- 1College of Life Science, Baicheng Normal University, Baicheng, China
- 2Terra Research and Teaching Centre, Microbial Processes and Interactions (MiPI), Gembloux Agro-Bio Tech, University of Liège, Gembloux, Belgium
- 3Key Laboratory of Development and Application of Rural Renewable Energy, Biogas Institute of Ministry of Agriculture and Rural Affairs, Chengdu, China
- 4Institute of Grassland Research, Chinese Academy of Agricultural Sciences, Hohhot, China
Urtica species is an angiosperm plant in the Urticaceae family. It serves as a traditional food and medicinal herb, possessing high nutritional value and various bioactive compounds, including polysaccharides, flavonoids, and polyphenolic compounds. In the realm of animal feeds, Urtica spp. can replace traditional protein feed sources and high-quality forage, thereby reducing feed costs. Moreover, Urtica spp. extract exhibits antioxidant and anti-inflammatory properties and boosts immune regulation. Hence, Urtica spp. plays a beneficial role in enhancing animal performance and improving their immune function. Recently, with the development of sustainable farming techniques, the demand for feed additives that prioritize safety, the absence of drug residues, and environmental friendliness have grown. Consequently, Urtica spp. and its extracts have received widespread attention in animal production. This article summarizes the biological functions of Urtica spp. and its application in animal husbandry while also outlining future prospects for its application. It will provide a scientific basis and reference point for the application of Urtica spp. in animal health and breeding.
1 Introduction
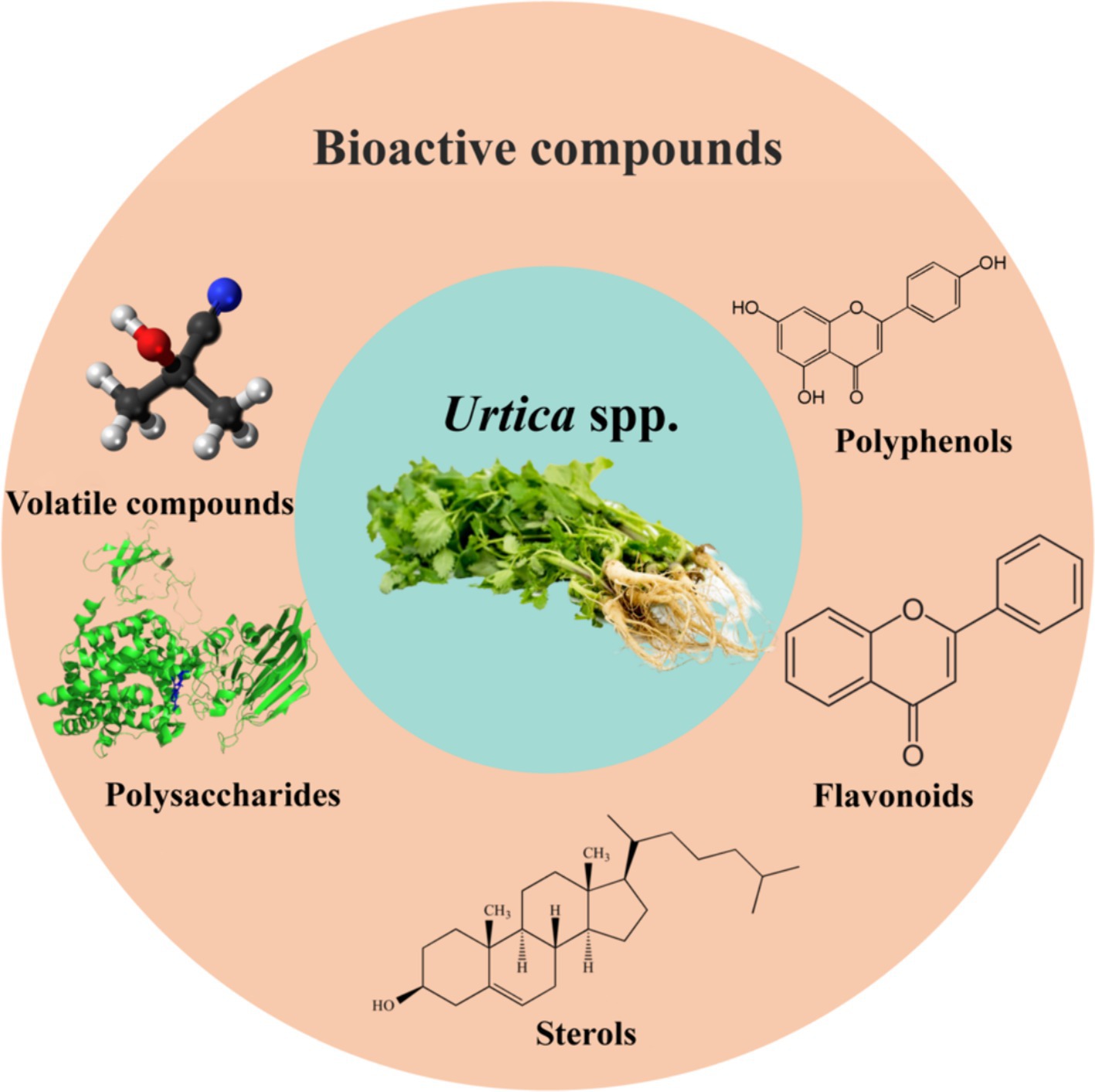
Recently, the heightened emphasis on food safety and nutritional health among human beings has led to a surge in widespread interest in the use of animal feed additives. With the ban on feed antibiotics, the quest for safe and efficient alternatives has gained increasing importance among researchers (1). For agricultural countries, the breeding industry holds a crucial position in the national economy. Owing to the lack of protein resources, essential protein feeds, such as tadpoles and soybean meal, have long relied on imports. Simultaneously, the intensification of environmental problems like global climate change has propelled the protection of the ecological environment and sustainable development into a subject of global concern (2). Under these circumstances, finding a new high-efficiency feed additive to replace traditional protein feeds has emerged as a critical issue for the breeding industry in large agricultural countries. Urtica spp., a traditional ingredient and Chinese medicinal material, boasts a rich composition of carotene, protein, cellulose, vitamins, minerals, and an array of biologically active compounds, including polyphenols, flavonoids, polysaccharides, pyrumin compounds, and sterol, as illustrated in Figure 1 (3–5). Research has unveiled that these substances exhibit numerous biological activities such as antioxidant, antibacterial, hypoglycemic, and blood lipid-reducing properties, hence playing an important role in regulating animal immunity and growth performance (6, 7). Furthermore, Urtica spp. is characterized by a high protein content and a balanced amino acid profile. The presence of high-quality protein can fulfill the demand for animal growth and development (8). The protein content of Urtica spp. is higher compared to soybean meal, while the levels of anti-nutritional factors are low, thus improving the digestive and absorptive abilities of animals (9). Nonetheless, Urtica spp. has immense potential for application in animal husbandry. However, the application of Urtica spp. as feed components is still in its nascent stage, necessitating in-depth exploration of its application methods and security. Consequently, this article aims to summarize the biological functions, action mechanisms, and application in animal husbandry of Urtica spp. with the intention of providing the scientific basis and reference for the utilization of Urtica spp. in ensuring the safety of animal production and improving the sustainability of agriculture.
2 Overview of the Urtica spp
2.1 Introduction and geographical distribution of Urtica spp
The genus Urtica belongs to the family Urticaceae in the major group Angiosperms (flowering plants). The most prominent members of the genus are the stinging nettle Urtica dioica L. and the small nettle U. urens L., which are native to Europe, Africa, Asia and North America (10). Plants belonging to the genus Urtica are herbaceous perennials and can grow up to 2 m tall. Serrated leaves are attached in pairs opposite each other to the stem. The soft leaves and the rest of the plant are coated in hairs, some of which sting. The serrated, hairy leaves and sting are generally recognized characteristics of this plant (11). Urtica spp. is a perennial herbaceous plant that serves as an important taxonomic unit in the Urticaceae family. It is highly valued for its versatility in the fields of food, feed, and medicine. Regarding culinary uses, the tender leaves and stems of Urtica spp. can be eaten as vegetables that are rich in proteins, vitamins, and minerals. Additionally, nettle tea is a common drink known for its heat-clearing and detoxifying properties. In the field of feed, Urtica spp. serves as a valuable source of nutrition for livestock and aquatic animals, promoting growth performance and enhancing immunity. Furthermore, in the pharmaceutical field, Urtica spp. is an important Chinese medicinal ingredient with anti-inflammatory properties, often used to treat rheumatism and arthritis (12).
2.2 Nutritional and phytochemical composition of Urtica spp
Different factors affect the chemical composition of nettle plants, such as the variety, genotype, climate, soil, vegetative stage, harvest time, storage, processing and treatment (13). Stinging nettles are a rich source of nutrients. A comprehensive proximate analysis showed that harvested up-growths contained approximately 90% moisture, up to 3.7% proteins, 0.6% fat, 2.1% ash, 6.4% dietary fiber and 7.1% carbohydrates. On the other hand, nettle leaf powders contain on average 30% proteins, 4% fats, 40% non-nitrogen compounds, 10% fiber and 15% ash (8). The protein content in Urtica spp. is well-balanced, with mineral and vitamin levels surpassing other nutrients. Protein can account for 30% of dry matter, with levels in the leaves typically around 23%, in the stems around 14%, and in the roots around 10%. Minerals make up 20% of the dry matter, along with trace elements such as zinc, iron, cobalt, potassium, nickel, and molybdenum and vitamins like vitamin A and vitamin C (14). Research indicates that Urtica spp. is abundant in bioactive compounds, including pyrodhumin compounds, flavonoids, carotene, fatty acids, and polyphenols, found in the leaves (15). However, the content of biologically active substances in roots is notably lower compared to leaves, particularly polyphenols. Roots also contain other active compounds like Scopoletin, fatty acids, polysaccharides, and plant hemorchin (16). The seeds mainly included saturated and unsaturated fatty acids, carotene, and β-carotene (17). The bioactive compounds present in the leaves, roots, and seeds of Urtica spp. are illustrated in Table 1.
3 The biological functions of Urtica spp
3.1 Antioxidant capacity
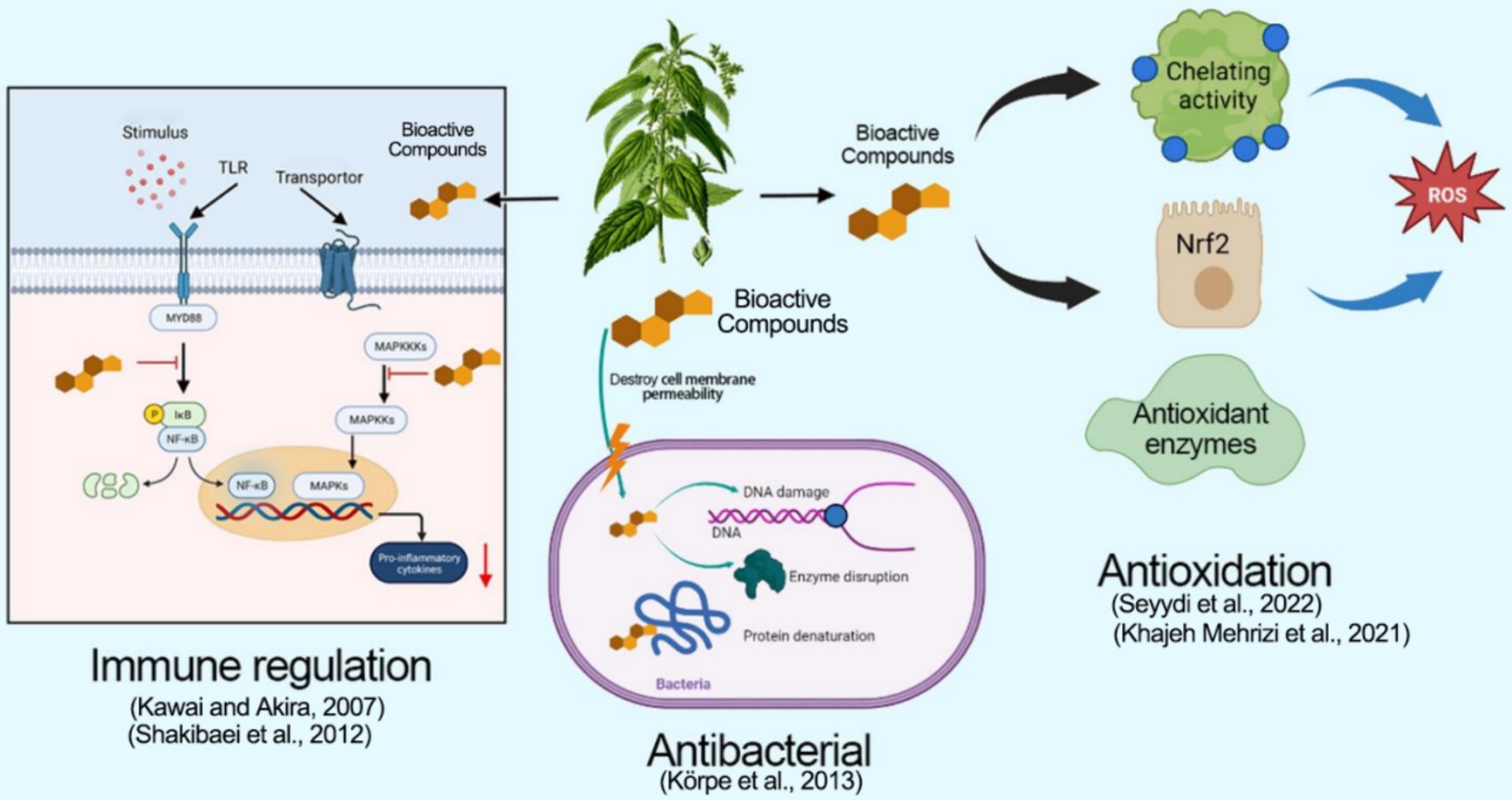
Animal oxidation can be triggered by different factors, such as radiation, inflammation, and infection. During the process of oxidative stress, an excess of reactive oxygen species (ROS) and nitric oxide free-radicals (NO2) can cause lipid peroxidation, protein oxidation, and DNA damage, thus affecting cellular function (18). Cells eliminate excessive oxygen free radicals through endogenous and exogenous antioxidant defense systems. The endogenous antioxidant defense system comprises superoxide dismutase (SOD), glutathione peroxidase (GSH-PX), and cysteine. Exogenous antioxidant defense systems include antioxidant compounds from in vitro sources (19, 20). Urtica spp. exhibits a range of biological activities, with its antioxidant properties vital for pharmacological activity. Bio-active compounds like flavonoids, polyphenols, phenolic acids, amino acids, and vitamins found in Urtica spp. can directly remove free radicals and mitigate oxidative stress reactions (21).
In addition, the bioactive compounds present in Urtica spp. can form stable complexes with iron ions through chelation, thereby inhibiting the formation of hydroxyl free radicals to reduce the production of ROS and mitigate cellular oxidative damage (22). Concurrently, the compounds in Urtica spp. facilitate the transfer of iron ions, leading to reduced accumulation within the body and decreased oxidative damage to cells (22). Research has demonstrated that leaf extracts of Urtica dioica can be chelated through iron ions, resulting in a significant reduction in oxidative stress and lipid peroxidation in the liver and kidney tissues of rats, hence shielding these organs from damage and ameliorating abnormal nerve behavior (23). Additionally, extracts of Urtica dioica seed can lower oxidative stress levels in the liver tissues of rats post-radiation exposure, improve their antioxidant capacity, and protect liver tissue from radiation damage (24). Moreover, the antioxidant properties of Urtica spp. are closely associated with the NrF2 signaling pathway. Studies have revealed that compounds in Urtica dioica can activate the NrF2 signaling pathway, thereby enhancing the activity of antioxidant enzymes (SOD and GSH-PX) in the cells and reducing mitochondrial oxidative stress reactions (25). Vajic et al. found that the extracts from Urtica spp. leaves can significantly enhance the SOD and GSH-PX activity in the serum of hypertensive rats, leading to lower levels of oxidative stress and protecting cardiovascular health (26). With the addition of Urtica spp. extract to the cellular and rat models, the NrF2 signaling pathway is triggered. As a result, the activities of SOD, GSH-PX, and glutathione reduction enzymes improve significantly, consequently reducing the level of oxidative stress in macrophages and enhancing the mitochondrial function of myocardial tissues (26, 27). Consequently, Urtica spp. exhibits diverse antioxidant properties and can serve as a natural antioxidant in animal husbandry.
3.2 Immune regulation
There is a direct relationship between the nutritional supply of animals and their immunity. Improving the nutritional quality of animals has potential value in improving their health (28). Natural nutrients can keep the immune system functioning efficiently. Therefore, improving the immunity of animals can be achieved by improving their nutritional levels (29). Urtica spp. has a positive effect on boosting immune function by regulating immune cells. Research has demonstrated that Urtica spp. extracts can regulate the function of various immune cells, including macrophages, T lymphocytes, and B lymphocytes (30). The presence of polysaccharides, flavonoids, and alkaloids in Urtica spp. can improve the activity of immune cells (31). Moreover, studies have revealed that Urtica spp. bolsters its phagocytic ability by stimulating macrophages (32). Furthermore, Urtica spp. extracts can promote the proliferation and differentiation of T lymphocytes while simultaneously boosting the production of antibodies by B lymphocytes (33). Additionally, Urtica spp. regulates the inflammatory response through MyD88-NF-κB-MAPKs signaling pathways. MyD88 plays an important role in the inflammatory responses of Toll-like receptors (TLR). It can affect the NF-κB pathway and regulate the production of inflammatory factors. MAPKs are an integral component of intracellular signal transformation, including extracellular regulating kinase (ERK), c-Jun NH(2)-terminal kinase (JNK), and P38, which can also participate in regulating inflammatory responses and apoptosis (34). Franciskovic et al. demonstrated that Urtica spp. extracts interact with TLRs, utilizing MyD88 as a receptor to regulate the MyD88/NF-κB pathway and inhibiting the phosphorylation of JNK and P38. This inhibition promotes the regulation of the MAPK pathway to reduce the inflammatory response of intestinal epithelial cells induced by LPS (35). Wagner et al. confirmed that polysaccharides derived from Urtica spp. can inhibit foot swelling in rats, indicating their anti-inflammatory properties (36). Moreover, Urtica spp. extracts can reduce the activation of the NF-κB signaling pathway induced by IL-1β, reducing pain and improving cartilage tissue lesions (37). Consequently, Urtica spp. holds promising applications in preventing and treating immune diseases and improving animal immunity, although its mechanisms to improve immune function require further investigation in different animal models.
3.3 Anti-bacterial
With the ban on antibiotics, more and more natural active substances have been discovered to replace antibiotics (38). Improving the antibacterial ability of animals can improve their growth performance (39). Urtica spp. extracts exhibit a broad spectrum of antibacterial, anti-fungal, and anti-viral properties. The inhibition of microbial growth is attributed to the synergistic action of multiple bioactive constituents. Research has demonstrated that the addition of 150 g/mL Urtica spp. extracts can effectively suppress the growth of Bacillus, Golden Polychiococcus, Salmonella, and Viblus. Notably, the key bioactive compounds that are responsible for this activity are flavonoids (40). Urtica spp. also contains various phenolic compounds, like cinnamonic acids, which display a significant inhibitory effect on Pseudomonas fragi and Campylobacter jejuni (41). Furthermore, the widespread polysaccharides in Urtica spp. exhibit inhibitory effects on foodborne and plant-pathogenic bacteria (42). Studies have revealed that the positively charged components in Urtica spp. can interact with negatively charged components on the microbial cell membrane, leading to changes in its permeability, causing hydrolysis, and subsequent binding with DNA in microbial cells, thus inhibiting microbial protein synthesis. This inhibition results in protein degeneration or loss of enzyme activity, ultimately causing bacteriostatic effects (43). Urtica spp. extracts can also inhibit bacterial biofilm formation, consequently reducing bacterial resistance. Research indicates that adding 2–20 mg/mL Urtica spp. extract can inhibit the biofilm formation in Salmonella, thereby enhancing bacterial sensitivity to antibiotics (44). Recently, nanoparticles derived from Urtica spp. can disrupt cell membranes, causing microbial membrane damage and inhibiting the growth of bacteria and fungus cells (45, 46). Furthermore, the latest research has identified the separated urticine cohesion in the root system of Urtica spp. as a monomeric protein that can bind to the spike protein on the surface of the COVID-19 virus, thus inhibiting the CoV-2 variants (47). In conclusion, the primary biological function of Urtica spp. is anti-oxidation, immune regulation, and antimicrobial activity. The mechanism of its function is illustrated in Figure 2.
4 The application of Urtica spp. in animal husbandry
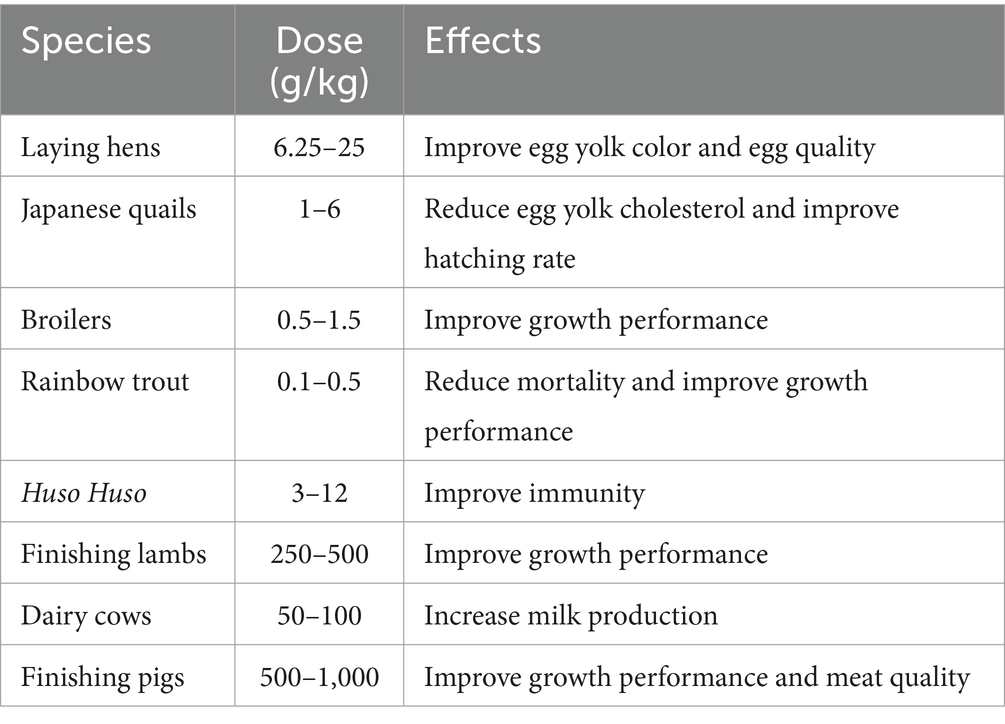
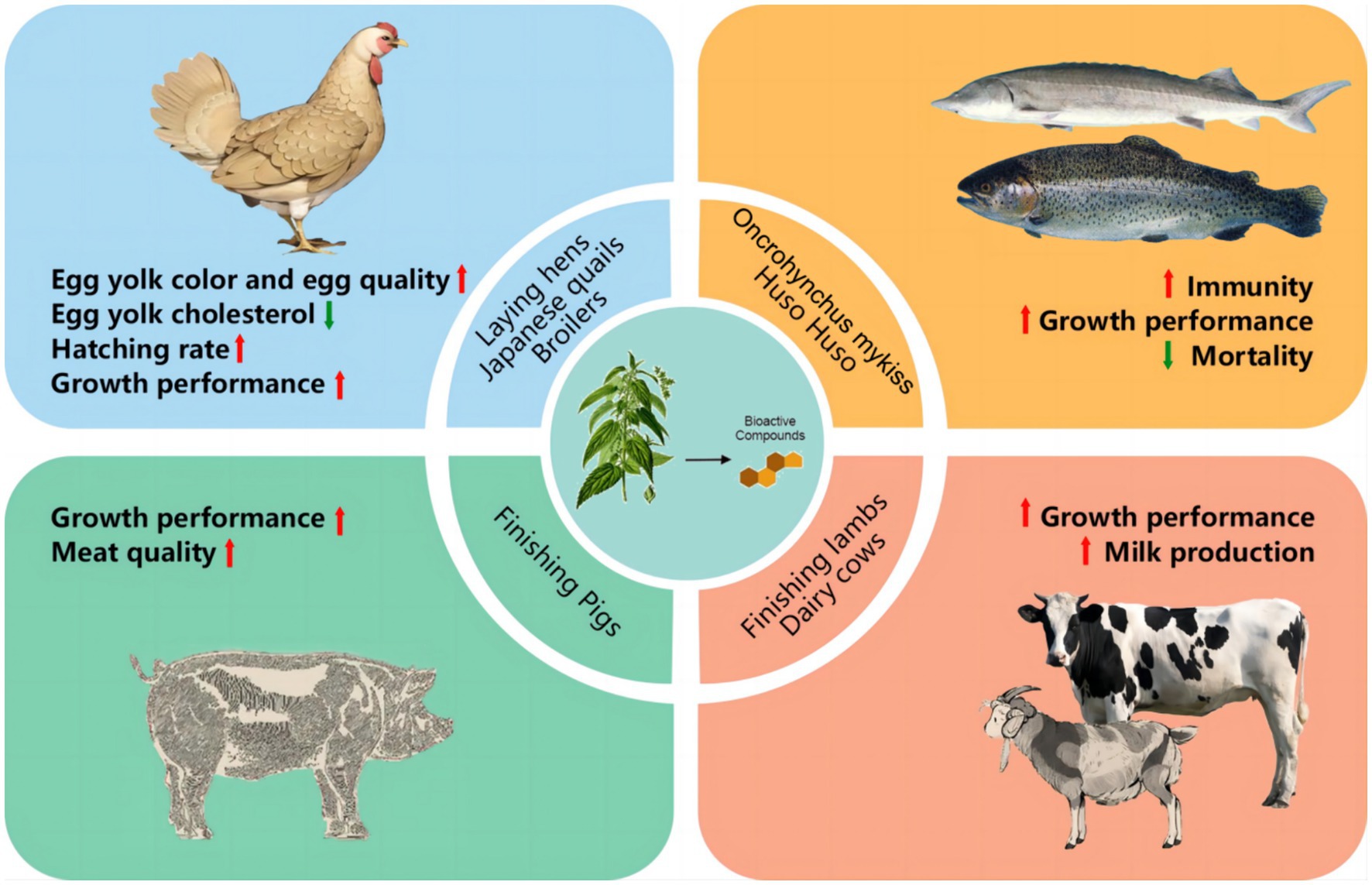
Currently, Urtica spp. is mainly used in feed for poultry, aquatic animals, ruminants and pigs. The dosage and effects of Urtica spp. in different species are detailed in Table 2 and Figure 3.
4.1 Application in poultry production
Loetscher et al. discovered that supplementing 6.25, 12.5, and 25 Urtica spp. to 70-week-old laying hens, respectively, resulted in enhanced egg yolk color with the increasing concentration of Urtica spp. after 4 weeks of trial time. Similar effects were observed in the group that added artificial pigmentation; however, the content of natural vitamin E in the egg yolk in the Urtica spp. group was significantly higher than the CON group without any negative effects on the productivity and quality of eggs of laying hens (48). Similarly, 15% Urtica spp. was supplemented with 28-week-old laying hens as a substitute for soybean meal. The results demonstrated a notable increase in the n-3 polyunsaturated fatty acids, egg yolk color, and eggshell thickness, along with reduced cholesterol levels in the serum and yolk (49). Moula et al. (50) also revealed that adding 6% of Urtica spp. to the diet of 10-week-old Japanese quail led to a significant decrease in the level of yolk cholesterol, blood cholesterol, and triglyceride levels without any adverse effect on productive performance. Furthermore, in older Japanese quails (52 weeks old), adding Urtica spp. to the basal diet not only increased egg production but also increased reproductive and incubation rates (51). Evidently, the addition of Urtica spp. to poultry diets has a positive impact on improving egg quality, lipid metabolism, and egg production performance.
Ahmadipour et al. (52) added 0.5, 1, and 1.5% Urtica spp. to the diet of 1-day-old broilers (Ross308) in high-altitude regions. The results revealed a significant increase in relative overexpression of catalase (CAT) and superoxide dismutase 1 (SOD1) in the liver and lung of the chickens that were fed Urtica spp. Lipid peroxidation was significantly suppressed, as reflected in reduced levels of malondialdehyde (MDA) in the circulatory system. Adding 4% Urtica spp. to the broiler diet mitigated cortisol levels, total cholesterol concentration, and tissue damage markers such as creatine kinase induced by heat stress (53). In standard breeding conditions, adding 0.5 g/kg root extract of Urtica spp. or 0.05% nanoencapsulated Urtica spp. resulted in enhanced growth performance parameters such as body weight gain, feed conversion rate, and carcass weight (54). In conclusion, supplementing poultry diets with Urtica spp. can substitute soybean meal, improve egg quality, boost stress resistance, and promote growth performance.
4.2 Application in aquatic animals
Microbial infection is the primary cause of the slow growth and death of aquatic animals. Urtica spp. serves as a growth promoter and immune booster, widely used in the intensive aquaculture industry. Rainbow trout currently stands as the main aquaculture fish globally, boosting an annual production of up to 762,000 tons with high economic value. It has emerged as the main research focus of Urtica spp. in aquaculture applications. Studies have indicated that feeding rainbow trout with 1% Urtica spp. for 14 days led to decreased mortality following exposure to Aeromonas hydrophila. Furthermore, there was a significant enhancement in serum bactericidal activity, respiratory burst, and lysozyme activity in the treatment groups compared to the control group (32). Saeidi et al. demonstrated that adding 3% Urtica spp. to the rainbow trout diet significantly improved hematological parameters while improving body growth and feed conversion rate. The mortality rate is significantly lower compared to the control group (55). Bilen et al. manifested that adding 0.1 and 0.5 g/kg Urtica spp. to the rainbow Trout diet, respectively, resulted in higher fish weight and specific growth rate. The 30-day feeding trial revealed that all treated groups outperformed the control group when challenged with the bacterial pathogen A. hydrophila (56). These findings suggest that adding Urtica spp. to the diet can improve the growth performance and immune function of rainbow trout. Beluga (Huso Huso) is a large sturgeon fish with high economic value. Due to excessive fishing in recent years, its wild resources are now endangered. Presently, the population is primarily maintained through artificial reproduction, yet microbial infections in the breeding process can lead to substantial losses. Binaii et al. investigated the effects of adding 3, 6, and 12% Urtica spp. to the diets of juvenile Beluga, respectively. After an eight-week trial period, the level of neutral granulocytes and hemoglobin in the blood increased significantly compared with the control group, with these indicators directly related to the levels of Urtica spp. supplementation. The group fed with 12% exhibited a highly significant difference in total red blood cells, total protein, and total immunoglobulin (57). Research has demonstrated that adding Urtica spp. to the basal diet can enhance thermal, biochemical, and immune function in fish. This can be attributed to the following reasons: First, Urtica spp. is rich in vitamin C and iron. Vitamin C facilitates iron absorption in the fish intestine, thereby improving blood biochemical indicators. Second, the flavonoids present in Urtica spp. can activate neutral granulocytes and produce ROS to play a bactericidal effect. Third, the feed of Urtica spp. can increase immunoglobulin by increasing the total protein levels of fish (58). In conclusion, adding an appropriate amount of Urtica spp. to the diet can significantly enhance the growth performance and immune function of various fish species; however, the mechanism still needs to be further explored.
4.3 Application in the production of ruminants
The addition of Urtica spp. to the sheep diet can promote growth performance and improve nutritional metabolism. By adding 250 and 500 g/kg Urtica spp. to the diet of finishing lambs, respectively, an increase in blood glucose level was observed, accompanied by a decrease in total cholesterol and triglyceride levels. The antioxidant parameters had also improved significantly. The contents of unsaturated fatty acids in the treatment group were significantly higher than the control group, particularly in the 500 g/kg group (59). The addition of 250 and 500 g/kg Urtica spp. to diets of growth lamb led to a significant increase in the apparent digestion of crude protein, neutral, and acidic washing fibers compared to the control group. This increase could be attributed to the presence of saponin in Urtica spp., which can stimulate the smooth muscle contraction of the abomasum and secrete digestive enzymes that improve the efficiency of the digestion and absorption of feeds (60). Feeding sheep with urticaries changes the composition of the microbiota, decreases the pH rumen value, and elevates propionic acid and volatile fatty acid concentrations, thus enhancing the production performance of sheep (59, 61). Furthermore, adding 12% Urtica spp. to the dairy cow diet as a substitute for lucern has been shown to have no detrimental effect on production performance. It improves the composition of fatty acids and amino acids, immune function, and antioxidant capacity (62). Humphries et al. added 50 and 100 g/kg Urtica spp. to the diet of dairy cows instead of ryegrass. The findings indicated no adverse effect on milk production while also regulating the pH value of the rumen to effectively prevent ruminal acidosis that may be induced by high-grain diets (63). In conclusion, Urtica spp. can be used as a substitute for pastoral grass. It can not only reduce breeding costs but also enhance the production performance of ruminants. The bio-active substances can also play a significant role in enhancing immunity and antioxidant capabilities.
4.4 Application in the swine industry
Szewczyk et al. added 500 and 1,000 mg/kg of Urtica dioica extract in the dietary phases of fattening pigs (60–112 kg), respectively. The results demonstrated an increase in the loin eye area, with the supplementation with Urtica dioica affecting the fatty acid profile in the loin fat. This led to a reduction in short-chain fatty acids and an elevation in the proportion of multi-unsaturated fatty acids (64). By adding Urtica spp. to the basal diet of finishing pigs (60–110 kg), the protein content in the longissimus muscle increased while the fat content decreased in the treatment group compared to the control group. Additionally, the supplementation of Urtica spp. also increased the lightness of meat and maintained the color stability for 6 months when stored at 20°C. Furthermore, it slightly improved the meat’s oxidative stability during frozen storage and boosted polyunsaturated fatty acid (PUFA) contents, mainly due to reducing monounsaturated fatty acid (MUFA) contents (65). The addition of 500 mg/kg Urtica extract to the diets of fattening pigs (60–112 kg) resulted in a significant improvement in the meat quality and a reduction in cholesterol content in the pork; however, it displayed no significant impact on the growth performance (66). Supplementing Urtica extracts to the diet of piglets can significantly improve growth performance, with significantly higher villi height of the ileum in the treatment group compared to the control group (67). In conclusion, Urtica spp. can serve as feed additives to improve growth performance during the growing stage and meat quality during the fattening stage of pigs. However, there is limited research on using Urtica spp. in the swine industry, especially concerning local breeds. Consequently, the mechanism of Urtica spp. needs to be explored further in terms of its role in regulating pork meat quality.
5 Conclusion and perspectives
Urtica spp. is being considered a substitute for traditional protein feed and high-quality pasture due to its abundance of various nutrients and bio-active compounds, thereby reducing the cost of feed. Furthermore, it also possesses antioxidant, anti-inflammatory, and immune-regulating properties, which positively impact animal production. Presently, research on Urtica spp. primarily focuses on food processing and pharmacological studies. However, there are still some limitations in the use of Urtica spp. For example, excessive consumption of Urtica spp. can cause damage to the respiratory system and skin of animals. Therefore, the appropriate additive dosage in animal feed is very important. At the same time, the literature in the field of animal nutrition is limited, necessitating a thorough exploration of the key bio-active constituents in Urtica spp. In the future, the application of Urtica spp. should be expanded to replace traditional protein feed and high-quality pastoral grass in animal production, particularly regarding safety alternatives and growth performance. Regarding the extraction of Urtica spp., the key bio-active compounds and their mechanisms in animals should be further explored to establish the theoretical basis for the application of Urtica spp. in animal husbandry.
Author contributions
YG: Funding acquisition, Writing – original draft. XY: Supervision, Writing – original draft. BC: Supervision, Writing – original draft. HL: Software, Writing – review & editing. JZ: Funding acquisition, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research review was supported by the funding of Baicheng Normal University Doctoral Research Initiation Fund Project (90024169041) and Natural Science Foundation of Inner Mongolia (2022LHMS03005).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Millet, S, and Maertens, L. The European ban on antibiotic growth promoters in animal feed: from challenges to opportunities. Vet J. (2011) 187:143–4. doi: 10.1016/j.tvjl.2010.05.001
2. Garcia-Bustos, V, Cabañero-Navalon, MD, Ruiz-Gaitán, A, Salavert, M, Tormo-Mas, M, and Pemán, J. Climate change, animals, and Candida auris: insights into the ecological niche of a new species from a one health approach. Clin Microbiol Infect. (2023) 29:858–62. doi: 10.1016/j.cmi.2023.03.016
3. Devkota, HP, Paudel, KR, Khanal, S, Baral, A, Panth, N, Adhikari-Devkota, A, et al. Stinging nettle (Urtica dioica L.): nutritional composition, bioactive compounds, and food functional properties. Molecules. (2022) 27:5219. doi: 10.3390/molecules27165219
4. Orčić, D, Francišković, M, Bekvalac, K, Svirčev, E, Beara, I, Lesjak, M, et al. Quantitative determination of plant phenolics in Urtica dioica extracts by high-performance liquid chromatography coupled with tandem mass spectrometric detection. Food Chem. (2014) 143:48–53. doi: 10.1016/j.foodchem.2013.07.097
5. Rutto, LK, Xu, Y, Ramirez, E, and Brandt, M. Mineral properties and dietary value of raw and processed stinging nettle (Urtica dioica L.). Int J Food Sci. (2013) 2013:857120. doi: 10.1155/2013/857120
6. El Haouari, M, and Rosado, JA. Phytochemical, anti-diabetic and cardiovascular properties of Urtica dioica L. (Urticaceae): a review. Mini Rev Med Chem. (2019) 19:63–71. doi: 10.2174/1389557518666180924121528
7. Gülçin, I, Küfrevioglu, OI, Oktay, M, and Büyükokuroglu, ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol. (2004) 90:205–15. doi: 10.1016/j.jep.2003.09.028
8. Adhikari, BM, Bajracharya, A, and Shrestha, AK. Comparison of nutritional properties of stinging nettle (Urtica dioica) flour with wheat and barley flours. Food Sci Nutr. (2016) 4:119–24. doi: 10.1002/fsn3.259
9. Bekele, B, Melesse, A, Beyan, M, and Berihun, K. The effect of feeding stinging nettle (Urtica simensis S.) leaf meal on feed intake, growth performance and carcass characteristics of Hubbard broiler chickens. Glob J Sci Front Res D Agric Vet. (2015) 15:1–20.
10. Kregiel, D, Pawlikowska, E, and Antolak, H. Urtica spp.: ordinary plants with extraordinary properties. Molecules. (2018) 23:1664. doi: 10.3390/molecules23071664
11. Zhang, M, Li, JJ, Wu, HY, Geng, YH, and Han, WL. First report of Chaetomella raphigera causing leaf spot on Rosa chinensis in China. Plant Dis. (2014) 98:569. doi: 10.1094/pdis-08-13-0810-pdn
12. Jan, KN, Zarafshan, K, and Singh, S. Stinging nettle (Urtica dioica L.): a reservoir of nutrition and bioactive components with great functional potential. J Food Meas Charact. (2017) 11:423–33.
13. Pastore, G, Sant'Ana, AS, Cazarin, CBB, Bicas, JL, and Junior, MRM. Editorial on food science and its impact on a changing world. Food Res Int. (2019) 124:108486. doi: 10.1016/j.foodres.2019.05.034
14. Đurović, S, Micić, D, Šorgić, S, Popov, S, Gašić, U, Tosti, T, et al. Recovery of polyphenolic compounds and vitamins from the stinging nettle leaves: thermal and behavior and biological activity of obtained extracts. Molecules. (2023) 28:2278. doi: 10.3390/molecules28052278
15. Kukrić, ZZ, Topalić-Trivunović, LN, Kukavica, BM, Matoš, SB, Pavičić, SS, Boroja, MM, et al. Characterization of antioxidant and antimicrobial activities of nettle leaves (Urtica dioica L.). Acta Periodica Technol. (2012) 43:257–72. doi: 10.2298/APT1243257K
16. Pinelli, P, Ieri, F, Vignolini, P, Bacci, L, Baronti, S, and Romani, A. Extraction and HPLC analysis of phenolic compounds in leaves, stalks, and textile fibers of Urtica dioica L. J Agric Food Chem. (2008) 56:9127–32. doi: 10.1021/jf801552d
17. Đurović, S, Pezo, L, Gašić, U, Gorjanović, S, Pastor, F, Bazarnova, JG, et al. Recovery of biologically active compounds from stinging nettle leaves part II: processing of exhausted plant material after supercritical fluid extraction. Food Secur. (2023) 12:809. doi: 10.3390/foods12040809
18. Sharifi-Rad, M, Anil Kumar, NV, Zucca, P, Varoni, EM, Dini, L, Panzarini, E, et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front Physiol. (2020) 11:694. doi: 10.3389/fphys.2020.00694
19. Wang, Z, Cai, L, Li, H, Liang, M, Zhang, Y, Wu, Q, et al. Rice protein stimulates endogenous antioxidant response attributed to methionine availability in growing rats. J Food Biochem. (2020) 44:e13180. doi: 10.1111/jfbc.13180
20. Xu, Z, Liu, G, Huang, J, and Wu, J. Novel glucose-responsive antioxidant hybrid hydrogel for enhanced diabetic wound repair. ACS Appl Mater Interfaces. (2022) 14:7680–9. doi: 10.1021/acsami.1c23461
21. Jaiswal, V, and Lee, HJ. Antioxidant activity of Urtica dioica: an important property contributing to multiple biological activities. Antioxidants. (2022) 11:2494. doi: 10.3390/antiox11122494
22. Khajeh Mehrizi, R, Mozaffari-Khosravi, H, and Aboee, P. The effect of Urtica Dioica extract on blood lipids profile in patients with type 2 diabetes: a randomized double-blinded clinical trial. J Nutr Food Sec. (2021) 6:315–20. doi: 10.18502/jnfs.v6i4.7615
23. Dhouibi, R, Affes, H, Ben Salem, M, Charfi, S, Marekchi, R, Hammami, S, et al. Protective effect of Urtica dioica in induced neurobehavioral changes, nephrotoxicity and hepatotoxicity after chronic exposure to potassium bromate in rats. Environ Pollut. (2021) 287:117657. doi: 10.1016/j.envpol.2021.117657
24. Yıldızhan, K, Demirtaş, ÖC, Uyar, A, Huyut, Z, Çakir, T, Keleş, ÖF, et al. Protective effects of Urtica dioica L. seed extract on liver tissue injury and antioxidant capacity in irradiated rats. Braz J Pharm Sci. (2020) 56:e18354. doi: 10.1590/s2175-97902019000318354
25. Seyydi, SM, Tofighi, A, Rahmati, M, and Tolouei Azar, J. Exercise and Urtica Dioica extract ameliorate mitochondrial function and the expression of cardiac muscle nuclear respiratory factor 2 and peroxisome proliferator-activated receptor gamma coactivator 1-alpha in STZ-induced diabetic rats. Gene. (2022) 822:146351. doi: 10.1016/j.gene.2022.146351
26. Vajic, UJ, Grujic-Milanovic, J, Miloradovic, Z, Jovovic, D, Ivanov, M, Karanovic, D, et al. Urtica dioica L. leaf extract modulates blood pressure and oxidative stress in spontaneously hypertensive rats. Phytomedicine. (2018) 46:39–45. doi: 10.1016/j.phymed.2018.04.037
27. Jakubczyk, K, Łukomska, A, Baranowska-Bosiacka, I, Goschorska, M, Dec, K, Wolska, J, et al. The influence of extracts from the seeds of the common nettle (Urtica dioica L.) on the activity of antioxidative enzymes in macrophages incubated with sodium fluoride. Fluoride. (2018) 51:65–76.
28. Hasan, M, Oster, M, Reyer, H, Wimmers, K, and Fischer, DC. Efficacy of dietary vitamin D(3) and 25(OH)D(3) on reproductive capacities, growth performance, immunity and bone development in pigs. Br J Nutr. (2023) 130:1298–307. doi: 10.1017/s0007114523000442
29. Tu, W, Nie, W, Yao, X, Zhang, J, Zhang, H, Di, D, et al. Growth performance, lipid metabolism, and systemic immunity of weaned piglets were altered by buckwheat protein through the modulation of gut microbiota. Mol Gen Genomics. (2024) 299:15. doi: 10.1007/s00438-024-02103-y
30. Herrera, SB, Rodriguez, L, Garca, M, Flores, JL, and Velasco, R. Effects of extract of Urtica dioica L. (stinging nettle) on the immune response of rats with severe malnutrition. J Complement Med Res. (2018) 9:63–3. doi: 10.5455/jcmr.20180508052039
31. Dhouibi, R, Affes, H, Ben Salem, M, Hammami, S, Sahnoun, Z, Zeghal, KM, et al. Screening of pharmacological uses of Urtica dioica and others benefits. Prog Biophys Mol Biol. (2020) 150:67–77. doi: 10.1016/j.pbiomolbio.2019.05.008
32. Awad, E, and Austin, B. Use of lupin, Lupinus perennis, mango, Mangifera indica, and stinging nettle, Urtica dioica, as feed additives to prevent Aeromonas hydrophila infection in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis. (2010) 33:413–20. doi: 10.1111/j.1365-2761.2009.01133.x
33. Sharma, S, Singh, DK, Gurung, YB, Shrestha, SP, and Pantha, C. Immunomodulatory effect of stinging nettle (Urtica dioica) and Aloe vera (Aloe barbadensis) in broiler chickens. Vet Anim Sci. (2018) 6:56–63. doi: 10.1016/j.vas.2018.07.002
34. Kawai, T, and Akira, S. Signaling to NF-kappaB by toll-like receptors. Trends Mol Med. (2007) 13:460–9. doi: 10.1016/j.molmed.2007.09.002
35. Francišković, M, Gonzalez-Pérez, R, Orčić, D, Sánchez de Medina, F, Martínez-Augustin, O, Svirčev, E, et al. Chemical composition and Immuno-modulatory effects of Urtica dioica L. (stinging nettle) extracts. Phytother Res. (2017) 31:1183–91. doi: 10.1002/ptr.5836
36. Wagner, H, Willer, F, Samtleben, R, and Boos, G. Search for the antiprostatic principle of stinging nettle (Urtica dioica) roots. Phytomedicine. (1994) 1:213–24. doi: 10.1016/s0944-7113(11)80068-1
37. Shakibaei, M, Allaway, D, Nebrich, S, and Mobasheri, A. Botanical extracts from rosehip (Rosa canina), willow bark (Salix alba), and nettle leaf (Urtica dioica) suppress IL-1β-induced NF-κB activation in canine articular chondrocytes. Evid Based Complement Alternat Med. (2012) 2012:509383. doi: 10.1155/2012/509383
38. Casewell, M, Friis, C, Marco, E, McMullin, P, and Phillips, I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J Antimicrob Chemother. (2003) 52:159–61. doi: 10.1093/jac/dkg313
39. Bao, H, She, R, Liu, T, Zhang, Y, Peng, KS, Luo, D, et al. Effects of pig antibacterial peptides on growth performance and intestine mucosal immune of broiler chickens. Poult Sci. (2009) 88:291–7. doi: 10.3382/ps.2008-00330
40. Mzid, M, Ben Khedir, S, Ben Salem, M, Regaieg, W, and Rebai, T. Antioxidant and antimicrobial activities of ethanol and aqueous extracts from Urtica urens. Pharm Biol. (2017) 55:775–81. doi: 10.1080/13880209.2016.1275025
41. Elez Garofulić, I, Malin, V, Repajić, M, Zorić, Z, Pedisić, S, Sterniša, M, et al. Phenolic profile, antioxidant capacity and antimicrobial activity of nettle leaves extracts obtained by advanced extraction techniques. Molecules. (2021) 26:6153. doi: 10.3390/molecules26206153
42. Körpe, DA, İşerİ, ÖD, Sahin, FI, Cabi, E, and Haberal, M. High-antibacterial activity of Urtica spp. seed extracts on food and plant pathogenic bacteria. Int J Food Sci Nutr. (2013) 64:355–62. doi: 10.3109/09637486.2012.734290
43. Alirezalu, K, Hesari, J, Nemati, Z, Munekata, PES, Barba, FJ, and Lorenzo, JM. Combined effect of natural antioxidants and antimicrobial compounds during refrigerated storage of nitrite-free frankfurter-type sausage. Food Res Int. (2019) 120:839–50. doi: 10.1016/j.foodres.2018.11.048
44. Cesur, A, and Soyer, Y. Determination of antimicrobial effect of the aqueous extract of stinging nettle (Urtica dioica) on biofilm formation of Salmonella enterica serovars. Gıda. (2021) 46:324–38. doi: 10.15237/gida.GD21016
45. Hashem, AH, and Salem, SS. Green and ecofriendly biosynthesis of selenium nanoparticles using Urtica dioica (stinging nettle) leaf extract: antimicrobial and anticancer activity. Biotechnol J. (2022) 17:e2100432. doi: 10.1002/biot.202100432
46. Kalia, A, Kaur, M, Shami, A, Jawandha, SK, Alghuthaymi, MA, Thakur, A, et al. Nettle-leaf extract derived ZnO/CuO nanoparticle-biopolymer-based antioxidant and antimicrobial nanocomposite packaging films and their impact on extending the post-harvest shelf life of guava fruit. Biomol Ther. (2021) 11:224. doi: 10.3390/biom11020224
47. Vanhulle, E, D'Huys, T, Provinciael, B, Stroobants, J, Camps, A, Noppen, S, et al. Carbohydrate-binding protein from stinging nettle as fusion inhibitor for SARS-CoV-2 variants of concern. Front Cell Infect Microbiol. (2022) 12:989534. doi: 10.3389/fcimb.2022.989534
48. Loetscher, Y, Kreuzer, M, and Messikommer, R. Utility of nettle (Urtica dioica) in layer diets as a natural yellow colorant for egg yolk. Anim Feed Sci Technol. (2013) 186:158–68. doi: 10.1016/j.anifeedsci.2013.10.006
49. Zhang, J, Na, T, Jin, Y, Zhang, X, Qu, H, and Zhang, Q. Thicker Shell eggs with enriched N-3 polyunsaturated fatty acids and lower yolk cholesterol contents, as affected by dietary nettle (Urtica cannabina) supplementation in laying hens. Animals. (2020) 10:1994. doi: 10.3390/ani10111994
50. Moula, N, Sadoudi, A, Touazi, L, Leroy, P, and Geda, F. Effects of stinging nettle (Urtica dioica) powder on laying performance, egg quality, and serum biochemical parameters of Japanese quails. Anim Nutr. (2019) 5:410–5. doi: 10.1016/j.aninu.2019.05.002
51. Sharokhyan Rezaee, M, Farzinpour, A, Farshad, A, and Hatfaludi, T. The regulative effect of Urtica dioica on sex hormones imbalance: elevated follicle-stimulating hormone/luteinizing hormone ratio ≥4.5 is associated with low performance in aged breeder quails. Ital J Anim Sci. (2022) 21:142–52. doi: 10.1080/1828051X.2021.2007801
52. Ahmadipour, B, and Khajali, F. Expression of antioxidant genes in broiler chickens fed nettle (Urtica dioica) and its link with pulmonary hypertension. Anim Nutr. (2019) 5:264–9. doi: 10.1016/j.aninu.2019.04.004
53. Mirsaiidi Farahani, M, and Hosseinian, SA. Effects of dietary stinging nettle (Urtica dioica) on hormone stress and selected serum biochemical parameters of broilers subjected to chronic heat stress. Vet Med Sci. (2022) 8:660–7. doi: 10.1002/vms3.721
54. Tabari, M. A., Ghazvinian, K., Irani, M., and Molaei, R.. Effects of dietary supplementation of nettle root extract and pumpkin seed oil on production traits and intestinal microflora in broiler chickens. Bulg. J. Vet. Med. (2016) 19:108–116. doi: 10.5547/bjvm.879
55. Saeidi Asl, MR, Adel, M, Caipang, CMA, and Dawood, MAO. Immunological responses and disease resistance of rainbow trout (Oncorhynchus mykiss) juveniles following dietary administration of stinging nettle (Urtica dioica). Fish Shellfish Immunol. (2017) 71:230–8. doi: 10.1016/j.fsi.2017.10.016
56. Bilen, S, Ünal, S, and Güvensoy, H. Effects of oyster mushroom (Pleurotus ostreatus) and nettle (Urtica dioica) methanolic extracts on immune responses and resistance to Aeromonas hydrophila in rainbow trout (Oncorhynchus mykiss). Aquaculture. (2016) 454:90–4. doi: 10.1016/j.aquaculture.2015.12.010
57. Binaii, M, Ghiasi, M, Farabi, SM, Pourgholam, R, Fazli, H, Safari, R, et al. Biochemical and hemato-immunological parameters in juvenile beluga (Huso huso) following the diet supplemented with nettle (Urtica dioica). Fish Shellfish Immunol. (2014) 36:46–51. doi: 10.1016/j.fsi.2013.10.001
58. De Vico, G, Guida, V, and Carella, F. Urtica dioica (stinging nettle): a neglected plant with emerging growth promoter/Immunostimulant properties for farmed fish. Front Physiol. (2018) 9:285. doi: 10.3389/fphys.2018.00285
59. Zhang, X, Jiang, C, Jin, Y, Li, P, and Zhong, J. The effect of substitution of mixed grass hay with Urtica cannabina hay and/or Leymus chinensis hay on blood biochemical profile, carcass traits, and intramuscular fatty acid composition in finishing lambs. Anim Feed Sci Technol. (2021) 272:114780. doi: 10.1016/j.anifeedsci.2020.114780
60. Jin, Y, Jiang, C, Zhang, X, Shi, L, and Wang, M. Effect of dietary Urtica cannabina on the growth performance, apparent digestibility, rumen fermentation and gastrointestinal morphology of growing lambs. Anim Feed Sci Technol. (2018) 243:1–9. doi: 10.1016/j.anifeedsci.2018.06.014
61. Rahchamani, R, Faramarzi, M, Moslemipor, F, and Bayat Kohsar, J. Effect of supplementing sheep diet with Glycyrrhiza glabra and Urtica dioica powder on growth performance, rumen bacterial community and some blood biochemical constituents. Iranian J Appl Anim Sci. (2019) 9:95–103.
62. Romanazzi, G, Orçonneau, Y, Moumni, M, Davillerd, Y, and Marchand, PA. Basic substances, a sustainable tool to complement and eventually replace synthetic pesticides in the management of pre and postharvest diseases: reviewed instructions for users. Molecules. (2022) 27:3484. doi: 10.3390/molecules27113484
63. Humphries, DJ, and Reynolds, CK. The effect of adding stinging nettle (Urtica dioica) haylage to a total mixed ration on performance and rumen function of lactating dairy cows. Anim Feed Sci Technol. (2014) 189:72–81. doi: 10.1016/j.anifeedsci.2014.01.006
64. Szewczyk, A, Hanczakowska, E, and Swiatkiewicz, M. The effect of nettle (<i>Urtica dioica</i>) extract on fattening performance and fatty acid profile in the meat and serum lipids of pigs. J Anim Feed Sci. (2006) 15:81–4. doi: 10.22358/jafs/70148/2006
65. Hanczakowska, E, Wiytkiewicz, M, and Szewczyk, A. Effect of dietary nettle extract on pig meat quality. Med Wet. (2007) 63:525–7.
66. Hanczakowska, E, Świątkiewicz, M, and Grela, ER. Effect of dietary inclusion of a herbal extract mixture and different oils on pig performance and meat quality. Meat Sci. (2015) 108:61–6. doi: 10.1016/j.meatsci.2015.05.020
67. Hanczakowska, E., and Swiatkiewicz, M.. Effect of herbal extracts on piglet performance and small intestinal epithelial villi. (2012).
68. Manai-Djebali, H, Yeddes, W, Hammami, M, Nait-Mohamed, S, Habachi, E, Msaada, K, et al. Exploring the synergistic potential of wild nettle and olive oil: bioactive compounds, antioxidant capacity, and antibacterial properties. Int J Environ Health Res. (2023) 34:3046–55. doi: 10.1080/09603123.2023.2287589
69. Mitrović, J, Nikolić, N, Ristić, I, Karabegović, I, Savić, S, Šimurina, O, et al. The chemical characterisation of nettle (Urtica dioica L.) seed oil. Nat Prod Res. (2023). doi: 10.1080/14786419.2023.2250525, [Online ahead of print]
70. Semwal, P, Rauf, A, Olatunde, A, Singh, P, Zaky, MY, Islam, MM, et al. The medicinal chemistry of Urtica dioica L.: from preliminary evidence to clinical studies supporting its neuroprotective activity. Nat Prod Bioprospect. (2023) 13:16. doi: 10.1007/s13659-023-00380-5
71. Tarasevičienė, Ž, Vitkauskaitė, M, Paulauskienė, A, and Černiauskienė, J. Wild stinging nettle (Urtica dioica L.) leaves and roots chemical composition and phenols extraction. Plants. (2023) 12:309. doi: 10.3390/plants12020309
72. Wolska, J, Janda, K, Szkyrpan, S, and Gutowska, I. The influence of stinging nettle (Urtica dioica L.) extracts on the activity of catalase in THP1 monocytes/macrophages. Pomeranian J Life Sci. (2015) 61:315–8. doi: 10.21164/pomjlifesci.129
73. Pałka, SE, Drąg-Kozak, E, Migdał, Ł, and Kmiecik, M. Effect of a diet supplemented with nettle (Urtica dioica L.) or fenugreek (Trigonella Foenum-graecum L.) on the content of selected heavy metals in liver and rabbit meat. Animals. (2022) 12:827. doi: 10.3390/ani12070827
74. Siabi, S, Torbati, M, Azadmard-Damirchi, S, Naebi, M, and Savage, GP. Extraction of oil from Roman nettle seed by cold press and evaluation of its quality during storage. Eur J Lipid Sci Technol. (2023) 125:2200071. doi: 10.1002/ejlt.202200071
75. Aksoylu Özbek, Z, Kawata, K, Zhou, H, Chung, C, Park, JH, and McClements, DJ. Isolation and characterization of nettle (Urtica dioica L.) seed proteins: conversion of underutilized by-products of the edible oil industry into food emulsifiers. Food Chem. (2024) 456:139878. doi: 10.1016/j.foodchem.2024.139878
76. Hekmat, S, Sharifzadeh, M, Toliyat, T, Savary Kouzehkonan, R, Mehri Ardestani, M, Tabarrai, M, et al. Urtica pilulifera L. seed extract promotes folliculogenesis and alleviates the diminished ovarian reserve in the Balb/c mice model: an experimental study. Int J Reprod Biomed. (2024) 22:111–26. doi: 10.18502/ijrm.v22i2.15708
Keywords: Urtica spp., biological functions, action mechanisms, application, animals
Citation: Gao Y, Yang X, Chen B, Leng H and Zhang J (2024) The biological function of Urtica spp. and its application in poultry, fish and livestock. Front. Vet. Sci. 11:1430362. doi: 10.3389/fvets.2024.1430362
Edited by:
Hany M. R. Abdel-Latif, Alexandria University, EgyptReviewed by:
Sengul Uysal, Erciyes University, TürkiyeSameh A. Abdelnour, Zagazig University, Egypt
Copyright © 2024 Gao, Yang, Chen, Leng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jize Zhang, emhhbmdqaXplQGNhYXMuY24=; Huan Leng, bGVuZ2h1YW5AY2Fhcy5jbg==
 Yang Gao
Yang Gao Xuexi Yang1
Xuexi Yang1 Jize Zhang
Jize Zhang