- 1Veterinary Teaching Hospital, Clinical Sciences, Colorado State University, Fort Collins, CO, United States
- 2Small Animal Hospital, Small Animal Clinical Sciences, University of Florida, Gainesville, FL, United States
Introduction: Locomotion improvement without serious complications is the main goal of treatment for feline aortic thromboembolism (FATE). We aimed to describe the survival and functional recovery of cardiogenic FATE treated with tissue plasminogen activator (rtPA) continuous rate infusion (CRI) and/or pentoxifylline (PTX) and/ or cyproheptadine (CYP), and to identify non-survivor characteristics.
Methods: This is a retrospective, bicentric case series. Inclusion criteria were cardiogenic FATE cats receiving any of the medications described. Proportions of locomotion recovery, survival to discharge, reperfusion injury (RI) and acute kidney injury (AKI) were described. Admission and outcome characteristics were compared between survivors and non-survivors.
Results: Nine cats were identified, 8/9 (88.9%) with bilateral FATE. Median age was 8.2 years (5.3–12.5). Median weight was 5.3 kg (4.1–7.1). Admission rectal temperature, affected limb lactate, creatinine and potassium were 36.9°C (35.4–38.8), 14.2 mmol/L (7.1–18.8), 114.9 umol/L (53–185.6) and 3.7 mmol/L (3.5–44.), respectively. No significant differences were found between survivors and non-survivors for relevant admission characteristics. Seven (77.8%) cats received tPA-CRI, 7/9 (77.8%) cats received pentoxifylline and 5/9 (55.6%) cats received cyproheptadine. Three (33.3%) cats received monotherapy (two rtPA-CRI, one PTX), 2/9 (22.2%) cats received dual therapy (one rtPA-CRI/PTX, one PTX/ CYP) and 4/9 (44.4%) cats received triple therapy. All study cats (100%) had an improvement in locomotion, and 4/9 (44.4%) survived to discharge. Reperfusion injury and AKI were documented in 3/9 (33.3%) and 4/9 (44.4%) of cats, respectively. Non-survivors had a greater proportion of AKI than survivors (4/5 (80%) and 0/4 (0%), respectively). No other outcome characteristics were different between survivors and non-survivors.
Discussion: rtPA CRI, PTX and/or CYP could be considered for treatment of FATE.
1 Introduction
Cardiogenic feline aortic thromboembolism (FATE), defined as the migration of a left atrial thrombus into systemic arteries, is a commonly encountered disease in cats. Bilateral FATE has a guarded prognosis with a 30–40% survival to discharge proportion in cats treated with conventional therapy (1–5). Previous studies have identified effective ways of preventing further embolization through the use of antiplatelet and anticoagulant medications (6–8). This has allowed some cats with cardiogenic FATE to have low re-embolization rates (three out of 18 (16.7%) in one study) and median survival times of 502 days, provided they survive to discharge (8). Therapy aimed at effectively and safely removing the clot while minimizing complications in the hospital, allowing survival to discharge, is therefore desirable.
The Consensus on the Rational Use of Antithrombotics in Veterinary Critical Care (CURATIVE) domain 6 guidelines, published to guide the use of thrombolytic therapy in dogs and cats, recently suggested that thrombolysis can be attempted in acute (i.e., <6 h) FATE, although no specific protocol was recommended, and that strong evidence-based recommendations are lacking (9). Alteplase, a recombinant form of tissue plasminogen activator (rtPA), is a frequently used thrombolytic. Alteplase has a half-life of less than 5 min and has minimal persistent effects on the body, making it ideal for constant-rate infusion (10). A commonly used thrombolytic protocol in FATE (11, 12) is based on acute ischemic stroke (AIS) protocols in people (13); although AIS differs from FATE in etiology, organ(s) affected the amount of ischemic tissue and complications. Acute ischemic stroke has been shown to be most effectively treated when rtPA is given within 4.5 h of the event (14, 15). It is licensed in people in North America for administration of 0.9 mg/kg, with 10% of this dose administered as an initial bolus followed by an infusion of the remaining dose over 1 h.1 Sudden aortic thromboembolism is rare in people, is most often seen in neonates associated with umbilical catheterization, and is most commonly treated with rtPA continuous rate infusion (rtPA-CRI) and thromboprophylaxis, although clear recommendation and treatment consensus is lacking (16–18). In veterinary medicine, rtPA-CRI in six FATE cats was described in 1987 abstract, reporting a three out of six (50%) survival rate (19).
Common complications seen during FATE treatment, regardless of the use of thrombolytics, include reperfusion injury (RI) and acute kidney injury (AKI) (11, 12). Reperfusion injury occurs during recanalization of the clot, with subsequent flooding of the body with cytotoxic and inflammatory mediators (20). AKI has historically been thought to be related to occlusion of the renal vessels with clots; other contributing factors may include the release of vasoconstrictive substances, a lack of collateral circulation, and dehydration (11, 21). These comorbidities can be life-threatening, and identifying therapy to minimize them alongside maximizing recanalization of the aorta represents a significant target of FATE treatment. The development of collateral circulation is especially important, as experimental data showed that complete ligation of the aorta does not induce clinical paralysis in cats, whereas the use of vasoactive substances in the distal aorta without a physical occlusion would result in paralysis and lack of perfusion in the pelvic limbs (21).
Pentoxifylline (PTX) and cyproheptadine (CYP) are drug candidates to help mitigate AKI and RI. Pentoxifylline is a methylxanthine class medication that may aid kidney perfusion and reduce inflammation (22, 23), and studies evaluating its use in human neonatal vasospasm and thrombosis have been promising (24). Cyproheptadine is a serotonin receptor inhibitor capable of reducing platelet aggregation (25), and may oppose the vasoconstrictive and platelet-aggregatory effects of serotonin in experimental FATE if given prior to a FATE event (26). The use of these medications has never been studied in naturally occurring FATE.
Our primary aim was to describe the survival and functional recovery outcomes of cats presenting with cardiogenic FATE and treated with rtPA-CRI and/or pentoxifylline and/or cyproheptadine. Our secondary aim was to identify non-survivor characteristics. We hypothesized that treatment with an rtPA-CRI and/or pentoxifylline and/or cyproheptadine would result in a similar proportion of return to limb function with a similar proportion of RI, AKI and survival compared to historical studies on FATE treatment.
2 Materials and methods
2.1 Case identification, inclusion and exclusion
Cats in this retrospective case series were identified using the clinician’s personal case log at the Colorado State University (CSU) James L. Voss Veterinary Teaching Hospital (JG) or an rtPA prescription pharmacy search at the University of Florida (UF) Small Animal Hospital (JW) emergency rooms. Ethical approval was not required by the Institutional Animal Care and Use Committee due to the retrospective nature of this study. Cats with a diagnosis of cardiogenic FATE that received treatment including at least one of rtPA-CRI, PTX or CYP were included. FATE was diagnosed based on clinical observations of any limb affected by the following five criteria: pale, cold, pulseless, painful, and lack of motor function (11, 12). Cardiogenic FATE was then diagnosed based on left-atrial to aorta ratio > 1.6:1 on a right parasternal short axis cardiac point-of-care ultrasound view or by complete echocardiography performed or supervised by a diplomate of the American College of Veterinary Internal Medicine (Cardiology) (27). Cases were excluded if they did not receive at least one of the three previously mentioned therapies.
2.2 Data collection
Data collected were as follows: demographic (age, sex, and breed); FATE characteristics (i.e., FATE limb location and time of event to admission); physiological characteristics (i.e., admission body weight/rectal temperature/pulse rate/respiratory rate/Doppler blood pressure); clinicopathological variables (i.e., admission lactate (non-affected limb, averaged affected limb, differential lactate), admission systemic blood glucose/creatinine/potassium/platelet count, and maximum creatinine); disease processes (i.e., type of cardiac disease and comorbidities); treatment (i.e., rtPA use and dose, admission time and FATE onset time to rtPA treatment time, PTX use and dose, CYP use and dose, other in-hospital medications, and pre-hospital medications); measured outcomes (i.e., reperfusion injury and timing, AKI and timing, locomotion improvement; survival, length of hospitalization); and euthanasia (yes/no). Any other pertinent details to each case were recorded in a qualitative manner. All data were stored in commercially available software.2
Reperfusion injury was defined as a rise in serum potassium over the reference range for the used biochemistry analyzer. AKI was defined as per the Risk, Injury, Failure, Loss, End-Stage Renal Failure (RIFLE) ‘injury’ score [i.e., a ≥ 2 fold (100%) increase in serum creatinine from baseline (admission) using point-of-care blood gas analyzers, as previously described (11, 12, 28, 29)]. This definition, away from the IRIS classification (30), was used to limit capturing AKI from furosemide use (31) and make AKI diagnosis more reflective of underlying disease, as recently published (11, 12). Locomotion improvement was defined as mild, moderate, or marked based on regain of motor function without autonomous ambulation; with slow and intermittent autonomous locomotion; and with strong ambulation, respectively. Locomotion improvement was established retrospectively and independently by two of the authors, and conflicts were resolved through consensus.
Clinicopathological variables were measured using ALB800 FLEX (CSU, UF) and ABL90 FLEX PLUS (CSU).3,4 Values out of the reference range were recorded as follows: respiratory rate ‘panting’ as 150 breaths per minute, ‘low’ temperature as 32.2°C (90°F), creatinine above the reference range as 1,503 umol/L (17 mg/dL).
2.3 Statistical analysis
Normality was assessed using the Shapiro–Wilk test. Descriptive statistics for the study group were reported as median (min–max) for all continuous data due to the small sample size. Categorical data were presented as frequencies and percentages.
Mann–Whitney U and Fisher’s exact tests were used to assess differences between survivors’ and non-survivor’ admission characteristics, cumulative rtPA dose and outcome characteristics. All tests were run two-sided where appropriate, with exact p-values reported. Significance was set at p ≤ 0.05. All statistics were performed in SPSS.5
3 Results
Nine cats were identified from November 2021 to August 2024, with 6/9 (66.7%) coming from CSU and 3/9 (33.3%) from UF (Table 1). The breeds in the study group were domestic, with various coat lengths [5/9 (55.6%)], Bengal [2/9 (22.2%)], and 1/9 (11.1%) each of Sphynx and Turkish Angora.
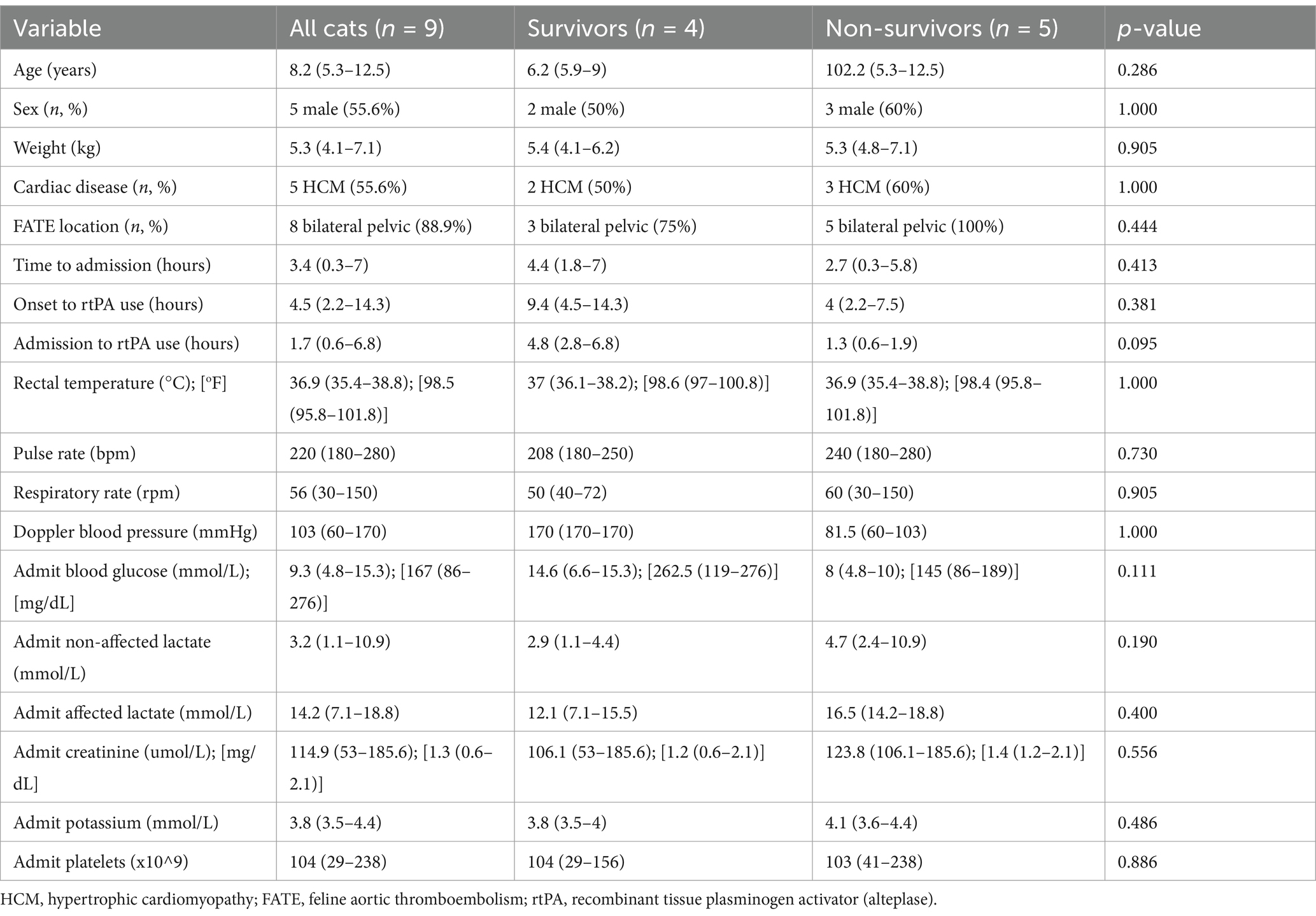
Table 1. Admission characteristics of nine study cats treated with a combination (or not) of alteplase constant rate infusion, pentoxifylline or cyproheptadine, and comparison of survivor and non-survivor characteristics.
One (11.1%) cat had a unilateral left forelimb embolism. Two (22.2%) cats had previously been diagnosed with heart disease (one restrictive cardiomyopathy phenotype and one undiagnosed bi-atrial enlargement). Three (33.3%) cats had non-cardiac comorbidity: exercise intolerance, mixed hepatopathy and chronic enteropathy/exocrine pancreatic insufficiency, and lower airway disease. Seven (77.8%) cats had at least one pre-hospital medication, as follows: methadone [3/9 (33.3%)], furosemide [2/9 (22.2%)], and 1/9 (11.1%) each of clopidogrel, ketamine, buprenorphine, gabapentin, diphenhydramine, pancreatic enzymes, and cobalamin. All pre-hospital medications were acutely prescribed by the referring veterinarian except one case each of furosemide, diphenhydramine, pancreatic enzymes, and cobalamin. Five (55.6%) cats were referrals, of which 4/5 (80%) were presented to CSU and 1/5 (20%) was presented to UF.
3.1 Treatments
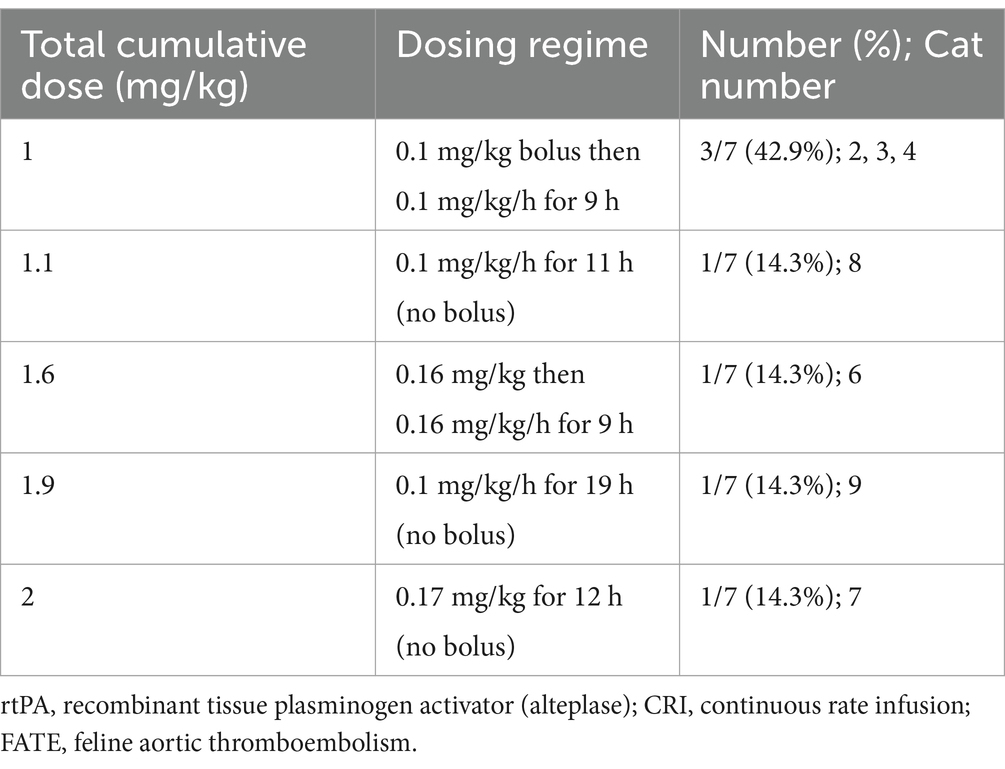
Seven (77.8%) cats received rtPA-CRI, 7/9 (77.8%) cats received PTX and 5/9 (55.6%) cats received CYP (Table 2). Per individual patients, 3/9 (33.3%) cats received monotherapy: 2/9 (22.2%) rtPA-CRI and 1/9 (11.1%) PTX. One (11.1%) cat received rtPA/PTX dual therapy, 1/9 (11.1%) cat received CYP/PTX dual therapy, and 4/9 (44.4%) cats received rtPA/PTX/CYP triple therapy. The average PTX dose was 100 mg (range: 60–100 mg) every 12 h, with an average time to start PTX after admission of 14 h (range: 4–22 h). All cases that received CYP did so at a 2-mg dose every 12 h, with an average time to start CYP after admission of 12 h (range: 5–25 h). Specific rtPA doses used are shown in Table 3, with an average onset to rtPA administration of 4.5 h (range: 2.2–14.3 h). The average cumulative rtPA dose was 1.1 mg/kg (range: 1–2 mg/kg), and there was no difference in cumulative rtPA dose between survivors and non-survivors (p = 1.000).
All cats (100%) received thromboprophylaxis with clopidogrel and analgesia with a full μ-agonist during hospitalization. Seven cats (77.8%) received a 75 mg-loading dose of clopidogrel. Five (55.5%) cats received enoxaparin, and 3/9 (33.3%) different cats received rivaroxaban. Other in-hospital treatments included as follows: furosemide (n = 8); dextrose and maropitant (n = 5); regular insulin, calcium gluconate, lactated ringers, 0.45% sodium chloride and gabapentin (n = 4); potassium chloride, sodium bicarbonate, pimobendan, mirtazapine and n-acetylcysteine (n = 2); and one each of metoclopramide, ondansetron, spironolactone, buprenorphine, inhaled salbutamol, terbutaline, peritoneal dialysis, diltiazem, packed red blood cell transfusion, mannitol, hypertonic saline, potassium gluconate, s-adenosylmethionine, and pancreatic enzymes.
3.2 Outcomes
All (n = 9) cats had some degree of locomotor improvement, and 4/9 (44.4%) survived to discharge (Table 2). All non-survivors (n = 5) were euthanized. Admission characteristics between survivors and non-survivors were not different (Table 1). Three (33.3%) cats developed RI, and 4/9 (44.4%) cats developed an AKI. All cats that developed AKI had received furosemide. One cat was euthanized due to a repeat FATE event alongside a suspected cerebral ischemic event 24 h after stopping the rtPA-CRI and was the only case suspected of having a repeat thromboembolic event (1/9, 11.1%). This cat received 0.1-mg/kg/h rtPA-CRI for 19 h. The time from admission to development of RI and time from the first blood sample to AKI were 11 h (range: 7.6–14 h) and 12.3 h (range: 11–39 h), respectively. The degree of locomotor recovery was mild [5/9 (55.6%)], moderate [3/9 (33.3%)], and marked [1/9 (11.1%)]. The median length of hospitalization was 2.4 days (range: 1–3.7 days).
In order to investigate possible causes of euthanasia, comparisons of locomotion recovery, RI and AKI proportions between survivors and non-survivors were performed. There was a greater proportion of AKI in non-survivors (4/5 (80%)) than in survivors (0/5 (0%)) (p = 0.048). There was no difference in locomotion recovery (p = 0.087) or RI (p = 1.000) between survivors and non-survivors. The length of hospitalization (days) was significantly longer in survivors versus non-survivors (2.9 days (2.4–3.7) versus 1.3 days (1–2.8), p = 0.050).
4 Discussion
Our retrospective bicenter study described the treatment of a series of cats with cardiogenic FATE treated with rtPA-CRI and/or pentoxifylline and/or cyproheptadine. All cats in our case series regained motor function. Cats that did not survive were more likely to have an AKI.
Patient characteristics in our study were similar to previously reported FATE studies regarding age, weight, admission rectal temperature, heart rate, respiratory rate, hypertrophic cardiomyopathy (HCM) diagnosis, bilateral hindlimb FATE, and previously diagnosed heart disease (1, 2, 5, 7, 8, 12, 19, 32–34). Sex and breed characteristics in the study group were found to be 55.6% male and 50% domestic breed demographic. This differs from previous studies where males have typically been greatly over-represented and domestic breed frequency is over 60%; however, those differences are likely due to our small sample size.
Systemic blood glucose at admission in our study group was elevated, although lower than the largest prospective study in FATE cats (11). The significance of this finding is unclear. In dogs and people, hyperglycemia has been associated with worse outcomes, but not in cats (35–37). Hyperglycemia has been reported to be common in FATE cats (2); however, as in the general feline population, hyperglycemia has not been identified as a prognostic indicator in FATE. Admission blood glucose were not different between survivors and non-survivors in our study.
The novel rtPA protocol described in our study uses a CRI as performed in human neonatal aortic thromboembolism (16, 18), although the dosing (0.1–0.5 mg/kg/h, repeated if no effect), time frame (6-h infusion) and adjunct drugs (heparin; 10 U/kg/h) differ, and a CRI was chosen to try to optimize previously attempted treatment regimens (4, 11, 12). Studies on the use of rtPA in FATE have tested similar treatment protocols for people with AIS and failed to find benefits in survival or adverse outcomes compared to placebo; however, due to patient deaths, these studies became underpowered (11, 12). The most recent, a multicenter randomized control trial comparing a 1-h 1-mg/kg rtPA infusion to placebo failed to find a difference in AKI, RI or survival (11). Although non-significant, limb scores were consistently better in the rtPA group as was survival for 48 h. Type 2 error was likely a key factor in the lack of significant treatment effect found.
Our results are comparable to a previous clinical veterinary study, presented in an abstract form in 1987, that investigated the use of rtPA-CRI in cardiogenic FATE (19). Complications in the 1987 abstract were reported as being minor (limited to mild bleeding at the catheter site during rtPA infusion); however, the non-survivors [3/6 (50%)] were all reported to have died from reperfusion injury (defined as acidosis and/or hyperkalemia) (19). In a book chapter written by the same author, they mentioned that RI was not expected following FATE treatment and suggested it may have been due to rtPA use (38). That study enrolled six cats to receive rtPA-CRI without a placebo group, with an overall 50% (3/6) survival rate versus ours with 44% (4/9) survival and 100% (3/3) improvement in limb function in all surviving cats as we also found. Their study found a 50% (3/6) RI rate versus ours with a 33% (3/9) RI rate. Doses up to 8 mg/kg and timing between event and treatment up to approximately 30 h in the 1987 study may explain the higher RI rate in that study. Nevertheless, it provided the first evidence that rtPA may be successful in the treatment of cardiogenic FATE.
Another study (34) of 11 cats investigated two 5-mg/cat protocols, a 1.5-h rtPA infusion and a 4-h rtPA-CRI group, although the authors combined both protocols in the results. Among seven survivors 24 h after rtPA, 57% (4/7) of cats had motor function post-rtPA versus 28% (3/11) pre-rtPA. There was 64% (7/11) 24-h survival; however, only 27% (3/11) of the cats survived to discharge, and the study was terminated early due to high adverse events. This is compared to the 44% survival to discharge in our study. The high degree of adverse events was attributed to rtPA administration; however, the study did not include a control group. It should be noted that 36% (4/11) of the cats in that study received a second dose of the 5-mg/cat protocol, three of which died. This narrative of rtPA leading to poor outcomes (19, 34) is now being challenged, with a similar frequency of adverse events such as azotemia, hyperkalemia, and cardiac arrest in thromboprophylaxis-only protocols, and similar between rtPA group and control groups (11, 12).
The treatment modality in our study used an rtPA-CRI, as is reported for neonatal distal aortic thromboembolism (16, 18). Recommendation in neonatal medicine is an rtPA-CRI (0.1–0.5 mg/kg/h) for 6 h alongside a 10-U/kg/h heparin CRI in cases of major thrombosis, defined as absent femoral pulse alongside severe ischemia, defined as gangrene, tissue loss and/or paralysis (18). Moderate thrombosis, defined as absent femoral pulses and/or evidence of peripheral ischaemia (cool, pale, mottled extremities, and poor capillary refill time), is treated with rtPA-CRI if no improvement is noted with heparin only. If there is no clinical or radiological improvement after 6 h, a repeat infusion at the same dose and time frame is recommended (18). A surgical consultation for thrombectomy is also recommended at this later stage. However, evidence-based thrombolytic treatment in neonates is lacking (18). Future FATE studies may benefit from investigating neonatal rtPA protocols to improve outcomes.
Minimizing complications, such as AKI and RI, and promoting collateral circulation is paramount for treating clinical FATE. Experimental studies have shown that ligation of the distal aorta in normal cats does not create the clinical syndrome of FATE due to the development of collateral circulation. Instead, vasoactive substances injected in the distal aorta, even without physical obstruction, mimic the clinical syndrome of FATE (21, 26). The use of medications to promote collateral circulation has been suggested for more than 35 years, but our study is the first report of clinical use (38). Our study investigated the candidate drugs PTX and CYP based on the mitigation of vasoconstrictive effects of serotonin with CYP in the feline distal aorta (21, 26) and the vasodilatory effects of PTX (39, 40). One of the desired outcomes of using these medications is to improve functional recovery, and another desired outcome is to improve kidney perfusion, decrease the proportion of AKI, and decrease the proportion of RI.
Pentoxifylline is a methylxanthine drug that improves red blood cell rheology and induces vasodilation, and it has been shown to positively impact the restoration of blood flow in several experimental models (20, 40–43). It has been shown to maintain cerebral perfusion following ischemia in cats post-cardiac arrest compared to control without the drug if given pre-arrest, and it has some vasodilatory effects in feline cerebral and pulmonary vasculature that may mitigate renal arteriolar vasoconstriction (39, 40, 44). Cyproheptadine is a serotonin receptor inhibitor (26). Serotonin has been experimentally shown to have a role in vasoconstriction and the development of paralysis in the distal aorta of cats (21), and serotonin agonism has been shown to increase the risk of coronary stenosis and thrombosis in animal models (45, 46) and people (47). Cyproheptadine was an effective agent for preventing paresis or paralysis in an experimental model of FATE (i.e., double aortic ligation and injection of a mixture of cat blood and glass beads in the space created between the two ligatures) if given prior to the induced FATE event (26). Additionally, it has been shown to reduce serotonin-related vasospasm and platelet aggregation in the canine pulmonary vasculature (48) and may reduce serotonin-related vasospasm in human cerebral vasculature (49). Non-cyproheptadine serotonin antagonists have been shown to reduce coronary thrombosis in canine models when given prior to clot formation (50).
Our study is the first to report the use of PTX and CYP in clinical cardiogenic FATE and found locomotion improvement in all cats. Our study has higher locomotion recovery proportions than previous studies, although a direct comparison cannot be made (4, 11, 12, 34). The average cumulative rtPA doses in these studies and ours are similar, suggesting that the use of a CRI or the addition of PTX or CYP should be considered. Specifically, a 2022 abstract from France showed a significantly higher proportion of arterial recanalization (54.5% vs. 20.9%) and locomotion recovery (26.1% vs. 13.8%) with a 1-h, 1 mg/kg rtPA protocol (n = 52) than supportive care only (n = 65), with similar survival (35.5% vs. 34.7%) (4). This is an important consideration, as locomotion recovery is likely a major desired outcome in FATE. Locomotion recovery was not different between survivors and non-survivors in our study and may suggest reasons for euthanasia in this population are not related to locomotion recovery.
Compared to the most recent retrospective study on rtPA in FATE, our study found lower RI proportions [5/10 (50%) and 3/9 (33.3%), respectively] and higher AKI proportions [3/10 (30%) and 4/9 (44.4%), respectively] (12). The most recent prospective study on rtPA in FATE also found lower AKI rates (39%) but similar RI rates (33%) in our study (11). Further studies with a larger sample size are needed to investigate the impact of PTX or CYP or the use of rtPA as a CRI on RI and AKI. In our study, PTX and CYP were given more than 12 h after FATE onset and sometimes after starting rtPA. Major mechanisms for the development of RI are calcium overload, reactive oxygen species production, complement activation, and leukocyte migration (20). Flooding of the systemic circulation with these factors following occlusion relief leads to myocardial stunning and arrhythmia, and even once macroscopic blood flow is restored, an organ can continue to remain under-perfused (no-reflow phenomenon). Pentoxifylline, in particular, is an ideal candidate for reducing RI, as there is promising evidence for PTX’s ability to limit RI and hypoperfusion in experimental animal models if given before the RI event (40–42), including in a renal ischemia canine model (43) and hindlimb ischemia rabbit model (20).
Acute kidney injury was seen in 4/9 (44.4%) of the study population. This finding may indicate that thrombolysis and subsequent re-cannulation are associated with an increased risk of AKI; however, a small sample size limits the conclusion. It is unlikely that PTX and CYP contributed to the higher AKI rate based on their mechanisms of action. Our AKI rate is lower than that of a previously published study assessing AKI using IRIS criteria after furosemide use in cats with CHF (31). An altered definition of AKI, away from IRIS guidelines (30), was chosen in our study to limit the effect of furosemide use and may explain this difference; however, cases of AKI in our study likely still included furosemide-induced and transient AKI. Data are lacking regarding the incidence of AKI during treatment of any type for FATE. Many cats with FATE present with azotemia (2, 5), which differs from our study group, although low study numbers make this hard to interpret. Causes of AKI in FATE are likely multifactorial: thrombo-occlusive disease, mediators released as part of clot formation and subsequent vasospasm, and furosemide administration may all contribute (11). Serotonin release from platelet granules may be responsible for vasospasm (21). The presence of thrombi elsewhere in the body, such as pulmonary embolism, has been associated with AKI development in people (51). The rationale given for thrombotic-induced AKI is a mix of hemodynamic instability and renal congestion. Significantly more non-survivors developed AKI than survivors in the study group [4/5 (80%) vs. 0/4 (0%), respectively], and it is possible that greater study numbers would present a reduced survival benefit from AKI development in FATE. The use of thrombolytic therapy itself has also been associated with AKI development, specifically for the treatment of iliofemoral deep vein thrombosis in humans via catheter-directed thrombolysis; however, this risk has been attributed to other factors (bilateral and extensive thrombosis, single-session thrombolysis and female sex) rather than rtPA use itself (52). In contrast to this, similar AKI rates have been seen in FATE treatment both with and without rtPA use, and human AIS studies have failed to show thrombolysis as a risk factor for AKI (11, 12, 53).
Our study had 4/9 (44.4%) survivors and mirrors findings from prospective and retrospective studies that included a control group and failed to find a significant improvement in survival (11, 12). It is possible our study population represented a sicker group of cats compared to these previous studies: mean lactate concentration in the study group (14.2 mmol/L) was higher and above the previously identified cut-off associated with a worse outcome (11.5 mmol/L) (11). Although some of these findings can be due to the small sample size, they may represent a direct (through RI or AKI) or indirect (through survival bias) effect of thrombolysis treatment. This is illustrated by the fact that all non-survivor cats in our study group were euthanized despite having documented improvement in their locomotion. While finances may have played a role in the decision to euthanize, all non-survivors had a significantly shorter length of hospitalization than survivors, suggesting ethical and prognostic concerns may have been a primary motivator of euthanasia. A significantly lower proportion of survivors in the study group developed AKI versus non-survivors, a finding which may have led to a choice of euthanasia by clients. Future studies should document the reason for euthanasia in these cats to identify possible targets to reduce the high euthanasia rate, such as complications or perceived poor prognosis.
It is important to note the difference in survival compared to other studies using thrombolytic agents, such as streptokinase, rtPA, and urokinase; these studies had discharge rates similar to or lower than our study group (4/9 vs. 15/46, 3/11 and 5/12, respectively) (33, 34, 54). The more recently reported improved outcomes, both in the present study and others (4), may be influenced by improvement in standard treatment for FATE (7, 8), with some more recent evidence that in-hospital use of antithrombotics may aid in short-term clinical sign resolution (32). Finally, all but one cat in our study had bilateral hindlimb FATE, which has been associated with worse outcomes versus single limb FATE (33).
4.1 Limitations
Our study had a number of limitations. The lack of a control group and heterogeneous treatments means that conclusions that are drawn from this should be taken cautiously and should be considered hypothesis-generating. Bias may have been present in the dataset, given the data were collected over a 3-year period and at different institutions. One example is treatment bias, as the different centers involved in data collection may have various levels of experience treating FATE, which has been anecdotally associated with survival (2, 11). Clinicians were not blinded to the use of rtPA, which may have impacted their assessment of prognosis past the initial treatment phase based on motor improvement and pulse palpation. This may have led to unidentifiable effects of survival in each population. The sample size of our study group was low, likely increasing the chances of type 2 errors. Another very important limitation to consider is the difficulty in discerning treatment effect, as the cats in our study received a heterogenous treatment regimen. The rtPA dose in this study was non-standardized (as seen in the range of rtPA doses). There was also no standardization of medication administration, as not all cats received rtPA, PTX, and CYP (only three cases received all three medications). The pooling of these different treatment types limits the ability to make any conclusions about treatment effects. Furthermore, studies evaluating the use of adjunctive medications in FATE suggest that these medications must be given prior to ischemia and/or reperfusion, which was not the case in our study (26). Clopidogrel is reported to reduce platelet serotonin release and vascular smooth muscle response to serotonin (55, 56). Although all cats in our study received clopidogrel, not all were loaded, which may have impacted platelet kinetics and impacted the clinical course of these cats. A multicenter prospective approach would be ideal to maximize study numbers while standardizing treatments used and treatment timing (i.e., PTX and CYP prior to reperfusion) to allow conclusions on treatment effects to be made. A definition of AKI in our study differs from the IRIS guidelines, limiting the comparison to other studies, but was chosen to take into account the impact of furosemide on serum creatinine. Diagnosis of RI relied solely on the rise in serum potassium and does not account for other aspects of RI, such as reduced nitric oxide release and increased reactive oxygen species production, as well as increased creatine kinase, acidemia, arrhythmia and multiorgan dysfunction syndrome (20, 57, 58). However, diagnostic criteria for RI are not well defined in human or veterinary medicine, and potassium is a commonly used biomarker for RI (59, 60). Finally, the locomotion recovery assessment was subjective and based on retrospective data. The use of a subjective assessment makes it hard to accurately apply the results of this study and may have led to both type 1 and type 2 errors, depending on the assessor.
4.2 Conclusion
A combination of an rtPA CRI over 9 h and/or oral pentoxifylline and/or oral cyproheptadine may improve locomotion function in cats with cardiogenic FATE. Population numbers and data heterogeneity make any findings from this study difficult to interpret, and future studies should explore prospective evaluations of these medications alongside greater study numbers via a multicenter approach.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies involving animals in accordance with the local legislation and institutional requirements because this is a retrospective study and ethical approval was not required by the local IACUC. Written informed consent was not obtained from the owners for the participation of their animals in this study because this is a retrospective study with no identifiable data.
Author contributions
CR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. JW: Data curation, Investigation, Validation, Writing – review & editing. JG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
A preliminary version of this study was presented at the 2024 European Veterinary Emergency and Critical Care Congress as a poster abstract (61).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AIS, acute ischemic stroke; AKI, acute kidney injury; CSU, Colorado State University; CYP, cyproheptadine; FATE, feline aortic thromboembolism; RI, reperfusion injury; rtPA, recombinant tissue plasminogen activator (alteplase); rtPA-CRI, recombinant tissue plasminogen activator continuous rate infusion; PTX, pentoxifylline; UF, University of Florida.
Footnotes
1. ^ACTIVASE (alteplase) [package insert]. South San Francisco, CA 94080–4990: Genetech (2022).
2. ^Microsoft Corporation, 2018. Microsoft Excel, RRID:SCR_016137. Available at: https://office.microsoft.com/excel.
3. ^ABL800 FLEX, Radiometer Medical ApS, Bronshoj, Denmark, RRID:SCR_025871.
4. ^ABL90 FLEX, Radiometer Medical ApS, Bronshoj, Denmark, RRID:SCR_025872.
5. ^IBM Corp. Released 2022. IBM SPSS Statistics for Windows, Version 29.0. Armonk, NY: IBM Corp, RRID:SCR_016479.
References
1. Borgeat, K, Wright, J, Garrod, O, Payne, JR, and Fuentes, VL. Arterial thromboembolism in 250 cats in general practice: 2004–2012. J Vet Intern Med. (2014) 28:102–8. doi: 10.1111/jvim.12249
2. Smith, SA, Tobias, AH, Jacob, KA, Fine, DM, and Grumbles, PL. Arterial thromboembolism in cats: acute crisis in 127 cases (1992–2001) and long-term management with low-dose aspirin in 24 cases. J Vet Intern Med. (2003) 17:73–83. doi: 10.1111/j.1939-1676.2003.tb01326.x
3. Pion, PD. Feline aortic thromboemboli and the potential utility of thrombolytic therapy with tissue plasminogen activator. Vet Clin North Am Small Anim Pract. (1988) 18:79–86. doi: 10.1016/s0195-5616(88)50008-6
4. Cambournac, M, Damoiseaux, C, Guillaumin, J, Goy-Thollot, I, and Pouzot-Nevoret, C. Multicentric aortic thromboembolism retrospective study in 158 cats: the maters study. In: D Chan, editor. Abstracts from the International Veterinary Emergency and Critical Care Symposium, the European Veterinary Emergency and Critical Care Annual Congress, and the ACVECC VetCOT Veterinary Trauma & Critical Care Conference 2022; 2022 Sept 07–11; Ghent, Belgium. Wiley (2022).
5. Schoeman, JP. Feline distal aortic thromboembolism: a review of 44 cases (1990–1998). J Feline Med Surg. (1999) 1:221–31. doi: 10.1053/jfms.1999.0049
6. Blais, MC, Bianco, D, Goggs, R, Lynch, AM, Palmer, L, Ralph, A, et al. Consensus on the rational use of Antithrombotics in veterinary critical care (CURATIVE): domain 3-defining antithrombotic protocols. J Vet Emerg Crit Care (San Antonio). (2019) 29:60–74. doi: 10.1111/vec.12795
7. Hogan, DF, Fox, PR, Jacob, K, Keene, B, Laste, NJ, Rosenthal, S, et al. Secondary prevention of cardiogenic arterial thromboembolism in the CAT: the double-blind, randomized, positive-controlled feline arterial thromboembolism; clopidogrel vs. aspirin trial (FAT CAT). J Vet Cardiol. (2015) 17:S306–17. doi: 10.1016/j.jvc.2015.10.004
8. Lo, ST, Walker, AL, Georges, CJ, Li, RH, and Stern, JA. Dual therapy with clopidogrel and rivaroxaban in cats with thromboembolic disease. J Feline Med Surg. (2022) 24:277–83. doi: 10.1177/1098612x211013736
9. Sharp, CR, Blais, MC, Boyd, CJ, Brainard, BM, Chan, DL, de Laforcade, A, et al. 2022 update of the consensus on the rational use of Antithrombotics and Thrombolytics in veterinary critical care (CURATIVE) domain 6: defining rational use of thrombolytics. J Vet Emerg Crit Care (San Antonio). (2022) 32:446–70. doi: 10.1111/vec.13227
10. Acheampong, P, and Ford, GA. Pharmacokinetics of alteplase in the treatment of ischaemic stroke. Expert Opin Drug Metab Toxicol. (2012) 8:271–81. doi: 10.1517/17425255.2012.652615
11. Guillaumin, J, DeFrancesco, TC, Scansen, BA, Quinn, R, Whelan, M, Hanel, R, et al. Bilateral lysis of aortic saddle thrombus with early tissue plasminogen activator (BLASTT): a prospective, randomized, placebo-controlled study in feline acute aortic thromboembolism. J Feline Med Surg. (2022) 24:e535–45. doi: 10.1177/1098612x221135105
12. Guillaumin, J, Gibson, RM, Goy-Thollot, I, and Bonagura, JD. Thrombolysis with tissue plasminogen activator (TPA) in feline acute aortic thromboembolism: a retrospective study of 16 cases. J Feline Med Surg. (2019) 21:340–6. doi: 10.1177/1098612x18778157
13. Pu, L, Wang, L, Zhang, R, Zhao, T, Jiang, Y, and Han, L. Projected global trends in ischemic stroke incidence, deaths and disability-adjusted life years from 2020 to 2030. Stroke. (2023) 54:1330–9. doi: 10.1161/STROKEAHA.122.040073
14. Lansberg, MG, Bluhmki, E, and Thijs, VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke. Stroke. (2009) 40:2438–41. doi: 10.1161/STROKEAHA.109.552547
15. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. (1995) 333:1581–8. doi: 10.1056/NEJM199512143332401
16. Mulcaire-Jones, JP, Bailly, DK, Frank, DU, Verma, AR, Barney, BJ, and Siefkes, HM. Spontaneous aortic thrombosis in neonates: a case report and review of literature. Cardiol Young. (2020) 30:95–9. doi: 10.1017/s1047951119003093
17. Levy, M, Benson, LN, Burrows, PE, Bentur, Y, Strong, DK, Smith, J, et al. Tissue plasminogen activator for the treatment of thromboembolism in infants and children. J Pediatr. (1991) 118:467–72. doi: 10.1016/S0022-3476(05)82170-5
18. Nagel, K, Tuckuviene, R, Paes, B, and Chan, AK. Neonatal aortic thrombosis: a comprehensive review. Klin Padiatr. (2010) 222:134–9. doi: 10.1055/s-0030-1249662
19. Pion, P, Kittleson, M, and Peterson, S. Thrombolysis with recombinant tissue-type plasminogen activator (rt-PA) in feline aortic thromboembolism: clinical and experimental data. In: AM Ledendre, editor. Proceedings of the 5th American College of Veterinary Internal Medicine forum; (1987) San Diego, California, USA. Wiley.
20. Collard, CD, and Gelman, S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology. (2001) 94:1133–8. doi: 10.1097/00000542-200106000-00030
21. Butler, HC. An investigation into the relationship of an aortic embolus to posterior paralysis in the cat. J Small Anim Pract. (1971) 12:141–58. doi: 10.1111/j.1748-5827.1971.tb06212.x
22. Wen, WX, Lee, SY, Siang, R, and Koh, RY. Repurposing Pentoxifylline for the treatment of fibrosis: an overview. Adv Ther. (2017) 34:1245–69. doi: 10.1007/s12325-017-0547-2
23. Frampton, JE, and Brogden, RN. Pentoxifylline (Oxpentifylline). Drugs Aging. (1995) 7:480–503. doi: 10.2165/00002512-199507060-00007
24. Bayraktar, S, and Tanyeri-Bayraktar, B. Pentoxifylline in the treatment of neonatal vasospasm and thromboembolism: an observational case series study. J Clin Pharm Ther. (2022) 47:455–61. doi: 10.1111/jcpt.13565
25. Gaut, ZN. Influence of various substances which induce and inhibit aggregation on the uptake of deoxyglucose by human blood platelets. J Pharmacol Exp Ther. (1974) 190:180–6. doi: 10.1016/S0022-3565(25)29907-4
26. Olmstead, ML, and Butler, HC. Five-hydroxytryptamine antagonists and feline aortic embolism. J Small Anim Pract. (1977) 18:247–59. doi: 10.1111/j.1748-5827.1977.tb05878.x
27. Duler, L, Scollan, KF, and LeBlanc, NL. Left atrial size and volume in cats with primary cardiomyopathy with and without congestive heart failure. J Vet Cardiol. (2019) 24:36–47. doi: 10.1016/j.jvc.2019.04.003
28. Lee, Y-J, Chang, C-C, Chan, JP-W, Hsu, W-L, Lin, K-W, and Wong, M-L. Prognosis of acute kidney injury in dogs using RIFLE (risk, injury, failure, loss and end-stage renal failure)-like criteria. Vet Rec. (2011) 168:264. doi: 10.1136/vr.c6234
29. Thoen, ME, and Kerl, ME. Characterization of acute kidney injury in hospitalized dogs and evaluation of a veterinary acute kidney injury staging system. J Vet Emerg Crit Care. (2011) 21:648–57. doi: 10.1111/j.1476-4431.2011.00689.x
30. Segev, G, Cortellini, S, Foster, JD, Francey, T, Langston, C, Londoño, L, et al. International renal interest society best practice consensus guidelines for the diagnosis and management of acute kidney injury in cats and dogs. Vet J. (2024) 305:106068. doi: 10.1016/j.tvjl.2024.106068
31. Rogg, S, Mochel, JP, Kundu, D, Tropf, MA, Masters, AK, Adin, DB, et al. Frequency and progression of azotemia during acute and chronic treatment of congestive heart failure in cats. J Vet Intern Med. (2025) 39:e17254. doi: 10.1111/jvim.17254
32. Hassan, MH, Abu-Seida, AM, Torad, FA, and Hassan, EA. Feline aortic thromboembolism: presentation, diagnosis, and treatment outcomes of 15 cats. Open Vet J. (2020) 10:340–6. doi: 10.4314/ovj.v10i3.13
33. Moore, K, Morris, N, Dhupa, N, Murtaugh, R, and Rush, J. Retrospective study of streptokinase administration in 46 cats with arterial thromboembolism. J Vet Emerg Crit Care. (2000) 10:245–57. doi: 10.1111/j.1476-4431.2000.tb00010.x
34. Welch, KM, Rozanski, EA, Freeman, LM, and Rush, JE. Prospective evaluation of tissue plasminogen activator in 11 cats with arterial thromboembolism. J Feline Med Surg. (2010) 12:122–8. doi: 10.1016/j.jfms.2009.08.001
35. Lee, TF, Drake, SM, Roberts, GW, Bersten, A, Stranks, SN, Heilbronn, LK, et al. Relative hyperglycemia is an independent determinant of in-hospital mortality in patients with critical illness. Crit Care Med. (2020) 48:e115–22. doi: 10.1097/ccm.0000000000004133
36. Torre, DM, DeLaforcade, AM, and Chan, DL. Incidence and clinical relevance of hyperglycemia in critically ill dogs. J Vet Intern Med. (2007) 21:971–5. doi: 10.1111/j.1939-1676.2007.tb03051.x
37. Ray, CC, Callahan-Clark, J, Beckel, NF, and Walters, PC. The prevalence and significance of hyperglycemia in hospitalized cats. J Vet Emerg Crit Care. (2009) 19:347–51. doi: 10.1111/j.1476-4431.2009.00435.x
38. Pion, PD, and Kittleson, MD. Therapy for feline aortic thromboembolism In: RW Kirk, editor. Current veterinary therapy X: Small animal practice. 10th ed. Independence Square West, Philadelphia, PA: The Curtis Center, W. B. Saunders Company (1989). 295–302.
39. Kaye, AD, Ibrahim, IN, Kadowitz, PJ, and Nossaman, BD. Analysis of responses to pentoxifylline in the pulmonary vascular bed of the cat. Crit Care Med. (1996) 24:263–7. doi: 10.1097/00003246-199602000-00014
40. Tanahashi, N, Fukuuchi, Y, Tomita, M, Kobari, M, Takeda, H, and Yokoyama, M. Pentoxifylline ameliorates postischemic delayed hypoperfusion of the cerebral cortex following cardiac arrest in cats. J Neurol Sci. (1995) 132:105–9. doi: 10.1016/0022-510X(95)00153-S
41. Sener, G, Akgün, U, Satiroğlu, H, Topaloğlu, U, and Keyer-Uysal, M. The effect of pentoxifylline on intestinal ischemia/reperfusion injury. Fundam Clin Pharmacol. (2001) 15:19–22. doi: 10.1046/j.1472-8206.2001.00007
42. Savaş, Ç, Aras, T, Çakmak, M, Bilgehan, A, Ataoǧlu, Ö, Türközkan, N, et al. Pentoxifylline inhibits overflow and reduces intestinal reperfusion injury. J Pediatr Surg. (1997) 32:905–10. doi: 10.1016/S0022-3468(97)90648-5
43. Doustar, Y, Davuod, K, Habibolah, N, Hossein, Z, and Saeid, S. The role of pentoxifylline in renal ischemic reperfusion cell injury and inflammatory reaction. Adv Environ Biol. (2011) 5:1522–6. doi: 10.1016/j.jphs.2021.03.011
44. Steen, PA, Milde, JH, and Michenfelder, JD. Pentoxifylline in regional cerebral ischemia in cats. Acta Anaesthesiol Scand. (1982) 26:39–43. doi: 10.1111/j.1399-6576.1982.tb01722
45. Pakala, R, Willerson, JT, and Benedict, CR. Effect of serotonin, thromboxane A2, and specific receptor antagonists on vascular smooth muscle cell proliferation. Circulation. (1997) 96:2280–6. doi: 10.1161/01.cir.96.7.2280
46. Willerson, JT, Eidt, JF, McNatt, J, Yao, S-K, Golino, P, Anderson, HV, et al. Role of thromboxane and serotonin as mediators in the development of spontaneous alterations in coronary blood flow and neointimal proliferation in canine models with chronic coronary artery stenoses and endothelial injury. J Am Coll Cardiol. (1991) 17:101–10. doi: 10.1016/0735-1097(91)90945-6
47. Sanner, JE, and Frazier, L. The role of serotonin in depression and clotting in the coronary artery disease population. J Cardiovasc Nurs. (2011) 26:423–9. doi: 10.1097/JCN.0b013e3182076a81
48. Nemir, P Jr, Hamilton, WM, and Brody, JI. Intravenous cyproheptadine hydrochloride in the treatment of pulmonary embolism: an experimental study. Surgery. (1972) 72:920–32. doi: 10.5555/uri:pii:0039606072901948
49. Chima, J, Mullaguri, N, Fan, T, George, P, and Newey, CR. Cyproheptadine in the treatment of reversible cerebral vasoconstriction syndrome. Acta Neurol Belg. (2021) 121:1871–4. doi: 10.1007/s13760-020-01487-z
50. Fleming, JS, Buchanan, JO, Yocca, FD, Iben, LG, and Antonaccio, MJ. Effect of encainide, ODE, MODE, and flecainide on ADP/5-HT induced platelet aggregation and in the anesthetized dog coronary artery stenosis-occlusion model of intravascular thrombosis. Cardiovasc Drugs Ther. (1991) 5:763–8. doi: 10.1007/BF03029752
51. Murgier, M, Bertoletti, L, Darmon, M, Zeni, F, Valle, R, Del Toro, J, et al. Frequency and prognostic impact of acute kidney injury in patients with acute pulmonary embolism. Data from the RIETE registry. Int J Cardiol. (2019) 291:121–6. doi: 10.1016/j.ijcard.2019.04.083
52. Salem, KM, Saadeddin, Z, Go, C, Malak, OA, Eslami, MH, Hager, E, et al. Risk factors for acute kidney injury after pharmacomechanical thrombolysis for acute deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. (2021) 9:868–73. doi: 10.1016/j.jvsv.2020.11.005
53. Gadalean, F, Simu, M, Parv, F, Vorovenci, R, Tudor, R, Schiller, A, et al. The impact of acute kidney injury on in-hospital mortality in acute ischemic stroke patients undergoing intravenous thrombolysis. PLoS One. (2017) 12:e0185589. doi: 10.1371/journal.pone.0185589
54. Whelan, MF, O'Toole, TE, Chan, DL, and Rush, JE. Retrospective evaluation of Urokinase use in cats with arterial thromboembolism (abstract). J Vet Emerg Crit Care. (2005) 15:S1–S13. doi: 10.1111/j.1476-4431.2005.00155.x
55. Hogan, DF, Andrews, DA, Green, HW, Talbott, KK, Ward, MP, and Calloway, BM. Antiplatelet effects and pharmacodynamics of clopidogrel in cats. J Am Vet Med Assoc. (2004) 225:1406–11. doi: 10.2460/javma.2004.225.1406
56. Yang, LH, Hoppensteadt, D, and Fareed, J. Modulation of vasoconstriction by Clopidogrel and Ticlopidine. Thromb Res. (1998) 92:83–9. doi: 10.1016/S0049-3848(98)00114-5
57. Apichartpiyakul, P, Shinlapawittayatorn, K, Rerkasem, K, Chattipakorn, SC, and Chattipakorn, N. Mechanisms and interventions on acute lower limb ischemia/reperfusion injury: a review and insights from cell to clinical investigations. Ann Vasc Surg. (2022) 86:452–81. doi: 10.1016/j.avsg.2022.04.040
58. Conner, B, and McMichael, M. Ischemia-reperfusion injury. Stand Care Emerg Crit Care Med. (2008) 10:1–4.
59. Beyersdorf, F, Matheis, G, Krüger, S, Hanselmann, A, Freisleben, HG, Zimmer, G, et al. Avoiding reperfusion injury after limb revascularization: experimental observations and recommendations for clinical application. J Vasc Surg. (1989) 9:757–66. doi: 10.1016/0741-5214(89)90081-5
60. Patrono, D, and Romagnoli, R. Postreperfusion syndrome, hyperkalemia and machine perfusion in liver transplantation. Translational. Gastroenterol Hepatol. (2019) 4:4. doi: 10.21037/tgh.2019.08.12
61. Ray, CC, Wolf, J, and Guillaumin, J. Ancillary treatments with Thrombolytics or medications aimed at improving functional recovery or both in acute feline aortic thromboembolism. In: JM Burkitt-Creedon, editor. Abstracts from the international veterinary emergency and critical care symposium and the European veterinary emergency and critical care annual congress 2024; (2024). Wiley 34. p. S41–S42.
Keywords: tPA, rtPA, tissue plasminogen activator, arterial, thrombosis, reperfusion, acute kidney injury
Citation: Ray CC, Wolf J and Guillaumin J (2025) Use of alteplase continuous rate infusion, pentoxifylline, and cyproheptadine in association or not, in acute feline aortic thromboembolism: a study of nine cats. Front. Vet. Sci. 12:1512649. doi: 10.3389/fvets.2025.1512649
Edited by:
Benjamin M. Brainard, University of Georgia, United StatesReviewed by:
Esther Hassdenteufel, University of Giessen, GermanyDominic Barfield, Royal Veterinary College (RVC), United Kingdom
Copyright © 2025 Ray, Wolf and Guillaumin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julien Guillaumin, anVsaWVuLmd1aWxsYXVtaW5AY29sb3N0YXRlLmVkdQ==
 Christopher C. Ray
Christopher C. Ray Jacob Wolf
Jacob Wolf Julien Guillaumin
Julien Guillaumin