- 1College of Veterinary Medicine, Shanxi Agricultural University, Jinzhong, China
- 2China Animal Health and Epidemiology Center, Qingdao, China
- 3New Hope Liuhe Co., Ltd., Chengdu, China
Senecaviurs A (SVA) infection, an emerging infectious disease in pig populations, is characterized by vesicular lesions predominantly affecting the mouth, snout, and hooves of infected pigs, similar to the symptoms of Foot and Mouth Disease Virus (FMDV). This disease first spread into China in 2015, causing great panic in the pig breeding industry. To determine the prevalence of SVA in pig herds in China from 2018 to 2021, a total of 4,901 pig tissue samples were collected from 18 provinces, autonomous regions and municipalities (P.A.M.s) for epidemiological investigation, virus isolation and genetic analysis. In 2021, the individual positive rates (IPRs) from the perspective of spatial distribution in East China, South China, Central China, North China, Southwest China, Northwest China, and Northeast China were 0, 0, 1.69, 0.94, 11.70, 3.31 and 2.21%, respectively. The herd positive rates (HPRs) were 0, 0, 9.52, 9.09, 50.00, 7.69 and 23.08%. From the perspective of temporal distribution, the IPR showed an overall downwards trend from 2018 to 2021, with only a slight increase in 2020. Moreover, the HPR decreased from 36.63 to 10.07%. From the perspective of population distribution in 2021, the IPR (2.62%) and HPR (12.00%) in apparently healthy pig herds (slaughterhouses) were greater than those in non-healthy pig herds (2.10 and 5.13%, respectively), consistent with the results in 2019. To characterize the prevalent strains, 10 SVA strains isolated from positive samples in 2019 were clustered in Clades I and VII; SVA-FJ039-2019, SVA-HuN032-2019, SVA-GX011-2019, SVA-FJ036-2019, SVA-GXF011-2019 and SVA-GXF053-2019 were clustered in Clade I; and SVA-FJ018-2019, SVA-SD069-2019, SVA-SD072-2019, and SVA-SD074-2019 were clustered in Clade VII. In conclusion, until 2021, the prevalence of SVA in pig herds in China was still relatively high, the contaminated area was still large, and there were a number of hidden infections. In the future, the epidemic status of SVA in pig herds in China must be closely monitored and the prevention and control measures must be adjusted in a timely manner.
1 Introduction
Senecavirus A (SVA) belongs to the genus Senecavirus in the family Picornaviridae. It is the only SVA serotype that has been identified (1). The virus was first discovered in a human embryonic retinal cell line (PRE. C6) by American researchers in 2002 and was named SVV-001 (2). The viral particle has a diameter of 25–30 nm and exhibits an icosahedral structure with no envelope (3). It is a single-stranded positive-sense RNA virus with a genome length of approximately 7300 nt, featuring a typical “L-4-3-4” structure of the small RNA virus family (4).
SVA can infect pig herds of different types and ages. Reports have confirmed that SVA is associated with porcine idiopathic vesicular disease (PIVD) and increased mortality in newborn piglets (5, 6). After infection, piglets, finishing pigs, and multiparous sows may experience ulcers in the nasal and oral cavities, as well as red blisters in the nose. Additional clinical symptoms may include occasional fever, lameness, and diarrhea. Acute death can occur in some cases (7). Notably, SVA shows similar transmission characteristics and clinical symptoms to Foot and Mouth Disease Virus (FMDV), and it is difficult to differentiate these two diseases with clinical diagnosis (2). Currently, neither vaccines nor drugs are commercially available for preventing SVA. Expensive laboratory diagnostics, especially qRT–PCR and ELISA, are required for diagnosis, making the prevention and control of SVA significantly challenging (5).
The first outbreak of SVA occurred in Canada in 2007. Furthermore, it has spread to multiple countries worldwide, including Brazil, Canada, the United States, and Thailand, and has become a globally prevalent virus infecting pigs (8). SVA first appeared in Guangdong, China, in March 2015 (9). Subsequently, outbreaks of SVA were reported in Fujian, Henan, and other regions from 2016 to 2017 (10). Ding et al. (11) reported a significant epidemiological correlation between successive SVA outbreaks that occurred in Guangdong and Hubei Provinces during retrospective monitoring of the SVA epidemic from 2015 to 2016. Zhang et al. (12) conducted retrospective monitoring of porcine SVA infection in seven provinces in China, including Liaoning and Fujian Provinces, as early as 2016. Since then, many epidemiological investigations have been conducted, but the majority of these studies are limited to local areas. In this study, we conducted an epidemiological study in 18 P.A.M.s of China from 2018 to 2021 to investigate the prevalence of SVA in China. Additionally, a phylogenetic analysis of 12 SVA strains was also conducted to analyse the prevalent strains (12).
2 Methods and materials
2.1 Experimental design and sample collection
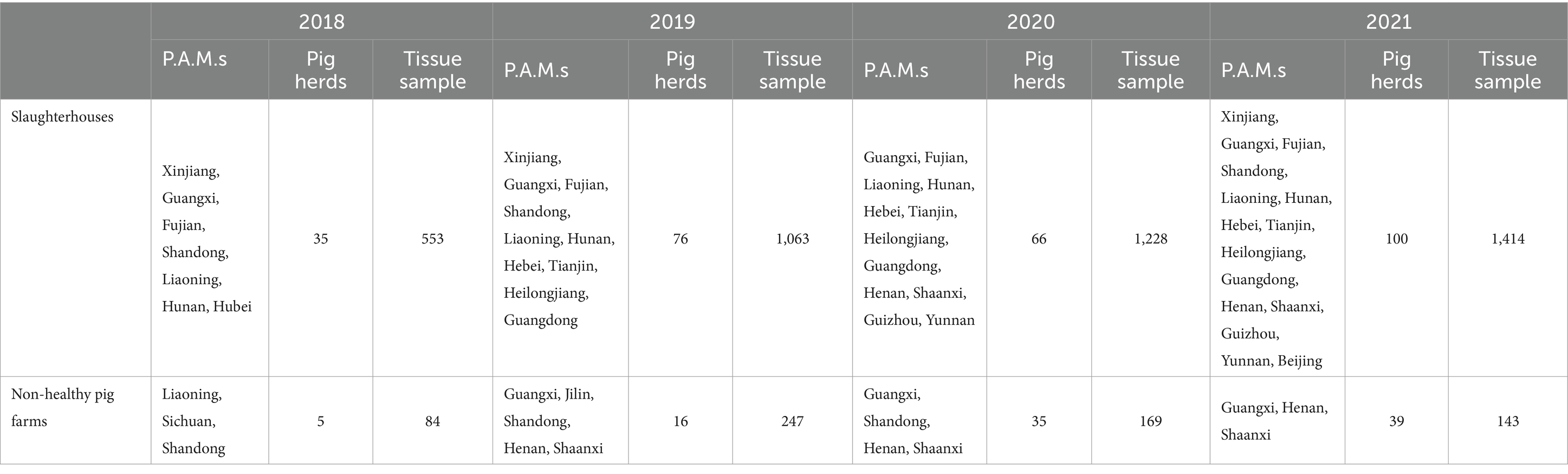
An epidemiological investigation was designed and conducted to investigate the prevalence of SVA in Chinese pig herds. In 2018, only 8 primary pig-producing provinces, autonomous regions and municipalities (P.A.M.s) were investigated. As the area contaminated with SVA progressively expanded, the number of P.A.M.s included in the investigation gradually increased and reached 15 by 2021 (Table 1). The samples were collected from two types of pig herds: samples from slaughterhouses representing healthy pig herds and samples from sick pigs and dead pigs (not specifically caused by SVA infection) from non-healthy pig farms representing non-healthy pig herds. The samples were collected by the veterinary authorities of the respective P.A.M.s and then sent to the China Animal Health and Epidemiology Center (CAHEC) for further analysis. A total of 4,901 tissue samples (each from the tonsils, spleen, kidneys, lymph nodes, lungs, etc., from one pig) were collected from 277 slaughterhouses and 95 non-healthy pig farms in 18 P.A.M.s (Table 1).
2.2 Tissue sample processing
The tissue samples were resuspended in 1 mL of PBS and then ground for 10 min. Subsequently, centrifugation was performed at 12,000 rpm for 10 min by utilizing a high-speed centrifuge at 4°C. The supernatant was used for RNA extraction following the instructions provided with the magnetic bead virus RNA/DNA extraction kit (Xi’an Tianlong Science and Technology Co., Ltd.).
2.3 qRT–PCR
qRT–PCR was performed using the following primer sequences: SVA-F, 5’-CTGCGCTGGGACCGTATCTCA-3′; SVA-R, 5’-CGCCCCACCCTCATT-3′; and SVA-P, 5’-FAM-TCGCCCGTAAGCGCGCACCGAGAG-BHQ1. The amplification program for qRT–PCR was as follows: reverse transcription at 55°C for 15 min, predenaturation at 95°C for 30 s, denaturation at 95°C for 10 s, and annealing and extension at 60°C for 30 s for a total of 45 cycles. The judgment criteria for the results were as follows: Ct < 35 was considered to be positive, 35 ≤ Ct ≤ 37 was considered suspected, and Ct > 37 was regarded as negative. The suspected samples were confirmed before being recorded as positive. The suspected or negative results after confirmation were recorded as negative.
2.4 SVA isolation
SVA was isolated from the BHK-21 cell line (stored in CAHEC) using Dulbecco’s modified Eagle’s medium (Vivacell, #C3113-0500) supplemented with 2% fetal bovine serum (Opcel, #BS-1101) and 1% penicillin–streptomycin (Vivacell. #C3423-0100). Ten distinct SVA strains were successfully isolated from qRT–PCR-positive samples.
2.5 SVA fragment RT–PCR amplification
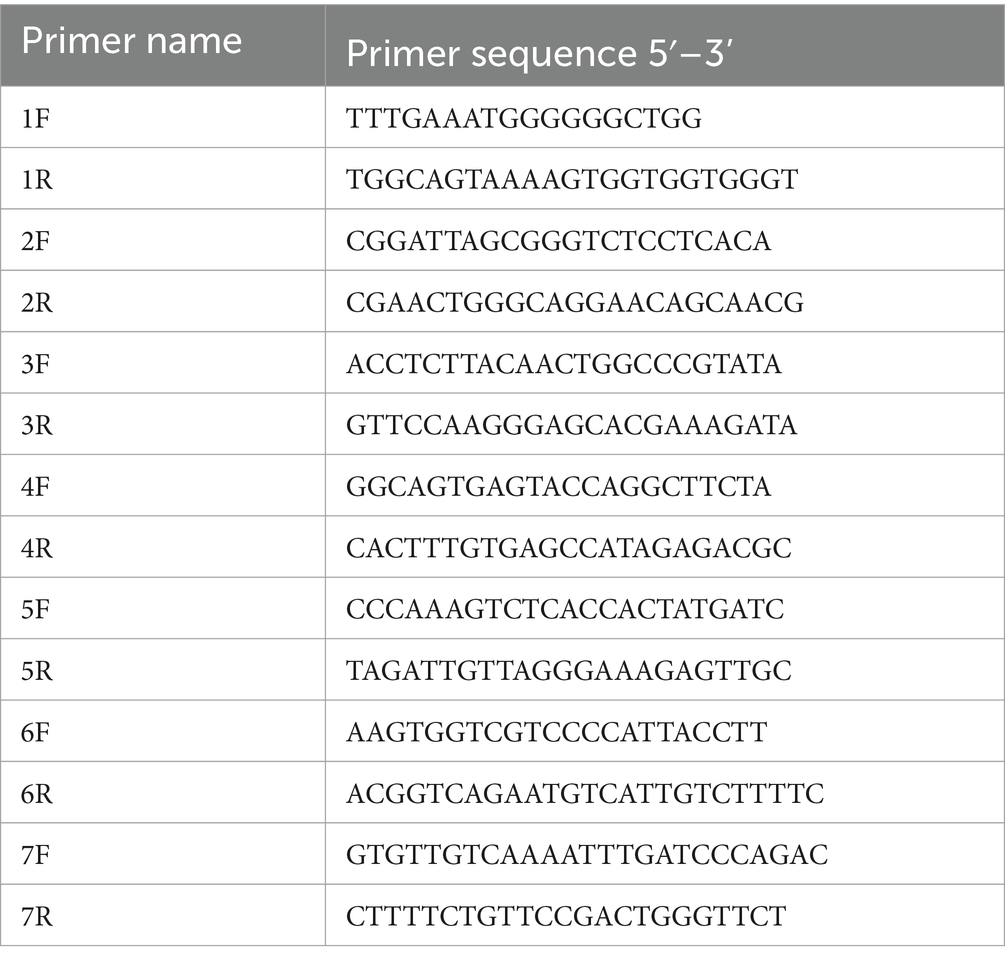
The nucleic acid samples from the 2019 samples were reverse transcribed by HiScript® III RT Supermix for qPCR (+cDNA Wiper), after which the whole-genome sequence of SVA was amplified in 7 segments (Table 2) by using Phanta® Max as a template. The amplification program was as follows: predenaturation at 94°C for 2 min, 30 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min. After the reaction, the PCR products were identified and detected by 1% agarose gel electrophoresis; the PCR products were subsequently sent to Shenggong Bioengineering (Qingdao) Co., Ltd., for sequencing and sequence assembly.
2.6 Data processing
Due to the inconsistency in the P.A.M.s where samples are collected each year, we conducted a prevalence analysis based on seven geographical regions of China, namely, East China (Shandong Province, Fujian Province, etc.), South China (Guangdong Province, Guangxi Zhuang Autonomous Region, etc.), Central China (Henan Province, Hunan Province, Hubei Province, etc.), North China (Tianjin, Beijing, Hebei Province, Inner Mongolia Autonomous Region, etc.), Southwest China (Yunnan Province, Guizhou Province, etc.), Northwest China (Xinjiang Uygur Autonomous Region, Ningxia Hui Autonomous Region, Gansu Province, Shaanxi Province, etc.) and Northeast China (Heilongjiang Province, Liaoning Province, etc.).
2.7 SVA genome assembly and phylogenetic analysis
SVA genome assembly was carried out by SeqMan, and the 7-segment sequences of each strain were assembled into a whole-genome sequence. Ten isolated SVA sequences and 157 reference SVA sequences from eight clades were included as previously reported (13). The Neighbor-Joining (NJ) tree was applied to the SVA sequences using Mega 11. The homology analysis was performed with Megalign.
3 Results
3.1 Spatial distribution
In 2021, the overall individual positive rate (IPR) in P.A.M.s was 2.57%, while the herd positive rate (HPR) was 10.07%. The IPRs in East China, South China, Central China, North China, Southwest China, Northwest China, and Northeast China were 0, 0, 1.69, 0.94, 11.70, 3.31 and 2.21%, respectively. The HPR of Southwest China reached 50.00%. Northwest China had a positive rate of 7.69%, followed by Central China with 9.52%, North China with 9.09%, and Northeast China with 23.08%. No SVA-positive samples were detected in East China or South China (Figure 1).
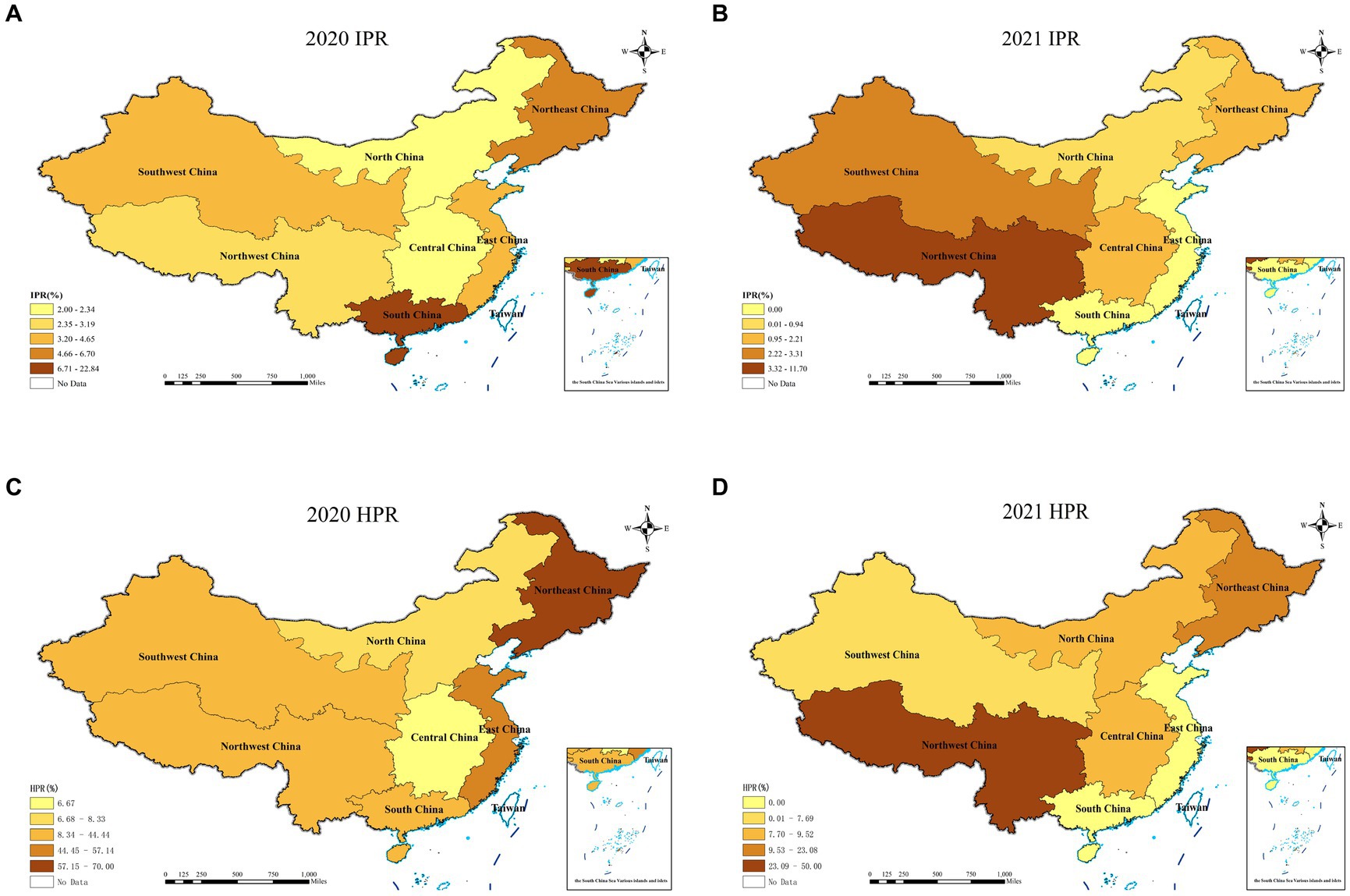
Figure 1. Individual positive rates and herd positive rates of SVA detected in each region from 2020 to 2021.
3.2 Temporal distribution
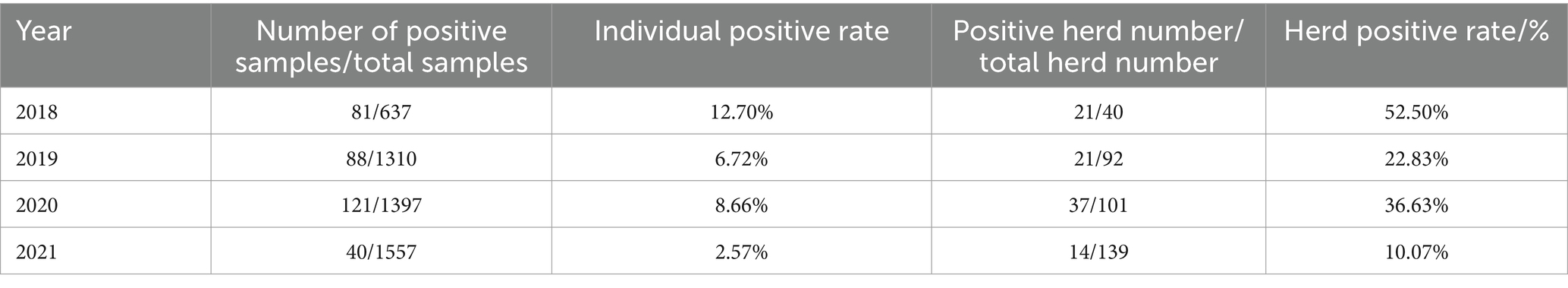
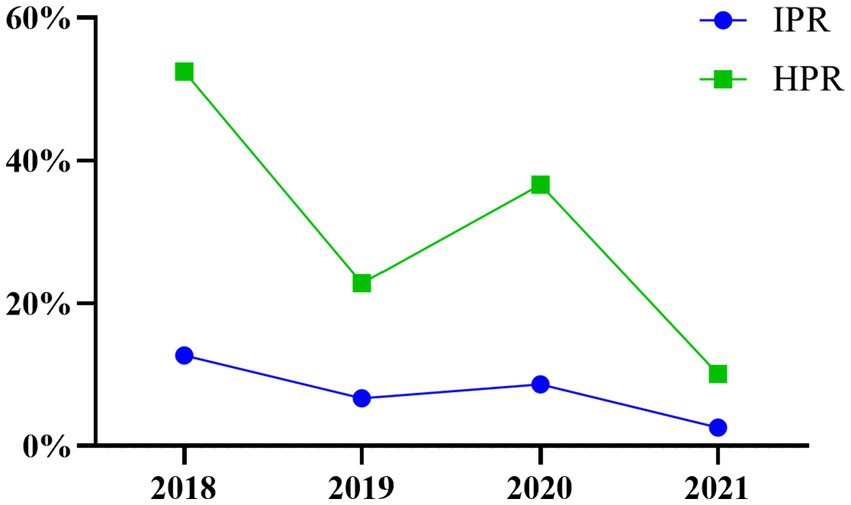
The IPR and HPR of the SVA exhibited a fluctuating declining trend from 2018 to 2021 in China (Table 3; Figure 2). Specifically, compared to 2020, the IPR decreased from 8.66 to 2.57%, and the HPR decreased from 36.63 to 10.07%. These findings suggested a notable decline in the prevalence of SVA from 2018 to 2021. When analysing the IPRs and HPRs in different regions of China (Figure 1), the IPRs in the seven regions in 2021 exhibited varying trends compared to those in 2020. Among these regions, the majority (6 out of 7) showed a decrease in the IPR, except for Southwest China. Similarly, the HPRs also demonstrated a downwards trend in 4 out of 7 regions, but an increasing trend was observed in the HPRs in North China, Central China, and Southwest China in 2021.
3.3 Population distribution
As shown in Figure 3, the IPR in slaughterhouses in 2021 was 2.62%, while in non-healthy pig farms, it was slightly lower at 2.10%. Moreover, the HPR in slaughterhouses in 2021 was 12.00%, which was higher than the 5.13% observed in non-healthy pig farms. These results were consistent with the data from 2019, in which the slaughterhouses exhibited greater IPRs and HPRs than did non-healthy pig farms. However, there was a change in this trend in 2020 for the IPR. During this year, the incidence of SVA was greater in non-healthy pig farms than in slaughterhouses.
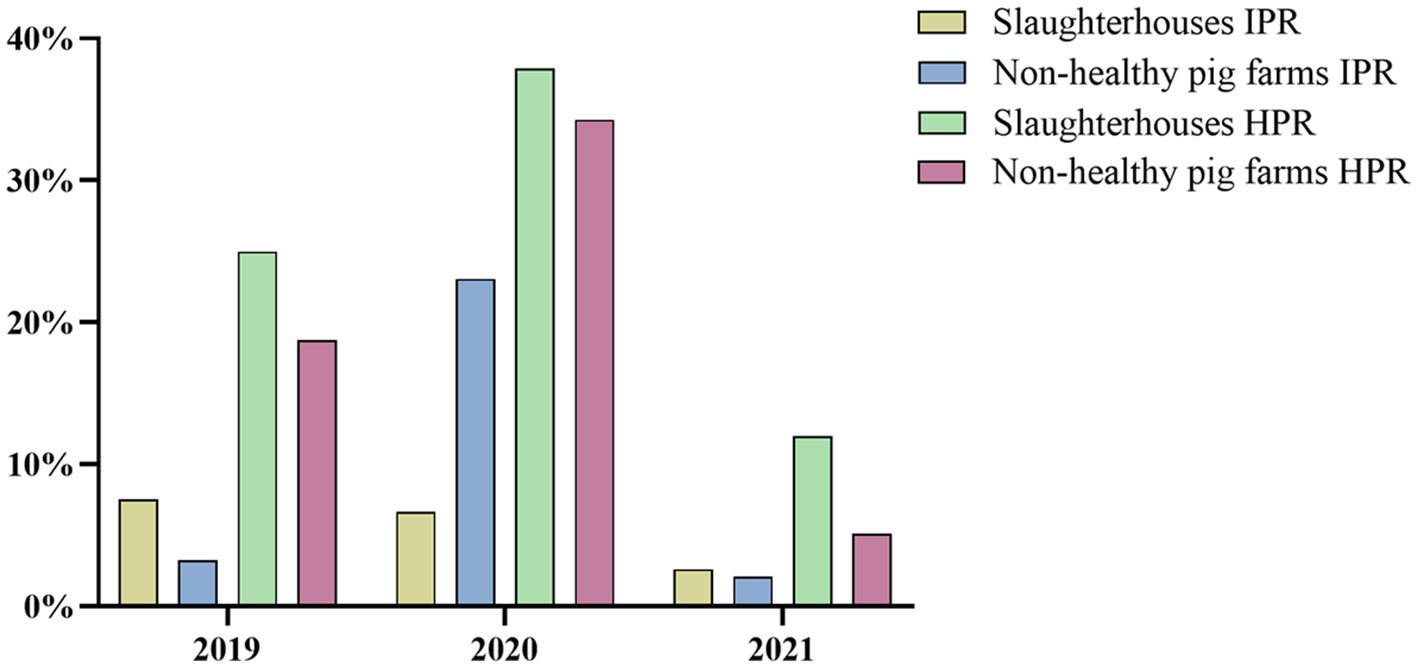
Figure 3. Comparison of IPR and HPR between slaughterhouse and non-healthy pig farms from 2019 to 2021.
3.4 Genetic evolution virus isolation and homology analysis
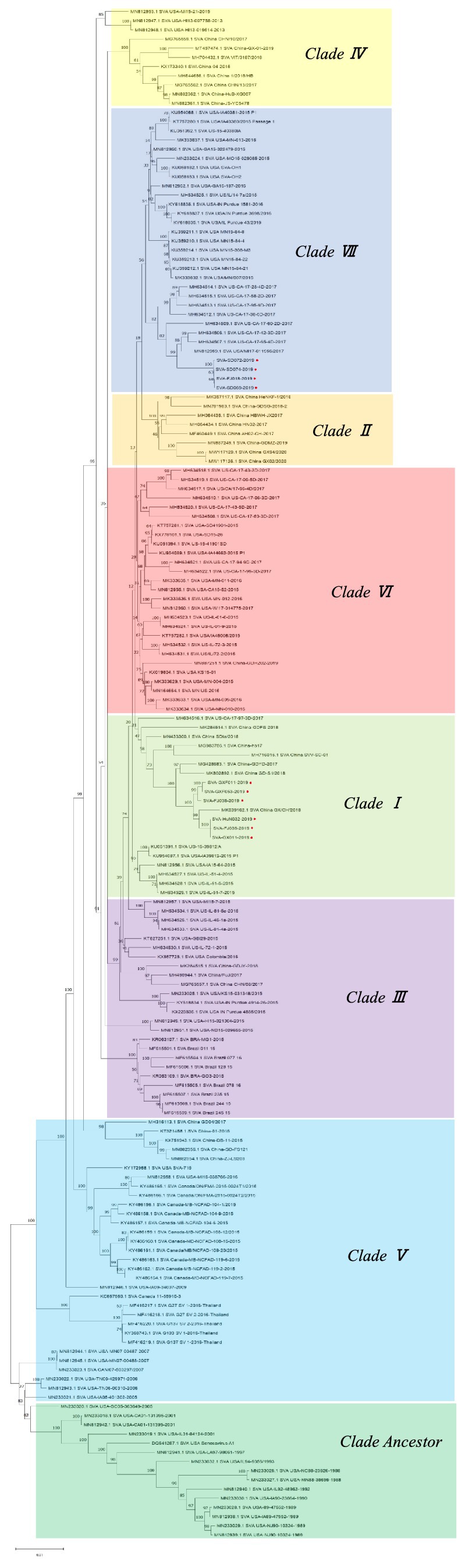
Ten SVA strains isolated from SVA-positive samples in 2019 were sequenced, and phylogenetic tree analysis was conducted using the Neighbor-Joining (NJ) method in MEGA11. Ten SVA isolates were clustered in Clade I or Clade VII. SVA-FJ018-2019, SVA-SD069-2019, SVA-SD072-2019, and SVA-SD074-2019 were clustered in Clade VII, showing a genetic association with the USA/MI17-011956/2017 strain, which was identified in the United States. Additionally, the isolates SVA-FJ039-2019, SVA-HuN032-2019, SVA-GX011-2019, SVA-FJ036-2019, SVA-GXF011-2019 and SVA-GXF053-2019 were clustered in Clade I (Figure 4).
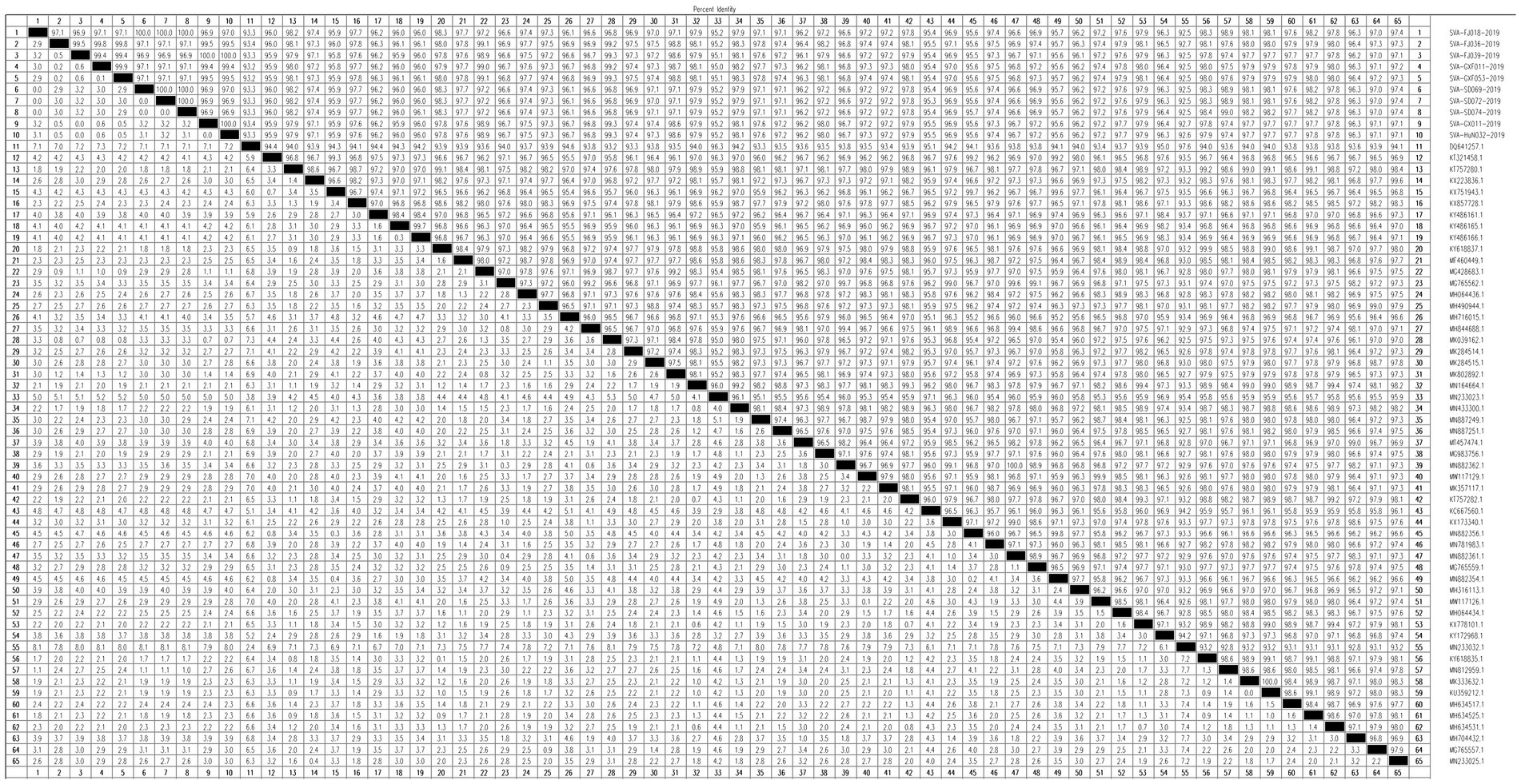
The homology analysis was performed with Megalign. The percentages of isolates SVA-FJ018-2019, SVA-SD069-2019, SVA-SD072-2019, SVA-SD074-2019, and the American isolate MI17-011956/2017 were approximately 98.90, 98.90, 98.90, and 99.00%, respectively. SVA-GXF011-2019, SVA-GXF053-2019, SVA-FJ036-2019, SVA-FJ039-2019, SVA-GX011-2019, and SVA-HuN032-2019 had the highest homology with the Chinese strain SVA/GX/CH/2018, ranging from 99.20 to 99.30% (Figure 5).
4 Discussion
The first SVA case was found in Guangdong Province, China, in 2015, which raised considerable concerns in the pig industry. Since then, a series of epidemiological studies on SVA have been performed in China. However, most of these investigations were disparate and localized, and a comprehensive epidemiological survey was lacking (14). To address this issue, an epidemiological survey was conducted in 18 P.A.M.s of China from 2018 to 2019. A total of 4,901 tissue samples were collected from 293 slaughterhouses and 104 pig farms. Due to variations in sampling across different years, it is more convenient to analyse the prevalence of SVA based on 7 geographical regions of China.
In our study, the IPRs of SVA in different regions varied significantly, ranging from as low as 0 to 11.70%. Notably, the IPR of SVA in Southwest China exceeded 10.00%, indicating a comparatively severe infection outbreak in this area. For the HPR, which was even more variable, spanning from 0% to a striking 50.00%, Southwest China once again showed the highest prevalence at 50.00%, while Northeast China experienced a lower but still concerning rate of 23.08%. These findings suggest that SVA contamination is widespread and particularly severe in some regions. Integrating our results with previous epidemiological studies conducted in the provinces of Hubei, Henan, and Hainan (15–20), it becomes apparent that a number of provinces across China have encountered SVA outbreaks. The temporal distribution of the SVA data between 2018 and 2021 reveals a fluctuating downwards trend in both the IPR and HPR. We suspected that this may be associated with the outbreak of African swine fever virus (ASFV) in China, after which national-level policies for prevention and control were introduced and effectively implemented, leading to a continuous decrease in the transmission of various epidemic diseases (21). The structure of pig breeding has gradually shifted from traditional small-scale household operations to large-scale farms, and the level of biosecurity protection in pig farms has continuously increased. This improvement not only helped in controlling ASFV (22) but also effectively prevented other epidemic diseases, such as SVA. However, we found that there was a sudden increase in the positivity rate observed in 2020, which we believe is primarily due to the rapid escalation of the pig herd population in China. In 2020, there was a significant increase in the number of reproductive sows and the total pig population, with year-on-year increases of 35 and 31%, respectively, compared to 2019. This increase implies greater movements within the pig population and higher stocking densities, contributing to an increased incidence of infectious diseases in pigs. Zhang et al. (23) carried out an epidemiological investigation of SVA prevalence in pig populations across Zhejiang Province. Their findings indicated fluctuating rates for SVA between 2018 and 2020, consistent with the trends observed in our research. The results for the population distribution indicated that there was a greater IPR and HPR in pigs from slaughterhouses than in those from non-healthy pig farms during the period from 2019–2021. Therefore, the health condition of the pig population is not the primary factor affecting SVA infection, and SVA should be transmitted and infected during the transportation process and preslaughter husbandry practices. Since SVA is prevalent in apparently healthy pig herds, there might be a subclinical infection of this virus in pig herds. Compared to the results of tissue sample detection, the IPRs and HPRs identified through serological testing in Northwest China are both much greater (data not shown), implying that the actual scope of SVA infection among pig populations might be broader than what tissue-based diagnostics alone have revealed.
Previous reports have indicated the presence of Clade Ancestor and Clade I-VII isolates in China (13). In this study, 10 epidemic strains were successfully isolated; the isolates SVA-FJ018-2019, SVA-SD069-2019, SVA-SD072-2019, and SVA-SD074-2019 were clustered in Clade VII, which was closely related to the American strain USA/MI17-011956/2017, suggesting the potential introduction of this strain through international trade, and further research is needed. Genomic sequencing has revealed marginal variance, with only approximately 20 amino acid variations, between attenuated and pathogenic strains of SVA (24). Given the potential for ongoing genetic evolution to enhance the virulence of prevalent strains of SVA in China, it is imperative to maintain vigilant monitoring and surveillance of SVA infection (25) and to develop accurate and specific differential diagnostic techniques to reduce the impact of SVA on the pig industry.
5 Conclusion
In conclusion, we conducted an epidemiological investigation of SVA in China from 2018 to 2021 to explore the prevalence of SVA in China. This epidemiological investigation provides a foundation for implementing effective SVA prevention and control measures to ultimately curb its propagation.
Data availability statement
The datasets presented in this study can be found in online repositories (https://www.ncbi.nlm.nih.gov/genbank/). The accession numbers are as follows: SVA-FJ018-2019, PP888073; SVA-FJ036-2019, PP888074; SVA-FJ039-2019, PP888075; SVA-GXF011-2019, PP888076; SVA-GXF053-2019, PP888077; SVA-SD069-2019, PP888078; SVA-SD072-2019, PP888079; SVA-SD074-2019, PP888080; SVA-GX011-2019, PP888081; SVA-HuN032-2019, PP888082.
Author contributions
CL: Investigation, Writing – original draft. CG: Investigation, Writing – original draft. LT: Investigation, Writing – original draft. JC: Data curation, Writing – review & editing. HZha: Data curation, Writing – review & editing. HZhe:Resources, Writing – review & editing. RW: Supervision, Writing –review & editing. SG: Methodology, Supervision, Writing – review & editing. ZS: Supervision, Writing – review & editing. BN: Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key R&D Program for the 14th Five-Year Plan (2021YFD1800301-04).
Acknowledgments
We thank the other members of our group for their help in sample collection and testing.
Conflict of interest
LT was employed by New Hope Liuhe Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Venkataraman, S, Reddy, S, Loo, J, Idamakanti, N, Hallenbeck, PL, and Reddy, VS. Crystallization and preliminary X-ray diffraction studies of Seneca Valley virus-001, a new member of the Picornaviridae family. Acta Crystallogr Sect F Struct Biol Cryst Commun. (2008) 64:293–6. doi: 10.1107/S1744309108006921
2. Houston, E, Temeeyasen, G, and Piñeyro, PE. Comprehensive review on immunopathogenesis, diagnostic and epidemiology of Senecavirus A. Virus Res. (2020) 286:198038. doi: 10.1016/j.virusres.2020.198038
3. Hole, K, Ambagala, T, and Nfon, C. Vesicular disease in pigs inoculated with a recent Canadian isolate of Senecavirus A. Can J Vet Res. (2019) 83:242–7.
4. Buckley, A. C. (2020). Pathogenesis and control of Senecavirus A in swine(Doctoral dissertation, Iowa State University). Available at: https://www.proquest.com/openview/9ba94e5834546f3caa545061da5d5cdc/1?pq-origsite=gscholar&cbl=18750&diss=y
5. Zhang, J, Piñeyro, P, Chen, Q, Zheng, Y, Li, G, Rademacher, C, et al. Full-length genome sequences of Senecavirus A from recent idiopathic vesicular disease outbreaks in U.S. Pig Genome Announc. (2015) 3:e01270–15. doi: 10.1128/genomeA.01270-15
6. Liu, C, Liu, C, Liu, Q, Wang, J, Li, X, Cui, S, et al. Research progress in the etiology, epidemiology, and diagnosis of type a Seneca virus.Pig industry. Science. (2018) 35:104–8. doi: 10.1016/j.virol.2016.07.003
7. Fan, X, Chi, T, Zhao, Y, Wu, X, and Wang, Z. Research progress of cereal virus disease in Senecavirus A. Chin J.Prev Vet Med. (2016) 38:831–4. doi: 10.3969/j.issn.1008-0425.2016.10.16
8. Pasma, T, Davidson, S, and Shaw, SL. Idiopathic vesicular disease in pig in Manitoba. Can Vet J. (2008) 49:84–5.
9. Wu, Q, Zhao, X, Bai, Y, Sun, B, Xie, Q, and Ma, J. The first identification and complete genome of Senecavirus A affecting pig with idiopathic vesicular disease in China. Transbound Emerg Dis. (2017) 64:1633–40. doi: 10.1111/tbed.12557
10. Zhao, Z, Zhu, Z, Yang, F, Cao, W, Liu, X, Yang, X, et al. Advance in Seneca valley virus epidemiology, diagnosis and control. Microbiol China. (2017) 44:3024–30. doi: 10.13344/j.microbiol.china.170546
11. Ding, K, Ma, X, Huang, J, Liu, X, and Sun, P. Advances in epidemiology and diagnosis technologies of Senecavirus A in China. Chin Vet Sci. (2023) 53:631–40. doi: 10.16656/j.issn.1673-4696.2023.0082
12. Zhang, Z, Zhang, L, Zhang, F, Liu, S, Dong, Y, Zhang, H, et al. Retrospective surveillance for Senecavirus A in porcine samples in China from 2016 to 2018. China Ani Health Inspec. (2019) 36:1–6. doi: 10.3969/j.issn.1005-944X.2019.01.001
13. Gao, H, Chen, YJ, Xu, XQ, Xu, ZY, Xu, SJ, Xing, JB, et al. Comprehensive phylogeographic and phylodynamic analyses of global Senecavirus A. Front Microbiol. (2022) 13:980862. doi: 10.3389/fmicb.2022.980862
14. Ran, X, Hu, Z, Wang, J, Yang, Z, Li, Z, and Wen, X. Prevalence of Senecavirus A in pigs from 2014 to 2020: a global systematic review and meta-analysis. J Vet Sci. (2023) 24:e48. doi: 10.4142/jvs.22307
15. Singh, K, Corner, S, Clark, SG, Scherba, G, and Fredrickson, R. Seneca valley virus and vesicular lesions in a pig with idiopathic vesicular disease. J Vet Sci Technol. (2012) 03:123. doi: 10.4172/2157-7579.1000123
16. Qian, S, Fan, W, Qian, P, Chen, H, and Li, X. Isolation and full-genome sequencing of Seneca Valley virus in piglets from China. Virol J. (2016):13. doi: 10.1186/s12985-016-0631-2
17. Wang, H, Li, C, Zhao, B, Yuan, T, Yang, D, Zhou, G, et al. Complete genome sequence and phylogenetic analysis of Senecavirus A isolated in Northeast China in 2016. Arch Virol. (2017) 162:3173–6. doi: 10.1007/s00705-017-3480-4
18. Zhang, X, Xiao, J, Ba, L, Wang, F, Gao, D, Zhang, J, et al. Identification and genomic characterization of the emerging Senecavirus A in Southeast China, 2017. Transbound Emerg Dis. (2018) 65:297–302. doi: 10.1111/tbed.12750
19. Ruan, H, Guo, Z, Geng, R, Weng, M, Qiao, S, and Zhang, G. Isolation and identification ofa Seneca valley virus strain. Chin Vet Sci. (2020) 50:1135–41. doi: 10.16656/j.issn.1673-4696.2020.0152
20. Mu, G, Lu, S, Zhang, Z, and Lin, H. Monitoring and early warning analysis of the prevalence of Seneca virus in Jilin Province. Jilin Ani Hus Vet Med. (2019) 40:73–4. doi: 10.3969/j.issn.1672-2078.2019.12.055
21. Liu, Y, Zhang, X, Qi, W, Yang, Y, Liu, Z, An, T, et al. Prevention and control strategies of African pig fever and Progress on pig farm repopulation in China. Viruses. (2021) 13:2552. doi: 10.3390/v13122552
22. Bellini, S, Rutili, D, and Guberti, V. Preventive measures aimed at minimizing the risk of African pig fever virus spread in pig farming systems. Acta Vet Scand. (2016):58. doi: 10.1186/s13028-016-0264-x
23. Zhang, H, Wang, Y, Huang, J, Zhang, L, Dong, J, Zhao, L, et al. Epidemiological investigation on infection with pig Senecavirus A in Zhejiang Province. China Ani Health Inspec. (2022) 39:1–6. doi: 10.3969/j.issn.1005-944X.2022.10.001
24. Zhang, H, Chen, P, Hao, G, Liu, W, Chen, H, Qian, P, et al. Comparison of the pathogenicity of two different branches of Senecavirus A strain in China. Pathogens. (2020):9. doi: 10.3390/pathogens9010039
Keywords: SVA, pig populations, epidemiological study, individual positive rate, herd positive rate
Citation: Li C, Gao C, Tao L, Cui J, Zhang H, Zheng H, Wei R, Gu S, Sha Z and Ni B (2024) Epidemiological investigation of Senecavirus A infection in pig herds in China from 2018 to 2021. Front. Vet. Sci. 11:1391513. doi: 10.3389/fvets.2024.1391513
Edited by:
Ping Qian, Huazhong Agricultural University, ChinaReviewed by:
Fuxiao Liu, Qingdao Agricultural University, ChinaJuan Bai, Nanjing Agricultural University, China
Copyright © 2024 Li, Gao, Tao, Cui, Zhang, Zheng, Wei, Gu, Sha and Ni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Ni, bmlib0BjYWhlYy5jbg==; Zhou Sha, c2hhemhvdUBjYWhlYy5jbg==; Shaopeng Gu, c2hwZ3VAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Chenyu Li
Chenyu Li Chunliu Gao2†
Chunliu Gao2† Shaopeng Gu
Shaopeng Gu Bo Ni
Bo Ni