- Department of Small Mammal, Reptile and Avian Medicine and Surgery, University of Veterinary Medicine Hannover, Foundation, Hannover, Germany
Introduction: Fecal microbiota transplantation (FMT) is the process of transferring fecal microbiota from a healthy donor into the gastrointestinal tract of a recipient. Although many mechanisms of FMT are still not completely understood at present, it has been described that the treatment of various gastrointestinal diseases in different species, including humans, is significantly improved by FMT therapy. Since the first report on FMT therapy in veterinary medicine in small mammals numerous cases have been reported, but little information has been published on the therapeutic effects of FMT treatment in reptiles. The present case report describes the effects of orally administered fecal microbiota transplantation in a Mediterranean spur-thighed tortoise (Testudo graeca) suffering from chronic gastrointestinal disorders.
Case presentation: A nine-year-old, 330 g, intact female Mediterranean spur-thighed tortoise (Testudo graeca) from the animal owner’s own offspring was presented for consultation due to decreased general condition, anorexia and sialorrhea following oral intake of a lettuce species (Lactuca virosa) known for its poisonous plant ingredients (sesquiterpene lactones) 3 weeks prior to presentation. Pre-existing conditions were not reported. Clinical examination revealed sialorrhea and a reduced general condition. Diagnostic procedures included blood chemistry, radiography and ultrasonography. Despite repeated treatment attempts with various medical regimes over 158 days, the tortoise continued showing variable recurring gastrointestinal symptoms. An orally administered FMT was initiated and continued for a total of 3 weeks. Gastrointestinal signs improved rapidly within 1 week and resolved completely after 3 weeks. Over a follow up period of 9 months, no symptom recurrence or adverse effects were monitored.
Conclusion: This case report describes the first successful trial of fecal microbiota transplantation in chelonians. The outcome indicates that this therapeutic approach may be beneficial not only to small animals but also for the therapy of gastrointestinal disorders in reptiles, especially those cases with insufficient conventional therapy results.
Introduction
Fecal microbiota transplantation (FMT) is a treatment option that involves introducing fecal microbiota from a healthy donor into the recipient’s gastrointestinal tract (1). The treatment aims to positively influence the microbiome (and metabolom) and restore its balance (2, 3). The microbiota encompasses all microorganisms residing in the gastrointestinal tract (4, 5). These microorganisms have a crucial role in various physiological processes and form a symbiotic relationship with the host (6). Microbiome imbalances (dysbioses) are associated with various diseases, including acute and chronic gastrointestinal disorders (5). Although not all of the mechanisms of FMT are fully understood at present, it has been described that the treatment of various gastrointestinal diseases in different species, including humans, is significantly improved by FMT therapy (7–9). Anecdotally, FMT has been used as therapeutic approach in human medicine as early as the 4th century AD (10) and emerged as an important therapeutic tool in the 20th century, particularly for infections with Clostridium difficile (11). In veterinary medicine, the first case report of FMT treatment in a dog with Inflammatory Bowel Disease (IBD) was published in 2013 (12). Since then, numerous case reports and case series have been published for cats and dogs (13, 14), and also for other domestic animals such as horses (15). In the field of reptile medicine, a study evaluating the evolution of the microbiota in relation to behavioral aspects of green iguana hatchlings (Iguana iguana) was published as early as 1984 (16). In recent years, several studies have explored various aspects of microbiome research (17–19). The intestinal flora of wild and captive species of turtles has been described (20, 21). Another publication showed metagenomic differences in fecal microbiota between wild, released, and farmed individuals of a single turtle species (22). A recent study described host-microbial interactions in a desert lizard (Eremias multiocellata) and also assessed the biological impact of climate conditions on the gut microbiota (23). This study was also the first to perform fecal microbiota transplantation experiments in reptiles. However, no case reports or studies have examined the effects of FMT therapy in chelonians. The present report describes the treatment process, clinical outcome and follow-up of a tortoise with chronic gastrointestinal disease using FMT.
Case presentation
Clinical history
A nine-year-old, 330 g, intact female Mediterranean spur-thighed tortoise (Testudo graeca) bred from the owner’s own stock has been kept in a 25 m2 sized outdoor enclosure for the last 5 years alongside three siblings (one male, two females). Temperature, UVB-light and hibernation management were species-appropriate. The animals were fed daily, receiving hay (available ad libitum) and a variety of herbs and leafy green. The owner did not report any pre-existing conditions and the siblings were assessed as healthy. The tortoise was presented with a decreased general condition, anorexia and sialorrhea following oral intake of a lettuce species (Lactuca virosa) 3 weeks prior to presentation. Clinical symptoms developed within 1 day after oral ingestion and showed no improvement following soaking the tortoise repeatedly in warm water.
Clinical findings and investigations
Clinical examination revealed a moderately softened plastron and minor chronic shell deformities. Sialorrhea and a moderately reduced general condition of the tortoise were also noted during the general examination. Initial diagnostic procedures included blood chemistry, radiography and ultrasonography. Blood chemistry examination was performed in an in-house laboratory (Cobas C 311, La Roche Ltd., Basel, Switzerland) 15 min after venipuncture (dorsal coccygeal vein) and revealed moderate hyperuricemia (6.5 mg/dL; reference range 0–5.2 mg/dL) (24). Radiographic examination (digital X-ray; detector system: Fujifilm Console Advance DR-ID 300 CL, Fujifilm Europe GmbH, Düsseldorf, Germany; tube system: Gierth X-ray International GmbH, Riesa, Germany; film focus distance 60 cm, 50 kV, 5 mA) under manual restraint in dorsoventral (vertical radiation beam direction) and lateral (horizontal radiation beam direction) projections showed a prominent filling of the digestive tract with ingesta. Ultrasonography (GE Vivid 7 Dimension, Micro curved array transducer, 5–9 MHz; GE Healthcare GmbH, Solingen, Germany) performed under manual restraint confirmed the sex diagnosis by clearly showing ovarian follicles but revealed no other abnormalities. Fecal samples were used for a microbiological examination including sensitivity testing conducted in a commercial veterinary laboratory at the time of starting antibiotic treatment. Testing revealed the growth of 2 gram-negative bacteria (Cronobacter spp. and Citrobacter braakii). Both bacteria showed intermediate susceptibility to enrofloxacine.
Treatment and follow-up
The animal received allopurinol (50 mg/kg SID PO; Allopurinol AL 100, Aliud Pharma, Laichingen, Germany) according to treatment recommendation (25) and parenteral fluids (10 mL/kg SID SC, Sterofundin, ISO 1/1 E; B. Braun AG, Melsungen, Germany). However, the tortoise’s general condition remained unchanged after 10 days of treatment and the animal was presented again. Neither a stationary therapy for 9 days nor several treatment approaches using various protocols including antibiotics, analgesics, probiotics, devolatilizing and gastroprotective drugs led to a sustainable and lasting therapy success in the following 149 days. Table 1 provides details on the respective gastrointestinal signs, diagnostic methods (including radiography of Figures 1–3), therapy attempts and outcomes of the follow-up history.
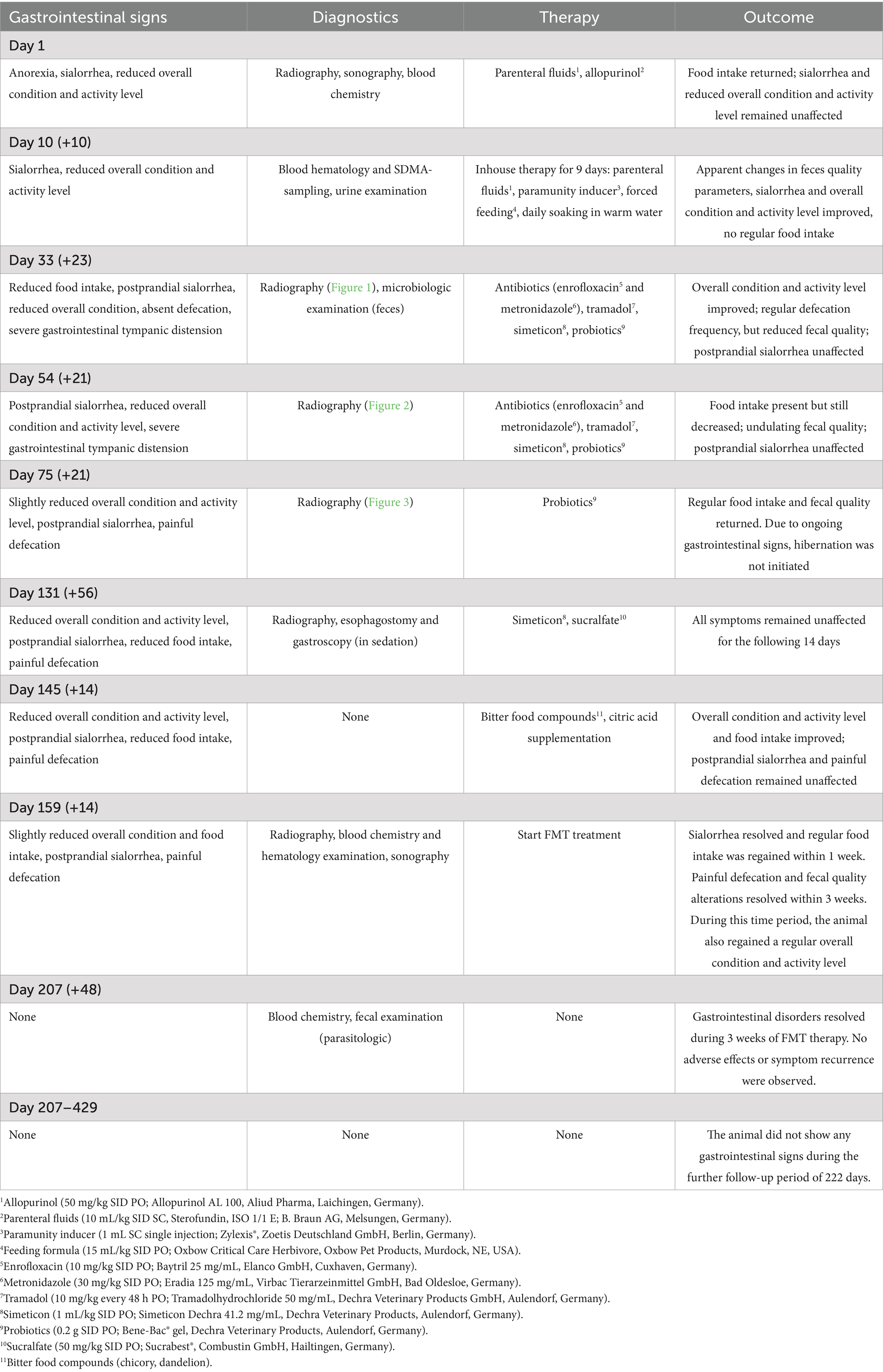
Table 1. Detailed information on the evolution of gastrointestinal signs and the corresponding diagnostic methods, therapies and outcomes of the according follow-ups during the entire case history.
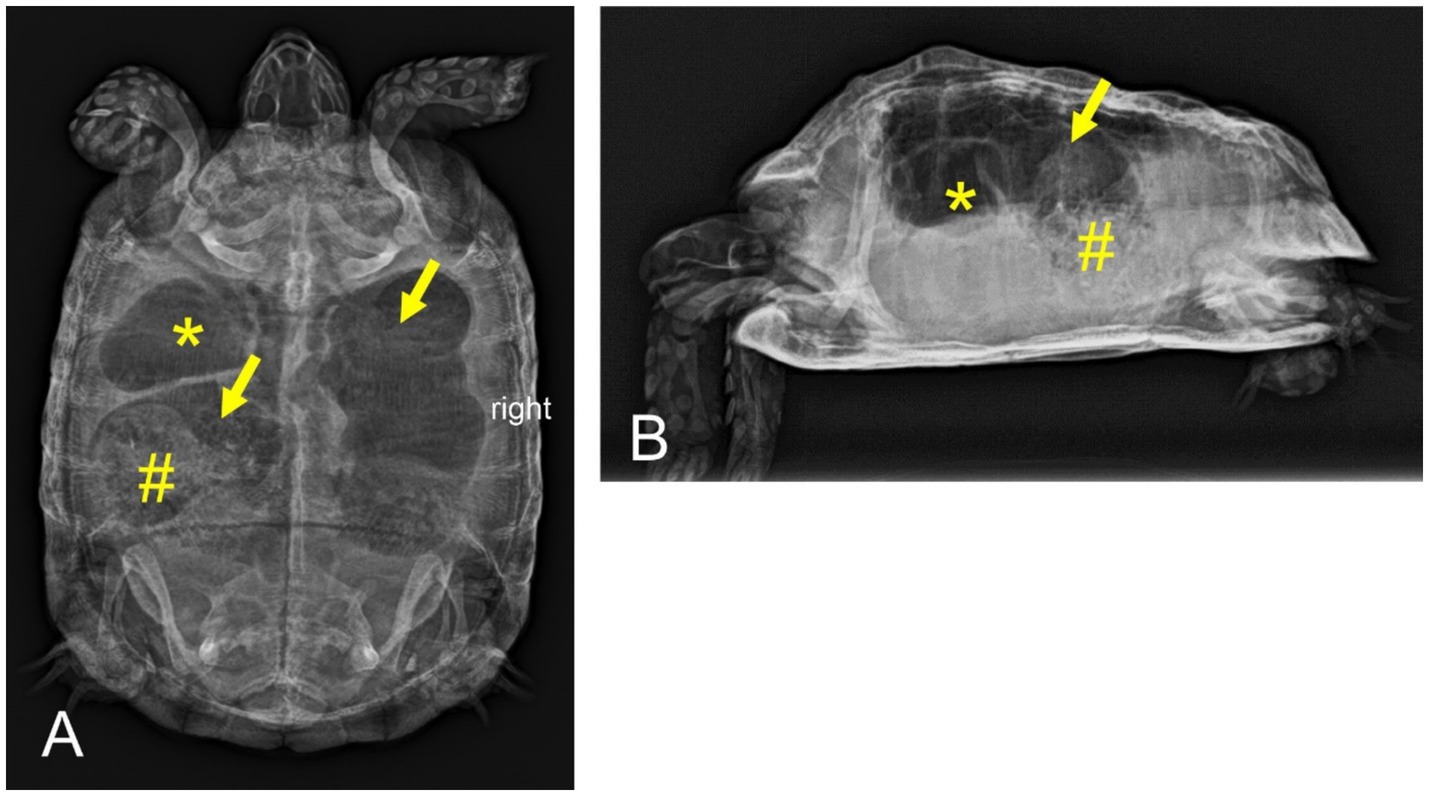
Figure 1. Dorsoventral (A) and horizontal-beam right lateral (B) radiographs of a nine-year-old Mediterranean spur-thighed tortoise (Testudo graeca) demonstrating severe gas accumulation throughout the gastrointestinal tract 33 days after initial presentation. The stomach (asterisk) and parts of the large intestine (arrows) appear to be filled only with gaseous contents. Parts of the large intestine, though, contain structured material (ingesta, number sign).
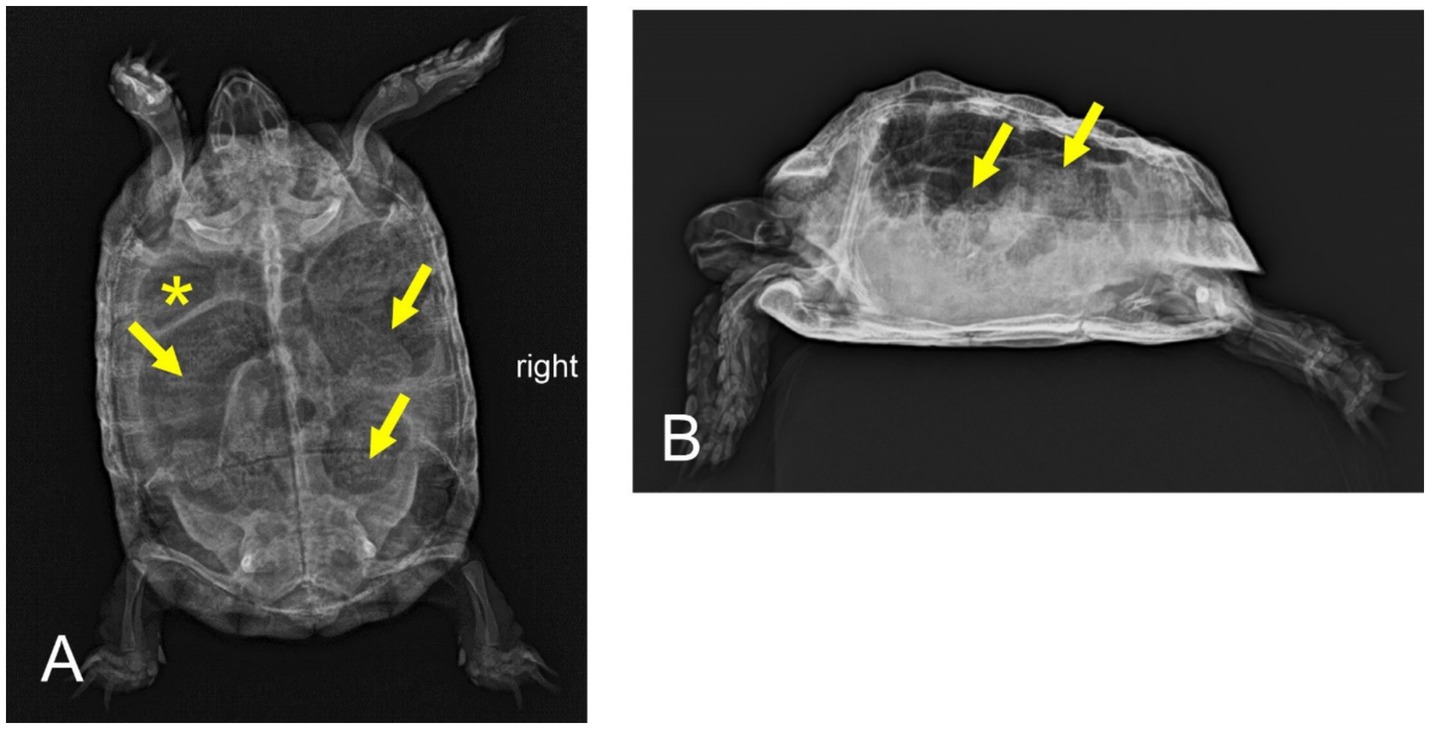
Figure 2. Dorsoventral (A) and horizontal-beam right lateral (B) radiographs of a nine-year-old Mediterranean spur-thighed tortoise (Testudo graeca) with persistent severe gas accumulation throughout the gastrointestinal tract 54 days after initial presentation. However, major parts of the gastrointestinal tract, including the stomach (asterisk) and various parts of the large intestine (arrows), clearly contain structured ingesta material.
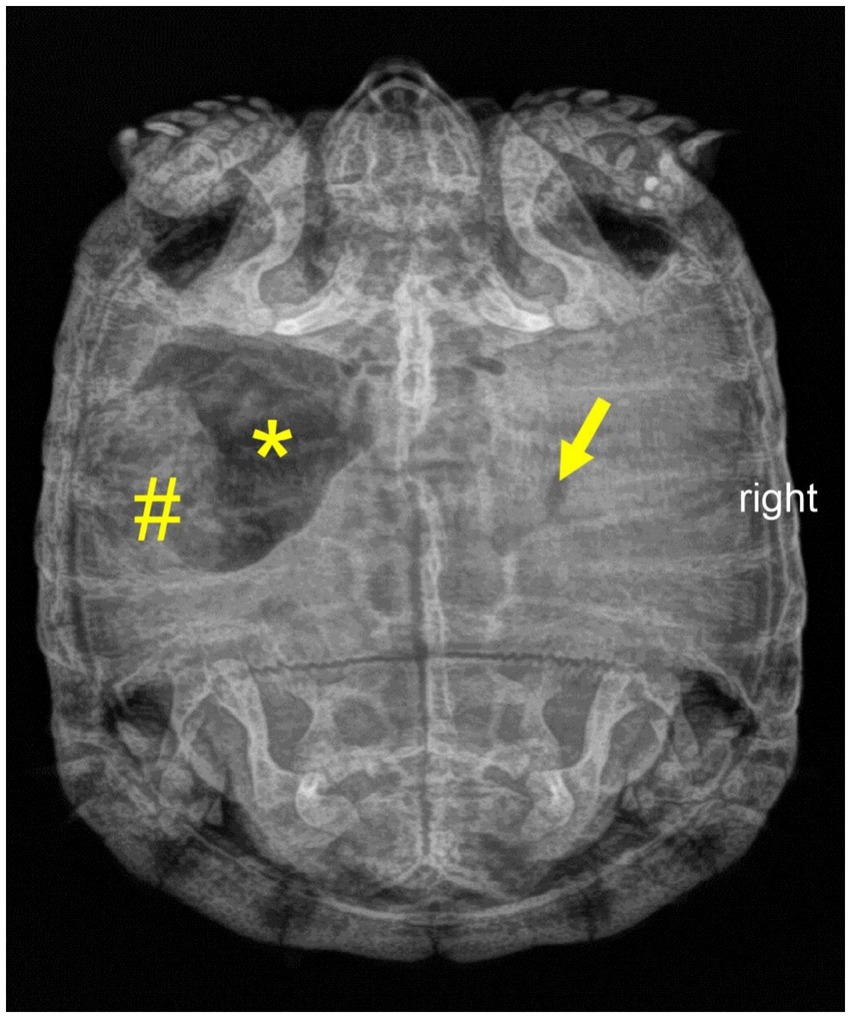
Figure 3. Dorsoventral radiograph of a nine-year-old Mediterranean spur-thighed tortoise (Testudo graeca) demonstrating mixed contents of gas (asterisk) and structured ingesta material (number sign) within the stomach 75 days after initial presentation. Note that only minor gas accumulation is still present in the intestine (arrow).
FMT treatment
An orally administered fecal microbiota transplantation (FMT) was initiated as new therapeutic approach 181 days after the oral intake of lettuce and 159 days after the initial presentation. The feces were obtained from three clinically healthy siblings that had been housed and fed under identical conditions for the last 5 years. Fecal samples were macroscopically normal and tested negative for endoparasitic infections on two separate occasions prior to FMT. All four animals tested negative for herpesvirus (PCR and serology), reovirus (PCR) and mycoplasma (PCR) 3 years before. A total of 15 grams of fresh feces was mixed with 10 mL sodium chloride (sodium chloride 0.9%, B. Braun Melsungen AG, Melsungen, Germany) to a paste-like consistency. A total volume of 9 mL of dispersed fecal mixture was orally administered into the stomach in a two-step procedure (two-hour breaks between administrations) using a metal probe (diameter 6.4 mm, length 102 mm; Eickemeyer, Tuttlingen, Germany). The animal owner continued to administer fresh feces from the sibling animals through manual feeding every 2–3 days for a total period of 3 weeks. The fecal samples were mixed with sodium chloride and coated with the animal’s favorite salad. Using this procedure, voluntary oral ingestion of the transplants by the tortoise was achieved. Gastrointestinal signs resolved within 1 week (sialorrhea, reduced food intake) to 3 weeks (painful defecation) after the initial fecal transplantation. The tortoise’s defecations became more consolidated, displaying the physiological characteristics of normal feces. In addition, the animal regained a physiologic level of activity that had not been achieved during the entire former therapy period. Over the following 6 weeks, the animal did not exhibit any of the preceding gastrointestinal signs and continued food intake and defecation on a regular basis. No adverse effects were observed during this time period.
A final on-site follow-up 48 days after the initial fecal transplantation revealed a healthy overall condition. Regular food intake before and defecation with physiological fecal characteristics during the examination could be verified within the scope of the clinical follow-up. Blood chemistry parameters and parasitologic fecal sampling were normal. The tortoise was deemed to be free of any gastrointestinal signs, and the animal owner began preparations for a regular hibernation.
The animal was monitored for a total of 9 months following the initial FMT treatment. According to personal communication with the animal owner, the tortoise remained free of any gastrointestinal signs or adverse effects both before and after hibernation.
Discussion
Gastrointestinal dysbiosis is considered a disruption of the normal physiological colonization of the gastrointestinal tract by microorganisms (5). FMT aims to correct an existing dysbiosis and consecutive metabolic imbalances (26). The detailed mechanisms of FMT in mammals are not yet fully understood, though various complex interactions between the host and its microbiota are assumed (3, 27, 28). The microbiota stimulates the development of the immune system and acts as a protective barrier against infectious agents (29), either through direct cell-to-cell contact mechanisms or through metabolites produced by the microbiota (30). Whenever there is a direct or indirect perturbation of the microbiota, the host becomes more susceptible to gastrointestinal infections (30). In human medicine, FMT treatment has been shown to have beneficial effects, not only for gastrointestinal disorders (31), but also for extragastrointestinal indications such as metabolic syndrome (32), autism, stereotypy, or speech disorders (33). FMT transplantation studies in small animals evaluated that fecal transplantation increased the number of bacterial diversity and particularly useful bacteria like, e.g., Peptacetobacter hiranonis and Fusobacteria ssp. (34, 35).
In addition, FMT treatment has been introduced to a number of wildlife species in recent years, bringing valuable therapy experience to more exotic, and possibly endangered species, for which novel treatment methods are needed (36–38). Despite the growing evidence supporting the application of FMTs to a wide range of animal species, there is a dearth of research on FMT, especially for carnivores (39).
In reptiles, a recent study described host-microbial interactions in a desert lizard (Eremias multiocellata) and also assessed the biological impacts of climate conditions on the gut microbiota (23). Interestingly, this study also included the first fecal microbiota transplantation experiments in reptiles showing that FMT enhanced antibacterial activity and host immune response of the lizards. This study provides useful initial data for future prospective evaluations of this emerging topic.
Detailed theoretical specifications have been published for the required characteristics of donor animals in small animal medicine (3, 26). In our case, three possible donor animals were available and considered to be suitable donors. The sibling tortoises were evaluated to be clinically healthy, showed regular food intake and defecation, and had no reported previous gastrointestinal disorders. Infections with intranuclear coccidiosis in tortoises (TINC) have been first described in radiated tortoises (Astrochelys radiata) (40). TINC can result in systemic disease, which has been described in several chelonian species (41), but is more common in tropical tortoises. However, TINC represents a chronic disease and there is a risk of transmission from asymptomatic carrier animals shedding coccidia for life (42). Therefore, sampling the siblings prior to FMT would have extended the donor assessment.
In general, FMT can be performed via the upper or lower gastrointestinal tract (43). In small animals, enemas, endoscopic transplantation into the intestine, and oral administration (capsule or dilution) have been described (9, 44–46). Nonetheless, no studies have evaluated any route of administration and their efficacy in reptiles. In the present case report, the authors chose oral administration, as tube-feeding with a metal probe is a common and low-risk procedure in the treatment of tortoises. In contrast, an incorrectly placed cloacal FMT may lead to a too rapid discharge from the rectum, which should be avoided.
FMT treatment protocols vary widely regarding the number and frequency of repeated administrations (26, 34, 47, 48). Chronicity of disease, route of administration and course of treatment represent important factors for the number of repeated FMT doses (26). The authors of this report aimed to establish a feasible treatment plan for continuing FMT therapy after the initial administration, considering defecation frequency of the siblings, suspected gastrointestinal transit time and animal habits (usual defecation time). Continuation of FMT may have been appropriate if the tortoise’s gastrointestinal signs had persisted.
Assessment of treatment efficacy should represent an important part of FMT evaluation. In several small animal studies, the short-term efficacy of FMT treatment was rated good to excellent (34, 35, 48–50). Often, medium-term efficacy remained unclear due to a lack of follow-up data. In our case, we considered a nine-month follow-up period after FMT initiation to be highly valuable to assess the long-term effect of the FMT procedure on the digestive system and the overall clinical outcome.
Studies evaluating the adverse effects of FMT have been published in human medicine (51) and, more recently, in small animal medicine (26, 52). Short- and medium-term adverse effects generally were rare and mostly mild and self-limiting (26, 48, 53). The FMT technique, transplant quality (54), potential comorbidities and immune competence of the recipient as well as the health of the donor were among the factors that needed to be considered for a safe FMT (26).
Gut microbial communities are often characteristic of specific dietary modes (55, 56). Obligate herbivory in mammals is associated with increased microbial diversity compared to other dietary modes (57). Herbivore reptiles, such as Galápagos tortoises and iguanids, share a similar digestive mode and gut morphology with mammalian hindgut fermenters (58). However, little is known about species-specific factors that influence the composition of gut microbiota in reptiles. A study evaluating gut microbial diversity in gopher tortoises (Gopherus polyphemus) found that fine-scale spatial structure, inbreeding, degree of relatedness and possibly ontogeny shape patterns of diversity in fecal microbiomes (59). Coprophagy may serve as another factor affecting the composition of the gut microbiome. Some species of herbivore reptiles have been described to exhibit coprophagy as normal behavior during microbiota development in juvenile life stages (16). Other species show regular coprophagy even in the presence of a good range of usual dietary components (60, 61). Although it is unknown, whether this behavior is the primary route of colonization for critical gut symbionts (59), evidence of coprophagy in wildlife may indicate that this behavior may be a physiological route to (re)establish a healthy microbiome, even in diseased individuals.
In general, it should be critically discussed whether the ingested lettuce plant actually caused the gastrointestinal symptoms. The temporal context and the available information on the toxicity of the plant for humans (62), other mammals (63) and reptiles (64) strengthen the assumption that the lettuce intake was the initial cause of the ongoing gastrointestinal disorders. Lactuca virosa is known for its poisonous plant ingredients (sesquiterpene lactones) (62, 64), which cause central nervous signs, but also gastrointestinal disorders in mammals (62, 63). However, no toxicological tests, such as spectrophotometric or chromatographic methods (65, 66), were carried out in this case to confirm the presence and amount of sesquiterpene lactones such as lactucerin, actucic acid, lactucopicrin and lactucin. Therefore, clear evidence of any association between the oral consumption of Lactuca virosa and gastrointestinal signs remains uncertain and speculative.
In the present report, husbandry parameters have been assessed as species-appropriate based on information from the animal owner, and all siblings were assessed without any abnormalities. However, moderate softening and deformities of the shell and uricemia are common signs of nephropathy (67, 68) and may also indicate possible nephropathy in this case. Intoxication with sesquiterpene lactones may have exacerbated renal disease, facilitating non-specific symptoms such as lethargy and reduced activity.
The conducted long-term gastrointestinal therapy prior to FMT treatment needs a critical reflection. The various treatment approaches using supportive therapy, including parenteral fluids, probiotics, gastroprotective therapy, antibiotics, or combinations of these treatments temporarily affected the various signs. Gastrointestinal tympanic distension and reduced food intake improved during antibiotic treatment. Antibiotics may have significantly altered the microbial composition and also reduced the amount of gas-forming gut bacteria, which can lead to tympanic distension. Probiotics may have positively altered the microbial composition and fecal quality. Also, by reducing the gastrointestinal pain of tympanic distension, the analgesics used may facilitated defecation frequency. Bitter food compounds also led to improved gastrointestinal signs, which may be related to the diverse immunomodulating, anti-inflammatory and digestive properties that bitter substances are known for (69, 70). However, other gastrointestinal signs, such as sialorrhea, remained largely unaffected, gastrointestinal signs recurred and no lasting improvement in clinical status was achieved. Subsequently, the conditions did improve rapidly after FMT initiation. However, recovery cannot be attributed solely to FMT, as the microbiome status and gastrointestinal signs were already influenced over a long period by the pre-treatments and their multiple (synergistic) effects as described above.
This case report also needs a critical review of antibiotic treatment regimes. In this report, antibiotic treatment was started using enrofloxacine and metronidazole when signs of gastrointestinal disease – suspected dysbiosis causing extended gastrointestinal tympania - were diagnosed. Fecal samples were sent to a laboratory for bacteriological examination and consecutive sensitivity testing. Especially the use of enrofloxacine is discussed critically for different reasons and is not recommended clearly as a first-line antibiotic in reptiles (71). However, in Germany enrofloxacine is approved for the treatment of gastrointestinal disease in reptiles, as the only antibiotic available. For this reason, the treatment regime was started and continued following an initial improvement of some gastrointestinal signs despite the laboratory results (intermediate sensitivity of enrofloxacine). Retrospectively, this measure needs to be viewed critically, as no lasting effect was seen and the use of both antibiotics in the present case may have had a significant (negative) impact on the tortoise’s microbiome. It is the authors’ experience and also reported in literature that antibiotic treatments of gastrointestinal disease in reptiles are often not effective (71). This case report is a more or less typical example of different common but unsuccessful treatment regimes. As treatment options for gastrointestinal disorders are not as versatile as in other companion animals, it was the authors’ aim to invent a new treatment option in this case, which could not be resolved using conventional therapies. Therefore, despite using antibiotics in the course of the treatment described here, this report is clearly intended to point out a new therapy alternative in FMT and minimize antibiotic use.
As it is inherent in case reports, several limitations arise due to individual circumstances. Most importantly, we are not able to associate the different clinical stages with the corresponding microbiome. Microbiota analysis would have been highly beneficial, especially comparing the gastrointestinal flora before and after FMT administration. Initial treatment was started before laboratory results were available, and treatment regimens were adapted individually to the clinical situation, without lasting effects. Although the animal was regularly presented to the clinic, some health assessments were conducted by the owner, and the interpretation of these could be subjective and potentially misleading. However, it is the nature of an individual case report that diagnostic and treatment procedures are case-sensitive and dependent on the owner’s compliance, which was excellent in this case. Finally, extrinsic or intrinsic factors other than FMT may have influenced clinical improvement. However, the tortoise’s rapid improvement after starting FMT, following several months of chronic, fluctuating gastrointestinal signs, suggests that FMT treatment likely had a positive impact on the therapy outcome.
Therefore, the scientific value of this report lies exclusively in the effective use of FMT as an alternative treatment option in a chronic case of gastrointestinal disease and pretreatment failures.
Conclusion
This case report provides an evidence-based example of the use of FMT in a tortoise suffering from chronic gastrointestinal disease. Oral FMT proved to be a safe and non-invasive therapy approach. Following FMT therapy, chronic gastrointestinal symptoms vanished with no relapses over a nine-month follow-up period. Further studies are needed on the therapeutic efficacy of FMT in chelonians and all reptile orders.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and institutional approval were waived for this report since this case included the examination and treatment of an animal with clinical disease. No animal was killed for the purpose of this study. Written informed consent has been obtained from the owner of the animals involved in this study. Written informed consent was obtained from the participants for the publication of this case report.
Author contributions
JH: Conceptualization, Data curation, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. MP: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We acknowledge financial support by the Open Access Publication Fund of the University of Veterinary Medicine Hannover, Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Borody, TJ, and Khoruts, A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. (2012) 9:88–96. doi: 10.1038/nrgastro.2011.244
2. Cammarota, G, Ianiro, G, Tilg, H, Rajilić-Stojanović, M, Kump, P, Satokari, R, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. (2017) 66:569–80. doi: 10.1136/gutjnl-2016-313017
3. Chaitman, J, and Gaschen, F. Fecal microbiota transplantation in dogs. Vet Clin North Am Small Anim Pract. (2021) 51:219–33. doi: 10.1016/j.cvsm.2020.09.012
4. Swanson, KS, Dowd, SE, Suchodolski, JS, Middelbos, IS, Vester, BM, Barry, KA, et al. Phylogenetic and gene-centric metagenomics of the canine intestinal microbiome reveals similarities with humans and mice. ISME J. (2011) 5:639–49. doi: 10.1038/ismej.2010.162
5. Suchodolski, JS. Analysis of the gut microbiome in dogs and cats. Vet Clin Pathol. (2022) 50:6–17. doi: 10.1111/vcp.13031
6. Ziese, AL, and Suchodolski, JS. Impact of changes in gastrointestinal microbiota in canine and feline digestive diseases. Vet Clin North Am Small Anim Pract. (2021) 51:155–69. doi: 10.1016/j.cvsm.2020.09.004
7. Sugita, K, Yanuma, N, Ohno, H, Takahashi, K, Kawano, K, Morita, H, et al. Oral faecal microbiota transplantation for the treatment of Clostridium difficile-associated diarrhoea in a dog: a case report. BMC Vet Res. (2019) 15:11–4. doi: 10.1186/s12917-018-1754-z
8. Borody, TJ, Eslick, GD, and Clancy, RL. Fecal microbiota transplantation as a new therapy: from Clostridioides difficile infection to inflammatory bowel disease, irritable bowel syndrome, and colon cancer. Curr Opin Pharmacol. (2019) 49:43–51. doi: 10.1016/j.coph.2019.04.017
9. Cerquetella, M, Marchegiani, A, Rossi, G, Trabalza-Marinucci, M, Passamonti, F, Isidori, M, et al. Case report: Oral fecal microbiota transplantation in a dog suffering from relapsing chronic diarrhea—clinical outcome and follow-up. Front Vet Sci. (2022) 9:893342. doi: 10.3389/fvets.2022.893342
10. Zhang, F, Cui, B, He, X, Nie, Y, Wu, K, Fan, D, et al. Microbiota transplantation: concept, methodology and strategy for its modernization. Protein Cell. (2018) 9:462–73. doi: 10.1007/s13238-018-0541-8
11. Eiseman, B, Silen, W, Bascom, GS, and Kauvar, AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. (1958) 44:854–9.
12. Weese, JS, Costa, MC, and Webb, JA. Preliminary clinical and microbiome assessment of stool transplantation in the dog and cat. J Vet Intern Med. (2013) 3:705–5.
13. Furmanski, S, and Mor, T. First case report of fecal microbiota transplantation in a cat in Israel. Isr J Vet Med. (2017) 72:35–41.
14. Sugita, K, Shima, A, Takahashi, K, Ishihara, G, Kawano, K, and Ohmori, K. Pilot evaluation of a single oral fecal microbiota transplantation for canine atopic dermatitis. Sci Rep. (2023) 13:8824. doi: 10.1038/s41598-023-35565-y
15. Mullen, KR, Yasuda, K, Divers, TJ, and Weese, JS. Equine faecal microbiota transplant: current knowledge, proposed guidelines and future directions. Equine Vet Educ. (2018) 30:151–60. doi: 10.1111/eve.12559
16. Troyer, K. Behavioral acquisition of the hindgut fermentation system by hatchling Iguana iguana. Behav Ecol Sociobiol. (1984) 14:189–93. doi: 10.1007/BF00299618
17. Abdelrhman, KF, Bacci, G, Mancusi, C, Mengoni, A, Serena, F, and Ugolini, A. A first insight into the gut microbiota of the sea turtle Caretta caretta. Front Microbiol. (2016) 7:1060. doi: 10.3389/fmicb.2016.01060
18. Siddiqui, R, Maciver, SK, and Khan, NA. Gut microbiome–immune system interaction in reptiles. J Appl Microbiol. (2022) 132:2558–71. doi: 10.1111/jam.15438
19. Hoffbeck, C, Middleton, DM, Nelson, NJ, and Taylor, MW. 16S rRNA gene-based meta-analysis of the reptile gut microbiota reveals environmental effects, host influences and a limited core microbiota. Mol Ecol. (2023) 32:6044–58. doi: 10.1111/mec.17153
20. Fong, JJ, Sung, YH, and Ding, L. Comparative analysis of the fecal microbiota of wild and captive beal’s eyed turtle (Sacalia bealei) by 16S rRNA gene sequencing. Front Microbiol. (2020) 11:570890. doi: 10.3389/fmicb.2020.570890
21. Niu, X, Lin, L, Zhang, T, An, X, Li, Y, Yu, Y, et al. Comparison of the intestinal flora of wild and artificial breeding green turtles (Chelonia mydas). Front Microbiol. (2024) 15:1412015. doi: 10.3389/fmicb.2024.1412015
22. Khan, I, Bu, R, Ali, Z, Iqbal, MS, Shi, H, Ding, L, et al. Metagenomics analysis reveals the composition and functional differences of fecal microbiota in wild, farm, and released Chinese three-keeled pond turtles (Mauremys reevesii). Animals. (2024) 14:1750. doi: 10.3390/ani14121750
23. Yang, J, Liu, W, Han, X, Hao, X, Yao, Q, and Du, W. Gut microbiota modulation enhances the immune capacity of lizards under climate warming. Microbiome. (2024) 12:37. doi: 10.1186/s40168-023-01736-2
24. Andreani, G, Carpene, E, Cannavacciuolo, A, Di Girolamo, N, Ferlizza, E, and Isani, G. Reference values for hematology and plasma biochemistry variables, and protein electrophoresis of healthy Hermann’s tortoises (Testudo hermanni ssp.). Vet Clin Pathol. (2014) 43:573–83. doi: 10.1111/vcp.12203
25. Koelle, P. Efficacy of allopurinol in European tortoises with hyperuricemia. Proc ARAV. (2001) 16:185–6.
26. Winston, JA, Suchodolski, J, Gaschen, FP, Busch, KB, Marsilio, S, Costa, MC, et al. Clinical guidelines for fecal microbiota transplantation in companion animals. Adv Small Anim Care. (2024) 5:79–107. doi: 10.1016/j.yasa.2024.06.006
27. Kelly, CR, Kahn, S, Kashyap, P, Laine, L, Rubin, D, Atreja, A, et al. Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology. (2015) 149:223–37. doi: 10.1053/j.gastro.2015.05.008
28. Khoruts, A, and Sadowsky, MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol. (2016) 13:508–16. doi: 10.1038/nrgastro.2016.98
29. Pilla, R, and Suchodolski, JS. The role of the canine gut microbiome and metabolome in health and gastrointestinal disease. Front Vet Sci. (2020) 6:498. doi: 10.3389/fvets.2019.00498
30. Agus, A, Planchais, J, and Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. (2018) 23:716–24. doi: 10.1016/j.chom.2018.05.003
31. Khanna, S, Vazquez-Baeza, Y, González, A, Weiss, S, Schmidt, B, Muñiz-Pedrogo, DA, et al. Changes in microbial ecology after fecal microbiota transplantation for recurrent C. difficile infection affected by underlying inflammatory bowel disease. Microbiome. (2017) 5:1–8. doi: 10.1186/s40168-017-0269-3
32. Zhang, Z, Mocanu, V, Cai, C, Dang, J, Slater, L, Deehan, EC, et al. Impact of fecal microbiota transplantation on obesity and metabolic syndrome—a systematic review. Nutrients. (2019) 11:2291. doi: 10.3390/nu11102291
33. Vendrik, KE, Ooijevaar, RE, De Jong, PR, Laman, JD, Van Oosten, BW, Van Hilten, JJ, et al. Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol. (2020) 10:98. doi: 10.3389/fcimb.2020.00098
34. Chaitman, J, Guard, B, Sarwar, F, Lidbury, J, Steiner, J, and Suchodolski, J. Fecal microbial transplantation decreases the dysbiosis index in dogs presenting with chronic diarrhea. J Vet Intern Med. (2017) 31:1287.
35. Niina, A, Kibe, R, Suzuki, R, Yuchi, Y, Teshima, T, Matsumoto, H, et al. Fecal microbiota transplantation as a new treatment for canine inflammatory bowel disease. Biosci Microbiota Food Health. (2021) 40:98–104. doi: 10.12938/bmfh.2020-049
36. Thacher, PR, Kendrick, EL, Maslanka, M, Muletz-Wolz, CR, and Bornbusch, SL. Fecal microbiota transplants modulate the gut microbiome of a two-toed sloth (Choloepus didactylus). Zoo Biol. (2023) 42:453–8. doi: 10.1002/zoo.21751
37. Bornbusch, SL, Harris, RL, Grebe, NM, Roche, K, Dimac-Stohl, K, and Drea, CM. Antibiotics and fecal transfaunation differentially affect microbiota recovery, associations, and antibiotic resistance in lemur guts. Anim Microbiome. (2021) 3:65. doi: 10.1186/s42523-021-00126-z
38. Kohl, KD, Weiss, RB, Cox, J, Dale, C, and Dearing, MD. Gut microbes of mammalian herbivores facilitate intake of plant toxins. Ecol Lett. (2014) 17:1238–46. doi: 10.1111/ele.12329
39. Bornbusch, SL, Crosier, A, Gentry, L, Delaski, KM, Maslanka, M, and Muletz-Wolz, CR. Fecal microbiota transplants facilitate post-antibiotic recovery of gut microbiota in cheetahs (Acinonyx jubatus). Commun Biol. (2024) 7:1689. doi: 10.1038/s42003-024-07361-5
40. Jacobse, ER, Schuhmacher, J, Telford, SR, Greiner, EC, Buergelt, CD, and Gardiner, CH. Intranuclear coccidiosis in radiated tortoises (Geochelone radiata). J Zoo Wildl Med. (1994) 25:95–102.
41. Kolesnik, E, Dietz, J, Heckers, KO, and Marschang, RE. Detection of intranuclear coccidiosis in tortoises in Europe and China. J Zoo Wildl Med. (2017) 48:328–34. doi: 10.1638/2016-0054R1.1
42. Wellehan, JF, Jacobsen, E, Stilwell, J, Gibbons, PM, Garner, MM, Rosenoff, E, et al. Testudine Intranuclear coccidiosis (TINC). J Herpetol. (2022) 32:144–54. doi: 10.5818/JHMS-D-20-00024
43. Kelly, BJ, and Tebas, P. Clinical practice and infrastructure review of fecal microbiota transplantation for Clostridium difficile infection. Chest. (2018) 153:266–77. doi: 10.1016/j.chest.2017.09.002
44. Schmitz, SS. Observational study of small animal practitioners’ awareness, clinical practice and experience with fecal microbiota transplantation in dogs. Top Companion Anim Med. (2022) 47:100630. doi: 10.1016/j.tcam.2022.100630
45. Gal, A, Barko, PC, Biggs, PJ, Gedye, KR, Midwinter, AC, Williams, DA, et al. One dog’s waste is another dog’s wealth: a pilot study of fecal microbiota transplantation in dogs with acute hemorrhagic diarrhea syndrome. PLoS One. (2021) 16:e0250344. doi: 10.1371/journal.pone.0250344
46. Berlanda, M, Innocente, G, Simionati, B, Di Camillo, B, Facchin, S, Giron, MC, et al. Faecal microbiome transplantation as a solution to chronic enteropathies in dogs: a case study of beneficial microbial evolution. Animals. (2021) 11:1433. doi: 10.3390/ani11051433
47. Pereira, GQ, Gomes, LA, Santos, IS, Alfieri, AF, Weese, JS, and Costa, MC. Fecal microbiota transplantation in puppies with canine parvovirus infection. J Vet Intern Med. (2018) 32:707–11. doi: 10.1111/jvim.15072
48. Toresson, L, Spillmann, T, Pilla, R, Ludvigsson, U, Hellgren, J, Olmedal, G, et al. Clinical effects of faecal microbiota transplantation as adjunctive therapy in dogs with chronic enteropathies—a retrospective case series of 41 dogs. Vet Sci. (2023) 10:271. doi: 10.3390/vetsci10040271
49. Bottero, E, Benvenuti, E, and Ruggiero, P. Fecal microbiota transplantation (FMT) in 16 dogs with idiopatic IBD. Veterinaria. (2017) 31:31–45. doi: 10.5555/20173098113
50. Niina, A, Kibe, R, Suzuki, R, Yuchi, Y, Teshima, T, Matsumoto, H, et al. Improvement in clinical symptoms and fecal microbiome after fecal microbiota transplantation in a dog with inflammatory bowel disease. Vet Med. (2019) 10:197–201. doi: 10.2147/VMRR.S230862
51. Michailidis, L, Currier, AC, Le, M, and Flomenhoft, DR. Adverse events of fecal microbiota transplantation: a meta-analysis of high-quality studies. Ann Gastroenterol. (2021) 34:802–14. doi: 10.20524/aog.2021.0655
52. Lee, MA, Questa, M, Wanakumjorn, P, Kol, A, McLaughlin, B, Weimer, BC, et al. Safety profile and effects on the peripheral immune response of fecal microbiota transplantation in clinically healthy dogs. J Vet Intern Med. (2024) 38:1425–36. doi: 10.1111/jvim.17061
53. Rojas, CA, Entrolezo, Z, Jarett, JK, Jospin, G, Kingsbury, DD, Martin, A, et al. Microbiome responses to fecal microbiota transplantation in cats with chronic digestive issues. Vet Sci. (2023) 10:561. doi: 10.3390/vetsci10090561
54. Takáčová, M, Bomba, A, Tóthová, C, Micháľová, A, and Turňa, H. Any future for faecal microbiota transplantation as a novel strategy for gut microbiota modulation in human and veterinary medicine? Life. (2022) 12:723. doi: 10.3390/life12050723
55. Muegge, BD, Kuczynski, J, Knights, D, Clemente, JC, Gonzalez, A, Fontana, L, et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. (2011) 332:970–4. doi: 10.1126/science.1198719
56. Delsuc, F, Metcalf, JL, Wegener Parfrey, L, Song, SJ, González, A, and Knight, R. Convergence of gut microbiomes in myrmecophagous mammals. Mol Ecol. (2014) 23:1301–17. doi: 10.1111/mec.12501
57. Ley, RE, Hamady, M, Lozupone, C, Turnbaugh, PJ, Ramey, RR, Bircher, JS, et al. Evolution of mammals and their gut microbes. Science. (2008) 320:1647–51. doi: 10.1126/science.1155725
58. Bjorndal, KA. Fermentation in reptiles and amphibians In: RI Mackie and BA White, editors. Gastrointestinal Microbiology. Boston, MA: Springer (1997). 199–230.
59. Yuan, ML, Dean, SH, Longo, AV, Rothermel, BB, Tuberville, TD, and Zamudio, KR. Kinship, inbreeding and fine-scale spatial structure influence gut microbiota in a hindgut-fermenting tortoise. Mol Ecol. (2015) 24:2521–36. doi: 10.1111/mec.13169
60. Lance, VA, and Morafka, DJ. Post natal lecithotroph: a new age class in the ontogeny of reptiles. Herpetol Monogr. (2001) 15:124–34. doi: 10.2307/1467040
61. Joshua, QI, Hofmeyr, MD, and Henen, BT. Seasonal and site variation in angulate tortoise diet and activity. J Herpetol. (2010) 44:124–34. doi: 10.1670/08-306R1.1
62. Besharat, S, Besharat, M, and Jabbari, A. Wild lettuce (Lactuca virosa) toxicity. BMJ Case Rep. (2009) 2009:bcr0620080134. doi: 10.1136/bcr.06.2008.0134
63. Nozohour, Y, Jalilzadeh-Amin, G, and Maham, M. First case report of toxicity with Lactuca virosa in a lamb. Fut Nat Prod. (2021) 6:56–62.
64. Schramm, R. LandschildkrötenFutterpflanzen: das Bestimmungsbuch im Taschenformat / Ricarda Schramm. Grebenhain: Tartaruga-Verlag (2017).
65. Salapovic, H, Geier, J, and Reznicek, G. Quantification of sesquiterpene lactones in Asteraceae plant extracts: evaluation of their allergenic potential. Sci Pharm. (2013) 81:807–18. doi: 10.3797/scipharm.1306-17
66. Merfort, I. Review of the analytical techniques for sesquiterpenes and sesquiterpene lactones. J Chromatogr A. (2002) 967:115–30. doi: 10.1016/S0021-9673(01)01560-6
67. Selleri, P, and Hernandez-Divers, SJ. Renal diseases of reptiles. Vet Clin North Am Exot Anim Pract. (2006) 9:161–74. doi: 10.1016/j.cvex.2005.10.008
68. Holz, P. Diseases of the urinary tract In: B Doneley, D Monks, and BC Robert Johnson, editors. Reptile medicine and surgery in clinical practice. Hoboken, NJ: John Wiley & Sons (2017). 323–30.
69. Rezaie, P, Bitarafan, V, Horowitz, M, and Feinle-Bisset, C. Effects of bitter substances on GI function, energy intake and glycaemia-do preclinical findings translate to outcomes in humans? Nutrients. (2021) 13:1317. doi: 10.3390/nu13041317
70. Qiao, K, Zhao, M, Huang, Y, Liang, L, and Zhang, Y. Bitter perception and effects of foods rich in bitter compounds on human health: a comprehensive review. Food Secur. (2024) 13:3747. doi: 10.3390/foods13233747
Keywords: reptile, tortoise, fecal microbiota transplantation, FMT, chronic gastrointestinal disease
Citation: Hetterich J and Pees M (2025) Case Report: Oral fecal microbiota transplantation in a Mediterranean spur-thighed tortoise (Testudo graeca) suffering from chronic gastrointestinal disease—procedure, clinical outcome and follow-up. Front. Vet. Sci. 12:1560689. doi: 10.3389/fvets.2025.1560689
Edited by:
Ferran Solanes Vilanova, Ghent University, BelgiumReviewed by:
Alessandro Vetere, University of Parma, ItalyFranziska Sandmeier, Colorado State University Pueblo, United States
Copyright © 2025 Hetterich and Pees. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johannes Hetterich, am9oYW5uZXMuaGV0dGVyaWNoQHRpaG8taGFubm92ZXIuZGU=
 Johannes Hetterich
Johannes Hetterich Michael Pees
Michael Pees