- 1Carlson College of Veterinary Medicine, Oregon State University, Corvallis, OR, United States
- 2American Bird Conservancy, The Plains, VA, United States
- 3Department of Forest Engineering, Resources, and Management, Oregon State University, Corvallis, OR, United States
- 4Department of Forest, Ecosystems and Society, Oregon State University, Corvallis, OR, United States
- 5Department of Fisheries, Wildlife, and Conservation Sciences, Oregon State University, Corvallis, OR, United States
Introduction: Estimated white blood cell (WBC) counts are a valuable tool for assessing individual and population health in wildlife and domestic animals due to their role in the response to environmental stressors and disease. These measures are infrequently used in the study of wild seabird species, despite their utility when used alongside other common health assays - such as infectious disease testing, body condition, and population monitoring efforts. The Marbled Murrelet (Brachyramphus marmoratus) is a seabird of conservation concern that is federally listed as threatened by the states of Oregon, Washington, and California, thus necessitating the evaluation of its physiological health.
Methods: We evaluated the utility of estimated WBC counts as measures of health, asking whether counts changed by measures of individual health (i.e., sex, L. marmoratii hemoparasite burden, body condition index, and nesting propensity) and population health (i.e., changes in counts by year). We used blood smears collected from over 350 murrelets captured along the Oregon Coast between April and June of 2017-2019 and 2021-2022 to estimate total WBC and differential counts.
Results: Estimated WBC counts were found to appear lower in years with more favorable ocean conditions, when nesting propensity was relatively high. Male murrelets, individuals less likely to nest, and individuals with greater L. marmoratii burden had significantly lower estimated WBC counts, whereas individuals with a lower body condition index had elevated estimated WBC counts.
Discussion: These results demonstrate the utility of estimated WBC counts to further assess health at the individual and population levels in the study of species of heightened conservation concern and should be considered as an addition to research plans.
1 Introduction
Common methods of studying wild seabird species generally focus on the collection of nesting, survival, and morphometric data (e.g., body measurements and mass) to assess population health and trends, species behavior, and factors affecting recruitment (1–8). A method that is less frequently used to assess wild seabird population health is the field of hematology, the study of blood and its cellular characteristics (9, 10). Factors such as insufficient sample size, small populations, or difficulty in sampling may limit use of this method in wild species (11). Nevertheless, blood-based metrics can be an informative way to further assess health in wild seabird populations, due to the immune system activities of the individual white blood cell types. The five types of white blood cells found in birds are categorized in two ways. In the first, there are three types of granulocytes; the heterophil, which functions through phagocytosis of infectious agents in the acute inflammatory response; and the basophil and eosinophil, both of which function in the hypersensitivity response, with eosinophils also functioning in some parasitic infections. In the second, there are two types of mononuclear cells; the lymphocyte, which functions through directing the immune response in the body via the action of T cells and B cells; and the monocyte, which functions in phagocytosis and antigen presentation to lymphocytes (12). Variations in leukocyte number may be present due to environmental or physiologic stress; lymphopenia or relative decrease in lymphocyte count and heterophilia or relative increase in heterophil count, may be seen as part of the stress response in the body (13). The study of the quantity and quality of these cell types gives valuable insight as to the immune system response in the body caused by stress or disease.
White blood cell count reference intervals for wild species are valuable because they allow comparison and detection of changes in individual and population health over time. Establishment of reference intervals for wild species allows a deeper assessment of individual health in these settings. Comparing obtained cell counts from an individual to an established reference interval for the population allows for a deeper assessment of individual health alongside other parameters, including physical appearance. Additionally, concerning their utility in monitoring population health over time, changes in reference intervals for a given population may be detected if studied over time, allowing further investigation as to the cause of these changes and opportunity for response.
The Marbled Murrelet (Brachyramphus marmoratus, hereafter murrelet) is a small, non-migratory diving seabird in the auk family (Alcidae), occurring from Alaska to central California along the Pacific coast of North America (14, 15). The species is listed as threatened in California, Oregon, and Washington under the U.S. Endangered Species Act (16, 17), thereby making the murrelet a species of conservation concern throughout its range in the contiguous United States. Murrelets were once common throughout their range, but studies investigating abundance of murrelets have reported that populations have continued to decline annually across their range (18, 19). Recent reports suggest that at-sea abundance of murrelets appears to be decreasing in the northern part of their range and increasing in the southern part of their range; however, the cause of these trends is likely multifactorial (20, 21).
Murrelets have an unusual breeding strategy, which makes them challenging to study. They forage for schooling fish and invertebrates in nearshore (within 5 km) marine waters, and they nest arboreally within mature coastal forests with occasional nesting on the ground and on rock ledges in the northern part of their range (22–25). This species can fly long distances inland (>80 km) as it socializes, searches for nest sites, and travels to and from nesting areas (15, 26, 27), though factors such as poor ocean conditions can cause murrelets to travel great distances (>500 km) away from their terrestrial nesting habitat in their selection of adequate marine habitat (28). Murrelet nests have been historically difficult to locate due to their small body size and cryptic nesting behavior of the species, and placement of nests high in trees or on cliffs located in rugged terrain (29–31). Murrelet pairs select a nesting location and work together to incubate a single egg. The pair will trade off incubation duties approximately every 24 h, leaving one at the nest and the other to forage at sea (25, 32). Although marine factors such as prey availability and quality being impacted by shifts in climate over time or acute elevations in ocean temperatures seasonally have likely contributed to murrelet population declines (33–35), the major cause is thought to be sustained low recruitment resulting from the loss of quality nesting sites and high rates of nest failure from predation related to edge effects (16, 18, 20, 30, 36–40).
Recently, the Oregon population of murrelets was found to harbor a previously undocumented species of Leucocytozoon hemoparasite (Leucocytozoon marmoratii) that was detected with a prevalence of 62% for the population (41). Protozoans in the Leucocytozoon genus belong to the avian Haemosporida order of vector-borne parasites which are part of the Apicomplexa phylum. Leucocytozoon hemoparasites have been identified and described in a number of avian species, such as raptors, songbirds, and poultry; however, they have rarely been found in seabirds (42–49). Michlanski (41) found that increased parasite burden (described as the number of L. marmoratii detected per 100 white blood cells) was associated with a reduction in nesting propensity, suggesting that the burden of L. marmoratii may be affecting murrelet health. It has not been investigated whether L. marmoratii burden is associated with changes in white blood cell differential counts in murrelets, although in other species Leucocytozoon hemoparasites may have impacts on immunologic, physiologic, and reproductive health (43, 44, 47). Thus, investigation to assess any changes in white blood cell counts due to L. marmoratii burden in murrelets is important.
There are several studies concerning the population health of the murrelet, the majority of which are focused on the collection of nesting, survival, and morphometric data (19, 30, 50, 51). There are limited studies concerning blood-based measures to evaluate individual and population health for this species (9). This study sought to assess the utility of white blood cell count estimations alongside other commonly collected parameters included in population and individual health assessments: sex, body condition index, nesting propensity, and L. marmoratii parasite burden. We also sought to generate reference intervals for estimated total white blood cell and differential count data. The goals of this project were as follows: (1) To determine if estimated white blood cell counts change relative to changing environmental conditions or other stressors affecting this population; (2) To use estimated white blood cell counts alongside other covariates (i.e., sex, body condition index, nesting attempt, and L. marmoratii burden) to investigate relationships that may show the utility of white blood cell count estimations as an additional assay that can be used to assess population and individual health in murrelets and other seabird species. We did this by assessing whether estimated white blood cell counts correlated to measures of individual health (sex, body condition index, L. marmoratii burden), as well as whether counts covaried with environmental conditions that influenced nesting propensity; (3) Use estimated white blood cell counts from blood smears to construct white blood cell count reference intervals for the Oregon population to be used as an additional method of monitoring the population for changes over time, as well as in rehabilitation and medical environments to assess the health of an individual animal.
2 Materials and methods
Field data collection occurred from late April to early June during 2017–2019 and 2021–2022 as part of a large-scale, long-term study investigation of murrelet nesting ecology in the coastal forests of central Oregon led by researchers in the College of Forestry at Oregon State University. To do this, teams departed from Newport, Oregon and undertook at-sea captures overnight within nearshore areas 35 km to the north or south. Working from a large vessel, a small inflatable boat was offloaded at sea to search for birds with a high-powered spotlight, and a large dip net was used to capture birds (52). Immediately after capture, birds were moved into plastic transport containers and transported to the research vessel for assessment and processing. Once on the research vessel, a brief physical examination was performed by experienced personnel, assessing relative stress levels and overall body condition of each bird. Most birds were healthy enough to continue, but if a bird was undergoing severe stress (panting severely), it was released prior to sampling. If considered healthy enough to proceed then a uniquely numbered metal identification band was placed on one of the bird’s legs and each individual measured for body mass (± 1.0 g) and culmen length (± 0.1 mm). A small sample of blood from the medial metatarsal vein was collected, between 0.6–1.0 mL per bird based upon body mass, using a heparinized 3 milliliter syringe (BD Luer-Lok disposable) with a 27 or 25 gage butterfly catheter (Terumo Surflo winged-infusion sets). The samples were used to make blood smears for white blood cell count estimates and parasite assessment, and a drop from each sample was placed on a Whatman FTA card for DNA sexing, as murrelets are not sexually dimorphic in size and plumage. For individuals that weighed ≥200 g, a small VHF telemetry tag (model A4330, 2.5 g, Advanced Telemetry Systems, Isanti, MN, US) was attached to the upper back using a subcutaneous anchor (53). In 2019 only, tail-mounted VHF telemetry tags were employed on a small number of individuals (n = 7); however, this approach was ineffective and discontinued due to poor tag retention. All birds were released within 1 h of capture and within 1 km of their original capture location.
After release, radio-tagged birds were tracked by fixed wing aircraft and via 72 ground-based, fixed telemetry stations that were located every 2–3 km along the coast across the 135 km study area, stretching from Pacific City, Oregon southward to Florence, Oregon. Using both methods, birds were tracked on a near-daily basis and thus we were able to detect the unique inland movement patterns murrelets exhibit during incubation that signaled an active nest (29, 32). In the analysis we call this variable nesting propensity, which is a binomial variable indicating whether a bird attempted to nest or not.
The process to obtain estimates for white blood cell count data was accomplished through manual counting from blood smears collected from captured individual murrelets. Blood smears were made on the research vessel immediately following blood collection and allowed to air dry and then transferred to the OSU Oregon Veterinary Diagnostic Laboratory for analysis. Prepared slides were stained using a modified Wright stain for preservation and to highlight blood cells and their contents. 100-cell white blood cell differential counts were performed, and total white blood cell counts were estimated using a standard manual blood smear technique (54). The five types of white blood cells found in birds and identified in differential counting are the heterophil, lymphocyte, monocyte, basophil, and eosinophil. Two observers (JJ and SP) performed the hematology evaluation. When evaluating for estimated white blood cell counts as well as parasite presence and burden, all blood smears were analyzed blind with respect to the main factors analyzed in this study, including birds that were male vs. female, body condition index, or birds that attempted a nest vs. those that did not. Minimal inter-observer variation was confirmed by both observers via comparison of WBC estimated counts and differentials on 20 samples. Although it has been demonstrated that manual WBC estimated counts and differential counts determined from blood smears may have a wider coefficient of variation when compared to automated methods (55), due to sample size, remote location of blood sample collection, and availability of equipment, manual methods, and thus WBC count estimation, was determined to be adequate for this project. Blood smears are used as the standard method for estimating WBC counts for non-mammalian species in the Oregon State University Veterinary Diagnostic Laboratory.
Evaluation for L. marmoratii burden was completed using the same blood smear slides that were used for estimating white blood cell counts, using the methods described by Michlanski (41). Parasite burden was determined by scanning the monolayer of each blood smear and counting the number of L. marmoratii detected per 100 white blood cells. In individuals where no parasites were detected in the monolayer, the feathered edge was also scanned in an attempt to identify individuals with low burden.
Sex was determined molecularly from samples cut from the center of a blood spot on a Whatman FTA card which were used for the extraction of genomic DNA. Samples were stored at room temperature until time of analysis. Amplification of the chromo-helicase-DNA-binding protein genes of the Z and W chromosomes was completed using a standard method for DNA sexing of bird species (56). Each sample was amplified in a minimum of three independent PCR replicates to verify results. Female samples produced two bands at approximately 400 bp and 600 bp, while male samples showed only a 600 bp band.
To estimate body condition index (BCI), we calculated residual values from a regression of body mass to tarsus length in GraphPad Prism, with residuals representing each bird’s BCI, with positive values indicating better condition (i.e., larger body mass than expected body size) and negative values indicative of worse condition (i.e., smaller body mass than expected body size) Residuals can be confounded by sex due to variation in BCI between males and females (57, 58), so we performed linear analysis and calculated residuals separately for males and females given that some females were likely in the process of developing eggs (personal observations, BB) when captured, and given that the capture period of the study took place in the breeding to early nesting period for the species (32).
Generation of reference intervals were completed using the guidelines for veterinary species set forth by the American Society of Veterinary Clinical Pathologists (ASVCP) Quality Assurance and Laboratory Standards Committee, which advise standards on sample collection, methods for identifying outliers, and minimum sample size (11). Only samples from clinically healthy birds were included for reference intervals, based on a brief physical examination at time of capture. Upon initial assessment of the blood smear slides, we found that L. marmoratii were detected in 233 of the 374 birds that were sampled (41). Microfilariae were also observed in five samples. Uniquely numbered metal leg bands allowed identification of recaptured individuals, which occurred in eight instances; a randomly selected blood smear was used for individuals who were captured in multiple years. The omission of these samples, as well as samples where L. marmoratii and Microfilariae were detected, reserved 130 samples from which non-parametric reference intervals could be generated (11). We used all 5 sample years in the generation of the reference intervals to give a representative range of counts for a wild population. Reference Value Advisor, a macro instruction for Microsoft Excel was used to calculate reference limits (59). This test allowed for the detection of outliers using the Tukey and Dixon-Reed tests. Upon identification of potential outliers by the program, we determined through slide reassessment that these values may not be errors, but instead could be normal variation in white blood cell counts and were not removed.
Analyses evaluating correlations between white blood cell counts estimates and year, sex, body condition index, nesting status, and Leucocytozoon burden were completed using R and GraphPad Prism software. Eosinophils are uncommon in many avian species, which was found to be true for murrelets in this study. Therefore, we decided to not include eosinophils in further analyses. In comparing white blood cell counts by year, a Kruskal-Wallis ANOVA test with Dunn’s correction for multiple comparisons was completed in GraphPad Prism for each cell type. To assess whether sex, body condition index, parasite burden, or nesting propensity correlated to estimated white blood cell counts, we used a generalized linear model with quasi-Poisson distribution in R, accounting for year using the lme4 and lmer.test packages (60). The dependent variable for the model was each white blood cell count (one model each for total white blood cells count and the differential counts for the separate cell types, excluding eosinophils), and the independent variables were sex, body condition index, parasite burden, and nesting propensity. Independent variables that were not binomial (parasite burden and body condition index) were rescaled using the scale function in the MASS package in R to allow comparisons of the beta estimates from outputs (61).
3 Results
3.1 Interannual variation in murrelet white blood cell count estimates
Estimated total white blood cell counts varied significantly among years (Kruskal-Wallis: H = 15.12, p = 0.0045); however, post hoc comparisons showed that the significant difference was primarily driven by differences between 2021 and 2022, while other pairwise comparisons were not significant (Figure 1A; Table 1). For each blood cell type, heterophils and basophils varied significantly between many years but lymphocytes and monocytes were more stable with the exception of one small difference each (Figure 1). Estimated heterophil counts varied significantly by year (Kruskal-Wallis ANOVA; h = 24.59, p < 0.0001) (Figure 1B). Differences by year were explained by mean rank counts for 2021 being significantly lower in comparison to several other years: 2017 (p < 0.0001), 2018 (p = 0.0066), and 2022 (p = 0.0092), and 2017 being significantly higher than 2019 (p = 0.0409) (Table 1; Figure 1B). Estimated lymphocyte counts varied significantly by year (Kruskal-Wallis ANOVA; h = 11.31, p = 0.0233), driven primarily by 2018 being lower than 2022 (p = 0.0199; Table 1; Figure 1C). Estimated monocyte counts varied significantly by year (Kruskal-Wallis ANOVA; h = 10.51, p = 0.0327), driven primarily by 2021 being lower than 2018 (p = 0.0403; Table 1; Figure 1D). Estimated basophil counts varied significantly by year (Kruskal-Wallis ANOVA; h = 35.93, p < 0.0001) (Figure 1E). Differences by year were driven by mean rank counts for 2017 being significantly higher in comparison to several other years: 2018 (p = 0.0486), 2021 (p < 0.0001), and 2022 (p < 0.0001), and 2021 being significantly lower than 2018 (p = 0.0231) and 2019 (p = 0.0488) (Table 1; Figure 1E).
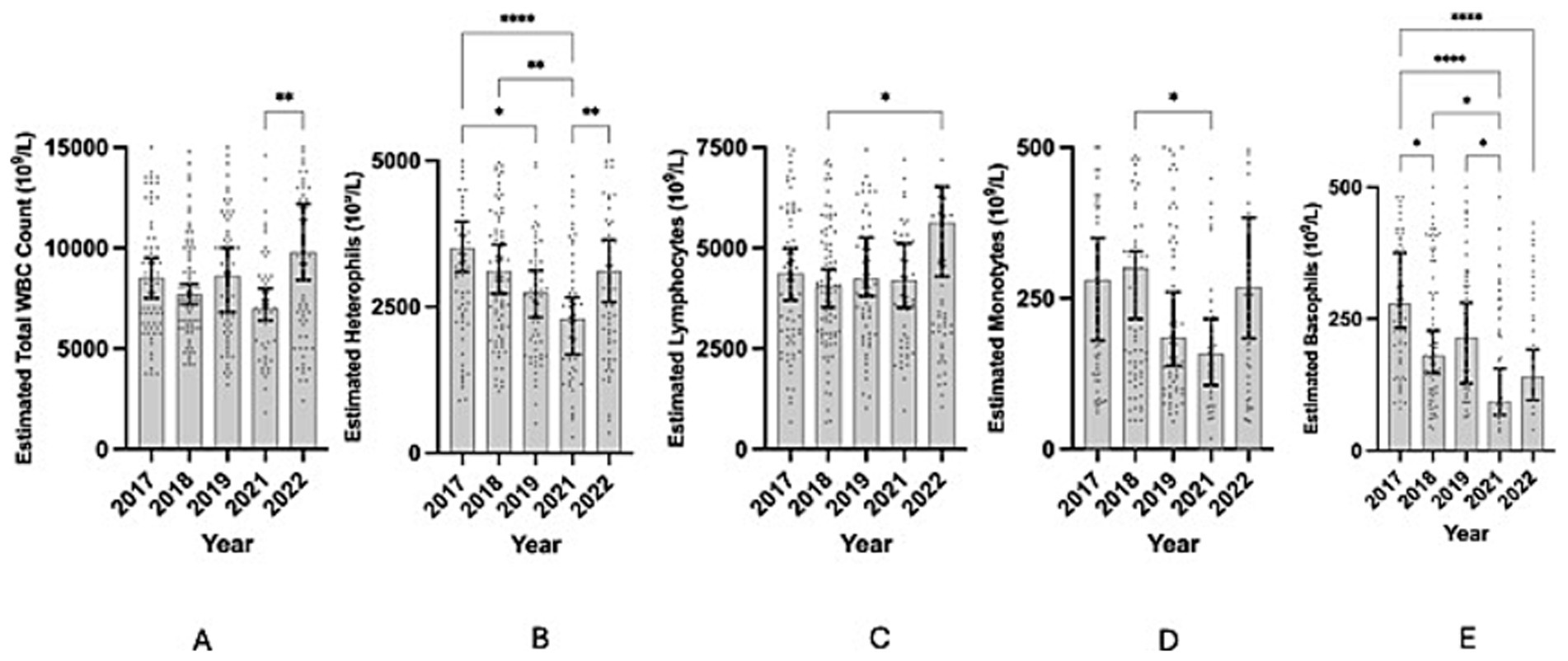
Figure 1. Scatter plots showing estimated white blood cell counts by cell type for each year that samples were collected during the project. Top of the shaded area reaches the sample median. Black error bars mark the 95% confidence interval for the estimated white blood cell count sample median for each year. Asterisks mark significance of the difference between different years with more numerous symbols corresponding to a more significant relationship (based upon a p-value of less than or equal to 0.05). (A) Estimated total white blood cell counts vs. year. (B) Estimated heterophil counts vs. year (C) Estimated lymphocyte counts vs. year (D) Estimated monocyte counts vs. year. (E) Estimated basophil counts vs. year. Y-axis scale is reduced to allow best visualization for each plot, where some data points are omitted in each plot as a result.

Table 1. A representation of the values obtained for estimated white blood cells count comparisons by year.
3.2 Estimated white blood cell count variation with sex, body condition index, nesting propensity, and Leucocytozoon burden
We found that estimated counts of several white blood cells varied significantly by sex, with males having significantly lower estimated total white blood cell, heterophil, and lymphocyte counts than females (p = 0.0009, 0.0009, and 0.0330) (Table 2). No relationship was found in estimated monocyte and basophil counts between the sexes during the sample period (Table 2). We found that estimated monocyte counts were strongly related to BCI, with more negative BCI values in individuals who had higher estimated monocyte counts (p = 0.0249) (Table 2). Estimated total white blood cell, heterophil, lymphocyte, and basophil counts, did not show a significant relationship when compared to BCI (Table 2). Murrelets that did not attempt to nest had significantly higher estimated lymphocyte counts than those that did nest, (p = 0.0328), however no correlations with total white blood cell count, heterophil count, monocyte count or basophil count were noted (Table 2). Murrelets with a higher Leucocytozoon burden had significantly lower estimated total white blood cell and heterophil counts (p = 0.0147 and 0.0358), but no changes in lymphocyte, monocyte or basophil counts (Table 2).
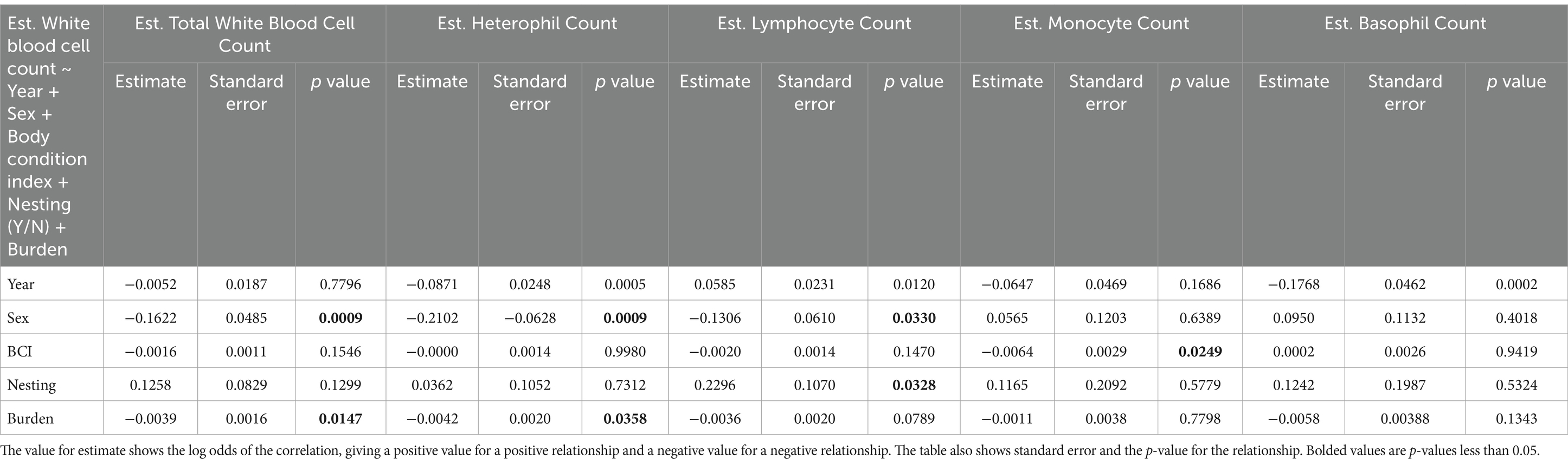
Table 2. A representation of the values obtained for estimated white blood cells count comparisons by sex, BCI, nesting attempt, and L. marmoratii burden after taking into account variations present by year and all other covariates using a multiple linear regression analysis with a quasi-Poisson distribution.
3.3 Reference intervals
Reference Intervals were generated from blood samples taken from 130 murrelets that were clinically healthy and showed no L. marmoratii burden during 2017–2019 and 2021–2022, with the sample period being late April through early June of each year (Table 3). Table 3 shows the values obtained for estimated total white blood cell count, percent of each white blood cell type that makes up the total count, and the differential counts for each cell type. The 90% confidence intervals displayed in the table were calculated for the upper and lower limits of each reference interval. Examples of each type of cell are shown in Figure 2.

Table 3. A representation of the values obtained for estimated total white blood cell count, relative percent of each white blood cell type that makes up the total count, and the estimated differential counts for each cell type from n = 130 murrelets between 2017–2019 and 2021–2022.
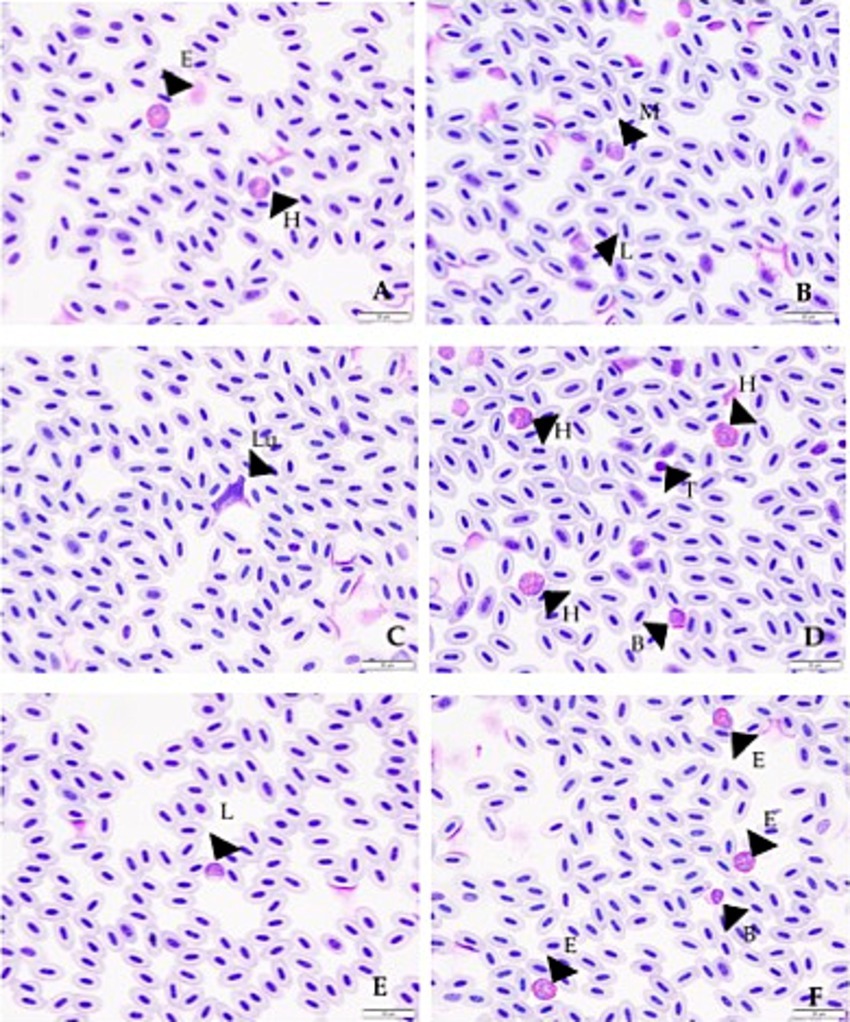
Figure 2. Photographs showing examples of the different white blood cells as found for Marbled Murrelets in this study. Each arrow with letter corresponds to a cell or other finding. (A) Arrow with E shows an eosinophil and arrow with H shows a heterophil. (B) Arrow with M shows a monocyte and arrow with L shows a lymphocyte. (C) Arrow with Lu shows a Leucocytozoon (L. marmoratii) within a cell. (D) Arrows with H’s show heterophils, arrow with B shows a basophil, and arrow with T shows a thrombocyte. (E) Arrow with L shows a lymphocyte. (F) Arrows with E’s show eosinophils and arrow with B shows basophil.
4 Discussion
Our study demonstrated the value of hematological analysis in addition to more traditionally collected covariates used in the study of seabirds, such as sex, body condition index, nesting propensity, and L. marmoratii burden, in the evaluation of individual and population health. We found that estimated white blood cell counts varied compared to several factors, inducing across years, and by sex, body condition index, nesting propensity, and L. marmoratii burden.
We found that estimated white blood cell counts varied by year in this study. Wild bird species respond to the seasonal changes in their environments and leukocyte profiles may reflect these changes throughout the year or over time (62). There are many additional factors that may influence white blood cell counts in wild avian species such as environmental contaminants, prey abundance, and habitat quality, all of which can affect immune function (63–66). This effect of stressors on immune function may result in changes in white blood cell counts and may also explain why we found that estimated white blood cell counts appear to vary across years. It is particularly intriguing that the granulocytes showed significant variation across all the years, suggesting they may be more responsive to environmental changes than the monocytes and lymphocytes – which would make them important to monitor in populations of interest. Additionally, in 2021 multiple white blood cell counts were significantly lower than in other years (Figure 1), perhaps indicating that the birds were suffering from reduced parasite and pathogen challenges that year, which is also the year in which our study population experienced the most favorable ocean conditions and exhibited the greater nesting propensity (37%, unpublished data, JWR) (34, 67). This suggests that murrelets may be subject to energy-based trade-offs between stressors, immune function, disease, and reproductive effort, as has been found for other species (68–70).
We noted a significant relationship between Leucocytozoon burden and estimated white blood cell counts where murrelets with a larger burden of L. marmoratii had a significant decrease in both estimated total white blood cell and estimated heterophil counts. At this time, the blood cell type(s) that L. marmoratii merozoites invade is unknown; however, we know that different species of Leucocytozoon can invade either red blood cells or mononuclear white blood cells such as monocytes and lymphocytes (71). Other species, including raptors, are often hosts for Leucocytozoon species, and experience decreased lymphocyte and monocyte counts and anemia, which is in contrast with our findings in murrelets (72, 73). The difference in the relationship we found between estimated white blood cell counts and parasite burden in murrelets compared with other species may indicate that the effect of parasitemia on murrelets is more driven by additional factors than those we investigated and that additional research on this topic is warranted. In particular, investigation as to the pathophysiology of L. marmoratii and murrelets would allow further characterization of the impact that parasitism may have on murrelets.
We noted differences between males and females when comparing sex to estimated white blood cell counts in this study. Estimated total white blood cell, heterophil, and lymphocyte counts were significantly higher for female birds compared to male birds, during the sample period. This is perhaps not surprising, as a recent meta-analysis suggested that seasonal and sex differences in the immune system may be common in birds in general, and that differences between males and females appear to be stronger during the breeding period than in other times of the year (74). Nevertheless, it is difficult to interpret the reason why estimated white blood cell counts differed between males and females in our study; however, we can speculate based upon our knowledge of white blood cell function and the conditions that may cause a change in their relative numbers. In general, a decrease in lymphocyte counts may be related to a reduction in immune system energy allocation and function associated with stressors, and can vary in number based upon immune response in the identification and response to pathogens (75). It seems unlikely that the difference in estimated mean white blood cell counts between the sexes that we observed is due to a pathogen, as we would expect both sexes to be affected equally if that was the case. Related findings were published by Ots and Hõrak in 1998 during their study of sex-specific clinical profiles of Great Tits (Parus major) (76). In this study, male tits showed lower lymphocyte counts in the breeding season, which was speculated to be a result of the increase in testosterone and corticosterone hormones that are present in the breeding period for this species. Additionally, in the Little Auk (Alle alle), another small seabird species in the family Alcidae that has a very similar breeding ecology as murrelets (i.e., monogamous, shared incubation), it was demonstrated that males had lower lymphocyte counts in the early incubation period compared to females (77). Behavioral observations of Little Auks in this study showed that, while males and females appeared to share incubation duties on the nest equally, that males appeared to be engaging in other activities other than foraging while they were off the nest, including aggressive interactions with other members of the colony. These social interactions may explain the differences in white blood cell counts in male Little Auks vs. females in the early incubation phase (77). Murrelets are non-colonial, and it is unknown if males engage in territorial behavior when off the nest at this time (15). However, it is possible that at this time of year during the breeding season and before egg laying, male murrelets are experiencing stressors that are apparent through changes in white blood cell counts compared to females, that we do not understand at this time.
We found that as BCI decreased, estimated lymphocyte counts and monocyte counts increased. Lymphocytosis, the relative increase in quantity of lymphocytes in the peripheral blood in birds, can be caused by infections that cause antigenic stimulation, such as viral infections (75). Monocytosis, the relative increase in quantity of monocytes in the peripheral blood in birds, can be caused by chronic diseases such as bacterial or fungal disease, or in some cases associated with mineral deficient diets (75). Immune system stimulation can be energetically expensive and may cause a decrease in fitness leading to decreased body condition (78). At this time, the exact mechanism explaining the correlation between white blood cell counts and body condition index for murrelets remains unknown. However, these findings further support the use of the white blood cell counts in the field of wildlife research, as the association between BCI and estimated white blood cell count elevations may correlate to the development of disease within a population or an individual, warranting further investigation as to the cause.
We found that nesting propensity was significantly correlated to estimated lymphocyte counts; individuals that were less likely to attempt to nest tended to have significantly higher estimated lymphocyte counts than birds that did attempt to nest. It has been found in other seabird species that during the pre-laying and laying phases of the breeding period the stress and effort associated with breeding may cause alterations in white blood cell counts, namely a decrease in lymphocytes and an increase in heterophils (74, 75, 79, 80). Our modeling accounted for several covariates, including sex; therefore, this decrease in estimated lymphocytes appears to be affecting both sexes. It is possible that this decrease in estimated lymphocytes in birds more likely to attempt a nest, seen in both sexes, could be attributed to changing hormone levels or stressors during the breeding season associated with nesting, and that birds that are not nesting are not experiencing these changes in hormone levels or other stressors associated with breeding to as great of an extent, maintaining their lymphocyte counts at higher levels (81–84). During the time of year that our sampling occurred, murrelets were likely in the pre-laying and/or early laying period of their breeding season (32) therefore, the effect of hormones or the energetic costs of egg laying in the female could be causing the decrease in estimated lymphocyte counts we are seeing (79).
A separate published study, conducted in Alaska and published in 1997 by Newman et al., sought to publish reference intervals for a variety of seabird species, including the murrelet population in that area (9). In that study Marbled Murrelets were sampled, and samples were collected in June of 1990 from the Shumigan Islands, Alaska. The mean counts derived from these samples appear to generally fall within the reference intervals that were obtained in this study and were collected during a similar time of year; however, the sample sizes used for analysis varied widely between the two studies (n = 11 by Newman et al. (9), and n = 130 in this study). Although these two studies were conducted in two separate geographical areas, comparison of reference intervals within the same species over time is a helpful tool in identifying trends and changes in population health that may be developing. This is because the study of blood from living animals over time provides an additional benefit in proactive management of individual or population health, rather than reactive management in response to a health event, such as high mortality or failure of recruitment (9). When estimating reference intervals, the larger the sample size, the less degree of uncertainty and the greater the likelihood that the sample is representative of the population as a whole (11). Thus, the large sample analyzed in our study is beneficial in providing a large number of specimens to produce statistically significant reference intervals that are representative of the population we have investigated.
5 Conclusion
We found that changes in estimated white blood cell counts can be correlated to morphologic, seasonal, infectious, and external factors. Importantly, we found that estimated white blood cell counts varied by year with several parameters being significantly lower in 2021, the year that was found to have the best ocean conditions and nesting propensity compared to other years. It would be valuable to evaluate white blood cell counts across a wide range of ocean conditions to better understand the drivers of this pattern. This study also established reference intervals for white blood cell count estimates for the Oregon population of the Marbled Murrelet. These reference intervals can provide information about the health of this population and can be used over time to look for deviations that may be associated with challenges affecting the species. The relationship between changing environmental conditions and white blood cell parameters is significant from a conservation standpoint, because poor and variable ocean conditions are expected to increase in the future, and the monitoring of blood values over time can help us further understand the immunological effects that these changes are having on murrelets and other seabird species.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The animal study was approved by Oregon State University Animal Care Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
KR: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. LA: Data curation, Investigation, Writing – original draft, Writing – review & editing. MB: Funding acquisition, Writing – original draft, Writing – review & editing. JD: Data curation, Investigation, Writing – original draft, Writing – review & editing. JJ: Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. MM: Data curation, Investigation, Writing – original draft, Writing – review & editing. SN: Conceptualization, Funding acquisition, Project administration, Resources, Writing – original draft, Writing – review & editing. SP: Investigation, Writing – original draft, Writing – review & editing. JR: Conceptualization, Funding acquisition, Project administration, Resources, Writing – original draft, Writing – review & editing. DR: Funding acquisition, Writing – original draft, Writing – review & editing. EW: Data curation, Investigation, Writing – original draft, Writing – review & editing. BB: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the College of Forestry at Oregon State University and the USDA National Institute of Food and Agriculture, McIntire Stennis project #1014995. Parasite diagnostics were funded through funding to B. Beechler from the Carlson College of Veterinary Medicine Department of Biomedical Sciences. K. Ryan was partially supported by Carlson College of Veterinary Medicine’s Department of Biomedical Sciences fellowship funding at Oregon State University.
Acknowledgments
For assistance with murrelet capture and tagging we thank N. Parker, S. Newman, J. Adams, H. Carter, M. Parker, D. Whitworth, T. Whitworth, J. Felis, S. B. Barbaree, M. Martinez, S. Thomsen, B. Lovelace, P. Hebert, S. Collar, A. DuVall, B. Beechler, J. Koepke, C. Strong, T. Marcella, A. Peck-Richardson, K. Bixler, Y. Suzuki, M. Bancroft, OSU Ship Operations, the R/V Pacific Storm and crew, the R/V Coral Sea and crew, the F/V Western Breeze and crew, and the F/V Tauny Ann and crew. We also thank Gold Aero, Owyhee Air Research, and Lighthawk Conservation Flying for aerial telemetry support. Finally, we thank C. Horton, D. Arnold, R. Bath-Rosenfeld, G. Case, S. Fety, C. Hutton, E. Matechak, M. Monnin, B. Nahorney, B. Popp, A. Powell, K. Ray, C. Rose, J. Rothe, S. Rossiter, N. Trejo, and M. Wilson for extensive logistical support throughout the course of this project. We thank R. Crowhurst in the Epps Lab at Oregon State University for processing the DNA to determine the sex of individuals used in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bustnes, JO, Bourgeon, S, Leat, EHK, Magnusdóttir, E, Strøm, H, Hanssen, SA, et al. Multiple stressors in a top predator seabird: potential ecological consequences of environmental contaminants, population health and breeding conditions. PLoS One. (2015) 10:e0131769. doi: 10.1371/journal.pone.0131769
2. Hovinen, JEH, Tarroux, A, Ramírez, F, Forero, M, and Descamps, S. Relationships between isotopic ratios, body condition and breeding success in a high Arctic seabird community. Mar Ecol Prog Ser. (2019) 613:183–95. doi: 10.3354/meps12886
3. Salas, R, Müller, W, Vercruijsse, H, Lens, L, and Stienen, E. Forced nest site relocations negatively affect reproductive investment in a colonial seabird species. Biol Conserv. (2020) 246:108550. doi: 10.1016/j.biocon.2020.108550
4. Woodworth, BK, Fuller, RA, Hemson, G, McDougall, A, Congdon, BC, and Low, M. Trends in seabird breeding populations across the great barrier reef. Conserv Biol. (2021) 35:846–58. doi: 10.1111/cobi.13630
5. Strøm, H, Descamps, S, Ekker, M, Fauchald, P, and Moe, B. Tracking the movements of North Atlantic seabirds: steps towards a better understanding of population dynamics and marine ecosystem conservation. Mar Ecol Prog Ser. (2021) 676:97–116. doi: 10.3354/meps13801
6. Fayet, AL, Clucas, GV, Anker-Nilssen, T, Syposz, M, and Hansen, ES. Local prey shortages drive foraging costs and breeding success in a declining seabird, the Atlantic puffin. J Anim Ecol. (2021) 90:1152–64. doi: 10.1111/1365-2656.13442
7. Emmerson, L, and Southwell, C. Environment-triggered demographic changes cascade and compound to propel a dramatic decline of an Antarctic seabird metapopulation. Glob Chang Biol. (2022) 28:7234–49. doi: 10.1111/gcb.16437
8. Kentie, R, Shamoun-Baranes, J, Spaans, AL, and Camphuysen, K. Spatial patterns in age-and colony-specific survival in a long-lived seabird across 14 contrasting colonies. Ibis. (2023) 165:82–95. doi: 10.1111/ibi.13120
9. Newman, SH, Piatt, JF, and White, J. Hematological and plasma biochemical reference ranges of Alaskan seabirds: their ecological significance and clinical importance. Colon Waterbirds. (1997) 20:492–504. doi: 10.2307/1521600
10. Silva, A, Mujica, P, Valdés, E, and Cañon-Jones, H. Hematology and blood chemistry reference values of captive adult black-faced Ibis (Theristicus melanopis melanopis). Animals. (2020) 10:2227. doi: 10.3390/ani10122227
11. Arnold, JE, Camus, M, Freeman, KP, Giori, L, Hooijberg, E, Jeffery, U, et al. ASVCP guidelines: principles of quality assurance and standards for veterinary clinical pathology (version 3.0): developed by the American society for veterinary clinical pathology’s (ASVCP) quality assurance and laboratory standards (QALS) committee. Vet Clin Pathol. (2019) 48:542–618. doi: 10.1111/vcp.12810
12. Haile, Y, and Chanie, M. Comparative aspects of the clinical hematology of birds: a review. Br J Poult Sci. (2012) 3:88–95. doi: 10.20372/NADRE:1547201109.99
14. Sealy, SG. Breeding phenology and clutch size in the marbled Murrelet. Auk. (1974) 91:10–23. doi: 10.2307/4084657
15. Nelson, SK. Marbled Murrelet (Brachyramphus marmoratus) In: AAFG Poole, editor. The Birds of North America. Illinois: American Ornithologists’ Union; Academy of Natural Sciences (1997)
16. U.S. Fish and Wildlife Service. Endangered and threatened wildlife and plants; determination of threatened status for the Washington, Oregon and California population of the Marbled Murrelet. Oregon: USDI Fish and Wildlife Service (1992).
17. U.S. Fish and Wildlife Service. Recovery plan for the marbled Murrelet (Brachyramphus marmoratus) in Washington, Oregon and California. Portland, OR: Oregon Field Office (1997). 203 p.
18. Miller, SL, Raphael, MG, Falxa, GA, Strong, C, Baldwin, J, Bloxton, T, et al. Recent population decline of the marbled Murrelet in the Pacific Northwest. Condor. (2012) 114:771–81. doi: 10.1525/cond.2012.110084
19. Falxa, GA, and Raphael, MG. Northwest Forest plan--the first 20 years (1994–2013): Status and trend of marbled Murrelet populations and nesting habitat. Oregon: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station (2016). 132 p.
20. McIver, WR, Pearson, SF, Strong, C, Lance, MM, Baldwin, J, Lynch, D, et al. Status and trend of marbled Murrelet populations in the Northwest Forest plan area, 2000 to 2018. Portland, Oregon: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station (2021).
21. McIver, WR, Baldwin, J, Lance, MM, Pearson, SF, Strong, C, Raphael, MG, et al. (2024). Marbled murrelet effectiveness monitoring, Northwest Forest plan −2023. https://www.fs.usda.gov/r6/reo/monitoring/downloads/murrelet/20240221-nwfpemp-mamu-summary-report-2023-final.pdf (Accessed April 30 2024).
22. Bradley, RW, and Cooke, F. Clief and deciduous tree nests of marbled Murrelets in southwestern British Columbia. Northwest Nat. (2001) 82:52–7. doi: 10.2307/3536786
23. Carter, HR, and Sealy, SG. Who solved the mystery of the Marbled Murrelet? Northwestern. Naturalist. (2005) 86:2–11.
24. Barbaree, BA, Nelson, SK, Dugger, BD, Roby, DD, Carter, HR, Whitworth, DL, et al. Nesting ecology of marbled Murrelets at a remote mainland fjord in Southeast Alaska. Condor. (2014) 116:173–84. doi: 10.1650/CONDOR-13-116.1
25. Nelson, SK. Marbled Murrelet (Brachyramphus marmoratus) In: F Poole and FB Gill, editors. Birds of the World. Ithaca, NY: Cornell Lab of Ornithology (2020)
26. Day, RH, Oakley, KL, and Barnard, DR. Nest sites and eggs of Kittlitz’s and marbled Murrelets. Condor. (1983) 85:265–73. doi: 10.2307/1367058
27. Kim Nelson, S, and Hamer, TE. Nesting biology and behavior of the marbled murrelet In: RC John and LH George Jr, editors. Ecology and conservation of the Marbled Murrelet. Albany, CA: Pacific Southwest Research Station, Forest Service, U.S. Department of Agriculture (1995)
28. Garcia-Heras, M-S, Wolf, C, Guerrero, JAB, Adrean, LJ, Nelson, SK, Roby, DD, et al. Marine habitat use and movement in response to ocean warming by a threatened forest-nesting seabird. Glob Ecol Conserv. (2024) 50:e02857. doi: 10.1016/j.gecco.2024.e02857
29. Bradley, RW, Cooke, F, Lougheed, LW, and Boyd, WS. Inferring breeding success through radiotelemetry in the marbled murrelet. J Wildl Manag. (2004) 68:318–31. doi: 10.2193/0022-541X(2004)068[0318:IBSTRI]2.0.CO;2
30. Peery, MZ, Beissinger, SR, Newman, SH, Burkett, EB, and Williams, TD. Applying the declining population paradigm: diagnosing causes of poor reproduction in the marbled murrelet. Conserv Biol. (2004) 18:1088–98. doi: 10.1111/j.1523-1739.2004.00134.x
31. Baker, LM, Peery, MZ, Burkett, EE, Singer, SW, Suddjian, DL, and Beissinger, SR. Nesting habitat characteristics of the marbled murrelet in Central California redwood forests. J Wildl Manag. (2006) 70:939–46. doi: 10.2193/0022-541X(2006)70[939:NHCOTM]2.0.CO;2
32. Hamer, TE, and Nelson, SK. Nesting chronology of the marbled Murrelet In: RC John and LH George Jr, editors. Ecology and conservation of the Marbled Murrelet. Albany, CA: Pacific Southwest Research Station, Forest Service, U.S. Department of Agriculture (1995). 49–56.
33. Becker, BH, and Beissinger, SR. Centennial decline in the trophic level of an endangered seabird after fisheries decline. Conserv Biol. (2006) 20:470–9. doi: 10.1111/j.1523-1739.2006.00379.x
34. Betts, MG, Northrup, JM, Guerrero, JAB, Adrean, LJ, Nelson, SK, Fisher, JL, et al. Squeezed by a habitat split: warm ocean conditions and old-forest loss interact to reduce long-term occupancy of a threatened seabird. Conserv Lett. (2020) 13:745. doi: 10.1111/conl.12745
35. Becker, BH, Peery, MZ, and Beissinger, SR. Ocean climate and prey availability affect the trophic level and reproductive success of the marbled murrelet, an endangered seabird. Mar Ecol Prog Ser. (2007) 329:267–79. doi: 10.3354/meps329267
36. Nelson, SK, and Hamer, TE. Nest success and the effects of predation on marbled Murrelets In: RC John and LH George Jr, editors. Ecology and conservation of the Marbled Murrelet. Albany, CA: Pacific Southwest Research Station, Forest Service, U.S. Department of Agriculture (1995). 89–98.
37. Hébert, PN, and Golightly, RT. Observations of predation by corvids at a marbled Murrelet nest. J Field Ornithol. (2007) 78:221–4. doi: 10.1111/j.1557-9263.2007.00105.x
38. Malt, JM, and Lank, DB. Marbled murrelet nest predation risk in managed forest landscapes: dynamic fragmentation effects at multiple scales. Ecol Appl. (2009) 19:1274–87. doi: 10.1890/08-0598.1
39. Raphael, MG, Shirk, A, Falxa, GA, and Young, RD. Factors influencing status and trend of marbled Murrelet populations: an integrated perspective In: GA Falxa and MG Raphael, editors. Status and Trend of Marbled Murrelet Populations and Nesting Habitat. Portland, Oregon: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station (2016). 95–120.
40. Valente, JJ, Rivers, JW, Yang, Z, Nelson, SK, Northrup, JM, Roby, DD, et al. Fragmentation effects on an endangered species across a gradient from the interior to edge of its range. Conserv Biol. (2023) 37:e14091. doi: 10.1111/cobi.14091
41. Michlanski, M. The epidemiology of a novel Leucocytozoon parasite in an endangered population of marbled Murrelets (Brachyramphus marmoratus) on the Oregon coast. Oregon: Oregon State University (2024).
42. Morii, T. A review of Leucocytozoon caulleryi infection in chickens. J Protozool Res. (1992) 2:128–33.
43. Marzal, A, de Lope, F, Navarro, C, and Møller, AP. Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia. (2005) 142:541–5. doi: 10.1007/s00442-004-1757-2
44. Norte, AC, Araujo, PM, Sampaio, HL, Sousa, JP, and Ramos, JA. Haematozoa infections in a great tit Parus major population in Central Portugal: relationships with breeding effort and health. Ibis. (2009) 151:677–88. doi: 10.1111/j.1474-919X.2009.00960.x
45. Argilla, LS, Howe, L, Gartrell, BD, and Alley, MR. High prevalence of Leucocytozoon spp. in the endangered yellow-eyed penguin (Megadyptes antipodes) in the sub-Antarctic regions of New Zealand. Parasitology. (2013) 140:672–82. doi: 10.1017/S0031182012002089
46. Hanel, J, Doležalová, J, Stehlíková, Š, Modrý, D, Chudoba, J, Synek, P, et al. Blood parasites in northern goshawk (Accipiter gentilis) with an emphasis to Leucocytozoon toddi. Parasitol Res. (2016) 115:263–70. doi: 10.1007/s00436-015-4743-1
47. Granthon, C, and Williams, DA. Avian malaria, body condition, and blood parameters in four species of songbirds. Wilson J Ornithol. (2017) 129:492–508. doi: 10.1676/16-060.1
48. Parsons, NJ, Voogt, NM, Schaefer, AM, Peirce, MA, and Vanstreels, RET. Occurrence of blood parasites in seabirds admitted for rehabilitation in the Western cape, South Africa, 2001–2013. Vet Parasitol. (2017) 233:52–61. doi: 10.1016/j.vetpar.2016.12.001
49. Kleinschmidt, B, Dorsch, M, Heinänen, S, Morkūnas, J, Schumm, YR, Žydelis, R, et al. Prevalence of Haemosporidian parasites in an Arctic breeding seabird species—the red-throated diver (Gavia stellata). Microorganisms. (2022) 10:2147. doi: 10.3390/microorganisms10112147
50. Cam, E, Lougheed, L, Bradley, R, and Cooke, F. Demographic assessment of a marbled Murrelet population from capture-recapture data. Conserv Biol. (2003) 17:1118–26. doi: 10.1046/j.1523-1739.2003.01287.x
51. Loehle, C, Verschuyl, J, and Solarik, KA. Population trends and vital rates for marbled murrelet (brachyramphus marmoratus) in the pacific Northwest. N W Nat. (2022) 103:20–9. doi: 10.1898/1051-1733-103.1.20
52. Whitworth, DL, Takekawa, JY, Carter, HR, and McIver, WR. A night-lighting technique for At-Sea capture of Xantus’ Murrelets. Colon Waterbirds. (1997) 20:525–31. doi: 10.2307/1521603
53. Newman, SH, Takekawa, JY, Whitworth, DL, and Burkett, EE. Subcutaneous anchor attachment increases retention of radio transmitters on Xantus’ and marbled Murrelets (Conector Subcutáneo de Tipo Ancla Aumenta la Retención de Radiotransmisores en Synthliboramphus hypoleucus y Brachyramphus marmoratus). J Field Ornithol. (1999) 70:520–34.
54. ECLINPATH (2013) WBC counts. Available online at:. (https://eclinpath.com/hematology/tests/wbc-count/)
55. Carisch, L, Stirn, M, Hatt, JM, Federer, K, Hofmann-Lehmann, R, and Riond, B. White blood cell count in birds: evaluation of a commercially available method. BMC Vet Res. (2019) 15:93. doi: 10.1186/s12917-019-1834-8
56. Fridolfsson, A-K, and Ellegren, H. A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol. (1999) 30:116–21. doi: 10.2307/3677252
57. Green, AJ. Mass/length residuals: measures of body condition or generators of spurious results? Ecology. (2001) 82:–1483. doi: 10.1890/0012-9658(2001)082[1473:MLRMOB]2.0.CO;2
58. Labocha, MK, and Hayes, JP. Morphometric indices of body condition in birds: a review. J Ornithol. (2012) 153:1–22. doi: 10.1007/s10336-011-0706-1
59. Geffré, A, Concordet, D, Braun, J-P, and Trumel, C. Reference value advisor: a new freeware set of macroinstructions to calculate reference intervals with Microsoft excel. Vet Clin Pathol. (2011) 40:107–12. doi: 10.1111/j.1939-165X.2011.00287.x
60. Douglas Bates, MM, Bolker, B, and Walker, S. Fitting linear mixed-effects models using lme 4. J Stat Softw. (2015) 67:1–48. doi: 10.18637/jss.v067.i01
61. Ripley, B, Venables, B, Bates, DM, Hornik, K, Gebhardt, A, Firth, D, et al. Package ‘mass. Cran R. (2013) 538:113–20.
62. Hegemann, A, Matson, KD, Both, C, and Tieleman, BI. Immune function in a free-living bird varies over the annual cycle, but seasonal patterns differ between years. Oecologia. (2012) 170:605–18. doi: 10.1007/s00442-012-2339-3
63. Strandin, T, Babayan, SA, and Forbes, KM. Reviewing the effects of food provisioning on wildlife immunity. Philos Trans R Soc Lond Ser B Biol Sci. (2018) 373:20170088. doi: 10.1098/rstb.2017.0088
64. Roast, MJ, Aulsebrook, AE, Fan, M, Hidalgo Aranzamendi, N, Teunissen, N, and Peters, A. Short-term climate variation drives baseline innate immune function and stress in a tropical bird: a reactive scope perspective. Physiol Biochem Zool. (2019) 92:140–51. doi: 10.1086/702310
65. Nimra, S, Kayani, AR, Irfan, M, and Ahmed, MS. Seasonal changes in hematological parameters in house sparrows of subtropical Pakistan. Integr Org Biol. (2023) 5:obad 027. doi: 10.1093/iob/obad027
66. Bustnes, JO, Hanssen, SA, Folstad, I, Erikstad, KE, Hasselquist, D, and Skaare, JU. Immune function and organochlorine pollutants in Arctic breeding glaucous gulls. Arch Environ Contam Toxicol. (2004) 47:530–41. doi: 10.1007/s00244-003-3203-6
67. Peterson, WT, Fisher, JL, Peterson, JO, Morgan, CA, Burke, BJ, and Fresh, KL. Ecosystem indicators of ocean conditions inform fisheries Management in the California Current. Oceanography. (2014) 27:80–9. doi: 10.5670/oceanog.2014.88
68. Friedl, TWP, and Edler, R. Stress-dependent trade-off between immunological condition and reproductive performance in the polygynous red bishop (Euplectes orix). Evol Ecol. (2005) 19:221–39. doi: 10.1007/s10682-005-0509-z
69. French, SS, DeNardo, DF, and Moore, MC. Trade-offs between the reproductive and immune systems: facultative responses to resources or obligate responses to reproduction? Am Nat. (2007) 170:79–89. doi: 10.1086/518569
70. Carlton, ED, Cooper, CL, and Demas, GE. Metabolic stressors and signals differentially affect energy allocation between reproduction and immune function. Gen Comp Endocrinol. (2014) 208:21–9. doi: 10.1016/j.ygcen.2014.08.004
71. Valkiūnas, G, and Iezhova, TA. Insights into the biology of Leucocytozoon species (Haemosporida, Leucocytozoidae): why is there slow research Progress on agents of leucocytozoonosis? Microorganisms. (2023) 11:1251. doi: 10.3390/microorganisms11051251
72. Wiegmann, A, Springer, A, Rinaud, T, Ottensmann, M, Legler, M, Krüger, O, et al. The prevalence of Leucocytozoon spp. in nestlings of three wild raptor species including implications on haematological and blood chemistry values. Int J Parasitol Parasites Wildl. (2021) 16:236–43. doi: 10.1016/j.ijppaw.2021.10.009
73. Martín-Maldonado, B, Mencía-Gutiérrez, A, Andreu-Vázquez, C, Fernández, R, Pastor-Tiburón, N, Alvarado, A, et al. A four-year survey of Hemoparasites from nocturnal raptors (Strigiformes) confirms a relation between Leucocytozoon and Low hematocrit and body condition scores of parasitized birds. Vet Sci. (2023) 10:54. doi: 10.3390/vetsci10010054
74. Valdebenito, JO, Halimubieke, N, Lendvai, ÁZ, Figuerola, J, Eichhorn, G, and Székely, T. Seasonal variation in sex-specific immunity in wild birds. Sci Rep. (2021) 11:1349. doi: 10.1038/s41598-020-80030-9
75. Campbell, TW. Hematology In: BW Ritchie, GJ Harrison, and LR Harrison, editors. Avian medicine: Principles and application. Post office box 6863. Lake Worth, Florida: Wingers Publishing, Inc (1994). 176–98.
76. Ots, I, Murum Ägi, A, and Hõrak, P. Haematological health state indices of reproducing great tits: methodology and sources of natural variation. Funct Ecol. (1998) 12:700–7. doi: 10.1046/j.1365-2435.1998.00219.x
77. Jakubas, D, Wojczulanis-Jakubas, K, and Kreft, R. Sex differences in body condition and hematological parameters in Little auk Alle alle during the incubation period. Ornis Fenn. (2008) 85:90–7.
78. Martin, LB 2nd, Scheuerlein, A, and Wikelski, M. Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc Biol Sci. (2003) 270:153–8. doi: 10.1098/rspb.2002.2185
79. Wojczulanis-Jakubas, K, Jakubas, D, Kulaszewicz, I, Kidawa, D, and Taylor, JRE. Influence of primary reproductive investments on blood biochemistry, leukocyte profile, and body mass in a small Arctic seabird. Auk. (2014) 131:743–55. doi: 10.1642/AUK-14-62.1
80. Mallory, ML, Little, CM, Boyd, ES, Ballard, J, Elliott, KH, Gilchrist, HG, et al. Leucocyte profiles of Arctic marine birds: correlates of migration and breeding phenology. Conserv Physiol. (2015) 3:cov028. doi: 10.1093/conphys/cov028
81. Kern, MD, De Graw, WA, and King, JR. Effects of gonadal hormones on the blood composition of white-crowned sparrows. Gen Comp Endocrinol. (1972) 18:43–53. doi: 10.1016/0016-6480(72)90078-0
82. Ketterson, ED, and Nolan, V Jr. Adaptation, exaptation, and constraint: a hormonal perspective. Am Nat. (1999) 154:S4–S25. doi: 10.1086/303280
83. Salvante, KG. Techniques for studying integrated immune function in birds. Auk. (2006) 123:575–86. doi: 10.1093/auk/123.2.575
Keywords: Marbled Murrelet, seabird, wildlife, reference interval, white blood cell count, population health, hematology, clinical pathology
Citation: Ryan K, Adrean LJ, Betts MG, Dachenhaus J, Johns J, Michlanski M, Nelson SK, Phelps S, Rivers JW, Roby DD, Woodis E and Beechler BR (2025) White blood cell estimates correlate to measures of population and individual health in an endangered population of Marbled Murrelets (Brachyramphus marmoratus). Front. Vet. Sci. 12:1545905. doi: 10.3389/fvets.2025.1545905
Edited by:
Alberto Muñoz, University of Murcia, SpainReviewed by:
O. Alejandro Aleuy, University of Calgary, CanadaManti Debnath, Noida International University, India
Copyright © 2025 Ryan, Adrean, Betts, Dachenhaus, Johns, Michlanski, Nelson, Phelps, Rivers, Roby, Woodis and Beechler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kelsey Ryan, a2Vsc2V5cnlhbi5kdm1AZ21haWwuY29t; Brianna R. Beechler, QnJpYW5uYS5iZWVjaGxlckBvcmVnb25zdGF0ZS5lZHU=
 Kelsey Ryan1*
Kelsey Ryan1* Jennifer Johns
Jennifer Johns James W. Rivers
James W. Rivers Brianna R. Beechler
Brianna R. Beechler