- 1Department of Quality Management and Inspection and Quarantine, Yibin University, Yibin, China
- 2YibinShangougou Agricultural Science and Technology Co., Ltd, Yibin, China
- 3Institute of Animal Nutrition, Key Laboratory for Animal Disease-Resistance Nutrition of China Ministry of Education, Sichuan Agricultural University, Chengdu, China
- 4Yibin Agricultural Product Quality Inspection and Testing Center, Yibin, China
Introduction: Theaflavins (TF), natural compounds extracted from black tea, demonstrate various beneficial functions including antioxidant properties and lipid metabolism regulation. However, their effects on laying hens remained unclear. This study investigated the effects of different TF levels on production performance, egg quality, antioxidant capacity, lipid metabolism in laying hens, and egg antioxidant capacity.
Methods: A total of 512 twenty-nine-week-old Lohmann commercial laying hens were randomly divided into four dietary treatments (0, 250, 500, or 1000 mg/kg TF) in a completely randomized design. Each treatment consisted of eight replicates of 16 birds, and the experiment lasted for 8 weeks. Data were analyzed using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test, with quadratic polynomial contrasts applied to evaluate dose-response relationships.
Results: Results showed that TF supplementation quadratically increased (p < 0.05) egg production, egg yolk color, serum total antioxidant capacity (T-AOC), hepatic T-AOC, uterine T-AOC, hepatic superoxide dismutase activity, serum total cholesterol (TC), hepatic TC, hepatic triglycerides, and the expression of antioxidant-related and lipid metabolism-related genes. It also enhanced the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging capacity, T-AOC, tyrosine, and tryptophan levels in egg yolks. TF supplementation significantly decreased (p < 0.05) malondialdehyde (MDA) levels in serum, liver, ovaries, and egg yolks.
Conclusion: These findings suggest that TF improves laying performance by enhancing antioxidant capacity and regulating lipid metabolism, while simultaneously boosting egg antioxidant potential. Based on the quadratic regression analysis, the optimal TF supplementation level was determined to be 500 mg/kg.
1 Introduction
Theaflavins (TF) are key functional phytochemicals formed through the enzymatic action of polyphenol oxidase during the processing of black tea. They constitute 2–20 g/kg of the dry weight of solids in brewed black tea (1). The main structure of TF features a seven-membered benzotropolone ring, followed by decarboxylation and simultaneous fusion with the epicatechin ring B or epicatechin gallate (2). Over 25 types of TF have been identified, with the major characterized structures being theaflavin (TF1), theaflavin 3-gallate (TF2A), theaflavin-3′-allate (TF2B), and theaflavin-3,3′-digallate (TF3).
TF have been widely reported to resist the oxidative stress and regulated the lipid metabolism. Firstly, TF have excellent antioxidant capacity. Yang et al. report that TF exhibit the highest antioxidant activity in the Fenton reaction system, 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, and H2O2-mediated oxidative damage in HPF-1 cells, compared to that of other oxidized phenolic compounds in black tea (3). The hydroxyl radical-scavenging ability and DPPH scavenging ability follow the order TF3 > TF2 > TF1 > epigallocatechin-3-gallate (EGCG) (4). Research on diabetic mice reveals that TF could counteract the increase and decrease of malondialdehyde (MDA) and glutathione peroxidase (GSH-Px) levels in the liver and kidney tissues, respectively (5). The nuclear factor erythroid 2-related factor 2 (Nrf2) pathway is a key regulator of oxidative stress (6). TF upregulate the expression of total Nrf2, nuclear Nrf2, and its downstream targets heme oxygenase 1 (HO-1) and NAD(P)H quinone dehydrogenase 1 (NQO1), protecting the liver and kidneys of mice from oxidative damage (4). Second, TF regulate lipid metabolism, improving hyperlipidemia and regulating body composition in human studies. TF decrease the serum levels of total cholesterol (TC), triglyceride (TG), and low-density lipoprotein cholesterol in obese rats by 26.5, 50.8, and 71.7%, respectively (7). This effect is mainly attributed to the ability of TF to inhibit lipid synthesis and catabolism. TF decrease the mRNA and protein expression levels of fatty acid synthase (FAS), acetyl-CoA carboxylase (ACC), and HMG-CoA reductase while increasing the expression levels of carnitine palmitoyl transferase 1 (CPT1), thereby inhibiting fatty acid and cholesterol synthesis and decreasing lipid accumulation (8, 9). In our previous study, dietary supplementation with 600 mg/kg tea polyphenols was observed to alleviate oxidative stress induced by aging corn in laying hens. However, the potential for TF to demonstrate superior antioxidant efficacy in this context remains unclear, warranting further comparative investigation (10).
Currently, the research on the physiological functions of TF is already very extensive, and it has been demonstrated that they have the effects of enhancing antioxidant capacity and regulating lipid metabolism. However, it is unclear whether TF can be applied in laying hens, what their effects are on the production performance of laying hens and the antioxidant capacity of eggs, and what the optimal level of addition is for laying hens. Thereby, this study was conducted to investigate the effects of different levels of TF on production performance, egg quality, antioxidant capacity, lipid metabolism in laying hens, and the antioxidant capacity of eggs. We hypothesized that 500 mg/kg TF would increase the performance, egg quality, antioxidant status and egg antioxidant capacity of laying hens.
2 Materials and methods
2.1 Theaflavins
TF were purchased from Dannisi Technology Co., Ltd. (Shan’xi province, China), with 62.9% purity. The composition includes 40.67% TF1, 8.91% TF3, 7.14% TF2A, and 6.18% TF2B.
2.2 Animals and diets
This study was performed on a poultry farm in Yi’bin, China, during the summer (Latitude: 28.7599°N, Longitude: 104.6399°E). All experimental procedures were approved by the Animal Care and Use Committee of Yibin University (No. 202402). Twenty-nine-week-old (n = 512) Lohmann commercial laying hens were randomly allocated to one of the four dietary treatments. Each treatment included eight replicates, with 16 hens per replicate, housed in two cages containing eight birds each. The housing temperature and relative humidity were 20 ± 4°C and 50–65%, respectively. The four treatment groups consisted of a control group (basal diet) and three experimental groups with different levels of TF addition (basal diet + 250, 500, 1,000 mg/kg TF). The diets (Table 1) were based on corn and soybean meal, with nutrient levels aligned with the Agricultural Trade Standardization of China (11). Feed and water were supplied ad libitum, and a 16L:8D photoperiod was used during the 8-week laying hens trial.
2.3 Hen performance
Daily records were kept of egg production, egg weight, and the quantity of dirty and broken eggs. The laying rate, average egg weight, and rates of dirty and broken eggs were then computed. Weekly feed intake (ADFI) was monitored, and the feed conversion rate (FCR) was determined.
2.4 Egg collection
At the end of the trial, a total of 128 eggs for each treatment, with four eggs in each replicate, were gathered and split into two groups. One half of the eggs was utilized for assessing egg quality. While the other half were used for measuring the antioxidant capacity of the egg yolks (two pooled egg yolks per replicate).
2.5 Sample collection
In the final days of the trial, 10 mL blood samples (n = 8) were taken from the brachial vein of one laying hen per replicate and placed in lithium heparinized vacutainers. The serum was separated at 4°C and then stored at −20°C for analysis of antioxidant status. After the blood collection, the chicks were euthanized by cervical dislocation, and the liver and ovaries were promptly extracted, frozen at −80°C, and stored for antioxidant status analysis.
2.6 Egg quality
The Multi-tester (EMT-7300, Robotmation Co., Ltd., Tokyo, Japan) was utilized to measure the albumen height, Haugh unit, and color of the egg yolk. The yolk and eggshell (which had been dried at room temperature for 3 days) were meticulously separated and weighed. The ratios of the eggshell, albumen, and yolk within the egg were then calculated. The thickness of the eggshell (excluding the inner and outer membranes) was measured using Vernier calipers, with the average taken from three measurements at different locations (top, middle, and bottom).
2.7 Antioxidant status of laying hens
Antioxidant enzyme activity. Liver and ovarian tissue samples (n = 8) were homogenized in PBS and centrifuged at 1,699×g for 10 min. The supernatant was then collected for the determination of correlation indices. The activities of SOD, total antioxidant capacity (T-AOC), malondialdehyde (MDA) levels in the liver, ovarian tissue, and serum, as well as TC and TG in the liver and serum, were analyzed using reagent kits from Jiancheng Bioengineering Institute in Jiangsu, China. SOD activity was assessed using the hydroxylamine method, where superoxide anions generated from the xanthine–xanthine oxidase system oxidized hydroxylamine to produce nitrite, which was measured at 550 nm using a colorimetric approach. The production of nitrite was inhibited by SOD, indicating the activity of superoxide anions. One unit of SOD is defined as the amount of sample that inhibits 50% of the activity under the assay conditions, and SOD activity was reported as units per gram of tissue. T-AOC was evaluated using the ferric reducing-antioxidant power assay, conducted within a 5-min timeframe at 37°C and measured at 593 nm. MDA levels were determined using the thiobarbituric acid method, where 1 mL of the homogenate was treated with thiobarbituric acid, mixed quickly, and heated in a boiling water bath for 40 min. The samples were then centrifuged at 10,621×g for 10 min, and the supernatants were placed in a 96-well plate and measured at 532 nm.
Antioxidant-related gene expression. Total RNA was extracted from liver tissue using Trizol reagent (Takara, Dalian, China), according to the protocol of the manufacturer. The absorbance ratio at 260–280 nm was calculated for each sample. cDNA synthesis was conducted using the primeScript RT reagent kit (Takara), and real-time PCR was performed using the SYBR Premix Ex Tap (Takara). The PCRs were conducted on an Applied Biosystems 7900HT Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The amplification cycles included an initial denaturation at 95°C for 35 s, followed by 40 cycles of denaturation at 95°C for 5 s, annealing at 60°C for 34 s, and extension at 95°C for 15 s, and a final elongation step at 60°C for 15 s. Table 2 presents the primer sequences for antioxidant-related genes.
2.8 Lipid metabolism index of laying hens
TG and TC. TC and TG (n = 8) in liver and serum were analyzed using reagent kits (Jiancheng Bioengineering Institute, Jiangsu, China), and the supernatant was used for antioxidant status analysis. TG and TC were determined using the glycerol-3-phosphate oxidase peroxidase method and measured at 500 nm.
Lipid metabolism-related gene expression. The liver FAS, ACC, HMGCR, and CPT1 were measured according to the methods described for antioxidant-related gene expression analysis. Table 2 shows the primer sequences for lipid metabolism-related genes.
2.9 Antioxidant capacity of eggs
Free radical-scavenging activity on 1,1-diphenyl-2-picrylhydrazyl (DPPH). The scavenging effect of the DPPH free radical (n = 8) was measured using a previously described method (12). A sample solution (1 mL, containing 10 mg of egg yolk per mL; with ethanol as a control) was combined with an equal volume of a DPPH (0.1 mM) solution, mixed thoroughly, and left to incubate for 30 min at room temperature. The absorbance was measured at 517 nm. The scavenging effect was calculated using this formula: DPPH scavenging activity (%) = [(Blank absorbance − Sample absorbance) /Blank absorbance] × 100.
MDA. The egg yolk samples (n = 8) were homogenized in PBS and centrifuged at 1,699×g for 10 min. The resulting supernatant was collected for MDA determination. MDA content in egg yolk was measured as described in the tissue antioxidant status analysis.
SOD and T-AOC. SOD activity and T-AOC (n = 8) in egg yolks were measured as described in the tissue analysis methods.
2.10 Egg composition
Free amino acid. Free amino acids were determined using the method described by Nimalaratne et al. (13) with slight modifications (n = 8). About 100 mg of freeze-dried egg yolk was mixed with 1 mL of a 10% sulfosalicylic acid solution, vortexed, and then centrifuged at 15,294×g for 10 min using a Centrifuge 5430/R from Eppendorf in Hamburg, Germany. The resulting supernatant was passed through a 0.22-μm nylon syringe filter prior to analysis with an automated AA analyzer (L-8900, Hitachi, Tokyo, Japan) equipped with a lithium high-performance column.
Fatty acid profile. The analysis of the egg yolk (n = 8) was measured according to the national standard method (14). Fatty acid methyl esters were analyzed using a gas chromatograph (7890A, Agilent Technologies, Santa Clara, CA, USA) that featured a flame ionization detector and a silica capillary column (Supelco SP-2560; 100 × 0.25 mm i.d., 0.20 μm film thickness). Nitrogen was used as the carrier gas at a pressure of 0.5 kg/cm2. The injector and detector temperatures were kept at 250°C. The findings were reported as percentages (%) of the total fatty acids.
2.11 Statistical analysis
The results were statistically analyzed using one-way ANOVA followed by Duncan’s multiple range tests of SPSS 27.0 (SPSS Inc., Chicago, IL, USA). Regression analysis was subsequently performed to evaluate linear and quadratic effects. Differences among treatments were considered significant at p < 0.05, and results were reported as mean and standard error mean (SEM).
3 Results
3.1 Hen performance
Dietary TF levels had no significant effect on hen performance, including egg weight, ADFI, FCR, as well as dirty and broken egg rate (Table 3). Increasing TF levels quadratically (p < 0.05) increased egg production throughout the entire laying period.
3.2 Egg quality
Dietary TF levels had no significant effect on egg quality, including eggshell color, egg strength, eggshell thickness, albumen height, and Haugh units (Table 4). However, increasing TF levels quadratically (p < 0.05) increased the egg yolk color.
3.3 Antioxidant status of laying hens
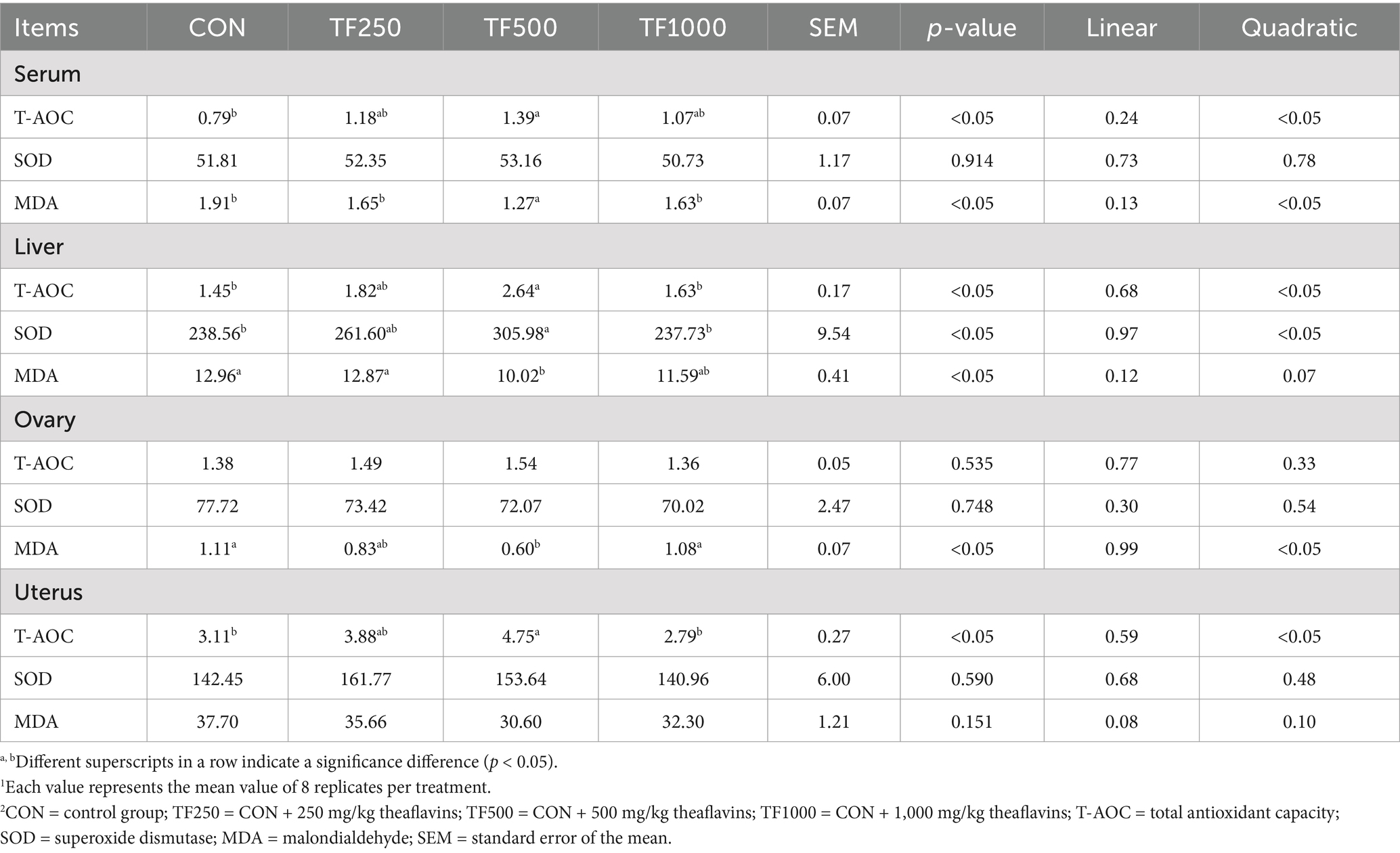
Antioxidant enzyme activity assay. TF significantly enhanced the antioxidant capacity of laying hens (Table 5). Increasing TF levels quadratically (p < 0.05) increased the serum T-AOC, liver T-AOC, uterus T-AOC, and liver SOD, while it quadratically (p < 0.05) decreased the MDA levels in the serum, liver, and ovary of laying hens.
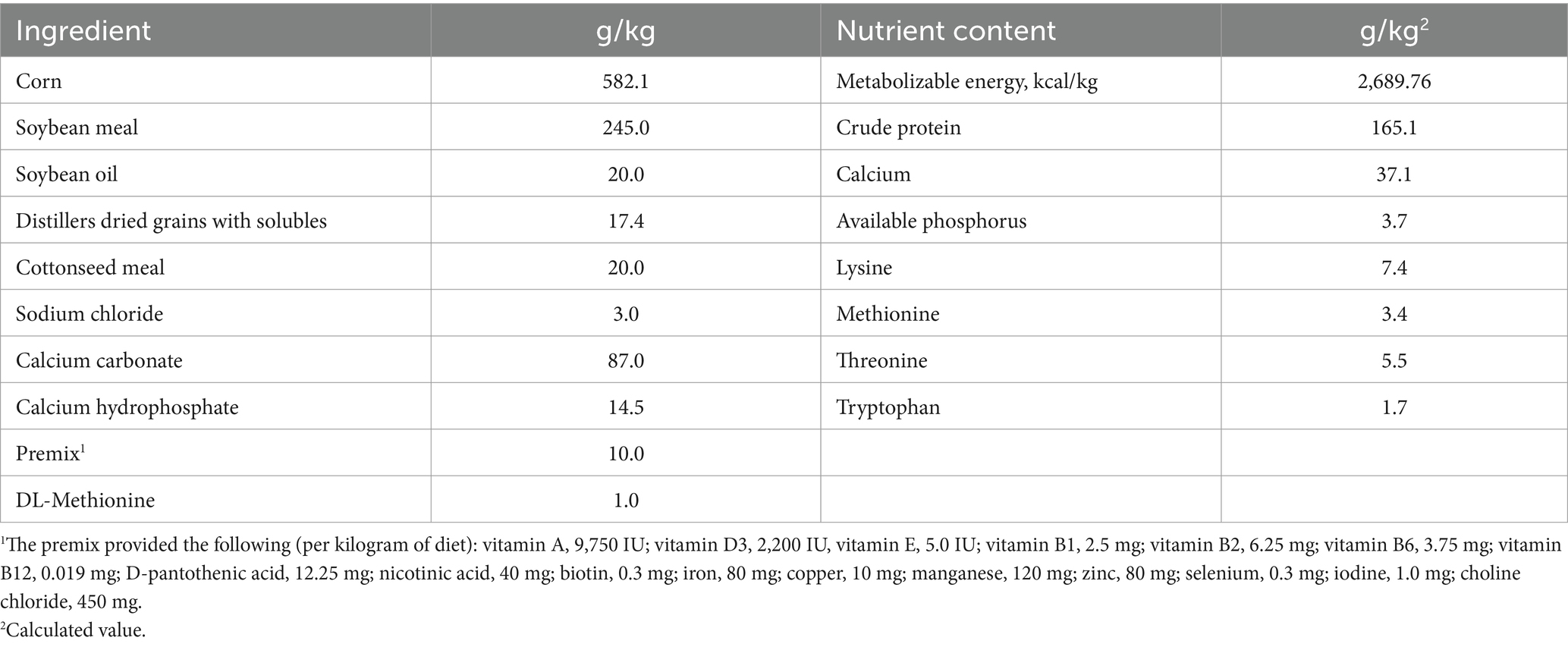
Antioxidant-related gene expression. Figure 1 illustrates the expression of antioxidant-related genes. Increasing TF levels quadratically (p < 0.05) increased the expression of Nrf2, HO-1, and NQO1 in the liver of laying hens.
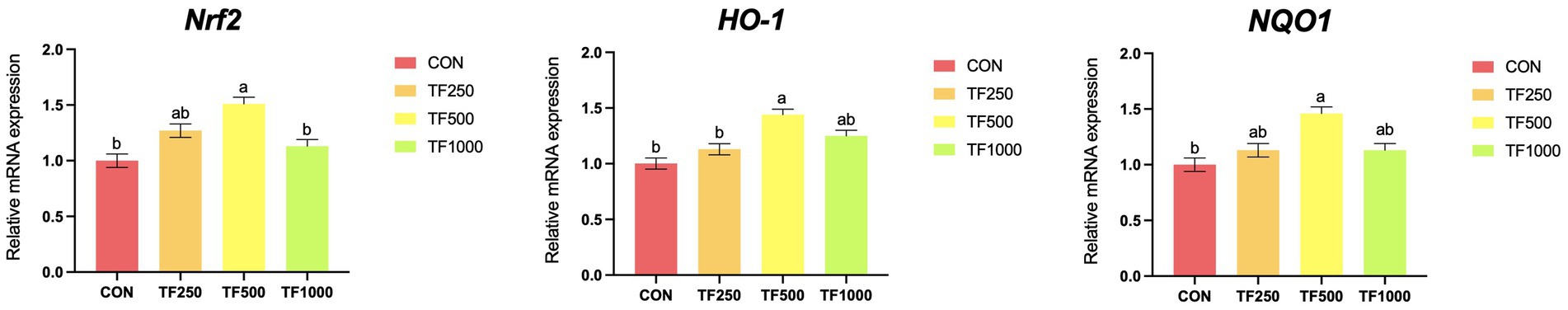
Figure 1. Antioxidant-related gene expression of laying hens at different theaflavin levels. a,bDifferent superscripts indicate a significance difference (p < 0.05). Nrf2 = nuclear factor erythroid 2-related 2; NQO1 = NAD(P)H: quinone dehydrogenase 1; HO-1 = heme oxygenase-1. 1Values are means ± SEM. Each value represents the mean value of 8 replicates per treatment. 2All the data were acquired using real-time PCR.
3.4 Lipid metabolism indexes of laying hen
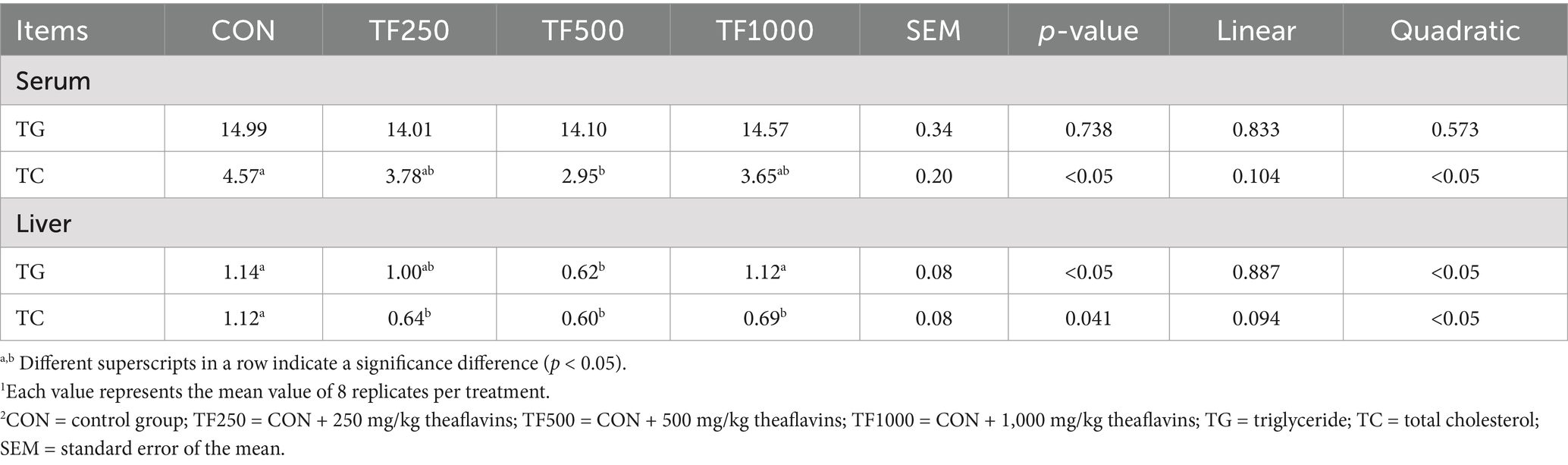
TG and TC. Table 6 shows the lipid metabolism indices in serum and liver. Increasing TF levels quadratically (p < 0.05) decreased the serum TC, as well as the liver TC and TG content in laying hens.
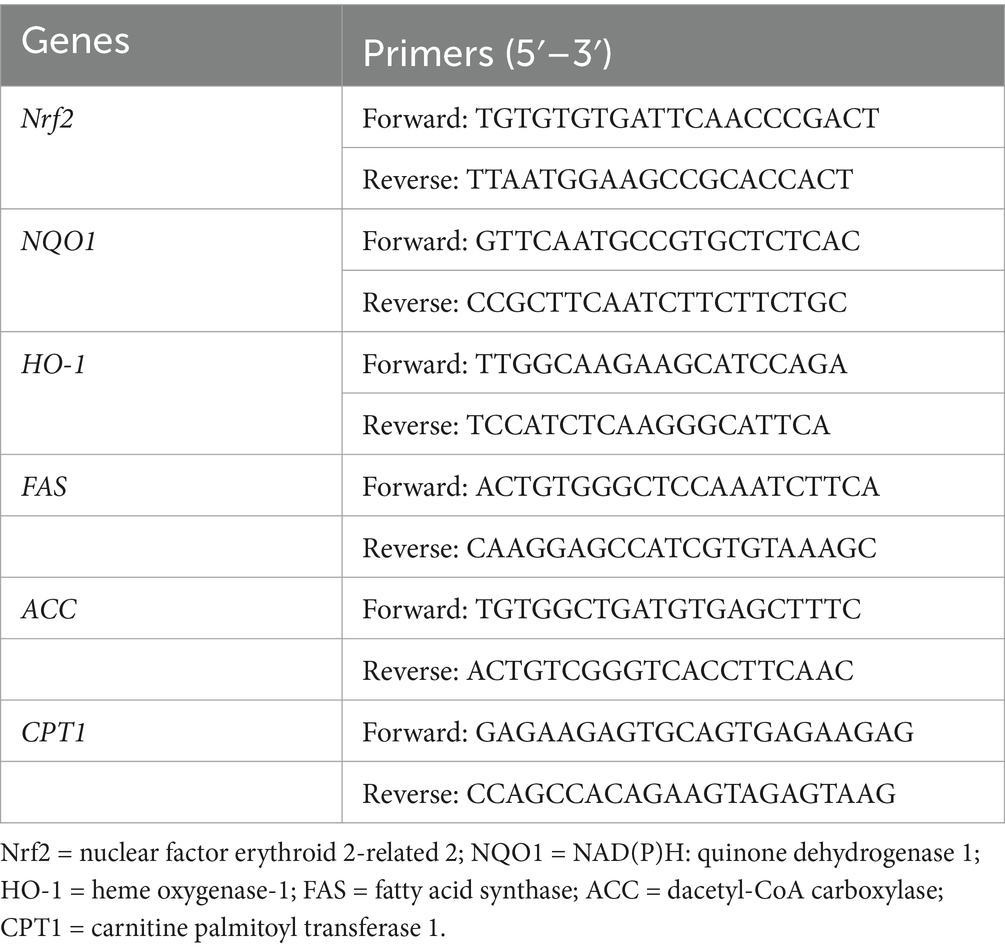
Lipid metabolism-related gene expression. Figure 2 depicts the expression of lipid metabolism-related genes. Increasing TF levels quadratically (p < 0.05) decreased the expression of FAS and ACC while increasing the expression of CPT1 (p < 0.05) in the liver of laying hens.
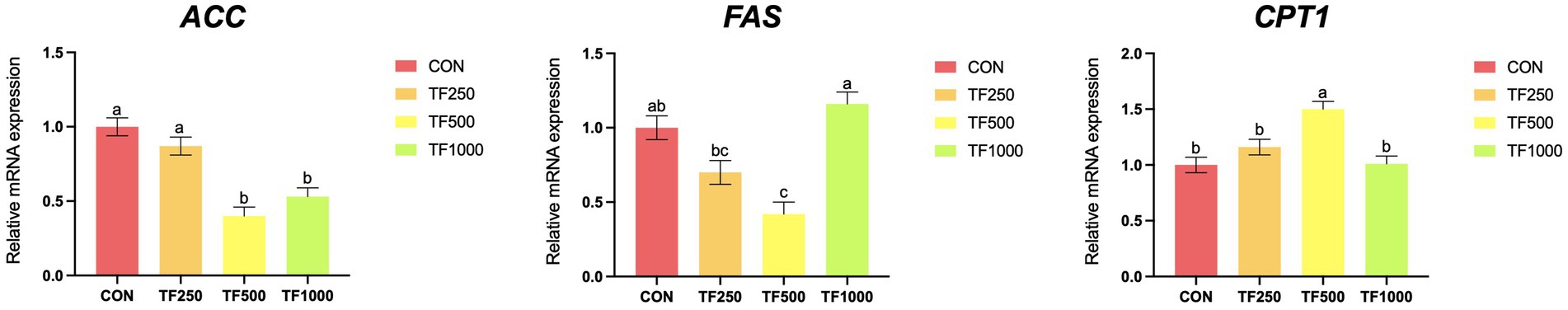
Figure 2. Lipid metabolism-related gene expression of laying hens at different theaflavin levels. a,bDifferent superscripts indicate a significance difference (p < 0.05). ACC = acetyl-CoA carboxylase; FAS = fatty acid synthase; CPT1 = carnitine palmitoyl transferase 1. 1Values are means ± SEM. Each value represents the mean value of 8 replicates per treatment. 2All the data were acquired using real-time PCR.
3.5 Antioxidant capacity of eggs
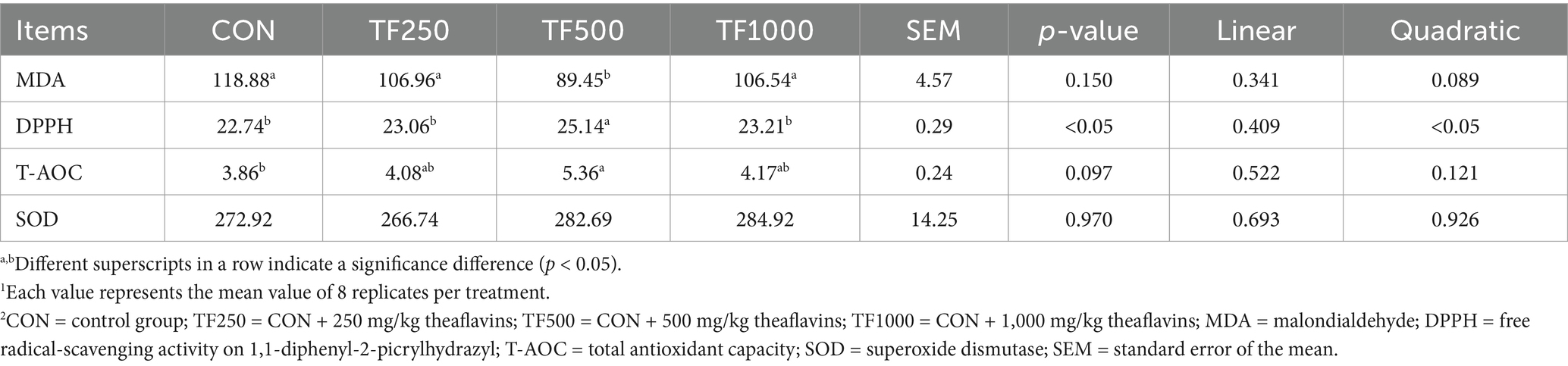
Table 7 shows the antioxidant capacity of eggs. Increasing TF levels quadratically (p < 0.05) increased the DPPH value of egg yolks. TF (500 mg/kg) significantly (p < 0.05) decreased the egg yolk MDA content compared to those of the other groups and significantly (p < 0.05) increased the egg yolk T-AOC compared to that of the control group.
3.6 Egg composition
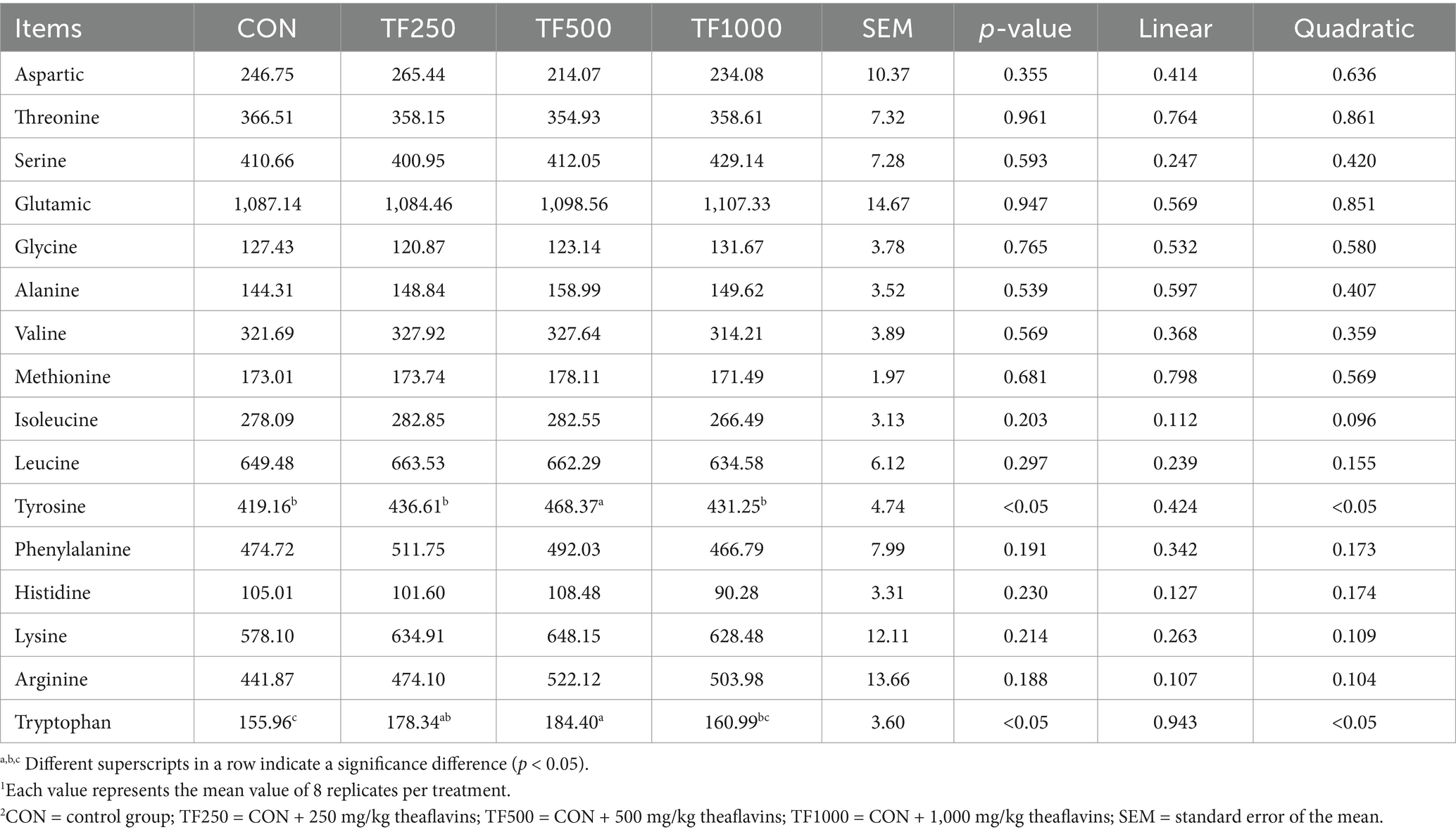
Free amino acids. Table 8 shows the free amino acids in egg yolks. Increasing TF levels quadratically (p < 0.05) increased the tyrosine and tryptophan content in egg yolks.
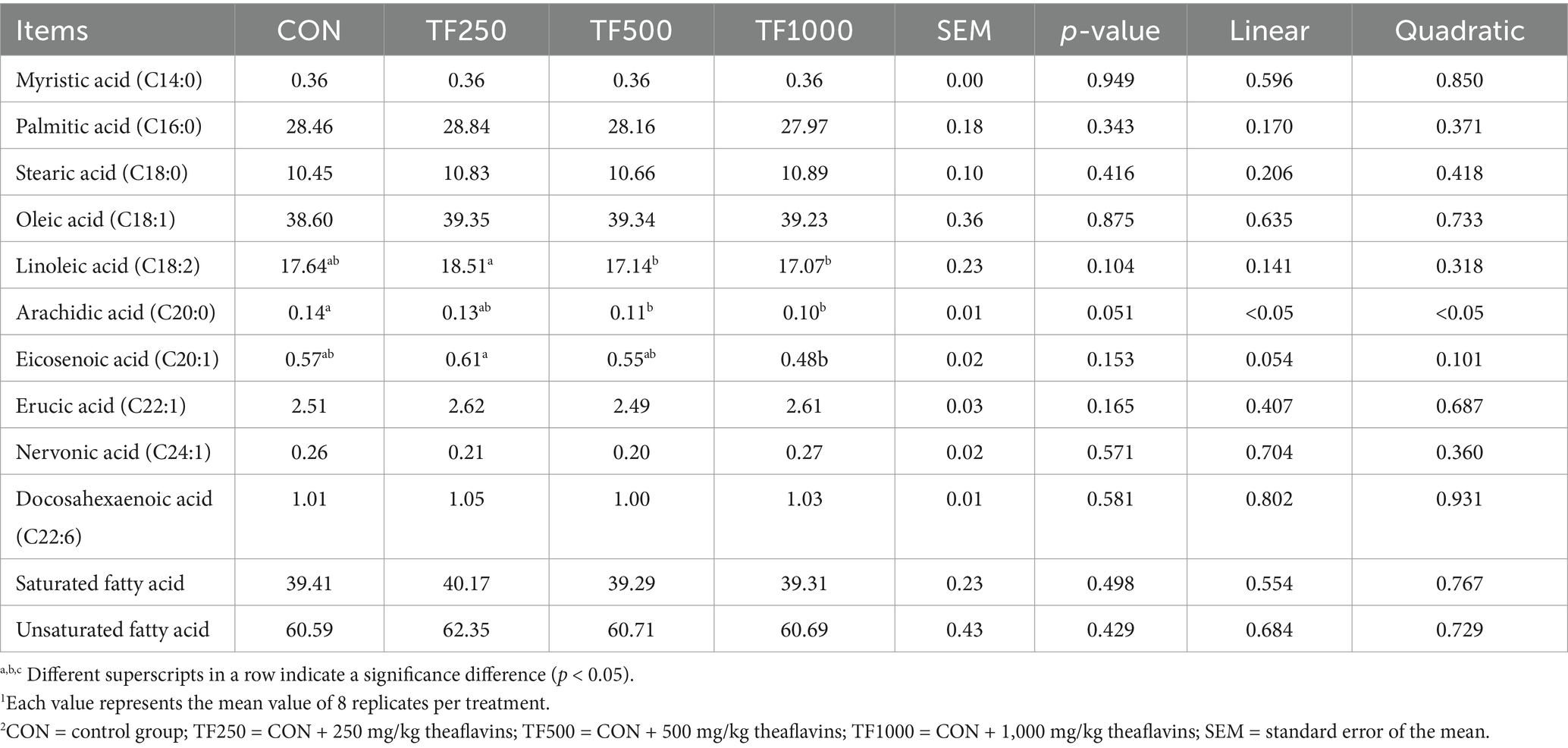
Fatty acid profile. Table 9 shows the fatty acid profile in egg yolks. TF (250 mg/kg) significantly (p < 0.05) increased the linoleic acid content compared to that of the 500 and 1,000 mg/kg TF group and increased the eicosenoic acid content compared to that of the 1,000 mg/kg TF group of egg yolks. Additionally, TF linearly (p < 0.05) decreased the arachidic acid content in egg yolks.
4 Discussion
TF, present in black, red, and oolong teas, exhibit various biological activities and health benefits (15). In this study, we observed that 500 mg/kg TF significantly enhanced the production performance of laying hens. Although reports on the application of TF in laying hens are unknown, other tea extracts, such as theabrownins (TB) and EGCG, have been widely investigated. Studies show that 200–400 mg/kg TB and EGCG enhance the production performance of laying hens (16–18). Furthermore, tea polyphenols (600 mg/kg) could counteract the adverse effects of aging corn and heavy metals on the production performance and egg albumen quality of laying hens (10, 19, 20). Both TF and TBs are key pigments in black tea, derived from the oxidation of catechins and their gallates during the fermentation process. Structurally, TF are dimeric polyphenols with a benzotropolone core (C17H16O6), enabling stronger electron delocalization and antioxidant capacity than the polymeric TBs (irregular catechol-quinone frameworks) or monomeric EGCG (C22H18O11) (21). Therefore, TF exhibit higher antioxidant activity than TBs and hold greater potential for application in the egg production industry (4).
Egg yolk color plays a significant role in the purchasing decisions of consumers. The typical yellow color results from the accumulation of lutein and carotenoids in the diet (22). Tea leaves contain chlorophyll, lutein, carotenoids, and other pigments (22). The TF obtained in this experiment may contain residual lutein and carotenoids that were present during the extraction process of TF, which could increase their deposition in eggs. In China, consumers prefer eggs with a more intense yolk color. The increased yolk color indicates that TF-fed layers may be more preferred.
TF is the main antioxidant active components of black tea and have been shown to effectively improve the antioxidant status in different cell cultures and animal models (23). Our results showed that TF quadratically increased the activities of antioxidant enzymes and decreased the MDA content in laying hens. Ai et al. report that TF could increase the activities of SOD and decrease the MDA levels in serum, thereby alleviating oxidative stress caused by ovariectomy in mice (24). Zeng et al. report that TF administration significantly decreases MDA levels, increases SOD activity, and alleviates oxidative damage in the aorta caused by high-fat diet exposure in mice. Our findings align with that of a previous study demonstrating that TF intake enhances antioxidant capacity in laying hens (5).
Nrf2 is a key transcription factor that regulates cellular antioxidant defenses against oxidative stress by controlling the expression of various antioxidant and detoxifying enzymes. NQO1 and HO-1, downstream genes of Nrf2, are widely recognized for their strong antioxidant properties. Reactive oxygen species (ROS) are highly unstable oxygen-free radicals. TF could suppress receptor activators of nuclear factor-kappa B ligand-induced ROS generation by upregulating the expression of antioxidant genes, such as Nrf2, HO-1, and catalase in osteoclasts (5, 25, 26). In this study, TF levels quadratically (p < 0.05) increased the expression of Nrf2, HO-1, and NQO1. These findings indicate that TF may exert antioxidant effects by upregulating the expression of HO-1 and NQO1 genes, with the Nrf2 signaling pathway likely serving as an underlying mechanism.
The role of TF in preventing diabetes has been extensively investigated. TF could regulate energy metabolism, enhance fatty acid oxidation, and reduce de novo lipogenesis through the AMP-activated protein kinase (AMPK) signaling pathway (27). In this study, TF quadratically decreased the serum TC, liver TC, and TG content. In obese mice, TF reduce the adiposity index by 24.5% and significantly decrease serum levels of TC and TG by 26.5 and 50.8%, respectively (28). Clinical trials show that oral administration of TF reduces serum levels of TC, TG, and LDL in patients with hypercholesterolemia. TF also improve body composition by proportionally decreasing total and subcutaneous fat while increasing skeletal muscle (29–31). Our finding was consistent with that of a previous study showing that the intake of TF by laying hens regulates energy metabolism and reduces TG and TC content in the serum and liver. By reducing lipid deposition, TF may redirect metabolic energy toward egg synthesis rather than adipose storage, thereby improving feed efficiency and egg production (32). Additionally, enhanced fatty acid oxidation via AMPK activation could maintain hepatic health, ensuring sufficient yolk precursor (VLDL) synthesis for egg formation (33). This dual modulation of energy partitioning and reproductive resource allocation may explain the improved laying performance observed in TF-supplemented hens.
AMPK serves as a key regulator of lipid metabolism, controlling whole-body metabolism by downregulating the ACC and CPT-1 pathways, which regulate beta-oxidation (34). Our results showed that TF quadratically decreased the expression of FAS and ACC while increasing CPT1 expression. TF reduced the gene expression of ACC and FAS while increasing CPT1 expression in mice liver. This regulation inhibits fatty acids and cholesterol synthesis, decreases lipid accumulation, and promotes fatty acid oxidation (8, 9, 35). Our findings align with this, indicating that TF regulate lipid metabolism in laying hens through the modulation of ACC, FAS, and CPT1, with AMPK serving as a potential signaling pathway.
The antioxidant activity of eggs was determined using the DPPH, T-AOC and MDA contents. The DPPH radical scavenging model and T-AOC are widely used to evaluate the free radical scavenging ability of natural compounds (36). In our study, TF (500 mg/kg) increased the DPPH value and T-AOC while decreasing the MDA content in egg yolks. Studies show that tea extracts increase the antioxidant capacity of eggs. Zhou et al. and Wang et al. report that 600 mg/kg tea polyphenols increased the DPPH value and most free amino acids (isoleucine, leucine, tyrosine, phenylalanine, tryptophan, lysine, threonine, serine, glutamic acid, valine, and methionine) in egg yolks (10, 16). Wang et al. report that 400 mg/kg TBs improved the DPPH value, reducing power value, T-AOC, and levels of tryptophan, cysteine, methionine, and histidine in egg yolks while decreasing the MDA content (17). Our findings are consistent with those of previous studies, which show that antioxidative compounds such as egg-derived peptides, tryptophan, tyrosine, vitamin E, and carotenoids contribute to the T-AOC of egg yolks (13). Our results showed that 500 mg/kg of TF increased the tyrosine and tryptophan content in egg yolks. This indicates that TF enhance the antioxidant capacity of eggs by increasing the content of tyrosine and tryptophan in egg yolks. However, the mechanism by which TF increase the content of tyrosine and tryptophan in egg yolks remains unclear and requires further research. Based on our findings, TF (250 mg/kg) significantly increased the linoleic acid content compared to that of the 500 and 1,000 mg/kg TF group. Studies show that high antioxidant capacity in the yolk preserves lipid quality by preventing oxidation and inhibiting the degradation of certain unsaturated fatty acids (37, 38). This indicates that TF can inhibit the degradation of unsaturated fatty acids by enhancing the antioxidant capacity of eggs.
5 Conclusion
TF improved the laying rate of laying hens by enhancing their antioxidant capacity and regulating lipid metabolism. TF also enhanced the antioxidant capacity of eggs by increasing the content of tyrosine and tryptophan levels in egg yolks. The optimal supplementation level of TF in laying hens is 500 mg/kg.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The animal studies were approved by the Animal Care and Use Committee of Yibin University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
LZ: Conceptualization, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. PZ: Conceptualization, Supervision, Writing – review & editing. JinZ: Conceptualization, Data curation, Investigation, Writing – review & editing. JieZ: Conceptualization, Formal analysis, Investigation, Writing – review & editing. YR: Investigation, Resources, Software, Writing – review & editing. JT: Data curation, Formal analysis, Project administration, Writing – review & editing. LJ: Conceptualization, Funding acquisition, Writing – review & editing. FC: Funding acquisition, Project administration, Writing – review & editing. LC: Funding acquisition, Project administration, Writing – review & editing. KZ: Funding acquisition, Project administration, Supervision, Writing – review & editing. OW: Writing – review & editing, Supervision. JP: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was financially supported by the Research Project of Yibin university (2022QH04); Youths Fund of Natural Science Foundation in Sichuan Province (2023NSFSC1137); Expert Workstation-Yibin.
Conflict of interest
LJ, FC, and LC were employed by YibinShangougou Agricultural Science and Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shi, MLY, Wu, J, Zheng, Z, Lv, C, Ye, J, Qin, S, et al. Beneficial effects of Theaflavins on metabolic syndrome: from molecular evidence to gut microbiome. Int J Mol Sci. (2022) 23:7595. doi: 10.3390/ijms23147595
2. Wang, M, Xu, J, Han, T, and Tang, L. Effects of theaflavins on the structure and function of bovine lactoferrin. Food Chem. (2021) 338:128048. doi: 10.1016/j.foodchem.2020.128048
3. Yang, Z, Jie, G, Dong, F, Xu, Y, Watanabe, N, and Tu, Y. Radical-scavenging abilities and antioxidant properties of theaflavins and their gallate esters in H2O2-mediated oxidative damage system in the HPF-1 cells. Toxicol In Vitro. (2008) 22:1250–6. doi: 10.1016/j.tiv.2008.04.007
4. Yang, Z, Tu, Y, Xia, H, Jie, G, Chen, X, and He, P. Suppression of free-radicals and protection against H2O2-induced oxidative damage in HPF-1 cell by oxidized phenolic compounds present in black tea. Food Chem. (2007) 105:1349–56. doi: 10.1016/j.foodchem.2007.05.006
5. Zeng, J, Deng, Z, Zou, Y, Liu, C, Fu, H, Gu, Y, et al. Theaflavin alleviates oxidative injury and atherosclerosis progress via activating micro RNA-24-mediated Nrf2/HO-1 signal. Phytother Res. (2021) 35:3418–27. doi: 10.1002/ptr.7064
6. Xu, X, Luo, P, Wang, Y, Cui, Y, and Miao, L. Nuclear factor (erythroid-derived 2)-like 2 (NFE2L2) is a novel therapeutic target for diabetic complications. J Int Med Res. (2013) 41:13–9. doi: 10.1177/0300060513477004
7. Jin, D, Xu, Y, Mei, X, Meng, Q, Gao, Y, Li, B, et al. Antiobesity and lipid lowering effects of theaflavins on high-fat diet induced obese rats. J Funct Foods. (2013) 5:1142–50. doi: 10.1016/j.jff.2013.03.011
8. Huang, HC, and Lin, JK. Pu-erh tea, green tea, and black tea suppresses hyperlipidemia, hyperleptinemia and fatty acid synthase through activating AMPK in rats fed a high-fructose diet. Food Funct. (2012) 3:170–7. doi: 10.1039/C1FO10157A
9. Cai, X, Liu, Z, Dong, X, Wang, Y, Zhu, L, Li, M, et al. Hypoglycemic and lipid lowering effects of theaflavins in high-fat diet-induced obese mice. Food Funct. (2021) 12:9922–31. doi: 10.1039/D1FO01966J
10. Zhou, L, Ding, X, Wang, J, Bai, S, Zeng, Q, Su, Z, et al. Tea polyphenols increase the antioxidant status of laying hens fed diets with different levels of ageing corn. Anim Nutr. (2021) 7:650–60. doi: 10.1016/j.aninu.2020.08.013
11. Ministry of Agriculture of the People’s Republic of China. Chicken feeding standard (NY/T33-2004). Beijing, China: China Standard Press (2004).
12. Zhang, T, Li, Y, Miao, M, and Jiang, B. Purification and characterisation of a new antioxidant peptide from chickpea (Cicer arietium L.) protein hydrolysates. Food Chem. (2011) 128:28–33. doi: 10.1016/j.foodchem.2011.02.072
13. Nimalaratne, C, Schieber, A, and Wu, J. Effects of storage and cooking on the antioxidant capacity of laying hen eggs. Food Chem. (2016) 194:111–6. doi: 10.1016/j.foodchem.2015.07.116
14. Ministry of Health of the People’s Republic of China and Standardization Administration of China. Determination of fatty acid profile in foods (GB 5009.168-2016). Beijing, China: China Standard Press (2016).
15. Fu, H, He, J, Li, C, and Chang, H. Theaflavin-3, 3'-Digallate protects liver and kidney functions in diabetic rats by up-regulating Circ-ITCH and Nrf2 signaling pathway. J Agric Food Chem. (2024) 72:14630–9. doi: 10.1021/acs.jafc.3c08251
16. Wang, J, Jia, R, Celi, P, Ding, X, Bai, S, Zeng, Q, et al. Green tea polyphenol epigallocatechin-3-gallate improves the antioxidant capacity of eggs. Food Funct. (2020) 11:534–43. doi: 10.1039/C9FO02157D
17. Wang, J, Zhang, T, Wan, C, Lai, Z, Li, J, Chen, L, et al. The effect of theabrownins on the amino acid composition and antioxidant properties of hen eggs. Poult Sci. (2023) 102:102717. doi: 10.1016/j.psj.2023.102717
18. Wang, J, Yuan, Z, Zhang, K, Ding, X, Bai, S, Zeng, Q, et al. Epigallocatechin-3-gallate protected vanadium-induced eggshell depigmentation via P38MAPK-Nrf2/HO-1 signaling pathway in laying hens. Poult Sci. (2018) 97:3109–18. doi: 10.3382/ps/pey165
19. Yuan, ZH, Zhang, KY, Ding, XM, Luo, YH, Bai, SP, Zeng, QF, et al. Effect of tea polyphenols on production performance, egg quality, and hepatic antioxidant status of laying hens in vanadium-containing diets. Poult Sci. (2016) 95:1709–17. doi: 10.3382/ps/pew097
20. Wang, J, Yang, Z, Celi, P, Yan, L, Ding, X, Bai, S, et al. Alteration of the antioxidant capacity and gut microbiota under high levels of molybdenum and green tea polyphenols in laying hens. Antioxidants (Basel). (2019) 8:503. doi: 10.3390/antiox8100503
21. Lin, X, Chen, Z, Zhang, Y, Luo, W, Tang, H, Deng, B, et al. Comparative characterisation of green tea and black tea cream: physicochemical and phytochemical nature. Food Chem. (2015) 173:432–40. doi: 10.1016/j.foodchem.2014.10.048
22. Chen, Y, Han, Y, He, S, Cheng, Q, and Tong, H. Differential metabolic profiles of pigment constituents affecting leaf color in different albino tea resources. Food Chem. (2025) 467:142290. doi: 10.1016/j.foodchem.2024.142290
23. Wang, W, Le, T, Wang, W, Yu, L, Yang, L, and Jiang, H. Effects of key components on the antioxidant activity of black tea. Food Secur. (2023) 12:3134. doi: 10.3390/foods12163134
24. Ai, Z, Wu, YO, Yu, M, Li, J, and Li, S. Theaflavin-3, 3′-Digallate suppresses RANKL-induced osteoclastogenesis and attenuates ovariectomy-induced bone loss in mice. Front Pharmacol. (2020) 11:803. doi: 10.3389/fphar.2020.00803
25. Xu, C, Ni, S, Xu, N, Yin, G, Yu, Y, Zhou, B, et al. Theaflavin-3, 3'-Digallate inhibits Erastin-induced chondrocytes ferroptosis via the Nrf2/GPX4 signaling pathway in osteoarthritis. Oxidative Med Cell Longev. (2022) 2022:1–17. doi: 10.1155/2022/3531995
26. Li, X, Zhang, Y, Zhao, W, Ren, T, Wang, X, and Hu, X. Extracts from Tartary buckwheat sprouts restricts oxidative injury induced by hydrogen peroxide in Hep G2 by upregulating the redox system. Food Secur. (2024) 13:3726. doi: 10.3390/foods13233726
27. Xu, S, Chen, Y, and Gong, Y. Improvement of Theaflavins on glucose and lipid metabolism in diabetes mellitus. Food Secur. (2024) 13:1763. doi: 10.3390/foods13111763
28. Tung, YC, Liang, ZR, Yang, MJ, Ho, CT, and Pan, MH. Oolong tea extract alleviates weight gain in high-fat diet-induced obese rats by regulating lipid metabolism and modulating gut microbiota. Food Funct. (2022) 13:2846–56. doi: 10.1039/D1FO03356E
29. Hartley, L, Flowers, N, Holmes, J, Clarke, A, Stranges, S, Hooper, L, et al. Green and black tea for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. (2013) 2013:Cd009934. doi: 10.1002/14651858.CD009934.pub2
30. Maron, DJ, Lu, GP, Cai, NS, Wu, ZG, Li, YH, Chen, H, et al. Cholesterol-lowering effect of a theaflavin-enriched green tea extract: a randomized controlled trial. Arch Intern Med. (2003) 163:1448–53. doi: 10.1001/archinte.163.12.1448
31. Aizawa, T, Yamamoto, A, and Ueno, T. Effect of oral theaflavin administration on body weight, fat, and muscle in healthy subjects: a randomized pilot study. Biosci Biotechnol Biochem. (2017) 81:311–5. doi: 10.1080/09168451.2016.1246170
32. Wei, F, Yang, X, Zhang, M, Xu, C, Hu, Y, and Liu, D. Akkermansia muciniphila enhances egg quality and the lipid profile of egg yolk by improving lipid metabolism. Front Microbiol. (2022) 13:927245. doi: 10.3389/fmicb.2022.927245
33. Yue, HY, Wang, J, Qi, XL, Ji, F, Liu, MF, Wu, SG, et al. Effects of dietary oxidized oil on laying performance, lipid metabolism, and apolipoprotein gene expression in laying hens. Poult Sci. (2011) 90:1728–36. doi: 10.3382/ps.2011-01354
34. Oh, J, Ahn, S, Zhou, X, Lim, YJ, Hong, S, and Kim, HS. Effects of cinnamon (Cinnamomum zeylanicum) extract on adipocyte differentiation in 3T3-L1 cells and lipid accumulation in mice fed a high-fat diet. Nutrients. (2023) 15:15 (24). doi: 10.3390/nu15245110
35. Lin, CL, Huang, HC, and Lin, JK. Theaflavins attenuate hepatic lipid accumulation through activating AMPK in human Hep G2 cells. J Lipid Res. (2007) 48:2334–43. doi: 10.1194/jlr.M700128-JLR200
36. Kallel, F, Driss, D, Bouaziz, F, Belghith, L, Zouari-Ellouzi, S, Chaari, F, et al. Polysaccharide from garlic straw: extraction, structural data, biological properties and application to beef meat preservation. RSC Adv. (2014) 5:6728–41. doi: 10.1039/C4RA11045E
37. Tufarelli, V, Ceci, E, and Laudadio, V. 2-Hydroxy-4-methylselenobutanoic acid as new organic selenium dietary supplement to produce selenium-enriched eggs. Biol Trace Elem Res. (2016) 171:453–8. doi: 10.1007/s12011-015-0548-4
Keywords: theaflavins, production performance, egg antioxidant capacity, antioxidant status, lipid metabolism, laying hen
Citation: Zhou L, Zhao P, Zhang J, Zhong J, Rao Y, Tang J, Jiang L, Chen F, Chen L, Zhang K, Ouyang W and Peng J (2025) Theaflavins enhances laying hen production performance and egg antioxidant capacity via improving antioxidant status and regulating lipid metabolism. Front. Vet. Sci. 12:1566580. doi: 10.3389/fvets.2025.1566580
Edited by:
Moyosore Joseph Adegbeye, University of Africa, NigeriaReviewed by:
Olajide Mark Sogunle, Federal University of Agriculture, Abeokuta, NigeriaYujia Tian, Tianjin Agricultural University, China
Copyright © 2025 Zhou, Zhao, Zhang, Zhong, Rao, Tang, Jiang, Chen, Chen, Zhang, Ouyang and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jialong Peng, OTQzNzY2Nzg1QHFxLmNvbQ==
 Ling Zhou
Ling Zhou Pinyao Zhao1
Pinyao Zhao1 Keying Zhang
Keying Zhang