- 1National Pathogen Resource Center, Chinese Center for Disease Control and Prevention, Beijing, China
- 2Smoke-Free and Healthy Life Association of Macau, Macao, Macao SAR, China
- 3Hainan International Medical Center, Shanghai Jiao Tong University School of Medicine, Boao Hope City, Boao, Hainan, China
- 4State Key Laboratory of Oncogenes and Related Genes, Center for Single-Cell Omics, School of Public Health, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 5International Agency for Research on Cancer, World Health Organization, Lyon, France
Introduction
Biobanking, defined as the systematic collection of biological samples and associated data under a defined governance framework, has become a foundational activity in medical research, facilitating the development of personalized medicine research through the provision of high-quality, research-ready materials and linked data. Such global frameworks include the ISO20387:Biobanking, the Nagoya protocol, and others (1, 2). Furthermore, biobanking is emerging as a core activity outside of medical research and precision medicine (3) to include animal, plant, environmental and other types of activities that would fall under One Health and Global Health contexts (4, 5). Finally, as several experimental approaches are increasingly based on high-throughput ‘-omics’ technologies, biobanking offers a practical solution to the provision of standardized biological material and data at scale.
However, while biobanks are becoming indispensable to scientific research, they face significant evolving intersectoral challenges across current and long-term horizons (6, 7). These challenges can be more structural, e.g., ageing infrastructure and the need for continuous training of staff as new technologies are introduced, and also operational, e.g., including standardization, data-sharing, interoperability and integration of operations, and others. Furthermore, such challenges can often be magnified in low- and middle-income countries (LMIC), and while the introduction of digital solutions may offer powerful tools for improving the real-time control and efficiency of operations, it can also introduce further complexities relating to power dynamics and access (8, 9). It is the authors’ opinion that the most effective way in overcoming such challenges in biobanking, in particular within the One Health and Global Health contexts, lies in the fostering of robust, equitable and collaborative networks. This manuscript provides an overview of the existing and anticipated challenges, as well as a prospective for the way forward.
Current challenges: foundations under strain
The ongoing data collection for banked samples from diverse sources, as is often the case in One Health projects, can lead to a heterogeneous information repository, particularly when various subsets of a biobank’s collection are used for different purposes—generating and occasionally returning distinct types of data. We believe that this situation is exacerbated further by the lack of uniform protocols on sample collection, processing, storage, and quality control. Standardization efforts (10) and practical propositions for biological samples storage and use, especially for diverse pathogen sources across environments (e.g., pathogens coming from human or non-human sources, and particularly when the same pathogen is taken from different environments (11, 12)), are addressing these issues. Recording such collections requires the implementation of Laboratory Information Management Systems (LIMS), tracking sample provenance and ensuring quality control (13), reflecting the efforts of standardization from the biological to the digital aspect (14). However, for LMICs, the absence or inaccessibility of robust digital solutions can amplify the standardization challenge, making quality control and adherence to standards more difficult and reverting to the need for manual control. A limited number of projects have attempted to address this latter point, e.g., the European Union (EU)-funded ‘Bridging Biobanking and Biomedical Research across Europe and Africa’ (B3 Africa), that did create an open-source LIMS biobank software (in a box [BIBOX]) designed from the beginning with LIMC specificities into account (15). In our view, such projects are few and their frequency does not concur with the level of need.
An additional current challenge relates to data-sharing, which encompasses many aspects, whether data is collected for healthcare or environmental research, and is thus impacting biobanking. The regulatory landscape is evolving continuously in response to the technological progress and the possibilities the latter affords. For example, in the EU there has been the introduction of General Data Protection Regulation (GDPR) (16, 17), and globally the Nagoya protocol has been implemented for non-human samples (18). Most recently the World Health Organization (WHO) adopted the Pathogen access and benefit sharing agreement (PABS), which also outlines obligations and expectations in terms of data-sharing for pathogens specifically (19). It becomes clear from the above that the data-sharing functions relating to biobanking in One Health and Global Health are governed by several frameworks at the same time (often overseen by different ministries, e.g., health and agriculture), and this can pose ongoing stresses to such work moving forward. From a technological point of view, there are solutions that can offer adaptation to data-sharing restrictions, such as federated (20) and block-chain approaches (21), however these have not been tested at scale as yet. Lastly, data sharing requires clarity as regards the legal concerns such as intellectual protection, and these have been described in detail (22) though not necessarily addressed in full. Prior experience from such work, e.g., during the zika virus outbreak (23), exposed legal/governance gaps, power imbalances and trust issues, highlighting the need for data sharing and the complexities of participating LMIC where such outbreaks often occur.
Interoperability and integration: bridging the gaps
Interoperability and integration are two further challenges requiring attention. Interoperability is contingent on the existence of standardized metadata, common data models and IT infrastructure, to allow for seamless exchange of data with successful examples available for imaging, genomic and clinical data (24). The interoperability challenge is impacting LMIC biobanks more, as those in high-income settings are better able to modernise existing IT infrastructure. In comparison, LMIC biobank IT systems are often legacy systems from different projects, with limited access to robust LIMS. The control over data formats -directly affecting interoperability- often resides with the proprietary software, which may be difficult to change or adapt for LMIC settings. Integration is a complex challenge in One Health and Global Health biobanking as it entails the integration of biobank data with other critical health data sources (e.g., Electronic Health Records (EHRs), environmental and animal health databases, etc.). Significant steps are the integration of complex ‘-omic’ derived datasets, as a technical pre-requisite for One Health approaches (25). The next step towards One Health, is the integration of entire digital platforms, as opposed to unique collections (26). However, doing so infers the implementation of complex digital solutions for data management and control (27), and the closer integration of systems locally, so that they can be integrated at the global stage (28). The current LMIC biobank status is that of fragmented information systems, thus, limiting the participation in large-scale One Health and Global Health initiatives. Recent One Health examples have demonstrated the urgency of implementing sufficient technical infrastructure to overcome these challenges (29, 30).
Future grand challenges
We believe that one of the major challenges in biobanking for One Health and Global Health is addressing the lack of diversity in existing biobank collections. This holds true both for the human diversity, as well as for the environmental sampling, where high-income settings tend to be over-represented, and thus limiting the generalizability of research findings (31–34). Even with the ongoing efforts considered, there still needs to be a direct LMIC perspective more visible on efforts to achieve equity in biological and data sampling representation. Equally critical is the question of long-term sustainability, as biobanking requires stable and predictable resources to maintain infrastructure, quality standards, and trained personnel over time. Current funding models are fragmented, short-term, or project-based, which creates vulnerabilities in continuity and undermines the capacity of biobanks to serve as reliable research partners for One Health and Global Health. Addressing these challenges will require innovative and diversified funding approaches adapted to LMIC needs, that combine public and charitable investment, international cooperation, and local stakeholder engagement.
Furthermore, biobanks are predicted to play a critical role in the implementation of Artificial Intelligence (AI) in future research studies, as they hold increasingly growing volumes of systematically recorded, and curated data (25). These data can be leveraged by researchers, in particular in the fields of One Health and Global Health, where the questions may require quite complex methodologies to be answered (35, 36), though, the implementation of AI in research based on biobanked data, is still in its early stages. LMICs could draw on existing ethical AI frameworks for responsible and equitable AI implementation, such as WHO’s guidance on AI in health, which emphasizes transparency, inclusiveness, accountability, and data protection (37). While there is great promise, there are questions over the interpretation – for example, the algorithmic bias and explainability of the algorithms that are implemented (13). Moreover, the control over AI development is often concentrated in high-income countries that can carry the risks of: bias amplification (if the AI model is trained on non-representative data); lack of local capacity building within LMICs, and ethical oversight challenges (when the algorithm is designed and applied in two distinctly different contexts).
The power of networks
As part of the quest for improved quality in the biobanked samples, the creation of networks has been catalytical to the development of biobanking by enabling standardized practices, resource sharing, and collaborative research across institutions and borders (38). These networks foster trust, data harmonization, and scalability, which are essential for large-scale, high-impact biomedical and translational research (Figure 1). Indeed, addressing the complex, intersectoral challenges within One Health and Global Health requires a shift from isolated biobanks to interconnected, collaborative networks that allow at a minimum the sharing of best practices and protocols. Several biobanking networks in recent years have emerged with the aim of providing resilience, through standardization and capacity building, to the individual biobanks that are their members. These networks can be national, i.e., supported by the government or national associations (39, 40),; the result of a crisis such as the COVID-19 pandemic (41); or based on a shared linguistic background and -by extension- mutual cultural understanding (42). The Lusophone biobank network for tropical health (42), is an example of regional and LMIC-focused collaborative network that can be leveraged to address One Health research. Importantly, such networks have the potential to ensure capacity building, training and technology transfer both in physical and digital operations, thus accelerating the access to larger, more diverse datasets with greater local control over the AI deployment. It is through these networks that LMIC biobanks in One Health and Global Health are more likely to contribute, benefit and have agency in the digital research ecosystem. It is important to note that there are also fewer, yet successful examples of biobanks achieving interoperability without reliance to extensive networks, such as the Golestan cancer biobank in Iran (43), and the King Hussein Cancer Centre Biobank in Jordan (44).
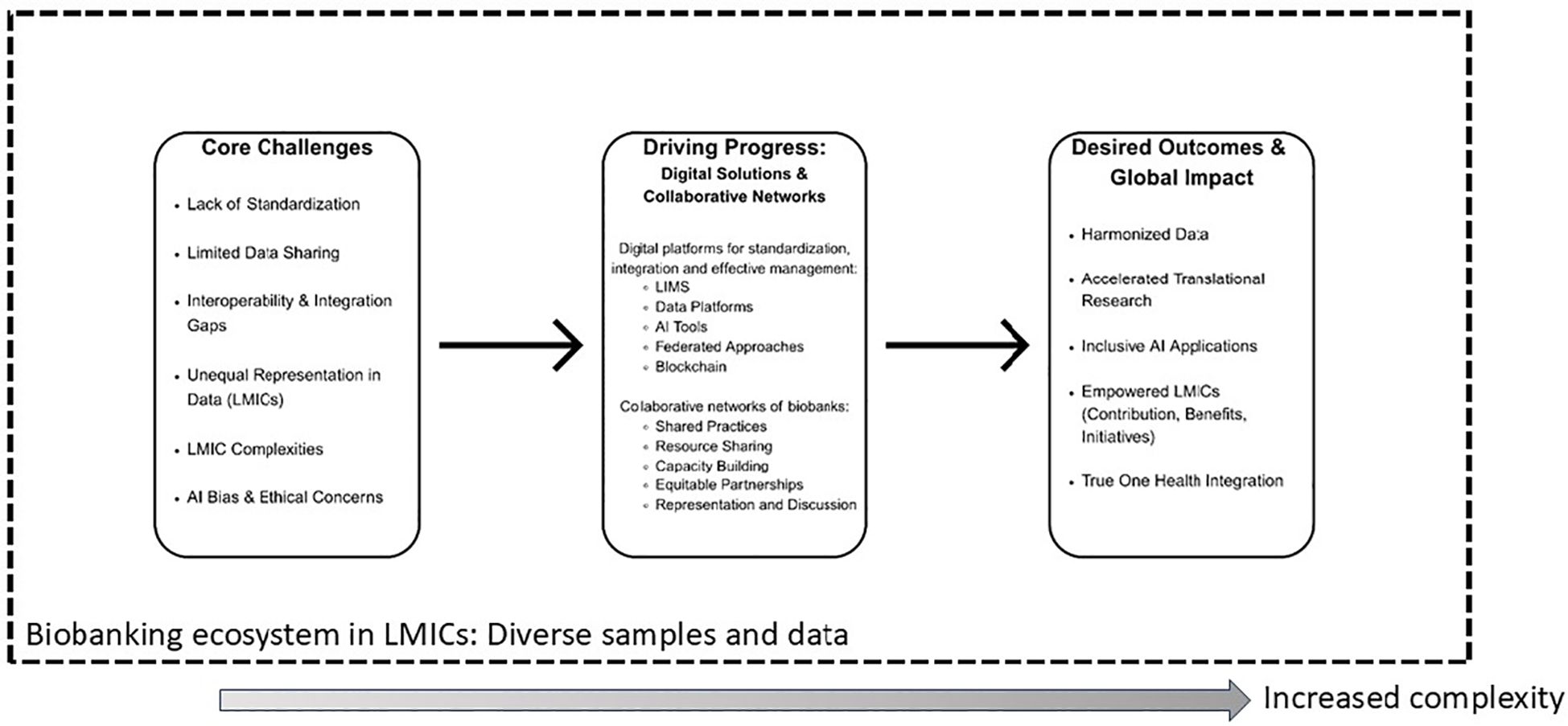
Figure 1. Biobanking’s path towards effective One Health and Global Health equity. The figure illustrates how core intersectoral challenges in biobanking can be addressed by leveraging the power of strategic digital solutions and collaborative networks, leading to impactful and equitable global health outcomes within a One Health framework.
Conclusion
Biobanking plays a foundational role in current research through the provision of high-quality, standardized, research-ready samples and data. It can do so at scale, thus supporting many of the ‘-omics’ research initiatives globally. From this perspective, it is anticipated to play a critical role in addressing challenges within the One Health and Global Health frameworks. However, these challenges include the need for standardization of sample and data, the implementation of quality controls, and the ability for extensive data-sharing.
As One Health and Global Health questions are complex, they would lead to a need for greater interoperability and integration of existing biobanking capacities. While this is technically challenging, it is not impossible, as recent experiences and some early successes demonstrate. One of the main considerations remains the degree to which collections and biobanks in LMIC settings are able to contribute to such complex research, entailing the potential danger of bias for the interpretation and generalizability of results. Biobanks have the opportunity to respond to such challenges through the strategic development of and strengthening of collaborative, equitable, and intersectoral networks that leverage digital solutions to empower local stakeholders and ensure shared, rather than centralized, control. To achieve this, policymakers must create enabling governance frameworks and funding mechanisms that prioritize equity and inclusion, biobank managers must commit to adopting interoperable standards and ethical AI practices, and funders must support capacity-building initiatives that empower LMIC biobanks to be full partners in international networks.
Author contributions
QW: Writing – original draft, Investigation, Conceptualization. JL: Investigation, Visualization, Writing – original draft, Methodology. IC: Conceptualization, Investigation, Supervision, Writing – review & editing. ZK: Writing – review & editing, Conceptualization, Supervision.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/WHO, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/WHO.
References
1. ISO. International Standard ISO 20387:2018 - Biotechnology - Biobanking - General requirements for biobanking, First Edit (2018). Available online at: https://www.iso.org/standard/67888.html. (Accessed September 02, 2025).
2. Knauf S, Abel L, and Hallmaier-Wacker LK. The Nagoya protocol and research on emerging infectious diseases. Bull World Health Organ. (2019) 97:379. doi: 10.2471/BLT.19.232173
3. Annaratone L, De Palma G, Bonizzi G, Sapino A, Botti G, Berrino E, et al. Basic principles of biobanking: from biological samples to precision medicine for patients. Virchows Archiv. (2021) 479:233–46. doi: 10.1007/s00428-021-03151-0
4. Fujihara M and Comizzoli P. Human and wildlife biobanks of germplasms and reproductive tissues can contribute to a broader concept of One Health. F&S Rep. (2025) 6:63–6. doi: 10.1016/j.xfre.2025.01.004
5. Capps B and Lederman Z. One Health and paradigms of public biobanking. J Med Ethics. (2015) 41:258–62. doi: 10.1136/medethics-2013-101828
6. Kinkorová J. Biobanks in the era of personalized medicine: objectives, challenges, and innovation: overview. EPMA J. (2016) 7:1–2. doi: 10.1186/s13167-016-0053-7
7. Goisauf M, Martin G, Bentzen HB, Budin-Ljøsne I, Ursin L, Durnová A, et al. Data in question: A survey of European biobank professionals on ethical, legal and societal challenges of biobank research. PloS One. (2019) 14:e0221496. doi: 10.1371/journal.pone.0221496
8. Mohammadzadeh A, Farjaminejad S, Patel P, Nanyonga S, Ahmad R, Stavropoulou C, et al. Biobanking in Sub-Saharan Africa: A review of data protection frameworks. Biopreservation Biobanking. (2025) 23(3):177–85. doi: 10.1089/bio.2024.0086
9. Kozlakidis Z. Biobanks and biobank-based artificial intelligence (AI) implementation through an international lens. In: Artificial intelligence and machine learning for digital pathology: State-of-the-art and future challenges. Springer International Publishing, Cham (2020). p. 195–203.
10. Dagher G. Quality matters: International standards for biobanking. Cell Proliferation. (2022) 55:e13282. doi: 10.1111/cpr.13282
11. Lajaunie C and Ho CW. Pathogens collections, biobanks and related-data in a One Health legal and ethical perspective. Parasitology. (2018) 145:688–96. doi: 10.1017/S0031182017001986
12. Kozlakidis Z, Cheong IH, and Wei Q. Supporting the scientific advancement from pathogenic microorganisms biobank. Biosafety Health. (2022) 4:283–4. doi: 10.1016/j.bsheal.2022.09.002
13. Bukreeva AS, Malsagova KA, Petrovskiy DV, Butkova TV, Nakhod VI, Rudnev VR, et al. Biobank digitalization: from data acquisition to efficient use. Biology. (2024) 13:957. doi: 10.3390/biology13120957
14. Alkhatib R and Gaede KI. Data management in biobanking: strategies, challenges, and future directions. BioTech. (2024) 13:34. doi: 10.3390/biotech13030034
15. Klingström T, Mendy M, Meunier D, Berger A, Reichel J, Christoffels A, et al. (2016). Supporting the development of biobanks in low and medium income countries, in: 2016 IST-Africa Week Conference, South Africa: IEEE. pp. 1–10.
16. Slokenberga S, Tzortzatou O, and Reichel J. GDPR and biobanking: Individual rights, public interest and research regulation across Europe. Switzerland: Springer Nature (2021).
17. Morrison M, Bell J, George C, Harmon S, Munsie M, and Kaye J. The European General Data Protection Regulation: challenges and considerations for iPSC researchers and biobanks. Regenerative Med. (2017) 12:693–703. doi: 10.2217/rme-2017-0068
18. Lajaunie C and Morand S. Nagoya protocol and infectious diseases: hindrance or opportunity? Front Public Health. (2020) 8:238. doi: 10.3389/fpubh.2020.00238
19. Switzer S, Eccleston-Turner M, Rourke M, and Hampton AR. Comments on Article 12: Pathogen Access and Benefit Sharing (PABS) of the REVISED Draft of the negotiating text of the WHO Pandemic Agreement, 13th March 2024. (2024). doi: 10.2139/ssrn.4760373
20. Rujano MA, Boiten JW, Ohmann C, Canham S, Contrino S, David R, et al. Sharing sensitive data in life sciences: an overview of centralized and federated approaches. Briefings Bioinf. (2024) 25:bbae262. doi: 10.1093/bib/bbae262
21. Ortiz-Lizcano MI, Arias-Antunez E, Bravo ÁH, Caminero MB, Guillen TR, and Cha SH. Increasing the security and traceability of biological samples in biobanks by blockchain technology. Comput Methods Programs Biomedicine. (2023) 231:107379. doi: 10.1016/j.cmpb.2023.107379
22. Corradi A, Bonizzi G, Sajjadi E, Pavan F, Fumagalli M, Molendini LO, et al. The regulatory landscape of biobanks in europe: from accreditation to intellectual property. Curr Genomics. (2025) 26:15–23. doi: 10.2174/0113892029313697240729091922
23. Peeling RW, Fongwen NT, Guzman MG, Méndez-Rico JA, Avumegah MS, Jaenisch T, et al. Specimen and data sharing to advance research and development on Zika virus. Lancet Microbe. (2025) 6(6):101057. doi: 10.1016/j.lanmic.2024.101057
24. Brancato V, Esposito G, Coppola L, Cavaliere C, Mirabelli P, Scapicchio C, et al. Standardizing digital biobanks: integrating imaging, genomic, and clinical data for precision medicine. J Transl Med. (2024) 22:136. doi: 10.1186/s12967-024-04891-8
25. Nam Y, Kim J, Jung SH, Woerner J, Suh EH, Lee DG, et al. Harnessing AI in multi-modal omics data integration: paving the path for the next frontier in precision medicine. Annu Rev BioMed Data Sci. (2024) 7:225–50. doi: 10.1146/annurev-biodatasci-102523-103801
26. Huang L, He J, Zhang C, Liu J, Guo Z, Lv S, et al. China’s One Health governance system: the framework and its application. Sci One Health. (2023) 2:100039. doi: 10.1016/j.soh.2023.100039
27. Panel OH, Hayman DT, Adisasmito WB, Almuhairi S, Behravesh CB, Bilivogui P, et al. Developing One Health surveillance systems. One Health. (2023) 17:100617. doi: 10.1016/j.onehlt.2023.100617
28. Guo ZY, Zheng J, Li SZ, and Zhou XN. Orientation of One Health development: think globally and act locally. Sci One Health. (2023) 2:100042. doi: 10.1016/j.soh.2023.100042
29. Adnyana IMDM, Utomo B, Eljatin DS, and Sudaryati NLG. One Health approach and zoonotic diseases in Indonesia: Urgency of implementation and challenges. Narra J. (2023) 3:e257. doi: 10.52225/narra.v3i3.257
30. Moriyón I, Blasco JM, Letesson JJ, De Massis F, and Moreno E. Brucellosis and one health: inherited and future challenges. Microorganisms. (2023) 11:2070. doi: 10.3390/microorganisms11082070
31. Lieb W, Strathmann EA, Röder C, Jacobs G, Gaede KI, Richter G, et al. Population-based biobanking. Genes. (2024) 15:66. doi: 10.3390/genes15010066
32. Liu H, Liu Y, Zhao Y, Ma Y, Chen Q, Xu H, et al. A scoping review of human genetic resources management policies and databases in high- and middle-low-income countries. BMC Med Ethics. (2025) 26:37. doi: 10.1186/s12910-025-01192-7
33. Ho CW. Operationalizing “one health” as “one digital health” through a global framework that emphasizes fair and equitable sharing of benefits from the use of artificial intelligence and related digital technologies. Front Public Health. (2022) 10:768977. doi: 10.3389/fpubh.2022.768977
34. Astorga F, Groom Q, Shimabukuro PH, Manguin S, Noesgaard D, Orrell T, et al. Biodiversity data supports research on human infectious diseases: global trends, challenges, and opportunities. One Health. (2023) 16:100484. doi: 10.1016/j.onehlt.2023.100484
35. Venturini P, Faria PL, and Cordeiro JV. AI and omics technologies in biobanking: Applications and challenges for public health. Public Health. (2025) 243:105726. doi: 10.1016/j.puhe.2025.105726
36. Battineni G, Hossain MA, Chintalapudi N, and Amenta F. A survey on the role of artificial intelligence in biobanking studies: A systematic review. Diagnostics (Basel). (2022) 12:1179. doi: 10.3390/diagnostics12051179
37. Guidance WH. Ethics and governance of artificial intelligence for health. Geneva, Switzerland: World Health Organization (2021). Available online at: https://iris.who.int/bitstream/handle/10665/341996/9789240029200-eng.pdf. (Accessed September 03, 2025).
38. Henderson MK and Kozlakidis Z. ISBER and the biobanking and cohort network (BCNet): A strengthened partnership. Biopreservation Biobanking. (2018) 16:393–4. doi: 10.1089/bio.2018.29043.mkh
39. Kim Y, Cheong HM, Choi G, Choi KM, Chung EJ, Kim A, et al. Strengthening the korean network of microbial culture collections in the microbiome era. Mycobiology. (2024) 52:207–13. doi: 10.1080/12298093.2024.2372917
40. Omae Y, Goto YI, and Tokunaga K. National center biobank network. Hum Genome Var. (2022) 9:38. doi: 10.1038/s41439-022-00217-6
41. Peeling RW, Boeras D, Wilder-Smith A, Sall A, and Nkengasong J. Need for sustainable biobanking networks for COVID-19 and other diseases of epidemic potential. Lancet Infect Dis. (2020) 20:e268–73. doi: 10.1016/S1473-3099(20)30461-8
42. Arez AP, Souto A, Da Silva M, do Nascimento CR, Couto I, Belo S, et al. Biobanking for tropical health: leveraging collaborative initiatives in the Lusophone world. Front Trop Dis. (2024) 5:1438842. doi: 10.3389/fitd.2024.1438842
43. Ghasemi-Kebria F, Jafari-Delouie N, Amiriani T, Norouzi A, Abedi-Ardekani B, Nasrollahzadeh D, et al. Building a cancer biobank in a low-resource setting in Northern Iran: the Golestan cancer biobank. Arch Iranian Med. (2021) 24:526–33. doi: 10.34172/aim.2021.75
Keywords: biobanking, One Health, Global Health, data integration, standardization
Citation: Wei Q, Luong JCH, Cheong IH and Kozlakidis Z (2025) The intersectoral challenges facing biobanking in One Health and Global Health. Front. Trop. Dis. 6:1653226. doi: 10.3389/fitd.2025.1653226
Received: 24 June 2025; Accepted: 03 September 2025;
Published: 18 September 2025.
Edited by:
Ana Paula Arez, New University of Lisbon, PortugalReviewed by:
Radha Ambalavanan, The Self Research Institute, United StatesCopyright © 2025 Wei, Luong, Cheong and Kozlakidis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zisis Kozlakidis, a296bGFraWRpc3pAd2hvLmludA==
 Qiang Wei1
Qiang Wei1 Io Hong Cheong
Io Hong Cheong Zisis Kozlakidis
Zisis Kozlakidis