- 1AAU Health and Biomedical Research Center, Al Ain University, Abu Dhabi Campus, Abu Dhabi, United Arab Emirates
- 2Department of Pharmaceutical Sciences, College of Pharmacy, Al Ain University, Al Ain Campus, Al Ain, United Arab Emirates
- 3School of Biochemistry and Biotechnology, University of the Punjab, Lahore, Pakistan
- 4Chief Operations Office, Sheikh Shakhbout Medical City (SSMC), PureHealth, Abu Dhabi, United Arab Emirates
Introduction: One of the major challenges hindering blue agri-economy of Pakistan, is the extensive use of antibiotics and chemotherapeutics in aquaculture. A sustainable alternative is the supplementation of fish feed with non-pathogenic and non-invasive probiotics. In this study, bacteria associated with gastrointestinal tract (GIT) of fish Solea solea were isolated and characterized for probiotic potential.
Methods: Bacterial isolation was conducted from the gut using serial dilution method and Mueller-Hinton Agar (MHA) medium. Isolates were characterized through biochemical analysis and 16S rRNA gene sequencing. Analysis of, intestinal cell adhesion efficiency, tolerance to bile salts, NaCl and pH, survivability in simulated gastric conditions, antibiotic sensitivity profiling, heat shock tolerance, antimicrobial activity of bacteria against Staphylococcus aureus and Pseudomonas aeruginosa, hemolytic activity, cholesterol assimilation potential and resistance against antibiotics. i.e., azithromycin, erythromycin, amoxil, ciprofloxacin and velosef, was performed.
Results: Five isolates were identified as Lacticaseibacillus rhamnosus, Enterococcus faecium, Bacillus amyloliquefaciens, Bacillus subtilis, and Bacillus cereus. All bacteria were fast growing. Optimal growth was observed at pH 5. All isolates demonstrated growth in simulated gastric medium. They exhibited γ-hemolysis, survived heat shock treatment at 100°C, and showed good cholesterol degradation efficiency (ranging between 26.77 and 83.44 mg/dL). Optimum cell adhesion potential was recorded at 90 min. i.e., 119–129 CFUs. All isolates were sensitive to antibiotics with sensitivity order velosef > ciprofloxacin > amoxil > erythromycin and azithromycin.
Conclusion: Due to these probiotic characteristics, current study bacteria might be good candidates for antibiotics replacement in aquaculture.
1 Introduction
According to the Pakistan Economic Survey 2023–2024, aquaculture contributed approximately 2.4% to the country's gross domestic product (GDP). Recognizing its economic potential, provincial governments have allocated significant resources, including 429 million Pakistani rupees (PKR) for projects in Gwadar, 9.7 billion PKR in Punjab, and 5 million PKR in Balochistan (1). The sector also plays a crucial role in foreign exchange earnings, with fish import revenue fluctuating between 296 million USD (2009–2010) and 394 million USD (2016–2017) (2). In response to rising population demands, aquaculture has emerged as an essential source of animal protein, employing approximately 390,000 individuals across Pakistan (3). The country currently has a total fish pond area of 60.47 thousand hectares and 13,000 fish farms (3).
Despite these contributions, Pakistan's aquaculture sector faces considerable challenges, including outdated and unhygienic technologies, poor management practices, and environmental concerns. These deficiencies have prompted intermittent export bans by the European Union (4). Additionally, the sector lags behind regional counterparts, with an annual growth rate of just 2.5% compared to India's 8% (5). Key impediments include marine pollution, inadequate infrastructure (6), weak policy frameworks, overfishing, climate change, untreated sewage, lack of awareness, and unfair trade practices (6, 7). The cumulative impact of these issues has led to an estimated 80% reduction in Pakistan's fish population (4).
Another area of concern is the excessive use of antibiotics and chemicals to control fish diseases (8). Frequent and misuse of antibiotics is linked to antibiotic and multidrug resistance (MDR) in bacteria inhabiting the gastrointestinal tract (GIT) of fish (9). A study reported two antimicrobial-resistant genes (ARGs)—blaCTX − M−55 and QnrVC5—and mutations in the gyrA, gyrB, and parC genes in Vibrio vulnificus, a bacterial pathogen isolated from Lates calcarifer (Asian sea bass). These genes contributed to the evolution of MDR in this bacterium (10). Another bacterium, Edwardsiella tarda isolated from the fishes Oreochromis niloticus (Nile Tilapia) and Clarias gariepinus (African catfish), exhibited MDR against six classes of antimicrobials: sulfonamides, tetracyclins, aminoglycosides, fluoroquinolones, lincosamides, and penicillin (11). Additionally, bacterium B. cereus in Mugil seheli (Bluespot mullet) was shown to carry MDR genes bla1, bla2, tetA, and ermA (12).
This resistance is a threat to aquatic ecosystems and the higher trophic levels of the food chain through the processes of bioaccumulation, biomagnification, and the spread of antibiotic resistance genes (13–15). Recent studies have highlighted the detrimental impact of MDR on aquaculture sustainability, calling for stringent regulations on antibiotic use and the promotion of alternative approaches (16–20). In light of these concerns, sustainable alternatives to antibiotics are urgently needed. Feed supplements, particularly probiotics, prebiotics, and synbiotics, offer a promising, eco-friendly, and cost-effective solution (21–23). These supplements have been reported to contribute to the immune response, resistance to disease, improvement of digestion, growth and survival, protection against pathogens, and better pond water quality in aquaculture (24–26). Two studies documented the positive effects of synbiotics (Lacto Forte), as well as β-1,3-glucan and fructooligosaccharides, on the histological and hematologic profiles, growth, immunity, and antimicrobial resistance in O. niloticus and Litopenaeus vannamei (Pacific white shrimp), respectively (27, 28).
With reference to aquaculture, probiotics can be defined as the live whole microbes or components of microbes that, when administered either as a feed supplement or rearing water, provide health benefits by improving gut microbiota composition and function (29–31). Their mechanisms of action include competitive exclusion of pathogens, production of inhibitory compounds, enhancement of digestion, stimulation of host immunity, and modulation of quorum sensing (QS) (31). In aquaculture, maintaining a stable microbial ecosystem is essential due to the continuous interaction of farmed fish with their aquatic environment, which influences their health and growth (21, 32).
Several probiotic species have been identified from the gut microbiota of fish, including Pediococcus acidilactici, P. pentosaceus, Levilactobacillus brevis, Lactobacillus acidophilus, Lactiplantibacillus pentosus, and L. plantarum (33); Enterococcus sp., Weissella cibaria, Lactococcus lactis, and Limosilactobacillus fermentum (34); Lactococcus garvieae (35); Shewanella sp., Proteus sp., and Alcaligenes sp. (36); L. lactis CLFP 101, L. plantarum CLFP 238, and L. fermentum CLFP 242 (37).
Common sole or Solea solea inhabits muddy and sandy seabeds, coastal areas, and shallow waters and exhibits seasonal migration to deeper waters in winter. It is a nocturnal predator that feeds on small crustaceans and benthic invertebrates (38). In Pakistan, it is a commercially important fish and, along with other marine fishes, constitutes 0.51 million tons of total fisheries production (https://agro.tdap.gov.pk/pakistan-seafood). Being a part of the broader fisheries sector, it contributes to the economy of the country through employment opportunities, export earnings, value-added products, and income generation (39). In Pakistan, due to manufacturing costs, labor, economic pressures, fuel prices, and need and supply dynamics, the price of this fish increased significantly by 2024—for example, in Karachi (1,800 PKR) and in Lahore and Islamabad (789 to 1,579 PKR per pound). Being part of coastal cultural festivities and customs, as well as a selected staple food, it is considered a cultural and culinary treasure of Pakistan. However, the species faces economic difficulties (40). For its preservation, there is a crucial need for sustainable practices like feed improvement with probiotic supplementation.
Recent studies suggest that probiotics application in aquaculture not only enhances fish health as being alternative to antibiotics and vaccines, inhibiting the pathogens' growth, accelerating the resistance to diseases, and improving the digestibility of fats, but also improves water quality by reducing harmful bacterial loads and nitrogenous wastes (24, 41). Additionally, within the context of Pakistan's aquaculture sector, there are several limitations of probiotics, such as the dependency of probiotics' efficacy on regional environmental factors and water quality, poor optimization of already commercialized probiotics, and inaccessibility of smaller fish farms to costly probiotics available in the Pakistani market. Given the cultural importance and economic difficulties associated with S. solea, the vital role of probiotics in aquaculture sustainability, and barriers in the successful implementation of probiotics in Pakistan, this study was initiated to identify the native probiotic strains from healthy S. solea fish through comprehensive in vitro screening strategies.
In addition to biochemical and genomic characterization, the safety and antagonistic properties of these isolates against fish pathogens were evaluated. Following in vivo validation, these probiotics could serve as viable alternatives to antibiotics, promoting sustainable and resilient aquaculture practices.
2 Materials and methods
2.1 Fish guts collection and preparation
In vitro characterization was performed at experimental facilities of the School of Biochemistry and Biotechnology (SBB), Punjab University (PU), Lahore. Ten healthy S. solea fish, commonly known as sole fish, were bought from a commercial supplier located at Qadimi Shehar, Lahore. Healthy and fresh fish with no apparent pathological symptoms were purchased. Length and weight of fish ranged between 25 and 30 cm and between 170 and 230 g, respectively. These values are consistent with the weight and length reported for healthy S. solea fish by IFCA North West and the Food and Agriculture Organization (https://www.nw-ifca.gov.uk/managing-sustainable-fisheries/species/fish/sole) and the Food and Agriculture Organization of the United Nations (https://www.fao.org/fishery/en/culturedspecies/solea_spp/en). Fish specimens that exhibited signs of illness or were in bad condition were not considered for purchase.
For fish handling, transport, and dissection, the Guidelines of CPCSEA for Experimentation on Fishes were followed (42, 43). To minimize the tissue damage, dead fish were handled gently. To prevent contamination with pathogenic bacteria, personal protective equipment (PPE) was used. Specimens were cushioned with sterilized foam and quickly transported in an insulated cooler. Fish were stored in the refrigerator for a few hours. Fish were dissected by a veterinarian under sterile conditions to remove the gut. Fish carcasses were disposed of according to waste disposal protocols (https://www.msdvetmanual.com). Fish guts were thoroughly washed with sterilized distilled water and phosphate buffer saline (PBS) buffer (100 mL) to remove the mucus, grimes, and feed materials (44). The posterior part of the intestine was cut from the cleaned gut and homogenized using sterile glass pestles in autoclaved 100 mL Ringer solution (45). To remove fungal contaminants, the previously reported method of boiling homogenate at 80°C for 15–20 min was employed (46). Following this, the homogenate was incubated at 25°C up to 6 h to facilitate bacterial isolation.
2.2 Bacterial isolation and stock preparation
The isolation of bacteria associated with the fish gut was performed using Mueller-Hinton Agar (MHA) medium (47). The gut-homogenized Ringer solution was serially diluted up to 6-fold, and each dilution was spread onto MHA plates. These plates were incubated at 37°C for 24 h to allow bacterial growth (48). The following day, bacterial colonies were analyzed, and the colony-forming units (CFU) per mL were calculated using the following formula:
The streak plate method was used to isolate purified colonies, which were then stored in 50% glycerol stock solution at −20°C for long-term preservation (49). Bacterial morphological analysis was performed by examining the shape, color, size, and texture of the colonies.
2.3 Biochemical characterization
To characterize the bacterial isolates biochemically, several tests were conducted, including the catalase test, mannitol fermentation test, glucose, lactose, and fructose fermentation tests, as well as assays for hydrogen cyanide (HCN) production, cellulose degradation, chitinase, and pectinase production. For the catalase test, a freshly grown overnight bacterial culture was used to prepare the smear. A few drops of hydrogen peroxide were then added to the fixed smear, and the results were compared with a negative control.
For the mannitol fermentation test, autoclaved medium was used as a substrate to assess the ability of the isolates to ferment mannitol sugar. An overnight culture was streaked onto MSA plates and incubated at 37°C for 24 h. After incubation, color changes in the medium were observed and compared with a negative control (50).
For the glucose fermentation test, 5 mL of syringe-filtered glucose medium was added to a sterile test tube and inoculated with 20 μL of freshly grown overnight culture. The culture was incubated overnight at 37°C in a shaking incubator (150 rpm), and the color change in the medium was recorded and compared with a negative control. For the sucrose fermentation test, 5 mL of syringe-filtered sucrose medium was used as a substrate in a sterile test tube. The medium was inoculated with 20 μL of freshly grown overnight culture and incubated overnight at 37°C in a shaking incubator (150 rpm). A color change in the medium indicated the bacterial ability to ferment sucrose, with results compared with a negative control (51).
For the lactose fermentation test, 5 mL of syringe-filtered lactose medium was inoculated with 20 μL of freshly grown overnight culture, incubated at 37°C in a shaking incubator (150 rpm), and the color change in the medium was recorded and compared with a negative control (51).
For the HCN production test, 25 mL of sterile glycine agar medium was poured into Petri plates. Once solidified, a freshly grown overnight culture was spread onto the agar surface. Autoclaved filter paper dipped in picric acid solution (used as an indicator) was placed on a sterile Petri plate lid, and the plate was sealed with parafilm. The plates were incubated at 37°C for 48 h. A color change in the filter paper indicated HCN production, while no change indicated the absence of HCN. The results were compared with a negative control (52).
For the cellulase production test, autoclaved carboxy-methyl cellulose (CMC) medium was used. Autoclaved solidified media plates were prepared, and agar wells were formed. A synchronized overnight culture was inoculated into these wells and incubated at 37°C for 24 h. The zone of inhibition was assessed using iodine solution as an indicator, and the results were compared with a negative control (53).
For the chitinase production test, autoclaved chitin medium was used. Solidified agar plates were prepared, and agar wells were formed. A synchronized overnight culture was introduced into these wells and incubated at 37°C for 24 h. The zone of inhibition was determined using iodine solution, and the results were compared with a negative control. For the pectinase production test, autoclaved pectin media was used. Similar to the previous assays, solidified agar plates were prepared, and agar wells were formed. A synchronized overnight bacterial culture was poured into these wells and incubated at 37°C for 24 h. The zone of inhibition was checked using iodine solution, and the results were compared with a negative control (54).
2.4 Molecular characterization
The organic method was used for DNA extraction by following a previously documented protocol. This method involved the use of phenol:chloroform:isoamyl alcohol (55). DNA integrity was confirmed by agarose gel electrophoresis at 90 V for 45 min. Afterward, gel visualization was performed in a gel documentation system under UV. DNA was stored for future use at −20°C (56).
Polymerase chain reaction (PCR) was performed to amplify the 16S rRNA gene in isolated DNA. Previously documented 16S rRNA gene-specific primers were used (57). Sequences, melting temperatures (Tm), and GC content (the proportion of guanine and cytosine bases) of forward and reverse primers were AGAGTTTGATCCTGGCTCAG, 55°C, and 50% and AAGGAGGTGATCCAGCCGCA, 60°C, and 50%, respectively. The PCR amplicon size was 1500 bp. The PCR reaction mixture (25 μL) was prepared using 0.5 μL Template DNA, 1 μL forward primer, 1 μL reverse primer, 12.5 μL PCR master mix (DNA polymerase, dNTPs, MgCl2, and PCR buffer), 9.5 μL PCR water, and 0.5 μL Taq polymerase. All these components were added to the PCR tube by placing the tube in an ice bucket. PCR reaction was carried out under optimized conditions: initial denaturation (95°C for 5 min), cyclic denaturation (95°C for 45 seconds), annealing (58°C for 40 seconds), and cyclic extension (92°C for 1 min and final extension (72°C for 10 min). The number of cycles was 35X (58).
DNA purification from post-PCR agarose gel bands was performed using the FavorPrepTM gel purification mini kit (Cat # FAGCK 001). The purified DNA samples were then shipped to Macrogen, Korea, for Sanger sequencing analysis.
Sequencing results were obtained in the FASTA format. On the NCBI platform, the basic local alignment search tool (BLAST) was used to determine the similarity index between the obtained bacterial sequences and existing bacterial species in the database. Multiple sequence alignment was performed using the Clustal Omega Multiple Sequence Alignment Tool (https://www.genome.jp/tools-bin/clustalw) (59). Gaps were removed from aligned sequences. Ungapped sequences were used to construct the phylogenetic tree. To construct a phylogenetic representation of evolutionary relationships, the neighbor-joining statistical method was used. The lengths of the branches in the tree directly represented comparisons between different sequences at the genetic level.
Bacterial DNA sequences were submitted to the publicly accessible NCBI GenBank database to obtain the assigned accession numbers (60). The accession numbers assigned to five bacteria, L. rhamnosus SBBPro6, E. faecium SBBPro7, B. amyloliquefaciens SBBPro8, B. subtilis SBBPro9, and B. cereus SBBPro10, were PQ002180, PQ002492, PQ002184, PQ002187, and PQ002188, respectively.
2.5 Growth curve analysis
Bacterial growth analysis was performed in triplicate. For this analysis, synchronized cultures with OD600 = 0.1 were prepared and used to inoculate the medium. The control consisted of uninoculated medium. Initially, the OD600 of the control and experimental tubes was measured at 0 h. Following this, tubes were incubated (37°C and 150 rpm), and OD600 was measured at different time intervals: 3, 6, 24, 27, 30, 48, 51, and 54 h. Time was plotted against OD600 to generate the growth curve (61).
2.6 Probiotic assays
2.6.1 Bile salt tolerance assay
Autoclaved De Man-Rogosa-Sharpe (MRS) broth supplemented with 0.3 g bile salt (Himedia, Cat # RM008-500G) was inoculated with the isolates from this study. Control contained MRS and inoculum without bile salts. Following this, bacteria were cultured at 37°C and 150 rpm until the exponential phase was achieved. Then, OD600 of the culture was measured at 0 and 24 h. A bar graph was plotted to compare the growth of bacteria in the presence of bile salts, which reflected the bacterial resistance against bile salts (62).
2.6.2 NaCl tolerance assay
Bacteria were grown for 24 h to prepare the synchronized cultures. Culture of each bacterium with OD600 = 1 was used to inoculate three MRS media containing different NaCl (WEL GENE Precision SolutionTM, Cat # ML 011-01) concentrations: 0.2%, 2%, and 5%. Afterward, OD600 was measured at 0 h. All test tubes were then kept in a shaking incubator (37°C and 150 rpm) for efficient growth. After 24 h, OD600 was measured again to check the NaCl tolerance potential of isolates (63).
2.6.3 pH tolerance assay
MRS media adjusted at pH values 2, 3, and 5 using 1N HCl and 1N NaOH were prepared. Overnight-grown cultures with OD600 = 1 were inoculated into the medium to synchronize the cultures. A negative control, MRS medium without any inoculum, was used. The OD600 of control and experimental cultures was measured at 0 h. All test tubes were then kept in a shaking incubator (37°C and 150 rpm) for efficient growth. After 24 h, OD600 was assessed again to check the growth of isolates at different pH values (64). Bar graphs were plotted to analyze and compare the growth potential of bacteria at different pH levels.
2.6.4 Survival potential of isolates in simulated gastric conditions
To investigate the survival potential of the isolates from this study under gastric conditions, simulated gastric medium was synthesized. Previously reported composition of simulated gastric fluid was used with slight modifications: KCl (0.0256 g), KH2PO4 (0.0061 g), NaHCO3 (0.105 g), NaCl (0.1375 g), MgCl2.(H2O)6 (0.0012 g), (NH4)2CO3 (0.0024 g), HCl (0.0284 g), and CaCl2 (H2O)2 (0.0011g) per 50 mL of distilled water, and filtered through a microporous membrane (0.2 μm pore size) (65). The 15 mL of simulated gastric fluid was supplemented with pepsin solution (3.2 mL), 0.3M CaCl2 (0.01 mL), and distilled water (1.8 mL). The pH was adjusted to 2. Overnight-grown fresh bacterial cultures were centrifuged at 6,000 × g, 4°C for 20 min to pellet down the cells. Following this, cells were suspended in 0.9% saline solution (1 mL). Saline solution was used as an alternative to broth. The broth (1 mL) was inoculated into gastric medium (9 mL) and incubated for 3 h under shaking conditions (150 rpm). After 3 h, each isolate sample was taken from the respective tube and streaked on MHA agar plates, followed by incubation at 37°C for 24 h. Growth of bacteria was observed (66).
2.6.5 Cell adhesion assay
To remove the surface mucous, the ileum was washed in PBS for 30 min at 4°C. Following this, small pieces of ~1 cm of the ileum were cut and placed in an overnight-grown bacterial cell suspension for different time intervals: 30, 60, and 90 min. These pieces were macerated and seeded on freshly prepared autoclaved MHA agar plates. Afterward, plates were incubated at 37°C for 24 h. Growth was observed through enumerating CFUs of each isolate at different time intervals (67).
2.6.6 Hemolytic assay
Blood agar was prepared using peptone (0.5%), yeast or beef extract (1.5%), agar (1.5%), NaCl (0.5%), distilled water, and sheep blood (5%). The medium was autoclaved before the addition of blood. Blood agar was poured into the Petri plates and solidified. Each isolate was streaked on a blood agar media plate and incubated at 37°C for 24 h. Following this, the hemolytic activity/type (α, β, and ⋎) of each isolate was observed (68).
2.6.7 Antimicrobial test
The antibacterial potential of the isolates from this study was assessed using Pseudomonas aeruginosa and Staphylococcus aureus. Overnight culture (200 μL) of each strain was added to a freshly prepared MRS media plate using the spread plate method (69). Freshly grown pathogenic culture was added to wells made in solidified MRS agar. All plates were then kept in an incubator at 37°C for 24 h (70). After the incubation, zones of inhibition and colony formation around the wells were observed and recorded to assess the antibacterial potential of each fish gut-borne bacterium against the pathogens used.
2.6.8 Heat shock tolerance assay
Bacterial cells were pelleted down by centrifugation at 4,000 rpm at 4°C for 5–7 min, washed with 1 mL of PBS solution, and subjected to heat shock at 100°C for 2–3 min. Pellet was suspended in growth medium (5 mL) and incubated at 37°C and 150 rpm overnight. OD600 of each strain was measured to assess the heat shock tolerance potential of bacteria (71).
2.6.9 Cholesterol assimilation test
Isolates properties of cholesterol breakdown were checked by cholesterol liquicolor kit (PATHOZYMES DIAGNOSTICS, India) using the CHOD-PAP method (72). Cholesterol standard (10 μL) was added to a cuvette containing 1 mL of culture. Mixed thoroughly and incubated for 5 min at 20–25°C. A cuvette containing a cholesterol standard without culture was used as a standard. Following incubation, the absorbance at 505 nm (OD505) was measured. The cholesterol concentration of standard and test samples was estimated using the following formula:
2.6.10 Antibiotic sensitivity test
Different antibiotics—erythromycin (250 mg), azithromycin (250 mg), ciprofloxacin (500 mg), amoxil (500 mg), and ampicillin (250 mg)—were used to perform the antibiotic sensitivity profiling of the isolates. The disk diffusion method was used to perform this test (73). Disks were prepared manually using dried Whatman filter paper no. 3. Antibiotic stock solutions were prepared, added to disks in a predefined amount, and allowed to absorb for 10 min. Disks were dried in an oven for 15 min at 37°C. MRS agar plates were prepared and inoculated with an overnight-grown fresh culture of bacteria via spreading. Then, antibiotic disks were evenly placed on the plates using sterilized forceps. Different dilutions of antibiotics (10, 20, and 30 μL) were used to assess the extent of sensitivity. The effect of antibiotics on the isolates was determined by measuring the diameter of the zones of inhibition (mm). The zone size indicated the bacterial susceptibility against antibiotics.
2.7 Statistical analysis
Each experiment was performed in triplicate, and the results were represented as mean ± SD. To evaluate the probiotic potential of the five isolates, statistical analyses were conducted using SPSS version 20 (74). One-way ANOVA was performed to determine the significance status of the p-value (75). Dunnett's test was performed for NaCl and bile salt tolerance tests and temperature and pH resistance tests to determine whether the growth of each probiotic strain significantly differed from the control group. Dunnett's test was specifically chosen as a post hoc analysis to compare each probiotic strain directly with the control, rather than comparing the probiotics with each other (76). For the antibiotic tolerance test and cell adhesion test, a one-sample t-test was applied (77). As the control group had a value of zero, it was not suitable to apply the one-way ANOVA and Dunnett's test. Based on the statistical analyses, significant differences in probiotic performance were accurately identified.
3 Results
3.1 Bacterial isolation
Bacterial colonies were observed on an MHA agar plate after 24 h of incubation. CFUs of isolates were calculated as follows: Lacticaseibacillus rhamnosus SBBPro6 and Enterococcus faecium SBBPro7 (19 × 10−4 per mL), Bacillus amyloliquefaciens SBBPro8 and Bacillus subtilis SBBPro9 (36 × 10−5 per mL), and Bacillus cereus SBBPro10 (11 × 10−6 per mL) (Supplementary Figure S1).
3.2 Morphological analysis
Isolates were examined through colony features such as texture, shape, color, margins, and elevation. All colonies showed off-white color with creamy texture. Gram-positive character, spindle shape, full margins, and raised elevation were exhibited by L. rhamnosus SBBPro6 and B. amyloliquefaciens SBBPro8. Irregular shape, lobate margins, and raised elevation were observed in E. faecium SBBPro7. B. subtilis SBBPro9 and B. cereus SBBPro10 also showed Gram-positive features with rod shape, entire margins, and convex elevation (Supplementary Figure S2).
3.3 Biochemical characterization
In catalase production activity, instant effervescence was recorded in all isolates, which reflected their antioxidant property (Supplementary Figure S3). In the mannitol fermentation test, L. rhamnosus SBBPro6, E. faecium SBBPro7, and B. amyloliquefaciens SBBPro8 showed maximum potential to ferment mannitol, as the media color changed from pink to yellow, while B. subtilis SBBPro9 showed no growth and B. cereus SBBPro10 exhibited mild growth (Supplementary Figure S4). Each isolate exhibited the potential to ferment glucose, lactose, and fructose (Supplementary Figure S5).
All isolates were positive for HCN, cellulase, and chitinase production tests (Supplementary Figures S6–S8). All bacteria were negative for the pectinase production test (Supplementary Figure S9, Supplementary Table S1).
3.4 Molecular characterization and phylogenetic tree construction
Extracted DNA and PCR amplicons were visualized on a gel to check their quality (Supplementary Figures S10, S11). FASTA sequences were submitted to GenBank and assigned the following accession numbers: L. rhamnosus SBBPro6 (PQ002180), E. faecium SBBPro7 (PQ002492), B. amyloliquefaciens SBBPro8 (PQ002184), B. subtilis SBBPro9 (PQ002187), and B. cereus SBBPro10 (PQ002188).
The phylogram displayed Lacticaseibacillus rhamnosus strain SBBPro6, L. rhamnosus, and L. casei strains as a cohesive group with minimal phylogenetic distinctions among them. The phylogenetic analysis confirmed that L. rhamnosus SBBPro6 fits perfectly into the Lacticaseibacillus genus, making it a prototypical representative. The position of Enterococcus faecium strain SBBPro7 showed complete affiliation with a group consisting of E. faecium strains exclusively. The close position to strains LMG_11423 and JR38 demonstrated evolutionary continuity. Genetic diversity in this clade is moderate. Strain Bacillus amyloliquefaciens SBBPro8 formed a tight cluster with additional strains of its species, such as KC1 and AUC2, because of their short branch lengths, which suggested similarities in their genetic sequences. This tight clustering demonstrated evolutionary conservation. Bacillus subtilis strain SBBPro9 belonged to the B. subtilis complex, where it stood nearest to strains BS01 and WES3. The genetic makeup of SBBPro9 showed only moderate variation from its genus specifications, yet it still stayed cohesive with its genus groups. The two Bacillus cereus strains SBBPro10 and SBBPT2 existed as members of the extensive B. cereus phylogenetic group. Genetic variations within B. cereus appeared to affect the branch length of SBBPro10 compared to SBBPT2, as the second strain exhibited more similarity to other B. cereus strains. Their near position demonstrated shared evolutionary beginnings, although they could exhibit distinct characteristics at the strain level for functional or ecological behavior (Figure 1).
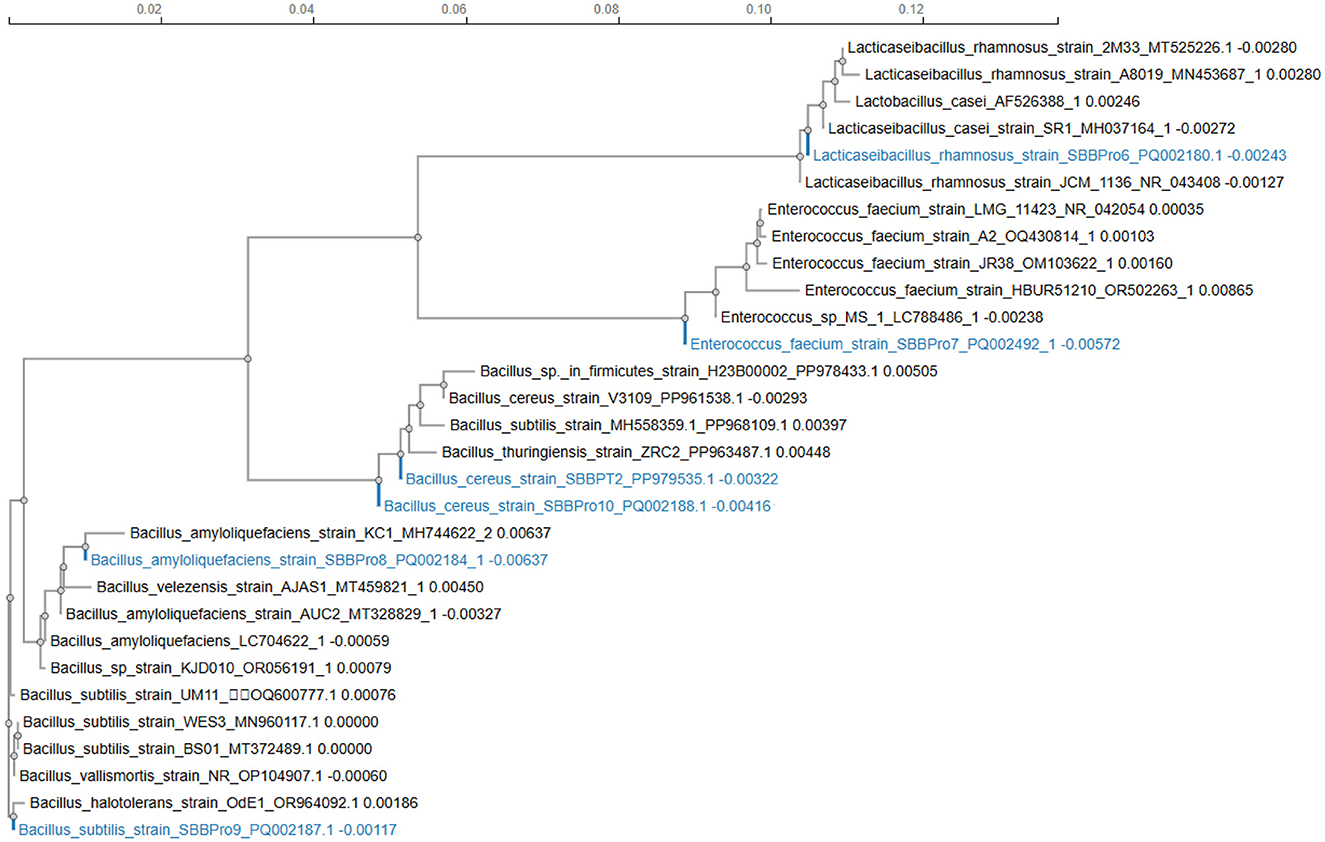
Figure 1. Phylogenetic tree construction for bacteria isolated in the current study using the neighbor-joining statistical method through the ClustalW package.
3.5 Growth curve study
Each isolate exhibited an efficient growth rate. L. rhamnosus SBBPro6 and E. faecium SBBPro7 were the fastest growing, with the log phase starting at 27 h, as both of these showed optimum OD600 at this time interval, i.e., 1.127 ± 0.024 and 1.490 ± 0.003, respectively. In B. amyloliquefaciens SBBPro8, B. subtilis SBBPro9, and B. cereus SBBPro10, the maximum growth was recorded at 30 h with OD600 = 1.172 ± 0.008, 1.356 ± 0.008, and 1.146 ± 0.01, respectively (Supplementary Table S2, Figure 2).
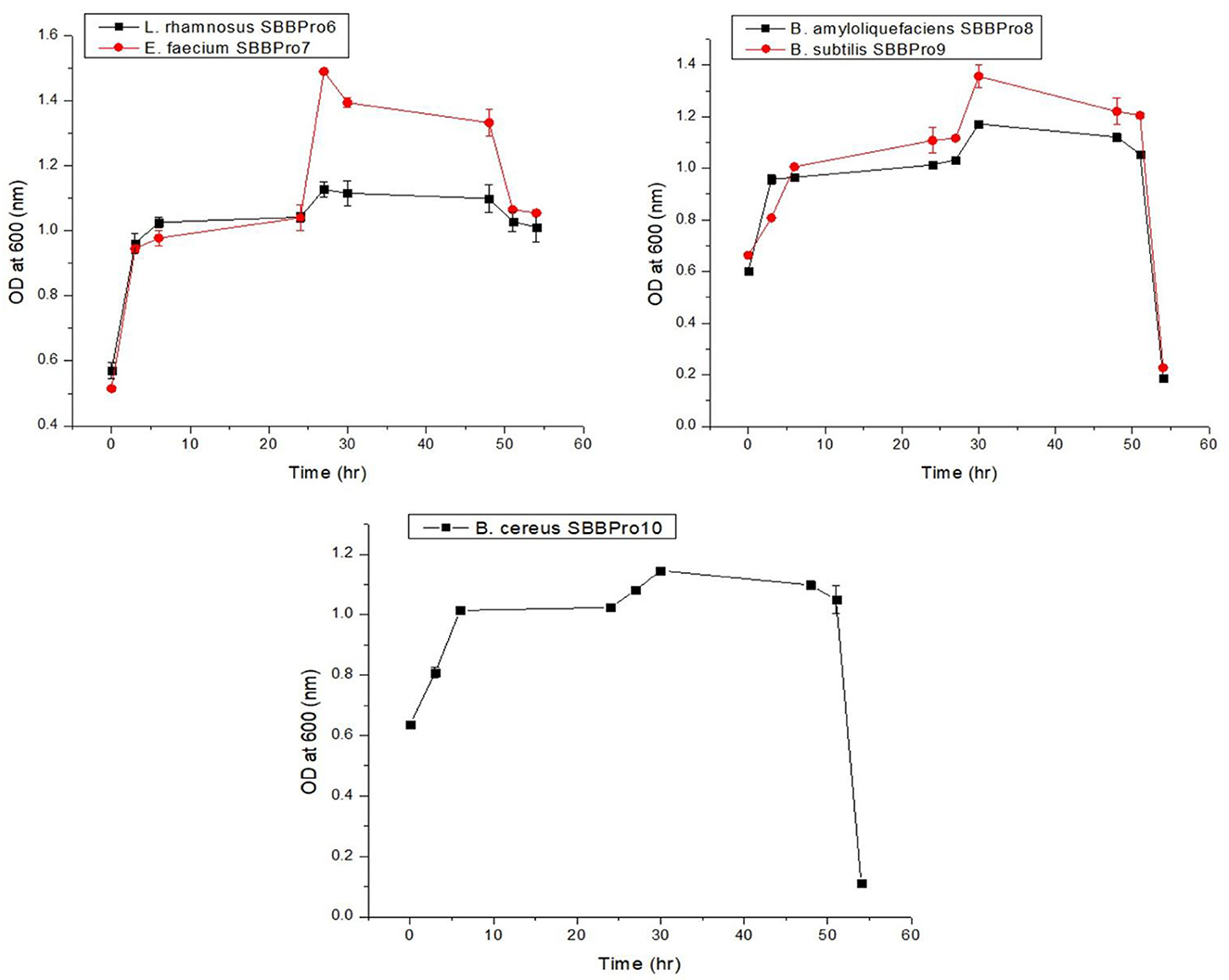
Figure 2. Growth curves of bacteria isolated in the current project obtained by plotting time vs. OD600.
3.6 Bacterial characterization through probiotic assays
3.6.1 Bile salt tolerance assay
The isolates from this study showed maximum tolerance for bile salts. Among all the isolates, B. cereus SBBPro10 showed maximum tolerance against bile salt with the highest OD600, i.e., 2.10 ± 0.07, while E. faecium SBBPro7 exhibited minimum growth with the lowest OD600, i.e., 1.91 ± 0.02. The results were found significant with a p-value < 0.05 (Supplementary Tables S3, S4, Figure 3).
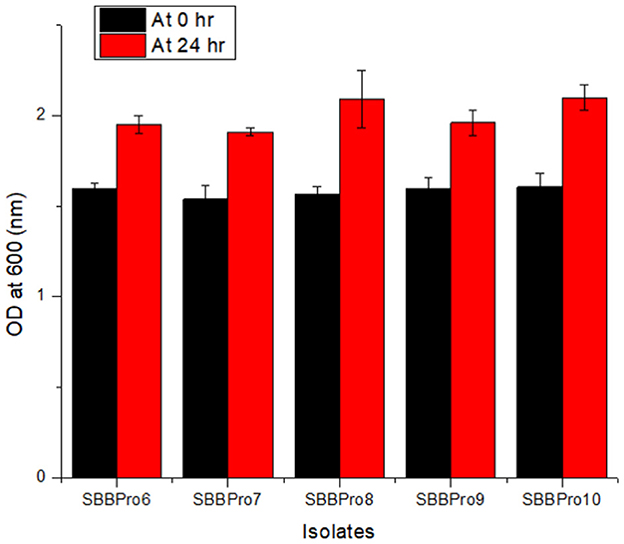
Figure 3. Bile salt tolerance assay of bacteria isolated in the current study performed at 0.3% bile salt concentration: L. rhamnosus SBBPro6, E. faecium SBBPro7, B. amyloliquefaciens SBBPro8, B. subtilis SBBPro9, and B. cereus SBBPro10.
3.6.2 NaCl tolerance assay
The growth of bacteria was found to drop slightly with an increase in NaCl concentration. In all the isolates from this study, OD600 was highest at 0.2%, followed by 2%. At 0.2%, the OD600 was highest (1.86 ± 0.04) in L. rhamnosus strain SBBPro6 and lowest (1.61 ± 0.11) in B. cereus SBBPro10. At 2% concentration, maximum and minimum OD600 values were observed in L. rhamnosus SBBPro6 (1.62 ± 0.15) and B. subtilis SBBPro9 (1.45 ± 0.03), respectively. At 5% concentration, maximum OD600 was observed in L. rhamnosus SBBPro6, i.e., 1.44 ± 0.01, and minimum was observed in E. faecium SBBPro7, i.e., 1.15 ± 0.04. The results were significant with a p-value < 0.05 (Supplementary Tables S5, S6, Figure 4).
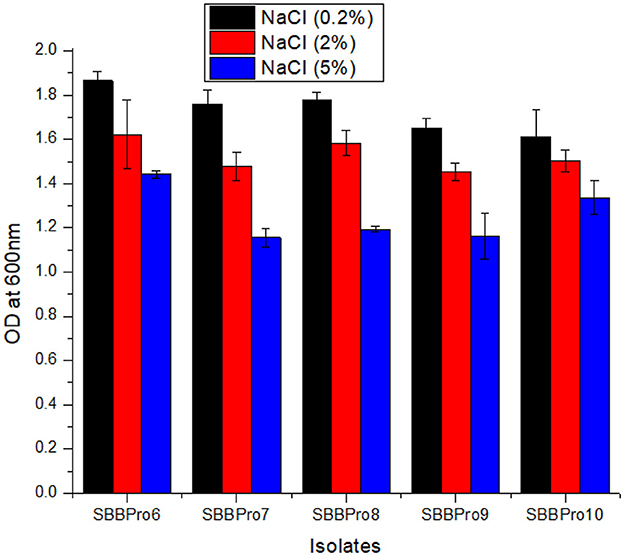
Figure 4. Growth of isolated bacteria at 0.2%, 2%, and 5% concentrations of NaCl analyzed through measurement of OD600 at logarithmic phase: L. rhamnosus SBBPro6, E. faecium SBBPro7, B. amyloliquefaciens SBBPro8, B. subtilis SBBPro9, and B. cereus SBBPro10.
3.6.3 pH tolerance assay
The isolates from this study exhibited relatively efficient growth at pH 5, followed by pH 3. Highest OD600 values were observed in B. amyloliquefaciens SBBPro8 (0.48 ± 0.009) and B. cereus SBBPro10 (0.84 ± 0.019) at pH = 2 and 3, respectively. At pH = 5, OD600 was observed in the range of 0.85 ± 0.044 and 1.07 ± 0.082. The highest was recorded in B. amyloliquefaciens SBBPro8 and the lowest in E. faecium SBBPro7, respectively. The results were significant with p-values recorded as follows: pH 2 (0.002973), pH 3 (0.000003), and pH 5 (0.001647). At pH 2 and 3, Dennett's test indicated that all probiotic strains showed significantly higher tolerance than the control, with B. cereus SBBPro10 demonstrating the highest mean difference at pH = 2 (Supplementary Tables S7, S8a-f, Figure 5).
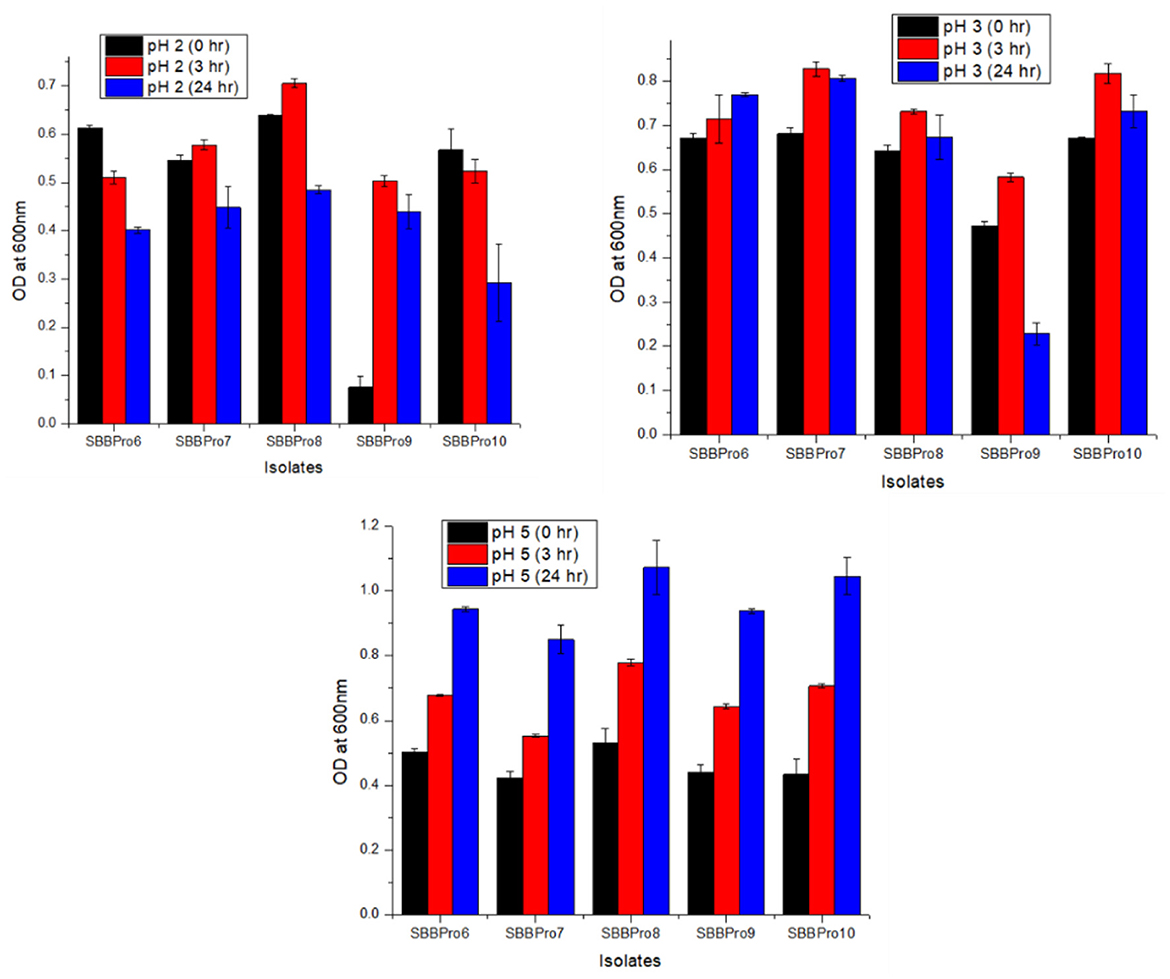
Figure 5. Assessment of pH tolerance potential of bacteria isolated in the current study, at pH values 2, 3, and 5: L. rhamnosus SBBPro6, E. faecium SBBPro7, B. amyloliquefaciens SBBPro8, B. subtilis SBBPro9, and B. cereus SBBPro10.
3.6.4 Simulated gastric conditions survivability assay
All isolates exhibited growth on synthetic gastric fluid media, which showed their potential to tolerate gastrointestinal (GI) conditions of the host body. This confirmed their safety profile as probiotics (Supplementary Figure S12).
3.6.5 Cell adhesion test
Each isolate exhibited the capability of adhesion to epithelial cells of the ileum, and CFUs increased with the incubation time from 30 to 90 min. After 30 min of incubation, L. rhamnosus SBBPro6 and B. subtilis SBBPro9 showed the maximum CFUs, with a count of 41, while E. faecium SBBPro7, B. amyloliquefaciens SBBPro8, and B. cereus SBBPro10 showed CFUs of 40, 39, and 38, respectively. At 60 min of incubation, B. amyloliquefaciens SBBPro8 showed the maximum CFUs of 81, while L. rhamnosus SBBPro6, E. faecium SBBPro7, B. subtilis SBBPro9, and B. cereus SBBPro10 showed CFUs of 59, 57, 52, and 47, respectively. At 90 min of incubation, B. subtilis SBBPro9 showed CFUs of 129, while L. rhamnosus SBBPro6, B. amyloliquefaciens SBBPro8, B. cereus SBBPro10, and E. faecium SBBPro7 showed CFUs of 128, 125, 120, and 119, respectively (Supplementary Figure S13, Table 1, Figure 6). All the isolates demonstrated high cell adhesion efficiency, with a significant p-value of < 0.05 (Supplementary Tables S9a–c).
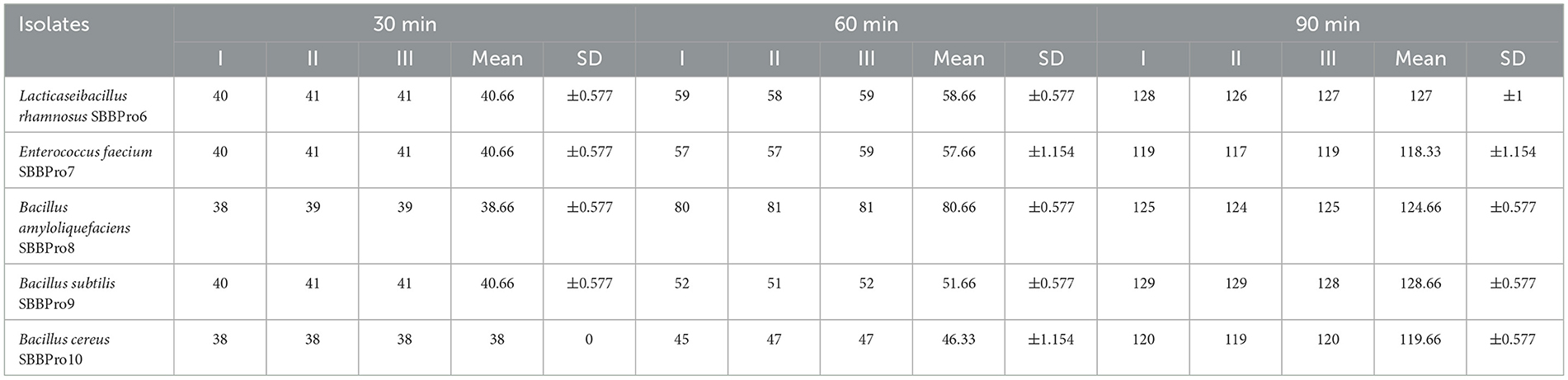
Table 1. Assessment of adhesion potential of fish gut-borne bacteria with intestinal cells at three different time intervals.
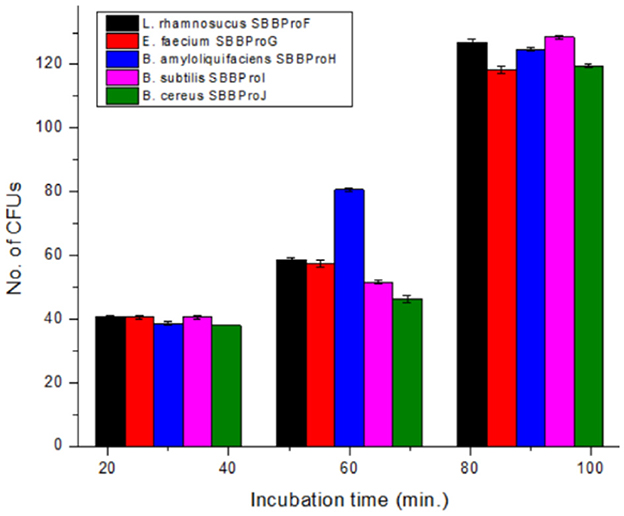
Figure 6. Assessment of cell adhesion potential of bacteria isolated in the current study by cell adhesion assay performed at 30, 60, and 90 min.
3.6.6 Hemolytic assay
All bacterial isolates showed gamma (γ) hemolysis, i.e., no zone was observed around colonies, which confirmed the safety profile of probiotics (Supplementary Figure S14).
3.6.7 Antimicrobial resistance assay
Among the isolates from this study, L. rhamnosus SBBPro6, E. faecium SBBPro7, B. amyloliquefaciens SBBPro8, and B. subtilis SBBPro9 showed resistance against S. aureus. In case of P. aeruginosa, L. rhamnosus SBBPro6, and B. subtilis SBBPro9 were slightly sensitive (Supplementary Figure S15).
3.6.8 Heat shock tolerance assay
All isolates survived the heat shock treatment. L. rhamnosus SBBPro6 showed maximum growth with OD600 = 1.40 ± 0.02, and B. amyloliquefaciens SBBPro8 exhibited minimum growth with an OD600 value of 0.95 ± 0.01. The results were significant with a p-value < 0.05 (Supplementary Tables S10, S11, Figure 7).
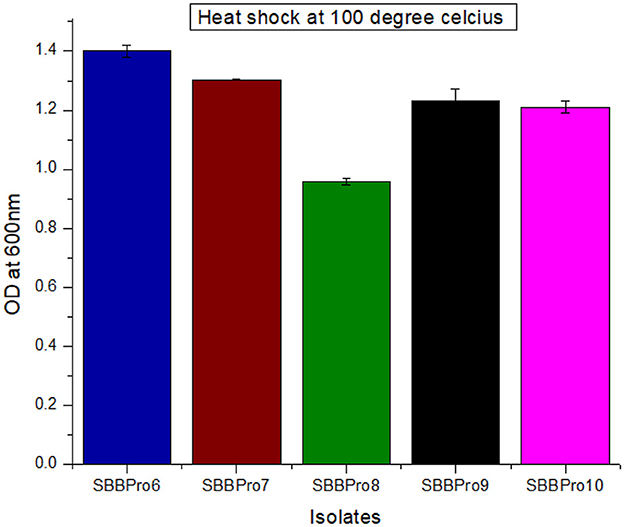
Figure 7. Assessment of heat shock tolerance of bacteria isolated in the current study through measurement of OD600 at log phase after heat shock treatment at 100°C.
3.6.9 Assessment of cholesterol assimilation potential
All isolates were capable of cholesterol degradation. Compared with the standard, maximum cholesterol degradation was observed in E. faecium SBBPro7, i.e., 83.44 mg/dL. Minimum cholesterol degradation was exhibited by L. rhamnosus SBBPro6, i.e., 26.768 mg/dL (Table 2, Figure 8).
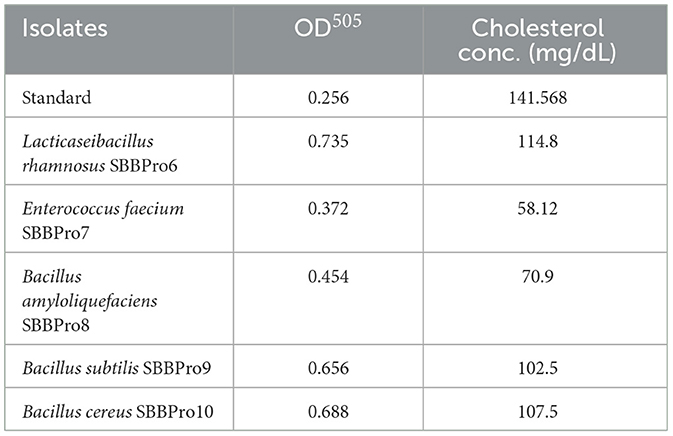
Table 2. Estimation of cholesterol assimilation efficiencies of the current study bacteria based on measurement of OD505 through cholesterol liquicolor kit using CHOD-PAP method.
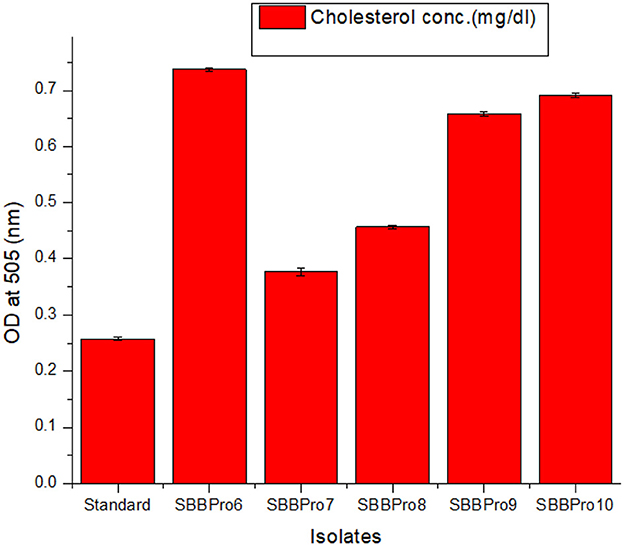
Figure 8. Comparison of cholesterol assimilation efficiencies of the current study documented bacteria through measurement of OD505 of standard (STD) and test samples: L. rhamnosus SBBPro6, E. faecium SBBPro7, B. amyloliquefaciens SBBPro8, B. subtilis SBBPro9, and B. cereus SBBPro10.
3.6.10 Antibiotic sensitivity test
Each isolated strain was efficiently sensitive to antibiotics and showed inhibition zones. All of them showed the highest inhibitory zones against Velosef, measuring 31.5, 31.6, 33.5, 35.3, and 34 mm, respectively. In case of amoxil and ciprofloxacin, B. amyloliquefaciens SBBPro8 showed maximum zone of inhibition, 12.5 and 16.8 mm, respectively. Minimum inhibitory zones observed in E. faecium SBBPro7 and B. subtilis SBBPro9 were 11.5 and 12.8 mm against amoxil and ciprofloxacin, respectively. In case of azithromycin and erythromycin, L. rhamnosus SBBPro6 showed maximum zones of inhibition, 11.33 and 11 mm, respectively, while minimum inhibitory zones were exhibited by B. amyloliquefaciens SBBPro8 (7.5 mm) and E. faecium SBBPro7 (6 mm) (Supplementary Table S12, Figure 9). All the probiotics demonstrated statistically significant sensitivity against all the antibiotics at all three concentrations with p-values < 0.005 in each case (Supplementary Tables S13a–e).
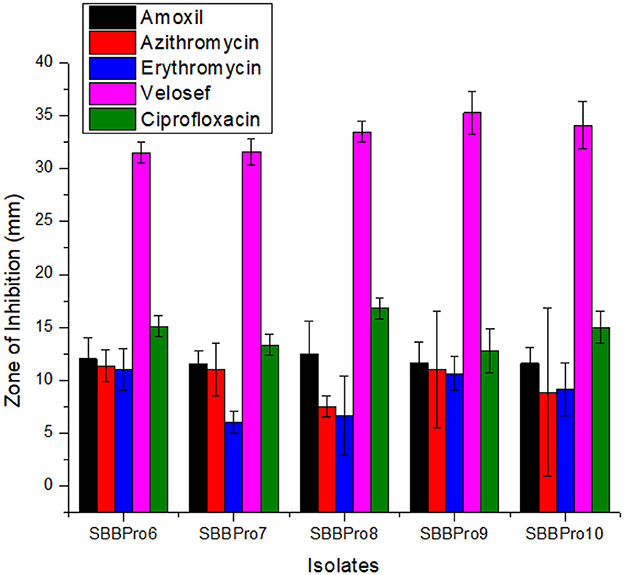
Figure 9. Antibiotic sensitivity profiling of bacteria isolated in the current study against antibiotics amoxil, azithromycin, erythromycin, Velosef, and ciprofloxacin through measurement of zones of inhibition: L. rhamnosus SBBPro6, E. faecium SBBPro7, B. amyloliquefaciens SBBPro8, B. subtilis SBBPro9, and B. cereus SBBPro10.
4 Discussion
The five isolates characterized in this study were identified as L. rhamnosus, E. faecium, B. amyloliquefaciens, B. subtilis, and B. cereus. Lactobacillus, which has been granted Generally Recognized as Safe (GRAS) status, is of particular interest due to its indigenous origin from the gut, enhancing its potential application as a probiotic in aquaculture. Several studies have previously documented L. rhamnosus (78), E. faecium (79–82), B. amyloliquefaciens (83, 84), B. subtilis (85–87), and B. cereus (88) as fish gut-associated bacteria, supporting the findings of this study. Additionally, the Gram-positive nature of the identified isolates is consistent with current literature (89, 90). Earlier explored GIT-associated probiotics from marine fishes documented so far include genera of Lactobacillus, Bacillus, and Enterococcus (69, 91, 92). Hence, this study is in accordance with the previously published study.
A study has documented fermentation potentials for different carbohydrates in gut-borne lactic acid bacterial strains in marine fishes, such as mannitol (strains LB41 and LC1333) and glucose and fructose (strains LB411, LE823, LC1132, LC1333, LC1334, LC1342, and LC1344) (69). These findings are consistent with the current study's results.
Tolerance to bile salts and an acidic environment are two of the major prerequisites for bacteria to survive in the fish gut during transit through the stomach and intestine. The isolates from this study demonstrated growth at pH values of 3 and 5 and at a bile concentration of 0.3 g. This suggests that these bacteria do have the potential to survive well in the fish gut environment. Consistent with our finding, L. plantarum LB411 from the marine fish gut has been documented to exhibit optimal growth at pH = 3. In contrast, Enterococcus faecium EN936 and E. gallinarum EN10113 have been reported to grow only at near-neutral pH (69). The response of the bacterial isolates to varying pH levels aligns with previous literature, as most gut microbes have been documented to exhibit more tolerance to pH > 3 (89). Additionally, all isolates in our study exhibited significant growth at the exponential phase, ranging between 1.91 ± 0.02 and 2.10 ± 0.07 at 0.3% bile salt concentration. However, this finding does not appear to be consistent with previous literature (89). Optimal bile salt tolerance in marine fish gut bacteria is reported as 1% in L. mesenteroides LE823, L. plantarum LB411, and E. gallinarum EN10113, while the least tolerance is reported at 0.1% concentration in Lactococci strains (69).
All isolates from this study exhibited the highest tolerance at 0.2 (OD600 of 1.61 to 1.86) and 2% (OD600 of 1.45 to 1.62) NaCl concentration. In contrast, previous studies have reported optimal bacterial growth in fish gut isolates at 0.1% (OD600 of 0.25 to 0.1) and markedly reduced growth (OD600 of 0.025 to 0.125) at 2% salt concentration (90). Hence, the findings of this study contradict those previously reported.
All isolates demonstrated tolerance to synthetic gastric fluid, and this indicates their ability to withstand gastrointestinal conditions in the host, further confirming their safety profile as probiotics. This finding is also consistent with earlier reported literature (66, 93).
In the current study, isolates exhibited optimum growth at 2 and 3 pH. However, as the pH approached neutrality (pH 5), growth decreased, and this finding contradicts previous literature reporting optimal growth at near-neutral pH values (5 and 9). A previous study also documented optimal growth at bile salt concentrations of 0.5% and 1%, which is partially consistent with this study, where isolates showed tolerance at 0.3% bile salt concentration (69). Additionally, another study reported the optimal growth of fish gut probiotics at neutral pH values (pH 6 and 7) (90), whereas a separate study documented the highest survival rates at pH 2 and 3 with 2% bile salt concentration (34). These findings align with the current study (94).
The ⋎ hemolysis exhibited by the isolates from this study is consistent with previously published research (34, 95, 96). A study on marine fish gut-borne bacteria also showed no hemolysis (97). However, contrary to these findings, some bacterial isolates have been reported to exhibit beta hemolysis (98).
Several studies have evaluated the antimicrobial potential of fish gut bacteria against pathogens that were identified in this study (34). One study classified these bacteria among major fish food-associated pathogens and further documented the antimicrobial activity of fish gut bacteria against P. aeruginosa and S. aureus (99). While the majority of bacteria were insensitive to S. aureus and P. aeruginosa, the findings of the current study are consistent with previous research documenting fish gut probiotics with resistance to S. aureus (100–102). Consistent with current findings, another study has reported antagonistic activity in marine fish gut-borne bacteria against P. aeruginosa and S. aureus (97).
The isolates from this study exhibited resistance to heat shock at 100°C. However, previous literature has reported fish-borne bacteria tolerating temperatures only up to 30°C and 37°C (90). Consistent with the present findings, other studies have reported fish-borne bacteria tolerating heat shocks at 80°C, 90°C, and 100°C (96).
Probiotic bacteria with strong potential must be capable of reducing cholesterol levels to help maintain a healthy threshold in the host (103). In this study, the isolates B. cereus, B. subtilis, and B. amyloliquefaciens exhibited cholesterol assimilation potentials of 34 mg/dL, 39 mg/dL, and 70 mg/dL, respectively. These findings are supported by previous studies that reported cholesterol metabolism roles of these species in Atlantic salmon, Amur minnow, and rohu (104–106). The isolates from this study exhibited cholesterol assimilation within a range of 26.76 to 83.44 mg/dL, which contradicts another study that documented cholesterol assimilation efficiencies between 1.2 and 4.3 mg/dL in fish gut probiotics (107).
The transfer of ARGs remains a major safety concern in using probiotics as feed supplements (108). However, all isolated strains in this study were highly sensitive to antibiotics, as demonstrated by the clear inhibition zones observed. Sensitivity to erythromycin was particularly consistent with existing literature (89, 109). Furthermore, various studies have reported antibiotic sensitivity in freshwater as well as marine fish gut bacterial species (34, 97). The use of these bacterial isolates in aquaculture could prevent the dispersion and enrichment of ARGs within aquatic systems.
Literature data regarding the gut bacteria of S. solea is limited. However, several articles have reported the effect of probiotic supplementation on larviculture improvement of S. solea (110) and juvenile intestine function and growth, immune response, gut morphology, host defense, ecology of digestive tract, and gut microbial diversity in Solea senegalensis (111–114). To the best of our knowledge, only two studies so far have reported the isolation and characterization of S. solea gut-borne bacteria internationally. On the other hand, in Pakistan, this is the first ever study reporting gut microbes of this marine fish (115, 116). Hence, this study documents the information regarding the probiotic potential of gut microbes from a least-explored fish.
5 Conclusion
The isolates characterized in this study demonstrated strong probiotic potential based on their excellent in vitro characterization. Antibiotic sensitivity of these isolates is a significant finding of the current project and indicates the potential of documented bacteria as a safe alternative to antibiotics. Additionally, the results from the simulated gastric medium survival assay and optimal growth at pH 2 and 3 suggested that these isolates could adapt to the gastric conditions in the host. Keeping in view these probiotic attributes, bacterial strains might be recommended for in vivo assessment of their impact on fish growth, body weight, and meat quality through their in-feed administration.
Data availability statement
The FASTA sequences of present study probiotics are available at NCBI database. The accession numbers assigned are PQ002180, PQ002492, PQ002184, PQ002187, and PQ002188.
Ethics statement
The animal study was approved by the Punjab University Institutional Ethics Reviews Board. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
NH: Writing – original draft. AM: Formal analysis, Writing – review & editing. FM: Writing – original draft, Conceptualization, Methodology, Supervision. AH: Formal analysis, Writing – review & editing. MA: Formal analysis, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to extend their gratitude to Al Ain University, United Arab Emirates.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1581675/full#supplementary-material
References
1. Rasool S. Corporate window: Nurturing Pakistan's blue economy. (2024). Available online at: https://www.dawn.com/news/1846991
2. Shahzad SM. Economic opportunities for Islamic financing-from green to blue economy. Int J Multidisc Curr Res. (2020) 8:205–14. doi: 10.14741/ijmcr/v.8.2.9
3. Aslam MU, Nadeem N, Baig IA, Ahmed UI. Economic analysis of fish farming in Punjab, Pakistan. Rev Econ Dev Stud. (2020) 6:625–37. doi: 10.47067/reads.v6i3.251
4. Alam S, Azam M. Challenges and prospects of blue economy for Pakistan. J Asian Dev Stud. (2023) 12:1516–27. Available online at: https://poverty.com.pk/index.php/Journal/article/view/222
5. Lam RD, Lazo DPL, Barman BK, Khatun Z, Parvin L, Choudhury A, et al. Sustainability impacts of ecosystem approaches to small-scale aquaculture in Bangladesh. Sustain Sci. (2022) 17:295–313. doi: 10.1007/s11625-021-01076-w
6. Syed R, Safdar A. Revisiting blue economy: challenges and prospects for the maritime sector of Pakistan. J Contempor Stud. (2021) 10:16–37. doi: 10.54690/jcs.v10i2.191
7. Askari MU, Tahir M, Shaheen N. Blue economy of Pakistan: challenges and prospects. J Punjab Univ Histor Soc. (2020) 33:1–14. Available online at: https://pu.edu.pk/images/journal/HistoryPStudies/PDF_Files4_v33_2_2019.pdf
8. Luthman O, Robb DHF, Henriksson PJG, Jørgensen PS, Troell M. Global overview of national regulations for antibiotic use in aquaculture production. Aquaculture Int. (2024) 32:9253–70. doi: 10.1007/s10499-024-01614-0
9. Algammal A, Hetta HF, Mabrok M, Behzadi P. Emerging multidrug-resistant bacterial pathogens “superbugs”: a rising public health threat. Front Microbiol. (2023) 14:1135614. doi: 10.3389/fmicb.2023.1135614
10. Raharjo HM, Budiyansah H, Mursalim MF, Chokmangmeepisarn P, Sakulworakan R, Debnath PP, et al. The first evidence of blaCTX-M-55, QnrVC5, and novel insight into the genome of MDR Vibrio vulnificus isolated from Asian sea bass (Lates calcarifer) identified by resistome analysis. Aquaculture. (2023) 571:739500. doi: 10.1016/j.aquaculture.2023.739500
11. Algammal AM, Mabrok M, Ezzat M, Alfifi KJ, Esawy AM, Elmasry N, et al. Prevalence, antimicrobial resistance (AMR) pattern, virulence determinant and AMR genes of emerging multi-drug resistant Edwardsiella tarda in Nile tilapia and African catfish. Aquaculture. (2022) 548:737643. doi: 10.1016/j.aquaculture.2021.737643
12. Algammal AM, Alfifi KJ, Mabrok M, Alatawy M, Abdel-Moneam DA, Alghamdi S, et al. Newly emerging MDR B. cereus in Mugil Seheli as the First Report Commonly Harbor nhe, hbl, cyt K, and pc-Plc virulence genes and bla 1, bla 2, tet A, and erm a resistance genes. Infect Drug Resist. (2022) 15:2167–85. doi: 10.2147/IDR.S365254
13. Salma U, Shafiujjaman M, Zahid MA, Faruque MH, Habibullah-Al-Mamun M, Hossain A. Widespread use of antibiotics, pesticides, and other aqua-chemicals in finfish aquaculture in Rajshahi District of Bangladesh. Sustainability. (2022) 14:17038. doi: 10.3390/su142417038
14. Ren X, Qin Y, Zhang Y, Xie J, Diao X, Altaf MM. Regional distribution differences of antibiotics in tropical marine aquaculture area: Insights into antibiotic management and risk assessment. Sci Total Environ. (2024) 954:176391. doi: 10.1016/j.scitotenv.2024.176391
15. Milijasevic M, Veskovic-Moracanin S, Babic Milijasevic J, Petrovic J, Nastasijevic I. Antimicrobial resistance in aquaculture: risk mitigation within the one health context. Foods. (2024) 13:2448. doi: 10.3390/foods13152448
16. Preena PG, Swaminathan TR, Kumar VJR, Singh ISB. Antimicrobial resistance in aquaculture: a crisis for concern. Biologia. (2020) 75:1497–517. doi: 10.2478/s11756-020-00456-4
17. Milijasevic M, Veskovic-Moracanin S, Babic Milijasevic J, Petrovic J, Nastasijevic I. Antimicrobial resistance in aquaculture: risk mitigation within the one health context. Foods. (2024) 13:2448. doi: 10.3390/foods13152448
18. Schar D, Zhao C, Wang Y, Larsson DGJ, Gilbert M, Van Boeckel TP. Twenty-year trends in antimicrobial resistance from aquaculture and fisheries in Asia. Nat Commun. (2021) 12:5384. doi: 10.1038/s41467-021-25655-8
19. Ramos-Vivas J, Superio J, Galindo-Villegas J, Acosta F. Phage therapy as a focused management strategy in aquaculture. Int J Mol Sci. (2021) 22:10436. doi: 10.3390/ijms221910436
20. Bhat R, Altinok I. Antimicrobial resistance (AMR) and alternative strategies for combating AMR in aquaculture. Turkish J Fisher Aquatic Sci. (2023) 23:TRJFAS24068. doi: 10.4194/TRJFAS24068
21. El-Saadony MT, Alagawany M, Patra AK, Kar I, Tiwari R, Dawood MAO, et al. The functionality of probiotics in aquaculture: An overview. Fish Shellfish Immunol. (2021) 117:36–52. doi: 10.1016/j.fsi.2021.07.007
22. Subedi B, Shrestha A. A review: application of probiotics in aquaculture. Int J Animal Fisher Res. (2020) 4:1–34. doi: 10.22161/ijfaf.4.5.1
23. Kiliç N, Gültekin G. Sustainable approaches in aquaculture: Pharmacological and natural alternatives to antibiotics. Mar Sci Technol Bull. (2024) 13:239–50. doi: 10.33714/masteb.1488998
24. Butt UD, Lin N, Akhter N, Siddiqui T, Li S, Wu B. Overview of the latest developments in the role of probiotics, prebiotics and synbiotics in shrimp aquaculture. Fish Shellfish Immunol. (2021) 114:263–81. doi: 10.1016/j.fsi.2021.05.003
25. Amenyogbe E, Chen G, Wang Z, Huang JS, Huang B, Li H. The exploitation of probiotics, prebiotics and synbiotics in aquaculture: present study, limitations and future directions: a review. Aquac Int. (2020) 28:1017–41. doi: 10.1007/s10499-020-00509-0
26. Srirengaraj V, Razafindralambo HL, Rabetafika HN, Nguyen HT, Sun YZ. Synbiotic agents and their active components for sustainable aquaculture: concepts, action mechanisms, and applications. Biology. (2023) 12:1498. doi: 10.3390/biology12121498
27. Ismail M, Wahdan A, Mohamed SY, Metwally E, Mabrok M. Effect of dietary supplementation with a synbiotic (Lacto Forte) on growth performance, haematological and histological profiles, the innate immune response and resistance to bacterial disease in Oreochromis niloticus. Aquac Res. (2019) 50:2545–62. doi: 10.1111/are.14212
28. Eissa ESH, Ahmed RA, Elghany NAA, Elfeky A, Saadony S, Ahmed NH, et al. Potential symbiotic effects of β-1, 3 glucan, and fructooligosaccharides on the growth performance, immune response, redox status, and resistance of Pacific white shrimp, Litopenaeus vannamei to Fusarium solani infection. Fishes. (2023) 8:105. doi: 10.3390/fishes8020105
29. da Silva TF, Glória RA, Americo MF, Freitas ADS, de Jesus LCL, Barroso FAL, et al. Unlocking the potential of probiotics: a comprehensive review on research, production, and regulation of probiotics. Probiotics Antimicrob Proteins. (2024) 16:1687–723. doi: 10.1007/s12602-024-10247-x
30. Merrifield DL, Dimitroglou A, Foey A, Davies SJ, Baker RT, Bøgwald J, et al. The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture. (2010) 302:1–18. doi: 10.1016/j.aquaculture.2010.02.007
31. Torres-Maravilla E, Parra M, Maisey K, Vargas RA, Cabezas-Cruz A, Gonzalez A, et al. Importance of Probiotics in Fish Aquaculture: Towards the Identification and Design of Novel Probiotics. Microorganisms. (2024) 12:626. doi: 10.3390/microorganisms12030626
32. Swain HS, Baisakhi B, Ramteke MH, Kumar V, Upadhyay A. Application of probiotics in aquaculture, in biotechnological tools in fisheries and aquatic health management. In: Biotechnological Tools in Fisheries and Aquatic Health Management. Springer (2023). p. 217–230. doi: 10.1007/978-981-99-2981-8_11
33. Mazlumi A, Panahi B, Hejazi MA, Nami Y. Probiotic potential characterization and clustering using unsupervised algorithms of lactic acid bacteria from saltwater fish samples. Sci Rep. (2022) 12:11952. doi: 10.1038/s41598-022-16322-z
34. Govindaraj K, Samayanpaulraj V, Narayanadoss V, Uthandakalaipandian R. Isolation of lactic acid bacteria from intestine of freshwater fishes and elucidation of probiotic potential for aquaculture application. Probiotics Antimicrob Proteins. (2021) 13:1598–610. doi: 10.1007/s12602-021-09811-6
35. Patel P, Patel B, Amaresan N, Joshi B, Shah R, Krishnamurthy R. Isolation and characterization of Lactococcus garvieae from the fish gut for in vitro fermentation with carbohydrates from agro-industrial waste. Biotechnol Rep. (2020) 28:e00555. doi: 10.1016/j.btre.2020.e00555
36. Gutiérrez-Falcón A, Padilla D, Sosa MJR, Barrasa JLM, Acosta-Hernández B, Henao AS, et al. Characterization in vitro of new bacterial strains showing potentially probiotic crossed effect against vibriosis in relevant fish species for marine aquaculture. J Appl Anim Res. (2020) 48:553–8. doi: 10.1080/09712119.2020.1844714
37. Balcázar JL, Vendrell D, Blas Id, Ruiz-Zarzuela I, Muzquiz JL, Girones O. Characterization of probiotic properties of lactic acid bacteria isolated from intestinal microbiota of fish. Aquaculture. (2008) 278:188–91. doi: 10.1016/j.aquaculture.2008.03.014
38. Sardi AE, Bégout ML, Lalles AL, Cousin X, Budzinski H. Temperature and feeding frequency impact the survival, growth, and metamorphosis success of Solea solea larvae. PLoS ONE. (2023) 18:e0281193. doi: 10.1371/journal.pone.0281193
39. Salma N. TDAP-Fisheries. In:Salman, R., , editor. Fisheries: Potential of Pakistan. Pakistan: Trade Development Authority of Pakistan (2021).
40. Muhammad I. Increasing costs and cultural importance: Sole fish in Pakistan. In: Pakistan National Fish. Pakistan (2024).
41. Vulla KE, Mmanda FP, Nyangoko BP, Makule EE. Unlocking potential benefits on applications of probiotics in Inland aquaculture industry: a review. Aquac Fish Fisher. (2024) 4:e70027. doi: 10.1002/aff2.70027
42. Stoskopf M, Posner LP. Anesthesia and restraint of laboratory fish. Anesthe Analge Lab Animals. (2008) 2:519–34. doi: 10.1016/B978-012373898-1.50025-5
43. Anapana G, Gudivada M. A Review On Ethical Issues Of Animal Experimentation India. Available at: SSRN 5078215 (2020).
44. Thirunavukkarasu R, Pandi P, Balaraman D, Albalawi F, Ahmad N, Panagal M, et al. Influence of extracellular protein isolated from fish gut associated bacteria as an enhancer of growth and innate immune system in Mugil cephalus. Sci Rep. (2022) 12:3217. doi: 10.1038/s41598-022-05779-7
45. Gupta S, Fečkaninová A, Lokesh J, Koščová J, Sørensen M, Fernandes J, et al. Lactobacillus dominate in the intestine of Atlantic salmon fed dietary probiotics. Front Microbiol. (2019) 9:3247. doi: 10.3389/fmicb.2018.03247
46. Kavitha M, Raja M, Perumal P. Evaluation of probiotic potential of Bacillus spp. isolated from the digestive tract of freshwater fish Labeo calbasu (Hamilton, 1822). Aquac Rep. (2018) 11:59–69. doi: 10.1016/j.aqrep.2018.07.001
47. Soemarie Y.B, Milanda T., Barliana M.I. Isolation, characterization, and identification candidate of probiotic bacteria isolated from Wadi Papuyu (Anabas testudineus Bloch.) a fermented fish product from Central Kalimantan, Indonesia. Int J Food Sci. (2022) 2022:4241531. doi: 10.1155/2022/4241531
48. Hakkinen M, Faqih AR, Prihanto AA, Anitasari S. Isolation of lactic acid bacteria as potential probiotic candidates from the digestive tract of Gobiopterus sp. Biodiversitas. J Biol. Diver. (2025) 26:49. doi: 10.13057/biodiv/d260249
49. Trisiswanti Arista AM, Sugimin S, Rizquita EA, Oxi R. Effectiveness of bacterial culture preservative techniques in the microbiology laboratory. in Proceeding of International Joint Conference on UNESA 2023 (2024)
50. Saleena LAK, Chandran D, Geetha R, Radha R, Sathian CT, Kumar M, et al. Optimization and identification of lactic acid bacteria with higher mannitol production Potential. Indian J Animal Res. (2023) 57:1644–1651. doi: 10.18805/IJAR.B-4759
51. Hedberg M, Hasslöf P, Sjöström I, Twetman S, Stecksén-Blicks C. Sugar fermentation in probiotic bacteria–an in vitro study. Oral Microbiol Immunol. (2008) 23:482–5. doi: 10.1111/j.1399-302X.2008.00457.x
52. Wu H, Wang L, Kang L, Liu C, Li M. Identification and growth promotion effect of rhizosphere probiotics in kiwifruit. Bangladesh J Botany. (2025) 54:67–74. doi: 10.3329/bjb.v54i1.80749
53. Saha S, Ray AK, Roy RN, Sen SK. Characterization of cellulase-producing bacteria from the digestive tract of tilapia, Oreochromis mossambica (Peters) and grass carp, Ctenopharyngodon idella (Valenciennes). Aquac Res. (2006) 37:380–8. doi: 10.1111/j.1365-2109.2006.01442.x
54. Sasi A, Duraipandiyan N, Marikani K, Dhanasekaran S, Al-Dayan N, Venugopal D. Identification and characterization of a newly isolated chitinase-producing strain Bacillus licheniformis SSCL-10 for chitin degradation. Archaea. (2020) 2020:8844811. doi: 10.1155/2020/8844811
55. Javadi A, Shamaei M, Mohammadi Ziazi L, Pourabdollah M, Dorudinia A, Seyedmehdi SM, et al. Qualification study of two genomic DNA extraction methods in different clinical samples. Tanaffos. (2014) 13:41. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC4386014/
56. Green MR, Sambrook J. Analysis of DNA by agarose gel electrophoresis. Cold Spring Harb Protoc. (2019) 2019:100388. doi: 10.1101/pdb.top100388
57. Hussain N, Mohiuddin F, Muccee F, Bunny SM, Al Haddad AHI. Isolation, molecular, and metabolic profiling of benzene-remediating bacteria inhabiting the tannery industry soil. Polish J Microbiol. (2025) 74:3. doi: 10.33073/pjm-2025-003
58. Green M, Sambrook J. Polymerase chain reaction. Cold Spring Harb Protoc. (2019) 2019:95109. doi: 10.1101/pdb.top095109
59. Sievers F, Higgins DG. The clustal omega multiple alignment package. In: Multiple Sequence Alignment: Methods and Protocols (2021). p. 3–16. doi: 10.1007/978-1-0716-1036-7_1
60. Sayers EW, Cavanaugh M, Clark K, Ostell J, Pruitt KD, Karsch-Mizrachi I. GenBank. Nucleic Acids Res. (2019) 47:D94–9. doi: 10.1093/nar/gky989
61. Shahbaz F, Muccee F, Shahab A, Safi SZ, Alomar SY, Qadeer A. Isolation and in vitro assessment of chicken gut microbes for probiotic potential. Front Microbiol. (2024) 15:1278439. doi: 10.3389/fmicb.2024.1278439
62. Nawaz AN, Jagadeesh K, Krishnaraj P. Isolation and screening of lactic acid bacteria for acidic pH and bile tolerance. Int J Curr Microbiol Appl Sci. (2017) 6:3975–80. doi: 10.20546/ijcmas.2017.607.411
63. Rocha-Ramírez LM, Hernández-Chiñas U, Moreno-Guerrero SS, Ramírez-Pacheco A, Eslava CA. Probiotic properties and immunomodulatory activity of Lactobacillus strains isolated from dairy products. Microorganisms. (2021) 9:825. doi: 10.3390/microorganisms9040825
64. Chen Z, Leng X, Zhou F, Shen W, Zhang H, Yu Q, et al. Screening and identification of probiotic Lactobacilli from the infant gut microbiota to alleviate lead toxicity. Probiotics Antimicrob Proteins. (2023) 15:821–31. doi: 10.1007/s12602-021-09895-0
65. Mulet-Cabero A. Electronic supplementary material (ESI) for food and function. In: The Royal Society of Chemistry (2020).
66. Guan X, Lin B, Wang Q, Zhao D, Huang J, Yang Y, et al. Characterization and in vitro assessment of probiotic potential of Lactiplantibacillus plantarum BXM2 from fermented honey passion fruit beverage. Food Front. (2023) 4:1372–81. doi: 10.1002/fft2.240
67. Reuben RC, Roy PC, Sarkar SL, Alam RU, Jahid IK. Isolation, characterization, and assessment of lactic acid bacteria toward their selection as poultry probiotics. BMC Microbiol. (2019) 19:1–20. doi: 10.1186/s12866-019-1626-0
68. Kumari V B C, Huligere SS, Alotaibi G, Al Mouslem AK, Bahauddin AA, Shivanandappa TB, et al. Antidiabetic activity of potential probiotics limosilactobacillus spp, levilactobacillus spp, and lacticaseibacillus spp. isolated from fermented sugarcane juice: a comprehensive in vitro and in silico study. Nutrients. (2023) 15:1882. doi: 10.3390/nu15081882
69. Alonso S, Carmen Castro M, Berdasco M, de la Banda IG, Moreno-Ventas X, de Rojas AH. Isolation and partial characterization of lactic acid bacteria from the gut microbiota of marine fishes for potential application as probiotics in aquaculture. Probiot Antimic Proteins. (2019) 11:569–79. doi: 10.1007/s12602-018-9439-2
70. Cizeikiene D, Jagelaviciute J. Investigation of antibacterial activity and probiotic properties of strains belonging to Lactobacillus and Bifidobacterium genera for their potential application in functional food and feed products. Probiot Antimic Proteins. (2021) 13:1387–403. doi: 10.1007/s12602-021-09777-5
71. Mulak V, Tailliez R, Eb P, Becel P. Heat resistance of bacteria isolated from preparations based on seafood products. J Food Prot. (1995) 58:49–53. doi: 10.4315/0362-028X-58.1.49
72. Jomehzadeh N, Javaherizadeh H, Amin M, Saki M, Al-Ouqaili MTS, Hamidi H, et al. Isolation and identification of potential probiotic Lactobacillus species from feces of infants in southwest Iran. Int J Infect Dis. (2020) 96:524–30. doi: 10.1016/j.ijid.2020.05.034
73. Sharma R, Otieno S, Otieno S. Kirby Bauer disc diffusion method for antibiotic susceptibility testing. Microbe Notes (2022).
75. Kim TK. Understanding one-way ANOVA using conceptual figures. Korean J Anesthesiol. (2017) 70:22. doi: 10.4097/kjae.2017.70.1.22
76. Shun Z, Silverberg A, Chang CK, Ouyang P. Dunnett's many-to-one test and least square means. J Biopharm Stat. (2003) 13:17–28. doi: 10.1081/BIP-120017723
77. Muhammed Al-Kassab M. The use of one sample t-test in the real data. J Adv Mathem. (2022) 21:9279. doi: 10.24297/jam.v21i.9279
78. Ngamkala S, Satchasataporn K, Setthawongsin C, Raksajit W. Histopathological study and intestinal mucous cell responses against Aeromonas hydrophila in Nile tilapia administered with Lactobacillus rhamnosus GG. Veter World. (2020) 13:967. doi: 10.14202/vetworld.2020.967-974
79. Kanmani P, Suganya K, Kumar RS, Yuvaraj N, Pattukumar V, Paari KA, et al. Synthesis and functional characterization of antibiofilm exopolysaccharide produced by Enterococcus faecium MC13 isolated from the gut of fish. Appl Biochem Biotechnol. (2013) 169:1001–15. doi: 10.1007/s12010-012-0074-1
80. Tilwani YM, Lakra AK, Domdi L, Jha N, Arul V. Characterization of potential probiotic bacteria Enterococcus faecium MC-5 isolated from the gut content of Cyprinus carpio specularis. Microb Pathog. (2022) 172:105783. doi: 10.1016/j.micpath.2022.105783
81. Mohammed AA, Jassim AY, Farner KW. Isolation and Identification of Enterococcus faecium from the Gastrointestinal Tract of the Common Carp (Cyprinus carpio) and the Nile Tilapia (Oreochromis niloticus). Egyptian J Aquatic Biol Fisher. (2024) 28:380432. doi: 10.21608/ejabf.2024.380432
82. Mao Q, Sun X, Sun J, Zhang F, Lv A, Hu X, et al. A candidate probiotic strain of Enterococcus faecium from the intestine of the crucian carp Carassius auratus. AMB Express. (2020) 10:1–9. doi: 10.1186/s13568-020-00973-0
83. Xu R, Li M, Wang T, Zhao YW, Shan CJ, Qiao F, et al. Bacillus amyloliquefaciens ameliorates high-carbohydrate diet-induced metabolic phenotypes by restoration of intestinal acetate-producing bacteria in Nile Tilapia. Br J Nutr. (2022) 127:653–65. doi: 10.1017/S0007114521001318
84. Reda RM, El-Hady MA, Selim KM, El-Sayed HM. Comparative study of three predominant gut Bacillus strains and a commercial B. amyloliquefaciens as probiotics on the performance of Clarias gariepinus. Fish Shellfish Immunol. (2018) 80:416–25. doi: 10.1016/j.fsi.2018.06.031
85. Wang X, Yao Y, Ge H, Zhang J, Zhang J, Yan Q. Isolation and identification of probiotic Bacillus subtilis AJQ03 from the intestinal tract of Anguilla japonica (Japanese eel). Front Microbiol. (2024) 15:1446299. doi: 10.3389/fmicb.2024.1446299
86. Saravanakumar S, Prabakaran NN, Ashokkumar R, Jamuna S. Unlocking the gut's treasure: lipase-producing Bacillus subtilis probiotic from the intestine of microstomus kitt (lemon sole). Appl Biochem Biotechnol. (2024) 196:4273–86. doi: 10.1007/s12010-023-04749-7
87. Dhayalan A, Velramar B, Govindasamy B, Ramalingam KR, Dilipkumar A, Pachiappan P. Isolation of a bacterial strain from the gut of the fish, Systomus sarana, identification of the isolated strain, optimized production of its protease, the enzyme purification, and partial structural characterization. J Genetic Eng Biotechnol. (2022) 20:24. doi: 10.1186/s43141-022-00299-3
88. Feliatra F, Batubara UM, Nurulita Y, Lukistyowati I, Setiaji J. The potentials of secondary metabolites from Bacillus cereus SN7 and Vagococcus fluvialis CT21 against fish pathogenic bacteria. Microb Pathog. (2021) 158:105062. doi: 10.1016/j.micpath.2021.105062
89. Muthukumar P, Kandeepan C. Isolation, identification and characterization of probiotic organisms from intestine of fresh water fishes. Int J Curr Microbiol Appl Sci. (2015) 4:607–16. Available online at: https://researchgate.net/publication/354200934
90. Ganguly A, Banerjee A, Mandal A, Khan MA, Mohapatra PKD. Isolation and characterization of bacteria from the intestine of Clarias batrachus for probiotic organism. in Proceedings of the Zoological Society. Springer (2019). doi: 10.1007/s12595-018-0283-x
91. Santos RA, Oliva-Teles A, Pousão-Ferreira P, Jerusik R, Saavedra MJ, Enes P, et al. Isolation and characterization of fish-gut Bacillus spp. as source of natural antimicrobial compounds to fight aquaculture bacterial diseases. Mar Biotechnol. (2021) 23:276–93. doi: 10.1007/s10126-021-10022-x
92. Hovda MB, Lunestad BT, Fontanillas R, Rosnes JT. Molecular characterisation of the intestinal microbiota of farmed Atlantic salmon (Salmo salar L.). Aquaculture. (2007) 272:581–8. doi: 10.1016/j.aquaculture.2007.08.045
93. Yao Y, Wang X, Yan Q, Lin X, Wu J, Wang P, et al. Isolation and characterization of probiotic Lysinibacillus species from the gastrointestinal tract of large yellow croaker (Larimichthys crocea). Front Mar Sci. (2024) 11:1408979. doi: 10.3389/fmars.2024.1408979
94. Jilani AK, Haider MN, Hasan A, Mahfuz MA, Rifat MNI, Hossain MM, et al. Isolation, identification, and potentiality of gut-derived probiotic bacteria from Heteropneustes fossilis, stinging catfish. J Adv Veter Animal Res. (2024) 11:560. doi: 10.5455/javar.2024.k806
95. Nandi A, Dan SK, Banerjee G, Ghosh P, Ghosh K, Ringø E, et al. Probiotic potential of autochthonous bacteria isolated from the gastrointestinal tract of four freshwater teleosts. Probiot Antimic Proteins. (2017) 9:12–21. doi: 10.1007/s12602-016-9228-8
96. Kuebutornye FKA, Lu Y, Abarike ED, Wang Z, Li Y, Sakyi ME. In vitro assessment of the probiotic characteristics of three Bacillus species from the gut of Nile tilapia, Oreochromis niloticus. Probiot Antimic Proteins. (2020) 12:412–24. doi: 10.1007/s12602-019-09562-5
97. Network EM. Technological characterization of lactic acid bacteria isolated from intestinal microbiota of marine fish in the Oran Algeria coast. African J Microbiol Res. (2012) 6:3125–3133. doi: 10.5897/AJMR11.1175
98. Jiang Y, Liang M, Yang Y, Xue J, Suo H. Probiotic Lactobacillus plantarum SHY21-2 protected zebrafish against Aeromonas hydrophila infection by maintaining intestinal barrier integrity, inhibiting inflammatory and oxidative stress responses, and regulating intestinal microbiome. Aquaculture. (2024) 582:740506. doi: 10.1016/j.aquaculture.2023.740506
99. Mumbo MT, Nyaboga EN, Kinyua J, Muge EK, Mathenge SGK, Muriira G, et al. Prevalence and antimicrobial resistance profile of bacterial foodborne pathogens in Nile tilapia fish (Oreochromis niloticus) at points of retail sale in Nairobi, Kenya. Front Antibiot. (2023) 2:1156258. doi: 10.3389/frabi.2023.1156258
100. Sugita H, Shibuya K, Hanada H, Deguchi Y. Antibacterial abilities of intestinal microflora of the river fish. Fisher Sci. (1997) 63:378–83. doi: 10.2331/fishsci.63.378
101. Floris R, Sanna G, Mura L, Fiori M, Culurgioni J, Diciotti R, et al. Isolation and identification of bacteria with surface and antibacterial activity from the gut of Mediterranean grey mullets. Microorganisms. (2021) 9:2555. doi: 10.3390/microorganisms9122555
102. Uniacke-Lowe S, Collins FWJ, Hill C, Ross RP. Bioactivity screening and genomic analysis reveals deep-sea fish microbiome isolates as sources of novel antimicrobials. Mar Drugs. (2023) 21:444. doi: 10.3390/md21080444
103. Wu S, Pan M, Zan Z, Zhao W, Zou H, Wang G, et al. Regulation of lipid metabolism by gut microbiota in aquatic animals. Rev Aquacult. (2024) 16:34–46. doi: 10.1111/raq.12819
104. Mukherjee A, Dutta D, Banerjee S, Ghosh K, Ringø E, Breines EM, et al. Culturable autochthonous gut bacteria in rohu, Labeo rohita. In vitro growth inhibition against pathogenic Aeromonas spp., stability in gut, bio-safety and identification by 16S rRNA gene sequencing. Symbiosis. (2017) 73:165–77. doi: 10.1007/s13199-017-0474-7
105. Wu Z. Screening and adhesion characteristics analysis of enzyme producing probiotics from intestine of Rhynchocypris lagowskii. M. Sc, Jilin Agricultural University (2020).
106. Askarian F, Zhou Z, Olsen RE, Sperstad S, Ringø E. Culturable autochthonous gut bacteria in Atlantic salmon (Salmo salar L.) fed diets with or without chitin. Characterization by 16S rRNA gene sequencing ability to produce enzymes and in vitro growth inhibition of four fish pathogens. Aquaculture. (2012) 326:1–8. doi: 10.1016/j.aquaculture.2011.10.016
107. Le B, Yang S-H. Identification of a novel potential probiotic Lactobacillus plantarum FB003 isolated from salted-fermented shrimp and its effect on cholesterol absorption by regulation of NPC1L1 and PPARα. Probiot Antimicr Proteins. (2019) 11:785–93. doi: 10.1007/s12602-018-9469-9
108. Wang Y, Jiang Y, Deng Y, Yi C, Wang Y, Ding M, et al. Probiotic supplements: hope or hype? Front Microbiol. (2020) 11:160. doi: 10.3389/fmicb.2020.00160
109. Kong Y, Li M, Li R, Shan X, Wang G. Evaluation of cholesterol lowering property and antibacterial activity of two potential lactic acid bacteria isolated from the intestine of snakehead fish (Channa argus). Aquac Rep. (2020) 17:100342. doi: 10.1016/j.aqrep.2020.100342
110. Avella MA, Olivotto I, Silvi S, Ribecco C, Cresci A, Palermo F, et al. Use of Enterococcus faecium to improve common sole (Solea solea) larviculture. Aquaculture. (2011) 315:384–93. doi: 10.1016/j.aquaculture.2011.02.046
111. RodrigÁÑez MASD, AlarcÓn FJ, Cara JB, Moyano FJ, DÍaz-Rosales P, ChabrillÓn M, et al. Effect of dietary administration of probiotics on growth and intestine functionality of juvenile Senegalese sole (Solea senegalensis, Kaup 1858). Aquac Nutr. (2009) 15:177–185. doi: 10.1111/j.1365-2095.2008.00581.x
112. Tapia-Paniagua S, Lobo C, Moreno-Ventas X, de la Banda IG, Moriñigo MA, Balebona MC. Probiotic supplementation influences the diversity of the intestinal microbiota during early stages of farmed Senegalese sole (Solea senegalensis, Kaup 1858). Marine Biotechnol. (2014) 16:716–728. doi: 10.1007/s10126-014-9588-6
113. Batista S, Valente LM, Ramos MA, Rema P, Ozório RO, Pires MA, et al. Immune responses and gut morphology of Senegalese sole (Solea senegalensis, Kaup 1858) fed monospecies and multispecies probiotics. Aquac Nutr. (2015) 12:191. doi: 10.1111/anu.12191
114. Medina A, García-Márquez J, Moriñigo MÁ, Arijo S. Effect of the Potential Probiotic Vibrio proteolyticus DCF12.2 on the Immune System of Solea senegalensis and Protection against Photobacterium damselae subsp piscicida and Vibrio harveyi. Fishes. (2023) 8:344. doi: 10.3390/fishes8070344
115. Campbell A, Buswell J. The intestinal microflora of farmed Dover sole (Solea solea) at different stages of fish development. J Appl Bacteriol. (1983) 55:215–223. doi: 10.1111/j.1365-2672.1983.tb01318.x
Keywords: aquaculture, bile salts, cell adhesion, hemolysis, blue agri-economy
Citation: Hussain N, Mirza AF, Muccee F, Haddad AHIA and Afzal MI (2025) In vitro analysis of probiotic characteristics of gut-associated bacteria from Solea solea. Front. Vet. Sci. 12:1581675. doi: 10.3389/fvets.2025.1581675
Received: 22 February 2025; Accepted: 09 May 2025;
Published: 04 June 2025.
Edited by:
Hosam Elsaied, National Institute of Oceanography and Fisheries (NIOF), EgyptReviewed by:
Mostafa Mohamed El-Sheekh, Tanta University, EgyptMahmoud Mabrok, Suez Canal University, Egypt
Amel Mohamed El Asely, Benha University, Egypt
Copyright © 2025 Hussain, Mirza, Muccee, Haddad and Afzal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fatima Muccee, ZmF0aW1hLnNiYkBwdS5lZHUucGs=
†ORCID: Fatima Muccee orcid.org/0000-0001-8149-6279
 Nadia Hussain1,2
Nadia Hussain1,2 Fatima Muccee
Fatima Muccee