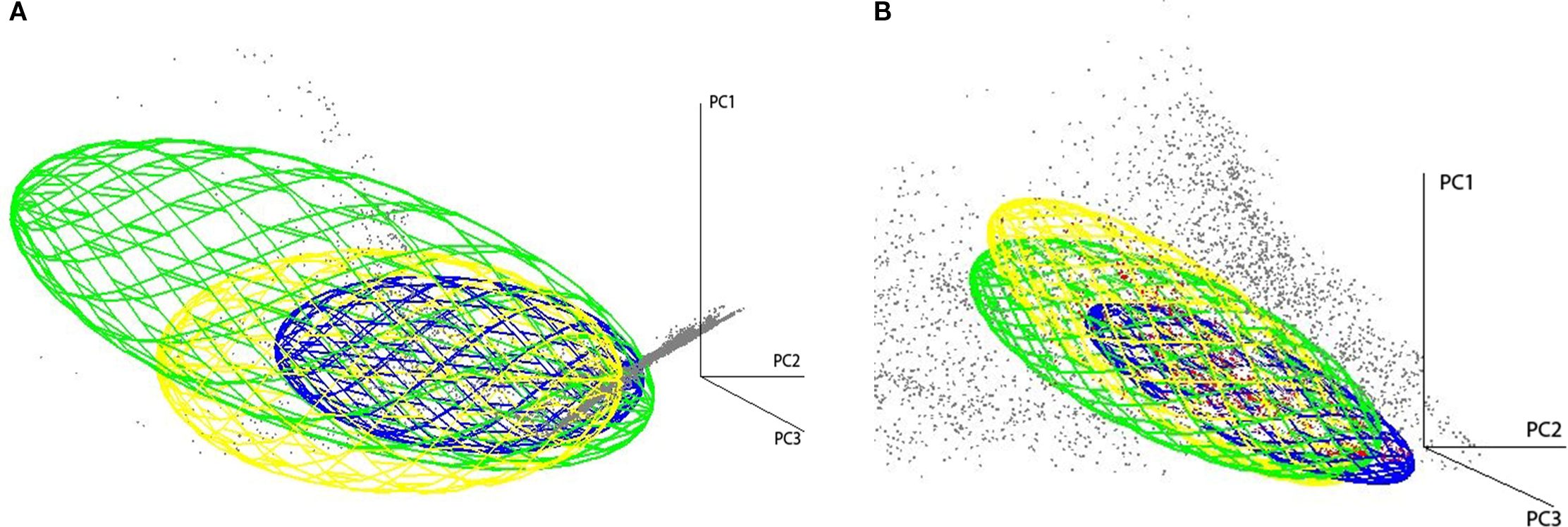
Visualization of L. major, P. papatasi, and M. shawi virtual ecological niches in 3D environmantal space (PC1, PC2, and PC3). Gray points represents environmental background under present-day (A) and future climate conditions (B). Bleu ellipsoid represents L. major, Yelow ellipsoid represent M. shawi and Green ellipsoid represent P. papatasi.
4 Discussion
This study is the first to look at the full ecological niche of the L. major transmission cycle in Morocco. It combines current and future locations of the parasite, its sand fly vector (P. papatasi), and its rodent reservoir host (M. shawi). Our findings reveal significant spatiotemporal shifts in habitat suitability, which are driven by climate change. These shifts have implications for the future epidemiology of zoonotic cutaneous leishmaniasis (ZCL) in North Africa.
In line with previous reports, the current prevalence of ZCL is predominantly concentrated in the southeastern and eastern regions of Morocco (17, 31). However, the models indicate a considerable future expansion of suitable habitats for L. major, particularly under higher-emission RCP scenarios (RCP 6.0 and 8.5). This suggests that warming temperatures and altered precipitation patterns may facilitate the establishment of transmission cycles in currently non-endemic areas, including parts of the High Atlas and Rif Mountains (32, 33).
Projections of the habitat expansion of P. papatasi, particularly under RCP 6.0 and 8.5, are congruent with the prevailing ecological hypothesis that sand flies exhibit enhanced vitality in warmer, more humid environments (34, 35). Notably, the relatively low contraction rates and consistent gains across all Representative Concentration Pathways (RCPs) suggest that this species possesses ecological plasticity, supporting its potential as a persistent and expanding vector in a warming climate. These findings are consistent with the findings of studies that demonstrate the northward movement of phlebotomine species in Europe and North Africa under climate change (36, 37).
Furthermore, the potential distribution of M. shawi, a crucial reservoir host, exhibits substantial ecological resilience, with forecasts indicating expansion under all simulated climate scenarios. These trends are particularly worrisome, as they imply that the three components of the ZCL transmission cycle parasite, vector, and reservoir may converge spatially in broader geographic zones, increasing the risk of sustained local transmission (38, 39). The 3D niche visualization employed in this study underscores the observed overlap, particularly within the geographical regions of central and southern Morocco. This observation serves to reinforce the prevailing concern that the phenomenon of climate convergence is likely to favor the co-occurrence of the three species, thereby giving rise to transmission zones characterized by elevated levels of risk.
These findings underscore the importance of ecological niche modeling in public health forecasting, particularly in regions like Morocco where zoonotic diseases intersect with vulnerable ecological systems. The outcomes of this study align with the mounting imperative for One Health surveillance strategies that integrate environmental monitoring with epidemiological and entomological data to proactively identify emerging disease hotspots (40, 41).
In addition, the utilization of a multifaceted modeling approach, encompassing partial ROC (receiver operating characteristic) validation, consensus among multiple GCMs, and fine-scale regional analysis, substantiates the reliability of our projections with a high degree of confidence (26). The limited uncertainty across the scenarios examined herein underscores the pressing need for prompt policy responses. Such responses must prioritize the implementation of effective vector control measures, the cultivation of community awareness programs, and the construction of essential infrastructure for the timely diagnosis and treatment of emerging cases in previously unaffected regions.
It is imperative to acknowledge the profound psychological and socioeconomic ramifications of ZCL in Morocco, which underscore the necessity for comprehensive policy interventions and social interventions to mitigate the adverse impact of this phenomenon. As numerous studies have previously documented, visible skin lesions frequently result in stigmatization, particularly among women and children, with long-term mental health consequences (22, 23, 42). It is imperative to acknowledge that anticipating the expansion of ZCL risk is not solely a matter of infectious disease control; it is also a matter of protecting community well-being.
5 Conclusion
Our study provides robust projections on how climate change will reshape the distribution of L. major and its vector P. papatasi and reservoir host M. shawi, across Morocco under all climate scenarios (RCP 2.6, 4.5, 6.0, and 8.5). The models predict a clear expansion of suitable habitats, with L. major gaining 1.548–1.637% in range (from RCP 2.6 to 8.5), particularly in eastern, High Atlas, and Rif regions. Meanwhile, P. papatasi (3.489–3.807%) and M. shawi (5.562–5.872%) show even greater adaptability across central and southern Morocco. These shifts are most pronounced under high-emission scenarios (RCP 6.0 and 8.5), suggesting that rising temperatures and changing precipitation patterns will drive the parasite, vector, and host into new overlapping zones, elevating ZCL transmission risks in previously unaffected areas.
The ecological resilience of P. papatasi and M. shawi, evidenced by minimal habitat loss (<0.6% across all RCPs), poses a significant challenge for disease control. Their ability to thrive under diverse climatic conditions implies that ZCL transmission could persist and even intensify in endemic regions while spreading to new territories. This expansion is particularly concerning for Morocco’s highland and northern regions, where human populations may have limited immunity to L. major. Public health strategies must prioritize these emerging risk zones through enhanced surveillance, early diagnosis, and targeted vector control measures.
To mitigate the growing threat of ZCL, a One Health approach is essential. Integrating environmental monitoring, veterinary surveillance, and human health data will be critical for predicting outbreaks and implementing timely interventions across all climate scenarios. Future research should further refine these models by incorporating land-use changes, urbanization trends, and human migration patterns. By aligning predictive modeling with proactive policy, Morocco can reduce the future burden of ZCL and safeguard vulnerable communities from this climate-sensitive disease.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
MD: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AO: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DO: Writing – original draft, Writing – review & editing. MA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MB: Writing – original draft, Writing – review & editing. AA: Writing – original draft, Writing – review & editing. MN: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank Élodie Breton for her assistance in reviewing the English language of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2025.1629454/full#supplementary-material
References
1
Pörtner H-O Roberts D Tignor M Poloczanska E Mintenbeck K Alegría A et al . Climate change 2022: impacts, adaptation and vulnerability working group II contribution to the sixth assessment report of the intergovernmental panel on climate change. (2022). [Research Report] GIEC; IPCC. 2022:ffhal-03774939. doi: 10.1017/9781009325844
2
WHO . Leishmaniasis. (2023). Available online at: https://www.who.int/newsroom/fact-sheets/detail/leishmaniasis. (Accessed January 04, 2025).
3
Daoui O Bennaid H Kbaich MA Mhaidi I Aderdour N Rhinane\ H et al . (2022). Environmental, Climatic, and Parasite Molecular Factors Impacting the Incidence of Cutaneous Leishmaniasis Due to Leishmania tropica in Three Moroccan Foci. Microorganisms, 10(9):1712. doi: 10.3390/microorganisms10091712
4
El Omari H Chahlaoui A Talbi FZ Chlouchi A El-Akhal F Lahouiti K et al . Entomological survey and impact of climatic factors on the dynamics of sandflies in central Morocco. Sci World J. (2023) 2023:6952992. doi: 10.1155/2023/6952992
5
Amane M El Mazini S Echchakery M Hafidi M Lemrani M Boussaa S . Entomological, parasitological and molecular investigations in a new focus of cutaneous leishmaniasis in Youssoufia region, Morocco. Zoonoses Public Health. (2024) 71:248–57. doi: 10.1111/zph.13105
6
McDowell MA Rafati S Ramalho-Ortigao M Ben Salah A . Leishmaniasis: Middle East and North Africa research and development priorities. PloS Negl Trop Dis. (2011) 5:e1219. doi: 10.1371/journal.pntd.0001219
7
Zinsstag J Schelling E Waltner-Toews D Tanner M . From "one medicine" to "one health" and systemic approaches to health and well-being. Prev Vet Med. (2011) 101:148–56. doi: 10.1016/j.prevetmed.2010.07.003
8
Kamhawi S . Phlebotomine sand flies and Leishmania parasites: friends or foes? Trends Parasitol. (2006) 22:439–45. doi: 10.1016/j.pt.2006.06.012
9
Trájer AJ Grmasha RA . The potential effects of climate change on the climatic suitability patterns of the Western Asian vectors and parasites of cutaneous leishmaniasis in the mid- and late twenty-first century. Theor Appl Climatology. (2024) 155:1897–914. doi: 10.1007/s00704-023-04726-4
10
Paz S . The potential of climatic suitability indicator for Leishmania transmission modelling in Europe: insights and suggested directions. Lancet Regional Health – Europe. (2024) 43:100995. doi: 10.1016/j.lanepe.2024.100995
11
Laboudi M Sahibi H Elabandouni M Nhammi H Ait Hamou S Sadak A . A review of cutaneous leishmaniasis in Morocco: A vertical analysisto determine appropriate interventions for control and prevention. Acta Trop. (2018) 187:275–83. doi: 10.1016/j.actatropica.2018.07.019
12
Ready PD . Leishmaniasis emergence in europe. Euro Surveill. (2010) 15:19505. doi: 10.2807/ese.15.10.19505-en
13
Elith J Kearney M Phillips S . The art of modeling range-shifted species. Methods Ecol Evol. (2010) 1:330–42. doi: 10.1111/j.2041-210X.2010.00036.x
14
Peterson A . Uses and requirements of ecological niche models and related distributional models. Biodiversity Inf. (2006) 3. doi: 10.17161/bi.v3i0.29
15
Githeko AK Lindsay SW Confalonieri UE Patz JA . Climate change and vector-borne diseases: a regional analysis. Bull World Health Organ. (2000) 78:1136–47.
16
Tatem AJ Hay SI Rogers DJ . Global traffic and disease vector dispersal. Proc Natl Acad Sci U.S.A. (2006) 103:6242–7. doi: 10.1073/pnas.0508391103
17
Rhajaoui M Sebti F Fellah H Alam MZ Nasereddin A Abbasi I et al . Identification of the causative agent of cutaneous leishmaniasis in Chichaoua province, Morocco. Parasite (Paris France). (2012) 19:81–4. doi: 10.1051/parasite/2012191081
18
Escobar LE Qiao H Cabello J Peterson AT . Ecological niche modeling re-examined: A case study with the Darwin's fox. Ecol Evol. (2018) 8:4757–70. doi: 10.1002/ece3.2018.8.issue-10
19
Chalghaf B Chemkhi J Mayala B Harrabi M Benie GB Michael E et al . Ecological niche modeling predicting the potential distribution of Leishmania vectors in the Mediterranean basin: impact of climate change. Parasites Vectors. (2018) 11:461. doi: 10.1186/s13071-018-3019-x
20
Kilpatrick AM Randolph SE . Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet. (2012) 380:1946–55. doi: 10.1016/S0140-6736(12)61151-9
21
Semenza JC Suk JE . Vector-borne diseases and climate change: a European perspective. FEMS Microbiol Lett. (2018) 365(2):fnx244. doi: 10.1093/femsle/fnx244
22
Bennis I Thys S Filali H De Brouwere V Sahibi H Boelaert M . Psychosocial impact of scars due to cutaneous leishmaniasis on high school students in Errachidia province, Morocco. Infect Dis Poverty. (2017) 6:46. doi: 10.1186/s40249-017-0267-5
23
Fenniche S Souissi A Benmously R Ben Jannet S Marrak H Mokhtar I . Childhood cutaneous leishmaniasis in Tunisia: retrospective study of 60 cases. Med Trop (Mars). (2006) 66:456–60.
24
Kamal M Kenawy MA Rady MH Khaled AS Samy AM . Mapping the global potential distributions of two arboviral vectors Aedes aEgypti and Ae. albopictus under changing climate PloS One. (2018) 13:e0210122. doi: 10.1371/journal.pone.0210122
25
Qiao H Peterson AT Campbell LP Soberón J Ji L Escobar LE . NicheA: creating virtual species and ecological niches in multivariate environmental scenarios. Ecography. (2016) 39:805–13. doi: 10.1111/ecog.2016.v39.i8
26
Outammassine A Zouhair S Loqman S . Global potential distribution of three underappreciated arboviruses vectors (Aedes japonicus, Aedes vexans and Aedes vittatus) under current and future climate conditions. Transbound Emerg Dis. (2022) 69:e1160–71. doi: 10.1111/tbed.14404
27
Phillips SJ Anderson RP Schapire RE . Maximum entropy modeling of species geographic distributions. Ecol Model. (2006) 190:231–59. doi: 10.1016/j.ecolmodel.2005.03.026
28
Merow C Smith M Silander J . A practical guide to Maxent: What it does, and why inputs and settings matter. Ecography. (2013) 36:1–12. doi: 10.1111/j.1600-0587.2013.07872.x
29
Pearson R Raxworthy C Nakamura M Peterson A . ORIGINAL ARTICLE: Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J Biogeography. (2007) 34:102–17. doi: 10.1111/j.1365-2699.2006.01594.x
30
Brown JL Bennett JR French CM . SDMtoolbox 2.0: the next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ. (2017) 5:e4095. doi: 10.7717/peerj.4095
31
Kahime K Boussaa S Idrissi A Nhammi H Boumezzough A . Epidemiological study on acute cutaneous leishmaniasis in Morocco. J Acute Dis. (2015) 5(1):41–5. doi: 10.1016/j.joad.2015.08.004
32
Kholoud K Bounoua L Sereno D El Hidan M Messouli M . Emerging and re-emerging leishmaniases in the mediterranean area: what can be learned from a retrospective review analysis of the situation in Morocco during 1990 to 2010? Microorganisms. (2020) 8(10):1511. doi: 10.3390/microorganisms8101511
33
Kahime K Boussaa S Nhammi H Boumezzough A . Urbanization of human visceral leishmaniasis in Morocco. Parasite Epidemiol Control. (2017) 2:1–6. doi: 10.1016/j.parepi.2017.07.001
34
Gálvez R Descalzo MA Miró G Jiménez MI Martín O Dos Santos-Brandao F et al . Seasonal trends and spatial relations between environmental/meteorological factors and leishmaniosis sand fly vector abundances in Central Spain. Acta Tropica. (2010) 115:95–102. doi: 10.1016/j.actatropica.2010.02.009
35
Vivero R Duque-Granda D Rader J Stuckert A Santander R Cadavid-Restrepo G et al . Humidity and temperature preference in two Neotropical species of sand flies. Parasites Vectors. (2024) 17(1):246. doi: 10.1186/s13071-024-06325-2
36
Trájer AJ Kniha E . Climatic and meteorological factors shaping the potential activity season of sand flies in Southeast Europe. Acta Tropica. (2025) 261:107486. doi: 10.1016/j.actatropica.2024.107486
37
González C Calderón JM López AM Bernardini I Gradoni L Pombi M et al . Species-specific variation in predicted distribution and habitat suitability of phlebotomine sand flies in Italy under different climate change scenarios. Sci Rep. (2025) 15:13297. doi: 10.1038/s41598-025-96296-w
38
Ben Salah A Kamarianakis Y Chlif S Alaya N Prastacos P . Zoonotic cutaneous leishmaniasis in central Tunisia: Spatio-temporal dynamics. Int J Epidemiol. (2007) 36:991–1000. doi: 10.1093/ije/dym125
39
Karmaoui A Sereno D Maia C Campino L El Jaafari S Taybi AF et al . A conceptual model for understanding the zoonotic cutaneous leishmaniasis transmission risk in the Moroccan pre-Saharan area. Parasite Epidemiol Control. (2022) 17:e00243. doi: 10.1016/j.parepi.2022.e00243
40
Hong A Zampieri RA Shaw JJ Floeter-Winter LM Laranjeira-Silva MF . One health approach to leishmaniases: understanding the disease dynamics through diagnostic tools. Pathogens. (2020) 9(10):809. doi: 10.3390/pathogens9100809
41
Palatnik-de-Sousa CB Day MJ . One Health: The global challenge of epidemic and endemic leishmaniasis. Parasites Vectors. (2011) 4:197. doi: 10.1186/1756-3305-4-197
42
Nuwangi H Agampodi TC Price HP Shepherd T Weerakoon KG Agampodi SB . Stigma associated with cutaneous and mucocutaneous leishmaniasis: A systematic review. PloS Negl Trop Dis. (2023) 17:e0011818. doi: 10.1371/journal.pntd.0011818
Summary
Keywords
Leishmania major , Meriones shawi , Phlebotomus papatasi , ecological niche modeling, climate change, Morocco, Vector-borne diseases
Citation
Daoudi M, Outammassine A, Olivier D, Amane M, Beaulieu M, Akarid A, Ndao M, Hafidi M, Boussaa S and Boumezzough A (2025) Modeling the impact of climate change for the potential distribution of the main vector and reservoirs of zoonotic cutaneous leishmaniasis due to leishmania major in Morocco. Front. Trop. Dis. 6:1629454. doi: 10.3389/fitd.2025.1629454
Received
15 May 2025
Accepted
27 August 2025
Published
26 September 2025
Volume
6 - 2025
Edited by
Rafael Gutiérrez LÓPEZ, Carlos III Health Institute (ISCIII), Spain
Reviewed by
Aldemir Branco de Oliveira Filho, Federal University of Pará, Brazil
Estela Gonzalez, Animal and Plant Health Agency (United Kingdom), United Kingdom
Updates
Copyright
© 2025 Daoudi, Outammassine, Olivier, Amane, Beaulieu, Akarid, Ndao, Hafidi, Boussaa and Boumezzough.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed Daoudi, mohamed.daoudi@mail.mcgill.ca
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.