Abstract
A complete postmortem examination, including a computed tomography scan “virtopsy” (virtual necropsy), gross necropsy, cytology, histology, and molecular diagnostics were performed to investigate the cause of death of a deceased adult male Atlantic spotted dolphin (Stenella frontalis) that stranded on Pensacola Beach, Florida, USA in February 2020. Significant findings included chronic inflammation of the meninges, brain, and spinal cord with intralesional protozoa (identified as Sarcocystis speeri via 18S rRNA and ITS-1 sequences), suppurative fungal tracheitis and bronchopneumonia (identified as Aspergillus fumigatus via ITS-2 gene sequence) and ulcerative bacterial glossitis (associated with a novel Treponema species, Candidatus Treponema stenella, identified via 23S rRNA gene sequence). This is the first reported case of S. speeri in a marine mammal. Little is understood about the epidemiology of S. speeri, including the identity of its intermediate hosts. The findings of this case suggest that S. frontalis may be a capable aberrant host and experience morbidity and mortality from this parasite. It is suspected that the novel Treponema and Aspergillus fumigatus infections were opportunistic or secondary to immunosuppression, either due to S. speeri infection or other co-morbidities.
1. Introduction
Atlantic spotted dolphins (Stenella frontalis) are a relatively abundant species of dolphin, endemic to the Atlantic Ocean waters of West Africa, the Caribbean, South America, and the southeastern USA (1, 2). Some subpopulations of S. frontalis are pelagic, but other subpopulations native to the southeastern USA and the Bahamas are usually found in shallower, coastal waters (1). Causes of morbidity and mortality previously reported for this species include fishing interactions, infections such as Erysipelothrix rhusiopathiae and Aspergillus fumigatus, as well as cases of neoplastic diseases (1, 3–7).
Aspergillus species are ubiquitous fungi, commonly reported as causes of fungal infections in marine mammals but are often opportunistic or secondary to systemic disease such as cetacean morbillivirus (5, 8, 9). There is one report of an Aspergillus fumigatus bronchopneumonia associated mortality in a S. frontalis in Brazil (5).
Sarcocystis species are apicomplexan protozoan parasites that have obligate two-host life cycles. Sexual reproduction (gametogony) occurs only in the definitive host and asexual reproduction (schizogony) occurs in the intermediate host. There is typically greater specificity for definitive hosts. Sarcocystis spp. have been reported in all groups of marine mammals except for sirenians (10). Sarcocysts may be considered incidental findings in the skeletal muscles of marine mammals, due to exposure to feces from terrestrial definitive hosts (11–15). Infection of the central nervous system is more likely to be clinically significant. Sarcocystis speeri is closely related to S. neurona, and opossums in the genus Didelphis are the definitive hosts for both species (16, 17). The epidemiology and intermediate hosts of S. speeri are largely unknown, although there is a recent report of S. speeri found in the brain and heart of a blue-fronted Amazon parrot (Amazona aestiva) (18). Unspeciated intramuscular sarcocysts have been identified incidentally in S. frontalis in the Canary Islands (11). There is also a recent report of meningoencephalitis associated with an unidentified Sarcocystis-like organism in two striped dolphins (Stenella coeruleoalba) off the Ligurian coast of Italy (12).
Treponema species are bacteria in the phylum Spirochaetes well-characterized for their role as pathogens. Treponema spp. have been identified as the causative agents in many animal diseases, including digital dermatitis in cattle and sheep and periodontal disease in humans and dogs (19–26). However, some Treponema spp. are considered commensal in animals ranging from termites to cattle (21, 26). Treponema spp. have been characterized as part of the free-ranging Atlantic bottlenose dolphin (Tursiops truncatus) microbiome, but association with disease has not been reported to date (27).
The case report herein describes a stranded cetacean from the Western hemisphere infected with Sarcocystis sp. cysts in the central nervous system, which were identified as S. speeri via polymerase chain reaction (PCR) and Sanger sequencing. This animal also had ulcerative glossitis associated with a novel Treponema species (Candidatus Treponema stenella), also identified by PCR and subsequent sequencing.
2. Case description
An adult male S. frontalis stranded alive on Pensacola Beach, Florida, USA on the 24th of February 2020. Members of the public made multiple unauthorized attempts to push the animal back into the Gulf of Mexico. The animal was found deceased in the water later that day. The carcass was recovered and transported in sternal position packed in ice to the University of Florida College of Veterinary Medicine for postmortem examination.
Prior to necropsy, a postmortem computed tomography scan (CT), or “virtopsy,” was performed on the whole body using a Toshiba Aquilion Prime 160-slice helical scanner (Cannon Medical Systems, Tustin, CA, USA) (8). The images were reconstructed into 0.5-mm thick overlapping slices in transverse, sagittal, and dorsal planes with soft tissue, lung, and bone algorithms. Images were reviewed on two commercially available picture archiving and communications systems: Merge PACSTM (IBM Watson Healthcare, Cambridge, MA, USA) and HorosTM (Purview, Annapolis, MD, USA). Three-dimensional reformations of the transverse images were generated using a volumetric display.
Endotracheal intubation for lung expansion was not performed prior to imaging due to the technical difficulty of intubation in a dolphin of this size. CT images revealed that the pulmonary parenchyma had severely increased soft tissue attenuation bilaterally (Figure 1). The diffuse changes in the pulmonary parenchyma were attributed to postmortem atelectasis. Within the lungs there were numerous small, poorly-defined, rounded soft-tissue attenuating nodules, some with mineral attenuating foci (Figure 1). The nodules measured up to 2 centimeters in diameter. These represented antemortem lesions, and the differential diagnosis included chronic granulomatous pneumonia of fungal, parasitic and/or bacterial etiologies. Visible abnormalities were absent from the central nervous system.
Figure 1
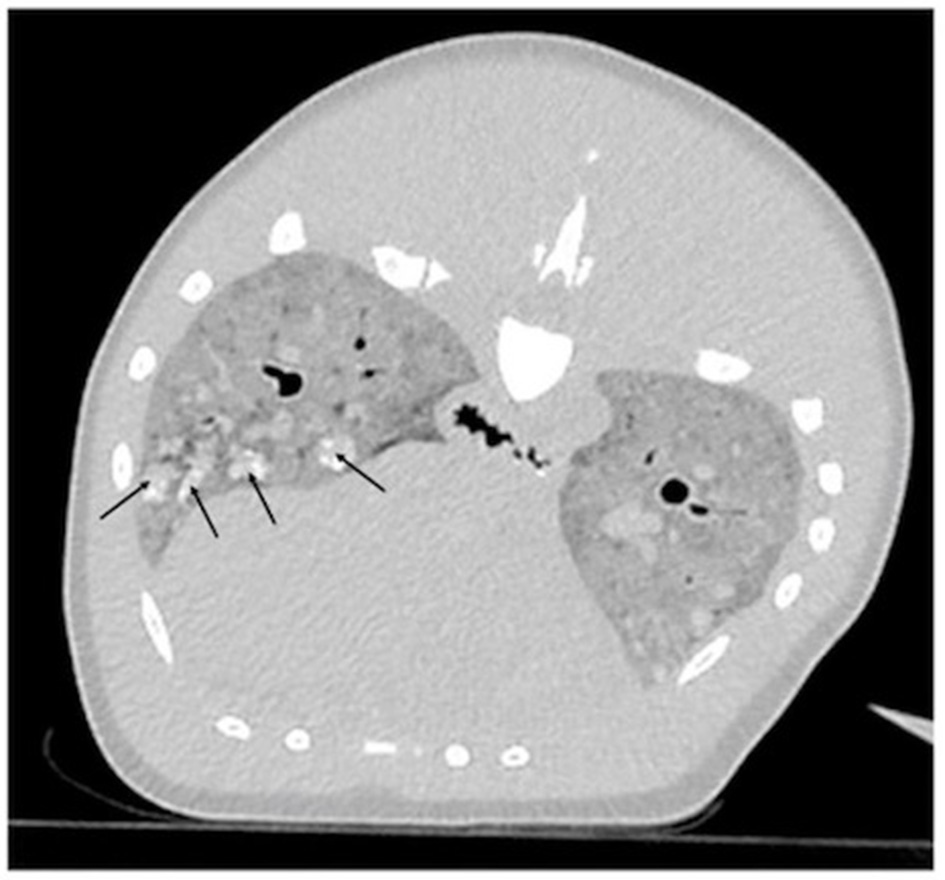
Computed tomographic (CT) transverse image centered on the mid-thorax reconstructed in a lung algorithm of an adult male Stenella frontalis. Diffusely, the pulmonary parenchyma has a severe increase in soft tissue attenuation and contains multifocal and ill-defined soft tissue attenuating nodules, some of which have mineral foci (black arrows).
On gross necropsy, the animal was thin, with decreased nuchal fat. There were no notable external lesions or indications of human interaction present. Prescapular and mesenteric lymphadenopathy was noted. An ulcerated lesion was present on the ventral aspect of the tongue (Figure 2A). The gastrointestinal tract was empty. There were multifocal to coalescing regions of white, palpable nodules in the lungs (Figure 2B) that correlated to the nodules identified on CT, as well as thick mucus and white material in the anterior trachea (Figure 2C) and secondary bronchi. There were no gross lesions noted in the brain or spinal cord.
Figure 2
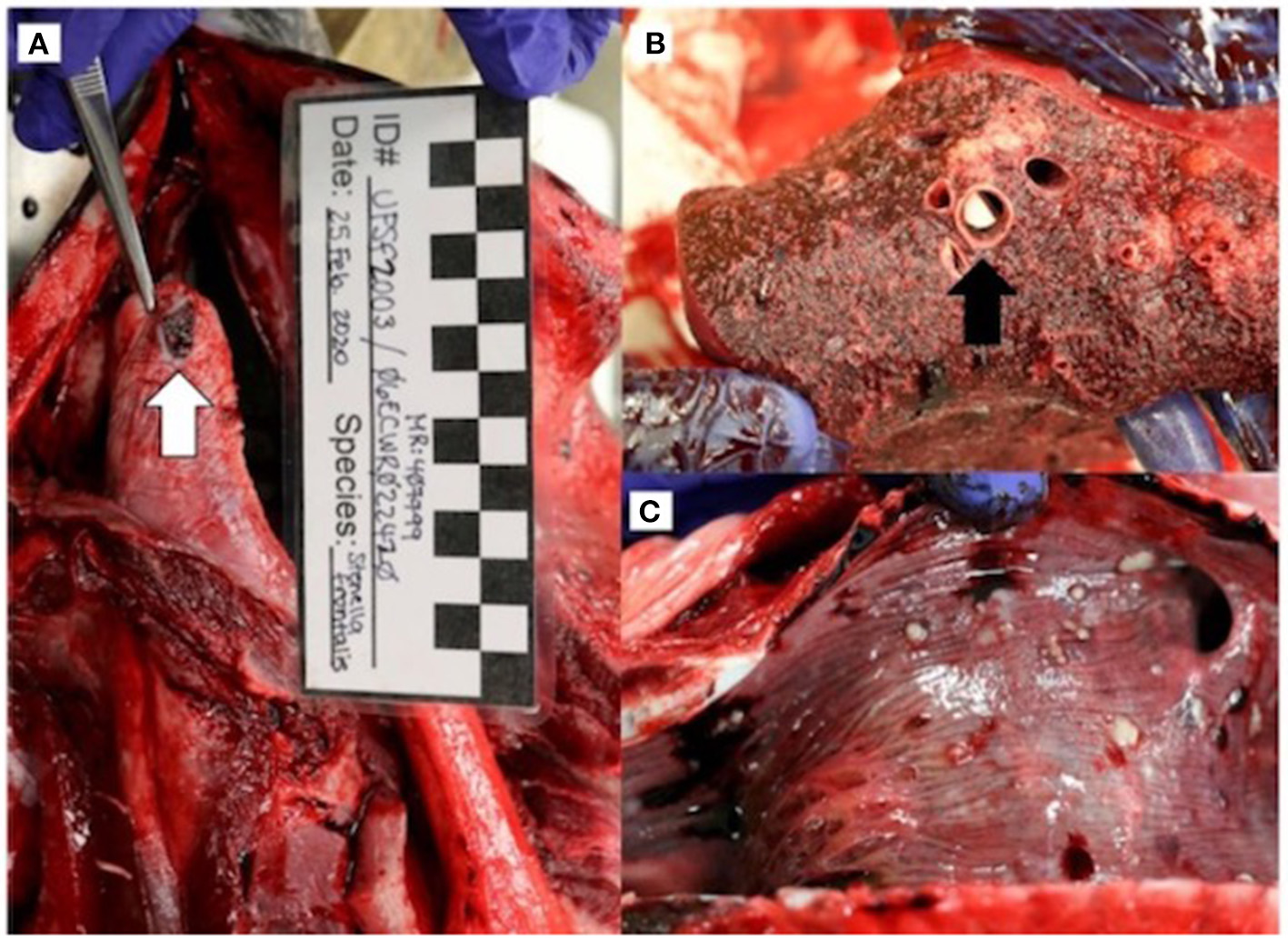
Gross necropsy findings in an adult male Stenella frontalis included a 1.0 x 0.8 cm ulcerated lesion (white arrow) on the ventral aspect of the tongue (A), lungs with multifocal to coalescing white raised nodules (B) with necrotic debris within bronchi (black arrow), and thick mucus and 1 to 4 mm free floating white debris in the anterior trachea (C).
Cytologic findings based on tissue imprints stained with Wright-Giemsa (Harleco® EMD Millipore, Billerica, MA, USA) included reactive hyperplasia of the prescapular lymph node, and suppurative inflammation with squamous cell hyperplasia and mixed bacterial infection of the tongue lesion with abundant extracellular and phagocytized bacilli, diplococci, and spirilliform bacilli (Figure 3A). The tracheal mucosa had suppurative inflammation with respiratory epithelial cell hyperplasia, mixed bacilli, and dense mats of branching fungal hyphae (Figure 3B). No cytologic abnormalities were noted on evaluation of direct smears of cerebrospinal fluid. Gastric fluid direct smear showed suppurative inflammation with epithelial cell hyperplasia and mixed bacteria.
Figure 3
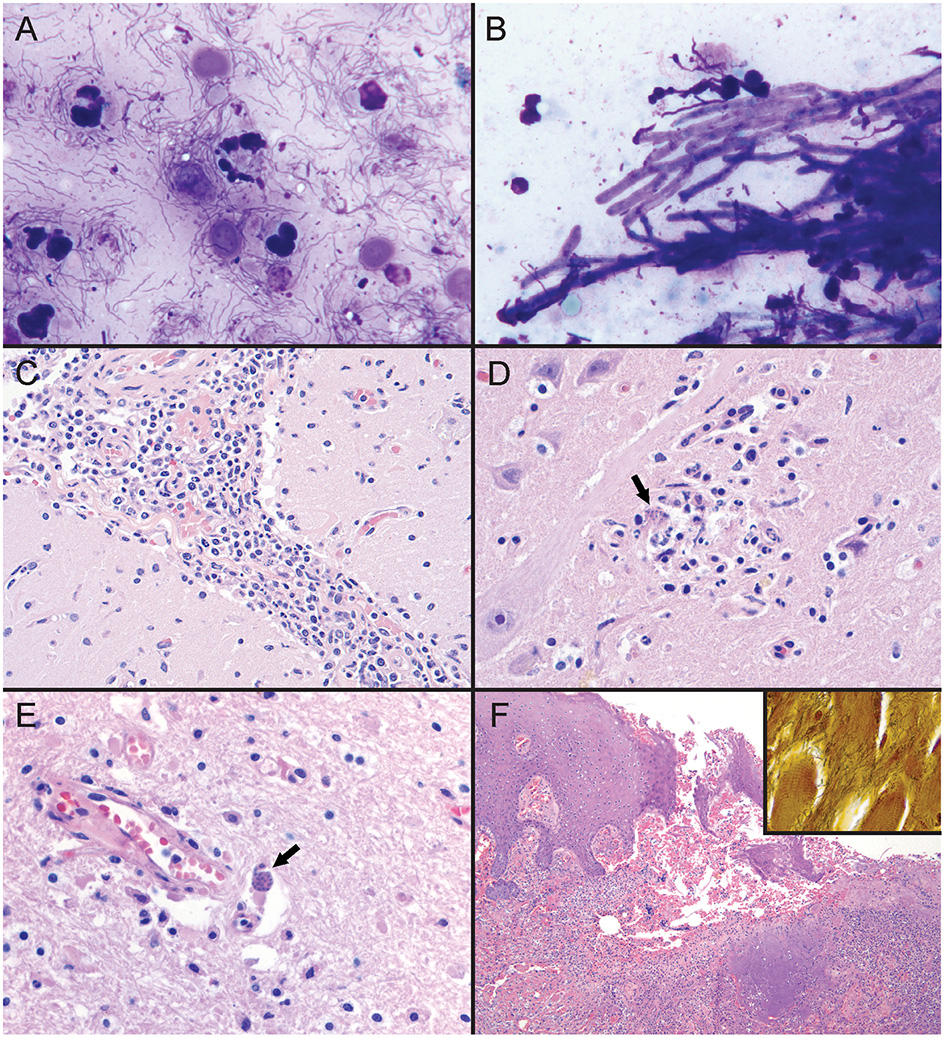
Tissue sections and imprint cytology from an adult male Stenella frontalis. (A) Imprint of tongue lesion. Mixed bacterial infection with suppurative inflammation, characterized by extracellular and phagocytosed mixed bacilli, diplococci, and spirilliform bacteria (1000x; hematoxylin and eosin stain [HE]). (B) Imprint of tracheal mucosa. There is a dense mat of septate branching fungal hyphae and mixed bacilli (1000x; HE). PCR on frozen lung tissue identified the fungus as Aspergillus fumigatus. (C) Photomicrograph of leptomeninges in cerebral sulcus. Mixed leukocytes, with a predominant population of lymphocytes and histiocytes expand the leptomeninges (400x; HE). (D) Photomicrograph of the right midbrain. Apicomplexan protozoa (black arrow) are present in a focus of malacia and gliosis (600x; HE). (E) Photomicrograph of right frontal cortex. Apicomplexan bradyzoites (black arrow) are adjacent to a vessel in the gray matter. Increased numbers of glial cells are scattered throughout the neuroparenchyma (600x; hematoxylin and eosin stain). PCR on frozen central nervous system tissue identified Sarcocystis speeri within the samples. (F) Photomicrograph of tongue lesion. There is regional ulceration of the glossal mucosa with a underlying bed of cellular and necrotic debris, and intact and degenerate neutrophils. HE; 100x. [Inset] Large numbers of spirilliform bacteria are present throughout the ulcerative lesion (600x; Warthin-Starry stain). PCR on frozen tongue tissue identified the bacteria as a Treponema sp.
Histologically, using standard hematoxylin and eosin staining methods, there was severe, chronic-active, suppurative bronchopneumonia with bronchiectasis and associated mixed bacilli and fungal hyphae, as well as chronic, lymphohistiocytic, meningoencephalomyelitis and gliosis with protozoa and rare protozoal cysts (Figures 3C–E). Marked chronic-active suppurative glossitis with ulceration and mixed bacteria, moderate lingual sialadenitis, and severe chronic-active suppurative prostatitis were identified, but considered of questionable significance regarding their role as contributing factors to cause of death (Figure 3F). Warthin-Starry staining of the tongue lesion highlighted the spirilliform bacteria noted on cytology that were not identifiable on routine hematoxylin and eosin stained sections (Figure 3F inset). These bacteria were associated with the suppurative inflammation noted in the tongue lesion. There were no protozoa, protozoal cysts, or other lesions identified on histologic evaluation of skeletal muscle.
Morbilliviruses were absent via PCR of brain and lung tissue (University of Georgia® Veterinary Diagnostic Laboratories, Athens, GA, USA) using previously described methods (28). Brucella spp. were absent via PCR of cerebrospinal fluid and brain (Veterinary Diagnostic Laboratory at the University of Illinois College of Veterinary Medicine, Urbana, IL, USA) using previously described methods (29). At the time of necropsy, a fungal culture of a lung nodule was performed on potato flake agar and inhibitory mold agar incubated at 25°C for 26 days (University of Florida Veterinary Diagnostic Laboratories, Gainesville, FL, USA), but no fungi were isolated. Nucleic acids were extracted from frozen lung tissue (University of Florida Zoological Medicine Diagnostic Laboratory [UF ZMDxL], Gainesville, FL, USA) using a DNeasy blood and tissue kit (Qiagen, Germantown, MD, USA) following the manufacturer's instructions. PCR amplification of the internal transcribed segment 2 (ITS-2) was performed using previously described methods (30). PCR products, as well as positive (DNA extract from animal case of Fusarium sp. infection) and negative (water) controls, were resolved on a 1.5% agarose gel with ethidium bromide and were visualized under ultraviolet light. An amplicon of appropriate size was excised and purified using the QIAquick® Gel Extraction kit (Qiagen) following the manufacturer's instructions. The purified amplicon was bidirectionally, commercially (Genewiz, South Plainfield, NJ, USA) sequenced. Sequences were assembled, edited, analyzed, and compared with sequences previously entered in GenBank by nucleotide basic local alignment search tool (BLASTN) (31, 32). The sequence was 100% identical to Aspergillus fumigatus (Supplementary Table S1).
Immunohistochemistry for Toxoplasma gondii performed on formalin-fixed paraffin embedded brain tissue was negative (Animal Health Diagnostic Center, Cornell University, Ithaca, NY, USA) utilizing a purified rabbit polyclonal IgG anti-Toxoplasma gondii antibody (T8075) according to the manufacturer's instructions (US Biological, Salem, MA, USA). For protozoal identification, nucleic acids were extracted at the UF ZMDxL as above from frozen samples of cerebellum, cerebrum, spinal cord, and cranial nerve. Extracts were tested by conventional PCR using both pan-apicomplexan and Sarcocystis-specific assays targeting the 18S rRNA and internal transcribed spacer 1 (ITS-1) regions, respectively, using previously described methods (33, 34). Amplification products were resolved, gel purified, and bidirectionally, commercially sequenced, assembled, edited, and analyzed as above. All sequences were identical to each other, and 100% identical to Sarcocystis speeri (Genbank accession KT207458; Supplementary Tables S2, S3). Although T. gondii infections has been reported in S. frontalis, no evidence of coinfection with S. speeri was found in this case (35–37).
For genetic characterization of the glossal spirilliform bacteria, a frozen sample of the tongue lesion was submitted to the UF ZMDxL, nucleic acids were extracted as described above and PCR was performed using primers Spirochaete23SF (5'-TCGACCAGTGAGCTRTTACGCAC-3') and Spirochaete23SR (5'-KTACCAAACYCARYYAAACTCCG-3') to target the 23S rRNA region. Amplicons were resolved and analyzed as above, resulting in a 173 base pair product. This product was compared to others in the GenBank database (National Center for Biotechnology Information, Bethesda, MD, USA) database using the BLASTN tool, and was determined to be a novel Treponema species most closely related (97.1% nucleotide identity) to Treponema vincentii (Genbank accession CP051197); the next two most closely related Treponema included T. phagedenis (Genbank accession CP054692) and T. medium (Genbank accession CP031393) at 96.5% nucleotide identity. The novel sequence was submitted to GenBank (accession MZ267537) and is hereafter referred to as Candidatus Treponema stenella.
Phylogenetic analysis of the novel Treponema was performed by first downloading homologous 170 to 173 base pair sequences of 23S rRNA from Spirochaetes closely related to Candidatus Treponema stenella were downloaded from GenBank and aligning them using MAFFT (38). Bayesian analysis was then performed with MrBayes on the CIPRES server, utilizing 2,000,000 iterations, a time-reversible model, gamma-distributed proportion of invariant sites and rate of variation (39–41). The outgroup used was Spirochaeta thermophila (Genbank accession CP002903). A maximum likelihood analysis of the phylogeny was performed with RAxML on the CIPRES server, utilizing a time-reversible model, gamma-distributed proportion of invariant sites and rate of variation, 1,000 resamplings for bootstrap analysis, and Spirochaeta thermophila as the outgroup (42, 43). Maximum likelihood bootstrap values are shown on the Bayesian tree (Figure 4).
Figure 4
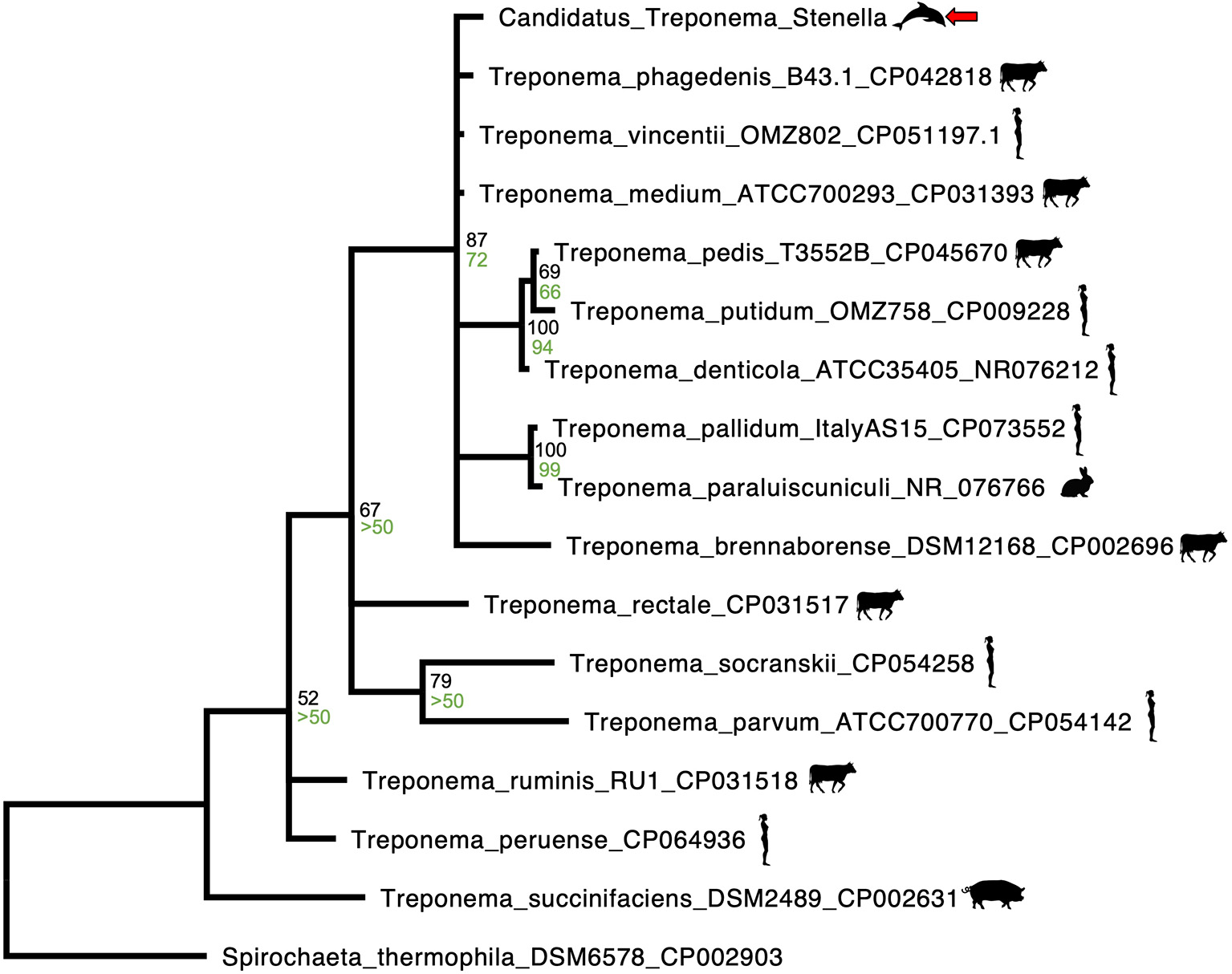
MAFFT alignment Bayesian phylogenetic tree representative of homologous 170 to 173 base pair 23S rRNA sequences of Spirochaetes closely related to a novel Treponema species identified from an ulcerative tongue lesion in a Stenella frontalis. The outgroup used was Spirochaeta thermophila (Genbank accession CP002903). Bayesian posterior probabilities are written in black, above the maximum likelihood bootstrap values, which are written in green. Both posterior probabilities and maximum likelihood bootstrap values were rounded to the nearest whole percent. The novel Treponema species (Candidatus Treponema stenella) is identified by a red arrow, Treponema spp. associated with bovids, humans, pigs or rabbits are identified by accompanying graphics of those species.
3. Discussion and conclusions
This is the first report of S. speeri infection in the central nervous system of S. frontalis, as well as the first documented infection of S. speeri in any marine mammal (5, 11, 44). An unknown Sarcocystis species has been reported as an incidental finding in two S. frontalis that stranded on the Canary Islands, consistent with postulations that dolphins may act as intermediate hosts for Sarcocystis parasites (10, 11). No sarcocysts were identified in the skeletal muscle in the present case, and in contrast with the previous report, the finding of S. speeri along with inflammation in the central nervous system suggests not only that S. speeri was a cause for morbidity, but also that dolphins may be aberrant intermediate hosts for these parasites, similar to S. canis hepatitis in sea lions (14, 16, 44). It is possible that previous cases of S. speeri have been overlooked in marine mammals due to the genetic analysis required for speciation (17).
Historically, water runoff, sewage, and consumption of benthic invertebrates have been hypothesized to increase the risk for Sarcocystis species infection in marine mammals (13, 14). This may put S. frontalis that inhabit shallower coastal waters, such as in the Gulf of Mexico, at increased risk (1). S. frontalis eat both fishes and benthic invertebrates throughout their range, so pelagic subpopulations could also be at risk if benthic invertebrates are capable transport hosts for S. speeri (1, 13).
As Morbillivirus and Brucella infections were not detected in this case, it is possible that S. speeri infection may have led to immunosuppression and secondary Aspergillus fumigatus pneumonia, or alternatively, though considered less likely, another unidentified systemic disease or stressor may have caused susceptibility to S. speeri and fungal pneumonia. A. fumigatus bronchopneumonia was reported as the primary cause of death in the only previous report of A. fumigatus in a S. frontalis (5). S. frontalis may be susceptible to severe disease from pulmonary aspergillosis.
Magnetic resonance imaging (MRI) is a more sensitive imaging modality for identification of central nervous system lesions than CT in cetaceans and other animals, though time, accessibility, and additional expense can influence its use (45). However, it is possible that central nervous system abnormalities would not have been identified in this case if an MRI was performed because intravenous contrast enhancing agents cannot be used postmortem. Although “virtopsy” is an emerging tool to help locate lesions and guide necropsy sample collection, this case demonstrates the importance of conducting complete histopathologic investigations, in concert with saving representative frozen tissue samples, even in the absence of visible lesions (8).
The sequence of the spirochete found in the tongue of this dolphin was most closely related to Treponema spp., with T. vincentii having the highest sequence identity at 97.1%. Differences in this region of the 23S gene of <1% are observed in the closely related T. vincentii, T. phagedenis, and T. denticola. As such, it is likely that the spirochete identified in the tongue of this dolphin represents a novel species, Candidatus Treponema stenella. The Bayesian phylogenetic analysis performed (Figure 4) revealed close relatedness of Candidatus Treponema stenella to multiple Treponema species that have been previously associated with either periodontitis in humans (T. medium, T. putidum, T. denticola, and T. parvum) or digital dermatitis in cattle and sheep (T. phagedenis and T. phagedenis-like, T. brennaborense, and T. pedis) (21–26). These two groups do not cluster separately, consistent with previous reports of overlap (e.g., T. medium has been reported in periodontal abscesses in humans, and T. medium and T. medium-like species have been reported in bovine and ovine digital dermatitis) (21–24). This is interesting, given cetaceans' phylogenetic relationship to ruminants (12). A study of dental disease in odontocetes found that S. frontalis had a comparatively high rate of dental disease (46).
There is evidence that certain treponemes may be more pathogenic than others, and complex relationships exist between Treponema spp. and their hosts (21, 26). For example, symbiotic or commensal Treponema spp. act as pathogens in periodontal disease in humans and digital dermatitis (21, 26). It is possible that Candidatus T. stenella may be a commensal organism that was an opportunistic pathogen in this case with the infection localized to chronic glossitis. Treponema spp. have been described as part of the Atlantic bottlenose dolphin microbiome (Tursiops truncatus), with significantly higher abundance in the genital region relative to the oral cavity or other body regions (27). Although disease associated with these Treponema spp. have not been reported in dolphins, it is noteworthy that one of the two recently identified Stenella coeruleoalba cases from Italy with Sarcocystis-like meningoencephalitis had ulcerative glossitis, and it is possible that immunosuppression secondary to Sarcocystis and Sarcocystis-like infections could put dolphins at risk for opportunistic infections from commensal Treponema spp. or other pathogens, though immunocompromise may be multifactorial (12, 27). More research is needed to elucidate the role of Treponema spp. as oral pathogens in cetaceans, especially because Treponema are difficult to culture, leaving many uncharacterized and poorly understood (19, 26). Thus, this case highlights the importance of non-culture based methods for identification and characterization of potential pathogens, and also raises concern that Treponema spp. (either pathogenic or commensal flora) may have the potential to cause clinically relevant disease in S. frontalis or other dolphins that are immunocompromised due to concurrent morbidities.
Further investigation is required to understand the epidemiology, clinical relevance, and pathologic significance of S. speeri as an emerging disease in S. frontalis and other marine mammals. Careful handling and thorough postmortem diagnostic work up (including imaging, necropsy, tissue fixation, cytology, histology, and collection of frozen tissues for molecular diagnostics) may increase the potential for discovering clinically significant findings in stranded marine animals.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for this study because rescue activities and diagnostic investigation were performed under a stranding agreement between National Marine Fisheries Service and the University of Florida's Marine Animal Rescue, and in accordance with general animal welfare standards.
Author contributions
SB and MW contributed to the pursuit and interpretation of diagnostic work up. EH and AG provided interpretation of the CT scan. RO contributed to preparation and interpretation of histopathology samples. AC performed molecular diagnostics (PCR). RO and JW contributed to preparation, design, and interpretation of molecular diagnostics. JW and SB contributed to the creation of the Candidatus Treponema stenella phylogenetic tree. NS conducted preparation and interpretation of clinical pathology samples. BB, LA, and MW participated in carcass recovery, animal transport, and performed the initial necropsy. BD managed submission of tissue samples. All authors contributed to manuscript writing and editing.
Funding
This study was funded by the Florida Fish and Wildlife Conservation Commission.
Acknowledgments
The authors thank the Florida Fish and Wildlife Conservation Commission for funding this work, as well as the staff of the Emerald Coast Wildlife Refuge, Escambia Sheriff's Department, Pensacola Beach Lifeguards, and the University of Florida's Aquatic Animal Health program for their efforts. The authors are also grateful for the contributions of Salvatore Frasca, Andrew Allison, the University of Florida College of Veterinary Medicine's Radiology Service faculty, Mary Wilson, and the radiology technician team. Special thanks to the Zoological Pathology Program at University of Illinois at Urbana-Champaign College of Veterinary Medicine, the University of Georgia® Veterinary Diagnostic Laboratories, and the Animal Health Diagnostic Center at Cornell University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1132161/full#supplementary-material
References
1.
Braulik G Jefferson TA . Stenella frontalis. The IUCN Red List of Endangered Species 2018:e.T20732A50375312. (2018). Available online at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T20732A50375312.en (accessed October 7, 2022).
2.
Herzing DL Perrin WF . Atlantic Spotted Dolphin. In:WürsigBThewissenJGMKovacsK, editors. Encyclopedia of Marine Mammals, Third Edition. London, United Kingdom: Elsevier. (2018). p. 40-41.
3.
Puig-Lozano R Fernández A Sierra E Saavedra P Suárez-Santana CM De la Fuente J et al . Retrospective study of fishery interactions in stranded cetaceans, Canary Islands. Front Vet Sci. (2020) 7:567258. 10.3389/fvets.2020.567258
4.
Díaz-Delgado J Arbelo M Sierra E Vela A Domínguez M Paz Y et al . Fatal Erysipelothrix rhusiopathiae septicemia in two Atlantic dolphins (Stenella frontalis and Tursiops truncatus). Dis Aquat Org. (2015) 116:75–81. 10.3354/dao02900
5.
Groch KR Díaz-Delgado J Sacristán C Oliveira DE Souza G Sánchez-Sarmiento AM et al . Pulmonary and systemic fungal infections in an Atlantic spotted dolphin and a Bryde's whale, Brazil. Dis Aquat Org. (2018) 128:1. 10.3354/dao03207
6.
Díaz-Delgado J Sierra E Arbelo M Suárez-Bonnet A Suárez-Santana C Grau-Bassas E et al . Primary uterine T-cell lymphoma with metastasis in an Atlantic spotted dolphin. (Stenella frontalis), Canary Islands, Spain J Wildl Dis. (2015) 51:538–41. 10.7589/2014-08-199
7.
Estep JS Baumgartner RE Townsend F Pabst DA McLellan WA Friedlaender A et al . Malignant seminoma with metastasis, Sertoli cell tumor, and pheochromocytoma in a spotted dolphin (Stenella frontalis) and malignant seminoma with metastasis in a bottlenose dolphin (Tursiops truncatus). Vet Pathol. (2005) 42:357–9. 10.1354/vp.42-3-357
8.
Hamel PE Giglio RF Cassle SE Farina LL Leone AM Walsh MT . Postmortem computed tomography and magnetic resonance imaging findings in a case of coinfection of dolphin morbillivirus and Aspergillus fumigatus in a juvenile bottlenose dolphin (Tursiops truncatus). J Zoo Wildl Med. (2020) 51:2. 10.1638/2019-0087
9.
Reidarson TH García-Párraga D Wiederhold NP . Marine Mammal Mycoses. In: Gulland FMD, Dierauf LA, Whitman KL. CRC Handbook of Marine Mammal Medicine, Third Edition. Boca Raton, FL: CRC Press. (2018). p. 389-416.
10.
Miller M Shapiro K Murray MJ Haulena M Raverty S . Protozoan Parasites of Marine Mammals. In: Gulland FMD, Dierauf LA, Whitman KL. CRC Handbook of Marine Mammal Medicine, Third Edition. Boca Raton, FL: CRC Press (2018). p. 425-439.
11.
Sierra E Espinosa de Los Monteros A Fernández A Díaz-Delgado J Suárez-Santana C Arbelo M et al . Muscle pathology in free-ranging stranded cetaceans. Vet Pathol. (2017) 54:2. 10.1177/0300985816660747
12.
Giorda F Romani-Cremaschi U Marsh AE Grattarola C Iulini B Pautasso A et al . Evidence for unknown sarcocystis-like infection in stranded striped dolphins (Stenella coeruleoalba) from the Ligurian Sea, Italy. Animals. (2021) 11:5. 10.3390/ani11051201
13.
Barbosa L Johnson CK Lambourn DM Gibson AK Haman KH Huggins JL et al . A novel Sarcocystis neurona genotype XIII is associated with severe encephalitis in an unexpectedly broad range of marine mammals from the northeastern Pacific Ocean. Int J Parasitol. (2015) 45:9–10. 10.1016/j.ijpara.2015.02.013
14.
Dubey JP Zarnke R Thomas NJ Wong SK Van Bonn W Briggs M et al . Toxoplasma gondii, Neospora caninum, Sarcocystis neurona, and Sarcocystis canis-like infections in marine mammals. Vet Parasitol. (2003) 116:4. 10.1016/S0304-4017(03)00263-2
15.
Ewing R Zaias J Stamper MA Bossart GD Dubey JP . Prevalence of Sarcocystis sp. in stranded Atlantic white-sided dolphins (Lagenorhynchus acutus). J Wildl Dis. (2002) 38:2. 10.7589/0090-3558-38.2.291
16.
Calero-Bernal R Mauroo NF Hui SW Kuiken T van de Bildt MW de Jong AW et al . Acute fatal sarcocystosis hepatitis in an Indo-Pacific bottlenose dolphin. (Tursiops aduncus) in Hong Kong. Vet Parasitol. (2017) 235:1. 10.1016/j.vetpar.2017.01.001
17.
Dubey JP Verma SK Dunams D Calero-Bernal R Rosenthal BM . Molecular characterization and development of Sarcocystis speeri sarcocysts in gamma interferon gene knockout mice. J Parasitol. (2015) 142:13. 10.1017/S0031182015001109
18.
Alves ME Fernandes FD Bräunig P Murer L Minuzzi CE Santos HF et al . Toxoplasma gondii, Neospora caninum, and Sarcocystis spp. in species of naturally infected birds. Pesqui Vet Bras. (2022) 1:42. 10.1590/1678-5150-PVB-7026
19.
Nises J Rosander A Pettersson A Backhans A . The occurrence of Treponema spp. in gingival plaque from dogs with varying degree of periodontal disease. PLoS ONE. (2018) 13:8. 10.1371/journal.pone.0201888
20.
Yousefi L Leylabadlo HE Pourlak T Eslami H Taghizadeh S Ganbarov K et al . Oral spirochetes: pathogenic mechanisms in periodontal disease. Microb Pathog. (2020) 144:4193. 10.1016/j.micpath.2020.104193
21.
You M Mo S Leung WK Watt RM . Comparative analysis of oral treponemes associated with periodontal health and disease. BMC Infect Dis. (2013) 13:1. 10.1186/1471-2334-13-174
22.
Kuhnert P Brodard I Alsaaod M Steiner A Stoffel MH Jores J . Treponema phagedenis. (Ex noguchi 1912) brumpt 1922 sp. nov. nom rev, isolated from bovine digital dermatitis. Int J Syst Evol Microbiol. (2020) 70:3. 10.1099/ijsem.0.004027
23.
Newbrook K Carter SD Crosby-Durrani H Evans NJ . Challenge of bovine foot skin fibroblasts with digital dermatitis treponemes identifies distinct pathogenic mechanisms. Front Cell Infect. (2021) 10:8591. 10.3389/fcimb.2020.538591
24.
Angell JW Clegg SR Sullivan LE Duncan JS Grove-White DH Carter SD et al . In vitro susceptibility of contagious ovine digital dermatitis associated Treponema spp. isolates to antimicrobial agents in the UK. Vet Dermatol. (2015) 26:6. 10.1111/vde.12269
25.
Buyuktimkin B Zafar H Saier MH Jr . Comparative genomics of the transportome of Ten Treponema species. Microb Pathog. (2019) 132:34. 10.1016/j.micpath.2019.04.034
26.
Klitgaard K Boye M Capion N Jensen TK . Evidence of multiple Treponema phylotypes involved in bovine digital dermatitis as shown by 16S rRNA gene analysis and fluorescence in situ hybridization. J Clin Microbiol. (2008) 46:9. 10.1128/JCM.00670-08
27.
Robles-Malagamba MJ Walsh MT Ahasan MS Thompson P Wells RS Jobin C et al . Characterization of the bacterial microbiome among free-ranging bottlenose dolphins (Tursiops truncatus). Heliyon. (2020) 6:e03944. 10.1016/j.heliyon.2020.e03944
28.
Sierra E Fernández A Suárez-Santana C Xuriach A Zucca D de Quirós YB et al . Morbillivirus and pilot whale deaths, Canary Islands, Spain, 2015. Emerg Infect Dis. (2016) 22:740. 10.3201/eid2204.150954
29.
Colegrove KM Venn-Watson S Litz J Kinsel MJ Terio KA Fougeres E et al . Fetal distress and in utero pneumonia in perinatal dolphins during the Northern Gulf of Mexico unusual mortality event. Dis Aquat Org. (2016) 119:1–6. 10.3354/dao02969
30.
Turenne CY Sanche SE Hoban DJ Karlowsky JA Kabani AM . Rapid identification of fungi by using the ITS2 genetic region and an automated fluorescent capillary electrophoresis system. J Clinical Microbiol. (1999) 38:2. 10.1128/JCM.37.6.1846-1851.1999
31.
Altschul SF Madden TL Schäffer AA Zhang J Zhang Z Miller W et al . Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nuc Acids Res Spec Publ. (1997) 25:3389–402.
32.
Johnson M Zaretskaya I Raytselis Y Merezhuk Y McGinnis S Madden TL . NCBI BLAST: a better web interface. Nucl Acids Res Spec Publ. (2008) 36(suppl_2):W5–9. 10.1093/nar/gkn201
33.
Prakas P Oksanen A Butkauskas D Sruoga A Kutkiene L ŠvaŽas S et al . Identification and intraspecific genetic diversity of Sarcocystis rileyi from ducks, Anas spp, in Lithuania and Finland. J Parasitol. (2014) 100:5. 10.1645/13-395
34.
Sledge DG Bolin SR Lim A Kaloustian LL Heller RL Carmona FM et al . Outbreaks of severe enteric disease associated with Eimeria furonis infection in ferrets (Mustela putorius furo) of 3 densely populated groups. J Am Vet Med Assoc. (2011) 239:12. 10.2460/javma.239.12.1584
35.
Arbelo M de Los Monteros AE Herráez P Andrada M Sierra E Rodríguez F et al . Pathology and causes of death of stranded cetaceans in the Canary Islands. (1999–2005). Dis Aquat Org. (2013) 103:87–99. 10.3354/dao02558
36.
Díaz-Delgado J Fernández A Sierra E Sacchini S Andrada M Vela AI et al . Pathologic findings and causes of death of stranded cetaceans in the Canary Islands. (2006–2012). PLoS ONE. (2018) 5:e0204444. 10.1371/journal.pone.0204444
37.
Sierra E Fernández A Felipe-Jiménez I Zucca D Díaz-Delgado J Puig-Lozano R et al . Histopathological differential diagnosis of meningoencephalitis in cetaceans: Morbillivirus, Herpesvirus, Toxoplasma gondii, Brucella sp, and Nasitrema sp. Front Vet Sci. (2020) 7:650. 10.3389/fvets.2020.00650
38.
Katoh K Toh H . Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform. (2008) 9:286–98. 10.1093/bib/bbn013
39.
Huelsenbeck JP Ronquist F MRBAYES . Bayesian inference of phylogenetic trees. J Bioinform. (2001) 17:754–5. 10.1093/bioinformatics
40.
Miller MA Schwartz T Pickett BE He S Klem EB Scheuermann RH et al . A RESTful API for access to phylogenetic tools via the CIPRES science gateway. Evol Bioinform Online. (2015) 11:43–8. 10.4137/EBO.S21501
41.
Ronquist F Teslenko M van der Mark P Ayres DL Darling A Hohna S et al . MrBayes 32: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. (2012) 61:539–42. 10.1093/sysbio/sys029
42.
Stamatakis A Hoover P Rougemont J . A rapid bootstrap algorithm for the RAxML web servers. Syst Biol. (2008) 57:758–71. 10.1080/10635150802429642
43.
Felsenstein J . Confidence limits on phylogenies: an approach using the bootstrap. Evol. (1985) 39:783–91.
44.
Resendes A Juan-Sallés C Almeria S Majo N Domingo M Dubey JP . Hepatic sarcocystosis in a striped dolphin (Stenella coeruleoalba) from the Spanish Mediterranean coast. J Parasitol. (2002) 88:1. 10.1645/0022-3395(2002)088[0206:HSIASD]2.0.CO;2
45.
Kot BCW Tsui HCL Chung T Lau APY . Postmortem neuroimaging of cetacean brains using computed tomography and magnetic resonance imaging. Front Mar Sci. (2020) 5:7. 10.3389/fmars.2020.544037
46.
Loch C Grando LJ Kieser JA Simões-Lopes PC . Dental pathology in dolphins (Cetacea: Delphinidae) from the southern coast of Brazil Dis Aquat Organ. (2011) 94:3. 10.3354/dao02339
Summary
Keywords
apicomplexa, case report, cetacean·marine mammal, encephalitis, glossitis, pneumonia, spirochaetaceae, trichocomaceae
Citation
Balik SE, Ossiboff RJ, Stacy NI, Wellehan JFX, Huguet EE, Gallastegui A, Childress AL, Baldrica BE, Dolan BA, Adler LE and Walsh MT (2023) Case report: Sarcocystis speeri, Aspergillus fumigatus, and novel Treponema sp. infections in an adult Atlantic spotted dolphin (Stenella frontalis). Front. Vet. Sci. 10:1132161. doi: 10.3389/fvets.2023.1132161
Received
26 December 2022
Accepted
15 March 2023
Published
03 April 2023
Volume
10 - 2023
Edited by
Fernando Costa Ferreira, University of Lisbon, Portugal
Reviewed by
Eva Sierra, University of Las Palmas de Gran Canaria, Spain; Veronica Risco-Castillo, INRA École Nationale Vétérinaire d'Alfort (ENVA), France
Updates
Copyright
© 2023 Balik, Ossiboff, Stacy, Wellehan, Huguet, Gallastegui, Childress, Baldrica, Dolan, Adler and Walsh.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah Emily Balik seb295@cornell.edu
†Present addresses: Sarah Emily Balik, National Aquarium, Baltimore, MD, United States
This article was submitted to Veterinary Experimental and Diagnostic Pathology, a section of the journal Frontiers in Veterinary Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.