- Department of Public Health, Pharmacology and Toxicology, University of Nairobi, Nairobi, Kenya
Introduction: Donkeys are vital to livelihoods in Kenya, yet their exclusion from national disease surveillance leaves potential health risks underexplored. Brucellosis, a significant zoonosis, remains poorly characterized in donkeys despite frequent close contact with humans. This study aimed to determine the seroprevalence, molecular detection, and risk factors for Brucella spp. infection in donkeys, and to assess owner knowledge, attitudes, and practices (KAP) across seven Kenyan counties representing diverse production systems.
Methods: Between October 2024 and February 2025, a cross-sectional survey sampled 392 donkeys. Serum was tested using the Rose Bengal Plate Test (RBPT) and indirect ELISA (iELISA). Donkeys testing seropositive on either test (n = 42) had their corresponding whole blood samples subjected to DNA extraction for PCR analysis, targeting Brucella abortus and Brucella melitensis. Structured interviews with owners were conducted to assess knowledge, attitudes, and practices (KAP). Mixed-effects logistic regression in R was used to identify risk factors.
Results: Overall seroprevalence was 10.7% by RBPT, 2.0% by iELISA, and 0.0% by PCR. All iELISA-positive cases (n = 8) were from Turkana (4), Narok (3), and Nairobi (1). Young donkeys (<3 years) had significantly higher odds of being seropositive (aOR = 11.8; 95% CI: 1.70–81.99; p = 0.013). Owner knowledge was low—only 25.3% had heard of brucellosis and risky practices were common, with 91.1% assisting foaling without protective equipment and 19.4% consuming donkey products, often raw.
Conclusion: Donkeys in Kenya may contribute to Brucella transmission within mixed livestock systems and to humans. Inclusion of donkeys in brucellosis surveillance, targeted community education, and improved diagnostics are recommended. These findings provide the first field-based evidence of donkey brucellosis in Kenya and underscore the importance of integrating donkeys into One Health strategies to reduce zoonotic risk.
1 Introduction
Donkeys are vital working animals in many developing countries, providing affordable transport, supporting agriculture, and generating income for millions of households (1). The global donkey population is estimated at about 50 million, with roughly half in Asia and more than a quarter in Africa. Ethiopia has the largest national herd, while smaller populations are distributed across West, Southern, and East Africa, including Kenya (2, 3). In Kenya, the most recent census recorded 1,176,293 donkeys (4), concentrated in diverse production systems such as arid, semi-arid, urban, and high-potential (5). Their adaptability to harsh environments (6) and cost-effectiveness compared to mechanized transport make them indispensable, particularly for women whose livelihoods depend on them (1).
Brucellosis, caused mainly by Brucella abortus and Brucella melitensis, is a zoonotic disease of economic and public health importance. In donkeys, infection can result in abortion, infertility, orchitis, and epididymitis, leading to productivity losses (7, 8). The disease is endemic in East Africa (9) and transmitted through direct animal contact, ingestion of contaminated materials, or inhalation of aerosols (10).
In Kenya, brucellosis contributes to major livestock losses from infertility, abortion, and extended calving intervals (11). Human brucellosis is also significant, with seroprevalence estimates ranging from 10.8% in Marsabit (12) to 16.5% in Kajiado (13), and incidence rates exceeding 40% among febrile patients (14). Risk factors include raw milk consumption, handling of birth materials, and frequent livestock contact (15). In rural and peri-urban areas where donkeys play central roles, the potential for zoonotic transmission is particularly high (16).
Globally, donkey brucellosis prevalence averages 10.2% but varies widely (0–63.7%) depending on geography, management, and diagnostic methods (17). Pastoral systems generally report higher prevalence due to herd mixing, communal grazing, and wildlife contact (18, 19). Despite this, donkeys remain largely excluded from surveillance and control programs, which may underestimate their reservoir role (17).
Control of brucellosis relies on One Health strategies such as vaccination, surveillance, biosecurity, test-and-slaughter, and public education. While near-eradication has been achieved in some high-income countries (20), progressing Africa is constrained by limited resources, weak veterinary infrastructure, and low community awareness (21). In Kenya, most brucellosis research has focused on cattle, camels, goats, and sheep, with little epidemiological data available for donkeys (17, 22).
To address this gap, we applied a One Health approach to investigate brucellosis in donkeys across seven Kenyan counties representing different production systems. Specifically, we aimed to: (i) estimate seroprevalence, (ii) detect Brucella species using PCR, (iii) identify associated risk factors, and (iv) assess community perceptions of the disease.
2 Materials and methods
2.1 Study design and data collection
This study employed a cross-sectional design conducted between October 2024 and February 2025 across seven counties in Kenya: Turkana, Kitui, Narok, Nakuru, Nairobi, Kiambu, and Bungoma (Figure 1). These counties were purposively selected to represent diverse donkey production systems, including arid, semi-arid, urban, and high-potential agro-ecological zones. The selected communities are characterized by informal animal health systems, close interspecies contact, and reliance on donkeys for daily livelihoods.
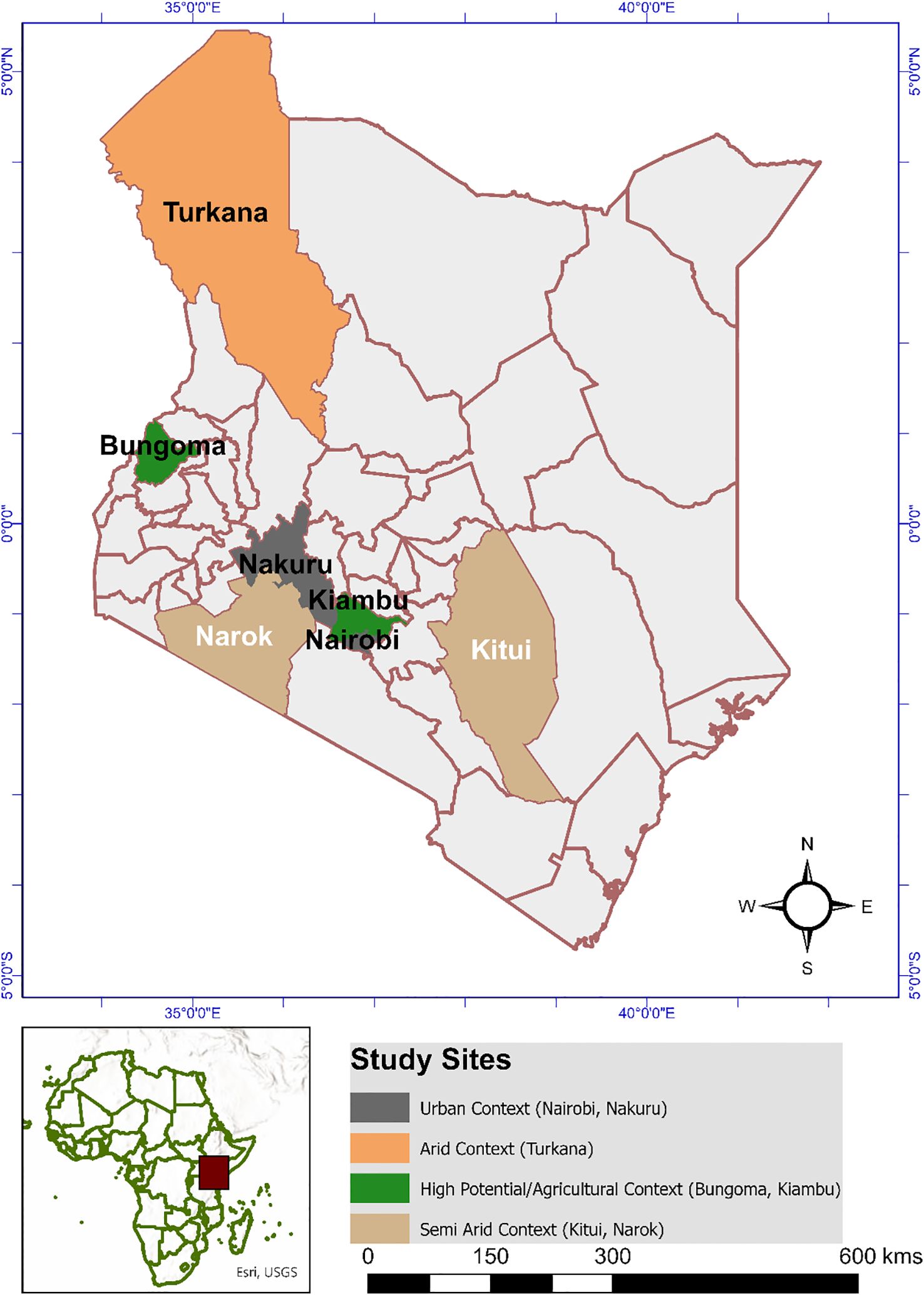
Figure 1. A map of Kenya showing study counties and categorized into production systems (Urban, Semi-arid, high potential and Arid. [Source: The map was generated using ArcMap version 10.8.2., ESRI, California, USA]).
The study population comprised donkeys aged six months and above, managed under traditional husbandry systems. Donkeys younger than six months or belonging to owners who declined consent were excluded. A sample size of 384 was calculated using the (23) formula, assuming a 50% expected prevalence, 5% margin of error, and 95% confidence level. To improve representativeness, samples were proportionally distributed across counties based on donkey population size. At the end of the exercise, 392 donkeys were sampled. Within each county, one or more sub-counties were purposively selected based on security, logistical access, and presence of active donkey-owning communities. Donkey welfare groups were identified with support from local non-governmental organisations, and within each group, households were randomly selected. Where an owner had more than one donkey, one animal was randomly chosen for sampling.
Data collection involved a combination of structured questionnaires and blood sampling. The questionnaire was developed using previously published KAP tools on zoonoses, adapted to donkeys, and pre-tested in one county to check clarity. It was then digitized, and administered via Google Forms on mobile devices by trained enumerators. Responses were scored and grouped into knowledge, attitudes, and practices domains. The tool captured information on owner demographics, knowledge, attitudes, and practices related to brucellosis, as well as donkey characteristics, reproductive history, and husbandry practices. Interviews were conducted in English or local languages, depending on participant preference.
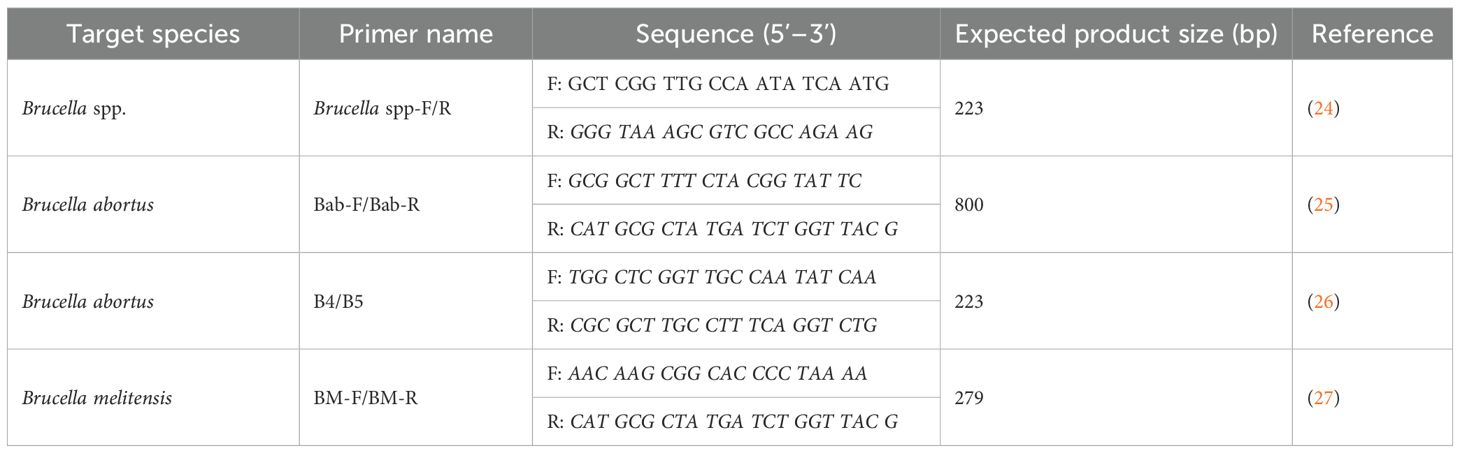
Following the interview, approximately 10 ml of blood was aseptically collected from the donkey’s jugular vein using sterile vacutainer tubes. Samples were stored in cool boxes (4–8 °C) and processed at county laboratories, where serum was separated for serological testing and EDTA blood retained for molecular analysis. All samples were tested in parallel using the Rose Bengal Plate Test (Pourquier® Rose Bengal antigen - Innovative Diagnostics [IDVET], France), valued for its sensitivity, and indirect ELISA (ID Screen® Brucellosis Serum Indirect Multi-species kit (IDVET, France), known for specificity. Samples positive on either test were further analyzed by conventional PCR (QIAamp® DNA Blood Mini Kit (Qiagen, cat. no. 51106, Hilden, Germany) to detect and differentiate Brucella abortus and Brucella melitensis. Laboratory protocols followed international standards, with appropriate positive and negative controls.
2.2 Ethics
Ethical approval for the study was obtained from the University of Nairobi Faculty of Veterinary Medicine Biosafety, Animal Use and Ethics Committee (Ref: FVM BAUEC/2024/117). Research authorization and permits were also granted by the National Commission for Science, Technology and Innovation (NACOSTI), Permit No. NACOSTI/P/24/38872.
All animal handling and blood collection were performed humanely and non-invasively, using minimal restraint to avoid distress, and in accordance with approved animal welfare and biosafety protocols. Participation in the accompanying questionnaire survey was voluntary, and written informed consent was obtained from all donkey owners prior to interviews after explaining the purpose and confidentiality of the study.
2.3 Data management and analysis
Data from questionnaires and laboratory results were merged into a single dataset, cleaned for consistency, and analysed using R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria). Descriptive statistics were used to estimate seroprevalence with 95% confidence intervals. Associations between potential risk factors and Brucella seropositivity were initially assessed using Chi-square tests. Mixed-effects multilevel logistic regression models were then used to identify independent predictors, accounting for clustering by county. Variables with p-values <0.20 in univariable analysis were considered for multivariable models.
3 Results
3.1 Seroprevalence of donkey brucellosis and by county
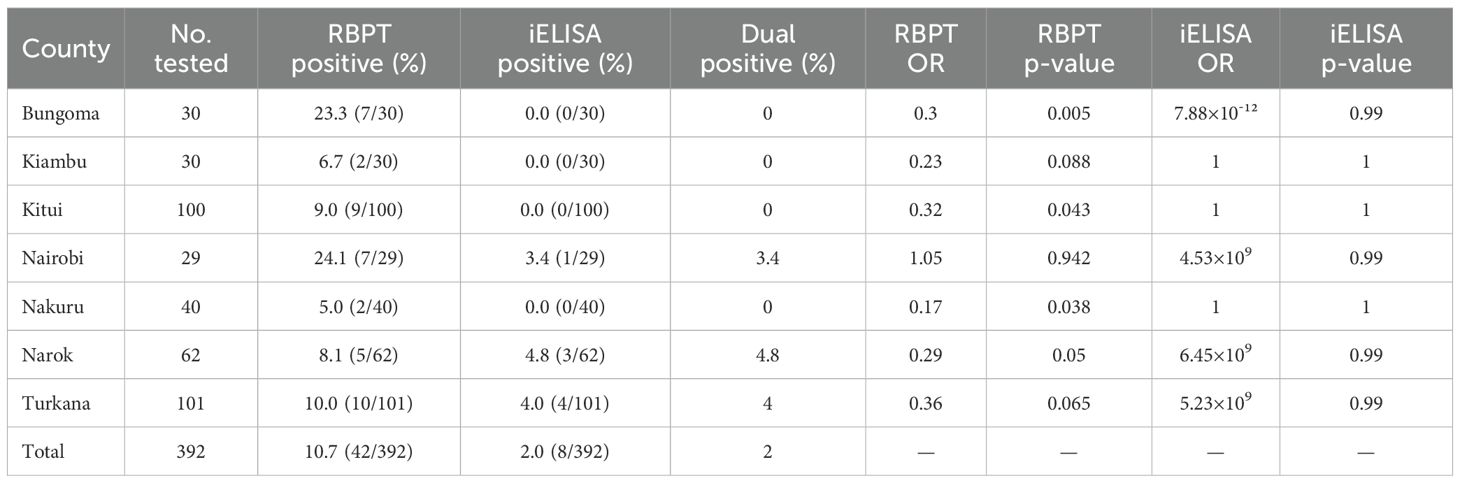
A total of 392 donkey serum samples were collected across seven Kenyan counties; Bungoma, Kiambu, Kitui, Nairobi, Nakuru, Narok, and Turkana to assess the seroprevalence of Brucella spp. infection. Using RBPT, 42 donkeys (10.7%) tested positive, while iELISA confirmed only 8 donkeys (2.0%). All iELISA-positive samples were also RBPT-positive, indicating concordance in true positives but highlighting the lower specificity of RBPT. The iELISA-derived prevalence (2.0%) is considered the more accurate estimate of Brucella exposure in donkeys, while RBPT results are best regarded as preliminary screening. Substantial variation in seroprevalence was observed across counties. Nairobi (24.1%) and Bungoma (23.3%) reported the highest RBPT prevalence, followed by Turkana (10.0%) and Kitui (9.0%). Lower RBPT seroprevalence was observed in Narok (8.1%), Kiambu (6.7%), and Nakuru (5.0%). iELISA detected positives in Nairobi (3.4%), Narok (4.8%), and Turkana (4.0%) only. The proportion of dual-positive samples mirrored the iELISA results, reflecting the concordance between assays.
A Chi-square test indicated a statistically significant association between county and RBPT seroprevalence (χ² = 13.12, df = 6, p = 0.041). Cohen’s Kappa statistic revealed a fair agreement between RBPT and iELISA (κ = 0.30), suggesting some degree of diagnostic discordance, likely due to differences in test sensitivity and specificity as detailed in Table 1 below. However, none of the 42 seropositive samples yielded Brucella DNA by PCR, indicating absence of detectable infection in blood at the time of sampling.
3.2 Seroprevalence by sex, age, and body condition score
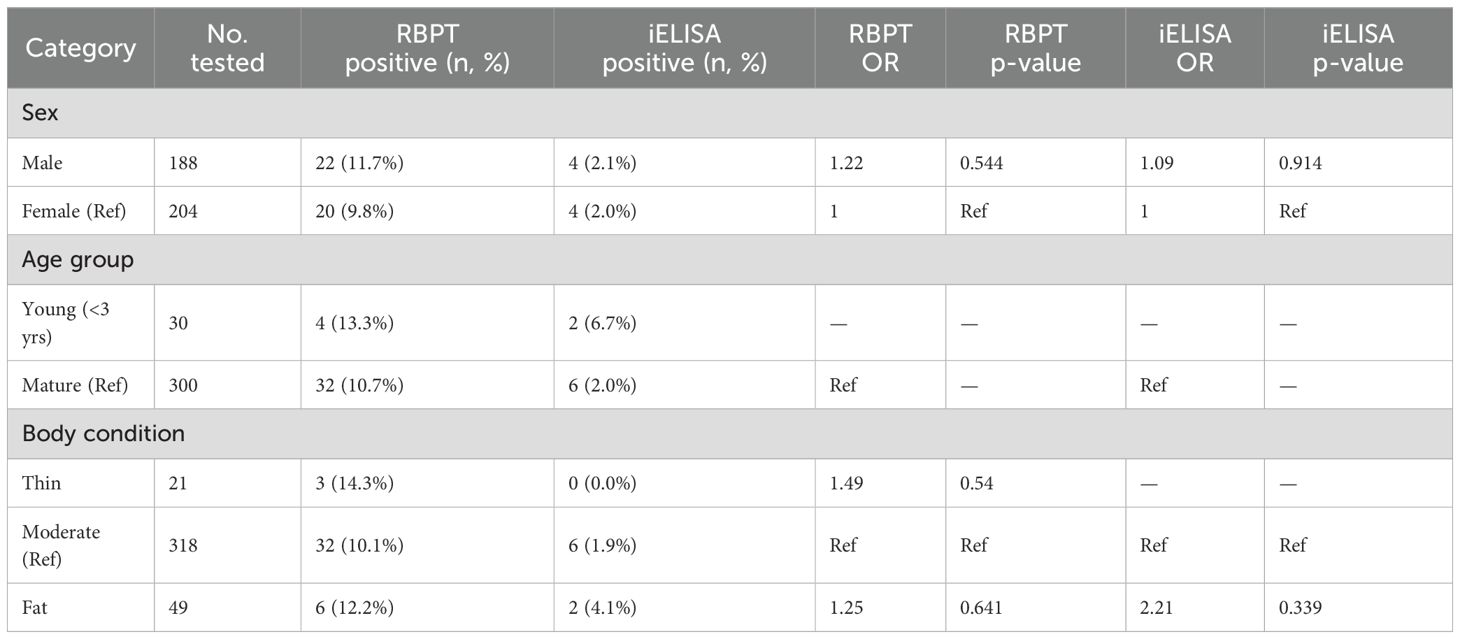
Among the 392 donkeys sampled, 188 were male and 204 female. Seroprevalence based on the RBPT was slightly higher in males (11.7%) compared to females (9.8%). Both sexes recorded 4 positive cases by iELISA, yielding nearly identical prevalence rates (2.1% in males vs. 2.0% in females). Logistic regression showed no significant difference by sex for either RBPT (OR = 1.22, p = 0.544) or iELISA (OR = 1.09, p = 0.914).
Age-based analysis revealed that young donkeys (<3 years) had the highest RBPT (13.3%) and iELISA (6.7%) positivity, followed by mature (10.7%, 2.0%) and old donkeys (9.7%, 0.0%). The low number of positive iELISA results limited statistical inference, and no associations were statistically significant.
Body condition also showed modest variation. RBPT seroprevalence was highest in very fat donkeys (25.0%), followed by thin (14.3%), fat (12.2%), and moderate (10.1%) animals. For iELISA, only moderate (1.9%) and fat (4.1%) donkeys recorded positive results. Odds ratios comparing fat to moderate donkeys were elevated (OR = 2.21, p = 0.3394), though not statistically significant (Table 2).
3.3 Molecular detection of Brucella species by PCR
A total of 42 seropositive samples, identified by RBPT and/or iELISA, were subjected to conventional polymerase chain reaction (PCR) to confirm the presence of Brucella DNA and determine species identity. The analysis was performed at the Department of Public Health, Pharmacology and Toxicology, University of Nairobi, using species-specific primers targeting Brucella abortus and Brucella melitensis (Table 3).
DNA was successfully extracted from all samples, and PCR amplification was conducted in triplicate to enhance detection reliability. Despite this, none of the samples yielded amplification products for either B. abortus or B. melitensis, indicating no detectable Brucella DNA in the tested sera.
3.4 Analysis of risk factors for donkey brucellosis
A total of 392 donkeys were tested using iELISA, yielding an overall seroprevalence of 2.04%. Univariable logistic regression was used to explore associations between Brucella seropositivity and key variables: sex, age, county, body condition score (BCS), and recent movement history.
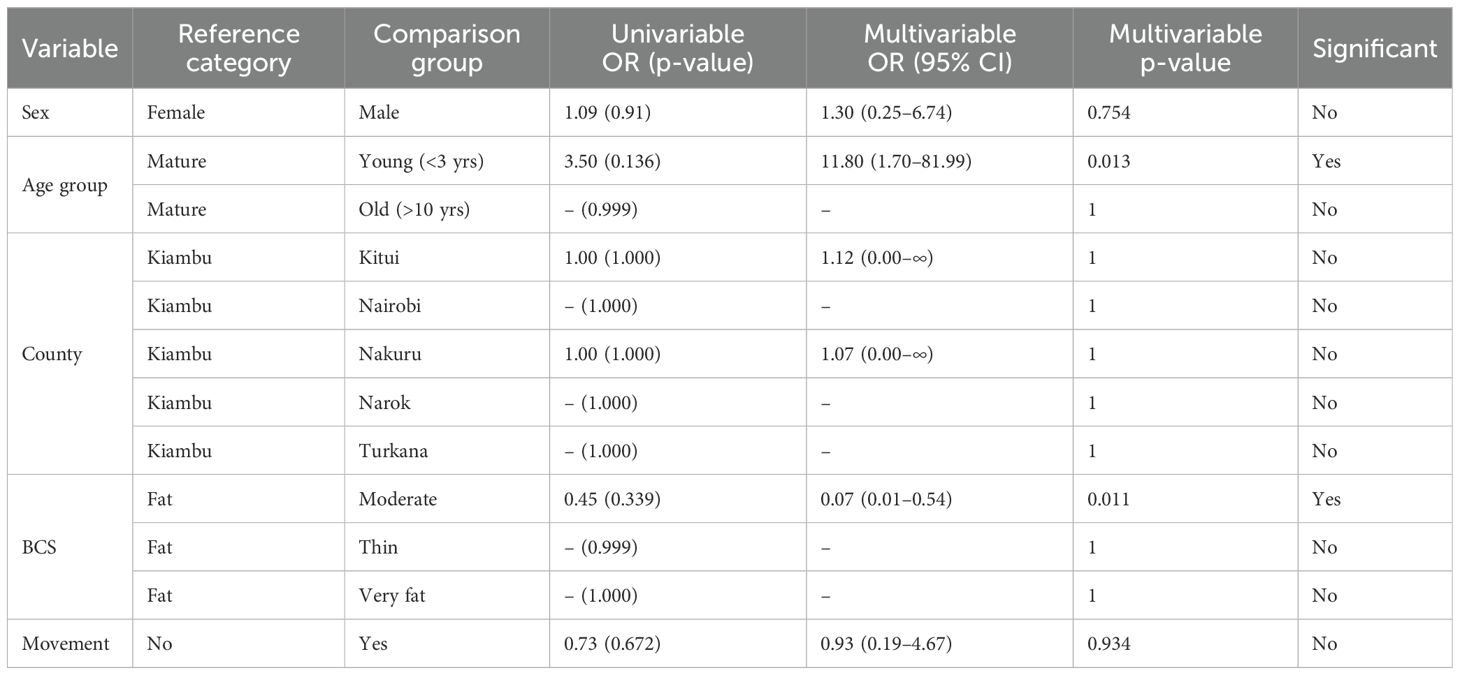
In univariable analysis, none of the variables reached statistical significance. However, younger donkeys (below 3 years) showed higher odds of testing positive (OR = 3.5; p = 0.136), suggesting possible recent exposure or age-related susceptibility. Seropositivity clustered in Turkana, Narok, and Nairobi counties, but the odds ratios were unstable due to zero cases in other counties. Movement history and BCS showed no significant association.
Multivariable logistic regression was conducted including all the variables. Only age group remained statistically significant: young donkeys had an adjusted odds ratio (aOR) of 11.8 (95% CI: 1.70–81.99; p = 0.013), confirming that younger age was independently associated with higher risk. Body condition (moderate vs. fat) also showed significance (aOR = 0.07; p = 0.011), though caution is needed due to small sample sizes in some BCS categories.
All other variables (sex, county, movement) were not significant in the multivariable model. The findings highlight young age as the strongest and most consistent predictor of Brucella exposure in donkeys (Table 4).
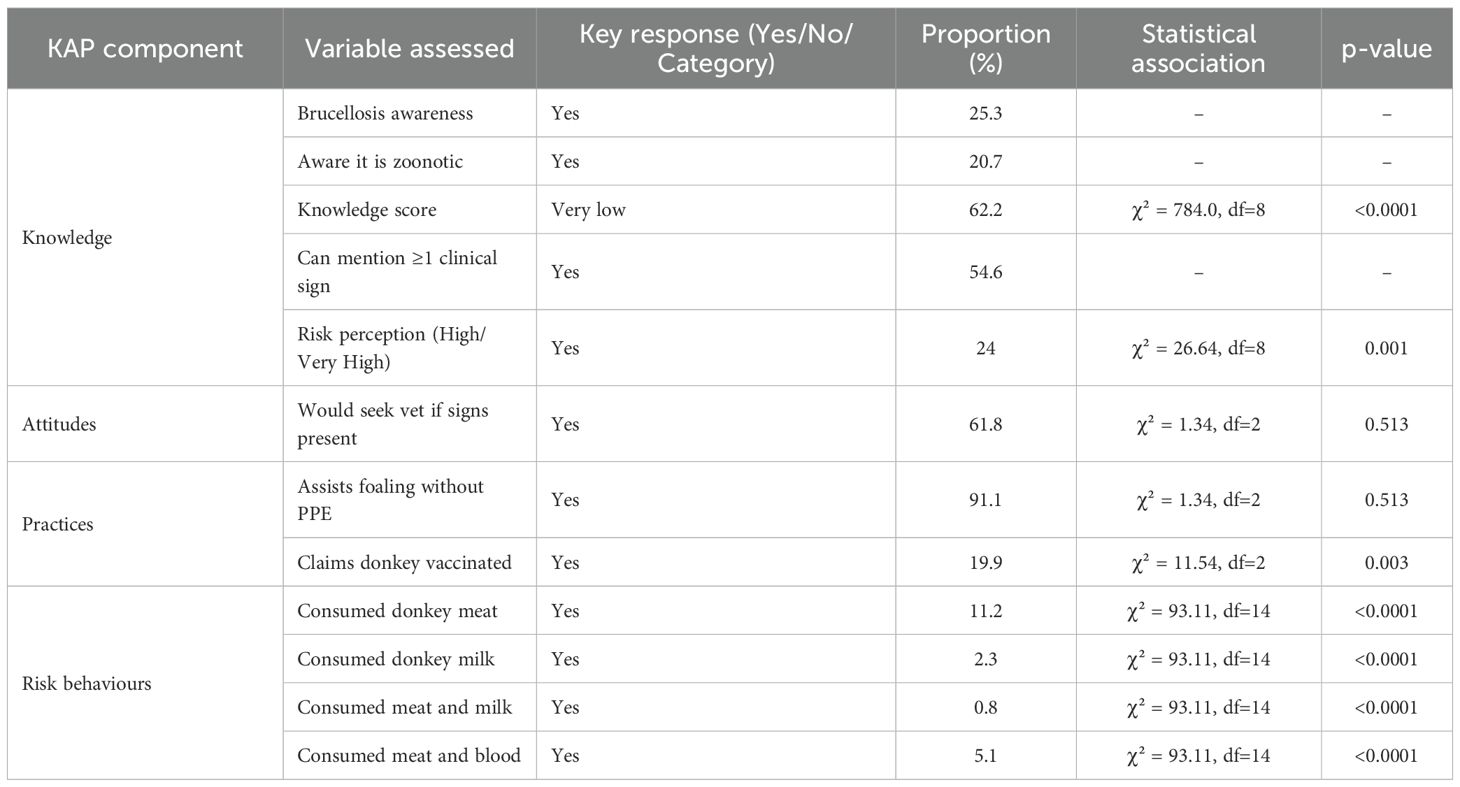
3.5 Knowledge, attitude, and practices of donkey owners on brucellosis
A cross-sectional survey involving 392 donkey owners across seven counties in Kenya was conducted to evaluate their knowledge, attitudes, and practices (KAP) related to brucellosis a neglected but important zoonotic disease. The demographic profile of the respondents showed an even gender distribution (50% male and 50% female), with the majority aged between 19 and 49 years. Educational attainment was generally low: 69% had only completed primary school, 28.1% had secondary education, and less than 4% had attained post-secondary qualifications. This background provides important context for understanding the observed knowledge and behavioural gaps.
The level of awareness about brucellosis among donkey owners was strikingly low. Only 25.3% had ever heard of the disease, and fewer (20.7%) recognized its potential to transmit to humans. Just over half (54.6%) could identify at least one clinical sign suggestive of the disease in donkeys, such as abortion or infertility. When knowledge was measured using a structured scoring scale, the majority (62.2%) fell into the “very low” category, and only 2.0% attained a “very high” score. This lack of knowledge was significantly associated with several factors. Higher education levels were positively linked to better understanding (χ² = 17.57, p = 0.007), as were county of residence (χ² = 52.78, p < 0.001), awareness of donkey vaccination history (χ² = 11.54, p = 0.003) and movement history (χ² = 10.35, p = 0.006). Variables such as respondent age and gender showed no statistically significant associations with knowledge levels.
Attitudes toward brucellosis risk were similarly concerning. A significant proportion of owners underestimated the potential impact of the disease, with 63.5% rating it as a “low” or “very low” risk. Only 24% of respondents perceived brucellosis as a “high” or “very high” threat. Individuals who had previously observed signs suggestive of brucellosis in their donkeys (such as abortion or joint swelling) were more likely to report a willingness to seek veterinary care (61.8%) compared to those without such experiences (28.9%). This suggests that firsthand experience, rather than formal knowledge, plays a major role in shaping risk perception and response behaviour.
Risky practices were widespread across the study population. An overwhelming 91.1% of owners reported assisting donkeys during foaling without any personal protective equipment, and none reported routine use of disinfectants during animal handling or within donkey shelters. These practices greatly increase the risk of zoonotic transmission, especially in the absence of formal training or veterinary oversight. About 19.9% of owners claimed their donkeys had been vaccinated against brucellosis, although no such vaccine is currently licensed or administered for donkeys in Kenya. This likely reflects a misunderstanding or confusion with other livestock vaccination programs.
Consumption of donkey products also emerged as a potential route of human infection. While not widespread, a notable proportion of respondents reported consuming donkey meat (11.2%), donkey blood (5.1%), and raw milk (2.3%). Others had consumed combinations of these products, further compounding risk. Such practices, when combined with poor hygiene and low awareness, pose a significant public health threat.
Environmental factors further exacerbated these risks. Donkeys were commonly reported to share grazing areas (84.2%), water sources (79.5%), and shelters (62.3%) with other livestock, particularly cattle, goats, and sheep. These close interspecies interactions, in the absence of routine health checks and preventive measures, create an ideal environment for the transmission of brucellosis within and between herds (Table 5).
4 Discussion
This study provides the first comprehensive One Health oriented investigation of Brucella spp. seroprevalence in donkeys across seven diverse Kenyan counties namely Turkana, Kitui, Narok, Nakuru, Bungoma, Nairobi, and Kiambu, integrating serological data with donkey owner Knowledge–Attitudes–Practices (KAP). Donkeys, while integral to livelihoods in pastoral, peri-urban, and rural settings, remain largely excluded from brucellosis surveillance and control efforts (28, 29).
The marked disparity in seroprevalence, 2.0% by indirect iELISA compared with 10.7% by RBPT, with almost no agreement (κ ≈ 0), highlights the well-recognised limitations of RBPT, particularly its lower specificity and potential cross-reactivity with non-Brucella bacteria. In contrast, iELISA has been validated as a more specific assay in equids and other livestock and is therefore recommended for epidemiological studies (28, 30). In this study, iELISA-derived seroprevalence is considered the more reliable estimate of Brucella seroprevalence in donkeys, while RBPT findings are best interpreted as preliminary screening. Similar test discrepancies have been reported in livestock across East Africa, further underscoring the importance of confirmatory testing. For example, in Amibara, Ethiopia, camel brucellosis prevalence was 7.6% by RBPT but only 3.2% when confirmed by the complement fixation test (31). Likewise, in Kajiado County, Kenya, iELISA outperformed RBPT in detecting bovine seropositive animals (32). These findings strengthen the case for prioritizing iELISA or other confirmatory assays when assessing brucellosis in donkeys.
Despite identifying 42 seropositive donkeys, none yielded positive PCR results for Brucella abortus or B. melitensis. Similar discrepancies between serological and molecular findings have been reported elsewhere, including studies in Ethiopia where livestock that tested seropositive by RBPT or ELISA were PCR-negative when only blood samples were analyzed, even though Brucella organisms were later isolated from reproductive tissues and lymph nodes of the same animals (33). Such variation between serology and PCR outcomes can be attributed to both biological and methodological factors. Serological positivity often reflects previous exposure or infection that has already been cleared bacteriologically, resulting in the persistence of antibodies despite the absence of detectable bacteria in circulation (34). In addition, during chronic or latent stages of brucellosis, bacteraemia is typically intermittent or absent, and Brucella organisms tend to localize in reproductive organs, lymph nodes, or bone marrow rather than in peripheral blood (35). The possibility of intermittent bacteremia, particularly in chronic infections, further reduces the likelihood of detecting Brucella DNA in single blood samples and may partly explain the PCR negativity observed in this study. Therefore, blood may not always be the most suitable specimen for molecular detection of Brucella DNA, particularly when bacterial loads are low or the infection is inactive at the time of sampling (36). Indeed, reviews have reported that brucellosis in equines (horses, donkeys, and mules) presents as abscesses in tendons, bursae, and joints, while rreproductive disorders seen in other livestock are rare occurrence (10). The limited sensitivity of conventional PCR assays, coupled with low bacterial concentrations in peripheral blood, likely contributed to the absence of detectable DNA in this study. Future investigations should therefore include alternative specimens such as reproductive tissues, milk, vaginal swabs, and lymph nodes and employ more sensitive diagnostic approaches, including real-time PCR or culture-based techniques, to improve detection and confirm active infection (33). But it is also argued that tests currently used in the diagnosis of brucellosis in other livestock have not been validated for equines (10). Globally, donkey brucellosis seroprevalence varies from 0% to 65%, with a pooled mean of approximately 10.3% (17). Our ELISA-derived prevalence aligns with reports from Nigeria (2.5–11.4%) (37, 38) but is lower than values reported in other African contexts such as Ethiopia (14.5%) (39). These differences likely reflect variation in diagnostic approaches, ecological conditions, animal movement, and national control measures (40).
Age was a significant independent predictor of seropositivity, with donkeys under three years old being over thirteen times more likely to test positive than adults (aOR = 13.21, p = 0.008). This observation, consistent with findings by Jansen et al. (41), differs from the classical pattern in many livestock species, where seroprevalence typically rises with sexual maturity and reproductive activity (42). Several explanations may account for this deviation. Younger donkeys may experience early exposure through communal grazing and shared watering points, which are well-known pathways for cross-species transmission of Brucella in pastoral systems (43). For example, in Nigeria, A study done to determine prevalence of equine brucellosis reported a higher seroprevalence of 9.6% for donkeys’ aged 4–6 years which could explain the high rate of infection in young donkeys; this was in comparison to 6.8% for pregnant donkey’s and 3.8% for non-pregnant ones (44). Therefore, young donkeys grazed within infected environments may be exposed to infections by Brucella organisms. Infection may also occur through ingestion of contaminated maternal milk or close nursing contact, since Brucella spp. can be shed in milk and persist within dairy secretions (41). Additionally, variation in maternal antibody transfer and its gradual decline can affect serological detection, as foals acquire antibodies solely through colostrum and these wane over time, complicating interpretation of seropositivity in very young animals (45). Management practices such as young donkeys accompanying their dams and other livestock to communal grazing areas may further increase their contact with infectious materials (46). Given the small number of iELISA-positive animals, the wide 95% confidence interval around the odds ratio suggests statistical instability, and the observed association should therefore be interpreted with caution. To clarify these uncertainties, larger age-stratified and longitudinal studies combining serology with culture or molecular testing of milk, reproductive tissues, and environmental samples are recommended, alongside measurements of maternal antibody dynamics to distinguish passive immunity from true infection. This pattern mirrors findings in goats in Qatar (47) but contrasts with reports in Nigerian donkeys, where older animals showed higher prevalence (38). A possible explanation is that younger animals may be more susceptible due to immature immune systems or acute infections generating stronger antibody responses, whereas older animals may have declining antibody titre following past exposure (34, 35).
Interestingly, donkeys with moderate body condition had significantly lower odds of seropositivity compared to those in good condition (aOR = 0.081, 95% CI: 0.011–0.57, p = 0.012). Although counterintuitive, similar associations have been attributed to differences in mobility, workload, and exposure opportunities (48). However, the small number of seropositive cases within these subgroups may have influenced the association, and the finding should therefore be interpreted with caution until verified in larger studies.
Geographical clustering of iELISA-positive cases in Turkana (3.96%), Narok (4.84%), and Nairobi (3.45%) suggests spatial heterogeneity in transmission risk. Such patterns have also been reported in Algeria, where pastoral and peri-urban environments with intense interspecies interactions were linked to higher prevalence (40). In our study, over 80% of donkey owners reported shared grazing and watering points with cattle, goats, and sheep; well-recognized risk factors for cross-species transmission of Brucella spp. (49–51). However, the relatively small and uneven sample sizes across counties particularly in Nairobi (n = 29) limit the robustness of inter-county comparisons and may exaggerate apparent clustering. Larger, more evenly distributed sampling will be needed to confirm true geographical patterns.
The KAP survey revealed critical public health gaps. Most respondents (91.1%) assisted donkeys during foaling without protective equipment, and 19.4% consumed donkey products often raw, mirroring risky practices reported in Ethiopia and northern Kenya (29, 39, 52). Awareness of brucellosis was low, with only 25.3% having heard of the disease and 20.7% recognizing its zoonotic potential, similar to patterns reported across pastoral regions in Africa and the Middle East (53, 54). Notably, 19.9% of respondents reported vaccinating donkeys against brucellosis, despite no official vaccination ever recorded for the donkeys in Kenya, suggesting confusion with cattle vaccination or misinterpretation of the question. Such findings underscore possible biases in self-reported data, including recall errors, misunderstanding of questions, and social desirability bias, which should be considered when interpreting the results.
In Kenya, brucellosis surveillance has predominantly focused on ruminants, with prevalence estimates reaching 19.5% in camels in Isiolo County (29), while donkeys remain largely excluded from national disease reporting frameworks. Yet, their close and frequent contact with humans, particularly women and youth engaged in transport and animal husbandry, suggests that donkeys could represent overlooked link in the epidemiology of brucellosis. Although equids are generally regarded as incidental hosts for Brucella spp., the concurrent circulation of B. abortus and B. melitensis among multiple livestock species and humans in Kenyan pastoral systems, coupled with shared grazing areas, water sources, and close interspecies interactions, makes it plausible that donkeys may function as transient carriers or “bridge” hosts facilitating spill-over between species. Direct evidence demonstrating that donkeys independently maintain Brucella transmission is, however, still lacking (10, 55a; 12). Brucella in equines has previously been reported in cattle farms in Minas Gerais, a state in Brazil where bovine brucellosis was widespread (56). This infection of donkeys could support the hypothesis of cross infection especially if the environment is contaminated birth products by the Brucella spp. However, other studies conducted to test this hypothesis that presence of other farm animals that commingled with herds and/or flocks are potential hosts that could maintain the disease in the farm. In this study, 10 donkeys and 2 dogs which were in close proximity to cattle herds raised in areas which was known to have endemic brucellosis for ruminants did not show evidence of cross infection between donkeys dogs and the other ruminants (57). To date, there is also no evidence of direct donkey-to-human transmission of Brucella spp., which underscores their likely incidental rather than reservoir role and defines the current epidemiological limits of transmission (58). Targeted research incorporating bacterial isolation and molecular typing from reproductive tissues, milk, and environmental samples is therefore needed to determine whether donkeys can sustain local transmission cycles or simply serve as occasional spill-over hosts within multi-host ecosystems.
From a diagnostic perspective, our findings highlight the limitations of relying solely on blood-based PCR in donkeys. Incorporating more sensitive real-time PCR assays, reproductive tissue sampling, or bacteriological culture could improve detection, particularly in chronic infections (35, 36).
This study reinforces the need to include donkeys in brucellosis surveillance and control programs. Targeted public health messaging, improved diagnostic strategies, and community engagement should form part of integrated One Health interventions to mitigate both animal health and zoonotic risks.
5 Conclusion
This study provides the first integrated assessment of serological, molecular, and behavioural aspects of donkey brucellosis in Kenya. We report a low seroprevalence of 2.0% by iELISA, with RBPT screening indicating a higher but less specific estimate of 10.7%. Confirmed seropositive cases were clustered in Turkana, Narok, and Nairobi. No Brucella DNA was detected in blood samples by PCR, likely reflecting chronic or past infections with minimal or absent bacteraemia. Younger donkeys and those in good body condition had higher odds of seropositivity though these associations should be interpreted with caution given wide confidence intervals and small subgroup sizes. Widespread high-risk practices, such as unprotected foaling assistance and consumption of raw donkey products, were documented, yet awareness of the zoonotic potential of brucellosis remained critically low.
These findings provide evidence of past exposure to Brucella spp. in donkeys rather than confirmed active infection. They underscore the need to include donkeys in national brucellosis surveillance systems, strengthen community education, and expand diagnostic capacity. Embedding these measures within a coordinated One Health framework will be essential to sustain progress and to reduce both the animal health burden and the risk of zoonotic transmission.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal studies were approved by Ethical clearance for this study was obtained from the Biosafety, Animal Use, and Ethics Committee of the Faculty of Veterinary Medicine, University of Nairobi, under reference number BAUEC/2024/549 (Appendix 9); National Commission for Science, Technology and innovation Licence No: NACOSTI/P/24/36654 (Appendix 10). Additional ethical clearance was obtained from Brooke’s Animal Welfare Ethical Review Board (Appendix 11), and formal permission to carry out the study was granted by the respective County Directors of Veterinary Services and local administrative authorities in each study area. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
JK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. TW: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – review & editing. JO: Conceptualization, Data curation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by Brooke East Africa under the donkey welfare program funding reference No BHAKEN 06/08/2024. The research was conducted under the University of Nairobi registration number JPH81/61956/2023. The funder had no role in the study design, data collection, analysis, interpretation, or manuscript preparation.
Acknowledgments
We thank the donkey owners and county veterinary officers in the seven study counties for their cooperation. Appreciation goes to the University of Nairobi, Faculty of Veterinary Medicine, Department of Veterinary Public Health, Pharmacology and Toxicology Laboratory teams led by Mr Alfred Mainga for technical and laboratory support. We also acknowledge Brooke East Africa field staff and all data collectors for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ravichandran T, Perumal RK, Vijayalakshmy K, Raw Z, Cooke F, Baltenweck I, et al. Means of livelihood, clean environment to women empowerment: the multi-faceted role of donkeys. Animals. (2023) 13:1927. doi: 10.3390/ani13121927
2. Norris SL, Little HA, Ryding J, and Raw Z. Global donkey and mule populations: Figures and trends. PloS One. (2021) 16:e0247830. doi: 10.1371/journal.pone.0247830
3. Pearson RA, Smith DG, Alemayehu M, and Shiferaw Y. Managing the working donkey in Ethiopia to assist poor people make the most of their resources. Proc Br Soc Anim. Sci. (2005) 2005:34–4. doi: 10.1017/S1752756200009455
4. KNBS. Kenya national population and housing census. Nairobi, Kenya: Kenya Government Printer (2019). 366–86.
5. Gichure M, Onono J, Wahome R, and Gathura P. Analysis of the benefits and production challenges of working donkeys in smallholder farming systems in Kenya. Vet World. (2020) 13:2346–52. doi: 10.14202/vetworld.2020.2346-2352
6. Proops L, Osthaus B, Bell N, Long S, Hayday K, and Burden F. Shelter-seeking behavior of donkeys and horses in a temperate climate. J Vet Behav. (2019) 32:16–23. doi: 10.1016/j.jveb.2019.03.008
7. Altissimi C, Noé-Nordberg C, Ranucci D, and Paulsen P. Presence of foodborne bacteria in wild boar and wild boar meat—A literature survey for the period 2012–2022. Foods. (2023) 12:1689. doi: 10.3390/foods12081689
8. Bamaiyi PH, Hassan L, Khairani-Bejo S, ZainalAbidin M, Ramlan M, Adzhar A, et al. The prevalence and distribution of Brucella melitensis in goats in Malaysia from 2000 to 2009. Prev Vet Med. (2015) 119:232–6. doi: 10.1016/j.prevetmed.2015.02.001
9. Djangwani J, Ooko Abong’ G, Gicuku Njue L, and Kaindi DWM. Brucellosis: Prevalence with reference to East African community countries – A rapid review. Vet Med Sci. (2021) 7:851–67. doi: 10.1002/vms3.425
10. Dorneles EMS, Santana JA, Costa A.C.T.R.B., Junqueira DG, Heinemann MB, and Lage AP. Equine brucellosis: current understanding and challenges. J Equine Vet Sci. (2023) 127:104298. doi: 10.1016/j.jevs.2023.104298
11. Akoko J, Mwatondo A, Muturi M, Wambua L, Abkallo HM, Nyamota R, et al. Mapping brucellosis risk in Kenya and its implications for control strategies in sub-Saharan Africa. Sci Rep. (2023) 13:20192. doi: 10.1038/s41598-023-47628-1
12. Muema J, Oboge H, Mutono N, Makori A, Oyugi J, Bukania Z, et al. Sero – epidemiology of brucellosis in people and their livestock: A linked human – animal cross-sectional study in a pastoralist community in Kenya. Front Vet Sci. (2022) 9:1031639. doi: 10.3389/fvets.2022.1031639
13. Munyua P, Osoro E, Hunsperger E, Ngere I, Muturi M, Mwatondo A, et al. High incidence of human brucellosis in a rural Pastoralist community in Keny. PloS Negl Trop Dis. (2021) 15:e0009049. doi: 10.1371/journal.pntd.0009049
14. Kahariri SM, Kitala PM, Muchemi GM, Njenga K, and Nanyingi M. Sero-prevalence and risk factors for human brucellosis in Marsabit county, Kenya, (2014). PAMJ - One Health. (2021) 4(9):1–14. doi: 10.11604/pamj-oh.2021.4.9.27024
15. Waringa NMA, Waiboci LW, Bebora L, Kinyanjui PW, Kosgei P, Kiambi S, et al. Human brucellosis in Baringo County, Kenya: Evaluating the diagnostic kits used and identifying infecting Brucella species. PloS One. (2023) 18:e0269831. doi: 10.1371/journal.pone.0269831
16. Foddai A, Kelly L, McGiven J, Grace K, and Evans S. Quantitative assessment of the probability of introducing bovine brucellosis into English cattle herds by imported live cattle. Microb Risk Anal. (2020) 16:100130. doi: 10.1016/j.mran.2020.100130
17. Kithuka JM, Wachira TM, Onono JO, and Ngetich W. The burden of brucellosis in donkeys and its implications for public health and animal welfare: A systematic review and meta-analysis. Vet World. (2025) 18(2):367–78. doi: 10.14202/vetworld.2025.367-378
18. Megersa B, Biffa D, Niguse F, Rufael T, Asmare K, and Skjerve E. Cattle brucellosis in traditional livestock husbandry practice in Southern and Eastern Ethiopia, and its zoonotic implication. Acta Vet Scand. (2011) 53:24. doi: 10.1186/1751-0147-53-24
19. Njeru J, Wareth G, Melzer F, Henning K, Pletz MW, Heller R, et al. Systematic review of brucellosis in Kenya: disease frequency in humans and animals and risk factors for human infection. BMC Public Health. (2016) 16:853. doi: 10.1186/s12889-016-3532-9
20. Memish ZA and Balkhy HH. Brucellosis and international travel. J Travel Med. (2004) 11:49–55. doi: 10.2310/7060.2004.13551
21. Njeru J, Nthiwa D, Akoko J, Oyas H, and Bett B. Incidence of Brucella infection in various livestock species raised under the pastoral production system in Isiolo County, Kenya. BMC Vet Res. (2021) 17:342. doi: 10.1186/s12917-021-03036-z
22. Osoro EM, Munyua P, Omulo S, Ogola E, Ade F, Mbatha P, et al. Strong association between human and animal brucella seropositivity in a linked study in Kenya 2012–2013. Am Soc Trop Med Hyg. (2015) 93:224–31. doi: 10.4269/ajtmh.15-0113
23. Wayne D and Chad. Biostatistics: A foundation for Analysis in the health sciences. Danvers, Massachusetts, USA: John Wiley & Sons. Inc. (1999).
24. Zerva L, Bourantas K, Mitka S, Kansouzidou A, and Legakis NJ. Serum is the preferred clinical specimen for diagnosis of human brucellosis by PCR. J Clin Microbiol. (2001) 39:1661–4. doi: 10.1128/JCM.39.4.1661-1664.2001
25. Song K, Li Z, Kim T-H, Wang X, Bu Z, Li S, et al. Ultra-fast detection and differentiation of Brucella genus bacteria, B. abortus, B. melitensis and B. suis, using multiplex convection polymerase chain reaction. Hokkaido, Japan: Faculty of Veterinary Medicine, Hokkaido University (2019). doi: 10.14943/jjvr.67.1.51.
26. Gupta VK, Shivasharanappa N, Kumar V, and Kumar A. Diagnostic evaluation of serological assays and different gene based PCR for detection of Brucella melitensis in goat. Small Rumin. Res. (2014) 117:94–102. doi: 10.1016/j.smallrumres.2013.11.022
27. Mutnal MB, Purwar S, Metgud SC, Nagmoti MB, and Patil CS. PCR confirmation of cutaneous manifestation due to Brucella melitensis. J Med Microbiol. (2007) 56:283–5. doi: 10.1099/jmm.0.46927-0
28. Matope G, Muma JB, Toft N, Gori E, Lund A, Nielsen K, et al. Evaluation of sensitivity and specificity of RBT, c-ELISA and fluorescence polarisation assay for diagnosis of brucellosis in cattle using latent class analysis. Vet Immunol Immunopathol. (2022) 141:58–63. doi: 10.1016/j.vetimm.2011.02.005
29. Mwatondo A, Muturi M, Akoko J, Nyamota R, Nthiwa D, Maina J, et al. Seroprevalence and related risk factors of Brucella spp. in livestock and humans in Garbatula subcounty, Isiolo county, Kenya. PloS Negl Trop Dis. (2023) 17:e0011682. doi: 10.1371/journal.pntd.0011682
30. Loubet P, Magnan C, Salipante F, Pastre T, Keriel A, O’Callaghan D, et al. Diagnosis of brucellosis: Combining tests to improve performance. PloS Negl Trop Dis. (2024) 18:e0012442. doi: 10.1371/journal.pntd.0012442
31. Wegi FG, Amenu K, Chalchisa A, and Mamo G. Brucellosis in camels and humans: seroprevalence and associated risk factors in amibara district of afar region, Ethiopia. Vet Med Int. (2021) 2021:1–10. doi: 10.1155/2021/5482725
32. Odongo M, Bebora L, Gathumbi J, Aboge G, Waiboci L, and Erume J. Seroprevalence and spatial distribution of livestock brucellosis using three serological tests in Kajiado County, Kenya. Open Vet J. (2023) 13:1583. doi: 10.5455/OVJ.2023.v13.i12.8
33. Wakjira BS, Jorga E, Lakew M, Olani A, Tadesse B, Tuli G, et al. Animal brucellosis: seropositivity rates, isolation and molecular detection in southern and central Ethiopia. Vet Med Res Rep Volume. (2022) 13:201–11. doi: 10.2147/VMRR.S372455
34. Nielsen K. Diagnosis of brucellosis by serology. Vet Microbiol. (2002) 90:447–59. doi: 10.1016/S0378-1135(02)00229-8
35. Gwida MM, El-Gohary AH, Melzer F, Tomaso H, Rösler U, Wernery U, et al. Comparison of diagnostic tests for the detection of Brucella spp. in camel sera. BMC Res Notes. (2011) 4:525. doi: 10.1186/1756-0500-4-525
36. OIE. OIE reference laboratory reports activities activities in 2021. Surrey, UK: OIE Reference Laboratories (2021).
37. Adamu SG, Hassan M, and Ardo MB. Seroprevalence of Brucella antibodies in Donkeys (Equus asinus) in Yobe south senatorial zone, Northeastern Nigeria. J Equine Sci. (2020) 31:5–10. doi: 10.1294/jes.31.5
38. Nathaniel J. Seroprevalence of brucellosis in donkeys (Equus asinus) and assessment of donkey management practices in Gamawa local government area, Bauchi state, Nigeria. Lagos, Nigeria: Animal Science Association of Nigeria (2021).
39. Edao BM, Ameni G, Assefa Z, Berg S, Whatmore AM, and Wood JLN. Brucellosis in ruminants and pastoralists in Borena, Southern Ethiopia. PloS Negl Trop Dis. (2020) 14:e0008461. doi: 10.1371/journal.pntd.0008461
40. Lounes N, Melzer F, Sayour AE, Maamar HT, Rahal K, Benamrouche N, et al. Identification, geographic distribution and risk factors of Brucella abortus and Brucella melitensis infection in cattle in Algeria. Vet Microbiol. (2021) 254:109004. doi: 10.1016/j.vetmic.2021.109004
41. Jansen W, Demars A, Nicaise C, Godfroid J, De Bolle X, Reboul A, et al. Shedding of Brucella melitensis happens through milk macrophages in the murine model of infection. Sci Rep. (2020) 10:9421. doi: 10.1038/s41598-020-65760-0
42. Hussen AM, Alemu F, Hasan Hussen A, Mohamed AH, and Gebremeskel HF. Herd and animal level seroprevalence and associated risk factors of small ruminant brucellosis in the Korahey zone, Somali regional state, eastern Ethiopia. Front Vet Sci. (2023) 10:1236494. doi: 10.3389/fvets.2023.1236494
43. Bosco Ntirandekura J, Eliaimringi Matemba L, Iddi Kimera S, Bwayla Muma J, and Daniel Karimuribo E. Brucellosis and its associated risk factors to humans and domestic ruminants in Kagera Ecosystem, Tanzania. Afr. Health Sci. (2021) 21:523–30. doi: 10.4314/ahs.v21i2.6
44. Tijjani AO, Junaidu AU, Salihu MD, Farouq AA, Faleke OO, Adamu SG, et al. Serological survey for Brucella antibodies in donkeys of north-eastern Nigeria. Trop Anim. Health Prod. (2017) 49:1211–6. doi: 10.1007/s11250-017-1318-4
45. Eertink LG, Swope M, Uprety T, Sreenivasan C, Page AE, Adam EN, et al. Characteristics of maternal antibodies transferred to foals raised through maternal equine rotavirus A vaccination. Vet Microbiol. (2024) 299:110304. doi: 10.1016/j.vetmic.2024.110304
46. Tschopp R, GebreGiorgis A, Abdulkadir O, Molla W, Hamid M, Tassachew Y, et al. Risk factors for Brucellosis and knowledge-attitude practice among pastoralists in Afar and Somali regions of Ethiopia. Prev Vet Med. (2022) 199:105557. doi: 10.1016/j.prevetmed.2021.105557
47. Alhussain H, Gawish A, Zughaier S, Al-Zeyara AM, Yassine HM, Al Thani A, et al. Seroprevalence of small ruminant brucellosis in the state of Qatar. Vet Med Sci. (2024) 10:e1355. doi: 10.1002/vms3.1355
48. Jokar M, Rahmanian V, Golestani N, Raziee Y, and Farhoodi M. The global seroprevalence of equine brucellosis: A systematic review and meta-analysis based on publications from 1990 to 2022. J Equine Vet Sci. (2023) 123:104227. doi: 10.1016/j.jevs.2023.104227
49. Efrem GH, Mihreteab B, Ghebremariam MK, Okbamichael T, Ghebresilasie Y, Mor SM, et al. Prevalence of brucellosis and associated risk factors in dairy cattle in Maekel and Debub Regions, Eritrea. Front Vet Sci. (2023) 10:1177572. doi: 10.3389/fvets.2023.1177572
50. Robi DT, Urge B, Bogale A, Aleme M, and Temteme S. Herd and animal level seroprevalence and associated risk factors of bovine brucellosis in different agro-ecologies of southwest Ethiopia. Heliyon. (2023) 9:e16852. doi: 10.1016/j.heliyon.2023.e16852
51. Sadiq MA, Tijjani A-N, Auwal MS, Mustapha AR, and Gulani I. Serological Prevalence of Brucellosis among Donkeys (Equus asinus) in Some Local Government Areas of Yobe State. Equine Vet Sci. (2013) 33:150–4. doi: 10.1016/j.jevs.2012.05.071
52. Wainaina M, Aboge GO, Omwenga I, Ngaywa C, Ngwili N, Kiara H, et al. Detection of Brucella spp. in raw milk from various livestock species raised under pastoral production systems in Isiolo and Marsabit Counties, northern Kenya. Trop Anim. Health Prod. (2020) 52:3537–44. doi: 10.1007/s11250-020-02389-1
53. Abbasi-Ghahramanloo A, Ebrahimoghli R, Ebrahimnejad M, Gholizadeh N, and Moradi-Asl E. Knowledge, attitudes, and practices regarding brucellosis in a rural population: A cross-sectional study. Heliyon. (2024) 10:e28041. doi: 10.1016/j.heliyon.2024.e28041
54. Alqahtani YA, Shati AA, Al-Qahtani SM, Asseri AA, Alhanshani AA, Alqahtani FM, et al. Knowledge, attitudes, and practices regarding brucellosis among parents in aseer region, southwestern Saudi Arabia. Healthcare. (2021) 9:1541. doi: 10.3390/healthcare9111541
55. Akoko JM, Pelle R, Lukambagire AS, Machuka EM, Nthiwa D, Mathew C, et al. Molecular epidemiology of Brucella species in mixed livestock-human ecosystems in Kenya. Sci Rep. (2021) 11:8881. doi: 10.1038/s41598-021-88327-z
56. Junqueira DG, Dorneles EMS, Salvador Picão Gonçalves V, Santana JA, de Andrade Almeida VM, Nicolino RR, et al. Brucellosis in working equines of cattle farms from Minas Gerais State, Brazil. Prev Vet Med. (2015) 121:380–5. doi: 10.1016/j.prevetmed.2015.06.008
57. Diez JG, Coelho AM, and Anna C. Brucellosis prevalence in dogs and donkeys commingled with large and small ruminants. Berlin, Germany: ResearchGates (2015).
Keywords: brucellosis, donkeys, Kenya, One Health, zoonosis
Citation: Kithuka JM, Wachira TM and Onono JO (2025) Seroprevalence, molecular detection, risk factor analysis and public health perceptions of brucellosis in donkeys across different production systems in Kenya. Front. Trop. Dis. 6:1694016. doi: 10.3389/fitd.2025.1694016
Received: 27 August 2025; Accepted: 08 October 2025;
Published: 22 October 2025.
Edited by:
Lucas Sousa Magalhães, Federal University of Alagoas, BrazilReviewed by:
Ehsan Javanmard, Tehran University of Medical Sciences, IranYahia Achour, Universite Saad Dahlab Blida 1, Algeria
Copyright © 2025 Kithuka, Wachira and Onono. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: James Mutiiria Kithuka, amFtZXNta2l0aHVrYUBnbWFpbC5jb20=
†ORCID: James Mutiiria Kithuka, orcid.org/0009-0004-8071-056X
Timothy Muthui Wachira, orcid.org/0009-0004-8071-056X
Joshua Orungo Onono, orcid.org/0000-0003-4245-1232
 James Mutiiria Kithuka
James Mutiiria Kithuka Timothy Muthui Wachira†
Timothy Muthui Wachira† Joshua Orungo Onono
Joshua Orungo Onono