- Section of Parasitology, Department of Biology, Health, and Environment, Faculty of Pharmacy and Food Science, University of Barcelona, Barcelona, Spain
Leishmaniosis infection begins when a phlebotomine sand fly vector inoculates pathogenic protozoan parasites of the genus Leishmania into a mammalian host. In the case of Leishmania infantum, the domestic dog is considered to be the main parasite reservoir, and canine leishmaniosis (CanL) has a high mortality rate in untreated dogs. Hundreds of cases of human leishmaniosis (HL) are reported in the world each year, the incidence in Europe being relatively low. Leishmaniosis control is primarily focused on the dog, combining methods that prevent sand fly bites and boost host resistance to infection. However, these measures are only partially effective and new solutions need to be found. One of the main factors limiting CanL and HL control is the existence of a sylvatic Leishmania transmission cycle that interacts with the domestic cycle maintained by dogs. It is suspected that the main reservoir of infection in wildlife are rodents, whose expansion and rapid population growth worldwide is increasing the risk of human and zoonotic pathogen transfer. The aim of this review is therefore to analyze reports in the literature that may shed light on the potential role of rodents in the leishmaniosis transmission cycle in the Mediterranean area. Following the general methodology recommended for reviews, six databases (Google Scholar, Ovid, PubMed, Science Direct, Scopus and Web of Science) were explored for the period January 1995 to December 2020. The results extracted from 39 publications that met the established inclusion criteria were analyzed. It was found that 23 species of rodents have been studied in nine countries of the Mediterranean basin. Of the 3,643 specimens studied, 302 tested positive for L. infantum infection by serology, microscopy and/or molecular techniques.
Introduction
Leishmaniosis is a parasitic vector-borne disease caused by Leishmania spp. affecting humans and other mammals. In Europe, leishmaniosis (caused mainly by Leishmania infantum) is an emerging zoonosis, with 700 new cases appearing annually (1). Of the four Leishmania species present in the Mediterranean basin, L. infantum is predominant and the causative agent of the human form of visceral (VL), cutaneous (CL), and mucocutaneous leishmaniosis (MCL). The others are Leishmania major (North Africa and the Middle East; CL), Leishmania tropica (Greece, Turkey, the Middle East and North Africa; CL), and Leishmania donovani (Cyprus; VL and CL) (2, 3).
These Leishmania species are capable of spreading to new geographical areas that have sufficient numbers of suitable sand fly vectors and favorable ecological conditions (4).
In the Mediterranean basin and surrounding areas, where L. infantum is endemic (5–8), dogs are currently considered to be the main reservoir. Thanks to the application of molecular tools and serology, Leishmania has been detected in clinically healthy and seronegative mammals, not only dogs but also other domestic/peridomestic and wild mammal species, including rodents (9–13). The long list of potential reservoir hosts suggests that Leishmania can be transmitted to a diverse range of mammals through sand fly bites and that wild mammals can suffer frequent and non-specific infection (14). The abundance and widespread distribution of rodents, together with their longevity, which allows them to survive an entire sand fly season, makes them likely candidates for infection with Leishmania species, including L. infantum (15). Furthermore, rodents are known to remain asymptomatic carriers for very long periods (16–19). Consequently, it can be hypothesized that rodent populations, as well as other wild animals, can maintain the permanent circulation of the parasite in an endemic area.
Many interacting host species fulfill the criteria that define a reservoir of Leishmania (abundance; attracting and infecting sand flies; evidence of long-term infection at the individual or species level) (20). Nevertheless, their categorization as primary, secondary, or accidental reservoirs depends on local ecological and epidemiological conditions. Although, it has not been demonstrated that rodents (and wildlife in general) act as a reservoir for Leishmania, some species of rodents are known to contribute to maintaining the circulation of L. infantum in certain areas of southern Europe (11). Studies employing xenodiagnosis could help to determine the role of wildlife in the current epidemiology of leishmaniosis, as demonstrated in an outbreak in Fuenlabrada (Madrid) (21), where this approach incriminated lagomorphs as the source of human infection spread by phlebotomine sand flies. Unfortunately, these types of studies are difficult to carry out in wildlife.
The aim of this review is to provide an overview of studies dealing with the potential role of rodents in the life cycle of L. infantum and the current epidemiological status of leishmaniosis in the Mediterranean basin.
Materials and Methods
Search and Eligibility Criteria
A bibliographic search was carried out in the databases of Google Scholar, Ovid, Pubmed, ScienceDirect, Scopus, and Web of Science. The general terms “leishmania infantum,” “epidemiology,” and “detection” were used, together with the MeSH term “rodentia.” If the latter was not accepted, it was replaced by the general term “rodent.” The selected articles were those dealing with studies on rodent species as leishmaniosis reservoirs in the Mediterranean area. Other inclusion criteria were the language (English) and date of publication (between January 1, 1995 and December 31, 2020). This review was carried out essentially based on guidelines outlined in the study published in Research Synthesis Methods (22).
Restricting the review to studies published in English may be considered a limitation, as the Mediterranean area has a wide diversity of languages. Nevertheless, as all the studies included here have been published in indexed journals, their rigor is ensured. We have also referenced the four articles found in the search that have an abstract in English but were excluded from the review as otherwise they are written in Turkish (one) (23) and French (one) (24).
Statistics
The SPSS program, version 25.0 (SPSS, Chicago, IL, USA), and the GraphPad Prism Software program, version 8.0 (La Jolla, CA, USA) were used for the different statistical analyses performed. The normality of the different variables studied was verified with the Kolmogorov-Smirnov test (P ≥ 0.200). The differences in medians were compared using the Mann-Whitney U test (for two independent variables) or the Kruskal-Wallis test (for more than two independent variables), because they were non-parametric variables. The frequencies of the different variables studied were compared using the X2 test. In all cases, P-values < 0.05 were reported as statistically significant.
Results and Discussion
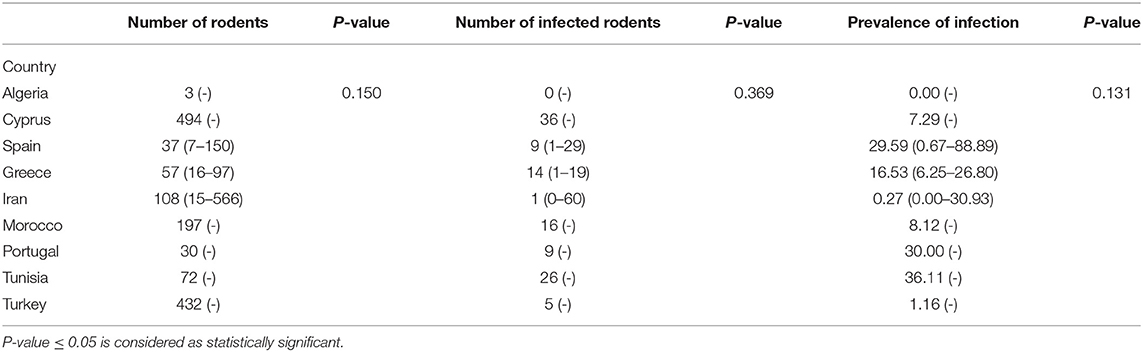
A total of 39 articles were included for review (11, 13, 14, 21, 25–59). The number of rodents examined in all the reported studies was 3,643, 302 of which were infected, implying an infection prevalence of 8.3%, (IC95% 7.4–9.2) (Supplementary Data Sheets 1, 2). Infection was detected in different sample types using molecular techniques, and only four studies also included serological methods [Dabaghmanesh et al. (29), Tsakmakidis et al. (59), Othman et al. (27), Alcover et al. (13)].
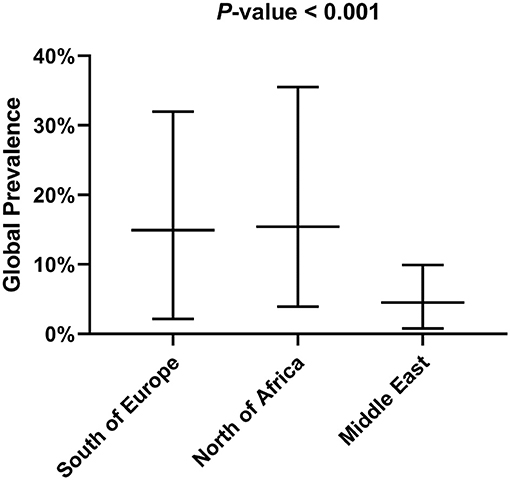
The selected articles were classified as clinical, epidemiological and review studies, the great majority (75%) being epidemiological (Figure 1A). In the last 25 years, the possible role of rodents in the L. infantum cycle has been assessed in nine countries of the Mediterranean basin, with the highest number of studies being carried out in Iran. Seven studies were performed in more than one of these nine countries (11, 14, 25, 41, 44, 45, 52) (Figure 1B). If the Mediterranean basin is divided into three large geographical areas, Southern Europe, North Africa and the Middle East, the latter concentrates the greatest number of studies (Figure 1C).
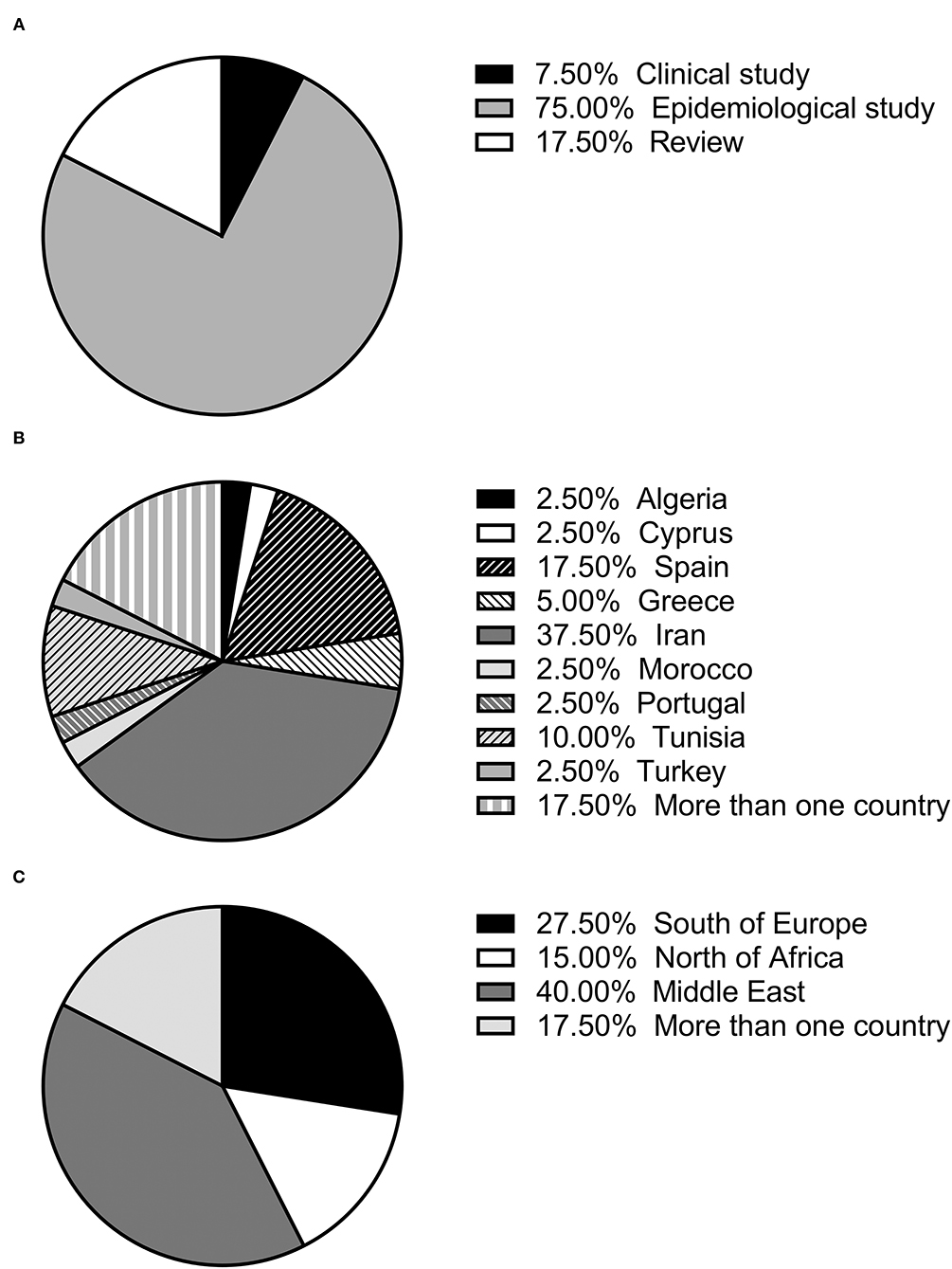
Figure 1. (A) Percentage of each type of study (review, clinical or epidemiological); (B) percentage of studies published in each country; (C) percentage of studies published in each geographical area.
Tables 1, 2 show the number of animals studied in each country and geographical area, as well as the number of infected specimens and prevalence of infection. Statistically significant differences in the proportion of infected hosts were found only between geographical areas, with the highest rate in Southern Europe, above all in Portugal and Spain (more than 25%). When considering the overall prevalence of infection in rodents, a significant difference (P-value of Chi-Square < 0.001) is again apparent between the Middle East (4.5%), North Africa (15.4%), and Southern Europe (14.9%) (Figure 2). The differences observed between these geographical areas may be due to a variable diversity of Leishmania species (25, 32), and that rodents have been reported to host more than one species (26). When drawing conclusions from the data reviewed here, it should therefore be taken into account that an animal may be parasitized by more than one species of Leishmania or that identification at species level could be absent.
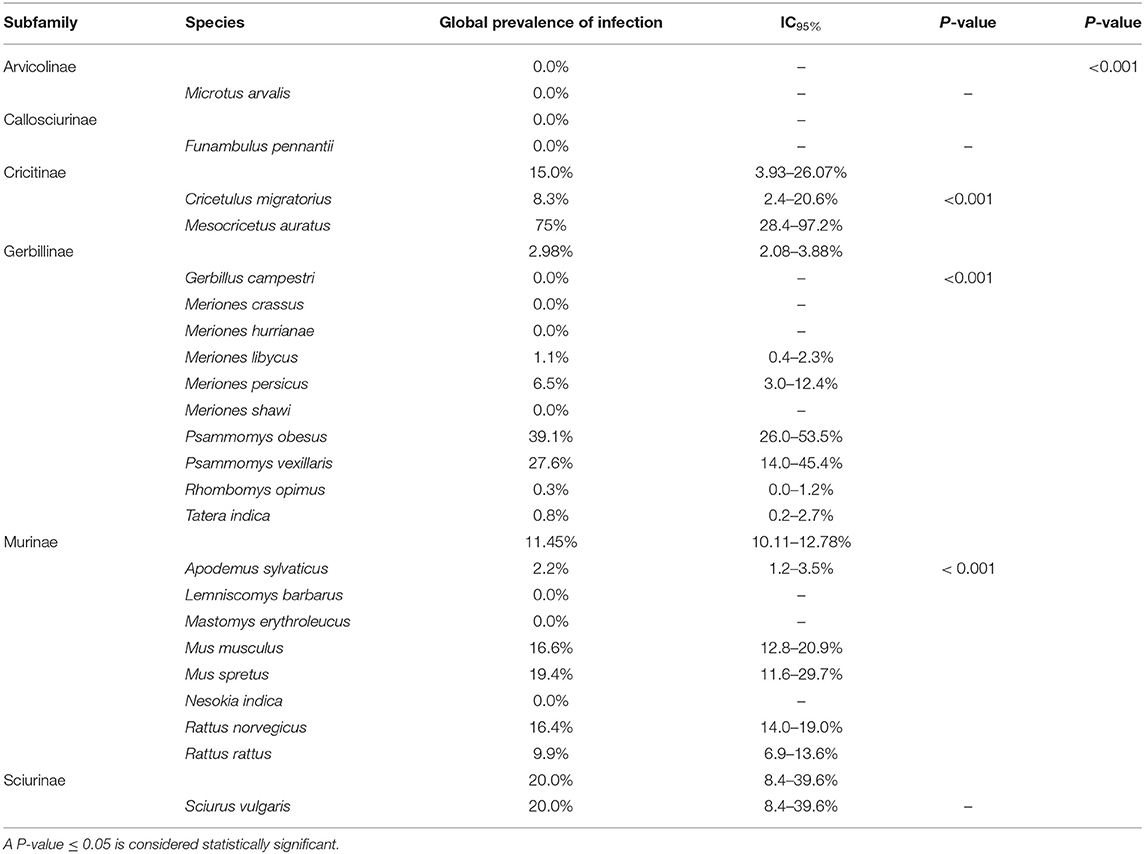
No statistically significant differences were found for the three variables studied (the number of animals captured, number of infected animals, and the median prevalence of infection for each family/subfamily/species) (Figures 1, 3). However, when analyzing the overall prevalence, statistically significant differences become apparent for each species and subfamily, as shown in Table 3. The highest overall infection rate is found in Mesocricetus auratus (44), whereas, 10 of the 25 studied species have 0% infection.
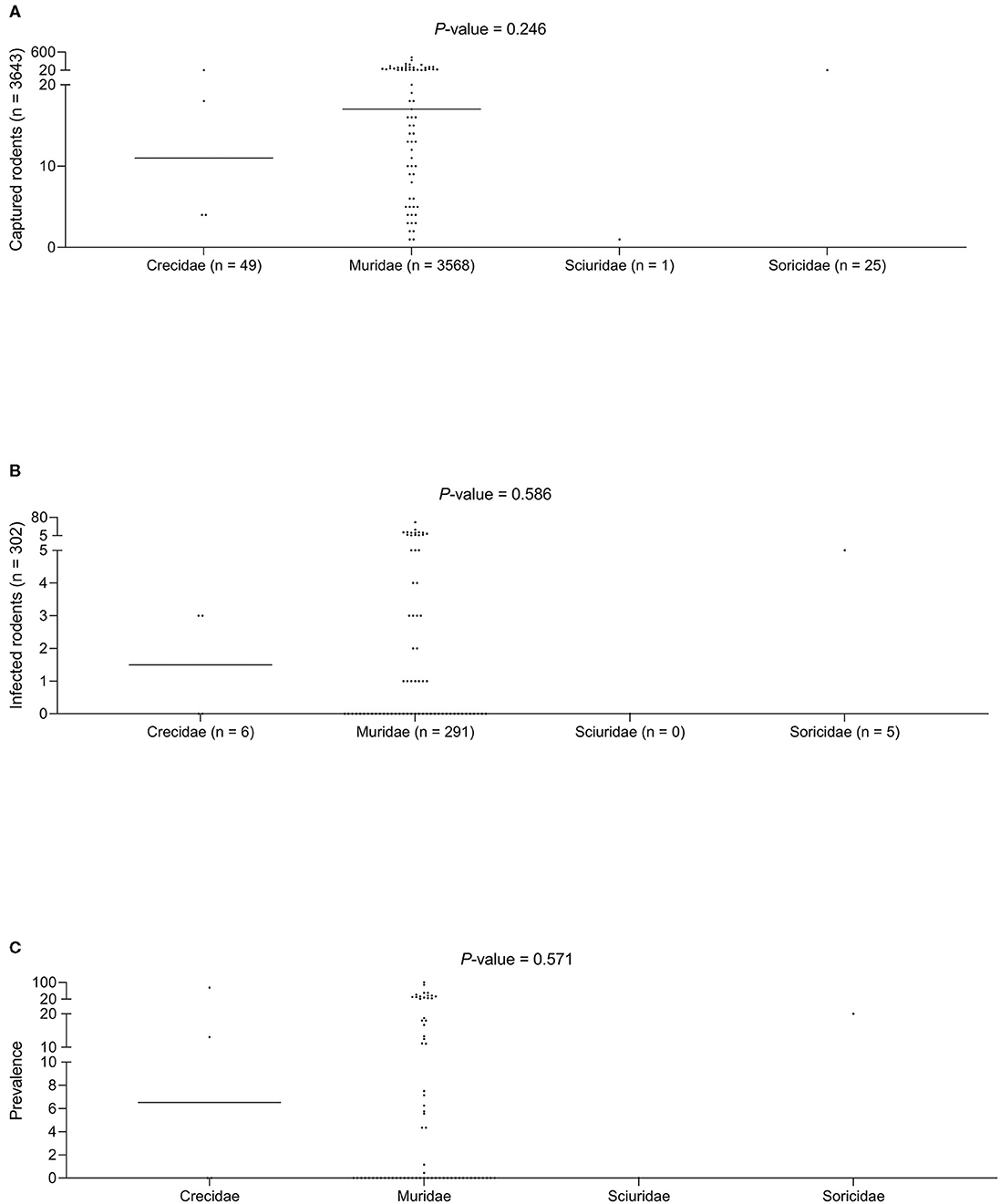
Figure 3. (A) Captured rodents. (B) Infected rodents. (C) Prevalence of infection among different rodent families.
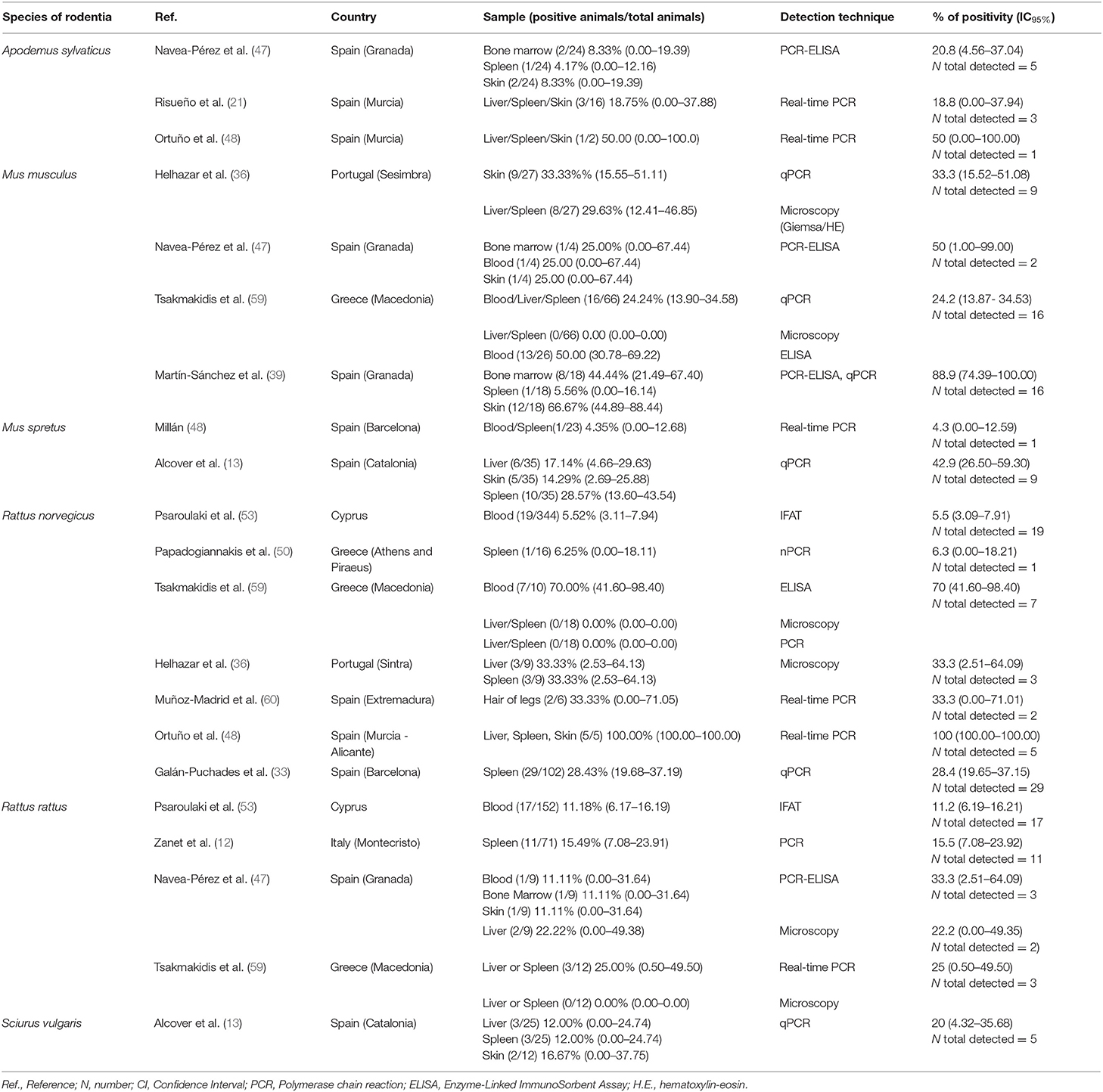
In studies carried out in Southern Europe published between January 2001 and December 2020 (Tables 4, 5), six rodent species were examined in five countries. No statistically significant differences were found related to the different types of sample analyzed (blood, bone marrow, hair, liver, skin, and spleen).
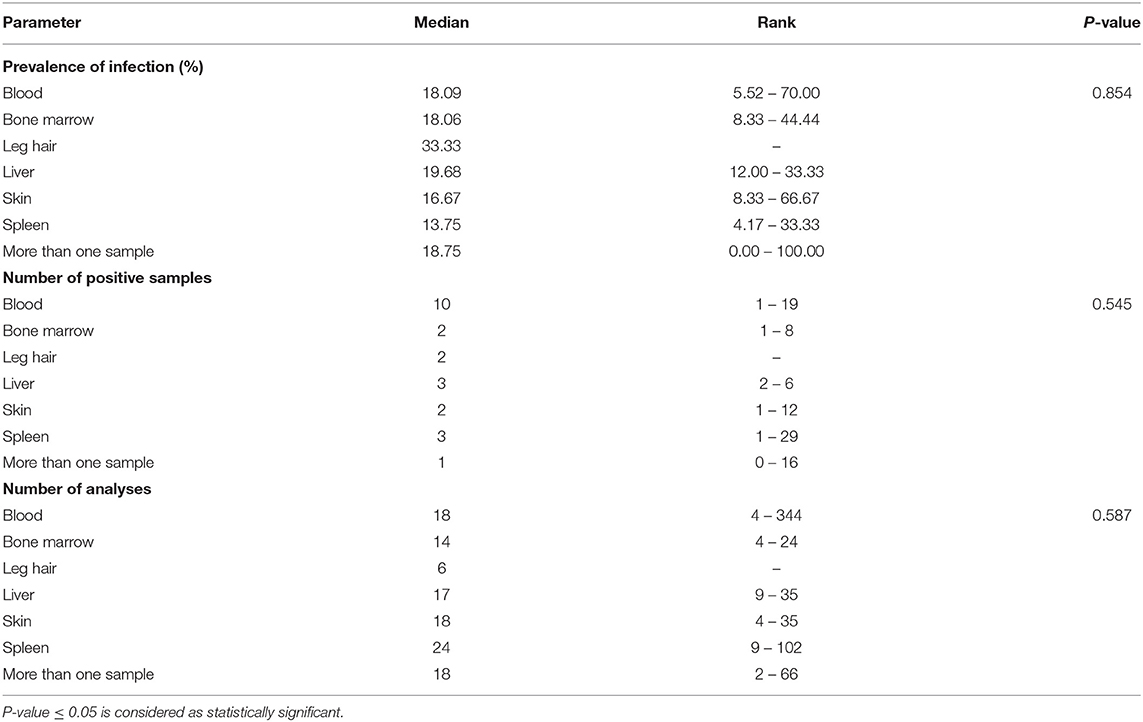
Table 4. Median and rank of infection prevalence in the different tissue samples analyzed, the number of positive samples and th number of analyses in Southern Europe between January 2001 and December 2020.
In the studies covered by this review, only some of the criteria outlined by the WHO for defining a species as a reservoir of Leishmania (61) have been met. The reservoir must be sufficiently abundant and long-lived, and there should be continuous contact between the host and vector. Some species of Phlebotomus are described as opportunistic, feeding on the most accessible animals (31, 62). Therefore, if the density of rodents is high, they may be expected to have an increased risk of exposure to the bite of the vector. However, given the complexity of the interactions between the different actors of the transmission cycle (protozoan–vector–mammal), the link between the vector and animal host is difficult to prove (63, 64).
In a reservoir population, the prevalence of Leishmania infantum should be >20%, which has been found for the following rodent species: Cricetulus migratorius (44), Mesocricetus auratus (43, 44), Mus musculus (31, 36, 47, 59), Mus spretus (13), Psammomys obesus (27), Psammomys vexillaris (27), and Sciurus vulgaris (13). The house mouse (M. musculus), which is native to southwestern Asia (65), is an invasive rodent with a dramatic impact on biodiversity, and human health and activities (66). Asian rodents of the genus Rattus have been implicated in the emergence and spread of infectious diseases affecting human health (67, 68). In the Mediterranean region, the global prevalence of Leishmania infection in the Norway or brown rat (Rattus norvegicus), and the black or roof rat (Rattus rattus) is below 20% but not negligible (9.9 and 16.4%, respectively) (Table 3). Both species merit special attention due to their readiness to colonize urban environments worldwide (69), and their serious impact on global health. Among several publications reporting cases of Leishmania infection in rodents, a study using molecular analysis in an urban area of Brazil found that 16.7% of R. norvegicus, 10% of M. musculus and up to 25% of Cerradomys subflavus, a species native to Brazil, tested positive for L. infantum (70). These results provide evidence that the control of leishmaniosis in urban areas should take into consideration the potential transmission role of rodents, especially those species that live alongside humans.
Another relevant factor is the course of infection, which must be non-pathogenic and long enough to allow parasites to survive a season without transmission. Our review shows that rodents with clinical manifestations of Leishmania infection, such as splenomegaly or hepatomegaly, have not been observed in most studies. Finally, parasites must be accessible in the skin or blood of the host in sufficient quantities to be ingested by a sand fly, which was not demonstrated in the reviewed studies. Table 5 details the rank of the median of infection prevalence in the different tissue samples analyzed, which ranges between 13.75% (spleen) and 33.33% (leg hair). In the case of skin samples, the range of infection prevalence is 8.33–66.67%.
The complexity of the transmission cycle is increased by the diversity of the rodent hosts, as this may give rise to a dilution effect. This hypothesis holds that for vector-borne parasites, the presence of less competent host species may reduce the prevalence of infection in the main host (71) and the relative contribution of each rodent species to the cumulative reservoir can differ (14). Therefore, the equilibrium of each stage of this complex system of ecological and epidemiological interactions between different hosts, pathogens and vectors is affected by the density of rodent populations.
In total, the studies included in this review investigated 23 species of rodents. The number of specimens per species varies considerably, ranging from one of Funambulus pennanti to 807 of Apodemus sylvaticus. In the latter, 13 specimens (1.6%) were found to be infected (IC95% 0.9–2.8). The species with the highest number of infected specimens was Rattus norvegicus: 80 out of 659 animals (9.9%) (IC95% 12.1–14.9). The highest rate of infection was found in Mesocricetus auratus, but only four specimens were analyzed, three testing positive (75%) (IC95% of 67.7%). These results indicate that there is still insuficient data available to define which species may have a significant impact on the transmission cycle of the parasite.
Conclusion
The detection of Leishmania infection in rodents, regardless of the species, suggests that these animals may contribute to maintaining the life cycle of L. infantum. Further, studies employing xenodiagnosis should shed more light on this role. Additionally, experimental and analytical studies are necessary to evaluate which type of sample and technique are the most suitable to detect the infection. Despite the challenging nature of rodent control, more information about this zoonotic parasite carried by rodent populations in the Mediterranean basin is required to develop suitable surveillance plans and intervention strategies.
Author Contributions
MA and RF designed the research study and wrote the manuscript. MA, MR, and RF contributed with data analysis and interpretation. All authors read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a shared affiliation with the authors MA, MR, and RF at time of review.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank ATP biostatistical consulting for technical support on statistical testing. Grateful acknowledgment to Lucy Brzoska for her advice on the English preparation of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.702687/full#supplementary-material
References
1. Hotez PJ, Gurwith M. Europe's neglected infections of poverty. Int J Infect Dis. (2011) 15:e611–9. doi: 10.1016/j.ijid.2011.05.006
2. Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. (2012) 7:e35671. doi: 10.1371/journal.pone.0035671
3. Antoniou M, Gramiccia M, Molina R, Dvorak V, Volf P. The role of indigenous phlebotomine sandflies and mammals in the spreading of leishmaniasis agents in the Mediterranean region. Euro Surveill. (2013) 18:20540. doi: 10.2807/1560-7917.ES2013.18.30.20540
4. Ready PD. Leishmaniasis emergence in Europe. Euro Surveill. (2010) 15:19505. doi: 10.2807/ese.15.10.19505-en
5. Mary Ch, Faraut F, Lascombe L, Dumon H. Quantification of Leishmania infantum DNA by a real-time PCR assay with high sensitivity. J Clin Microbiol. (2004) 42:5249–55. doi: 10.1128/JCM.42.11.5249-5255.2004
6. Gómez-Saladín E, Doud CW, Maroli M. Short report: surveillance of Leishmania sp. among sand flies in Sicily (Italy) using a fluorogenic real-time polymerase chain reaction. Am J Trop Med Hyg. (2005) 72:138–41. doi: 10.4269/ajtmh.2005.72.138
7. Solano-Gallego L, Rodríguez-Cortés A, Iniesta L, Quintana J, Pastor J, Espada Y, et al. Cross-sectional serosurvey of feline leishmaniasis in ecoregions around the Northwestern Mediterranean. Am J Trop Med Hyg. (2007) 76:676–80. doi: 10.4269/ajtmh.2007.76.676
8. Dujardin JC, Campino L, Cañavate C, Dedet JP, Gradoni L, Soteriadou K, et al. Spread of vector-borne diseases and neglect of Leishmaniasis, Europe. Emerg Infect Dis. (2008) 14:1013–8. doi: 10.3201/eid1407.071589
9. Rioux JA, Albaret JL, Houin R, Dedet JP, Lanotte G. Ecologie des leishmanioses dans le sud de la France. 2. Les reservoirs selvatiques. Infestation spontanee de renard (Vulpes vulpes L.). Ann Parasitol Hum Comp. (1968) 43:421–28. doi: 10.1051/parasite/1968434421
10. Sobrino R, Ferroglio E, Oleaga A, Romano A, Millan J, Revilla M, et al. Characterization of widespread canine leishmaniasis among wild carnivores from Spain. Vet Parasitol. (2008) 155:198–203. doi: 10.1016/j.vetpar.2008.05.003
11. Millán J, Ferroglio E, Solano-Gallego L. Role of wildlife in the epidemiology of Leishmania infantum infection in Europe. Parasitol Res. (2014) 113:2005–14. doi: 10.1007/s00436-014-3929-2
12. Zanet S, Sposimo P, Trisciuoglio A, Giannini F, Strumia F, Ferroglio E. Epidemiology of Leishmania infantum, Toxoplasma gondii, and Neospora caninum in Rattus rattus in absence of domestic reservoir and definitive hosts. Vet Parasitol. (2014) 199:247–9. doi: 10.1016/j.vetpar.2013.10.023
13. Alcover MM, Ribas A, Guillén MC, Berenguer D, Tomás-Pérez M, Riera C, et al. Wild mammals as potential silent reservoirs of Leishmania infantum in a Mediterranean area. Prev Vet Med. (2020) 175:104874. doi: 10.1016/j.prevetmed.2019.104874
14. Meerburg BG, Singleton GR, Kijlstra A. Rodent-borne diseases and their risks for public health. Crit Rev Microbiol. (2009) 35:221–70. doi: 10.1080/10408410902989837
15. Schenk JJ, Rowe KC, Steppan SJ. Ecological opportunity and incumbency in the diversification of repeated continental colonizations by muroid rodents. Syst Biol. (2013) 62:837–64. doi: 10.1093/sysbio/syt050
16. Pozio E, Maroli M, Gradoni L, Gramiccia M. Laboratory transmission of Leishmania infantum to Rattus rattus by the bite of experimentally infected Phlebotomus perniciosus. Trans R Soc Trop Med Hyg. (1985) 79:524–6. doi: 10.1016/0035-9203(85)90085-9
17. Svobodová M, Votýpka J. Experimental transmission of Leishmania tropica to hamsters and mice by the bite of Phlebotomus sergenti. Microbes Infect. (2003) 5:471–4. doi: 10.1016/S1286-4579(03)00066-2
18. Svobodová M, Votýpka J, Nicolas L, Volf P. Leishmania tropica in the black rat (Rattus rattus): persistence and transmission from asymptomatic host to sand fly vector Phlebotomus sergenti. Microbes Infect. (2003) 5:361–4. doi: 10.1016/S1286-4579(03)00046-7
19. Gramiccia M, Gradoni L. The current status of zoonotic leishmaniases and approaches to disease control. Int J Parasitol. (2005) 35:1169–80. doi: 10.1016/j.ijpara.2005.07.001
20. Hallmaier-Wacker LK, Munster VJ, Knauf S. Disease reservoirs: from conceptual frameworks to applicable criteria. Emerg Microbes Infect. (2017) 6:e79. doi: 10.1038/emi.2017.65
21. Risueño J, Ortuño M, Pérez-Cutillas P, Goyena E, Maia C, Cortes S, et al. Epidemiological and genetic studies suggest a common Leishmania infantum transmission cycle in wildlife, dogs and humans associated to vector abundance in Southeast Spain. Vet Parasitol. (2018) 259:61–67. doi: 10.1016/j.vetpar.2018.05.012
22. Polanin JR, Piggot TD, Espelage DL, Grotpeter JK. Best practice guidelines for abstract screening large-evidence systematic reviews and meta-analyses. Res Syn Meth. (2019) 10:330–42. doi: 10.1002/jrsm.1354
23. Özbilgin A, Çavuş I, Yildirim A, Gündüz C. Do the rodents have a role in transmission of cutaneous Leishmaniasis in Turkey? Mikrobiyol Bul. (2018) 52:259–72. doi: 10.5578/mb.66828
24. Derbali M, Chelbi I, Ben Hadj Ahmed S, Zhioua E. Leishmania major Yakimoff et Schokhor, 1914 (Kinetoplastida: Trypanosomatidae) in Meriones shawi Duvernoy, 1842 (Rodentia: Gerbillidae): persistence of the infection in meriones and its infectivity for the sand fly vector (Phlebotomus) papatasi Scopoli, 1786 (Diptera: Psychodidae). Bull Soc Pathol Exot. (2012) 105:399–402. doi: 10.1007/s13149-012-0259-4
25. Aoun K, Bouratbine A. Cutaneous Leishmaniasis in North Africa: a review. Parasite. (2014) 21:14. doi: 10.1051/parasite/2014014
26. Azimi T, Reza Pourmand M, Fallah F, Karimi A, Mansour-Ghanaie R, Hoseini-Alfatemi SM, et al. Serosurvey and molecular detection of the main zoonotic parasites carried by commensal Rattus norvegicus population in Tehran, Iran. Trop Med Health. (2020) 48:60. doi: 10.1186/s41182-020-00246-3
27. Othman SB, Ghawar W, Ghaouch M, Ayari Ch, Chemkhi J, Cancino-Faure B, et al. First detection of Leishmania DNA in Psammomys obesus and Psammomys vexillaris: their potential involvement in the epidemiology of leishmaniasis in Tunisia. Infect Genet Evol. (2018) 59:7–15. doi: 10.1016/j.meegid.2018.01.013
28. Chemkhi J, Souguir H, Hadj Ali IB, Driss M, Guizani I, Guerbouj S. Natural infection of Algerian hedgehog, Atelerix algirus (Lereboullet 1842) with Leishmania parasites in Tunisia. Acta Trop. (2015) 150:42–51. doi: 10.1016/j.actatropica.2015.06.009
29. Dabaghmanesh T, Asgarib Q, Moemenbellah-Fardc MD, Soltanic Azizic K. Natural transovarial and transstadial transmission of Leishmania infantum by naïve Rhipicephalus sanguineus ticks blood feeding on an endemically infected dog in Shiraz, south of Iran. Trans R Soc Trop Med Hyg. (2016) 110:408–13. doi: 10.1093/trstmh/trw041
30. Davami MH, Motazedian MH, Kalantari M, Asgari Q, Mohammadpou I, Sotoodeh-Jahromi A, et al. Molecular survey on detection of Leishmania infection in rodent reservoirs in Jahron District, Southern Iran. J Arthropod Borne Dis. (2014) 8:139–46.
31. Echchakery M, Chicharro C, Boussaa S, Nieto J, Carrillo E, Sheila O, et al. Molecular detection of Leishmania infantum and Leishmania tropica in rodent species from endemic cutaneous leishmaniasis areas in Morocco. Parasit Vectors. (2017) 10:454. doi: 10.1186/s13071-017-2398-8
32. Foroutan F, Khademvatan S, Majidiani H, Khalkhali H, Hedayati-Rad F, Khashaveh S, et al. Prevalence of Leishmania species in rodents: a systematic review and metanalysis in Iran. Acta Trop. (2017) 172:164–72. doi: 10.1016/j.actatropica.2017.04.022
33. Galán-Puchades MT, Gómez-Samblás M, Suárez-Morán JM, Osuna A, Sanxis-Furió J, Pascual J, et al. Leishmaniasis in Norway rats in sewers, Barcelona, Spain. Emerg Infect Dis. (2019) 25:1222–4. doi: 10.3201/eid2506.181027
34. Hajjaran H, Mohebali M, Mamishi S, Vasigheh F, Oshaghi MA, Naddaf SR, et al. Molecular identification and polymorphism determination of cutaneous and visceral leishmaniasis agents isolated from human and animal hosts in Iran. Biomed Res Int. (2013) 2013:789326. doi: 10.1155/2013/789326
35. Harrat Z, Pratlong F, Belazzoug S, Dereure J, Deniau M, Rioux JA, et al. Leishmania infantum and L. major in Algeria. Trans R Soc Trop Med Hyg. (1996) 90:625–9. doi: 10.1016/S0035-9203(96)90410-1
36. Helhazar M, Leitão J, Duarte A, Tavares L, Pereira da Fonseca I. Natural infection of synathropic rodent species Mus musculus and Rattus norvegicus by Leishmania infantum in Sesimbra and Sintra-Portugal. Parasit Vectors. (2013) 6:88. doi: 10.1186/1756-3305-6-88
37. Karakuş M, Öktem MA, Sözen M, Matur F, Çolak F, Nalçaci M, Özbel Y, Töz S. First molecular detection and identification of Leishmania species in small wild rodents from Turkey. Parasitology. (2020) 147:1088–93. doi: 10.1017/S0031182020000803
38. Mahmoudzadeh-Niknam H, Ajdary S, Riazi-Rad F, Mirzadegan E, Rezaeian A, Khaze V, et al. Molecular epidemiology of cutaneous leishmaniasis and heterogeneity of Leishmania major strains in Iran. Trop Med Int Health. (2012) 17:1335–44. doi: 10.1111/j.1365-3156.2012.03078.x
39. Martín-Sánchez J, Torres-Medina N, Corpas-López V, Morillas-Márquez F, Díaz-Sáez V. Vertical transmission may play a greater role in the spread of Leishmania infantum in synanthropic Mus musculus rodents than previously believed. Transbound Emerg Dis. (2020) 67:1113–18. doi: 10.1111/tbed.13436
40. Masoumeh A, Kourosh A, Mohsen K, Hossein MM, Qasem A, Djaefar MFM, et al. Laboratory based diagnosis of leishmaniasis in rodents as the reservoir hosts in southern Iran, 2012. Asian Pac J Trop Biomed. (2014) 4:S575–80. doi: 10.12980/APJTB.4.2014APJTB-2014-0199
41. Millán J. Molecular investigation of vector-borne parasites in wild micromammals, Barcelona (Spain). Parasitol Res. (2018) 117:3015–18. doi: 10.1007/s00436-018-5971-y
42. Mirzaei A, Schweynoch C, Rouhani S, Parvizi P, Schönian G. Diversity of Leishmania species and of strains of Leishmania major isolated from desert rodents in different foci of cutaneous leishmaniasis in Iran. Trans R Soc Trop Med Hyg. (2014) 108:502–12. doi: 10.1093/trstmh/tru085
43. Mohebali M, Javadian E, Yaghoobi-Ershadi MR, Akhavan AA, Hajjaran H, Abaei MR. Characterization of Leishmania infection in rodents from endemic areas of the Islamic Republic of Iran. East Mediterr Health J. (2004) 10:591–9.
44. Mohebali M. Visceral leishmaniasis in Iran: review of the epidemiological and clinical features. Iran J Parasitol. (2013) 8:348–58.
45. Moradi M, Rassi Y, Abai MR, Zahraei Ramazani A, Mohebali M, Rafizadeh S. Some epidemiological aspects of cutaneous leishmaniasis with emphasis on vectors and reservoirs of disease in the borderline of Iran and Iraq. J Parasit Dis. (2018) 42:243–51. doi: 10.1007/s12639-018-0991-1
46. Mostafa BH, Souha BA, Sabeh F, Noureddine Ch, Riadh BS. Evidence for the existence of two distinct species: Psammomys obesus and Psammomys vexillaris within the sand rats (Rodentia, Gerbillinae), reservoirs of cutaneous leishmaniasis in Tunisia. Infect Genet Evol. (2006) 6:301–8. doi: 10.1016/j.meegid.2005.09.002
47. Navea-Pérez HM, Díaz-Sáez V, Corpas-López V, Merino-Espinosa G, Morillas-Márquez F, Martín-Sánchez J. Leishmania infantum in wild rodents: reservoirs or just irrelevant incidental hosts? Parasitol Res. (2015) 114:2363–70. doi: 10.1007/s00436-015-4434-y
48. Ortuño M, Latrofa MS, Iborra MA, Pérez-Cutillas P, Bernal LJ, Risueño J, et al. Genetic diversity and phylogenetic relationships between Leishmania infantum from dogs, humans and wildlife in south-east Spain. Zoonoses Public Health. (2019) 66:961–73. doi: 10.1111/zph.12646
49. O'Shea B, Rebollar-Tellez E, WardR D, Hamilton JGC, el Naiem D, Polwart A. Enhanced sandfly attraction to Leishmania-infected hosts. Trans R Soc Trop Med Hyg. (2002) 96:117–8. doi: 10.1016/S0035-9203(02)90273-7
50. Papadogiannakis E, Spanakos G, Kontos V, Menounos PG, Tegos N, Vakalis N. Molecular detection of Leishmania infantum in wild rodents (Rattus norvegicus) in Greece. Zoonoses Public Health. (2010) 57:e23–5. doi: 10.1111/j.1863-2378.2009.01264.x
51. Hajjaran H, Mohebali M, Teimouri A, Oshaghi MA, Mirjalali H, Kazemi-Rad E, et al. Identification and phylogenetic relationship of Iranian strains of various Leishmania species isolated from cutaneous and visceral cases of leishmaniasis based on N-acetylglucosamine-1-phosphate transferase gene. Infect Genet Evol. (2014) 26:203–12. doi: 10.1016/j.meegid.2014.05.026
52. Pratlong F, Dereure J, Ravel C, Lami P, Balard Y, Serres G, et al. Geographical distribution and epidemiological features of Old World cutaneous leishmaniasis foci, based on the isoenzyme analysis of 1048 strains. Trop Med Int Health. (2009) 14:1071–85. doi: 10.1111/j.1365-3156.2009.02336.x
53. Psaroulaki A, Antoniou M, Toumazos P, Mazeris A, Ioannou I, Chochlakis D, et al. Rats as indicators of the presence and dispersal of six zoonotic microbial agents in Cyprus, an island ecosystem: a seroepidemiological study. Trans R Soc Trop Med Hyg. (2010) 104:733–9. doi: 10.1016/j.trstmh.2010.08.005
54. Quinnell RJ, Courtenay O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology. (2009) 136:1915–34. doi: 10.1017/S0031182009991156
55. Rassi Y, Gassemi MM, Javadian E, Rafizadeh S, Motazedian H, Vatandoost H. Vectors and reservoirs of cutaneous leishmaniasis in Marvdasht district, southern Islamic Republic of Iran. East Mediterr Health J. (2007) 13:686–93.
56. Sofizadeh A, Hanafi-Bojd AA, Shoraka HR. Modeling spatial distribution of Rhombomys opimus as the main reservoir host of zoonotic cutaneous leishmaniasis in northeastern Iran. J Vector Borne Dis. (2018) 55:297–304. doi: 10.4103/0972-9062.256565
57. Souguir-Omrani H, Chemkhi J, Fathallah-Mili A, Saadi-BenAoun Y, BelHadjAli Y, Guizani I, et al. Paraechinus aethiopicus (Ehrenberg 1832) and Atelerix algirus (Lereboullet 1842) hedgehogs: possible reservoirs of endemic leishmaniases in Tunisia. Infect Genet Evol. (2018) 63:219–30. doi: 10.1016/j.meegid.2018.05.029
58. Tomassone L, Berriatua E, De Sousa R, Duscher GG, Mihalca AD, Silaghi C, et al. Neglected vector-borne zoonoses in Europe: into the wild. Vet Parasitol. (2018) 251:17–26. doi: 10.1016/j.vetpar.2017.12.018
59. Tsakmakidis I, Angelopoulou K, Dovas Cl, Dokianakis E, Tamvakis A, Symeonidou I, et al. Leishmania infection in rodents in Greece. Trop Med Int Health. (2017) 22:1523–32. doi: 10.1111/tmi.12982
60. Muñoz-Madrid R, Belinchón-Lorenzo S, Iniesta V, Fernández-Cotrina J, Parejo JC, Serrano FJ, et al. First detection of Leishmania infantum kinetoplast DNA in hair of wild mammals: Application of qPCR method to determine potential parasite reservoirs. Acta Tropica. (2013) 128:706–9. doi: 10.1016/j.actatropica.2013.08.009
61. World Health Organization. Control of the leishmaniases. WHO Technical Report Series no. 949. Geneva: The Organization (2010).
62. de Colmenares M, Portús M, Botet J, Dobaño C, Gállego M, Wolff M, et al. Identification of blood meals of Phlebotomus perniciosus (Diptera: psychodidae) in Spain by competitive enzyme-linked immunoadsorbent assay biotin/avidin method. J Med Entomol. (1995) 32:229–33. doi: 10.1093/jmedent/32.3.229
63. Glass GE, Cheek JE, Patz JA, Shields TM, Doyle TJ, Thoroughman DA, et al. Using remotely sensed data to identify areas at risk for hantavirus pulmonary syndrome. Emerg Infect Dis. (2000) 6:238–47. doi: 10.3201/eid0603.000303
64. Ostfeld RS, Holt RD. Are predators good for your health? Evaluating evidence for top-down regulation of zoonotic disease reservoirs. Front Ecol Environ. (2004) 2:13–20. doi: 10.1890/1540-9295(2004)002[0013:APGFYH]2.0.CO;2
65. Suzuki H, Nunome M, Kinoshita G, Aplin KP, Vogel P, Kryukov AP, et al. Evolutionary and dispersal history of Eurasian house mice Mus musculus clarified by more extensive geographic sampling of mitochondrial DNA. Heredity (Edinb). (2013) 111:375–90. doi: 10.1038/hdy.2013.60
66. Singleton GR, Hinds LA, Krebs CJ, Spratt DM. Rats, Mice and People: Rodent Biology and Management. Canberra, ACT: ACIAR Monograph 96 Australian Centre for International Agricultural Research (2003).
67. Morand S, Bordes F, Hsuan-Wien C, Julien C, Cosson JJF, Maxime G, et al. Global parasite and Rattus rodent invasions: the consequences for rodent-borne diseases. Integr. Zool. (2015) 10:409–23. doi: 10.1111/1749-4877.12143
68. Wells K, O'Hara RB, Morand S, Lessard JP, Ribas A. The importance of parasite geography and spillover effects for global patterns of host-parasite associations in two invasive species. Divers Distrib. (2015) 21:477–86. doi: 10.1111/ddi.12297
69. Aplin KP, Suzuki H, Chinen AA, Chesser RT, Have JT, Donnellan CS, et al. Multiple geographic origins of commensalism and complex dispersal history of Black Rats. PLoS ONE. (2011) 6:e26357. doi: 10.1371/journal.pone.0026357
70. de Castro Ferreira E, Cruz I, Cañavate C, de Melo LA, Sampaio Pereira AA, Madeira FAM, et al. Mixed infection of Leishmania infantum and Leishmania braziliensis in rodents from endemic urban area of the New World. Vet Res. (2015) 11:71. doi: 10.1186/s12917-015-0392-y
Keywords: Leishmania infantum, zoonosis, rodentia, reservoir, wildlife, Mediterranean basin
Citation: Alcover MM, Riera MC and Fisa R (2021) Leishmaniosis in Rodents Caused by Leishmania infantum: A Review of Studies in the Mediterranean Area. Front. Vet. Sci. 8:702687. doi: 10.3389/fvets.2021.702687
Received: 29 April 2021; Accepted: 13 July 2021;
Published: 06 August 2021.
Edited by:
Alexis Ribas Salvador, University of Barcelona, SpainReviewed by:
Laia Solano-Gallego, Universitat Autònoma de Barcelona, SpainDavid Bruce Conn, Berry College, United States
Copyright © 2021 Alcover, Riera and Fisa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Magdalena Alcover, bW1hZ2RhbGVuYWFsY292ZXJhbWVuZ3VhbEB1Yi5lZHU=
 M. Magdalena Alcover
M. Magdalena Alcover M. Cristina Riera
M. Cristina Riera