- 1Department of Surgical and Radiological Sciences, School of Veterinary Medicine, University of California, Davis, Davis, CA, United States
- 2College of Veterinary Medicine, Texas A&M University, College Station, TX, United States
- 3Essex Animal Hospital, Essex, ON, Canada
- 4Department of Veterinary Clinical Sciences, Washington State University, Pullman, WA, United States
- 5Veterinary Referral Associates, Gaithersburg, MD, United States
- 6Red Sage Integrative Veterinary Partners, Fort Collins, CO, United States
- 7Vetoquinol United States, Fort Worth, TX, United States
Osteoarthritis (OA) is a ubiquitous problem affecting dog joints, particularly the hip, elbow, stifle, and spine. OA most often results from developmental orthopedic problems such as hip dysplasia, elbow dysplasia, and patellar luxation and from injuries to the cranial cruciate ligament. Several management approaches have been proposed to manage OA, including steps to modulate growth, physical activity, and exercise, nutrition and nutritional supplementation, medications, physical rehabilitation, and surgical procedures. This article is the first in a series of articles that propose steps for practical OA management in dogs at various life stages. The review presented here focuses on growing dogs. The text describes the early pathophysiology and diagnosis of OA. The physical, nutritional, analgesic, and surgical management options of OA in growing dogs are presented. The application of these management options is described for three dogs. The overall approach to the management of OA in growing dogs is discussed.
Introduction
Clients wish for a full and unrestricted life for their dogs. Osteoarthritis (OA) threatens their quality of life because it is the most common orthopedic condition observed in dogs and it is often associated with severe chronic joint pain (1). In a large study of more than 450,000 dogs, the annual period prevalence of OA was 2.5% (2). In that study, risk was increased when dogs were neutered, heavier, and older than 8 years.
The impact of OA in dogs can be physical, behavioral, and social (3). Physically, limb use, mobility, and function during daily activities can be compromised (4). Behaviorally, dogs with chronic pain often express problem behavior (5). Socially, OA can create major life challenges for the owner-animal bond because it negatively impacts the owners’ lives, including creating physical and mental hardships and financial challenges (6). The career longevity of working and sporting dogs with OA is also negatively impacted (7–10). The lifespan of dogs with OA is also shortened by approximately 2 years (11).
No single solution can be used to manage or cure OA and its impact. However, practical management steps and whole-body wellness can positively impact the life of dogs with OA. Whole-body wellness for dogs can be defined as increased owner mindfulness about general aspects of their dog’s health, including the dog’s lifestyle, nutrition, and exercise (4, 12). Whole-body wellness is relevant at all life stages: puppyhood, adulthood, and life as senior dogs. Beyond its positive impact on the life of all dogs and their owners, whole-body wellness enhances the management of OA. This is because lifestyle, nutrition, and exercise lead to increased owner awareness of their dog and increased dog fitness and strength. For dogs with OA, a multimodal management plan is more effective than any single management step (13). Multimodal therapy can include whole-body wellness, physical rehabilitation, nutraceutical and supplements, and pharmaceuticals, as well as surgical intervention, when appropriate (14). Pharmaceuticals may be administered orally, systemically or intraarticularly depending on the stage of OA and the specific needs of the patient.
This review presents practical guidance for preventing and managing OA in dogs during the first life stage. The text describes and discusses the use of all OA management options, including physical, nutritional, and analgesic management of OA in growing dogs. The application of these management options in 3 classic cases is also included.
OA pathophysiology
Canine osteoarthritis (OA) is a common problem in dogs characterized by irreversible and progressive degenerative changes to joints leading to pain and disability (15). The prevalence of OA in dogs is not known. An estimated OA prevalence of 20% is reported in the literature, based on the results of a survey of 200 veterinarians conducted in 1996 (15). OA is a complex syndrome that can result from mechanical and biological factors. It has been described as the consequence of abnormal forces applied to joints with normal physiology or from normal forces applied to joints with abnormal physiology (16). OA includes inflammation (17), the degeneration of articular surfaces, changes in the synovial membrane (18), changes in subchondral bone (19), and bone production at the edges of the articular surface and at the origin of intraarticular tendons (20). The combination of these changes leads to loss of joint function and disability. Loss of joint function may include pain, changes in joint alignment, and changes in joint motion. Disability may result from chronic pain (21), loss of strength (22), loss of fitness, and loss of mobility resulting from the loss of joint function (23). The progression rate and clinical signs of OA in dogs vary widely. Clinical signs can be severe at a young age in some dogs.
Overall, the discovery of OA in growing dogs is relatively uncommon because OA is seldomly detected based on its common triggers (joint subluxation or joint instability). Instead, OA appears to be discovered because of the synovial or subchondral bone inflammation that results from joint subluxation and instability. Inflammation leads to pain. Acute pain leads to limb disuse and chronic pain (16). Chronic pain leads to a long-term physical and functional signs, including a change in demeanor (loss of playfulness, increased introversion, loss of willingness to exercise), a loss of limb function, and in severe cases, a loss of mobility (24). Subjectively in growing dogs, the signs of OA are most often linked to inflammation, acute pain, and limb disuse.
Diagnosis of OA
The early diagnosis of OA in dogs is challenging but important. The early diagnosis of OA in dogs presents key advantages over a late diagnosis. From a pathophysiologic viewpoint, the inflammatory component of OA may be severe in the early stage of the problem. An early diagnosis of OA brings the opportunity to alleviate joint inflammation. This may limit the progression of the problem. The diagnosis of OA most often relies on the presence of osteophytes and enthesophytes (new bone formation at the insertion site of musculoskeletal soft tissues). However, in puppies with joint disease, the main abnormal finding in the joint is often joint subluxation. Subluxation often leads to the development of OA within weeks to months (25, 26). Therefore, it is logical to diagnose clinical OA as soon as the presence of subluxation is observed.
Some forms of OA appear to be more readily diagnosed than others. For example, OA from an osteochondral fragment in the stifle or tibiotarsal joint is likely to be diagnosed promptly because of severe lameness and joint effusion. Other forms of OA are less likely to be diagnosed promptly. Hip dysplasia in dogs with transient hip subluxation and bilateral elbow dysplasia may not have a severe impact on gait in the short term and may not be readily confirmed on radiographs because hip subluxation is intermittent and because elbow subluxation may be impossible to document on radiographs before the development of osteophytes. Delays in the identification of OA may reflect a certain lack of attention to the dog’s gait and joint comfort. The use of the Ortolani sign during hip joint palpation may be required to diagnose hip subluxation, before the onset of hip OA. For dogs with patellar luxation, the OA diagnosis may be delayed when the luxation is more intermittent. Dogs with severe luxation are readily identified because they may be non-weight bearing (unilateral luxation) or may have a crouched posture and limited use of their pelvic limbs (bilateral luxation). Conversely, dogs with milder forms of patellar luxation are diagnosed later in life and have more severe OA than younger dogs with patellar luxation (27).
From a therapeutic viewpoint, clinical signs are much more limited in early OA compared to chronic OA and are, therefore, easier to control. Pain in early OA is most often acute rather than chronic. The impact of pain, referred to in pain physiology as the neuropathobiological signature of pain, is simpler and more limited in acute pain than chronic pain. Acute pain is focal. Chronic pain is focally more severe, due to local sensitization and allodynia. Central pain is present due to central sensitization (28). Practically, the simpler neuropathobiological pain signature means that fewer drugs and fewer non-drug-based pain-relieving steps (such as manual therapy and electrophysical modalities) will likely be required to control pain and medical therapy will be required for a shorter period. Pain may reemerge after the end of medical therapy and pulsed medical therapy may be required. Also, mobility and function are rarely compromised in puppies with OA. Therefore, ambulation assistance and advanced therapeutic exercises such as underwater treadmill therapy are unlikely to be needed when OA is diagnosed early and managed promptly. The opportunity to modulate growth by adjusting food intake, to control the timing of neutering, and to prevent excessive weight gains are other key advantages of the early diagnosis of OA in dogs. Also, and subjectively, owner education regarding OA pathophysiology and management can be more progressive when the disease is diagnosed early. Early OA and its management are simpler than late-stage OA for owners to understand and manage. It may also be easier to adopt a healthy long-term exercise strategy when OA is diagnosed in a puppy because bad habits may be harder to break, and good habits may be harder to learn when OA is diagnosed or managed later in life.
Whole body wellness
Whole-body wellness is important in puppies, it requires regular physical examinations, deworming, protection against ectoparasites and close monitoring of growth and body weight (29). It is critically important not to overfeed puppies because overfeeding (i.e., excess energy intake) accelerates growth and promotes excess weight, leading to an increase in the severity and impact of OA in dogs (30). Increased body weight could promote the progression of OA through multiple mechanisms, it increases the forces resisted by joints, it alters joint motion, and it leads to a systemic inflammatory response (31, 32).
Studies have demonstrated that rapid weight gain during the first year of life is detrimental to skeletal development (33–35). Neutering (ovariohysterectomy or castration) should be discussed, particularly when a puppy has OA because of a genetic disease, like hip or elbow dysplasia, and should therefore not be bred. Neutering offers clear health benefits to dogs, including a lower risk of developing several tumor types (36). However, neutering is also associated with an increased risk of weight gain after surgery (29) and with an increased risk of being diagnosed with orthopedic problems such as a cruciate ligament injury (37) or OA (38). The mechanisms linking neutering and the risk of OA are not clear. They may be mediated by excess weight, hormonal changes, or other mechanisms. Consequently, there is no optimal age to neuter dogs and cats. The decision is based upon breed, body size, predisposition to specific diseases, and other factors (39).
Because OA progresses more rapidly in overweight dogs, the prevention of excess weight is key to lowering the likelihood of rapid OA progression (24). Food plays a complex role in the relationship between owners and their pets (40, 41), it is therefore important to set up a healthy nutrition pattern in puppies (42). Also, because weight loss programs do not always succeed, an emphasis must be placed on avoiding the initial gain of excess weight. A large-scale study of client owned puppies showed that puppies younger than 6 months had energy intake requirements that were approximately 80% of the 2006 National Research Council (NRC) recommendations and approximately 88% of the NRC recommendations in older puppies, suggesting that the NRC guidelines may overestimate nutritional needs (30).
Multiple complete and balanced diets exist for the feeding of puppies. Despite this, some owners elect to make their pet’s food at home often without the consultation of a veterinarian, or veterinary nutritionist. Home prepared diets carry the risk of nutrient insufficiencies, excesses, and imbalances (43). This is particularly concerning for large breed puppies who are at risk of developmental orthopedic disease (DOD). Hip dysplasia and osteochondrosis make up many musculoskeletal diseases, with a possible nutritional etiology reported in the literature (43, 44). The most critical period for the development of DOD occurs before epiphyseal closure during the growth phase. Risk factors that increase the risk of DOD in young dogs include unrestricted feeding, overfeeding high energy foods, feeding foods, treats, or supplements with excess calcium, and being a large or giant breed (43).
Puppies have higher protein, essential fatty acid (EFA) and mineral requirements (44). High protein diets that have been balanced for large breed puppies do not negatively affect skeletal development but will mildly affect growth rate (44). Increased levels of docosahexaenoic acid (DHA) can improve retinal development, learning, and memory in puppies. Particularly for large breed puppies, it is recommended to feed a diet that controls calcium and calorie intake and maintains a 2:1 to 1:1 calcium to phosphorus ratio. Unbalanced diets, diets with too much or too little calcium, or all meat diets without bone elements predispose puppies to DOD, osteopenia, and pathological fractures. In general, large-breed puppy food should contain a range of 2.0 to 4.5 g calcium/1000 kcal (45). Virtually all commercial diets will contain this minimum amount of calcium; however, some all-life stage diets contain significantly more than the maximum. Although when reading a bag or can of dog food, adult foods look similar to many large breed puppy formulations, however, feeding adult maintenance diets to puppies is not recommended as these foods will be a deficient in trace minerals or vitamins or have excessive calcium that may affect growth (44).
When feeding large or giant breed puppies, the goal should be moderate energy restriction. From weaning to 4 months, puppies should be fed three times their resting energy requirement (RER), then two to 2.5 times the RER until 6 to 12 months of age, depending on the breed (45). In general, most puppies kept in good body condition will escalate calorie consumption until 4 to 6 months of age at which time they reach peak caloric consumption and rarely need more calories to complete proper growth to adulthood (45, 46). This escalating caloric consumption likely extends to 8 to 10 months in giant breed dogs. All breeds can be switched to adult maintenance formulas at 1 year of age. Ad libitum feeding and overnutrition contribute to the development of OA, as this places excess stress on potentially genetically abnormal joints (43, 44). Controlled caloric intake should continue for life of the dog to avoid abnormal stresses on joints.
Maintenance of lean body weight throughout the pet’s life, starting with the growth phase, is critical for longevity and delaying the development of OA. A 14-year lifespan study in Labrador Retrievers showed that, when fed to maintain a lean body condition from puppyhood, and throughout life, dogs lived on average 1.8 years (15%) longer, than their free fed littermates (4, 11). Maintaining optimal body condition throughout life can delay the onset and reduce the severity of OA in dogs. For example, in the lifelong study of 7 litters of Labrador retrievers, when dogs were evaluated for the presence of OA in the shoulder and hip joints, 68% of the heavier dogs had hip or shoulder OA compared to 10% of slender dogs. Also, 77% of the heavier dogs had OA in 2 or 3 joints (among their shoulders and hips) compared to 10% of the slender dogs (24). Lean dogs also showed delayed onset of other chronic and age-related diseases (24). Scoring body condition using a 9-point or 5-point scale is done routinely in practice (47, 48). However, veterinarians (and owners) may underestimate body condition (49, 50) or may decide to overlook excess weight to avoid a challenging conversation with owners. Societal norms and the standards for certain dog breeds make it more challenging to keep dogs slender, complicating the issue for dogs with OA.
In early OA, clinical signs are relatively discreet and mostly result from acute joint inflammation and pain. Clinically and subjectively, dogs with early OA usually do not have a loss of muscle mass or loss of joint motion, unlike dogs with chronic OA (51). The signs of early OA tend to be more transient and easier to control than the signs of chronic OA. Osteoarthritis in growing dogs often results from hip or elbow dysplasia. In the hip, early OA most often results from hip laxity, subluxation, and secondary joint inflammation, followed by focal cartilage damage to the dorsal acetabular rim and femoral head. In the elbow, the trigger of early OA is less clear. Some attribute it to abnormal geometry of the ulnar notch and others to radioulnar subluxation. Both abnormalities lead to damage to the articular surface of the ulna and, to a lesser extent, to the medial aspect of the humeral condyle. Elbow subluxation and secondary elbow OA are also seen in chondrodystrophic dogs that have impaired growth of their long bones, particularly in the antebrachium (52). Less often, OA may result from the presence of an osteochondral fragment in a joint, such as, with osteochondritis dissecans or because of an intraarticular fracture. Clinically, it appears that OA is more likely to be detected in puppies when dogs are larger, are affected in multiple limbs, or are severely affected because the negative impact of OA is more immediate and severe in these situations. The detection and management of OA in puppies, however challenging, is particularly important because it is more effective to preserve joint health than to attempt to recover it once it is lost. Also, chronic joint inflammation and chronic joint pain have complex physiologic and physical consequences that may only be partially reversible.
In conclusion, OA in the growing dog is most often caused by hip or elbow dysplasia. Signs of acute OA are more transient and more responsive to conservative multimodal therapy than signs of chronic OA. When managing OA, multimodal management is more effective than any single management strategy. Nutritional errors and imbalances promote the development of OA in dogs. Maintaining lean body condition delays the onset and slows the progression of OA. It also increases longevity and is essential for overall quality of life.
Physical wellness
A healthy daily routine should be established for each puppy based on their general demeanor and their future purpose as a pet, working dog, or sporting dog. A consistent daily routine will maximize training and compliance and will minimize fear, frustration, and anxiety. The implementation of a healthy daily routine takes time, effort, and consistency. Success is more likely when owners develop a long-term relationship with a trusted caregiver and advisor (53).
For growing dogs with OA, the initial clinic visit is the foundation of a long-term relationship with the owners and the beginning of long-term OA management. In dogs at risk of OA, it is wise to set aside time to gather information from owners and to discuss OA management with owners. Information about the dog’s functional ability is collected using one or more validated client questionnaires such as the Canine Brief Pain Inventory (CBPI), the Liverpool OsteoArthritis in Dogs (LOAD), the Helsinki Chronic Pain Index, and the Canine Orthopedic Index (COI) (54–57). Information about function particularly as it relates to daily activities (for example, the ability to climb a set of stairs or perform a small jump) is also collected during the period of observation preceding the physical examination. Functional information is critically important because it serves as a baseline for future evaluations of disease progression and response to therapy (13). A set of time- and place-specific daily activities can be used to develop a set of client-specific outcome measures (CSOM). Just like standard validated questionnaires, this CSOM set will be used to monitor response to therapy and disease progression. A change in any of the scores is suggestive of a change in limb function or pain, with a proportionality between the amplitude of the score change and the amplitude of the change in pain perceived and function. The minimal clinically important difference (MCID) of a questionnaire score can be determined. Rather than being a numerical change with unclear clinical impact, the MCID is the score difference that represents a clinically impactful change: a clinically significant positive response to therapy or a clinically significant increase in disease severity. In one study, the MCID for the LOAD and the COI, were 4 and 14, respectively (58). Notably, none of the client questionnaires were designed to evaluate function and disability in puppies and questionnaires are not currently validated when used to evaluate puppies (59, 60).
Dog owners should understand what joints and limbs are affected by OA, what physiologic, physical, and functional responses are observed at the time of evaluation and anticipated in the future. Changes in demeanor (e.g., a loss of playfulness) and mobility (e.g., a loss of ability to jump into a motor vehicle or a loss of willingness to go on a walk) are particularly important, as they are likely surrogate markers of the presence of chronic pain (61, 62). Observing for changes in demeanor (playfulness) is particularly relevant in puppies with OA because the problem is acute and recent. However, loss of function (mobility) is less likely because OA rarely alters strength or joint motion in the near term.
A better client understanding of the impact of OA on dogs will help clients notice changes more promptly when signs recur after therapy or when new signs emerge. Clients can then report these changes to the clinical team immediately. In turn, the clinical team will be able to provide advice or therapy more rapidly. The owner and caregiver should be aligned on the owner’s preferred approach to manage OA. This includes the owners’ level of involvement, the willingness to try therapeutic options supported by varying levels of scientific evidence, the cost of care, whether care is delivered by owners or medical professionals, and what to do when facing a symptom flare. In other words, care should be patient centered rather than clinician centered (53). To facilitate contact with owners, communications should consistently take place with the same clinical team who know the dog and the owner. Veterinary technicians make great case coordinators.
The foundation of OA management includes appropriate nutrition, regular leash walks and play, and a safe and effective pain-relieving strategy when joint pain is observed. Intensity, duration, and frequency should match patients’ needs for all exercises. Exercise programs often start with slow walks lasting 5 to 10 min and happening 3 to 5 times a week. If dogs do well, the duration, the intensity, and the frequency increase progressively and additional exercises are introduced (63). When dogs experience a symptom flare, exercise is curtailed while pain is managed.
In conclusion, establishing a relationship and good rapport between the owner and the clinical team is critical to the long-term successful management of OA. Client questionnaires and pain scales can be used to determine the patient baseline and track treatment response and disease progression. A consistent daily routine will maximize training and compliance and will minimize fear, frustration, and anxiety.
Nutritional supplements
Several nutritional supplements are commonly used by dog owners for the prevention or treatment of OA. Nutritional supplementation is one of the areas of pet care growing most rapidly. Supplements may provide nutritional or therapeutic benefits. They include vitamins, minerals, amino acids, enzymes, herbals, or botanicals. Dietary supplements may be added to food to make it nutritionally complete or to balance the diet. A nutraceutical is a therapeutic supplement that provides potential medical or health benefits.
In general, puppies eating a reputable, complete, and balanced diet need few supplements. B-vitamins, digestive enzymes, probiotics, or antioxidants may be needed during times of stress. For puppies with OA, proactive management using nutrition or supplements starting in puppyhood is often recommended. Many clients are interested in preventing the progression of OA in their puppies, particularly when their breed or conformation suggests an increased risk of OA, when they are intended to become a sporting dog, or when they already show signs of OA. Puppies can be divided into three groups: healthy with low OA risk, healthy with high OA risk, and early OA onset.
Healthy puppies with conformational abnormalities, puppies who will engage in high-intensity athletic activity, and puppies from breeds known to get early onset OA are considered high risk for developing OA. In puppies with OA, joint supplementation aims to lower inflammation and mitigate the development of OA, working synergistically with pain medications. When managing OA, nutraceuticals have multiple physiologic benefits, such as decreasing inflammatory prostaglandins (PGE2), decreasing the production of matrix metalloproteinases (MMP-2, MMP-9) responsible for cartilage damage, and increasing the concentration of tissue inhibitor of MMP (TIMP2) (64). Nutritional supplements should be made of high-quality ingredients. Quality can be ensured through certification, for example through certification by the National Animal Supplement Council (NASC) and in compliance with current Good Manufacturing Practices (cGMP). Scientific evidence of efficacy should also be available in the peer-review scientific literature. Several families of supplements exist for the management of OA in companion animals. Scientific evidence is stronger for some supplements than others (65). Common veterinary supplements used for joint support and the evidence for their use is listed below.
Omega-3 Fatty Acids – Omega-3 fatty acids, also named eicosanoids, are metabolically active compounds derived from 20-carbon fatty acids, usually arachidonic acid. Lipoxygenase (LOX) and cyclooxygenase (COX) enzymes are the rate-limiting steps in the production of leukotriene B4, thromboxane (TX) A2 (TXA2), and prostaglandin E2 (PGE2). In inflammatory conditions such as OA, PGE2 production can be increased up to 50-fold (66). Leukotriene B4 has a potent chemotactic effect and promotes further inflammation. PGE2 and TXA2 promote the release of tumor necrosis factor alpha and interleukin 1β, which further promote inflammation and, in joints, stimulate the production of MMP, collagen-destroying enzymes that promotes the breakdown of articular cartilage in joints with OA. Further, PGE2 is a potent stimulator of pain receptors, therefore contributing to the pain resulting from OA. Eicosapentaenoic acid (EPA) can be used by COX and LOX enzymes to produce the eicosanoids PGE3, TXA3, and leukotriene B5, which are less active and are anti-inflammatory relative to their counterparts produced from arachidonic acid. Fish oil is a primary source of omega-3 fatty acids. The scientific evidence supporting the use of omega-3 fatty acids to alleviate the signs of OA is strong (65). In dogs with OA, a diet containing approximately 3.5% omega-3 fatty acids decreased pain and lameness, improved weight bearing, and decreased the perceived need for NSAIDs (67, 68). Approximately 480 mg/kg of fish oil (50 to 100 mg/kg of EPA) would be required as a supplement to match the amounts available in the therapeutic food discussed above (69). In a recent 16-week clinical trial in dogs with OA, fish oil supplementation (90 mg/kg EPA and 20 mg/kg DHA) added to a non-fish-based food led to improvement of indicators of pain and quality of life (70). Perna canaliculus, the green-lipped mussel (GLM) found in the waters around Australia and New Zealand, contains several omega-3 fatty acids: EPA, docosahexaenoic acid (DHA), and eicosatetraenoic acid (ETA). It also contains glycoproteins and glycosaminoglycans (GAG). A clinical trial in dogs with chronic OA pain reported improved mobility in dogs receiving 50 mg/kg of GLM (71).
Undenatured Type II Collagen – Undenatured Type II collagen is a form of collagen obtained through low temperature processing of chicken sternum. Several clinical studies support the safety and efficacy of undenatured Type II collagen in modulating joint discomfort resulting from OA or rheumatoid arthritis in humans. Also, several studies designed to assess the efficacy and tolerability of undenatured Type II collagen in moderating joint function and joint pain due to strenuous exercise in healthy subjects showed a positive impact. One study showed that undenatured Type II collagen decreased inflammation in healthy young dogs following strenuous exercise (72). In dogs with OA, two clinical trials investigated the effects of undenatured Type II collagen using objective measures of gait. Decreased pain was observed in these trials (73, 74). The positive impact of undenatured Type II collagen in dogs with OA appears to be dose dependent, with a stronger response noted in dogs receiving 80 mg per day than 40 mg per day (75).
Glucosamine and Chondroitin – Glucosamine and chondroitin sulphate (GCS)-based supplements were historically the most common joint supplements used in companion animals. GCS has been shown to decrease systemic inflammation in humans (76). Several systematic reviews documented their efficacy when managing knee OA (77, 78). In companion animals, GCS are safe. However, less is known about their long-term impact on cartilage health and their clinical efficacy (65, 79–81). Some studies in dogs identified benefits to receiving GCS supplements. In one clinical trial with 35 client-owned dogs, pain on palpation was lower after GCS administration (82). Other studies failed to identify clinical improvement in dogs receiving a GCS supplement (83, 84), possibly because OA has wide-ranging consequences and because GCS would not eliminate all consequences of OA. Because of their anti-inflammatory activity, GCS is likely most beneficial to puppies with early OA compared to older dogs with chronic OA.
Cannabis supplementation – Cannabis-derived extracts (phytocannabinoids) are regulated as a supplement in the United States and other jurisdictions, but they are regulated as a drug in several countries. Phytocannabinoids have emerged as a potential approach to alleviate chronic pain in humans and dogs (85). There are no known clinical studies to the efficacy and safety of the use of phytocannabinoids in growing dogs.
Fortetropin® – Fortetropin is a supplement made from pasteurized, freeze dried, fertilized egg yolk. It has been shown to increase lean body mass in humans and dogs. Fortetropin has not been clinically studied for safety or efficacy in the growing dog.
In conclusion, nutraceuticals are therapeutic supplements intended to provide medical or health benefits. When managing OA, supplements can provide multiple benefits to the body through various proposed mechanisms of action. Strong scientific evidence supports the use of omega-3 fatty acids and undenatured Type II collagen.
Pharmaceutical management of OA
The choice of medications to manage OA in dogs is based on the observed level of pain and disability. Since the perception of pain is highly individual, medical therapy should be individualized. Medical therapy is also influenced by the severity of inflammation and the chronicity of OA. Medical therapy may not fully alleviate the pain experienced by dogs with OA: pain decreases but may not be eliminated. Importantly, a treatment that may be considered a “failure” because it only partially alleviates the signs of OA, may in fact bring relief to a puppy with OA. That is why, whenever possible, the medical management of OA should include medications with scientifically proven benefits. Since a single medication often does not fully alleviate OA pain, medications with varying modes of action are routinely combined when managing OA in dogs. Medications are also combined with non-medical pain-relieving strategies such as manual therapy or electrophysical modalities (low-level heat or cold, for example) to maximize pain relief. Because the response to therapy in dogs with OA is multifaceted—affecting their limb use, mobility, willingness to exercise, playfulness, appetite, sleeping habits, and demeanor, the assessment of the response to therapy will also be multifaceted. Revaluations should include the assessment of changes in behavior and activity, observation of gait, palpation, and a client questionnaire.
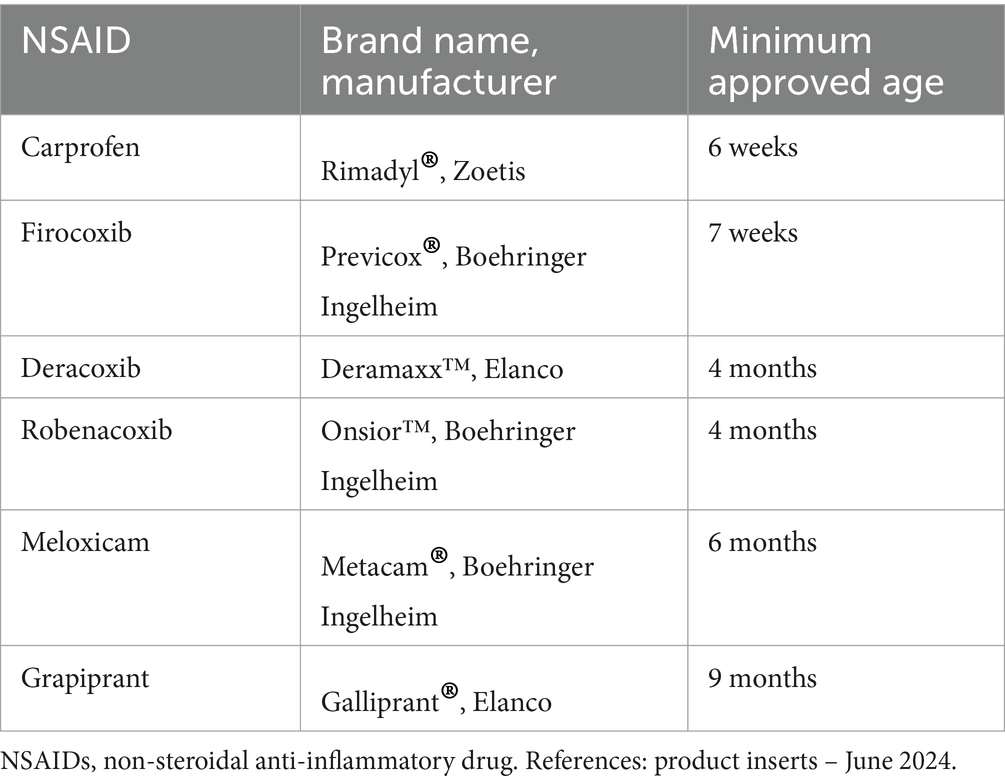
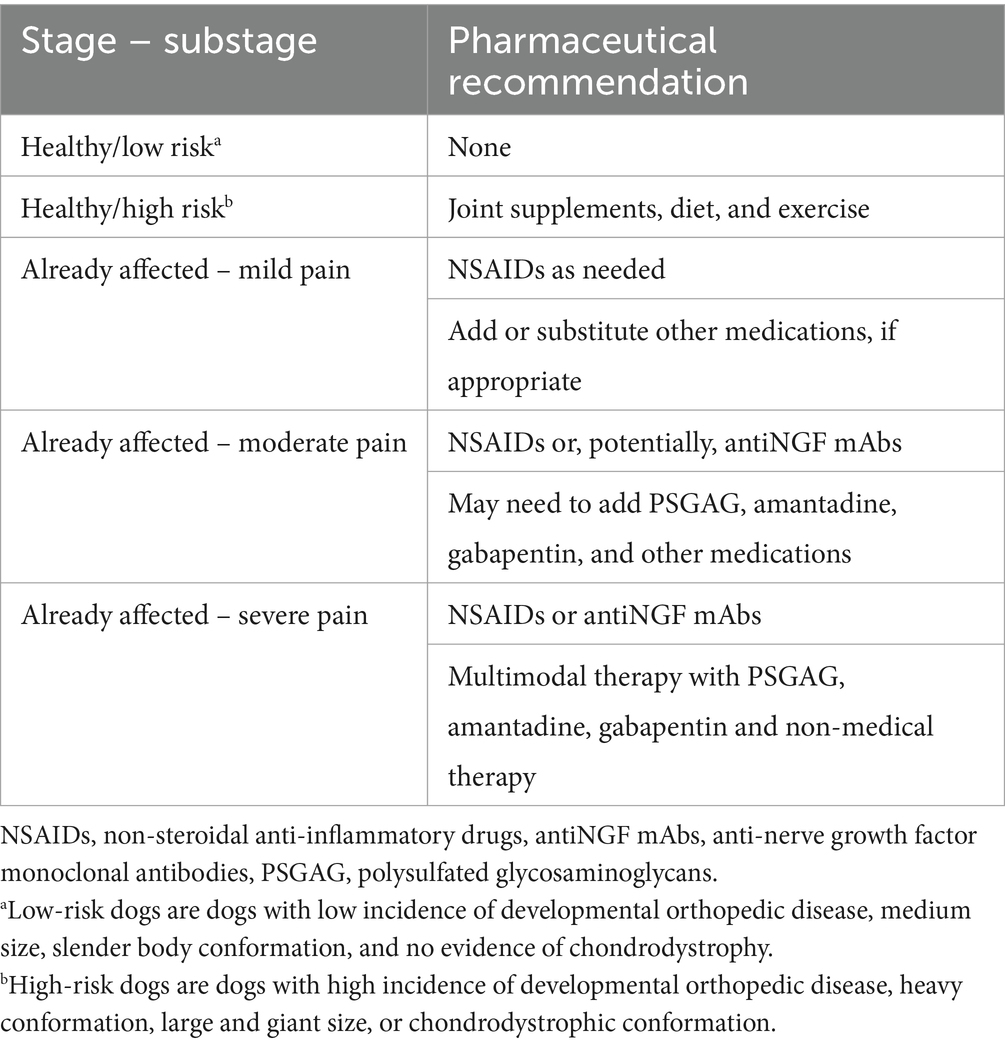
Most of the research on the safety and effectiveness of medications to manage OA in puppies focused on non-steroidal anti-inflammatory drugs (NSAIDs). Consequently, NSAIDs are the most widely use pain medication in puppies with OA. In the United States, several NSAIDs have been approved for use in puppies, at ages ranging from 6 weeks to 9 months, depending on the NSAID (Table 1). In puppies whose OA pain is not fully controlled by NSAIDs, adjunctive pain medications can be considered (Table 2). However, research on medical management using medication classes other than NSAIDs is mostly absent from the peer-reviewed literature. This is in part because few clinical trials overall have included medications other than NSAIDs (79) and also because clinical trials of dogs with OA rarely focus on puppies. Practically, NSAIDs should be the first treatment choice because the available data supporting their efficacy is stronger than other pain medications. However, NSAIDs and some other drugs anecdotally used in puppies (gabapentinoids and NMDA-receptor antagonists) are primarily cleared via hepatic metabolism and immature hepatic function in puppies less than 4 to 5 months of age could result in higher serum drug concentrations, potentially leading to an increased magnitude of effects and duration of action (86). However, NSAIDs can be used down to the approved minimum age. Also, other hepatically-metabolized drugs can be administered at the low end of the dosing range, at the discretion of the prescribing veterinarian. Injectable polysulfated glycosaminoglycans have been shown to decrease inflammation and pain and to slow cartilage degeneration in puppies with hip laxity.
While anti-nerve growth factor (anti-NGF) monoclonal antibodies are used to alleviate OA pain in adult dogs, their safety and efficacy have not been evaluated in dogs less than 12 months of age (87). The impact of anti-nerve growth factor on the developing nervous system is therefore unknown. Since the nervous system is largely mature by 6-weeks of age in puppies (88), an anti-NGF drug such as bedinvetmab could potentially be used at the veterinarian’s discretion in puppies with OA pain.
In conclusion, the perception and expression of pain is highly individual. Medical therapy should be individualized, accounting for the severity of inflammation and the chronicity of OA. NSAIDs are the main medication used to manage OA pain in puppies because of safety studies in that age group. Few clinical trials have studied the effects of medications from pharmaceutical classes other than NSAIDs and polysulfated glycosaminoglycans, but other drugs (e.g., antiNGF mAbs, gabapentinoids, and NMDA-receptor antagonists) can be used at the discretion of the veterinarian.
Intra-articular injections in puppies
Intraarticular (IA) injections and specific surgical procedures may also be part of the management of OA. The use of IA injections is often considered when non-surgical therapy fails or in combination with some surgical procedures. These injections can be used to control symptom flares, acute episodes of exacerbation of joint pain, or to manage patients who are at increased risk of adverse event from NSAID therapy or have a history of adverse events from NSAIDs.
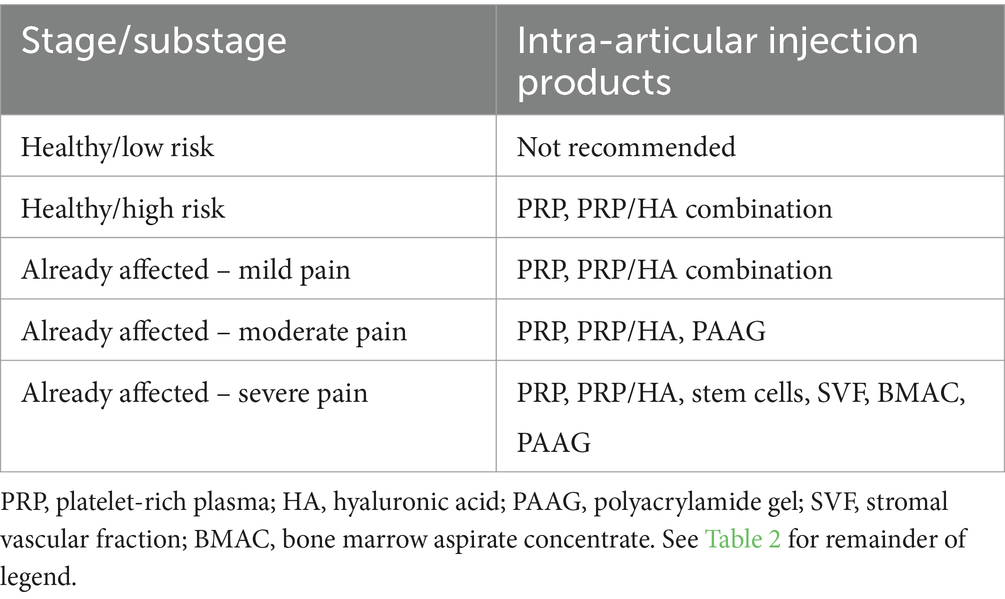
Intra-articular (IA) injections, also named therapeutic joint injections, have been used in humans and horses for the management of OA with acceptable safety and efficacy (89, 90). The IA products currently used for OA management in humans, horses, and dogs include regenerative medicine injections (e.g., platelet rich plasma, platelet rich factor, bone marrow aspirate concentrate, autogenous stem cells, stromal vascular fraction cells), viscosupplementation (hyaluronic acid, hydrogels), radiosynoviorthesis, and glucocorticoids (corticosteroids). IA injections have been used less commonly in dogs than in horses and human beings. Evidence of efficacy is limited to a few clinical reports and none of those focused on puppies (91). Intra-articular injections appear safe overall (92, 93). Glucocorticoids are no longer routinely used to manage OA in dogs and would not be expected to be used in puppies because they appear to accelerate cartilage breakdown in joints with OA (94).
In puppies with severe joint problems that led to OA or will lead to OA, surgical procedures should be considered before IA injections. Once a joint has been managed with surgery, or when surgery is not an option, IA therapy can be considered for its anti-inflammatory and anabolic effects on inflamed joints with early OA (Table 3). IA therapy is often considered after a surgical procedure (for example after arthroscopy) where joint disease is confirmed. For example, to manage a 6-month-old large-breed dog with elbow dysplasia, a PRP IA injection may be considered at the time of surgery and several weeks after surgery if joint effusion (a feature of inflammation) and lameness (a feature of pain) persist. Preference is given to IA injections that have potential regenerative properties (e.g., platelet rich plasma, platelet rich factor) over IA injections that have palliative properties (e.g., hydrogel, radiosynoviorthesis).
Viscosupplementation is a type of IA injection done to increase the viscosity of synovial fluid with the intent to restore the physical properties of joint fluid. The objectives of viscosupplementation include aiding in joint lubrication, decreasing inflammation and cartilage degradation, and assisting cartilage repair (95). Viscosupplementation was initially achieved using low or high molecular weight hyaluronic acid (96). More recently, collagen elastin hydrogel microparticles (CEHM) and cross-linked polyamide hydrogels have been used (97).
Hyaluronic acid (sodium hyaluronate, hyaluronan, HA) is a fluid present in all living organisms. Intra-articular HA is a form of viscosupplementation used to manage OA in humans and horses. No study has focused on the use of HA in growing dogs with OA.
Polyacrylamide hydrogels are non-soluble, non-immunogenic, non-toxic and highly viscous 2.5% or 4% solution of cross-linked polyacrylamide in sterile water. There are no known studies in puppies, all current canine studies have included skeletally mature dogs.
Regenerative medicine
There is increasing use of regenerative medical therapy, also named orthobiologics, in humans, horses, and dogs. Orthobiologic products include platelet rich plasma (PRP), autologous or allogenic mesenchymal stem cell (MSC) therapy, stromal vascular fraction (SVF) therapy, and bone marrow aspirate concentrate (BMAC). Safety and efficacy have been reported in humans, horses, and dogs with OA (98). Intra-articular PRP has been shown to positively impact dogs with OA (99) and promote tendon and ligament healing. Several PRP systems are commercially available and produce PRP with varying parameters (100, 101). The IA injection of PRP usually includes one to three injections weeks to months apart. To minimize the risk of negative interaction, some have recommended avoiding NSAIDs one week before to two weeks after PRP injection and avoiding cold therapy after injection. The combined use of IA PRP and HA has also been reported (102).
Stem cells are characterized by their ability to self-renew and differentiate along multiple lineage pathways, contributing to generating new tissues. Stem cells are chemotactic for progenitor cells, supply growth factors, make extracellular matrix, promote angiogenesis, are anti-apoptotic, anti-inflammatory, and anti-fibrotic (103). When used in regenerative therapy, millions to billions of cells are injected. These cells are usually autologous cells harvested using a minimally invasive procedure, differentiated along multiple cell lineage pathways in a regulated and reproducible manner, transplanted to either an autologous or allogeneic host, and manufactured in accordance with current GMP guidelines.
In dogs, autogenous sources of stem cells include bone marrow and fat. Cells are isolated, expanded, and returned to the patient. Bone marrow aspirates contain hematopoietic stem cells, which can form all types of blood cells, and MSC which can generate bone, cartilage, fat, and connective tissue. Bone marrow aspirate can be processed in-house through a series of centrifugation steps to yield BMAC (104, 105). Adipose tissue contains MSC. Adipose-derived stem cells can also be processed in-house through a series of steps involving enzymatic tissue digestion and centrifugation to obtain the SVF (106). Several clinical studies suggest that IA stem cell therapy is safe and beneficial to dogs with OA (107, 108). The combination of stem cells and PRP also has reported benefits to manage OA and for tissue healing (109–111).
The IA injection of a colloid containing a radioactive tin (tin-117 m) is commercially available to manage the signs of OA (Synovetin OA®). This process has not been studied in puppies, only in skeletally mature animals, where no deleterious effects to the joint were detected.
In conclusion, the goals of intra-articular therapy include improving joint lubrication, decreasing inflammation and cartilage degradation, and improving cartilage repair. IA injections can be used as an alternative to surgery in patients who are poor surgical candidates or after surgery, in patients with persistent joint effusion and pain. Studies evaluating the efficacy and long-term safety of IA in puppies are lacking.
Surgical management of OA in puppies
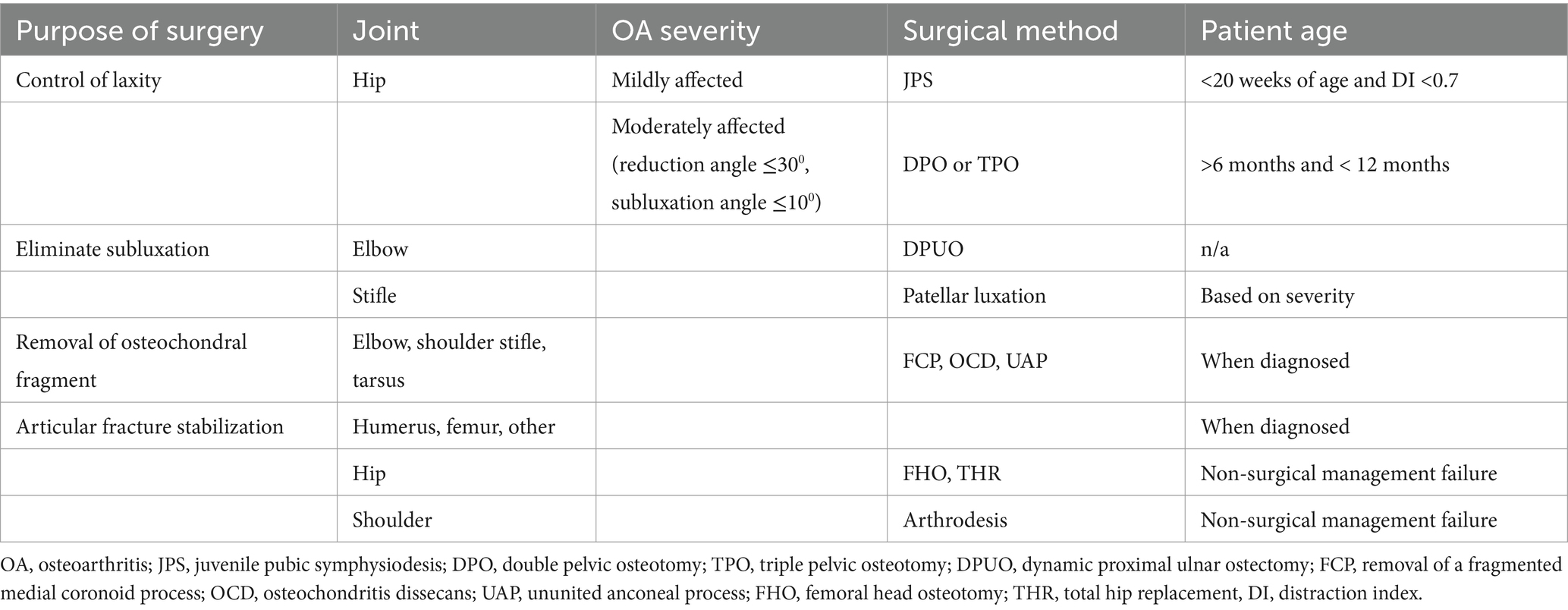
Surgical procedures may be warranted to manage OA in puppies. Procedures are intended to protect joints from OA or to decrease the impact and progression of OA. In puppies, OA most often results from joint instability or joint subluxation, from the intraarticular presence of an osteochondral fragment, or from an articular fracture. Surgical procedures to manage OA in puppies aim to minimize joint instability, eliminate joint subluxation, remove osteochondral fragments, rigidly stabilize articular fractures, and potentially restore articular surfaces with autogenous or exogenous grafts (Table 4).
Canine hip dysplasia is the most common orthopedic disease in dogs. Dysplastic hip joints develop laxity after birth. Laxity is influenced by several factors, including the geometry of the hip joint, pelvic muscle mass, and the ligament of the femoral head. Subluxation results in abnormal forces being exerted at the acetabular rim and on the femoral head. The result is abnormal hip joint conformation, joint incongruity, increased hip joint laxity, and cartilage damage. During growth, pain arises from cartilage damage, subchondral bone inflammation, and joint capsule strain. In dogs with hip laxity, surgical intervention aims to increase coverage of the femoral head by acetabular rotation and lateralization to decrease femoral head subluxation.
In growing dogs between 16 and 20 weeks of age, alteration of the acetabular orientation can be achieved by limiting growth of the pubis though premature closure induced using electrocautery or mechanical crushing (112). The procedure is named juvenile pubic symphysiodesis (JPS). The JPS procedure appears to help dogs with hip laxity (113), but its outcome relative to non-surgical measures has not been evaluated in a long-term clinical trial. In dogs older than 26 weeks, alteration of the acetabular orientation can be achieved by rotating the acetabulum using a double or triple pelvic osteotomy (DPO or TPO, respectively). DPO and TPO are much more invasive than JPS and have similar clinical results. They appear to benefit dogs with hip laxity, without being curative (113). Total hip replacement (THR) is rarely done in growing dogs. A THR is recommended in dogs that fail to improve with non-surgical management and that are too severely affected to benefit from a JPS or DPO. Most of the dogs who receive a THR as puppies have luxoid hip dysplasia, a severe form of dysplasia with early dorsal luxation (114). In dogs with severe hip pain non-responsive to non-surgical management and when a THR is not considered as an option, a femoral head ostectomy (FHO) can be considered. With the FHO, the femoral head and neck are cut, and the head is removed, forming a fibrous joint. Mild pain and clinical dysfunction often persist following FHO, but dogs are often less painful once they recover from surgery.
Elbow dysplasia is an umbrella term describing several problems including radioulnar incongruity, fragmentation, or cartilage damage to the medial coronoid process. Elbow dysplasia is most common in large-breed dogs. It starts during growth and is commonly diagnosed between 6 and 10 months of age. The medial aspect of the elbow joint is more vulnerable to OA secondary to elbow dysplasia than the lateral aspect of the joint. Some dogs have osteochondritis dissecans (OCD) of the medial aspect of the humeral condyle. Elbow dysplasia is managed conservatively or with surgery. Surgical procedures include the removal of osteochondral fragments form the medial coronoid process or humeral condyle, potentially combined with an ulnar osteotomy (bi-oblique ulnar osteotomy) to improve joint congruity.
Corrective osteotomies that alter the direction of forces traveling across the elbow joint and increase loads resisted by central and lateral aspects of the joint have been reported, including the sliding humeral osteotomy and the proximal abducting ulnar osteotomy. The long-term benefits of these surgical procedures are unclear. Most dogs with elbow dysplasia develop OA (115), which progresses over time. Focal joint resurfacing and partial or total elbow replacement may be considered in dogs with severe chronic lameness non-responsive to conservative management or other surgical procedures. In some dogs, the anconeal process, which develops from a secondary center of ossification that normally ossifies with the ulna at approximately 6 months of age, does not fuse with the ulna, leading to an ununited anconeal process. Surgery is recommended for management. Non-displaced processes can be managed with a bi-oblique ulnar osteotomy. Displaced processes can be reduced and secured to the ulna with a bone screw and protected with a bi-oblique ulnar osteotomy. Deformed anconeal processes are excised.
Perthes disease is a developmental problem affecting the femoral head and hip joint. With Perthes disease, growing small-breed dogs experience a collapse of their femoral head presumably resulting from damage to the blood supply to the proximal femoral epiphysis. The collapsed femoral head, visible on a ventrodorsal radiograph, is a source of joint pain and OA. Diagnosis may be delayed by weeks to months, possibly because owners and veterinarians may not anticipate the presence of hip joint problems in small breed dogs. The non-surgical management of OA secondary to Perthes disease is implemented and surgery (THR or FHO) is recommended for patients that do not respond to conservative management.
Patellar luxation is a developmental problem secondary to skeletal abnormalities to the femur and tibia that alter the direction of forces exerted by the quadriceps femoris muscle on the patella. Early luxation of the patella may further alter the growth of the distal portion of the femur and proximal portion of the tibia, and, in severe cases, can lead to a loss of stifle joint extension. Luxations are often medial but can be lateral. The asymmetric forces generated by the luxated patella on the physes, particularly on the distal femoral physis, may lead to increased growth in the side under tension and decreased growth on the side under compression (Hueter-Volkmann law) (116). Luxations that occur earlier in life likely result in more severe displacement, growth abnormalities, and tissue changes than luxation that occur near adulthood. Patella luxation has been classified as grade 1 to 4. With grade 1, the patella can be luxated with digital pressure and returns when released. With grade 2, the patella can be luxated and does not return immediately when released. With grade 3, the patella is found luxated but can be reduced to its normal position. With grade 4, the patella is luxated and cannot be reduced.
Mild forms of patellar luxation may be manageable without surgery. Severe forms of patellar luxation require surgery. Most small-breed dogs with patellar luxation have OA at the time of presentation for surgery, and the severity of OA is greater when the dogs are older (27), suggesting that OA progresses even when patellar luxation is managed conservatively.
The objectives of surgery most often include deepening the trochlear groove and aligning the quadriceps femoris with the trochlea. This can be achieved with a range of methods whose complexity are proportional to the severity of the problem. Dogs that develop grade 4 patellar luxation early in growth may lose stifle joint extension, because the quadriceps no longer acts as an extensor of the stifle. These dogs need surgery as early as possible to prevent further loss of extension and to restore limb use.
Cranial cruciate ligament injury is common in dogs and sometimes occurs in puppies. Subjectively, the problem is most prevalent in working and sporting dogs that begin training at 5 or 6 months of age, before skeletal maturity (117). The ligament can tear or can avulse at its femoral origin or tibial insertion. The diagnosis of a partial or complete cranial cruciate ligament rupture may be achieved using arthroscopy. Small diameter scopes (< 2 mm) are increasingly used to diagnose these injuries. Dogs with (minor) partial ligament tears may be managed with an intraarticular injection of PRP, combined with rest. Dogs with cranial cruciate ligament injury are often managed with surgery and most of them have OA at the time of presentation (27, 118). Osteoarthritis in dogs with cranial cruciate ligament injury is more severe than in dogs with patellar luxation, possibly because cranial cruciate ligament injury is more inflammatory than patellar luxation. Several surgical procedures are used to manage cranial cruciate ligament injury, particularly tibial plateau leveling osteotomies. It may be possible to repair some cranial cruciate ligament avulsions. Some surgical procedures interfere with the proximal tibial growth plate and should probably be avoided in puppies.
Osteochondral fragments occur in joints because of osteochondritis dissecans (OCD). These fragments are found on the axial aspect of the lateral femoral condyle in the stifle joint and in the shoulder, elbow, and tarsus. The condition is identified most often in male, large, or giant dog breeds between 6 and 10 months of age. Osteochondral fragments lead to pain, lameness, and OA. Dogs that do not respond to conservative management undergo surgery. Historically, the bone fragment was removed from the joint. This appears to work well in the shoulder joint. Alternative procedures such as osteochondral autograft or synthetic (metal-backed polyurethane implants) have emerged. In the tarsus, OCD fragments often involve the proximal-central zone of the medial ridge of the talus. Most joints with OCD develop OA. Some dogs, generally small-breed dogs, have glenoid dysplasia, an abnormal development of the glenoid articular surface, possibly secondary to laxity or luxation early in growth. Conservative management may not help dogs with joint luxation. An arthrodesis of the shoulder joint may be recommended.
Conformational abnormalities are common in dogs, particularly deformities of the antebrachium. Most conformational abnormalities result from genetic developmental disease such as chondrodystrophy or chondrodysplasia (52). Abnormal growth of the distal ulnar physis leads to a curvature of the radius and elbow incongruity. The problem affects both forelimbs. In contrast, unilateral deformities generally result from an injury to a growth plate of the ulna or radius. Radial head subluxation or luxation can occur. Mild cases are monitored and managed conservatively. Surgery may be recommended to manage severe problems. Corrective osteotomies can decrease angulation and torsion, restore limb length, and improve joint subluxation. In one report, half of the dogs with antebrachial deformities had OA at the time of presentation (119). In that report, the presence of OA and the severity of lameness at the time of presentation were factors that negatively impacted the outcome of surgery, suggesting that antebrachial deformities should be managed without delay in puppies, before the onset of OA.
In conclusion, OA most often results from joint instability or joint subluxation, from the intraarticular presence of an osteochondral fragment, or from an articular fracture. The purpose of surgical procedures is to protect joints from OA or to decrease the impact and progression of OA.
Case examples
6-month-old male Labrador retriever with hip dysplasia
A 6-month-old male Labrador retriever presents with hip dysplasia diagnosed based on a ventrodorsal radiograph that shows bilateral hip subluxation and mild osteophytosis. Upon observation, the dog has swaying gait in pelvis limbs. On palpation, a moderate pain response to extension of both hip joints is observed. The owner considers surgical options. The dog is too old for a JPS and not sufficiently painful or debilitated for a DPO, THR, or FHO. The initial management of OA is conservative. The owner fills out a baseline CBPI questionnaire, which shows minimal pain severity and pain interference. An NSAID is selected as baseline pain medication and prescribed as needed (PRN). See Table 1 for minimal age for various NSAIDs. Growth and weight optimization are discussed. Nutritional advice includes continuing with large breed puppy food and maintaining an optimal body condition of 5/9. A decision to postpone castration until a year of age is made to protect the dog against weight gain after castration. Daily walks are recommended and an exercise program of 30-min leash walks four times a week is started. Nutritional supplementation with omega-3 fatty acids and Undenatured Type II collagen is implemented. The anticipated signs suggestive of symptom flares and OA progression are discussed with the owner. The owner is tasked to keep a log of OA signs, including the activities preceding any increase in clinical signs. A reevaluation after 3 months is scheduled, with the understanding that the dog will be reevaluated sooner if clinical signs increase. Strengthening exercises are discussed, including weight-shifting, sit-to-stand, and backward walking exercises.
6-month-old female French Bulldog with elbow subluxation and OA
A 6-month-old female French Bulldog presents with a forelimb lameness and OA of both elbow joints, diagnosed on mediolateral radiographs of the elbow joint that show humero-ulnar subluxation and mild osteophytosis. Upon observation, the dog is hesitant to walk down steps. When resting, the dog flexes his elbow and wrist joints, possibly to relieve pain. On palpation, a mild pain response to full flexion of the elbow joint is present. A computed tomography scan is discussed to rule out the presence of coronoid process fragmentation, delayed ossification of the humeral condyle, and other abnormalities potentially invisible on radiographs. The owner fills a baseline CBPI questionnaire. Ovariohysterectomy is discussed and scheduled. The importance of maintaining a lean body condition is discussed. Surgical options, including arthroscopy and DPUO, are discussed. The owners select conservative management. An NSAID is prescribed after baseline bloodwork. An exercise program is initiated with 10- to 15-min-long leash walks done twice daily, whenever possible based on weather and owner availability. A reevaluation is planned after 30 days, unless clinical signs warrant an earlier evaluation.
9-month-old male Bernadoodle with elbow dysplasia
A 9-month-old castrated male Bernadoodle presents with elbow dysplasia diagnosed based on mediolateral radiographs. A baseline CBPI is filled by the owners, indicating mild severity and minimal interference. The dog’s body condition is rated as slightly overweight (6/9). The dog has a mild lameness of his forelimbs resembling a slightly hypermetric gait, suggesting elbow pain. On palpation, a mild pain response to flexion of the elbow joints is observed. A weight loss program to reach optimal body condition (5/9) is initiated. Nutritional supplements (omega-3 fatty acids from fish oil and Undenatured Type II collagen) are recommended. An exercise program based on leash walks and gentle play is initiated. Exercises are initiated, including down-to-up, waving, high-five, and weight-shifting, with a target of 20 min every other day at the beginning to be increased to 30 min per day after 1 month. Several sessions of weekly hydrotherapy, with underwater treadmill walk and swim, are prescribed.
Integrating all approaches to manage OA in puppies
Puppies commonly have OA. The most common causes of OA that develop during the first year of life appears to be hip dysplasia (120), elbow dysplasia, and patellar luxation. Injuries to the cranial cruciate ligament are relatively uncommon in puppies. Few surgical procedures have been shown to positively impact the course of OA over a lifetime and, to be maximally protective, these procedures should ideally be done before the development of OA. Since the development of OA is rapid and often insidious, protective surgical procedures are rarely done and most growing dogs with OA are managed conservatively.
Despite how common OA is in puppies, the management of OA in growing dogs has not received much coverage in the scientific literature. That dearth of literature results from the fact that the impact of OA in growing dogs is less severe than in older dogs. In growing dogs, OA mostly leads to acute pain and in adult dogs OA leads to chronic pain combined with loss of muscle mass, joint motion, and fitness and potentially leads to behavioral changes. Subjectively, puppies with OA may also show mild behavioral changes, they may be more introverted and less playful than puppies without OA. This suggests that OA could negative impact socialization and training. Further research is warranted on that issue.
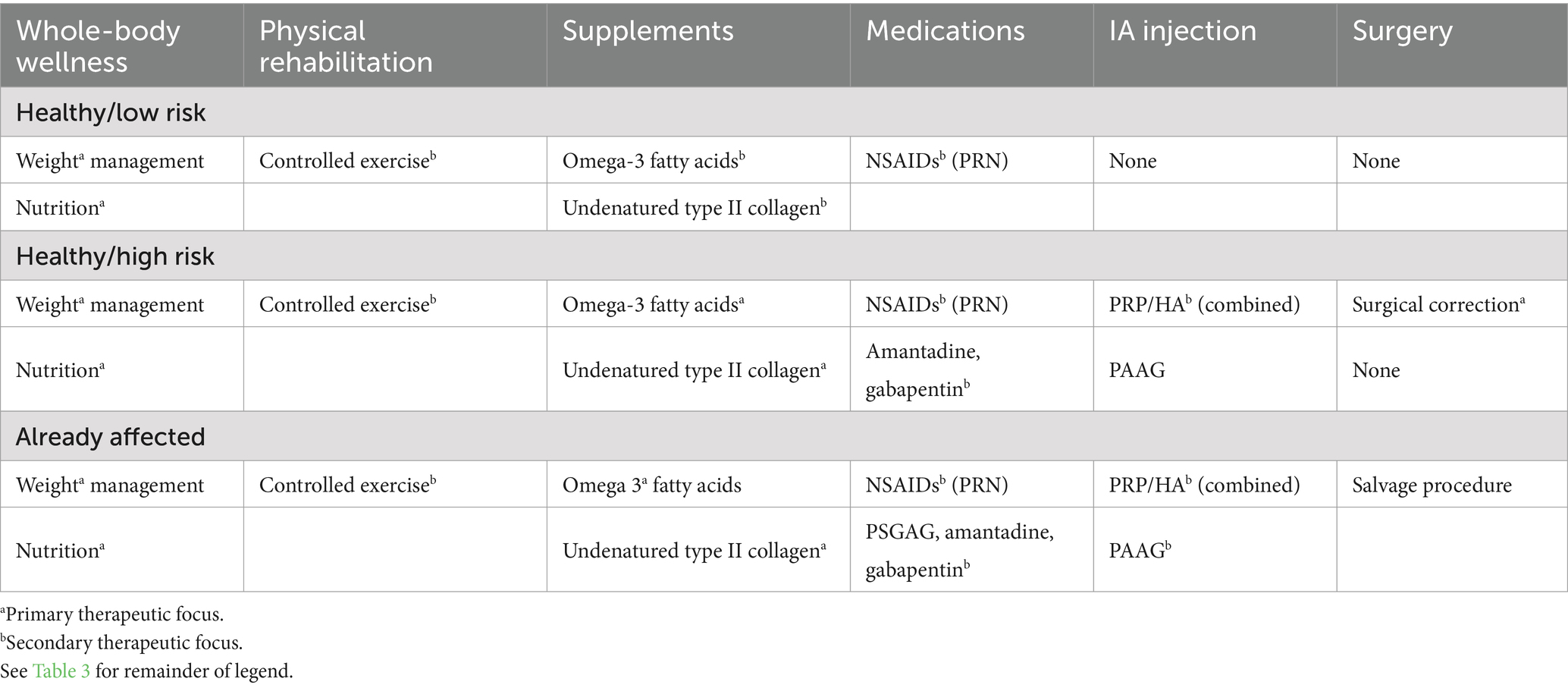
Because OA management will be required over the dog’s lifetime, it is often daunting to owners and clinicians to work-up, discuss, and manage OA when it is diagnosed in a puppy. It is important to prioritize management options that does not overburden or overwhelm owners (financially or logistically) in the short term and are sustainable in the long term (Table 5). Safe and effective management that are affordable and convenient are prioritized. Beyond the initial management plan, a management plan for symptom flares should also be developed early so that owners are prepared to deal with periodic recurrences of clinical signs. Symptom flare plans generally include the identification of flare triggers, rest, and anti-inflammatory and pain-relieving steps. To ensure the short-term and long-term success of OA management, communication between owner and the clinical team should be simple and sustained (53). Therapy should be individualized to the needs of the patient and the preferences of the owner (53). The efficacy of OA management steps is particularly important when treating early OA in puppies because management steps with minimal efficacy are unlikely to be sustained over the long term by owners. Also, ineffective therapy may compromise owner trust and could endanger the long-term relationship that is required to manage OA over a lifetime.
While OA is described as a progressive disease, few studies have evaluated the progression of OA over time (53). There is a lack of knowledge about how rapidly OA might transition in dogs from a physiologic problem to a physical problem, and to a functional disability. The speed of these transitions appears to vary based on the cause of OA, its initial severity, the number of joints and limbs involved, the dog size, demeanor, body condition, and living conditions (53, 121). Based on changes in osteophyte size, the progression rate of radiographic OA is slow (53). In one study, progression of radiographic OA was more rapid in puppies than adults and in spayed females (121). However, the correlation between the size of osteophytes and joint inflammation in dogs with OA appears to be poor (122). In other words, some dogs have mild radiographic signs of OA but have severe clinical signs and other dogs have severe radiographic. OA but show few clinical signs and have no functional impairment. The progression of pain is most relevant for dogs with OA. The pain resulting from OA also varies widely among dogs based on the cause of OA, its initial severity, the number of joints and limbs involved, the dog size, demeanor, body condition, and living conditions (51). While puppies are vulnerable to trauma, counterintuitively in one studies of large-breed puppies, dogs with hip dysplasia showed fewer clinical signs when they engaged in off leash activity early in life than when they did not have off leash activity (123).
The primary management goals of OA in puppies are to alleviate pain and decrease joint inflammation. Little is known about the inflammatory burden of various forms of OA in dogs. For dogs with chronic hip dysplasia, inflammation is present in the joint capsule, joint fluid, and subchondral bone (124). The fact that OA has an impact that varies widely among dogs suggests differences in inflammation. For example, among dogs presenting for the surgical management of patellar luxation and cranial cruciate ligament injury, OA from cruciate ligament rupture is more severe than OA from patellar luxation, suggesting that OA from cruciate ligament rupture carries a heavier inflammatory burden (27). Pain relief in puppies is often achieved using NSAIDs, rest, and low-intensity exercise. Other therapeutic goals include being able to achieve proper socialization and training and maintaining limb strength. Optimizing growth, avoiding excess body weight, and cautiously timing neutering are also priorities. The extent and complexity of OA management in puppies should be based on severity of the problem and initial response to therapy. Therapy is often introduced in phases with an initial focus on nutrition, nutritional supplementation, and activity oversight, followed by an NSAID and other medications, followed by joint injections, followed by a surgical procedure. Salvage surgical procedures can have a transformative impact on painful joints with OA in puppies, but they are used as a last resort. Osteoarthritis management programs are individualized. They incorporate owners’ therapeutic philosophy and goals. They take into account the dog’s demeanor and limitations (53). Because individual results vary with all types of therapy, objective reevaluations are critically important. Clinicians should be mentally prepared to change therapeutic course based on patient response to therapy and disease progression. The detection and management of symptom flares is also important. The source of flares should be identified whenever possible. In puppies with OA, good days should vastly outnumber bad days.
In conclusion, OA often affects growing dogs. Its clinical signs often result from joint inflammation and acute pain. The early diagnosis of OA in growing dogs can be challenging because changes are less severe than in older dogs. The management of OA in growing dogs relies on whole body wellness, nutritional oversight and supplementation, medications, electrophysical physical modalities, and exercise. Intra-articular injections and regenerative medicine are used less often in growing dogs than in adult dogs in part because simpler measures are often sufficient to control clinical signs and less is known about their impact in growing dogs than in adult dogs. Surgery is used in growing dogs to improve the long-term joint health or manage pain that cannot be controlled with non-surgical steps. To be sustainable in the long term, the early management of OA in growing dogs should carry a low practical and financial burden for owners, in addition to being safe and effective.
Author contributions
DM-L: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing. DH: Writing – original draft, Writing – review & editing. JH: Writing – original draft, Writing – review & editing. TG: Writing – original draft, Writing – review & editing, Formal analysis. MB: Writing – original draft, Writing – review & editing, Formal analysis. AM: Writing – original draft, Writing – review & editing, Formal analysis. BF: Data curation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank Dr. Joseph J. Wakshlag, Cornell University, Dr. Debra Canapp, Veterinary Orthopedic and Sports Medicine Group, Dr. Sherman O. Canapp, Veterinary Orthopedic and Sports Medicine Group, Dr. Chris Lewis, Vetoquinol USA, Dr. Brian McLaughlin, Vetoquinol USA for their input.
Conflict of interest
BF was employed by Vetoquinol United States. The authors were members of Vetoquinol USA Osteoarthritis Advisory Group, Fort Worth, TX, United States.
The authors declare that this study received funding from Vetoquinol United States. Vetoquinol USA funded travel and honorariums for participation in advisory group meetings for the preparation of this article.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jones, GMC, Pitsillides, AA, and Meeson, RL. Moving beyond the limits of detection: the past, the present, and the future of diagnostic imaging in canine osteoarthritis. Front Vet Sci. (2022) 9:789898. doi: 10.3389/fvets.2022.789898
2. Hart, BL, Hart, LA, Thigpen, AP, and Willits, NH. Assisting decision-daking on age of neutering for 35 breeds of dogs: associated joint disorders, cancers, and urinary incontinence. Front Vet Sci. (2020) 7:388. doi: 10.3389/fvets.2020.00388
3. Adams, LM, and Turk, DC. Central sensitization and the biopsychosocial approach to understanding pain. J Appl Biobehav Res. (2018) 23:1–18. doi: 10.1111/jabr.12125
4. Kealy, RD, Lawler, DF, Ballam, JM, Mantz, SL, Biery, DN, Greeley, EH, et al. Effects of diet restriction on life span and age-related changes in dogs. J Am Vet Med Assoc. (2002) 220:1315–20. doi: 10.2460/javma.2002.220.1315
5. Mills, DS, Demontigny-Bédard, I, Gruen, M, Klinck, MP, McPeake, KJ, Barcelos, AM, et al. Pain and problem behavior in cats and dogs. Animals (Basel). (2020) 10:318. doi: 10.3390/ani10020318
6. Belshaw, Z, Dean, R, and Asher, L. "you can be blind because of loving them so much": the impact on owners in the United Kingdom of living with a dog with osteoarthritis. BMC Vet Res. (2020) 16:190. doi: 10.1186/s12917-020-02404-5
7. Bray, EE, Otto, CM, Udell, MAR, Hall, NJ, Johnston, AM, and MacLean, EL. Enhancing the selection and performance of working dogs. Front Vet Sci. (2021) 8:644431. doi: 10.3389/fvets.2021.644431
8. O'Neill, DG, Rooney, NJ, Brock, C, Church, DB, Brodbelt, DC, and Pegram, C. Greyhounds under general veterinary care in the UK during 2016: demography and common disorders. Canine Genet Epidemiol. (2019) 6:4. doi: 10.1186/s40575-019-0072-5
9. Houlton, JEF. Survey investigating the reasons why UK-based gundogs ceased working between 2010 and 2019. Vet Rec. (2022) 190:e1080. doi: 10.1002/vetr.1080
10. Isaksen, KE, Linney, L, Williamson, H, Norman, EJ, Cave, NJ, and Cogger, N. TeamMate: a longitudinal study of New Zealand working farm dogs. III. Factors affecting the risk of dogs being lost from the workforce. Animals (Basel). (2021) 11:1602. doi: 10.3390/ani11061602
11. Lawler, DF, Evans, RH, Larson, BT, Spitznagel, EL, Ellersieck, MR, and Kealy, RD. Influence of lifetime food restriction on causes, time, and predictors of death in dogs. J Am Vet Med Assoc. (2005) 226:225–31. doi: 10.2460/javma.2005.226.225
12. Corrigan, VK, Newman, RL, Richmond, P, Strand, EB, and Vaisman, JM. The future of flourishing in veterinary medicine: a systems-informed positive psychology approach in veterinary education. Front Vet Sci. (2024) 11:1484412. doi: 10.3389/fvets.2024.1484412
13. Mosley, C, Edwards, T, Romano, L, Truchetti, G, Dunbar, L, Schiller, T, et al. Proposed Canadian consensus guidelines on osteoarthritis treatment based on OA-COAST stages 1-4. Front Vet Sci. (2022) 9:830098. doi: 10.3389/fvets.2022.830098
14. Gruen, ME, Lascelles, BDX, Colleran, E, Gottlieb, A, Johnson, J, Lotsikas, P, et al. 2022 AAHA pain management guidelines for dogs and cats. J Am Anim Hosp Assoc. (2022) 58:55–76. doi: 10.5326/JAAHA-MS-7292
15. Johnston, SA. Osteoarthritis. Joint anatomy, physiology, and pathobiology. Vet Clin North Am Small Anim Pract. (1997) 27:699–723. doi: 10.1016/S0195-5616(97)50076-3
16. Guilak, F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol. (2011) 25:815–23. doi: 10.1016/j.berh.2011.11.013
17. Shen, J, Abu-Amer, Y, O’Keefe, RJ, and McAlinden, A. Inflammation and epigenetic regulation in osteoarthritis. Connect Tissue Res. (2017) 58:49–63. doi: 10.1080/03008207.2016.1208655
18. Sellam, J, and Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. (2010) 6:625–35. doi: 10.1038/nrrheum.2010.159
19. Li, G, Yin, J, Gao, J, Cheng, TS, Pavlos, NJ, Zhang, C, et al. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. (2013) 15:223. doi: 10.1186/ar4405
20. Moskowitz, RW, and Goldberg, VM. Studies of osteophyte pathogenesis in experimentally induced osteoarthritis. J Rheumatol. (1987) 14:311–20.
21. Fingleton, C, Smart, K, Moloney, N, Fullen, BM, and Doody, C. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthr Cartil. (2015) 23:1043–56. doi: 10.1016/j.joca.2015.02.163
22. Culvenor, AG, Ruhdorfer, A, Juhl, C, Eckstein, F, and Øiestad, BE. Knee extensor strength and risk of structural, symptomatic, and functional decline in knee osteoarthritis: a systematic review and Meta-analysis. Arthritis Care Res (Hoboken). (2017) 69:649–58. doi: 10.1002/acr.23005
23. Enomoto, M, de Castro, N, Hash, J, Thomson, A, Nakanishi-Hester, A, Perry, E, et al. Prevalence of radiographic appendicular osteoarthritis and associated clinical signs in young dogs. Sci Rep. (2024) 14:2827. doi: 10.1038/s41598-024-52324-9
24. Kealy, RD, Lawler, DF, Ballam, JM, Lust, G, Biery, DN, Smith, GK, et al. Evaluation of the effect of limited food consumption on radiographic evidence of osteoarthritis in dogs. J Am Vet Med Assoc. (2000) 217:1678–80. doi: 10.2460/javma.2000.217.1678
25. Lust, G, Williams, AJ, Burton-Wurster, N, Beck, KA, and Rubin, G. Effects of intramuscular administration of glycosaminoglycan polysulfates on signs of incipient hip dysplasia in growing pups. Am J Vet Res. (1992) 53:1836–43. doi: 10.2460/ajvr.1992.53.10.1836
26. Olson, NC, Carrig, CB, and Brinker, WO. Asynchronous growth of the canine radius and ulna: effects of retardation of longitudinal growth of the radius. Am J Vet Res. (1979) 40:351–5.
27. Villatoro, AS, Langenbach, A, Yoon, J, Garcia, TC, and Marcellin-Little, DJ. Stifle joint osteoarthritis in small-breed and medium-breed dogs is more severe after cranial cruciate ligament injury than medial patellar luxation. Vet Radiol Ultrasound. (2023) 64:385–92. doi: 10.1111/vru.13207
28. Latremoliere, A, and Woolf, CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. (2009) 10:895–926. doi: 10.1016/j.jpain.2009.06.012
29. de Godoy, MRC. Pancosma comparative gut physiology symposium: all about appetite regulation: effects of diet and gonadal steroids on appetite regulation and food intake of companion animals. J Anim Sci. (2018) 96:3526–36. doi: 10.1093/jas/sky146
30. Klein, C, Thes, M, Böswald, LF, and Kienzle, E. Metabolisable energy intake and growth of privately owned growing dogs in comparison with official recommendations on the growth curve and energy supply. J Anim Physiol Anim Nutr (Berl). (2019) 103:1952–8. doi: 10.1111/jpn.13191
31. Brady, RB, Sidiropoulos, AN, Bennett, HJ, Rider, PM, Marcellin-Little, DJ, and Devita, P. Evaluation of gait-related variables in lean and obese dogs at a trot. Am J Vet Res. (2013) 74:757–62. doi: 10.2460/ajvr.74.5.757
32. De Roover, A, Escribano-Núñez, A, Monteagudo, S, and Lories, R. Fundamentals of osteoarthritis: inflammatory mediators in osteoarthritis. Osteoarthr Cartil. (2023) 31:1303–11. doi: 10.1016/j.joca.2023.06.005
33. Salt, C, Morris, PJ, Butterwick, RF, Lund, EM, Cole, TJ, and German, AJ. Comparison of growth patterns in healthy dogs and dogs in abnormal body condition using growth standards. PLoS One. (2020) 15:e0238521. doi: 10.1371/journal.pone.0238521
34. Kasström, H. Nutrition, weight gain and development of hip dysplasia. An experimental investigation in growing dogs with special reference to the effect of feeding intensity. Acta Radiol Suppl. (1975) 344:135–79.
35. Dobenecker, B, Endres, V, and Kienzle, E. Energy requirements of puppies of two different breeds for ideal growth from weaning to 28 weeks of age. J Anim Physiol Anim Nutr (Berl). (2013) 97:190–6. doi: 10.1111/j.1439-0396.2011.01257.x
36. Kustritz, MV. Determining the optimal age for gonadectomy of dogs and cats. J Am Vet Med Assoc. (2007) 231:1665–75. doi: 10.2460/javma.231.11.1665
37. Torres de la Riva, G, Hart, BL, Farver, TB, Oberbauer, AM, Messam, LL, Willits, N, et al. Neutering dogs: effects on joint disorders and cancers in golden retrievers. PLoS One. (2013) 8:e55937. doi: 10.1371/journal.pone.0055937
38. Anderson, KL, Zulch, H, O'Neill, DG, Meeson, RL, and Collins, LM. Risk factors for canine osteoarthritis and its predisposing arthropathies: a systematic review. Front Vet Sci. (2020) 7:220. doi: 10.3389/fvets.2020.00220
39. Howe, LM. Current perspectives on the optimal age to spay/castrate dogs and cats. Vet Med (Auckl). (2015) 6:171–80. doi: 10.2147/VMRR.S53264
40. Kienzle, E, Bergler, R, and Mandernach, A. A comparison of the feeding behavior and the human-animal relationship in owners of normal and obese dogs. J Nutr. (1998) 128:S2779–82. doi: 10.1093/jn/128.12.2779S
41. Munoz-Prieto, A, Nielsen, LR, Dąbrowski, R, Bjørnvad, CR, Söder, J, Lamy, E, et al. European dog owner perceptions of obesity and factors associated with human and canine obesity. Sci Rep. (2018) 8:13353. doi: 10.1038/s41598-018-31532-0
42. Brooks, D, Churchill, J, Fein, K, Linder, D, Michel, KE, Tudor, K, et al. 2014 AAHA weight management guidelines for dogs and cats. J Am Anim Hosp Assoc. (2014) 50:1–11. doi: 10.5326/JAAHA-MS-6331
43. Cline, MG, Burns, KM, Coe, JB, Downing, R, Durzi, T, Murphy, M, et al. 2021 AAHA nutrition and weight management guidelines for dogs and cats. J Am Anim Hosp Assoc. (2021) 57:153–78. doi: 10.5326/JAAHA-MS-7232
44. Wakshlag, J, and Shmalberg, J. Nutrition for working and service dogs. Vet Clin North Am Small Anim Pract. (2014) 44:719–40, vi. doi: 10.1016/j.cvsm.2014.03.008
45. National Research Council, Division on Earth, Life Studies, Committee on Animal Nutrition, Subcommittee on Dog, & Cat Nutrition. Nutrient requirements of dogs and cats. Washington, D.C.: The National Academies Press (2006).
46. Debraekeleer, JGK, and Zicker, SC. Chapter 17 – feeding growing puppies: Postweaning to adulthood. In: MS Hand, CD Thatcher, RL Remillard, P Roudebush, and BJ Novotny, editors. Small animal clinical nutrition. 5th ed. Topeka: KS Mark Morris Institute (2010)
47. Laflamme, DP. Development and validation of a body condition score system for dogs. Canine Pract. (1997) 22:10–5.
48. Mawby, DI, Bartges, JW, d’Avignon, A, Laflamme, DP, Moyers, TD, and Cottrell, T. Comparison of various methods for estimating body fat in dogs. J Am Anim Hosp Assoc. (2004) 40:109–14. doi: 10.5326/0400109
49. Wolf, JM, and Drobatz, KJ. Body condition and hair coat length impact weight estimation in dogs and cats presented to an emergency department. J Am Vet Med Assoc. (2022) 261:353–7. doi: 10.2460/javma.22.08.0341
50. Eastland-Jones, RC, German, AJ, Holden, SL, Biourge, V, and Pickavance, LC. Owner misperception of canine body condition persists despite use of a body condition score chart. J Nutr Sci. (2014) 3:e45. doi: 10.1017/jns.2014.25
51. Greene, LM, Marcellin-Little, DJ, and Lascelles, BD. Associations among exercise duration, lameness severity, and hip joint range of motion in Labrador retrievers with hip dysplasia. J Am Vet Med Assoc. (2013) 242:1528–33. doi: 10.2460/javma.242.11.1528
52. Bannasch, D, Batcher, K, Leuthard, F, Bannasch, M, Hug, P, Marcellin-Little, DJ, et al. The effects of FGF4 retrogenes on canine morphology. Genes (Basel). (2022) 13:325. doi: 10.3390/genes13020325
53. Pechette Markley, A, Kieves, NR, Levine, D, and Marcellin-Little, DJ. Patient-centered physical rehabilitation in companion animals. Adv Sm Anim Care. (2023) 4:21–35. doi: 10.1016/j.yasa.2023.05.002
54. Brown, DC, Boston, RC, Coyne, JC, and Farrar, JT. Ability of the canine brief pain inventory to detect response to treatment in dogs with osteoarthritis. J Am Vet Med Assoc. (2008) 233:1278–83. doi: 10.2460/javma.233.8.1278
55. Pappa, E, Maddox, TW, Crystal, E, Comerford, EJ, and Tomlinson, AW. Recall bias in client-reported outcomes in canine orthopaedic patients using clinical metrology instruments. Vet Comp Orthop Traumatol. (2023) 36:302–10. doi: 10.1055/s-0043-1771032
56. Alves, JC, Santos, A, Jorge, P, Lavrador, C, and Carreira, LM. Evaluation of four clinical metrology instruments for the assessment of osteoarthritis in dogs. Animals (Basel). (2022) 12:2808. doi: 10.3390/ani12202808
57. Muller, C, Gaines, B, Gruen, M, Case, B, Arrufat, K, Innes, J, et al. Evaluation of clinical metrology instrument in dogs with osteoarthritis. J Vet Intern Med. (2016) 30:836–46. doi: 10.1111/jvim.13923
58. Innes, JF, Morton, MA, and Lascelles, BDX. Minimal clinically-important differences for the 'Liverpool osteoarthritis in Dogs' (LOAD) and the 'Canine orthopedic Index' (COI) client-reported outcomes measures. PLoS One. (2023) 18:e0280912. doi: 10.1371/journal.pone.0280912
59. Hyytiäinen, HK, Levine, D, and Marcellin-Little, DJ. Clinical instruments for the evaluation of orthopedic problems in dogs and human patients, a review. Adv Small Anim Care. (2023) 4:37–52. doi: 10.1016/j.yasa.2023.05.007
60. Hielm-Bjorkman, AK, Rita, H, and Tulamo, RM. Psychometric testing of the Helsinki chronic pain index by completion of a questionnaire in Finnish by owners of dogs with chronic signs of pain caused by osteoarthritis. Am J Vet Res. (2009) 70:727–34. doi: 10.2460/ajvr.70.6.727
61. Ryan, WG, Moldave, K, and Carithers, D. Clinical effectiveness and safety of a new NSAID, firocoxib: a 1,000 dog study. Vet Ther. (2006) 7:119–26.
62. Lascelles, BD, Gaynor, JS, Smith, ES, Roe, SC, Marcellin-Little, DJ, Davidson, G, et al. Amantadine in a multimodal analgesic regimen for alleviation of refractory osteoarthritis pain in dogs. J Vet Intern Med. (2008) 22:53–9. doi: 10.1111/j.1939-1676.2007.0014.x
63. Marcellin-Little, DJ, Levine, D, and Taylor, R. Rehabilitation and conditioning of sporting dogs. Vet Clin North Am Small Anim Pract. (2005) 35:1427–39, ix. doi: 10.1016/j.cvsm.2005.08.002
64. Henrotin, Y, Lambert, C, Couchourel, D, Ripoll, C, and Chiotelli, E. Nutraceuticals: do they represent a new era in the management of osteoarthritis? – a narrative review from the lessons taken with five products. Osteoarthr Cartil. (2011) 19:1–21. doi: 10.1016/j.joca.2010.10.017
65. Barbeau-Gregoire, M, Otis, C, Cournoyer, A, Moreau, M, Lussier, B, and Troncy, E. A 2022 systematic review and Meta-analysis of enriched therapeutic diets and nutraceuticals in canine and feline osteoarthritis. Int J Mol Sci. (2022) 23:10384. doi: 10.3390/ijms231810384
66. Trumble, TN, Billinghurst, RC, and McIlwraith, CW. Correlation of prostaglandin E2 concentrations in synovial fluid with ground reaction forces and clinical variables for pain or inflammation in dogs with osteoarthritis induced by transection of the cranial cruciate ligament. Am J Vet Res. (2004) 65:1269–75. doi: 10.2460/ajvr.2004.65.1269
67. Roush, JK, Cross, AR, Renberg, WC, Dodd, CE, Sixby, KA, Fritsch, DA, et al. Evaluation of the effects of dietary supplementation with fish oil omega-3 fatty acids on weight bearing in dogs with osteoarthritis. J Am Vet Med Assoc. (2010) 236:67–73. doi: 10.2460/javma.236.1.67
68. Roush, JK, Dodd, CE, Fritsch, DA, Allen, TA, Jewell, DE, Schoenherr, WD, et al. Multicenter veterinary practice assessment of the effects of omega-3 fatty acids on osteoarthritis in dogs. J Am Vet Med Assoc. (2010) 236:59–66. doi: 10.2460/javma.236.1.59
69. Bauer, JE. Therapeutic use of fish oils in companion animals. J Am Vet Med Assoc. (2011) 239:1441–51. doi: 10.2460/javma.239.11.1441
70. Mehler, SJ, May, LR, King, C, Harris, WS, and Shah, Z. A prospective, randomized, double blind, placebo-controlled evaluation of the effects of eicosapentaenoic acid and docosahexaenoic acid on the clinical signs and erythrocyte membrane polyunsaturated fatty acid concentrations in dogs with osteoarthritis. Prostaglandins Leukot Essent Fatty Acids. (2016) 109:1–7. doi: 10.1016/j.plefa.2016.03.015
71. Rialland, P, Bichot, S, Lussier, B, Moreau, M, Beaudry, F, del Castillo, J, et al. Effect of a diet enriched with green-lipped mussel on pain behavior and functioning in dogs with clinical osteoarthritis. Can J Vet Res. (2013) 77:66–74.
72. Varney, JL, Fowler, JW, and Coon, CN. Undenatured type II collagen mitigates inflammation and cartilage degeneration in healthy Labrador retrievers during an exercise regimen. Transl. Anim Sci. (2021) 5:txab084. doi: 10.1093/tas/txab084
73. Gupta, RC, Canerdy, TD, Lindley, J, Konemann, M, Minniear, J, Carroll, BA, et al. Comparative therapeutic efficacy and safety of type-II collagen (UC-II), glucosamine and chondroitin in arthritic dogs: pain evaluation by ground force plate. J Anim Physiol Anim Nutr (Berl). (2012) 96:770–7. doi: 10.1111/j.1439-0396.2011.01166.x
74. Stabile, M, Samarelli, R, Trerotoli, P, Fracassi, L, Lacitignola, L, Crovace, A, et al. Evaluation of the effects of undenatured type II collagen (UC-II) as compared to robenacoxib on the mobility impairment induced by osteoarthritis in dogs. Vet Sci. (2019) 6:72. doi: 10.3390/vetsci6030072
75. Sahin, E, Orhan, C, Erten, F, Saiyed, Z, Azari, EK, Durkee, S, et al. The effect of oral administration of undenatured type II collagen on monosodium iodoacetate-induced osteoarthritis in young and old rats. Sci Rep. (2023) 13:6499. doi: 10.1038/s41598-023-33763-2
76. Navarro, SL, White, E, Kantor, ED, Zhang, Y, Rho, J, Song, X, et al. Randomized trial of glucosamine and chondroitin supplementation on inflammation and oxidative stress biomarkers and plasma proteomics profiles in healthy humans. PLoS One. (2015) 10:e0117534. doi: 10.1371/journal.pone.0117534
77. Meng, Z, Liu, J, and Zhou, N. Efficacy and safety of the combination of glucosamine and chondroitin for knee osteoarthritis: a systematic review and meta-analysis. Arch Orthop Trauma Surg. (2023) 143:409–21. doi: 10.1007/s00402-021-04326-9
78. Yang, W, Sun, C, He, SQ, Chen, JY, Wang, Y, and Zhuo, Q. The efficacy and safety of disease-modifying osteoarthritis drugs for knee and hip osteoarthritis-a systematic review and network Meta-analysis. J Gen Intern Med. (2021) 36:2085–93. doi: 10.1007/s11606-021-06755-z
79. Aragon, CL, Hofmeister, EH, and Budsberg, SC. Systematic review of clinical trials of treatments for osteoarthritis in dogs. J Am Vet Med Assoc. (2007) 230:514–21. doi: 10.2460/javma.230.4.514
80. Vandeweerd, JM, Coisnon, C, Clegg, P, Cambier, C, Pierson, A, Hontoir, F, et al. Systematic review of efficacy of nutraceuticals to alleviate clinical signs of osteoarthritis. J Vet Intern Med. (2012) 26:448–56. doi: 10.1111/j.1939-1676.2012.00901.x
81. Sanderson, RO, Beata, C, Flipo, RM, Genevois, JP, Macias, C, Tacke, S, et al. Systematic review of the management of canine osteoarthritis. Vet Rec. (2009) 164:418–24. doi: 10.1136/vr.164.14.418
82. McCarthy, G, O'Donovan, J, Jones, B, McAllister, H, Seed, M, and Mooney, C. Randomised double-blind, positive-controlled trial to assess the efficacy of glucosamine/chondroitin sulfate for the treatment of dogs with osteoarthritis. Vet J. (2007) 174:54–61. doi: 10.1016/j.tvjl.2006.02.015
83. Moreau, M, Dupuis, J, Bonneau, NH, and Desnoyers, M. Clinical evaluation of a nutraceutical, carprofen and meloxicam for the treatment of dogs with osteoarthritis. Vet Rec. (2003) 152:323–9. doi: 10.1136/vr.152.11.323
84. Scott, RM, Evans, R, and Conzemius, MG. Efficacy of an oral nutraceutical for the treatment of canine osteoarthritis. A double-blind, randomized, placebo-controlled prospective clinical trial. Vet Comp Orthop Traumatol. (2017) 30:318–23. doi: 10.3415/VCOT-17-02-0020
85. Ukai, M, McGrath, S, and Wakshlag, J. The clinical use of cannabidiol and cannabidiolic acid-rich hemp in veterinary medicine and lessons from human medicine. J Am Vet Med Assoc. (2023) 261:623–31. doi: 10.2460/javma.23.02.0064
86. Grundy, SA. Clinically relevant physiology of the neonate. Vet Clin North Am Small Anim Pract. (2006) 36:443–59, v. doi: 10.1016/j.cvsm.2005.12.002
87. Zoetis. Librela presribing information. (2024). Available online at: https://www.zoetisus.com/content/pages/products/dogs/Librela/Resources/documents/Librela-prescribing-information.pdf?_ga=2.30757311.167200569.1718821062-1126290968.1718821062 (accessed June 19, 2024).
88. Hoskins, JD, and Shelton, OD. Chapter 19 – the nervous and neuromuscular systems. In: JD Hoskins, editor. veterinary pediatrics: dogs and cats from birth to six months Philadelphia: W.B. Saunders: Elsevier (2001). 425–62.
89. Xue, Y, Wang, X, Wang, X, Huang, L, Yao, A, and Xue, Y. A comparative study of the efficacy of intra-articular injection of different drugs in the treatment of mild to moderate knee osteoarthritis: a network meta-analysis. Medicine (Baltimore). (2023) 102:e33339. doi: 10.1097/MD.0000000000033339
90. Goodrich, LR, and Nixon, AJ. Medical treatment of osteoarthritis in the horse – a review. Vet J. (2006) 171:51–69. doi: 10.1016/j.tvjl.2004.07.008
91. Lotsikas, PJ. Intra-articular injectates: what to use and why. Vet Clin North Am Small Anim Pract. (2022) 52:967–75. doi: 10.1016/j.cvsm.2022.03.004
92. Di Salvo, A, Chiaradia, E, Nannarone, S, and Della Rocca, G. Intra-articular use of analgesic/antinflammatory drugs in dogs and horses. Res Vet Sci. (2021) 134:159–70. doi: 10.1016/j.rvsc.2020.12.014
93. Miller, AV, Carney, PC, Markmann, A, and Frye, CW. Retrospective analysis describes safety of therapeutic joint injections in dogs. J Am Vet Med Assoc. (2023) 261:397–402. doi: 10.2460/javma.22.11.0483
94. Wernecke, C, Braun, HJ, and Dragoo, JL. The effect of intra-articular corticosteroids on articular cartilage: a systematic review. Orthop J Sports Med. (2015) 3:2325967115581163. doi: 10.1177/2325967115581163
95. Balazs, EA, and Denlinger, JL. Viscosupplementation: a new concept in the treatment of osteoarthritis. J Rheumatol Suppl. (1993) 39:3–9.
96. Jevsevar, D, Donnelly, P, Brown, GA, and Cummins, DS. Viscosupplementation for osteoarthritis of the knee: a systematic review of the evidence. J Bone Joint Surg Am. (2015) 97:2047–60. doi: 10.2106/JBJS.N.00743
97. Wang, S, Qiu, Y, Qu, L, Wang, Q, and Zhou, Q. Hydrogels for treatment of different degrees of osteoarthritis. Front Bioeng Biotechnol. (2022) 10:858656. doi: 10.3389/fbioe.2022.858656
98. Kaneps, AJ. A one-health perspective: use of hemoderivative regenerative therapies in canine and equine patients. J Am Vet Med Assoc. (2023) 261:301–8. doi: 10.2460/javma.22.12.0556
99. Carr, BJ, Miller, AV, Colbath, AC, Peralta, S, and Frye, CW. Literature review details and supports the application of platelet-rich plasma products in canine medicine, particularly as an orthobiologic agent for osteoarthritis. J Am Vet Med Assoc. (2024) 262:S8–S15. doi: 10.2460/javma.23.12.0692
100. Carr, BJ, Canapp, SO Jr, Mason, DR, Cox, C, and Hess, T. Canine platelet-rich plasma systems: a prospective analysis. Front Vet Sci. (2015) 2:73. doi: 10.3389/fvets.2015.00073
101. Franklin, SP, Garner, BC, and Cook, JL. Characteristics of canine platelet-rich plasma prepared with five commercially available systems. Am J Vet Res. (2015) 76:822–7. doi: 10.2460/ajvr.76.9.822
102. Lee, MI, Kim, JH, Kwak, HH, Woo, HM, Han, JH, Yayon, A, et al. A placebo-controlled study comparing the efficacy of intra-articular injections of hyaluronic acid and a novel hyaluronic acid-platelet-rich plasma conjugate in a canine model of osteoarthritis. J Orthop Surg Res. (2019) 14:314. doi: 10.1186/s13018-019-1352-1
103. Vilar, JM, Cuervo, B, Rubio, M, Sopena, J, Domínguez, JM, Santana, A, et al. Effect of intraarticular inoculation of mesenchymal stem cells in dogs with hip osteoarthritis by means of objective force platform gait analysis: concordance with numeric subjective scoring scales. BMC Vet Res. (2016) 12:223. doi: 10.1186/s12917-016-0852-z
104. Fortier, LA. Equine bone marrow aspirate concentrate. Vet Clin North Am Equine Pract. (2023) 39:453–9. doi: 10.1016/j.cveq.2023.05.002
105. Kim, GB, Seo, MS, Park, WT, and Lee, GW. Bone marrow aspirate concentrate: its uses in osteoarthritis. Int J Mol Sci. (2020) 21:3224. doi: 10.3390/ijms21093224
106. Schroers, M, Bruns, Y, Waselau, AC, Steigmeier-Raith, S, and Meyer-Lindenberg, A. Autologous point-of-care stromal vascular fraction transplantation in dogs with advanced osteoarthritis of the knee and hip joints. Aust Vet J. (2024) 102:41–6. doi: 10.1111/avj.13303
107. Brondeel, C, Pauwelyn, G, de Bakker, E, Saunders, J, Samoy, Y, and Spaas, JH. Review: mesenchymal stem cell therapy in canine osteoarthritis research: "Experientia docet" (experience will teach us). Front Vet Sci. (2021) 8:668881. doi: 10.3389/fvets.2021.668881
108. Cabon, Q, Febre, M, Gomez, N, Cachon, T, Pillard, P, Carozzo, C, et al. Long-term safety and efficacy of single or repeated intra-articular injection of allogeneic neonatal mesenchymal stromal cells for managing pain and lameness in moderate to severe canine osteoarthritis without anti-inflammatory pharmacological support: pilot clinical study. Front Vet Sci. (2019) 6:10. doi: 10.3389/fvets.2019.00010
109. Sanghani-Kerai, A, Black, C, Cheng, SO, Collins, L, Schneider, N, Blunn, G, et al. Clinical outcomes following intra-articular injection of autologous adipose-derived mesenchymal stem cells for the treatment of osteoarthritis in dogs characterized by weight-bearing asymmetry. Bone Joint Res. (2021) 10:650–8. doi: 10.1302/2046-3758.1010.BJR-2020-0540.R1
110. Mehranfar, S, Abdi Rad, I, Mostafavi, E, and Akbarzadeh, A. The use of stromal vascular fraction (SVF), platelet-rich plasma (PRP) and stem cells in the treatment of osteoarthritis: an overview of clinical trials. Artif Cells Nanomed Biotechnol. (2019) 47:882–90. doi: 10.1080/21691401.2019.1576710
111. Jevotovsky, DS, Alfonso, AR, Einhorn, TA, and Chiu, ES. Osteoarthritis and stem cell therapy in humans: a systematic review. Osteoarthr Cartil. (2018) 26:711–29. doi: 10.1016/j.joca.2018.02.906
112. Dunlap, AE, Mathews, KG, Walters, BL, Bruner, KA, Ru, H, and Marcellin-Little, DJ. Three-dimensional assessment of the influence of juvenile pubic symphysiodesis on the pelvic geometry of dogs. Am J Vet Res. (2018) 79:1217–25. doi: 10.2460/ajvr.79.11.1217
113. Manley, PA, Adams, WM, Danielson, KC, Dueland, RT, and Linn, KA. Long-term outcome of juvenile pubic symphysiodesis and triple pelvic osteotomy in dogs with hip dysplasia. J Am Vet Med Assoc. (2007) 230:206–10. doi: 10.2460/javma.230.2.206
114. Horwood, C, Carvajal, JL, Pozzi, A, and Kim, SE. Complications and outcomes of total hip arthroplasty in dogs with luxoid hip dysplasia: 18 cases (2010-2022). Vet Surg. (2024) 53:620–9. doi: 10.1111/vsu.14087
115. Shubert, MP, Filliquist, B, Chou, PY, Kapatkin, AS, Spriet, M, Kim, SY, et al. Results of using multiplanar reconstructed CT images for assessing elbow joint osteoarthritis in dogs are consistent with results of radiographic assessment. Am J Vet Res. (2022) 83:ajvr.22.04.0066. doi: 10.2460/ajvr.22.04.0066
116. Stokes, IA. Mechanical effects on skeletal growth. J Musculoskelet Neuronal Interact. (2002) 2:277–80.
117. Sellon, DC, and Marcellin-Little, DJ. Risk factors for cranial cruciate ligament rupture in dogs participating in canine agility. BMC Vet Res. (2022) 18:39. doi: 10.1186/s12917-022-03146-2
118. Gilbert, S, Langenbach, A, Marcellin-Little, DJ, Pease, AP, and Ru, H. Stifle joint osteoarthritis at the time of diagnosis of cranial cruciate ligament injury is higher in boxers and in dogs weighing more than 35 kilograms. Vet Radiol Ultrasound. (2019) 60:280–8. doi: 10.1111/vru.12718
119. Theyse, LF, Voorhout, G, and Hazewinkel, HA. Prognostic factors in treating antebrachial growth deformities with a lengthening procedure using a circular external skeletal fixation system in dogs. Vet Surg. (2005) 34:424–35. doi: 10.1111/j.1532-950X.2005.00064.x
120. Johnson, J, Austin, C, and Breur, GJ. Incidence of canine appendicular musculoskeletal disorders in 16 veterinary teaching hospitals from 1980 through 1989. Vet Comp Orthop Traumatol. (1994) 7:56–69. doi: 10.1055/s-0038-1633097
121. Enomoto, M, Baines, EA, Roe, SC, Marcellin-Little, DJ, and Lascelles, BDX. Defining the rate of, and factors influencing, radiographic progression of osteoarthritis of the canine hip joint. Vet Rec. (2021) 189:e516. doi: 10.1002/vetr.516
122. McLarty, E, Spriet, M, Beylin, D, Chou, PY, Filliquist, B, Marcellin-Little, DJ, et al. Comparison of 18F-sodium fluoride positron emission tomography and CT: an exploratory study in 12 dogs with elbow pain. Vet Radiol Ultrasound. (2021) 62:498–506. doi: 10.1111/vru.12967
123. Krontveit, RI, Trangerud, C, Sævik, BK, Skogmo, HK, and Nødtvedt, A. Risk factors for hip-related clinical signs in a prospective cohort study of four large dog breeds in Norway. Prev Vet Med. (2012) 103:219–27. doi: 10.1016/j.prevetmed.2011.09.018
Keywords: osteoarthritis, dog, physical rehabilitation, weight management, non-steroidal anti-inflammatory drugs, nutraceuticals, nutritional supplements, medical management guidelines
Citation: Marcellin-Little DJ, Hulse DA, Huntingford JL, Grubb T, Brunke MW, Markley AP and Frank B (2025) A proposed framework for practical multimodal management of osteoarthritis in growing dogs. Front. Vet. Sci. 12:1565922. doi: 10.3389/fvets.2025.1565922
Edited by:
Ismael Hernández Avalos, National Autonomous University of Mexico, MexicoReviewed by:
Giorgia della Rocca, University of Perugia, ItalyAlejandro Casas Alvarado, Autonomous Metropolitan University, Mexico
Copyright © 2025 Marcellin-Little, Hulse, Huntingford, Grubb, Brunke, Pechette Markley and Frank. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Denis J. Marcellin-Little, ZGptYXJjZWxAdWNkYXZpcy5lZHU=
 Denis J. Marcellin-Little
Denis J. Marcellin-Little Donald A. Hulse
Donald A. Hulse Janice L. Huntingford
Janice L. Huntingford Tamara Grubb4
Tamara Grubb4 Matthew W. Brunke
Matthew W. Brunke Arielle Pechette Markley
Arielle Pechette Markley Bethany Frank
Bethany Frank