- 1Department of Zoology, Namrup College, Dibrugarh, Assam, India
- 2Consultant Anesthesiologist, Demow Rural Community Health Centre, Sivasagar, Assam, India
- 3Department of Paediatrics, Demow Rural Community Health Centre, Sivasagar, Assam, India
- 4Department of Paediatrics, Boko Rural Community Health Centre, Kamrup Rural, Assam, India
- 5Department of Molecular Biology and Biotechnology, Tezpur University, Tezpur, Assam, India
Background: Envenoming by venomous snakes may induce serious pathophysiological manifestations and death in victims if timely treatment is not provided. Such abilities of snakes remain intact even after death and decapitation. In this article, three cases of envenoming caused by dead snakes that required antivenom therapy are presented from Assam, India.
Case description: Two incidents of envenoming by heads from decapitated Naja kaouthia were referred to the Demow Rural Community Health Centre, Sivasagar, Assam. Victims were clinically examined, and the necessary antivenom was administered. Another case of envenoming by a dead black krait was referred to the Boko 30-bed Community Health Centre/First Referral Unit, Kamrup, Assam. The victim exhibited neurotoxic symptoms and required ventilatory support. Antivenom was immediately administered, along with doses of neostigmine, glycopyrrolate, and calcium gluconate. The victim recovered and was discharged after 43 h of hospitalization.
Conclusion: These instances of envenoming by dead or decapitated snakes highlight the potential danger posed by venomous snakes even after death, emphasizing the necessity of extreme caution while handling them.
1 Introduction
Snakes have inspired extreme fear and fright in humans since primeval times. Ancient stories involving snakebite and resulting death have been an integral part of various mythological books and folktales (1). Envenoming and death resulting from snakebite and the perception created by such stories have fostered an enormous fear of snakebite in humans. Although approximately 80% of snakes in India are non-venomous (2), a lack of knowledge about snake diversity and distribution, combined with this widespread fear, often leads to snakes being killed in rural households, regardless of the danger they represent. Additionally, there is a general belief that the snake’s head should be smashed as it might bite even after death.
Snakebite is a general concern in rural communities of tropical and sub-tropical countries, causing significant physical, mental, and socioeconomic challenges. An estimated 1.2–5.5 million envenomings occur annually, with 125,000 fatalities and 3–4 times as many permanent disabilities (3). India recorded approximately 1.2 million snakebite deaths alone (58,000 per annum) between 2000 and 2019 (4). Based on the verbal autopsies of a community-based survey (Million Death Study) and a systematic literature review, eight states were marked as highest-burden states, which exhibit high prevalence of the Big-Four snake species (Naja naja, Daboia russelii, Echis carinatus, and Bungarus caeruleus), considered to be the most medically significant (4, 5). Northeastern India accounted for a total of 37 fatalities as per the Million Death Study (MDS) from 2001 to 2014 (4); however, there is a dearth of actual epidemiological figures due to underreporting of envenoming cases and deaths in this geographical region (6).
Assam, a state in the northeastern part of India, has a diverse geography and demography as well as rich biodiversity. The state officially recorded approximately 11,000 snakebite cases in 2024, along with 36 deaths (7). Most snakebites have been reported during the monsoon season. Venomous snakes of Assam responsible for these reported incidences are cobras, kraits, and pit vipers. Epidemiological data from the Demow Community Health Centre in Assam show a total of 1,011 snakebite cases between 2018 and 2022. Dry bites and bites from non-venomous snakes accounted for 872 cases. Among the 139 venomous bites, snakes belonging to the family of Elapidae, such as the Indian monocled cobra, banded krait, and lesser/greater black krait, inflicted envenoming in 30 patients (25.5%), which required antivenom treatment (8). These snakes are considered to be crucial from a clinical perspective in Assam, as they are responsible for substantial mortality and morbidity in envenomated patients (8). The clinical manifestations caused by monocled cobras (Naja kaouthia) have been well reported in the literature, highlighting their severity post-bite (8, 9). Furthermore, envenomings by various species of krait (Bungarus spp.) have also been reported to cause pathophysiological abnormalities in victims (8, 10, 11). Moreover, the lesser-known pit vipers from northeastern India, collectively known as green pit vipers due to their high morphological similarity, account for the highest number of envenoming cases in Assam (66% of total venomous bites) (8, 12). Although non-fatal, the envenoming causes severe pain, ecchymosis, tissue necrosis, and prolonged coagulopathy in victims (12, 13). However, no reports of envenoming caused by dead cobras or kraits are available in the literature so far from this region.
A few reports of envenoming from dead or presumed to be dead snakes have been reported previously. Klouber reported that a rattlesnake head can remain active for 20–60 min after decapitation (14). Freeze-dried fangs of venomous snakes have also been reported to cause envenoming after weeks and months, requiring antivenom treatment (15). Moreover, Suchard et al. reported on five envenoming cases involving freshly killed rattlesnakes that required antivenom treatment (16). Fatality due to a dead snake bite was first reported by Wilhite and his team in 2018, in which the victim was envenomated by a freshly severed prairie rattlesnake (Crotalus viridis) and developed a bradysystolic cardiac arrest (17). Another report of coagulopathy by a freshly killed copperhead (Agkistrodon contortrix) was reported by Emswiler et al., which required eight vials of antivenom for treatment (18). Although reports of envenoming by presumably dead snakes remain scarce in the scientific literature, a number of press articles have reported such instances, which have been recently reviewed by Naik (19). A fatal envenoming by a freshly decapitated Indochinese spitting cobra while preparing a delicacy soup in a restaurant was reported from China (20). Also, an envenoming by a red-bellied black snake (Pseudechis porphyriacus), which had been killed for 45 min, was reported from Australia, which required antivenom treatment (21). Another report from India presented the case of two inebriated individuals who ate a dead and half-burnt Bungarus caeruleus (22). Nevertheless, there is a dearth of reported incidents of such envenoming for other venomous snakes of India.
Herein, we report the first case of envenoming resulting from bites by two dead monocled cobras and a lesser black krait from Assam, India, with their symptoms and management. The article is intended to add to the limited knowledge repository of envenoming caused by dead venomous snakes. The article also incorporates the therapeutic interventions associated with the envenoming, which would provide a framework for future management of such cases.
2 Case descriptions and therapeutic interventions
2.1 Case 1
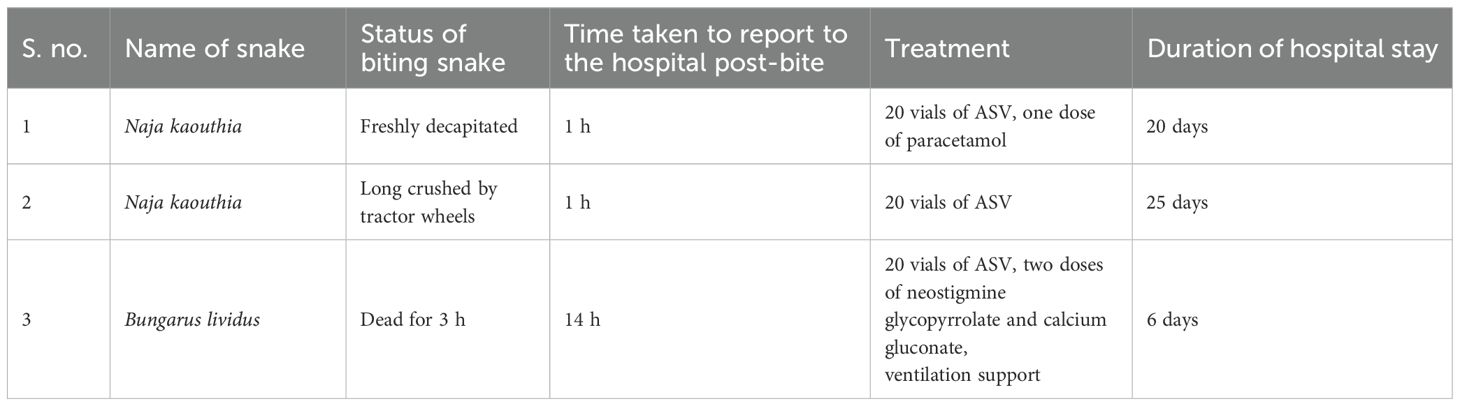
A 45-year-old man encountered a black-colored snake eating chickens in his house at 9:30 p.m. in Sivasagar, Assam. He killed the snake by beheading it, and while trying to discard the body, he was bitten by the snake on his right thumb. The bite was followed by severe pain at the bite site, radiating up to his shoulder. He reported to the nearby community hospital (Demow Rural Community Health Centre, Sivasagar, Assam) at 10:30 p.m. He had multiple episodes of vomiting (five to six times) on the way to the hospital, along with unbearable pain, which was radiating up to his shoulders. Upon initial examination, his pulse rate and blood pressure were observed to be 110/min and 130/80 mmHg. The patient was conscious and responsive to verbal commands. Examination of the bite site revealed blackening of the affected area (Figure 1b). The snake was identified to be a monocled cobra (N. kaouthia) based on the photograph shown (Figure 1a). The attending physician immediately administered 20 vials of polyvalent antivenom intravenously along with a dose of paracetamol (1 g) infusion for pain relief. Magnesium sulfate (MgSO4) glycerin dressing was applied to the wound. Antibiotics, trypsin, and paracetamol infusion were prescribed along with pantoprazole and MgSO4 glycerin dressing. The pain significantly decreased following this treatment. The patient did not develop any symptoms of neurotoxicity. However, the bite resulted in some blebs that were aspirated, followed by sloughing and finally developed in the form of a profound wound. Extensive debridement was performed with hydrogen peroxide and papain-urea ointment, and dressing was continued with Placentrex extract gel over the course of a month (Figure 1c). Upon normalization of all the laboratory parameters on the 20th day, the patient was discharged with a regular follow-up for wound dressing. The wound was completely healed without any residual effect on the 40th day.
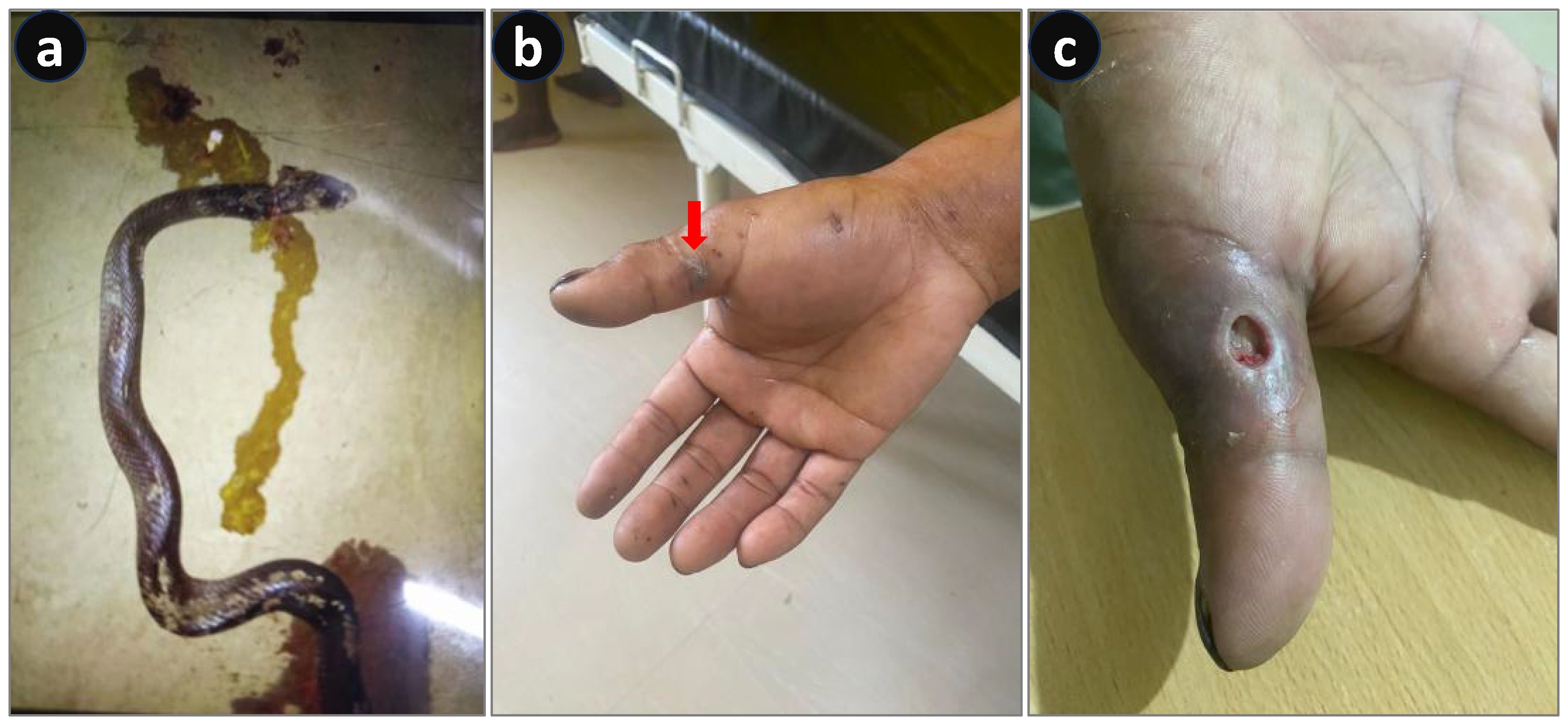
Figure 1. Envenoming by a decapitated monocled cobra (Naja kaouthia). (a) Photograph of a dead snake. (b) Blackened bite area. (c) A cytotoxic ulcer developed at the bite site.
2.2 Case 2
A farmer in Sivasagar, Assam, working in a paddy field, unknowingly crushed a snake with the wheels of his tractor. Upon dismounting his vehicle at the end of his work, the farmer was bitten by the snake on his foot at approximately 7:30 a.m. He observed that it was a monocled cobra (locally known as “feti,” Figure 2a), which was presumed to be dead. He started feeling pain at the bite site, which was progressive in nature; hence, he immediately rushed to a nearby hospital (Demow Rural Community Health Centre, Sivasagar, Assam). The patient complained of severe pain, progressive swelling, and noticeable color change at the bite site. He had two episodes of vomiting at approximately 8:30 a.m. in the hospital. Based on the photograph shown by the patient and the vital signs, envenoming was suspected and 20 vials of polyvalent antivenom were immediately administered. The patient was also prescribed antibiotics, trypsin, paracetamol infusion, and pantoprazole, along with MgSO4 glycerin dressing and limb immobilization. Following the treatment, vomiting gradually stopped and pain was significantly reduced. Although he did not develop any signs of neurotoxicity, severe cytotoxicity led to an ulcer, which required extensive debridement with hydrogen peroxide and papain-urea ointment and Placentrex extract dressing. The patient was discharged on the 25th day after normalization of all the laboratory parameters. Moreover, he was asked to regularly visit the hospital for wound dressing. The wound was completely healed without any residual effect by the 65th day.
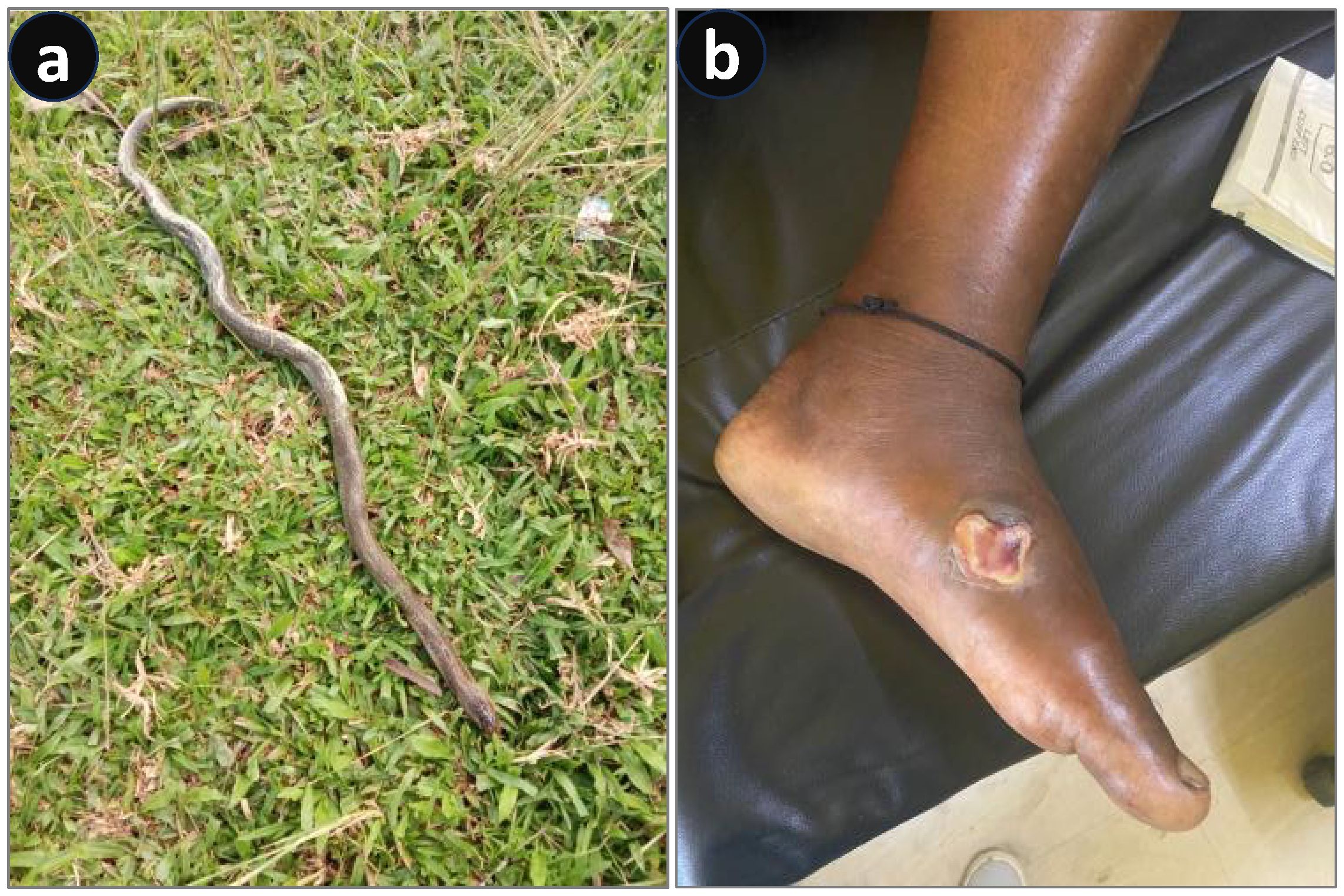
Figure 2. Envenoming by a dead monocled cobra (Naja kaouthia). (a) Photograph of a dead snake. (b) Bite area showing tissue necrosis.
2.3 Case 3
A black-colored snake entered a household at 6:30 p.m. in a village in Kamrup, Assam. The owners killed the snake and discarded the dead body in the backyard of the house. Due to curiosity, one of their neighbors visited the household to see the dead snake at approximately 9:30 p.m. Upon noticing that the snake was dead, he tried to hold its head, but during the process, his little finger of the right hand was envenomed. Since there was no pain or swelling, and considering the snake was already dead, he and his family members initially ignored the bite. However, the victim started to feel uneasiness, sleeplessness, and body pain and gradually became restless by 2:00 a.m. He first sought treatment from a faith healer, but it did not help. He began experiencing difficulty in swallowing and developed drooping eyelids. Finally, at approximately 12:30 p.m. the following day, he reported to a nearby community hospital (Boko 30-bed Community Health Centre/First Referral Unit, Kamrup, Assam). On initial examination, his pulse rate and blood pressure were recorded at 90/min and 140/80 mmHg, respectively. He exhibited ptosis, dysphagia, throat pain, and body aches (Figure 3B). The snake was identified as a black krait (Bungarus sp., likely to be either B. lividus or B. niger) (Figure 3A), as the dead snake had been brought to the hospital by the family members. Twenty vials of polyvalent antivenom were immediately administered, along with a loading dose of AN [atropine (0.01 mg/kg) and neostigmine glycopyrrolate (0.04 mg/kg)], followed by two maintenance doses of the same. Non-responsiveness to three AN doses confirmed the photographic identification of a krait bite (Standard Treatment Guideline 2017, Ministry of Health and Family Welfare). The patient was infused with calcium gluconate IV (2 mg/kg) every 6 h. However, his condition further deteriorated, and he was moved to the ICU for monitoring. He gradually became quadriplegic and, after 1 h of ASV infusion, became unresponsive to verbal commands. Hypercapnia (PCO2 was 70 mmHg) developed, and mechanical ventilation of the lungs was immediately initiated. His condition improved over time, and after 43 h of respiratory support, the trachea was extubated, meeting all the vital extubation criteria. However, ptosis and dysphagia persisted for 3 and 5 days, respectively. The patient was later discharged in good health on the 6th day.
Figure 3. Envenoming by a black krait (Bungarus sp.). (A) Photograph of the dead snake. (B) Ptosis on the day of hospital admission.
3 Discussion
Snakes are ectothermic limbless reptiles and feed on small rodents, frogs, lizards, insects, fish, birds, eggs, etc. (23). They are mostly shy creatures and avoid human interaction while hunting for their prey. However, frequent encounters with farmers do happen during rainy seasons when the digs and burrows are flooded with water (24). An accidental bite from a venomous snake can cause severe complications, requiring emergency medical treatment and sometimes leading to fatalities. Such incidents lead to human–snake conflict, with the latter being killed whenever spotted. Furthermore, accidental death of snakes is also common due to road accidents, agricultural practices, etc. (25). Despite sustaining fatal injuries, venomous snakes retain the capacity to envenom, posing severe complications.
The monocled cobra (N. kaouthia) is the most prevalent cobra species found in northeast India. It has an “O”-shaped mark on its hood, which serves as the identification mark of the snake. It is popularly known as “feti” in the local Assamese language. The monocled cobra is responsible for the majority of envenomings in northeast India and Bangladesh (26, 27). Clinical manifestations of N. kaouthia bite include neurotoxicity causing bilateral ptosis, dysphagia, hypotension, and cranial neuropathy, along with cytotoxic symptoms like local blistering, tissue necrosis, ecchymosis, etc. (9). In the present article, envenoming by a monocled cobra presumed to be dead was reported in cases 1 and 2 (Table 1). Case 1 described an instance of envenoming by a beheaded snake while discarding the freshly killed body. The victim began to develop symptoms of envenoming; however, early administration of antivenom prevented neurotoxicity. Nevertheless, cytotoxic wounds developed, which required prolonged care (Figure 1c). The most lethal protein of monocled cobra venom is the non-enzymatic three-finger toxins (3FTXs), which constitute various neurotoxic, cytotoxic, cardiotoxic, and myotoxic components (28). The cytotoxins (CTXs) are known to disrupt the cell membrane, thereby causing tissue necrosis and wounds in victims (28, 29). Similarly, a case of a bite from a dead monocled cobra was described in case 2. Despite being crushed and presumed to be dead for several hours, the snake was capable of delivering a venomous bite, requiring antivenom treatment along with extended wound care (Figure 2b).
Northeastern India is home to various species of kraits that are medically important; among them, the banded krait (Bungarus fasciatus), the lesser black krait (B. lividus), and the greater black krait (B. niger) are found in Assam. Bungarus fasciatus is a morphologically distinct krait species with alternate black and yellow bands on its triangular cross-sectioned body (30). The lesser and greater black kraits are nocturnal snakes characterized by their shiny black coloration and a slender body with a triangular cross-section (31). Bungarus lividus shows high morphological similarity with B. niger; the only visible difference lies in the relative size of hexagonal vertebral scales on the dorsal surface of the body, which is larger than the bordering scales in B. niger compared to B. lividus (31, 32). Such resemblance makes them difficult to be identified at the species level, and these species are therefore referred to as “black” krait when spotted. Very few cases of envenoming by greater and lesser black kraits have been reported in the literature (8, 10, 11, 33, 34). Bites from these snakes are generally characterized by abdominal and chest pain, ptosis, burning sensation, vomiting, slurred speech, and progressive neuromuscular paralysis leading to respiratory distress (33, 34). Although the specific venom composition of the greater or lesser black krait is not known, their venom largely consists of presynaptic neurotoxins belonging to the phospholipase A2 superfamily, which causes severe neuromuscular paralysis in victims (35). The victims usually remain unaware of the envenoming, as there is usually no pain or insignificant pain during the bite (35). However, patients may experience restlessness, speechlessness, and ptosis followed by respiratory distress (33, 34). In the present report, envenoming by a dead black krait has been presented in case 3 (Table 1). Based on the geographical distribution of kraits, the snake responsible for envenoming could be either the greater or the lesser black krait; however, a close observation of the dead body, especially the midventral scale of the snake, suggests it to be a lesser black krait (B. lividus). The victim was envenomated by a snake that had been dead for 3 h. Symptoms of envenoming occurred approximately 5 h after the bite and were treated with the administration of 20 vials of antivenom and respiratory support (ventilation) for 43 h.
The potential to bite even after death and decapitation lies in the structural organization of the venom apparatus and fangs of front-fanged snakes (families Elapidae, Viperidae, and Atractaspididae) (19). The venom gland consists of a large basal lumen for storage of secreted venom, which is connected to a long hollow fang (36). Venom is rapidly injected into the target, either like a hypodermic syringe or via the groove of the fang by contraction of the compressor muscle (37). An inadvertent envenoming may occur due to accidental pressing of the venom gland of the dead snake while handling the severed head. Additionally, some residual venom, expelled from the venom glands but retained within the lumen of the fangs, might just seep into the pierced tissue and cause envenoming (15). Furthermore, the delivery of venom is controlled by the snake, which may modulate the amount of venom injected based on the size and type of the target (38). They can pierce through the target tissue and inject the entire venom present in the venom sac, or when needed, they might just retrieve the fangs to avoid envenoming (dry bites) (39). This property is completely lost in a dead snake; hence, the entire quantity of venom present in the venom delivery system may just be transferred to the target tissue, leading to severe envenoming (40). Additionally, bites from dead snakes can be attributed to their inherent potential to respond reflexively even after death (14, 41). In the cases highlighted here, such envenomings lead to clinical symptoms comparable to those produced by live snakes, hence requiring antivenom to mitigate the pathophysiological complications. Furthermore, the extent of envenoming might vary depending on the amount of venom present in the sac as well as the structural stability of the venom components. Therefore, vigilant clinical observation is required during antivenom administration and management of the patient.
Previous studies have reported the prolonged stability of snake venom in the absence of air exposure, emphasizing that the pharmacological stability of venom of a dead snake remains virtually similar when compared to live snakes (19, 42). This indicates that venom retained within the venom delivery system remains biologically active for several hours after death and may cause envenoming. In case of pooled venom, factors like air exposure, temperature, and storage condition reduced venom stability over time, thereby reducing its potency (43, 44). However, exactly how long dead and/or decapitated snakes retain the ability to involuntarily deliver a venomous bite remains unknown.
4 Conclusion
Accidental envenoming may occur while handling the corpse or the severed head of a snake. Such envenoming can lead to clinical complications similar to those caused by live snakes requiring antivenom therapy. Such cases of envenoming by a dead venomous snake or its severed head highlight the critical necessity for utmost care while handling the snake, whether dead or alive. Moreover, the post-mortem envenoming ability of a venomous snake constitutes a critical element of snakebite-related knowledge and should be integrated as a core component into awareness and training programs targeting both the general public and the healthcare providers for effective management of snakebite. Furthermore, research oriented toward understanding the duration and effectiveness of venom activity after snake death would provide a comprehensive understanding of dead snake envenoming and its management.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Joint Director of Health Services, Sivasagar, Govt. of Assam vide letter no. JT.DHS/SIV/Snake Bite/2025/2614 dated 27/03/2025. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ST: Writing – original draft, Writing – review & editing, Formal Analysis, Investigation, Methodology. SG: Data curation, Resources, Writing – review & editing. GC: Data curation, Writing – review & editing. HN: Data curation, Writing – review & editing. RD: Conceptualization, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to acknowledge the Joint Director, Health, Sivasagar, Assam, and the Deputy Superintendent, Boko CHC, Kamrup, Assam, for the approval of the publication of case reports.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Whitaker R, Captain A, and Ahmed F. Snakes of India: Draco books. Tamil Nadu, India: Draco books (2008).
3. Afroz A, Siddiquea BN, Chowdhury HA, Jackson TN, and Watt AD. Snakebite envenoming: A systematic review and meta-analysis of global morbidity and mortality. PloS Negl Trop diseases. (2024) 18:e0012080. doi: 10.1371/journal.pntd.0012080
4. Suraweera W, Warrell D, Whitaker R, Menon G, Rodrigues R, Fu SH, et al. Trends in snakebite deaths in India from 2000 to 2019 in a nationally representative mortality study. Elife. (2020) 9:e54076. doi: 10.7554/eLife.54076.sa2
5. Mohapatra B, Warrell DA, Suraweera W, Bhatia P, Dhingra N, Jotkar RM, et al. Snakebite mortality in India: a nationally representative mortality survey. PloS Negl Trop diseases. (2011) 5:e1018. doi: 10.1371/journal.pntd.0001018
6. Rai A, Chettri M, Dewan S, Khandelwal B, and Chettri B. Epidemiological study of snakebite cases in Sikkim: Risk modeling with regard to the habitat suitability of common venomous snakes. PloS Negl Trop diseases. (2021) 15:e0009800. doi: 10.1371/journal.pntd.0009800
7. The Assam Tribune. Assam sees 11,000 snakebites in 2024, experts push for urgent awareness, action. Assam, India: The Assam Tribune (2025).
8. Kakati H, Giri S, Patra A, Taye SJ, Agarwalla D, Boruah H, et al. A retrospective analysis of epidemiology, clinical features of envenomation, and in-patient management of snakebites in a model secondary hospital of Assam, North-east India. Toxicon. (2023) 230:107175. doi: 10.1016/j.toxicon.2023.107175
9. Faiz M, Ahsan M, Ghose A, Rahman M, Amin R, Hossain M, et al. Bites by the monocled cobra, Naja kaouthia, in chittagong division, Bangladesh: Epidemiology, clinical features of envenoming and management of 70 identified cases. Am J Trop Med Hygiene. (2017) 96:876. doi: 10.4269/ajtmh.16-0842
10. Wall F. Notes on snakes collected in Upper Assam. Part II. J Bombay Natural History Soc. (1910) 19:825–45.
11. Faiz MA, Ghose A, Ahsan MF, Rahman MR, Amin MR, Hassan MMU, et al. The greater black krait (Bungarus Niger), a newly recognized cause of neuro-myotoxic snake bite envenoming in Bangladesh. Brain. (2010) 133:3181–93. doi: 10.1093/brain/awq265
12. Thakur S, Giri S, Lalremsenga H, and Doley R. Indian green pit vipers: A lesser-known snake group of north-east India. Toxicon. (2024) 242:107689. doi: 10.1016/j.toxicon.2024.107689
13. Thakur S, Malhotra A, Giri S, Lalremsanga H, Bharti OK, Santra V, et al. Venom of several Indian green pit vipers: Comparison of biochemical activities and cross-reactivity with antivenoms. Toxicon. (2022) 210:66–77. doi: 10.1016/j.toxicon.2022.02.014
14. Klauber LM. Rattlesnakes: their habits, life histories, and influence on mankind. California, US: Univ of California Press (1982).
15. Griffen D and Donovan JW. Significant envenomation from a preserved rattlesnake head (in a patient with a history of immediate hypersensitivity to antivenin). Ann Emergency Med. (1986) 15:955–8. doi: 10.1016/S0196-0644(86)80685-0
16. Suchard JR and LoVecchio F. Envenomations by rattlesnakes thought to be dead. New Engl J Med. (1999) 340:1930–. doi: 10.1056/NEJM199906173402420
17. Willhite LA, Willenbring BA, Orozco BS, and Cole JB. Death after bite from severed snake head. Clin Toxicol. (2018) 56:864–5. doi: 10.1080/15563650.2018.1439951
18. Emswiler MP, Griffith FP4, and Cumpston KL. Clinically significant envenomation from postmortem copperhead (Agkistrodon contortrix). Wilderness Environ Med. (2017) 28:43–5. doi: 10.1016/j.wem.2016.09.007
19. Naik BS. Envenomation by ‘dead’snakes: A review. Trans R Soc Trop Med Hygiene. (2025), trae125. doi: 10.1093/trstmh/trae125
20. Rkaina S. Chef cooking snake dies after cobra bites him – 20 minutes AFTER head was cut off. London, UK: Mirror (2014). Available online at: https://www.mirror.co.uk/news/world-news/chef-cooking-snake-dies-after-4088634 (Accessed May 26, 2025).
21. Daily Telegraph. Jake Thomas thought a long-dead snake couldn’t bite. He was wrong. Sydney: Daily Telegraph (2014). Available online at: https://www.dailytelegraph.com.au/news/jake-thomas-thought-a-longdead-wsnake-couldnt-bite-he-was-wrong/news-story/2866268f48a98b4d600b43958084cf17 (Accessed May 26, 2025).
22. India.com. Two drunkards eat poisonous snake in Chhattisgarh’s Korba to avenge frequent snake bites in village. Uttar Pradesh, India: India.com (2021). Available online at: https://www.India.com/viral/two-drunkards-eat-poisonous-snake-in-chhattisgarhs-korba-to-avenge-frequent-snake-bites-in-village-4938436/ (Accessed May 26, 2025).
23. Greene HW. Dietary correlates of the origin and radiation of snakes. Am Zoologist. (1983) 23:431–41. doi: 10.1093/icb/23.2.431
24. Kuttalam S, Owens JB, Santra V, Ahmed MT, Das B, Das S, et al. Utilizing snake rescue data to understand snake–human conflict in Hooghly, West Bengal, India. Trans R Soc Trop Med Hygiene. (2025), trae124. doi: 10.1093/trstmh/trae124
25. Chittaragi JB and Hosetti B. Road kill mortality of snakes (Squamata: Serpentes) in different land cover areas of Semi-Malnad region, Mid Western Ghats, Shimoga, India. Current Biotica. (2014) 8:57–65.
26. Rahman R, Faiz MA, Selim S, Rahman B, Basher A, Jones A, et al. Annual incidence of snake bite in rural Bangladesh. PloS Negl Trop diseases. (2010) 4:e860. doi: 10.1371/journal.pntd.0000860
27. Deka A, Reza MA, Hoque KMF, Deka K, Saha S, and Doley R. Comparative analysis of Naja kaouthia venom from North-East India and Bangladesh and its cross reactivity with Indian polyvalent antivenoms. Toxicon. (2019) 164:31–43. doi: 10.1016/j.toxicon.2019.03.025
28. Deka A, Gogoi A, Das D, Purkayastha J, and Doley R. Proteomics of Naja kaouthia venom from North East India and assessment of Indian polyvalent antivenom by third generation antivenomics. J proteomics. (2019) 207:103463. doi: 10.1016/j.jprot.2019.103463
29. Chong HP, Tan KY, Liu B-S, Sung W-C, and Tan CH. Cytotoxicity of venoms and cytotoxins from Asiatic cobras (Naja kaouthia, Naja sumatrana, Naja atra) and neutralization by antivenoms from Thailand, Vietnam, and Taiwan. Toxins. (2022) 14:334. doi: 10.3390/toxins14050334
30. Talukdar A, Giri S, and Doley R. Kraits of Indian subcontinent: Natural History, Risks, Venom Variation, Lethality and Treatment Strategies-A comprehensive review. Toxicon. (2025) 262:108406. doi: 10.1016/j.toxicon.2025.108406
31. Biakzuala L, Purkayastha J, Rathee YS, and Lalremsanga HT. New data on the distribution, morphology, and molecular systematics of two venomous snakes, Bungarus Niger and Bungarus lividus (Serpentes: Elapidae), from north-east India. Salamandra. (2021) 57:219–28.
32. Sharma S, Pandey D, Shah K, Tillack F, Chappuis F, Thapa C, et al. Venomous snakes of Nepal. A Photographic Guide. Dharan, Nepal: BP Koirala Institute of Health Sciences (2013).
33. Kuch U, Sharma SK, Alirol E, and Chappuis F. Fatal neurotoxic envenomation from the bite of a Lesser Black Krait (Bungarus lividus) in Nepal. Southeast Asian J Trop Med Public Health. (2011) 42:960–4. doi: 10.1186/s40409-016-0073-8
34. Pandey DP, Sharma SK, Alirol E, Chappuis F, and Kuch U. Fatal neurotoxic envenomation following the bite of a greater black krait (Bungarus Niger) in Nepal: a case report. J Venomous Anim Toxins including Trop Diseases. (2016) 22:19. doi: 10.1186/s40409-016-0073-8
35. Rashmi U, Bhatia S, Nayak M, Khochare S, and Sunagar K. Elusive elapids: biogeographic venom variation in Indian kraits and its repercussion on snakebite therapy. Front Pharmacol. (2024) 15:1443073. doi: 10.3389/fphar.2024.1443073
37. Pucca MB, Knudsen C S, Oliveira I, Rimbault C, Cerni F A, FH W, et al. Current knowledge on snake dry bites. Toxins. (2020) 12:668. doi: 10.3390/toxins12110668
38. Hayes WK, Herbert SS, Rehling GC, and Gennaro JF. Factors that influence venom expenditure in viperids and other snake species during predatory and defensive contexts. In: Biology of the Vipers. Eagle mountain, Utah: Eagle Mountain (2002). p. 207–33.
39. Young BA and Zahn K. Venom flow in rattlesnakes: mechanics and metering. J Exp Biol. (2001) 204:4345–51. doi: 10.1242/jeb.204.24.4345
40. Kardong KV. Predatory strike behavior of the rattlesnake, Crotalus viridis oreganus. J Comp Psychol. (1986) 100:304. doi: 10.1037/0735-7036.100.3.304
41. Gold BS, Dart RC, and Barish RA. Bites of venomous snakes. New Engl J Med. (2002) 347:347–56. doi: 10.1056/NEJMra013477
42. Jesupret C, Baumann K, Jackson TN, Ali SA, Yang DC, Greisman L, et al. Vintage venoms: proteomic and pharmacological stability of snake venoms stored for up to eight decades. J Proteomics. (2014) 105:285–94. doi: 10.1016/j.jprot.2014.01.004
43. Almeida JR, Mendes B, Patiño RS, Pico J, Laines J, Terán M, et al. Assessing the stability of historical and desiccated snake venoms from a medically important Ecuadorian collection. Comp Biochem Physiol Part C: Toxicol Pharmacol. (2020) 230:108702. doi: 10.1016/j.cbpc.2020.108702
Keywords: case report, dead snake, envenoming, Naja kaouthia, krait
Citation: Thakur S, Giri S, Choudhary G, Nath H and Doley R (2025) Death to bite: a case report of dead snake envenoming and treatment. Front. Trop. Dis. 6:1644239. doi: 10.3389/fitd.2025.1644239
Received: 10 June 2025; Accepted: 22 July 2025;
Published: 19 August 2025.
Edited by:
Naoual Oukkache, Institut Pasteur du Maroc, MoroccoReviewed by:
Michel M. Dugon, University of Galway, IrelandAbdellah Moustaghfir, Mohammed V University of Rabat, Morocco
Jed Jebali, Institut Pasteur de Tunis, Tunisia
Copyright © 2025 Thakur, Giri, Choudhary, Nath and Doley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robin Doley, ZG9sZXlAdGV6dS5lcm5ldC5pbg==
 Susmita Thakur1
Susmita Thakur1 Surajit Giri
Surajit Giri Gaurav Choudhary
Gaurav Choudhary Robin Doley
Robin Doley