- 1Momo Group Inc, Seongnam-si, Republic of Korea
- 2CORbio, Seoul, Republic of Korea
- 3Seijo Kobayashi Veterinary Clinic, Setagaya, Tokyo, Japan
- 4School of Veterinary Medicine, Nippon Veterinary and Life Science University, Musashino, Tokyo, Japan
Introduction: The prevalence of lipid metabolism disorders, including obesity, increases with age in cats and humans. Obesity is a condition characterized by systemic low-grade inflammation and oxidative stress caused by excessive visceral fat accumulation. Resveratrol (RSV), a natural plant polyphenol, modulates the expression of anti-inflammatory factors. This study aimed to investigate the effects of resveratrol supplementation on lipid metabolism in both healthy and obese cats and assess its potential as a dietary supplement for improving lipid metabolism disorders in this population.
Methods: Plasma metabolite and hormone concentrations, and enzyme activities were measured in healthy, obese, and overweight cats supplemented with RSV for 4 weeks. RVS was supplemented at 1 mg/kg body weight/day (low dose) and 5 mg/kg/day (high dose) in capsules for 4 weeks.
Results: Body weight, body condition score, BUN, and insulin concentrations did not change in obese or overweight cats with RSV supplementation for 4 weeks. Plasma triglyceride, free fatty acids, and serum amyloid A (SAA) concentrations and lactate dehydrogenase (LDH) activities decreased, and adiponectin concentrations increased markedly in obese and overweight cats after RSV supplementation.
Discussion: Decreased plasma SAA concentrations and LDH activities and increased plasma adiponectin concentrations in obese and overweight cats seem to be induced by the improvement in liver function and the anti-inflammatory effect of RSV. Moreover, RSV supplementation may be useful in treating lipid metabolism disorders, including obesity, in cats.
1 Introduction
The prevalence of lipid metabolism disorders, including obesity, increases with age in cats and humans (1, 2). Obesity is due to excessive triglyceride (TG) accumulation in the adipose tissue (AT) caused by an energy imbalance in which energy intake exceeds energy expenditure. When AT reaches its maximum capacity for energy storage, it releases free fatty acids (FFA), causing ectopic lipid deposition in other tissues, such as the liver, skeletal muscle, and vasculature. AT is characterized by increased macrophage infiltration during obesity development (3, 4). Consequently, AT macrophages secrete high levels of proinflammatory cytokines, resulting in obesity-associated chronic low-grade inflammation and impaired insulin signaling (5). Obesity and insulin resistance change with advancing age and are linked to chronic low-grade inflammation, leading to age-related systemic metabolic dysfunction, physical limitation, and frailty (6).
Cats are more prone to obesity than dogs because of their unique glucose and lipid metabolic characteristics (7, 8). In feline livers, glucokinase, the rate-limiting enzyme in glycolysis, is lacking (7), and gluconeogenic enzyme activities are higher than that in canine livers (9). Additionally, the expression levels of mRNA associated with insulin signaling pathway, including insulin receptor substrate (IRS)-1, IRS-2, phosphatidylinositol 3-kinase (PI3K) P-85 α, are significantly lower in cats than those in dogs (8). Furthermore, the secretion of adiponectin, an adipokine that improves insulin sensitivity, is lower in cats in the normal state (8) and with weight gain (10).
Obesity is a condition characterized by systemic low-grade inflammation and oxidative stress caused by excessive visceral fat accumulation. Weight reduction through energy restriction and increased physical activity are key strategies for obesity prevention (11). Moreover, the consumption of foods rich in bioactive anti-inflammatory compounds such as polyphenols has been shown to reduce inflammation (12). Resveratrol (RSV), a natural polyphenol found in plants such as peanuts, grapes, and strawberries (13), modulates the expression of pro-and anti-apoptotic factors, neutralizes free radical species, affects mitochondrial function, chelates redox-active transition metal ions, and prevents protein aggregation (14).
This study aimed to investigate the effects of resveratrol supplementation on lipid metabolism in both healthy and obese cats and assess its potential as a dietary supplement for improving lipid metabolism disorders in this population.
2 Methods
2.1 Animals
Cats are kept in two branches, Nabiya Sarang Ha and Dong Go Dong Rak, of Korean Private Shelters (Seoul, South Korea). Nabiya Sarangha Branch has 300 cats (70% male, 30% female; domestic short-haired cats, 2–11 years old), and Dong Go Dong Rak Branch has 150 cats (80% male, 20% female; domestic short-haired cats, 2–113 years old). All the cats examined were kept individually in cages and provided the same condition for 4 weeks with environmental maintained at 25.0 ± 2.0°C and 55.0 ± 10.0% relative humidity, and on a 13:11h, light: dark cycle (light on 8 a.m. to 9:00 p.m.). All cats were fed with a commercial diet, Royal Canin Feline Health Nutrition Indoor 27 Cat Dry Food (Royal Canin Korea, Seoul, South Korea), two times a day at 9:00 a.m. and 8:00 p.m. and free access to water. Body weight (BW) and body condition score (BCS) were measured before (week 0) and 4 weeks after supplementation (week 4). BCS was assessed using a 5-point scale system (15, 16) as follows: BCS1, very thin; BCS2, underweight; BCS3, ideal; BCS4, overweight; BCS5, obese. A total of 12 clinically healthy cats (domestic short-haired cats, six females and six males, aged 3–7 years) and 4 obese cats (domestic short-haired cats, three males and one female, aged 7–8 years) were used in this study.
2.2 Resveratrol supplementation
Healthy cats were divided into three groups: control without RVS supplementation (2 males, 2 females; 4–6 years old), RSV low-dose group (1 mg/kg body weight/day; 2 males, 2 females; 4–6 years old), and RSV high-dose group (5 mg/kg/day; 2 males, 2 females; 3–5 years old). Powdered RSV (Trans Resveratrol Powder Pure Bulk) was purchased from PureBulk Inc. (Roseburg, OR, USA), and packed in capsules (Suheung Capsule Co., Ltd., Seoul, South Korea). Capsule filled with and without the required amount of powdered RSV were administered orally to each cat with diet at 9:00 a.m. every day for 4 weeks.
2.3 Blood sampling and metabolite, hormone, and enzyme assay
A total of 1.5 mL of blood was collected from the jugular vein of each animal into heparinized tubes before (0 weeks) and 4 weeks after RVS supplementation. Blood collection was performed before the morning feeding and the collected samples were centrifuged at 2,000 × g for 5 min at 4°C to obtain plasma. The plasma samples were stored at−25°C until use. Plasma glucose, TG, total cholesterol (TC), blood urea nitrogen (BUN), creatinine, alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) activities were measured using an autoanalyzer (Catalyst One, IDXX, Westbrook, Maine 04092 USA). Plasma-free fatty acid (FFA) and serum amyloid A (SAA) concentrations were measured using the NEFA Test Wako (Wako Pure Chemical, Tokyo, Japan) and VET-SAA kits (Eiken Chemical Co., Tokyo, Japan), respectively. The SAA concentration is an inflammatory marker in cats (17). Adiponectin and insulin concentrations were measured using commercial ELISA kits a mouse/rat adiponectin ELISA kit (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) and a rat insulin ELISA kit (AKRIN-010T; Shibayagi Co., Gunma, Japan), respectively.
2.4 Statistical analysis
All measured values are expressed as means ± standard deviation (SD). Statistical significance was determined using the paired t-test. The significance level was set at p < 0.05. Statistical analyses were performed using Microsoft Excel.
3 Results
Table 1 shows the changes in metabolite and hormone concentrations and enzyme activities in the plasma of healthy cats supplemented with RSV for 4 weeks. All values measured in healthy control cats without RSV supplementation were maintained within the reference values for 4 weeks. BW, BCS, glucose, TG, TC, BUN, creatinine, insulin concentrations, and ALT activities in healthy cats (low-dose and high-dose groups) did not change after 4 weeks of RVS supplementation. Plasma FFA and SAA concentrations and LDH activities decreased, whereas plasma adiponectin concentrations increased significantly in the RSV low-dose group. These changes were not in dose-dependent manner upon RSV supplementation in healthy cats.
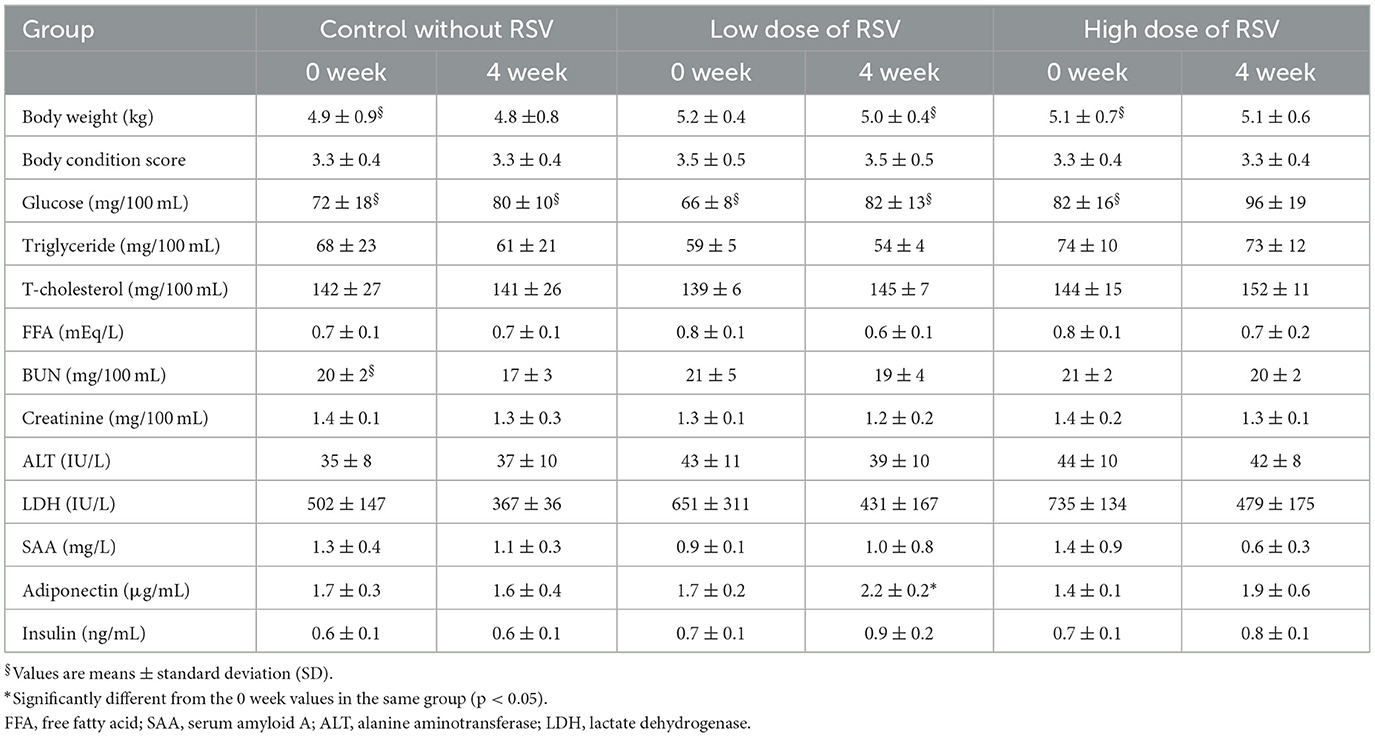
Table 1. Changes in fasting plasma metabolites and hormone concentrations and enzyme activities in healthy cats supplemented with resveratrol.
Table 2 shows the changes in metabolite and hormone concentrations and enzyme activities in the plasma of obese and overweight cats supplemented with RSV. BW, BCS, BUN, and insulin concentrations did not change in obese and overweight cats supplemented with RSV at 5 mg/kg/day (high dose) for 4 weeks. Plasma glucose concentrations were increased in three cats and creatinine concentrations were decreased in all four cats. Plasma TG, FFA, and SAA concentrations and LDH activities decreased remarkably in the four cats after RSV supplementation. In contrast, the plasma adiponectin concentrations increased remarkably in the four cats after RSV supplementation.
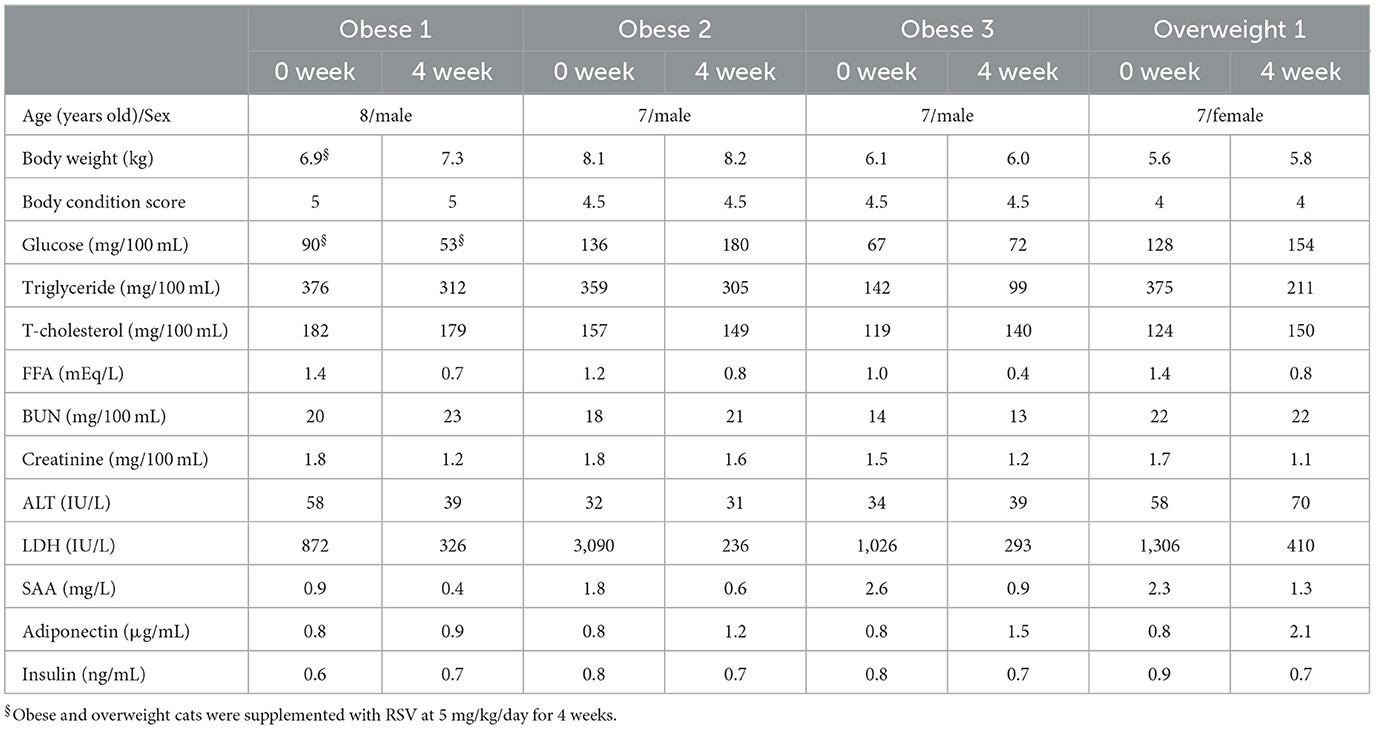
Table 2. Changes in fasting plasma metabolites and hormones concentrations and enzyme activities in obese cats supplemented with resveratrol.
4 Discussion
This study aimed to investigate the effects of resveratrol supplementation on lipid metabolism in both healthy and obese cats and assess its potential as a dietary supplement for improving lipid metabolism disorders in this population. Remarkable changes in LDH activities and an increase in adiponectin concentrations were observed in healthy cats supplemented with RSV. TG and FFA concentrations were maintained within the reference ranges. These results indicate that RSV supplementation improves liver function and lipid metabolism in cats. The response to RSV supplementation was not in dose-dependent manner. In obese and overweight cats, RSV supplementation markedly improved lipid metabolism (decreased plasma TG and FFA concentrations) and exerted anti-inflammatory effects (decreased plasma SAA concentration). These changes are thought to be due to liver function improvement (decrease in plasma LDH activity) and an increase in plasma adiponectin concentrations.
Resveratrol has anti-inflammatory (18), antioxidant (19), anti-obesogenic (20, 21), and anti-diabetic effects (22, 23). Furthermore, RSV is hypothesized to be potentially useful in protecting against factors that increase the susceptibility to metabolic syndrome (24, 25). A moderate dose of resveratrol activates Sirtuin 1 (NAD-dependent deacetylase sirtuin-1) followed by activating AMP activated protein kinase (AMPK), whereas a high dose of RSV activates AMPK in a Sirtuin 1-independent manner (26). AMPK activation induces amelioration of glucose and lipid metabolism in livers resulting in reduction of glucose and TG concentrations in obese animals (27, 28). RSV supplementation induces decreasing in the plasma glucose and TG concentrations in obese animals, but in cats supplemented with RSV, plasma glucose concentrations increased in this study. This change was not statistically significant. On the other hand, in some types of cancer cells, Sirtuin 1 activation is able to inhibit glucose uptake (29). The reason of increased plasma glucose concentrations observed in cats supplemented with RSV should be further studied in many cats including expression of associated genes.
In obese and overweight cats, a clear improvement in liver function, such as a decrease in LDH activity, was observed after RSV supplementation. RSV supplementation ameliorates liver function in animals with severe liver dysfunction like non-alcoholic fatty liver disease (NAFLD) (30, 31). Changes in cholesterol concentrations and ALT activities may associate with liver function improvement caused by RSV supplementation. Moreover, there are many reports that RSV supplementation improves hypertension and kidney dysfunction in rodents with chronic kidney disease (CKD) (32, 33). RSV supplementation may ameliorate kidney function leading decreased plasma creatinine concentrations in the obese cat.
RSV supplementation increases plasma adiponectin concentrations in obese and overweight cats. Obesity induces adipocyte senescence followed by a senescence-associated secretory phenotype (SASP) like decreased adiponectin secretion, which triggers a cascade of reactions leading to inflammation, insulin signaling disruption, insulin resistance, and severe metabolic disorders (34, 35). In contrast, RSV shows potent anti-SASP and anti-inflammatory activities (36, 37) and improves hepatic function (38). Decreased plasma SAA concentrations and LDH activities and increased plasma adiponectin concentrations in obese cats seemed to be induced by the improvement in liver function and the anti-SASP effect of RSV (39). Moreover, RSV supplementation may be useful for treating lipid metabolism disorders, including obesity, and may be effective in preventing age-related diseases in cats.
This study is preliminary, and its limitations include the small number of samples and biological and environmental variables (age, sex, diet, and others). The optimal dose and duration of RSV supplementation for cats have not been sufficiently examined. More animal studies are needed to assess the precise effect of RSV supplementation. In particular, long-term effect of RSV supplementation in obese cats should be investigated in detail in animals with different severities of obesity. Moreover, further studies are needed to evaluate the usefulness of RSV as anti-obesity supplement in a large number of animals.
5 Conclusion
Healthy, obese, and overweight cats were supplemented with RSV for 4 weeks. Plasma metabolite and hormone concentrations, and enzyme activities were measured in cats before (0 weeks) and 4 weeks after RSV supplementation. RVS was administered as a capsule at a dose of 1 mg/kg body weight/day (low-dose group) or 5 mg/kg/day (high-dose group) for 4 weeks. Body weight, BCS, BUN, and insulin concentrations did not change in obese or overweight cats with RSV supplementation (high dose, 5 mg/kg/day) for 4 weeks. Plasma TG, FFA, and SAA concentrations, and LDH activity decreased remarkably in obese and overweight cats after RSV supplementation. Plasma adiponectin concentrations increased markedly in all four cats after RSV supplementation. Decreased plasma SAA concentrations and LDH activity and increased plasma adiponectin concentrations in obese and overweight cats seemed to be induced by the improvement in liver function and the anti-SASP effect of RSV. Moreover, RSV supplementation may be useful in the treatment of lipid metabolism disorders, including obesity, in cats.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Momo Group Research Animal Ethical Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JEY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft. SRK: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft. JYK: Investigation, Resources, Writing – review & editing. HJK: Formal analysis, Investigation, Methodology, Writing – review & editing. MK: Investigation, Validation, Writing – review & editing. TA: Conceptualization, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank the staff at Nabiya Sarang Ha Branch and Dong Go Dong Rak Branch (Korean Privet Shelter, Seoul, Republic of Korea) for their careful monitoring of the animals to ensure their safety and for blood sampling.
Conflict of interest
JEY, SRK, and JYK were employed by Momo Group Inc. HJK was employed by company CORbio.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lund EM, Armstrong PJ, Kirk CA, Klausner JS. Prevalence and risk factor for obesity in adult dogs from private US veterinary practices. Int Appl Res Vetmed. (2006) 4:177–86.
2. Chandler M, Cunningham S, Lund EM, Khanna C, Naramore R, Patel A, et al. Obesity and associated comorbidities in people and companion animals. A one health perspective. J Comp Pathol. (2017) 156:296–309. doi: 10.1016/j.jcpa.2017.03.006
3. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. (2003) 112:1821–30. doi: 10.1172/JCI200319451
4. Castoldi A, Naffah de. Souza C, Câmara NOS, Moraes-Vieira PM. The macrophage switch in obesity development. Front Immunol. (2015) 6:637. doi: 10.3389/fimmu.2015.00637
5. Gao D, Madi M, Ding C, Fok M, Steele T, Ford C, et al. Interleukin-1beta mediated macrophage-induced impairment of insulin signaling in human primary adipocytes. Am J Physiol Endocrinol Metab. (2014) 307:E289–304. doi: 10.1152/ajpendo.00430.2013
6. Stout MB, Justice JN, Nicklas BJ, Kirkland JL. Physiological aging: links among adipose tissue dysfunction, diabetes and frailty. Physiology. (2017) 32:9–19. doi: 10.1152/physiol.00012.2016
7. Tanaka A, Inoue A, Takeguchi A, Washizu T, Bonkobara M, Arai T. Comparison of expression of glucokinase gene and activities of enzymes related to glucose metabolism in livers between dogs and cats. Vet Res Commun. (2005) 29:477–85. doi: 10.1007/s11259-005-1868-1
8. Mori A, Lee P, Takemitsu H, Sako T, Arai T. Comparison of insulin signaling gene expression in insulin sensitive tissues between cats and dogs. Vet Res Commun. (2009) 33:211–26. doi: 10.1007/s11259-008-9168-1
9. Washizu T, Tanaka A, Sako T, Washizu M, Arai T. Comparison of the activities of enzymes related to glycolysis and gluconeogenesis in the liver of dogs and cats. Res Vet Sci. (1999) 67:205–6. doi: 10.1053/rvsc.1998.0305
10. Verkest KR, Rand JS, Fleeman LM, Morton JM, Richards AA, Rose FJ, et al. Distinct adiponectin profiles might contribute to differences in susceptibility to type 2 diabetes in dogs and humans. Domest Anim Endocrinol. (2011) 41:67–73. doi: 10.1016/j.domaniend.2011.03.003
11. Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch Intern Med. (2002) 162:1286–92. doi: 10.1001/archinte.162.11.1286
12. Grosso G, Laudisio D, Frias-Toral E, Barrea L, Muscogiuri G, Savastano S, et al. Anti-inflammatory nutrients and obesity-associated metabolic-inflammation: state of the art and future direction. Nutrients. (2022) 14:1137. doi: 10.3390/nu14061137
13. Zhang D, Zhang J, Zeng J, Li Z, Zuo H, Huang C, et al. Nano-gold loaded with resveratrol enhance the anti-hepatoma effect of resveratrol in vitro and in vivo. J Biomed Nanotechnol. (2019) 15:288–300. doi: 10.1166/jbn.2019.2682
14. Yessenkyzy A, Saliev T, Zhanaliyeva M, Masoud AR, Umbayev B, Sergazy S, et al. Polyphenols as caloric-restriction mimetics and autophagy inducers in aging research. Nutrients. (2020) 12:1344. doi: 10.3390/nu12051344
15. Rand JS. Current understanding of feline diabetes mellitus: part 1, pathogenesis. J Feline Med Surg. (1999) 1:143–53. doi: 10.1016/S1098-612X(99)90203-6
16. Nelson RW, Reusch CE. Animal models of disease: Classification and etiology of diabetes in dogs and cats. J Endocrinol. (2014) 222:T1–9. doi: 10.1530/JOE-14-0202
17. Yuki M, Inden T, Hirano T, Naito E, Taira H, Yokota S, et al. Comparison of polyclonal and monoclonal antibody assays for serum amyloid A in cats: a study based on an automated turbidimetric immunoassay in a primary care veterinary hospital. Am J Vet Res. (2024) 85:ajvr:ajvr.24.03.0067. doi: 10.2460/ajvr.24.03.0067
18. Meng T, Xiao D, Muhammed A, Deng J, Chen L, He J. Anti-inflammatory action and mechanisms of resveratrol. Molecules. (2021) 26:229. doi: 10.3390/molecules26010229
19. Truong V-L, Jun M, Jeong W-S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. BioFactors. (2018) 44:36–49. doi: 10.1002/biof.1399
20. Franco JG, Lisboa PC, da Silva Lima N, Peixoto-Silva N, Maia LA, Oliveira E, et al. Resveratrol prevents hyperleptinemia and central leptin resistance in adult rats programmed by early weaning. Horm Metab Res. (2014) 46:728–35. doi: 10.1055/s-0034-1375688
21. Martel J, Ojcius DM, Chang C-J, Lin C-S, Lu C-C, Ko Y-F, et al. Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nat Rev Endocrinol. (2017) 13:149–60. doi: 10.1038/nrendo.2016.142
22. Zhu X, Wu C, Qiu S, Yuan X, Li L. Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: systematic review and meta-analysis. Nutr Metab. (2017) 14:60. doi: 10.1186/s12986-017-0217-z
23. Zheng S, Feng Q, Cheng J, Zheng J. Maternal resveratrol consumption and its programming effects on metabolic health offspring mechanisms and potential implications. Biosci Rep. (2018) 38:BSR20171741. doi: 10.1042/BSR20171741
24. Tain Y-L, Hsu C-N. Developmental programming of the metabolic syndrome: can we reprogram with resveratrol? Int J Mol Sci. (2018) 19:2584. doi: 10.3390/ijms19092584
25. Hsu M-H, Chen Y-C, Sheen J-M, Huang L-T. Maternal obesity programs offspring development and resveratrol potentially reprograms the effects of maternal obesity. Int J Environ Res Public Health. (2020) 17:1610. doi: 10.3390/ijerph17051610
26. Price NL, Gomes AP, Ling AJY, Duarte FV, Martin-Montalvo A, North BJ, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. (2012) 15:675–90. doi: 10.1016/j.cmet.2012.04.003
27. Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. (2016) 48:e245. doi: 10.1038/emm.2016.81
28. Musi N, Goodyear LJ. AMP-activated protein kinase and muscle glucose uptake. Acta Physiol Scand. (2003) 178:337–45. doi: 10.1046/j.1365-201X.2003.01168.x
29. Azevedo C, Correia-Brando A, Araujo JR, Guimaraes JT, Keating E. Martel F. The chemopreventive effect of the dietary compound kaempferol on the MCF-7 human breast cancer cell line is dependent on inhibition of glucose cellular uptake. Nutr Cancer. (2015) 67:504–13. doi: 10.1080/01635581.2015.1002625
30. Jakubczyk K, Skonieczna-Żydecka K, Kałduńska K, Stachowska E, Gutowska I, Janda K. Effects of resveratrol supplementation in patients with non-alcoholic fatty liver disease—A meta-analysis. Nutrients. (2020) 12:2435. doi: 10.3390/nu12082435
31. Yang K, Chen J, Zhang T, Yuan X, Ge A, Wang S, et al. Efficacy and safety of dietary polyphenol supplementation in the treatment of non-alcoholic fatty liver disease: a systemic review and meta-analysis. Front Immunol. (2022) 13:949746. doi: 10.3389/fimmu.2022.949746
32. Tain Y-L, Chang C-I, Hou C-Y, Cang-Chien G-P, Lin S, Hsu C-N. Dietary resveratrol butyrate monoester supplement improves hypertension and kidney dysfunction in a young rat chronic kidney disease model. Nutrients. (2023) 15:635. doi: 10.3390/nu15030635
33. Zheng C-M, Hou Y-C, Tsai K-W, Hu W-C, Yang H-C, Liao M-T, et al. Resveratrol mitigates uremic toxin-induced intestinal barrier dysfunction inchronic kidney disease by promoting mitophagy and inhibiting apoptosis pathways. Int J Mol Sci. (2024) 21:2437–49. doi: 10.7150/ijms.100963
34. Ohtani N. The roles and mechanisms of senescence-associated secretory phenotype (SASP): can it be controlled by senolysis? Inflamm Regen. (2022) 42:11. doi: 10.1186/s41232-022-00197-8
35. Shimi G, Sohouli MH, Ghorbani A, Shakery A, Zand H. The interplay between obesity, immunosenescence, and insulin resistance. Immun Ageing. (2024) 21:13. doi: 10.1186/s12979-024-00414-7
36. Menicacci B, Margheri F, Laurenzana A, Chillà A, Del Rosso M, Giovannelli L, et al. Chronic resveratrol treatment reduces the pro-angiogenic effect of human fibroblast “senescent-associated secretory phenotype” on endothelial colony-forming cells: the role of IL8. J Gerontol A Biol Sci Med Sci. (2019) 74:625–33. doi: 10.1093/gerona/gly175
37. Matacchione G, Gurău F, Silvestrini A, Tiboni M, Mancini L, Valli D, et al. Anti-SASP and anti-inflammatory activity of resveratrol curcumin and β-caryophyllene association on human endothelial and monocytic cells. Biogerontology. (2021) 22:297–313. doi: 10.1007/s10522-021-09915-0
38. Ran Q, Song D, Wang Q, Wang D, Chen X, Zhang A, et al. Resveratrol alleviates arsenic exposure induced liver fibrosis in rats by inhibiting hepatocyte senescence. Biol Trace Elem Res. (2024). doi: 10.1007/s12011-024-04255-9
Keywords: adiponectin, cat, lipid metabolism, obesity, resveratrol, serum amyloid A
Citation: Yun JE, Kang SR, Kim JY, Kim HJ, Kobayashi M and Arai T (2025) Effect of resveratrol supplementation on lipid metabolism in healthy and obese cats. Front. Vet. Sci. 12:1565367. doi: 10.3389/fvets.2025.1565367
Received: 23 January 2025; Accepted: 31 March 2025;
Published: 16 April 2025.
Edited by:
Anna Maria Giudetti, University of Salento, ItalyReviewed by:
Anmol Bhandari, Guru Nanak Dev University, IndiaIlaria Serra, Sapienza University of Rome, Italy
Copyright © 2025 Yun, Kang, Kim, Kim, Kobayashi and Arai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Toshiro Arai, dG9zaGlhcmFpNzRAZ21haWwuY29t
 Jung Eun Yun1
Jung Eun Yun1 Motoo Kobayashi
Motoo Kobayashi Toshiro Arai
Toshiro Arai