- College of Animal Science, Inner Mongolia Agricultural University, Hohhot, China
Introduction: Artemisia annua L., an herbaceous plant, belong to the Artemisia genus within the Asteraceae family. Due to its significant medicinal properties, it has emerged as a focal point of research in the field of animal production. In the present study, the responses of intestinal immune and antioxidative indexes, and the related gene expression to water extracts of Artemisia annua L. (WEAA) supplementation in diet were profiled in lambs.
Methods: In total, 32 female lambs (Dorper × Han), with eight replicates per group, were randomly assigned to four treatment groups. These groups were created by supplementing 0, 500, 1,000, and 1,500 mg/kg WEAA to the basal diet, respectively.
Results: The results showed that WEAA addition increased sIgA, IgG, IL-1β, IL-2 and IL-4 levels in the duodenal and jejunal mucosa in a manner that was dependent on the dosage (p < 0.05). Moreover, WEAA promoted the expression of factors (TLR4, MyD88, IKKβ, IκBα, NF-κB p50, NF-κB p65, IL-1β and IL-4) related with the TLR4/NF-κB pathway, thus improving small intestinal immune function, thereby showing peak effects in the 1,000 mg/kg WEAA group. Additionally, WEAA supplementation also enhanced antioxidative function through the Nrf2/Keap1 pathway in the small intestinal mucosa, particularly by increasing GSH-Px and CAT concentrations and decreasing MDA content in a manner that was dependent on the dosage (p < 0.05), with maximal effects observed in the 1,000 mg/kg group. Furthermore, expressions levels of Nrf2, GSH-Px and HO-1 in the small intestine increased quadratically (p < 0.05), while Keap1 expression levels exhibited a downward quadratic trend (p < 0.10).
Conclusion: In summary, the optimal dietary addition of 1,000 mg/kg WEAA significantly enhanced intestinal immune function, antioxidant capacity, and the expression of related genes in the intestinal mucosa of lambs.
1 Introduction
In recent decades, the global sheep industry has undergone a significant transformation from traditional extensive grazing systems to modern intensive production models. Due to increased demand for lamb and its by-products, land degradation problem is increasingly serious along with the pursuit of industry to improve the market competitiveness of livestock products and other issues (1, 2). In contemporary sheep production systems, this shift has brought about substantial changes to the natural habitat and innate behavior of sheep, which are likely to have negative effects on their metabolism, immune function and health (3, 4). These changes are different from the natural conditions to which sheep have been adapted for a long time (5). In addition, sheep health and productivity are heavily influenced by their diet composition, which holds a key position in growth performance, body development, and immune function, but the nutritional requirements of sheep are often easily overlooked in intensive production systems (6). Therefore, there has been a growing interest in improving diet nutrition strategies to enhance lamb health and performance. Among these strategies, natural plant-derived feed additives have potential to be a suitable alternative to synthetic growth promoters and alternatives to antibiotics in animal production in recent years (7–9).
The natural additives have been shown to have safe and reliable positive effects in livestock and poultry production, to have high consumer acceptance, and have the potential to promote animal growth and health without the developing resistance (10). Artemisia annua L. (A. annua), a member of the family Asteraceae which is considered native to Asia with its origin in China. A. annua is commonly known as sweet wormwood (11). This plant has a long history of being used as a traditional herbal remedy for malaria in China. Extensive research has been conducted on the A. annua and its extracts owing to their rich phytochemical profile and diverse biological activities, including polysaccharides, flavonoids, sesquiterpenes (artemisinin), phenols, essential oils, etc. (10, 12). Artemisinin is globally recognized for its effectiveness against malaria. In addition to its anti-malaria properties, A. annua also exhibits various other significant biological activities, including antibacterial, anti-inflammatory, anti-tumor, and antioxidant effects (13, 14).
In a series of studies involving different species, the multiple benefits of A. annua and its extracts on animal nutrition and health have been scientifically validated. Studies using rodents and poultry as models have shown that adding an appropriate amount of A. annua extract to the feed can significantly improve growth performance, optimize intestinal health, and enhance antioxidant status. Zhang et al. (15) evaluated immunomodulatory and antioxidant capabilities of polysaccharides derived from A. annua. These polysaccharides showed significant immunostimulatory activity and antioxidant effects, promoting the synthesis of TNF-α and IL-6 in mouse macrophages. Cui et al. (16) studied the effect of 1% A. annua supplemented to the diet on antioxidant capacity in serum and jejunum of geese. The results showed improved antioxidant enzyme activity (CAT, GSH-Px, SOD, T-AOC) and decreased MDA contents. Guo et al. (17) reported that the supplementing polysaccharides extracted from A. annua to the diet could significantly increase the expression of CAT and Nrf2 in the small intestinal mucosa of broilers. This suggest that the enhancement of antioxidant enzyme activities may be through the Nrf2 signaling pathway. Similarly, in the field of aquaculture, A. annua leaf extract has been found to increased serum immunoglobulin levels, and activity of CAT and GSH-Px, while decreasing the content of malondialdehyde (MDA) in serum of Common Carp (18). In a study on weaned pigs, Niu et al. (19) found that the enzyme-treated A. annua (EA) affected the intestinal immune ability in weaned pigs, and the EA dosage of 2 g/kg decreased levels of IL-1β, IL-6, and TNF-αin the jejunum and increased the concentration of immunoglobulin (Ig) A and IgG in the ileum compared to the CON group. Song et al. (20) demonstrated that EA the intestinal inflammatory response of heat-stressed broilers, and found that EA could significantly increase IL-10 production and decrease TLR4 mRNA expression in both the jejunum and ileum, suggesting that EA can inhibit TLR4/NF-κB pathway activation in heat-stressed conditions, thereby reducing inflammation. In addition, studies have shown that the regulatory mechanisms of Artemisia plants and their extracts are related to recognition of bio-active compounds by TLR4 on the surfaces of immune cells. This recognition triggers a series of signaling pathways, among which the NF-κB pathway is an extremely important pathway in cellular signaling and plays a key role in regulating the body’s immune inflammatory response (21, 22). The results indicate that A. annua may have a relatively positive effect in different livestock species, including sheep. The intestinal tissue serves as both a physical and immune barrier against pathogens and toxins, playing a crucial role in nutrient absorption and immune system (23). Enhancing intestinal immunity and antioxidant function through dietary interventions may improve lambs’ health and productivity.
The potential advantages of A. annua in animal nutrition have been demonstrated across several animal species. However, there is limited research exploring its role in sheep nutrition. Based on the unique physiological and nutritional demands of lambs, as well as the special challenges associated with intensive farming models, it has become increasingly important to study the effectiveness of A. annua supplements. Consequently, there is demand for further research into the impact of supplementing with WEAA on the intestinal immunity and antioxidant ability of lambs. These studies will provide valuable insights into the potential of A. annua as a natural feed additive in intensive sheep production, and may offer a sustainable and effective approach to improving lamb health and productivity. This research endeavor aims to fill this knowledge gap by evaluating the effects of WEAA supplementation on the intestinal immunity, antioxidant function, and their underlying molecular regulation in lambs, all within the context of conventional feeding practices.
2 Materials and methods
The animals used in the study were from a sheep farm in Hohhot, Inner Mongolia, China. All experimental protocols were approved by the Animal Care and Use Committee of Inner Mongolia Agricultural University (approval no. NND2021097) and followed the ethical guidelines for animal research established by the Laboratory Animal Sciences and Technical Committee (SAC/TC281) of the Standardization Administration of China, in accordance with Laboratory animal—Guideline for ethical review of animal welfare (GB/T 35892-2018) (24, 56).
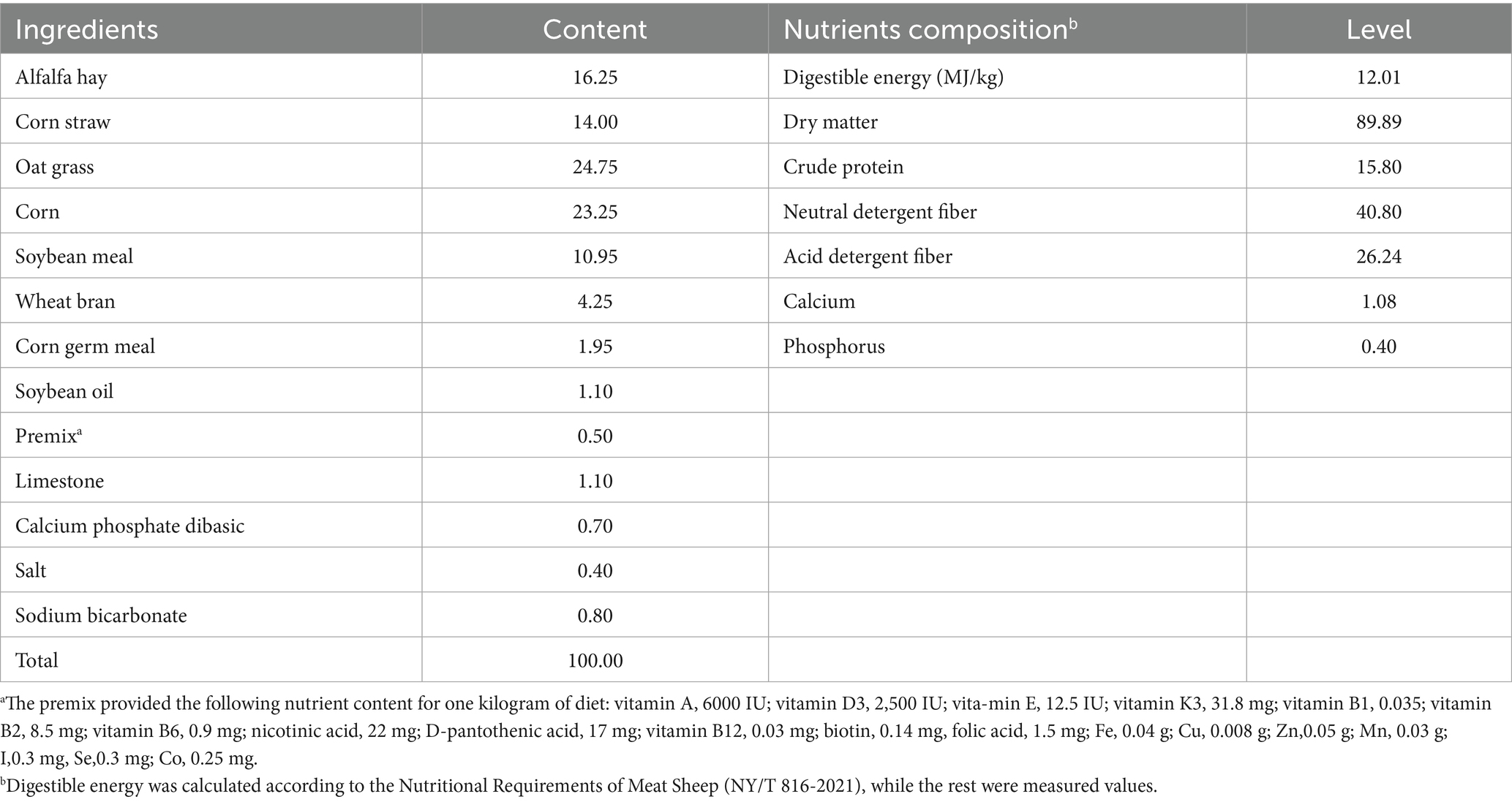
2.1 Animals, diets, and design
In total, 32 female lambs (Dorper × Han) aged 3 months, weighing 24 ± 0.09 kg each, were recruited to the study. These lambs were divided into 4 groups, with 8 lambs in each group. The control group was fed solely a basal diet, whereas the treatment groups were provided with the same basal diet supplemented with different amounts of WEAA: 500, 1,000, and 1,500 mg/kg, respectively. The feeding trial lasted for 75 days (adaptation phase: 15d; formal experimental phase: 60d). The lambs were provided with pelleted feed twice a day (at 08:00 and 16:00 h) with ad libitum access to experimental diets and water. Each of the lambs was kept in individual pen, and their feed intake was carefully monitored to make sure that any leftover feed was over 5% of the total supply. The basic diet was designed to fulfill the Nutritional Requirements of Meat-type Sheep by NY/T 816–2021 (57). In Table 1, the feed components and the chemical makeup of the basal diet are outlined. Small intestinal (duodenum, jejunum and ileum) tissue samples were gathered to assess immune and antioxidative factors, along with the expression of related genes.
2.2 Preparation of WEAA
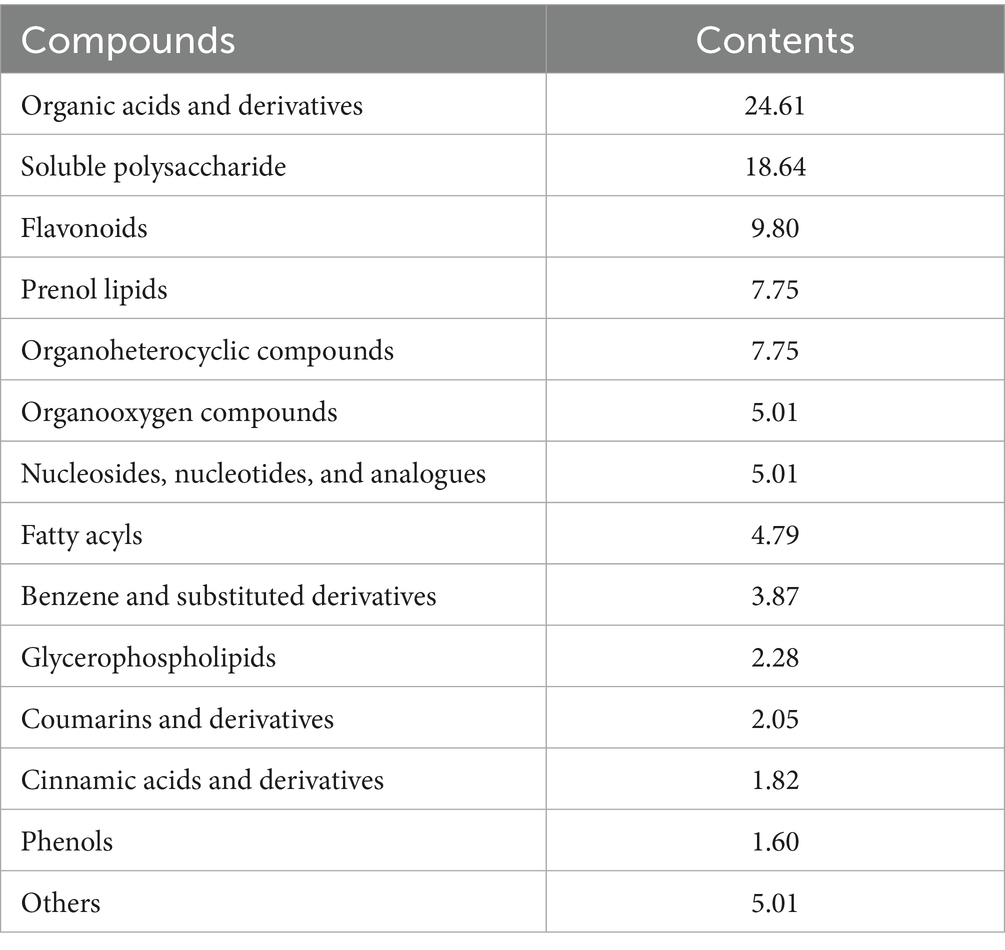
The extract utilized in this research originated from A. annua gathered in Hohhot, Inner Mongolia. The WEAA was produced according to the protocol detailed by Guo et al. (25). Briefly, the A. annua were shade-dried, cut into small pieces, and then processed through decoction, concentration, and finally, freeze-drying. The extraction process involved incubating the plant material in water (1:25 ratio) at 80°C for 7 h. Subsequently, the mixture underwent vacuum filtration, and the filtrate was concentrated at a temperature of 80°C by a rotary evaporator. The concentrated solution was then freeze-dried using an ALPHA1-2LD plus freeze-drying machine (Christ, Germany) until a powdered extract was obtained. All extracts were stored at 4°C for future use. We used UPLC-Orbitrap-MS system to identify the structures of the active ingredients of WEAA, according to the method used by Xin et al. (26). The results are shown in Table 2.
2.3 Sample collection and preparation of intestinal homogenate
On day 60 of the experiment, all 32 lambs (4 groups × 8 replicates, 1 lamb per replicate) were fasted for 12 h and then slaughtered at a commercial slaughterhouse by professionals, following the National Standard Operating Procedures (GB/T 43562-2023, Code of Practice for Livestock and Poultry Slaughtering Operation—Sheep, China). Each lamb was rendered unconscious using an electrical stunning method, exsanguinated via the jugular vein to induce death, and subsequently had their intestinal tissue collected. Samples from the duodenum, jejunum, and ileum were finely minced and homogenized using a hand-held homogenizer was employed in a 0.9% sodium chloride solution, kept ice-cold at 4°C, with a mixture ratio of 10% (w/v). After homogenization, the mixture was centrifuged at 3000 rpm for a duration of 10 min at 4°C. The supernatant obtained was then gathered for further analysis (27).
2.4 Analyses of intestinal immune and antioxidative indicators
Immune and antioxidant markers were evaluated through commercially available assays. Levels of IgA, IgG, IgM, IL-1β, IL-2, IL-4, IL-6, and TNF-α in the duodenum, jejunum, and ileum were determined with ELISA kits (Wuhan Colorful Gene Biological Technology Co., Ltd., Wuhan, China; 96 T per kit each), following the instructions set forth by the manufacturer. Additionally, antioxidant indicators (SOD, CAT, GSH-Px, MDA and T-AOC) were assessed with a V-1000 spectrophotometer (AOE Instruments Shanghai Co., Ltd., Shanghai, China) in the duodenum, jejunum, and ileum. These measurements kits were purchased from Jiancheng Bioengineering Institute, located in Nanjing, China. All procedures were performed in accordance with the manufacturer’s guidelines, and samples were assayed in duplicate.
2.5 Reverse transcriptase-quantitative PCR (RT-qPCR)
2.5.1 RNA extraction and cDNA synthesis
Total RNA was isolated from small intestine tissues (duodenum, jejunum, ileum) using TRIzol reagent (TaKaRa Biotechnology, Dalian, China). RNA concentration was quantified using a Pultton P200 + spectrophotometer (Plextech, San Jose, CA, United States), and purity was assessed by A₂₆₀/A₂₈₀ ratio (1.8–2.1). RNA integrity was verified via 1% agarose gel electrophoresis. Genomic DNA contamination was eliminated by DNase I treatment (TaKaRa). cDNA was synthesized using the PrimeScript RT Master Mix kit (TaKaRa) on a LifeECO thermal cycler (Bori Technology, Hangzhou, China), following the protocol: 37°C for 15 min, 85°C for 5 s.
2.5.2 Quantitative real-time PCR (qRT-PCR)
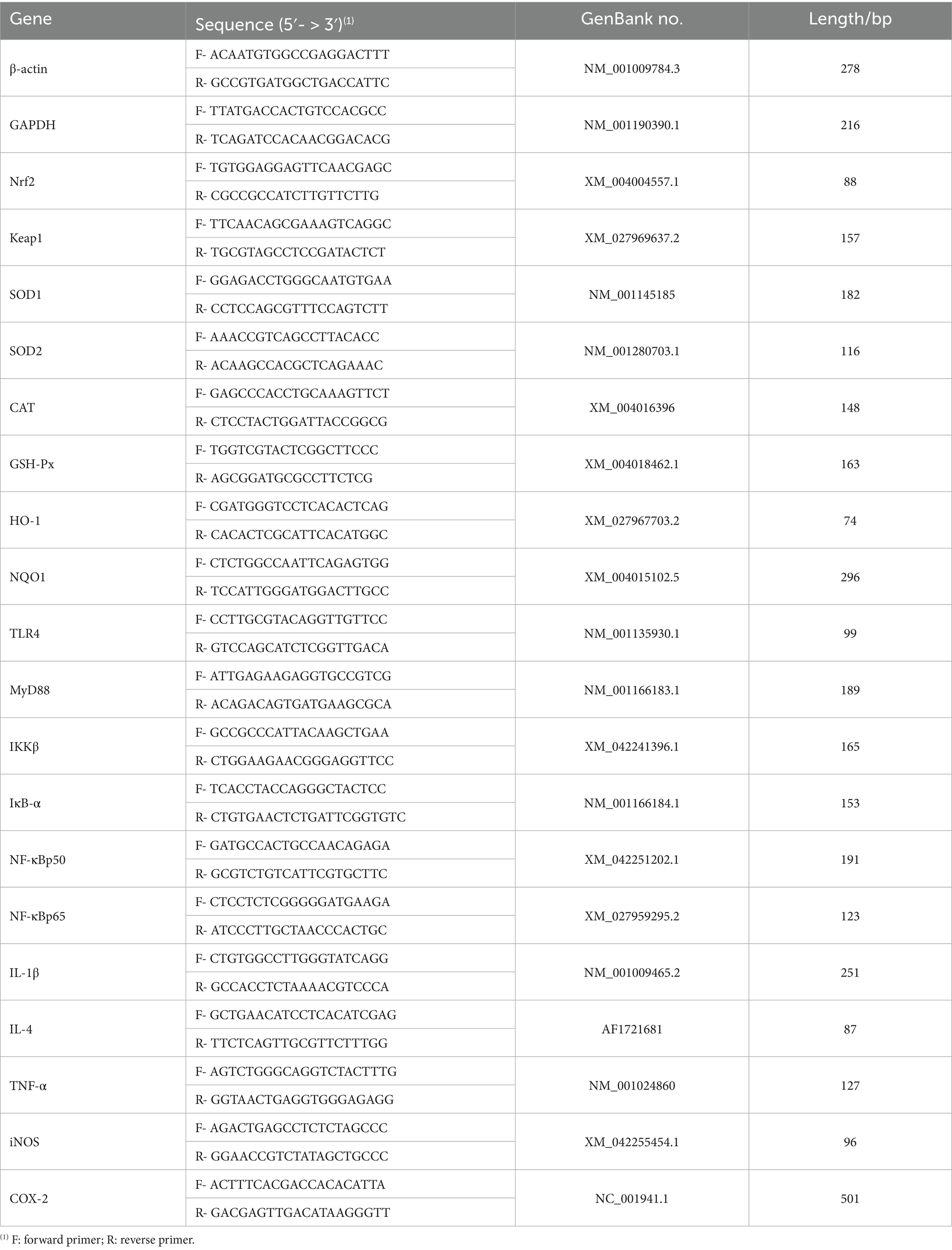
The target genes and their specific primer sequences can be found in Table 3. Quantitative real-time PCR (qRT-PCR) was performed using the LightCycler® 96 System (Roche Diagnostics, Switzerland) and the TB® Premix Ex Taq™ Kit (TaKaRa). qPCR Cycling Conditions: pre—denaturation at 95°C for 30 s; followed by 45 cycles of denaturation at 95°C for 5 s; annealing at 60°C for 30 s; extension at 72°C for 20 s; followed by melting curve analysis (95°C for 15 s, 60°C for 1 min, 95°C for 15 s). All reactions were performed in strict accordance with the manufacturers’ protocols (28).
2.5.3 Data analysis
Melting curves were analyzed to verify single peaks for product specificity. Outliers in triplicate Ct values (n = 3) were excluded, and the relative fold difference in mRNA expression levels was calculated using the 2-∆∆Ct method (58).
2.6 Statistical analysis
All data were first processed using Microsoft Excel 2021 and then subjected to quadratic polynomial regression analysis with SAS Version 9.2 (2008, SAS Institute, Cary, NC, United States). Specifically, the analysis evaluated both linear and quadratic effects of dietary WEAA levels on various indices, in order to determine of dose-dependent relationships. Results were reported as mean and standard error of the mean (SEM), with p < 0.05 as the threshold for statistical significance. Additionally, a p-value within the range of 0.05 ≤ p < 0.10 was considered as a tendency.
3 Result
3.1 Small intestine inflammatory cytokines and immunoglobulins
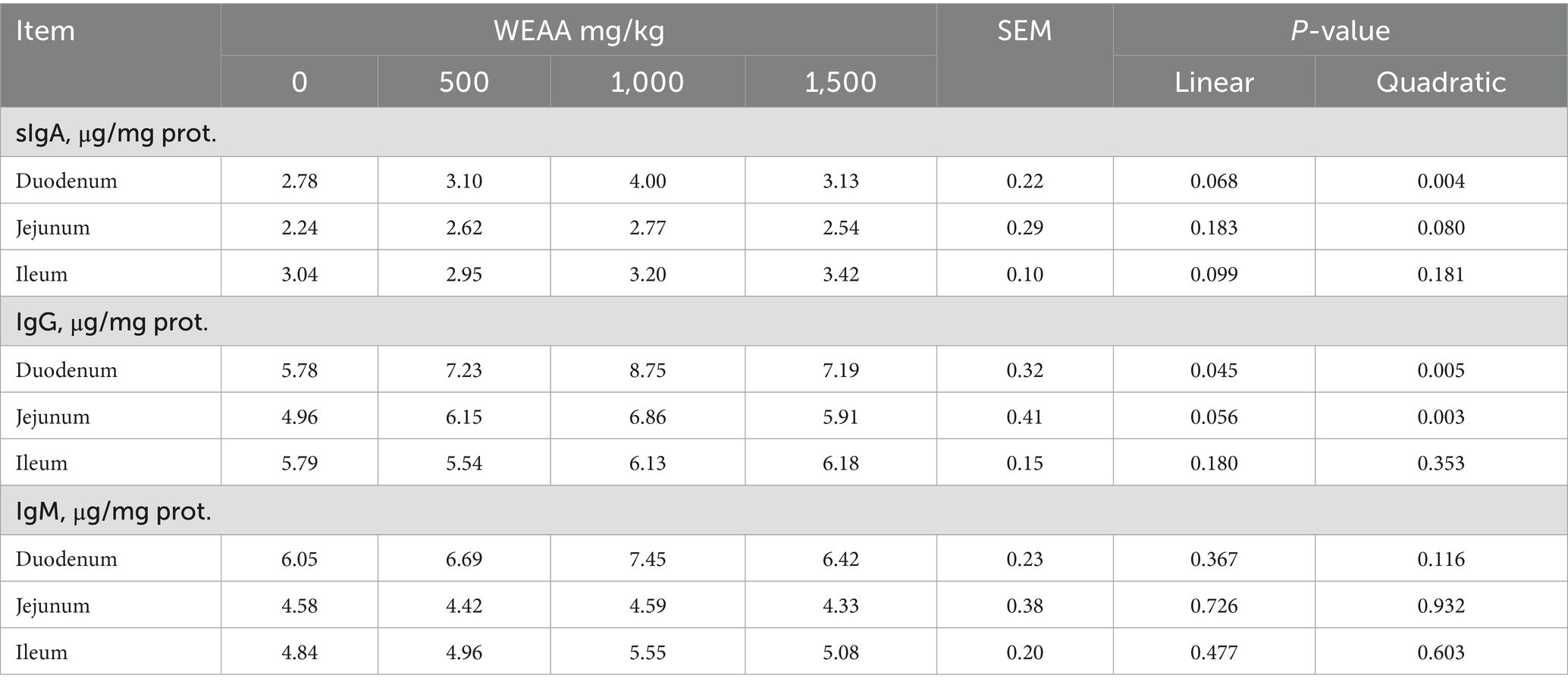
The influence of WEAA supplementation on immunoglobulin levels in the small intestine of lambs is shown in Table 4. With increasing WEAA supplementation, the duodenal sIgA level demonstrated a linear upward trend (p < 0.10) and a significant quadratic increase (p < 0.05), whereas the IgG level exhibited a linear or quadratic increase (p < 0.05). The jejunal sIgA level demonstrated a quadratic increasing trend (p < 0.10), while IgG levels exhibited a linear upward trend (p < 0.10) and a significant quadratic increase (p < 0.05), peaking at 1000 mg/kg WEAA group. Additionally, WEAA supplementation did not significantly affect immunoglobulin levels in ileum tissue (p > 0.10).
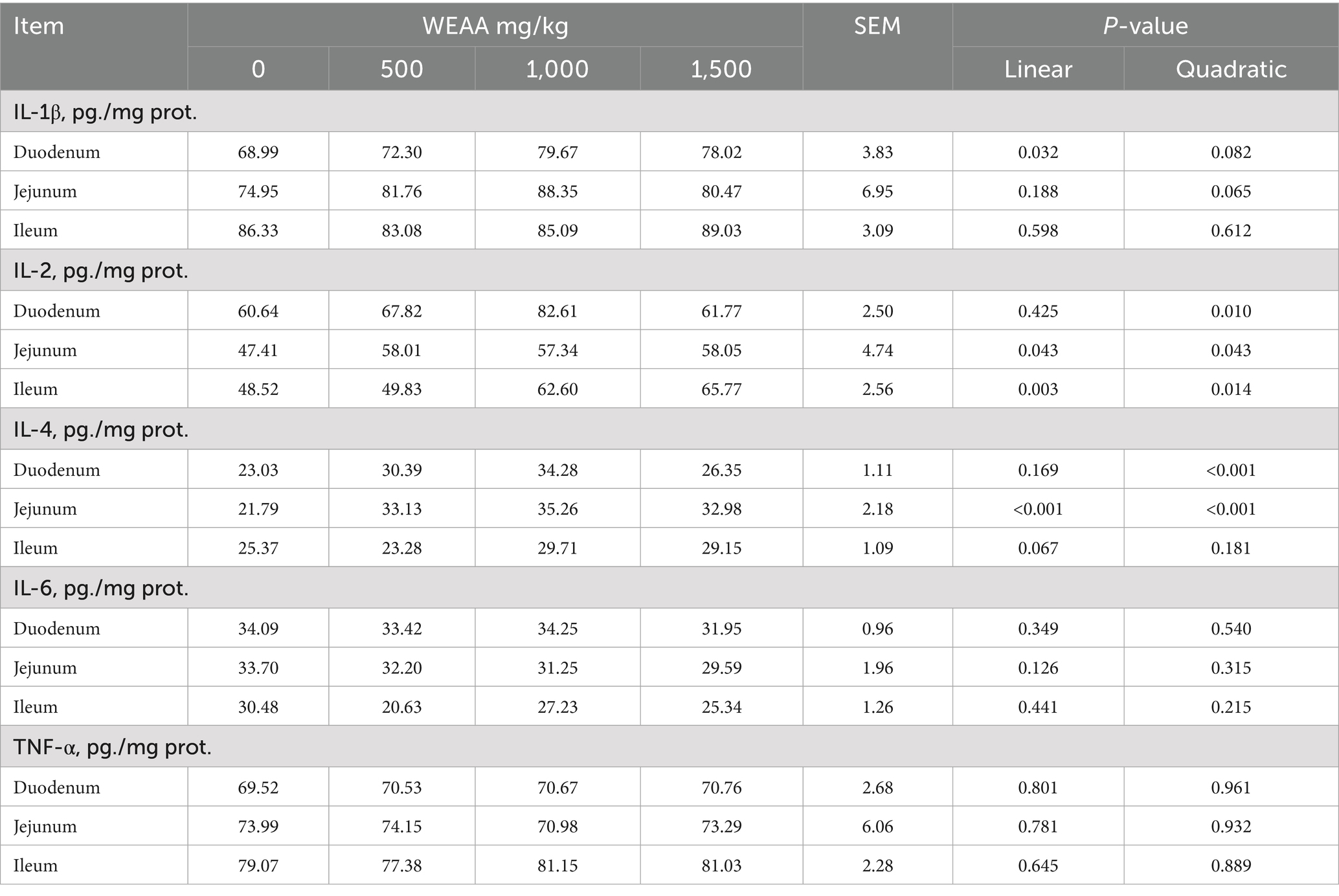
In Table 5, intestinal inflammatory cytokine contents were shown. With increasing WEAA supplementation, IL-1β content of duodenum tissue demonstrated a significant linear increase (p < 0.05) and a quadratic trend (p < 0.10), while jejunal IL-1β level exhibited a quadratic upward trend (p < 0.10). Additionally, the duodenal IL-2 and IL-4 levels exhibited a significant quadratic increase (p < 0.05), and the jejunal and ileal IL-2 levels showed a linear or quadratic increase (p < 0.05), while jejunal IL-4 level exhibited a significant linear or quadratic increase (p < 0.05). Meanwhile, the ileal IL-4 content tended to increase linearly (p < 0.10).
3.2 Small intestine antioxidative indexes
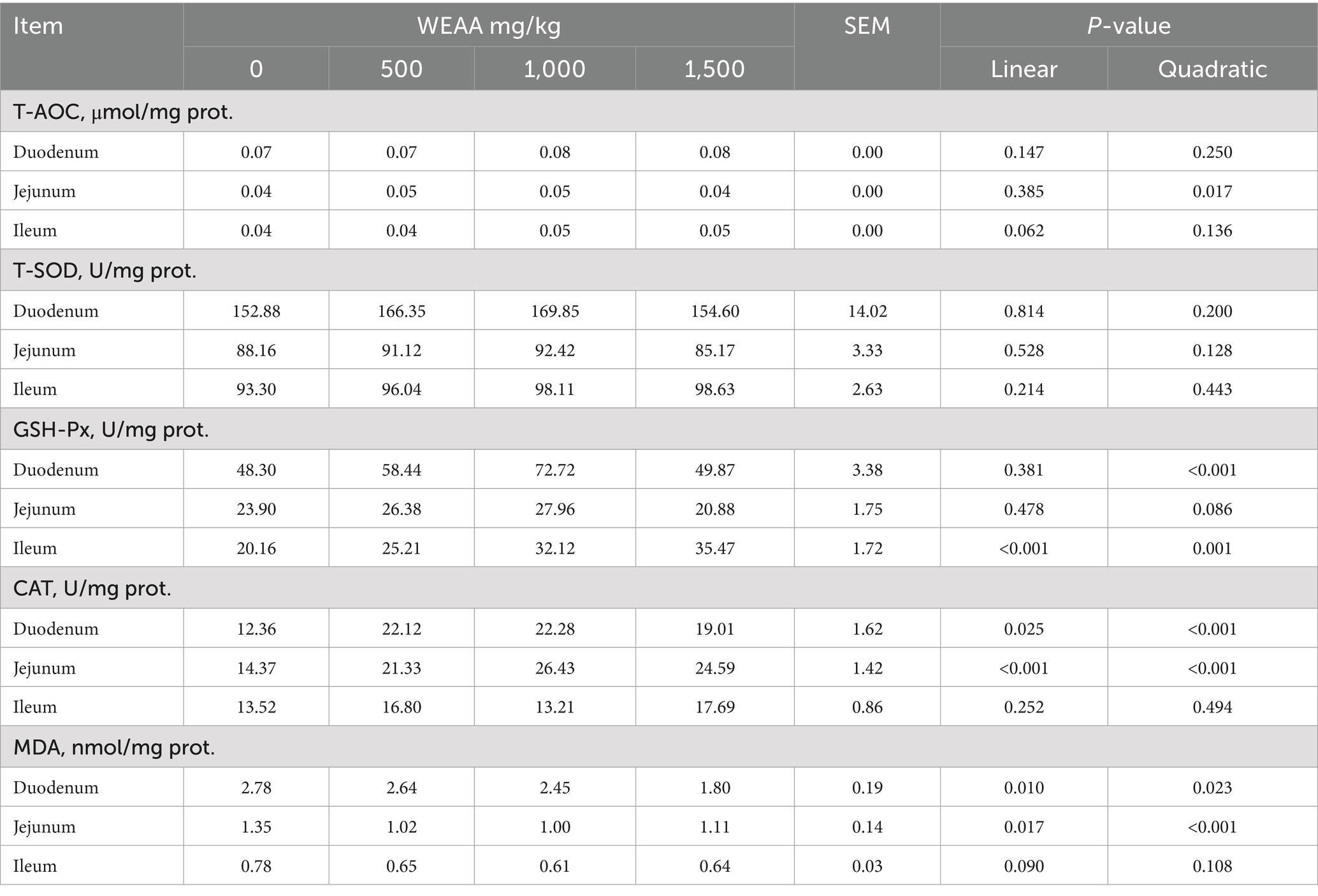
The effects of WEAA on the antioxidative indexes in the intestinal tissue of lambs is showed in Table 6. With increased WEAA supplementation, the jejunal T-AOC demonstrated a significant quadratic increase (p < 0.05), while the ileal T-AOC exhibited a linear increasing trend (p < 0.10). The duodenal GSH-Px concentration demonstrated a significant quadratic increase (p < 0.05), and jejunal GSH-Px concentration tended to increase quadratically (p < 0.10). Moreover, with the increase of WEAA dose, the ileal GSH-Px, duodenal and jejunal CAT concentration revealed a significant linear or quadratic increase effect (p < 0.05), while the duodenal and jejunal MDA content demonstrated a linear or quadratic decrease effect (p < 0.05), in addition, the concentration of MDA in the ileal tended to decline linearly (p < 0.10).
3.3 Duodenum TLR4/NF-κB and Nrf2/Keap1 pathway related gene expression
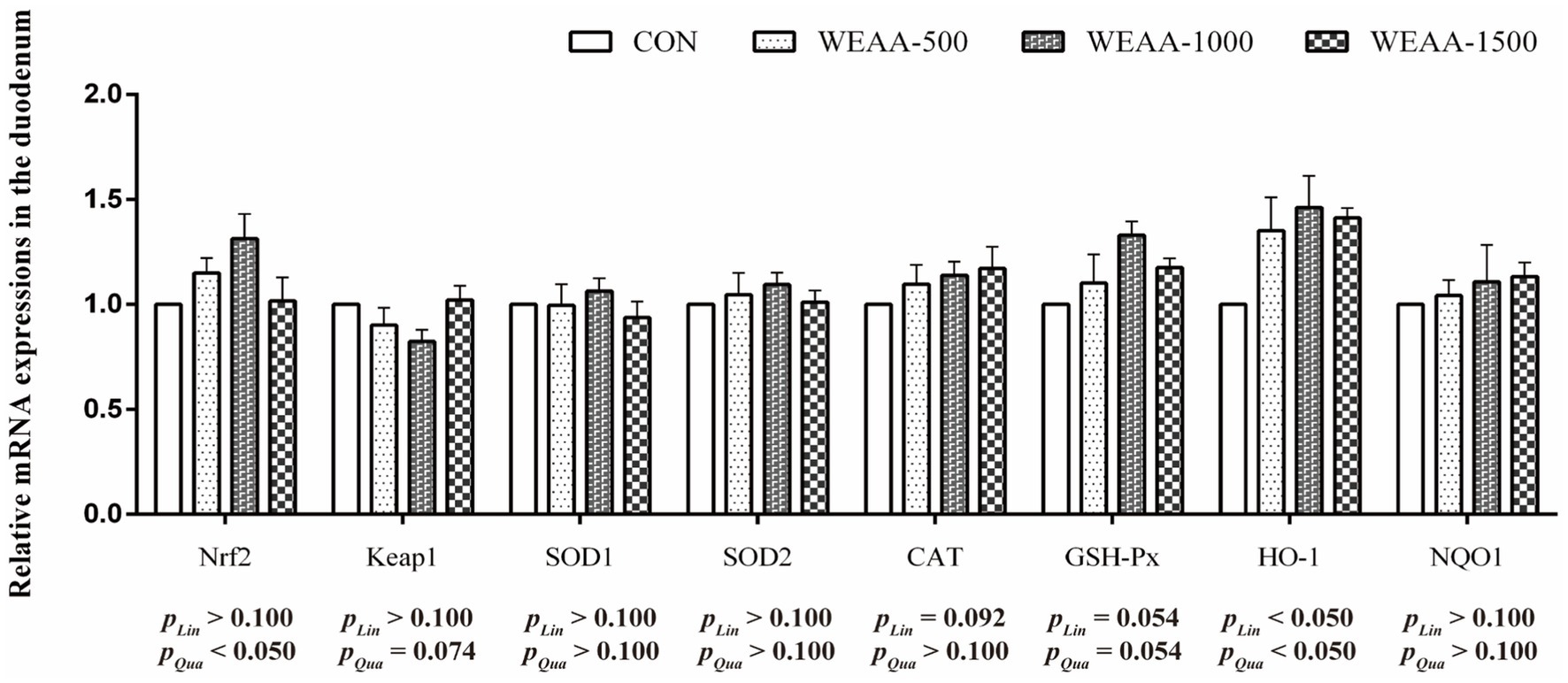
Figure 1 shows the levels of mRNA expressions of immune-related genes in the duodenum. With the supplementation of WEAA increased, the expression levels of TLR4, NF-κBp50 and IL-1β demonstrated a significant linear or quadratic increase effect (p < 0.05). The expression levels of MyD88 exhibited a linear upward trend (p < 0.10). The expression levels of IKKβ demonstrated a linear increase (p < 0.05). Meanwhile, the expression levels of IκB-α exhibited a linear increase (p < 0.05) and a quadratic increasing trend (p < 0.10). In addition, the IL-4 expression exhibited a linear increasing trend (p < 0.10), and a significant quadratic increase (p < 0.05).
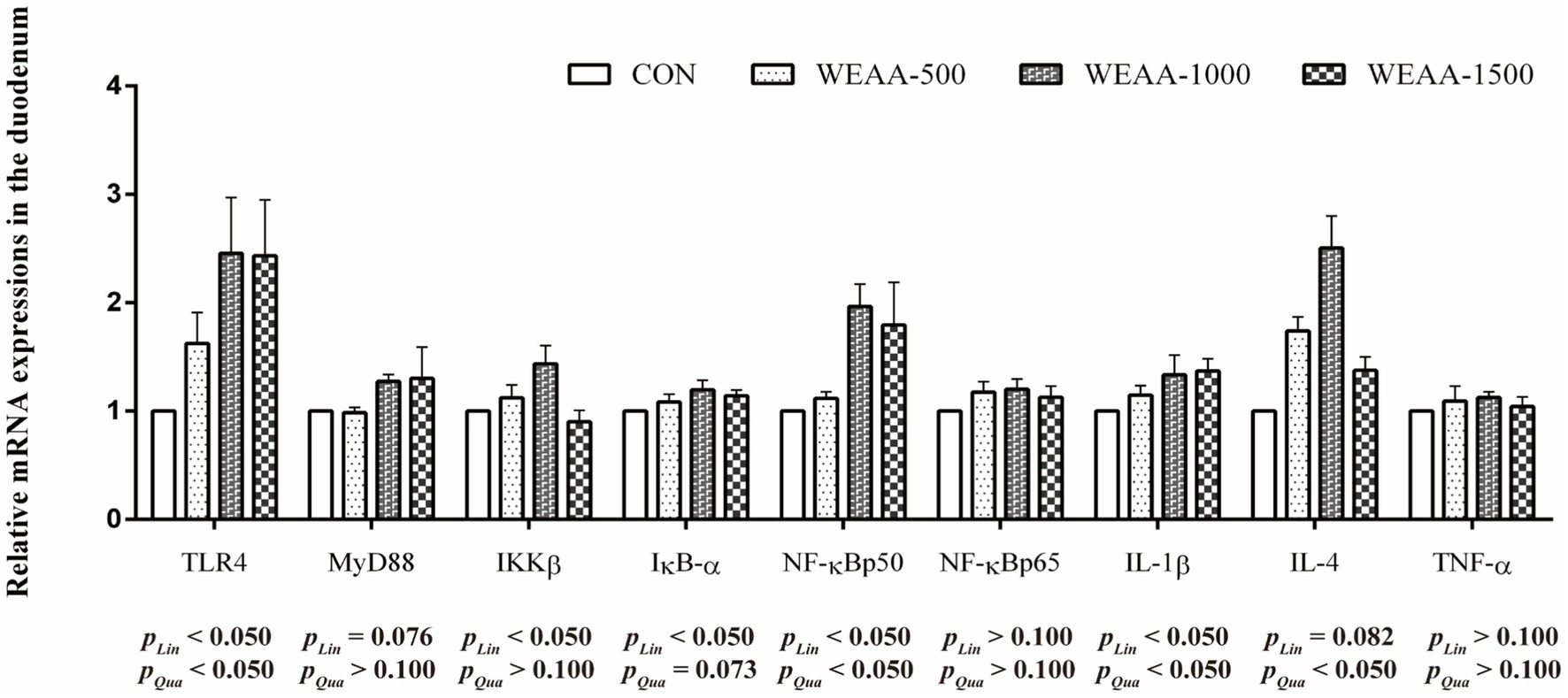
Figure 1. Effects of WEAA on expression of immune-related genes in the duodenum of lambs. Dose-dependent effects of WEAA (Lin: Linear; Qua: Quadratic) and the same as below.
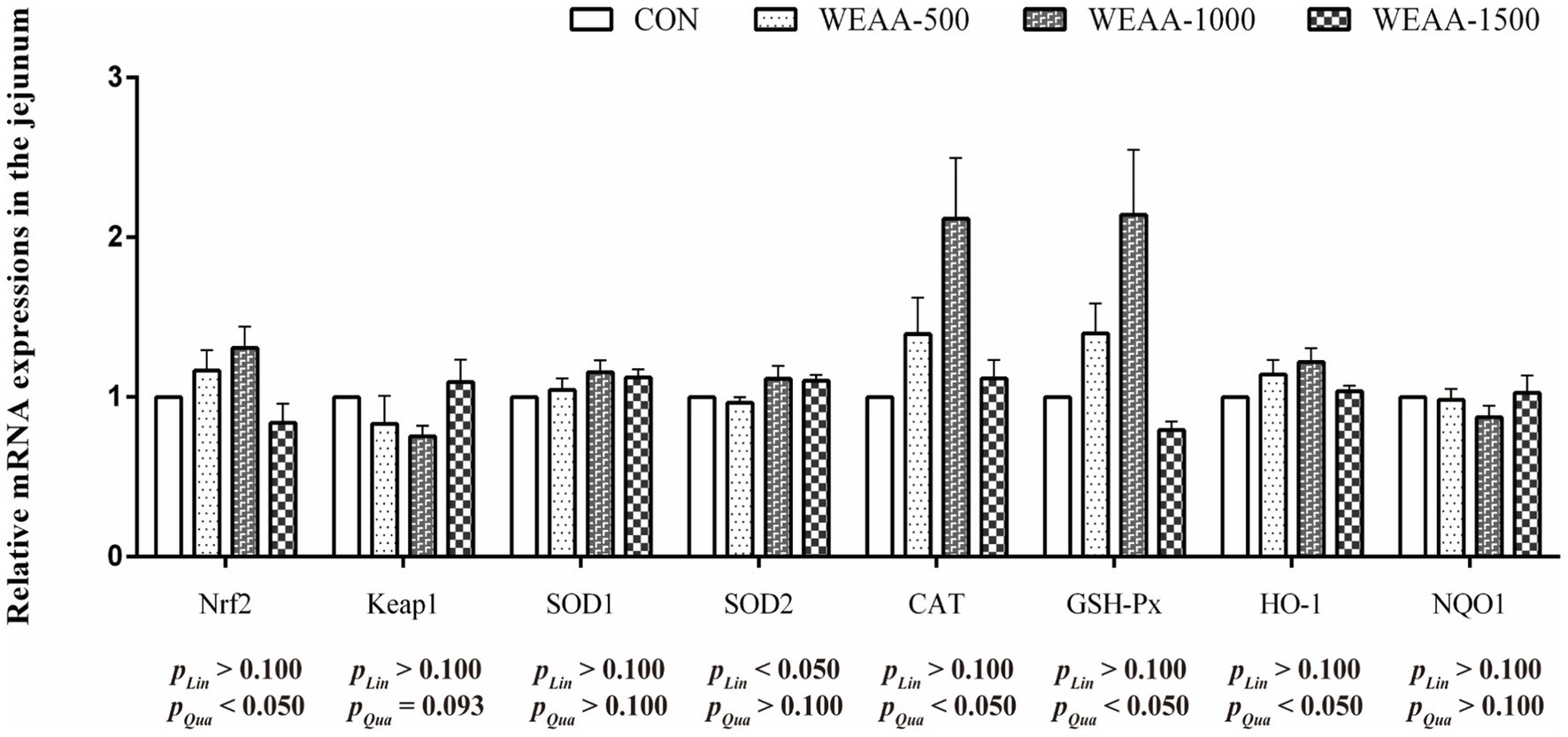
Figure 2 showed the levels of mRNA expression related to antioxidant genes within the duodenum. With the supplementation of WEAA increased, the expression levels of Nrf2 revealed an effect of increasing quadratically (p < 0.05). The expression levels of Keap1 demonstrated a significant quadratic downward trend (p < 0.10), while the expression levels of CAT and GSH-Px revealed a linear upward trend (p < 0.10) and GSH-Px gene expression revealed a quadratic increasing trend (p < 0.10). Moreover, with the supplementation of WEAA increased, the expression level of HO-1 revealed a significant linear or quadratic increase (p < 0.05).
3.4 Jejunum TLR4/NF-κB and Nrf2/Keap1 pathway related gene expression
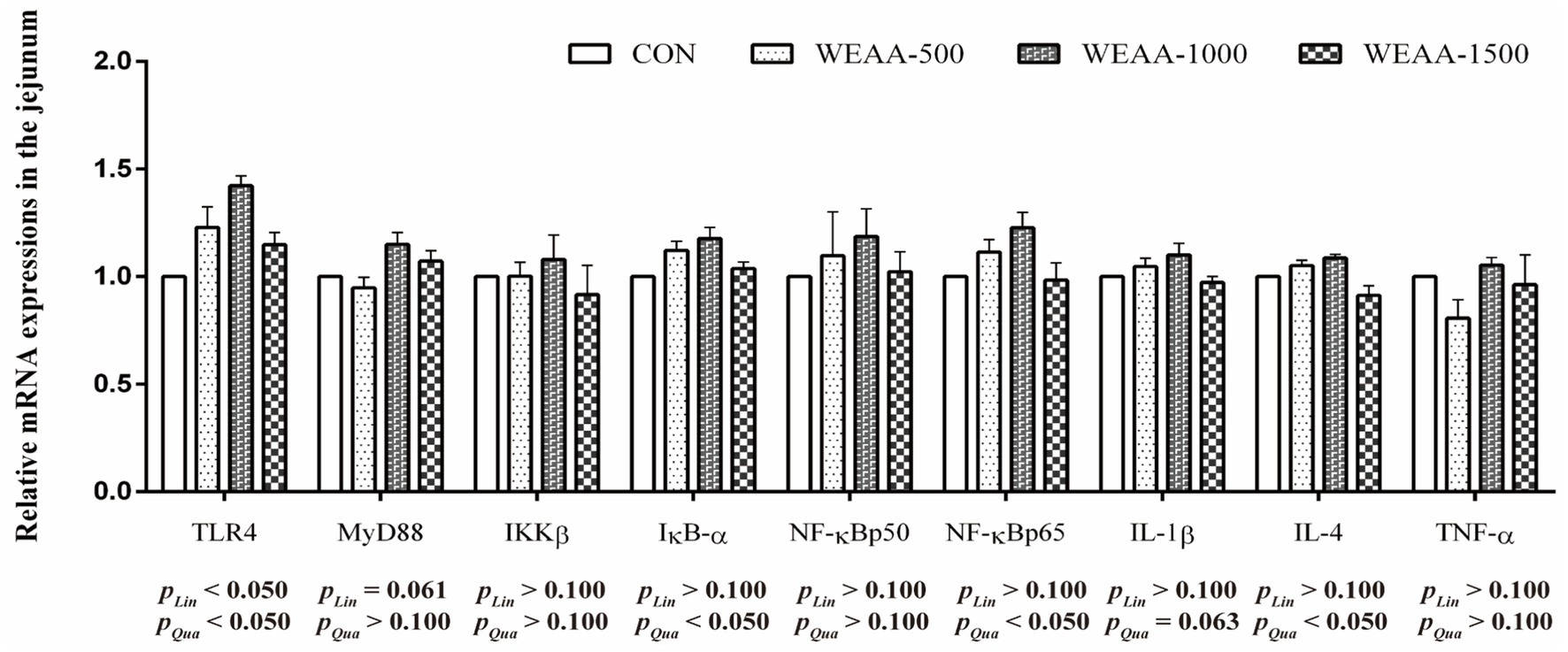
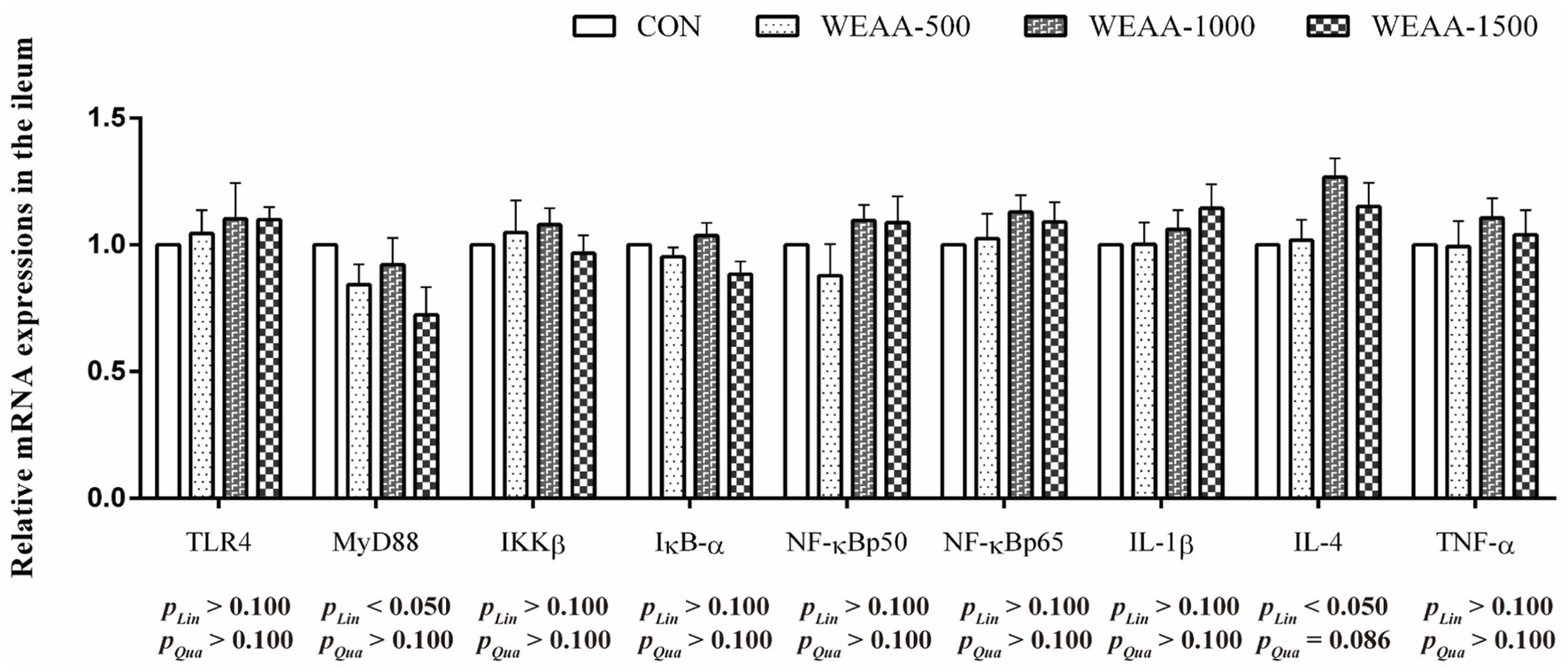
Figure 3 revealed the relative mRNA expressions levels of immune-related genes in the jejunum. With the supplementation of WEAA increased, the expression of TLR4 demonstrated a significant linear or quadratic increase (p < 0.05). The expression levels of MyD88 demonstrated a linear increasing trend (p < 0.10). Meanwhile, the expression of IκB-α, NF-κBp65 and IL-4 demonstrated a significant quadratic increase (p < 0.05), and the expression of IL-1β revealed a quadratic upward trend (p < 0.10).
Figure 4 showed the relative mRNA expressions of relative antioxidant genes in the jejunum. With the supplementation of WEAA increased, Nrf2, CAT, GSH-Px and HO-1 demonstrated a significant quadratic increase (p < 0.05). The expression levels of Keap1 revealed a quadratic downward trend (p < 0.10). Meanwhile, the expression of SOD2 demonstrated a significant linear increase effect (p < 0.05).
3.5 Ileum TLR4/NF-κB and Nrf2/Keap1 pathway related gene expression
Figure 5 demonstrated the relative expressions of immune-related genes in the ileum. With the supplementation of WEAA increased, the expression of MyD88 and IL-4 revealed a significant linear increase (p < 0.05). Meanwhile, the IL-4 gene expression demonstrated a quadratic increasing trend (p < 0.10).
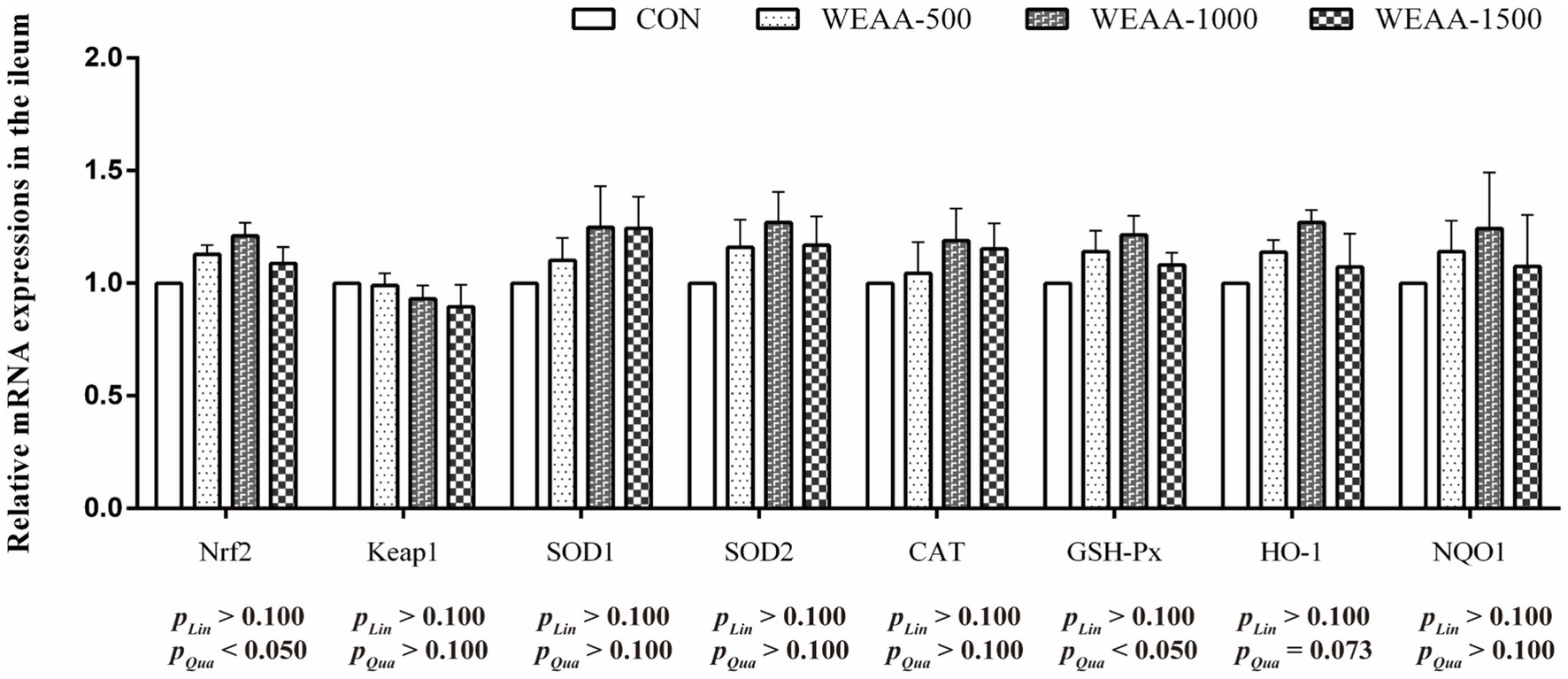
Figure 6 showed the relative expressions levels of relative antioxidant genes in the ileum. With the supplementation of WEAA increased, the expression levels of Nrf2 and GSH-Px revealed a significant quadratic increase (p < 0.05). Meanwhile, the HO-1 expression demonstrated a quadratic upward trend (p < 0.10).
4 Discussion
A. annua is a traditional medicinal plant from China, well-known for its significant biological effectiveness and minimal toxicity. It is typically prepared as decoctions to break down the plant cell wall, thus aiding in the release of bioactive constituents (29). Notably, among these constituents are polysaccharides, flavonoids, and sesquiterpenes, which have particular relevance in the field of immunopharmacology (22) A. annua contains a variety of active components, such as flavonoids, polysaccharides, and sesquiterpenes (17, 30). In the present study, the identification of active ingredients by structure analysis for WEAA indicated the presence of organic acids, polysaccharide, flavonoids and prenol lipids, which were the dominant substances (Table 2). Previous research indicated that flavonoids from Artemisia ordosica that significantly boost antioxidant enzyme activity when added to broiler diets (31). Moreover, extracts from A. annua have shown antioxidative properties in mouse models (32). Additionally, polysaccharides obtained from the leaves of Artemisia argyi have been found to possess antioxidant and immune-boosting abilities (33, 34).
This study observed elevated sIgA and IgG concentrations in the duodenum and jejunum of lambs as dietary intake of WEAA increased. This result agrees with the findings of Guo et al. (35), who demonstrated that a diet high in WEAA increased the concentrations of sIgA, IgG, and IgM in the small intestine of broiler chickens. Immunoglobulins, which are antigen-specific receptors, are essential for humoral immunity as they bind to specific antigens, such as flavonoids and plant polysaccharides, thereby triggering immune cell signaling that initiates effective adaptive immune responses (36). Specifically, sIgA is essential for the maintenance of intestinal homeostasis, that is, maintaining the integrity of biological barriers and limiting microorganism and antigen exposure. Moreover, the immune response is directly influenced by IgG, which is produced by B cells (37). Supporting these findings, Yang et al. (38) demonstrated that the inclusion of alcohol extracts from Artemisia argyi in the diet mitigated the adverse effects induced by LPS challenges. This effect is attributed to an increase in immunoglobulin levels (IgA, IgG, and IgM) in the small intestine of broiler chickens, suggesting an enhanced immune response in the intestinal tract. Moreover, Zhang et al. (39) investigated impact of water extracts from Artemisia rupestris L. (AEAR) in both in vivo and in vitro, showing that AEAR significantly increased IgG titers in ICR mice, partly by promoting dendritic cell maturation via the TLR4 signaling pathway, thereby facilitating immune cell proliferation, differentiation, and autoantibody production.
TNF-α, IL-1β, and IL-6 are crucial inflammatory cytokines in the intestinal immune system, where they activate adaptive immune responses to combat pathogenic bacterial invasion. However, their excessive expression can damage mucosal cells. Song et al. (23) demonstrated that exposure to heat enhanced the TLR4 and pro-inflammatory cytokines expression in the intestinal of broiler chickens. However, pretreatment with EA mitigated the increase of IL-1β expression levels of TLR4 and IL-6 in the intestinal regions under heat stress. Additionally, a dietary supplement of 0.1% A. annua extract led to a decrease in both the content and expression of inflammatory cytokines within the small intestine of both neonatal and post-weaning piglets (15). These results suggest that A. annua plays a beneficial role in alleviating intestinal inflammation. Yang et al. (40) also demonstrated that AEAR stimulated the content of IL-1β and IL-6 through the activation of TLR2/4 signaling pathways in dendritic cells, without inducing toxicity. Du et al. (41) demonstrated that polysaccharides derived from Artemisia ordosica significantly stimulated inflammatory cytokine production in the small intestine with a dose-dependent manner, thereby improved the immune function of broilers under conventional feeding conditions. Similarly, Artemisia argyi polysaccharide at an optimal dosage regulated immune functions, elevating cytokine levels (IL-1β, IL-2, and IL-4) and IFN-γ in broiler serum (42). The present study suggested that supplementing the diet with WEAA might enhance the content and gene expression of IL-1β, IL-2, and IL-4 in the duodenum and jejunum of lambs. These results suggested that WEAA could play a significant role in modulating immune responses.
In order to delve deeper into the molecular mechanisms underlying the impact of WEAA addition on intestinal immunoregulation, we detected the expression levels of crucial mRNAs associated with the TLR4/NF-κB signaling cascades. A. annua, a well-known medicinal plant, primarily comprises polysaccharides, flavonoids, and artemisinin as its main components which are noted for their structural diversity and enhance their affinity with various pattern recognition receptors (PRRs), thereby initiating signal pathways related to immune response. TLR4, a pattern recognition receptor belonging to the TLR family. This receptor is essential for immune responses and is present in nearly all types of intestinal epithelial cells. Research identified that water-soluble polysaccharide from Artemisia rupestris L. interacted with TLR2/4 on dendritic cells, facilitating cytokine production and signal transduction processes. This interaction not only enhanced the phosphorylation levels of IKKβ and NF-κBp65 but also stimulated the degradation of IκB-α in the cytoplasm and promoted the translocation of NF-κBp65 into the nucleus (39). These mechanism insights suggest that the elicitation of NF-κB via TLR2/4 pathways by these polysaccharides is crucial for cytokine synthesis. Further studies by Ando et al. (43) uncovered that safflower polysaccharides could activate TLR4/NF-κB pathway, thereby inducing cytokine production in macrophages. Additionally, Guo et al. (44) found that A. annua polysaccharide alleviated excessive release of intestinal pro-inflammatory factors induced by E. coli challenge in broilers via inhibiting TLR4/MyD88/NF-κB signaling pathway, and up-regulated anti-inflammatory factor IL-4 and immunoglobulins (IgA, IgG, IgM) to synergistically enhance immune regulation; moreover, A. annua polysaccharide also increased serum IgA in non-challenged broilers, reversed E. coli-induced decrease in serum IgM and jejunal IgG/IgM, and promoted IL-4 secretion, furthermore, enhanced anti-inflammatory effect. These results suggest that A. annua polysaccharide coordinates immune regulation by modulating TLR4/NF-κB pathway and immune effector molecules. The present study found that dietary supplementation with WEAA could significantly enhance TLR4 expression in the duodenum and jejunum of lambs, and up-regulated the gene expression levels of its downstream signaling molecules MyD88, IKKβ, IκBα, NF-κBp50 and NF-κBp65. The results suggest that WEAA may recognize extracellular signals through activating TLR4 receptors, thereby initiating the cascade activation of the TLR4/MyD88/NF-κB signaling pathway. It was noteworthy that the significant increase in IκBα expression might be due to the transcription and expression of IκBα induced by the pathway to form negative feedback regulation to limit the excessive activation of NF-κB (45), thereby avoiding excessive inflammatory response in intestinal tissues. Generally, the activation of the NF-κB signaling pathway promotes the pathway’s binding to the κB response element of target genes, and initiates the transcription of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, mediates inflammatory responses and immune responses, and indirectly promotes the production of immunoglobulins (46), regulates the secretion of T cell-related cytokines (IL-2), and affects the secretion of IL-4 by Th2 cells, thereby enhancing the body’s immune function from multiple dimensions (47). Some studies found that AEAR significantly increased the gene expression of IL-1β and IL-2 and the protein expression level of NF-κB in dendritic cells, which significantly enhanced the immunomodulatory activity of dendritic cells (40). Furthermore, polysaccharides derived from Artemisia ordosica were reported to enhance the contents of immunoglobulins (IgG, IgA, IgM) and cytokines (IL-1β, IL-2, IL-4), also to increase the mRNA expression of the TLR4, MyD88, NF-κBp50, and IL-1β in the small intestine of broilers (41). Similarly, the current study revealed that supplementing WEAA into the diet up-regulated the mRNA expression of IL-1β in the duodenum and jejunum of lambs, which was consistent with the increase in its content. Additionally, the trend of its change was similar to the expression of NF-κB. Meanwhile, the content of IL-4 and its mRNA expression level were also significantly increased, demonstrating the anti-inflammatory activity of WEAA. Based on the above results, it is speculated that under normal physiological conditions, WEAA can synergistically improve the immune regulation function of the body by regulating the dynamic balance of the TLR4/NF-κB signaling pathway. In addition, a relatively significant change in relative expressions of the related factors was noted in the small intestine that is located in its proximal and middle regions of lambs.
Reactive oxygen species (ROS) are produced throughout the normal growth and metabolic processes of mammals. They play a crucial and continuous role in several functions, including intracellular signal transduction and the modulation and activation of immune responses. If these radicals are not promptly scavenged, they accumulate in the body, damaging cellular morphology and function. The peroxidation of unsaturated lipids within biological membranes results in the formation of lipid peroxides and malondialdehyde, which precipitate a cascade of inflammatory changes (48). The antioxidant defense system has a variety of endogenous antioxidant enzymes in cells, which are the first line of defense against oxidative stress. Specifically, Mn-SOD catalyzes the conversion of O2− to e H2O2 and plays a significant role in activating associated signaling pathways that promote the activity of SOD1, CAT, GSH-Px, HO-1, thereby facilitating the detoxification of H2O2 into H2O (49). In the current research, dietary WEAA was found to improve the activity of CAT and GSH-Px in the duodenum. Meanwhile, it increased the activity of T-AOC, CAT, and GSH-Px in the jejunum, and the activity of GSH-Px in the ileum of lambs. This finding aligned with a prior study by Zhang et al. (50) on broilers, which stated that dietary Artemisia argyi powder could enhance intestinal antioxidative enzyme activities. Moreover, this study found that an increase in the relative gene expressions of CAT and GSH-Px were observed in the small intestine, and the changes in relative gene expression were consistent with the change in enzymatic activity. These results suggest that WEAA may improve small intestinal antioxidant capacity by regulating gene expression and activity of the antioxidant enzymes. By contrast, MDA is a reliable biomarker for the assessment of oxidative stress (51). The current study demonstrated that the dietary supplementation of WEAA to lambs MDA levels were found to decrease in duodenum and jejunum of lambs. The observation of Wan et al. (8) that A. annua leaves and EA can enhance antioxidant enzyme activities, while simultaneously reducing MDA levels in both the serum and liver of broilers support our research findings. This finding underscores the potential for in vivo antioxidant defense. Furthermore, Song et al. (23) reported that A. annua was rich in polysaccharides, phenolic and flavonoids. Their researches demonstrated that dietary addition with EA could alleviate the elevation of MDA concentration in the intestine induced by heat stress and relieve the stress response in broilers following such stress. As we all know, under the normal physiological conditions or the status with low levels of lipid peroxidation, organisms have an inherent capacity to regulate cellular metabolism. This regulatory mechanism via the activation of related signaling pathways to effectively enhance the activity of antioxidant enzymes. Consequently, this leads to an adaptive stress response that helps maintain cellular homeostasis (52). Nrf2 is a key regulatory factor with intracellular antioxidant and anti-inflammatory damage. Nrf2 dissociates from Keap1 into the nucleus and binds to antioxidant response elements (ARE), promoting the transcription of cell protective factors and upregulating the expression of related antioxidant genes. This leads to an increase in intracellular antioxidant enzyme levels, thereby clearing free radicals and reactive oxygen species, and resistance to oxidative damage (53). In addition, the activation of the Nrf2-ARE signaling pathway not only has the effect of anti-oxidative stress, but also enhance the body’s immune response (54). Therefore, the Nrf2/Keap1 signaling pathway, as a key protective pathway, has important biological significance for the immune and antioxidant functions of the body. Niu et al. (55) when they fed weaned piglets with EA, it could improve the intestinal antioxidant status. Specifically, the mRNA expression of Nrf2 and HO-1 was raised. Xing et al. (46) further suggested that the effect of adding polysaccharides derived from Artemisia ordosica to diet on lipopolysaccharide induced oxidative stress in broilers, and found can alleviate oxidative stress in broilers by regulating the Nrf2 signaling pathway and increasing the production of antioxidant enzymes. The current research demonstrated that WEAA greatly enhanced Nrf2 and HO-1 expression levels in intestine, accompanied by a significant decrease in Keap1 gene expression. These findings showed that WEAA might enhance the antioxidant activity of intestinal tissue by activating Nrf2-mediated pathway related mRNA expressions. Therefore, WEAA emerges as a promising green feed additive capable of enhancing immunomodulatory and antioxidant capabilities within the intestines of lambs.
5 Conclusion
To summarize, this research showed that dietary supplementation of WEAA in lambs effectively modulated the TLR4/NF-κB signaling pathway in the duodenum and jejunum, promoting the production of immunoglobulins (sIgA, IgG) and cytokines (IL-1β, IL-2, IL-4). Concurrently, WEAA significantly upregulated Nrf2/HO-1 expression while downregulating Keap1, thereby activating the antioxidant pathway and enhancing antioxidant enzyme (CAT, GSH-Px) activity. The study determined that the optimal dosage of WEAA in lamb’s diet was 1,000 mg per kg of feed. Future studies should explore the molecular mechanisms for WEAA regulating TLR4/NF-κB and Keap1/Nrf2 pathways.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The animal study was approved by Animal Care and Use Committee of Inner Mongolia Agricultural University (approval code: NND2021097). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
GG: Conceptualization, Formal analysis, Methodology, Visualization, Writing – original draft. RG: Resources, Writing – review & editing. HZ: Investigation, Writing – review & editing. XJ: Data curation, Writing – review & editing. YXi: Validation, Writing – review & editing. LH: Supervision, Writing – review & editing. SY: Conceptualization, Supervision, Writing – review & editing. YXu: Supervision, Validation, Writing – review & editing. BS: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Basic Research Fund for Universities in Inner Mongolia Autonomous Region (grant no. BR22-01-35) and the Basic Research Fund for Universities in Inner Mongolia Autonomous Region (grant no. BR22-13-13).
Acknowledgments
We would like to thank our laboratory colleagues for their assistance in the data and sample collection, and laboratory analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kasapidou, E, Basdagianni, Z, Papadopoulos, V, Karaiskou, C, Kesidis, A, and Tsiotsias, A. Effects of intensive and semi-intensive production on sheep Milk chemical composition, physicochemical characteristics, fatty acid profile, and nutritional indices. Animals. (2021) 11:2578. doi: 10.3390/ani11092578
2. Rigby, RJ, Carr, J, Orgel, K, King, SL, Lund, PK, and Dekaney, CM. Intestinal bacteria are necessary for doxorubicin-induced intestinal damage but not for doxorubicin-induced apoptosis. Gut Microbes. (2016) 7:414–23. doi: 10.1080/19490976.2016.1215806
3. Ding, W, Lu, Y, Xu, B, Chen, P, Li, A, Jian, F, et al. Meat of sheep: insights into mutton evaluation, nutritive value, influential factors, and interventions. Agriculture. (2024) 14:1060. doi: 10.3390/agriculture14071060
4. Zhang, Q, Zhao, Y, Li, Y, Guo, X, Guo, Y, Ma, G, et al. Dietary polysaccharide-rich extract from noni (Morinda citrifolia L.) fruit modified ruminal fermentation, ruminal bacterial community and nutrient digestion in cashmere goats. Animals. (2023) 13:221. doi: 10.3390/ani13020221
5. Shyer, AE, Huycke, TR, Lee, C, Mahadevan, L, and Tabin, CJ. Bending gradients: how the intestinal stem cell gets its home. Cell. (2015) 161:569–80. doi: 10.1016/j.cell.2015.03.041
6. Prathap, P, Chauhan, SS, Flavel, M, Mitchell, S, Cottrell, JJ, Leury, BJ, et al. Effects of sugarcane-derived polyphenol supplementation on methane production and rumen microbial diversity of second-cross lambs. Animals. (2024) 14:905. doi: 10.3390/ani14060905
7. Faryabi, R, Mousaie, A, Bahrampour, J, and Barazandeh, A. The effect of dietary inclusion of Artemisia sieberi leaves on growth performance, feeding behaviors, ruminal fermentation, feed digestibility, and blood hemato-biochemical profile of growing male lambs. Trop Anim Health Prod. (2023) 55:41. doi: 10.1007/s11250-023-03455-0
8. Wan, XL, Niu, Y, Zheng, XC, Huang, Q, Su, WP, Zhang, JF, et al. Antioxidant capacities of Artemisia annua L. leaves and enzymatically treated Artemisia annua L. in vitro and in broilers. Anim Feed Sci Technol. (2016) 221:27–34. doi: 10.1016/j.anifeedsci.2016.08.017
9. Wang, J, Deng, L, Chen, M, Che, Y, Li, L, Zhu, L, et al. Phytogenic feed additives as natural antibiotic alternatives in animal health and production: a review of the literature of the last decade. Anim Nutr. (2024) 17:244–64. doi: 10.1016/j.aninu.2024.01.012
10. Iqbal, S, Younas, U, Chan, KW, Zia-Ul-Haq, M, and Ismail, M. Chemical composition of Artemisia annua L. leaves and antioxidant potential of extracts as a function of extraction solvents. Molecules. (2012) 17:6020–32. doi: 10.3390/molecules17056020
11. Skowyra, M, Gallego, MG, Segovia, F, and Almajano, MP. Antioxidant properties of Artemisia annua extracts in model food emulsions. Antioxidants. (2014) 3:116–28. doi: 10.3390/antiox3010116
12. Zibaee, A, and Bandani, AR. Effects of Artemisia annua L. (Asteracea) on the digestive enzymatic profiles and the cellular immune reactions of the Sunn pest, Eurygaster integriceps (Heteroptera: Scutellaridae), against Beauveria bassiana. Bull Entomol Res. (2010) 100:185–96. doi: 10.1017/S0007485309990149
13. El-Sawy, SA, Amin, YA, El-Naggar, SA, and Abdelsadik, A. Artemisia annua L. (sweet wormwood) leaf extract attenuates high-fat diet-induced testicular dysfunctions and improves spermatogenesis in obese rats. J Ethnopharmacol. (2023) 313:116528. doi: 10.1016/j.jep.2023.116528
14. Juteau, F, Masotti, V, Bessière, JM, Dherbomez, M, and Viano, J. Antibacterial and antioxidant activities of Artemisia annua essential oil. Fitoterapia. (2002) 73:532–5. doi: 10.1016/S0367-326X(02)00175-2
15. Zhang, SA-O, Xiong, LA-O, Cui, C, Zhao, H, Zhang, Y, Tian, Z, et al. Maternal supplementation with Artemisia annua L. ameliorates intestinal inflammation via inhibiting the TLR4/NF-κB and MAPK pathways and improves the oxidative stability of offspring. Food Funct. (2022) 13:9311–23. doi: 10.1039/d2fo00675h
16. Cui, Y, Leng, X, Zhao, Y, Zhao, Y, and Wang, Q. Effects of dietary Artemisia annua supplementation on growth performance, antioxidant capacity, immune function, and gut microbiota of geese. Poult Sci. (2024) 103:103594. doi: 10.1016/j.psj.2024.103594
17. Guo, S, Ma, J, Xing, Y, Xu, Y, Jin, X, Yan, S, et al. Effects of Artemisia annua L. water extract on growth performance and intestinal related indicators in broilers. J Poult Sci. (2023) 60:2023024. doi: 10.2141/jpsa.2023024
18. Sarhadi, I, Alizadeh, E, Ahmadifar, E, Adineh, H, and Dawood, MAO. Skin mucosal, serum immunity and antioxidant capacity of common carp (Cyprinus carpio) fed Artemisia annua (). Ann Anim Sci. (2020) 20:1011–27. doi: 10.2478/aoas-2020-0011
19. Niu, YJ, Zhao, Y, He, J, Yun, Y, Shi, Y, Zhang, L, et al. Effect of diet supplemented with enzymatically treated Artemisia annua L. on intestinal digestive function and immunity in weaned pigs. Ital J Anim Sci. (2020) 19:1170–9. doi: 10.1080/1828051X.2020.1826364
20. Song, Z, Cheng, K, Zhang, L, and Wang, T. Dietary supplementation of enzymatically treated Artemisia annua could alleviate the intestinal inflammatory response in heat-stressed broilers. J Therm Biol. (2017) 69:184–90. doi: 10.1016/j.jtherbio.2017.07.015
21. Zeng, KW, Wang, S, Dong, X, Jiang, Y, and Tu, PF. Sesquiterpene dimer (DSF-52) from Artemisia argyi inhibits microglia-mediated neuroinflammation via suppression of NF-κB, JNK/p38 MAPKs and Jak2/Stat3 signaling pathways. Phytomedicine. (2014) 21:298–306. doi: 10.1016/j.phymed.2013.08.016
22. Zhao, XAO, Huang, X, Yang, C, Jiang, Y, Zhou, W, and Zheng, W. Artemisinin attenuates amyloid-induced brain inflammation and memory impairments by modulating TLR4/NF-κB signaling. Int J Mol Sci. (2022) 23:6354. doi: 10.3390/ijms23116354
23. Song, ZH, Cheng, K, Zheng, XC, Ahmad, H, Zhang, LL, and Wang, T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult Sci. (2018) 97:430–7. doi: 10.3382/ps/pex312
24. Clark, AJ, and Sun, D. Guidelines for the ethical review of laboratory animal welfare People's Republic of China National Standard GB/T 35892-2018 [issued 6 February 2018 effective from 1 September 2018]. Anim Models Exp Med. (2020) 3:103–13. doi: 10.1002/ame2.12111
25. Guo, S, Ma, J, Xing, Y, Xu, Y, Jin, X, Yan, S, et al. Artemisia annua L. aqueous extract as an alternative to antibiotics improving growth performance and antioxidant function in broilers. Ital J Anim Sci. (2020) 19:399–409. doi: 10.1080/1828051X.2020.1745696
26. Xin, Z, Ma, S, Ren, D, Liu, W, Han, B, Zhang, Y, et al. UPLC–Orbitrap–MS/MS combined with chemometrics establishes variations in chemical components in green tea from Yunnan and Hunan origins. Food Chem. (2018) 266:534–44. doi: 10.1016/j.foodchem.2018.06.056
27. Shi, L, Guo, Y, Cheng, Y, Xing, Y, Guo, S, Zhang, L, et al. An Artemisia ordosica extract: effects on growth performance, immune, and inflammatory response in lipopolysaccharide-challenged broilers. Front Vet Sci. (2022) 9:980690. doi: 10.3389/fvets.2022.980690
28. Gang, G, Gao, R, Zhao, H, Xu, Y, Xing, Y, Jin, X, et al. Effects of water extracts of Artemisia annua L. on rumen immune and antioxidative indexes, fermentation parameters and microbials diversity in lambs. Front Microbiol. (2024) 15:1485882. doi: 10.3389/fmicb.2024.1485882
29. Nair, MS, Huang, Y, Wang, M, and Weathers, PJ. SARS-CoV-2 omicron variants are susceptible in vitro to Artemisia annua hot water extracts. J Ethnopharmacol. (2023) 308:116291. doi: 10.1016/j.jep.2023.116291
30. Zhao, Y, Zhu, L, Yang, L, Chen, M, Sun, P, Ma, Y, et al. In vitro and in vivo anti-eczema effect of Artemisia annua aqueous extract and its component profiling. J Ethnopharmacol. (2024) 318:117065. doi: 10.1016/j.jep.2023.117065
31. Shi, L, Jin, X, Xu, Y, Xing, Y, Yan, S, Guo, Y, et al. Effects of Total flavonoids of Artemisia ordosica on growth performance, oxidative stress, and antioxidant status of lipopolysaccharide-challenged broilers. Antioxidants. (2022) 11:1985. doi: 10.3390/antiox11101985
32. Kim, MH, Seo, JY, Liu, KH, and Kim, JS. Protective effect of Artemisia annua L. extract against galactose-induced oxidative stress in mice. PLoS One. (2014) 9:e101486. doi: 10.1371/journal.pone.0101486
33. Lan, MB, Zhang, YH, Zheng, Y, Yuan, HH, Zhao, HL, and Gao, F. Antioxidant and immunomodulatory activities of polysaccharides from moxa (Artemisia argyi) leaf. Food Sci Biotechnol. (2010) 19:1463–9. doi: 10.1007/s10068-010-0209-5
34. Soares, MP, Cardoso, IL, Ishikawa, MM, de Oliveira, A d SS, Sartoratto, A, Jonsson, CM, et al. Effects of Artemisia annua alcohol extract on physiological and innate immunity of Nile tilapia (Oreochromis niloticus) to improve health status. Fish Shellfish Immunol. (2020) 105:369–77. doi: 10.1016/j.fsi.2020.07.035
35. Guo, S, Ma, J, Xing, Y, Shi, L, Zhang, L, Xu, Y, et al. Artemisia annua L. aqueous extract promotes intestine immunity and antioxidant function in broilers. Front Vet Sci. (2022) 9:934021. doi: 10.3389/fvets.2022.934021
36. Wu, Z, Yang, K, Zhang, A, Chang, W, Zheng, A, Chen, Z, et al. Effects of Lactobacillus acidophilus on the growth performance, immune response, and intestinal barrier function of broiler chickens challenged with Escherichia coli O157. Poult Sci. (2021) 100:101323–3171. doi: 10.1016/j.psj.2021.101323
37. Maghsoudi, A, Vaziri, E, Feizabadi, M, and Mehri, M. Fifty years of sheep red blood cells to monitor humoral immunity in poultry: a scientometric evaluation. Poult Sci. (2020) 99:4758–68. doi: 10.1016/j.psj.2020.06.058
38. Yang, S, Zhang, J, Jiang, Y, Xu, YQ, Jin, X, Yan, S, et al. Effects of dietary supplementation with Artemisia argyi alcohol extract on growth performance, blood biochemical properties and small intestinal immune markers of broilers challenged with lipopolysaccharide. Anim Prod Sci. (2021) 62:234–47. doi: 10.1071/an21157
39. Zhang, A, Yang, Y, Wang, Y, Zhao, G, Yang, X, Wang, D, et al. Adjuvant-active aqueous extracts from Artemisia rupestris L. improve immune responses through TLR4 signaling pathway. Vaccine. (2017) 35:1037–45. doi: 10.1016/j.vaccine.2017.01.002
40. Yang, Y, Wang, D, Li, Q, He, J, Wang, B, Li, JA-O, et al. Immune-enhancing activity of aqueous extracts from Artemisia rupestris L. via MAPK and NF-kB pathways of TLR4/TLR2 downstream in dendritic cells. Vaccine. (2020) 8:525. doi: 10.3390/vaccines8030525
41. Du, H, Xing, Y, Xu, Y, Jin, X, Yan, SA-O, and Shi, BA-O. Dietary Artemisia Ordosica polysaccharide enhances spleen and intestinal immune response of broiler chickens. Biology. (2023) 12:2079–7737. doi: 10.3390/biology12111390
42. Zhang, L, Xing, Y, Shi, L, Guo, S, Jin, X, Xu, YQ, et al. The effects of dietary supplementation of Artemisia argyi polysaccharide on immune and antioxidative functions in broilers. J Appl Anim Res. (2022) 50:587–97. doi: 10.1080/09712119.2022.2119982
43. Ando, I, Tsukumo, Y, Wakabayashi, T, Akashi, S, Miyake, K, Kataoka, T, et al. Safflower polysaccharides activate the transcription factor NF-kappa B via toll-like receptor 4 and induce cytokine production by macrophages. Int Immunopharmacol. (2002) 2:1155–62. doi: 10.1016/s1567-5769(02)00076-0
44. Guo, S, Shi, B, Xing, Y, Xu, Y, Jin, X, Hong, L, et al. Artemisia annua L. polysaccharide improves the growth performance and intestinal barrier function of broilers challenged with Escherichia coli. Front Microbiol. (2024) 15:1390815. doi: 10.3389/fmicb.2024.1390815
45. Fagerlund, R, Behar, M, Fortmann, KT, Lin, YE, Vargas, JD, and Hoffmann, A. Anatomy of a negative feedback loop: the case of Iκbα. J R Soc Interface. (2015) 12:0262. doi: 10.1098/rsif.2015.0262
46. Xing, YY, Zheng, YK, Yang, S, Zhang, LH, Guo, SW, Shi, LL, et al. Artemisia ordosica polysaccharide alleviated lipopolysaccharide-induced oxidative stress of broilers via Nrf2/Keap1 and TLR4/NF-κB pathway. Ecotoxicol Environ Saf. (2021) 223:112566. doi: 10.1016/j.ecoenv.2021.112566
47. Cheng, Y, Xie, Y, Shi, L, Xing, Y, Guo, S, Gao, Y, et al. Effects of rare earth-chitosan chelate on growth performance, antioxidative and immune function in broilers. Ital J Anim Sci. (2022) 21:303–13. doi: 10.1080/1828051x.2022.2028589
48. Apel, K, and Hirt, H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. (2004) 55:373–99. doi: 10.1146/annurev.arplant.55.031903.141701
49. Wu, JQ, Kosten, TR, and Zhang, XY. Free radicals, antioxidant defense systems, and schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. (2013) 46:200–6. doi: 10.1016/j.pnpbp.2013.02.015
50. Zhang, P, Chen, H, Shi, B, Zhao, F, Guo, X, Jin, X, et al. In vitro antioxidant activity of Artemisia argyi powder and the effect on hepatic and intestinal antioxidant indices in broiler chickens. Ann Anim Sci. (2020) 20:1085–99. doi: 10.2478/aoas-2020-0029
51. Giera, M, Lingeman, H, and Niessen, WM. Recent advancements in the LC- and GC-based analysis of malondialdehyde (MDA): a brief overview. Chromatographia. (2012) 75:433–40. doi: 10.1007/s10337-012-2237-1
52. Ahmad, MM, Chatha, SAS, Iqbal, Y, Hussain, AI, Khan, I, and Xie, F. Recent trends in extraction, purification, and antioxidant activity evaluation of plant leaf-extract polysaccharides. Biofuels. (2022) 16:1820–48. doi: 10.1002/bbb.2405
53. Kim, J, Cha, Y-N, and Surh, Y-J. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res. (2010) 690:12–23. doi: 10.1016/j.mrfmmm.2009.09.007
54. Sajjad, NAO, Wani, A, Sharma, A, Ali, R, Hassan, S, Hamid, R, et al. Artemisia amygdalina upregulates Nrf2 and protects neurons against oxidative stress in Alzheimer disease. Cell Mol Neurobiol. (2019) 39:387–99. doi: 10.1007/s10571-019-00656-w
55. Niu, Y, He, JT, Zhao, YW, Gan, ZD, Shen, MM, Zhang, LL, et al. Dietary enzymatically treated Artemisia annua L. supplementation improved growth performance and intestinal antioxidant capacity of weaned piglets. Livest Sci. (2020) 232:103937. doi: 10.1016/j.livsci.2020.103937
56. National Standardization Technical Committee on Slaughter and Processing (SAC/TC 516). GB/T 43562–2023. State Administration for Market Regulation Standardization Administration of China. Beijing: Standards Press of China (2023).
57. National Technical Committee of Standardization on Animal Husbandry. Nutritional Requirements of Meat-type Sheep (NY/T 816–2021). Animal Husbandry and Veterinary Administration [S]. Beijing: Standards Press of China (2021).
Keywords: water extracts of Artemisia annua L, lamb, small intestine, immune index, antioxidative index
Citation: Gang G, Gao R, Zhao H, Jin X, Xing Y, Hong L, Yan S, Xu Y and Shi B (2025) Effects of diet supplemented with water extracts of Artemisia annua L. on small intestinal immune and antioxidative indexes in lambs. Front. Vet. Sci. 12:1545729. doi: 10.3389/fvets.2025.1545729
Edited by:
Valiollah Palangi, Ege University, TürkiyeReviewed by:
Misbah Ijaz, University of Agriculture, PakistanVasiliki Makri, Aristotle University of Thessaloniki, Greece
Copyright © 2025 Gang, Gao, Zhao, Jin, Xing, Hong, Yan, Xu and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanqing Xu, aGFwcHl4eXFAeWVhaC5uZXQ=; Binlin Shi, c2hpYmlubGluQHllYWgubmV0
 Gen Gang
Gen Gang Sumei Yan
Sumei Yan Yuanqing Xu
Yuanqing Xu Binlin Shi
Binlin Shi