Abstract
Malaria is still a primary cause of morbidity and death in Sub-Saharan Africa, disproportionately impacting children under five and pregnant women. In 2023, the worldwide malaria cases and death were estimated to be 263 million and 597,000, respectively. The introduction of recently approved malaria vaccines, RTS, S, and R21/Matrix-M, by World Health Organization represents a significant breakthrough as malaria continues to pose a serious threat to public health in Africa. However, a major obstacle to obtaining optimum coverage of immunization programs is vaccine hesitancy. This paper explores the underlying causes of vaccine hesitancy in malaria-endemic African regions by analyzing two significant events—the 1996 Pfizer Trovan clinical trial controversy in Kano, Nigeria, and the most recent COVID-19 vaccination campaigns. We find recurrent patterns of medical mistrust, false information, and socio-cultural resistance by using these cases. Drawing from these insights, we offer a paradigm, Three C Model for Trust and Acceptance, that stresses ethical standards, community-driven participation, and culturally relevant communication strategies to promote public trust, malaria vaccine literacy and acceptability especially as Africa expands its malaria vaccination programs.
Introduction
Malaria is one of the most challenging public health concerns in sub-Saharan Africa. In 2023, worldwide malaria cases and deaths were estimated to be 263 million and 597,000, respectively. With an estimated 94% of global malaria cases occurring in the World Health Organization’s (WHO) African Region, the region continues to bear the greatest burden of the disease. Ethiopia (4%), Mozambique (4%), Uganda (5%), The Democratic Republic of the Congo (13%), and Nigeria (26%), were the top five countries with the highest estimated burden of malaria cases in 2023 (1).
Despite tremendous advances in prevention and control, malaria is still prevalent in many regions of the continent (2). A variety of antimalarials developed over the years are failing sequel to the emergence of drug resistant malaria parasites. Added to this is the delay in the development of potent malaria vaccines until recently when the WHO formally approved two malaria vaccines, namely; RTS, S/AS01 (RTS,S) and R21/Matrix-M (R21) (1, 3, 4) (Table 1). The two malaria vaccines are recommended for use especially in malaria endemic areas with moderate to high transmission rates (1). Both vaccines target the circumsporozoite protein (CSP) of pre-erythrocytic Plasmodium falciparum sporozoites (5, 6).
Table 1
| Vaccine | WHO vaccine recommendation/approval | Pilot phases | Rollout status |
|---|---|---|---|
| RTS,S | 2021 (WHO recommended for broader use in children in Africa) | Piloted in Ghana, Kenya, and RTS,S Malawi between 2019-2021 with over 2 million Africa from 2022 doses administered | Transitioned from pilot to broader rollout across Africa from 2022 onwards |
| R21/Matrix-M | 2023 (WHO recommended for use in children in malaria endemic regions) | Clinical trials and pilot evaluations mainly in Burkina Faso and other African sites | Large-scale introduction began in 2024, complementing RTS,S rollout |
Status of malaria vaccine recommended by WHO.
The RTS,S vaccine has been tested in Ghana, Kenya, and Malawi, leading to its wider rollout across Africa in 2021. In 2023, the WHO recommended the R21 vaccine, with a large-scale implementation in 2024 following successful pilot stages and regulatory evaluations (1) (Table 1). With the World Health Organization’s (WHO) approval of these malaria vaccines, there is fresh optimism for malaria control and eventual eradication in high-burden areas (7, 8).
The success of scientific advances depends on public acceptance, which is influenced by past experiences. The public acceptance of the novel vaccines is essentially impacted by past experiences, cultural attitudes, and trust in healthcare systems. The mandate of SAGE Working Group on vaccine hesitancy (WG) were to propose a definition of hesitancy and its scope and to construct a model to classify the behavioral elements that influence the choice to accept a vaccine. Therefore, the SAGE Working Group defines vaccine hesitancy as the delay in acceptance or refusal to vaccinate despite the availability of vaccines (9). This phenomenon has emerged as a major threat to world health (10). In Africa, vaccine hesitancy is shaped by distinct historical and sociopolitical situations. This paper discusses how memories of the Pfizer Trovan event in Nigeria, as well as subsequent issues experienced during COVID-19 vaccination efforts, might give useful insights for malaria vaccine program delivery.
The legacy of the Pfizer case in Kano
In the midst of a meningitis outbreak in Kano, Nigeria, Pfizer conducted a clinical trial involving an experimental drug, Trovan, on more than 100 children without appropriate informed consent (11). Tragically, 11 children lost their lives allegedly due to the trial, while others suffered serious health effects (12). Concerns were raised regarding the trial’s lack of ethical endorsement and parental permission (13).
The event sparked widespread national and international condemnation, leading to legal battles and symbolizing unethical medical practices in Africa. The legal battles culminated in a $75 million out-of-court settlement in 2009 to compensate affected families and establish health initiatives. Decades later, the repercussions of the Pfizer-Kano incident persist in shaping public attitudes toward foreign medical assistance, particularly in the realm of vaccinations (14). Based on conspiracy theory, many individuals in northern Nigeria and beyond harbor suspicions that vaccinations are purportedly utilized for exploitative Western agendas, population management, or covert sterilization objectives.
The Pfizer-Kano incident demonstrates the enormous impact that unethical medical practices may have on public trust. It demonstrates how a single violation of ethical conduct can cause long-term damage, driving reluctance, skepticism and opposition to life-saving therapies. As malaria vaccinations become available, this historical distrust and skepticism must be acknowledged and addressed proactively.
COVID-19 vaccination campaigns: a recent parallel
The COVID-19 pandemic caused a worldwide health emergency that necessitated urgent vaccine development and distribution. The COVID-19 vaccinations were not widely accepted and adopted in Africa due to widespread mistrust and skepticism (15–17). In addition, logistical problems emanating from insecurity, and inadequate cold-chain infrastructure, limited rural outreach, and last-mile delivery obstacles impeded access and discouraged people who might otherwise were willing to get vaccinated (18–20). Last-mile delivery obstacles include poor infrastructures (poor road condition), lack of qualified personnel, traffic congestion etc (19). Several reasons led to the observed vaccination reluctance during the COVID-19 pandemic.
-
▪ Misinformation and conspiracy theories spread on social media, often using accessible and shareable formats such as WhatsApp forwards, Facebook posts, and X (formerly Twitter) threads. These statements are circulated in closed community groups, where repetition and familiarity confer credibility as seen during the COVID-19 pandemic. For instance, during COVID-19, suspicions surfaced that COVID-19 vaccinations were purportedly aimed to diminish African populations (21, 22).
-
▪ Doubts regarding the safety and need of COVID-19 vaccinations were raised due to skepticism regarding government’s intentions and foreign aid (23).
-
▪ Religious leaders in certain communities opposed COVID-19 vaccination, seeing it as against divine will or morality (24–26).
-
▪ According to Dickson et al. (27), health authorities sometimes failed to communicate vaccination safety and efficacy in a clear, accurate, and culturally relevant manner.
Discussion
The recent COVID-19 vaccination resistance, particularly in rural and conservative areas, is similar to the reluctance found during previous polio and meningitis campaigns (28). These problems provide current insights into the roadblocks that, if not addressed successfully, may stymie malaria vaccine implementation. Understanding the root causes of vaccination reluctance in Africa is critical for designing feasible strategies. Key elements include:
-
Mistrust in foreign-led health initiatives stems from historical events such as the Trovan trial and historical medical abuses during colonial times (14, 29). Under colonial rule, African populations were subjected to forced medical experiments, clinical trials without consent, and coercive public health campaigns, such as mass smallpox vaccination drives, and sleeping sickness control programs. These purported experiences left deep suspicions and continue to influence community attitudes towards current vaccination efforts (30, 31)
-
Social-cultural resistance: Traditional beliefs sometimes conflict with scientific explanations, thereby constraining vaccine policy implementation. Consequently, vaccination may be viewed with skepticism, particularly when offered without adequate community engagement (32).
-
With increased internet access, misinformation spreads rapidly, often filling gaps where credible sources of health education are absent (33).
-
Weak Health Systems: Inconsistent vaccine supplies, inadequate infrastructure, and a shortage of trained workers reduce trust in immunization efforts (34).
According to Otundo Richard (35), top-down strategies that disregard local perspectives and/or inputs are usually ineffective among targeted communities. Especially in very heterogeneous countries like Nigeria, National Health Authorities’ mandates often fail to consider local languages, beliefs, or communication channels, leading to resistance rather than cooperation. The 2003–2004 polio vaccine boycott in northern Nigeria illustrates the consequences of not including religious and traditional leaders in planning stages (36). When stakeholders, especially those of recipient communities, are not involved at the onset of planning and mapping of implementation strategies, it often elicits cascade of doubts that may jeopardize acceptability of vaccination process and targets (37, 38).
The question remains, what are the viable lessons for malaria vaccine rollout? To improve malaria vaccine acceptability, stakeholders must learn from previous and continuing experiences. The foremost lesson emphasizes the importance of ethics and transparency. Ethical standards must be followed throughout clinical studies and vaccination distribution. According to Pahuja (38), establishing trust requires informed consent, community participation, and openness.
Also, since trust is not assumed but earned, engaging trusted local figures such as religious leaders, traditional healers, and community elders might help overcome mistrust (39). According to Solis Arce et al. (15), community health professionals recruited from within the community often outperform external personnel because they live with the people, and understand their prevailing circumstances and lifestyles.
Another vital lesson is the need to prioritize culturally sensitive communication. Health information should be communicated in local languages and tailored to cultural circumstances (40, 41). Ekezie et al. (42) suggest that visual aids, narrative, and radio programs can be effective in low-literacy situations. Furthermore, crucial to vaccine acceptance is community involvement. Communal involvement includes setting up a community advisory boards, enlisting religious and traditional leaders as malaria vaccine champions, and hosting participatory town-hall meetings to discuss local issues, training community health volunteers as peer educators, establishing feedback systems (suggestion boxes or hotlines), and collaboration with youth and women’s groups can be implemented. According to Larson et al. (37), communities should be involved in the planning stage to co-create strategies rather than seemingly imposing them.
Based on insights gained from previous campaigns, we proposed a three-part model to guide malaria vaccine acceptance strategies (Figure 1). This approach, which we named the Three C Model for Trust and Acceptance, focuses on ethical standards, community-driven participation, and culturally relevant communication strategies that promote public trust, malaria vaccine literacy and acceptability.
Figure 1
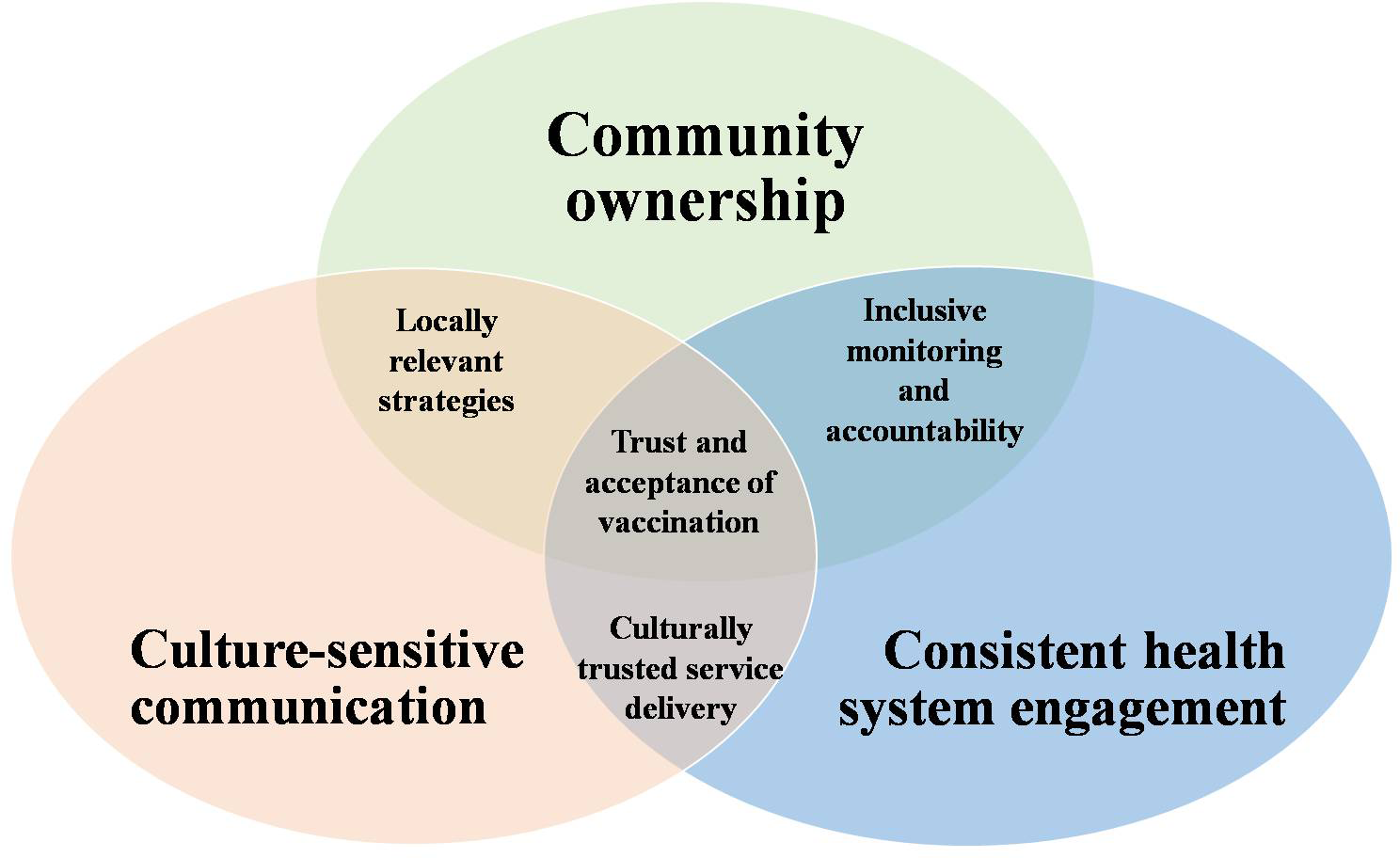
Three C model for trust and acceptance of malaria vaccine.
Community ownership
Community ownership facilitates engagement and participatory techniques to empower local stakeholders. This involves setting up community advisory boards and local champions that play complementary roles in ensuring trust and acceptance of vaccine through interconnected functions (Figure 2). The community advisory boards ensure inclusivity and equitable representation of diverse voices in vaccination campaigns. They co-design campaigns, address conflict, monitor vaccine distribution, and provide feedback loops. They ensure equitable distribution, ensure disadvantaged groups are not excluded and bridge the gap between national strategies and local realities. On the other hand, local champions are trusted role models who influence community norms and behaviors by modeling trust, culturally mediating and using storytelling to build confidence in vaccination. They also act as first responders to rumors and encourage communities to see vaccination as a shared responsibility for collective health. This dual approach in turn promotes the adoption of culturally relevant strategies, improve allocation of resources as well as improve implementation of monitoring and evaluation of vaccination programs.
Figure 2
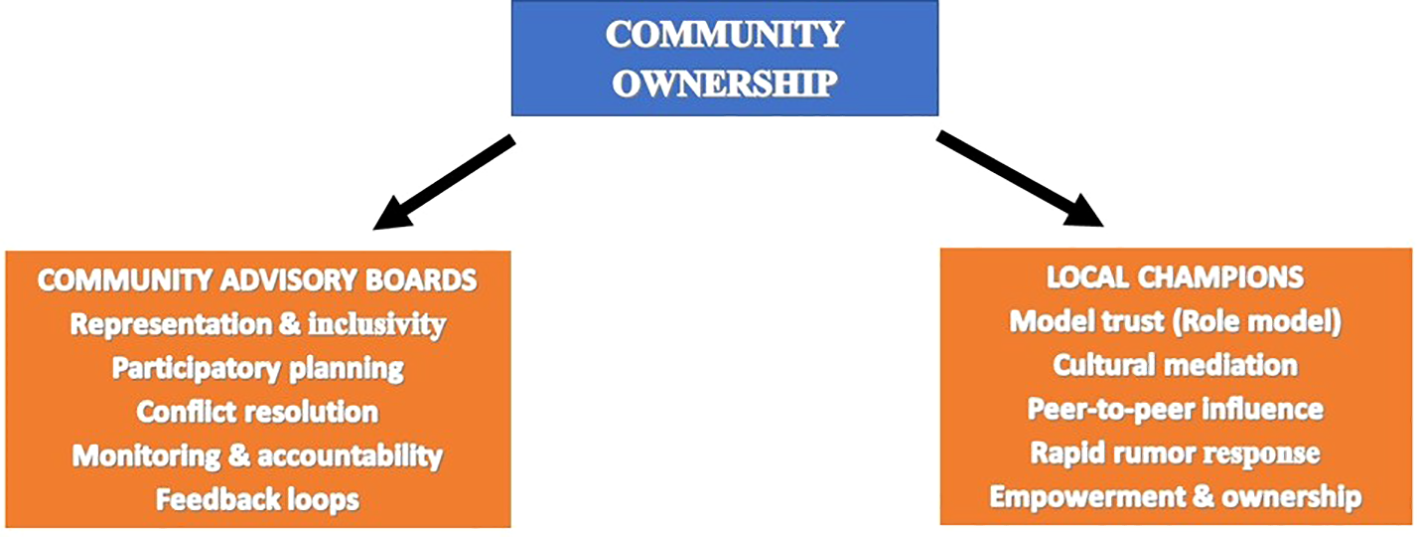
Community ownership framework.
Culture-sensitive communication
Skilled educators who understand local beliefs, appreciate and respect indigenous customs should incorporate local idioms and metaphors while using traditional media outlets. Practical strategies that can include health messages into well-known cultural genres include folk music, radio plays, storytelling, and community theater. Local audiences respond well to such strategies, particularly in rural areas with low literacy rates. There should also be employment of feedback channels to address local issues.
Consistent health system engagement
Malaria vaccine distribution should be integrated with other vital health services, such as prenatal and postnatal care, growth monitoring, nutrition programs (e.g. vitamin A supplementation), family planning services, health education campaigns and routine childhood vaccinations (e.g. polio, measles etc) to ensure consistent health system engagement. Alignment of malaria vaccine with these well-established and dependable health care services will support consistent health system engagement. In turn, consistent and reliable services will boost trust in the healthcare system and diminish the negative perception of malaria vaccine.
Conclusion
In conclusion, the success of malaria immunization initiatives in Africa, particularly Nigeria, depends on both scientific innovation and societal preparedness. The Pfizer-Kano incident and COVID-19 immunization issues highlight the need to maintain community trust. Increasing malaria vaccine adoption requires a holistic strategy that is based on ethics, empathy, and the involvement of recipient communities (32, 43). More so, a long-term monitoring and assessment structure is crucial for tracking malaria vaccine uptake, gauging community attitudes, and identifying new obstacles. This includes ongoing feedback loops, recurring surveys and open reporting procedures, ensuring flexible plans and sensitivity to changing needs. Sustained supervision is essential for maintaining confidence and ensuring the long-term viability of malaria vaccine campaigns. The recommended 3C approach is crucial for improving the acceptability, sustainability and impact of vaccination programs in communities where vaccine hesitancy is prevalent. By learning from the past and listening to communities, stakeholders can work together to develop an inclusive, respectful, and effective road to a malaria-free future.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SS: Resources, Writing – original draft, Methodology. CU: Writing – review & editing, Conceptualization, Formal analysis.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
World Health Organization . World malaria report 2024: addressing inequity in the global malaria response. Geneva, Switzerland: World Health Organization (2024).
2
Li J Docile HJ Fisher D Pronyuk K Zhao L . Current status of malaria control and elimination in Africa: epidemiology, diagnosis, treatment, progress and challenges. J Epidemiol Global Health. (2024) 14:561–79. doi: 10.1007/s44197-024-00228-2
3
World Health Organization . World malaria report 2023 (2023). Available online at: https://iris.who.int/bitstream/handle/10665/374472/9789240086173-eng.pdf?sequence=1 (Accessed September 17, 2025).
4
World Health Organization . WHO recommends R21/Matrix-M vaccine for malaria prevention in updated advice on immunization (2023). Available online at: https://www.who.int/news/item/02-10-2023-who-recommends-r21-matrix-m-vaccine-for-malaria-prevention-in-updated-advice-on-immunization (Accessed September 17, 2025).
5
Chen J Wang Q He X Yang B . Malaria vaccines: current achievements and path forward. Vaccines. (2025) 13:542. doi: 10.3390/vaccines13050542
6
Macià D Pons-Salort M Moncunill G Dobaño C . The effect of disease transmission on time-aggregated treatment efficacy estimates: a critical analysis of factors influencing the RTS,S and R21 malaria vaccine phase 3 trials. Lancet Infect Dis. (2025) 25:e516–26. doi: 10.1016/S1473-3099(25)00090-8
7
Hammershaimb EA Berry AA . Pre-erythrocytic malaria vaccines: RTS,S, R21, and beyond. Expert Rev Vaccines. (2023) 23:49–52. doi: 10.1080/14760584.2023.2292204
8
Sallam M Al-Khatib AO Al-Mahzoum KS Abdelaziz DH Sallam M . Current developments in malaria vaccination: A concise review on implementation, challenges, and future directions. Clin.Pharmacol. Adv Appl. (2025) 17:29–47. doi: 10.2147/CPAA.S513282
9
MacDonald NE . The SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy definition, scope and determinants. Vaccine. (2015) 33:4161–4. doi: 10.1016/j.vaccine.2015.04.036
10
World Health Organization . Ten threats to global health in 2019 (2019). Available online at: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (Accessed August 15, 2025).
11
Trovalla U . The necessity of suspicion: Treading with caution through a Nigerian medical landscape. Nordic J Afr Stud. (2022) 31:187–206. doi: 10.53228/njas.v31i3.924
12
Kovac C . Nigerians to sue US drug company over meningitis treatment. BMJ: Br Med J. (2001) 323:592. doi: 10.1136/bmj.323.7313.592b
13
Ahmad K . Drug company sued over research trial in Nigeria. Lancet. (2001) 358:815. doi: 10.1016/s0140-6736(01)06011-1
14
Okonta PI . Ethics of clinical trials in Nigeria. Nigerian Med J. (2014) 55:188–94. doi: 10.4103/0300-1652.132035
15
Solís Arce JS Warren SS Meriggi NF Scacco A McMurry N Voors M et al . COVID-19 vaccine acceptance and hesitancy in low-and middle-income countries. Nat Med. (2021) 27:1385–94. doi: 10.1038/s41591-021-01454-y
16
Troiano G Nardi A . Vaccine hesitancy in the era of COVID-19. Public Health. (2021) 194:245–51. doi: 10.1016/j.puhe.2021.02.025
17
Wu Z Huang J Zuo K . Oscillation of doubt: a historical odyssey of vaccine hesitancy and the evolution of public trust. Hist. Philos Med. (2025) 7:4. doi: 10.53388/HPM2025004
18
Wollburg P Markhof Y Kanyanda S Zezza A . Assessing COVID-19 vaccine hesitancy and barriers to uptake in Sub-Saharan Africa. Commun Med. (2023) 3:121. doi: 10.1038/s43856-023-00330-9
19
Bakkabulindi P Wafula ST Ssebagereka A Sekibira R Mutebi A Ameny J et al . Improving the last mile delivery of vaccines through an informed push model: Experiences, opportunities and costs based on an implementation study in a rural district in Uganda. PloS Glob Public Health. (2024) 4:e0002647. doi: 10.1371/journal.pgph.0002647
20
Fox A . Market Failure, State Failure: The political economy of supply chain strengthening to ensure equitable access to vaccines and medicines in low- and middle-income countries. J Health Polit Policy Law. (2024) 49:43–72. doi: 10.1215/03616878-10910242
21
Wilson SL Wiysonge C . Social media and vaccine hesitancy. BMJ Global Health. (2020) 5:e004206. doi: 10.1136/bmjgh-2020-004206
22
Mmadu-Okoli C Ifeanyi N . WhatsApp, COVID-19 related misinformation in Africa and the need for continuous infoveillance. HPHR Journal. (2021) 39. doi: 10.54111/0001/MM8
23
Dzinamarira T Nachipo B Phiri B Musuka G . COVID-19 vaccine roll-out in South Africa and Zimbabwe: urgent need to address community preparedness, fears and hesitancy. Vaccines. (2021) 9:250. doi: 10.3390/vaccines9030250
24
Chimuanya L Igwebuike EE . From COVID-19 to COVID-666: Quasi-religious mentality and ideologies in Nigerian coronavirus pandemic discourse. J Afr Media Stud. (2021) 13:399–416. doi: 10.1386/jams_00056_1
25
Ossai EC . ‘It is the antichrist. Can’t you see?’Perceptions of COVID-19 among Nigeria’s Christians and the Religion—Health Debate. Stud World Christianity. (2021) 27:48–64. doi: 10.3366/swc.2021.0325
26
Oloruntobi T . ““The battle is the lord’s”: social media, faith-based organizations, and challenges with COVID-19/vaccine misinformation in Nigeria”. In: CalafellBMEguchiS, editors. The routledge handbook of ethnicity and race in communication. Routledge, NY: Taylor and Francis Group (2023). doi: 10.4324/9780367748586
27
Dickson K Aboltins C Pelly J Jessup RL . Effective communication of COVID-19 vaccine information to recently-arrived culturally and linguistically diverse communities from the perspective of community engagement and partnership organisations: a qualitative study. BMC Health Serv Res. (2023) 23:877. doi: 10.1186/s12913-023-09836-3
28
Eddy JJ Smith HA Abrams JE . Historical lessons on vaccine hesitancy: smallpox, polio, and measles, and implications for COVID-19. Perspect Biol Med. (2023) 66:145–59. doi: 10.1353/pbm.2023.0008
29
Wang D Chukwu A Mwanyika-Sando M Abubakari SW Assefa N Madzorera I et al . COVID-19 vaccine hesitancy and its determinants among sub-Saharan African adolescents. PloS Global Public Health. (2022) 2:e0000611. doi: 10.1371/journal.pgph.0000611
30
Tilley H . Medicine, empires, and ethics in colonial africa. AMA J Ethics. (2016) 18:743–53. doi: 10.1001/journalofethics.2016.18.7.mhst1-1607
31
Lowes S Montero E . The legacy of colonial medicine in central africa. Am Economic Rev. (2021) 111:1284–314. doi: 10.1257/aer.20180284
32
Kuatewo M Ebelin W Doegah PT Aberese-Ako M Lissah S Kpordorlor AG et al . Fake news, misinformation, vaccine hesitancy and the role of community engagement in COVID-19 vaccine acceptance in Southern Ghana. PloS One. (2025) 20:e0316969. doi: 10.1371/journal.pone.0316969
33
Wiysonge CS Ndwandwe D Ryan J Jaca A Batouré O Anya BM et al . Vaccine hesitancy in the era of COVID-19: could lessons from the past help in divining the future? Hum Vaccin Immunother. (2022) 18(1):1–3. doi: 10.1080/21645515.2021.1893062
34
Guignard A Praet N Jusot V Bakker M Baril L . Introducing new vaccines in low-and middle-income countries: challenges and approaches. Expert Rev Vaccines. (2019) 18:119–31. doi: 10.1080/14760584.2019.1574224
35
Otundo Richard M . Assessing the ineffectiveness of top-down developmental approaches: A case study of NGOs in Kwale, Mombasa, and Kilifi Counties, Kenya. (2024). doi: 10.2139/ssrn.4956481
36
Yahya M . Polio vaccines—”no thank you!” barriers to polio eradication in Northern Nigeria. African Affairs. (2007) 106:185–204. doi: 10.1093/afraf/adm016.
37
Larson HJ Jarrett C Eckersberger E Smith DM Paterson P . Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature 2007–2012. Vaccine. (2014) 32:2150–9. doi: 10.1016/j.vaccine.2014.01.081
38
Pahuja R . Ethical dimensions of informed consent: principles, processes, and case studies. Ethical Frameworks Special Education: A Guide Researchers. (2024) 2nd:116.
39
Unfried K Priebe J . Vaccine hesitancy and trust in sub-Saharan Africa. Sci Rep. (2024) 14:10860. doi: 10.1038/s41598-024-61205-0
40
Dubé E Laberge C Guay M Bramadat P Roy R Bettinger J . Vaccine hesitancy: an overview. Hum Vaccin Immunother. (2013) 9(8):1763–73. doi: 10.4161/hv.24657
41
Griffith DM Efird CR Baskin ML Webb Hooper M Davis RE Resnicow K . Cultural sensitivity and cultural tailoring: lessons learned and refinements after two decades of incorporating culture in health communication research. Annu Rev Public Health. (2024) 45:195–212. doi: 10.1146/annurev-publhealth-060722-031158
42
Ekezie W Igein B Varughese J Butt A Ukoha-Kalu BO Ikhile I et al . Vaccination communication strategies and uptake in Africa: A systematic review. Vaccines. (2024) 12:1333. doi: 10.3390/vaccines12121333
43
Ogugua JO Anyanwu EC Olorunsogo T Maduka CP Ayo-Farai O . Ethics and strategy in vaccination: A review of public health policies and practices. Int J Sci Res Arch. (2024) 11:883–95. doi: 10.30574/ijsra.2024.11.1.0141
Summary
Keywords
vaccine hesitancy, malaria, Nigeria, COVID-19, 3C
Citation
Suru SM and Ugwu CE (2025) Addressing malaria vaccine hesitancy in Nigeria: lessons from the Pfizer-Kano incident and COVID-19 vaccination. Front. Trop. Dis. 6:1691239. doi: 10.3389/fitd.2025.1691239
Received
23 August 2025
Accepted
26 September 2025
Published
14 October 2025
Volume
6 - 2025
Edited by
Janet Masaku, Kenya Medical Research Institute (KEMRI), Kenya
Reviewed by
Joyeeta Talukdar, All India Institute of Medical Sciences, India
Updates
Copyright
© 2025 Suru and Ugwu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chidiebere Emmanuel Ugwu, ce.ugwu@unizik.edu.ng
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.