- 1Centre Interdisciplinaire de Recherches Médicales de Franceville, Franceville, Gabon
- 2EpiPointe, Cary, NC, United States
- 3National institute of Allergy and Infectious Diseases, Bethesda, MD, United States
Editorial on the Research Topic
Enhancing global access to diagnostic tools for emerging tropical diseases in resource-limited settings
The persistent emergence and re-emergence of tropical diseases represent a major global health challenge, particularly in regions with fragile health systems and constrained diagnostic capabilities (1). The COVID-19 pandemic has starkly underscored that timely access to reliable diagnostics is a cornerstone of effective outbreak response and control (2, 3). However, despite this global lesson, a critical diagnostic gap persists for numerous infections. This is especially true for arboviruses, parasitic infections, and neglected tropical diseases, which lack adequate surveillance strategies and diagnostic tools (4). This Research Topic aims to address this disparity by highlighting original research and comprehensive reviews that investigate these diagnostic challenges across diverse geographical and epidemiological settings. The collective findings will provide valuable insights and strategies for strengthening diagnostic capacities in resource-limited environments.
Appropriate diagnostics at the human-vector interface are important
Effective diagnostics at the human-vector interface are essential for detecting and interrupting the transmission cycles of vector-borne diseases. This may require the detection of pathogens in atypical biological matrices, which frequently presents a diagnostic challenge. A notable example is demonstrated with the Zika virus outbreak in Brazil, where Ferraz et al. analyzed semen samples from sperm banks. Their research revealed that, although serological screening is common, it does not reliably predict viral shedding in semen. This finding further highlights the significant risk of relying exclusively on antibody tests in reproductive health settings, and emphasizes the potential benefit of other approaches to diagnostics such as the use of molecular assays (i.e., Nucleic Acid Amplification Tests) for managing the Zika outbreak. That is, it is critical to employ the appropriate diagnostic matched to the corresponding clinical matrix to effect positive public health objectives.
Beyond human diagnostics, surveillance at the vector level presents its own complexities. In a comprehensive review of mosquito-borne diseases in East African urban areas, Joseph et al. documented the widespread co-occurrence of Aedes, Culex, and Anopheles species. A key insight from their work is that the lack of standardized definitions for ‘urban’ environments hinders the comparability of entomological data. They consequently advocate harmonized surveillance protocols and the application of molecular xenomonitoring detecting pathogen DNA or RNA in vectors to accurately assess and monitor vectorial capacity across diverse ecological settings.
Lessons from the epidemic response
Diagnostics are critically important during acute epidemics, where the speed and accuracy of the public health response are paramount. An after-action review of the 2023 dengue epidemic in Burkina Faso by Diao et al. illustrates this complex reality. While the country demonstrated improved preparedness compared to previous outbreaks, the review identified persistent, critical gaps in leadership, strategic planning, and laboratory logistics. This analysis underscores a fundamental lesson: sustainable access to diagnostics is inextricably linked to robust governance, effective coordination, and resilient supply chains.
The challenge of diagnostic reliability extends beyond systemic issues with the public health enterprise, and can include the biological characteristics of pathogens themselves. Chukwudi et al. documented this phenomenon in their study on rapid diagnostic tests (RDTs) for human African trypanosomiasis (HAT) in Nigeria. They found that despite the practical advantages of RDTs, their sensitivity was compromised by the local antigenic diversity of Trypanosoma brucei strains, leading to several false-negative results in confirmed cases. Their study highlights a broader implication, diagnostic tools developed for a pathogen could depend on the geographical or epidemiological context. One cannot assume an assay will be universally effective in detecting a given pathogen, especially if it was not specifically designed for inclusivity of known divergence of strains. Further, it emphasizes the importance of rigorous validation against local circulating strains to ensure clinical utility.
Strengthening laboratory systems to ensure sustained diagnostic access
Building resilient diagnostic access requires structural investments that extend beyond disease-specific innovations to strengthen entire laboratory systems. A compelling case study by Kohar et al. examines Liberia’s systematic efforts to rebuild its national diagnostic network following decades of conflict and the devastating Ebola outbreak. Their research documents how strategic reforms in governance, specialized workforce training, and the implementation of robust quality management systems led to measurable improvements in diagnostic capacity. Crucially, however, the authors emphasize that sustaining these hard-won gains is contingent upon three interdependent factors-continuous financial investment, full integration of laboratory data into national surveillance systems, and unwavering long-term political commitment. This analysis underscores that systemic diagnostic resilience is not a one-time achievement but a continuous process dependent on foundational health system support.
Cross-cutting lessons and future perspectives
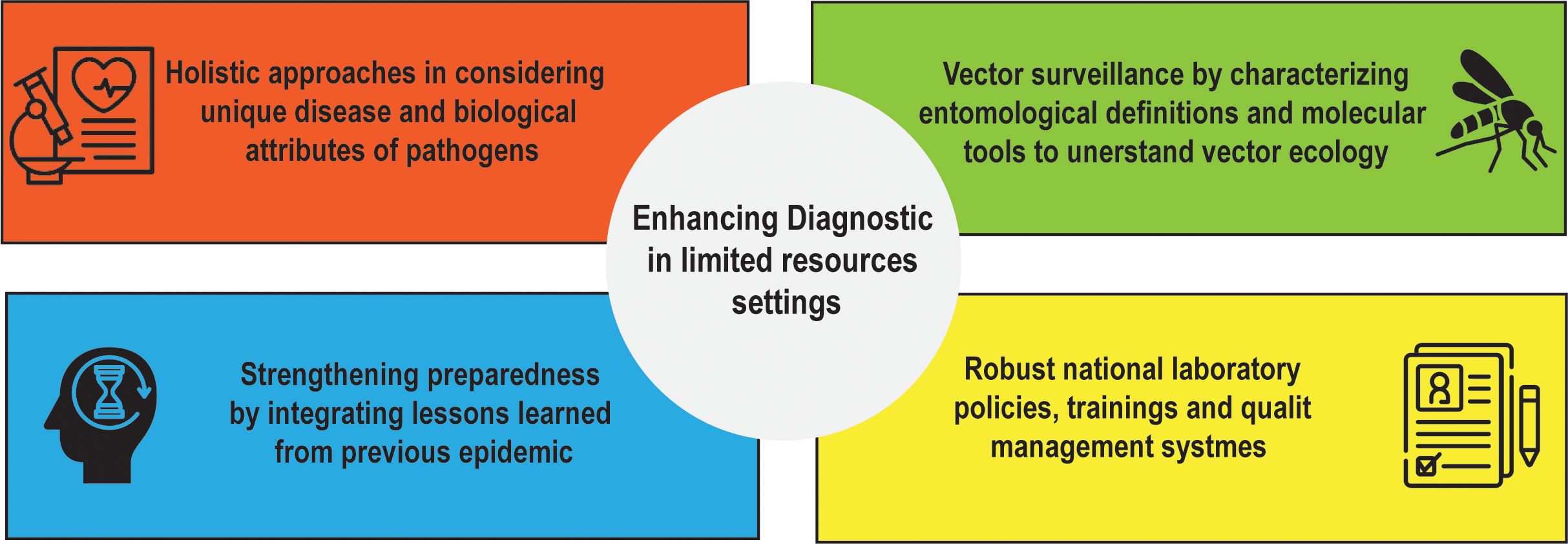
Taken together, the contributions to this Research Topic highlight four interdependent lessons (Figure 1):
1. Assay approaches must be holistic in considering unique disease and biological attributes of pathogens. As demonstrated with Zika virus semen testing, the appropriate diagnostic technology matched to an unconventional sample type may be necessary for management of disease outbreak and accomplishing public health goals. With trypanosomiasis RDTs, diagnostic platforms should be validated against local epidemiological and genetic diversity of pathogens of interest prior to deployment.
2. Vector surveillance requires diagnostic innovation: standardized entomological definitions and molecular tools are needed to capture the complexities of urban vector ecology.
3. Preparedness relies on diagnostics being fully integrated with other aspects of public health systems. Tools alone are insufficient without strong governance, supply chains, funds and risk communication strategies.
4. Robust national laboratory policies, training and quality management systems are essential for sustained access to diagnostics.
These points are reinforced by broader literature. For example, frameworks for the rapid development of diagnostics during outbreaks emphasize iterative validation and integration with surveillance networks (5). Reviews of point-of-care diagnostics in settings with limited resources highlight both opportunities and persistent implementation challenges (6). While experiences with mobile health and AI-assisted diagnostic tools show promise, they also emphasize the need for local adaptation and user-centered design (7).
Conclusion
This Research Topic collectively demonstrates that improving diagnostic access for emerging tropical diseases demands a multifaceted approach that extends far beyond technological innovation alone. Achieving accessible diagnostics necessitates their seamless integration into broader health systems, rigorous validation across diverse ecological and epidemiological contexts, and the strengthening of laboratory governance and supply chains. Consequently, diagnostic accessibility is not just a technical hurdle that must be overcome but also an urgent public health need. Ultimately, closing the diagnostic gap and fortifying global health security will depend on sustained, integrated collaboration that unites assay developers, scientists, laboratory professionals, clinicians, and public health policymakers.
Author contributions
LB: Conceptualization, Validation, Visualization, Writing – original draft, Writing – review & editing. FP: Writing – review & editing. RS: Writing – review & editing. IM: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing.
Acknowledgments
We would like to acknowledge all authors who have contributed with high-quality original articles and very interesting reviews.
Conflict of interest
Author FP was employed by company EpiPointe.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Lond Engl. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
2. Mercer TR and Salit M. Testing at scale during the COVID-19 pandemic. Nat Rev Genet. (2021) 22:415–26. doi: 10.1038/s41576-021-00360-w
3. Peeling RW, Wedderburn CJ, Garcia PJ, Boeras D, Fongwen N, Nkengasong J, et al. Serology testing in the COVID-19 pandemic response. Lancet Infect Dis. (2020) 20:e245–9. doi: 10.1016/S1473-3099(20)30517-X
4. Wilder-Smith A, Gubler DJ, Weaver SC, Monath TP, Heymann DL, and Scott TW. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis. (2017) 17:e101–6. doi: 10.1016/S1473-3099(16)30518-7
5. Chaturvedi M, Köster D, Bossuyt PM, Gerke O, Jurke A, Kretzschmar ME, et al. A unified framework for diagnostic test development and evaluation during outbreaks of emerging infections. Commun Med. (2024) 4:263. doi: 10.1038/s43856-024-00691-9
6. Heidt B, Siqueira WF, Eersels K, Diliën H, van Grinsven B, Fujiwara RT, et al. Point of care diagnostics in resource-limited settings: A review of the present and future of PoC in its most needed environment. Biosensors. oct. (2020) 10:133. doi: 10.3390/bios10100133
Keywords: infectious diseases, diagnostics, laboratory sustainability, disease control, vector surveillance, limited resources settings
Citation: Boundenga L, Parekh FK, Soong R and Mombo IM (2025) Editorial: Enhancing global access to diagnostic tools for emerging tropical diseases in resource-limited settings. Front. Trop. Dis. 6:1716391. doi: 10.3389/fitd.2025.1716391
Received: 30 September 2025; Accepted: 02 October 2025;
Published: 15 October 2025.
Edited and reviewed by:
Alfonso J. Rodriguez-Morales, Fundacion Universitaria Autónoma de las Américas, ColombiaCopyright © 2025 Boundenga, Parekh, Soong and Mombo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Illich Manfred Mombo, bW9tYm8uaWxsaWNoQGdtYWlsLmNvbQ==
 Larson Boundenga
Larson Boundenga Falgunee K. Parekh
Falgunee K. Parekh Ricky Soong
Ricky Soong Illich Manfred Mombo
Illich Manfred Mombo