- 1Key Laboratory of Forage Breeding-by-Design and Utilization, Key Laboratory for Agro-Ecological Processes in Subtropical Region, National Engineering Laboratory for Pollution Control and Waste Utilization in Livestock and Poultry Production, and Hunan Provincial Key Laboratory of Animal Nutritional Physiology and Metabolic Process, Institute of Subtropical Agriculture, the Chinese Academy of Sciences, Changsha, China
- 2Liuyang Liu'an Agricultural Technology Comprehensive Development Co., Ltd., Liuyang, China
- 3Department of Pathobiochemistry, Faculty of Pharmacy, Meijo University, Nagoya, Japan
- 4Department of Husbandry and Development of Animal Wealth, Faculty of Veterinary Medicine, Menoufia University, Shebin Alkom, Egypt
- 5Department of Animal Nutrition and Husbandry, University of Veterinary Medicine and Pharmacy, Košice, Slovakia
- 6Facultad de Medicina Veterinaria y Zootecnia, Universidad Autónoma del Estado de México, Toluca, Mexico
- 7Dipartimento di Scienze del Suolo, della Pianta e degli Alimenti (Di.S.S.P.A.), Università degli Studi di Bari, Bari, Italy
Introduction: As a low-cost, high-fibre biomass resource, Phragmites australis (reed) has significant potential for feed applications, particularly as a partial replacement for conventional roughage in ruminant diets.
Methods: This study investigated the effects of integrating Bacillus subtilis (B. subtilis BNCC109047) with homofermentative/ heterofermentative lactic acid bacteria (LAB) consortia on the fermentation and nutritional quality of Phragmites australis (reed) silage. Five treatments were evaluated: a Control (CK, without inoculum) and four inoculants—LAB (1.5 × 108 CFU/kg LAB, 1:4 homofermentative (Lentilactobacillus plantarum BNCC 336421 and Pediococcus pentosaceus BNCC 135034 in a ratio of 1:1): heterofermentative (L. buchneri BNCC 187961) ratio), LAB-BS2.5 (LAB plus 2.5 × 107 CFU/kg B. subtilis), LAB-BS5.0 (LAB plus 5.0 × 107 CFU/kg B. subtilis), and LAB-BS10.0 (LAB plus 1.0 × 108 CFU/kg B. subtilis)—with triplicate samples per group. Silage fermentation was conducted for 90 days.
Results: LAB-BS10.0 demonstrated superior fermentation outcomes, achieving the highest lactic acid-to-total acid ratio (62.3%, p < 0.05) and the lowest ammonia nitrogen (NH3-N) content (0.60 ± 0.09 g/kg, p < 0.05). Acetic and butyric acid concentrations were significantly reduced (p < 0.05), while neutral detergent fiber (NDF) decreased by 5.9% compared to the Control. Ether extract (EE) increased to 4.76% (p < 0.01), highlighting enhanced lipid preservation.
Conclusion: These results emphasize the synergistic potential of B. subtilis and LAB to optimize P. australis silage, providing a sustainable strategy to enhance forage quality and tackle global feed shortages.
Introduction
Phragmites australis (reed) is a perennial aquatic grass with global distribution, recognized for its high biomass yield and adaptability to wetland ecosystems (1). Its structural components—roots, stems, and leaves—have been utilized in diverse applications, ranging from construction materials to phytoremediation (2, 3). Recently, P. australis has gained attention as a high-biomass forage candidate, offering a sustainable alternative to conventional fodder crops (4, 5). As a promising unconventional silage material, reed shows considerable potential for forage applications. However, similar to other non-traditional feedstocks such as sorghum, barley, and oats, its use in silage systems remains poorly understood, particularly in optimizing fermentation efficiency and nutrient retention (6). The plant’s high lignocellulosic fiber content promotes undesirable microbial activity during ensiling, leading to excessive butyric acid production, pH instability, and nutrient loss (7–9). These factors compromise silage quality, limiting its adoption in livestock feed systems (10, 11).
Recent advances in silage microbiology highlight the potential of microbial inoculants to enhance fermentation quality (12, 13). Homofermentative lactic acid bacteria (LAB), such as Lentilactobacillus plantarum (14), excel in lactic acid (LA) production, effectively acidifying various lignocellulosic substrates, including sweet sorghum bagasse, barley, P. australis, corn, and rice (15). This rapid acidification process significantly suppresses spoilage microorganisms (16–18). Heterofermentative LAB, including Lactobacillus buchneri, play a complementary role in the silage fermentation of various forage crops, including lucerne, maize, and Napier grass. These bacteria convert residual carbohydrates to acetic acid through their unique metabolic pathway, providing an additional antimicrobial barrier against spoilage microorganisms such as molds and yeasts (19–22). However, the limited fiber-degrading capacity of LAB often hinders the utilization of lignocellulose-rich forages like P. australis.
To address this limitation, Bacillus subtilis has emerged as a promising adjunct. This bacterium produces bacteriocins that inhibit undesirable microbes while secreting cellulases and xylanases to break down recalcitrant fibers (23). Synergistically, B. subtilis enhances nutrient availability, elevates flavor compounds, and improves silage palatability (24–27). Despite these benefits, research on B. subtilis-LAB consortia in unconventional forage silage, particularly P. australis, remains scarce.
Current research on optimizing P. australis silage through microbial interventions remains sparse. While LAB is widely used to enhance silage fermentation, its synergy with fiber-degrading bacteria like Bacillus subtilis (B. subtilis) remains a mystery. This study investigates the novel combination of homofermentative/heterofermentative LAB consortia (L. plantarum, Pediococcus pentosaceus, and L. buchneri) with incremental doses of B. subtilis. We aimed to (1) evaluate the effects on fermentation parameters (e.g., LA, NH3-N), (2) assess fiber degradation (e.g., NDF, ADF), and (3) establish an optimal inoculant ratio for enhancing the nutritional quality and digestibility of P. australis silage, offering a sustainable solution to mitigate the growing feed supply–demand imbalance.
Materials and methods
Plant materials preparation
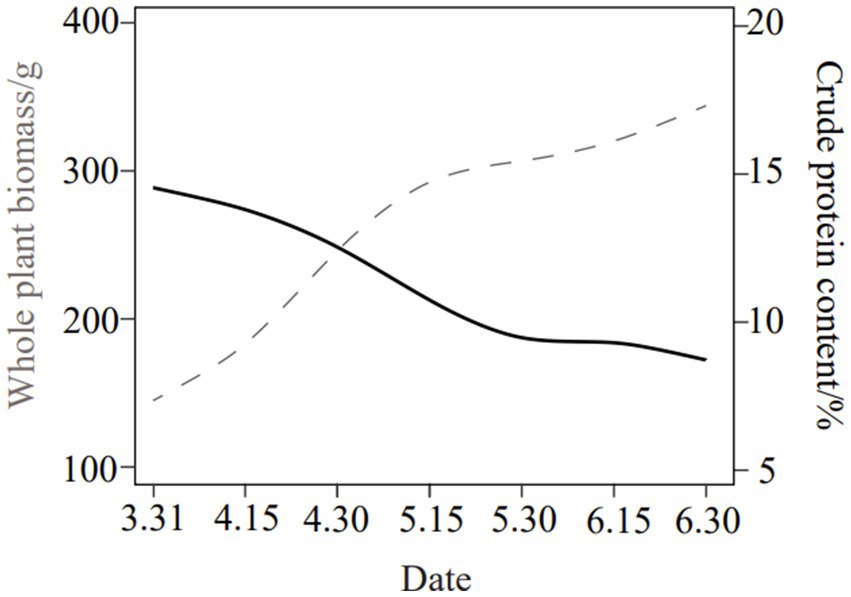
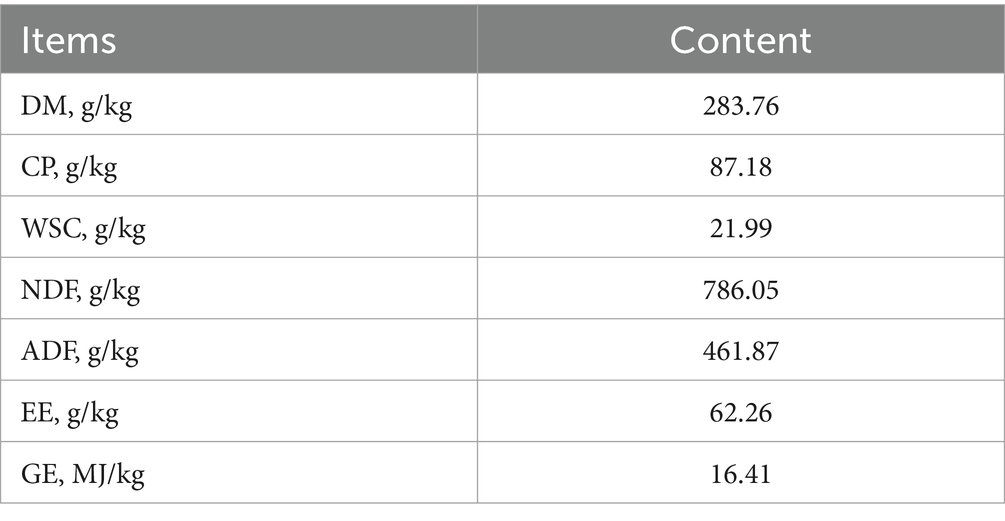
Phragmites australis (reed) was harvested at the vegetative growth stage from Dongting Lake District, Yueyang City, Hunan Province, China, in 2021. The biomass and protein content simulation of P. australis plant (containing stems and leaves) at the early vegetative growth stage is presented in Figure 1. The plants were chopped into 2–3 cm segments, and surface moisture was air-dried. Baseline nutrient composition of raw material was analyzed (Table 1), including dry matter (DM), crude protein (CP), water-soluble carbohydrates (WSC), neutral detergent fiber (NDF), acid detergent fiber (ADF), ether extract (EE), and gross energy (GE).
Microbial inoculants and experimental design
Microbial strains
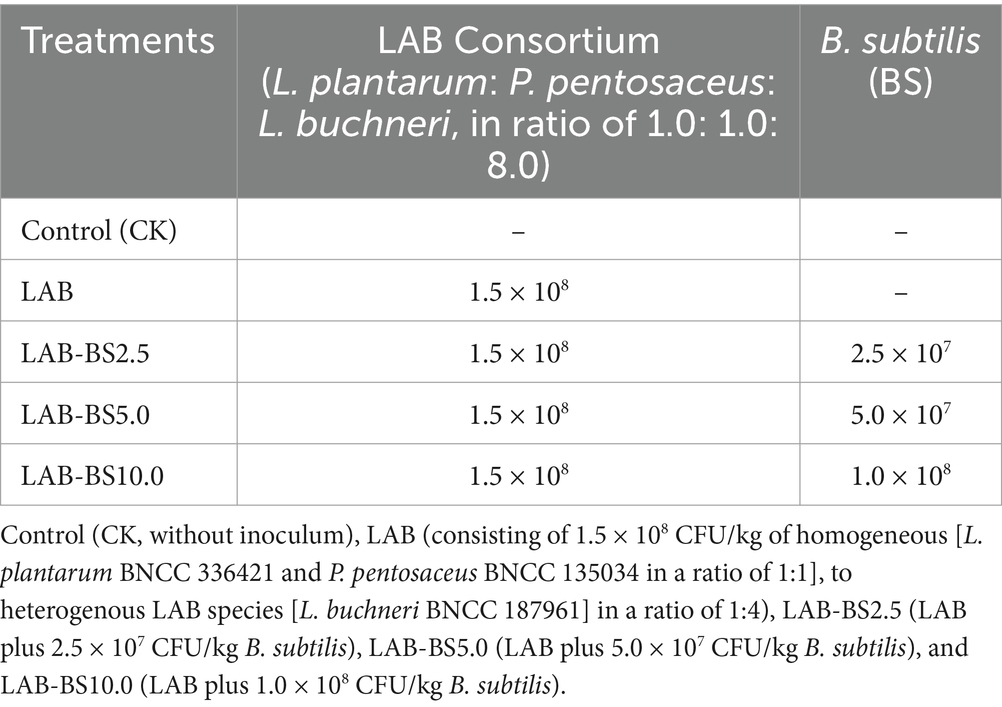
Homogenenous LAB: L. plantarum (3.0 × 109 CFU/g, BNCC 336421), P. pentosaceus (1.0 × 1010 CFU/g, NBCC 135034). Heterogeneous LAB: L. buchneri (1.0 × 1010 CFU/g, BNCC 187961). Functional bacteria: B. subtilis (5.0 × 1010 CFU/g, BNCC 109047). All strains were procured from the BeNa Culture Collection (Beijing, China).
Silage treatments
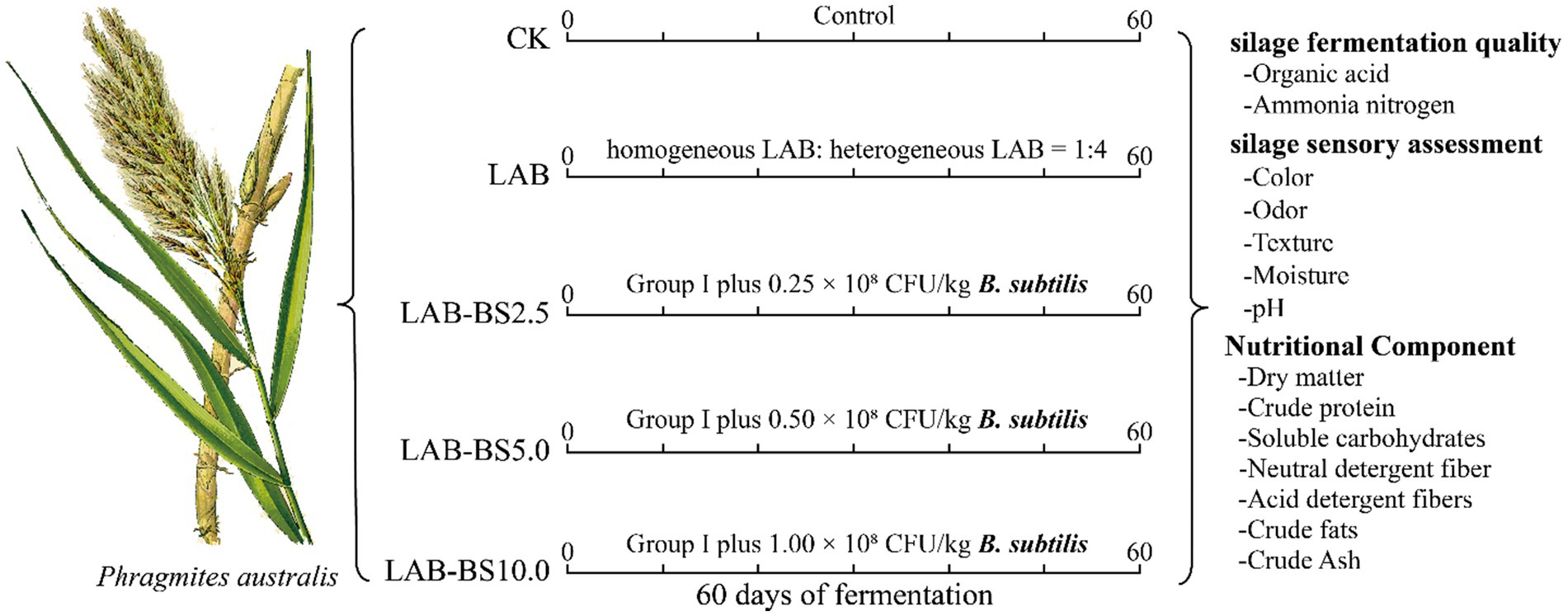
Five groups with three replicates each were established, including the control group (CK), inoculum LAB, inoculum LAB-BS2.5, inoculum LAB-BS5.0, and inoculum LAB-BS10.0 groups. The control group (CK) was P. australis silage without inoculants. The inoculum LAB group was the P. australis with homogenous LAB (L. plantarum + L. pentosaceus at a 1:1 ratio) combined with heterogeneous LAB (L. buchneri) at a 1:4 ratio (total LAB: 1.5 × 108 CFU/kg fresh silage). The inoculum LAB-BS2.5, LAB-BS5.0, and LAB-BS10.0 were LAB consortium plus B. subtilis with 2.5 × 107, 5.0 × 107, and 1.0 × 108 CFU/kg, respectively. The details are presented in Table 2. The fermentation process and the determination index of silage are shown in Figure 2. The B. subtilis gradient (2.5 × 107 to 1.0 × 108 CFU/kg) was selected to evaluate dose-dependent effects on fiber degradation and fermentation efficiency.
Silage preparation and fermentation
Inoculation
Microbial suspensions were prepared by dissolving strains in distilled water (10 mL/kg fresh weight), activated at 30°C for 2 h, and uniformly sprayed onto chopped P. australis.
Packing and storage
reated material was packed into 40 cm × 60 cm polyethylene bags (1 kg/bag), vacuum-sealed, and stored at 25–30°C for 90 days (18). This duration was based on preliminary trials confirming pH stabilization and organic acid equilibrium.
Silage quality assessment
Silage fermentation quality
After homogenizing P. australis silage, 50 g of the sample was combined with 450 mL of distilled water in a sealed 500 mL Erlenmeyer flask. The mixture was refrigerated at 4°C for 24 h, filtered through polyester cloth, and pressed to extract the residual liquid. The filtrate was further purified using qualitative filter paper and stored in 15 mL centrifuge tubes for subsequent analyses.
pH and organic acid analysis
The pH value was measured using a calibrated pH meter (HI2211, Hanna Instruments). Lactic acid (LA) was quantified using high-performance liquid chromatography (HPLC; Agilent 1,290, USA). Volatile fatty acids, such as acetic acid (AA), butyric acid (BA), and propionic acid (PA), were analyzed using gas chromatography (GC; Agilent 7890A, USA) equipped with a flame ionization detector (FID) and an FFAP capillary column (15 m × 0.32 mm × 0.25 μm). Operational parameters included helium carrier gas (19.991 kPa), injector temperature (250°C), and a 2 μL injection volume.
Ammonia nitrogen (NH3-N) determination
NH3-N content was assessed using phenol-sodium hypochlorite colorimetry. Specifically, a 1.5 mL aliquot of extract was mixed with 0.15 mL of 25% metaphosphoric acid (10,1, v/v), stabilized for 30 min, and centrifuged (1,500 rpm, 4°C, 15 min). The supernatant was filtered through a 0.45 μm filter membrane prior to spectrophotometric analysis.
Nutritional component determination
Silage samples (200 g) were oven-dried at 65°C to constant weight, ground through a 40 mesh sieve, and stored in airtight containers. DM was determined by further drying at 105°C. Nutritional parameters were analyzed as follows: CP: Kjeldahl nitrogen method; WSC: anthraquinone-sulfuric acid colorimetry; NDF and ADF: sequential detergent filtration (Fan’s method); EE: Sohren’s extraction with petroleum ether; Crude ash: high-temperature incineration (550°C, 6 h) (24, 28).
Silage evaluation standards
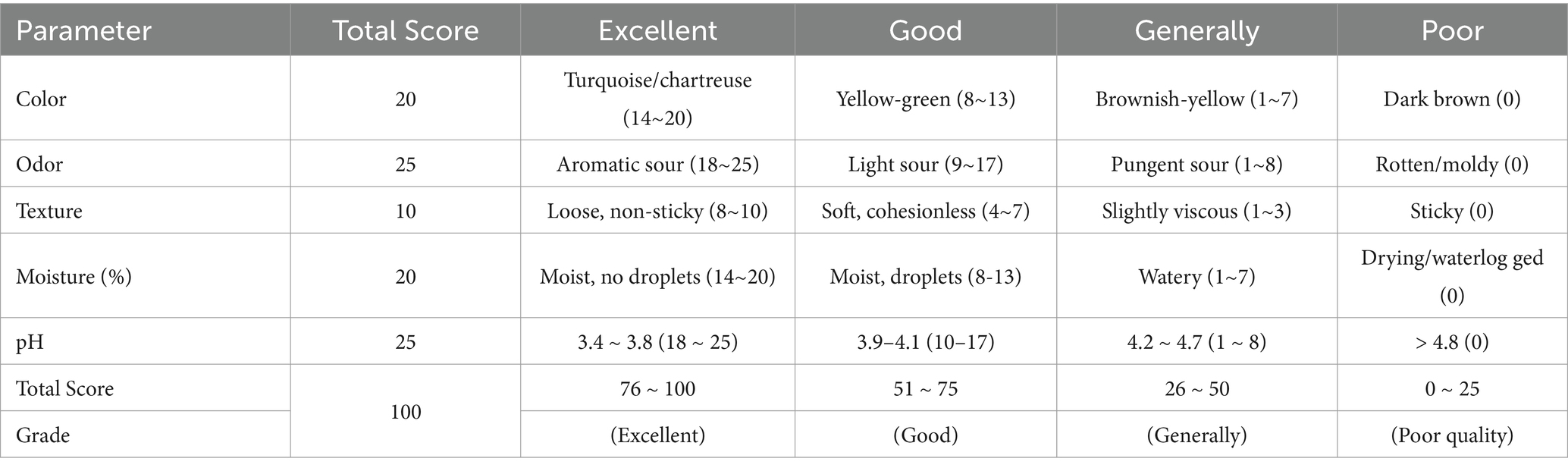
Silage sensory quality was graded per China’s Standard for silage quality evaluation (Table 3), assessing: color (turquoise to dark brown, score 0–20), odor (aromatic sour to moldy, score 0–25), texture (loose to sticky, score 0–10), moisture (compacted to watery, score 0–20), and pH values (3.4–4.8, score 0–25).
Statistical analysis
All experimental data were reported as mean ± standard error (SE), indicating variability around the mean estimate. Data normality was verified using the Shapiro–Wilk test (29). One-way ANOVA with Duncan’s post-hoc test compared treatment means was conducted in SPSS (v22.0), with significance thresholds at p < 0.05 (significant) and p < 0.01 (highly significant). Spearman’s correlation coefficients were calculated using the R package psych (v 2.4.1), and results were visualized with corrplot (v 0.92).
Results
Sensory assessment and pH score of P. australis silage
All silage treatments exhibited favorable sensory profiles, with no signs of mold or spoilage (Table 4). The silage color ranged from yellow-green to brownish-yellow, displaying a soft, non-sticky texture and optimal moisture content (14–17/20) without water droplet formation. A pronounced wine-like aroma was observed across treatments, particularly in LAB-BS5.0 and the Control (CK), which scored highest in odor (12/25). pH values ranged between 3.9 and 4.1, with LAB-BS5.0 (3.98) and LAB-BS10.0 (3.94) showing lower pH than CK (3.99), while LAB-BS2.5 (4.12) and LAB-BS2.5 (4.12) and LAB (4.02) had slightly higher values. Total sensory scores classified all treatments as “Good” (60–70/100), with LAB-BS 5.0 and CK achieving the highest scores (70/100).
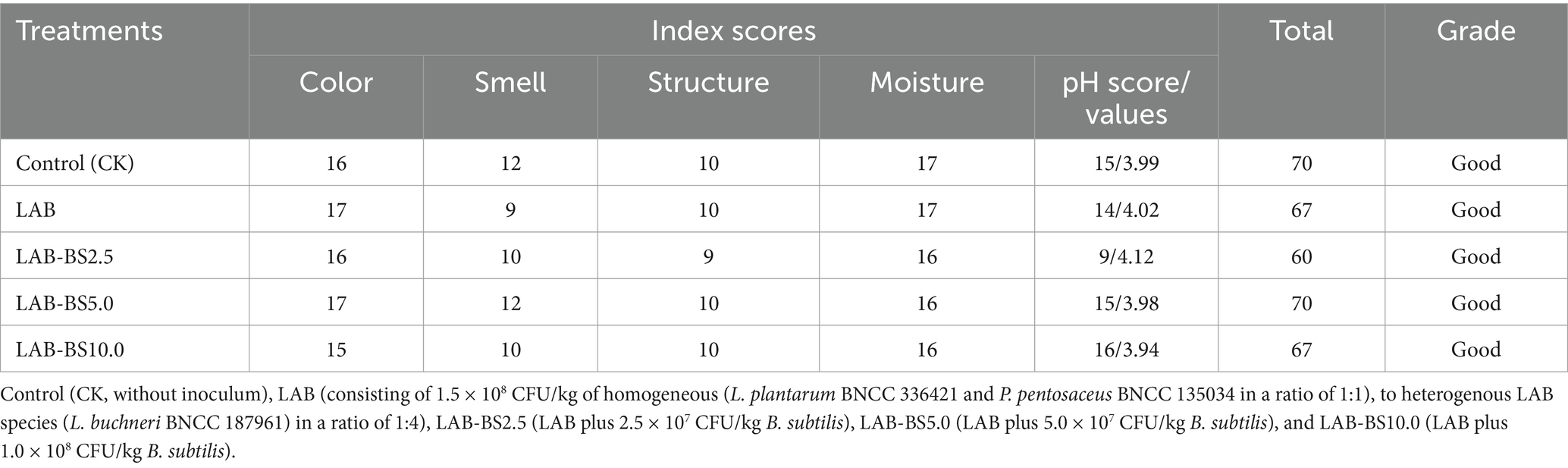
Table 4. Sensory evaluation scores of P. australis silage by introducing B. subtilis and LAB consortia.
Effects on organic acid profiles and ammonia nitrogen in P. australis silage by introducing B. subtilis and LAB consortia
The addition of B. subtilis significantly influenced organic acid profiles and NH3-N content (Table 5). While LAB increased LA content (1.86 ± 0.04 g/kg) compared to CK (1.76 ± 0.03 g/kg), the difference was non-significant. However, LAB-BS2.5 (2.00 ± 0.11 g/kg) and LAB-BS2.5 (1.93 ± 0.06 g/kg) showed significantly higher LA than CK (p < 0.05). BA and PA concentrations increased significantly in LAB (1.21 ± 0.05 g/kg and 0.43 ± 0.02 g/kg, respectively) compared to CK (p < 0.01). NH3-N decreased progressively with higher B. subtilis doses, reaching the lowest value in LAB-BS10.0 (0.60 ± 0.09 g/kg vs. CK: 0.89 ± 0.03 g/kg; p < 0.05). All treatments reduced acetic acid (AA) and PA compared to CK (p < 0.01), with LAB-BS10.0 showing the most pronounced reduction (AA: 2.01 ± 0.01 g/kg; PA: 0.17 ± 0.00 g/kg).
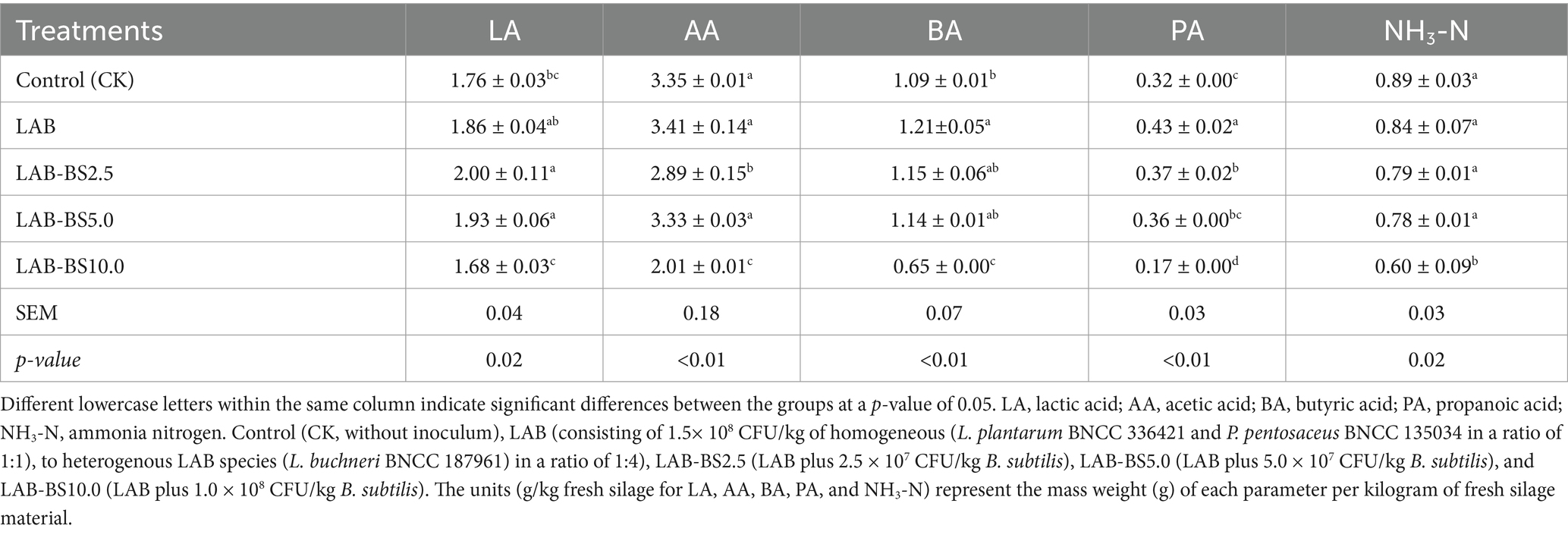
Table 5. Silage fermentation parameters for P. australis silage by introducing B. subtilis and LAB consortia.
In summary, LAB-BS10.0 achieved the highest LA-to-total acid ratio (62.3%, p < 0.01) and the lowest NH3-N content, indicating superior fermentation efficiency. LAB-BS2.5 and LAB-BS5.0 demonstrated a dose-dependent enhancement in LA production, highlighting B. subtilis’s role in acidification.
Effect on the nutrient content of P. australis silage
The inclusion of B. subtilis significantly influenced the nutritional profile of P. australis silage (Table 6). DM content decreased (p < 0.01) in all inoculated groups compared to CK, with the lowest values observed in LAB-BS2.5 (24.92%) and LAB-BS5.0 (24.99%). DM followed a descending order: LAB-BS10.0 (26.43%) > LAB-BS5.0 > LAB-BS2.5.
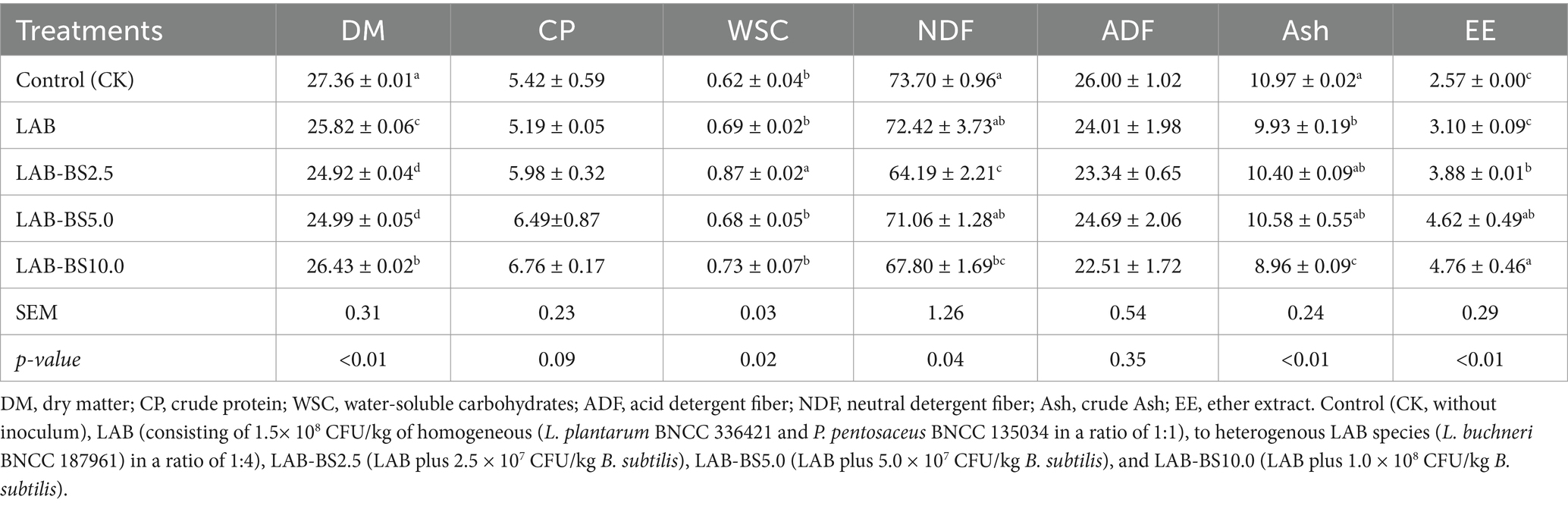
Table 6. Nutrient compositions of P. australis silage (%, DM basis) by introducing B. subtilis and LAB consortia.
CP content increased incrementally with higher B. subtilis doses, peaking in LAB-BS10.0 (6.76%), though differences from CK (5.42%) were non-significant (p = 0.09). LAB-BS2.5 exhibited the highest WSC (0.87%, p < 0.05), a 40.3% increase over CK. NDF decreased significantly in LAB-BS2.5 (64.19%, p < 0.05) and LAB-BS10.0 (67.80%, p < 0.05), representing reductions of 9.51 and 5.9%, respectively. ADF was lowest in LAB-BS10.0 (22.51%), though differences from CK (26.00%) were non-significant. Ash content decreased (p < 0.01) in LAB (9.93%) and LAB-BS10.0 (8.96%). EE increased progressively with B. subtilis dosage, reaching 4.76% in LAB-BS10.0 (p < 0.01). LAB-BS10.0 optimized fiber degradation (NDF: 67.80%) while enhancing CP (6.76%) and EE (4.76%). LAB-BA2.5 maximized WSC accumulation (0.87%), critical for microbial activity during fermentation.
Correlation analysis between nutrients and fermentation parameters by introducing B. subtilis
Key correlations between nutrients and fermentation parameters of P. australis silage were identified (Figure 3). Positive correlations between NDF/ADF and AA/TA (r = 0.82), BA (r = 0.76), and NH3-N (r = 0.68) suggest that fiber-rich substrates favor acetic/butyric acid production. Positive association of B. subtilis with CP (r = 0.71) and negative correlation with NH3-N (r = −0.65) indicate its role in converting ammonia to microbial protein. B. subtilis correlated positively with LA/TA (r = 0.89) and negatively with pH (r = −0.78), highlighting its dual role in acid production and fiber breakdown.
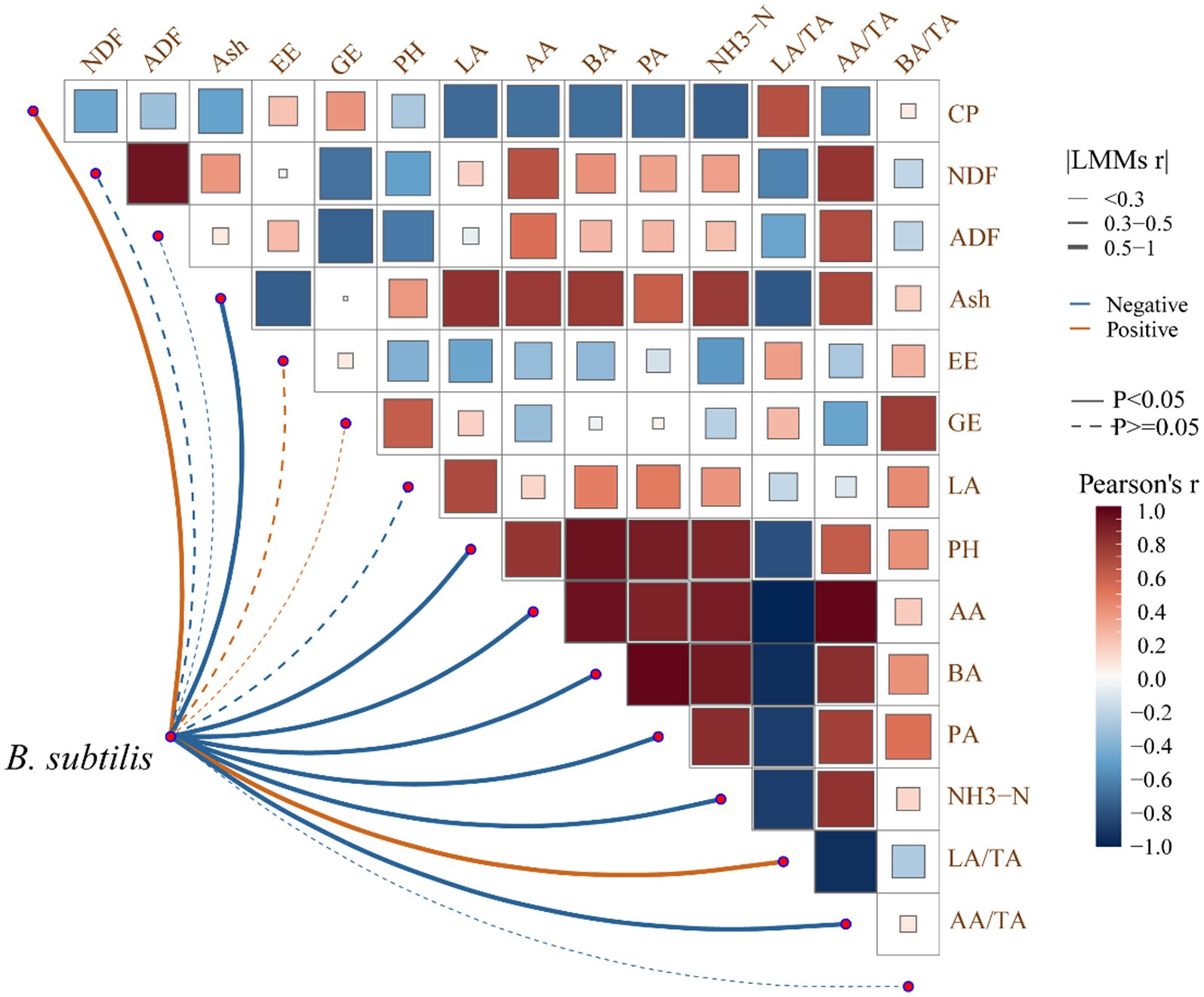
Figure 3. Correlation analysis between nutrients and fermentation parameters of P. australis by introducing B. subtilis in the LAB fermentation system.
B. subtilis enhances lactic acid dominance (LA/TA > 60%) while reducing NH3-N, critical for high-quality silage. Fiber degradation by B. subtilis improves digestibility, supporting P. australis as a viable unconventional forage.
Discussion
Effect on fermentation quality of P. australis silage
NH3-N, LA, and volatile fatty acids (VFAs) are critical indicators of silage fermentation quality (30). In this study, NH3-N content decreased progressively with increasing B. subtilis inoculation, reaching the lowest level in LAB-BS10.0 (0.60 ± 0.09 g/kg vs. Control: 0.89 ± 0.03 g/kg; p < 0.05). This aligns with prior findings that B. subtilis produces bacteriocin-like metabolites, suppressing yeasts and molds while enhancing aerobic stability and reducing NH3-N through proteolysis (27, 31–33). Additionally, B. subtilis-mediated acetolactate synthase activity likely catalyzed pyruvate conversion to acetolactic acid, improving both nutritional value and palatability (27, 32). Another factor affecting silage quality was the silage fermentation time, which ranged from 60 to 120 days, particularly 60 to 90 days (15, 18, 34).
LABs are well-documented for their role in rapid acidification, which preserves silage by inhibiting spoilage microorganisms (35–37). Homogeneous LAB (e.g., Lentilactobacillus plantarum) excel in LA production but offer limited inhibition of harmful bacteria (38, 39). Conversely, heterogeneous LAB (e.g., L. buchneri) metabolize residual sugars into AA, effectively suppressing molds (12). This study’s combination of homofermentative and heterofermentative LAB at a 1:4 ratio synergistically enhanced LA yield while maintaining AA-driven mold inhibition (Table 5).
Notably, LAB-BS10.0 achieved the highest LA-to-total acid ratio (62.3%, p < 0.01), despite a slight reduction in absolute LA content compared to LAB-BS2.5 (2.00 ± 0.11 g/kg). This paradox highlights B. subtilis’s dual role: (1) promoting fiber degradation to release fermentable substrates for LA synthesis and (2) redirecting metabolic pathways to prioritize LA over VFAs like BA and PA (40, 41). The progressive decline in AA, BA, and PA with increasing B. subtilis doses (p < 0.01) further underscores its ability to refine fermentation profiles, favoring LA dominance.
Effect on the nutritional value of P. australis silage
DM content is a critical indicator of silage preservation efficiency (42). In this study, DM decreased significantly (p < 0.01) in all inoculated groups compared to the Control (CK: 27.36%), likely due to microbial utilization of soluble carbohydrates during fermentation (Table 6). Notably, LAB-BS20.0 retained higher DM (26.43%) than other inoculated groups, suggesting B. subtilis moderates substrate consumption while enhancing fiber degradation.
CP content, a key nutritional metric (43), showed no significant differences between treatments (p = 0.09), though LAB-BS10.0 achieved the highest CP (6.76%). This aligns with Bai et al. (44), where B. subtilis improved protein retention in corn silage. Conversely, Bonaldi et al. (32) observed no CP enhancement with B. subtilis, possibly due to differences in substrate composition.
WSC peaked in LAB-BS2.5 (0.87%, p < 0.05), reflecting B. subtilis’s role in hydrolyzing structural carbohydrates. However, higher B. subtilis doses reduced WSC, likely due to accelerated microbial metabolism. B. subtilis’s cellulase activity (45) likely contributed to NDF reduction in LAB-BS2.5 (64.19%, p < 0.05) and LAB-BS10.0 (67.80%, p < 0.05), contrasting with Guo et al. (41), who reported minimal fiber impact.
Ash content decreased significantly (p < 0.01) in LAB (9.93%) and LAB+BS10.0 (8.96%), likely due to BS-driven mineral solubilization. Ether extract (EE) increased progressively with BS dosage, peaking at 4.76% in LAB+BS10.0 (p < 0.01), underscoring B. subtilis’s role in lipid preservation. B. subtilis synergizes with LAB to enhance fiber degradation (decrease NDF) and lipid retention (increase EE), though its dose-dependent effects on WSC and ash warrant further mechanistic exploration.
Conclusion
The integration of homogeneous LAB consortia with graded doses of B. subtilis significantly enhanced the fermentation and nutritional quality of P. australis silage. The optimal treatment, LAB-BS10.0 (1 × 108 CFU·kg-1 B. subtilis), demonstrated the highest lactic acid-to-total acid ratio (62.3%) alongside marked reductions in NH3-N (0.60 ± 0.09 g/kg) and NDF (67.80%). Concurrently, EE increased to 4.76%, emphasizing B. subtilis’s role in lipid preservation and fiber degradation. These findings validate the synergistic potential of LAB-BS consortia to improve P. australis silage quality, offering actionable insights for scaling its silage production as a sustainable feed resource. This study provides a technical framework for optimizing microbial inoculants in P. australis silage system, addressing local forage shortages through innovative biomass valorization.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YL: Conceptualization, Funding acquisition, Investigation, Writing – original draft. SZ: Formal analysis, Methodology, Writing – original draft. JL: Project administration, Software, Validation, Writing – original draft. NK: Data curation, Formal analysis, Resources, Writing – original draft. ST: Funding acquisition, Investigation, Resources, Writing – original draft. CZ: Project administration, Writing – original draft. ZT: Conceptualization, Data curation, Writing – original draft. AE: Conceptualization, Writing – original draft. IR: Funding acquisition, Writing – original draft. FZ: Funding acquisition, Writing – original draft. AS: Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The present project was supported by the National Key Research and Development Project of China (2023YFD1301705), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA26010301), the Natural Science Foundation of Guangxi Zhuang Autonomous Region (2022GXNSFAA035517), and the Natural Science Foundation of Hunan Province (2023JJ30613) and also the Slovak grant VEGA (no. 1/0162/23) and KEGA (no. 011UVLF-4/2024).
Acknowledgments
We thank all of the participants in the trial and their families. The silage experiment and sample analysis were conducted by Shibo Wang, Shuya Liang, and Jingjing Sun.
Conflict of interest
SZ was employed by Liuyang Liu'an Agricultural Technology Comprehensive Development Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views and opinions expressed in this article are solely those of the authors. The publisher remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
AA, acetic acid; ADF, acid detergent fiber; BA, butyric acid; BS, Bacillus subtilis; CP, crude protein; DM, dry matter; EE, ether extract; LA, lactic acid; LAB, lactic acid bacteria; LAB-BS, lactic acid bacteria combined with B. subtilis; NDF, neutral detergent fiber; NH3-N, ammonia nitrogen; PA, propionic acid; WSC, soluble carbohydrates; TN, total nitrogen.
References
1. Köbbing, J-F, Thevs, N, and Zerbe, S. The utilisation of reed (Phragmites australis): a review. Mires Peat. (2013) 13:1–14. Available at: http://www.mires-and-peat.net/pages/volumes/map13/map1301.php
2. Engloner, AI. Structure, growth dynamics and biomass of reed (Phragmites australis)–a review. Flora Morphol Distrib Funct Ecol Plants. (2009) 204:331–46. doi: 10.1016/j.flora.2008.05.001
3. Obreja, CD, Buruiana, DL, Mereuta, E, Muresan, A, Ceoromila, AM, Ghisman, V, et al. Detection of reed using Cnn method and analysis of the dry reed (Phragmites australis) for a sustainable Lake area. Plant Methods. (2023) 19:61. doi: 10.1186/s13007-023-01042-w
4. Beyzi, SB, Ülger, İ, and Konca, Y. Chemical, fermentative, nutritive and anti-nutritive composition of common reed (Phragmites australis) plant and silage. Waste Biomass Valori. (2023) 14:927–36. doi: 10.1007/s12649-022-01903-w
5. Tahir, MHN, Casler, MD, Moore, KJ, and Brummer, EC. Biomass yield and quality of reed canarygrass under five harvest management systems for bioenergy production. Bioenergy Res. (2011) 4:111–9. doi: 10.1007/s12155-010-9105-3
6. Sun, H, Kang, X, Tan, H, Cai, H, and Chen, D. Progress in fermented unconventional feed application in monogastric animal production in China. Fermentation. (2023) 9:947. doi: 10.3390/fermentation9110947
7. Jung, S-J, Kim, S-H, and Chung, I-M. Comparison of lignin, cellulose, and hemicellulose contents for biofuels utilization among 4 types of lignocellulosic crops. Biomass Bioenergy. (2015) 83:322–7. doi: 10.1016/j.biombioe.2015.10.007
8. Dunière, L, Sindou, J, Chaucheyras-Durand, F, Chevallier, I, and Thévenot-Sergentet, D. Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim Feed Sci Technol. (2013) 182:1–15. doi: 10.1016/j.anifeedsci.2013.04.006
9. Ge, X, Xu, F, Vasco-Correa, J, and Li, Y. Giant reed: a competitive energy crop in comparison with miscanthus. Renew Sust Energ Rev. (2016) 54:350–62. doi: 10.1016/j.rser.2015.10.010
10. Krause, DO, Denman, SE, Mackie, RI, Morrison, M, Rae, AL, Attwood, GT, et al. Opportunities to improve fiber degradation in the rumen: microbiology, ecology, and genomics. FEMS Microbiol Rev. (2003) 27:663–93. doi: 10.1016/S0168-6445(03)00072-X
11. Pasolli, E, De Filippis, F, Mauriello, IE, Cumbo, F, Walsh, AM, Leech, J, et al. Large-scale genome-wide analysis links lactic acid Bacteria from food with the gut microbiome. Nat Commun. (2020) 11:2610. doi: 10.1038/s41467-020-16438-8
12. Muck, RE, Nadeau, EMG, McAllister, TA, Contreras-Govea, FE, Santos, MC, and Kung, L Jr. Silage review: recent advances and future uses of silage additives. J Dairy Sci. (2018) 101:3980–4000. doi: 10.3168/jds.2017-13839
13. Duar, RM, Lin, XB, Zheng, J, Martino, ME, Grenier, T, Pérez-Muñoz, ME, et al. Lifestyles in transition: evolution and natural history of the genus Lactobacillus. FEMS Microbiol Rev. (2017) 41:S27–48. doi: 10.1093/femsre/fux030
14. Zheng, J, Wittouck, S, Salvetti, E, Franz, C, Harris, HMB, Mattarelli, P, et al. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. (2020) 70:2782–858. doi: 10.1099/ijsem.0.004107
15. Liu, Y, Zhou, Q, Ji, C, Mu, J, Wang, Y, Harrison, MT, et al. Microbial fermentation in co-ensiling forage-grain ratoon rice and maize to improve feed quality and enhance the sustainability of rice-based production systems. Resour Environ Sustain. (2025) 20:100205. doi: 10.1016/j.resenv.2025.100205
16. Dong, M, Li, Q, Xu, F, Wang, S, Chen, J, and Li, W. Effects of microbial inoculants on the fermentation characteristics and microbial communities of sweet Sorghum bagasse silage. Sci Rep. (2020) 10:837. doi: 10.1038/s41598-020-57628-0
17. Liu, B, Yang, Z, Huan, H, Gu, H, Xu, N, and Ding, C. Impact of molasses and microbial inoculants on fermentation quality, aerobic stability, and bacterial and fungal microbiomes of barley silage. Sci Rep. (2020) 10:5342. doi: 10.1038/s41598-020-62290-7
18. Liu, EY, Wang, S, Wang, S, Khan, NA, Zhou, X, Tang, S, et al. Bacterial inoculants and enzymes based silage cocktails boost the ensiling quality of biomasses from reed, corn and Rice straw. Chem Biol Technol Agric. (2024) 11:29. doi: 10.1186/s40538-024-00549-1
19. Rabelo, C, Basso, F, McAllister, T, Lage, J, Gonçalves, G, Lara, E, et al. Influence of Lactobacillus buchneri as silage additive and forage: concentrate ratio on the growth performance, fatty acid profile in longissimus muscle, and meat quality of beef cattle. Can J Anim Sci. (2016) 96:550–62. doi: 10.1139/cjas-2015-0161
20. Liu, Q, Shao, T, and Bai, Y. The effect of fibrolytic enzyme, Lactobacillus plantarum and two food antioxidants on the fermentation quality, alpha-tocopherol and Beta-carotene of high moisture napier grass silage ensiled at different temperatures. Anim Feed Sci Technol. (2016) 221:1–11. doi: 10.1016/j.anifeedsci.2016.08.020
21. Koç, F, Aksoy, SO, Okur, AA, Celikyurt, G, Korucu, D, and Ozduven, M. Effect of pre-fermented juice, Lactobacillus plantarum and Lactobacillus buchneri on the fermentation characteristics and aerobic stability of high dry matter alfalfa bale silage. J Anim Plant Sci. (2017) 27:1426–31.
22. Sawall, ZJ. Effects of forage preservation methods, dietary starch amount and supplementation with Propionibacterium freudenreichii strain P169 on transition cow health and production, and ketosis prediction tools. Minneapolis: University of Minnesota (2015) Available at: https://conservancy.umn.edu/handle/11299/173809.
23. Yin, H, Zhao, M, Pan, G, Zhang, H, Yang, R, Sun, J, et al. Effects of Bacillus Subtilis or Lentilactobacillus buchneri on aerobic stability, and the microbial community in aerobic exposure of whole plant corn silage. Front Microbiol. (2023) 14:1177031. doi: 10.3389/fmicb.2023.1177031
24. Pahlow, G, Muck, RE, Driehuis, F, Elferink, SJWHO, and Spoelstra, SF. Microbiology of ensiling. In Silage Science and Technology (2003) doi: 10.2134/agronmonogr42.c2
25. Bai, J, Xu, D, Xie, D, Wang, M, Li, Z, and Guo, X. Effects of antibacterial peptide-producing Bacillus subtilis and Lactobacillus buchneri on fermentation, aerobic stability, and microbial community of alfalfa silage. Bioresour Technol. (2020) 315:123881. doi: 10.1016/j.biortech.2020.123881
26. Sonenshein, AL. Control of key metabolic intersections in Bacillus subtilis. Nat Rev Microbiol. (2007) 5:917–27. doi: 10.1038/nrmicro1772
27. Bumbieris, VH, Guimarães, VAP, Fortaleza, APS, Massaro, FL, Moraes, GJ, and Meza, DAR. Aerobic stability in corn silage (Zea mays L.) ensiled with different microbial additives. Acta Sci Anim Sci. (2017) 39:357–62. doi: 10.4025/actascianimsci.v39i4.34426
28. Kiers, JL, Rombouts, F, and Nout, M. In vitro digestibility of Bacillus fermented soya bean. Int J Food Microbiol. (2000) 60:163–9. doi: 10.1016/S0168-1605(00)00308-1
29. Shapiro, SS, and Wilk, MB. An analysis of variance test for normality (complete samples). Biometrika. (1965) 52:591–611. doi: 10.1093/biomet/52.3-4.591
30. Zi, X, Li, M, Chen, Y, Lv, R, Zhou, H, and Tang, J. Effects of citric acid and Lactobacillus plantarum on silage quality and bacterial diversity of king grass silage. Front Microbiol. (2021) 12:631096. Epub 2021/03/16. doi: 10.3389/fmicb.2021.631096
31. Lara, EC, Basso, FC, de Assis, FB, Souza, FA, Berchielli, TT, and Reis, RA. Changes in the nutritive value and aerobic stability of corn silages inoculated with Bacillus subtilis alone or combined with Lactobacillus plantarum. Anim Prod Sci. (2015) 56:1867–74. doi: 10.1071/AN14686
32. Bonaldi, D, Carvalho, B, Ávila, C, and Silva, C. Effects of Bacillus subtilis and its metabolites on corn silage quality. Lett Appl Microbiol. (2021) 73:46–53. doi: 10.1111/lam.13474
33. Woolford, MK. The detrimental effects of air on silage. J Appl Bacteriol. (1990) 68:101–16. doi: 10.1111/j.1365-2672.1990.tb02554.x
34. Wu, Y, Xiao, Y, Okoye, CO, Gao, L, Chen, X, Wang, Y, et al. Fermentation profile and bioactive component retention in honeysuckle residue silages inoculated with lactic acid Bacteria: a promising feed additive for sustainable agriculture. Ind Crop Prod. (2025) 224:120315. doi: 10.1016/j.indcrop.2024.120315
35. Danner, H, Holzer, M, Mayrhuber, E, and Braun, R. Acetic acid increases stability of silage under aerobic conditions. Appl Environ Microbiol. (2003) 69:562–7. doi: 10.1128/AEM.69.1.562-567.2003
36. Okoye, CO, Wang, Y, Gao, L, Wu, Y, Li, X, Sun, J, et al. The performance of lactic acid Bacteria in silage production: a review of modern biotechnology for silage improvement. Microbiol Res. (2023) 266:127212. doi: 10.1016/j.micres.2022.127212
37. Jiang, FG, Cheng, HJ, Liu, D, Wei, C, An, WJ, Wang, YF, et al. Treatment of whole-plant corn silage with lactic acid Bacteria and organic acid enhances quality by elevating acid content, reducing Ph, and inhibiting undesirable microorganisms. Front Microbiol. (2020) 11:593088. doi: 10.3389/fmicb.2020.593088
38. Ranjit, NK, and Kung, L Jr. The effect of Lactobacillus buchneri, Lactobacillus plantarum, or a chemical preservative on the fermentation and aerobic stability of corn silage. J Dairy Sci. (2000) 83:526–35. doi: 10.3168/jds.S0022-0302(00)74912-5
39. Marco, ML, Sanders, ME, Gänzle, M, Arrieta, MC, Cotter, PD, De Vuyst, L, et al. The international scientific Association for Probiotics and Prebiotics (Isapp) consensus statement on fermented foods. Nat Rev Gastroenterol Hepatol. (2021) 18:196–208. doi: 10.1038/s41575-020-00390-5
40. Abdul Rahman, N, Abd Halim, MR, Mahawi, N, Hasnudin, H, Al-Obaidi, JR, and Abdullah, N. Determination of the use of Lactobacillus plantarum and Propionibacterium freudenreichii application on fermentation profile and chemical composition of corn silage. Biomed Res Int. (2017) 2017:1–8. doi: 10.1155/2017/2038062
41. Guo, X, Guo, W, Yang, M, Sun, Y, Wang, Y, Yan, Y, et al. Effect of Bacillus additives on fermentation quality and bacterial community during the ensiling process of whole-plant corn silage. PRO. (2022) 10:978. doi: 10.3390/pr10050978
42. Borreani, G, Tabacco, E, Schmidt, RJ, Holmes, BJ, and Muck, RE. Silage review: factors affecting dry matter and quality losses in silages. J Dairy Sci. (2018) 101:3952–79. doi: 10.3168/jds.2017-13837
43. Da Silva, L, Pereira, O, Da Silva, T, Valadares Filho, S, and Ribeiro, K. Effects of silage crop and dietary crude protein levels on digestibility, ruminal fermentation, nitrogen use efficiency, and performance of finishing beef cattle. Anim Feed Sci Technol. (2016) 220:22–33. doi: 10.1016/j.anifeedsci.2016.07.008
44. Bai, J, Franco, M, Ding, Z, Hao, L, Ke, W, Wang, M, et al. Effect of Bacillus amyloliquefaciens and Bacillus subtilis on fermentation, dynamics of bacterial community and their functional shifts of whole-plant corn silage. J Anim Sci Biotechnol. (2022) 13:1–14. doi: 10.1186/s40104-021-00649-0
Keywords: silage, microbial inoculant, homofermentative, heterofermentative, Lactobacillus , reed, silage quality
Citation: Liu Y, Zhang S, Liao J, Khan NA, Tang S, Zhou C, Tan Z, Elnagar A, Rehan IF, Zigo F and Salem AZM (2025) Enhancing fermentation quality and fiber decomposition of Phragmites australis silage by introducing Bacillus subtilis and lactic acid bacteria consortia. Front. Vet. Sci. 12:1557614. doi: 10.3389/fvets.2025.1557614
Edited by:
Izhar Hyder Qazi, South China Agricultural University, ChinaReviewed by:
Selim Esen, Republic of Turkey Ministry of Agriculture and Forestry, TürkiyeHuiyu Shi, Hainan University, China
Copyright © 2025 Liu, Zhang, Liao, Khan, Tang, Zhou, Tan, Elnagar, Rehan, Zigo and Salem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuanshe Zhou, emNzQGlzYS5hYy5jbg==; Abdelfattah Z. M. Salem, YWJkZWxmYXR0YWguc2FsZW1AdW5pYmEuaXQ=; František Zigo, ZnJhbnRpc2VrLnppZ29AdXZsZi5zaw==
 Yong Liu
Yong Liu Songbai Zhang2
Songbai Zhang2 Nazir Ahmad Khan
Nazir Ahmad Khan Shaoxun Tang
Shaoxun Tang Chuanshe Zhou
Chuanshe Zhou Zhiliang Tan
Zhiliang Tan Asmaa Elnagar
Asmaa Elnagar Ibrahim F. Rehan
Ibrahim F. Rehan František Zigo
František Zigo Abdelfattah Z. M. Salem
Abdelfattah Z. M. Salem