- 1Department of Parasitology, Bangladesh Agricultural University, Mymensingh, Bangladesh
- 2Institute of Parasitology, Justus Liebig University Giessen, Giessen, Germany
- 3International Livestock Research Institute, Nairobi, Kenya
- 4Royal (Dick) School of Veterinary Sciences, University of Edinburgh, Edinburgh, United Kingdom
Cysticercosis is a neglected zoonosis caused by Taenia solium, which involves pigs as intermediate hosts, leading to pig cysticercosis (PCC). Humans are the only definitive hosts, harbouring the mature tapeworm in the small intestines, but they can also act as intermediate hosts upon accidental ingestion of eggs, resulting in human cysticercosis (HCC), called neurocysticercosis (NCC) when the cysts lodge in the central nervous system. Diagnosis of HCC/NCC in humans is based on imaging technologies and serology. The gold standard method for PCC diagnosis is the full carcass dissection and recovery of cysts. However, tongue palpation and meat inspection are the most widely used methods in endemic countries. These methods are specific at the genus level but cannot distinguish mixed infection from other taeniids and are not sufficiently sensitive in pigs with low infection. Available serological tests for human and pig infection are based on parasite-specific immunoglobulin G (IgG). Still, most tests are either cross-reactive with other taeniids or not sensitive enough for single or inactive cysts, particularly for NCC patients. Here, we compare various serological techniques for PCC and NCC published since 2000 and discuss the benefit of IgE-based serodiagnosis as a potential alternative to traditional serology. Considering the diagnostic limitations described above and the need to identify endemic areas to prevent transmission between humans and pigs and monitor control efforts, the development of more sensitive and specific serological tests, followed by a field-applicable point-of-care (POC) test for cysticercosis, is of the utmost importance.
1 Introduction
Cysticercosis is a food-borne parasitosis caused by Taenia solium (T. solium) in humans and pigs. The World Health Organization (WHO) has identified cysticercosis as one of the twenty-one neglected tropical diseases (NTDs) worldwide (1), while the U. S. Centers for Disease Control and Prevention (CDC) has designated cysticercosis as one of the five neglected parasitic infections in the United States (2). This zoonotic parasite has been estimated to cause the highest number of foodborne Disability Adjusted Life Years (DALY) by the WHO Foodborne Disease Burden Epidemiology Reference Group (3), excluding viral and bacterial diseases (but including protozoan parasites). Furthermore, it is considered a significant public health problem in most endemic countries in Latin America, Africa, and Asia (4).
Human cysticercosis (HCC) is caused by the larvae of T. solium, which predominantly affect the central nervous system (CNS), accounting for 86% of cases (5). However, cysts may also develop in extra-CNS sites, including the eyes, muscles, skin, subcutaneous tissues, heart, lungs, and peritoneum (4, 6). These non-neural manifestations diaphragm are collectively referred to as extraneural HCC and can be further categorized into intramuscular, subcutaneous, cardiac, pulmonary, and ophthalmic forms (5, 7–11). The neural form of cysticercosis, known as neurocysticercosis (NCC), is a major neurological disorder in humans, estimated to account for up to 30% of avoidable epilepsy (12, 13). It evolves in endemic countries with low sanitation coverage conditions and a free-ranging pig production system. There are two types of NCC: Parenchymal NCC (occurring in the brain tissue) and extraparenchymal NCC (occurring in the intraventricular and subarachnoid spaces of the brain and spinal cord). Extraparenchymal NCC is the most severe form (14) and most resistant to drug treatment (15). Around 2.56–8.30 million people are affected by NCC, including symptomatic and asymptomatic cases (16). The larval/cysticercus stage of T. solium also causes pig cysticercosis (PCC). In pigs, T. solium larvae mostly remain in the host muscles but can also occur in other organs, including the eyes and tongue (17). This results in carcass condemnation and decreased value of pigs, with an estimated 20–60% production losses in pig-raising countries (18).
Diagnosing T. solium cysticercosis in humans and pigs depends on the organ affected and the severity of the infection. Additionally, the occurrence of other Taenia species (e.g., T. saginata, T. hydatigena, T. asiatica) in the same environment and cross-immunity among these species (19) could have moderated or modified the prevalence of T. solium in pigs and humans, as found in South East Asia and some African countries (20–22). This interspecific competition and cross-reactivity hinder the differential diagnosis of cysticercosis at the species level, leading to inadequate diagnostic capability and poor infection investigation. Therefore, in endemic countries where different Taenia species of pig (T. solium, T. hydatigena, T. asiatica) are prevalent, species-specific diagnosis for T. solium is difficult (22, 23). For NCC patients, a definitive diagnosis requires advanced neuro-imaging (magnetic resonance imaging [MRI] and a non-contrast computed tomography [CT] scan of the brain), brain or spinal cord biopsy, or in rare cases, visualization of subretinal cysts (24). HCC involving the intramuscular, cardiac, and pulmonary systems also requires imaging techniques as in NCC (25–27), whereas ophthalmic cysticercosis is mostly diagnosed using orbital ultrasonography (28). However, neuro-imaging techniques are expensive and often unavailable in endemic areas where tapeworm infection is frequent (29). For the diagnosis of PCC, the most widely used and cheapest methods are tongue palpation (Figure 1) and routine meat inspection of pigs (30, 31). Tongue palpation specificity is close to 100%, but its sensitivity is as low as 16.1% (95%CI: 5–34) (32), thus detecting only heavily infected pigs. Routine meat inspection showed high specificity (100%) with slightly higher sensitivity - 38.7% (95%CI: 22–58) than tongue palpation, but it is also insensitive in light infections (32). The gold standard method for diagnosing PCC is complete carcass muscle/brain dissection, as it has shown greater sensitivity for detecting T. solium cysts than the previous two methods (31, 33, 34). However, carcass dissection is not feasible as a routine method for detecting cysts because of the destruction of a valuable commercial commodity and time constraints (31).
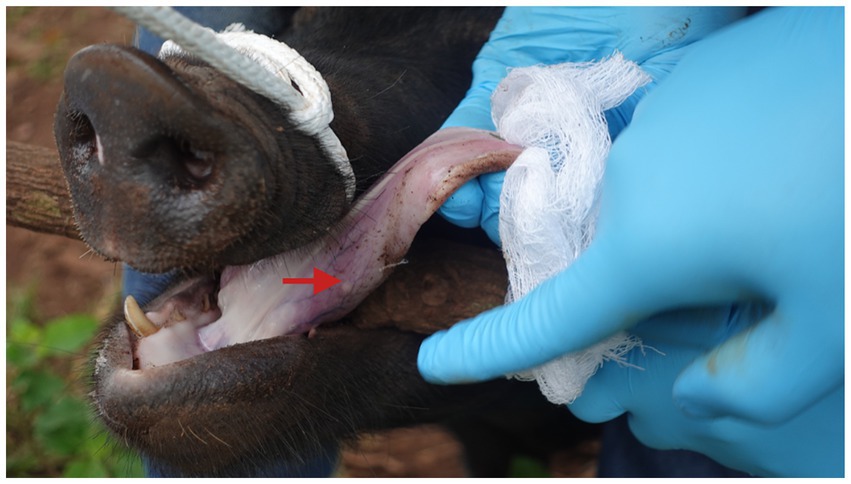
Figure 1. Tongue palpation of pig for testing cysticercosis in Uganda. The red arrow indicates a visible, palpable cyst. This method only works in heavily infected animals (photo: Md. Shahadat Hossain).
In addition to medical imaging in humans and the more ‘physical’ examination forms described above for pigs, a wide range of serological diagnostic methods have been published. Given the varied performance of the published serological tests, analyzing trends and singling out the best tests is difficult. To address this, we conducted a comparative analysis of the performance of serological tests used for NCC and PCC diagnosis published from 2000 to 2024, based on T. solium-specific IgG detection. The published literature in PubMed1 was searched with keywords “Taenia solium”, “neurocysticercosis”, “pig/porcine cysticercosis”, “serodiagnosis” with the Boolean operator AND, which identified relevant serology diagnostics-based studies. Studies which did not report both sensitivity and specificity were not included. Here, we discuss the various serological tests used for the diagnosis of cysticercosis, in humans and pigs, along with their limitations, and compare their sensitivity and specificity. We also describe the prospects of developing an IgE-based serological test to diagnose T. solium infections.
2 Available serological tests used for Taenia solium
Serological tests are the essential diagnostic tools in low-and middle-income countries (LMIC) for human and pig T. solium cysticercosis. These tests are based on detecting circulating T. solium antigens or anti-T. solium antibodies in serum (35), cerebrospinal fluid (CSF) (36), saliva (37) or urine (38). Serodiagnosis of cysticercosis through the detection of antibodies produced against T. solium has been based on different target antigenic preparations, ranging from total T. solium cysts extracts to more selected preparations, such as cyst fluid, scolex extract, or tegumental extract (39, 40). Additionally, lentil lectin-purified glycoproteins (LLGP) (41), recombinant and recombinantly expressed antigens (39, 42), monoclonal antibodies (mAb) (43, 44) of T. solium have been evaluated for serological diagnosis of cysticercosis. A critical issue is that existing serological tests show high levels of cross-reactivity with other taeniids in the field (45).
The two primary serological methods currently used for diagnosing cysticercosis in humans and pigs are the enzyme-linked immunosorbent assay (ELISA) and the enzyme-linked immune electro-transfer blot (EITB) based on lentil lectin-purified glycoproteins (LLGP) antigens and/or recombinant and synthetic antigens (12, 45, 46).
Antibody-capture ELISA techniques (Ab-ELISA) identify circulating T. solium antibodies in serum or CSF, indicating T. solium exposure in a population. The antibody-based EITB (LLGP) immunoblot was first introduced in 1989 based on seven diagnostic LLGP proteins of T. solium (41). In this assay, EITB combines sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and ELISA techniques to identify specific anti-T. solium antibodies in serum or CSF (45). This immunoblot method can detect antibodies to one or more of the seven LLGP antigens (GP 50 family-GP50; T24 family-GP42-39, GP24; 8 kDa family-GP21, GP18, GP14, and GP13) (46). The EITB (LLGP) assay is available through the CDC Parasitic Disease Reference Laboratory for clinical diagnosis of NCC in the United States (12). Recombinant EITB based on recombinant antigens of LLGP also showed promising results for diagnosing PCC/NCC (47, 48). EITB-based LDBIO cysticercosis Western blot kit (LDBIO, Lyon, France) has been developed to identify anti-T. solium IgG antibodies in human sera for diagnosing cysticercosis and NCC in humans (49). Furthermore, active/viable infection can be determined by detecting circulating T. solium cysticercus antigens using a monoclonal antibody (mAb)-based antigen-capture ELISA (Ag-ELISA). The HP10 (43) and B158/60 (44) mAbs are mostly used to detect T. solium infections in humans and pigs. The B158/B60 Ag-ELISA has been commercialized (apDia, Belgium) in the United States (12). Recently, the World Health Organization (WHO) has acknowledged a rapid test for cysticercosis detection that can be performed at the point of care (POC) (50). This rapid POC test or lateral flow assay (LFA) has been developed experimentally based on cyst fluid (51), recombinant antigens (52–56) or mAbs (57, 58) of T. solium using human serum/CSF/urine sample. In LFA, the liquid sample is placed on a solid phase-based platform (porous paper, microstructured polymer) that can transport fluid. The sample is run along the surface of the platform with reactive molecules that show a visual positive or negative result. The results are displayed within 5–30 min (59).
3 Pig cysticercosis (PCC) serodiagnosis
Serological assays for PCC have shown a higher sensitivity than traditional tongue palpation and carcass/meat inspection (31, 34, 60). The Ab-ELISA test was initially developed using antigens from crude worm extracts, cyst fluids, and excretory-secretory products of taeniid parasites. These unpurified complex antigenic mixtures have shown low sensitivities in Ab-ELISA-based diagnosis (35–67%) of PCC (32, 61, 62). EITB (LLGP) has initially reported 100% sensitivity and specificity in diagnosing PCC (63). However, the same test showed moderate sensitivity (88.9%) and unacceptably low specificity (48.3%) for identifying PCC under field conditions in Peru (64). This type of low specificity may be due to the cross-reaction of the GP50 band of the LLGP protein with T. hydatigena or other taeniids (65, 66). A recombinant T. solium antigen (rTs-p27)-based EITB was used to test naturally infected pigs in Mozambique, and the results showed disappointingly low sensitivity (29.7%) and specificity (71.7%) (67). EITB can produce more consistent results when the pigs have more cysts, and even in the absence of viable cysts, the test showed good reactivity if the pigs have a relatively large number of non-viable cysts (64). The sensitivity of the EITB (LLGP) assay has been compared with the Ag-ELISA test based on HP10 and B158/60 mAbs in a community-based study of pigs in South Africa. The results identified comparable sensitivities and specificities for B158/B60 Ag-ELISA (76.3 and 84.1%, respectively), HP10 Ag-ELISA (54.8 and 83.3%, respectively), and EITB (LLGP) (45.3 and 85.3%, respectively) (68). The apDia cysticercosis antigen (B158/B60) detection test showed 100% sensitivity (n = 31) and 99.6% specificity (n = 300) in experimentally infected pigs (69). B158/B60 Ag-ELISA showed similar sensitivity (82.7 and 82.9%) but variable specificity (86.3 and 96.8%) in naturally infected pigs of Tanzania and Peru, respectively (35, 70). In recent years, LFA has improved, using new technology in signal-amplification strategies, applications of new labels, improved quantification systems, and simultaneous detection (59). For instance, up-convert phosphor nanoparticles-based LFA was used for PCC. In this study, TSOL18 (oncospheral stage protein, the antigen used for vaccine development) and GP50 (cystic stage protein) antigens achieved higher sensitivity (93.5 and 97.4%, respectively) and specificity (100% for both) as compared to ELISA. Besides, the cystic larvae were significantly distinguished from those of T. asiatica, Toxoplasma gondii, Clonorchis sinensis, and Trichinella spiralis, suggesting a possible higher specificity of the test strip (71). However, this study did not include T. hydatigena and Echinococcus, which are important candidates for cross-reactivity in PCC diagnosis.
4 Human cysticercosis (HCC) serodiagnosis
Taenia solium larvae are capable of infecting both neural and extraneural tissues in humans. While human cysticercosis (HCC) predominantly involves the central nervous system (neurocysticercosis), dissemination to extraneural tissues can also occur, occasionally presenting as isolated cysts in sites such as muscle, subcutaneous tissue, and other organs (25, 26, 72, 73). Immunologically, extraneural cysticercosis is characterized by elevated levels of various immunoglobulins, including IgM, IgA, IgE, and IgG (74). Ab-ELISA based on IgG has been reported to diagnose pulmonary and intramuscular cysticercosis through serum samples, offering a supportive diagnostic tool in conjunction with imaging (25, 75). In a population-based study in Brazil, Moraes et al. (76) utilized Ab-ELISA to detect antibodies against T. solium metacestodes in both children and adults. The study reported a relatively high seroprevalence, attributed to cross-reactivity and prior exposure rather than active infection, highlighting a common limitation of IgG-based serological assays in endemic areas (76). Notably, IgA-ELISA has demonstrated 100% sensitivity in detecting IgA antibodies in tear samples for the diagnosis of ophthalmic cysticercosis in humans (77).
5 Human neurocysticercosis (NCC) serodiagnosis
NCC is known to result in different antibody response patterns during various phases of the disease (viable/active and inactive/degenerated cyst stage) (78). These variations in immune response have a significant impact on the diagnostic ability of serological tests. Ab-ELISA has been used for NCC diagnosis using T. solium antigens and recombinant antigens. Bueno et al. (79) reported that Ab-ELISA with T. solium total antigen for any NCC achieved 80% sensitivity and specificity in serum samples, whereas CSF samples yielded higher diagnostic accuracy, with 100% sensitivity and 90% specificity. Cyst fluid antigen-based Ab-ELISA were used in some studies, which demonstrated variability in diagnostic performance, with sensitivity ranging from 80 to 100% and specificity from 75 to 96% (80–83). Sahu et al. (84) used both somatic and excretory-secretory (ES) antigens from T. solium metacestode. Their findings indicated superior performance with CSF-derived ES antigens, yielding 88.2% sensitivity and 96.9% specificity (84). The recombinant antigens have further improved serodiagnostic accuracy. Lee et al. (85) evaluated an Ab-ELISA based on the recombinant T. solium metacestode protein rTsM10, which showed high sensitivity (94.3%) and specificity (96.4%) in both serum and CSF, particularly useful for detecting early-stage infections. Moreover, recombinant and synthetic antigens such as rT24H, rGP50, and Ts18var1 have been incorporated into the QuickELISA™ diagnostic platform to compare the performance of selected antigens. Among these, T24H QuickELISA™ showed the highest diagnostic performance, with a reported sensitivity of 96.3% and specificity of 99.2% (86). These findings were supported by Hernández-González et al. (87), who reported similar diagnostic accuracy using rT24H. Lee et al. studied low-molecular-weight proteins (ranging from 7–38 kDa) in T. solium cyst fluid for Ab-ELISA (88). These proteins were analysed from different geographical regions (Korea and Mexico) and showed diagnostic sensitivity and specificity of 97.7 and 98.7%, respectively, against the extraparenchymal NCC (89). The immunoblot, EITB (LLGP), showed 100% specificity and 95% sensitivity in patients with multiple active cysts, including intracranial lesions (41, 78, 90, 91). The GP50 band of EITB (LLGP) appeared first with initial exposure or cyst stage of NCC and remained even after the cyst resolution stage, while T24 responses are heterogeneous (46). Any response against the 8 kDa family indicated active infections or high antigen burden (78, 92). Recombinant and synthetic antigens (rT24H, rGP50, and sTsRS1)-based EITB showed very high sensitivity (99%) and specificity (98%) in patients with ≥2 viable cysts (48). This recombinant EITB method remained negative when they tested with hydatid echinococcosis positive sera, although the technique showed lower sensitivity for detecting NCC cases with a single viable cyst (56%, CI: 40–67) and a calcified cyst (78%, CI: 68–87). Handali et al. (93) developed a multiantigen printing immunoassay (MAPIA) based on six EITB diagnostic protein families (rGP50, rT24H, and peptides sTsRS1, sTs18var1, sTsRS2var1, and sTs14) which reached 97 and 99% sensitivity and specificity with both intra-and extraparenchymal NCC. Another MAPIA-based study using 3 diagnostic proteins (rGP50, rT24H, and sTs14) detected 97.5% parenchymal NCC cases and 100% subarachnoid cases, with 98.53% specificity (94). Tang et al. (95) reported a multiple triplex ELISA using rT24H, rGP50 and sTs18var3 in EITB-format; the highest sensitivity and specificity were observed for rGP50 (94 and 98%) with lower sensitivities obtained with rT24H (81, 92%) or sTs18var3 (93, 92%). A multiplex bead assay (MBA) based on two recombinant proteins (rT24H, and rTs8B2) also showed high sensitivity and specificity (96.1 and 96.5%, respectively) in cases with two or more viable cysts (87). However, in one study, five commercially available T. solium ELISA diagnostic tests (DRG™, Ridascreen™, Novatech™, Cypress™, and IVD™), developed from native or less purified versions of total and vesicular antigen of metacestodes, showed variable low sensitivity (42.9, 71.4, 42.9, 50, 42.9%) and specificity (93.7, 74.2, 95.6, 86.8, 90.6%), respectively. The result showed cross-reaction with all Echinococcus granulosus-positive sera and false-positive reactions to E. multilocularis positive sera. All the tests detected cross-reactions with other unrelated parasites such as Entamoeba histolytica, Schistosoma sp., Fasciola hepatica, Strongyloides stercoralis, Trichinella sp., and filarial worms (89).
Ag-ELISA has been reported as a highly sensitive and specific test for T. solium infections in humans with viable cysts (≥2) (96). The sensitivity of NCC diagnosis in humans using mAb-based HP10 and B158/B60 Ag-ELISA depends on the location of the cyst lesions. They can detect extra-parenchymal cysts more easily than intra-parenchymal cysts (15, 97). Moreover, these assays were reported to help support the diagnosis of NCC using different samples like CSF (36, 98), serum (97, 99), and even urine representing a non-invasive sampling approach (38, 46, 100). HP10 Ag-ELISA showed higher sensitivity (94.1%) and specificity (97.7%) in NCC patients with multiple viable cysts and inflammatory disease. However, the study was less sensitive (33.3%) for patients with non-inflammatory conditions and single viable cysts (36). The manufacturer of the commercialised B158/B60 Ag-ELISA (apDia, Belgium) reports a sensitivity of 94% (n = 100) and specificity of 99.3% (n = 300) (69). In one study, B158/B60 Ag-ELISA showed sensitivity and specificity of 100.0 and 84.0%, respectively, in diagnosing active infections in people with epilepsy (99). Two mAbs (TsW8 and TsW5) have currently been explored for accurately detecting antigen levels in serum and urine of NCC patients (46, 101). In other studies, QuickELISA™ was developed using rT24H, rGP50, and sTs18var1. The results identified better sensitivity and specificity with two or more viable cysts for rT24H (92.6, 97.8%) than rGP50 (89.8, 97.5%) and sTs18var1 (84.3, 93.4%) (86). This QuickELISA™ is a faster alternative to conventional ELISA. It relies on using two antigen conjugates, antigen-streptavidin and antigen-horseradish peroxidase, to capture and detect specific antibodies instead of secondary enzyme-labelled conjugates (102). One study reported 97% sensitivity and 95% specificity using single-chain viable fragments of antibodies (G10, A4, and B6), produced by phage display against T. solium total saline extraction (103). IgM-secreting hybridomas specific to T. solium (100) have been converted to mouse IgG, and resulted in a recombinant mAb (TsG10) with the highest affinity to crude Taenia antigen. The test achieved a 98% sensitivity and 100% specificity in detecting extraparenchymal NCC from serum, plasma, and CSF of patients. This assay could decrease the cost of mAb production and might be a more affordable option to detect circulating antigens (104). The good performance of the mAb-based Ag-ELISA test has initiated the development of LFA. Recombinant T. solium antigen (rT24H)-based LFA showed a good response (93.9% sensitivity and 98.9% specificity) for NCC diagnosis with two or more viable brain cysts (102). HP10 Ag-LFA showed high sensitivity and specificity (100%) using a CSF sample for detecting extraparenchymal NCC (57), but low or negative levels were observed in the CSF and serum samples from patients with parenchymal NCC (14). Quantum dots (Qdots)-labelled LFA reported 89% sensitivity and 99% specificity for identifying specific antibodies in sera of infected humans (105). The T. solium diagnostic project (SOLID) developed a two-strip T. solium point of care test (TS POC) prototype for the detection of antibodies against the adult stage (rES33), and larval stage (rT24H) of the parasite (56). This POC test was evaluated in two endemic countries, Zambia and Tanzania. In Zambia, the results showed low sensitivity and specificity (35 and 87%) in NCC patients as compared to rT24H-EITB (94 and 95%) and B158/B60 Ag-ELISA (36 and 87%) (53). In Tanzania, Stelzle et al. (54) compared the TS POC test with rT24H-EITB, LLGP-EITB, and Ag-ELISA and reported a sensitivity of 49% and a specificity of 91% for any type of NCC. The sensitivity of the TS POC test was comparable to Ag-ELISA (50%) and rT24H-EITB (44%) and higher than LLGP-EITB (23%). The researchers reported this test as very sensitive for NCC patients with vesicular lesions (54). LDBIO developed a commercial EITB kit called CYSTICERCOSIS Western Blot IgG (LDBIO, Lyon, France) for detecting HCC and NCC (49). Salazar-Anton et al. (106) compared the effectiveness of four immunodiagnostic techniques to diagnose NCC. The serum was tested with a commercial LDBIO EITB kit (Lyon, France) and later tested with an in-house immune-dot blot (107) with Tsol-p27 antigen, Ag-ELISA, and Western blot with Tsol-p27/TsolHSP36 antigens. The result showed the highest sensitivity and specificity for immunodot blot-Tsol-p27 (86.7 and 97.8%) compared to Ag-ELISA (86.7, 94.6%), and Western blot with Tsol_p27 (76.4, 95.6%) and Tsol HSP36 (61.9, 86.1%). A recent POC test based on TsW8/TsW5 mAbs in urine detected 97% of extraparenchymal NCC patients with a specificity rate of 100%. This rapid test can be performed in 15 min and could be a good option for low-resource areas (58). However, additional scrutiny is required for cross-reactivity with related parasites such as Echinococcus sp., which are frequently co-endemic with cysticercosis.
The differences in outcomes of serodiagnosis of NCC can be influenced by several factors, which may include the stage of T. solium infection, choice of antigens for serological assays, design of the study, location of cyst lesions in the brain, number of lesions, transient immunity among patients in endemic countries (34, 54), cross-reactivity with other parasite antigens, and prevalence and genetic variation across different endemic countries. The inclusion or omission of the close relative genus Echinococcus spp. in the study (as mentioned above), use of serum/CSF sample, and using sera with previously positive serology may be other potential influencing factors for human cysticercosis diagnostic test results, complicating their comparison and interpretation.
6 Limitations and critical appraisal
The available serological tests have shown significant variability in their sensitivity and specificity for diagnosing cysticercosis in humans and pigs (Table 1). ELISA is critical for identifying cysticercosis in endemic countries as it is cheaper (US$5-US$30/test) and more affordable than neuro-imaging (34, 65). However, the limitation of these tests is cross-reactivity with other taeniid parasites, particularly in cases where the diseases are co-endemic; furthermore, antibody detection tests cannot distinguish between active and inactive, past infections (108). The commercial apDia ELISA test for NCC has shown decreased sensitivity when the number of viable cysts is low, and cannot detect infections involving a single viable cyst. For PCC, the test does not allow the differentiation between infections of different Taenia species in pigs and is not cost-effective (€3.15/sample) (34). This specificity problem is significant in regions where T. hydatigena (Sub-Saharan Africa, South East Asia) (109, 110) and T. asiatica (Southeast Asia) (111) are prevalent alongside T. solium (69). Although EITB is widely preferred to detect anticysticercal antibodies, it is not field-friendly, not widely available, and does not exist commercially in point-of-care formats (102). In addition, EITB does not confirm central nervous system infection since it demonstrates a systemic antibody response (24) and has insufficient sensitivity with single intracranial cysts and calcified parasites (112, 113). In experimental studies, LFA tests have shown promising results for cysticercosis diagnosis; however, they are currently unavailable at commercial levels and can only detect active, viable cysts (4, 45, 93).
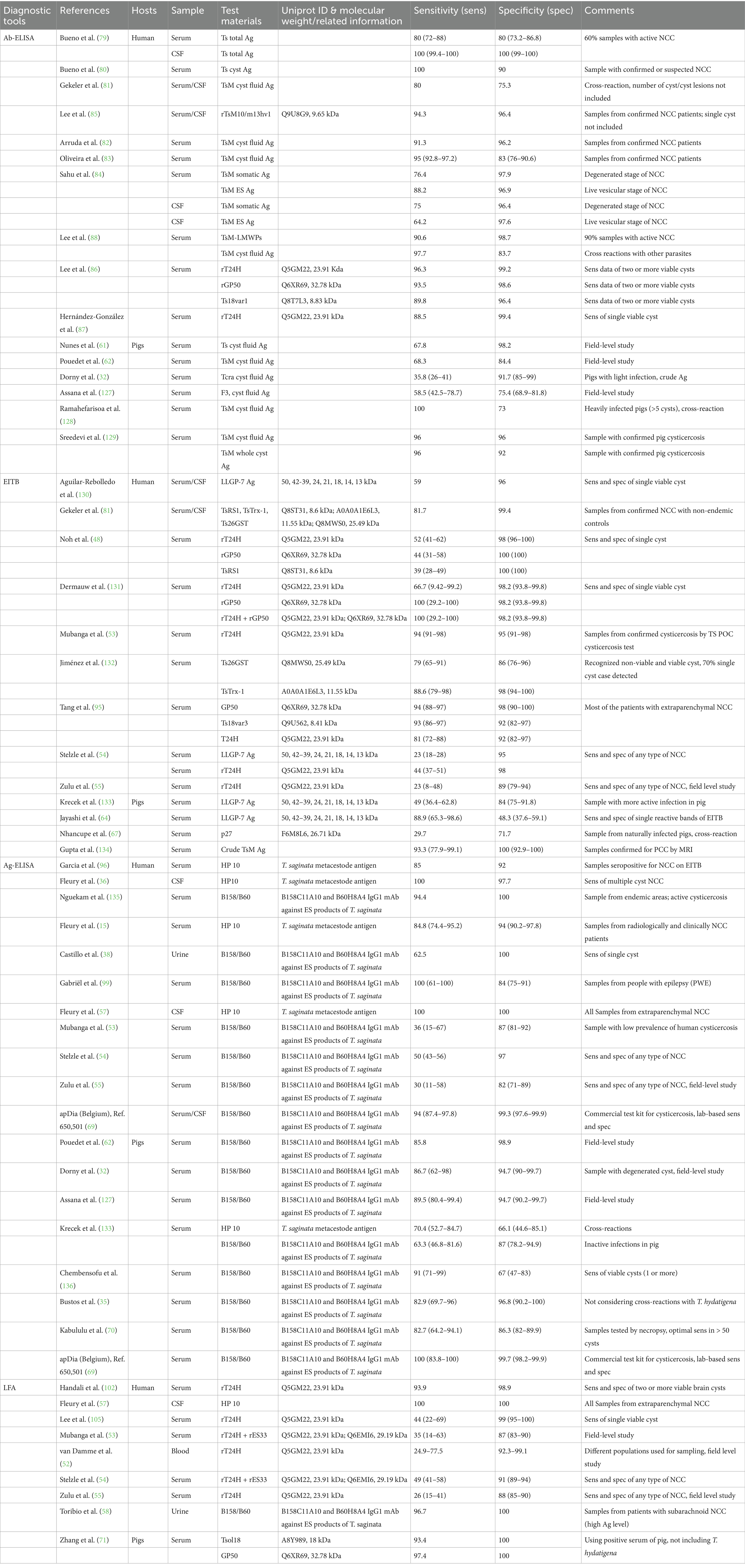
Table 1. Serological tests used for cysticercosis diagnosis in humans and pigs with estimated sensitivity and specificity.
In an effort to visualize trends underlying the development of the different serological techniques used, we used R software2 to create a bubble plot that compares the year of publication of the serological tools and the sensitivity/specificity of the test (Figure 2). This representation allows us to represent four parameters: sensitivity, specificity, year of publication, and the serodiagnosis technique used for humans or pigs.
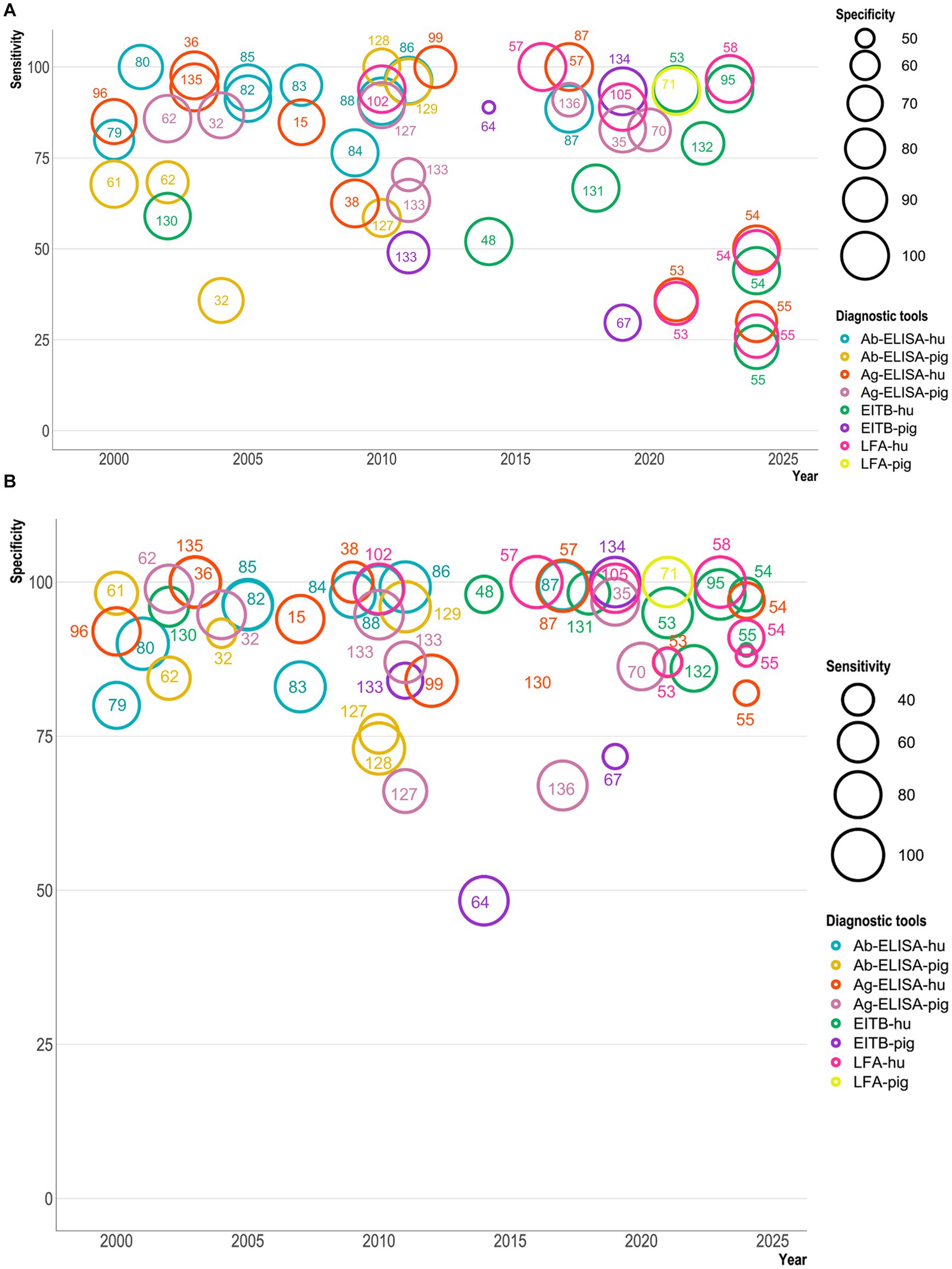
Figure 2. A bubble plot showing sensitivity and specificity of serological diagnostic tests for Taenia solium (Y-axis) published from 2000–2024 (X-axis). A represents the sensitivity plot, while B shows the specificity plot. The bubble size in A indicates the test’s specificity, while in B, it indicates the sensitivity. The color of the bubbles indicates different serological tests used for diagnosing cysticercosis in humans and pigs. The serological tests are Ab-ELISA (antibody ELISA), Ag-ELISA (antigen ELISA), EITB (enzyme-linked immunoelectrotransfer blot), and LFA (lateral flow assay), which have been marked with reference numbers with a test-specific color. These bubble plots include nine Ab-ELISA (79, 80, 82–88), eleven Ag-ELISA (15, 36, 38, 53–55, 57, 87, 96, 99, 135), eight EITB (48, 53–55, 95, 130–132), and seven LFA (53–55, 57, 58, 102, 105) tests for human NCC. In contrast, six Ab-ELISA (32, 61, 62, 127–129), eight Ag-ELISA (32, 35, 62, 68, 70, 127, 133, 136), four EITB (64, 67, 68, 133, 134), and one LFA (71) test have been used for PCC.
Our bubble plot analysis show that in serodiagnosis, sensitivity has not steadily improved over the years and has produced more scattered percentages (even ≤30%) than specificity percentages (≥ 50%) of tests. In Figure 2A, the sensitivity of serodiagnosis tests ranged between 25 and 100%, with few tests showing lower results (less than 25%). Ag-ELISA and Ab-ELISA tests have shown variability in their sensitivity and specificity. Ag-ELISA for humans showed less sensitivity (25–100%) than for pigs (50–100%). EITB tests for humans and pigs showed the lowest sensitivity (≤25–30%). The POC/LFA test sensitivity varied between 25 and 100% for humans, while one LFA study showed >90% sensitivity in pigs. In the specificity plot (Figure 2B), most serodiagnosis tests’ specificity ranges between 70 and 100%. Ab-ELISA and Ag-ELISA for humans and pigs showed 80–100% specificity, while one Ag-ELISA test showed around 70% specificity for pigs. EITB for humans has shown higher specificity (89–100%), but for pigs, the specificity is as low as around 50%. LFA for humans showed the best specificity, although sensitivity (even around 25%) is a problem. It is essential to note that not all studies included echinococcosis serum samples for human or other co-endemic Taenia species of pigs, which may have resulted in some cases with higher specificities than in field studies, as species-specific resolution of Taenia infection and distinction from Echinococcosis are notoriously challenging to achieve serologically.
7 Serodiagnosis of cysticercosis: investigating an IgE-based approach?
T. solium infection in humans and pigs induces specific antibody responses, especially Immunoglobulin G (IgG), and as discussed above, has been frequently used for serological diagnosis of cysticercosis. However, most metazoan parasitic infections typically also result in elevated levels of total and parasite-specific serum IgE in the infected host (114). IgE antibodies induce a protective immune response against invading parasites by initiating a Th2-biased response involving the high-affinity IgE receptor (FcεRI) (mast cells, basophils, dendritic cells, eosinophils) and the low-affinity IgE receptor (CD23) (eosinophils, dendritic cells, platelets, macrophages, and B cells) (115, 116).
NCC in humans is often associated with higher levels of parasite-specific antibodies (IgG1, IgG2, IgG4, IgE) (117). Flisser et al. (118) identified all immunoglobulin classes (IgG, IgM, IgE, IgA, and IgD) in decreasing order in NCC patients in serum, while Espinoza et al. (119) found IgG, IgA, and IgE in CSF. Cerebral cysticercosis has been reported with increased IgE levels in CSF or serum in infected patients (120). In Brazil, one study found elevated total IgE levels among all individuals with anti-T. solium cysticercus antibodies (42). Another group used T. solium and T. crassiceps-based Ag-ELISA to measure and compare immunoglobulins present in serum and CSF samples of NCC patients, in which IgE detection showed 24% sensitivity and 97.1% specificity for serum but was negative in CSF samples. This study also reported that IgE was more frequent in the patients with the inactive cyst form, with no degenerating cysts or immune-inflammatory processes (79). However, using the same method, Espinoza et al. (119) detected only 3% positivity for IgE in serum and CSF of NCC patients. For pigs, T. solium cysts showed detectable serum IgG levels in experimentally infected animals after 30 days of egg inoculation (60). However, IgE levels in PCC have not been studied yet.
Transgenic humanized reporter systems are extremely sensitive and can detect as little as 15 pg./mL of IgE because of their intrinsic signal amplification mechanisms (121). Using this reporter system may improve the early detection of cysticercosis (122). In human experimental infection with the hookworm Necator americanus, using basophil activation tests, we were able to detect binding of parasite-specific IgE to peripheral blood basophils as soon as 5–6 weeks after human infection, even in the absence of detectable amounts of free parasite-specific IgE in serum (123). This suggests that metazoan parasites with tissue migratory larval stages may be able to induce an early IgE response even in primary infections, possibly or in part due to the release of proteases during their migration (124). Combined with the high sensitivity of IgE reporter systems, this may enable the detection of small amounts of parasite-specific IgE at an early stage of infection. The diagnostic potential of parasite-specific IgE has also been explored, e.g., for toxoplasmosis (125) and strongyloidiasis (126), showing high sensitivity and specificity for diagnosing these parasitic infections, albeit not in a reporter cell format. One key advantage of using non-homologous reporter cell assays compared to, e.g., ELISA is that competing antibody isotypes (e.g., IgG4) are easily removed during a washing step, leaving only human IgE bound to the surface of the rat reporter cells, which do not bind human IgG. In contrast, for ELISA-type assays, to avoid competition, which will affect sensitivity, it is necessary to remove IgG from the serum samples by affinity chromatography, increasing cost and labour.
8 Conclusion
Cysticercosis is still a significant challenge for public health. Diagnosing cysticercosis in humans is progressing steadily, but PCC diagnostic test development is still lagging. The serological diagnosis of cysticercosis needs to be improved to avoid cross-reactivities and false negative results. T. hydatigena has been identified as one of the main cross-reactive species for pig cysticercosis serology. There is a lack of cross-reactivity studies on T. asiatica in co-endemic countries. The sensitivity of the serological tests still needs to be improved, as it is still unacceptably low in the case of low cyst numbers or calcified, inactive parasite stages. IgE-based serological tests may be a good alternative for laboratory-based diagnosis of cysticercosis. Difficulties persist regarding differential diagnosis of related parasitoses, such as cysticercosis and echinococcosis, particularly in areas where both parasites are co-endemic. Thus, a combination of serological and molecular technologies, such as loop-mediated isothermal amplification (LAMP) or recombinase polymerase amplification (RPA), may remain necessary.
Author contributions
MH: Formal analysis, Software, Visualization, Writing – original draft, Writing – review & editing. SS: Writing – review & editing. NN: Investigation, Writing – review & editing. LT: Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Writing – review & editing. FF: Funding acquisition, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. MSH is funded by the Academy for International Agricultural Research (ACINAR). ACINAR, commissioned by the German Federal Ministry for Economic Cooperation and Development (BMZ), is being carried out by ATSAF e.V. on behalf of the Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH. SS is funded via the German Academic Exchange Service (DAAD) and Higher education commission (HEC), Pakistan. LFT and NN is supported by the German Federal Ministry for Economic Cooperation and Development (BMZ) through the One Health Research, Education and Outreach Centre in Africa (OHRECA). FHF was supported by the LOEWE Centre DRUID within the Hessian Excellence Initiative (LOEWE/1/10/519/03/03.001(0016)/53). ILRI thanks all donors and organizations globally supporting its work through the CGIAR Fund (http://www.cgiar.org/funders/).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
EITB, enzyme-linked immune electro-transfer blot; HCC, human cysticercosis; LAMP, loop-mediated isothermal amplification; LFA, lateral flow assay; LLGP, lentil lectin-purified glycoproteins; NCC, neurocysticercosis; PCC, pig cysticercosis; POC, point of care test; RPA, recombinase polymerase amplification.
Footnotes
1. ^http://www.ncbi.nlm.nih.gov/pubmed/
2. ^https://cran.r-project.org/bin/windows/base/R-4.4.2-win.exe
References
2. CDC. Neglected parasitic infections in the United States (2020) Available online at: http://www.cdc.gov/parasites/npi.html
3. WHO. WHO estimates of the global burden of foodborne diseases: foodborne diseases burden epidemiology reference group 2007–2015 (2015). 265 p.
4. WHO. Taeniasis/Cysticercosis (2022). Available online at: https://www.who.int/news-room/fact-sheets/detail/taeniasis-cysticercosis
5. Ramraje, S, Bhatia, V, and Goel, A. Solitary intramuscular cysticercosis-a report of two cases. Australas Med J. (2011) 4:58–60. doi: 10.4066/AMJ.2011.483
6. Sciutto, E, Fragoso, G, Fleury, A, Laclette, JP, Sotelo, J, Aluja, A, et al. Taenia solium disease in humans and pigs: an ancient parasitosis disease rooted in developing countries and emerging as a major health problem of global dimensions. Microbes Infect. (2000) 2:1875–90. doi: 10.1016/S1286-4579(00)01336-8
7. Jankharia, BG, Chavhan, GB, Krishnan, P, and Jankharia, B. MRI and ultrasound in solitary muscular and soft tissue cysticercosis. Skeletal Radiol. (2005) 34:722–6. doi: 10.1007/s00256-005-0954-3
8. García, HH, Gonzalez, AE, Evans, CA, and Gilman, RH. Taenia solium cysticercosis. Lancet. (2003) 362:547–56. doi: 10.1016/S0140-6736(03)14117-7
9. Mauad, T, Battlehner, CN, Bedrikow, CL, Capelozzi, VL, and Saldiva, PH. Case report: massive cardiopulmonary cysticercosis in a leukemic patient. Pathol Res Pract. (1997) 193:527–9. doi: 10.1016/S0344-0338(97)80108-2
10. Discontools. DISCONTOOLS research gaps for Porcine cysticercosis (2021). Available online at: https://www.discontools.eu/database/77-cysticercosis.html
11. Lombardo, J. Subretinal cysticercosis. Optom Vis Sci. (2001) 78:188–94. doi: 10.1097/00006324-200104000-00007
12. Garcia, HH, O’Neal, SE, Noh, J, Handali, S, Gilman, RH, Gonzalez, AE, et al. Laboratory diagnosis of neurocysticercosis (Taenia solium). J Clin Microbiol. (2018) 56. doi: 10.1128/JCM.00424-18
13. Ndimubanzi, PC, Carabin, H, Budke, CM, Nguyen, H, Qian, Y-J, Rainwater, E, et al. A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis. (2010) 4:e870. doi: 10.1371/journal.pntd.0000870
14. Parkhouse, RM, Carpio, A, Campoverde, A, Sastre, P, Rojas, G, and Cortez, MM. Reciprocal contribution of clinical studies and the HP10 antigen ELISA for the diagnosis of extraparenchymal neurocysticercosis. Acta Trop. (2018) 178:119–23. doi: 10.1016/j.actatropica.2017.11.005
15. Fleury, A, Hernández, M, Avila, M, Cárdenas, G, Bobes, RJ, Huerta, M, et al. Detection of HP10 antigen in serum for diagnosis and follow-up of subarachnoidal and intraventricular human neurocysticercosis. J Neurol Neurosurg Psychiatry. (2007) 78:970–4. doi: 10.1136/jnnp.2006.107243
16. Lu, X, Miao, N, and Mannes, A. Neurocysticercosis: a rare cause of headache needing craniotomy. Transl Perioper Pain Med. (2022) 9:434–7. doi: 10.31480/2330-4871/154
17. Hossain, MS, Shabir, S, Toye, P, Thomas, LF, and Falcone, FH. Insights into the diagnosis, vaccines, and control of Taenia solium, a zoonotic, neglected parasite. Parasit Vectors. (2023) 16:380. doi: 10.1186/s13071-023-05989-6
18. Ouma, E, Dione, M, Mtimet, N, Lule, P, Colston, A, Adediran, S, et al. Demand for Taenia solium cysticercosis vaccine: lessons and insights from the pig production and trading nodes of the Uganda pig value chain. Front Vet Sci. (2021) 8. doi: 10.3389/fvets.2021.611166
19. Heath, DD, Lawrence, SB, and Yong, WK. Cross-protection between the cysts of Echinococcus granulosus, Taenia hydatigena and T ovis in lambs. Res Vet Sci. (1979) 27:210–2. doi: 10.1016/S0034-5288(18)32831-5
20. Conlan, JV, Vongxay, K, Fenwick, S, Blacksell, SD, and Thompson, RC. Does interspecific competition have a moderating effect on Taenia solium transmission dynamics in Southeast Asia? Trends Parasitol. (2009) 25:398–403. doi: 10.1016/j.pt.2009.06.005
21. Conlan, JV, Vongxay, K, Khamlome, B, Dorny, P, Sripa, B, Elliot, A, et al. A cross-sectional study of Taenia solium in a multiple taeniid-endemic region reveals competition may be protective. Am J Trop Med Hyg. (2012) 87:281–91. doi: 10.4269/ajtmh.2012.11-0106
22. Assana, E, Awah-Ndukum, J, Djonmaïla, JD, Djiatche, HD, Awé, C, Manchang, TK, et al. A comparison of Taenia solium and Taenia hydatigena infection in pigs using serological diagnosis and post-mortem inspection methods in Benoué division, North Cameroon. Vet Parasitol Reg Stud Reports. (2019) 17:100306. doi: 10.1016/j.vprsr.2019.100306
23. Nguyen, MT, Gabriël, S, Abatih, EN, and Dorny, P. A systematic review on the global occurrence of Taenia hydatigena in pigs and cattle. Vet Parasitol. (2016) 226:97–103. doi: 10.1016/j.vetpar.2016.06.034
24. Del Brutto, OH, Nash, TE, White, AC, Rajshekhar, V, Wilkins, PP, Singh, G, et al. Revised diagnostic criteria for neurocysticercosis. J Neurol Sci. (2017) 372:202–10. doi: 10.1016/J.JNS.2016.11.045
25. Mrigpuri, P, Gupta, VB, Menon, B, Spalgais, S, and Kumar, R. Isolated pulmonary cysticercosis presenting as mass lesion. Turk Thorac J. (2021) 22:418–21. doi: 10.5152/TurkThoracJ.2021.0027
26. Jain, BK, Sankhe, SS, Agrawal, MD, and Naphade, PS. Disseminated cysticercosis with pulmonary and cardiac involvement. Indian J Radiol Imag. (2010) 20:310–3. doi: 10.4103/0971-3026.73532
27. Bastos, AL, Marchiori, E, Gasparetto, EL, Andrade, BH, Junior, GC, Carvalho, RC, et al. Pulmonary and cardiac cysticercosis: helical CT findings. Br J Radiol. (2007) 80:e58–60. doi: 10.1259/bjr/43104295
28. Cardenas, F, Quiroz, H, Plancarte, A, Meza, A, Dalma, A, and Flisser, A. Taenia solium ocular cysticercosis: findings in 30 cases. Ann Ophthalmol. (1992) 24:25–8.
29. Deckers, N, and Dorny, P. Immunodiagnosis of Taenia solium taeniosis/cysticercosis. Trends Parasitol. (2010) 26:137–44. doi: 10.1016/j.pt.2009.12.008
30. Dermauw, V, Ganaba, R, Cissé, A, Ouedraogo, B, Millogo, A, Tarnagda, Z, et al. Taenia hydatigena in pigs in Burkina Faso: a cross-sectional abattoir study. Vet Parasitol. (2016) 230:9–13. doi: 10.1016/j.vetpar.2016.10.022
31. Sithole, MI, Bekker, JL, Tsotetsi-Khambule, AM, and Mukaratirwa, S. Ineffectiveness of meat inspection in the detection of Taenia solium cysticerci in pigs slaughtered at two abattoirs in the eastern Cape Province of South Africa. Vet Parasitol Reg Stud Reports. (2019) 17:100299. doi: 10.1016/j.vprsr.2019.100299
32. Dorny, P, Phiri, IK, Vercruysse, J, Gabriel, S, Willingham, AL, Brandt, J, et al. A Bayesian approach for estimating values for prevalence and diagnostic test characteristics of porcine cysticercosis. Int J Parasitol. (2004) 34:569–76. doi: 10.1016/j.ijpara.2003.11.014
33. Phiri, IK, Dorny, P, Gabriël, S, Willingham, AL, Sikasunge, C, Siziya, S, et al. Assessment of routine inspection methods for porcine cysticercosis in Zambian village pigs. J Helminthol. (2006) 80:69–72. doi: 10.1079/JOH2005314
34. Lightowlers, MW, Garcia, HH, Gauci, CG, Donadeu, M, and Abela-Ridder, B. Monitoring the outcomes of interventions against Taenia solium: options and suggestions. Parasite Immunol. (2016) 38:158–69. doi: 10.1111/PIM.12291
35. Bustos, JA, Ninaquispe, BE, Rodriguez, S, Castillo, Y, Yang, SY, Gilman, RH, et al. Performance of a Sandwich antigen-detection ELISA for the diagnosis of porcine Taenia solium Cysticercosis. Am J Trop Med Hyg. (2019) 100:604–8. doi: 10.4269/ajtmh.18-0697
36. Fleury, A, Hernández, M, Fragoso, G, Parkhouse, RM, Harrison, LJ, and Sciutto, E. Detection of secreted cysticercal antigen: a useful tool in the diagnosis of inflammatory neurocysticercosis. Trans R Soc Trop Med Hyg. (2003) 97:542–6. doi: 10.1016/s0035-9203(03)80019-6
37. Malla, N, Kaur, R, Ganguly, NK, Sawhney, IM, and Mahajan, RC. Utility of specific IgG4 response in saliva and serum samples for the diagnosis and follow up of human neurocysticercosis. Nepal Med Coll J. (2005) 7:1–9.
38. Castillo, Y, Rodriguez, S, García, HH, Brandt, J, van Hul, A, Silva, M, et al. Urine antigen detection for the diagnosis of human Neurocysticercosis. Am J Trop Med Hyg. (2009) 80:379–83. doi: 10.4269/ajtmh.2009.80.379
39. Parija, SC, and Gireesh, A. Cysticercus cellulosae antigens in the serodiagnosis of neurocysticercosis. Trop Parasitol. (2011) 1:64–72. doi: 10.4103/2229-5070.86932
40. Pinto, PS, Vaz, AJ, Germano, PM, and Nakamura, PM. ELISA test for the diagnosis of cysticercosis in pigs using antigens of Taenia solium and Taenia crassiceps cysticerci. Rev Inst Med Trop Sao Paulo. (2000) 42:71–9. doi: 10.1590/S0036-46652000000200003
41. Tsang, VC, Brand, JA, and Boyer, AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis. (1989) 159:50–9. doi: 10.1093/INFDIS/159.1.50
42. Prestes-Carneiro, LE, Freitas, Selma de Bastos Zambelide, Zago, SC, Miguel, NA, Primo, OB, Iha, AH, et al.. Taeniosis-cysticercosis complex in individuals of a peasants' settlement (Teodoro Sampaio, Pontal of Paranapanema, SP, Brazil). Mem Inst Oswaldo Cruz (2006) 101: 15–20. doi: 10.1590/s0074-02762006000100004
43. Harrison, LJ, Joshua, GW, Wright, SH, and Parkhouse, RM. Specific detection of circulating surface/secreted glycoproteins of viable cysticerci in Taenia saginata cysticercosis. Parasite Immunol. (1989) 11:351–70. doi: 10.1111/j.1365-3024.1989.tb00673.x
44. Dorny, P, Vercammen, F, Brandt, J, Vansteenkiste, W, Berkvens, D, and Geerts, S. Sero-epidemiological study of Taenia saginata cysticercosis in Belgian cattle. Vet Parasitol. (2000) 88:43–9. doi: 10.1016/s0304-4017(99)00196-x
45. Thomas, LF. Landscape analysis: Control of Taenia solium. Geneva: World Health Organization (2015). 47 p.
46. Toribio, LM, Bustos, JA, and Garcia, HH. From laboratory to clinical practice: an update of the immunological and molecular tools for neurocysticercosis diagnosis. Front Parasitol. (2024) 3. doi: 10.3389/fpara.2024.1394089
47. Ng-Nguyen, D, Noh, J, Breen, K, Stevenson, MA, Handali, S, and Traub, RJ. The epidemiology of porcine Taenia solium cysticercosis in communities of the central highlands in Vietnam. Parasit Vectors. (2018) 11:360. doi: 10.1186/s13071-018-2945-y
48. Noh, J, Rodriguez, S, Lee, Y-M, Handali, S, Gonzalez, AE, Gilman, RH, et al. Recombinant protein-and synthetic peptide-based immunoblot test for diagnosis of neurocysticercosis. J Clin Microbiol. (2014) 52:1429–34. doi: 10.1128/JCM.03260-13
49. Langa, I, Padama, F, Nhancupe, N, Pondja, A, Hlashwayo, D, Gouveia, L, et al. The burden of T. solium cysticercosis and selected neuropsychiatric disorders in Mocuba district, Zambézia province, Mozambique. PLoS Negl Trop Dis. (2022) 16:e0010606. doi: 10.1371/journal.pntd.0010606
50. Donadeu, M, Bote, K, Gasimov, E, Kim, S, Lin, Z, Lucianez, A, et al. WHO Taenia solium endemicity map – 2022 update. Wkly Epidemiol Rec. (2022) 97:169–72.
51. Sadaow, L, Boonroumkaew, P, Rodpai, R, Janwan, P, Sanpool, O, Thanchomnang, T, et al. Development and evaluation of an immunochromatography-based point-of-care test kit for a rapid diagnosis of human cysticercosis. Food Waterb Parasitol. (2023) 33:e00211. doi: 10.1016/j.fawpar.2023.e00211
52. Van Damme, I, Trevisan, C, Kabululu, M, Stelzle, D, Makasi, CE, Schmidt-Urbaneja, V, et al. Evaluation of a rapid lateral flow assay for the detection of taeniosis and cysticercosis at district hospital level in Tanzania: A prospective multicentre diagnostic accuracy study. PLoS Negl Trop Dis. (2025) 19:e0012310. doi: 10.1371/journal.pntd.001231010
53. Mubanga, C, van Damme, I, Trevisan, C, Schmidt, V, Phiri, IK, Zulu, G, et al. Evaluation of an antibody detecting point of care test for diagnosis of Taenia solium cysticercosis in a Zambian rural community: a prospective diagnostic accuracy study. Diagnostics. (2021) 11. doi: 10.3390/diagnostics11112121
54. Stelzle, D, Makasi, CE, Schmidt, V, van Damme, I, Trevisan, C, Ruether, C, et al. Evaluation of a point-of-care test for the diagnosis of Taenia solium neurocysticercosis in rural southern Tanzania: a diagnostic accuracy study. Lancet Infect Dis. (2024) 24:98–106. doi: 10.1016/S1473-3099(23)00378-X
55. Zulu, G, Stelzle, D, Mwape, KE, van Damme, I, Trevisan, C, Mubanga, C, et al. The performance of a point-of-care test for the diagnosis of Neurocysticercosis in a resource-poor community setting in Zambia—a diagnostic accuracy study. eClinicalMedicine. (2024) 77:102893. doi: 10.1016/j.eclinm.2024.102893
56. Trevisan, C, van Damme, I, Ngowi, B, Schmidt, V, Stelzle, D, Møller, KS, et al. Trial design of a prospective multicenter diagnostic accuracy study of a point-of-care test for the detection of Taenia solium taeniosis and neurocysticercosis in hospital-based settings in Tanzania. Diagnostics. (2021) 11:1528. doi: 10.3390/diagnostics11091528
57. Fleury, A, Sastre, P, Sciutto, E, Correia, S, Monedero, A, Toledo, A, et al. A lateral flow assay (LFA) for the rapid detection of extraparenchymal neurocysticercosis using cerebrospinal fluid. Exp Parasitol. (2016) 171:67–70. doi: 10.1016/J.EXPPARA.2016.10.016
58. Toribio, L, Handali, S, Marin, Y, Perez, E, Castillo, Y, Bustos, JA, et al. A rapid point-of-care assay for Cysticercosis antigen detection in urine samples. Am J Trop Med Hyg. (2023) 108:578–80. doi: 10.4269/ajtmh.22-0598
59. Koczula, KM, and Gallotta, A. Lateral flow assays. Essays Biochem. (2016) 60:111–20. doi: 10.1042/EBC20150012
60. Sato, M, Yamasaki, H, Sako, Y, Nakao, M, Nakaya, K, Plancarte, A, et al. Evaluation of tongue inspection and serology for diagnosis of Taenia solium cysticercosis in swine: usefulness of ELISA using purified glycoproteins and recombinant antigen. Vet Parasitol. (2003) 111:309–22. doi: 10.1016/S0304-4017(02)00383-7
61. Nunes, CM, Biondi, GF, Heinemann, MB, and Richtzenhain, LJ. Comparative evaluation of an indirect ELISA test for diagnosis of swine cysticercosis employing antigen from Taenia solium and Taenia crassiceps metacestodes. Vet Parasitol. (2000) 93:135–40. doi: 10.1016/s0304-4017(00)00355-1
62. Pouedet, MS, Zoli, AP, Nguekam, VL, Assana, E, Speybroeck, N, Berkvens, D, et al. Epidemiological survey of swine cysticercosis in two rural communities of West-Cameroon. Vet Parasitol. (2002) 106:45–54. doi: 10.1016/s0304-4017(02)00035-3
63. Gonzalez, AE, Cama, V, Gilman, RH, Tsang, VC, Pilcher, JB, Chavera, A, et al. Prevalence and comparison of serologic assays, necropsy, and tongue examination for the diagnosis of porcine cysticercosis in Peru. Am J Trop Med Hyg. (1990) 43:194–9. doi: 10.4269/ajtmh.1990.43.194
64. Jayashi, CM, Gonzalez, AE, Castillo Neyra, R, Rodríguez, S, García, HH, and Lightowlers, MW. Validity of the enzyme-linked immunoelectrotransfer blot (EITB) for naturally acquired porcine cysticercosis. Vet Parasitol. (2014) 199:42–9. doi: 10.1016/j.vetpar.2013.10.004
65. Muro, C, Gomez-Puerta, LA, Flecker, RH, Gamboa, R, Barreto, PV, Dorny, P, et al. Porcine Cysticercosis: possible cross-reactivity of Taenia hydatigena to GP50 antigen in the enzyme-linked Immunoelectrotransfer blot assay. Am J Trop Med Hyg. (2017) 97:1830–2. doi: 10.4269/ajtmh.17-0378
66. Gomez-Puerta, L, Vargas-Calla, A, Castillo, Y, Lopez-Urbina, MT, Dorny, P, Garcia, HH, et al. Evaluation of cross-reactivity to Taenia hydatigena and Echinococcus granulosus in the enzyme-linked immunoelectrotransfer blot assay for the diagnosis of porcine cysticercosis. Parasit Vectors. (2019) 12:57. doi: 10.1186/s13071-018-3279-5
67. Nhancupe, N, Noormahomed, EV, Afonso, S, Svard, S, and Lindh, J. Further evaluation of recombinant Tsol-p 27 by enzyme-linked immunoelectrotransfer blot for the serodiagnosis of cysticercosis in pigs from Mozambique. Parasit Vectors. (2019) 12:564. doi: 10.1186/s13071-019-3816-x
68. Krecek, RC, Michael, LM, Schantz, PM, Ntanjana, L, Smith, MF, Dorny, P, et al. Prevalence of Taenia solium cysticercosis in swine from a community-based study in 21 villages of the eastern Cape Province, South Africa. Vet Parasitol. (2008) 154:38–47. doi: 10.1016/j.vetpar.2008.03.005
69. Ap Dia. ELISA Cysticercosis Anitgen Kit. (2024) Available online at: https://apdiagroup.com/we-sell/elisa/elisa-apdia/cysticercosis-antigen/
70. Kabululu, ML, Johansen, MV, Mlangwa, JE, Mkupasi, EM, Braae, UC, Trevisan, C, et al. Performance of ag-ELISA in the diagnosis of Taenia solium cysticercosis in naturally infected pigs in Tanzania. Parasit Vectors. (2020) 13:534. doi: 10.1186/s13071-020-04416-4
71. Zhang, D, Qi, Y, Cui, Y, Song, W, Wang, X, Liu, M, et al. Rapid detection of Cysticercus cellulosae by an up-converting phosphor technology-based lateral-flow assay. Front Cell Infect Microbiol. (2021) 11:762472. doi: 10.3389/fcimb.2021.762472
72. Pandey, S, Malhotra, HS, Garg, RK, Malhotra, KP, Kumar, N, Rizvi, I, et al. Quantitative assessment of lesion load and efficacy of 3 cycles of albendazole in disseminated cysticercosis: a prospective evaluation. BMC Infect Dis. (2020) 20:220. doi: 10.1186/s12879-020-4891-5
73. Meena, D, Gupta, M, Jain, VK, and Arya, RK. Isolated intramuscular cysticercosis: Clinicopathological features, diagnosis and management—a review. J Clin Orthop Trauma. (2016) 7:243–9. doi: 10.1016/j.jcot.2016.06.016
74. Flisser, A, Espinoza, B, Tovar, A, Plancarte, A, and Correa, D. Host-parasite relationship in cysticercosis: immunologic study in different compartments of the host. Vet Parasitol. (1986) 20:95–102. doi: 10.1016/0304-4017(86)90094-4
75. Kumar, NS, Kumar, N, Rajakumar, K, and Basu, S. Intramuscular cysticercosis of the forearm mimicking a soft tissue tumour. BMJ Case Rep. (2024) 17:e259978. doi: 10.1136/bcr-2024-259978
76. Moraes, D, Santos, ÉA, Mendes, JA, Da Barcelos, IS, de Souza, JB, Fátima Gonçalves-Pires, MD, et al. Seropositivity to Cysticercosis in school-age children living in a low-income municipality in the Midwest region of Brazil. Iran J Parasitol. (2023) 18:211–6. doi: 10.18502/ijpa.v18i2.13187
77. Sahu, PS, Parija, SC, and Sahu, PK. Tear IgA-ELISA: a novel and sensitive method for diagnosis of ophthalmic cysticercosis. Acta Trop. (2008) 106:168–74. doi: 10.1016/j.actatropica.2008.03.004
78. Arroyo, G, Rodriguez, S, Lescano, AG, Alroy, KA, Bustos, JA, Santivañez, S, et al. Antibody banding patterns of the enzyme-linked Immunoelectrotransfer blot and brain imaging findings in patients with Neurocysticercosis. Clin Infect Dis. (2018) 66:282–8. doi: 10.1093/CID/CIX774
79. Bueno, EC, Vaz, AJ, Machado, LD, and Livramento, JA. Neurocysticercosis: detection of IgG, IgA and IgE antibodies in cerebrospinal fluid, serum and saliva samples by ELISA with Taenia solium and Taenia crassiceps antigens. Arq Neuropsiquiatr. (2000) 58:18–24. doi: 10.1590/S0004-282X2000000100003
80. Bueno, EC, Snege, M, Vaz, AJ, and Leser, PG. Serodiagnosis of human cysticercosis by using antigens from vesicular fluid of Taenia crassiceps cysticerci. Clin Diagn Lab Immunol. (2001) 8:1140–4. doi: 10.1128/CDLI.8.6.1140-1144.2001
81. Gekeler, F, Eichenlaub, S, Mendoza, EG, Sotelo, J, Hoelscher, M, and Löscher, T. Sensitivity and specificity of ELISA and immunoblot for diagnosing neurocysticercosis. Eur J Clin Microbiol Infect Dis. (2002) 21:227–9. doi: 10.1007/s10096-002-0695-3
82. Arruda, GC, Da Silva, AD, Quagliato, EM, Maretti, MA, and Rossi, CL. Evaluation of Taenia solium and Taenia crassiceps cysticercal antigens for the serodiagnosis of neurocysticercosis. Trop Med Int Health. (2005) 10:1005–12. doi: 10.1111/j.1365-3156.2005.01480.x
83. Oliveira, HB, Machado, GA, Cabral, DD, and Costa-Cruz, JM. Application of Taenia saginata metacestodes as an alternative antigen for the serological diagnosis of human neurocysticercosis. Parasitol Res. (2007) 101:1007–13. doi: 10.1007/s00436-007-0578-8
84. Sahu, PS, Parija, SC, Narayan, SK, and Kumar, D. Evaluation of an IgG-ELISA strategy using Taenia solium metacestode somatic and excretory-secretory antigens for diagnosis of neurocysticercosis revealing biological stage of the larvae. Acta Trop. (2009) 110:38–45. doi: 10.1016/j.actatropica.2009.01.002
85. Lee, E-G, Lee, M-Y, Chung, J-Y, Je, E-Y, Bae, Y-A, Na, B-K, et al. Feasibility of baculovirus-expressed recombinant 10-kDa antigen in the serodiagnosis of Taenia solium neurocysticercosis. Trans R Soc Trop Med Hyg. (2005) 99:919–26. doi: 10.1016/j.trstmh.2005.02.010
86. Lee, Y-M, Handali, S, Hancock, K, Pattabhi, S, Kovalenko, VA, Levin, A, et al. Serologic diagnosis of human Taenia solium cysticercosis by using recombinant and synthetic antigens in quick ELISA™. Am J Trop Med Hyg. (2011) 84:587–93. doi: 10.4269/ajtmh.2011.10-0079
87. Hernández-González, A, Noh, J, Perteguer, MJ, Gárate, T, and Handali, S. Comparison of T24H-his, GST-T24H and GST-Ts8B2 recombinant antigens in western blot, ELISA and multiplex bead-based assay for diagnosis of neurocysticercosis. Parasit Vectors. (2017) 10:237. doi: 10.1186/s13071-017-2160-2
88. Lee, E-G, Bae, Y-A, Kim, S-H, Díaz-Camacho, SP, Nawa, Y, and Kong, Y. Serodiagnostic reliability of single-step enriched low-molecular weight proteins of Taenia solium metacestode of American and Asian isolates. Trans R Soc Trop Med Hyg. (2010) 104:676–83. doi: 10.1016/j.trstmh.2010.07.011
89. Carod, JF, Randrianarison, M, Razafimahefa, J, Ramahefarisoa, RM, Rakotondrazaka, M, Debruyne, M, et al. Evaluation of the performance of 5 commercialized enzyme immunoassays for the detection of Taenia solium antibodies and for the diagnosis of neurocysticercosis. Diagn Microbiol Infect Dis. (2012) 72:85–9. doi: 10.1016/J.DIAGMICROBIO.2011.09.014
90. White, AC, Coyle, CM, Rajshekhar, V, Singh, G, Hauser, WA, Mohanty, A, et al. Diagnosis and treatment of neurocysticercosis: 2017 clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. (2018) 66:e49–75. doi: 10.1093/CID/CIX1084
91. Wilson, M, Bryan, RT, Fried, JA, Ware, DA, Schantz, PM, Pilcher, JB, et al. Clinical evaluation of the cysticercosis enzyme-linked immunoelectrotransfer blot in patients with neurocysticercosis. J Infect Dis. (1991) 164:1007–9. doi: 10.1093/infdis/164.5.1007
92. Garcia, HH, Rodriguez, S, Gilman, RH, Gonzalez, AE, and Tsang, VC. Neurocysticercosis: is serology useful in the absence of brain imaging? Trop Med Int Health. (2012) 17:1014–8. doi: 10.1111/j.1365-3156.2012.03037.x
93. Handali, S, Klarman, M, Gaspard, AN, Noh, J, Lee, Y-M, Rodriguez, S, et al. Multiantigen print immunoassay for comparison of diagnostic antigens for Taenia solium cysticercosis and taeniasis. Clin Vaccine Immunol. (2010) 17:68–72. doi: 10.1128/CVI.00339-09
94. Toribio, LM, Guzman, C, Noazin, S, Zimic-Sheen, A, Zimic, M, Gonzales, I, et al. Multiantigen print immunoassay (MAPIA) for the diagnosis of neurocysticercosis: a single-center diagnostic optimization and accuracy study in Lima, Peru. J Clin Microbiol. (2023) 61:e00760:–23. doi: 10.1128/jcm.00760-23
95. Tang, NL, Nash, TE, Corda, M, Nutman, TB, and O'Connell, EM. Triplex ELISA for assessing durability of Taenia solium Seropositivity after Neurocysticercosis cure. Emerg Infect Dis. (2023) 29:1340–8. doi: 10.3201/eid2907.230364
96. Garcia, HH, Parkhouse, RM, Gilman, RH, Montenegro, T, Bernal, T, Martinez, SM, et al. Serum antigen detection in the diagnosis, treatment, and follow-up of neurocysticercosis patients. Trans R Soc Trop Med Hyg. (2000) 94:673–6. doi: 10.1016/S0035-9203(00)90228-1
97. Rodriguez, S, Dorny, P, Tsang, VC, Pretell, EJ, Brandt, J, Lescano, AG, et al. Detection of Taenia solium antigens and anti-T. solium antibodies in paired serum and cerebrospinal fluid samples from patients with intraparenchymal or extraparenchymal neurocysticercosis. J Infect Dis. (2009) 199:1345–52. doi: 10.1086/597757
98. Correa, D, Sandoval, MA, Harrison, LJ, Parkhouse, RM, Plancarte, A, Meza-Lucas, A, et al. Human neurocysticercosis: comparison of enzyme immunoassay capture techniques based on monoclonal and polyclonal antibodies for the detection of parasite products in cerebrospinal fluid. Trans R Soc Trop Med Hyg. (1989) 83:814–6. doi: 10.1016/0035-9203(89)90340-4
99. Gabriël, S, Blocher, J, Dorny, P, Abatih, EN, Schmutzhard, E, Ombay, M, et al. Added value of antigen ELISA in the diagnosis of neurocysticercosis in resource poor settings. PLoS Negl Trop Dis. (2012) 6:e1851. doi: 10.1371/JOURNAL.PNTD.0001851
100. Paredes, A, Sáenz, P, Marzal, MW, Orrego, MA, Castillo, Y, Rivera, A, et al. Anti-Taenia solium monoclonal antibodies for the detection of parasite antigens in body fluids from patients with neurocysticercosis. Exp Parasitol. (2016) 166:37–43. doi: 10.1016/J.EXPPARA.2016.03.025
101. Castillo, Y, Toribio, LM, Guzman, C, Arroyo, G, Espinoza, C, Saavedra, H, et al. Consistent measurement of parasite-specific antigen levels in sera of patients with Neurocysticercosis using two different monoclonal antibody (mAb)-based enzyme-linked immunosorbent assays. Pathogens. (2023) 12:566. doi: 10.3390/pathogens12040566
102. Handali, S, Klarman, M, Gaspard, AN, Dong, XF, LaBorde, R, Noh, J, et al. Development and evaluation of a magnetic immunochromatographic test to detect Taenia solium, which causes taeniasis and neurocysticercosis in humans. Clin Vacc Immunol. (2010) 17:631–7. doi: 10.1128/CVI.00511-09
103. Da Ribeiro, VS, Araújo, TG, Gonzaga, HT, Nascimento, R, Goulart, LR, and Costa-Cruz, JM. Development of specific sc Fv antibodies to detect neurocysticercosis antigens and potential applications in immunodiagnosis. Immunol Lett. (2013) 156:59–67. doi: 10.1016/j.imlet.2013.09.005
104. Corda, M, Sciurba, J, Blaha, J, Mahanty, S, Paredes, A, Garcia, HH, et al. A recombinant monoclonal-based Taenia antigen assay that reflects disease activity in extra-parenchymal neurocysticercosis. PLoS Negl Trop Dis. (2022) 16:e0010442. doi: 10.1371/journal.pntd.0010442
105. Lee, C, Noh, J, O'Neal, SE, Gonzalez, AE, Garcia, HH, and Handali, S. Feasibility of a point-of-care test based on quantum dots with a mobile phone reader for detection of antibody responses. PLoS Negl Trop Dis. (2019) 13:e0007746. doi: 10.1371/journal.pntd.0007746
106. Salazar-Anton, F, Tellez, A, and Lindh, J. Evaluation of an immunodot blot technique for the detection of antibodies against Taenia solium larval antigens. Parasitol Res. (2012) 110:2187–91. doi: 10.1007/s00436-011-2747-z
107. Nhancupe, N, Salazar-Anton, F, Noormahomed, EV, Afonso, S, and Lindh, J. Further characterization of Tsol-p 27 as a diagnostic antigen in sub-Saharan Africa. Exp Parasitol. (2013) 135:573–9. doi: 10.1016/j.exppara.2013.09.006
108. FAO, OIE, WHO. Meeting to accelerate prevention and control of neglected foodborne parasitic zoonoses in selected Asian countries. Luang Prabang: WHO Regional Office for the Western Pacific (2018).
109. Gomez-Puerta, LA, Gonzalez, AE, Gavidia, C, Ayvar, V, Garcia, HH, and Lopez-Urbina, MT. Oxfendazole as successful treatment of Taenia hydatigena metacestodes in naturally infected pigs. Asian Pac J Trop Biomed. (2015) 5:971–3. doi: 10.1016/j.apjtb.2015.03.013
110. Nguyen, TT, Dermauw, V, Noh, J, Chien, NH, Dao, TT, Nguyen, TG, et al. Occurrence of Taenia species in pigs in slaughterhouses in Phu Tho province, northern Vietnam. J Helminthol. (2020) 94:e201. doi: 10.1017/S0022149X20000863
111. Ale, A, Victor, B, Praet, N, Gabriël, S, Speybroeck, N, Dorny, P, et al. Epidemiology and genetic diversity of Taenia asiatica: a systematic review. Parasit Vectors. (2014) 7:45. doi: 10.1186/1756-3305-7-45
112. Rajshekhar, V, and Oommen, A. Serological studies using ELISA and EITB in patients with solitary cysticercus granuloma and seizures. Neurol Infect Epidemiol. (1997) 2:177–80.
113. Moyano, LM, Saito, M, Montano, SM, Gonzalvez, G, Olaya, S, Ayvar, V, et al. Neurocysticercosis as a cause of epilepsy and seizures in two community-based studies in a cysticercosis-endemic region in Peru. PLoS Negl Trop Dis. (2014) 8:e 2692. doi: 10.1371/JOURNAL.PNTD.0002692
114. Pritchard, DI, Falcone, FH, and Mitchell, PD. The evolution of IgE-mediated type I hypersensitivity and its immunological value. Allergy. (2021) 76:1024–40. doi: 10.1111/all.14570
115. Kinet, JP. The high-affinity IgE receptor (fc epsilon RI): from physiology to pathology. Annu Rev Immunol. (1999) 17:931–72. doi: 10.1146/annurev.immunol.17.1.931
116. Gould, HJ, and Sutton, BJ. IgE in allergy and asthma today. Nat Rev Immunol. (2008) 8:205–17. doi: 10.1038/nri2273
117. Chavarría, A, Roger, B, Fragoso, G, Tapia, G, Fleury, A, Dumas, M, et al. TH2 profile in asymptomatic Taenia solium human neurocysticercosis. Microbes Infect. (2003) 5:1109–15. doi: 10.1016/s1286-4579(03)00206-5
118. Flisser, A, Woodhouse, E, and Larralde, C. Human cysticercosis: antigens, antibodies and non-responders. Clin Exp Immunol. (1980) 39:27–37.
119. Espinoza, B, Ruiz-Palacios, G, Tovar, A, Sandoval, MA, Plancarte, A, and Flisser, A. Characterization by enzyme-linked immunosorbent assay of the humoral immune response in patients with neurocysticercosis and its application in immunodiagnosis. J Clin Microbiol. (1986) 24:536–41. doi: 10.1128/jcm.24.4.536-541.1986
120. Goldberg, AS, Heiner, DC, Firemark, HM, and Goldberg, MA. Cerebrospinal fluid IgE and the diagnosis of cerebral cysticercosis. Bull Los Angel Neurol Soc. (1981) 46:21–5.
121. Wan, D, Ludolf, F, Alanine, DG, Stretton, O, Ali Ali, E, Al-Barwary, N, et al. Use of humanised rat basophilic leukaemia cell line RS-ATL8 for the assessment of allergenicity of Schistosoma mansoni proteins. PLoS Negl Trop Dis. (2014) 8:e3124. doi: 10.1371/journal.pntd.0003124
122. Prakash, PS, Weber, MH, van Hellemond, JJ, and Falcone, FH. Are humanized IgE reporter systems potential game changers in serological diagnosis of human parasitic infection? Parasitol Res. (2022) 121:1137–44. doi: 10.1007/s00436-021-07352-z
123. Falcone, FH, Telford, G, Hooi, D, Brown, AP, Seabra, R, Feary, J, et al. Antigen-driven basophil activation is indicative of early Necator americanus infection in IgE-seronegative patients. J Allergy Clin Immunol. (2009) 124:1343–50.e7. doi: 10.1016/j.jaci.2009.07.039
124. Falcone, FH, Loukas, A, Quinnell, RJ, and Pritchard, DI. The innate allergenicity of helminth parasites. Clin Rev Allergy Immunol. (2004) 26:61–72. doi: 10.1385/CRIAI:26:1:61
125. Matowicka-Karna, J, and Kemona, H. IgE antibodies in toxoplasmosis. Postepy Hig Med Dosw (Online). (2014) 68:597–602. doi: 10.5604/17322693.1102581
126. Ahmad, H, Arifin, N, Nolan, TJ, Lok, JB, Anuar, NS, and Noordin, R. Strongyloides-specific IgE phage cDNA clones and development of a novel ELISA for strongyloidiasis. Diagnostics. (2021) 11. doi: 10.3390/diagnostics11060985
127. Assana, E, Amadou, F, Thys, E, Lightowlers, MW, Zoli, AP, Dorny, P, et al. Pig-farming systems and porcine cysticercosis in the north of Cameroon. J Helminthol. (2010) 84:441–6. doi: 10.1017/S0022149X10000167
128. Ramahefarisoa, RM, Rakotondrazaka, M, Jambou, R, and Carod, J-F. Comparison of ELISA and PCR assays for the diagnosis of porcine cysticercosis. Vet Parasitol. (2010) 173:336–9. doi: 10.1016/j.vetpar.2010.05.002
129. Sreedevi, C, Hafeez, M, Subramanyam, KV, Kumar, AP, and Rayulu, CV. Development and evaluation of flow through assay for detection of antibodies against porcine cysticercosis. Trop Biomed. (2011) 28:160–70.
130. Aguilar-Rebolledo, F, Meza-Lucas, A, Torres, J, Cedillo-Rivera, R, Enciso, A, Garcia, RC, et al. Evaluation of the enzyme-linked immunoelectrotransfer blot assay for diagnosis of neurocysticercosis in children. J Child Neurol. (2002) 17:416–20. doi: 10.1177/088307380201700604
131. Dermauw, V, Carabin, H, Cissé, A, Millogo, A, Tarnagda, Z, Ganaba, R, et al. Evaluating the recombinant T24H enzyme-linked Immunoelectrotransfer blot assay for the diagnosis of Neurocysticercosis in a panel of samples from a large community-based randomized control trial in 60 villages in Burkina Faso. Am J Trop Med Hyg. (2018) 98:565–9. doi: 10.4269/ajtmh.17-0541
132. Jiménez, L, Castro-Nolasco, NK, Fleury, A, Díaz-Camacho, SP, Ochoa-Sánchez, A, and Landa, A. Evaluation of recombinant glutathione transferase 26 kDa, thioredoxin-1, and endophilin B1 of Taenia solium in the diagnosis of human neurocysticercosis. Acta Trop. (2022) 227:106294. doi: 10.1016/j.actatropica.2021.106294
133. Krecek, RC, Michael, LM, Schantz, PM, Ntanjana, L, Smith, MF, Dorny, P, et al. Corrigendum to “prevalence of Taenia solium cysticercosis in swine from a community-based study in 21 villages of the eastern Cape Province, South Africa”. Vet Parasitol. (2011) 183:198–200. doi: 10.1016/j.vetpar.2011.09.033
134. Gupta, KK, Singh, A, Singh, AK, Singh, SK, Tripathi, M, Gupta, RK, et al. Evaluation of enzyme-linked immunoelectrotransfer blot for diagnosis of cysticercosis in swine from North India. J Helminthol. (2019) 93:548–51. doi: 10.1017/S0022149X18000603
135. Nguekam, JP, Zoli, AP, Zogo, PO, Kamga, AC, Speybroeck, N, Dorny, P, et al. A seroepidemiological study of human cysticercosis in West Cameroon. Trop Med Int Health. (2003) 8:144–9. doi: 10.1046/J.1365-3156.2003.01000.X
Keywords: cysticercosis, human, pig, Taenia solium , serological tests, IgE
Citation: Hossain MS, Shabir S, Ngwili N, Thomas LF and Falcone FH (2025) Serological diagnosis of cysticercosis in humans and pigs: status, limitations, and prospects. Front. Vet. Sci. 12:1558555. doi: 10.3389/fvets.2025.1558555
Edited by:
Calin Mircea Gherman, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaReviewed by:
Calin Mircea Gherman, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaMwemezi Kabululu, Tanzania Livestock Research Institute (TALIRI), Tanzania
Copyright © 2025 Hossain, Shabir, Ngwili, Thomas and Falcone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Franco H. Falcone, RnJhbmNvLkZhbGNvbmVAdW5pLWdpZXNzZW4uZGU=
 Md. Shahadat Hossain
Md. Shahadat Hossain Shafqat Shabir
Shafqat Shabir Nicholas Ngwili
Nicholas Ngwili Lian F. Thomas
Lian F. Thomas Franco H. Falcone
Franco H. Falcone