- 1State Engineering Technology Institute for Karst Desertification Control, School of Karst Science, Guizhou Normal University, Guiyang, China
- 2Key Laboratory for Information System of Mountainous Areas and Protection of Ecological Environment, Guizhou Normal University, Guiyang, China
- 3College of Animal Science and Technology, Hunan Biological and Electromechanical Polytechnic, Changsha, China
The intestinal tract is essential for the overall health and productivity of animals, including poultry. Clostridium butyricum (C. butyricum) is a probiotic bacterium that has been shown to be a promising candidate for improving intestinal function and subsequently optimizing poultry growth. The beneficial effects of C. butyricum on intestinal health can be attributed to several key mechanisms. First, it helps maintain the balance of the intestinal microbiota by inhibiting the growth of harmful bacteria and promoting the proliferation of beneficial bacteria. This microbial homeostasis is essential for efficient nutrient digestion and absorption. Second, C. butyricum enhances the integrity of the intestinal barrier. It enhances the integrity of epithelial tight junctions, reducing the permeability of the intestinal mucosa and preventing the invasion of pathogenic substances. Furthermore, C. butyricum participates in the regulation of immune responses within the intestinal environment. It stimulates the production of immunoglobulins and cytokines, enhancing the immune defense mechanisms of the host. Additionally, C. butyricum influences the metabolism of nutrients in the intestine. It promotes the synthesis of short-chain fatty acids (SCFAs), which provide an energy source for intestinal cells and contribute to maintaining a healthy intestinal environment. Intestinal health is the basis of animal growth, and C. butyricum ultimately enhances production performance in poultry by regulating intestinal health. Studies have demonstrated that the administration of C. butyricum leads to improved feed conversion efficiency, increased weight gain, and enhanced overall production performance in poultry. However, further research is still needed to fully elucidate the complex interactions and precise molecular mechanisms underlying its beneficial effects. Understanding these mechanisms in detail will not only provide important insights for improving poultry production efficiency but also contribute to the development of more effective and sustainable strategies in the poultry industry.
Introduction
Poultry husbandry not only serves as a valuable source of protein for human consumption but also plays a crucial role in agricultural production, contributing significantly to agricultural economics and food security (1). Consequently, enhancing the efficiency of poultry production is vital for enhancing yields, lowering costs, and promoting sustainability. Current strategies to improve the efficiency of poultry production include genetic improvement and nutritional management (2, 3). Among these, the pivotal role of nutritional strategies in maximizing poultry productivity and promoting poultry health has received significant attention, as it addresses the increasing consumer demand for higher quality and safer food products (4, 5).
The intestinal tract of poultry is essential for nutrient digestion, absorption, and defense against pathogens (6). The intestinal health of poultry is closely associated with their growth, as a healthy gut is essential for enabling poultry to fully exploit nutrients in feedstuffs while maintaining normal growth patterns alongside resisting disease invasion (7–9). Therefore, maintaining the intestinal health of poultry is very important to improve the efficiency of poultry production. However, various factors including feed quality, housing environment conditions, and stress can all contribute to microbiota imbalance within the gut, malfunctioning intestinal barriers, and abnormal morphological changes within the intestines of poultry, resulting in adverse effects on their health and growth performance (10–13). Antibiotics have traditionally been used as growth promoters and gut health regulators in poultry. However, in recent years, with increasing emphasis on food safety and environmental protection, the pressing need for alternatives to antibiotics in livestock and poultry farming has emerged due to issues stemming from antibiotic misuse such as antibiotic resistance emergence, drug residues, and environmental pollution (14–16).
Probiotics, beneficial microorganisms, show significant potential in livestock and poultry farming (17–22). Previous studies have demonstrated that probiotics can inhibit bacterial infection, in-crease antioxidant capacity, alleviate toxicity and improve intestinal health in poultry production (23–26). The probiotics used in poultry production mainly include Bacillus (27), Lactobacillus (28), Bifidobacterium (29), and Enterococcus (30). Among these, Bacillus is more advantageous in regulating intestinal health and enhancing the growth performance of poultry as an alternative to antibiotics, due to its ability to produce resistance endospores, which confer heat resistance during feed granulation, low pH resistance in the stomach, and storage stability at ambient temperatures (22, 31, 32). Clostridium butyricum (C. butyricum) is a gram-positive obligate anaerobic bacterium belonging to Bacillus family and Clostridium genus that can produce butyric acid and lactic acid. It has the characteristics of Bacillus that can produce spores, making it resistant to heat, stomach acid, and bile salts, essential for its application in feed production (17, 33). Clostridium butyricum has attracted significant attention as a crucial probiotic due to its role in preserving gut health and enhancing the growth performance of poultry (23, 27, 34–36). This is attributed to the ability of C. butyricum to modulate intestinal flora balance and bolster immune function, while enhances nutrient absorption by producing diverse enzymes and vitamins (23, 36–38), thereby improving the growth performance of poultry. With its distinct biological characteristics and beneficial functions, C. butyricum has emerged as a potential alter-native to antibiotics.
However, Differences in digestion among poultry species, such as chickens, ducks, and geese, may influence the effects and mechanisms of C. butyricum. Therefore, although the application of C. butyricum in poultry production has received extensive attention, it is necessary to review its application effects on different poultry species. In this context, it is of great theoretical and practical significance to conduct indepth research on the effects of C. butyricum on the health of poultry intestines and its role in promoting growth performance. By understanding how C. butyricum works, we can offer more scientific and rational breeding strategies for the poultry sector, reducing reliance on antibiotics, enhancing breeding efficiency, and ensuring the quality and safety of poultry products to meet consumer demand for healthy food. This review aims to summarize the current understanding of how C. butyricum benefits poultry growth performance by regulating intestinal health.
Clostridium butyricum promotes intestinal health of poultry
The intestinal health of poultry is a complex and critical aspect of poultry production. A healthy intestinal system is essential for efficient nutrient digestion and absorption, immune function, and pathogen resistance (39). In actual production, poultry often face a variety of stressors, including heat stress (40), Eimeria spp. infection (41), Clostridium perfringens (C. perfringens) infection (42), mycotoxins (43), and other harmful effects, which can damage the structure of intestinal mucosa and intestinal barrier function. In recent years, the use of probiotics, such as C. butyricum, has gained attention as a potential strategy to enhance intestinal health and improve the performance of poultry (35–38).
Clostridium butyricum promotes intestinal development
Clostridium butyricum has been shown to have positive effects on the morphology and integrity of the intestinal mucosa in poultry including broiler chickens (38), laying hens (44), ducks (36), and goose (45). It promotes the development of villi, increases the intestinal villus height (VH), decreases the crypt depth (CD), and increases the VH-to-CD ratio (VCR) (38, 45–47). For instance, Liu et al. (36) showed that dietary C. butyricum supplementation significantly increased intestinal VH of Pekin ducks at 42 days. Obianwuna et al. (44) showed that dietary addition of C. butyricum (C. butyricum zlc-17, and C. butyricum lwc-13) significantly increased the VH, villus width, surface area, and VCR, and significantly reduced the CD of laying hens. Yu et al. (45) showed that dietary supplemented with C. butyricum significantly increased duodenal weight and absolute weight (weight/length), and significantly increased ileum VH of geese. Xu et al. (46) showed that C. perfringens destroys intestinal structure and deepens intestinal CD in broilers, while C. butyricum can improve intestinal morphological structure and decrease the CD. These structural improvements enhance the absorptive capacity of the intestine and facilitate better nutrient uptake.
The promoting effect of C. butyricum on intestinal morphology is primarily attributed to its strong capacity for butyric acid production, which serves as a direct energy source for the growth of intestinal villi, plays a pivotal nutritional role in the development of intestinal villi, and facilitates the repair of damaged intestinal epithelial cells (17, 48). Additionally, it is associated with its strong ability to promote the secretion of intestinal digestive enzymes, such as alkaline phosphatase, amylase, protease, trypsin, and lipase (38, 49, 50), which assist in the degradation of starch, fat, protein and anti-nutrient factors, and thus improve the digestion and absorption efficiency of nutrients. Therefore, the addition of C. butyricum to poultry diets can positively impact the development of the intestinal tract in poultry by enhancing intestinal morphology and structure, as well as stimulating the secretion of digestive enzymes. The inclusion of C. butyricum in poultry diets may therefore lead to overall enhancements in poultry health and productivity.
Clostridium butyricum enhances intestinal antioxidant capacity
Oxidative stress is a state of imbalance between the overproduction of reactive oxygen species (ROS) within the body and an inadequate function of the antioxidant defense system (51–53). ROS, such as superoxide anion, hydrogen peroxide, and hydroxyl radical, can cause lipid peroxidation, protein oxidation, and DNA damage (54). Malonaldehyde (MDA) is the main product of lipid peroxidation, protein carbonyl (PCO) is a marker of oxidative damage of protein, and 8-hydroxy-2 ‘-deoxyguanosine (8-OHdG) is a marker of oxidative damage of nucleic acid, which are generally considered as the markers of oxidative damage (55, 56). To counteract the harmful effects of ROS, cells possess a complex antioxidant defense system. This includes enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px), which convert ROS into less reactive species. Non-enzymatic antioxidants like vitamins C and E, glutathione, and carotenoids scavenge ROS and prevent their accumulation (57). The balance between ROS generation and antioxidant defense is critical for maintaining cellular homeostasis.
Clostridium butyricum is a beneficial bacterium that has been shown to exert multiple beneficial effects, including antioxidant function on the intestinal of poultry (45, 50). Studies have demonstrated that supplementation with C. butyricum can reduce the levels of oxidative stress markers in the intestinal tissue of poultry. For example, Xiang et al. (58) demonstrated that feeding a diet containing C. butyricum reduced ROS levels in the ileum (4.12 U/mL vs. 3.57 U/mL) and cecum (3.81 U/mL vs. 3.08 U/mL), and serum MDA levels (16.82 nmol/mL vs. 13.40 nmol/mL) of laying hens. Yu et al. (45) showed that the combination of C. butyricum and Bacillus subtilis (B. subtilis) significantly increased SOD levels in jejunum and T-AOC and GSH-Px levels in ileum of geese. Li et al. (50) demonstrated that supplementation of C. butyricum to broilers led to a significant increase in serum SOD (83.85 U/mL vs. 90.90 U/mL at 21 days; 117.2 U/mL vs. 128.1 U/mL at 42 days) and total antioxidant capacity (T-AOC; 0.68 U/mL vs. 0.71 U/mL at 42 days), as well as a notable reduction in MDA levels (4.15 nmol/mL vs. 3.67 nmol/mL at 42 days). Additionally, there was a significant upregulation in the mRNA expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and SOD1 in the jejunum mucosa (50). Nrf2 is a kind of transcription factor that closely related to cellular redox balance, which helps cells remain stable in response to oxidative stress. Under normal conditions, Nrf2 binds with Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm, where Keap1 promotes the ubiquitination and degradation of Nrf2 by the proteasome (51). When cells experience oxidative stress, ROS modify cysteine residues on Keap1, causing Nrf2 to dissociate from Keap1. The released Nrf2 then enters the nucleus, forms a heterodimer with small Maf proteins, and binds to the antioxidant response elements (ARE) in the promoter regions of target genes, thereby activating the transcription of downstream antioxidant genes, such as phase II metabolic enzymes (Heme Oxygenase-1 (HO-1) and NAD(P)H:Quinone Oxidoreductase 1 (NQO1)), and antioxidant enzymes (such as SOD, CAT, and GSH-Px) (51, 59). These antioxidant enzymes can eliminate ROS, reduce oxidative damage and maintain the redox balance of cells. It means that C. butyricum alleviates intestinal oxidative damage in poultry by activating the Nrf2 signaling pathway. Clostridium butyricum enhances intestinal mucosal barrier function.
The intestinal mucosal barrier in poultry is a complex and dynamic structure composed of multiple layers and components. The epithelial layer, which consists of enterocytes, goblet cells, and Paneth cells, forms the first line of defense (39). Tight junctions between enterocytes regulate the paracellular passage of substances and maintain the integrity of the physical barrier (22). The mucus layer secreted by goblet cells acts as a chemical barrier and traps pathogens (22). Therefore, the intestinal mucosal barrier, composed of a physical barrier and a chemical barrier, is very important for intestinal health. In production practice, poultry is susceptible to pathogens, such as Escherichia coli (E. coli) (38), Salmonella (60), and C. perfringens (46, 61), which can increase intestinal permeability and damage the intestinal mucosal barrier, resulting in significant economic losses in poultry farming. For instance, Zhang et al. (38) demonstrated that broilers exhibited a significant increase in serum endotoxin (0.252 EU/mL vs. 0.380 EU/mL at 21 days) and diamine oxidase (DAO; 0.819 U/mL vs. 3.952 U/mL at 21 days) levels following E. coli infection, indicating heightened intestinal permeability. Xu et al. (46) showed that necrotizing enteritis (NE) caused by C. perfringens infection led to reduced conductivity and short-circuit current values, along with increased fluorescein isothiocyanate–dextran flux, indicating heightened intestinal permeability in broilers. Furthermore, C. perfringens significantly downregulated the expression of Zonula Occludens-1 (ZO-1), Claudin-1, Occluding, and mucin 2 (MUC-2) genes in the small intestine of broilers, indicating that C. perfringens seriously damaged intestinal mucosal barrier function of broilers.
Numerous studies have shown that C. butyricum has a positive regulatory effect on the intestinal mucosal barrier of poultry (38, 46, 60, 61). Clostridium butyricum regulates the intestinal mucosal barrier of poultry mainly through the following ways:
Firstly, C. butyricum reduces intestinal permeability of poultry. Intestinal permeability, defined as the controlled passage of molecules across the intestinal epithelium, is a key determinant of barrier functions (17). An increased intestinal permeability can lead to increased translocation of pathogens and toxins, resulting in inflammation, impaired growth performance, and higher susceptibility to diseases (62). Therefore, understanding the factors that influence intestinal permeability and identifying effective strategies to maintain its integrity are of utmost importance in the poultry industry. Clostridium butyricum is a potential probiotic, and previous studies have provided convincing evidence that C. butyricum has a positive effect on intestinal permeability in poultry. For instance, Zhang et al. (38) demonstrated that the dietary inclusion of C. butyricum led to a notable reduction in endotoxin (0.380 EU/mL vs. 0.0.304 EU/mL at 21 days) and DAO levels (3.952 U/mL vs. 2.419 U/mL at 21 days), suggesting an amelioration of the intestinal mucosal damage caused by E. coli. Xu et al. (46) showed that dietary addition with C. butyricum led to increased conductivity and short-circuit current values, along with a decreased fluorescein isothiocyanate–dextran flux in intestinal of broilers infection with C. perfringens, which means that C. butyricum decreased intestinal permeability in broilers. A decrease in intestinal permeability means an enhanced intestinal barrier function, which can more effectively prevent harmful substances (such as bacteria, toxins, and incompletely digested proteins) from entering the bloodstream while promoting the absorption of nutrients, improving feed conversion rates and reducing mortality (63, 64).
Secondly, C. butyricum enhances the expression of intestinal tight junction proteins in poultry. Tight junctions, including ZO-1, Claudin-1, Occluding, are essential components of the intestinal mucosal barrier that protect against harmful pathogens (22). Previous studies have shown that C. butyricum can enhance the expression of intestinal tight junction protein in poultry, so as to maintain the integrity of the physical barrier and ensure its normal function (36, 60, 61, 65–67). For instance, Zhao et al. (60) showed that treatment with C. butyricum significantly upregulated the expression of intestinal ZO-1 gene in Salmonella enteritis-infected broilers (6 days post-infection) and in Chicken intestinal epithelial cells (IECs) after 6 h post-infection with Salmonella enteritis. Li et al. (65) showed that dietary supplemented with C. butyricum (1 × 109 cfu/kg) for 42 days significantly upregulated the expression of claudin-1 and ZO-1 mRNA in ileum of broilers.
Thirdly, C. butyricum stimulates the differentiation of intestinal goblet cells and promote the secretion of intestinal mucins (MUCs) (60, 68). For example, Liu et al. (36) showed that feeding 200 mg/kg, 400 mg/kg and 600 mg/kg C. butyricum for 42 days could significantly increase intestinal MUC2 gene expression in Pekin ducks. Zhao et al. (60) showed that C. butyricum could alleviate intestinal damage of Salmonella enteritis-infected broilers by increasing the expression of MUC-2 mRNA. Xu et al. (68) showed that broilers fed with C. butyricum (1 × 109 cfu/kg) for 28 days significantly increased the number, length and density of goblet cells, as well as upregulated the expression of MUC-2 mRNA in in ileum of broilers.
Clostridium butyricum strengthens intestinal immune function and alleviates intestinal inflammation
The intestinal epithelium serves as not only a mechanical barrier in the intestine but also as one of the most crucial innate immune barriers. It plays a vital role in maintaining intestinal immune function by producing immune-related molecules and participating in the interaction of immune cells (22, 69). Within the intestinal epithelial mucosa, the lamina propria includes Peyer’s patches (PP), immune cells, antimicrobial peptides and secretory immunoglobulin A (sIgA), which are involved in protecting the host from invasion by foreign pathogens and maintaining the stability of the intestinal environment (22, 69–71). Many factors, such as heat stress (72) and pathogen infection (73), can cause intestinal inflammatory damage in poultry. Inflammatory response has a complex regulatory mechanism, and excessive inflammatory response will cause ir-reversible damage to the body. A large number of studies have confirmed that C. butyricum has an alleviating effect on intestinal inflammatory damage in poultry (38, 50, 65, 74).
When the intestinal mucosal barrier is damaged, Gram-negative bacteria may invade the intestinal mucosa, but the widespread presence of sIgA, an immunoglobulin secreted by the intestinal mucosal plasma cells, can effectively resist its invasion and destruction by interacting with these bacteria, thus playing a role in protecting the intestinal mucosal barrier (75, 76). Clear evidence supports the fact that C. butyricum can enhance the secretion of sIgA in the intestines of various animals, including rabbits (48, 77) and broiler chickens (74, 78). This suggests that one of the mechanisms by which C. butyricum regulates the intestinal immune barrier in poultry is by promoting the secretion of sIgA, which is particularly important in poultry, as intestinal health is directly linked to overall growth performance and disease resistance.
Anti-inflammatory cytokines such as interleukin (IL)-10 and transforming growth factor β (TGF-β) have been shown to downregulate pro-inflammatory cytokine production and promote tissue repair and homeostasis (79–81). By enhancing the production of anti-inflammatory cytokines, C. butyricum plays a crucial role in mitigating excessive inflammatory responses within the intestinal tract (46). This modulation helps maintain a balanced immune environment, which is essential for the overall health and productivity of poultry. For instance, Xu et al. (46) showed that infection with C. perfringens significantly induced intestinal inflammatory response in broilers, and dietary supplementation with C. butyricum alleviated intestinal inflammatory damage by promoting the secretion of anti-inflammatory factors IL-10 and TGF-β. Similarly, Huang et al. (61) showed that C. butyricum promoted intestinal IL-10 secretion in broilers with necrotizing enteritis caused by C. perfringens. At the same time, C. butyricum also had inhibitory effect on proinflammatory cytokines such as IL-1β, IL-6 and tumor necrosis factor α (TNF-α) in poultry intestines (60, 82). For instance, Zhao et al. (60) and Zhang et al. (83) showed that C. butyricum significantly inhibited the production of pro-inflammatory cytokines, such as IL-1β, IL-6, IL-8, interferon (IFN-γ) and TNF-α, thus alleviating the intestinal inflammatory damage caused by Salmonella in broilers. Similarly, Li et al. (65) showed that C. butyricum significantly decreased the expression levels of pro-inflammatory cytokines (IL-1β and TNF-α) to mitigate high stocking density-induced intestinal inflammatory damage in broilers. Although the immunomodulatory mechanisms of C. butyricum are general, their effects may be species-specific. That’s because the composition of the intestinal microbiota varies among different poultry breeds, due to the differences in genetic background, physiological characteristics and rearing methods (84), which can affect the colonization of exogenous microorganisms (such as C. butyricum) in the intestinal tract. For example, a certain type of C. butyricum may be more easily established in specific poultry breeds, producing more butyrate and thus exerting stronger immune-modulatory effects (85).
Studies have shown that C. butyricum can interact with related inflammatory signaling pathways to mitigate inflammatory pathological damage of poultry. For example, C. butyricum can inhibit toll-like receptor 4 (TLR4) signaling pathway, thus playing a protective role in Salmonella-induced intestinal inflammation of broilers (60, 86). Activation of nuclear factor-kappa B (NF-κB) signaling pathway induces the secretion of IL-1β, IL-8, TNF-α and IFN-γ and aggravates inflammation (87, 88). However, by inhibiting NF-κB signaling pathway, C. butyricum can reduce the levels of pro-inflammatory cytokines (IFN-γ, IL-1β, IL-6, IL-8, TNF-α), thereby reducing the inflammatory response of poultry (38, 60, 65, 86). For instance, Li et al. (50) showed that C. butyricum alleviated intestinal inflammatory response in broilers by inhibiting the activation of NF-κB, which was manifested as decreased expression of TNF-α and increased expression of anti-inflammatory cytokine IL-10. Zhao et al. (60, 86) showed that C. butyricum decreased the expression of pro-inflammatory factors (IFN-γ, IL-1β, IL-8, TNF-α) by inhibiting the TLR4-myeloid differentiation factor 88 (MyD88)-NF-κB pathway, thereby alleviating the intestinal inflammatory response of broilers caused by Salmonella infection. In summary, C. butyricum can reduce the expression of pro-inflammatory factors (TNF-α, IL-1β, IL-6 and IL-8), and promote the secretion of anti-inflammatory factors (IL-10 and TGF-β1) and immunoglobulin (sIgA) by inhibiting the TLR4-MyD88-NF-κb pathway, thereby reducing intestinal inflammation in poultry.
Clostridium butyricum regulates intestinal microbial balance
The intestinal microbiota of poultry is a complex ecosystem that consists of a diverse array of microorganisms, along with their metabolites, which form the intestinal microbial barrier (17). The intestinal microbiota of poultry is predominantly composed of Firmicutes, Bacteroides and Proteobacteria, which primarily inhabit the intestinal mucosa surface and play a pivotal role in intestinal nutrient metabolism, permeability, and immunity (89, 90). Under normal physiological conditions, the probiotics and pathogenic bacteria in the intestinal environment of poultry are in a dynamic balance state, but this balance is easily affected by exogenous and endogenous factors (91, 92), which would lead to a variety of diseases and cause great economic damage to poultry production. Thus, the maintenance of intestinal microbial dynamic balance is of great significance for promoting healthy poultry breeding.
Clostridium butyricum have significant effect in regulating the intestinal flora of poultry. It can stimulate the growth of beneficial bacteria (such as Lactobacillus and bifidobacterium) inherent in the intestinal tract of poultry, and inhibit the reproduction of harmful bacteria (Such as Salmonella, E. coli, Campylobacter, C. perfringens) (37, 93–95), thereby reducing dysentery in poultry. The probiotic effect of C. butyricum on poultry is attributed to its strong ability to produce short-chain fatty acids (SCFAs), particularly butyric acid, which can reduce intestinal pH and inhibits the growth of harmful bacteria thereby improving the structure of intestinal flora (36, 47, 49, 96–98). Additionally, butyric acid can stimulate the expression of the AVBD-9 gene in macrophages, bone marrow cells, and monocytes of poultry, and enhance the expression of host defense peptide genes such as AVBD-9, AVBD-10, AVBD-14, and Cath B1 in poultry gut, effectively suppressing the invasion of foreign microorganisms (99, 100). Furthermore, C. butyricum itself, as a probiotic, can compete with pathogenic microorganisms for adhesion sites, thereby inhibiting their adhesion and colonization in the gut (17, 101). For instance, Yang et al. (37) showed that dietary supplementation with 2 × 107 CFU/kg or 3 × 107 CFU/kg of C. butyricum significantly reduced the number of harmful bacteria (E. coli, Salmonella and C. perfringen) and increased the number of beneficial bacteria (C. butyricum, Lactobacillus and Bifidobacterium) in ileum of broilers.
Clostridium butyricum can also improve intestinal microbial diversity, regulate intestinal microbial structure, and maintain intestinal microbial dynamic balance. For instance, previous studies have demonstrated that dietary supplementation of C. butyricum significantly influenced the intestinal microbial α-diversity, as evidenced by a significant increase in the ACE index, Chao 1 index and Shannon index (36, 45, 65). At the phylum level, C. butyricum supplementation could increase the proportion of Firmicutes and decrease the proportion of Proteobacteria in the intestinal tract of poultry (68, 82,). At the genus level, C. butyricum supplementation could increase the proportion of some beneficial bacteria, such as Bacteroides (65, 96), Butyricicoccus (68), Lactobacillus (45, 68) and Barnesiella (102), while reduce the proportion of some harmful bacteria, such as Ralstonia (45), Escherichia-Shigella (96) and Klebsiella (96, 103) in the intestinal tract of poultry. In conclusion, C. butyricum can enhance the diversity of intestinal microbiota in poultry, optimize the composition and structure of intestinal microbiota, thereby maintaining a dynamic balance and promoting a healthy intestinal environment in poultry.
Clostridium butyricum promotes production performance of poultry
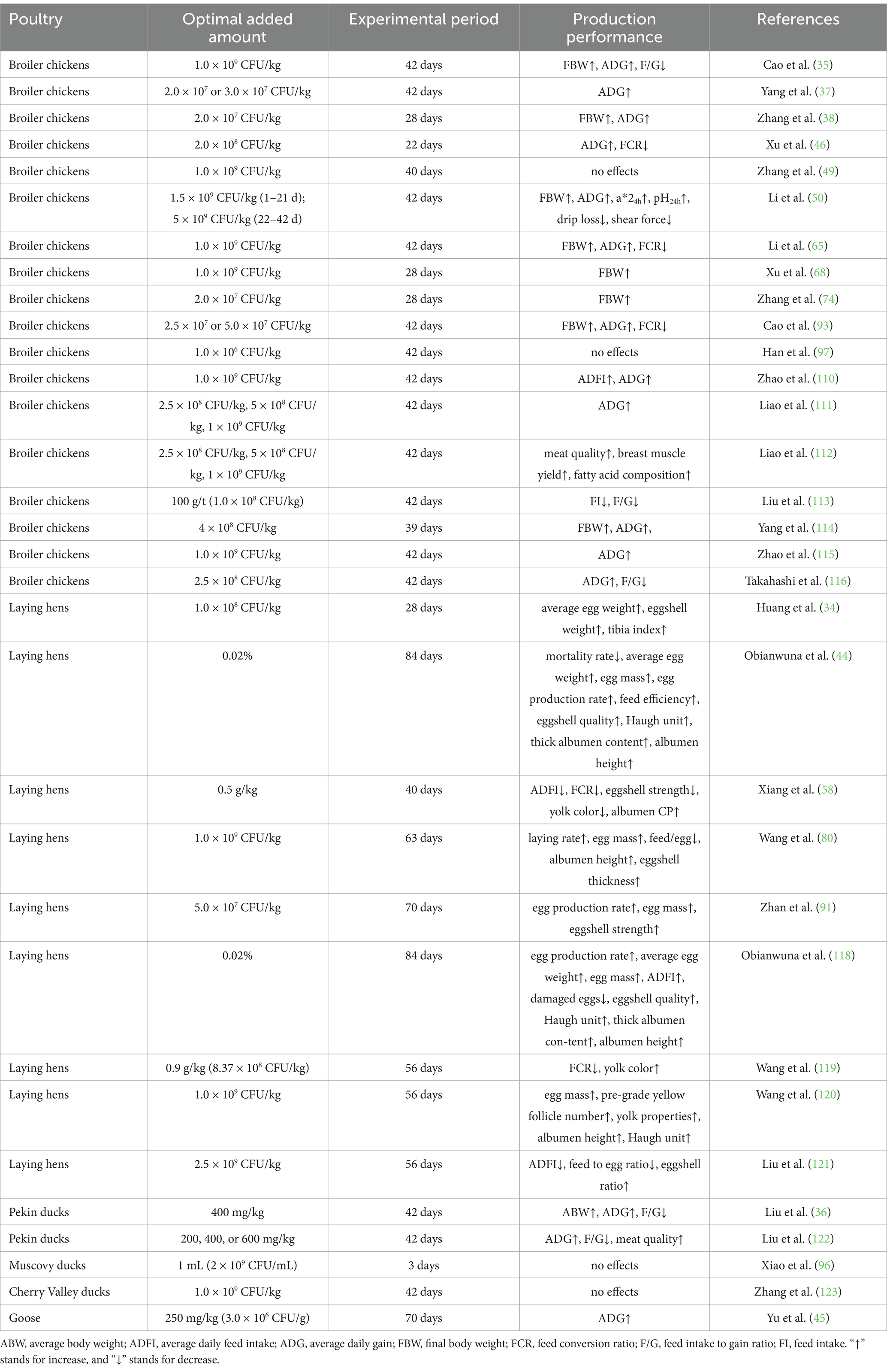
Intestinal health is very important for poultry growth and development. Optimal intestinal morphology and structure can facilitate the secretion of digestive enzymes, thereby enhancing the efficiency of nutrient digestion and absorption (38). A well-functioning intestinal barrier is essential for protecting poultry from various antigenic substances and ensuring their overall health and growth (104). Poultry is susceptible to heat stress (105), mycotoxin toxicity (106), and infections from pathogenic bacteria such as Salmonella (107), E. coli (108), and C. perfringens (109), which typically result in decreased feed intake and feed utilization efficiency, leading to a decline in overall production performance, thereby causing significant economic losses in the poultry industry. Many studies have shown that C. butyricum has a promoting effect on poultry production performance, including improving growth performance and product quality (Table 1).
From the summary table, it is evident that dietary addition of C. butyricum has a positive feeding effect on broiler chickens, laying hens, ducks and geese. In broilers, C. butyricum has been shown to increase average daily gain (ADG) and average daily feed intake (ADFI), improve feed conversion efficiency (decreased FCR or F/G), and improve meat quality and meat nutrient composition (35, 37, 38, 46, 49, 50, 65, 68, 74, 93, 97, 110–117). Similarly, for laying hens, C. butyricum has been found to improve laying rate, egg mass, average egg weight, and egg quality, while reducing the feed to egg ratio (34, 44, 58, 82, 95, 118–121). In the case of ducks, C. butyricum can increase ADG, decrease F/G, and improve meat quality (36, 122), but some studies believe that it has no effect on the production performance of ducks (96, 123). Although C. butyricum is less commonly used in goose production, limited studies suggest that it can increased the ADG of geese without affecting FCR (45). From this summary table, we can see that results for ducks and geese are less consistent than those for broilers or layers. This might be related to the fact that the digestive structure of ducks and geese is different from that of broilers and laying hens. Of course, the most important thing is that C. butyricum is used too little in ducks and geese. The limited data has a very significant impact on the accuracy of the conclusion, which requires further extensive research in the future with sufficient data support.
The promotion of poultry production performance by C. butyricum may be related to the following reasons: (i) it ferments and degrades undigested dietary fiber in the intestine to produce SCFAs, such as butyric acid (96–98), which directly supplies energy to intestinal epithelial cells, promotes intestinal villi growth, and enhances the digestion, absorption, and utilization of dietary nutrients in poultry, thus improving production performance. (ii) It stimulates the secretion of intestinal digestive enzymes (amylase, protease, lipase, and protease) (38, 49, 50) to break down macromolecular substances like carbohydrates, proteins, and lipids in the feed, thereby enhancing nutrient digestibility and improving feed conversion efficiency. (iii) It helps establish a healthy intestinal barrier for poultry (48, 60, 67, 100), which can effectively prevent the invasion of pathogenic substances and maintain the overall health of poultry, thereby improving the production performance of poultry.
Except for the addition of C. butyricum alone, the combined use of C. butyricum with other probiotics or functional substances has a synergistic promoting effect on the growth performance of poultry (47, 124). For instance, Zeng et al. (47) showed that dietary supplemented with a complex probiotic composed of C. butyricum, B. subtilis, and Bacillus licheniformis (B. licheniformis) can improve the overall health status of broilers, enhance their growth performance and immunity, and regulate the microbial community structure of the cecum. Zhang et al. (124) showed that dietary supplemented with a complex probiotic composed of C. butyricum and B. subtilis can effectively improve the growth performance and meat quality of broilers and regulate the microbial community structure in their excreta, which is of great significance to the poultry industry. However, it should be noted that the combined effect of probiotics may be influenced by multiple factors, including the type and dosage of probiotics, the breed of poultry, and the breeding environment. For instance, Yıldırım et al. (125) investigated the combined effects of tryptophan and probiotics on the growth and behavioral parameters of quail, it showed that supplementation with high-concentration tryptophan significantly reduced the weight gain of quails within 27 days, while probiotics had no obvious effect on this. Therefore, the combination of probiotics needs to be comprehensively considered in practical applications, and C. butyricum is no exception.
Conclusion
In conclusion, C. butyricum serves as an effective regulator of intestinal health and exhibits a prebiotic effect on the growth of poultry. Dietary addition of C. butyricum promotes intestinal development, reduces oxidative damage, maintains intestinal barrier function, enhances intestinal nutrient digestion and absorption, improves feed efficiency, and ultimately enhances poultry production performance (e.g., increase weight gain and feed intake, increase egg production rate, and improve the quality of muscle and eggs). However, further research is still needed to fully elucidate the complex interactions and precise molecular mechanisms underlying its beneficial effects. Understanding these mechanisms in detail will not only provide valuable insights for optimizing poultry production but also contribute to the development of more effective and sustainable strategies in the poultry industry.
Author contributions
XT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YZ: Project administration, Software, Writing – review & editing. ML: Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Guizhou Provincial Science and Technology Foundation (Qiankehe Jichu-ZK [2023] Yiban 267), the Natural Science Foundation of Hunan Province (2023JJ60228), and Guizhou Normal University Academic New Seedling Fund project (Qianshi Xinmiao [2021] B16).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Connolly, G, and Campbell, WW. Poultry consumption and human Cardiometabolic health-related outcomes: a narrative review. Nutrients. (2023) 15:3550. doi: 10.3390/nu15163550
2. Saeed, M, Yan, M, Ni, Z, Hussain, N, and Chen, H. Molecular strategies to enhance the keratinase gene expression and its potential implications in poultry feed industry. Poult Sci. (2024) 103:103606. doi: 10.1016/j.psj.2024.103606
3. Tang, X, Liu, X, and Liu, H. Effects of dietary probiotic (Bacillus subtilis) supplementation on carcass traits, meat quality, amino acid, and fatty acid profile of broiler chickens. Front Vet Sci. (2021) 8:767802. doi: 10.3389/fvets.2021.767802
4. Shastak, Y, and Pelletier, W. From metabolism to vitality: uncovering Riboflavin’s importance in poultry nutrition. Animals. (2023) 13:3554. doi: 10.3390/ani13223554
5. Yuan, H, Bai, G, Lin, Y, Yu, X, Yang, Q, Dou, R, et al. Effects of dietary Nisin on growth performance, immune function, and gut health of broilers challenged by Clostridium perfringens. J Anim Sci. (2024) 102:skae017. doi: 10.1093/jas/skae017
6. de Oliveira, MP, da Silva Pires, PG, Benetti Filho, V, Lima, ALF, Kindlein, L, Taschetto, D, et al. Intestinal health of broilers challenged with Eimeria spp. using functional oil blends in two physical forms with or without anticoccidials. Sci Rep. (2023) 13:14612. doi: 10.1038/s41598-023-41743-9
7. Saeed, M, Afzal, Z, Afzal, F, Khan, RU, Elnesr, SS, Alagawany, M, et al. Use of Postbiotic as growth promoter in poultry industry: a review of current knowledge and future prospects. Food Sci Anim Resour. (2023) 43:1111–27. doi: 10.5851/kosfa.2023.e52
8. Cao, J, Guo, Y, Luo, X, Ge, C, Hu, Z, Wu, L, et al. Interactions between enzyme preparations and trace element sources on growth performance and intestinal health of broiler chicks. Poult Sci. (2023) 102:103124. doi: 10.1016/j.psj.2023.103124
9. Song, W, Zou, Z, Chen, X, Tan, J, Liu, L, Wei, Q, et al. Effects of traditional Chinese herbal feed supplement on growth performance, immunity, antioxidant levels, and intestinal health in chickens: a study on Ningdu yellow chickens. Poult Sci. (2023) 102:102986. doi: 10.1016/j.psj.2023.102986
10. Kittelsen, KE, Vasdal, G, Moe, RO, Steinhoff, FS, and Tahamtani, FM. Health effects of feed dilution and roughage in Ross 308 broiler breeder cockerels. Poult Sci. (2023) 102:102985. doi: 10.1016/j.psj.2023.102985
11. Mao, J, Wang, Y, Duan, T, Yin, N, Dong, C, Ren, X, et al. Effect of fermented dandelion on productive performance, meat quality, immune function, and intestinal microbiota of broiler chickens. BMC Vet Res. (2023) 19:178. doi: 10.1186/s12917-023-03751-9
12. Kikusato, M, and Toyomizu, M. Mechanisms underlying the effects of heat stress on intestinal integrity, inflammation, and microbiota in chickens. J Poult Sci. (2023) 60:2023021. doi: 10.2141/jpsa.2023021
13. Liu, X, Ma, Z, Wang, Y, Jia, H, Wang, Z, and Zhang, L. Heat stress exposure cause alterations in intestinal microbiota, transcriptome, and metabolome of broilers. Front Microbiol. (2023) 14:1244004. doi: 10.3389/fmicb.2023.1244004
14. Subedi, M, Luitel, H, Devkota, B, Bhattarai, RK, Phuyal, S, Panthi, P, et al. Antibiotic resistance pattern and virulence genes content in avian pathogenic Escherichia coli (APEC) from broiler chickens in Chitwan, Nepal. BMC Vet Res. (2018) 14:113. doi: 10.1186/s12917-018-1442-z
15. Oseph, J, Zhang, L, Adhikari, P, Evans, JD, and Ramachandran, R. Avian pathogenic Escherichia coli (APEC) in broiler breeders: An overview. Pathogens. (2023) 12:1280. doi: 10.3390/pathogens12111280
16. Song, B, He, J, Pan, X, Kong, L, Xiao, C, Keerqin, C, et al. Dietary Macleaya cordata extract supplementation improves the growth performance and gut health of broiler chickens with necrotic enteritis. J Anim Sci Biotechnol. (2023) 14:113. doi: 10.1186/s40104-023-00916-2
17. Tang, X. Probiotic roles of Clostridium butyricum in piglets: considering aspects of intestinal barrier function. Animals. (2024) 14:1069. doi: 10.3390/ani14071069
18. Grandmont, A, Rhouma, M, Létourneau-Montminy, MP, Thériault, W, Mainville, I, Arcand, Y, et al. Characterization of the effects of a novel probiotic on Salmonella colonization of a piglet-derived intestinal microbiota using improved bioreactor. Animals. (2024) 14:787. doi: 10.3390/ani14050787
19. Hu, X, Lin, B, Luo, M, Zheng, X, and Zhang, H. The isolation, identification, physiological property of pig-isolate Clostridium butyricum LY33 using lactic acid and its effects on intestinal function of weaned piglets. Ital J Anim Sci. (2019) 18:910–21. doi: 10.1080/1828051X.2019.1603089
20. Grozina, AA, Ilina, LA, Laptev, GY, Yildirim, EA, Ponomareva, ES, Filippova, VA, et al. Probiotics as an alternative to antibiotics in modulating the intestinal microbiota and performance of broiler chickens. J Appl Microbiol. (2023) 134:lxad213. doi: 10.1093/jambio/lxad213
21. Tomczyk, G, Niczyporuk, JS, Kozdruń, W, Sawicka-Durkalec, A, Bocian, Ł, Barabasz, M, et al. Probiotic supplementation as an alternative to antibiotics in broiler chickens. J Vet Res. (2024) 68:147–54. doi: 10.2478/jvetres-2024-0009
22. Tang, X, Zeng, Y, Xiong, K, and Zhong, J. Bacillus spp. as potential probiotics: promoting piglet growth by improving intestinal health. Front Vet Sci. (2024) 11:1429233. doi: 10.3389/fvets.2024.1429233
23. Liu, M, Uyanga, VA, Cao, X, Liu, X, and Lin, H. Regulatory effects of the probiotic Clostridium butyricum on gut microbes, intestinal health, and growth performance of chickens. J Poult Sci. (2023) 60:2023011. doi: 10.2141/jpsa.2023011
24. Chang, J, Wang, T, Wang, P, Yin, Q, Liu, C, Zhu, Q, et al. Compound probiotics alleviating aflatoxin B1 and zearalenone toxic effects on broiler production performance and gut microbiota. Ecotoxicol Environ Saf. (2020) 194:110420. doi: 10.1016/j.ecoenv.2020.110420
25. Li, Z, Li, C, Lin, F, Yan, L, Wu, H, Zhou, H, et al. Duck compound probiotics fermented diet alters the growth performance by shaping the gut morphology, microbiota and metabolism. Poult Sci. (2024) 103:103647. doi: 10.1016/j.psj.2024.103647
26. Li, G, Wang, H, Yang, J, Qiu, Z, Liu, Y, Wang, X, et al. The protective effects of Lactobacillus SNK-6 on growth, organ health, and intestinal function in geese exposed to low concentration aflatoxin B1. Poult Sci. (2024) 103:103904. doi: 10.1016/j.psj.2024.103904
27. Dang, X, Zou, Q, Xu, Y, Cui, Y, Li, X, Xiao, Y, et al. Feeding broiler chicks with Bacillus subtilis, Clostridium butyricum, and Enterococcus faecalis mixture improves growth performance and regulates Cecal microbiota. Probiotics Antimicrob Proteins. (2024) 16:113–24. doi: 10.1007/s12602-022-10029-3
28. Shi, S, Ge, M, Xiong, Y, Zhang, Y, Li, W, Liu, Z, et al. The novel probiotic preparation based on Lactobacillus spp. mixture on the intestinal bacterial community structure of Cherry Valley duck. World J Microbiol Biotechnol. (2024) 40:194. doi: 10.1007/s11274-023-03859-y
29. Feng, Y, Wu, X, Hu, D, Wang, C, Chen, Q, and Ni, Y. Comparison of the effects of feeding compound probiotics and antibiotics on growth performance, gut microbiota, and small intestine morphology in yellow-feather broilers. Microorganisms. (2023) 11:2308. doi: 10.3390/microorganisms11092308
30. Wang, W, Cai, H, Zhang, A, Chen, Z, Chang, W, Liu, G, et al. Enterococcus faecium modulates the gut microbiota of broilers and enhances phosphorus absorption and utilization. Animals. (2020) 10:1232. doi: 10.3390/ani10071232
31. Poveda, J, and González-Andrés, F. Bacillus as a source of phytohormones for use in agriculture. Appl Microbiol Biotechnol. (2021) 105:8629–45. doi: 10.1007/s00253-021-11492-8
32. Cutting, SM. Bacillus probiotics. Food Microbiol. (2011) 28:214–20. doi: 10.1016/j.fm.2010.03.007
33. Kong, Q, He, GQ, Jia, JL, Zhu, QL, and Ruan, H. Oral administration of Clostridium butyricum for modulating gastrointestinal microflora in mice. Curr Microbiol. (2011) 62:512–7. doi: 10.1007/s00284-010-9737-8
34. Huang, J, Cui, L, Lin, H, Song, M, and Sun, S. Effects of Clostridium butyricum on production performance and bone development of laying hens. Vet Sci. (2024) 11:160. doi: 10.3390/vetsci11040160
35. Cao, G, Yu, Y, Wang, H, Yang, H, Tao, F, Yang, S, et al. Dietary Clostridium butyricum and 25-Hydroxyvitamin D3 modulate bone metabolism of broilers through the gut-brain axis. Poult Sci. (2024) 103:103966. doi: 10.1016/j.psj.2024.103966
36. Liu, Y, Liu, C, An, K, Gong, X, and Xia, Z. Effect of dietary Clostridium butyricum supplementation on growth performance, intestinal barrier function, immune function, and microbiota diversity of Pekin ducks. Animals. (2021) 11:2514. doi: 10.3390/ani11092514
37. Yang, CM, Cao, GT, Ferket, PR, Liu, TT, Zhou, L, Zhang, L, et al. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult Sci. (2012) 91:2121–9. doi: 10.3382/ps.2011-02131
38. Zhang, L, Zhang, L, Zhan, X, Zeng, X, Zhou, L, Cao, G, et al. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J Anim Sci Biotechnol. (2016) 7:3. doi: 10.1186/s40104-016-0061-4
39. Tang, X, Xiong, K, Fang, R, and Li, M. Weaning stress and intestinal health of piglets: a review. Front Immunol. (2022) 13:1042778. doi: 10.3389/fimmu.2022.1042778
40. Rostagno, MH. Effects of heat stress on the gut health of poultry. J Anim Sci. (2020) 98:skaa090. doi: 10.1093/jas/skaa090
41. López-Osorio, S, Chaparro-Gutiérrez, JJ, and Gómez-Osorio, LM. Overview of poultry Eimeria life cycle and host-parasite interactions. Front Vet Sci. (2020) 7:384. doi: 10.3389/fvets.2020.00384
42. Daneshmand, A, Kermanshahi, H, Mohammed, J, Sekhavati, MH, Javadmanesh, A, Ahmadian, M, et al. Intestinal changes and immune responses during Clostridium perfringens-induced necrotic enteritis in broiler chickens. Poult Sci. (2022) 101:101652. doi: 10.1016/j.psj.2021.101652
43. Wan, S, Sun, N, Li, H, Khan, A, Zheng, X, Sun, Y, et al. Deoxynivalenol damages the intestinal barrier and biota of the broiler chickens. BMC Vet Res. (2022) 18:311. doi: 10.1186/s12917-022-03392-4
44. Obianwuna, UE, Qiu, K, Chang, XY, Zhang, HJ, Wang, J, Qi, GH, et al. Enhancing egg production and quality by the supplementation of probiotic strains (Clostridium and Brevibacillus) via improved amino acid digestibility, intestinal health, immune response, and antioxidant activity. Front Microbiol. (2022) 13:987241. doi: 10.3389/fmicb.2022.987241
45. Yu, J, Dong, B, Zhao, M, Liu, L, Geng, T, Gong, D, et al. Dietary Clostridium butyricum and Bacillus subtilis promote goose growth by improving intestinal structure and function, Antioxidative Capacity and Microbial Composition. Animals. (2021) 11:3174. doi: 10.3390/ani11113174
46. Xu, X, Yang, S, Olajide, JS, Qu, Z, Gong, Z, Wang, J, et al. Clostridium butyricum supplement can ameliorate the intestinal barrier roles in broiler chickens experimentally infected with Clostridium perfringens. Front Physiol. (2021) 12:737481. doi: 10.3389/fphys.2021.737481
47. Zeng, X, Li, Q, Yang, C, Yu, Y, Fu, Z, Wang, H, et al. Effects of Clostridium butyricum-and Bacillus spp.-based potential probiotics on the growth performance, intestinal morphology, immune responses, and Caecal microbiota in broilers. Antibiotics. (2021) 10:624. doi: 10.3390/antibiotics10060624
48. Huang, P, Cui, X, Wang, Z, Xiao, C, Ji, Q, Wei, Q, et al. Effects of Clostridium butyricum and a bacteriophage cocktail on growth performance, serum biochemistry, digestive enzyme activities, intestinal morphology, immune responses, and the intestinal microbiota in rabbits. Antibiotics. (2021) 10:1347. doi: 10.3390/antibiotics10111347
49. Zhang, B, Yang, X, Guo, Y, and Long, F. Effects of dietary lipids and Clostridium butyricum on the performance and the digestive tract of broiler chickens. Arch Anim Nutr. (2011) 65:329–39. doi: 10.1080/1745039x.2011.568274
50. Li, Z, Long, L, Jin, X, Li, Y, Wu, Q, Chen, X, et al. Effects of Clostridium butyricum on growth performance, meat quality, and intestinal health of broilers. Front Vet Sci. (2023) 10:1107798. doi: 10.3389/fvets.2023.1107798
51. Tang, X, Xiong, K, Wassie, T, and Wu, X. Curcumin and intestinal oxidative stress of pigs with intrauterine growth retardation: a review. Front Nutr. (2022) 9:847673. doi: 10.3389/fnut.2022.847673
52. Li, S, Yuan, J, Che, S, Zhang, L, Ruan, Z, and Sun, X. Decabromodiphenyl ether induces ROS-mediated intestinal toxicity through the Keap1-Nrf2 pathway. J Biochem Mol Toxicol. (2022) 36:e22995. doi: 10.1002/jbt.22995
53. Yu, X, Chen, Y, and Tan, M. ROS-responsive carboxymethyl chitosan nanoparticles loaded with astaxanthin for alleviating oxidative damage in intestinal cells. Colloids Surf B Biointerfaces. (2024) 239:113960. doi: 10.1016/j.colsurfb.2024.113960
54. Damiano, S, Longobardi, C, Andretta, E, Prisco, F, Piegari, G, Squillacioti, C, et al. Antioxidative effects of curcumin on the hepatotoxicity induced by Ochratoxin a in rats. Antioxidants. (2021) 10:125. doi: 10.3390/antiox10010125
55. Mas-Bargues, C, Escrivá, C, Dromant, M, Borrás, C, and Viña, J. Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Arch Biochem Biophys. (2021) 709:108941. doi: 10.1016/j.abb.2021.108941
56. Yin, J, Ren, W, Liu, G, Duan, J, Yang, G, Wu, L, et al. Birth oxidative stress and the development of an antioxidant system in newborn piglets. Free Radic Res. (2013) 47:1027–35. doi: 10.3109/10715762.2013.848277
57. Demirci-Çekiç, S, Özkan, G, Avan, AN, Uzunboy, S, Çapanoğlu, E, and Apak, R. Biomarkers of oxidative stress and antioxidant defense. J Pharm Biomed Anal. (2022) 209:114477. doi: 10.1016/j.jpba.2021.114477
58. Xiang, Q, Wang, C, Zhang, H, Lai, W, Wei, H, and Peng, J. Effects of different probiotics on laying performance, egg quality, oxidative status, and gut health in laying hens. Animals. (2019) 9:1110. doi: 10.3390/ani9121110
59. Tang, X, Liu, B, Wang, X, Yu, Q, and Fang, R. Epidermal growth factor, through alleviating oxidative stress, protect IPEC-J2 cells from lipopolysaccharides-induced apoptosis. Int J Mol Sci. (2018) 19:848. doi: 10.3390/ijms19030848
60. Zhao, X, Yang, J, Ju, Z, Wu, J, Wang, L, Lin, H, et al. Clostridium butyricum ameliorates Salmonella enteritis induced inflammation by enhancing and improving immunity of the intestinal epithelial barrier at the intestinal mucosal level. Front Microbiol. (2020) 11:299. doi: 10.3389/fmicb.2020.00299
61. Huang, T, Peng, XY, Gao, B, Wei, QL, Xiang, R, Yuan, MG, et al. The effect of Clostridium butyricum on gut microbiota, immune response and intestinal barrier function during the development of necrotic enteritis in chickens. Front Microbiol. (2019) 10:2309. doi: 10.3389/fmicb.2019.02309
62. Hu, CH, Xiao, K, Luan, ZS, and Song, J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J Anim Sci. (2013) 91:1094–101. doi: 10.2527/jas.2012-5796
63. Obianwuna, UE, Agbai Kalu, N, Wang, J, Zhang, H, Qi, G, Qiu, K, et al. Recent trends on Mitigative effect of probiotics on oxidative-stress-induced gut dysfunction in broilers under necrotic enteritis challenge: a review. Antioxidants. (2023) 12:911. doi: 10.3390/antiox12040911
64. Van Nerom, S, Coleman, B, De Baets, R, Van Immerseel, F, Robbens, J, and Delezie, E. Microalgae as feed additives in poultry: a review on the health-promoting effects. Algal Res. (2024) 83:103733. doi: 10.1016/j.algal.2024.103733
65. Li, W, Xu, B, Wang, L, Sun, Q, Deng, W, Wei, F, et al. Effects of Clostridium butyricum on growth performance, gut microbiota and intestinal barrier function of broilers. Front Microbiol. (2021) 12:777456. doi: 10.3389/fmicb.2021.777456
66. Lyu, W, Yang, H, Li, N, Lu, L, Yang, C, Jin, P, et al. Molecular characterization, developmental expression, and modulation of occludin by early intervention with Clostridium butyricum in Muscovy ducks. Poult Sci. (2021) 100:101271. doi: 10.1016/j.psj.2021.101271
67. Du, M, Cheng, Y, Chen, Y, Wang, S, Zhao, H, Wen, C, et al. Dietary supplementation with synbiotics improves growth performance, antioxidant status, immune function, and intestinal barrier function in broilers subjected to cyclic heat stress. Environ Sci Pollut Res Int. (2023) 30:18026–38. doi: 10.1007/s11356-022-23385-y
68. Xu, L, Sun, X, Wan, X, Li, K, Jian, F, Li, W, et al. Dietary supplementation with Clostridium butyricum improves growth performance of broilers by regulating intestinal microbiota and mucosal epithelial cells. Anim Nutr. (2021) 7:1105–14. doi: 10.1016/j.aninu.2021.01.009
69. Tang, X, Xiong, K, Zeng, Y, and Fang, R. The mechanism of zinc oxide in alleviating diarrhea in piglets after weaning: a review from the perspective of intestinal barrier function. Int J Mol Sci. (2024) 25:10040. doi: 10.3390/ijms251810040
70. Zhou, X, Wang, L, Wang, Z, Zhu, P, Chen, Y, Yu, C, et al. Impacts of Eimeria coinfection on growth performance, intestinal health and immune responses of broiler chickens. Vet Parasitol. (2023) 322:110019. doi: 10.1016/j.vetpar.2023.110019
71. Wells, JM, Brummer, RJ, Derrien, M, MacDonald, TT, Troost, F, Cani, PD, et al. Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol. (2017) 312:G171–93. doi: 10.1152/ajpgi.00048.2015
72. Li, WH, Liu, YL, Lun, JC, He, YM, and Tang, LP. Heat stress inhibits TLR4-NF-κB and TLR4-TBK1 signaling pathways in broilers infected with Salmonella Typhimurium. Int J Biometeorol. (2021) 65:1895–903. doi: 10.1007/s00484-021-02146-5
73. Wu, Q, Wang, C, Liao, J, Hu, N, Cheng, B, Ma, Y, et al. Effects of dietary supplementation with glutamine on the immunity and intestinal barrier gene expression in broiler chickens infected with Salmonella Enteritidis. Animals. (2022) 12:2168. doi: 10.3390/ani12172168
74. Zhang, L, Cao, GT, Zeng, XF, Zhou, L, Ferket, PR, Xiao, YP, et al. Effects of Clostridium butyricum on growth performance, immune function, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult Sci. (2014) 93:46–53. doi: 10.3382/ps.2013-03412
75. De Palma, G, Nadal, I, Medina, M, Donat, E, Ribes-Koninckx, C, Calabuig, M, et al. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. BMC Microbiol. (2010) 10:63. doi: 10.1186/1471-2180-10-63
76. Tang, X, Liu, X, Zhong, J, and Fang, R. Potential application of Lonicera japonica extracts in animal production: from the perspective of intestinal health. Front Microbiol. (2021) 12:719877. doi: 10.3389/fmicb.2021.719877
77. Liu, L, Zeng, D, Yang, M, Wen, B, Lai, J, Zhou, Y, et al. Probiotic Clostridium butyricum improves the growth performance, immune function, and gut microbiota of weaning rex rabbits. Probiotics Antimicrob Proteins. (2019) 11:1278–92. doi: 10.1007/s12602-018-9476-x
78. Li, W, Xu, B, Wei, F, Deng, W, Ma, H, Wang, L, et al. Effects of Clostridium butyricum on growth, intestinal morphology and barrier function of broilers. J Henan Arg Sci. (2023) 52:144–53. doi: 10.15933/j.cnki.1004-3268.2023.01.015
79. Fang, D, and Zhu, J. Molecular switches for regulating the differentiation of inflammatory and IL-10-producing anti-inflammatory T-helper cells. Cell Mol Life Sci. (2020) 77:289–303. doi: 10.1007/s00018-019-03277-0
80. Nagata, K, and Nishiyama, C. IL-10 in mast cell-mediated immune responses: anti-inflammatory and Proinflammatory roles. Int J Mol Sci. (2021) 22:4972. doi: 10.3390/ijms22094972
81. Panahipour, L, Sordi, MB, Kargarpour, Z, and Gruber, R. TGF-β Signalling mediates the anti-inflammatory activity of enamel matrix derivative in vitro. Int J Mol Sci. (2022) 23:9778. doi: 10.3390/ijms23179778
82. Wang, Y, Wang, Y, Lin, X, Gou, Z, Fan, Q, and Jiang, S. Effects of Clostridium butyricum, sodium butyrate, and butyric acid glycerides on the reproductive performance, egg quality, intestinal health, and offspring performance of yellow-feathered breeder hens. Front Microbiol. (2021) 12:657542. doi: 10.3389/fmicb.2021.657542
83. Zhang, X, Song, M, Lv, P, Hao, G, and Sun, S. Effects of Clostridium butyricum on intestinal environment and gut microbiome under Salmonella infection. Poult Sci. (2022) 101:102077. doi: 10.1016/j.psj.2022.102077
84. Kogut, MH. The gut microbiota and host innate immunity: regulators of host metabolism and metabolic diseases in poultry? J Appl Poult Res. (2013) 22:637–46. doi: 10.3382/japr.2013-00741
85. Boranbayeva, G, Tekebayeva, Z, Temirkhanov, A, Temirbekova, A, Yevneyeva, D, Abilkhadirov, A, et al. Probiotic consortium from poultry strains for supporting gut immunity against pathogens. Microb Pathog. (2025) 204:107584. doi: 10.1016/j.micpath.2025.107584
86. Zhao, X, Yang, J, Wang, L, Lin, H, and Sun, S. Protection mechanism of Clostridium butyricum against Salmonella Enteritidis infection in broilers. Front Microbiol. (2017) 8:1523. doi: 10.3389/fmicb.2017.01523
87. Guo, Q, Jin, Y, Chen, X, Ye, X, Shen, X, Lin, M, et al. NF-κB in biology and targeted therapy: new insights and translational implications. Signal Transduct Target Ther. (2024) 9:53. doi: 10.1038/s41392-024-01757-9
88. Feng, AC, Thomas, BJ, Purbey, PK, de Melo, FM, Liu, X, Daly, AE, et al. The transcription factor NF-κB orchestrates nucleosome remodeling during the primary response to toll-like receptor 4 signaling. Immunity. (2024) 57:462–477.e9. doi: 10.1016/j.immuni.2024.02.004
89. Wei, S, Morrison, M, and Yu, Z. Bacterial census of poultry intestinal microbiome. Poult Sci. (2013) 92:671–83. doi: 10.3382/ps.2012-02822
90. Mancabelli, L, Ferrario, C, Milani, C, Mangifesta, M, Turroni, F, Duranti, S, et al. Insights into the biodiversity of the gut microbiota of broiler chickens. Environ Microbiol. (2016) 18:4727–38. doi: 10.1111/1462-2920.13363
91. Yuan, J, Li, Y, Sun, S, Wu, J, Zhou, J, and He, S. Response of growth performance and cecum microbial community to cyclic heat stress in broilers. Trop Anim Health Prod. (2023) 56:9. doi: 10.1007/s11250-023-03849-0
92. Nouri, A. Age-dependent development trends (models) of intestinal significant microbiota species and Eimeria oocysts in coccidia-challenged broiler chickens as affected by dietary encapsulated organic acids and anticoccidial drugs. Avian Pathol. (2024) 53:264–84. doi: 10.1080/03079457.2024.2319284
93. Cao, GT, Xiao, YP, Yang, CM, Chen, AG, Liu, TT, Zhou, L, et al. Effects of Clostridium butyricum on growth performance, nitrogen metabolism, intestinal morphology and cecal microflora in broiler chickens. J Anim Vet Adv. (2012) 11:2665–71. doi: 10.3923/javaa.2012.2665.2671
94. Yu, Y, Li, Q, Zhang, H, Wu, Y, Zhang, R, Yue, M, et al. Clostridium butyricum alone or combined with 1, 25-dihydroxyvitamin D3 improved early-stage broiler health by modulating intestinal flora. J Appl Microbiol. (2022) 132:155–66. doi: 10.1111/jam.15180
95. Zhan, HQ, Dong, XY, Li, LL, Zheng, YX, Gong, YJ, and Zou, XT. Effects of dietary supplementation with Clostridium butyricum on laying performance, egg quality, serum parameters, and cecal microflora of laying hens in the late phase of production. Poult Sci. (2019) 98:896–903. doi: 10.3382/ps/pey436.30285187
96. Xiao, X, Fu, Z, Li, N, Yang, H, Wang, W, and Lyu, W. Modulation of the intestinal microbiota by the early intervention with Clostridium Butyricum in Muscovy ducks. Antibiotics. (2021) 10:826. doi: 10.3390/antibiotics10070826
97. Han, J, Wang, Y, Song, D, Lu, Z, Dong, Z, Miao, H, et al. Effects of Clostridium butyricum and Lactobacillus plantarum on growth performance, immune function and volatile fatty acid level of caecal digesta in broilers. Food Agri Immunol. (2018) 29:797–807. doi: 10.1080/09540105.2018.1457013
98. Svejstil, R, Plachy, V, Joch, M, Salmonova, H, Duskova, D, Hautekiet, V, et al. Effect of probiotic Clostridium butyricum CBM 588 on microbiota and growth performance of broiler chickens. Czeh J Anim Sci. (2019) 64:387–94. doi: 10.17221/143/2019-CJAS
99. Sunkara, LT, Achanta, M, Schreiber, NB, Bommineni, YR, Dai, G, Jiang, W, et al. Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS One. (2011) 6:e27225. doi: 10.1371/journal.pone.0027225
100. Kim, DM, Liu, J, Whitmore, MA, Tobin, I, Zhao, Z, and Zhang, G. Two intestinal microbiota-derived metabolites, deoxycholic acid and butyrate, synergize to enhance host defense peptide synthesis and alleviate necrotic enteritis. J Anim Sci Biotechnol. (2024) 15:29. doi: 10.1186/s40104-024-00995-9
101. Wang, J, Qi, L, Mei, L, Wu, Z, and Wang, HC. Butyricum lipoteichoic acid inhibits the inflammatory response and apoptosis in HT-29 cells induced by S. aureus lipoteichoic acid. Int J Biol Macromol. (2016) 88:81–7. doi: 10.1016/j.ijbiomac.2016.03.054
102. Cai, H, Liao, S, Li, J, Liu, Q, Luo, S, Lv, M, et al. Single and combined effects of Clostridium butyricum and coccidiosis vaccine on growth performance and the intestinal microbiome of broiler chickens. Front Microbiol. (2022) 13:811428. doi: 10.3389/fmicb.2022.811428
103. Wang, WW, Wang, J, Zhang, HJ, Wu, SG, and Qi, GH. Supplemental Clostridium butyricum modulates lipid metabolism through shaping gut microbiota and bile acid profile of aged laying hens. Front Microbiol. (2020) 11:600. doi: 10.3389/fmicb.2020.00600
104. Hossain, M, Begum, M, and Kim, I. Effect of Bacillus subtilis, Clostridium butyricum and Lactobacillus acidophilus endospores on growth performance, nutrient digestibility, meat quality, relative organ weight, microbial shedding and excreta noxious gas emission in broilers. Vet Med. (2015) 60:77–86. doi: 10.17221/7981-VETMED
105. Liu, L, Ren, M, Ren, K, Jin, Y, and Yan, M. Heat stress impacts on broiler performance: a systematic review and meta-analysis. Poult Sci. (2020) 99:6205–11. doi: 10.1016/j.psj.2020.08.019
106. Ochieng, PE, Croubels, S, Kemboi, D, Okoth, S, De Baere, S, Cavalier, E, et al. Effects of aflatoxins and Fumonisins, alone or in combination, on performance, health, and safety of food products of broiler chickens, and mitigation efficacy of bentonite and Fumonisin esterase. J Agric Food Chem. (2023) 71:13462–73. doi: 10.1021/acs.jafc.3c01733
107. Logue, CM, De Cesare, A, Tast-Lahti, E, Chemaly, M, Payen, C, LeJeune, J, et al. Salmonella spp. in poultry production-a review of the role of interventions along the production continuum. Adv Food Nutr Res. (2024) 108:289–341. doi: 10.1016/bs.afnr.2023.11.001
108. Fadl, SE, El-Gammal, GA, Sakr, OA, Salah, AABS, Atia, AA, Prince, AM, et al. Impact of dietary Mannan-oligosaccharide and β-glucan supplementation on growth, histopathology, E-coli colonization and hepatic transcripts of TNF-α and NF-κB of broiler challenged with E. coli O78. BMC Vet Res. (2020) 16:204. doi: 10.1186/s12917-020-02423-2
109. Mora, ZV, Macías-Rodríguez, ME, Arratia-Quijada, J, Gonzalez-Torres, YS, Nuño, K, and Villarruel-López, A. Clostridium perfringens as foodborne pathogen in broiler production: pathophysiology and potential strategies for controlling necrotic enteritis. Animals. (2020) 10:1718. doi: 10.3390/ani10091718
110. Zhao, X, Guo, Y, Guo, S, and Tan, J. Effects of Clostridium butyricum and Enterococcus faecium on growth performance, lipid metabolism, and cecal microbiota of broiler chickens. Appl Microbiol Biotechnol. (2013) 97:6477–88. doi: 10.1007/s00253-013-4970-2
111. Liao, XD, Ma, G, Cai, J, Fu, Y, Yan, XY, Wei, XB, et al. Effects of Clostridium butyricum on growth performance, antioxidation, and immune function of broilers. Poult Sci. (2015) 94:662–7. doi: 10.3382/ps/pev038
112. Liao, X, Wu, R, Ma, G, Zhao, L, Zheng, Z, and Zhang, R. Effects of Clostridium butyricum on antioxidant properties, meat quality and fatty acid composition of broiler birds. Lipids Health Dis. (2015) 14:36. doi: 10.1186/s12944-015-0035-0
113. Liu, L, Ling, H, Zhang, W, Zhou, Y, Li, Y, Peng, N, et al. Functional comparison of Clostridium butyricum and sodium butyrate supplementation on growth, intestinal health, and the anti-inflammatory response of broilers. Front Microbiol. (2022) 13:914212. doi: 10.3389/fmicb.2022.914212
114. Yang, T, Du, M, Zhang, J, Ahmad, B, Cheng, Q, Wang, X, et al. Effects of Clostridium butyricum as an antibiotic alternative on growth performance, intestinal morphology, serum biochemical response, and immunity of broilers. Antibiotics. (2023) 12:433. doi: 10.3390/antibiotics12030433
115. Zhao, X, Ding, X, Yang, Z, Shen, Y, Zhang, S, and Shi, S. Effects of Clostridium butyricum on breast muscle lipid metabolism of broilers. Ital J Anim Sci. (2018) 17:1010–20. doi: 10.1080/1828051X.2018.1453758
116. Takahashi, M, McCartney, E, Knox, A, Francesch, M, Oka, K, Wada, K, et al. Effects of the butyric acid-producing strain Clostridium butyricum MIYAIRI 588 on broiler and piglet zootechnical performance and prevention of necrotic enteritis. Anim Sci J. (2018) 89:895–905. doi: 10.1111/asj.13006
117. Yang, T, Du, M, Wang, X, Wang, J, Li, J, Jiang, X, et al. Effects of dietary Clostridium Butyricum on carcass traits, antioxidant capacity, meat quality, and fatty acid composition of broilers. Agriculture. (2022) 12:1607. doi: 10.3390/agriculture12101607
118. Obianwuna, UE, Qiu, K, Wang, J, Wang, J, Zhang, HJ, Qi, GH, et al. Effects of dietary Clostridium butyricum and fructooligosaccharides, alone or in combination, on performance, egg quality, amino acid digestibility, jejunal morphology, immune function, and antioxidant capacity of laying hens. Front Microbiol. (2023) 14:1125897. doi: 10.3389/fmicb.2023.1125897
119. Wang, W, Wang, J, Zhang, H, Wu, S, and Qi, G. Effects of Clostridium butyricum on production performance and intestinal absorption function of laying hens in the late phase of production. Anim Feed Sci Technol. (2020) 264:114476. doi: 10.1016/j.anifeedsci.2020.114476
120. Wang, Y, Wang, Y, Wang, L, Wei, B, Lv, X, Huang, Y, et al. Dietary supplementation with Clostridium butyricum and its ferment substance improves the egg quality and ovarian function in laying hens from 50 to 58 weeks of age. Anim Sci J. (2023) 94:e13877. doi: 10.1111/asj.13877
121. Liu, Y, Zheng, M, Yang, Z, Zhang, G, Xu, W, Jiang, H, et al. Effects of dietary Clostridium butyricum and sodium butyrate on performance, egg quality and intestinal health of laying hens. Chin J Anim Nutr. (2023) 35:3025–40. doi: 10.12418/CJAN2024.423
122. Liu, Y, Li, Y, Feng, X, Wang, Z, and Xia, Z. Dietary supplementation with Clostridium butyricum modulates serum lipid metabolism, meat quality, and the amino acid and fatty acid composition of Peking ducks. Poult Sci. (2018) 97:3218–29. doi: 10.3382/ps/pey162
123. Zhuang, S, Jiang, F, Jia, ZX, and Yan, R. Clostridium butyricum can be used as a potential alternative for the antibiotic in Cherry Valley ducks. J Anim Plant Sci. (2015) 25:1227–32.
124. Zhang, Q, Cho, S, Kibria, S, and Kim, IH. Dietary Bacillus subtilis- and Clostridium butyricum-based probiotics supplement improves growth and meat quality, and alters microbiota in the excreta of broiler chickens. Can J Anim Sci. (2024) 104:200–6. doi: 10.1139/cjas-2023-0076
Keywords: Clostridium butyricum, growth performance, intestinal health, gut microbiota, poultry
Citation: Tang X, Zeng Y and Li M (2025) Clostridium butyricum: a promising approach to enhancing intestinal health in poultry. Front. Vet. Sci. 12:1544519. doi: 10.3389/fvets.2025.1544519
Edited by:
Mahmoud Madkour, National Research Centre, EgyptReviewed by:
Arda Yıldırım, Gaziosmanpaşa University, TürkiyeXiaokang Ma, Hunan Agricultural University, China
Ahmed Kewan, Al-Azhar University, Egypt
Copyright © 2025 Tang, Zeng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meijun Li, bGltZWlqdW5AaG5iZW1jLmVkdS5jbg==
 Xiaopeng Tang
Xiaopeng Tang Yan Zeng2
Yan Zeng2