- 1Department of Biochemistry, College of Science and Technology, Covenant University, Ota, Nigeria
- 2Covenant Applied Informatics and Communication, Africa Centre of Excellence (CApICACE), Covenant University, Ota, Nigeria
- 3Infectious Diseases and Antimicrobial Department, University of Buea, Buea, Cameroon
Introduction: Malaria remains a major public health concern in Nigeria, accounting for approximately 27% of global cases. In Ota, Ogun State, a recent study reported a 44% prevalence among symptomatic patients. This high burden underscores the importance of understanding community knowledge, attitudes, and behaviors, which are critical for designing targeted and effective control strategies.
Methods: A cross-sectional study was conducted between March and June 2025 among 483 residents of Canaanland, a semi-urban religious and educational community. Data were collected anonymously using semi-structured questionnaires in both hardcopy and KoboToolbox formats, with manual entry for paper responses. Descriptive analysis was performed using R software (version 4.3.1).
Results: Overall, 55% of respondents had moderate malaria knowledge, 60% exhibited poor preventive practices, and 75.8% believed malaria can be eliminated. Most respondents (80.5% students) demonstrated high awareness (98.0%), yet misconceptions about malaria causes (e.g., dirty water, contaminated food) persisted. Insecticide-treated net (ITN) use was suboptimal, with only 27.7% using ITNs nightly despite 66.9% ownership; postgraduate students were less likely to use ITNs. Preventive practice and attitude scores were significantly associated with gender and education (p < 0.05), with males and undergraduates exhibiting more suboptimal behaviors. While 67.7% sought prompt treatment, nearly one-quarter relied on self-diagnosis. More than half rated government malaria control efforts poorly, yet the majority supported increased sensitization. Key barriers identified included poor sanitation, limited ITN access, and financial constraints.
Discussion: These findings highlight that higher education alone does not ensure effective malaria-related behaviors. Persistent misconceptions, poor preventive practices, and systemic barriers call for behavior-focused, context-specific interventions alongside awareness campaigns in educated, semi-urban communities.
1 Introduction
Malaria remains one of the leading causes of morbidity and mortality across sub-Saharan Africa, with Nigeria bearing a disproportionate burden. According to the World Health Organization (WHO), Nigeria accounted for approximately 27% of global malaria cases in 2022, making it the country with the highest incidence globally (1). This translates not only into widespread illness but also into significant mortality, particularly among vulnerable populations such as children under five and pregnant women (2, 3). The persistence of malaria in Nigeria reflects a complex interplay of biological, socio-economic, and environmental determinants.
Despite global and national initiatives aimed at malaria control and elimination, Nigeria continues to experience high transmission rates (4). These patterns are shaped by a spectrum of transmission settings, from urban centers to rural and semi-urban communities, each presenting unique epidemiological and intervention challenges (5–7). Ogun State, particularly the semi-urban area of Ota, exemplifies the ongoing struggle against malaria transmission. Recent surveillance in Ota has reported a prevalence of approximately 44% among symptomatic patients, indicating sustained transmission despite the availability of diagnostic and therapeutic tools (8). This high prevalence suggests potential gaps in prevention, healthcare access, and awareness, as well as deeper social determinants of health (5, 9). Despite this high prevalence, little is known about the awareness–behavior gap in educated, semi-urban communities like Canaanland, where unique socio-religious dynamics may influence malaria prevention and control. This study addresses this gap to inform targeted interventions.
Semi-urban areas like Ota are particularly important for understanding malaria persistence due to their complex and evolving risk environments. These communities often face rapid population growth, ecological disruptions, increased human mobility, and insufficient health infrastructure, all of which compound the challenges of malaria control (10). In such settings, malaria prevention and treatment are shaped by heterogeneous behaviors, including varied levels of knowledge and attitudes toward disease prevention (11). Epidemiological studies have highlighted that malaria incidence in Ota and surrounding communities has remained stable or even increased over the past decade, despite Nigeria’s alignment with global malaria control strategies (6, 7, 12).
Treatment-seeking behavior in this region often includes both formal and informal pathways, with many individuals relying on self-medication and roadside chemists due to concerns about cost, convenience, and mistrust of formal healthcare systems (13–15). These practices have contributed to suboptimal treatment adherence, further complicating malaria control and increasing the risk of drug resistance. Hospital-based diagnosis, associated with higher adherence rates, underscores the importance of strengthening access to formal healthcare in these semi-urban communities (13).
This study aims to assess malaria-related knowledge, preventive practices, and perceptions among residents of Canaanland, a semi-urban religious and educational enclave in Ota, Nigeria. Specifically, we address the following research questions: (1) What is the prevalence of nightly insecticide-treated net (ITN) use? (2) What are the key misconceptions about malaria causes, and what factors are associated with ITN use? (3) What is the treatment-seeking behaviors and perceived barriers to malaria prevention in this community? These questions guide our cross-sectional survey to inform targeted, context-specific interventions. Treatment-seeking behaviors were assessed as a distinct objective from preventive practices in this study, as they represent critical post-infection responses that influence malaria control outcomes, such as timely diagnosis and treatment adherence, which are emphasized in national malaria control guidelines (16).
2 Methods
2.1 Study area
This study was conducted among residents of Canaanland and its adjoining communities in Ota, Ogun State, Nigeria. Canaanland, a prominent religious and educational enclave, is located in Ota, Ado-Odo/Ota Local Government Area, approximately 40 km from Lagos, Nigeria’s commercial capital. This 5,000-acre hub hosts the headquarters of Winners’ Chapel (Living Faith Church), Covenant University, Faith Academy Secondary School, and Kingdom Heritage Nursery/Primary School, with an estimated population of 11,000 residents, including over 2,000 church employees and 9,000 students. The area lies between latitudes 6°40′–6°42′N and longitudes 3°08′–3°10′E, bordered by Idiroko Road northwards, Sango-Ota in the south, Atan to the east, and Alade village to the west. Ota is a semi-urban area with a mix of residential, educational, and industrial activities. The climate features a tropical wet-and-dry pattern, with a prolonged wet season from April to October and a short dry season from November to March (17).
2.2 Justification of study period
The study was conducted between March and June 2025, spanning the onset and progression of the rainy season in southern Nigeria (18). This timeframe is epidemiologically significant, as increased rainfall creates favorable breeding conditions for Anopheles mosquitoes, often leading to a rise in malaria transmission.
2.3 Study design and sample size
The study was a community-based cross-sectional survey conducted among residents and workers in Canaanland and its surrounding areas, including students, staff, and other members of the semi-urban enclave. The sample size was determined using Cochran’s formula (19) for proportions, assuming a malaria prevalence of 44% (8), a 95% confidence level, and a 5% margin of error:
where Z = 1.96, p = 0.44, and e = 0.05, yielding n0 ≈ 378.
For the finite population (N = 11,000), the finite population correction was applied despite N = 11,000 being slightly above the conventional threshold of 10,000, as the population size was sufficiently small to warrant adjustment, consistent with Cochran’s methods and applied in similar studies (20).
To account for potential non-response, a buffer of approximately 30% was added (365 × 0.30 = 118), yielding a target sample size of 483. This target was fully achieved, thereby enhancing statistical power and robustness. A sensitivity analysis assuming a lower prevalence of 30% yielded n ≈ 350, further confirming the adequacy of the achieved sample size.
2.4 Sampling and recruitment
A convenience sampling approach was employed, targeting residents and workers in Canaanland and surrounding areas, as in previous community-based malaria studies in Nigeria (21, 22). Participants were recruited at Covenant University hostels, Cafeterias, shuttle stands, Faith Tabernacle and Chapel services, staff quarters, and the local market in nearby settlements. Eligibility was confirmed via verbal screening for age (≥16 years or <16 with consent) and residency/work duration (≥6 months). Of 525 invited individuals, 483 completed the survey (response rate: 92%).
2.5 Variables
The primary outcome was nightly ITN use (yes/no). Independent variables included gender, age, education, and residence, consistent with similar studies reported elsewhere (23). “Nightly ITN use” was defined as sleeping under an ITN every night, while “Prompt treatment” referred to seeking care within 24 hours of symptom onset, consistent with operational definitions used in the 2018 Nigeria Demographic and Health Survey (16). Covariates (gender, age, education) were selected based on their established associations with ITN use in prior studies (21, 24).
2.6 Inclusion and exclusion criteria
Participants were included if they were aged ≥16 years (or <16 with parental/guardian/school consent), had resided or worked in Canaanland for ≥6 months, and provided informed consent. Excluded were temporary visitors, individuals with <6 months’ presence, or those unable to provide consent.
2.7 Ethical and administrative considerations
Ethical approval (CU/HREC/OO & OA/432/24) was obtained from the Covenant Health Research Ethics Committee (CHREC). The questionnaire was administered in English, with verbal explanations provided in Pidgin English or Yoruba as needed; translations were not required due to high literacy rates. Reliability metrics (e.g., Cronbach’s α) were not calculated for single-item questions but were ensured through piloting for clarity and consistency.
2.8 Data collection
Data were collected from March to June 2025 using semi-structured hard-copy questionnaires administered to literate participants, capturing demographic details, malaria knowledge, prevention practices, treatment-seeking behaviors, and perceptions of control efforts. For respondents unable to write, trained interviewers used KoboToolbox (version 2.024.03; https://kf.kobotoolbox.org/#/projects/home) on mobile devices to record responses, ensuring consistency with the hard-copy questionnaire design. Hard-copy responses were manually entered into KoboToolbox for data consolidation. The dataset was exported to Microsoft Excel (version 16.0) for initial organization and cleaning, addressing missing values, inconsistencies, and outliers. The cleaned dataset was imported into R (version 4.3.1) for analysis.
2.9 Statistical analysis
Participant characteristics and malaria-related outcomes were summarized using descriptive statistics. Knowledge scores (range: 0–4) were calculated by assigning one point for each correct response to four core items: (1) identification of malaria causes, (2) recognition of common symptoms, (3) understanding of transmission mode, and (4) identification of at-risk populations. Practice and attitude scores were dichotomized as “poor” (<50% adherence to recommended behaviors or negative responses) or “good” (≥50% adherence or positive responses), based on established thresholds (22, 25). The questionnaire was piloted with 20 participants to ensure clarity and cultural appropriateness, with minor revisions to wording. The full questionnaire is provided in Supplementary File 1.
Chi-squared tests (or Fisher’s exact test for sparse cells) assessed associations between variables (e.g., diagnosis source and treatment adherence). Logistic regression evaluated factors influencing nightly ITN use (yes/no), with gender (reference: female), age (reference: <25 years), and education (reference: Undergraduate) as predictors. Age categories were collapsed (<25, 25–34, ≥35 years) to address sparse cells. Covariates were selected based on prior literature (11, 16), and multicollinearity was assessed (variance inflation factor <2). Adjusted odds ratios (AORs), 95% confidence intervals (CIs), and sample sizes per category are reported. Covariates were retained to adjust for potential confounding, with non-significant predictors included to ensure comprehensive adjustment. Statistical significance was set at p < 0.05.
3 Results
3.1 Demographic information of the participants
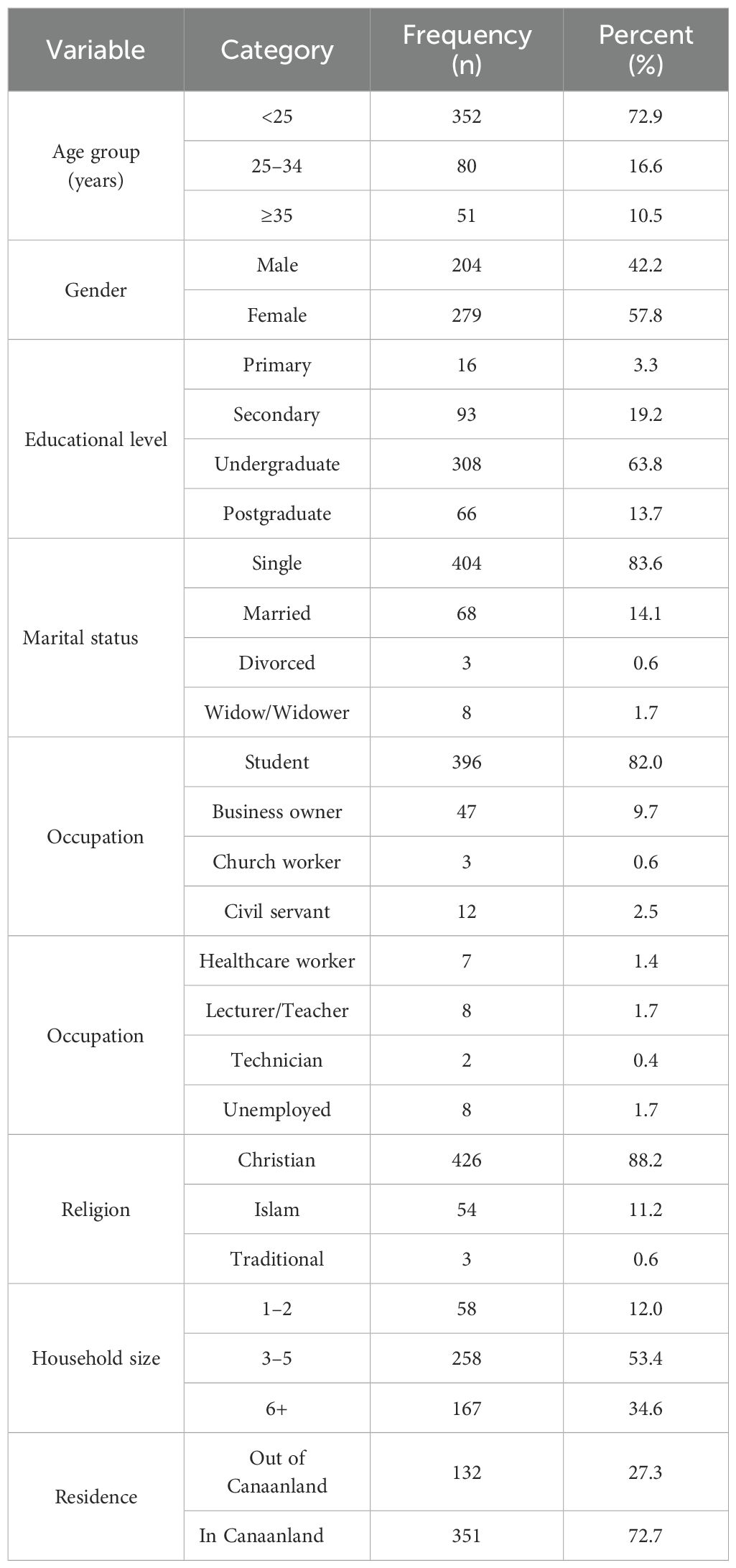
Of 483 participants, most were young (68.5% aged 16–24), female (57.8%), and students (82.0%) (Table 1).
3.2 Knowledge of malaria causes, transmission, and at-risk populations
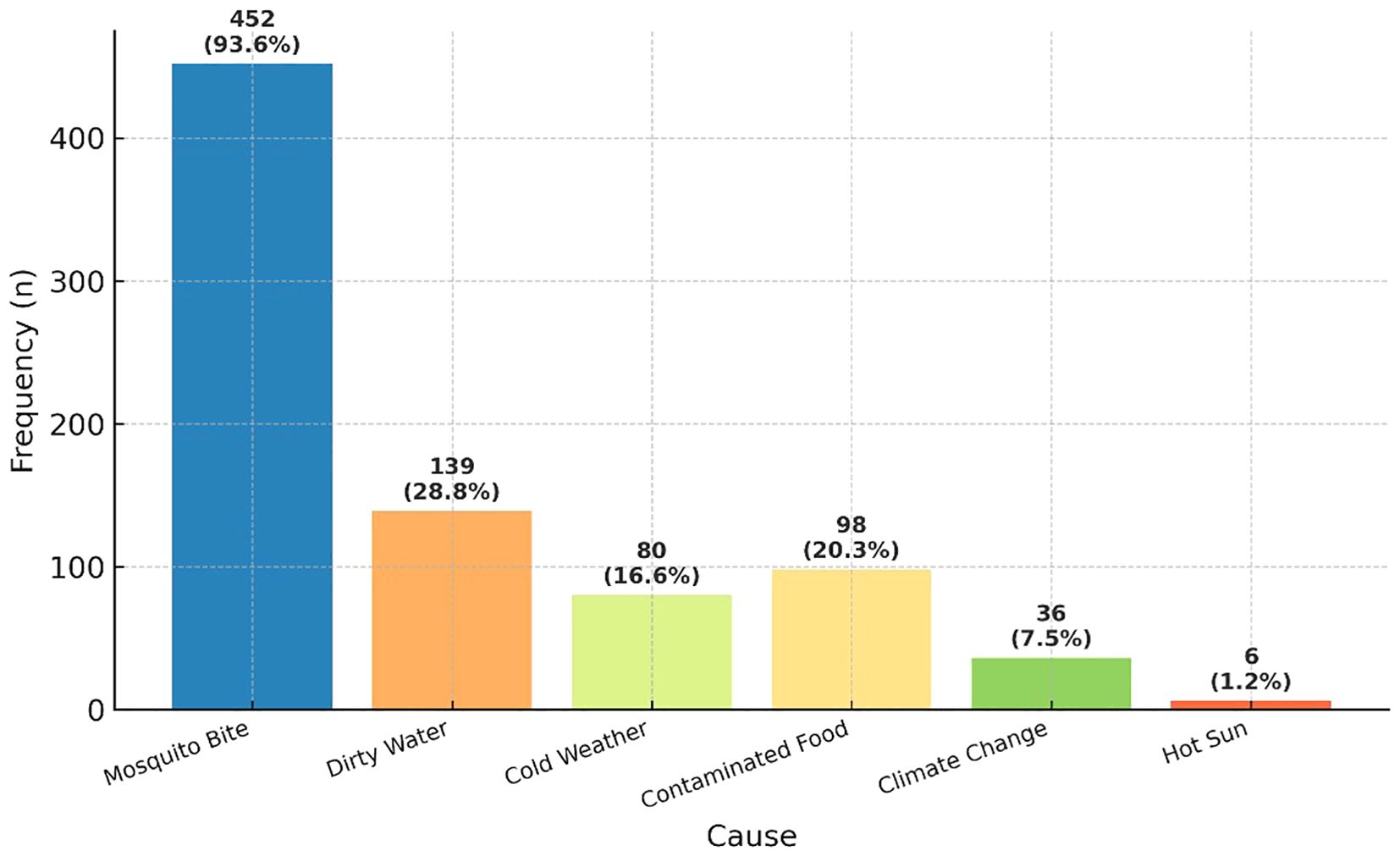
Malaria awareness was nearly universal, with 98.0% (n=475/483) of participants reporting familiarity with the disease. The majority (93.6%, n=452/483) correctly identified mosquito bites as the primary cause, though misconceptions persisted—28.8% (n=139) cited dirty water and 20.3% (n=98) contaminated food as possible causes (Figure 1).
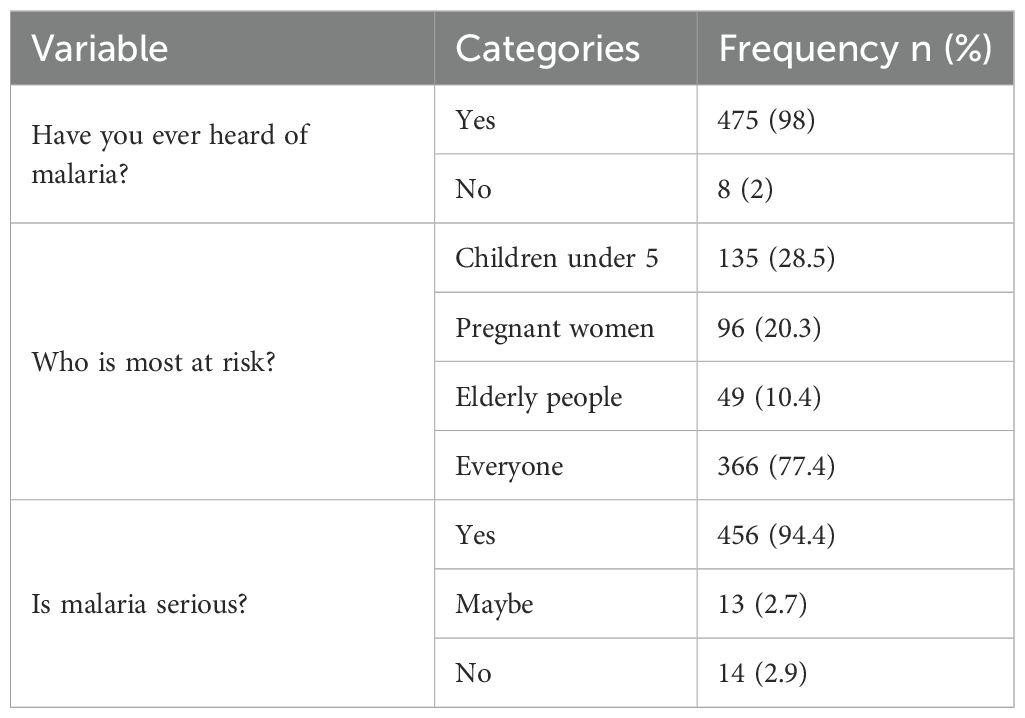
When asked about at-risk groups, most participants (77.4%) recognized that malaria affects everyone, though a considerable proportion specifically mentioned children under five (28.5%) and pregnant women (20.3%). Nearly all respondents (94.4%) considered malaria a serious illness (Table 2).
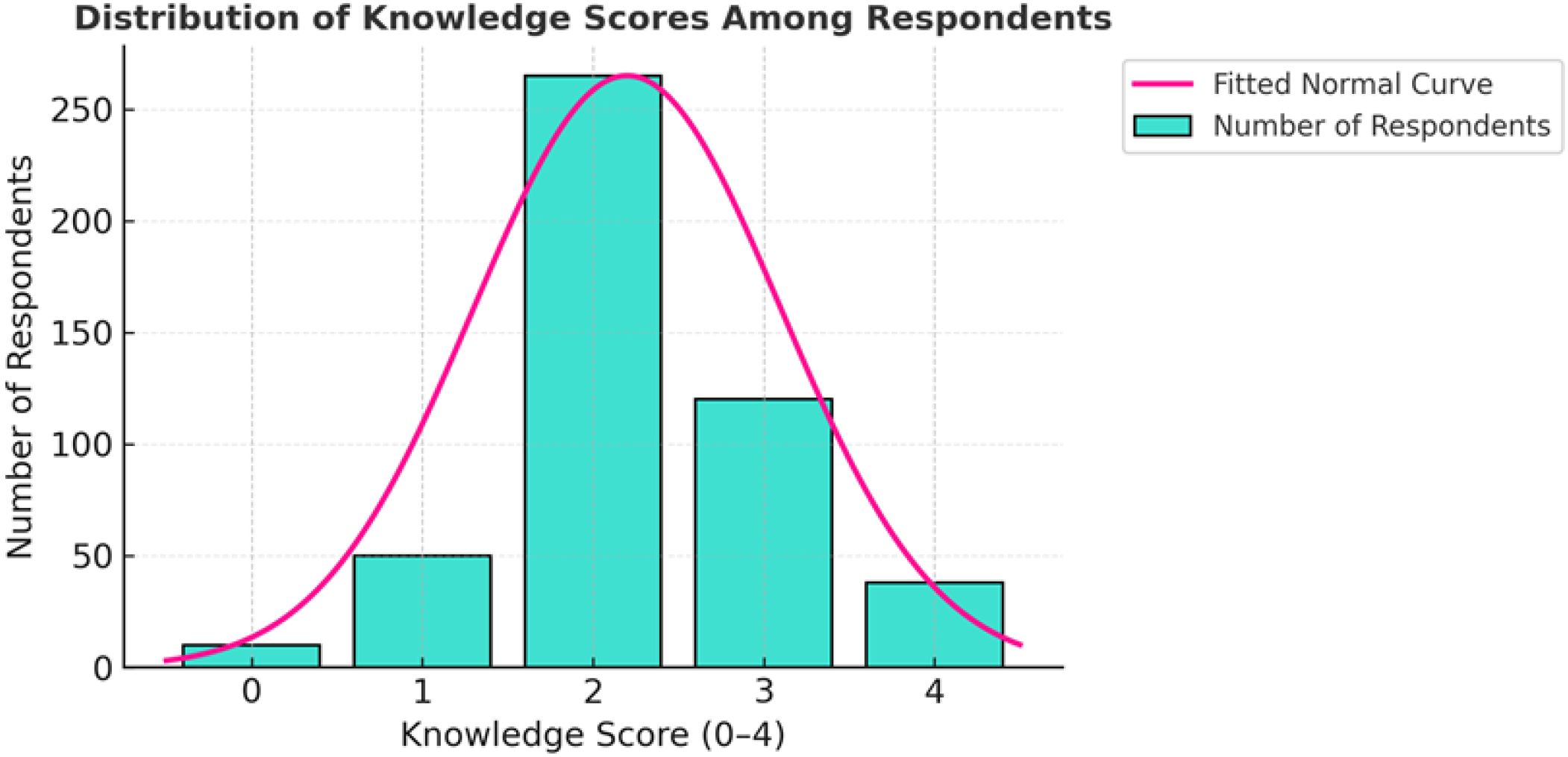
Despite high awareness, overall comprehension was moderate, with a mean knowledge score of 2.2 out of 4 (median = 2). More than half of respondents (55%) demonstrated moderate knowledge, as illustrated in Figure 2.
3.3 Malaria prevention practices
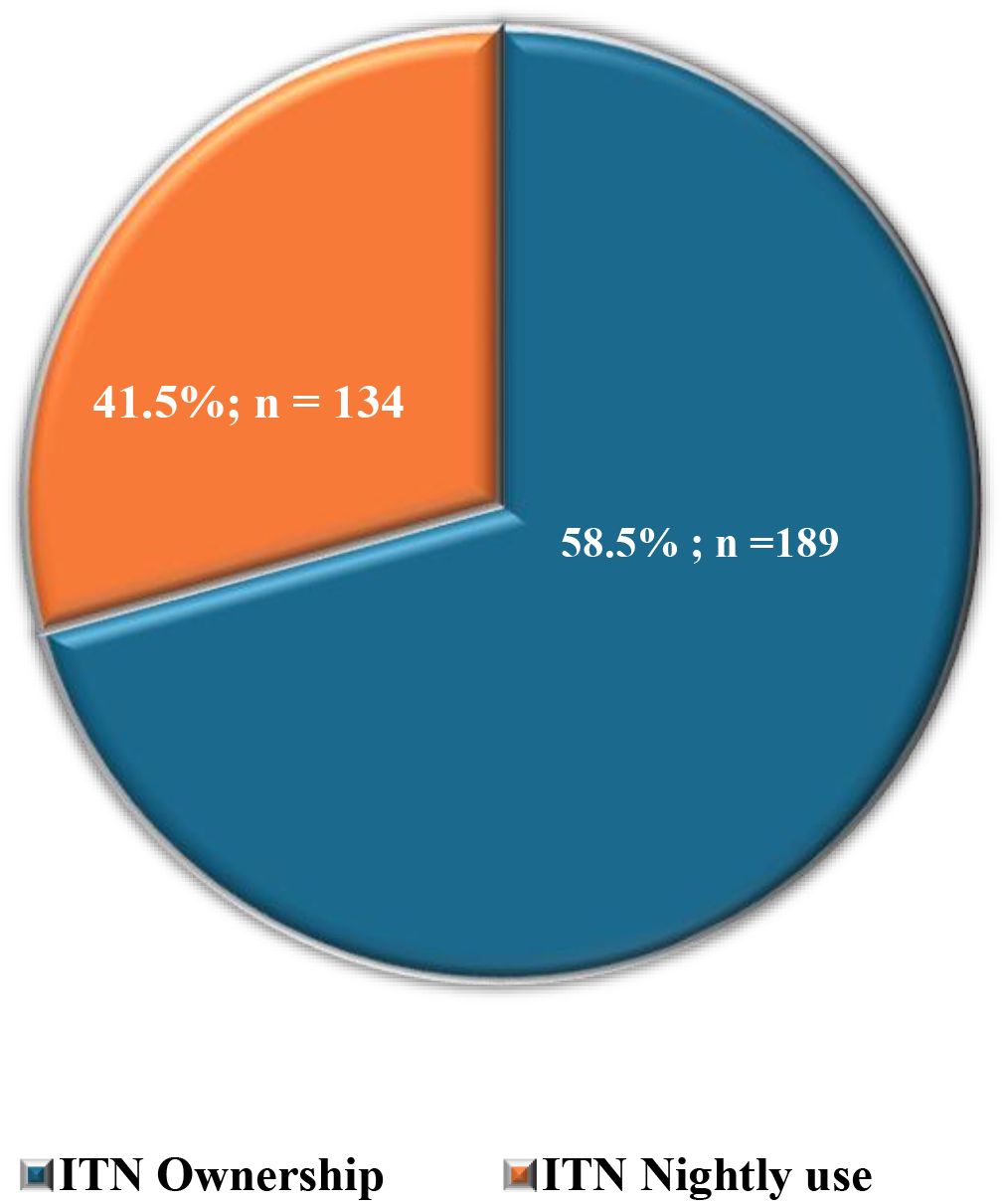
For malaria prevention practices, where participants could “select all that apply,” insecticide-treated net (ITN) ownership was most frequently reported (66.9%, n=323/483), followed by repellent use (60.5%, n=292/483), insecticide spraying (50.7%, n=245/483), and clearing stagnant water (49.5%). Less common measures included wearing protective clothing (28.2%) and using traditional methods (2.9%), while 4.6% reported taking no preventive action (Table 3). Among ITN owners (n=323), only 41.5% (n=134/323) reported using them nightly (Figure 3). Percentages exceed 100% due to multiple responses.
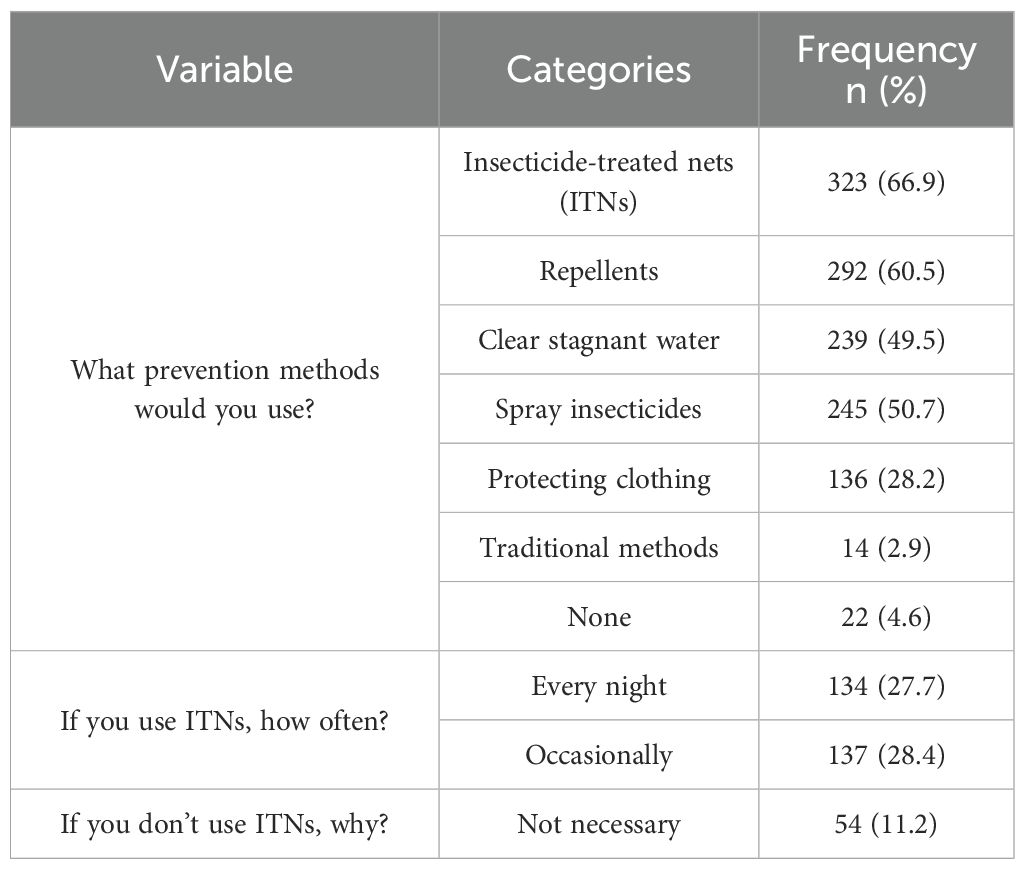
Table 3. The study participants’ responses to malaria prevention practice questions (multiple responses, n=483).
3.4 Factors influencing ITN usage
Logistic regression analysis identified gender, age, and education as predictors of ITN use (Table 4). Males were significantly more likely than females to use ITNs (AOR = 1.92, 95% CI: 1.31–2.84, p = 0.001). Age was a strong determinant: participants aged 25–34 years (AOR = 5.79, 95% CI: 1.49–24.36, p = 0.013) and ≥35 years (AOR = 7.89, 95% CI: 3.45–18.05, p < 0.001) had significantly higher odds of ITN usage compared to those <25 years. Regarding education, nightly ITN use was particularly low among postgraduate students (AOR = 0.34, 95% CI: 0.16–0.66, p = 0.002).
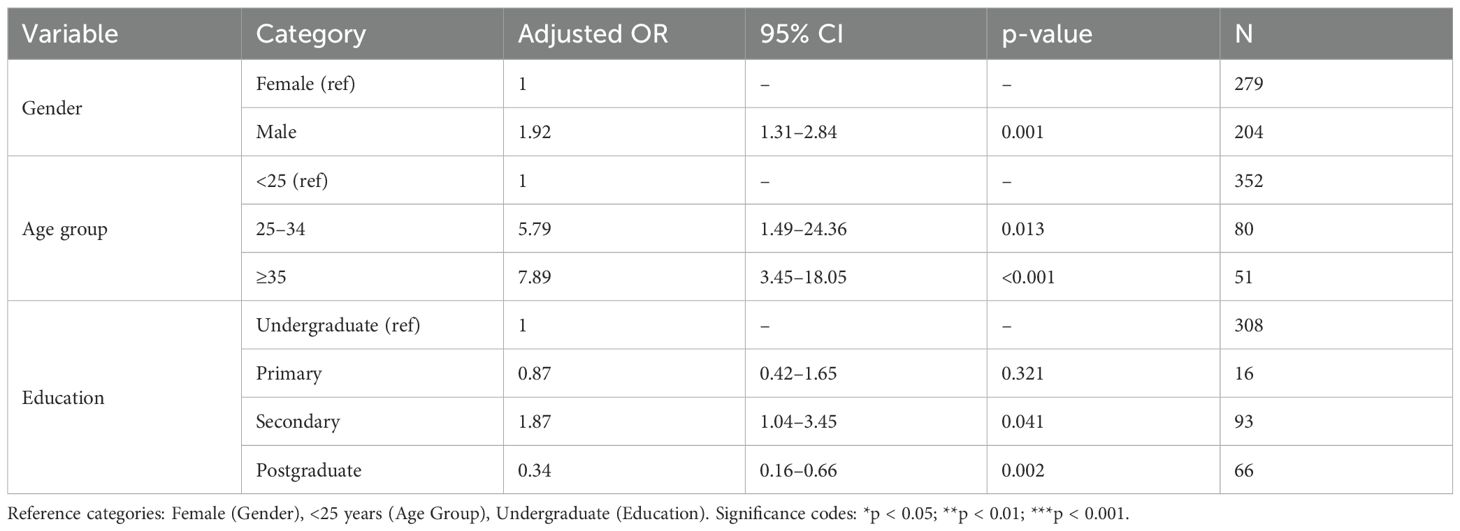
Table 4. Logistic regression analysis of factors influencing ITN usage (Adjusted Odds Ratios, AORs).
3.5 Treatment-seeking behaviors
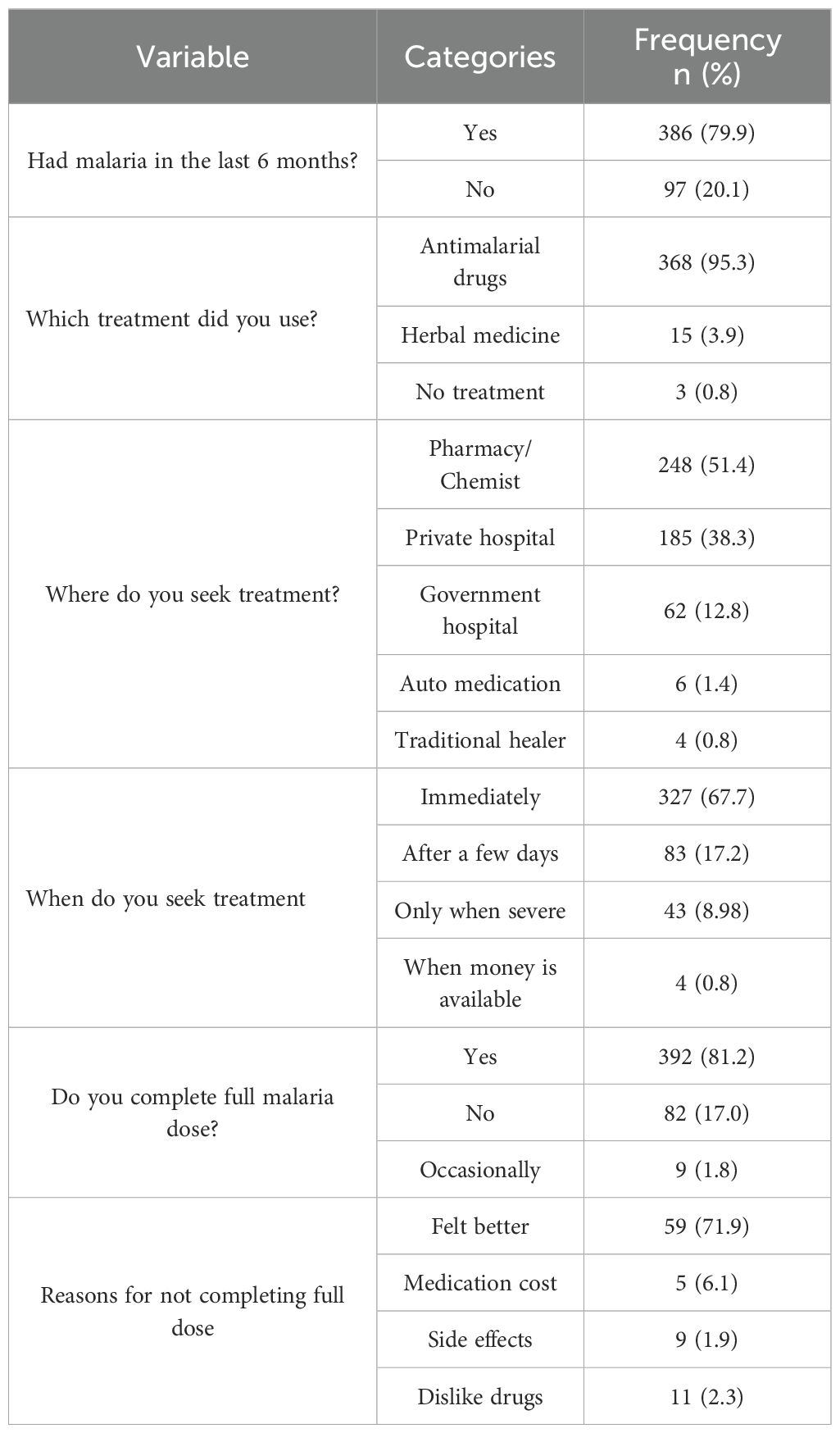
Among the 386 respondents reporting malaria in the past 6 months, 95.3% (n=368) used antimalarial drugs, 3.9% (n=15) used herbal medicine, and 0.8% (n=3) received no treatment (Table 5). Hospital/laboratory tests were used by 37.1% (n=143/386), self-diagnosis by 31.1% (n=120/386), and roadside chemists by 27.0% (n=104/386). “Select all that apply” where applicable were captured (Figure 4).
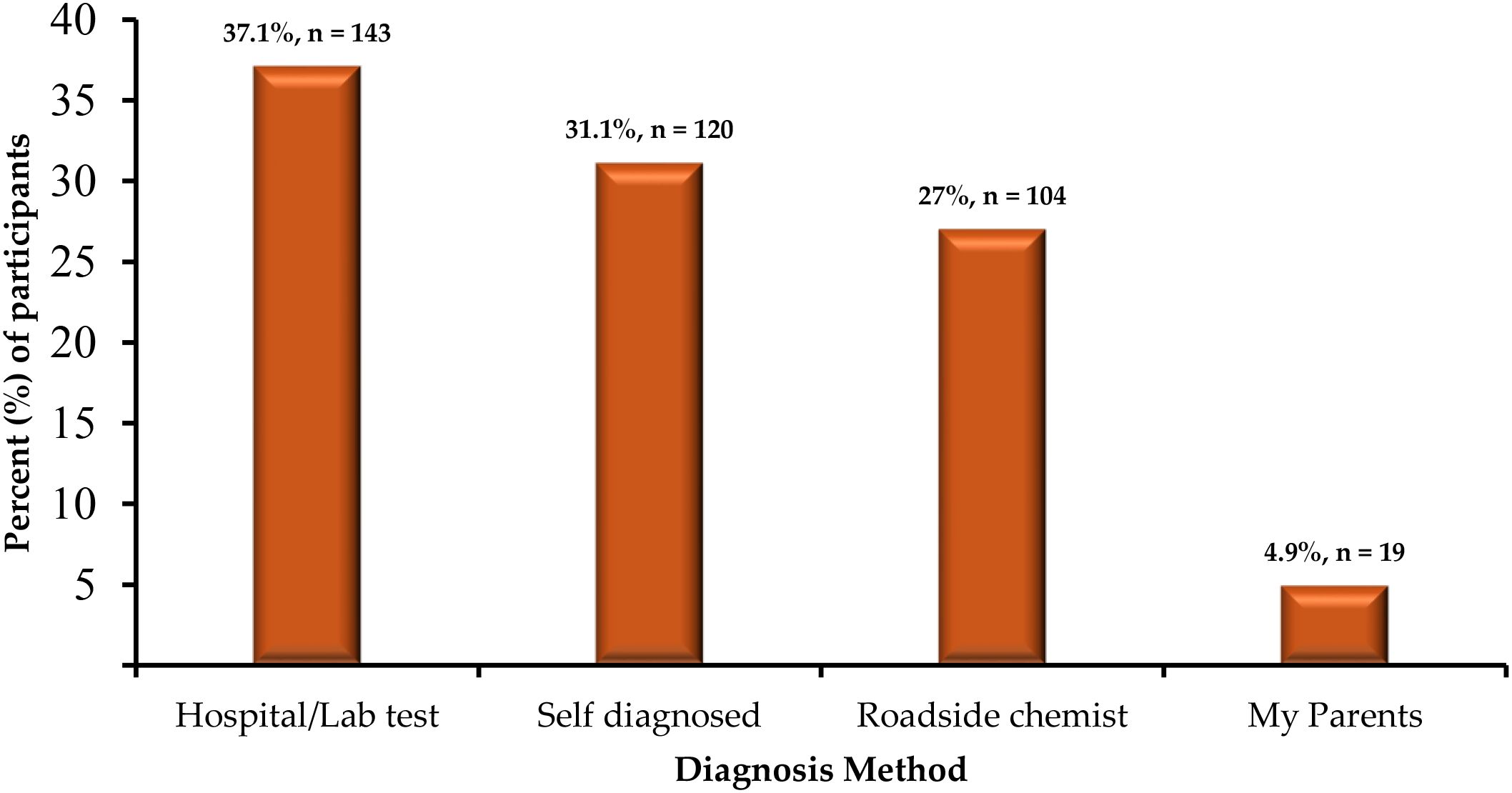
Figure 4. Distribution of diagnosis methods among respondents reporting malaria in the past 6 months (n=386; multiple responses allowed).
3.6 Associations with sociodemographic parameters
Chi-square analysis (or Fisher’s exact test for sparse cells) showed no significant associations between knowledge scores and sociodemographic variables (p > 0.05). Practice scores were associated with gender (χ² = 6.982, p = 0.008) and education (χ² = 8.006, p = 0.046), with males (62.6% poor) and undergraduates (66.1% poor) showing suboptimal practices. Attitude scores were associated with gender (χ² = 9.799, p = 0.002), education (χ² = 27.139, p < 0.001), household size (χ² = 13.712, p = 0.001), and residence (χ² = 5.357, p = 0.021) (Table 6).
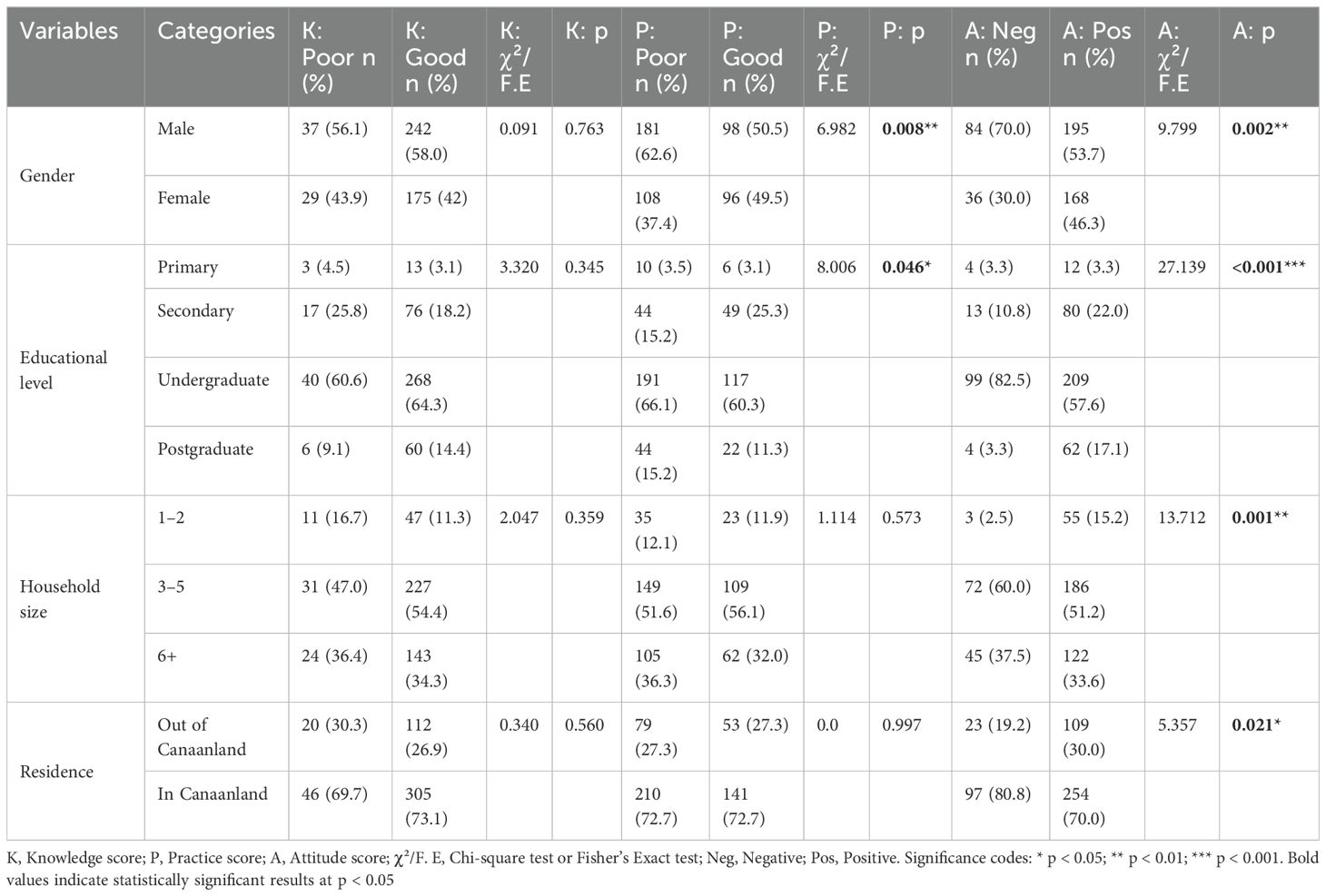
Table 6. Chi-square analysis of the overall association of knowledge, practice, and attitude scores with sociodemographic parameters of the participants.
The most commonly reported barrier was lack of mosquito nets (70.7%, n=342/483), followed by inadequate knowledge (50.5%, n=244/483) and limited healthcare access (47.4%, n=229/483) (Figure 5).
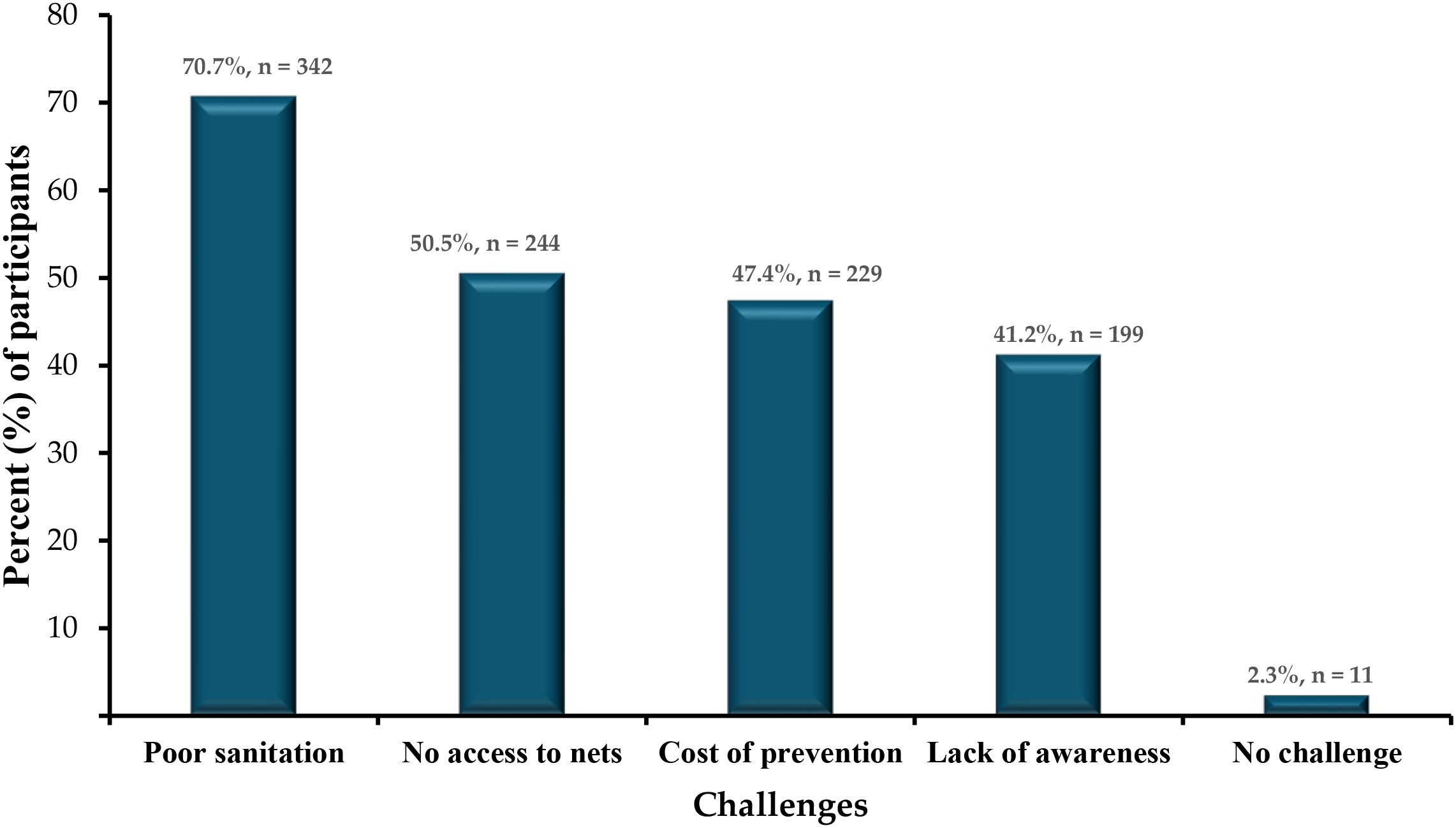
Figure 5. Distribution of reported barriers to malaria prevention (% of respondents, n=483; multiple responses allowed.
3.7 Respondents’ perceptions and attitudes
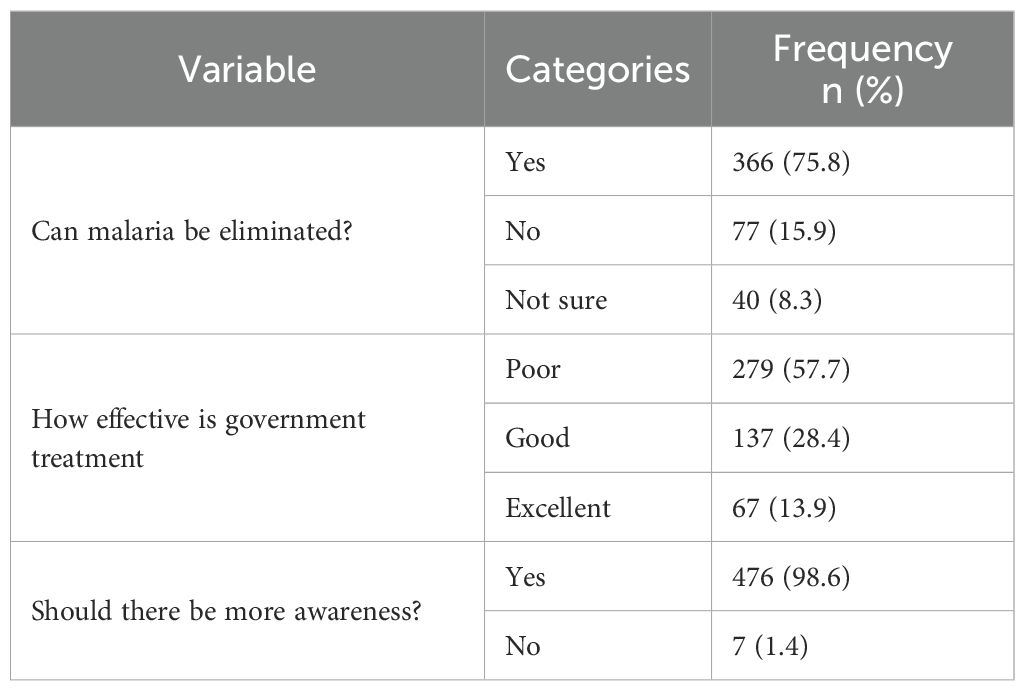
Evaluating how the Community dwellers perceive malaria, most respondents believed that malaria can be eliminated (75.8%), though 15.9% disagreed and 8.3% were unsure. Over half (57.7%) rated government control efforts as poor, while only 13.9% rated them as excellent. However, almost all participants (98.6%) strongly agree on the need for increased community awareness outreaches and sensitization. These findings are summarized in Table 7.
4 Discussion
This cross-sectional study conducted in Canaanland, a semi-urban religious and educational enclave in Ota, Ogun State, Nigeria, provides critical insights into the knowledge, preventive practices, and perceptions surrounding malaria among its residents. Even with high levels of awareness of malaria (98.0%) and comparatively strong educational attainment, the study highlights enduring gaps in knowledge, weaknesses in preventive practices, and barriers to effective malaria control.
The study found that while 93.6% of respondents correctly identified mosquito bites as the primary cause of malaria, misconceptions about the causes were prevalent, with 28.8% attributing malaria to dirty water and 20.3% to contaminated food. These findings are consistent with studies in Ghana and Uganda, where high awareness coexisted with myths about malaria etiology (26, 27). Such misconceptions can divert attention from evidence-based interventions like insecticide-treated nets (ITNs) and environmental management, undermining malaria control efforts.
Notably, only 28.5% and 20.3% of respondents recognized children under five and pregnant women as high-risk groups, respectively, despite the community’s educational profile (82.0% students). This limited recognition of vulnerable populations aligns with findings from Southeast Nigeria, where formal education did not fully translate into comprehensive malaria knowledge (28). These gaps suggest that educational curricula may lack targeted malaria education or that religious or cultural beliefs counteract formal instruction, necessitating context-specific health education campaigns.
Because mosquito bites are unpredictable and can occur at any time during the night, consistent use of insecticide-treated nets (ITNs) is essential for malaria prevention. In this study, although two-thirds of respondents reported owning an ITN (66.9%, n=323/483), only about one-quarter of the total sample (27.7%, n=134/483) reported using them nightly. When restricted to ITN owners, this translates to just 41.5% (n=134/323) achieving consistent use.
An interesting finding is the apparent contradiction between the high proportion of respondents reporting “No access to nets” as a barrier (70.7%) and the relatively high ownership rate of ITNs (66.9%) (Figure 5). This discrepancy likely reflects a perception-versus-reality issue. “Ownership” here refers to households possessing at least one ITN, while “access” may be interpreted by respondents as the availability of sufficient nets for all household members, the ability to replace damaged or worn nets, or ease of obtaining nets through distribution channels. Thus, even in households with at least one ITN, respondents may still perceive a lack of adequate access if the number of nets is insufficient for their family size or if replacement nets are not readily available (29, 30). This suggests that improving distribution strategies and ensuring equitable intra-household access are critical to closing the gap between ownership and effective use.
Nightly ITN use was particularly low among postgraduate students (AOR = 0.34, p = 0.002). This is in contrast to findings in Northeastern Nigeria, where over 90% of respondents in a higher education setting reported ITN nightly use (31). The lower ITN use in this group may reflect multiple interacting factors. One possibility is the heavy academic workload of postgraduate students, which could deprioritize preventive health practices. Another explanation is the perceived lower risk of malaria in campus-based housing, where structural protections or assumptions of safety may reduce motivation for ITN use. These findings align with research on health behaviors in academic environments, where students often engage in suboptimal preventive practices due to stress, competing demands, and a focus on immediate academic responsibilities rather than long-term health outcomes (32, 33). Moreover, studies of student populations in Nigeria and Kenya have shown that risk perception strongly shapes adherence to malaria prevention, with highly educated groups paradoxically underestimating their vulnerability (28, 31). This suggests that targeted interventions for postgraduate students should not only ensure ITN availability in residences but also address behavioral and perceptual barriers that reduce uptake. Though our findings are similar to results obtained from middle school students in Southeast Nigeria, where 96.6% of students were aware of ITNs but only 20.6% used them regularly (24), the exact barriers in this highly educated group remain hypothetical, and further studies are needed to confirm this hypothesis.
Education is often posited as a key determinant of improved health knowledge and behavioral change (33). The influence of educational attainment on the accuracy of malaria knowledge is complex. While the population sampled in Canaanland is predominantly students and thus highly educated, the mean malaria knowledge score was only moderate (2.2 out of a possible 4) (55%), indicating incomplete understanding despite formal education. This gap highlights that awareness alone does not equate to comprehensive knowledge or correct perceptions. Educational curricula may lack targeted, practical malaria education, or prevailing cultural beliefs may counteract formal instruction (34). This partly supports the findings by (35) who reported that people with better awareness of lower malaria transmission had 35% higher chances of knowing how malaria is diagnosed and 66.4% higher chances of knowing how to prevent it.
In contrast, older age groups (e.g., ≥35 years, AOR = 7.89, 95% CI: 3.45–18.05, p < 0.001) showed higher ITN usage, suggesting age-related differences in perceived risk or access (11). The low nightly ITN use contrasts with higher ownership rates reported in other Nigerian studies (23), highlighting the need for interventions that address barriers to consistent use, such as improving ITN availability in student hostels and integrating malaria education into university programs.
Other preventive practices were also suboptimal, with only 49.5% clearing stagnant water and 50.7% using insecticide spraying. Poor sanitation, cited by 66.5% of respondents as a barrier, likely exacerbates mosquito breeding, aligning with challenges noted in similar semi-urban settings (36). These findings pinpoint the importance of community-driven environmental management initiatives to complement ITN use.
Treatment-seeking behaviors revealed further challenges, with 31.1% of respondents relying on self-diagnosis and 27.0% seeking care from roadside chemists. These practices, driven by cost, accessibility, and trust issues, increase risks of misdiagnosis and drug resistance, consistent with findings in Ota and other Nigerian communities (6, 13, 14). Although 67.7% sought prompt treatment, 17.2% delayed care until symptoms worsened. There is a need to strengthen access to formal healthcare. The reliance on informal care sources reveals a critical gap in healthcare infrastructure, particularly in semi-urban areas like Canaanland, where proximity to educational and religious institutions might create an illusion of adequate health resources. The faith-based community centered around Winners’ Chapel may influence attitudes and behaviors differently compared to secular semi-urban settings.
This study revealed a mixed perception of malaria control efforts, with 57.7% rating government initiatives as poor, yet 98.6% supporting increased community sensitization. This strong community’s willingness to engage offers a valuable opportunity for intervention. Canaanland’s unique socio-religious context, centered around Winners’ Chapel headquarters and Covenant University, provides a platform for leveraging religious and academic leadership to promote malaria prevention. Faith-based health campaigns, as demonstrated in other settings (37), could dispel myths, encourage ITN use, and foster community-driven sanitation improvements. The positive attitude toward malaria elimination (75.8%) further suggests that residents are receptive to targeted interventions.
The findings highlight that high awareness and education alone do not ensure effective malaria-related behaviors. To bridge the knowledge–practice gap, we propose the following interventions tailored to Canaanland’s context:
i. Integration into academic curricula: Incorporate practical malaria education into Covenant University’s programs, focusing on correcting misconceptions, emphasizing vulnerable populations, and promoting ITN use, particularly among postgraduate students.
ii. Faith-based campaigns: Partner with Winners’ Chapel to deliver health campaigns during religious gatherings, leveraging the church’s influence to promote evidence-based prevention and dispel myths.
iii. Improved ITN access: Enhance ITN distribution in student and staff residences, addressing the low nightly usage rate (27.7%) despite high ownership (66.9%).
iv. Community-driven sanitation: Implement community-led initiatives to improve sanitation and eliminate mosquito breeding sites, addressing the 66.5% of respondents who cited poor sanitation as a barrier.
5 Strengths and limitations of the study
This study offers several important strengths, particularly its focus on a semi-urban, highly educated population—an often-overlooked group in malaria research—providing fresh insights into the knowledge–practice gap even among those with better access to information. The use of semi-structured questionnaires allowed for both quantitative data and richer qualitative perspectives, enabling a well-rounded understanding of malaria-related attitudes and behaviors. Additionally, the findings are valuable for designing localized malaria control interventions tailored to similar communities.
However, a few limitations should be noted. A noteworthy limitation is that this cross-sectional design, conducted during the rainy season (March–June 2025), may limit insights into dry-season behaviors, as ITN use and febrile illness prevalence may vary seasonally. The unique socio-religious and educational context of Canaanland may further limit the applicability of findings to other semi-urban communities with different demographic or cultural profiles. Additionally, the use of convenience sampling introduces the potential for selection bias, as participants may not fully represent the broader semi-urban Nigerian population. Furthermore, reliance on self-reported practices such as ITN use and treatment-seeking behaviors raises the possibility of social desirability bias, where respondents may over-report positive behaviors. These limitations should be considered when interpreting the findings. Nonetheless, these limitations do not diminish the relevance of the study’s contributions to malaria control efforts in semi-urban Nigeria.
6 Conclusion
This study highlights that although malaria awareness is nearly universal among Canaanland’s predominantly student population, significant knowledge gaps and suboptimal preventive behaviors persist. Misconceptions about malaria causes and limited recognition of high-risk groups could hinder effective prevention. Despite widespread ownership of insecticide-treated nets (ITNs), nightly use remains low, particularly among graduate students, and reliance on self-diagnosis and informal healthcare sources is prevalent. Barriers such as poor sanitation, limited ITN access, and financial constraints could further complicate control efforts. While these findings provide valuable insights, it is important to note that the study was conducted in a unique, highly educated, and religious semi-urban community using non-representative sampling. Therefore, the generalizability of results to other populations is limited. To bridge the knowledge–practice gap, we recommend integrating practical malaria education into academic curricula, collaborating with the church for faith-based health campaigns, enhancing ITN distribution in students’ and staff’s residential areas, and improving sanitation through community-led initiatives. These tailored, behavior-focused strategies can empower the Canaanland community to reduce malaria incidence and serve as a model for similar socio-religious settings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Covenant Health Research Ethics Committee (CHREC), Covenant University, Nigeria. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants OR participants legal guardian/next of kin.
Author contributions
TW: Conceptualization, Data curation, Formal Analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. CF: Data curation, Investigation, Writing – original draft, Writing – review & editing. IK: Data curation, Formal Analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. MJ: Writing – review & editing, Methodology, Formal analysis, Visualization, Software. SC: Conceptualization, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. IA: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded through the World Bank Impact Project, implemented via the Covenant Applied Informatics and Communication Africa Centre of Excellence (CAPIC-ACE), Covenant University, under a scholarship award granted to TN Wakai. The Article Processing Charges for this publication are supported by the Covenant University Centre for Research, Innovation and Discovery (CUCRID).
Acknowledgments
The authors sincerely thank the residents of Canaanland and the surrounding communities for their active participation in this study, as well as the research team for their invaluable support during data collection. The authors gratefully acknowledge the Centre for Applied Informatics and Communication African Centre of Excellence (CApIC-ACE), Covenant University, for the scholarship support provided to the lead author. We also extend our sincere appreciation to the Covenant University Centre for Research, Innovation and Discovery (CUCRID) for providing funding for the Article Processing Charge (APC).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2025.1686197/full#supplementary-material
Abbreviations
CApIC-ACE, Centre for Applied Informatics and Communication African Centre of Excellence; CHREC, Covenant Health Research Ethics Committee; CI, Confidence Interval; CUCRID, Covenant University Centre for Research, Innovation and Discovery; ITN, Insecticide-Treated Net; KAP, Knowledge, Attitudes, and Practices; OR, Odds Ratio; WHO, World Health Organization.
References
1. World Health Organization. Nigeria. Brazzaville, Republic of Congo: WHO Regional Office for Africa. (2023) Available online at: https://www.afro.who.int/countries/nigeria (Accessed August 14, 2025).
2. Berhe AD, Doritchamou JYA, and Duffy PE. Malaria in pregnancy: adverse pregnancy outcomes and the future of prevention. Front Trop Dis. (2023) 4:1229735. doi: 10.3389/fitd.2023.1229735
3. Isiko I, Nyegenye S, Mwesigwa A, Asingwire JM, Olot H, Mwesigye F, et al. Determinants of malaria spread among under-five children in Nigeria: results from a 2021 Nigerian malaria indicator cross-sectional survey. BMC Pediatr. (2024) 24:646. doi: 10.1186/s12887-024-05135-w
4. Usman M. Malaria still endemic in Nigeria despite multibillion naira funding. In: The ICIR- latest news, politics, governance, elections, investigation, factcheck, covid-19. (2024) Abuja, Nigeria: International Centre for Investigative Reporting (ICIR). Available online at: https://www.icirnigeria.org/malaria-still-endemic-in-nigeria-despite-multibillion-naira-funding/.
5. Andrade MV, Noronha K, Diniz BPC, Guedes G, Carvalho LR, Calazans JA, et al. The economic burden of malaria: a systematic review. Malaria J. (2022) 21:283. doi: 10.1186/s12936-022-04303-6
6. Olasehinde GI, Diji-Geske R, Fadinmade I, Arogundade D, Darby P, Adeleke A, et al. Epidemiology of Plasmodium falciparum infection and drug resistance markers in Ota Area, Southwestern Nigeria. Dovepress. (2019) 12:1941–9. doi: 10.2147/IDR.S190386
7. Omojuyigbe JO, Oluwadare OI, and Adeyemo OA. Malaria eradication in Nigeria: State of the nation and priorities for action. J Medicine Surgery Public Health. (2023) 1:100024. doi: 10.1016/j.glmedi.2023.100024
8. Oranusi SU, Mameh EO, Oyegbade SA, Balogun DO, Aririguzoh V-GO, and Atokolo A. Prevalence of malaria infection among symptomatic patients of selected healthcare centers in ota, ogun state Nigeria: proactive prevention strategies. In: Harnessing biotechnology tools for product development. Springer, Cham (2025). p. 333–44. doi: 10.1007/978-3-031-86002-7_19
9. GBD 2016 Healthcare Access and Quality Collaborators. Measuring performance on the Healthcare Access and Quality Index for 195 countries and territories and selected subnational locations: a systematic analysis from the Global Burden of Disease Study 2016. Lancet. (2018) 391:2236–71. doi: 10.1016/S0140-6736(18)30994-2
10. Adegbite G, Edeki S, Isewon I, Emmanuel J, Dokunmu T, Rotimi S, et al. Mathematical modeling of malaria transmission dynamics in humans with mobility and control states. Infect Dis Model. (2023) 8:1015–31. doi: 10.1016/j.idm.2023.08.005
11. Nyasa RB, Fomundam MF, Patrick CNW, and Damian AN. Prevalence of malaria and the use of long-lasting insecticide treated bed nets in households of rural and semi-urban communities in mbengwi health district, north west region, Cameroon. Int J Trop Dis Health. (2021) 42(20):23–35. doi: 10.9734/ijtdh/2021/v42i2030545
12. Olasehinde GI, Ajay AA, Taiwo SO, Adekeye BT, and Adeyeba OA. Prevalence and management of Falciparium malaria among infants and children in Ota, Ogun state, Southwestern Nigeria. Afr J Clin Exp Microbiol. (2010) 11(3):129–37. doi: 10.4314/ajcem.v11i3.57773
13. Bamgboye EA, Ogunwale AO, Al-Mukhtar AI, Musa B, Usman AM, Abdulraheem YJ, et al. Understanding malaria treatment patronage from informal healthcare providers in Nigerian urban settlements: insights from community members and providers. Malaria J. (2025) 24:26. doi: 10.1186/s12936-025-05255-3
14. Diji-geske IR, Olasehinde IG, Fadinad I, Arogundade D, and Darby P. Epidemiological data of falciparum malaria in Ado-Odo/Ota, Southwest Ogun State, Nigeria. Data Brief. (2018) 19:1398–402. doi: 10.1016/j.dib.2018.06.002
15. Shire QH, Sanni FO, and Tomori MO. Knowledge and perception regarding the risk associated with self-medication in Ado-Odo Ota local government area of Ogun State, Nigeria. MGM J Med Sci. (2023) 10:691. doi: 10.4103/mgmj.mgmj_240_23
16. National Population Commission (NPC) [Nigeria] and ICF. Nigeria demographic and health survey 2018. Abuja, Nigeria, and Rockville, Maryland, USA: NPC and ICF (2019). Available online at: https://dhsprogram.com/pubs/pdf/FR359/FR359.pdf (Accessed August 14, 2025).
17. Omole D, Bamgbelu O, Tenebe I, Emenike P, and Oniemayin B. Analysis of groundwater quality in a Nigerian community. JWRHE. (2017) 6:22–6. doi: 10.5963/JWRHE0602001
18. Ibrahim AO, Agbesanwa TA, Aremu SK, Odedina JO, Jimoh HO, Akintola OC, et al. Malaria infection and its association with socio-demographics, long lasting insecticide nets usage and hematological parameters among adolescent patients in rural Southwestern Nigeria. PloS One. (2023) 18:e0287723. doi: 10.1371/journal.pone.0287723
19. Onyeka AC, Izunobi CH, and Iwueze IS. Estimation of population ratio in post-stratified sampling using variable transformation. Open J Stat. (2015) 5:1–9. doi: 10.4236/ojs.2015.51001
20. Ladi-Akinyemi TW, Amoran OE, Ogunyemi AO, Kanma-Okafor OJ, and Onajole AT. Knowledge and implementation of the National Malaria Control Programme among health-care workers in primary health-care centers in Ogun State, Nigeria. J Clin Sci. (2018) 15:48. doi: 10.4103/jcls.jcls_55_17
21. Babylon P, Barka SJ, Emmanuel WB, Rhoda PSO, Aiyagbonrhule P, and Ingwu JA. Assessment of insecticide-treated nets (ITNs) devaluation: knowledge, attitude and practices regarding misuse of ITN in Taraba State of Nigeria. Direct Res J Public Health Environ Technol. (2025) 10:85–90. doi: 10.26765/DRJPHET36550914
22. Omale UI. Knowledge, attitude, and practice of the National Guidelines for Diagnosis and Treatment of Malaria among medical doctors in Ebonyi state, Nigeria: A cross-sectional survey. PloS One. (2021) 16:e0257600. doi: 10.1371/journal.pone.0257600
23. Ankomah A, Adebayo SB, Arogundade E, Ojo K, Ajumobi IO, Osofo D, et al. Determinants of insecticide-treated net ownership and utilization among pregnant women in Nigeria. BMC Public Health. (2012) 12:105. doi: 10.1186/1471-2458-12-105
24. JohnPaul Otuomasiri E, Michael Sichalwe M, and Ephraim Ibeabuchi E. Insecticide-treated nets utilization and influencing factors on middle school students in Southeast Nigeria. A cross-sectional study. Clin Epidemiol Global Health. (2025) 32:101952. doi: 10.1016/j.cegh.2025.101952
25. Ibrahim R, Al-hajje A, Khachman D, and Zein S. Comprehensive Assessment of Knowledge, Attitudes, and Practices, alongside Predictive Factors, Affecting Optimal Management of Gestational Diabetes in Pregnant Women across Multicenter Sites in Lebanon. Dr. Sulaiman Al Habib Med J. (2023) 5:138–44. doi: 10.1007/s44229-023-00038-x
26. Obol J, David Lagoro K, and Christopher Garimoi O. Knowledge and Misconceptions about Malaria among Pregnant Women in a Post-Conflict Internally Displaced Persons’ Camps in Gulu District, Northern Uganda. Malar Res Treat. (2011) 2011:107987. doi: 10.4061/2011/107987
27. Opare JKL, Boateng A, Gbogbo DK, and Ocansey DA. Community knowledge and perceptions on malaria and its prevention and control in the akwapim north municipality, Ghana. Int J Trop Dis Health. (2014) 4(1):82–93. doi: 10.9734/IJTDH/2014/6398
28. Dike N, Onwujekwe O, Ojukwu J, Ikeme A, Uzochukwu B, and Shu E. Influence of education and knowledge on perceptions and practices to control malaria in Southeast Nigeria. Soc Sci Med. (2006) 63:103–6. doi: 10.1016/j.socscimed.2005.11.061
29. Augustincic Polec L, Petkovic J, Welch V, Ueffing E, Tanjong Ghogomu E, Pardo JP, et al. Strategies to increase the ownership and use of insecticide-treated bednets to prevent malaria. Cochrane Database Syst Rev. (2015) 2015:CD009186. doi: 10.1002/14651858.CD009186.pub2
30. Hunter GC, Scandurra L, Acosta A, Koenker H, Obi E, and Weber R. ’We are supposed to take care of it’: a qualitative examination of care and repair behaviour of long-lasting, insecticide-treated nets in Nasarawa State, Nigeria. Malar J. (2014) 13:320. doi: 10.1186/1475-2875-13-320
31. Tula M. Ownership, usage, and perception of insecticide-treated nets (ITNs) for the prevention of malaria among students of a tertiary institution in northeastern Nigeria. Public Health Toxicol. (2020) 13:320. doi: 10.18332/PHT/162333
32. Atieli HE, Zhou G, Afrane YA, Lee M-C, Mwanzo I, Githeko AK, et al. Insecticide-treated net (ITN) ownership, usage, and malaria transmission in the highlands of western Kenya. Parasites Vectors. (2011) 4:113. doi: 10.1186/1756-3305-4-113
33. Enyi EO, Ikegbunam MN, Iroha IR, Udeagbala NT, and Esimone CO. Effects of formal education on malaria knowledge among people attending Federal Medical Centre, Owerri, Imo State, Nigeria. GSC Biol Pharm Sci. (2023) 25:053–60. doi: 10.30574/gscbps.2023.25.3.0514
34. Bello IS and Rehal S. A qualitative study exploring health seeking behaviour and cultural beliefs of mothers in paediatric malaria treatment decision-making process in Ile-Ife, South-West Nigeria. Malaria J. (2014) 13:P9. doi: 10.1186/1475-2875-13-S1-P9
35. Etukudoh N, Akpan E, Ocheola-Oki J, Essiet A, Udo C, Ekpenyong B, et al. Knowledge of malaria diagnosis and prevention linking awareness of low transmission to eradication efforts. Sci Rep. (2025) 15:23395. doi: 10.1038/s41598-025-89320-6
36. Adeneye AK, Jegede AS, Mafe MA, and Nwokocha EE. A pilot study to evaluate malaria control strategies in Ogun state, Nigeria. World Health Popul. (2007) 9:83–94. doi: 10.12927/whp.2007.19003
Keywords: malaria, knowledge, preventive practices, treatment-seeking, perceptions, Nigeria, semi-urban, health education
Citation: Wakai T, Fiamitia C, Kintung I, Johngwe M, Chinedu S and Afolabi I (2025) Knowledge, practices, and perceptions towards malaria prevention and control among Residents of Canaanland and surrounding areas in Ota, Ogun State, Nigeria: a cross-sectional study. Front. Trop. Dis. 6:1686197. doi: 10.3389/fitd.2025.1686197
Received: 15 August 2025; Accepted: 24 September 2025;
Published: 13 October 2025.
Edited by:
Martial Ndeffo, Texas A and M University, United StatesReviewed by:
Sheng-Qun Deng, Anhui Medical University, ChinaFufa Balcha Tolossa, Adama Hospital Medical College, Ethiopia
Copyright © 2025 Wakai, Fiamitia, Kintung, Johngwe, Chinedu and Afolabi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Theophilus Wakai, dGhlb3BoaWx1cy53YWthaXBnc0BzdHUuY3UuZWR1Lm5n
 Theophilus Wakai
Theophilus Wakai Carrin Fiamitia
Carrin Fiamitia Irrinus Kintung
Irrinus Kintung Mac Johngwe
Mac Johngwe Shalom Chinedu
Shalom Chinedu Israel Afolabi
Israel Afolabi