- 1College of Animal Science and Technology, Qingdao Agricultural University, Qingdao, China
- 2General Station of Animal Husbandry of Shandong Province, Jinan, China
Hesperidin or ascorbic acid is an effective antioxidant that can protect frozen-thawed sperm from oxidative damage. The aim of the present study was to investigate the cryoprotective effects of hesperidin (Hsd) and ascorbic acid (AsA) on boar sperm quality during cryopreservation. Sperm samples were collected once a week for 3 weeks. The ejaculated semen from eight boars in each of three replicates were pooled and split into seven groups with different doses of Hsd (0, 15, 45) μM and/or AsA (0, 100, 300) μM added to the freezing extender. The sperm motility, membrane integrity, acrosome integrity, mitochondrial membrane potentials, DNA integrity, reactive oxygen species (ROS), malondialdehyde (MDA) concentration, antioxidant enzyme activities, and protein expression were measured after freezing-thawing to evaluate the qualitative parameters of boar sperm. The results showed that the sperm motility, plasma membrane integrity, acrosomal integrity, DNA integrity, and mitochondrial membrane potential were significantly improved in the 15 μM Hsd + 100 μM AsA group compared with the control and other treatment groups (p < 0.01). It was observed that the supplementation in the 15 μM Hsd + 100 μM AsA group significantly improved the ATP content and antioxidant enzyme activities (SOD, CAT, GSH-Px, and PRODH) (p < 0.01). The levels of MDA and ROS were significantly reduced in the 15 μM Hsd + 100 μM AsA group (p < 0.01). Moreover, the supplementation of Hsd combined with AsA significantly increased the expression of the anti-apoptosis protein Bcl-2 and decreased the expression of the pro-apoptotic protein caspase-3, P53, Bax, and Cytochrome C (p < 0.05). These findings demonstrated that supplementing the freezing extender with both Hsd and AsA had a combined, beneficial effect on the quality of frozen-thawed boar sperm. The combination of 15 μM Hsd + 100 μM AsA showed greater potential in protecting the boar sperm during cryopreservation than the separate addition of either Hsd or AsA. The supplementation of Hsd and AsA could improve the quality of frozen-thawed sperm as an antioxidant.
1 Introduction
Cryopreservation of boar sperm is a reliable choice for long-term preservation of important genetic materials, and it is a convenient way to utilize outstanding individuals in the country and abroad (1, 2). However, a number of parameters, including diluents, the rate of freezing and thawing, cryoprotectants, breeds and individual boars, can easily alter the survival of frozen-thawed boar sperm (3). The efficiency of artificial insemination with frozen-thawed sperm is low, accounting for only 1% of swine insemination due to its poor efficiency (4). The polyunsaturated fatty acid (PUFA) content in the boar sperm membrane is particularly rich (5). This renders the boar sperm highly susceptible to lipid peroxidation (LPO), which occurs as a result of the oxidation of membrane lipids by partially reduced oxygen molecules, such as superoxide, hydrogen peroxide, and hydroxyl radicals (6). LPO influences the integrity and other properties of the sperm plasma membrane, even giving rise to the formation of malondialdehyde (MDA), the lipid peroxidation product of unsaturated fatty acid. During cryopreservation, freezing stress can disrupt the membrane of boar sperm and increase the production of reactive oxygen species (ROS). The release of ROS after thawing further aggravates oxidative stress. Oxidative stress has numerous detrimental effects on boar sperm, leading to DNA damage and impairing the production of ATP, which is essential for sperm motility. It also triggers the apoptosis pathway through ROS, leading to a decrease in the antioxidant capacity of boar sperm, making them more vulnerable to oxidative damage, finally depriving sperm of motility and viability (7). The quality of frozen-thawed sperm (e.g., sperm motility, membrane integrity, acrosome integrity, and mitochondria activity) may be influenced by a number of variables, such as species, ejaculates, diluent composition, cryopreservation techniques, and cryoprotective agents (8). Therefore, it is of great importance and urgency to select effective and nontoxic antioxidants to improve the boar sperm quality during cryopreservation. The addition of antioxidants such as hesperidin and ascorbic acid to freezing extenders has a positive effect on the cryopreservation quality of frozen sperm (9).
Flavonoids are the largest family of phenolic compounds, the majority of which are hesperidin (Hsd) (10). Hsd is widely distributed in various citrus fruits and plays an important role in many biological and physiological activities (11). Hsd may play an additive or synergistic role in anti-oxidation, anti-inflammatory, anticancer, and cardiovascular protection activities (12, 13). Hsd had greater antioxidative effects on fresh and frozen-thawed semen quality in Simmental bull (14). Ascorbic acid (AsA) is the most effective and least toxic antioxidant, involved in vital biological activities (15). It is an antioxidant that has showed positive effects during the vitrification of porcine embryos (16). Previous studies have shown that the supplementation of AsA (17) or AsA-derivative (2-O-α-glucoside, AA-2G) (18) improves sperm motility and survival and reduces MDA and DNA damage in boar.
The aim of this study was to determine the positive effects of the addition of Hsd and AsA (i.e., alone or combined) on boar frozen-thawed sperm quality, including sperm motility, membrane integrity, acrosome integrity, mitochondrial membrane potentials, DNA integrity, ROS level, MDA concentration, antioxidant enzyme activities [superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and proline dehydrogenase (PRODH)], and apoptotic factor protein expression.
2 Materials and methods
2.1 Animal and sperm collection
The ejaculate sperm samples used in this study were collected from eight healthy Duroc boars (aged 1.5 to 2.5 years) from a boar station. The boars were raised in separate limit bar (3 m long × 2.5 m wide) and fed 2.5 to 3.0 kg of feed twice a day with ad libitum water. The sperm was collected at 8:00 am after feeding for 1 h using the “gloved-hand” method once a week for three successive weeks. The gel particle was removed with four layers of sterile gauze, and only the sperm-rich fractions were brought to the laboratory within 60 min at 17°C. The sperm samples were assessed microscopically, and more than 90% of motile sperm and less than 10% of abnormal sperm were used in the trial. The ejaculated semen from eight boars was pooled to eliminate individual differences and divided into seven groups with three replicates per group.
2.2 Chemicals and extenders
Unless otherwise specified, all chemicals and reagents were purchased from Sinopharm Group in China. The Modena solution was prepared in the laboratory containing 153 mM D-glucose, 26.7 mM trisodium citrate, 11.9 mM sodium hydrogen carbonate, 15.1 mM citric acid, 6.3 mM ethylene diamine tetraacetic acid (EDTA)-2 Na, 46.6 mM Tris, 1,000 IU/mL penicillin G sodium salt (Solarbio Institute of Biotechnology, China), and 1 mg/mL streptomycin sesquisulfate (Solarbio Institute of Biotechnology, China), as described by Dong et al. (19). Extender I was composed of 11% lactose solution and egg yolk (4:1, v:v), containing seven groups of different concentrations of Hsd (97% purity; Sigma-Aldrich Co., St. Louis, Missouri, United States) and/or AsA (Cat: A8100, 99% purity; Solarbio Institute of Biotechnology, China). The concentrations were referred to Tahmasbian et al. (14) and Giaretta et al. (17) (Table 1). Extender II was composed of extender I (containing different concentrations of Hsd and/or AsA), glycerol, and Orvus Ex Paste™ (Minitube, Germany) (95.5%:3%:1.5%, v:v:v).
2.3 Sperm processing
According to Dong et al. (19), the fresh semen was diluted with Modena solution (v:v = 1:1) and incubated for 2 h at 17°C. It was centrifuged at 900 × g for 10 min to remove the Modena solution. The sperm pellets were resuspended with extender I that contain different concentrations of Hsd and AsA at a concentration of 2 × 109 sperm/mL and cooled slowly from 17 to 5°C for 2 h. Subsequently, the sperm was resuspended with the same volume of freezing extender II and packed into 0.5 mL plastic straws. The sealed straws were placed horizontally on a rack and frozen for 10 min in a vapour 3 cm above the liquid nitrogen, and then the straws were plunged into the liquid nitrogen for storage. After 2 weeks of storage, the extended semen was thawed in a water bath at 50°C for 10 s and then resuspended in Modena (1:4, v:v, 37°C) to evaluate the quality of sperm at 10 min after incubation at 37°C. Sperm samples were used to determine the qualitative parameters of boar sperm in the following analysis.
2.4 Assessments of sperm motility after freezing-thawing
Sperm motility was evaluated using the computer-assisted sperm analysis (CASA) system (QH-III, Spain). The associated parameters included the total motile sperm (TM, %), motile progressive sperm (PM, %), curvilinear velocity (VCL, μm/s), straight-line velocity (VSL, μm/s), average path velocity (VAP, μm/s), and linearity of the curvilinear trajectory (LIN, ratio of VSL/VCL, %), which were all evaluated. According to Dong et al. (19), 5 μL semen samples were placed on an analyzer’s Makler chamber and maintained at 37°C for 10 min during the analysis. Sperm motility was estimated by calculating the percentage of sperm showing progressive movement after viewing five different fields, examining over 500 sperm.
2.5 Measurement of sperm membrane integrity, acrosome integrity, and DNA integrity after freezing-thawing
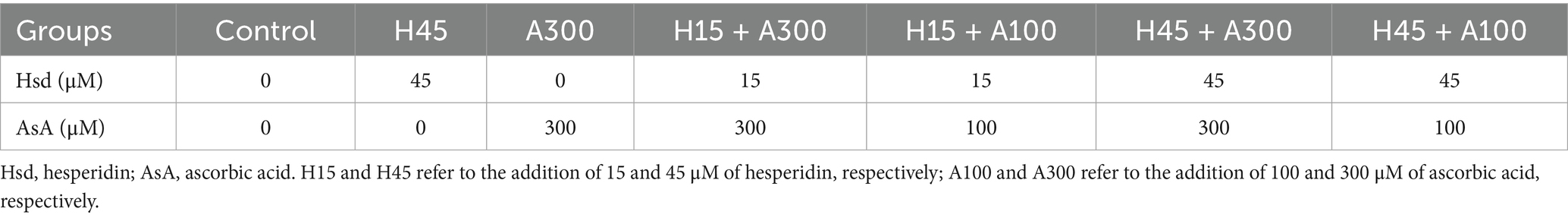
Membrane integrity was determined using the SYBR-14/PI sperm viability kit (L7011, Invitrogen™, Thermo Fisher Scientific) according to Zhang et al. (20). For this purpose, an aliquot of 250 μL sample was stained with 25 μL SYBR-14 working solution (100 mM in DMSO) in 36°C darkness for 10 min, followed by the addition of 12.5 μL PI stock solution (2.4 mM) and incubated for another 10 min. Staining was monitored and photographed using an epifluorescence microscope (DMi8, Leica, Germany) with a 400 × fold filter, and a total of 200 sperm in each slide was examined. Sperm were classified into two groups (Figure 1A), i.e., intact membranes and damaged membranes.
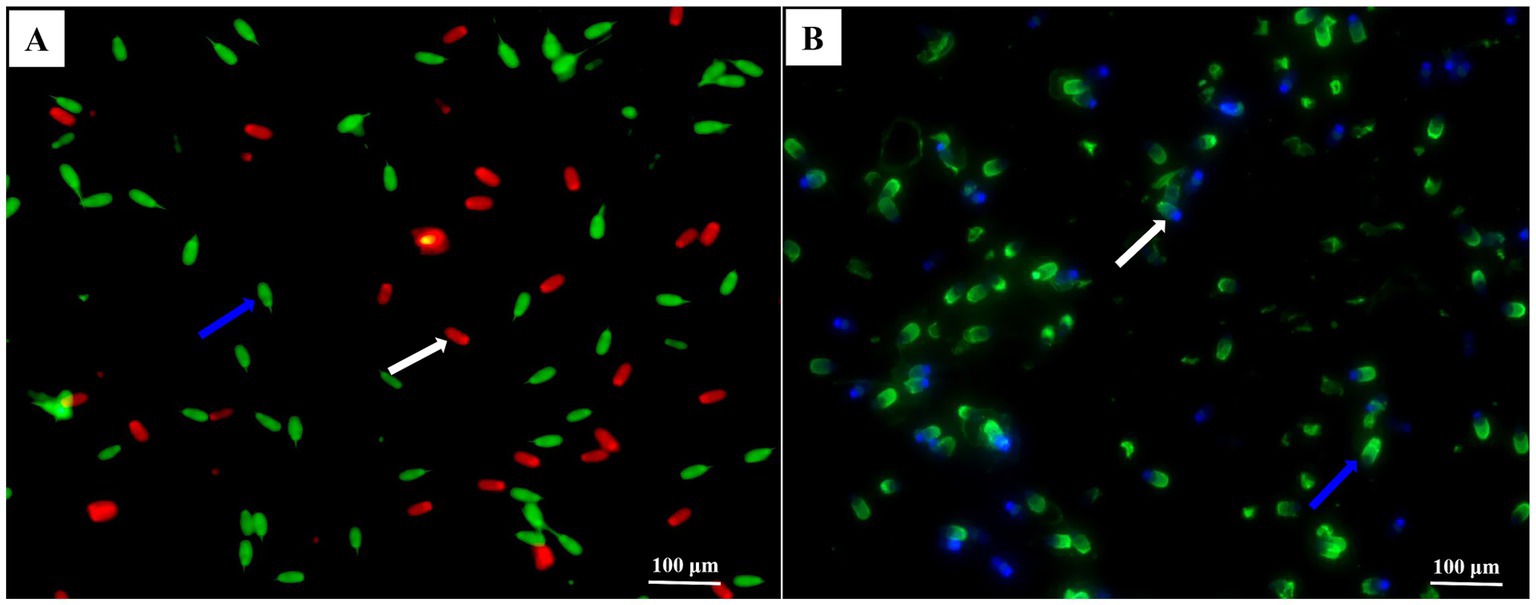
Figure 1. Photomicrographs of the post-thawed sperm stained with membrane using the SYBR-14/PI and acrosomes fluorescein isothiocyanate-peanut agglutinin (FITC-PNA). Images (A,B) obtained under a phase-contrast microscope. In image (A), the blue arrow indicated membrane integrity, and the white arrow indicated damaged membrane. In image (B), the blue arrow indicated intact acrosomes and sperm with intensively bright fluorescence of the acrosomal cap, which indicated by an intact outer acrosomal membrane. The white arrow indicated damaged acrosome and sperm with blue fluorescence, which indicated a loss of the outer acrosomal membrane (bars = 100 μm).
Acrosome integrity was evaluated using fluorescein isothiocyanate-peanut agglutinin (FITC-PNA, L7381, Sigma), as described by Zhang et al. (20). To do so, a 300 μL aliquot of each sperm sample was smeared onto a clean glass slide and then fixed with absolute methanol for 10 min. Each slide was then coated with 30 μL FITC-PNA solution (100 μg/mL) diluted with phosphate-buffered saline (PBS). The slides were incubated in a dark and moist chamber at 37°C for 30 min. Subsequently, the slides were rinsed with PBS, air-dried in the dark, and then mounted with 600 μL antifade solution to maintain fluorescence. Samples were monitored and photographed using an epifluorescence microscope (DMi8, Leica, Germany) with a 400 × fold filter, and a total of 200 sperm in each slide was examined. As shown in Figure 1B, fluorescence images of sperm stained with FITC-PNA could be classified into two groups, i.e., intact acrosomes (sperm with intensively bright fluorescence of the acrosomal cap) and damaged acrosomes (sperm without fluorescence).
DNA integrity was determined using acridine orange (AO) staining (CA1142, Solarbio Institute of Biotechnology, China). According to our previous study (19), an aliquot of a 30 μL sperm sample was washed with AO stain buffer (1×). The sperm concentration was adjusted to 106 sperm/mL by resuspension with an appropriate volume of AO stain buffer (1×). Appropriate amount of sperm cell suspension were mixed with AO staining (19:1) and incubated at 37°C for 15 min. The sperm were spread on each slide and photographed under an epifluorescence microscope (DMi8, Leica, Germany) with a 400× magnification filter. In each slide, 200 sperm were examined, and the percentage of sperm with healthy double-stranded DNA (normal, green fluorescent) or single-stranded DNA (abnormal, red fluorescent) was observed, as shown in Figure 2A.
Figure 2. Photomicrographs of the post-thawed sperm stained with MMP (mitochondrial membrane potentials) using JC-1 fluorescent probes and DNA using acridine orange (AO) staining. Images (A,B) were obtained under a phase-contrast microscope. In image (A), the blue arrow indicated intact DNA and sperm with intensively bright green fluorescence of the DNA, which indicated by an intact DNA integrity. The white arrow indicated damaged DNA and sperm with red fluorescence, which indicated DNA damage. In image (B), the blue arrow indicated sperm with a high level of MMP (high orange fluorescence level), and the white arrow indicated sperm with a low MMP level (low orange fluorescence level).
2.6 Assessments of sperm mitochondrial activity after freezing-thawing
Sperm mitochondrial activity was determined using a Mitochondrial Membrane Potential Detection Kit (C2003S, Beyotime Institute of Biotechnology, China), as described by Dong et al. (19). Each sperm sample (2 × 106 sperm/mL) was stained with a JC-1 working solution and incubated in the dark at 37°C for 30 min. They were centrifuged at 600 × g at 4°C for 5 min and then resuspended on ice with JC-1 buffer solution. The stained samples were immediately evaluated under a fluorescence microscope (DMi8, Leica, Germany) with a set of 400 × fold filters, and a total of 200 sperm in each slide was examined. High levels of orange fluorescence emission are associated with the sperm mid-piece (where the mitochondria are located), indicating high mitochondrial activity (Figure 2B). The heads of the sperm emit light yellow, indicating low mitochondrial activity.
2.7 Measurement of sperm intracellular ROS, MDA, and ATP content after freezing-thawing
The ROS content was measured using a Reactive Oxygen Species Assay Kit (D6470, Solarbio Institute of Biotechnology, China) according to our previous study (19). Semen samples were suspended in PBS containing 10 μM DCFH-DA, and incubated at 37°C in the dark for 20 min (5 × 106 sperm/mL). Relative fluorescence levels were qualified using a fluorospectro photometer at 488 nm excitation and 525 nm emission (F-4500, Implen, Japan).
The MDA content was measured using a Malondialdehyde Assay Kit (A003-1-2, Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions. Semen samples (108 sperm/group) were lysed in 100 μL of cell lysis reagent. After 10 min centrifugation at 10,000 × g, the supernatant was used to determine the MDA. The absorbance at 532 nm was recorded using a microplate reader (Infinite M Nano, Tecan, Switzerland).
ATP levels of sperm were assessed using an ATP Assay Kit (S0027, Beyotime Institute of Biotechnology, China). According to Dong et al. (19), sperm samples (107 sperm) were lysed with 200 μL of schizolysis solution and ultrasonic cell crusher (20 KHz, 750 W, operating at 40% power, five cycles of 3 s on and 5 s off), and centrifuged at 4°C at 12,000 × g for 10 min. The supernatants were immediately collected, transferred to 96-well plates, and measured using a luminometer (GloMax 20/20, Promega, United States).
2.8 Measurement of sperm GSH-Px, SOD, catalase and PRODH activities after freezing-thawing
The Glutathione Peroxidase Assay Kit (A005-1-2), Superoxide Dismutase Assay kit (A001-3-2), Catalase Assay kit (A007-2-1) (Nanjing Jiancheng Institute of Biotechnology), and Proline Dehydrogenase Assay Kit (BC4160, Solarbio Institute of Biotechnology, China) were used to measure the GSH-Px, SOD, CAT, and PRODH activities of sperm, respectively, according to the manufacturer’s instructions. The sperm samples were centrifuged at 1,000 × g at room temperature for 5 min. After being rinsed with PBS for 3 times and re-suspended to 1 mL PBS, it was lysed ultrasonically (20 KHz, 750 W, operating at 40% power, five cycles of 3 s on and 5 s off) on ice and centrifuged at 12,000 × g at 4°C for 10 min. The supernatants were transferred to a 96-well plate to detect the activities of GSH-Px, SOD, CAT, and PRODH with three replicates in each group.
2.9 Western blotting
Total protein was extracted from thawed sperm with a concentration of 2 × 109 sperm/mL, with protein lysis buffer composed of 20 mM Tris (pH7.5), 150 mM NaCl, 1% Triton X-100, PMSF [phenylmethanesulfonyl fluoride (long term storage to inhibit protease)] and the concentration was measured using a BCA Protein Assay Kit (P0012S, Beyotime Institute of Biotechnology, China) according to Zhu et al. (21). About 20 μg of total protein in each sample was fractionated using 10% SDS-PAGE. After transferring the resolved protein onto 0.2 μm PVDF membranes, the membrane was blocked with 5% nonfat milk and incubated with anti-α-tubulin (1:1000, Cat No. A6830), anti-B-cell lymphoma protein-2 (1:1000, Cat No. A21873), anti-BCL2-Associated X (1:1000, Cat No. A19648), anti-P53 (1:1000, Cat No. A19585), anti-caspase-3 (1:1000, Cat No. A19654), and anti-Cytochrome C (1:1000, Cat No. A4912) overnight at 4°C. All antibodies were type of polyclonal, host species of rabbit, purchased from ABclonzl, Wuhan, China. The membranes were washed with 1 × TBST 3 times and further incubated with HRP-conjugated secondary antibody (1:1000, type of polyclonal, host species of rabbit, Cat No. AS014) (ABclonzl, Wuhan, China) at room temperature for 1 h. Enhanced chemiluminescence (ECL) detection was performed using the ECL™ Prime Western Blotting Detection Reagents (P0018FS, Beyotime Institute of Biotechnology, China) according to the manufacturer’s instruction and appropriate exposure of blots to Fuji X-ray film (iBright FL1000, ABI, United States). Image acquisition and densitometric analysis of gels, blots and membranes were performed using ImageLab software (version 4.1, Bio-Rad).
2.10 Statistical analysis
Data were expressed as mean ± standard error of the mean (SEM). The effects of Hsd and/or AsA treatments were analyzed using one-way ANOVA analysis using general linear model (GLM) procedure in SAS (SAS Institute, Inc., Cary, NC). The Tukey’s post-hoc test was performed after a statistically significant omnibus test to compare multiple treatment groups (SAS Institute, Inc., Cary, NC). The level of statistical significance was set at p < 0.05.
3 Results
3.1 Effects of supplementation of Hsd and AsA on frozen-thawed boar sperm motility
Compared with the control group (CON), total and progressive sperm motilities were significantly increased in all treatment groups (p < 0.01, Table 2). Sperm motility after thawing with 15 μM Hsd + 100 μM AsA was significantly (p < 0.01) higher than that of other treatments. Similarly, the straight-line velocity (VSL) and curvilinear velocity (VCL) of frozen-thawed sperm in the addition of 15 μM Hsd + the 100 μM AsA group were significantly improved compared with control, Hsd, and AsA groups (p < 0.01). In addition, the average path velocity (VAP) and linearity of frozen-thawed sperm were significantly increased (p < 0.01), except for AsA.
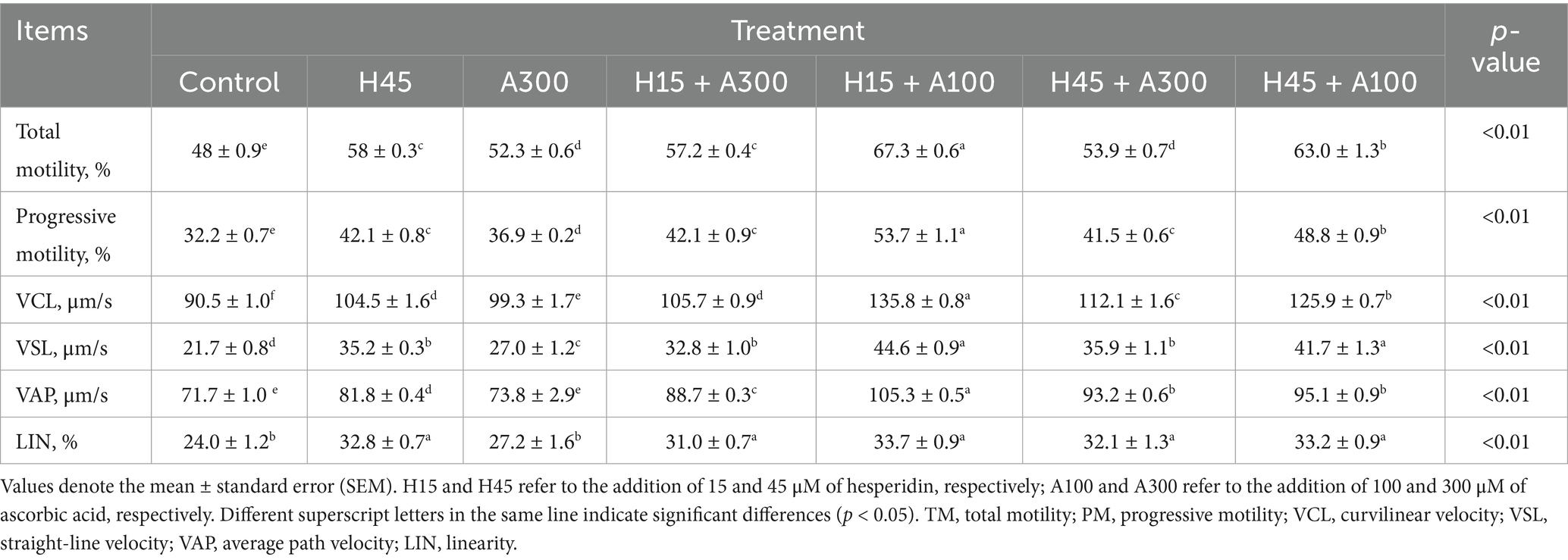
Table 2. Effects of different concentrations of hesperidin and ascorbic acid on post-thawed sperm motility parameters in control and at 10 min after thawing.
3.2 Effects of supplementation with Hsd and AsA on the physiological characteristics and ATP content of frozen-thawed boar sperm
The supplementation of Hsd and AsA to the extender significantly improved the membrane, acrosome, DNA integrity and the mitochondrial membrane potential of frozen-thawed sperm compared with the control group (Figure 3, p < 0.001). In addition, ATP levels were significantly (p < 0.01) greater in the Hsd-containing treatment compared with the control and groups that only contained AsA (Figure 3). Moreover, ATP content, mitochondrial membrane potential, membrane, acrosomal and DNA integrity of frozen-thawed sperm in the 15 μM Hsd + 100 μM AsA group were significantly (p < 0.01) higher than those in the control and other treatment groups (Figure 3).
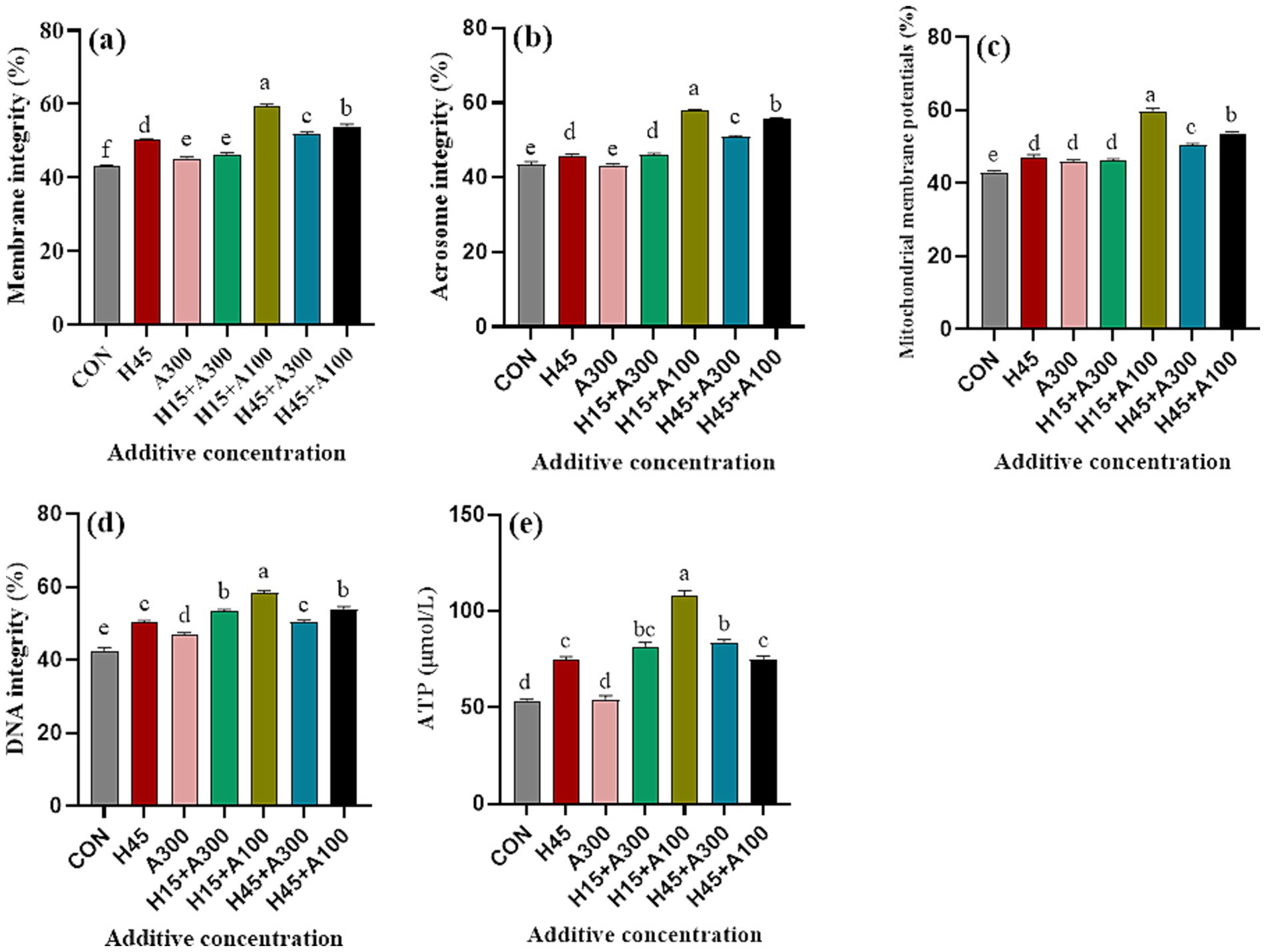
Figure 3. Effects of Hsd and AsA supplementation on the physiological characteristics and ATP content of frozen-thawed boar sperm in control and at 10 min after thawing. (a) Membrane integrity, (b) acrosome integrity, (c) mitochondrial membrane potentials, (d) DNA integrity, (e) ATP content.
3.3 Effects of supplementation with Hsd and AsA on antioxidant parameters of frozen-thawed boar sperm
As shown in Figure 4, SOD activity in Hsd treatment was significantly (p < 0.01) greater than that in control group. The addition of Hsd and AsA significantly (p < 0.01) increased CAT and GSH-Px activities compared with the control group. The SOD, CAT, and GSH-Px enzyme activities of 15 μM Hsd + 100 μM AsA group were significantly higher than those of the control and other groups. The PRODH activity in all treatments were significantly (p < 0.01) higher than that of the control group. Meanwhile, the difference between 15 μM Hsd + 100 μM AsA group and other treatment groups were significant (p < 0.01). Compared with the control group, the addition of Hsd and AsA significantly (p < 0.01) decreased the levels of MDA and ROS in frozen-thawed sperm. The ROS levels were lowest when 15 μM Hsd + 100 μM AsA were supplemented in the extender.
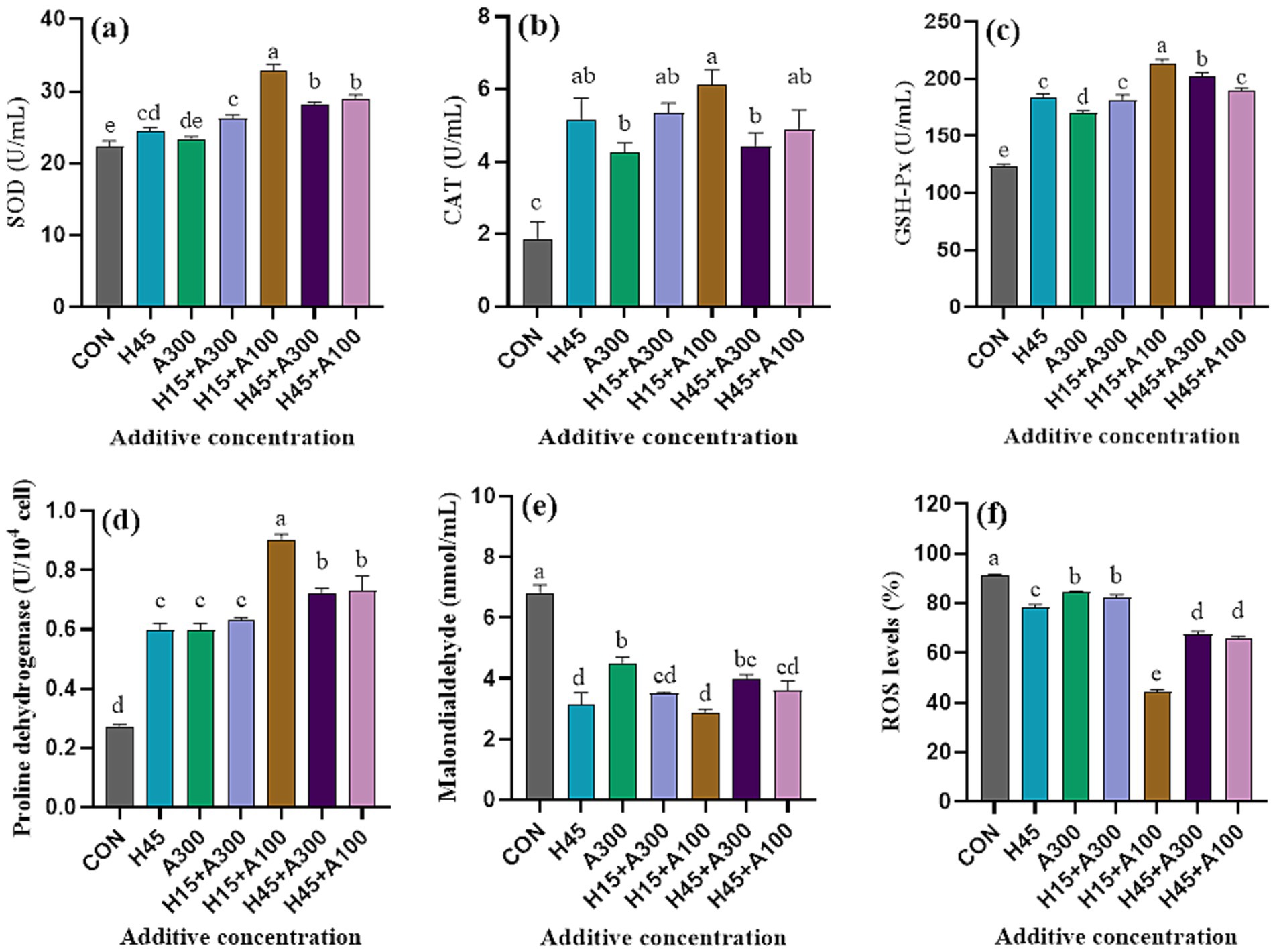
Figure 4. Effects of Hsd and AsA supplementation on the antioxidant parameters of frozen-thawed boar sperm in control and at 10 min after thawing. (a) SOD activities, (b) CAT activities, (c) GSH-Px activities, (d) PRODH activities, (e) MDA content, (f) ROS level.
3.4 Effect of supplementation with Hsd and AsA on protein expression of frozen-thawed boar sperm
As shown in Figure 5, part groups of the protein expression level of BCL-2 in the frozen-thawed sperm of Hsd combined with AsA group was significantly greater than that of Hsd group alone. Western blotting detected that the expression of apoptotic factor proteins Bax, Caspase-3, P53 and Cytochrome C in Hsd combined with AsA group were significantly decreased (p < 0.05, Figure 5B).
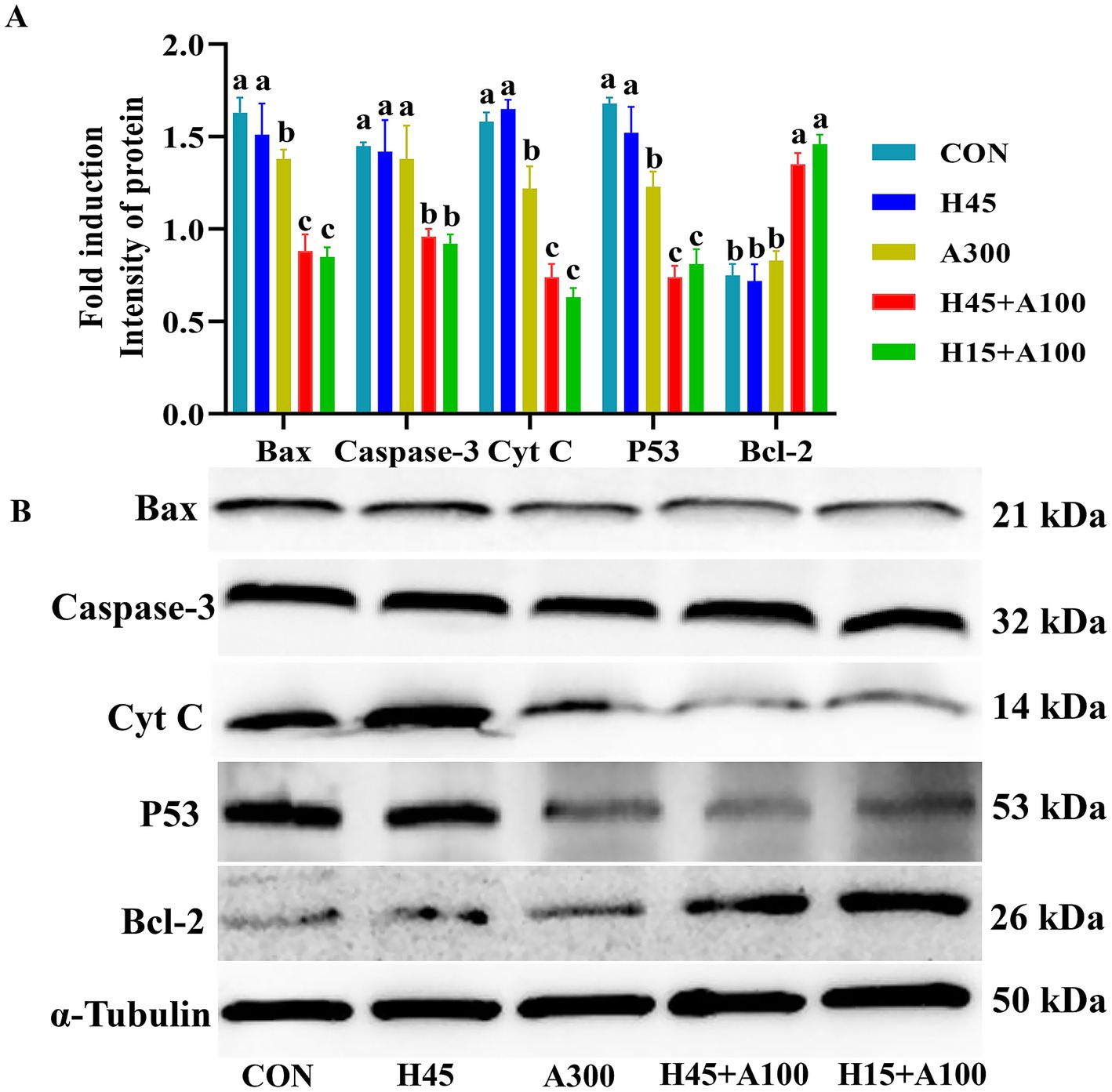
Figure 5. Effects of Hsd and AsA supplementation on part groups of the protein expression of frozen-thawed boar sperm in control and at 10 min after thawing. In image (A), relative quantitative intensity of protein expression of the frozen-thawed boar sperm. In image (B), protein expression of frozen-thawed boar sperm with western blot.
4 Discussion
China has the largest swine population in the world and accounts for roughly 45% of global pork production and 50% of the consumption of pork (22). African swine fever outbreaks have led to gross losses in the swine industry in China (23). The technology of cryopreservation in boar sperm is widely used to preserve the indigenous breeds, improve the preservation of superior breeds and the transport of genetic material without moving standing animals (2, 24). However, dramatic changes in temperature have induced oxidative damage to boar sperm during cryopreservation. Such damage is caused by the relatively high content of polyunsaturated phospholipids of the membrane and the relatively low antioxidant capacity of boar seminal plasma (6). The effective use of antioxidants during cryopreservation can improve survivability and minimize cryogenic damage. Some researchers have demonstrated the antioxidant function of both Hsd and ascorbic acid (25, 26). In addition, studies have shown that both AsA and other antioxidant combinations significantly improved boar sperm cryotolerance compared to when AsA was separately added to freezing and thawing media (17). This was the first report on the combined effect of Hsd and AsA on boar sperm quality during the freezing-thawing process. In the present study, the combined addition of Hsd and AsA to the boar sperm extender was evaluated. Our results showed that the combination of Hsd with AsA could improve boar sperm motility and velocity compared to when Hsd or AsA was separately added, as compared with the control. A recent study reported that semen motility parameters of Simmental cattle were significantly improved by Hsd supplementation (14). A previous study demonstrated that pig sperm frozen in an extender supplemented with 200 μM ascorbic acid 2-O-α-glucoside significantly increased sperm motility (18). Consistent with the results of the current study, the addition of both 15 μM Hsd and 100 μM of AsA to the freezing extender better protected the sperm from oxidative damage and significantly improved sperm motility.
Boar sperm is sensitive to the damage produced during the freezing-thawing process (27). In addition, because their plasma membranes contain a lot of polyunsaturated fatty acids, sperm cells are more susceptible to oxidative stress (28). It was reported that there was a significant correlation between antioxidant status and quality of frozen-thawed semen (29). The role of Hsd in the quality of frozen-thawed semen was evaluated in recent research, which indicated that Hsd significantly improved semen motility parameters in Simmental bull and reduced oxidative stress status in seminal plasma (14). To our knowledge, few studies have evaluated the effect of Hsd on semen quality after supplementation to an extender medium for frozen-thawed boar sperm. In the present study, treatment with 15 μM Hsd + 100 μM AsA had the greatest antioxidative enzyme activities of SOD, GPx and CAT in the frozen-thawed sperm among the tested groups. They play a crucial role in protecting sperm against the harmful effects of free radicals released due to mitochondrial metabolism. This protection is achieved through the actions of SOD and GSH-Px preventing oxidative attack during freezing. The SOD and GSH-Px are the main antioxidant enzyme defense systems in cells associated with antioxidant capacity. SOD is known to catalyze the decomposition of superoxide into hydrogen peroxide (H2O2) and oxygen (O2), and the increase of SOD activity can protect CAT and GSH-Px from inactivation of O2−, thus increasing the activity CAT and GSH-Px (29). Adding exogenous antioxidants to freezing media has been suggested as a strategy to counteract the effects of oxidative stress on cryopreserved sperm cells (30). Thus, the addition of Hsd + AsA to freezing and thawing media protects boar sperm against oxidative stress due to their antioxidant properties. Antioxidants possess the ability to combat free radicals by interrupting the reaction chain within the mitochondrial membrane. This interruption ultimately prevents oxidative damage. Consequently, we assume that Hsd and AsA play a significant role in the intracellular mechanism involved in cryopreservation. Cryopreservation is associated with an increase in the generation of reactive oxygen species (ROS) (31). In the reproduction system, high levels of ROS production are more vulnerable to oxidative damage in spite of their greater antioxidant capacity (32). Its cytotoxic effect on membrane phospholipids can lead to the production of malondialdehyde (MDA), which is a last product of unsaturated fatty acid lipid peroxidation (33). The combined addition of Hsd and AsA significantly reduced ROS levels and is more effective than the separate addition of either Hsd or AsA. They act directly or indirectly by minimizing the oxidative damage to lipids, proteins, and nucleic acids caused by ROS (34). Both Hsd and AsA may help increase fertility by reducing oxidative damage to DNA, proteins, and lipids. Membrane lipid peroxidation has been measured using malondialdehyde (MDA) formation. Supplementing freezing media with a Hsd and AsA combination significantly reduced lipid peroxidation. The structurally intact and functionally activated plasma membrane is crucial for the various physiological activities of sperm cells, including metabolism, capacitated acrosome reaction, attachment, and crossing of egg cells. For this reason, Hsd and AsA protect sperm from the detrimental effect of free oxidative radicals and have shown great sperm cryoprotective potential.
Oxidative stress occurs during freezing-thawing damage caused by an imbalance between the generation of ROS and antioxidants (35). Therefore, the intensity and duration of oxidative stress may influence the fate of cells, ranging from adaptive responses to apoptosis or necrosis (36). It can cause DNA damage in boar sperm. If the level of DNA damage exceeds the cells’ repair capabilities, it eventually leads to apoptosis. The decrease in mitochondrial membrane potential indicates early stages of apoptosis. Frozen-thawed sperm exhibited lower membrane integrity and sublethal dysfunction as a result of the cold shock, osmotic stress, and oxidative damage that occurred during cryopreservation. In this study, the supplementation of both Hsd and AsA significantly improved the integrity of the plasma membrane, acrosome, and DNA of frozen-thawed boar sperm compared with the control group. Additionally, the highest improvement was observed when the extender was supplemented with a combination of 15 μM of Hsd + 100 μM of AsA, which was greater when Hsd or AsA was added alone. This is in agreement with a previous study (17), in which combining reduced glutathione and AsA significantly improved acrosome integrity, sperm motility, and the integrity of nucleoprotein structure compared to the one that only contained reduced glutathione or AsA. The addition of a combination of Hsd and AsA to the extender produces a synergistic improvement in post-thawed sperm quality, preventing oxidative damage, lowering lipid peroxidation, DNA damage, and the expression of apoptotic factor proteins. However, this trial suffers from the limitations of the longer time evaluation after thawing.
5 Conclusion
In conclusion, the combined addition of Hsd and AsA to the extender enhanced the motility and integrities of the membrane, DNA, and acrosome of boar sperm. The combination of 15 μM Hsd + 100 μM AsA greatly increased mitochondrial and antioxidant functions while decreasing the levels of MDA and ROS and sperm apoptosis after freezing and thawing. The beneficial effect of Hsd combined with AsA on quality of frozen-thawed boar sperm was greater than that of Hsd or AsA alone.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Animal Care and Use Committee of Animal Science and Technology at Qingdao Agricultural University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
RD: Conceptualization, Formal analysis, Methodology, Writing – original draft. YD: Data curation, Software, Writing – original draft. MZ: Investigation, Methodology, Software, Validation, Writing – original draft. LL: Methodology, Software, Writing – original draft, Formal analysis. GY: Conceptualization, Funding acquisition, Project administration, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Key R&D Program of Shandong Province, China (Grant Nos. 2022LZGC003 and 2024LZGC017), and Natural Science Foundation of Shandong Province (Grant No. ZR2023MC047).
Acknowledgments
We gratefully acknowledge our colleagues at Qingdao Agricultural University and the General Station of Animal Husbandry of Shandong Province for their technical assistance and helpful comments on the manuscript. And we thank all of the staff for collecting samples.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yeste, M. Sperm cryopreservation update: cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology. (2016) 85:47–64. doi: 10.1016/j.theriogenology.2015.09.047
2. Bolarin, A, Berndtson, J, Tejerina, F, Cobos, S, Pomarino, C, D’Alessio, F, et al. Boar semen cryopreservation: state of the art, and international trade vision. Anim Reprod Sci. (2024) 269:107496. doi: 10.1016/j.anireprosci.2024.107496
3. Li, Y, Qin, S, Cui, W, Zhao, F, He, M, and Jiang, Z. Progress on the roles of zinc in sperm cryopreservation. Theriogenology. (2023) 211:134–41. doi: 10.1016/j.theriogenology.2023.08.014
4. Yeste, M. Recent advances in boar sperm cryopreservation: state of the art and current perspectives. Reprod Domest Anim. (2015) 50:71–9. doi: 10.1111/rda.12569
5. Mandal, R, Badyakar, D, and Chakrabarty, J. Role of membrane lipid fatty acids in sperm cryopreservation. Adv Androl. (2014) 2014:1–9. doi: 10.1155/2014/190542
6. Maldjian, A, Pizzi, F, Gliozzi, T, Cerolini, S, Penny, P, and Noble, R. Changes in sperm quality and lipid composition during cryopreservation of boar semen. Theriogenology. (2005) 63:411–21. doi: 10.1016/j.theriogenology.2004.09.021
7. Chanapiwat, P, and Kaeoket, K. The effect of Curcuma longa extracted (curcumin) on the quality of cryopreserved boar semen. Anim Sci J. (2015) 86:863–8. doi: 10.1111/asj.12395
8. Wang, SC, Wu, YY, and Chen, MC. Predicting the cryotolerance of boar sperm through antioxidant stress. Reprod Domest Anim. (2024) 59:e14554. doi: 10.1111/rda.14554
9. Zhang, S, Zhang, H, Liu, K, Xu, X, Qin, Y, Xiao, L, et al. Effect of cholesterol-loaded cyclodextrin treatment on boar sperm cryopreservation. Anim Biosci. (2024) 37:1558–67. doi: 10.5713/ab.24.0030
10. Tomasello, B, Malfa, GA, La Mantia, A, Miceli, N, Sferrazzo, G, Taviano, MF, et al. Anti-adipogenic and anti-oxidant effects of a standardised extract of Moro blood oranges (Citrus sinensis (L.) Osbeck) during adipocyte differentiation of 3T3-L1 preadipocytes. Nat Prod Res. (2021) 35:2660–7. doi: 10.1080/14786419.2019.1660337
11. Cebadera-Miranda, L, Domínguez, L, Dias, MI, Barros, L, Ferreira, IC, Igual, M, et al. Sanguinello and Tarocco (Citrus sinensis [L.] Osbeck): bioactive compounds and colour appearance of blood oranges. Food Chem. (2019) 270:395–402. doi: 10.1016/j.foodchem.2018.07.094
12. Buzdağlı, Y, Eyipınar, CD, Kacı, FN, and Tekin, A. Effects of hesperidin on anti-inflammatory and antioxidant response in healthy people: a meta-analysis and meta-regression. Int J Environ Health Res. (2023) 33:1390–405. doi: 10.1080/09603123.2022.2093841
13. Chen, J, Fan, X, Chen, J, Luo, X, Huang, X, Zhou, Z, et al. Effects of hesperidin on the histological structure, oxidative stress, and apoptosis in the liver and kidney induced by NiCl2. Front Vet Sci. (2024) 11:1424711. doi: 10.3389/fvets.2024.1424711
14. Tahmasbian, H, Ayen, E, and Khaki, A. Evaluation of the effects of hesperidin on fresh and frozen-thawed semen quality using two different cryopreservation methods in Simmental bull. Anim Reprod. (2022) 19:e20220042. doi: 10.1590/1984-3143-AR2022-0042
15. Celi, GEA, Gratão, PL, Lanza, MGDB, and Dos Reis, AR. Physiological and biochemical roles of ascorbic acid on mitigation of abiotic stresses in plants. Plant Physiol Biochem. (2023) 202:107970. doi: 10.1016/j.plaphy.2023.107970
16. Castillo-Martín, M, Yeste, M, Soler, A, Morató, R, and Bonet, S. Addition of L-ascorbic acid to culture and vitrification media of IVF porcine blastocysts improves survival and reduces HSPA1A levels of vitrified embryos. Reprod Fertil Dev. (2015) 27:1115–23. doi: 10.1071/RD14078
17. Giaretta, E, Estrada, E, Bucci, D, Spinaci, M, Rodríguez-Gil, JE, and Yeste, M. Combining reduced glutathione and ascorbic acid has supplementary beneficial effects on boar sperm cryotolerance. Theriogenology. (2015) 83:399–407. doi: 10.1016/j.theriogenology.2014.10.002
18. Yoshimoto, T, Nakamura, S, Yamauchi, S, Muto, N, Nakada, T, Ashizawa, K, et al. Improvement of the post-thaw qualities of Okinawan native pig spermatozoa frozen in an extender supplemented with ascorbic acid 2-O-α-glucoside. Cryobiology. (2008) 57:30–6. doi: 10.1016/j.cryobiol.2008.05.002
19. Dong, R, Luo, L, Liu, X, and Yu, G. Effects of riboflavin on boar sperm motility, sperm quality, enzyme activity and antioxidant status during cryopreservation. Vet Med Sci. (2022) 8:1509–18. doi: 10.1002/vms3.833
20. Zhang, W, Cui, H, Ding, K, Zhou, K, Li, Y, Hoque, SM, et al. Carboxylated ε-poly-l-lysine improves post-thaw quality, mitochondrial functions and antioxidant defense of goat cryopreserved sperm. Biology. (2023) 12:231. doi: 10.3390/biology12020231
21. Zhu, Z, Kawai, T, Umehara, T, Hoque, SM, Zeng, W, and Shimada, M. Negative effects of ROS generated during linear sperm motility on gene expression and ATP generation in boar sperm mitochondria. Free Radic Biol Med. (2019) 141:159–71. doi: 10.1016/j.freeradbiomed.2019.06.018
22. You, S, Liu, T, Zhang, M, Zhao, X, Dong, Y, Wu, B, et al. African swine fever outbreaks in China led to gross domestic product and economic losses. Nat Food. (2021) 2:802–8. doi: 10.1038/s43016-021-00362-1
23. Mason-D’Croz, D, Bogard, JR, Herrero, M, Robinson, S, Sulser, TB, Wiebe, K, et al. Modelling the global economic consequences of a major African swine fever outbreak in China. Nat Food. (2020) 1:221–8. doi: 10.1038/s43016-020-0057-2
24. Lee, S, Kim, Y-M, Cheong, H-T, Park, C-K, and Lee, S-H. Effect of magnetized freezing extender on membrane damages, motility, and fertility of boar sperm following cryopreservation. Animals. (2023) 13:634. doi: 10.3390/ani13040634
25. Pari, L, Karthikeyan, A, Karthika, P, and Rathinam, A. Protective effects of hesperidin on oxidative stress, dyslipidaemia and histological changes in iron-induced hepatic and renal toxicity in rats. Toxicol Rep. (2015) 2:46–55. doi: 10.1016/j.toxrep.2014.11.003
26. Njus, D, Kelley, PM, Tu, Y-J, and Schlegel, HB. Ascorbic acid: the chemistry underlying its antioxidant properties. Free Radic Biol Med. (2020) 159:37–43. doi: 10.1016/j.freeradbiomed.2020.07.013
27. Li, J, Li, J, Wang, S, Ju, H, Chen, S, Basioura, A, et al. Post-thaw storage temperature influenced boar sperm quality and lifespan through apoptosis and lipid peroxidation. Animals. (2023) 14:87. doi: 10.3390/ani14010087
28. Wysokińska, A, and Kondracki, S. Assessment of changes in sperm cell membrane integrity occurring during the storage of semen from genetically different males using two diagnostic methods. Can J Anim Sci. (2014) 94:601–6. doi: 10.4141/cjas2013-095
29. El-Khawagah, AR, Kandiel, MM, and Samir, H. Effect of quercetin supplementation in extender on sperm kinematics, extracellular enzymes release, and oxidative stress of Egyptian buffalo bulls frozen-thawed semen. Front Vet Sci. (2020) 7:604460. doi: 10.3389/fvets.2020.604460
30. Papas, M, Catalan, J, Barranco, I, Arroyo, L, Bassols, A, Yeste, M, et al. Total and specific activities of superoxide dismutase (SOD) in seminal plasma are related with the cryotolerance of jackass spermatozoa. Cryobiology. (2020) 92:109–16. doi: 10.1016/j.cryobiol.2019.11.043
31. Chatterjee, S, and Gagnon, C. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Mol Reprod Dev. (2001) 59:451–8. doi: 10.1002/mrd.1052
32. Simmons, LW, Lovegrove, M, and Lymbery, SJ. Dietary antioxidants, but not courtship effort, affect oxidative balance in the testes and muscles of crickets. J Exp Biol. (2018) 221:jeb184770. doi: 10.1242/jeb.184770
33. Kakkar, R, Kalra, J, Mantha, SV, and Prasad, K. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol Cell Biochem. (1995) 151:113–9. doi: 10.1007/BF01322333
34. Pezo, F, Yeste, M, Zambrano, F, Uribe, P, Risopatrón, J, and Sánchez, R. Antioxidants and their effect on the oxidative/nitrosative stress of frozen-thawed boar sperm. Cryobiology. (2021) 98:5–11. doi: 10.1016/j.cryobiol.2020.11.007
35. Rho, S, Chung, H-S, Kang, M, Lee, E, Cho, C, Kim, H, et al. Inhibition of production of reactive oxygen species and gene expression profile by treatment of ethanol extract of Moutan Cortex Radicis in oxidative stressed PC12 cells. Biol Pharm Bull. (2005) 28:661–6. doi: 10.1248/bpb.28.661
Keywords: antioxidant, ascorbic acid, boar sperm cryopreservation, hesperidin, sperm quality
Citation: Dong R, Dou Y Zhang M, Luo L and Yu G (2025) Effects of hesperidin and ascorbic acid combination on boar sperm quality during cryopreservation. Front. Vet. Sci. 12:1573983. doi: 10.3389/fvets.2025.1573983
Edited by:
Federica Turri, National Research Council (CNR), ItalyCopyright © 2025 Dong, Dou, Zhang, Luo and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guanghui Yu, Z3VhbmdodWl5dUBxYXUuZWR1LmNu
†ORCID: Ruilan Dong, orcid.org/0000-0003-0598-9455
Guanghui Yu, orcid.org/0000-0002-2068-5487
 Ruilan Dong1†
Ruilan Dong1† Guanghui Yu
Guanghui Yu