- Laboratory of Animal Pathology and Public Health, Key Laboratory of Zoonosis of Ministry of Agriculture, College of Veterinary Medicine, China Agricultural University, Beijing, China
Heat stress (HS) is a major concern in poultry production worldwide due to its adverse effects on feed intake, weight gain, carcass weight, and metabolic conditions. Several strategies have been explored to ameliorate the negative effects of HS in broiler chickens, among which antimicrobial peptides (AMPs) represent a promising approach. Previously, we isolated chicken hemoglobin antimicrobial peptides (CHAP) and further demonstrated that CHAP has strong bactericidal activity. However, whether CHAP can improve growth performance and maintain intestinal mucosal immunity under chronic HS conditions remains unclear. In the present study, a total of 141 one-day-old broilers were divided into two groups. A total of 36 broilers were used to establish a chronic HS model to evaluate the effects of CHAP on intestinal mucosal immunity, and the remaining 105 birds were used to monitor the inductive effects of CHAP on two vaccines, including Newcastle disease virus (NDV) and avian influenza virus (AIV) vaccines, in broilers. As expected, HS-stimulated broiler chickens supplemented with CHAP showed a significant increase in villus height in the duodenum (p < 0.01), jejunum (p < 0.05), and ileum (p < 0.01) compared to those who did not receive CHAP under chronic HS conditions. The levels of alkaline phosphatase (AKP) and the number of secretory IgA (sIgA)-producing cells were markedly decreased in the chronic HS group (p < 0.01), whereas both significantly recovered after CHAP administration (p < 0.01). CHAP administration improved the birds' body weight and average daily gain (ADG), as well as the feed utilization rate, under HS conditions. Moreover, CHAP effectively mitigated HS-induced bursa injury by inhibiting excessive bursal apoptosis through the downregulation of caspase-3 and Bax, as well as the upregulation of Bcl-2 (p < 0.01). Interestingly, CHAP supplementation enhanced the antibody titer of both NDV and AIV in the broilers. Finally, CHAP administration enhanced the proliferation of splenic lymphocytes. In summary, our data demonstrate that CHAP not only maintains intestinal stability to improve growth performance but also inhibits excessive apoptosis in immune organs and upregulates vaccination effects.
1 Introduction
Global warming has increased the frequency of extreme heat events, with heat stress (HS) being one of the most significant environmental challenges affecting poultry production (1, 2). Some pioneering studies have demonstrated that the poultry industry has become highly sensitive to environmental temperature changes, and thereby, HS has become a critical factor restricting the healthy and sustainable development of the livestock and poultry sectors (3, 4). Existing studies have demonstrated that chronic HS not only breaks the homeostasis of bile acid metabolism and gut-associated microbiota in broilers (4) but also induces lung injury in broiler chickens by disrupting the integrity of the blood–air barrier (5). Two studies from different groups have clarified that chronic HS not only significantly impairs growth performance and intestinal mucosal barrier parameters in broilers (6) but also causes liver damage by triggering ER stress-induced excessive apoptosis (7). Moreover, some reports have demonstrated that HS considerably reduces feed intake and production (8, 9), as well as decreases sperm fertility, leading to infertility (10). Our previous study also found that chronic HS markedly reduced average daily gain (ADG) and feed efficiency while causing severe intestinal injury in broilers (11). It is now widely recognized that heat stress is one of the most detrimental environmental challenges affecting poultry production and welfare, causing huge economic losses in the poultry industry worldwide (12).
Antimicrobial peptides (AMPs), originally described as natural microbicides that are selectively cytotoxic to bacteria while exhibiting minimal cytotoxicity to mammalian host cells, are an important part of the innate immune system of different vertebrate organisms (13–16). As a class of small peptides, AMPs not only exhibit stronger inhibitory effects against bacteria, fungi, parasites, and viruses (14, 17–20) but also have stronger regulatory effects during various pathophysiological processes (16, 21–24). With the successive discovery of a variety of AMPs, an increasing number of studies have shown that AMPs are actively involved in rapid chemoattraction and/or modulate host cells to engage in immunoregulatory processes. One kind of antimicrobial peptide derived from the sacculus rotundus of rabbits has been shown to maintain intestinal mucosal immunity and protect chickens from infection with very virulent infectious bursal disease virus (25, 26). Swine gut-derived antimicrobial peptides have been shown to improve growth performance and intestinal absorption capacity (27), as well as maintain the integrity of the intestinal mucosal surface, in chickens (28). NKHs27, originally derived from hyporthodus septemfasciatus, significantly improves the respiratory burst ability of macrophages in vitro and limits pathogen dissemination in vivo (29). A synthetic amphipathic molecule designed to mimic the properties of cationic antimicrobial peptides (cAMPs) could combat SARS-CoV-2 infection by upregulating the expression of type I interferons or IL-6 via an immunomodulatory strategy (30).
Recently, biologically active peptides obtained from food byproducts or wastewater from poultry slaughtering facilities have garnered increasing attention. Gallego et al. isolated a bioactive peptide from Spanish dry-cured ham bones and found that it positively impacts cardiovascular health (31). Wu et al. (32) identified an antioxidant peptide from the skin of Quasipaa spinosa and found that frog skin-derived peptide derivatives have potential applications as functional foods. A recent report found that steam-exploded tilapia skin-derived peptides exhibit strong ACE inhibitory effects and antihypertension activity (33). Saffron petals, a byproduct of the procession of the crude drug saffron, were also shown to effectively alleviate colon tissue damage and prevent body weight loss in a DSS-induced colitis mouse model (34). Previously, our research team successively established methods for the isolation and extraction of antimicrobial peptides from the swine intestine and blood (11, 27, 28), as well as from the sacculus rotundus of rabbits (25). In addition to isolating biological peptides from swine byproducts, we also established an optimized method to purify chicken hemoglobin-derived antimicrobial peptides (CHAP). We found that CHAP not only can combat 19 bacterial strains, including nine multidrug-resistant bacterial strains, but also exhibits lower embryotoxicity and high stability across different temperatures (35). Although CHAP has demonstrated potent antimicrobial activity, little is known about its immunoregulatory effects on chickens under heat-stress conditions. The present study aimed to determine the effects of CHAP on intestinal mucosal immunity under chronic heat stress and vaccination responses in broiler chickens.
2 Materials and methods
2.1 Preparation of CHAP
CHAP was isolated as previously described by Hu et al. (35). In brief, fresh chicken blood was harvested from Beijing Huadu Broiler Corporation (Beijing, China). It was mixed with a tissue masher to break the blood cells and then subjected to enzymatic hydrolysis with papain. Then, 5% acetic acid was added to the extract and incubated overnight at 4°C. Subsequently, the supernatant was centrifuged at 8,000 × g for 30 min at 4°C, and pH was adjusted to 6.0 ± 0.5 to obtain the crude extract. After measuring the concentration, the crude CHAP was loaded onto a 10 × 300 mm Sephadex G-100 column, and the purified elution was collected as previously described (35). The final fractions of interest were collected and stored as lyophilized powders at −20°C.
2.2 Establishment of a chronic HS model and CHAP intervention in the broiler chickens
In artificial climate chambers, 36 one-day-old, specific-pathogen-free (SPF), Arbor Acre (AA) male broiler chickens with similar BW were selected from Beijing Merial Vital Laboratory Animal Technology Co., Ltd (Beijing, China; https://en.bi-vital.com/). All birds were randomly divided into three groups (12 chicks per group): the Control group, the chronic HS group, and the heat-stress with CHAP intervention (CHAP&HS) group. A total of four chickens were placed in each cage, with three replicates per group. All chickens were kept at 20°C for 3-day acclimation before the trial. Each bird in the CHAP&HS group was given 0.2 mg of CHAP dissolved in 1 ml of sterile 0.9% NaCl solution by gavage every morning. The chickens in the other groups received the same volume of sterile 0.9% NaCl solution. During the 10-d experiment, the birds in the HS and CHAP&HS groups were exposed to 35°C to simulate high-temperature conditions, as previously established by Hu et al. (11). The control group was continuously maintained at 20°C until the end of this experiment. All birds received the same diet and were provided feed and water ad libitum. Body weight and feed intake were measured and recorded, and ADG, the feed conversion ratio (FCR), and average daily feed intake (ADFI) were calculated subsequently. This study was reviewed and approved by the Institutional Animal Care and Use Committee of China Agricultural University.
2.3 Effects of CHAP on the broiler chickens vaccinated against NDV and AIV
A total of 105 one-day-old, SPF, AA broiler chickens were obtained from Beijing Merial Vital Laboratory Animal Technology Co., Ltd (Beijing, China). All 105 birds were randomly assigned to three groups (35 chickens per group): the normal control group (CON), the CHAP intramuscularly injected group [CHAP(I)], and the CHAP drinking-administrated group [CHAP(D)]. Then, all chickens were immunized with the Newcastle disease virus (NDV) vaccine via nasal drops (0.05 ml/chicken) on days 3 and 15, and the avian influenza virus (AIV) vaccine was administered by myocardial injection (0.1 ml/bird) on day 15. For the CHAP(I) group, CHAP was intramuscularly injected at a dose of 0.1 mg/kg body weight on days 1, 7, 14, 21, 28, 35, 42, and 49. For the CHAP(D) group, CHAP was given at 20 mg/L per chicken at the same time points. All animals were provided with the same feed and water ad libitum. This study was reviewed and approved by the Institutional Animal Care and Use Committee of China Agricultural University.
2.4 Sample collection and processing
For the chronic HS model, the weight of all chickens was recorded daily throughout the experimental period. At the final time point of CHAP administration, all birds were euthanized and the blood samples were collected immediately. Then, the spleen, thymus, and bursa were quickly removed and weighed. Subsequently, different intestinal parts were removed and collected as previously described. A 1 cm section of the duodenum was obtained from the proximal duodenum (~4 cm after the pylorus), a 1 cm section of the jejunum was collected from the proximal jejunum (~2 cm after the yolk stalk), and a 1 cm section of the ileum was cut from the middle part between Meckel's diverticulum and the ileocecal junction (27, 36). Finally, all harvested tissues were immediately fixed in a 2.5% (vol/vol) glutaraldehyde-polyoxymethylene solution as previously described (37, 38).
To assess the induction effects of CHAP on the vaccination responses to NDV and AIV in chickens, five chickens from each group were randomly selected on days 7, 14, 21, 28, 35, 42, and 49. Then, each chicken was euthanized, weighed, and recorded. Blood samples were collected first, followed by the removal of the chicken's organs, including the spleen, thymus, and the bursa of Fabricius, to calculate the organ index using the following formula: organ index = Worgan/Wbody, where Worgan represents the weight of the organ (mg) and Wbody represents the weight of the body (g) (39).
2.5 Histology analysis
The fixed tissues, including immune organs such as the bursa, spleen, thymus, and different intestinal parts of the duodenum, jejunum, and ileum, were dehydrated and embedded in paraffin using our standard laboratory protocol (38, 40). Then, 5 μm serial paraffin sections were cut and kept at 37°C to dry thoroughly. Subsequently, the 5 μm sections were dewaxed with a graded alcohol solution and stained continuously with hematoxylin and eosin.
2.6 Measurement of villus height, AKP, and GCs in the intestine
Five micrometer histological sections of the duodenum, jejunum, and ileum were stained with hematoxylin and eosin. Then, five views per section and five sections per chicken were randomly selected and measured using the Motic Med 6.0 CMIAS Image Analysis System (MOTIC CHINA GROUP CO., LTD., China). Finally, the villus height values were obtained, and the average was calculated to determine the mean villus height for each section as per our established protocols (11, 25, 27, 28, 38). Goblet cells (GCs) in the intestine were identified using the improved periodic acid-Schiff (PAS) staining method as previously described (11, 28, 38). The profiles of intestinal alkaline phosphatase (AKP) were measured using our optimized Gomori's calcium-cobalt amendment method (11, 27, 38). After staining, five fields per section and five sections per chicken were randomly captured using the Motic Med6.0 CMIAS Image Analysis System (MOTIC CHINAGROUP CO., LTD., China). The number of GCs and the area density of AKP were calculated for statistical analysis (11, 38).
2.7 TUNEL staining
The 5 μm serial sections of the bursa, spleen, and thymus were prepared as previously described (11). Then, apoptotic cells in the histological sections—including the bursa, spleen, and thymus—were identified using our improved TUNEL staining method (41, 42). Deparaffinized and rehydrated sections were stained with an in-situ apoptosis detection kit (DeadEnd™ Colorimetric TUNEL System, Promega) according to the manufacturer's instructions. After counterstaining with hematoxylin, apoptotic cells with brown and blue nuclei were identified by visualization in the TUNEL-stained sections. Finally, all mounted sections were reviewed blindly under an Olympus microscope. The TUNEL-positive (apoptotic) cells were counted, and five fields per section and five sections per chicken were randomly captured using the Motic Med6.0 CMIAS Image Analysis System (MOTIC CHINAGROUP CO., LTD., China).
2.8 Effect of CHAP on the proliferation of splenic lymphocytes
Fresh spleen samples from the NDV- and/or AIV-vaccinated chickens were harvested and immediately transferred to an aseptic cabinet for isolating splenic lymphocytes, as described in a previous report (43). In brief, splenic lymphocytes were prepared by homogenizing the spleen of all groups of chickens with a grinder and suspending the cells in a lysis buffer (0.15 M NH4Cl, pH 7.4) for 10 min to remove erythrocytes. Then, the mixture was centrifuged at 1,500 rpm for 30 min, and the supernatant was discarded. The obtained precipitate was gently washed twice with Hanks' solution (Solarbio, Beijing, China) and re-suspended in RPMI-1640 medium supplemented with 10% FBS. Cell viability was measured by trypan blue staining according to the established protocol (44). Then, the cells were seeded into 96-well plates (1 × 106 cells/well) and treated with 20 μg/ml of Con A (20 μg/ml, Sigma Co., China) or LPS (20 μg/ml, Sigma Co., China) by adding 12 μl of the respective test solution. The control cultures received 12 μl of the culture medium without Con A or LPS. The cells were then cultured at 37°C in an atmosphere containing 5% CO2. After 24 h of incubation, cell viability was evaluated using a CCK-8 kit (Solarbio, Beijing, China). Finally, cell proliferation activity was measured using a commercialized WST-8 kit (Solarbio, Beijing, China) at 450 nm absorbance with a microplate reader. Cell proliferation activity was calculated as follows: Proliferation rate (%) = (P2 – P0)/(P1 – P0) × 100%, where P0 is the absorbance value of the medium containing Con A or LPS, P1 is the absorbance value of the control group, and P2 is the absorbance value of the CHAP-treated groups.
2.9 Immunological staining
The 5 μm histological sections of the duodenum, jejunum, and ileum were obtained and subjected to an avidin-biotin complex (ABC) immunohistochemistry examination for the detection of secretory IgA (sIgA, mouse-anti-chick IgA, Southern Technology Inc., Longwood, FL) and proliferating cell nuclear antigen (PCNA, rabbit anti-PCNA antibody, Beijing Biosynthesis Biotechnology CO., LTD., Beijing, China). Firstly, the histological sections were deparaffinized, rehydrated, and rinsed in PBS-T (0.01 mol/L PBS, pH 7.4). After being heated at 94°C in a citrate buffer solution (0.01 mol/L, pH 6.0) for antigen retrieval, the sections were allowed to cool naturally for 30 min, then immersed in 3% aqueous hydrogen peroxide at room temperature for 30 min for endogenous peroxidase ablation. The following steps were performed in a moist chamber. The sections were gently washed three times and incubated sequentially with the goat serum, the indicated primary antibody and its matched secondary antibody, and horseradish peroxidase-labeled avidin chain working fluid (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China). Finally, five fields per section and five sections per chicken were randomly captured using the Motic Med6.0 CMIAS Image Analysis System (MOTIC CHINAGROUP CO., LTD., China). The number and area density of sIgA and PCNA were quantified for statistical analysis (28, 40).
2.10 Statistical analysis
The experimental data were expressed as mean ± standard deviation (SD), as indicated in the matched figure legends. For comparisons between two groups, unpaired, two-tailed Student's t-test was used. Cellular data were analyzed using One-way ANOVA or two-way ANOVA for multiple comparisons, while clinical data were analyzed using the Mann–Whitney U-test. A p-value <0.05 was considered statistically significant. Graphs are representative of at least three independent experiments.
3 Results
3.1 Effects of CHAP on daily gain and the feed conversion rate in the chickens under chronic heat stress
Throughout the experimental period, the animals in the control group remained in good health, while chronic heat stress significantly reduced body weight and average daily gain and markedly increased the feed-to-gain ratio in the chickens. At the beginning of the study, there was no significant difference in body weight between the chronic HS group and the HS&CHAP group compared to the control group (p > 0.05; Figure 1A). After chronic HS was initiated in the broiler chickens, the body weight of the chickens in the HS group reduced significantly compared to the control group (p < 0.05). However, CHAP supplementation restored the body weight of the chickens, even under HS conditions (p < 0.05; Figure 1A). The animals in the chronic HS group exhibited lower average daily gain (ADG) and a higher feed conversion rate (FCR) compared to the control group (p < 0.05; p < 0.01). Unexpectedly, CHAP administration improved the ADG and reduced the FCR of the chickens under chronic HS conditions, showing a significant difference compared to the chronic HS group (p < 0.05; p < 0.01; Figures 1B, C). Surprisingly, there was no significant difference in the average daily feed intake (ADFI) of the chronic HS broilers compared to the control group or CHAP-intervened groups (Figure 1D). Moreover, superoxide dismutase (SOD) and ATPase levels were significantly reduced in the HS group compared to the control group, while CHAP intervention upregulated the levels of SOD and ATPase in the broilers, even under HS conditions (Figures 1E, F). The above results indicate that the chronic HS model had a significant inhibitory effect on the production performance of the chickens.
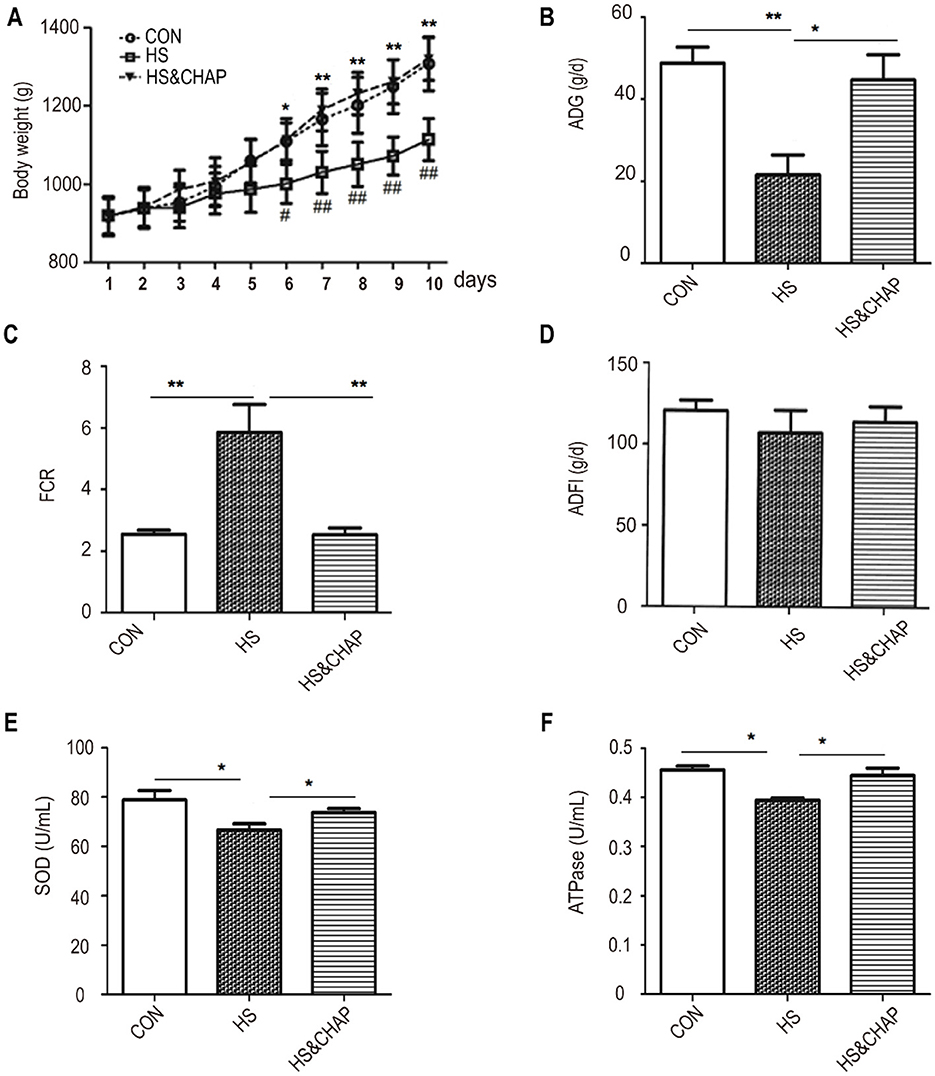
Figure 1. Effect of CHAP on the growth performance of the broiler chickens under chronic HS. (A) Dynamic changes in the body weight of the chickens under chronic HS conditions with or without CHAP supplementation. (B–D) Effects of CHAP on average daily gain (ADG), the feed conversion ratio (FCR), and average daily feed intake (ADFI) under chronic HS conditions, respectively. (E, F) Effects of CHAP on the expression levels of SOD and ATPase under chronic HS conditions, respectively. Data are presented as means ± SD. The * and ** indicate significant differences from the CON group at p < 0.05 and p < 0.01, respectively. The # and ## indicate significant differences between the HS and HS&CHAP groups at p < 0.05 and p < 0.01, respectively. CON, control group; HS, heat stress group; HS&CHAP, heat stress coupled with CHAP treated group.
3.2 Effects of CHAP on intestinal morphology under chronic conditions
Compared to the control group, the chickens under chronic HS had reduced villus height in the duodenum (p < 0.01; Figures 2A, B), jejunum (p < 0.05; Figures 2C, D), and ileum (p < 0.01; Figures 2E, F). Moreover, histopathological examination showed that the villus epithelium and the lamina propria of the duodenum and jejunum were edematous, and some villi were shed under chronic heat stress (Figures 2A, C). As expected, the chickens that were given CHAP showed an increase in villus height and recovered intestinal structure in the duodenum (p < 0.01; Figures 2A, B), jejunum (p < 0.01; Figures 2C, D) and ileum (p < 0.01; Figures 2E, F) compared to the chronic HS group.
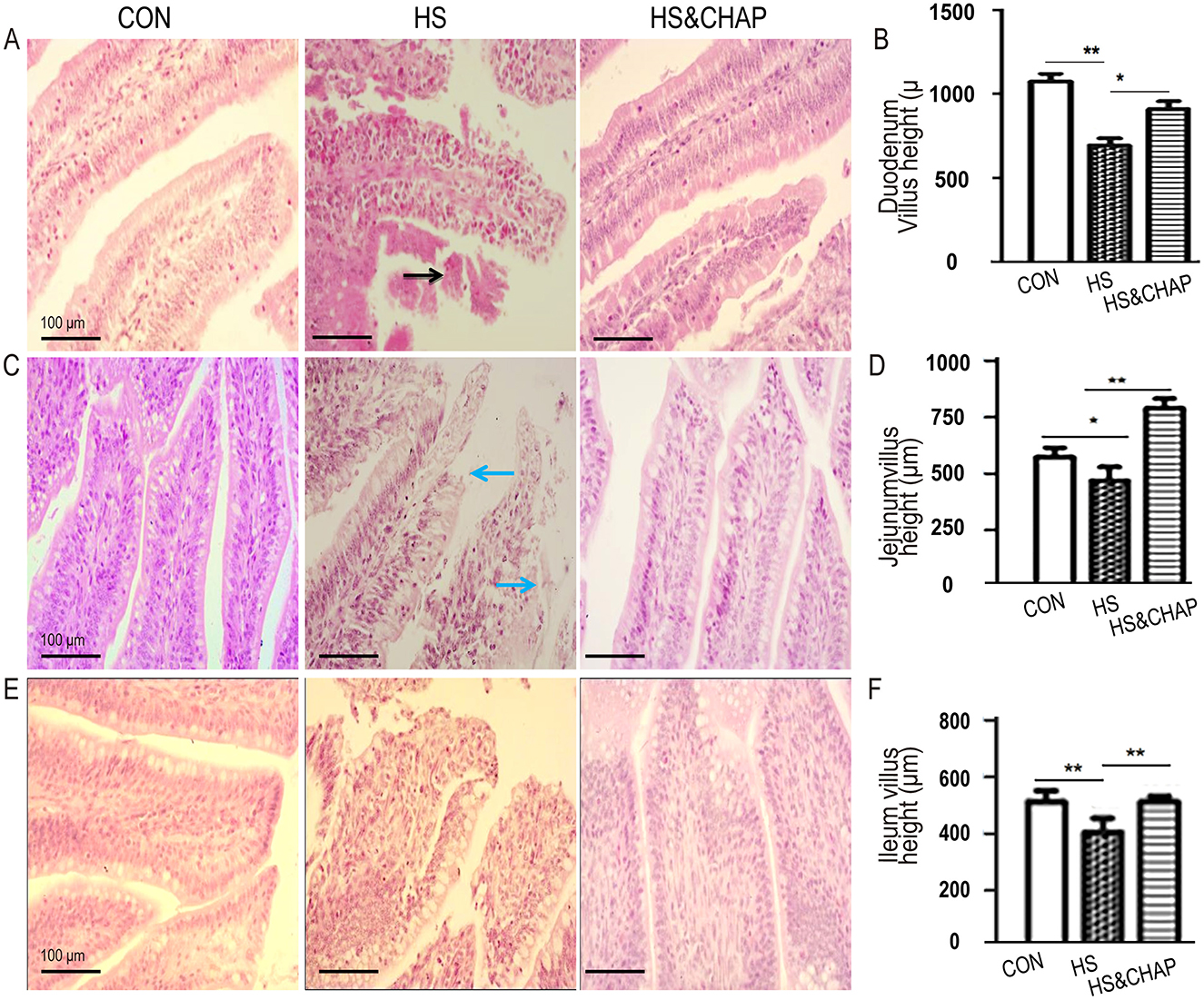
Figure 2. Effects of CHAP on villus height in different parts of the intestine. (A, B) Effects of CHAP on villus height in the duodenum under chronic HS stimulation. In the control group, the intestinal villus epithelium was arranged neatly and the villi were intact and longer. Under chronic HS conditions, the duodenal villi appeared disordered and shortened. Some villi were entangled in mucus, and hemorrhage occurred in the lamina propria and (or) submucosa (black arrow indicated). However, supplementation with CHAP in drinking water increased villus height in the duodenum. (C, D) CHAP administration effectively improved villus height in the jejunum, even under chronic HS conditions. (E, F) CHAP treatment alleviated the damage caused by chronic HS in the ileum. Data are presented as means ± SD. The * and ** indicate significant differences at p < 0.05 and p < 0.01, respectively. CON, control group; HS, heat stress group; HS&CHAP, heat stress coupled with CHAP treated group.
3.3 Effects of CHAP on the expression profile of AKP and sIgA under chronic conditions
Intestinal AKP is a resident brush-border enzyme that is crucial for the absorption and transport of nutrients and the maintenance of intestinal homeostasis (45–47). Considering that chronic heat stress reduced intestinal villus height, the AKP levels were measured. We found that AKP was highly expressed in the duodenum, but chronic heat stress significantly reduced its expression (p < 0.01; Figures 3A, B). Unexpectedly, AKP expression was restored in the duodenum of the chickens that received CHAP at 20 mg/L in the drinking water, even under chronic heat stress (p < 0.01; Figures 3A, B). Using established PAS staining (11, 27, 38), we found that the GCs displayed a typical goblet shape within the columnar cells. The number of GCs was significantly increased in the HS group, and there was a significant difference compared to the control group or HS&CHAP group (p < 0.01; Figures 3C, D). In each fragment of the intestinal samples, the sIgA-secreting cells were mainly distributed within the mucosa epithelium that featured a nucleus surrounded by a ring of yellow-brown cytoplasm (Figure 3E). In both the duodenum and jejunum, the area density of sIgA-producing cells in the chronic HS group was significantly reduced compared to the control group (p < 0.01; Figure 3F), while CHAP administration effectively recovered sIgA expression in the chickens under HS stimulation (p < 0.01; Figure 3F).
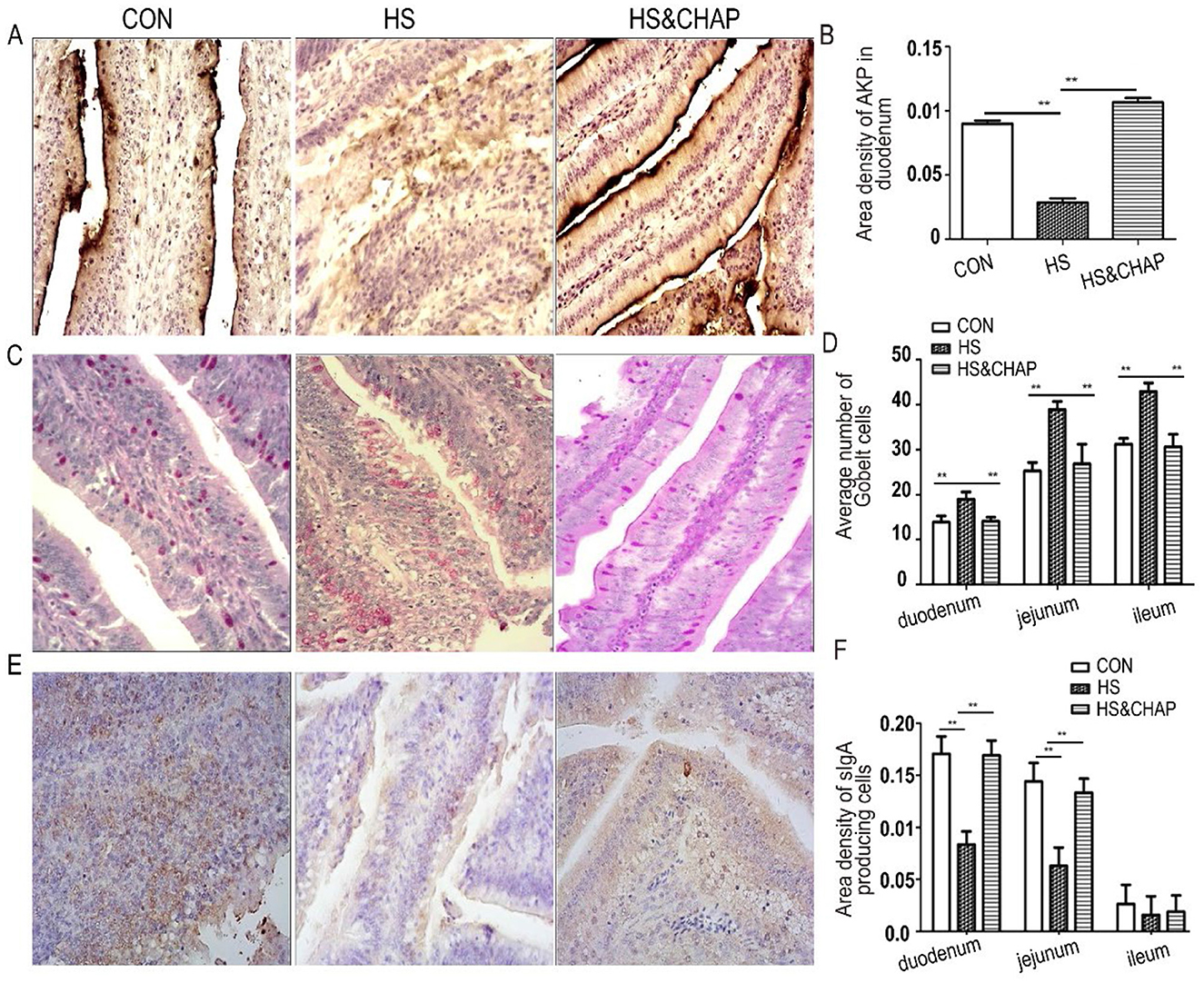
Figure 3. Effects of CHAP on intestinal AKP, GCs, and sIgA. (A, B) Gomori's calcium-cobalt staining found reduced expression of AKP in the HS group; however, CHAP administration significantly restored AKP production in the chickens, even under chronic HS conditions. (C, D) Improved PAS staining showed an increased density of GCs in the HS group; however, most of the GCs appeared incomplete, with their interior materials partially released. (E, F) Immunohistochemistry staining of sIgA and its quantitation. The ** indicate a significant differences at p < 0.01. CON, control group; HS, heat stress group; HS&CHAP, heat stress coupled with CHAP treated group.
3.4 Effects of CHAP on chronic HS-induced apoptosis in the chickens' immune organs
Histopathological examination showed that numerous follicular lymphocytes were absent in the bursa of Fabricius of the HS group (Figure 4A), statistically indicating that the organ index—including the bursa, spleen, and thymus—was significantly reduced in the HS group (Figures 4B–D) compared to the control and HS&CHAP groups (p < 0.05; p < 0.01). Administering CHAP to the chickens effectively restored those organs under chronic HS stimulation (Figures 4B–D). TUNEL staining revealed a large amount of brown signal distributed in the bursa of the animals under chronic HS conditions; however, fewer TUNEL-positive cells were observed in the control and HS&CHAP groups (Figure 4E). Statistical quantitation demonstrated that there was a significant difference in the HS group compared to the control and HS&CHAP groups (Figure 4F). Some critical pro-apoptotic or anti-apoptotic factors such as Caspase-3, Bcl2, and Bax were determined using immunohistochemistry staining. Consistent with the excessive TUNEL-positive cells in the HS group, Caspase-3 and Bax, both pro-apoptotic factors, were highly expressed in the bursa and showed a significant difference compared to the control group (p < 0.05; Figures 4G–J). However, CHAP intervention significantly reduced the expression of Caspase-3 and Bax, even under chronic HS conditions (p < 0.05; Figures 4G–J). Bcl-2, a well-known anti-apoptotic molecule, was highly expressed in both the control and HS&CHAP groups, whereas it was rarely detected in the bursal tissues of the chickens under chronic HS conditions (p < 0.05; Figures 4K, L).
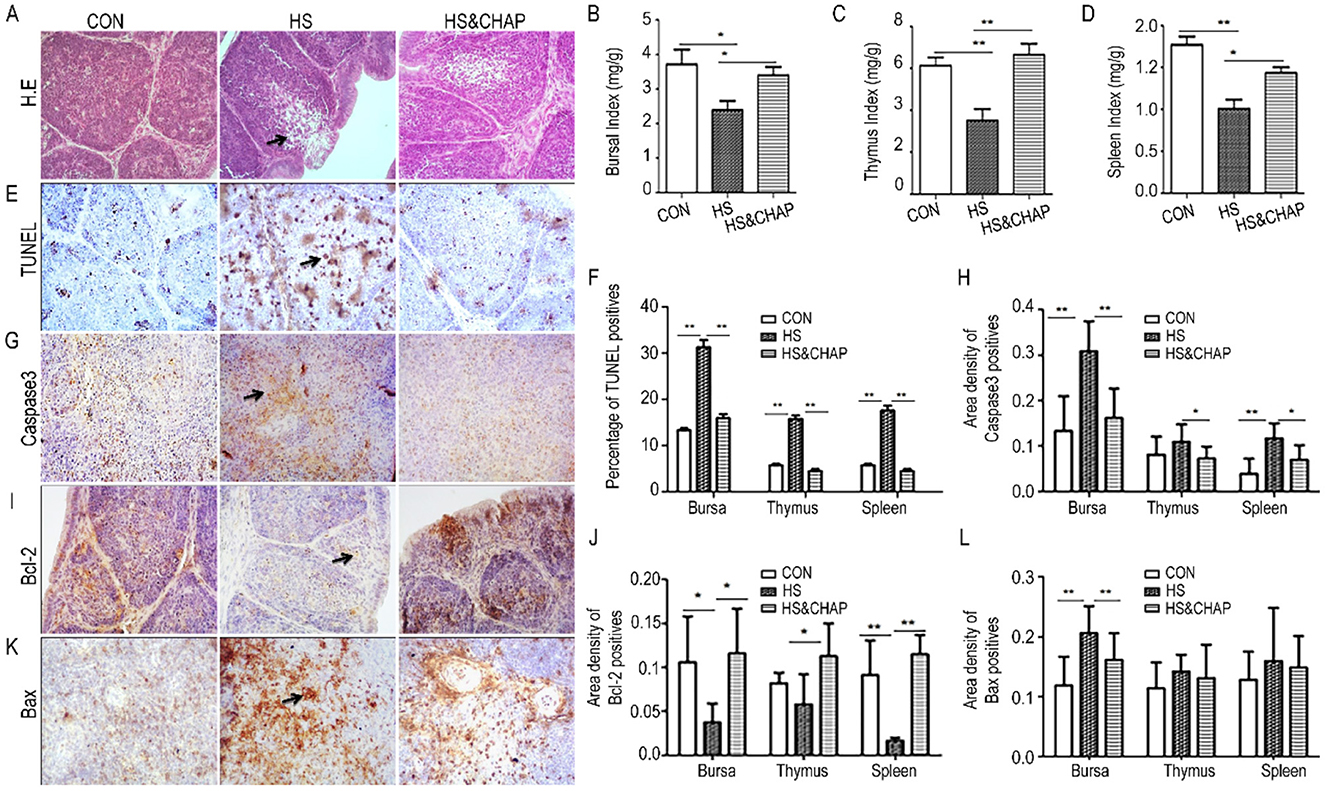
Figure 4. Effects of CHAP on apoptosis in the immune organs under chronic HS conditions. (A) H.E. staining found that the bursa of Fabricius was swollen and that a large number of necrotic lymphoid follicles and immune cells were present in the chronic HS group. (B–D) Quantitation demonstrated that chronic HS reduced the organ index of major immune organs, including the bursa, spleen, and thymus. (E, F) TUNEL staining showed excessive apoptotic positive cells in the chronic HS-treated group, while CHAP administration reduced apoptosis. (G, H) Immunohistochemistry staining demonstrated an increased expression of Caspase-3 in the bursal tissues of the HS group. (I, J) Bax, a pro-apoptotic protein, was significantly upregulated in the bursa under chronic HS conditions; however, it was dramatically decreased following CHAP treatment. (K, L) Bcl-2, an anti-apoptotic molecule, was downregulated in the HS group, but it was recovered after CHAP administration. The * and ** indicate significant differences at p < 0.05 and p < 0.01, respectively. CON, control group; HS, heat stress group; HS&CHAP, heat stress coupled with CHAP treated group.
3.5 Effects of CHAP on the immune response induced by NDV and AIV vaccination
The NDV antibody or AIV antibody was determined using the indicated commercial ELISA kit (Finde, Shenzhen, China) according to the manufacturer's instructions. As shown in Figure 5A, CHAP injected intramuscularly [CHAP(I)] significantly upregulated antibody titers in the broilers immunized with the NDV vaccine starting from day 21, compared to the chickens without CHAP (p < 0.05; p < 0.01). In contrast, the CHAP drinking group showed a significant difference on day 42 compared to the control (p < 0.05). For AIV vaccination shown in Figure 5B, both CHAP(D) and CHAP(I) enhanced induction in the broilers starting from day 21 compared to the matched control group (p < 0.05; p < 0.01).
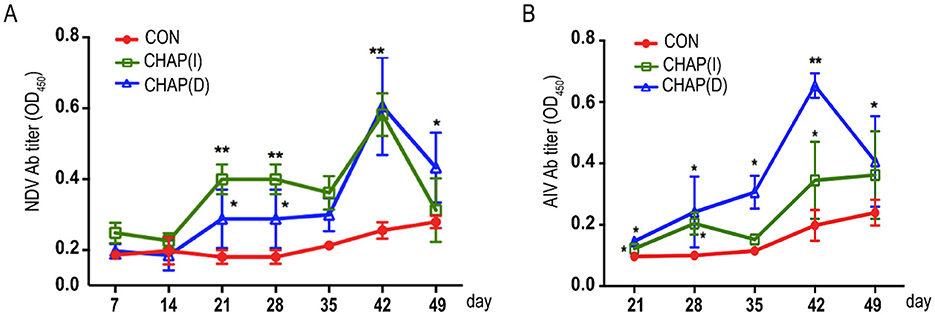
Figure 5. Effects of CHAP on the induction of vaccination responses to NDV and AIV. (A) The Newcastle disease virus (NDV) Ab titers were determined using a commercial ELISA kit (Finde, Shenzhen, China). The results showed that the NDV Ab titers in both CHAP-treated groups were higher compared to the control group (p < 0.05, p < 0.01). (B) CHAP administration significantly upregulated the concentration of the AIV antibody in the broiler chickens compared to the control group (p < 0.05, p < 0.01). Injected intramuscularly = CHAP(I), drinking water = CHAP(D). *p < 0.05, **p < 0.01.
3.6 Effects of CHAP on splenic lymphocytes proliferation
CHAP supplementation improved the chicken's spleen index compared to the control group (p < 0.05; Figure 6A). To further investigate the effect of CHAP on the proliferation of spleen lymphocytes, splenic lymphocytes were isolated from the spleen of all groups of chickens and treated with ConA or LPS to establish an inflammatory state. As shown in Figure 6B, after treatment with ConA, the splenic lymphocyte proliferation rate was increased in the CHAP-treated chickens starting from day 21, with a significant difference compared to the birds without CHAP supplementation (p < 0.05; p < 0.01). For LPS induction, the splenic lymphocyte proliferation rate was significantly increased on days 21, 35, 42, and 49 in the CHAP-treated group compared to the groups without CHAP (Figure 6C; p < 0.05; p < 0.01). These results indicate that CHAP could promote the proliferation of spleen lymphocytes in broiler chickens.
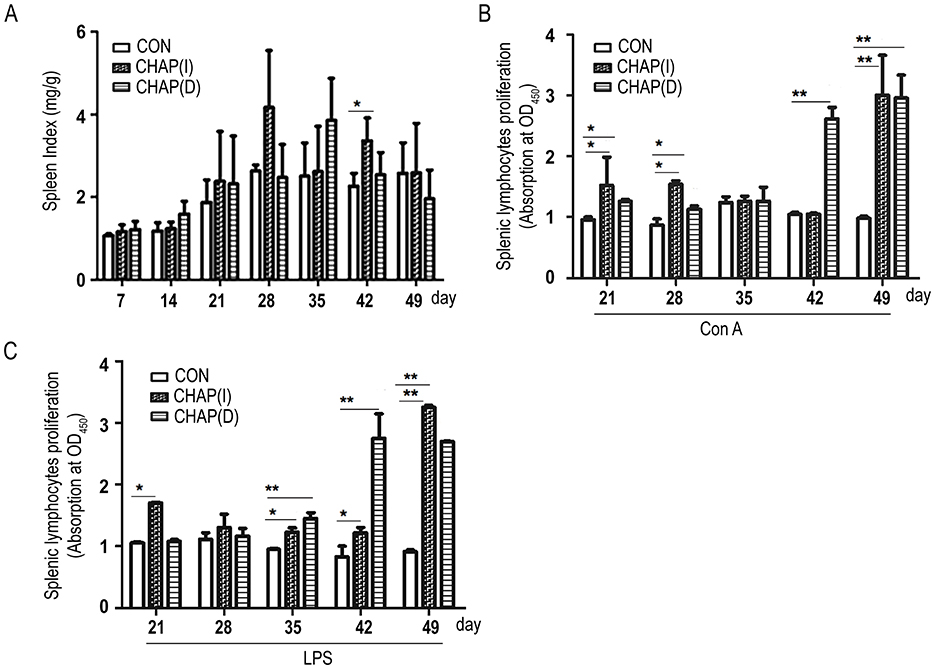
Figure 6. Effects of CHAP on the proliferation of splenic lymphocytes. (A) CHAP treatment improved the spleen index and showed a significant difference compared to the control group (p < 0.05). (B, C) CHAP-treated chicken splenic lymphocytes exhibited a higher proliferation rate compared to the control group under Con A or LPS stimulation (*p < 0.05, **p < 0.01).
4 Discussion
Rising global temperatures are leading to an increased frequency of extreme heat events, with heat stress being the most significant (1, 2, 10, 48, 49, 76). With the growing number of reports on the hazards of heat stress, its potentially significant impact on the livestock and poultry industry has attracted considerable attention. Numerous studies have found that chronic heat stress not only modifies physiological parameters and meat quality but also induces liver injury in broiler chickens (50, 51). Heat stress also decreases productivity and poses a significant negative impact on pig production (52). Moreover, chronic HS exposure not only mediates hepatic lipid deposition but also induces renal fibrosis and mitochondrial damage in growing laying hens (53, 54). Our previous study demonstrated that chronic HS negatively affected intestinal mucosal functions and reduced the growth performance of broiler chickens (55). To reduce the losses caused by heat stress in the poultry industry, numerous exploratory studies have been conducted. Yin et al. (54) found that dietary vitamin C supplementation effectively alleviated hepatic lipid deposition in broiler chickens under chronic HS exposure. A trial study implied that dietary supplementation with dimethyl itaconate could alleviate oxidative stress and inflammation in broilers under chronic heat stress (56). Similarly, a recent study demonstrated that resveratrol could improve liver antioxidant function and promote growth performance in broilers exposed to HS conditions (57). Our previous study found that an isolated antimicrobial peptide from the swine gut effectively maintained growth performance in broiler chickens even under chronic HS (11).
In the present study, we investigated the effects of CHAP on growth performance and intestinal immune parameters in broiler chickens under HS conditions. Consistent with previous findings, chronic HS exposure not only significantly reduced body weight, average daily gain, and the feed conversion ratio in the broiler chickens but also decreased villus height in the duodenum, jejunum, and ileum. Our findings are in line with published reports indicating that chronic HS could impair gastrointestinal histology and intestinal mucosal barrier function in broilers (6, 9). Furthermore, histological analysis demonstrated that CHAP supplementation, administered via drinking water, increased villus height and improved growth performance under chronic heat stress conditions. This finding is in agreement with our previous reports, which showed that an isolated antimicrobial peptide from the swine gut had similar effects on broiler chickens (11, 27).
Intestinal AKP, an endogenous protein secreted by the microvilli of the intestinal epithelium, plays an essential role in intestinal homeostasis via balanced intestinal inflammation and intestinal permeability (47, 58, 59). Reduced AKP expression has been observed not only in chickens infected with the very virulent infectious bursal disease virus (38) but also in various parts of the intestine in broiler chickens under chronic HS stimulation (11). In the current study, intestinal AKP expression was significantly decreased in the chronic HS-treated group. However, the administration of CHAP remarkably improved the intestinal AKP profile in the harmful HS environment, implying that CHAP could maintain normal intestinal morphology and ensure effective nutrient absorption for growth performance under HS conditions. In other words, the present study, which found that CHAP upregulated AKP expression, is consistent with our previous research, where a swine gut-derived antimicrobial peptide effectively enhanced AKP activity to potentiate digest function and absorption (27).
Our previous study found that sIgA expression in the duodenum, jejunum, and ileum was significantly reduced in SPF chickens infected with the very virulent infectious bursal disease virus (38). Goblet cells (GCs) and secretory IgA (sIgA) are major components and multifaceted players of the intestinal immune barrier in maintaining intestinal homeostasis (60–63, 77). GCs reside in the epithelium and serve as the primary site for synthesizing and secreting mucus, which protects the intestinal mucosal layer from dehydration, damage, and various stimuli (60, 61). In the present study, HS stimulation significantly upregulated the number of GCs, suggesting that HS-triggered changes in GCs led to an increase in their number, allowing for the secretion of large amounts of substances to adapt to gut activity under HS conditions. This finding is consistent with our previous reports showing that chronic HS increased the number of GCs (11), but it is in disagreement with the finding that decreased GC numbers were observed in Cobb broilers under HS conditions (64). This suggests that there are some intrinsic differences in the morphology and function of GCs among different kinds of chickens.
Although some previous studies have demonstrated that HS impairs the development of immune organs, including the bursa, spleen, and thymus, in broiler chickens (65, 66, 78, 79), the underlying mechanisms contributing to these processes are complicated and not yet fully understood. In the present study, our data demonstrated that the immune organ indexes of the bursa of Fabricius and thymus were significantly decreased following chronic HS stimulation, which is consistent with some published reports suggesting that HS impairs immune organ indexes to disrupt immune system function (66). Moreover, some reports suggested that HS induced higher expression levels of pro-apoptotic genes, including Caspase-3 and Bax, in broiler livers. However, these genes were downregulated after dietary supplementation with dimethyl itaconate (56). Consistent with established publications, we found that HS stimulation caused considerable apoptosis in the bursa of the chickens, in which TUNEL-positive cells, Caspase-3, and Bax were significantly upregulated. As important pro-apoptotic effectors, the highly expressed Caspase-3 and Bax proteins promote the occurrence of apoptosis (67). Unexpectedly, CHAP administration in the HS-stimulated broilers markedly reduced Caspase-3 and Bax in the bursa, while it promoted the expression of Bcl-2. In the present study, CHAP supplementation upregulated Bcl-2 to prevent excessive apoptosis in the bursa of the broilers under HS conditions. Meanwhile, Caspase-3 and Bax were markedly reduced after CHAP administration, which is consistent with a recently published report suggesting that rabbit sacculus rotundus-derived antimicrobial peptides could inhibit excessive apoptosis of bursal lymphocytes to reduce follicle depletion and destruction mediated by vvIBDV-infection (26). Collectively, CHAP could inhibit excessive apoptosis to mitigate immune organ injury by downregulating the expression of Caspase-3 and Bax, as well as enhancing Bcl-2 expression, in broiler chickens under HS stimulation. CHAP, a kind of antimicrobial peptide derived from chicken hemoglobin, was first isolated by our research team and has shown potent and rapid antimicrobial activity, along with lower toxicity and high stability under different temperature conditions (35). A recent report found that daily supplementation with melittin in ducks could alleviate HS-induced immune organ damage and improve growth performance (68). In addition, dried plum supplementation was reported to decrease the negative effects of HS in broiler chickens by upregulating heat-shock-associated genes and nutrient transporters (69). Cathelicidin LL-37, a widely studied antimicrobial 37-mer peptide with various ascribed functions, was reported to be able to prevent HS-induced intestinal damage and heat-related illnesses in rats (70). Our data showed that in the chronic HS model, CHAP was capable of maintaining normal intestinal morphology, particularly protecting villus height and the AKP expression profile against heat stress-induced injury, which is important for nutrient absorption and the growth performance of chickens. Collectively, the present data demonstrate that those bioactive peptides have positive effects on growth performance, intestinal mucosal immunity, and its functions, which are consistent with our previous findings (11, 25, 27, 28, 35, 80–84). Therefore, these beneficial effects of CHAP may lead to more successful management of systemic inflammation and multiple organ injury in broiler chickens under HS conditions.
Bioactive peptides were separated from chicken byproduct hydrolysates in slaughterhouses and exhibited biological activity, offering several health benefits (71, 72). Keratinase is obtained by effectively utilizing various poultry wastes, including feathers, and provides practical insights and novel approaches for recycling and degrading biomass (73). Moreover, some biological peptides isolated from fishery byproducts, such as frames, trimmings, and viscera, showed antioxidant activity (74). Considering the large amount of poultry blood produced each year, our research team previously isolated a bioactive peptide from chicken blood and established a practical protocol (35). The present data further demonstrate that CHAP has positive effects on growth performance, and the action mechanism of CHAP is further revealed.
5 Conclusion
In conclusion, this report provides a more in-depth study of our previous work on CHAP. CHAP administration improves growth performance, intestinal mucosal immunity, and vaccination responses in broilers under chronic HS. It protects against HS-induced immune organ damage by regulating apoptosis-related proteins and enhances nutrient absorption by maintaining intestinal integrity. These findings highlight the potential of CHAP as a therapeutic agent for improving poultry production under heat stress conditions.
6 Limitations of this study
Although the present study provides valuable insights into the effects of CHAP on growth performance, intestinal mucosal immunity, and immune organs, some limitations remain and should be clarified in future studies. For instance, although CHAP modulated apoptosis-associated genes, including Caspase-3, Bax, and Bcl-2, under HS stimulation, more evidence is needed to determine whether the interaction between them is direct or indirect. Although CHAP showed protective effects in broiler chickens under HS conditions, its potential adverse effects should be considered in future studies. According to a published report, Cathelicidin LL-37 acted as a double-edged sword, promoting wound healing but with some derivatives exhibiting higher hemolysis and cytotoxic effects during this process (75). Therefore, further evidence is required for the assessment of CHAP-derived derivatives under HS stimulation. Finally, although CHAP functionally mitigated immune organ damage and upregulated the antibody titers induced by NDV and AIV vaccination, these findings are based on laboratory data and require additional validation. More controlled experiments and validation data, especially from commercial chicken farms, are indeed needed.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the Institutional Animal Care and Use Committee of China Agricultural University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
DW: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. FH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Writing – original draft. HL: Conceptualization, Data curation, Formal analysis, Investigation, Resources, Writing – original draft. RS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. JT: Conceptualization, Formal analysis, Visualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant Nos. 31072110, 31272515, and 31472165 to Ruiping She), the Research Fund for the Doctoral Program of Higher Education of China (Grant No. 20070019035 to Ruiping She), and the Hubei Provincial Natural Science Foundation of China (Grant No. 2022CFB195 to Fengjiao Hu).
Acknowledgments
We would like to thank the National Natural Science Foundation of China (Grant Nos. 31072110, 31272515, and 31472165), the Research Fund for the Doctoral Program of Higher Education of China (Grant No. 20070019035), and the Hubei Provincial Natural Science Foundation of China (Grant No. 2022CFB195 to Fengjiao Hu) for providing financial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HS, heat stress; CHAP, chicken hemoglobin antimicrobial peptides; NDV, Newcastle disease virus; AIV, avian influenza virus; Con A, Concanavalin A; LPS, lipopolysaccharides; cAMPs, antimicrobial peptides; ADG, average daily gain; FCR, feed conversion ratio; ADFI, average daily feed intake; PAS, periodic acid-Schiff; AKP, alkaline phosphatase; ABC, avidin-biotin complex; SOD, superoxide dismutase; GCs, goblet cells; sIgA, secreting IgA; SRBC, sheep red blood cells; ROS, reactive oxygen species; SPF, specific-pathogen-free; AA, Arbor Acre; CON, control group; CHAP(I), CHAP intramuscularly injected group; CHAP(D), CHAP drinking-administrated group.
References
1. Ebi KL, Capon A, Berry P, Broderick C, de Dear R, Havenith G, et al. Hot weather and heat extremes: health risks. Lancet. (2021) 398:698–708. doi: 10.1016/S0140-6736(21)01208-3
2. Rau A, Tarr GAM, Baldomero AK, Wendt CH, Alexander BH, Berman JD, et al. Heat and cold wave-related mortality risk among United States veterans with chronic obstructive pulmonary disease: a case-crossover study. Environ Health Perspect. (2024) 132:27004. doi: 10.1289/EHP13176
3. Tang S, Li M, Sun Y, Liao Y, Wu X, Zhong R, et al. Effects of chronic heat stress on the immunophenotyping of lymphocytes in immune organs of growing pigs. J Anim Sci. (2022) 100:skac317. doi: 10.1093/jas/skac317
4. Zhang Y, Chen H, Cong W, Zhang K, Jia Y, Wu L, et al. Chronic heat stress affects bile acid profile and gut microbiota in broilers. Int J Mol Sci. (2023) 24:10238. doi: 10.3390/ijms241210238
5. Wu XY, Wang FY, Chen HX, Dong HL, Zhao ZQ, Si LF, et al. Chronic heat stress induces lung injury in broiler chickens by disrupting the pulmonary blood-air barrier and activating TLRs/NF-κB signaling pathway. Poult Sci. (2023) 102:103066. doi: 10.1016/j.psj.2023.103066
6. Peng XY, Xing T, Li JL, Zhang L, Jiang Y, Gao F, et al. Guanidinoacetic acid supplementation improves intestinal morphology, mucosal barrier function of broilers subjected to chronic heat stress. J Anim Sci. (2023) 101:skac355. doi: 10.1093/jas/skac355
7. Ma B, Xing T, Li J, Zhang L, Jiang Y, Gao F, et al. Chronic heat stress causes liver damage via endoplasmic reticulum stress-induced apoptosis in broilers. Poult Sci. (2022) 101:102063. doi: 10.1016/j.psj.2022.102063
8. Hu J, Xiong Y, Gates RS, Cheng HW. Perches as cooling devices for reducing heat stress in caged laying hens: a review. Animals. (2021) 11:3026. doi: 10.3390/ani11113026
9. Mazzoni M, Zampiga M, Clavenzani P, Lattanzio G, Tagliavia C, Sirri F, et al. Effect of chronic heat stress on gastrointestinal histology and expression of feed intake-regulatory hormones in broiler chickens. Animal. (2022) 16:100600. doi: 10.1016/j.animal.2022.100600
10. Gao Y, Wang C, Wang K, He C, Hu K, Liang M, et al. The effects and molecular mechanism of heat stress on spermatogenesis and the mitigation measures. Syst Biol Reprod Med. (2022) 68:331–47. doi: 10.1080/19396368.2022.2074325
11. Hu F, Gao X, She R, Chen J, Mao J, Xiao P, et al. Effects of antimicrobial peptides on growth performance and small intestinal function in broilers under chronic heat stress. Poult Sci. (2017) 96:798–806. doi: 10.3382/ps/pew379
12. Wankar AK, Rindhe SN, Doijad NS. Heat stress in dairy animals and current milk production trends, economics, and future perspectives: the global scenario. Trop Anim Health Prod. (2021) 53:70. doi: 10.1007/s11250-020-02541-x
13. Chung CR, Liou JT, Wu LC, Horng JT, Lee TY. Multi-label classification and features investigation of antimicrobial peptides with various functional classes. iScience. (2023) 26:108250. doi: 10.1016/j.isci.2023.108250
14. Lazzaro BP, Zasloff M, Rolff J. Antimicrobial peptides: application informed by evolution. Science. (2020) 368:eaau5480. doi: 10.1126/science.aau5480
15. Liu W, Han Y, An J, Yu S, Zhang M, Li L, et al. Alternation in sequence features and their influence on the anti-inflammatory activity of soy peptides during digestion and absorption in different enzymatic hydrolysis conditions. Food Chem. (2025) 471:142824. doi: 10.1016/j.foodchem.2025.142824
16. Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. (2002) 415:389–95. doi: 10.1038/415389a
17. Bai M, Liu H, Yan Y, Duan S, Szeto IM, He J, et al. Hydrolyzed protein formula improves the nutritional tolerance by increasing intestinal development and altering cecal microbiota in low-birth-weight piglets. Front Nutr. (2024) 11:1439110. doi: 10.3389/fnut.2024.1439110
18. Broekaert WF, Terras FR, Cammue BP, Osborn RW. Plant defensins: novel antimicrobial peptides as components of the host defense system. Plant Physiol. (1995) 108:1353–8. doi: 10.1104/pp.108.4.1353
19. Chen F, Wang Y, Wang K, Chen J, Jin K, Peng K, et al. Effects of Litsea cubeba essential oil on growth performance, blood antioxidation, immune function, apparent digestibility of nutrients, and fecal microflora of pigs. Front Pharmacol. (2023) 14:1166022. doi: 10.3389/fphar.2023.1166022
20. Ganz T, Lehrer RI. Antimicrobial peptides of vertebrates. Curr Opin Immunol. (1998) 10:41–4. doi: 10.1016/S0952-7915(98)80029-0
21. Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. (2005) 3:238–50. doi: 10.1038/nrmicro1098
22. Hancock RE, Scott MG. The role of antimicrobial peptides in animal defenses. Proc Natl Acad Sci U S A. (2000) 97:8856–61. doi: 10.1073/pnas.97.16.8856
23. Liu Y, Ding S, Shen J, Zhu K. Nonribosomal antibacterial peptides that target multidrug-resistant bacteria. Nat Prod Rep. (2019) 36:573–92. doi: 10.1039/C8NP00031J
24. Magana M, Pushpanathan M, Santos AL, Leanse L, Fernandez M, Ioannidis A, et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect Dis. (2020) 20:e216–30. doi: 10.1016/S1473-3099(20)30327-3
25. Liu T, She R, Wang K, Bao H, Zhang Y, Luo D, et al. Effects of rabbit sacculus rotundus antimicrobial peptides on the intestinal mucosal immunity in chickens. Poult Sci. (2008) 87:250–4. doi: 10.3382/ps.2007-00353
26. Wang D, Yu P, She R, Wang K. Protective effects of rabbit sacculus-derived antimicrobial peptides on SPF chicken against infection with very virulent infectious bursal disease virus. Poult Sci. (2024) 103:103797. doi: 10.1016/j.psj.2024.103797
27. Bao H, She R, Liu T, Zhang Y, Peng KS, Luo D, et al. Effects of pig antibacterial peptides on growth performance and intestine mucosal immune of broiler chickens. Poult Sci. (2009) 88:291–7. doi: 10.3382/ps.2008-00330
28. Wang D, Ma W, She R, Sun Q, Liu Y, Hu Y, et al. Effects of swine gut antimicrobial peptides on the intestinal mucosal immunity in specific-pathogen-free chickens. Poult Sci. (2009) 88:967–74. doi: 10.3382/ps.2008-00533
29. Wang CB, Yan X, Wang GH, Liu WQ, Wang Y, Hao DF, et al. NKHs27, a sevenband grouper NK-Lysin peptide that possesses immunoregulatory and antimicrobial activity. Fish Shellfish Immunol. (2023) 136:108715. doi: 10.1016/j.fsi.2023.108715
30. Suprewicz Ł, Szczepański A, Lenart M, Piktel E, Fiedoruk K, Barreto-Duran E, et al. Ceragenins exhibit antiviral activity against SARS-CoV-2 by increasing the expression and release of type I interferons upon activation of the host's immune response. Antiviral Res. (2023) 217:105676. doi: 10.1016/j.antiviral.2023.105676
31. Gallego M, Mora L, Hayes M, Reig M, Toldrá F. Peptides with potential cardioprotective effects derived from dry-cured ham byproducts. J Agric Food Chem. (2019) 67:1115–26. doi: 10.1021/acs.jafc.8b05888
32. Wu D, Cao Y, Su D, Karrar E, Zhang L, Chen C, et al. Preparation and identification of antioxidant peptides from Quasipaa spinosa skin through two-step enzymatic hydrolysis and molecular simulation. Food Chem. (2024) 445:138801. doi: 10.1016/j.foodchem.2024.138801
33. Dong Y, Yan W, Zhang YQ, Dai ZY. A novel angiotensin-converting enzyme (ACE) inhibitory peptide from tilapia skin: preparation, identification and its potential antihypertensive mechanism. Food Chem. (2024) 430:137074. doi: 10.1016/j.foodchem.2023.137074
34. Peng J, Deng H, Du B, Wu P, Duan L, Zhu R, et al. Saffron petal, an edible byproduct of saffron, alleviates dextran sulfate sodium-induced colitis by inhibiting macrophage activation and regulating gut microbiota. J Agric Food Chem. (2023) 71:10616–28. doi: 10.1021/acs.jafc.2c07915
35. Hu F, Wu Q, Song S, She R, Zhao Y, Yang Y, et al. Antimicrobial activity and safety evaluation of peptides isolated from the hemoglobin of chickens. BMC Microbiol. (2016) 16:287. doi: 10.1186/s12866-016-0904-3
36. Richards-Rios P, Fothergill J, Bernardeau M, Wigley P. Development of the ileal microbiota in three broiler breeds. Front Vet Sci. (2020) 7:17. doi: 10.3389/fvets.2020.00017
37. Wang D, Jia X, She R, Liu Y. Acute hypersensitive-like injury in specific-pathogen-free chickens after infection with very virulent infectious bursal disease virus. Poult Sci. (2012) 91:334–9. doi: 10.3382/ps.2010-01203
38. Wang D, Zhou X, She R, Xiong J, Sun Q, Peng K, et al. Impaired intestinal mucosal immunity in specific-pathogen-free chickens after infection with very virulent infectious bursal disease virus. Poult Sci. (2009) 88:1623–8. doi: 10.3382/ps.2009-00124
39. Wang G, Deng H, Wang T, Zheng X. Nutritional supplementation of breeding hens may promote embryonic development through the growth hormone-insulin like growth factor axis. Poult Sci. (2024) 103:103945. doi: 10.1016/j.psj.2024.103945
40. Wang D, Xiong J, She R, Liu L, Zhang Y, Luo D, et al. Mast cell mediated inflammatory response in chickens after infection with very virulent infectious bursal disease virus. Vet Immunol Immunopathol. (2008) 124:19–28. doi: 10.1016/j.vetimm.2008.01.005
41. Chang L, Wu Q, She R, Tong D. The pathologic lesions of liver caused by melamine alone or in combination with cyanuric acid in mice. Res Vet Sci. (2021) 136:230–8. doi: 10.1016/j.rvsc.2021.03.002
42. Yang Y, Shi R, Soomro MH, Hu F, Du F, She R, et al. Hepatitis E Virus induces hepatocyte apoptosis via mitochondrial pathway in mongolian gerbils. Front Microbiol. (2018) 9:460. doi: 10.3389/fmicb.2018.00460
43. Han AY, Zhang MH, Zuo XL, Zheng SS, Zhao CF, Feng JH, et al. Effect of acute heat stress on calcium concentration, proliferation, cell cycle, and interleukin-2 production in splenic lymphocytes from broiler chickens. Poult Sci. (2010) 89:2063–70. doi: 10.3382/ps.2010-00715
44. Nazir S, Charlesworth RPG, Moens P, Gerber PF. Evaluation of autofluorescence quenching techniques on formalin- fixed chicken tissues. J Immunol Methods. (2021) 496:113097. doi: 10.1016/j.jim.2021.113097
45. Alvarenga L, Cardozo L, Lindholm B, Stenvinkel P, Mafra D. Intestinal alkaline phosphatase modulation by food components: predictive, preventive, and personalized strategies for novel treatment options in chronic kidney disease. EPMA J. (2020) 11:565–79. doi: 10.1007/s13167-020-00228-9
46. Fink MP, Delude RL. Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit Care Clin. (2005) 21:177–96. doi: 10.1016/j.ccc.2005.01.005
47. Goldberg RF, Austen WG Jr, Zhang X, Munene G, Mostafa G, Biswas S, et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci U S A. (2008) 105:3551–6. doi: 10.1073/pnas.0712140105
48. John P, Jha V. Heat stress: a hazardous occupational risk for vulnerable workers. Kidney Int Rep. (2023) 8:1283–6. doi: 10.1016/j.ekir.2023.05.024
49. Wang H, Kim H, Park H, Ki JS. Temperature influences the content and biosynthesis gene expression of saxitoxins (STXs) in the toxigenic dinoflagellate Alexandrium pacificum. Sci Total Environ. (2022) 802:149801. doi: 10.1016/j.scitotenv.2021.149801
50. Cartoni Mancinelli A, Baldi G, Soglia F, Mattioli S, Sirri F, Petracci M, et al. Impact of chronic heat stress on behavior, oxidative status and meat quality traits of fast-growing broiler chickens. Front Physiol. (2023) 14:1242094. doi: 10.3389/fphys.2023.1242094
51. Tang LP, Liu YL, Zhang JX, Ding KN, Lu MH, He YM, et al. Heat stress in broilers of liver injury effects of heat stress on oxidative stress and autophagy in liver of broilers. Poult Sci. (2022) 101:102085. doi: 10.1016/j.psj.2022.102085
52. Niu K, Zhong J, Hu X. Impacts of climate change-induced heat stress on pig productivity in China. Sci Total Environ. (2024) 908:168215. doi: 10.1016/j.scitotenv.2023.168215
53. Nanto-Hara F, Yamazaki M, Murakami H, Ohtsu H. Chronic heat stress induces renal fibrosis and mitochondrial dysfunction in laying hens. J Anim Sci Biotechnol. (2023) 14:81. doi: 10.1186/s40104-023-00878-5
54. Yin C, Zhou C, Shi Y, Ge Y, Gao X, Wu C, et al. Effects and potential mechanism of dietary vitamin C supplementation on hepatic lipid metabolism in growing laying hens under chronic heat stress. J Anim Sci. (2023) 101:skad308. doi: 10.1093/jas/skad308
55. Hu Y, Jin H, Du X, Xiao C, Luo D, Wang B, et al. Effects of chronic heat stress on immune responses of the foot-and-mouth disease DNA vaccination. DNA Cell Biol. (2007) 26:619–26. doi: 10.1089/dna.2007.0581
56. Wang H, Yang Y, Huang B, Cui Z, Li L. Protective effects of dietary dimethyl itaconate supplementation on oxidative stress, inflammation, and apoptosis in broilers under chronic heat stress. J Anim Sci. (2023) 101:skad356. doi: 10.1093/jas/skad356
57. Ding KN, Lu MH, Guo YN, Liang SS, Mou RW, He YM, et al. Resveratrol relieves chronic heat stress-induced liver oxidative damage in broilers by activating the Nrf2-Keap1 signaling pathway. Ecotoxicol Environ Saf. (2023) 249:114411. doi: 10.1016/j.ecoenv.2022.114411
58. Estaki M, DeCoffe D, Gibson DL. Interplay between intestinal alkaline phosphatase, diet, gut microbes and immunity. World J Gastroenterol. (2014) 20:15650–6. doi: 10.3748/wjg.v20.i42.15650
59. Lallès JP. Intestinal alkaline phosphatase: novel functions and protective effects. Nutr Rev. (2014) 72:82–94. doi: 10.1111/nure.12082
60. Gustafsson JK, Johansson MEV. The role of goblet cells and mucus in intestinal homeostasis. Nat Rev Gastroenterol Hepatol. (2022) 19:785–803. doi: 10.1038/s41575-022-00675-x
61. Knoop KA, Newberry RD. Goblet cells: multifaceted players in immunity at mucosal surfaces. Mucosal Immunol. (2018) 11:1551–7. doi: 10.1038/s41385-018-0039-y
62. Pabst O. New concepts in the generation and functions of IgA. Nat Rev Immunol. (2012) 12:821–32. doi: 10.1038/nri3322
63. Tang W, Wei Y, Ni Z, Hou K, Luo XM, Wang H, et al. IgA-mediated control of host-microbial interaction during weaning reaction influences gut inflammation. Gut Microbes. (2024) 16:2323220. doi: 10.1080/19490976.2024.2323220
64. Zhang C, Zhao XH, Yang L, Chen XY, Jiang RS, Jin SH, et al. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult Sci. (2017) 96:4325–32. doi: 10.3382/ps/pex266
65. Li X, Bian J, Xing T, Zhao L, Li J, Zhang L, et al. Effects of guanidinoacetic acid supplementation on growth performance, hypothalamus-pituitary-adrenal axis, and immunity of broilers challenged with chronic heat stress. Poult Sci. (2023) 102:103114. doi: 10.1016/j.psj.2023.103114
66. Quinteiro-Filho WM, Ribeiro AV, Ferraz-de-Paula ML, Pinheiro M, Sakai LR, Sá AJ, et al. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult Sci. (2010) 89:1905–14. doi: 10.3382/ps.2010-00812
67. Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. (2002) 109(Suppl):S97–107. doi: 10.1016/S0092-8674(02)00704-3
68. Li Z, Qin F, Liu C, Zhao Z, Wu H, Li J, et al. Alleviating heat stress-induced immune organ damage in ducks: role of melittin. Trop Anim Health Prod. (2025) 57:57. doi: 10.1007/s11250-025-04303-z
69. Amaz SA, Wasti S, Adnan MR, Chaudhary A, Jha R, Mishra B, et al. Dried plum supplementation enhanced the expression of liver antioxidant capacity, metabolism, and epigenetic-related gene markers in broiler chickens under heat stress conditions: dried plum increased liver metabolism in broiler. Poult Sci. (2025) 104:104911. doi: 10.1016/j.psj.2025.104911
70. Shih CC, Liao WC, Ke HY, Kuo CW, Tsao CM, Tsai WC, et al. Antimicrobial peptide cathelicidin LL-37 preserves intestinal barrier and organ function in rats with heat stroke. Biomed Pharmacother. (2023) 161:114565. doi: 10.1016/j.biopha.2023.114565
71. Dibdiakova J, Matic J, Wubshet SG, Uhl W, Manamperuma LD, Rusten B, et al. Membrane separation of chicken byproduct hydrolysate for up-concentration of bioactive peptides. Membranes. (2024) 14:28. doi: 10.3390/membranes14020028
72. Zheng H, Zhao S, Lu Y, Zhang N, Soladoye OP, Zhang Y, et al. Toward the high-efficient utilization of poultry blood: insights into functionality, bioactivity and functional components. Crit Rev Food Sci Nutr. (2024) 64:10069–88. doi: 10.1080/10408398.2023.2220396
73. Saeed M, Yan M, Ni Z, Hussain N, Chen H. Molecular strategies to enhance the keratinase gene expression and its potential implications in poultry feed industry. Poult Sci. (2024) 103:103606. doi: 10.1016/j.psj.2024.103606
74. Nikoo M, Regenstein JM, Yasemi M. Protein hydrolysates from fishery processing by-products: production, characteristics, food applications, and challenges. Foods. (2023) 12:4470. doi: 10.20944/preprints202311.1798.v1
75. Ridyard KE, Overhage J. The potential of human peptide LL-37 as an antimicrobial and anti-biofilm agent. Antibiotics. (2021) 10:650. doi: 10.3390/antibiotics10060650
76. Qin Q, Li Z, Zhang M, Dai Y, Li S, Wu H, et al. Effects of melittin on production performance, antioxidant function, immune function, heat shock protein, intestinal morphology, and cecal microbiota in heat-stressed quails. Poult Sci. (2023) 102:102713. doi: 10.1016/j.psj.2023.102713
77. Bertolotti A. Keeping goblet cells unstressed: new insights into a general principle. EMBO J. (2024) 43:663–5. doi: 10.1038/s44318-024-00041-4
78. Sun W, Xu T, Lin H, Yin Y, Xu S. BPA and low-Se exacerbate apoptosis and autophagy in the chicken bursa of Fabricius by regulating the ROS/AKT/FOXO1 pathway. Sci Total Environ. (2024) 908:168424. doi: 10.1016/j.scitotenv.2023.168424
79. Sun S, Li B, Wu M, Deng Y, Li J, Xiong Y, et al. Effect of dietary supplemental vitamin C and betaine on the growth performance, humoral immunity, immune organ index, and antioxidant status of broilers under heat stress. Trop Anim Health Prod. (2023) 55:96. doi: 10.1007/s11250-023-03500-y
80. Liu L, Gong X, Zhang X, Zhang D, Tang Y, Liu J, et al. Resveratrol alleviates heat-stress-induced impairment of the jejunal mucosa through TLR4/MAPK signaling pathway in black-boned chicken. Poult Sci. (2024) 103:103242. doi: 10.1016/j.psj.2023.103242
81. Forbes SJ, Eschmann M, Mantis NJ. Inhibition of Salmonella enterica serovar typhimurium motility and entry into epithelial cells by a protective antilipopolysaccharide monoclonal immunoglobulin A antibody. Infect Immun. (2008) 76:4137–44. doi: 10.1128/IAI.00416-08
82. Li Y, Fu B, Zhang J, Wang G, Gong W, Tian J, et al. Effects of heat stress on the chemical composition, oxidative stability, muscle metabolism, and meat quality of Nile tilapia (Oreochromis niloticus). Food Chem. (2023) 426:136590. doi: 10.1016/j.foodchem.2023.136590
83. Liu YF, She RP, Li HJ, Jin H, Liang MZ, Liu YR, et al. The apoptosis and dynamic expression of Bax and Bcl-2 in lymphocytes of bursa of SPF chickens infected with SNJ93 strain. Sci Agric Sin. (2005) 38:405–9.
Keywords: antimicrobial peptides, heat stress, intestinal mucosal immunity, chicken, vaccination
Citation: Wang D, Hu F, Liu H, She R and Tian J (2025) Effects of chicken hemoglobin antimicrobial peptides on intestinal mucosal immunity under chronic heat stress and vaccination responses in broilers. Front. Vet. Sci. 12:1574513. doi: 10.3389/fvets.2025.1574513
Received: 11 February 2025; Accepted: 30 April 2025;
Published: 09 June 2025.
Edited by:
Lei Wang, Wuhan Polytechnic University, ChinaReviewed by:
Baseer Ahmad, Muhammad Nawaz Shareef University of Agriculture, PakistanYurong Yang, Henan Agricultural University, China
Lingling Chang, Northwest A&F University, China
Copyright © 2025 Wang, Hu, Liu, She and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruiping She, c2hlcnVpcGluZ0AxMjYuY29t
†Present addresses: Decheng Wang, Hubei Key Laboratory of Tumor Microenvironment and Immunotherapy, College of Basic Medical Sciences, Institute of Infection and Inflammation, College of Basic Medical Sciences, China Three Gorges University, Yichang, China
Fengjiao Hu, Medical Science Research Center, Zhongnan Hospital of Wuhan University, Wuhan, China
‡These authors have contributed equally to this work and share first authorship
§ORCID: Fengjiao Hu orcid.org/0000-0002-2235-6652
Hui Liu orcid.org/0009-0000-4300-6160
 Decheng Wang
Decheng Wang Fengjiao Hu†‡§
Fengjiao Hu†‡§ Ruiping She
Ruiping She Jijing Tian
Jijing Tian