Abstract
Introduction:
Angiostrongylus vasorum (A. vasorum) is a widely distributed gastropod-borne nematode, causing severe cardio-pulmonary disorders in dogs. In Romania, A. vasorum was detected in foxes and serologically confirmed in domestic dogs, but no clinical cases are currently diagnosed.
Methods:
Four dogs with clinical history of respiratory distress, originating from different geographical regions of Romania, were included in the study. One dog (case 1) was clinically evaluated using thoracic radiology and cardiac ultrasound; examination of feces and PCR were also performed for the etiologic diagnosis. The postmortem exam was performed in the other three cases, followed by parasitological and molecular analyses.
Results:
In the first case, parasitic pneumonia was suspected based on the radiographic examination of the thorax and the infection with A. vasorum was confirmed by L1 morphological identification and PCR. The main postmortem changes included large, coalescing, dark red areas of pulmonary consolidation (n = 3) and numerous adult nematodes in the pulmonary arteries (n = 2). The histopathological examination of the lungs showed necrotizing and granulomatous pneumonia with severe hemorrhages and chronic pulmonary arterial changes. Intralesional nematodes were seen in all necropsied cases. Additional inflammatory changes related to A. vasorum infection were identified in the brain and tracheobronchial and mediastinal lymph nodes (n = 2). Identification of larvae, histopathology and PCR confirmed the infection with A. vasorum.
Conclusions:
This study describes the first cases of canine cardiopulmonary angiostrongylosis in domestic dogs in Romania, and focuses on clinical presentation, pathological findings and molecular analysis. Angiostrongylosis should be included on the list of differential diagnoses of canine cardiopulmonary distress and/or haemorrhagic diathesis in Romania and awareness should be raised among clinicians to avoid post-mortem diagnosis in the future.
1. Introduction
Angiostrongylus vasorum (Nematoda: Metastrongyloidea) is a cardiovascular nematode, that resides in the pulmonary arteries and the right heart of domestic dogs and other carnivores (1). The biological life cycle is indirect with an intermediate obligatory host represented by terrestrial or aquatic gastropods, and may include paratenic hosts (frogs and domestic chickens) infected with the third larval stage (L3) (2). The natural definitive host is the red fox (Vulpes vulpes), an important reservoir that contributes to bridging infections in domestic animals (3, 4).
Infected dogs can develop severe thrombotic pulmonary endarteritis and pneumonia with varying clinical signs, ranging from minor respiratory distress to severe manifestations with a fatal outcome (5, 6). Respiratory symptoms (e.g., coughing, dyspnea), and exercise intolerance are caused by first stage larvae (L1) that migrate in the lungs (7, 8). In some cases, the lung pathology may progress to cor pulmonale associated with extrapulmonary lesions and clinical signs (9).
Clinical signs related to coagulation disorders were observed in some dogs infected with A. vasorum, and included hematochezia and haemorrhagic diathesis (5, 8).
Atypical clinical manifestations are produced by the larval migration in various organs or even adult stages in the eye, with panuveitis with hyphema, photophobia, and corneal edema (10).
Difficulties in making the specific diagnosis can arise when an animal exhibits non-specific symptoms such as weight loss, anorexia, lethargy or fever (8).
Neurological signs represent almost 4% of the clinical manifestations and, they can vary from ataxia, paresis, paralysis or seizures (5). Both hemorrhages due to coagulopathy (11) and larval migration (12) may cause neurological manifestations in dogs infected with A. vasorum.
Several diagnostic methods are available that can be used for the detection of canine angiostrongylosis. Until present, the “gold standard” method remains the modified Baermann technique for the detection of the L1 passed in the feces. Other tests like serological detection of circulating antigens (13, 14), or antibodies against A. vasorum (15), or isolating the parasites' DNA from different substrates (16, 17) are also available.
Since the first description in Toulouse (France) (18), this nematode gained a lot of interest among European parasitologists, mainly due to its broad and severe clinical picture. Two decades ago, the disease was sporadically reported in some parts of the United Kingdom, Denmark, France, Italy, and Spain (7). More recently, a sudden spread of the disease was observed in different locations in the same countries and in completely new geographical areas. Most likely, this expansion is correlated to the extended distribution of the red fox population (19).
Currently canine angiostrongylosis is an emerging disease reported in almost all European countries (1). Autochthonous infections by A. vasorum were identified in other parts of the world with three new different geographical regions reported in the last year (20–22).
In Romania, infection by A. vasorum was first confirmed in red foxes in 2017 (23) and 2 years later it was serologically confirmed also in domestic dogs (24). More recently, a nationwide survey among Romanian veterinarians proved that although still new for the country, A. vasorum infections were diagnosed in domestic dogs in different locations (25). However, one of the veterinarians that admitted diagnosing a case, treated it with melarsomine, a nematicide used for the treatment of Dirofilaria immitis (25).
Considering the known presence of the “French heartworm” in Romania, the severity of the disease and the challenges in diagnosing it, this study aimed to describe four lethal cases of canine angiostrongylosis, focusing on the clinical aspects and pathological findings, and to raise awareness among Romanian veterinarians.
To the best of our knowledge, no other clinical cases of canine angiostrongylosis have been previously reported in Romania.
2. Material and methods
2.1. Clinical history
Clinical signs were reported for all dogs and are summarized in Table 1.
Table 1
| Case | Breed | Age | Sex | Clinical signs |
|---|---|---|---|---|
| Case 1 | Yorkshire terrier | 10 years | M | Chronic dyspnea, coughing, low resistance to effort, cyanotic mucous membranes, crackling noise in lung area, grade II/VI systolic murmur |
| Case 2 | Caucasian shepherd | 8 years | F | Chronic dyspnea, coughing, low resistance to effort, apathy, vomiting |
| Case 3 | Poodle | 1.5 year | F | Acute dyspnea, coughing |
| Case 4 | Mixed breed | 5 months | F | Chronic dyspnea, coughing, apathy, anemia |
Signalment and clinical presentation of dogs infected with A. vasorum.
2.1.1. Case 1
A 10-year-old, intact male, Yorkshire terrier dog was referred to a private veterinary hospital from Bucharest (Pet Stuff Veterinary Hospital) on January 2021 with coughing, chronic dyspnea and cyanotic mucous membranes. The dog was previously diagnosed with degenerative mitral valve disease (stage B2) and pulmonary hypertension, for which it has received medication, but no deworming was performed within the last year. The dog was living in the periphery of the city, in a house with yard, near a lake. It was the only pet of the family and had no travel history. Upon physical examination, the animal showed increased respiratory rate, exercise intolerance, crackling noise in the pulmonary area, and obtunded mentation, besides abnormal grade II/VI systolic murmur. Point-of-care lung ultrasound (LUS) examination revealed numerous multifocal B-lines. Complete blood count (CBC) report showed marked reticulocytosis [205.2 K/μl reference interval (RI): 10.0–110.0] without evidence of anemia, most likely physiologic reticulocytosis (Hct: 43.0% RI: 37.3–61.7), inflammatory leukogram with a total white cell count of 18.57 K/μL (RI: 5.05–16.76) and neutrophilia with regenerative left-shift (14.01 K/μL, RI: 2.95 - 11.64), mild monocytosis (1.63 K/μL RI:0.16–1.12) suggesting stress leukogram and inflammatory response and non-reactive thrombocytosis (747 K/μL, RI:148–484) associated with inflammation and a redistribution mechanism. Routine serum biochemical profile revealed mild hyperglycemia, most likely stress induced (138 mg/dL; RI: 60–116 mg/dL), while lactate, blood urea nitrogen, creatinine, and electrolytes (sodium, potassium, chloride, calcium) were within normal limit values. The coagulation profile showed a prolonged prothrombin time (PT) (24.4 s; RI: 14–20 s) but normal activated partial thromboplastin time (aPTT) (120.1 s; RI: 94–123 s). A slightly increased PT with normal aPTT can suggest a disseminated intravascular coagulation (DIC) status. Quantitative D-dimer concentration was within reference intervals (0.27 μg/ml; RI: 0.023–0.65 μg/ml).
Following initial stabilization, a radiographic examination of the thorax and cardiac point of care ultrasound (POCUS) were performed. Radiology revealed small areas of interstitial pulmonary pattern and pronounced peribronchial infiltration around the large bronchi (Figures 1a, b). Parasitic pneumonia was suspected and a broncho-alveolar lavage was recommended but declined by the owner.
Figure 1
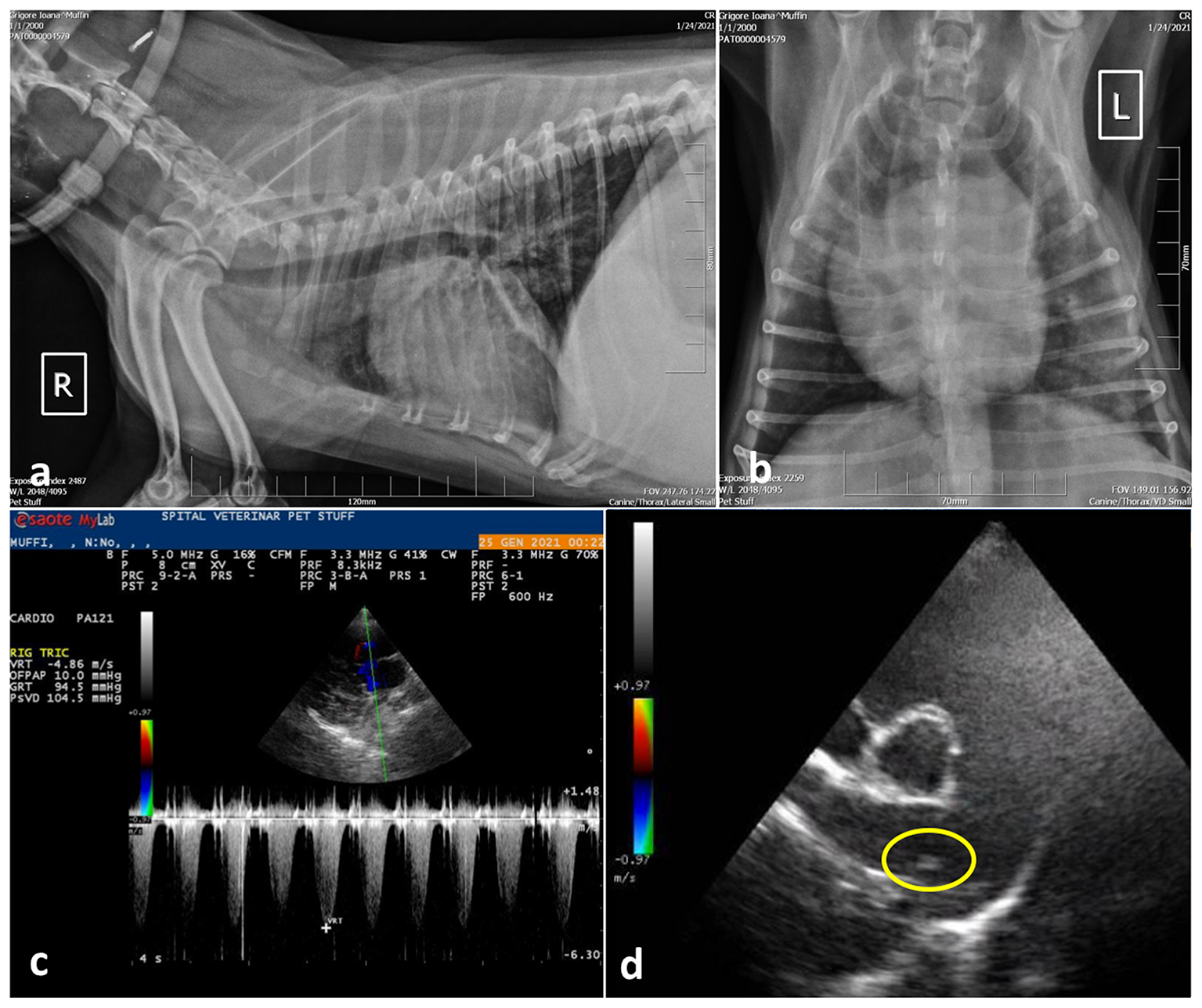
Case 1, thoracic radiographs: (a) Note the small distribution of areas with diffuse interstitial pulmonary pattern and pronounced peribronchial infiltration around the large bronchi; (b) Ventro-dorsal projection of the thorax radiography showing marked cardiomegaly with right ventricular enlargement; (c) Spectral Doppler study using continues wave revealed tricuspid regurgitant (TR) flow with a peak velocity >4.8 m/s suggesting severe PH (PG > 90 mmHg) and; (d) Right parasternal short axis view, slightly oblique for pulmonary trunk and right pulmonary artery optimal visualization. The main pulmonary artery is distended and parallel echogenic lines separated by a hypoechoic region were observed in the right main pulmonary artery (encircled area), suggesting a tubular structure, most probably the worm profile.
The cardiac POCUS images were reviewed by the cardiologist and showed signs of severe pulmonary hypertension (PH) with right ventricular enlargement, systolic flattening of the interventricular septum, the pulmonary artery to aorta ratio >1.0 and a peak tricuspid regurgitation velocity of ~4.86 m/s, PG 94 mmHg. In addition, parallel echogenic lines separated by a hypoechoic region were observed in the right main pulmonary artery in transverse and longitudinal views (right parasternal long axis 5 chambers and right parasternal short axis heart base) (Figures 1c, d). Type 5 PH was diagnosed, and further heartworm (Dirofilaria immitis) testing was performed by collecting a sample of peripheral blood, which was tested directly by means of semiquantitative PCR, and serologically by means of a rapid test (Idexx SNAP 4Dx Plus), respectively. Both exams came negative.
A sample of feces was tested using the Baermann-modified technique (26). Several collected larvae (n = 9) were morphologically identified as A. vasorum L1 based on their specific morphology and subsequently confirmed by PCR.
The dog was treated with a single dose of imidacloprid 10%, moxidectin 2.5% spot-on solution (Advocate; Bayer AG) in the clinic and the owner was advised to repeat the treatment at 4 weeks and to refer to a cardiologist but denied any other medical procedures or treatment. Four weeks after the initial treatment, via telephonic follow up the dog was better. The dog died 6 months after the initial diagnosis due to severe respiratory distress. A postmortem examination was not performed.
2.1.2. Case 2
The second case was an 8-year-old, intact female Caucasian shepherd dog from Cipău village (Mure? County). The dog was bought as a puppy at 4 months of age from Turda (Cluj county), and kept in the mentioned location since then. A representative part of Cipău village, 6.000 m2 (out of 10.000 m2) are fishing lakes populated with different fish species.
The dog showed low resistance to effort, chronic respiratory distress (dyspnea, coughing) and vomiting, and became more apathetic with each day. It was treated for pneumonia with different antibiotics (Fluoroquinolone, Doxycycline, and Amoxicillin-Clavulanic Acid) over a period of 10 months, without improvement. The clinical signs aggravated after ~10 days after weaning the puppies. The dog suddenly died 1 month later. The owners mentioned that the dog used to eat much dead or alive fish from the lakes. The last deworming was performed more than 6 months before the moment of death. There was another dog in the same household, which was tested using the modified Baermann funnel technique after the diagnosis was confirmed in the first animal, with negative results. The second dog did not consume fish.
2.1.3. Case 3
The third dog was a 1.5-year-old, intact female poodle, originating from Alba-Iulia (Alba County). The bitch developed acute dyspnea and coughing, and died after 20 min after the initial consult. There were other two mixed-breed dogs in the same household, which were tested by larval concentration with negative results.
2.1.4. Case 4
The fourth dog was a 5-month-old, intact female mixed breed originating from Reghin (Mure? county). The dog was referred for apathy, anemia and chronic respiratory distress (dyspnea, coughing) to a private veterinary clinic and died 1 h after the initial examination.
2.2. Postmortem examination
During postmortem examination, the pulmonary arteries were isolated, longitudinally opened and examined from the base of the heart to the narrowest branches at the margins of all lung lobes and scrutinized for parasites (cases 2 and 4) (9). Detected nematodes were collected and stored in 4% formalin-labeled tubes for morphological identification and absolute ethanol for molecular analysis. Samples from the lungs, tracheobronchial and mediastinal lymph nodes, liver, kidneys, gastric mucosa and brain (cerebral cortex, cerebellum, thalamus, brainstem) were collected for cytological, and histological analyses. In case 3, the necropsy was performed by the clinician and lung tissue samples were submitted to the Department of Anatomic Pathology.
2.3. Cytology
For cytological analysis, the imprints from tracheobronchial lymph nodes and lungs (cases 2 and 4) were stained by Dia Quick-Panoptic (DQP, Reagent Ltd., Budapest, Hungary).
2.4. Histopathology
For histology, tissue samples collected from the cases 2, 3 and 4 were fixed in 10 % phosphate-buffered formalin (pH = 7.0) for 24 h, routinely processed, embedded in paraffin wax, cut into 2–3 μm sections, and stained with hematoxylin and eosin (H&E). Samples were examined using an Olympus BX51 microscope. Photomicrographs and measurements of the parasites for morphologic identification were taken using an Olympus SP 350 digital camera and CellB basic imaging software (Olympus Corporation, Japan).
2.5. Parasitological examinations
The adult nematodes were collected from the pulmonary arteries (cases 2 and 4) and were cleaned in sterile saline (NaCl 0.9 %) solution. Several specimens were then microscopically examined in temporary mounts, clarified using lactophenol, and morphologically identified based on the specific descriptions (27).
For morphologic evaluation of the larvae (cases 2, 3, and 4), the remaining lung tissue samples were homogenized with saline solution on a magnetic stirrer, filtered and the solution was centrifuged in 50 ml corning tubes. One drop of Lugol solution was added to the sediment which was then examined.
2.6. DNA extraction and molecular identification
In the first case, DNA was extracted using the collected sediment containing larvae using a commercial kit (Qiagen, Germany). One ml of Baermann liquid was centrifuged at 5,000 rpm for 3 min and the supernatant was discarded. DNA purification was done with the QIAamp DNA Mini kit (Qiagen, Germany) was used according to the manufacturer's instructions, with elution of nucleic acids in 150 μl final volume.
PCR confirmation was performed using previously described primers and probe (121-bp product from ITS-2 region) (28), with minor modifications for best complementarity to all available sequences (data available upon request) and PerfeCta qPCR ToughMIx kit (Quantabio, USA) according to manufacturer's guidelines. Samples were loaded into a RotorGeneQ cycler (Qiagen, Germany) and PCR was performed: 2 min incubation at 95°C for polymerase activation and initial denaturation, followed by 40 PCR cycles of 95°C for 8 s and 60°C for 30 s with fluorescence data collection on green (FAM) channel.
In cases 2 and 4, genomic DNA was isolated from one nematode using a commercial kit (Isolate II Genomic DNA kit, meridian Bioscience, London, UK) according to the manufacturer's instructions. Subsequently, a fragment of the mitochondrial cytochrome c oxidase subunit 1 (cox1, ~700 bp) gene was amplified by conventional PCR, using the universal primers LCO1490/HCO2198, according to literature (29). The PCR product was excised from the gel, purified on a silica membrane spin column (Gel/PCR DNA Fragments Kit, Geneaid Biotech, Taiwan) and sequenced bidirectionally using an external service (performed by Macrogen Europe B.V., Amsterdam, The Netherlands). The sequences were assembled using Geneious software (biomatters LTD, New Zealand) and compared to other sequences available in the NCBI GenBank® database by Basic Local Alignment Search Tool (BLAST) analysis.
3. Results
3.1. Postmortem examination
3.1.1. Case 2
The main postmortem changes consisted of severe cyanosis and bilateral pulmonary densification with multiple areas of hemorrhage and necrosis (cobblestone appearance) (Figure 2a). The pulmonary arteries and right ventricle contained numerous adult nematodes of A. vasorum, measuring between 2 and 2.5 cm in length (Figure 2b). A single adult female of Dirofilaria immitis was found in the right ventricle of the heart. Cardiomegaly with hypertrophy of the right ventricular wall because of chronic pulmonary lesions and hypertension (cor pulmonale) was also observed. The tracheobronchial lymph nodes were edematous and hemorrhagic. The gastric mucosa from the body region showed granular to round white-gray nodules, varying between 2 and 5 mm, interpreted as nodular gastritis. No other significant gross changes were noticed.
Figure 2
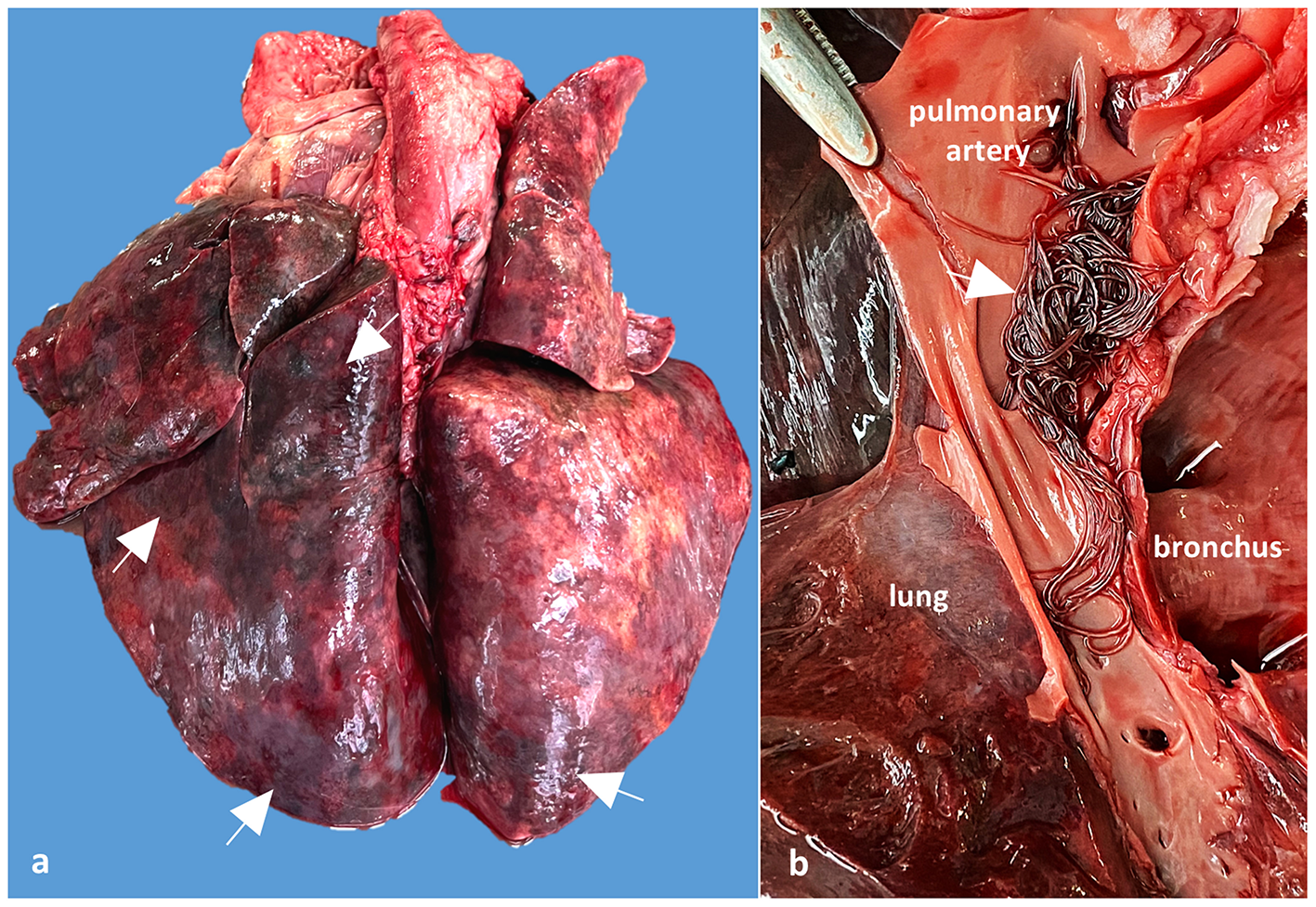
Case 2, Gross examination of the lungs and pulmonary arteries: (a) Lungs shows numerous dark red areas of densification, suggesting verminous pneumonia with necrosis and hemorrhages (arrows); (b) Detail of the opened pulmonary artery showing numerous adults of A. vasorum in the pulmonary artery (arrow).
3.1.2. Case 3
The clinician who performed the necropsy noted that the dog was in good body condition and the mucosae were pale. More than 100 ml of sero-hemorrhagic fluid was found within the thoracic cavity. The left lung failed to collapse and showed severe edema and multifocal dark red areas of densification due to necrosis and hemorrhages, affecting mostly the caudal lobe (Supplementary Figures 1a, b). The macroscopic evaluation of pulmonary arteries was not performed in this case.
3.1.3. Case 4
The dog was in a good body condition and the main postmortem changes were represented by bilateral and multifocal to coalescing red areas of consolidation within the lungs (Supplementary Figure 2a). The pulmonary arteries contained numerous thread-like adult nematodes, measuring between 2 and 2.5 cm in length (Supplementary Figure 2b). Cardiomegaly with hypertrophy of the right ventricular wall and right atrial dilation was a significant change.
3.2. Cytology
In cases 2 and 4, Dia Quick-Panoptic stained imprints showed numerous larvae of A. vasorum in both lungs and tracheobronchial lymph nodes (Supplementary Figures 3a, b). In addition, a mixed inflammatory cell population, composed of macrophages, small lymphocytes, plasma cells and scattered neutrophils, and eosinophils were identified in the examined samples.
3.3. Histopathology
Histologically, in all investigated cases (dogs 2, 3 and 4), the pulmonary parenchyma was multifocal to coalescing affected by variably-sized granulomatous nodules associated with hemorrhage, and centered on viable and fragmented parasitic organisms consisting of metastrongylid eggs and larvae (Figure 3a, a1). The granulomas were composed mainly of macrophages, multinucleated giant cells (foreign body and Langhans types), lymphocytes, plasma cells, and small numbers of eosinophils and neutrophils. The inflammatory nodules were occasionally rimmed by a thin fibrovascular tissue. The nematode eggs were thin-walled, ovoid, 50–70 μm in diameter, and contained a coarsely granulated basophilic material (morula) or a larva. The larvae were elongated and showed a thin acidophilic cuticle and a primitive intestinal tract. In some sections, the pulmonary arteries were markedly dilated and partially occluded by a thrombus, containing numerous longitudinal and cross sections of adult nematodes (Figure 3b). These parasites showed an eosinophilic cuticle, polymyarian-coelomyarian musculature, intestinal tract lined by multinucleated cells, and reproductive tract (Figure 3b). The wall of the affected arteries was severely and circumferentially thickened due to hypertrophy and hyperplasia of smooth muscle cells. The tunica intima was also thickened by fibrous tissue and inflammatory cells, represented by small lymphocytes, macrophages, and plasma cells. In other sections, the arterial lumen was occluded with organized thrombi, containing numerous blood-filled channels, fibroblasts and a few irregular collagen fibers (recanalization). The adjacent parenchyma was severely affected by coagulative necrosis associated with hemorrhage and neutrophilic infiltrates (pulmonary infarcts). Based on gross, cytological and histological findings a diagnosis of pulmonary angiostrongylosis was made.
Figure 3
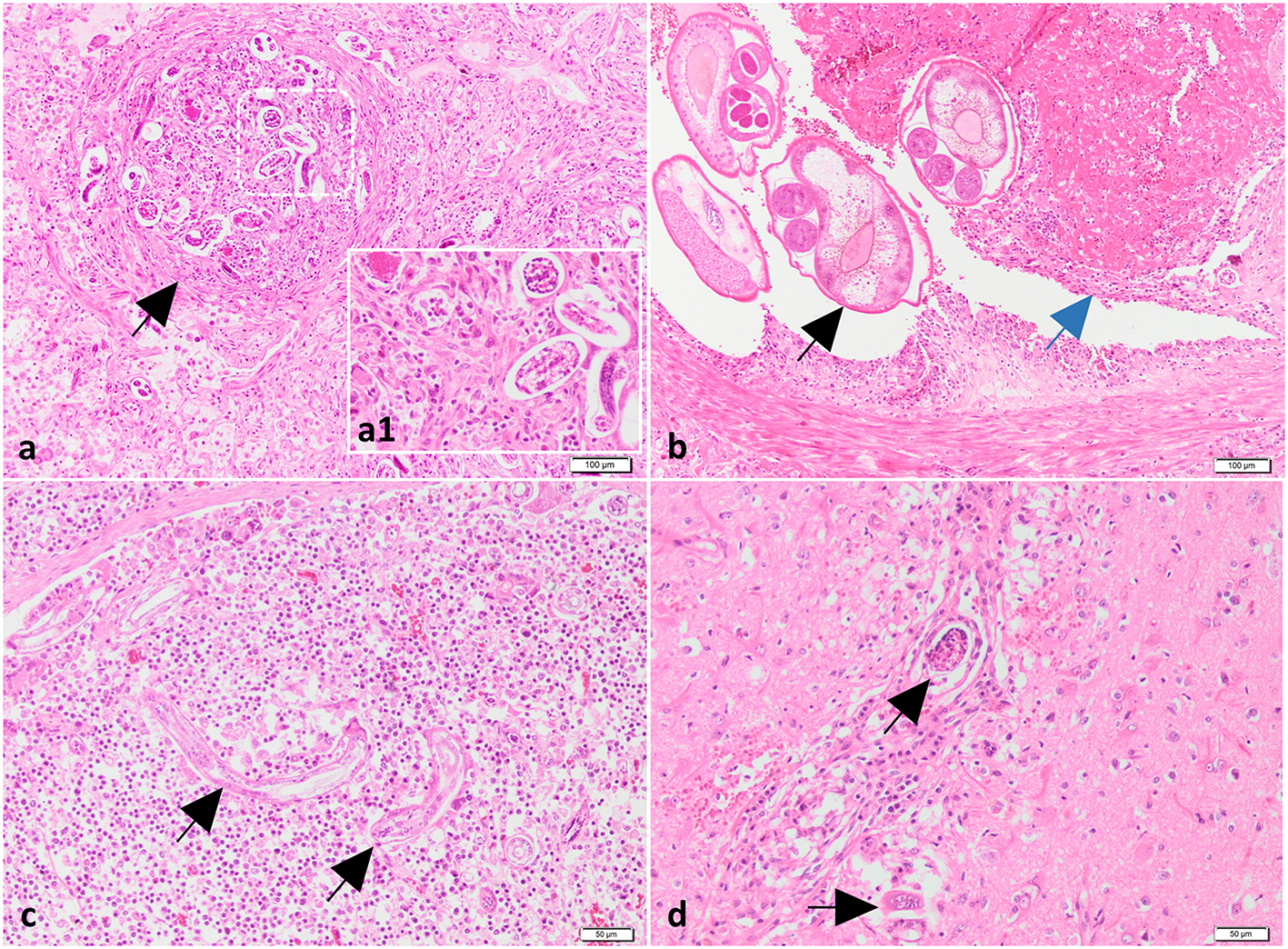
Histological findings of lungs, mediastinal lymph nodes and brain from case no. 2. (a) The lungs contain granulomas centered on nematode larvae and eggs (black arrow). H&E stain. Bar = 100 μm. Inset (higher magnification of figure a): nematode larvae and eggs surrounded by macrophages. (b) The pulmonary artery is partially occluded by a trombus (blue arrow) and numerous cross-sectioned adults of metastrongylid lungworms (black arrow). H&E stain. Bar = 100 μm. (c, d) Presence of parasites in the mediastinal lymph node (black arrow) (c) and neuroparenchyma (black arrows) (d) associated with a moderate inflammatory response. H&E stain. Bar = 50 μm.
The tracheobronchial and mediastinal lymph nodes were diffusely enlarged due to lymphoid hyperplasia and interfollicular and paracortical infiltration with macrophages, plasma cells and eosinophils. Nematode larvae were found in the subcapsular sinuses and cortex (Figure 3c). The cerebral cortex was multifocally affected by necrosis, vacuolation, hemorrhage, perivascular cuffs composed of lymphocytes, macrophages and neutrophils, and intralesional fragments of nematode larvae (cases 2 and 4) (Figure 3d). Moderate lymphoplasmacytic and eosinophilic gastritis with lymphoid follicle hyperplasia and mild colonization with Helicobacter-like organisms was found in the body region of the second dog. No significant changes were identified in the liver, kidneys and intestine of the investigated dogs.
3.4. Parasitological examinations
The obtained L1 were 330 to 360 μm in length with a non-rhabditiform esophagus, a terminal oral-opening and a waved tail with a dorsal spine and a notch consistent with the typical morphology of A. vasorum L1. All the examined adult nematodes collected from cases 2 and 4 were identified as A. vasorum (Figure 4).
Figure 4
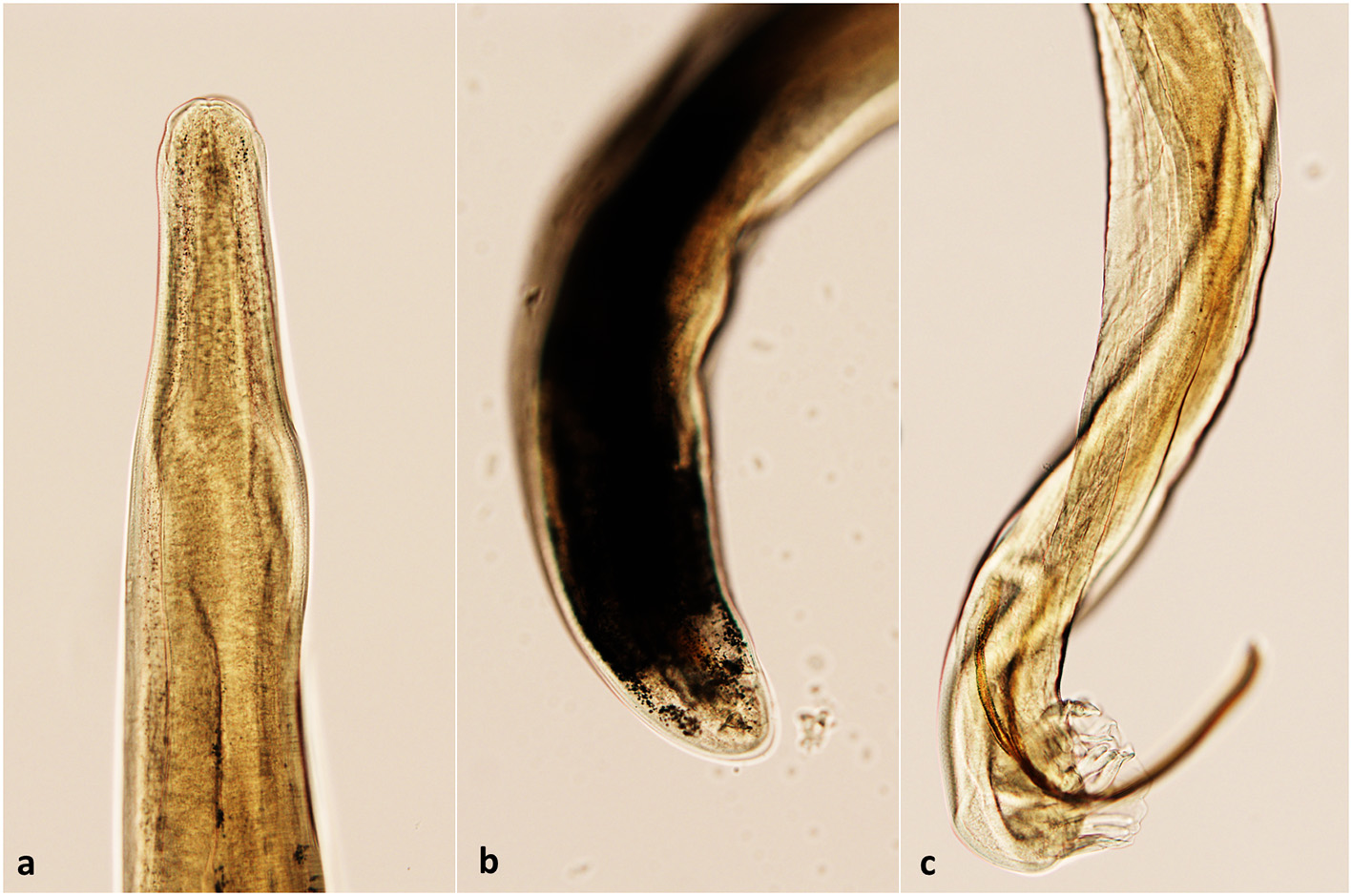
The morphological aspect of A. vasorum adult nematodes. (a) The anterior end (cephalic extremity); (b) Posterior end of a female A. vasorum; (c) Posterior end of a male A. vasorum with a short copulatory bursa and well-developed rays.
3.5. Molecular analysis
A specific amplification curve was obtained in case 1, demonstrating A. vasorum DNA in the analyzed sample. The Ct value obtained (22.76) might suggest a medium parasitic load into the sample.
A. vasorum was also amplified in cases 2 and 4. In case 2, The BLAST analysis revealed a 99.84% nucleotide identity (610/611 nucleotides) to two A. vasorum isolates, originating from red foxes in the UK (Accession numbers GQ982791 and GQ982741). The sequence was deposited in GenBank® under the Accession Number OQ210698. In case 4, The BLAST analysis indicated 100% nucleotide identity to numerous A. vasorum isolates (578 nucleotides) from red foxes from the UK (Accession Numbers: GQ982791, GQ982741, LT990053-LT990072). The sequence was deposited in GenBank® under the Accession Number OQ210699.
The phylogenetic analysis was performed using MEGA X software (30), using the Maximum Likelihood method, and Tamura and Nei model (31). Overall the analysis included 20 A. vasorum sequences obtained from domestic dogs and wild carnivores, and one A. cantonensis sequence as outgroup (Figure 5).
Figure 5

Phylogenetic tree constructed by Maximum Likelihood method and Tamura-Nei model. The tree with the highest log likelihood (-1039.46) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 21 nucleotide sequences. There were a total of 564 positions in the final dataset.
4. Discussion
The gastropod-borne nematode A. vasorum represents an important cause of cardio-pulmonary disease in dogs worldwide. Although more than 5 years passed since the first identification of A. vasorum in Romania, no clinical cases were yet diagnosed. The study aimed to describe the first clinical cases of canine angiostrongylosis in Romania (Figure 6). Angiostrongylus vasorum's distribution in Europe is characterized by hyperendemic foci in the proximity of low prevalence zones. The increase of A. vasorum reports in the last years could be due to wildlife movements, domestic dog relocations, and possibly climatic changes associated with an increasing number of gastropods (32, 33).
Figure 6
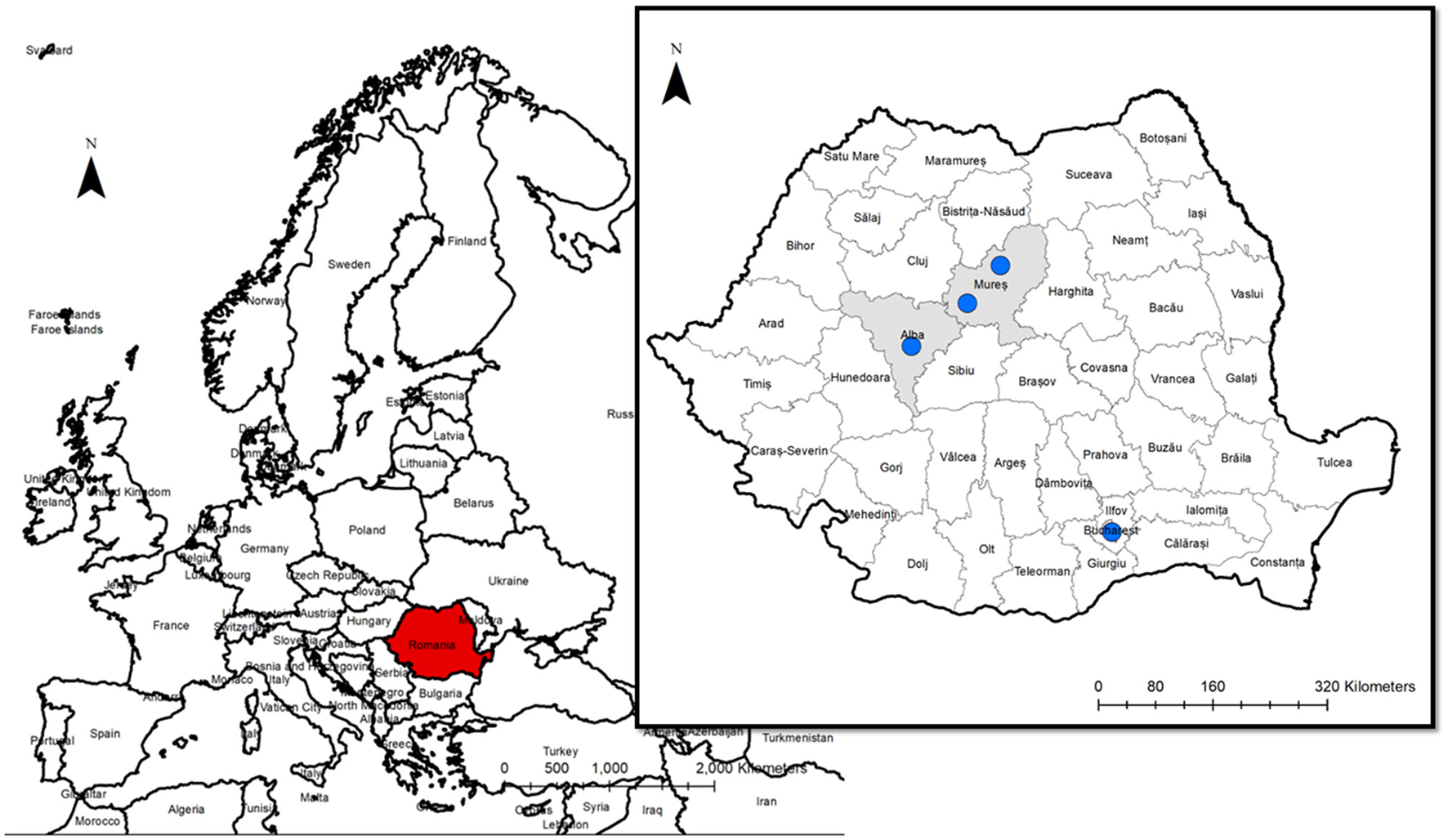
The geographical origin of the dogs infected by A. vasorum.
In a specific study aiming to determine the knowledge about canine angiostrongylosis among veterinarians, only 8.5% of them confirmed to have been diagnosed with a case, with the bulk of them hailing from Bucharest (25). In the same study, a lack of knowledge about this parasitic disease was detected particularly in rural areas where the veterinary focus is still centered mostly on farm animals. Besides a low interest from rural veterinarians in diagnosing and treating pet animals, the second problem is represented by the owners. In this study, only one dog (case 1) was correctly diagnosed intravitam and specific antiparasitic treatment was administered. However, due to the before-mentioned issue, many pet owners have a very low interest in treating their animals (personal observation) as in the present case when the dog died after 6 months due to respiratory distress.
Typically, infection by A. vasorum occurs through the consumption of intermediate or paratenic hosts. None of these dogs were seen to consume gastropods, however, the dog presented in case 2 was known to consume raw fish. Although fish are known as paratenic hosts for A. cantonensis (34), another species of the same genus, their role in the biology of A. vasorum is not known and further studies are needed to investigate the role of fish in the infection with canine angiostrongylosis.
Red foxes (Vulpes vulpes) are known as important reservoirs for parasitic diseases, including zoonoses (35). Experimental infections proved that A. vasorum can be transferred from foxes to dogs. Moreover, foxes seem to deal very well with infection and do not typically manifest clinical signs (36). In our case, only the dog from case 2 had free access to the environment and we can assume that infected foxes contaminated the pasture. Infection of urban dogs from cases 1, 3, and 4 can be associated with the presence of foxes in the urban environment which is frequently observed in Romania (personal observations).
The misdiagnosis of cases in Romania should not be related to the available diagnostic tests because there are several detection methods that can be easily used for the identification of canine angiostrongylosis, such as the “gold-standard” Baermann s technique, the AngioDetect rapid test (IDEXX Laboratories, Westbrook, Maine, USA) or genetic methods (28). In a study conducted by Bourque et al. (9), 3 out of 7 (42.9%) cases classified postmortem as positive for A. vasorum infection by larvae concentration methods, indicating that sometimes coproscopy is insufficient to provide a positive results. Another recent study comparing three methods for detection of A. vasorum in foxes showed that the sensitivity of dissection was higher than nested PCR of an 18S rRNA performed on bronchoalveolar lavage fluid, and canine A. vasorum antigen detection test (37).
In the present study, the coproparasitological examination was performed in only one case (case 1) suspected of cardiopulmonary angiostrongylosis based on clinical signs. Infection by A. vasorum was subsequently confirmed by PCR, using fluid resulting from the Baermann method. Unfortunately, in this case the postmortem examination was not carried out, and pulmonary lesions induced by these nematodes were not evaluated. As demonstrated by the three other lethal misdiagnosed cases (cases 2, 3 and 4), specific diagnosis methods (coproscopy) were not used due to the lack of knowledge related to A. vasorum infection in Romania. On the other hand, this scenario may lead to the use of ineffective treatment, endangering the animal's life, as in the described cases. Lack of awareness among veterinarians related to A. vasorum and non-specific clinical signs associated with infection (dyspnea, coughing, and exercise intolerance) can further complicate the diagnosis (38). However, angiostrongylosis should be considered as a potential cause in all dogs with respiratory symptoms, especially in those with free access to the environment. Interestingly, although common, no nervous signs were clinically observed in any of these cases. Moreover, as previously demonstrated, A. vasorum L1 and inflammatory response were detected in CNS (Case 2). The presence of L1 in the nervous system is considered to be related to neurological disorders (1).
The coagulation profile was available only for one of the dogs (case 1), and there was prolonged PT time, while aPTT time was within the normal reference interval, and platelet numbers were moderately increased, suggesting a disseminated intravascular coagulation (DIC) status. In most cases of canine angiostrongylosis, the platelet count is either within normal limits or more commonly slightly to moderately decreased (39, 40). Petechial hemorrhages associated with bleeding disorders were not recorded by the clinician in this dog. The link between infection by A. vasorum and hemostatic dysfunction is not fully elucidated, but the main theories proposed are as follows: (1) widespread immune complex deposition and complement fixation, which would lead to disseminated intravascular coagulation (41); (2) chronic infections may lead to downregulation of numerous proteins involved in homeostasis (42); and (3) hyperfibrinolysis (43).
Gross, cytological and histological changes were similar in all diagnosed cases, and in accordance with previously reported findings (9). Macroscopical findings of the lungs consisted in all cases of multifocal to coalescing dark-red densification areas, associated with hemorrhage and necrosis. The most consistent histological finding was granulomatous interstitial pneumonia associated with the intra-alveolar larvae and eggs. The presence of adult parasites was restricted to medium-sized and large arteries, and often they were embedded in a thrombotic mass and associated with proliferative endarteritis. Even though the main localization of the adult parasites is within the pulmonary arteries, they may be rarely encountered in unusual locations such as the pericardial sac, urinary bladder, kidney, and femoral artery (44–46). Larvae have been identified in numerous tissues, as follows: brain, cerebellum, liver, pancreas, skin, and kidney (44, 47). In this case series, the localization of the adult parasites was restricted to pulmonary arteries but in one case, numerous adult parasites were also identified in the right ventricle. From each case, several tissues were collected, but larvae and secondary inflammatory changes were identified only in the tracheobronchial lymph nodes and brain (cases 2 and 4). In only one case (case 4), nodular gastritis, affecting the body region, was identified. Nodular (follicular) gastritis defines marked mononuclear inflammatory infiltrates and the formation of lymphoid follicles with a germinal center in the lamina propria of the gastric mucosa. In dogs, the cause in still unknown, and some theories including Helicobacter-like organism infections and chronic respiratory distress especially in brachycephalic dogs (e.g., French bulldogs) have been proposed (48). In our case, no parasitic organisms were identified in the gastric nodular lesions.
Considering all these aspects, there is a strong need to raise awareness of this parasitosis in domestic canids in Romania among clinical veterinarians and dog owners.
5. Conclusions
This study reports the first clinical autochthonous case of infection with A. vasorum in a domestic dog from Romania and three other cases of fatal angiostrongylosis diagnosed post-mortem. We can confirm that respiratory signs represent the main clinical picture in infected dogs. Further national large-scale studies are needed for a complete picture of this disease in regard to its prevalence, distribution, biology and diagnostic methods in domestic dogs. Considering the severity of canine angiostrongylosis, national programs for continuous education could help advocate the importance of testing all symptomatic dogs and considering infection with A.vasorum as a part of differential diagnosis.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
Ethical review and approval was not required for the study on animals in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
MAT conceived and wrote the manuscript. MAT, CT, and AU performed the postmortem examination and sample prelevation. AN and IS performed the cytological examination. AI and MT performed the molecular work. AN and CG performed the histological examination. GD coordinated the study. GD, AI, and CG critically revised the manuscript. AB, AT, and IH did the clinical investigation and they were responsible for the follow-up. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by an internal grant of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca (Romania), Project No. 21863/04.10.2021. The funder had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Acknowledgments
MAT gratefully acknowledges the support of the Romanian Ministry of Research and Innovation, National Research Development Projects to finance excellence PFE-14/2022-2024.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1146713/full#supplementary-material
References
1.
Morgan ER Modry D Paredes-Esquivel C Foronda P Traversa D . Angiostrongylosis in animals and humans in Europe. Pathog. (2021) 10:1236. 10.3390/pathogens10101236
2.
Mozzer LR Lima WS . Gallus gallus domesticus: paratenic host of Angiostrongylus vasorum. Vet Parasitol. (2015) 207:81–4. 10.1016/j.vetpar.2014.11.003
3.
Schnyder M Fahrion A Riond B Ossent P Webster P Kranjc A et al . Clinical, laboratory and pathological findings in dogs experimentally infected with Angiostrongylus vasorum. Parasitol Res. (2010) 107:1471–80. 10.1007/s00436-010-2021-9
4.
Helm JR Morgan ER Jackson MW Wotton P Bell R . Canine angiostrongylosis: an emerging disease in Europe. J Vet Emerg Crit Care. (2010) 20:98–109. 10.1111/j.1476-4431.2009.00494.x
5.
Chapman PS Boag AK Guitian J Boswood A . Angiostrongylus vasorum infection in 23 dogs (1999-2002).J Small Anim Pract. (2004) 45:435–40. 10.1111/j.1748-5827.2004.tb00261.x
6.
Gallagher B Brennan SF Zarelli M Mooney CT . Geographical, clinical, clinicopathological and radiographic features of canine angiostrongylosis in irish dogs: a retrospective study. Ir Vet J. (2012) 65:1–10. 10.1186/2046-0481-65-5
7.
Koch J Willesen JL . Canine pulmonary angiostrongylosis: an update. Vet J. (2009) 179:348–59. 10.1016/j.tvjl.2007.11.014
8.
Colombo M Traversa D Grillotti E Pezzuto C De Tommaso C Pampurini F et al . Highly variable clinical pictures in dogs naturally infected with Angiostrongylus vasorum. Pathogens. (2021) 10:1372. 10.3390/pathogens10111372
9.
Bourque AC Conboy G Miller LM Whitney H . Pathological findings in dogs naturally infected with Angiostrongylus vasorum in newfoundland and labrador, Canada. J Vet Diagnostic Investig. (2008) 20:11–20. 10.1177/104063870802000103
10.
Colella V Lia RP Premont J Gilmore P Cervone M Latrofa MS et al . Angiostrongylus vasorum in the eye: new case reports and a review of the literature.Parasit Vectors. (2016) 9:1–8. 10.1186/s13071-016-1440-6
11.
Wessmann A Lu D Lamb CR Smyth B Mantis P Chandler K et al . Brain and spinal cord haemorrhages associated with Angiostrongylus vasorum infection in four dogs. Vet Rec. (2006) 158:858–63. 10.1136/vr.158.25.858
12.
Gredal H Willesen JL Jensen HE Nielsen OL Kristensen AT Koch J et al . Acute neurological signs as the predominant clinical manifestation in four dogs with Angiostrongylus vasorum infections in Denmark. Acta Vet Scand. (2011) 53:1–8. 10.1186/1751-0147-53-43
13.
Schnyder M Tanner I Webster P Barutzki D Deplazes P . An ELISA for sensitive and specific detection of circulating antigen of Angiostrongylus vasorum in serum samples of naturally and experimentally infected dogs. Vet Parasitol. (2011) 179:152–8. 10.1016/j.vetpar.2011.01.054
14.
Schnyder M Stebler K Naucke TJ Lorentz S Deplazes P . Evaluation of a rapid device for serological in-clinic diagnosis of canine angiostrongylosis. Parasit Vectors. (2014) 7:1–7. 10.1186/1756-3305-7-72
15.
Schucan A Schnyder M Tanner I Barutzki D Traversa D Deplazes P . Detection of specific antibodies in dogs infected with Angiostrongylus vasorum. Vet Parasitol. (2012) 185:216–24. 10.1016/j.vetpar.2011.09.040
16.
Schnyder M Jefferies R Schucan A Morgan ER Deplazes P . Comparison of coprological, immunological and molecular methods for the detection of dogs infected with Angiostrongylus vasorum before and after anthelmintic treatment. Parasitology. (2015) 142:1270–7. 10.1017/S0031182015000554
17.
Canonne AM Roels E Caron Y Losson B Bolen G Peters I et al . Detection of Angiostrongylus vasorum by quantitative PCR in bronchoalveolar lavage fluid in Belgian dogs. J Small Anim Pract. (2016) 57:130–4. 10.1111/jsap.12419
18.
Serres E . Entozoaires trouvés dans l'oreille droite, le ventricule correspondant et l'artère pulmonaire d'un chien. J Vét Midi. (1854) 7:70.
19.
Taylor CS Garcia Gato R Learmount J Aziz NA Montgomery C Rose H et al . Increased prevalence and geographic spread of the cardiopulmonary nematode Angiostrongylus vasorum in fox populations in Great Britain. Parasitology. (2015) 142:1190–5. 10.1017/S0031182015000463
20.
Idrissi H Khatat SEH Duchateau L Kachani M Azrib R Sahibi H . Canine cardiopulmonary nematodes in morocco: prevalence of dirofilaria immitis and report of the first autochthonous infection with Angiostrongylus vasorum. Moroc J Agric Sci. (2022) 3:99–105.
21.
Mahjoub HA Robbins WT Galeuzzi O Graham KF Jones MEB Buote MA et al . First report of Angiostrongylus vasorum (French heartworm) in red foxes (Vulpes vulpes) on Prince Edward Island. Can Vet J. (2022) 63:637–40.
22.
Uribe M Segeritz L Schnyder M Taubert A Hermosilla C López-Osorio S et al . Nationwide seroprevalence survey of Angiostrongylus vasorum-derived antigens and specific antibodies in dogs from Colombia. Microorganisms. (2022) 10:1565. 10.3390/microorganisms10081565
23.
Deak G Gherman CM Ionicǎ AM Vezendan AD D'Amico G Matei IA et al . Angiostrongylus vasorum in Romania: an extensive survey in red foxes, Vulpes vulpes.Parasit Vectors. (2017) 10:330. 10.1186/s13071-017-2270-x
24.
Deak G Gillis-Germitsch N Ionicǎ AM Mara A Pǎstrav IR Cazan CD et al . The first seroepidemiological survey for Angiostrongylus vasorum in domestic dogs from Romania. Parasit Vectors. (2019) 12:224. 10.1186/s13071-019-3481-0
25.
Deak G Berriatua E Mihalca AD . The current situation of Angiostrongylus vasorum in Romania: a national questionnaire-based survey. BMC Vet Res. (2021) 17:1–6. 10.1186/s12917-021-03034-1
26.
Conboy G Guselle N Schaper R . Spontaneous shedding of metastrongyloid third-stage larvae by experimentally infected limax maximus. Parasitol Res. (2017) 116:41–54. 10.1007/s00436-017-5490-2
27.
Costa JO Costa HM Guimarães M . Redescription of Angiostrongylus vasorum (Baillet, 1866) and systematic revision of species assigned to the genera angiostrongylus kamensky, 1905 and angiocaulus schulz, 1951. Rev Med Vet. (2003) 154:9–16.
28.
Jefferies R Morgan ER Helm J Robinson M Shaw SE . Improved detection of canine Angiostrongylus vasorum infection using real-time PCR and indirect ELISA. Parasitol Res. (2011) 109:1577–83. 10.1007/s00436-011-2414-4
29.
Folmer O Black M Hoeh W Lutz R Vrijenhoek R . DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. (1994) 3:294–9.
30.
Kumar S Stecher G Li M Knyaz C Tamura K Mega X . Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. (2018) 35:1547–9. 10.1093/molbev/msy096
31.
Tamura K Nei M . Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. (1993) 10:512–26.
32.
Morgan ER Jefferies R Krajewski M Ward P Shaw SE . Canine pulmonary angiostrongylosis: the influence of climate on parasite distribution. Parasitol Int. (2009) 58:406–10. 10.1016/j.parint.2009.08.003
33.
Traversa D Di Cesare A Conboy G . Canine and feline cardiopulmonary parasitic nematodes in Europe: emerging and underestimated. Parasit Vectors. (2010) 3:1–22. 10.1186/1756-3305-3-62
34.
Wallace GD Rosen L . Studies on eosinophilic meningitis. IV experimental infection of fresh-water and marine fish with Angiostrongylus cantonensis. Am J Epidemiol. (1967) 85:395–402. 10.1093/oxfordjournals.aje.a120701
35.
Veronesi F Deak G Diakou A . Wild mesocarnivores as reservoirs of endoparasites causing important zoonoses and emerging bridging infections across Europe. Pathog. (2023) 12:178. 10.3390/pathogens12020178
36.
Bolt G Monrad J Henriksen P Dietz HH Koch J Bindseil E et al . The Fox (Vulpes vulpes) as a reservoir for canine angiostrongylosis in Denmark: field survey and experimental infections. Acta Vet Scand. (1992) 33:357–62. 10.1186/BF03547302
37.
Houpin E McCarthy G Ferrand M De Waal T O'Neill EJ Zintl A . Comparison of three methods for the detection of Angiostrongylus vasorum in the final host. Vet Parasitol. (2016) 220:54–8. 10.1016/j.vetpar.2016.02.023
38.
Patteson MW Gibbs C Wotton PR Day MJ . Angiostrongylus vasorum infection in seven dogs.Vet Rec. (1993) 133:565–70.
39.
Sigrist NE Schefer RJJ Kutter APN . Characteristics of hyperfibrinolysis in dogs and cats demonstrated by rotational thromboelastometry (ROTEM). Vet J. (2018) 242:67–73. 10.1016/j.tvjl.2018.11.002
40.
Willesen JL Langhorn R Nielsen LN . Hemostatic dysfunction in dogs naturally infected with Angiostrongylus vasorum-a narrative review. Pathog. (2022) 11:249. 10.3390/pathogens11020249
41.
Caruso JP Prestwood AK . Immunopathogenesis of canine angistrongylosis: pulmonary effects of infection. Comp Immunol Microbiol Infect Dis. (1988) 11:85–92. 10.1016/0147-9571(88)90022-7
42.
Tritten L Gillis-Germitsch N Kockmann T Schnyder M . Quantitative proteomics analysis of Angiostrongylus vasorum-induced alterations in dog serum sheds light on the pathogenesis of canine angiostrongylosis. Sci Rep. (2021) 11:283. 10.1038/s41598-020-79459-9
43.
Sigrist NE Hofer-Inteeworn N Jud Schefer R Kuemmerle-Fraune C Schnyder M Kutter APN . Hyperfibrinolysis and hypofibrinogenemia diagnosed with rotational thromboelastometry in dogs naturally infected with Angiostrongylus vasorum. J Vet Intern Med. (2017) 31:1091–9. 10.1111/jvim.14723
44.
Oliveira-Júnior SD Barçante JMP Barçante TA Ribeiro VM Lima WS . Ectopic location of adult worms and first-stage larvae of Angiostrongylus vasorum in an infected dog. Vet Parasitol. (2004) 121:293–6. 10.1016/j.vetpar.2004.02.018
45.
Cury MC Lima WS . Ocorrência de Angiostrongylus vasorum no rim de cäo experimentalmente infectado. Arq bras med vet zootec. (1995) 47:593–5.
46.
Cury MC Lima WS . Rupture of femoral artery in a dog infected with Angiostrongylus vasorum. Vet Parasitol. (1996) 65:313–5. 10.1016/S0304-4017(96)00991-0
47.
Rinaldi L Cortese L Meomartino L Pagano TB Pepe P Cringoli G et al . Angiostrongylus vasorum: epidemiological, clinical and histopathological insights.BMC Vet Res. (2014) 10:236. 10.1186/s12917-014-0236-1
48.
Faucher MR Biourge V German AJ Freiche V . Comparison of clinical, endoscopic, and histologic features between dogs with chronic gastritis with and without lymphofollicular hyperplasia. J Am Vet Med Assoc. (2020) 256:906–13. 10.2460/javma.256.8.906
Summary
Keywords
Angiostrongylus vasorum , cardiopulmonary disorder, diagnosis, dogs, lungworms, pathology, Romania
Citation
Taulescu MA, Negoescu A, Ungur A, Toma C, Ionică AM, Gal C, Sandu I, Buzdea A, Tutuneanu A, Turcitu M, Horvat IE and Deak G (2023) Is the Angiostrongylus vasorum infection in domestic dogs underestimated or misdiagnosed? A comprehensive presentation of four lethal cases. Front. Vet. Sci. 10:1146713. doi: 10.3389/fvets.2023.1146713
Received
17 January 2023
Accepted
02 May 2023
Published
18 May 2023
Volume
10 - 2023
Edited by
Gabriele Rossi, Murdoch University, Australia
Reviewed by
J. Alberto Montoya-Alonso, University of Las Palmas de Gran Canaria, Spain; Manuela Schnyder, University of Zurich, Switzerland
Updates
Copyright
© 2023 Taulescu, Negoescu, Ungur, Toma, Ionică, Gal, Sandu, Buzdea, Tutuneanu, Turcitu, Horvat and Deak.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marian Aurel Taulescu marian.taulescu@usamvcluj.roGeorgiana Deak georgiana.deak@usamvcluj.ro
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.