- 1College of Veterinary Medicine, Jilin Agricultural University, Changchun, Jilin, China
- 2Ginseng and Antler Products Testing Center of the Ministry of Agricultural PRC, Jilin Agricultural University, Changchun, Jilin, China
- 3College of Chinese Medicine Materials, Jilin Agricultural University, Changchun, Jilin, China
- 4Department of Parasitology and Animal Diseases, Veterinary Research Institute, National Research Centre, Giza, Egypt
- 5Department of Veterinary Medicine, College of Agriculture, Yanbian University, Yanji, China
Background: Bovine tuberculosis (bTB) primarily caused by Mycobacterium bovis (M. bovis), is a globally prevalent zoonotic infectious disease of cattle and other livestock and wildlife species. Pakistan is the fourth-largest milk-producing country in the world, with approximately 212 million animals. Livestock farming provides a livelihood for almost 8 million families. Moreover, there is currently no effective control program and national data in place. Therefore, we constructed a first meta-analysis on the prevalence of bovine tuberculosis in Pakistan. This study aimed to provide an overview of bovine tuberculosis in this country and identify the risk factors associated with its prevalence.
Methods: We searched Science Direct, Pubmed, Base, Green File-Poly U Library, Google Scholar, and additional articles were also identified manually from reference lists of articles generated in database search, systemically for papers that presented bTB prevalence data, published in English language between January 1, 2000, and April 30 2024. A total of 35 published articles were selected for inclusion in the meta-analysis.
Results: The estimated overall prevalence of bTB was found to be 6.06% [95% CI: 4.67–7.87]. Cattle were more susceptible to infection, with a higher prevalence (6.44% [95% CI: 4.04–10.26]) compared to buffalo (5.54% [95% CI: 3.13–9.81]). The prevalence determined by PCR (5.65% [95% CI: 3.33–5.98]) was much similar to that of TST (5.61% [95% CI: 4.20–7.50]) with no significant difference. Milk samples showed the highest prevalence (14.66% [95% CI: 7.38–29.11]), particularly due to the consumption of unpasteurized milk, improper handling of dairy products and suckling by calves from the infected cows. Furthermore, the analysis considered effect of various potential risk factors (age, weight, breed, body condition score, herd size, animal status) along with different geographical factors (longitude, latitude, altitude, humidity, rainfall, temperature, climate) associated with bTB prevalence, which should be considered when developing future disease surveillance and control programs.
Conclusion: In Pakistan bTB was widely distributed throughout the country, as a neglected zoonotic disease. Long-term disease prevalence monitoring should be recommended along with the need to improve diagnostic techniques, enhance farm management practices, and implement targeted surveillance to protect both animal and public health.
1 Introduction
The farm animals are playing a marked role in keeping the global food security (1–3). They were subjected to different pathogens that affect their productivity (4–7). Bovine tuberculosis (bTB) is a chronic, debilitating granulomatous disease caused by Mycobacterium bovis (M. bovis) and belongs to the Mycobacterium tuberculosis complex (MTC) (8). It is also a zoonotic disease, with major infections including human tuberculosis (9). The infected animals exhibit asymptomatic phase in the early stages of infection, then gradually develop emaciation, low-grade fluctuating fever, enlarged draining lymph nodes, and udder induration can be observed in the later stages of the disease (10). When the digestive tract is affected, the clinical symptoms vary from diarrhea and constipation to cough and dyspnea (11).
Bovine tuberculosis is widespread throughout the world, with the highest prevalence observed in Asian and African countries, except Antarctica (12). According to the World Organization for Animal Health (WOAH), between 2017 and 2018, 44% of countries reported the disease occurrence. Of these nations, the majority (62%) reported illnesses in livestock alone, although 35% reported infections in both livestock and wildlife (13). Globally, the disease is estimated to impact more than 50 million cattle (14). The largest prevalence of infected herds were reported in India, where 7.3% of farm and dairy cattle have bovine tuberculosis (15). The following countries are free bTB, based on current statistics: Norway, Austria, Switzerland, Luxembourg, Jamaica, Latvia, Slovakia, Iceland, Estonia, Canada, Lithuania, Finland, Barbados, Singapore, Australia, Sweden, the Czech Republic and Denmark. Several European countries, as well as the United States, New Zealand, and Japan, have programs in place to eradicate bovine tuberculosis this disease (16). The main etiological agent is M. bovis (Supplementary Table S4), but other Mycobacterium species, such as M. tuberculosis, M. caprae (17), M. orygis (18), M. microti (19), and M. africanum can also infect various livestock and wildlife (20, 21). Although the principal reservoir host of bTB include cattle, it is prevalent in other species-like human, pigs, goats, buffaloes, primates, dogs, deers, possums, badgers, bison and wild animals (22, 23), also posing threat to some endangered species (24).
The transmission risk of bovine tuberculosis is influenced by pathogen, host, and environmental factors (25). Depending on the site of infection in the body, bacteria can be found in vaginal secretions, respiratory secretions, milk, feces, semen, urine, and exudates from lesions (such as lymph node drainage and certain skin lesions) (26–28). The predominate route of transmission among bovines is inhalation. Susceptible animals breathe in infectious aerosol droplets released in the respiratory secretions of infected animals, particularly in overcrowded or poorly ventilated environments (29). Direct contact with infected skin wounds and mucous membranes is another common route of transmission (30). Ingestion can also occur when animals consume feed, water or surfaces contaminated with M. bovis from infected secretions or excretions (31). Vertical transmission though rare, can occur in utero or postpartum (32). Calves are highly susceptible to infection through contaminated milk or colostrum from infected dams. The disease is primarily transmitted from bovines to humans through unpasteurized dairy products and direct contact with infected animals or their bodily fluids such as during handling or slaughtering (33, 34). Additionally, M. bovis could be transmitted by consumption of infected raw or undercooked meat and other animal-derived tissues (35).
The bovine tuberculosis results in considerable economic losses globally, causing an estimated loss of USD 3 billion annually in the form of decreased production along with higher mortality rates, culling, movement and trade restrictions. European Union (EU) legislation mandated that disease eradication is important for both public health and free trade facilitation of livestock products globally (36, 37). Azami and Zinsstag attributed the economic costs of bTB to several factors, including a 10–18% decrease in milk production, a 10–25% loss in productive efficiency, higher rates of edible organ condemnation, 15% reduction in meat production, and increased mortality (38). Throughout the history, humans consumed cattle meat and milk as basic food sources so zoonotic transmission were higher. Studies have identified the same strain of M. bovis, responsible for bTB, can be found in both humans and animals. This suggests potential “spillover” mechanism from animals to humans (39). Many developed countries have controlled the bTB, under active national control programs. Although maintaining bTB-free status and total eradication remains difficult because of spill-over possibility from animal-reservoir hosts (40).
The prevalence of bovine tuberculosis is influenced by various potential risk factors including both animal and herd levels. Animal-specific factors include sex, age, breed, body condition, weight and mode of transmission (41–43). Herd-level factors include herd size, biosecurity measures and overall farm management practices (16, 44). Prevalence is greatly influenced by geographic and environmental factors. Climatic conditions such as temperature, humidity and seasonal variations also play important role (45). Understanding these factors is crucial for the development of successful disease management and prevention strategies.
Pakistan faces significant challenge with bovine tuberculosis control. In 1969, the district of Faisalabad reported the first case of bTB with (6.72%) prevalence in dairy animals (46). Currently, Pakistan ranks 5th globally in the incidence of new human TB cases, with over than 500,000 cases reported annually. Within the WHO Eastern Mediterranean Region, this burden accounts for total of (61%) of all TB recorded cases. Moreover, 63% of population resides in rural areas of Pakistan, and a significant portion (62%) of population is directly or indirectly involved with livestock activities (47). The overlap between human-livestock contacts and high TB prevalence poses a significant public health concern. According to the Pakistan economic survey during 2023–2024, Pakistan reveals substantial bovine population with an estimation of 57.5 million cattle and 46.3 million buffaloes. Despite this huge significant livestock sector, data on bTB prevalence is still incomplete. To address this gap, we conducted this first-ever national meta-analysis to determine the prevalence of bTB in Pakistan. Furthermore, various factors that might influence the occurrence of the disease were also investigated, including geographical location, sampling year and season, detection methods, and animal characteristics (age, sex, and weight). The quality of the original studies was also assessed. Geographical factors including longitude, latitude, altitude, rainfall, humidity, temperature and climate, were also analyzed to assess their association with bTB infection.
2 Materials and methods
2.1 Search strategy
A meta-analysis was conducted following the guidelines of PRISMA (2009) (Supplementary Tables S1, S4) (48). We searched the published research literature on bovine tuberculosis through four databases and one search engine: Science Direct, PubMed, Base, Green File-Poly U Library, Google Scholar and additional articles were also identified manually from the reference lists of articles generated in the database search. All the relevant published literature on bovine tuberculosis in Pakistan was retrieved during the period from 1 January 2000 to 1 April 2024. This search was carried out on 3rd June 2024 and Endnote (version X.20) was used to record the retrieved articles.
2.1.1 Search terms
In Science Direct database, the keywords “Bovine,” “Cattle,” “Buffalo,” “Tuberculosis” and “Pakistan” were used for searching. In the base and Green File- Poly U Library, the used terms were “Cattle,” “Buffalo,” “Tuberculosis” and “Pakistan.” For Google Scholar, the words “Cattle,” “Bovine,” “Buffalo,” “Mycobacterium bovis,” “M. bovis,” “and bovine tuberculosis,” “Pakistan” were used.
The following formulas and MeSH terms “Cattle,” “Buffalo,” “Tuberculosis” and “Pakistan” were used in Pubmed. Boolean operators “AND” were used to connect MeSH terms and “OR” to connect the entry terms.
(“Cattle”[Mesh]) OR (Cow)) OR (Cows)) OR (Bos indicus)) OR (Bos indicus Cattle)) OR (Bos indicus Cattles)) OR (Cattle, Bos indicus)) OR (Cattles, Bos indicus)) OR (Indicine Cattle)) OR (Cattle, Indicine)) OR (Cattles, Indicine)) OR (Indicine Cattles)) OR (Zebu)) OR (Zebus)) OR (Holstein Cow)) OR (Cow, Holstein)) OR (Dairy Cow)) OR (Cow, Dairy)) OR (Dairy Cows)) OR (Beef Cow)) OR (Beef Cows)) OR (Cow, Beef)) OR (Bos grunniens)) OR (Yak)) OR (Yaks)) OR (Bos taurus)) OR (Taurine Cattle)) OR (Cattle, Taurine)) OR (Cattles, Taurine)) OR (Taurine Cattles)) OR (Taurus Cattle)) OR (Cattle, Taurus)) OR (Cattles, Taurus)) OR (Taurus Cattles)) OR (Cow, Domestic)) OR (Domestic Cow)) OR (Domestic Cows)).
AND (((((((“Buffaloes”[Mesh]) OR (Buffalo)) OR (Bubalus)) OR (Syncerus)) OR (Water Buffaloes)) OR (Water Buffalo)) OR (Buffalo, Water))).
AND ((((“Tuberculosis, Bovine”[Mesh]) OR (Bovine Tuberculoses)) OR (Bovine Tuberculosis)) OR (Tuberculoses, Bovine))).
AND ((“Pakistan” [Mesh]) OR (Islamic Republic of Pakistan)).
2.2 Selection criteria
Eligible studies were selected according to the following inclusion and exclusion criteria.
2.2.1 Inclusion criteria
• Studies specified for bovine tuberculosis in Pakistan.
• Published between 2000 and 2024.
• Data must include both (total sample size and bTB prevalence).
• Description of clear detection methods.
• Adequate sample size, >30 animals.
2.2.2 Exclusion criteria
• Studies investigating other than mentioned disease and conducted outside of Pakistan.
• Articles content that did not match with titles and abstracts.
• Hosts were not bovine.
• Full-text articles were not available.
• Data repetition in the articles.
2.3 Data extraction
Two reviewers XUL and WBL extracted data from qualified studies. The following information was reported in standardized forms using Microsoft Excel 2021: first author, publication year, number of samples (total and positive), study period, sample classification, detection method, characteristics of animal (age, gender, body condition score, weight, herd size), additional factors (animal status, lactation status, lactation length, parity), and geographical factors (longitude, latitude, altitude, rainfall, humidity, temperature, climate).
2.4 Quality assessment
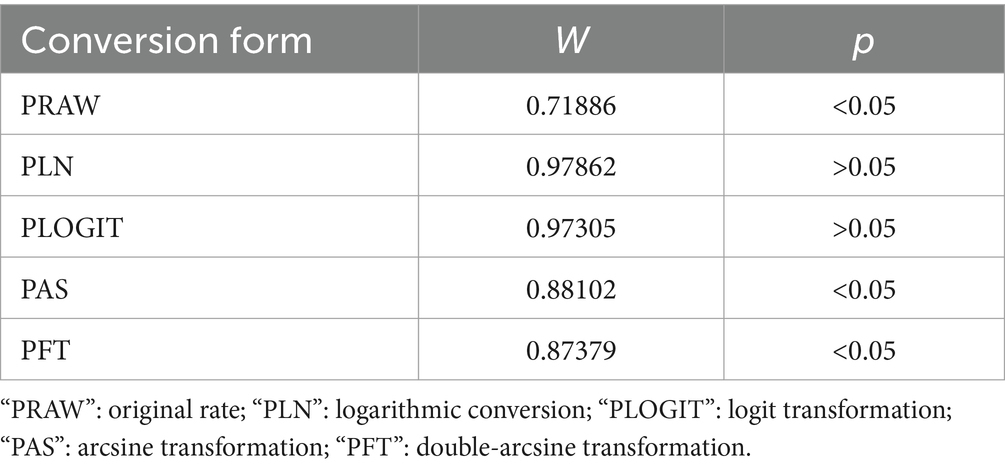
The quality of eligible publications was evaluated according to criteria derived from Grading of Recommendations Assessment, Development and Evaluation Method (GRADE) (49). Each study received one point if it met one of the following criteria: (clear detection method, random sampling, sampling method, sampling time, four or more risk factors). Based on scoring system, studies were assigned to three quality categories (Table 1). High quality as 3–4 points, medium quality 2 points and low quality 1 points.
2.5 Statistical analysis
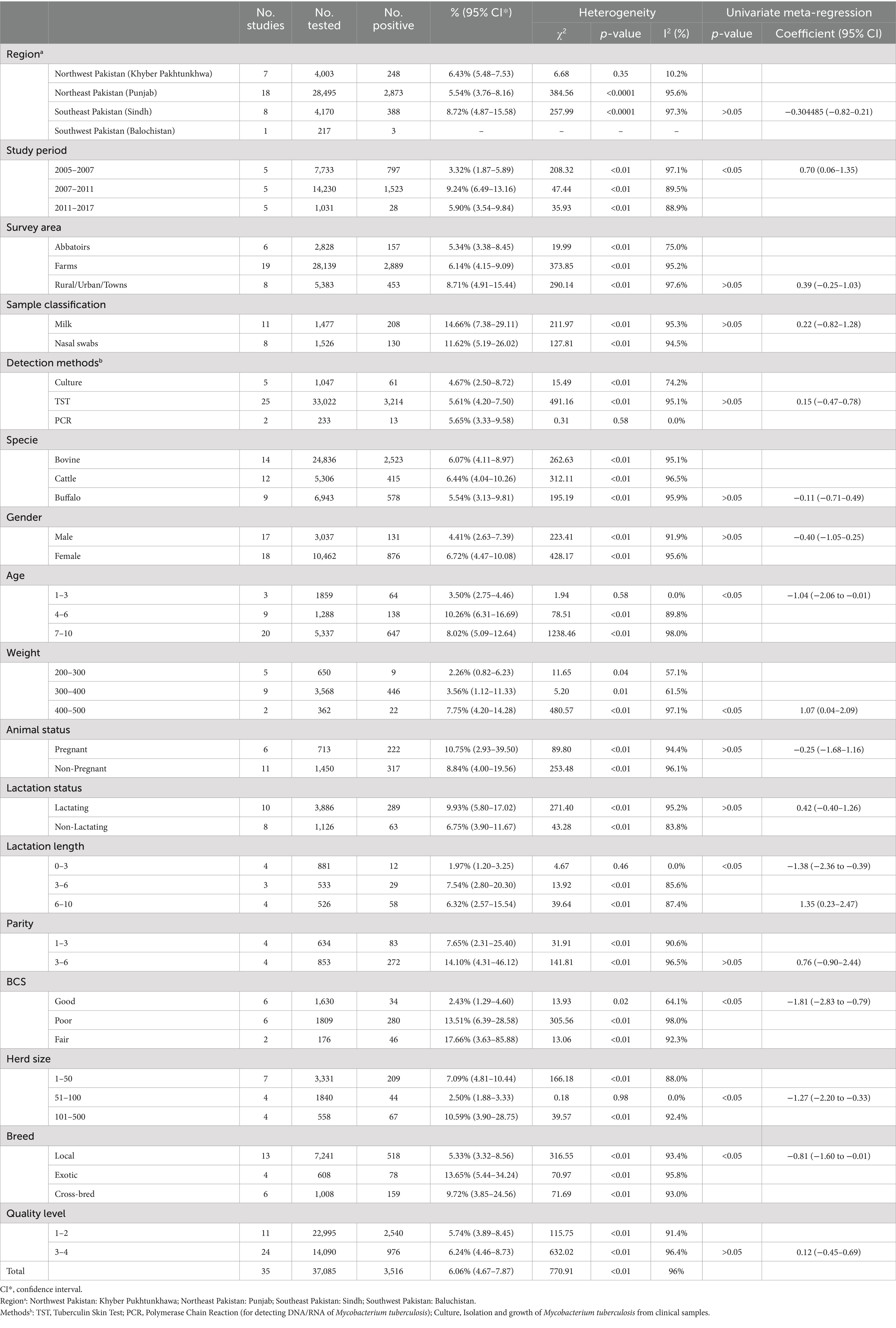
According to PRISMA statement, meta-analysis was performed and the “meta” package in R Studio (version 4.4.1) was used for the model’s estimation (45, 50). Before meta-analysis, four methods of data transformation were tested to bring the data closer to the normal distribution: original rate (PRAW), logarithmic conversion (PLN), logit transformation (PLOGIT), arsenic transformation (PAS) and double arsenic transformation (PFT) (Table 2). The conversion rate is based on the Shapiro–Wilk normality test. According to criteria, the p-value > 0.05 and W-value closer to 1, PLN was finally chosen for exchange conversion rate (W = 0.978, p = 0.713). Forest plots were used to visualize the results of analysis and to determine heterogeneity between studies. Forest plot gives the results of both common effect model as well as random effects model. Due to strong heterogeneity of the included studies, random-effects model was chosen for meta-analysis. To determine the statistical difference of heterogeneity between the included studies, Cochran’s Q-test (51), the I2 statistic and χ2 test (p < 0.05) were used. To verify presence of publication bias, a funnel plot, and an Eager’s test were used. Sensitivity analysis was performed to check the reliability of meta-analysis results. The code for R in meta-analysis is shown in (Supplementary Table S2).
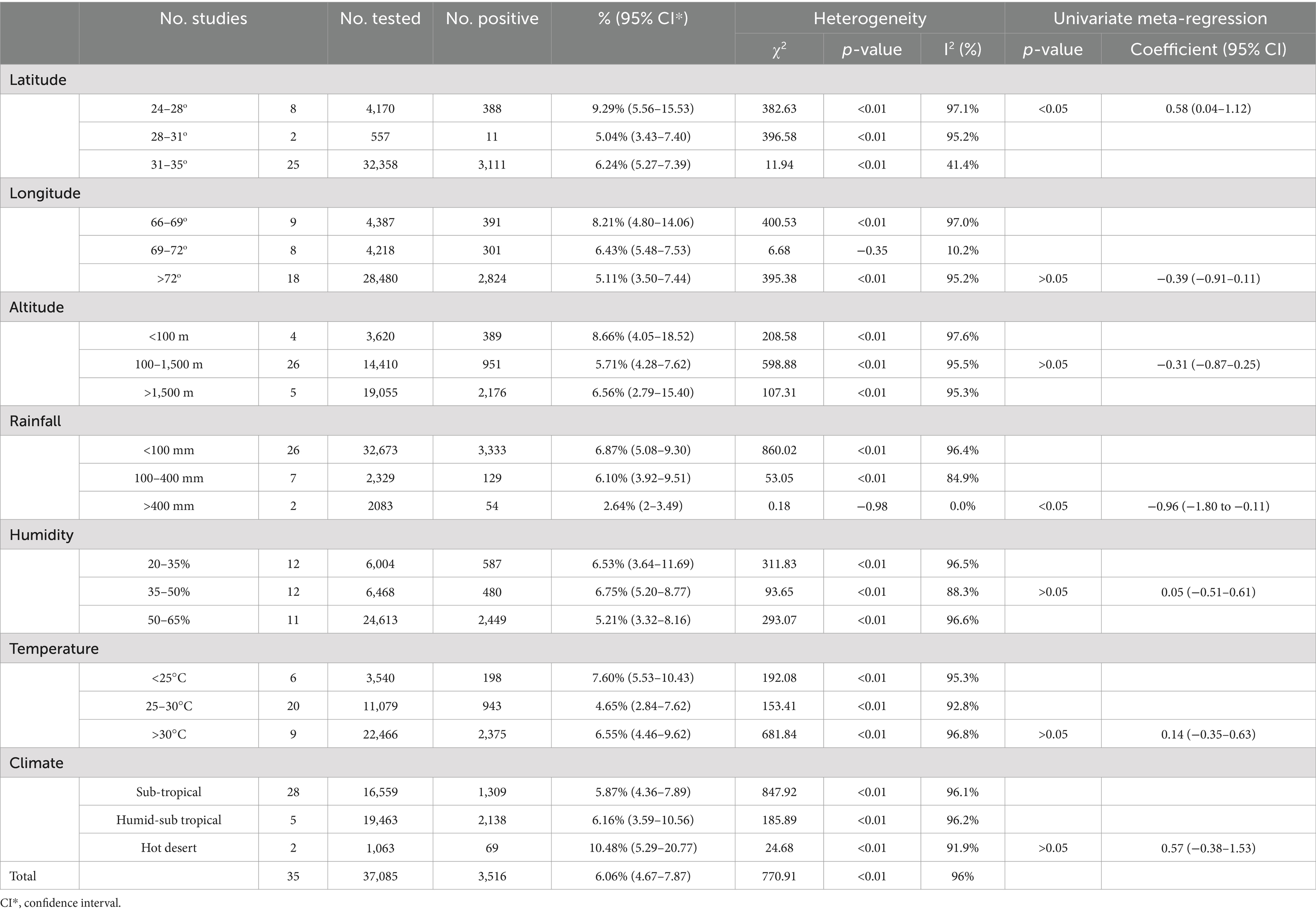
In meta-analyses, heterogeneity is a crucial factor to consider when evaluating studies. To explore the potential sources of heterogeneity, research data were analyzed using subgroup analyses and univariate meta-regression analyses. These analyses identified factors that contributed to heterogeneity among studies. These potential sources consist of study period, detection methods, age, gender, specie, breed, weight, animal status (pregnant, non-pregnant, lactating, non-lactating, parity), Body condition score (BCS), survey area, sample classification, quality of articles (Table 3). To further analyze the potential sources of heterogeneity, we also conduct an analysis of geographical factor sub-groups, including longitude, latitude, altitude, rainfall, humidity and climate (Table 4). Our meta-analysis is not registered in the Cochrane database as it does not include review agreement. Due to the absence of significant differences in some of the sub-group analyses, a point estimate will be used to determine bTB prevalence in our study.
3 Results
3.1 Literature search results and quality assessment of the included studies
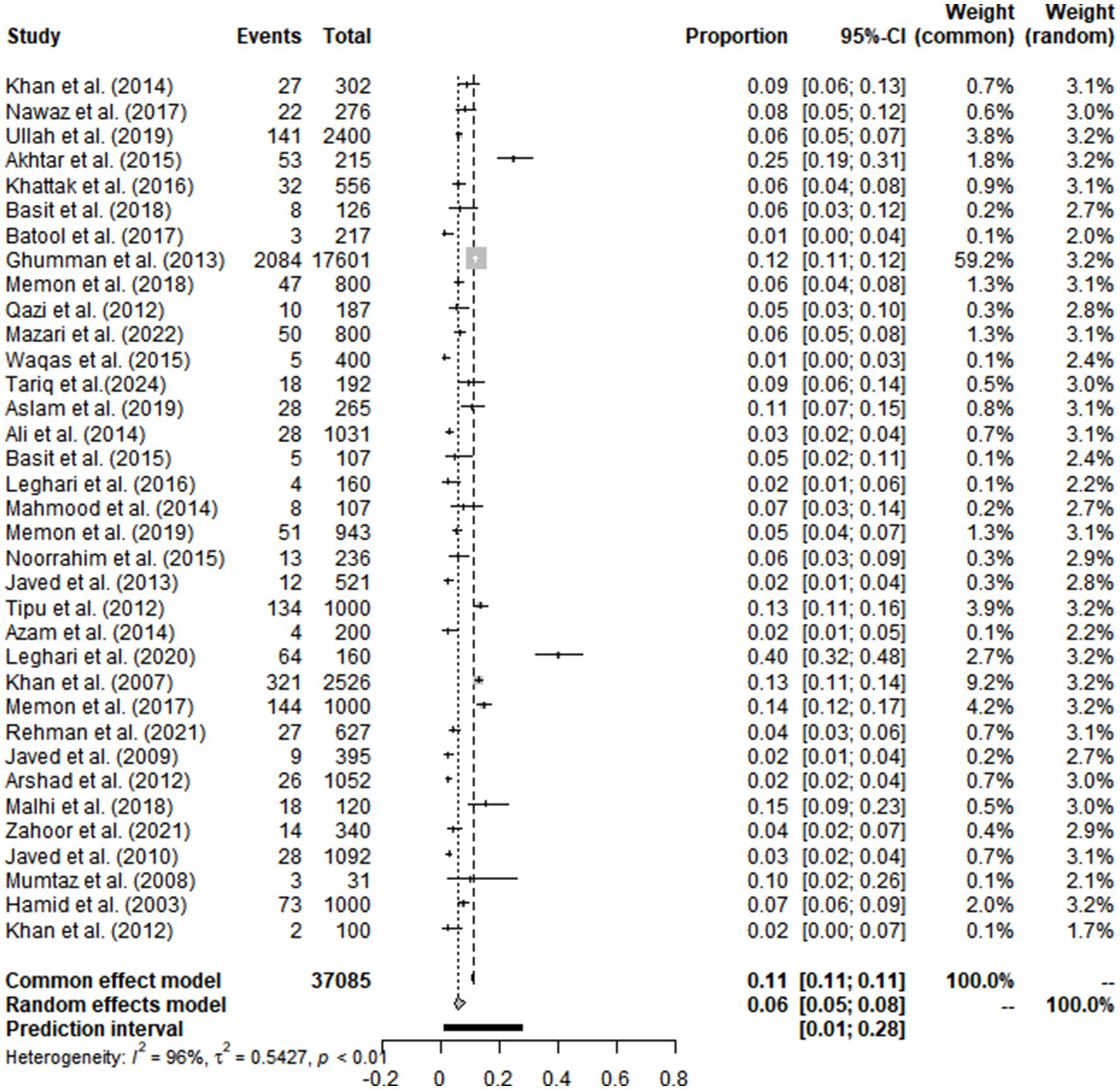
From 2000 to 2024, 851 articles were screened from the four databases, Google Scholar and publications cited in the published research (Figure 1). According to inclusion and exclusion criteria, 35 articles were included in this meta-analysis (Supplementary Table S5). The score of each article was shown separately to represent its evaluation in the analysis (Table 1). The meta-analysis of the 35 studies showed an overall prevalence of bTB (6.06% [95% CI: 4.67–7.87]), with 3,516 positive cases from a total of 37,085 samples and high heterogeneity between studies.
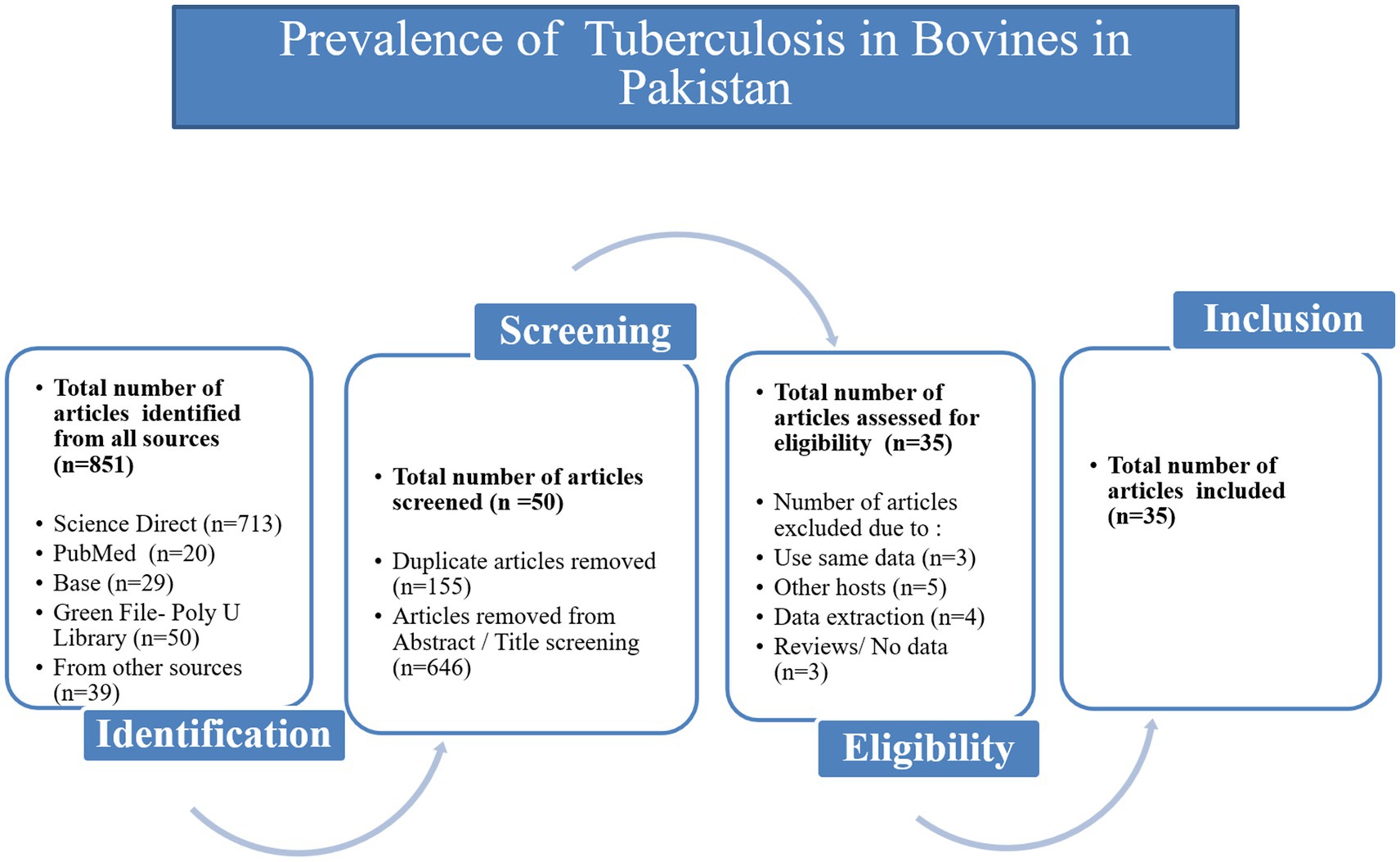
Figure 1. Flow diagram of the selection of eligible studies, based on the inclusion and exclusion criteria specific to this study.
3.2 Publication bias
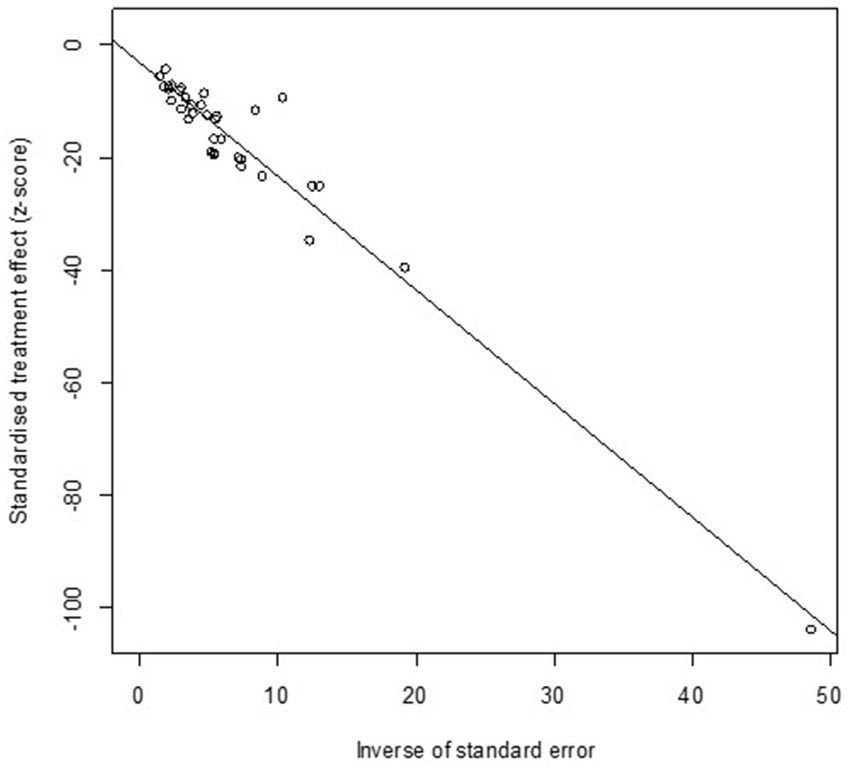
High heterogeneity was found in the included studies (I2 = 96%, p ≤ 0.01) (Figure 2), and PLN was used on the positive rate to make sure the total effect size data was closer to a normal distribution (Table 2). A funnel plot was used to identify publication bias. The asymmetrical scatter distribution suggests the possibility of publication bias or small sample size bias in the study (Figure 3). Publication bias was further confirmed using the Egger linear regression approach. Egger’s test (p = 0.001) also showed that there exists publication bias in our studies (Figure 4; Supplementary Table S3). The results of meta-analysis and publication bias of each subgroup are shown in Supplementary Figures S1–S34.
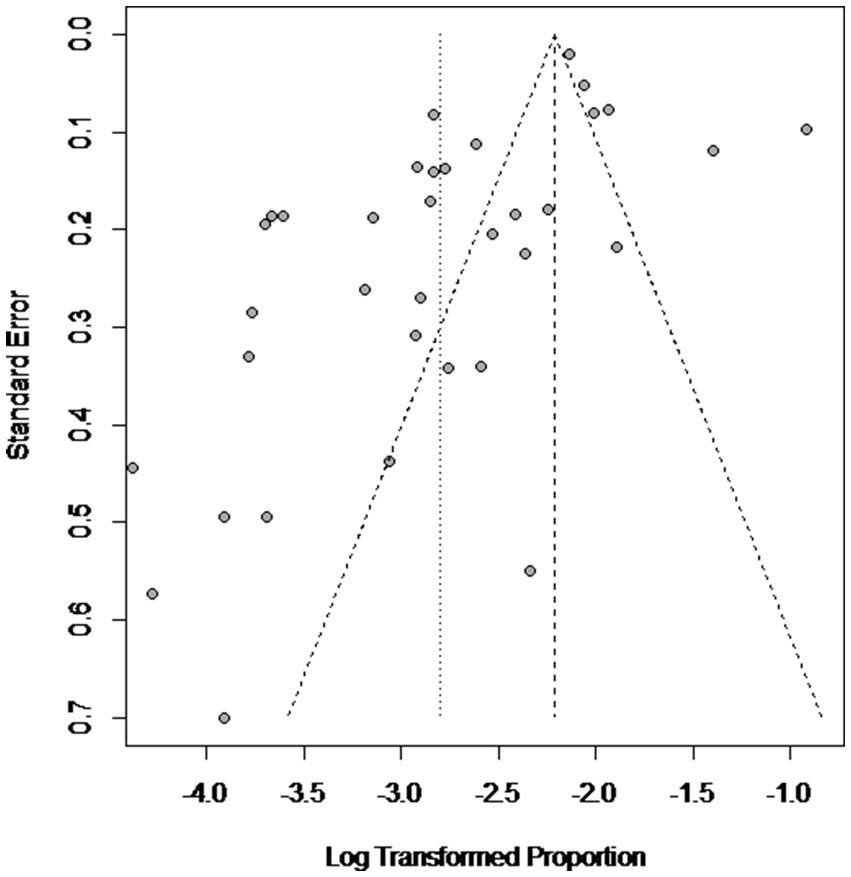
Figure 3. Funnel plot with pseudo 95% confidence interval limits for the examination of publication bias.
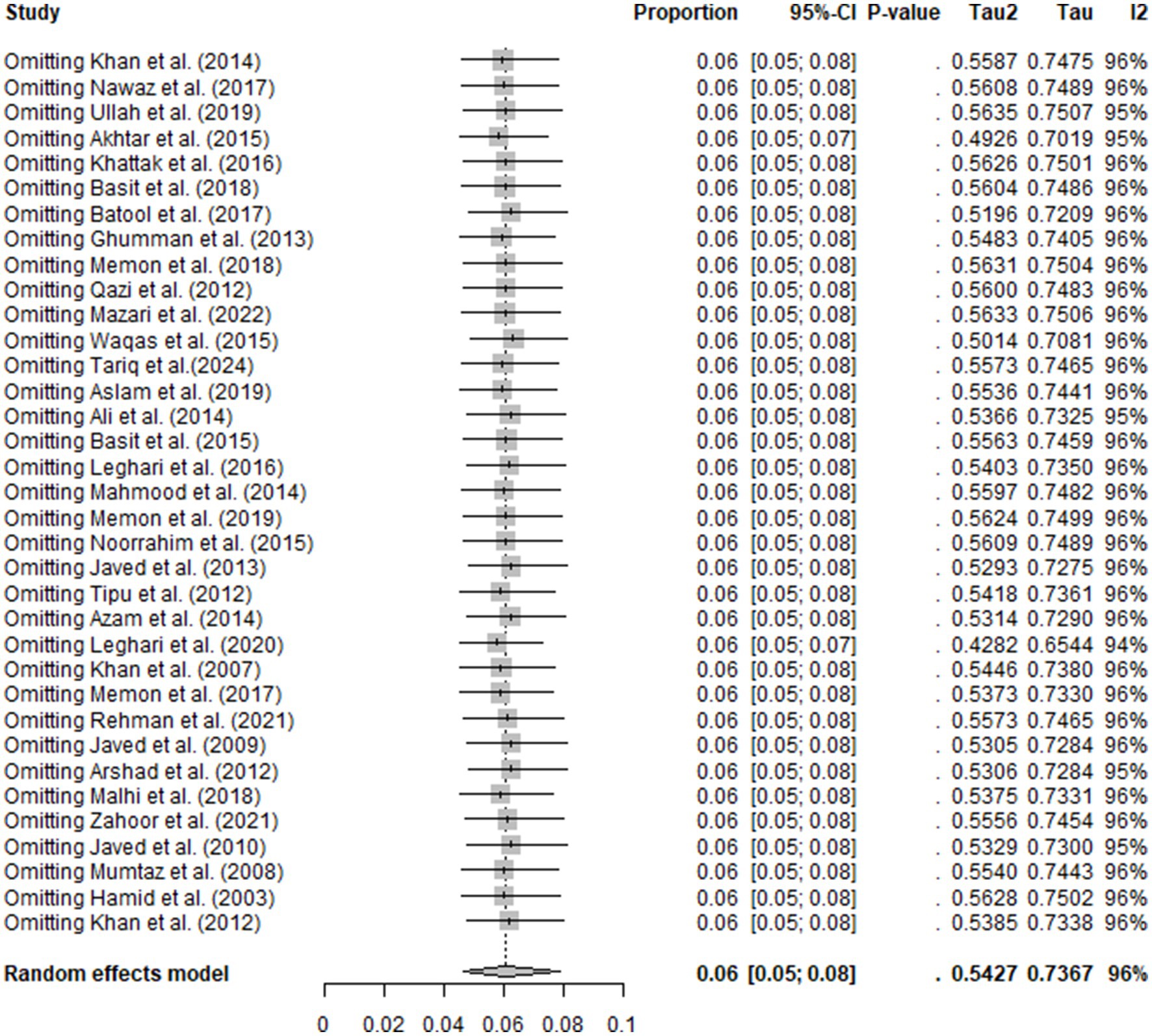
3.3 Sensitivity analysis
Sensitivity analysis confirmed the robustness of the overall study’s findings. The omission of any single study did not affect the results, and the remaining studies gave the same results. This finding confirmed stability and reliability of the meta-analysis (Figure 5).
3.4 Meta-analysis of bovine tuberculosis in Pakistan
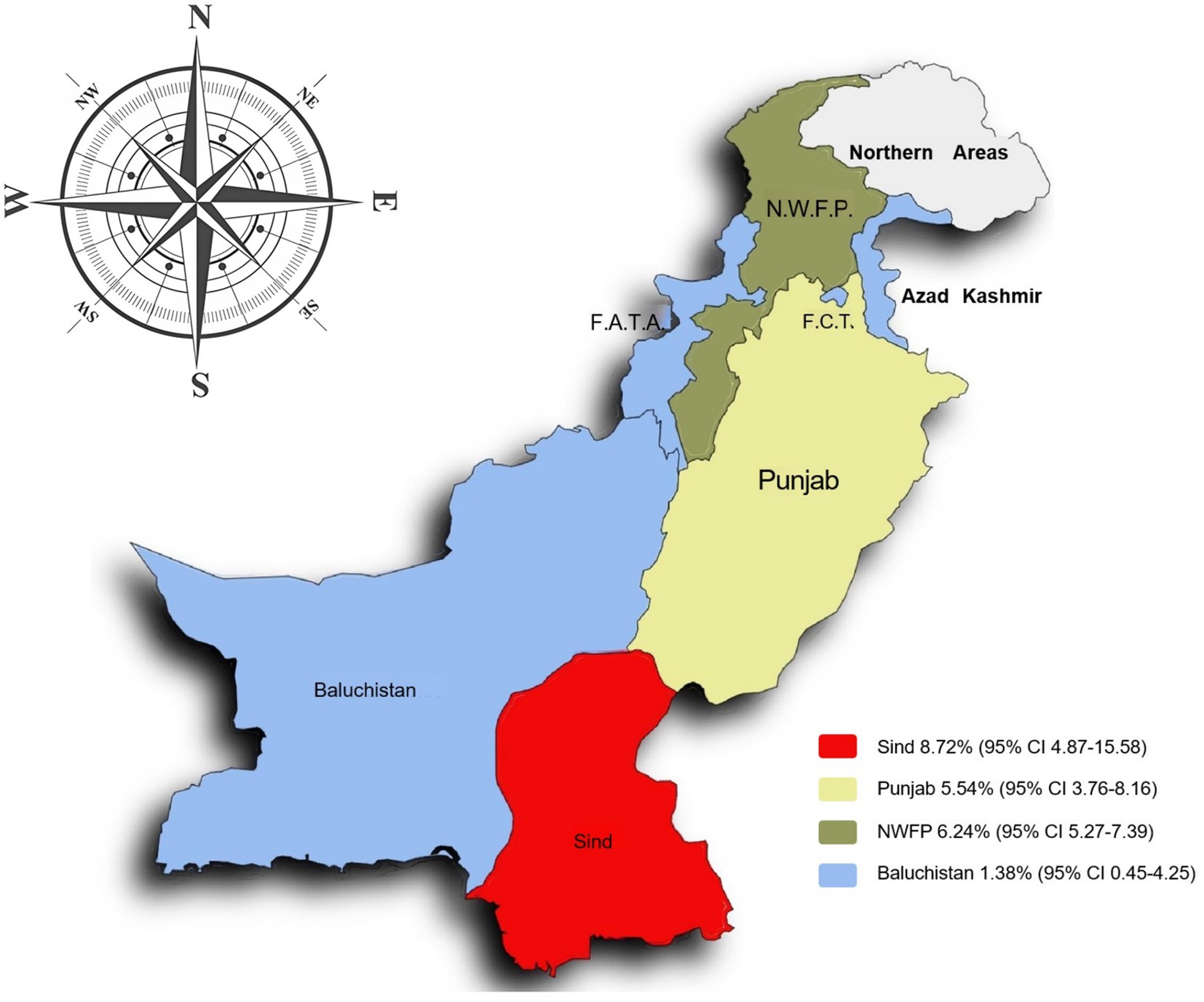
The prevalence of bovine tuberculosis (bTB) in different regions of Pakistan between 2000 and 2024 showed a significant variation. The highest prevalence was recorded in Southeast Pakistan (Sindh) at 8.72% [95% CI: 4.87–15.58]. In contrast the lowest prevalence was observed in Southwest Pakistan (Balochistan) at 1.38% [95% CI: 0.45–4.25]. However, as there is only one study available from Southwest Pakistan, the result should be interpreted with caution (Table 3; Figure 6) (52).
3.5 Factors associated with bovine tuberculosis
Based on subgroup analysis, the contribution of multiple parameters were assessed as risk factors for prevalence of bTB.
3.5.1 Factors related to the animal
Regarding animal species, cattle were the most susceptible one, with a highest prevalence of 6.44% [95% CI: 4.04–10.26], compared to bovine 6.07% [95% CI: 4.11–8.97] and buffaloes 5.54% [95% CI: 3.13–9.81] with no significant difference. Interestingly, the animal breed as found to be a significant risk factor as the exotic breeds showed a higher prevalence (13.65% [95% CI: 5.44–34.24]) compared to local breeds (5.33% [95% CI: 3.32–8.56]) and cross-bred (9.72% [95% CI: 3.85–24.56]) (Table 3).
Concerning the gender as a risk factor, females were found to have high positive rate (6.72% [95% CI: 4.47–10.08]) comparing to males (4.41% [95% CI: 2.63–7.39]) with no significant difference.
Regarding the pregnancy as a risk factor, prevalence was found to be high in the pregnant females (10.75% [95% CI: 2.93–39.50]) compared to the non-pregnant females (8.84% [95% CI: 4.00–19.56]) with no significant difference.
Lactating animals exhibited a higher prevalence rate (9.93% [95% CI: 5.80–17.02]) compared to non-lactating animals (6.75% [95% CI: 3.90–11.67]) with no significant difference.
Concerning the body condition score (BCS) (Supplementary Table S4), the animals with fair BCS showed the highest prevalence (17.66% [95% CI: 3.63–85.88]) compared to poor BCS (13.51% [95% CI: 6.39–28.58]) and good BCS (2.43% [95% CI: 1.29–4.60]) with significant difference.
The disease prevalence was differed according to animal age. It could be cleared that, animals of age (4–6 years) showed higher prevalence (10.26% [95% CI: 6.31–16.69]) compared to animals of age (7–10 years) (8.02% [95% CI; 5.09–12.64]) and animals of age (1–3 years) (3.50% [95% CI: 2.75–4.46]) with significant difference.
3.5.2 Factors related to the environment and climatic conditions
Geographical factors were found to have influence on bTB prevalence. Regarding latitude as a risk factor, regions with a low altitude (24–28°) showed higher prevalence (9.29% [95% CI: 5.56–15.53]) compared to regions of altitude 31–35° (6.24% [95% CI: 5.26–7.39]) and altitude 28–31° (5.04% [95% CI: 3.43–7.40]) with significant difference. Regarding longitude as risk factor, regions with a low longitude 66–69° showed the highest prevalence (8.21% [95% CI: 4.80–14.06]) compared to regions of longitude 69–72° (6.43% [95% CI: 5.48–7.53]) and > 72° (5.11% [95% CI: 3.50–7.44]) with no significant difference.
Studying of altitude as a risk factor revealed that, regions with a low altitude <100 m showed the highest prevalence (8.66% [95% CI: 4.05–18.52]) compared to regions with altitude >1,500 m (6.56% [95% CI: 2.79–15.40]) and altitude 100–1,500 m (5.71% [95% CI: 4.28–7.62]) with no significant difference.
The rainfall was evaluated as a risk factor, regions with low rainfall <100 mm showed highest prevalence (6.87% [95% CI; 5.08–9.30]) compared to regions with 100–400 mm rainfall (6.10% [95% CI: 3.92–9.51]) and with >400 mm rainfall (2.64% [95% CI: 2–3.49]) with significant difference.
Temperature as a risk factor was found to be a significant one, regions with an average annual temperature < 25°C had the highest prevalence (7.60% [95% CI: 5.53–10.43]) compared to regions with temperature > 30°C (6.55% [95% CI: 4.46–9.62]) and with temperature 25–30°C (4.65% [95% CI: 2.84–7.62]) with no significant difference.
Parallelly the regions of hot desert climate showed the highest prevalence (10.48% [95% CI: 5.29–20.77]) compared to regions of humid-sub tropical climate (6.16% [95% CI: 3.59–10.56]) and sub-tropical climate regions (5.87% [95% CI: 4.36–7.89]) with no significant difference (Table 4).
The regions with 35–50% humidity showed highest prevalence (6.75% [95% CI: 5.20–8.77]) compared to regions having 20–35% humidity (6.53% [95% CI: 3.64–11.69]) and regions with humidity 50–65% (5.21% [95% CI: 3.32–8.16]) with no significant difference.
3.5.3 Factors related to management
The significance of herd size as a risk factor was determined, highest prevalence rate was found in larger herds having (101–500 animals) (10.59% [95% CI: 3.90–28.75]) compared to small herds having (1–50 animals) (7.09% [95% CI:4.81–10.44]) and moderate herds (51–100 animals) (2.50% [95% CI: 1.88–3.33]) with significant difference.
Long lactation period (6–10 M) was found to be significantly (6.32% [95% CI: 2.57–15.54]) high compared to lactation length (3–6 M) (7.54% [95% CI: 2.80–20.30]) and lactation length (0–3 M) (1.97% [95% CI: 1.20–3.25]).
The heavy weighted animals (400–500 kg) showed higher prevalence (7.75% [95% CI: 4.20–14.28]) compared to animals having weight (300–400 kg) (3.56% [95% CI: 1.12–11.33]) and animals with weight (200–300 kg) (2.26% [95% CI: 0.82–6.23]) with significant difference.
3.5.4 Other risk factors
Regarding the study’s duration as risk factor, the time period from 2007 to 2011 showed highest prevalence (9.24% [95% CI: 6.49–13.16]) compared to 2011–2017 (5.90% [95% CI: 3.54–9.84]), and 2005–2007 (3.32% [95% CI: 1.87–5.89]) with no significant difference.
Although different methods were used for bTB, polymerase chain reaction (PCR) showed the highest prevalence (5.65% [95% CI: 3.33–9.58]), compared to the tuberculin skin test (TST) (5.61% [95% CI: 4.20–7.50]) and culture test (4.67% [95% CI: 2.50–8.72]) with no significant difference.
The sample type was an important factor to be evaluated. The highest prevalence was determined in milk samples (14.66% [95% CI: 7.38–29.11]) compared to nasal swabs (11.62% [95% CI: 5.19–26.02]) with no significant difference.
Concerning parity as a risk factor, the parity number 3–6 showed highest prevalence (14.10% [95% CI: 4.31–46.12]) compared to parity number 1–3 (7.65% [95% CI: 2.31–25.40]) with no significant difference.
Regarding quality level of studies as risk factor, it was found that, studies with higher quality scores (3–4) showed a higher prevalence (6.24% [95% CI: 4.46–8.73]) compared to quality scores (1–2) (5.74% [95% CI: 3.89–8.45]) (Table 3).
4 Discussion
Pakistan located in subtropical South Asia, has reported multiple cases of bTB across the country, primarily caused by M. bovis (53, 54). Although the majority of developed countries have successfully eliminated bTB through extensive application of test-and-slaughter programs, but the disease remains endemic in multiple areas of Africa, Asia, Latin America, and a large portion of the Middle East, posing serious public health and economic risks (40, 54). Low-and middle-income countries like Pakistan face additional challenges due to inadequate resources and incomplete data (53, 55, 56). On the human side, M. bovis is a neglected pathogen, excluded from the “National Guidelines for the Control of Tuberculosis in Pakistan-2019.” Despite efforts by the WHO (Supplementary Table S4) and other international organizations to tackle zoonotic TB, the country lacks an effective bTB surveillance program (57). Although small-scale studies have been conducted, national initiatives remain absent despite the availability of infrastructure from previous disease control efforts like Rinderpest eradication (56, 57).
The current meta-analysis showed an overall bTB prevalence of (6.06%) in Pakistan with highest prevalence in cattle (6.44%), as compared to buffalo (5.54%) and bovines (6.07%). These findings align with regional variations observed globally, including India (2018) (7.3%) (58), and much lower than those recorded in Ghana (19%) during (2011–2012) (59), (28%) in South Africa (2016–2017) (60), (22%) in Ethiopia (2016–2017) (61). The highest prevalence rate in Pakistan (9.24%) occurred between 2007 and 2011, significantly influenced by the devastating 2010 floods which caused severe losses to the agriculture and livestock sectors (62). Of the provinces affected by this damage, Sindh incurred (46%) of the total losses, followed by Punjab (36%), Khyber Pakhtunkhwa (8%), and Balochistan (8%). The overall estimated loss to this industry was approximately $5 billion (63). The 2010 summer floods resulted in the deaths of 274,334 domesticated animals due to lack of food and fodder, nutritional deficiencies, weakened immune systems and an increased susceptibility to diseases including bovine tuberculosis (64, 65). In Africa, increased TB prevalence is linked to flooding due to enforced contact between herds (66), and drought, which forces cattle to use communal water sources (67), and encourages large-scale movements (68). Ranking fifth in the 2019 Global Climate Risk Index (CRI), Pakistan has a high susceptibility to the impacts of climate change (69).
Detection methods in the meta-analysis primarily included the (TST, PCR and bacterial culture). PCR showed higher detection rate (5.65%) compared to the Tuberculin Skin Test (TST) (5.61%), the most preferred, and widely used diagnostic method (70) due to its cost-effectiveness and widespread availability. However, its sensitivity varies across regions and management conditions (71) with concerns about under-diagnosis and false negatives that could lead to disease re-emergence in cattle herds (16, 72). Although PCR has a comparable positive rate (5.65%), its application is limited in Pakistan due to the lack of advanced diagnostic facilities and small study sample sizes. On the other hand, the Culture test method, which is considered the gold standard test for bTB determination, requires BSL-3 laboratory-facilities that are unavailable in many developing countries. It is worth noting that molecular techniques were widely used for pathogen detection because of its high sensitivity, and less time consuming (22, 73, 74). Therefore, future researches in Pakistan should assess the effectiveness of TST in addition to PCR and culture, along with these, taking into account the need for more effective and accessible testing techniques, to increase the diagnostic accuracy and decrease false negatives (16).
In Pakistan, milk is mostly produced from bovines. So, it is important to determine the M. bovis status in both cattle and buffaloes to determine the bovine tuberculosis risk. Remarkably, the majority of previous studies conducted in Pakistan have focused on cattle. In contrast, the buffalo population, which constitutes a significant portion of country’s livestock and appears more susceptible to bovine tuberculosis (75), has been the subject of limited research, primarily restricted to regions such as districts of Okara and Faisalabad (76). Regarding animal species (cows vs. buffalo), the meta-analysis showed a higher prevalence in cows (6.44%) compared to buffalo and bovines. Similar results have been reported using RE model that might be due to management and biological factors (58). Higher prevalence in cows might be due to the high- density breeding environments in commercial dairy farms, where close contact between animals favors the spread of pathogens including M. bovis, in contrast buffaloes were raised in extensive systems with lower breeding densities, thereby reducing risk transmission (66, 77).
A high prevalence of bTB (11.7%) has been shown in several dairy cattle farms of Punjab, Pakistan (78). Cattle are biologically susceptible to M. bovis, frequently resulting in subclinical infections and transmission occurs either through inhalation or oral route (79). Milk samples showed the highest prevalence (14.66%) in our study indicating probability of zoonotic risk of transmission, especially in areas where dairy products are improperly handled and not pasteurized (80). Key factors contributed to the spread of bovine tuberculosis included consuming raw milk, close contact with animals, and poor hygiene standards on animal farms (81).
The primary way that bovine tuberculosis (bTB) is spread from cow to calf is through the consumption of contaminated milk or colostrum (43). M. bovis primarily affects the mammary glands of cattle and buffaloes, sub-clinically infected cows usually shed M. bovis at concentrations of about 103 colony forming unit (cfu/ml) (82). So, the bacteria are more likely to be excreted in the milk of the infected animals frequently without apparent clinical symptoms (82, 83). Depending on the immunological response, M. bovis can potentially spread by aerosol inhalation, which allows it to enter the lungs and be absorbed by alveolar macrophages, resulting in localized or systemic infection. Effective immunity blocks the infection and stops active shedding, but impaired immunity causes persistent lesions and active pulmonary tuberculosis, which spreads M. bovis through aerosol, feces, mucus, urine and milk (84). To determine the prevalence of M. bovis in milk, various diagnostic methods were employed in previous studies. The overall prevalence of M. bovis in milk samples was (5% [95% CI; 3–7%]). Among cows that tested positive for the tuberculin skin test (TST), the prevalence increased to (8% [95% CI; 4–13%]) likely due to the association between positive TST results and active or latent infections. Regardless of the herd TST infection status, the prevalence of M. bovis in bulk tank milk (BTM) was estimated at (5% [95% CI; 0–21%]) (85). These findings highlight milk transmission as an important risk factor. However, a separate meta-analysis of milk as a transmission factor was not conducted in this study due to the limited number of studies with sufficient data.
In the current study, prevalence rate was higher in females (6.72%) than in males (4.4.1%), which might be attributed to the differences in production systems and stress factors associated with females as pregnancy, parturition and lactation (86, 87). Males are closely related to beef farms and females are found on dairy farms (12). Therefore, prevalence might be higher in females due to the large number of samples collected particularly from dairy herds (60). However, some studies reported high prevalence in males as they were mainly used for oxen and kept in herds for longer time thus increasing their contact with infected herds (88). Management practices also differ between genders in both developing and developed countries. Due to close contact during milking and calving, dairy cows were more susceptible to bovine tuberculosis than males and they also achieve maturity earlier (89). This emphasizes the significance of routine bTB testing and efficient herd management to prevent the spread of disease within dairy herds.
Exotic breeds such as Holstein-Friesians were more prone to the disease and showed higher prevalence (13.65%) as compared to local and cross-bred due to difference in genetic resistance and adaptation to local environmental conditions (22). Moreover, research suggested association between the TauT gene (Taurine Transporter) in Holstein-Friesian cattle and bTB susceptibility (90), its presence leads to taurine deficiency and ultimately affecting immune system (91). Exotic breeds have been bred for high milk production in temperate climates; these breeds may lack the genetic adaptations for disease resistance prevalent in sub-tropical and tropical regions like Pakistan. This stress resulted in impairment of immunity, making them more vulnerable to bTB (92). The obtained results were aligned with previous studies showed a higher prevalence of bTB in exotic cattle (93, 94).
Age was the main individual risk factor in various studies in both developed and developing countries. Age and weight were interrelated factors contributed to higher prevalence of bovine tuberculosis in livestock (95). The prevalence of M. bovis was higher in animals aged 4–6 years (10.26%), followed by in animals aged 7–10 years (8.60%). Similarly, animals with an increased weight showed a prevalence of (7.75%). These patterns may result from the long incubation period and slow progression of the disease to detectable levels (96). The later decline in prevalence could be due to the development of an allergic state or a higher mortality rate among infected animals in the advanced stage of disease (97). Older animals often gain more weight, particularly due to increased fat deposits, which can lead to chronic-low grade inflammation and a weakened immune response. This impairment results in significantly higher rates of seropositivity. Animals may get infected at a young age but at adult stage they express disease clinically (98). Mycobacteria have the ability to remain in latent state for longer periods before being reactivated at old age (99). Scientists have not yet confirmed whether cattle can harbor a true dormant state of infection (100). Developing of experimental latency models in cattle is necessary to evaluate their implications, including the underdiagnosis of the disease, especially in the developed countries (89). Similar results have been found in studies that bTB infection increases with age, with older heavier animals more susceptible to disease (95).
In the current analysis, larger herds (101–500 animals) had more prevalence (10.59%) compared to the small ones. The same observation was previously recorded (101), especially with poor management practices (102). Factors such as defective ventilation (94%), improper waste disposal system (94.18%), cattle in same premises (92.59%) and poor floor sanitation (77.77%) were directly or indirectly related to the spread of bTB through coughing (103). As the dairy farmers continuously expand their herd size to increase farm yield, leading to overcrowding thus causing increased risk of animal-to-animal transmission (104).
In the present study, pregnant animals showed the highest prevalence (10.75%) compared to the non-pregnant ones, which correlate with other studies suggesting that pregnant cattle are more susceptible to infection (70). This could be due to many factors including the physiological, and hormonal changes during pregnancy which might suppress the immune system making them more susceptible at that time (105).
The study identified an increased prevalence of bTB (9.93%) in lactating animals, especially those with higher parity and longer lactation periods. The increased production stress in dairy cows endure and the gathering of cows during milking might increase the risk of disease transmission (106). Similar findings have been reported in previous studies that higher prevalence was observed in lactating as compared to non-lactating ones, mostly due to increased stress of high milk production (70, 107, 108). Furthermore, during lactation adult females may suffer from nutritional deficiency that can be a predisposing factor for bovine tuberculosis and might reduce the immunity against diseases (109, 110). Due to weakened immunity, animals become more vulnerable to infections especially when producing large quantities of milk over extended periods and through multiple pregnancies (111). Hence, the disease prevalence rate is directly proportional to the increase in milk production (112).
Our findings align with studies showing that animals with good BCS were less susceptible to bTB infection compared to those with fair (36, 61) or poor scores (113, 114). In contrast, some studies suggested a link between good BCS and high prevalence. This might be due to factors as increased milk production in well-nourished animals leading to weakened immune system thereby increasing infections as bTB (115).
In terms of regional and provinces sub-groups, Sindh group in southeastern of Pakistan, showed the highest prevalence (8.72%) of bTB infection. Flood-prone areas have been shown to favor the environmental persistence of other Mycobacteria species (116), because of the high moisture content that helps M. bovis to survive longer (117). The geographical regions with varied climates directly impact the environmental persistence and transmission dynamics of M. bovis (82, 118). Low altitude and decreased rainfall showed significant differences and highest prevalence in the meta-analysis. Sindh is also located at low altitude (<100 m) and latitude (24–28o), consisting of plain and flat areas with low rainfall (<100 mm) and hot climate. These conditions may facilitate bacterial survival and transmission, along with congregation of animals due to fewer water sources. Additionally, harsh environmental conditions, poor nutrition, and stress can weaken the animals’ immune systems, which increases their vulnerability to disease (119). All of these factors may contribute to the higher prevalence of M. bovis in this region.
The peri-urban areas, transitional zones between rural and urban settings, were susceptible to bTB due to presence of both extensive and intensive farming systems. Intensive livestock farming, poor sanitary conditions in rural farms and close contact at water farms favor the ideal environment for M. bovis infection (120). An increased incidence of bTB occurrences was related to intensive management approaches that enhance cattle-to-cattle contact within a herd (121). Farms and abattoirs having regulated environments where animals are rigorously screened for diseases, mostly implement strict biosecurity measures and more disease control programs including controlled breeding ones and vaccination schedules, which help in preventing the spread of disease as compared to less regulated environments in rural, urban and town settings. These findings were consistent with previous studies reporting higher prevalence in peri-urban, rural and urban settings (10, 122).
While our study included 24 high-quality and 11 average-quality articles. In most of the average articles, prevalence was low due to lack of clear sampling time and random sampling descriptions. The researchers must provide detailed relevant information about risk factors during epidemiological investigations thus providing reliable scientific data and effective follow-up research on bovine tuberculosis.
Our meta-analysis has some limitations including mainly the limited used databases. Accordingly, some eligible studies might be excluded. Also, some risk factors were examined in small number of studies, which could result in potentially unstable results due to small study effects. Most of included studies were from Northeast Pakistan, with only one study from Southwest Pakistan. Lastly, in interpreting the results of this meta-analysis, it is important to acknowledge the limitations associated with the use of univariate meta-regression.
We acknowledge the limitations of performing only univariable meta-regression in this study. While this method provides information on the independent association of each variable with tuberculosis prevalence, it does not account for confounding effects or correlations between variables. As such, results should be interpreted with caution, since associations found in isolation may become non-significant when adjusted for other variables in a multivariable model. A key limitation is the risk of multicollinearity, some study variables may be correlated, potentially leading to an over or underestimation of their true effects. Without a multivariable approach, it is difficult to distinguish direct effects from those influenced by confounding. Moreover, univariable meta-regression does not allow adjustment for multiple factors simultaneously, which can result in biased estimates, especially when variables interact or jointly affect tuberculosis prevalence.
These issues could be mitigated using a multivariable meta-regression model, which would control for key variables and reduce the impact of multicollinearity. However, due to limitations such as missing data and the small number of included studies, applying such a model risked overfitting and unreliable estimates. Given these constraints, we used univariable analyses as an initial step, with the understanding that future studies with larger datasets should employ multivariable meta-regression for more robust analysis. Despite these limitations, we interpreted our findings cautiously to minimize bias and provide meaningful preliminary insights. We emphasize that the associations reported here should be validated in future research using enhanced methodologies, including multivariable meta-regression, to improve result reliability.
5 Conclusion
This meta-analysis reveals the significant prevalence and risk factors associated with bTB in Pakistan, emphasizing its implications for livestock management, public health and economic sustainability. The overall prevalence of bTB (6.06%) aligns with regional trends but it is notably higher in certain areas and specific sub-groups such as dairy cows, lactating, pregnant animals, exotic breeds, and larger herds. Factors such as intensive farming, poor management practices and climate-induced stressors including flooding and drought, exacerbate the disease’s persistence and spread. The high prevalence of M. bovis in milk samples underscores the zoonotic risk of transmission in areas lacking pasteurization and proper hygiene standards. The study also highlights diagnostic challenges, with PCR showing comparable detection rates to TST but with limited use due to inadequate infrastructure. The gold standard culture test remains underutilized due to resource constraints, emphasizing the urgent need for accessible and accurate diagnostic tools.
Pakistan faces significant challenges in eradicating and controlling the bTB due to a lack of data, inadequate diagnostic and treatment facilities and limited awareness about the disease. Adopting a “One Health Approach” is crucial for effective control, integrating the livestock, agriculture, public health and food security sectors. Priorities include establishing comprehensive surveillance programs, improving diagnostic methods, focusing on underrepresented regions and buffalo populations, and addressing management-related issues. Strengthened biosecurity measures, effective herd management and farmer education are essential to reduce transmission. Coordinated national and regional efforts, supported by international organizations, are important for developing and implementing policies to protect public health and livestock industry.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SS: Writing – original draft, Writing – review & editing. X-TL: Data curation, Writing – review & editing. W-BL: Data curation, Writing – review & editing. S-YZ: Funding acquisition, Supervision, Writing – review & editing. EA: Writing – review & editing. G-GY: Methodology, Writing – review & editing. QW: Methodology, Writing – review & editing. F-LZ: Writing – review & editing. XL: Writing – review & editing. KS: Writing – review & editing. R-MA: Writing – review & editing. Q-LG: Writing – review & editing. Y-HS: Formal analysis, Software, Writing – review & editing. RD: Conceptualization, Funding acquisition, Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was funded by the National Natural Science Foundation of China Joint Fund (U23A20237) and Jilin Province Science and Technology Development Project (20220401115YY).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1525399/full#supplementary-material
References
1. Ge, G, Li, D, Ling, Q, Xu, L, Ata, EB, Wang, X, et al. IRF7-deficient MDBK cell based on CRISPR/Cas9 technology for enhancing IBRV replication. Front Microbiol. (2024) 15:1483527. doi: 10.3389/fmicb.2024.1483527
2. Kasem, S, Yu, MHH, Alkhalefa, N, Ata, EB, Nayel, M, Abdo, W, et al. Impact of equine herpesvirus-1 ORF15 (EUL45) on viral replication and neurovirulence. Vet Microbiol. (2024) 298:110234. doi: 10.1016/j.vetmic.2024.110234
3. Sha, W, Beshir Ata, E, Yan, M, Zhang, Z, and Fan, H. Swine Colibacillosis: Analysis of the Gut Bacterial Microbiome. Microorganisms. (2024) 12:1233. doi: 10.3390/microorganisms12061233
4. Ibrahiem, HS, Alsenosy, AA, El-Ktany, EM, Ata, EB, and Abas, OM. Anthelmintic Efficacy and Pharmcodynamic Effects of Levamisole-Oxyclozanide Combination as (Levanide®) in Fattening Calves. Egypt J Vet Sci. (2023) 54:1245–54. doi: 10.21608/ejvs.2023.219811.1532
5. Niu, T-MM, Yu, L-JJ, Zhao, J-HH, Zhang, R-RR, Ata, EB, Wang, N, et al. Characterization and pathogenicity of the porcine epidemic diarrhea virus isolated in China. Microb Pathog. (2023) 174:105924. doi: 10.1016/j.micpath.2022.105924
6. Shalaby, H, Kandil, O, Hendawy, S, Elsawy, BS, Ashry, HM, El-Namaky, A, et al. Dynamics of Haemonchus Contortus Coproantigen Appearance in Feces of Experimentally Infected Sheep. Egypt J Vet Sci. (2024) 55:1307–14. doi: 10.21608/ejvs.2024.251684.1693
7. Al-Ebshahy, EM, AboElkhair, M, Amer, S, Salama, A, Nayel, M, Abbas, R, et al. A study for Molecular Detection of Co-circulated Multiple FMDV Serotypes in Some Egyptian Governorates. Egypt J Vet Sci. (2025) 1–10. doi: 10.21608/ejvs.2024.337086.2501
8. Li, X, Xia, A, Xu, Z, Liu, J, Fu, S, Cao, Z, et al. Development and evaluation of a Mycobacterium bovis interferon-γ enzyme-linked immunospot (ELISpot) assay for detection of bovine tuberculosis. J Dairy Sci. (2022) 105:6021–9. doi: 10.3168/jds.2021-21301
9. Khalid, H, Pierneef, L, van Hooij, A, Zhou, Z, de Jong, D, Tjon Kon Fat, E, et al. Development of lateral flow assays to detect host proteins in cattle for improved diagnosis of bovine tuberculosis. Front Vet Sci. (2023) 10:10. doi: 10.3389/fvets.2023.1193332
10. Shirima, G, Kazwala, R, and Kambarage, D. Prevalence of bovine tuberculosis in cattle in different farming systems in the eastern zone of Tanzania. Prev Vet Med. (2003) 57:167–72. doi: 10.1016/S0167-5877(02)00214-3
11. Dametto, LL, Santos, ED, Santos, LR, and Dickel, EL. Bovine tuberculosis: diagnosis in dairy cattle through the association of analyzes. Pesqui Vet Bras. (2020) 40:12–6. doi: 10.1590/1678-5150-PVB-6294
12. Ramos, B, Pereira, AC, Reis, AC, and Cunha, MV. Estimates of the global and continental burden of animal tuberculosis in key livestock species worldwide: A meta-analysis study. One Health. (2020) 10:100169. doi: 10.1016/j.onehlt.2020.100169
13. World Organization for Animal Health (OIE), Food and Agriculture Organization of the United Nations (FAO). Roadmap for Zoonotic Tuberculosis. (2017). Available online at: https://bulletin.woah.org. (Accessed June 18, 2024).
14. Srinivasan, S, Conlan, AJ, Easterling, LA, Herrera, C, Dandapat, P, Veerasami, M, et al. A meta-analysis of the effect of Bacillus Calmette-Guérin vaccination against bovine tuberculosis: is perfect the enemy of good? Front Vet Sci. (2021) 8:637580. doi: 10.3389/fvets.2021.637580
15. Ramanujam, H, and Palaniyandi, K. Bovine tuberculosis in India: The need for one health approach and the way forward. One Health. (2023) 16:100495. doi: 10.1016/j.onehlt.2023.100495
16. Humblet, M-F, Boschiroli, ML, and Saegerman, C. Classification of worldwide bovine tuberculosis risk factors in cattle: a stratified approach. Vet Res. (2009) 40:50. doi: 10.1051/vetres/2009033
17. Cvetnic, Z, Katalinic-Jankovic, V, Sostaric, B, Spicic, S, Obrovac, M, Marjanovic, S, et al. Mycobacterium caprae in cattle and humans in Croatia. Int J Tuberc Lung Dis. (2007) 11:652–8.
18. Dawson, KL, Bell, A, Kawakami, RP, Coley, K, Yates, G, and Collins, DM. Transmission of Mycobacterium orygis (M. tuberculosis complex species) from a tuberculosis patient to a dairy cow in New Zealand. J Clin Microbiol. (2012) 50:3136–8. doi: 10.1128/jcm.01652-12
19. Michelet, L, de Cruz, K, Tambosco, J, Hénault, S, and Boschiroli, ML. Mycobacterium microti interferes with bovine tuberculosis surveillance. Microorganisms. (2020) 8:1850. doi: 10.3390/microorganisms8121850
20. Cadmus, SI, Yakubu, MK, Magaji, AA, Jenkins, AO, and van Soolingen, D. Mycobacterium bovis, but also M. africanum present in raw milk of pastoral cattle in north-central Nigeria. Trop Anim Health Prod. (2010) 42:1047–8. doi: 10.1007/s11250-010-9533-2
21. Ramanujam, H, Thiruvengadam, K, Singaraj, R, and Palaniyandi, K. Role of abattoir monitoring in determining the prevalence of bovine tuberculosis: A systematic review and meta‐analysis. Transbound Emerg Dis. (2022) 69:958–73. doi: 10.1111/tbed.14118
22. Refaya, AK, Bhargavi, G, Mathew, NC, Rajendran, A, Krishnamoorthy, R, Swaminathan, S, et al. A review on bovine tuberculosis in India. Tuberculosis. (2020) 122:101923. doi: 10.1016/j.tube.2020.101923
23. Du, Y, Qi, Y, Yu, L, Lin, J, Liu, S, Ni, H, et al. Molecular characterization of Mycobacterium tuberculosis complex (MTBC) isolated from cattle in northeast and northwest China. Res Vet Sci. (2011) 90:385–91. doi: 10.1016/j.rvsc.2010.07.020
24. Lantos, A, Niemann, S, Mezõsi, L, Sós, E, Erdélyi, K, Dávid, S, et al. Pulmonary tuberculosis due to Mycobacterium bovis subsp. caprae in captive Siberian tiger. Emerg Infect Dis. (2003) 9:1462–4. doi: 10.3201/eid0911.030297
25. Allen, AR, Ford, T, and Skuce, RA. Does Mycobacterium tuberculosis var. bovis survival in the environment confound bovine tuberculosis control and eradication? A literature review. Vet Med Int. (2021) 2021:8812898. doi: 10.1155/2021/8812898
26. Bolaños, CAD, Paula, CL, Guerra, ST, Franco, MMJ, and Ribeiro, MG. Diagnosis of mycobacteria in bovine milk: an overview. Rev Inst Med Trop Sao Paulo. (2017) 59:40. doi: 10.1590/S1678-9946201759040
27. Mohamed, A. Bovine tuberculosis at the human–livestock–wildlife interface and its control through one health approach in the Ethiopian Somali Pastoralists: A review. One Health. (2020) 9:100113. doi: 10.1016/j.onehlt.2019.100113
28. Broughan, J, Downs, S, Crawshaw, T, Upton, P, Brewer, J, and Clifton-Hadley, R. Mycobacterium bovis infections in domesticated non-bovine mammalian species. Part 1: review of epidemiology and laboratory submissions in Great Britain 2004–2010. Vet J. (2013) 198:339–45. doi: 10.1016/j.tvjl.2013.09.006
29. Bhembe, NL, Jaja, IF, Nwodo, UU, Okoh, AI, and Green, E. Prevalence of tuberculous lymphadenitis in slaughtered cattle in Eastern Cape, South Africa. Int J Infect Dis. (2017) 61:27–37. doi: 10.1016/j.ijid.2017.05.005
30. Phipps, E, McPhedran, K, Edwards, D, Russell, K, O'Connor, CM, Gunn-Moore, DA, et al. Bovine tuberculosis in working foxhounds: lessons learned from a complex public health investigation. Epidemiol Infect. (2019) 147:e24. doi: 10.1017/S0950268818002753
31. Sa’idu, A, Okolocha, E, Dzikwi, A, Gamawa, A, Ibrahim, S, Kwaga, J, et al. Public health implications and risk factors assessment of Mycobacterium bovis infections among abattoir personnel in Bauchi state, Nigeria. J Vet Med. (2015) 2015:718193. doi: 10.1155/2015/718193
32. Constable, PD, Hinchcliff, KW, Done, SH, and Grünberg, W. Veterinary medicine: a textbook of the diseases of cattle, horses, sheep, pigs and goats. 11th ed. St. Louis, MO: Elsevier (2016). 901 p.
33. Collins, ÁB, and More, SJ. Parameter estimates to support future risk assessment of Mycobacterium bovis in raw milk cheese. Microb Risk Anal. (2022) 21:100204. doi: 10.1016/j.mran.2022.100204
34. Serrano, M, Sevilla, IA, Fuertes, M, Geijo, M, Risalde, MÁ, Ruiz-Fons, JF, et al. Different lesion distribution in calves orally or intratracheally challenged with Mycobacterium bovis: implications for diagnosis. Vet Res. (2018) 49:74–10. doi: 10.1186/s13567-018-0566-2
35. Clausi, MT, Ciambrone, L, Zanoni, M, Costanzo, N, Pacciarini, M, and Casalinuovo, F. Evaluation of the presence and viability of Mycobacterium bovis in wild boar meat and meat-based preparations. Food Secur. (2021) 10:2410. doi: 10.3390/foods10102410
36. Waters, WR, Palmer, MV, Buddle, BM, and Vordermeier, HM. Bovine tuberculosis vaccine research: historical perspectives and recent advances. Vaccine. (2012) 30:2611–22. doi: 10.1016/j.vaccine.2012.02.018
37. Robinson, PA. Framing bovine tuberculosis: a ‘political ecology of health’ approach to circulation of knowledge (s) about animal disease control. Geogr J. (2017) 183:285–94. doi: 10.1111/geoj.12217
38. Caminiti, A. Panorama 2019-1: The socio-economic costs of bovine tuberculosis. Bulletin de l’OIE. (2019) 2019:1–2. doi: 10.20506/bull.2019.1.2916
39. Mukherjee, F, Bahekar, VS, Pasha, SY, Kannan, P, Prasad, A, Rana, SK, et al. Isolation and analysis of the molecular epidemiology and zoonotic significance of Mycobacterium tuberculosis in domestic and wildlife ruminants from three states in India. Rev Sci Tech. (2018) 37:999–1012. doi: 10.20506/rst.37.3.2902
40. Olmstead, AL, and Rhode, PW. An impossible undertaking: the eradication of bovine tuberculosis in the United States. J Econ Hist. (2004) 64:734–72. doi: 10.1017/S0022050704002955
41. Javed, MT, Irfan, M, Ali, I, Farooqi, FA, Wasiq, M, and Cagiola, M. Risk factors identified associated with tuberculosis in cattle at 11 livestock experiment stations of Punjab Pakistan. Acta Trop. (2011) 117:109–13. doi: 10.1016/j.actatropica.2010.10.009
42. Tschopp, R, Schelling, E, Hattendorf, J, Aseffa, A, and Zinsstag, J. Risk factors of bovine tuberculosis in cattle in rural livestock production systems of Ethiopia. Prev Vet Med. (2009) 89:205–11. doi: 10.1016/j.prevetmed.2009.02.006
43. Zanini, M, Moreira, E, Lopes, M, Mota, P, and Salas, C. Detection of Mycobacterium bovis in milk by polymerase chain reaction. Zentralbl Veterinarmed B. (1998) 45:473–9. doi: 10.1111/j.1439-0450.1998.tb00818.x
44. Aliraqi, OMM, Al-Jammaly, M, Al-Hankawi, O, Al-Farwachi, MI, and Dahl, MO. Preliminary prevalence and risk factors of Mycobacterium bovis in local and imported breeds of cattle and buffaloes in Mosul city, Iraq. Egypt J Vet Sci. (2020) 51:83–8. doi: 10.21608/ejvs.2019.17753.1102
45. Gong, Q-L, Chen, Y, Tian, T, Wen, X, Li, D, Song, Y-H, et al. Prevalence of bovine tuberculosis in dairy cattle in China during 2010–2019: A systematic review and meta-analysis. PLoS Negl Trop Dis. (2021) 15:e0009502. doi: 10.1371/journal.pntd.0009502
46. Javed, MT. Zoonotic Tuberculosis: Mycobacterium bovis and other pathogenic mycobacteria. In: Thoen CO, Steele JH, and Kaneene JB, editors. Zoonotic Tuberculosis: Mycobacterium bovis and other pathogenic mycobacteria. Hoboken, NJ: Wiley (2014). 181–90.
47. Ullah, I. Re-identifying the Rural/Urban: a case study of Pakistan. Espaço e Econ. (2022) 23. doi: 10.4000/espacoeconomia.22019
48. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DGPRISMA Group T. Preferred reporting items for systemic reviews and meta-analyses; the PRISMA statement. Ann Intern Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
49. Guyatt, GH, Oxman, AD, Vist, GE, Kunz, R, Falck-Ytter, Y, Alonso-Coello, P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
51. Cochran, WG. The combination of estimates from different experiments. Biometrics. (1954) 10:101–29. doi: 10.2307/3001666
52. Paint Maps. Pakistan map chart. (2024). Available online at: https://paintmaps.com/map-charts/174/Pakistan-map-chart (Accessed July 10, 2024).
53. Khattak, I, Mushtaq, M, Ahmad, M, Khan, M, and Haider, J. Zoonotic tuberculosis in occupationally exposed groups in Pakistan. J Occup Med. (2016) 66:371–6. doi: 10.1093/occmed/kqw039
54. More, SJ, and Good, M. Understanding and managing bTB risk: perspectives from Ireland. Vet Microbiol. (2015) 176:209–18. doi: 10.1016/j.vetmic.2015.01.026
55. Ehtisham-ul-Haque, S, Javed, MT, Ahmad, MZ, Ahmed, I, Rafique, MK, Irshad, I, et al. Monitoring the health status and herd-level Risk factors of tuberculosis in water buffalo (Bubalus bubalis) dairy farms in Pakistan. Pak Vet J. (2021) 41:552–6. doi: 10.29261/pakvetj/2021.051
56. Fareed, Z, Rana, A, Hadi, SA, Geluk, A, Hope, JC, and Khalid, H. A one health-focused literature review on bovine and zoonotic tuberculosis in Pakistan from the past two decades: challenges and way forward for control. One Health. (2024) 18:100763. doi: 10.1016/j.onehlt.2024.100763
57. Dean, AS, Forcella, S, Olea-Popelka, F, Idrissi, AE, Glaziou, P, Benyahia, A, et al. A roadmap for zoonotic tuberculosis: a one health approach to ending tuberculosis. Lancet Infect Dis. (2018) 18:137–8. doi: 10.1016/S1473-3099(18)30013-6
58. Srinivasan, S, Easterling, L, Rimal, B, Niu, XM, Conlan, AJ, Dudas, P, et al. Prevalence of bovine tuberculosis in India: a systematic review and meta‐analysis. Transbound Emerg Dis. (2018) 65:1627–40. doi: 10.1111/tbed.12915
59. Amemor, EA, Sackey, SO, Yebuah, N, Folitse, RD, Emikpe, BO, Afari, E, et al. The prevalence of tuberculosis in cattle and their handlers in north Tongu, Volta region, Ghana. Afr J Infect Dis. (2017) 11:12–7. doi: 10.21010/ajid.v11i1.2
60. Sichewo, PR, Etter, EMC, and Michel, AL. Prevalence of Mycobacterium bovis infection in traditionally managed cattle at the wildlife-livestock interface in South Africa in the absence of control measures. Vet Res Commun. (2019) 43:155–64. doi: 10.1007/s11259-019-09756-w
61. Mekonnen, GA, Conlan, AJ, Berg, S, Ayele, BT, Alemu, A, Guta, S, et al. Prevalence of bovine tuberculosis and its associated risk factors in the emerging dairy belts of regional cities in Ethiopia. Prev Vet Med. (2019) 168:81–9. doi: 10.1016/j.prevetmed.2019.04.010
62. Deen, S. Pakistan 2010 floods. Policy gaps in disaster preparedness and response. Int J Disaster Risk Reduct. (2015) 12:341–9. doi: 10.1016/j.ijdrr.2015.03.007
63. Floods, P. Preliminary damage and needs assessment. Asian Development Bank, Government of Pakistan, World Bank. (2010). Available online at: https://www.adb.org/sites/default/files (assessed December, 2010).
64. Bukhari, SI, and Rizvi, SH. Impact of 2010 floods on Pakistan’s Agriculture. J Environ Anal Toxicol. (2017) 7:1–4. doi: 10.4172/2161-0525.1000424
65. Abou-Zeina, HAA, Nasr, SM, Nassar, SA, Farag, TK, El-Bayoumy, MK, Ata, EB, et al. Beneficial effects of antioxidants in improving health conditions of sheep infected with foot-and-mouth disease. Trop Anim Health Prod. (2019) 51:2379–86. doi: 10.1007/s11250-019-01952-9
66. Cleaveland, S, Shaw, DJ, Mfinanga, SG, Shirima, G, Kazwala, RR, Eblate, E, et al. Mycobacterium bovis in rural Tanzania: risk factors for infection in human and cattle populations. Tuberculosis. (2007) 87:30–43. doi: 10.1016/j.tube.2006.03.001
67. Gumi, B, Schelling, E, Firdessa, R, Aseffa, A, Tschopp, R, Yamuah, L, et al. Prevalence of bovine tuberculosis in pastoral cattle herds in the Oromia region, southern Ethiopia. Trop Anim Health Prod. (2011) 43:1081–7. doi: 10.1007/s11250-010-9777-x
68. Tadeusz, H, and Bouazza, K. Control of bovine tuberculosis in the Kenitra district, Morocco; results, reflections and suggestions. Maghreb Vet. (1984) 68:13–16.
69. Eckstein, D, Hutfils, M-L, and Winges, M. Global climate risk index (2019). Available online at: https://www.burmalibrary.org/sites/burmalibrary.org/files (assessed December, 2018).
70. Khan, IA, Khan, A, Mubarak, A, and Ali, S. Factors affecting prevalence of bovine tuberculosis in nili ravi buffaloes. Pak Vet J. (2008) 28:155–158.
71. Vordermeier, M, Whelan, A, Ewer, K, Goodchild, T, Clifton-Hadley, R, Williams, J, et al. The BOVIGAM® assay as ancillary test to the tuberculin skin test. Gov Vet J. (2006) 171:229–44.
72. Akhtar, F, Javed, MT, Khan, MN, Akhtar, P, Hussain, SM, Aslam, MS, et al. The use of PCR technique in the identification of Mycobacterium species responsible for bovine tuberculosis in cattle and buffaloes in Pakistan. Trop Anim Health Prod. (2015) 47:1169–75. doi: 10.1007/s11250-015-0844-1
73. Ata, EB, Allam, AM, Elbayoumy, MK, and Mahmoud, MAE-F. Electron microscopy and phylogenetic analysis of Bovine papillomavirus infection in cattle from four Egyptian governorates. Trop Anim Health Prod. (2021) 53:160. doi: 10.1007/s11250-021-02607-4
74. Ata, EB, Mahmoud, MAE-F, and Madboli, AA. Molecular detection and immunopathological examination of Deltapapillomavirus 4 in skin and udder of Egyptian cattle. Vet World. (2018) 11:915–20. doi: 10.14202/vetworld.2018.915-920
75. Ali, S, Akhtar, R, Younus, M, Saleem, G, Nisa, QU, and Zahid, B. Comparative trends of bovine tuberculosis in cattle and buffalo population around Lahore, Pakistan. Eur J Environ Ecol. (2014) 1:7–11.
76. Javed, MT, Shahid, AL, Farooqi, FA, Akhtar, M, Cardenas, GA, Wasiq, M, et al. Risk factors associated with the presence of positive reactions in the SCCIT test in water buffalo around two cities in Punjab, Pakistan. Acta Trop. (2010) 115:242–7. doi: 10.1016/j.actatropica.2010.04.004
77. Ata, EB, Abdel-Aziz, TH, Abdel-Ghany, HSM, Elsawy, BSM, Abdullah, HHAM, Abouelsoued, D, et al. Molecular and serological diagnosis of the circulating Trypanosoma evansi in Egyptian livestock with risk factors assessment. Microb Pathog. (2024) 197:107073. doi: 10.1016/j.micpath.2024.107073
78. Ghumman, MA, Manzoor, AW, Naz, S, Ahmad, R, and Ahmad, R. Prevalence of tuberculosis in cattle and buffalo at various livestock farms in Punjab. Int J Vet Med Res Rep. (2013) 2013:1–4. doi: 10.5171/2013.145084
79. Winthrop, KL, Scott, J, Brown, D, Jay, M, Rios, R, Mase, S, et al. Investigation of human contacts: a Mycobacterium bovis outbreak among cattle at a California dairy. Int J Tuberc Lung Dis. (2005) 9:809–13.
80. Moda, G, Daborn, CJ, Grange, JM, and Cosivi, O. The zoonotic importance of Mycobacterium bovis. Int J Tuberc Lung Dis. (1996) 77:103–8. doi: 10.1016/S0962-8479(96)90022-2
81. Ullah, A, Sadique, U, Ayaz, S, Qureshi, MS, and Khan, FA. Bovine tuberculosis (bTB)-isolation and species-specific identification of Mycobacterium bovis from bovine raw milk in Pakistan. Sarhad J Agric. (2020) 36:489–98. doi: 10.17582/journal.sja/2020/36.2.489.498
82. Phillips, CJC, Foster, CRW, Morris, PA, and Teverson, R. The transmission of Mycobacterium bovis infection to cattle. Res Vet Sci. (2003) 74:1–15. doi: 10.1016/S0034-5288(02)00145-5
83. Michel, AL, Müller, B, and Van Helden, PD. Mycobacterium bovis at the animal–human interface: a problem, or not? Vet Microbiol. (2010) 140:371–81. doi: 10.1016/j.vetmic.2009.08.029
84. Quinn, PJ, Markey, BK, Leonard, FC, Hartigan, P, Fanning, S, and Fitzpatrick, E. Veterinary microbiology and microbial disease. UK: John Wiley & Sons (2011). 745 p.
85. Collins, ÁB, Floyd, S, Gordon, SV, and More, SJ. Prevalence of Mycobacterium bovis in milk on dairy cattle farms: an international systematic literature review and meta-analysis. Tuberculosis. (2022) 132:102166. doi: 10.1016/j.tube.2022.102166
86. De Bahir, DEE. Bovine pulmonary tuberculosis at Bahir Dar municipality abattoir, Ethiopia. J Anim Health Prod. (2008) 56:223–9. doi: 10.4314/bahpa.v56i3.43286
87. El Shanawany, E, Nassar, S, and Ata, E. Detection of Humoral and Cellular Immune Responses in Buffaloes Naturally Infected with Sarcocystosis with Risk Factor Assessment. Acta Vet Brno. (2019) 69:275–89. doi: 10.2478/acve-2019-0023
88. Kazwala, R, Kambarage, D, Daborn, C, Nyange, J, Jiwa, S, and Sharp, J. Risk factors associated with the occurrence of bovine tuberculosis in cattle in the southern highlands of Tanzania. Vet Res Commun. (2001) 25:609–14. doi: 10.1023/A:1012757011524
89. Tariq, A, Aslam, A, Tipu, Y, Ahmad, M, Sultan, R, and Anjum, A. A preliminary study on prevalence of bovine tuberculosis in cattle and buffalo in outskirts of Lahore, Pakistan. Wayamba J Anim Sci. (2024):1518–26.
90. Finlay, EK, Berry, DP, Wickham, B, Gormley, EP, and Bradley, DG. A genome wide association scan of bovine tuberculosis susceptibility in holstein-friesian dairy cattle. PLoS One. (2012) 7:30545. doi: 10.1371/journal.pone.0030545
91. Schuller-Levis, GB, and Park, E. Taurine and its chloramine: modulators of immunity. Neurochem Res. (2004) 29:117–26. doi: 10.1023/B:NERE.0000010440.37629.17
92. Leghari, A, Kamboh, AA, Lakho, SA, Khand, FM, Malhi, KK, Chandio, IB, et al. Prevalence and risk factors associated with bovine tuberculosis in cattle in hyderabad and tando allahyar districts, Sindh, Pakistan. Pak J Zool. (2020) 52:207. doi: 10.17582/journal.pjz/2020.52.1.207.212
93. Vordermeier, M, Ameni, G, Berg, S, Bishop, R, Robertson, BD, Aseffa, A, et al. The influence of cattle breed on susceptibility to bovine tuberculosis in Ethiopia. Comp Immunol Microbiol Infect Dis. (2012) 35:227–32. doi: 10.1016/j.cimid.2012.01.003
94. Romha, G, and Ameni, G. Assessment of bovine tuberculosis and its risk factors in cattle and humans, at and around Dilla town, southern Ethiopia. J Anim Vet Sci. (2014) 2:94–100. doi: 10.11648/j.avs.20140204.12
95. Wangmo, K, Gurung, RB, Choden, T, Letho, S, Pokhrel, N, Lungten, L, et al. Seroprevalence and risk factors associated with bovine tuberculosis in cattle in Eastern Bhutan. PLoS Negl Trop Dis. (2024) 18:e0012223. doi: 10.1371/journal.pntd.0012223
96. Qazi, IH, Lochi, GM, Mandan, AH, Shah, IA, Korejo, RA, Kalhoro, A, et al. Prevalence of bovine tuberculosis in rural areas of district Tando Allahyar. IJAVMS. (2024) 6:345–8. doi: 10.5455/ijavms.157
97. Hamed, YK, Nasr, E, Azooz, MF, and Youssef, HM. Prevalence and risk factors of bovine tuberculosis in dairy cattle farms in Egypt. Iraqi J Vet Sci. (2021) 35:351–9. doi: 10.33899/ijvs.2020.126850.1399
98. Griffin, JM, Martin, SW, Thorburn, MA, Eves, JA, and Hammond, RF. A case-control study on the association of selected risk factors with the occurrence of bovine tuberculosis in the Republic of Ireland. Prev Vet Med. (1996) 27:75–87. doi: 10.1016/0167-5877(95)00548-X
99. Pollock, JM, and Neill, DS. Mycobacterium bovis infection and tuberculosis in cattle. Vet J. (2002) 163:115–27. doi: 10.1053/tvjl.2001.0655
100. Van Rhijn, I, Godfroid, J, Michel, A, and Rutten, V. Bovine tuberculosis as a model for human tuberculosis: advantages over small animal models. Microbes Infect. (2008) 10:711–5. doi: 10.1016/j.micinf.2008.04.005
101. Ameni, G, Aseffa, A, Engers, H, Young, D, Hewinson, G, and Vordermeier, M. Cattle husbandry in Ethiopia is a predominant factor affecting the pathology of bovine tuberculosis and gamma interferon responses to mycobacterial antigens. Clin Vaccine Immunol. (2006) 13:1030–6. doi: 10.1128/CVI.00134-06
102. Dejene, SW, Heitkönig, IM, Prins, HH, Lemma, FA, Mekonnen, DA, Alemu, ZE, et al. Risk factors for bovine tuberculosis (bTB) in cattle in Ethiopia. PLoS One. (2016) 11:e0159083. doi: 10.1371/journal.pone.0159083
103. Islam, MN, Khan, MK, Khan, MFR, Kostoulas, P, Rahman, AA, and Alam, MM. Risk factors and true prevalence of bovine tuberculosis in Bangladesh. PLoS One. (2021) 16:e0247838. doi: 10.1371/journal.pone.0247838
104. Ghebremariam, M, Michel, AL, Nielen, M, Vernooij, J, and Rutten, VP. Farm‐level risk factors associated with bovine tuberculosis in the dairy sector in Eritrea. Transbound Emerg Dis. (2018) 65:105–13. doi: 10.1111/tbed.12622
105. Radostits, O, Blood, D, Gay, C, and Arundel, J. Veterinary Medicine: a text book of the diseases of cattle, sheep, pigs, goats and horses. London: Bailliere Tindall (1997).
106. Barlow, N, Kean, J, Hickling, G, Livingstone, P, and Robson, A. A simulation model for the spread of bovine tuberculosis within New Zealand cattle herds. Prev Vet Med. (1997) 32:57–75. doi: 10.1016/S0167-5877(97)00002-0
107. Sayin, Z, and Erganis, O. Diagnosis of bovine tuberculosis by PPD-ELISA and sonication-ELISA. Israel J Vet Med. (2013) 68:180–184.
108. Jalil, H, Das, P, and Suleman, A. Bovine tuberculosis in dairy animals at Lahore, threat to the public health. Metropolitan Corporation Lahore, Pakistan. (2003); 11:1–11.
109. Arshad, M, Ifrahim, M, Ashraf, M, Rehman, S, and Khan, H. Epidemiological studies on tuberculosis in buffalo population in villages around Faisalabad. JAPS. (2012) 22:246–9.
110. Bonsu, O, Laing, E, and Akanmori, B. Prevalence of tuberculosis in cattle in the dangme-west district of Ghana, public health implications. Acta Trop. (2000) 76:9–14. doi: 10.1016/S0001-706X(00)00082-6
111. Javed, MT, Usman, M, Irfan, M, and Cagiola, M. A study on tuberculosis in buffaloes: some epidemiological aspects, along with hematological and serum protein changes. Vet Arh. (2006) 76:193–206.
112. Hussain, R, Javed, MT, Khan, A, and Muhammad, G. Risks factors associated with subclinical mastitis in water buffaloes in Pakistan. Trop Anim Health Prod. (2013) 45:1723–9. doi: 10.1007/s11250-013-0421-4
113. Waters, WR, Vordermeier, HM, Rhodes, S, Khatri, B, Palmer, MV, Maggioli, MF, et al. Potential for rapid antibody detection to identify tuberculous cattle with non-reactive tuberculin skin test results. BMC Vet Res. (2017) 13:164–7. doi: 10.1186/s12917-017-1085-5
114. McCallan, L, Brooks, C, Barry, C, Couzens, C, Young, FJ, McNair, J, et al. Serological test performance for bovine tuberculosis in cattle from herds with evidence of on-going infection in Northern Ireland. PLoS One. (2021) 16:e0245655. doi: 10.1371/journal.pone.0245655
115. Katale, BZ, Mbugi, EV, Karimuribo, ED, Keyyu, JD, Kendall, S, Kibiki, GS, et al. Prevalence and risk factors for infection of bovine tuberculosis in indigenous cattle in the serengeti ecosystem, Tanzania. BMC Vet Res. (2013) 9:1–11. doi: 10.1186/1746-6148-9-267
116. Ashford, D, Whitney, E, Raghunathan, P, and Cosivi, O. Epidemiology of selected mycobacteria that infect humans and other animals. Sci Tech Rev. (2001) 20:325–37. doi: 10.20506/rst.20.1.1266
117. Wray, C. Survival and spread of pathogenic bacteria of veterinary importance within the environment. Int Bull Vet Med. (1975) 45:543–550.
118. Kemal, J, Sibhat, B, Abraham, A, Terefe, Y, Tulu, KT, Welay, K, et al. Bovine tuberculosis in eastern Ethiopia: prevalence, risk factors and its public health importance. BMC Infect Dis. (2019) 19:39–9. doi: 10.1186/s12879-018-3628-1
119. Braam, DH, Chandio, R, Jephcott, FL, Tasker, A, and Wood, JL. Disaster displacement and zoonotic disease dynamics: the impact of structural and chronic drivers in Sindh, Pakistan. PLOS Glob Public Health. (2021) 1:0000068. doi: 10.1371/journal.pgph.0000068
120. Mshelia, I, Atsanda, N, Bitrus, A, Adam, B, Fika, I, Balami, S, et al. Retrospective study of selected endemic viral diseases of poultry diagnosed in Maiduguri North-Eastern Nigeria. J Anim Health Prod. (2016) 4:60–4. doi: 10.14737/journal.jahp/2016/4.2.60.64
121. Winkler, B, and Mathews, F. Environmental risk factors associated with bovine tuberculosis among cattle in high-risk areas. Biol Lett. (2015) 11:20150536. doi: 10.1098/rsbl.2015.0536
122. Chauhan, AS, George, MS, Lindahl, J, Grace, D, and Kakkar, M. Community, system and policy level drivers of bovine tuberculosis in smallholder periurban dairy farms in India: a qualitative enquiry. BMC Public Health. (2019) 19:1–10. doi: 10.1186/s12889-019-6634-3
123. Jalil, H. Bovine tuberculosis in Dairy Animals at Lahore, Threat to the Public Health. Metropolitan Corporation Lahore, Pakistan. United Kingdom: Priory Lodge Education Ltd. (2003);11:1–11.
124. Khan, IA, and Khan, A. Prevalence and risk factors of bovine tuberculosis in Nili Ravi buffaloes in the Punjab, Pakistan. Ital J Anim Sci. (2007) 6:817–20. doi: 10.4081/ijas.2007.s2.817
125. Mumtaz, N, Chaudhry, ZI, Mahmood, N, and Shakoori, AR. Reliability of PCR for detection of bovine tuberculosis in Pakistan. Pak J Zool. (2008) 40:347–51.
126. Javed, MT, Farooqi, AF, and Ullah, H. Epidemiological basis of bovine tuberculosis in buffaloes. Pak J Zool. (2009) 9:417–20.
127. Khan, A, Chaudhry, ZI, Shakoori, AR, Mahmood, N, Ijaz, M, Khan, MZU, et al. Detection of Mycobacterium bovis in buffaloes blood through polymerase chain reaction (PCR) and tuberculin test. JAPS. (2012) 22:237–41.
128. Tipu, MY, Chaudhary, ZI, Younus, M, and Rabbani, M. A cross sectional study of Mycobacterium bovis in dairy cattle in and around Lahore city, Pakistan. Pak J Zoo. (2012) 44:393–8.
129. Javed, MT, Wasiq, M, Farooqi, FA, Shahid, AL, Kausar, R, and Cagiola, M. Brief communication (Original). Certain risk factors associated with positive SCCIT test for tuberculosis in cattle at two cities in Pakistan. Asian Biomed. (2013) 7:267–74. doi: 10.5372/1905-7415.0702.175
130. Mahmood, F, Khan, A, Hussain, R, and Khan, IA. Molecular based epidemiology of bovine pulmonary tuberculosis–a mortal foe. Pak Vet J. (2014) 34:185–8.
131. Waqas, A, Javed, MT, Ashfaque, K, and Mehwish, Q. An Abattoir Based Study on Brucellosis, Bovine Tuberculosis and Paratuberculosis in Buffaloes and Cattle at Faisalabad, Pakistan. Int J Vet Health Sci Res. (2015) 3:34–8.
132. Aslam, MS, Javed, MT, Khan, A, and Iqbal, Z. Bacterial and PCR based diagnosis of naturally occurring bovine tuberculosis in cattle and buffaloes. Pak J Agric Sci. (2019) 56:481–7. doi: 10.21162/PAKJAS/19.6608
133. Zahoor, M. Y. A cross-sectional study of bovine tuberculosis and its associated zoonotic risk factors in district Bahawalnagar, Punjab, Pakistan. Board of Reviewing Editors. Bangkok, Thailand. (2021);51:192–193.
134. Azam, A, Younas, U, Husna, A, Ullah, N, Ali, Q, and Akhter, S. Hematological studies among bovine tuberculosis suspected herds of cattle in suburb of Islamabad, Pakistan. Wayamba J Anim Sci. (2014) 6:921–6.
135. Khan, J, Ayaz, S, AbdElsalam, NM, Ullah, RR, and Shah, T. Prevalence of tuberculosis in buffalo and cattle. J Pure Appl Microbiol. (2014) 8:721–6.
136. Basit, A, Hussain, M, Ayaz, S, Shahid, M, Rahim, K, Ahmad, I, et al. Isolation and identification of Mycobacterium bovis and Mycobacterium tuberculosis from animal tissues by conventional and molecular method. Indian J Anim Res. (2015) 49:687–93. doi: 10.18805/ijar.5583
137. Noorrahim, MSK, Shahid, M, Shah, A, Shah, M, and Rafiullah, HA. Prevalence of tuberculosis in livestock population of district Charsadda by Tuberculin Skin Test (TST). J Entomol Zool Stud. (2015) 2:15–9.
138. Khattak, I, Mushtaq, MH, Ahmad, MUD, Khan, MS, Chaudhry, M, and Sadique, U. Risk factors associated with Mycobacterium bovis skin positivity in cattle and buffalo in Peshawar, Pakistan. Trop Anim Health Prod. (2016) 48:479–85. doi: 10.1007/s11250-015-0976-3
139. Nawaz, S., Qureshi, M. S., Khan, F. M., and Islam, Z. Prevalence and epidemiological parameters of bovine tuberculosis in cattle and buffaloes in district Peshawar, Pakistan : International Network for Natural Sciences. (2017).
140. Basit, A, Hussain, M, Shahid, M, Ayaz, S, Rahim, K, Ahmad, I, et al. Occurrence and Risk Factors Associated with Mycobacterium tuberculosis and Mycobacterium bovis in Milk Samples from North East of Pakistan. Pak Vet J. (2018) 38:199–203. doi: 10.29261/pakvetj/2018.038
141. Ullah, A, Khattak, US, Ayaz, S, Qureshi, MS, Khan, I, Jan, IU, et al. Bovine tuberculosis (bTB): prevalence and associated risk factors in large ruminants in the central zone of Khyber Pakhtunkhwa, Pakistan. Pak J Zool. (2019) 51:127–33. doi: 10.17582/journal.pjz/2019.51.1.127.133
142. Batool, BT, Tareen, A, Ahmed, SS, Ejaz, H, Kakar, MA, Rehman, SA, et al. Prevalence of zoonotic tuberculosis and brucellosis in animals of Quetta and Pishin Districts, Balochistan. Pak J Zool. (2017) 49:363–5. doi: 10.17582/journal.pjz/2017.49.1.sc4
143. Mujeeb-ur-Rahman Memon, AL, Bhutto, MGS, Baloch, J, Leghari, RA, and Soomro, SA. 4. Prevalence and pathological lesions of bovine tuberculosis assessment through routine procedures of meat inspection in infected cattle in Karachi metropolitan corporation abattoirs. Pure Appl Biol. (2019) 8:1909–18. doi: 10.19045/bspab.2019.80134
144. Qazi, IH, Lochi, GM, Mandan, AH, Shah, IA, Korejo, RA, Kalhoro, A, et al. Prevalence of Bovine Tuberculosis in Rural Areas of District Tando Allahyar. IJAVMS. (2012) 6:345–8.
145. Leghari, A, Kamboh, AA, Dewani, P, Abro, SH, Umrani, AP, Malhi, KK, et al. Isolation of Mycobacterium bovis from milk and nasal discharge samples of cattle from Hyderabad and Tando Allahyar districts. J Anim Health Prod. (2016) 4:105–10. doi: 10.14737/journal.jahp/2016/4.4.105.110
146. Memon, MR, Bhutto, AL, Khatri, P, Shah, MG, and Memon, MI. Prevalence and risk factor analysis of bovine tuberculosis in bovine population in Karachi. Pakistan J Anim Health Prod. (2017) 5:44–9. doi: 10.17582/journal.jahp/2017/5.2.44.49
147. Malhi, KK, Kamboh, AA, Dewani, P, Kumar, C, Abro, SH, Leghari, A, et al. Prevalence of bovine tuberculosis in buffaloes in Hyderabad and Tando Allahyar districts of Sindh province, Pakistan. Buffalo Bull. (2018) 37:545–58. doi: 10.18805/ijar.B-931
148. Memon, MR, Bhutto, AL, Memon, MI, Khatri, P, and Baloch, JA. Prevalence of Bovine tuberculosis in slaughtering animals at selected municipal slaughter houses: its impact on public health: Department of Veterinary Medicine, Sindh Agriculture University, Tandojam, Pakistan. Pak J Agric Agric Eng Vet Sci. (2018) 34:168–75.
Keywords: Pakistan, prevalence, raw milk, bovine tuberculosis, zoonosis
Citation: Sehrish S, Liu X-T, Lou W-B, Zhang S-Y, Ata EB, Yang G-G, Wang Q, Zeng F-L, Leng X, Shi K, Azeem R-M, Gong Q-L, Song Y-H and Du R (2025) Prevalence of tuberculosis in bovines in Pakistan during 2000–2024: a systematic review and meta-analysis. Front. Vet. Sci. 12:1525399. doi: 10.3389/fvets.2025.1525399
Edited by:
Zulqarnain Baloch, Kunming University of Science and Technology, ChinaReviewed by:
Sukolrat Boonyayatra, Long Island University, United StatesLerato Mabe, Council for Scientific and Industrial Research (CSIR), South Africa
Copyright © 2025 Sehrish, Liu, Lou, Zhang, Ata, Yang, Wang, Zeng, Leng, Shi, Azeem, Gong, Song and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu-Ying Zhang, emhhbmdzaHV5aW5nMTIwMUAxNjMuY29t; Qing-Long Gong, Z29uZ3Fpbmdsb25nMTAwMUAxNjMuY29t; Rui Du, ZHVydWkxOTcxMDFAc2luYS5jb20=
†These authors have contributed equally to this work and share first authorship
 Siddique Sehrish1†
Siddique Sehrish1† Emad Beshir Ata
Emad Beshir Ata Ge-Gui Yang
Ge-Gui Yang Qi Wang
Qi Wang Fan-Li Zeng
Fan-Li Zeng Xue Leng
Xue Leng Kun Shi
Kun Shi Qing-Long Gong
Qing-Long Gong Yu-Hao Song
Yu-Hao Song Rui Du
Rui Du