- 1College of Veterinary Medicine, Sichuan Agricultural University, Chengdu, China
- 2Giantstar Farming and Husbandry Co., Ltd., Chengdu, China
- 3Chengdu SG-Biotech Co., Ltd., Chengdu, China
Introduction: After being discovered for the first time in China in 2017, porcine reproductive and respiratory syndrome virus (PRRSV) NADC34-like strains have become the prevalent strain of PRRSV in certain regions of China. Our previous study showed that reduced Ingelvac PRRS MLV vaccination dosages against NADC30-like CF PRRSV had a better protection effect than the normal dosage. However, the protective effect of reduced dosages vaccination of Ingelvac PRRS MLV against NADC34-like PRRSV is unclear. Therefore, this study compared the effectiveness of 0.1 and 1 dosages against a NADC34-like PRRSV infection using commercial PRRSV vaccines, Ingelvac PRRS MLV, which have been widely utilized in China.
Methods: In this study, we immunized piglets with two different dosages of the MLV vaccine and infected piglets within a nasal way with NADC34-like CF PRRSV at 42 days post-vaccination. We observed the changes in growth performance before and after the NADC34-like PRRSV DX strain challenge and the protective effect of different vaccine dosages through multiple assays.
Results: After the challenge, the piglets from the challenge control group displayed clinical signs typical of PRRSV infection, including transient fever, high viremia, mild clinical symptoms, and histopathological changes in the lungs and lymph nodes, which indicates DX is a virulent virus. Without the challenge, the average daily gain of the non-immunized group at 5 weeks after the vaccination is greater than that of the 0.01 dosage group than that of the 1 dosage group, which proved that the commercial MLV vaccine has a negative effect on the growth performance of pigs and this effect may be dose-dependent. After the NADC34-like PRRSV challenge, there was no difference in average daily gain between the immunized pigs and pigs from the challenge control group. From the perspective of clinical score, gross lung lesions, and microscopic lesions, immunization with MLV vaccine can indeed relieve symptoms and lesions caused by the virus, and 0.1 dosage vaccination has a better effect in these aspects. Also, both dosages of MLV immunization shortened viremia with similar effects.
Discussion: Our research suggests that the MLV vaccine can provide piglets with some protection against NADC34-like PRRSV and the 0.1 dosage Ingelvac PRRS MLV vaccination showed greater benefits in our study. Therefore, considering the cost, side effects, and subsequent protective effects, we can adjust the immune dosage appropriately after further investigation to ensure safety, improve production efficiency, and reduce immunization costs.
Introduction
Porcine reproductive and respiratory syndrome (PRRS) is an acute infectious disease caused by the porcine reproductive and respiratory syndrome virus (PRRSV) (1, 2). The virus was an enveloped RNA virus with a genome length of approximately 15.3 kb and was first discovered in North Carolina, United States in 1987 (3, 4). For over 30 years, this disease has been occurring continuously and has currently become one of the major diseases affecting the healthy development of the global swine industry (5–7). Both vertical and horizontal transmission of the virus can result in reproductive problems such as stillbirth and abortion in pregnant sows, piglet mortality, and stunted growth and development (8).
PRRSV belongs to the genus Betaarterivirus, family Arteriviridae, and order Nidovirales. Based on the global PRRSV classification system, usually, PRRSV has two species: Betaarterivirus suid 1 (PRRSV-1, represented by Lelystad Virus strains) and Betaarterivirus suid 2 (PRRSV-2, represented by VR-2332 strains) (9). The main prevalent strain in China is PRRSV-2 (3, 10). PRRSV-2 strains 1–7-4 (IA/2014/NADC34, IA/2013/ISU1, and IN/2014/ISU-5) were first discovered in North Carolina and Iowa, United States in 2013 (11). Later in 2017, Zhang et al. reported that LNWK96 and LNWK130, which had a 100-amino-acid deletion in the Nsp2 region isolated from Liaoning pig farms in 2017, may have originated from the 1-7-4 strain prevalent in the United States, providing the first evidence of the emergence of NADC34-like PRRSV in China (12). The NADC34-like strain’s distribution started to progressively widen in 2019 (13–15). At present, NADC34-like PRRSV has become the main prevalent strain in some regions of China (10, 16–18).
At present, the most effective method to prevent PRRS is still vaccine immunization (19). In China, the most commonly used types of vaccine are modified-live-virus (MLV) vaccines (CH-1R, JXA1-R, VR-2332, etc.) (20). The live-attenuated PRRS vaccine can promote humoral immunity and cellular immunity (21, 22). Compared to the inactivated vaccine, it has a longer immune duration and a superior immune response (22). The common consensus is that vaccinations of PRRSV MLV against heterologous strains can help relieve symptoms (23–25). A study has suggested that current vaccinations may provide partial protection against PRRSV strains of linage 1 (NADC34-like, sublineage 1.5) (26).
According to our understanding, the industry is trying to reduce the cost of vaccines by lowering vaccination dosages. Additionally, in our previous research, piglets vaccinated with reduced dosages of Ingelvac PRRS MLV can provide better protection for the NADC30-like PRRSV (sublineage 1.8) challenge. Whether a reduced dosage of MLV vaccine can provide protection against NADC34-like strains and influence production performance remains to be investigated. We hope to comprehensively analyze and evaluate the cost and benefit of vaccine use from the perspective of its impact on growth performance. In this study, we investigate both the pathologic and productive effects of the 0.1 dosage and 1 dosage of this commercially available live-attenuated vaccine by animal experiments.
Materials and methods
Virus and MLV vaccine
Ingelvac PRRS MLV vaccine, purchased from Boehringer-Ingelheim, Germany, is one commercial live-modified PRRSV vaccine that is derived from the virus VR-2332 strain. The viral used in this work was DX (PQ217625), an isolated NADC34-like PRRSV provided by Chengdu SG-Biotech Co., Ltd.
Animal trials for vaccination and challenge
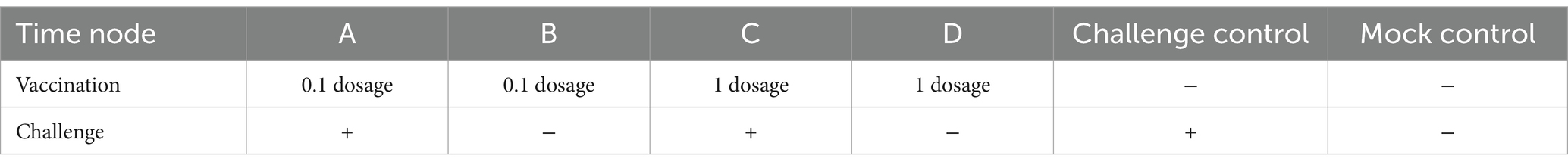
A total number of thirty-six 3-week-old piglets that were free of the porcine circovirus 2 (PCV2), classical swine fever virus (CSFV), pseudorabies virus (PRV), and PRRSV were randomly assigned to six groups, each consisting of six piglets. The Ingelvac PRRS MLV vaccine was diluted to 1 dosage (104.8 TCID50) and 0.1 dosage (103.8 TCID50). Piglets were intramuscularly (IM) inoculated with phosphate-buffered saline (PBS, including two groups), 1 dosage (including two groups), and 0.1 dosage of Ingelvac PRRS MLV vaccine (including two groups). Forty-two days after the first vaccination (42-day post-vaccination, 42 dpv), the piglets were intranasally challenged with the NADC34-like DX virus (2 mL total, 105.5 TCID50/ml, including three groups with different immune conditions), and the piglets from the other three negative control groups were treated with an equal amount of cell culture. The specific group information is shown in Table 1. All piglets were subject to free feeding during the experiment.
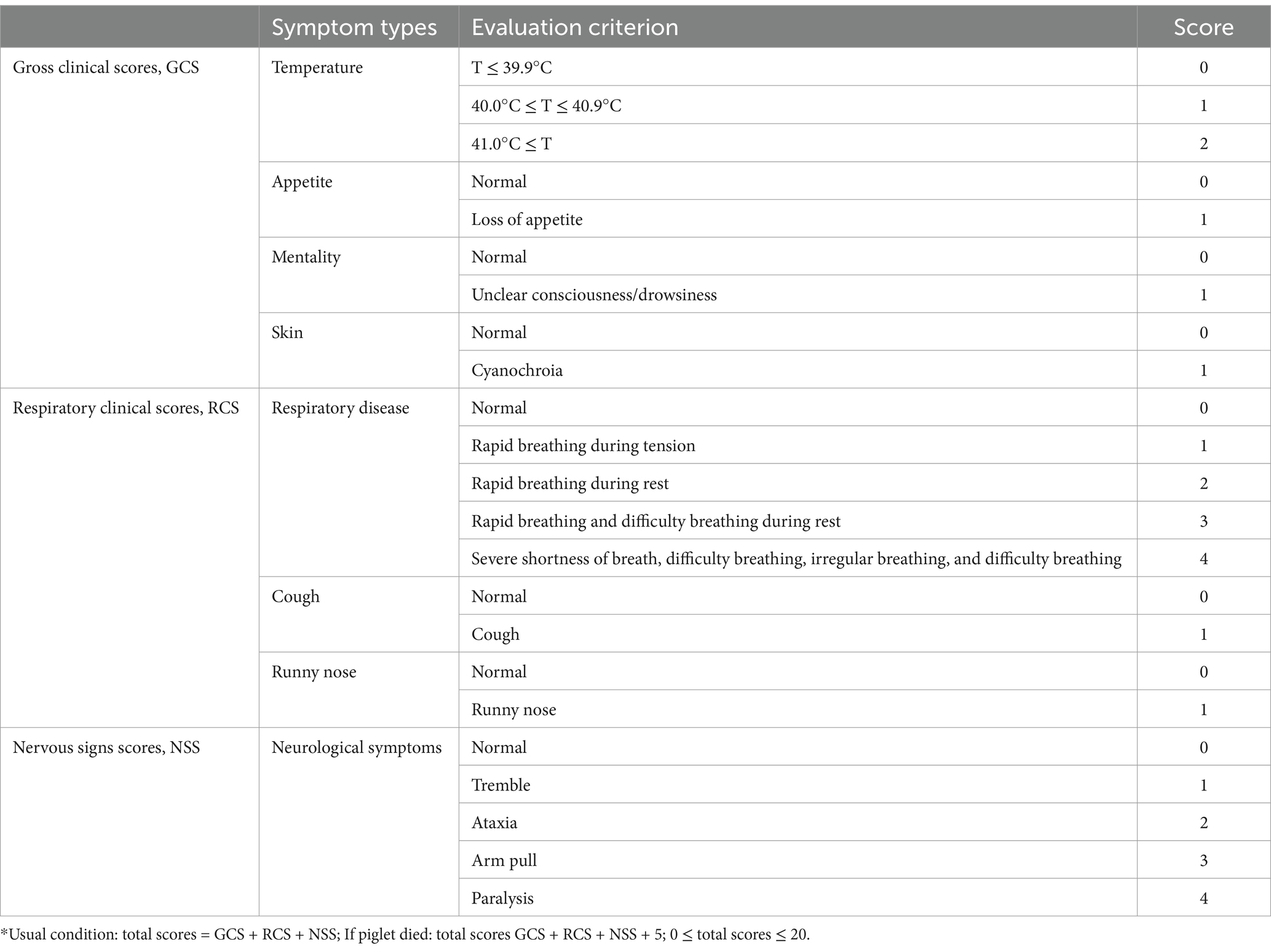
The rectal body temperatures were collected for 2 weeks after the vaccination. After the challenge, the rectal body temperatures and clinical signs of the piglets were recorded every day. During the experiment, the status of the piglets was recorded by scoring, including gross clinical scores (GCSs), respiratory clinical scores (RCSs), and nervous signs scores (NSSs). The specific scoring rules are shown in Table 2. All piglets were humanly euthanized at 21 dpc (days post-challenge). Body weight and total feed intake were measured every week throughout the experiment.
Serology and viremia test
The blood samples of piglets were drawn after immunization as well as at 21, 42 days post-vaccination, and 7, 14, and 21 days post-challenge to identify specific antibodies to PRRSV. The IDEXX PRRS 2XR Porcine Reproductive and Respiratory Syndrome Virus Antibody Test Kit (IDEXX Laboratories) was used as directed by the manufacturer to assess PRRSV-specific ELISA antibody titers. S/P ratios were used to report PRRSV-specific antibody titers, and the serum samples were deemed positive if the S/P ratio was 0.4 or greater.
Total RNA was extracted from the serum samples using the Virus DNA/RNA Extraction Kit 2.0 (Vazyme). After reverse transcription using the HiScript II Q RT SuperMix for qPCR (Vazyme), real-time PCR was conducted to detect the cDNA from samples from 1, 3, 5, 7, 10, 14, 21 days post-challenge using the AceQ qPCR Probe Master Mix (Vazyme). The qPCR primers and probe were designed according to the conserved sequence of the M gene by Chengdu SG-Biotech Co., Ltd. The primers of real-time PCR were PRRSV MF1: 5′-TCCAGATGCCGKTTGTGCTT-3′; MF2: 5′-TCCAGATRCCGGTTGTGCTT-3′; MF3: 5′-TCTAGATGCCGTTTGTGCYT-3′; PRRSV MR: 5′-ACGACAAATGCGTGGTTATCA-3′. The TaqMan probe was synthesized as 5′-FAM-CCCTGCCCACCACGT-MGB-3′. The conditions for amplification were 38°C for 2 min and 95°C for 5 min, then 40 cycles of 95°C for 10 s and 60°C for 30 s. The viral load of each sample was calculated by the equation (y = 40.18–3.25x) constructed previously by Chengdu SG-Biotech Co., Ltd.
Histopathology and immunohistochemistry examination
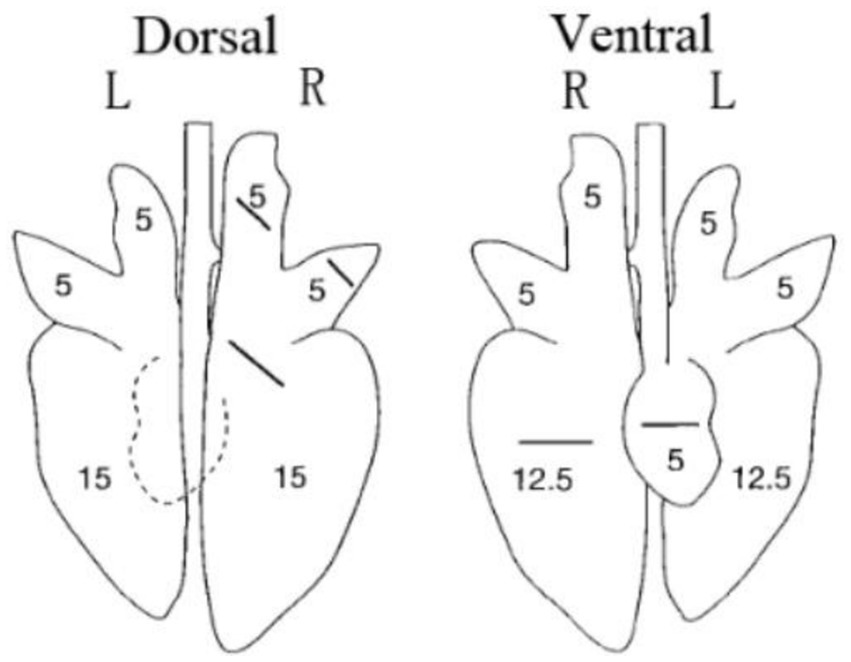
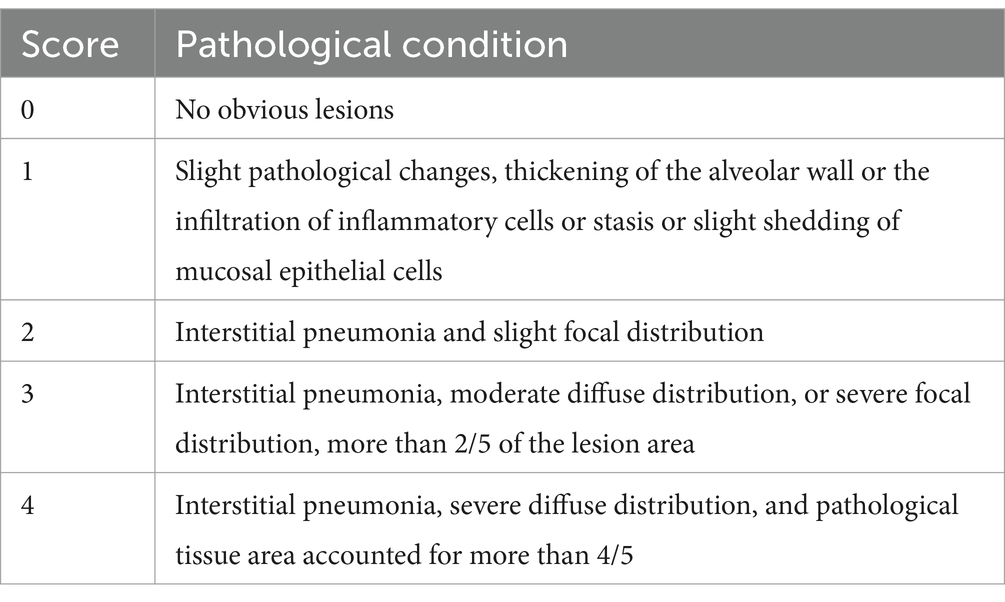
All piglets were humanly euthanized at 21 dpc. At necropsy, the lungs were observed and the lesions were recorded. The three parts of the lung were fixed in 10% buffered neutral formalin for hematoxylin and eosin (H&E) and immunohistochemistry staining. Photos taken with a 200× microscope were used to visualize the slides. The lungs were observed and the lesions were recorded and scored according to Figure 1 and Table 3.
Statistical analysis
All data were expressed as the mean value of 5 piglets ± SEM. Using the GraphPad Prism 9 program (San Diego, CA), statistical analyses were carried out by performing a two-way ANOVA and then Tukey’s t-test. When p < 0.05, differences were deemed statistically significant.
Results
Clinical presentation and piglet growth performance
As shown in Figure 2, after immunization, the body temperature was measured continuously for 2 weeks, and the body temperature of the immune group fluctuated but did not exceed 40°C.
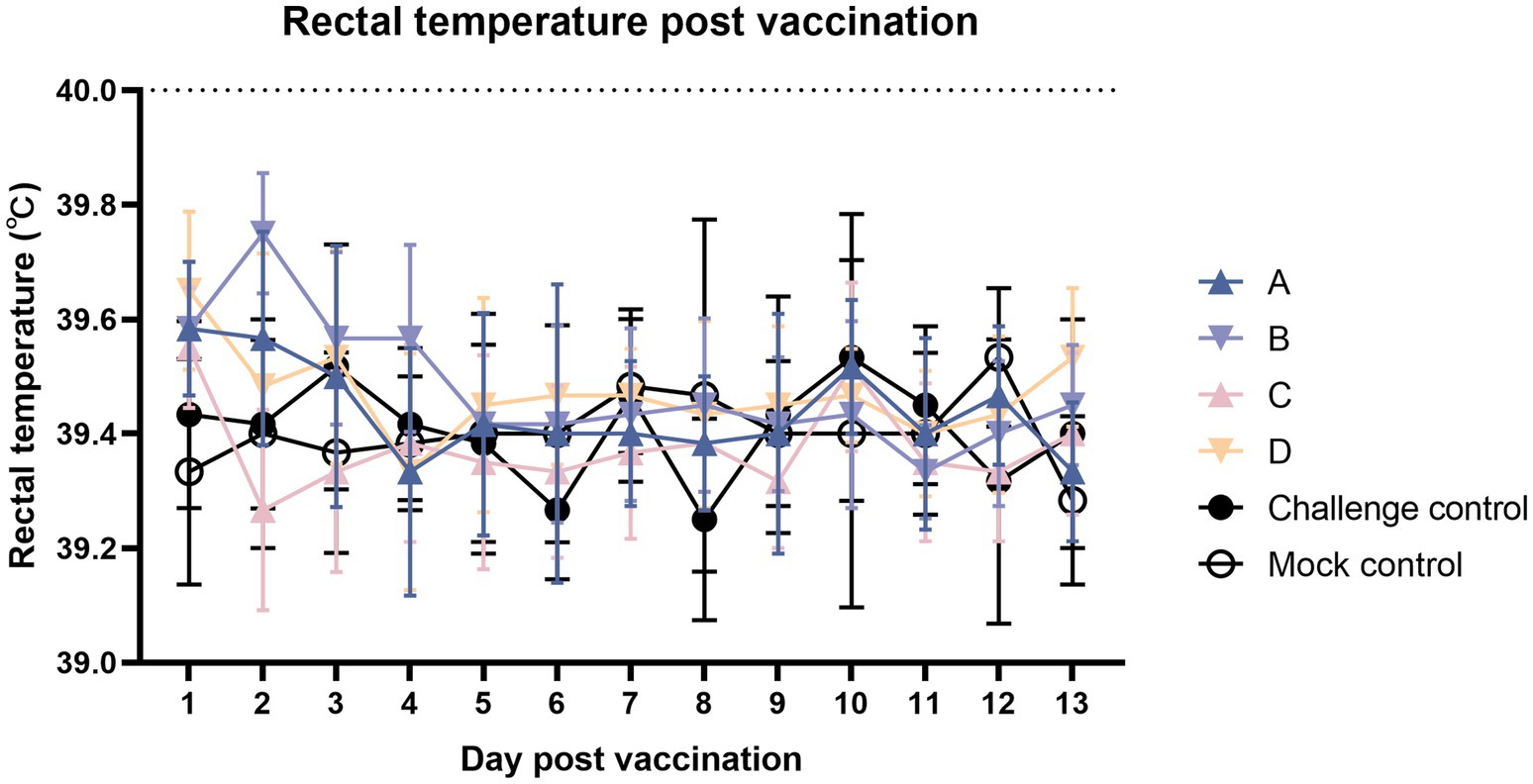
Figure 2. Comparison of mean rectal temperatures (±S.D.) after the vaccination. Clinical fever was set at 40°C.
According to Figure 3, after the challenge, each challenge group had different degrees of fever. The fever in the challenge group was the most critical in the first 1–3 days, followed by sporadic fever until the end of the experiment. The fever peak in the two vaccinated challenged groups was concentrated approximately 5 days after the challenge. The number of febrile animals from group C was more than group A, with sporadic fever continuing until the end of the experiment.
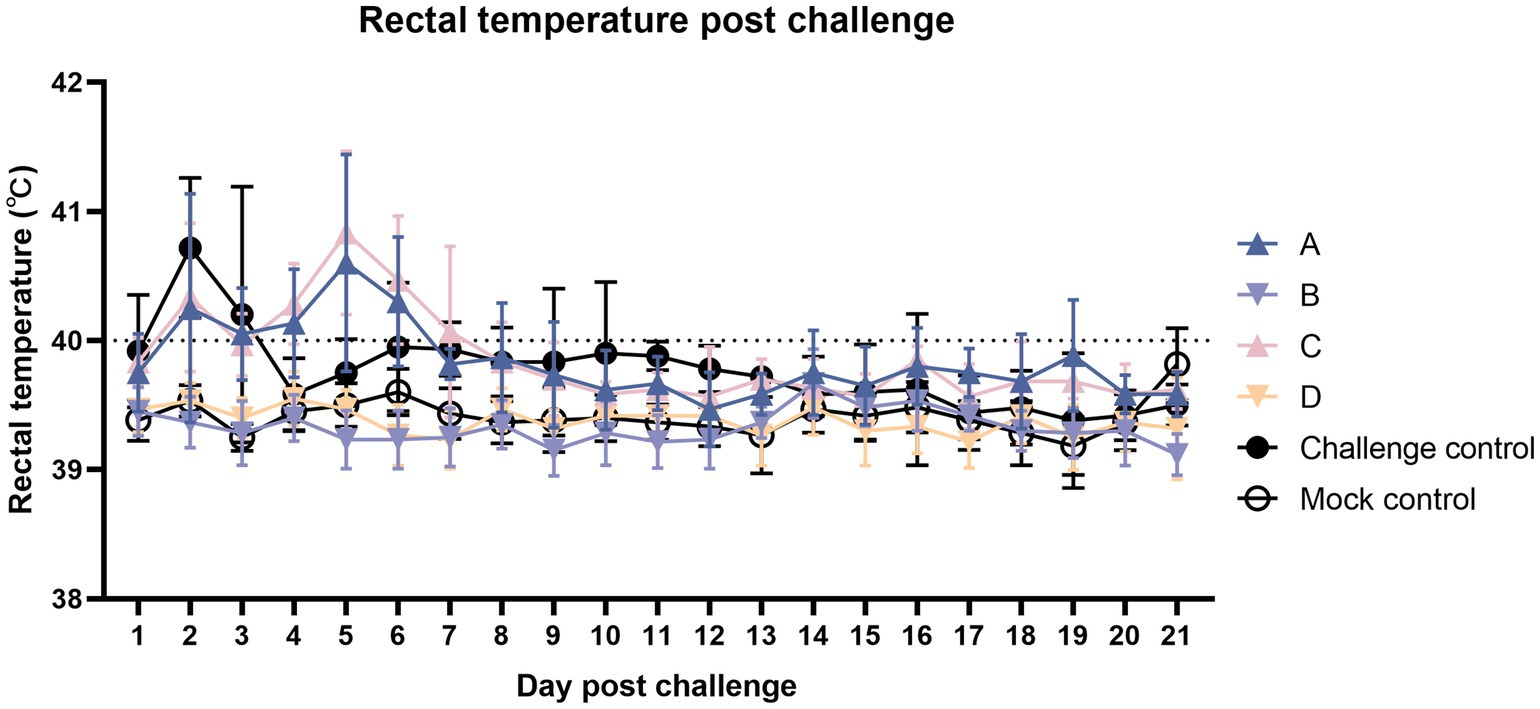
Figure 3. Comparison of mean rectal temperatures (±S.D.) after the challenge. Clinical fever was set at 40°C.
As shown in Figure 4A, at 1–3 days after the challenge, all six piglets in the challenge control group had fever, and their average clinical score was the highest, which was significantly higher than that of blank control and two vaccine immune control groups, and had no difference with other challenge groups. The score of group C, which had four piglets with temperatures over 40°C on the second day, was significantly higher than that of the blank control and two vaccine immunization control groups, and there was no difference with other challenge groups. Other than the above, no significant differences were found between the other groups not described.
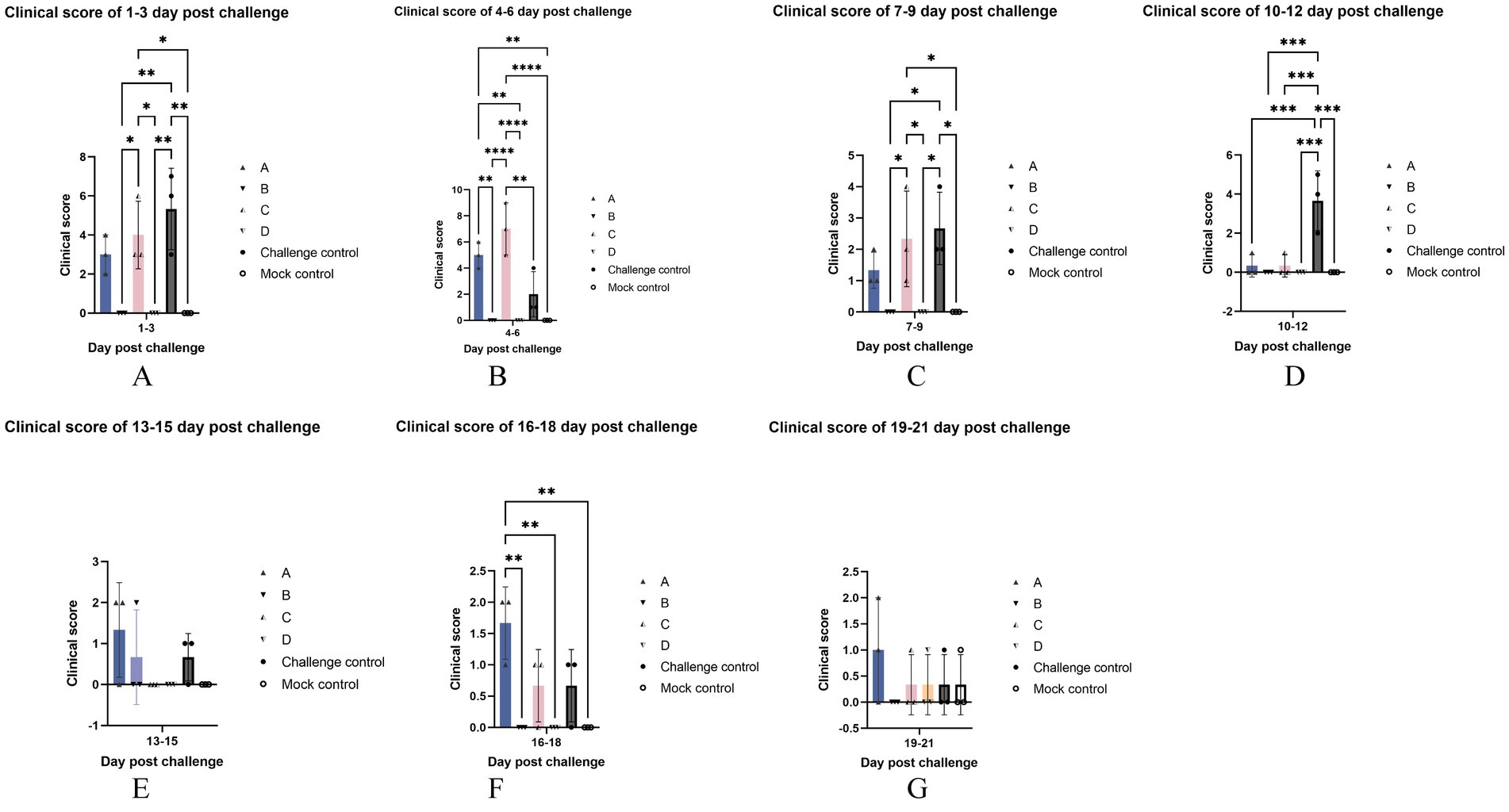
Figure 4. Clinical scores. The data are expressed as the mean ± S.D. of the number of pigs alive at the time of the sample collection. The clinical scores of each group were compared every three days after the challenge: 1–3 (A), 4–6 (B), 7–9 (C), 10–12 (D), 13–15 (E), 16–18 (F), 19–21 (F) day post challenge.* indicates a statistically significant difference (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001).
At 4–6 days after the challenge (Figure 4B), most of the piglets in group C were feverish, and one of them showed signs of lethargy on 6 dpc. The clinical score of group C was significantly higher than that of the control group but had no difference from that of group A. The clinical score of group A was significantly higher than that of blank control and two vaccine immunization control groups, and there was no significant difference between them and challenge control.
Cyanosis was present 7–9 days after the challenge (Figure 4C) in one of the piglets in the challenge control group with the highest clinical score. It was significantly higher than that of blank control and two vaccine immune control groups and had no difference with other challenged groups. Second, the score of group C was significantly higher than the blank control group and the two immune control groups, and there was no difference with group A.
10–12 days after the challenge (Figure 4D), the clinical score of the challenge control group was the highest due to the rectal prolapse of one of its piglets, which was significantly higher than that of all other experimental groups, and there was no significant difference between the other groups.
There was no significant difference in clinical scores among all groups at 13–15 days after the challenge (Figure 4E).
16–18 days after the challenge, the clinical score of group A was the highest (Figure 4F), which was significantly higher than that of blank control and two vaccine immune control groups, and there was no significant difference between the other groups. It is worth noting that a pig from the challenge control group was less active in food and the eating speed was significantly slowed down at 18 dpc.
There was no significant difference in clinical scores among all groups at 19–21 days after the challenge (Figure 4G).
The weight gain of each group of piglets was presented and compared by means of average weight, average daily weight gain, and cumulative average daily weight gain. As shown in Table 4, after adjusting for initial weight, statistical analysis showed that vaccine immunization had a negative effect on productivity growth. The average daily gain in the unvaccinated group was 55 g per day higher than that in the 1 dosage vaccinated group and 27 grams per day higher than that in the 0.1 dosage vaccinated group within 5 weeks after vaccination. Within 5 weeks after vaccination, the 0.1 dosage vaccinated group was 28 g per day higher than the 1 dosage group.
According to Table 5, there was no significant difference in cumulative daily gain between the unvaccinated challenged group and the vaccinated group with 1 or 0.1 dosage. Since the feeding environment of the blank control group and the immune control group were changed during the challenge, their weight data and cumulative daily gain data after the challenge were not statistically significant, and the results were for reference only (Supplementary Table S1). However, even with the presence of transition stress in the blank control group, the average body weight was 8.24 kg higher than that of the other groups at 9 weeks (21 dpc) after immunization, i.e., 88 days of age.
Pathological and histopathological examination
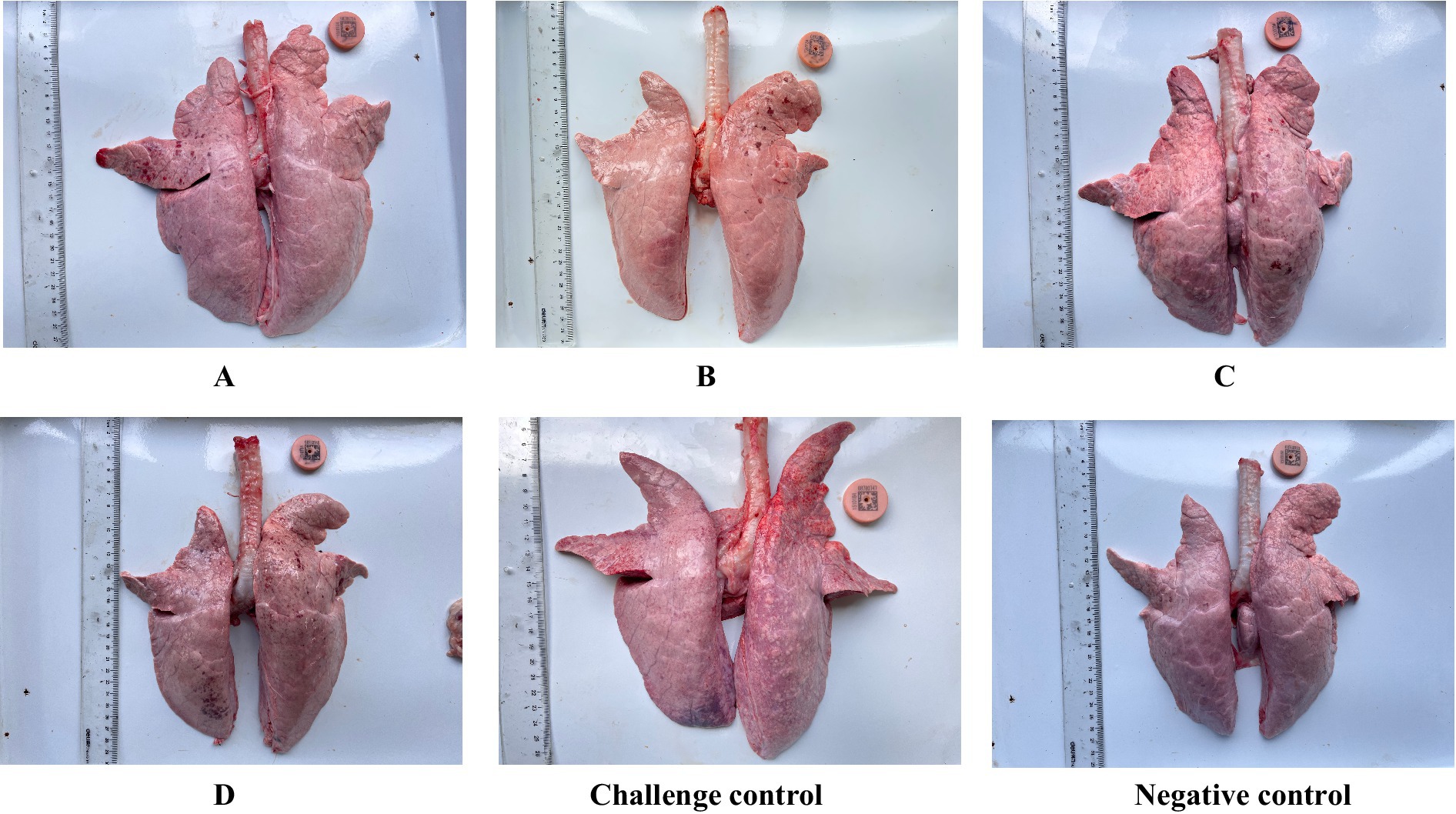
Twenty-one days after the challenge, all piglets were euthanized. At necropsy, the pathological changes in the Lungs were observed as shown in Figure 5. The pulmonary carnification of lungs from the challenge control group was more serious and the highest overall score was 6.06, compared to 3.5 in group C and 0.11 in group A. The degree of lung lesions was also scored. The challenge control group had the highest score and the most severe lesions, which was significantly higher than the other experimental groups except group C, and there was no significant difference between the other groups (Figure 6).
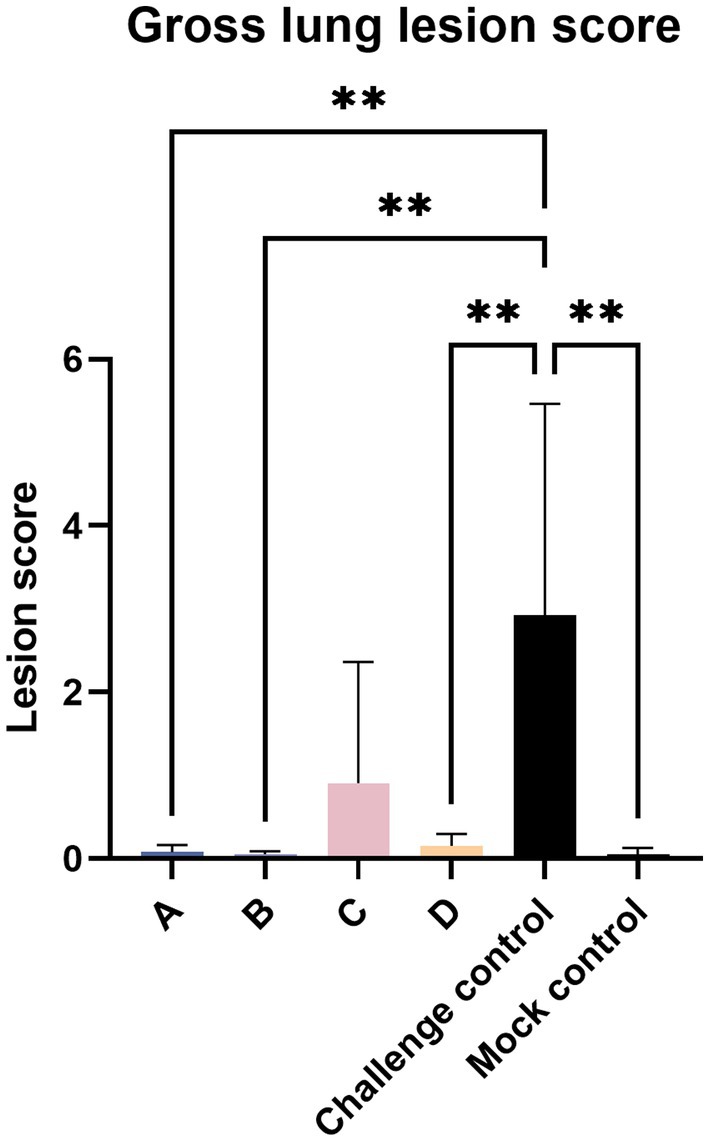
Figure 6. Gross lung lesion score. The data are expressed as the mean + S.D. * indicates a statistically significant difference (**p < 0.01).
Histopathological microscope observation showed that the lungs of the challenged control group showed a small amount of granulocyte infiltration, widened alveolar septum, narrowed alveolar cavity, a large amount of alveolar expansion, and macrophage infiltration was rare in the cavity. A small amount of lymphocyte infiltration is seen around the blood vessels, and bronchus, local bronchial hemorrhage, and exfoliated epithelial cells exist in the bronchial lumen (Figure 7). All immunized groups showed infiltration of alveolar wall granulocytes, a small amount of lymphocyte infiltration around blood vessels and bronchi, and the presence of shed epithelial cells in the bronchial lumen, while bronchial bleeding only existed in group A and group B of all the immunized groups.
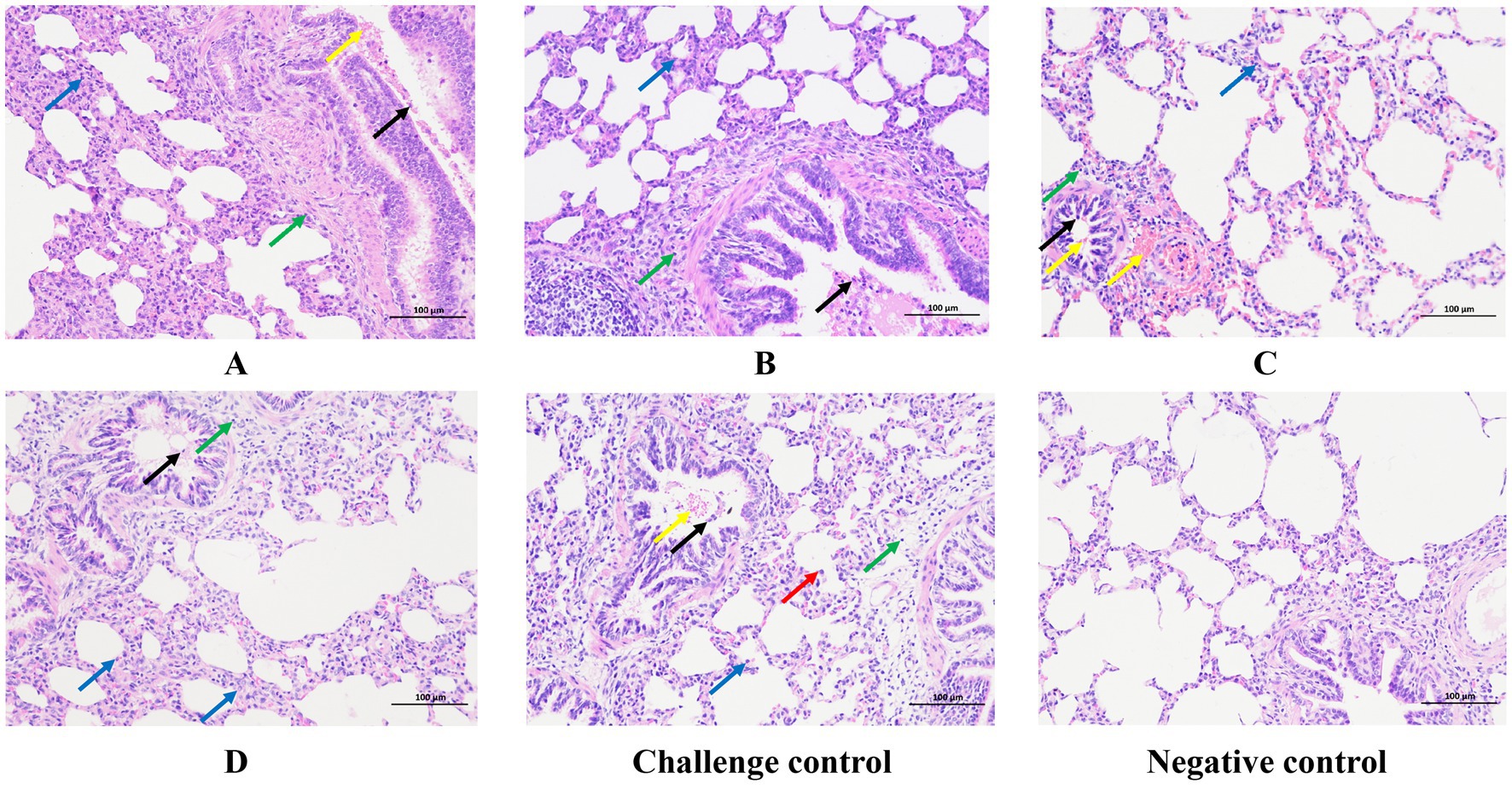
Figure 7. Typical HE manifestations of the lungs from each group. Blue arrows indicate granulocyte infiltration. Green arrows indicate lymphocyte infiltration. Black arrows indicate shed epithelial cells. Yellow arrows indicate hemorrhage. Red arrows indicate macrophage infiltration. Original magnification, 200 × .
Histopathologic sections were scored according to the preceding Table 3. The challenged control group had the majority of serious lesions, and its score was significantly higher than that of the other test groups; the score of the blank control group was the lowest, and there was no significant difference between the blank control group and other experimental groups except that the score was significantly lower than the challenge control group (Figure 8).
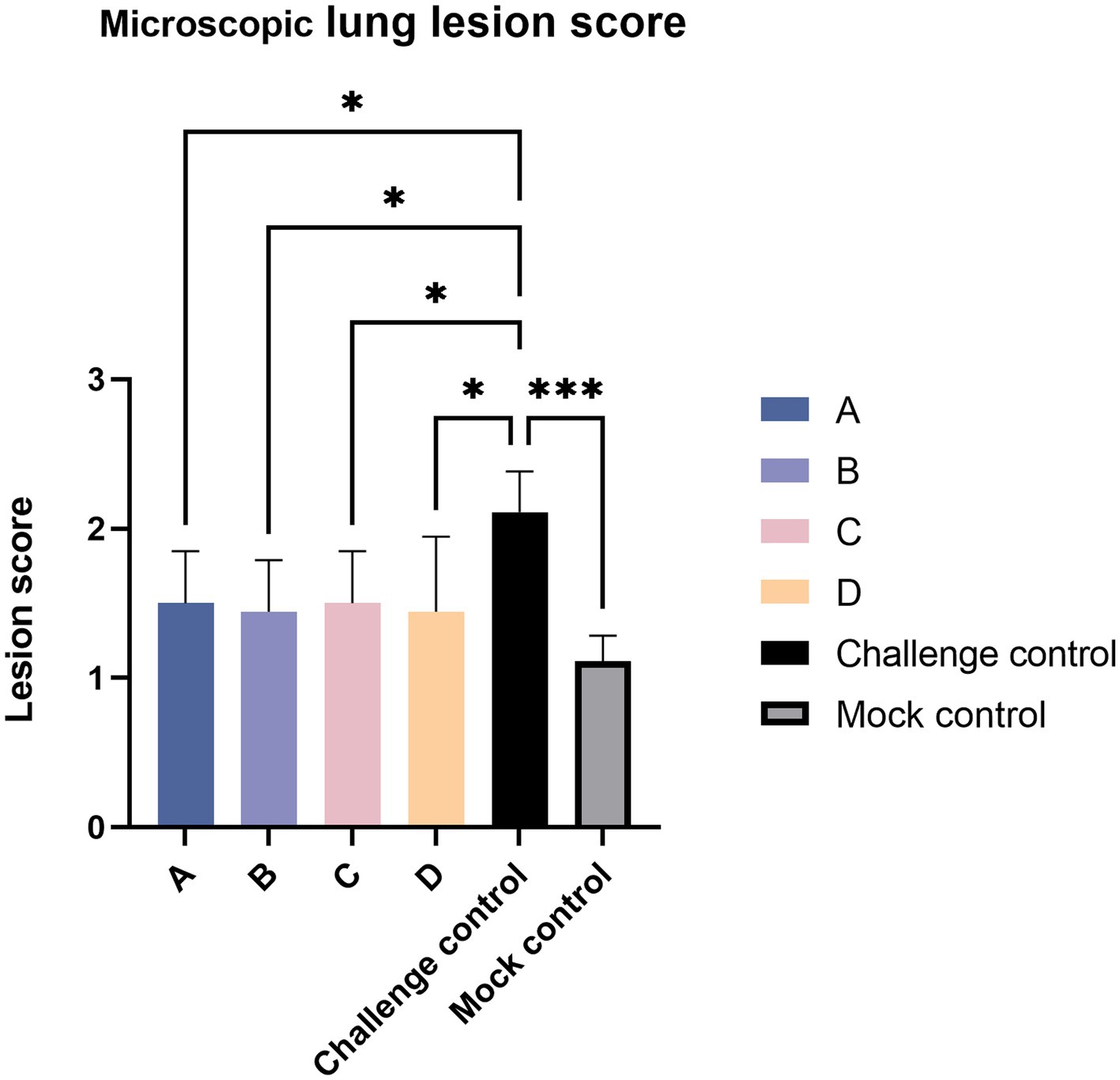
Figure 8. Lung tissue section score. The data are expressed as the mean + S.D. * indicates a statistically significant difference (*p < 0.05; ***p < 0.001).
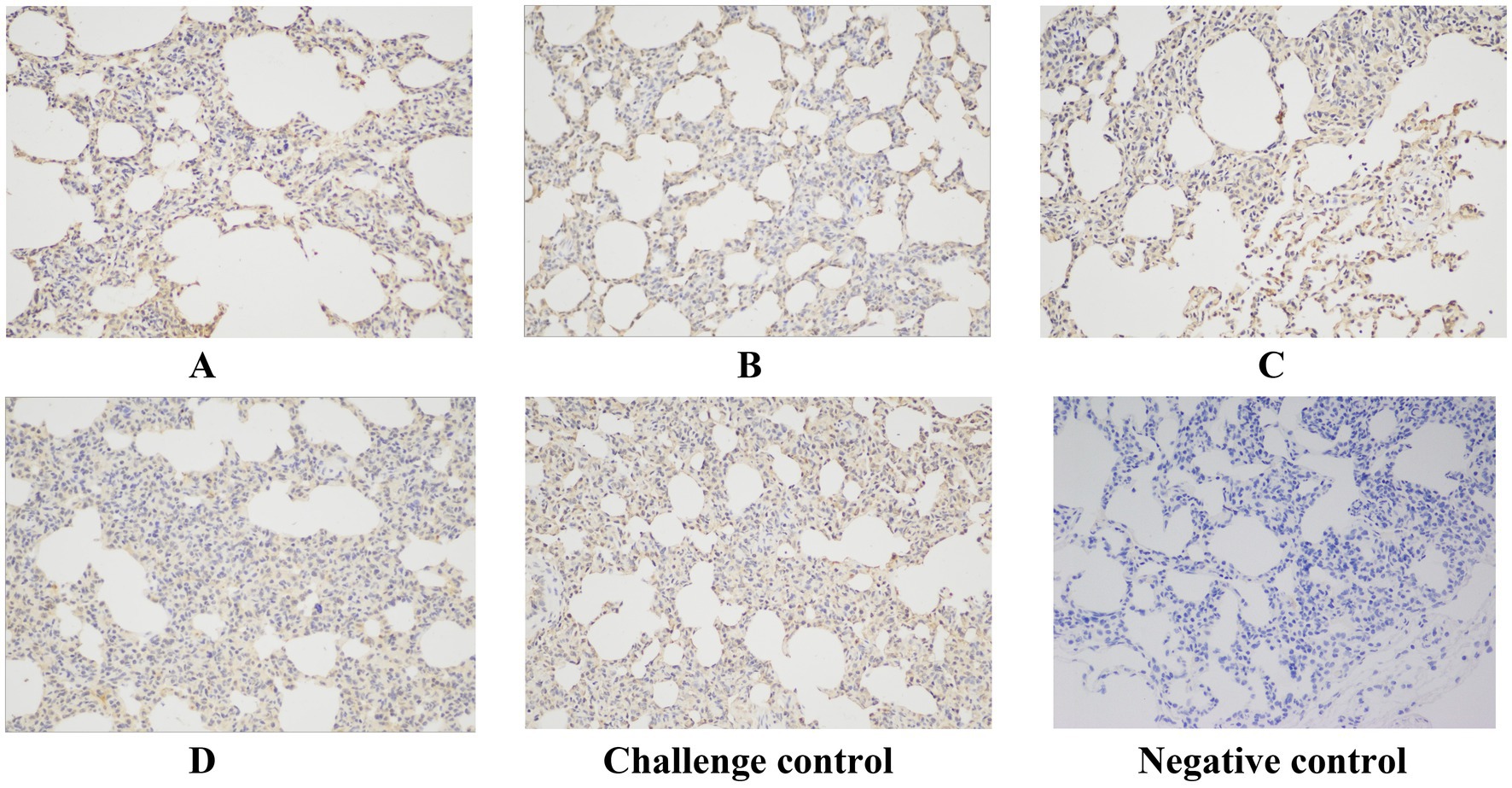
Immunohistochemistry (IHC) staining of the lung was also performed to detect the viral antigen, and typical IHC manifestations are shown in Figure 9. Positive cells were counted, and the positive cell rate was calculated (Figure 10). The positive cell rate of the blank control group was the lowest and significantly lower than that of the other experimental groups, and there was no significant difference between the other groups.
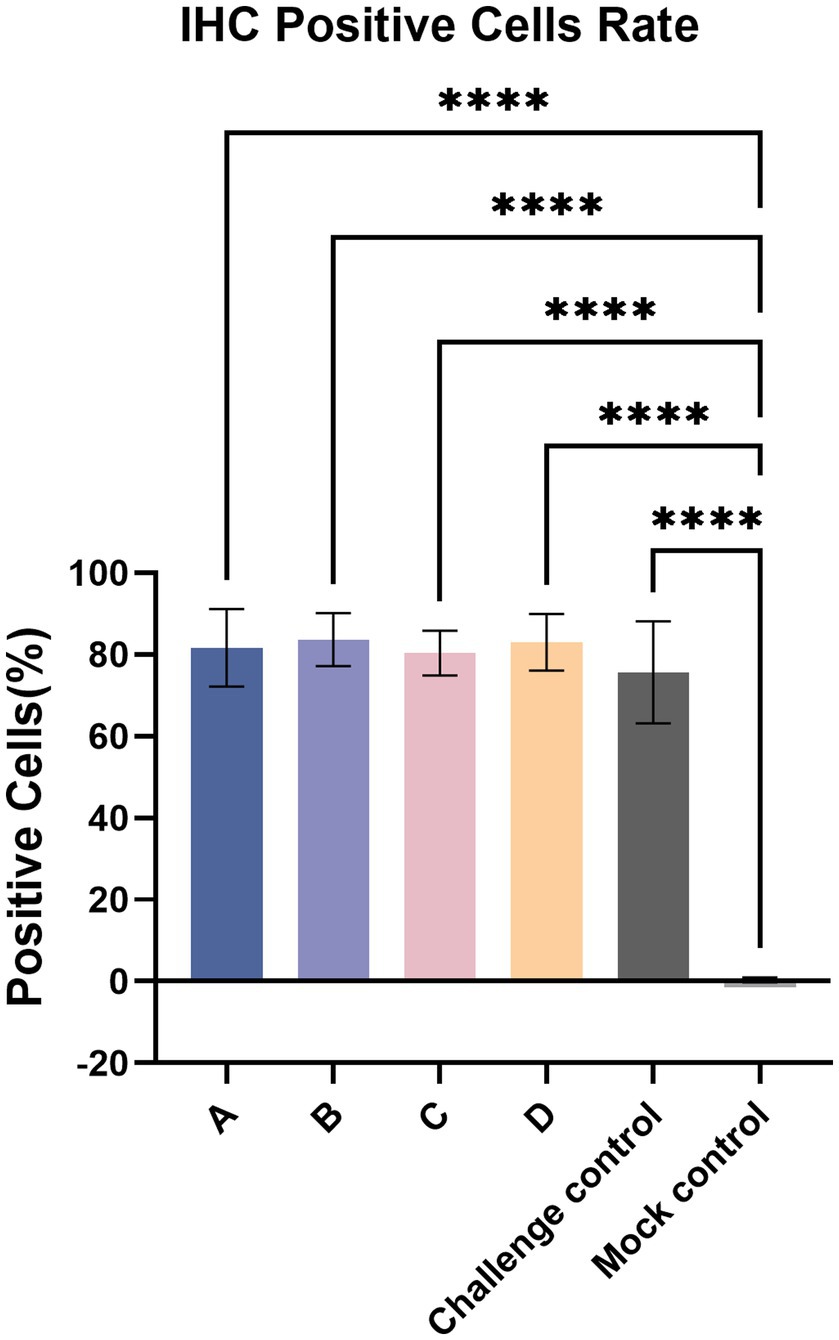
Figure 10. IHC-positive cells rate. The data are expressed as the mean ± S.D. * indicates a statistically significant difference (****p < 0.0001).
Viremia examination
Shortening the duration of viremia is a key index to evaluate the efficacy of vaccine immunity (Figure 11). Pig serum samples were taken 1, 3, 5, 7, 10, 14, and 21 dpc for viremia assessment. At 5 dpc, the virus load in the serum of the challenge control group was highest and significantly higher than that of the other experimental groups. There was no significant difference between the other groups. The effect of shortening the detoxification period in group C was similar to that in group A (The duration of viremia was slightly longer than or equal to 5 days).
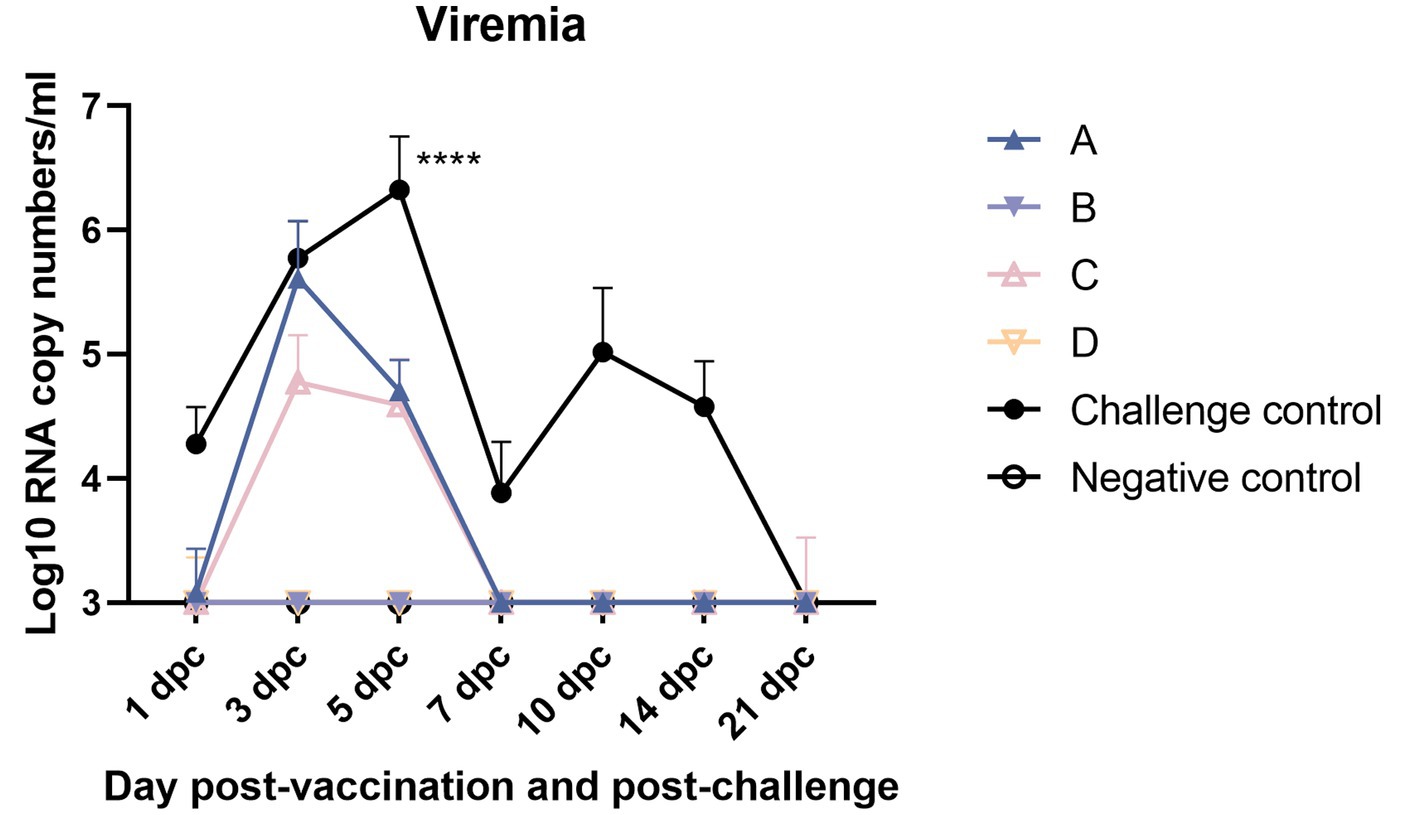
Figure 11. Viremia after NADC34-like DX PRRSV challenge (* indicates a statistically significant difference) (****p < 0.0001).
Serological test
The changes in PRRSV-specific antibodies were monitored from 21 dpv and assessed using an IDEXX ELISA kit. All vaccinated groups in 21 dpv turned positive and maintained an upward trend at 42 dpv (0 dpc) (Figure 12). At 7 dpc, the antibody level of group C decreased, and the average level was lower than that of other vaccine groups and then increased. By the end of the experiment, group B had the highest level of antibodies, followed by group A, group C, challenge control group, and group D.
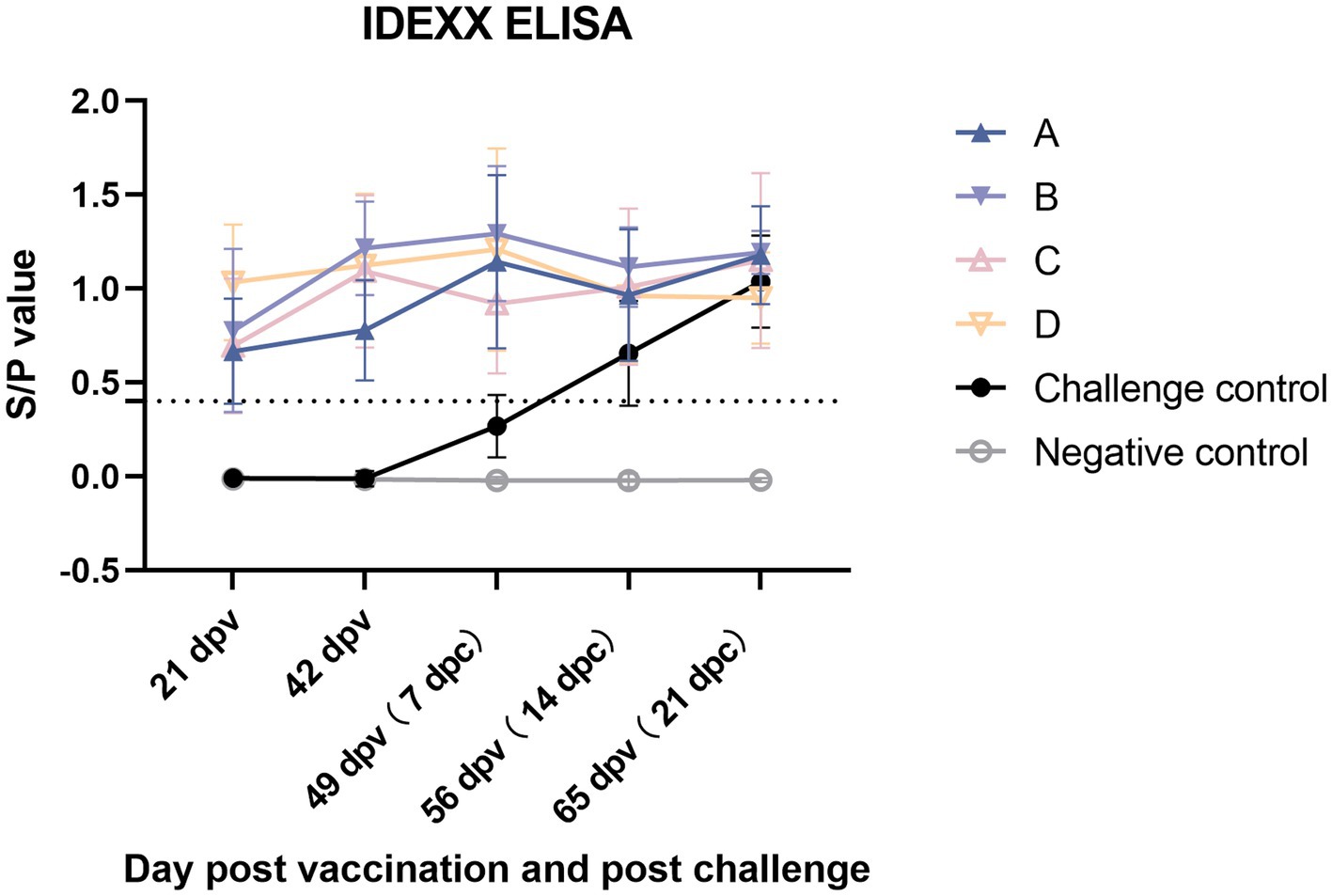
Figure 12. PRRSV-specific antibodies in each group following a PRRSV vaccination or challenge. The threshold for seroconversion was set at a sample-to-positive (s/p) ratio of 0.4 complying with the manufacturer’s guidelines. Each bar represents the average for five piglets ± SEM.
Discussion
The pig industry has suffered greatly as a result of NADC34-like PRRSV since it was first identified in the United States (5). Numerous publications from China have demonstrated the variable pathogenicity of PRRSV that resembles NADC34 (27–30). NADC34-like PRRSV DX strain, the strain used in this experiment, is the most stable and virulent of the isolated strains. In the challenge control group of this experiment, the typical clinical symptoms of PRRSV (transient fever, loss of appetite, etc.), and histopathological changes caused by it can also be seen.
The IA/2014/NADC34 prototype was proved highly pathogenic to piglets. While having a lower pathogenicity than the IA/2014/NADC34 strain, the DX strain can cause a longer duration of viremia and produce a larger serum viral load. It may have been reaffirmed that there is no positive correlation between viremia and the pathogenicity of NADC34-like PRRSV strains, and the use of viremia to determine the pathogenicity of NADC34-like PRRSV strains does not seem to be applicable (11).
In 2005, the vaccine against PRRSV was first released in China. For almost 20 years, China has used vaccines to prevent and control PRRSV. However, the current vaccines are not fully effective due to the repeated outbreaks of PRRS and the emergence of new PRRSV variants (9, 31), so the current clinical hope is to reduce the loss by reducing symptoms through immunization. Although safe, inactivated PRRSV vaccines are not effective against wild-type infections because they do not cause cell-mediated immunity (CMI) responses or the generation of particular PRRSV antibodies (32–34). The findings of a study showed that MLV offers significant cross-protection against the NADC30-like virus (35). It has come to our knowledge that Ingelvac PRRS MLV has been evaluated for protection against NADC34-like PRRSV (26). To date, several efficacy studies have been conducted on commercial PRRSV vaccines against different types of PRRSV (30, 31). In our research in the past, we have found that reduced dosages of Ingelvac PRRS MLV can provide better protection for NADC30-like PRRSV challenge. As a consequence, we tried different dosages to evaluate their protective effects against prevalent strains of the NADC34-like virus.
After the challenge, when comparing clinical scores, we found that the MLV vaccine immunization could relieve symptoms (Figure 4). As for gross lung lesion score and microscopic lung lesion score (Figures 6, 8), the score of the vaccine-immunized group was significantly lower than that of the challenge control group, and the performance of the reduced dosage group was better than that of the original dosage group. MLV vaccine immunization also shortened the detoxification period, and the effects of the two groups were similar (Figure 11). In our study, the Ingelvac PRRS MLV (VR-2332) vaccination still had certain toxic side effects and may be dose-dependent, which were reflected in increased body temperature, decreased daily gain, and aggravated lung lesions after immunization.
The majority of these studies talk about vaccine protection from a pathological point of view, so we also looked at the actual impact of vaccines on production. There was no significant difference in daily weight gain in the challenged groups (Table 5), that is, vaccine immunization could not improve the yield loss caused by the virus.
By using different assays, we verified again that the MLV vaccine can provide piglets with some protection against NADC34-like PRRSV. However, the 0.1 dosage Ingelvac PRRS MLV vaccination showed greater benefits in our study. Therefore, taking into account the cost, side effects, and subsequent protective effects, we can adjust the immune dosage appropriately after further investigation to ensure safety, improve production efficiency, and reduce immunization costs.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The animal study was approved by The procedures for the care and use of animals were approved by the Ethics Committee of the Sichuan Agricultural University Institutional Animal Care and Use Committee (protocol code 20230127 and July 1, 2023) and all applicable institutional and governmental regulations concerning the ethical use of animals were followed. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
XY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. FY: Methodology, Project administration, Resources, Supervision, Writing – original draft. YLin: Methodology, Project administration, Resources, Supervision, Writing – original draft. YLi: Supervision, Validation, Writing – original draft. WW: Supervision, Validation, Writing – original draft. SZ: Supervision, Writing – review & editing. XH: Supervision, Writing – review & editing. QZ: Supervision, Writing – review & editing. YW: Supervision, Writing – review & editing. SC: Supervision, Writing – review & editing. SD: Supervision, Writing – review & editing. NZ: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing. QY: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the Chengdu Major Science and Technology Application Demonstration Program (Giantstar Farming and Husbandry Co., Ltd), grant number 2022YF0900050SN.
Acknowledgments
Thanks to all the participants, collaborators, and researchers who contributed to this study. Special thanks to Chengdu SG-Biotech Co., Ltd. for assisting.
Conflict of interest
JL, YLi, WW, and NZ were employed by Giantstar Farming and Husbandry Co., Ltd. FY and YLin were employed by Chengdu SG-Biotech Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lunney, JK, Fang, Y, Ladinig, A, Chen, N, Li, Y, Rowland, B, et al. Porcine reproductive and respiratory syndrome virus (PRRSV): pathogenesis and interaction with the immune system. Annu Rev Anim Biosci. (2016) 4:129–54. doi: 10.1146/annurev-animal-022114-111025
2. Zhang, Z, Li, Z, Li, H, Yang, S, Ren, F, Bian, T, et al. The economic impact of porcine reproductive and respiratory syndrome outbreak in four Chinese farms: based on cost and revenue analysis. Front Vet Sci. (2022) 9:1024720. doi: 10.3389/fvets.2022.1024720
3. Guo, Z, Chen, XX, Li, R, Qiao, S, and Zhang, G. The prevalent status and genetic diversity of porcine reproductive and respiratory syndrome virus in China: a molecular epidemiological perspective. Virol J. (2018) 15:2. doi: 10.1186/s12985-017-0910-6
4. Johnson, CR, Griggs, TF, Gnanandarajah, J, and Murtaugh, MP. Novel structural protein in porcine reproductive and respiratory syndrome virus encoded by an alternative ORF5 present in all arteriviruses. J Gen Virol. (2011) 92:1107–16. doi: 10.1099/vir.0.030213-0
5. Dwivedi, V, Manickam, C, Binjawadagi, B, Linhares, D, Murtaugh, MP, and Renukaradhya, GJ. Evaluation of immune responses to porcine reproductive and respiratory syndrome virus in pigs during early stage of infection under farm conditions. Virol J. (2012) 9:45. doi: 10.1186/1743-422X-9-45
6. Neumann, EJ, Kliebenstein, JB, Johnson, CD, Mabry, JW, Bush, EJ, Seitzinger, AH, et al. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J Am Vet Med Assoc. (2005) 227:385–92. doi: 10.2460/javma.2005.227.385
7. Zhou, L, and Yang, H. Porcine reproductive and respiratory syndrome in China. Virus Res. (2010) 154:31–7. doi: 10.1016/j.virusres.2010.07.016
8. Albina, E. Le syndrome dysgénésique et respiratoire porcin: dix ans d’expérience (1986-1996) sur une infection virale insolite [Porcine reproductive and respiratory syndrome: ten years of experience (1986-1996) with this undesirable viral infection]. Vet Res. (1997) 28:305–52.
9. Zhou, L, Ge, X, and Yang, H. Porcine reproductive and respiratory syndrome modified live virus vaccine: a “leaky” vaccine with debatable efficacy and safety. Vaccine. (2021) 9:362. doi: 10.3390/vaccines9040362
10. Zhou, L, Yang, Y, Xia, Q, Guan, Z, Zhang, J, Li, B, et al. Genetic characterization of porcine reproductive and respiratory syndrome virus from eastern China during 2017-2022. Front Microbiol. (2022) 13:971817. doi: 10.3389/fmicb.2022.971817
11. van Geelen, AGM, Anderson, TK, Lager, KM, Das, PB, Otis, NJ, Montiel, NA, et al. Porcine reproductive and respiratory disease virus: evolution and recombination yields distinct ORF5 RFLP 1-7-4 viruses with individual pathogenicity. Virology. (2018) 513:168–79. doi: 10.1016/j.virol.2017.10.002
12. Zhang, HL, Zhang, WL, Xiang, LR, Leng, CL, Tian, ZJ, Tang, YD, et al. Emergence of novel porcine reproductive and respiratory syndrome viruses (ORF5 RFLP 1-7-4 viruses) in China. Vet Microbiol. (2018) 222:105–8. doi: 10.1016/j.vetmic.2018.06.017
13. Bao, H, and Li, X. Emergence and spread of NADC34-like PRRSV in China. Transbound Emerg Dis. (2021) 68:3005–8. doi: 10.1111/tbed.14316
14. Liu, J, Wei, C, Lin, Z, Xia, W, Ma, Y, Dai, A, et al. Full genome sequence analysis of a 1-7-4-like PRRSV strain in Fujian Province, China. PeerJ. (2019) 7:e7859. doi: 10.7717/peerj.7859
15. Xu, H, Song, S, Zhao, J, Leng, C, Fu, J, Li, C, et al. A potential endemic strain in China: NADC34-like porcine reproductive and respiratory syndrome virus. Transbound Emerg Dis. (2020) 67:1730–8. doi: 10.1111/tbed.13508
16. Xu, H, Li, C, Li, W, Zhao, J, Gong, B, Sun, Q, et al. Novel characteristics of Chinese NADC34-like PRRSV during 2020-2021. Transbound Emerg Dis. (2022) 69:e3215–24. doi: 10.1111/tbed.14485
17. Zhao, HZ, Wang, FX, Han, XY, Guo, H, Liu, CY, Hou, LN, et al. Recent advances in the study of NADC34-like porcine reproductive and respiratory syndrome virus in China. Front Microbiol. (2022) 13:950402. doi: 10.3389/fmicb.2022.950402
18. Li, P, Shen, Y, Wang, T, Li, J, Li, Y, Zhao, Y, et al. Epidemiological survey of PRRS and genetic variation analysis of the ORF5 gene in Shandong Province, 2020-2021. Front Vet Sci. (2022) 9:987667. doi: 10.3389/fvets.2022.987667
19. Canouï, E, and Launay, O. Histoire et principes de la vaccination [history and principles of vaccination]. Rev Mal Respir. (2019) 36:74–81. doi: 10.1016/j.rmr.2018.02.015
20. Zhang, H, Xiang, L, Xu, H, Li, C, Tang, YD, Gong, B, et al. Lineage 1 porcine reproductive and respiratory syndrome virus attenuated live vaccine provides broad cross-protection against homologous and heterologous NADC30-like virus challenge in piglets. Vaccine. (2022) 10:752. doi: 10.3390/vaccines10050752
21. Nielsen, HS, Oleksiewicz, MB, Forsberg, R, Stadejek, T, Bøtner, A, and Storgaard, T. Reversion of a live porcine reproductive and respiratory syndrome virus vaccine investigated by parallel mutations. J Gen Virol. (2001) 82:1263–72. doi: 10.1099/0022-1317-82-6-1263
22. Bøtner, A, Strandbygaard, B, Sørensen, KJ, Have, P, Madsen, KG, Madsen, ES, et al. Appearance of acute PRRS-like symptoms in sow herds after vaccination with a modified live PRRS vaccine. Vet Rec. (1997) 141:497–9. doi: 10.1136/vr.141.19.497
23. Charerntantanakul, W. Porcine reproductive and respiratory syndrome virus vaccines: immunogenicity, efficacy and safety aspects. World J Virol. (2012) 1:23–30. doi: 10.5501/wjv.v1.i1.23
24. Roca, M, Gimeno, M, Bruguera, S, Segalés, J, Díaz, I, Galindo-Cardiel, IJ, et al. Effects of challenge with a virulent genotype II strain of porcine reproductive and respiratory syndrome virus on piglets vaccinated with an attenuated genotype I strain vaccine. Vet J. (2012) 193:92–6. doi: 10.1016/j.tvjl.2011.11.019
25. Kick, AR, Amaral, AF, Cortes, LM, Fogle, JE, Crisci, E, Almond, GW, et al. The T-cell response to type 2 porcine reproductive and respiratory syndrome virus (PRRSV). Viruses. (2019) 11:796. doi: 10.3390/v11090796
26. Liu, J, Liu, C, Xu, Y, Yang, Y, Li, J, Dai, A, et al. Molecular characteristics and pathogenicity of a novel recombinant porcine reproductive and respiratory syndrome virus strain from NADC30-, NADC34-, and JXA1-like strains that emerged in China. Microbiol Spectr. (2022) 10:e0266722. doi: 10.1128/spectrum.02667-22
27. Song, S, Xu, H, Zhao, J, Leng, C, Xiang, L, Li, C, et al. Pathogenicity of NADC34-like PRRSV HLJDZD32-1901 isolated in China. Vet Microbiol. (2020) 246:108727. doi: 10.1016/j.vetmic.2020.108727
28. Yuan, L, Zhu, Z, Fan, J, Liu, P, Li, Y, Li, Q, et al. High pathogenicity of a Chinese NADC34-like PRRSV on pigs. Microbiol Spectr. (2022) 10:e0154122. doi: 10.1128/spectrum.01541-22
29. Xie, CZ, Ha, Z, Zhang, H, Zhang, Y, Xie, YB, Zhang, H, et al. Pathogenicity of porcine reproductive and respiratory syndrome virus (ORF5 RFLP 1-7-4 viruses) in China. Transbound Emerg Dis. (2020) 67:2065–72. doi: 10.1111/tbed.13549
30. Xu, H, Li, C, Gong, B, Li, W, Guo, Z, Sun, Q, et al. Protective efficacy of a candidate live-attenuated vaccine derived from the SD-R strain against NADC34-like porcine reproductive and respiratory syndrome virus. Vaccine. (2023) 11:1349. doi: 10.3390/vaccines11081349
31. Bai, X, Wang, Y, Xu, X, Sun, Z, Xiao, Y, Ji, G, et al. Commercial vaccines provide limited protection to NADC30-like PRRSV infection. Vaccine. (2016) 34:5540–5. doi: 10.1016/j.vaccine.2016.09.048
32. Bassaganya-Riera, J, Thacker, BJ, Yu, S, Strait, E, Wannemuehler, MJ, and Thacker, EL. Impact of immunizations with porcine reproductive and respiratory syndrome virus on lymphoproliferative recall responses of CD8+ T cells. Viral Immunol. (2004) 17:25–37. doi: 10.1089/088282404322875430
33. Kim, H, Kim, HK, Jung, JH, Choi, YJ, Kim, J, Um, CG, et al. The assessment of efficacy of porcine reproductive respiratory syndrome virus inactivated vaccine based on the viral quantity and inactivation methods. Virol J. (2011) 8:323. doi: 10.1186/1743-422X-8-323
34. Yu, HY, Qu, MS, Zhang, JL, Gan, L, Zhao, Y, Shan, XQ, et al. Recombinant porcine interferon alpha enhances immune responses to killed porcine reproductive and respiratory syndrome virus vaccine in pigs. Viral Immunol. (2019) 32:383–92. doi: 10.1089/vim.2019.0092
35. Li, C, Liu, Z, Chen, K, Qian, J, Hu, Y, Fang, S, et al. Efficacy of the synergy between live-attenuated and inactivated PRRSV vaccines against a NADC30-like strain of porcine reproductive and respiratory syndrome virus in 4-week piglets. Front Vet Sci. (2022) 9:812040. doi: 10.3389/fvets.2022.812040
Keywords: MLV vaccine, NADC34-like PRRSV, vaccine, efficacy, different dosages
Citation: Yan X, Liu J, Yue F, Lin Y, Li Y, Wu W, Zhao S, Huang X, Zhao Q, Wen Y, Cao S, Du S, Zeng N and Yan Q (2025) Efficacy of a reduced-dosage PRRS MLV vaccine against a NADC34-like strain of porcine reproductive and respiratory syndrome virus. Front. Vet. Sci. 11:1493384. doi: 10.3389/fvets.2024.1493384
Edited by:
Fanfan Zhang, Jiangxi Academy of Agricultural Sciences (CAAS), ChinaReviewed by:
Deping Song, Jiangxi Agricultural University, ChinaLi Wang, Northeast Agricultural University, China
Copyright © 2025 Yan, Liu, Yue, Lin, Li, Wu, Zhao, Huang, Zhao, Wen, Cao, Du, Zeng and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qigui Yan, eWFucWlndWlAMTI2LmNvbQ==; Nanfang Zeng, NDA5MjIwNjkzQHFxLmNvbQ==
†These authors have contributed equally to this work
 Xinyu Yan
Xinyu Yan Jiayu Liu2†
Jiayu Liu2† Wensi Wu
Wensi Wu Shan Zhao
Shan Zhao Xiaobo Huang
Xiaobo Huang Qin Zhao
Qin Zhao Sanjie Cao
Sanjie Cao Senyan Du
Senyan Du Qigui Yan
Qigui Yan