- Department of Animal Reproduction with Clinic, Faculty of Veterinary Medicine, University of Warmia and Mazury in Olsztyn, Olsztyn, Poland
The aim of this article is to provide a comprehensive review of the negative impact of benign prostatic hyperplasia (BPH) on fertility in dogs. BPH is the most common disease of the prostate gland and one of the major causes of infertility in dogs, associated with impaired semen quality. Hormonal imbalance, oxidative stress, biochemical changes in prostatic fluid and haematospermia have been discussed as causes of reduced semen quality in dogs with BPH. Chronic prostatitis often occurs concurrently with BPH and has an additive negative effect on semen quality and fertility. In breeding dogs with infertility associated with BPH, treatment with the anti-androgen osaterone acetate or the 5α-reductase inhibitor finasteride is recommended to reduce prostate size and clinical symptoms and to restore fertility. Combined occurrence of BPH and chronic prostatitis requires simultaneous treatment of both conditions, including microbials with good penetration into the prostate. The use of antioxidants in the supportive treatment of BPH seems reasonable.
1 Introduction
1.1 Anatomy of the canine prostate
The prostate is the only accessory sex gland in the canine genital tract. The function of the prostate is to produce prostatic fluid, which accounts for approximately 90% of the volume of seminal fluid (1). The canine prostate has an oval shape with a bilobed structure that surrounds the proximal part of the urethra at the neck of the bladder and is covered by a fibromuscular capsule (2). It is located in the pelvic cavity or caudal abdomen, depending on its size, dorsal to the pubic symphysis and ventral to the rectum (3). Prostatic fluid is secreted into the urethra through several prostatic ducts. The vas deferens passes through the prostate and enters the urethra (4).
1.2 Benign prostatic hyperplasia
Benign prostatic hyperplasia (BPH) is one of the most important problems in intact male dogs (2, 5). It accounts for more than 50% of prostate disease cases (6, 7). BPH is defined as an increase in the overall size of the prostate caused by hyperplasia (increase in number) and hypertrophy (increase in size) of epithelial cells (3, 6). The prostate volume of affected dogs is 2 to 6.5 times greater than that of normal dogs of similar weight (8).
BPH is an age-related disease, occurring in two thirds of dogs over 5 years of age, but can also be diagnosed in young breeding males (9, 10). DeKlerk (11) reported 25% incidence of BPH in young dogs aged 1–3 years and 88% in dogs aged 5–10 years. According to Berry et al. (12) approximately 80% of sexually intact dogs over 5 years of age and 95% of dogs over 9 years of age have gross or microscopic changes associated with BPH. Canine BPH has no breed predisposition and can occur in any intact male dog (3, 8).
Initially, BPH is often asymptomatic (10). With progressive enlargement of the prostate, sanguineous discharge from the urethra, haematuria, haematospermia, defecation dysfunction and, rarely, dysuria may occur. BPH is often associated with a decrease in libido and fertility (6, 9).
The diagnosis of clinical BPH is usually based on history, clinical examination and ultrasonography. Subclinical BPH can be diagnosed by cytology using fine-needle aspiration or prostate massage or by determination of canine prostate-specific esterase (CPSE) and acid phosphatase (6). Wieszczeczyński et al. (13) proposed micro-RNA-129 (miRNA-129) and vascular endothelial growth factor (VEGF) as new useful biomarkers for the diagnosis of BPH in dogs. Recently, Laurusevičius et al. (14) developed clinical scoring systems for early detection of BPH based on non-invasive techniques such as rectal palpation, ultrasonography and CPSE analysis.
1.3 BPH and infertility in dogs
Infertility in male dogs, defined as the inability to produce offspring, is becoming increasingly important in clinical practice. Little is known about the causes of male infertility. They can be divided into two main groups: congenital infertility and acquired infertility. Congenital infertility is caused by genetic abnormalities and is present at birth. Acquired infertility appears after the animal has been fertile (15). BPH is considered one of the major causes of acquired infertility in male dogs (10, 15–17). One study reported that 32.8% of infertile dogs were diagnosed with BPH (18). However, the mechanisms by which BPH affects canine fertility are not fully understood.
The aim of this article is to provide a comprehensive review of the impact of BPH on fertility in male dogs.
2 Pathogenesis of BPH
The pathogenesis of BPH is most likely multifactorial. BPH is thought to develop primarily under the influence of the androgen metabolite dihydrotestosterone (DHT) (19, 20). With age, testosterone concentrations decrease and estrogen concentrations increase. The altered ratio of estrogen to testosterone leads to increased concentration of the androgen receptor and increased conversion of testosterone to DHT by 5-α-reductase in the prostate (21, 22). DTH stimulates enlargement of the canine prostate by increasing the growth of glandular epithelial cells and, to a lesser extent, stromal cells (10). BPH begins as glandular hyperplasia and progresses to cystic hyperplasia with the formation of multiple small cysts in the parenchyma (12, 20). The vascularity of the prostate tissue increases, which may lead to haemorrhagic discharge from the urethra that is not associated with urination (23, 25).
Recently, oxidative stress has been suggested to play a potential role in the pathogenesis of canine BPH (25, 26). Oxidative stress is an imbalance between the production and scavenging of reactive oxygen species (ROS) (27). There is evidence that age-related hormonal changes induce a chronic inflammatory response in the prostate, leading to the accumulation of immunocompetent cells such as macrophages and neutrophils in the prostate (28, 29). This leads to excessive generation of ROS and oxidative stress. Oxidative DNA damage induces hyperplastic transformation of prostate cells (30, 31).
3 Effect of BPH on fertility
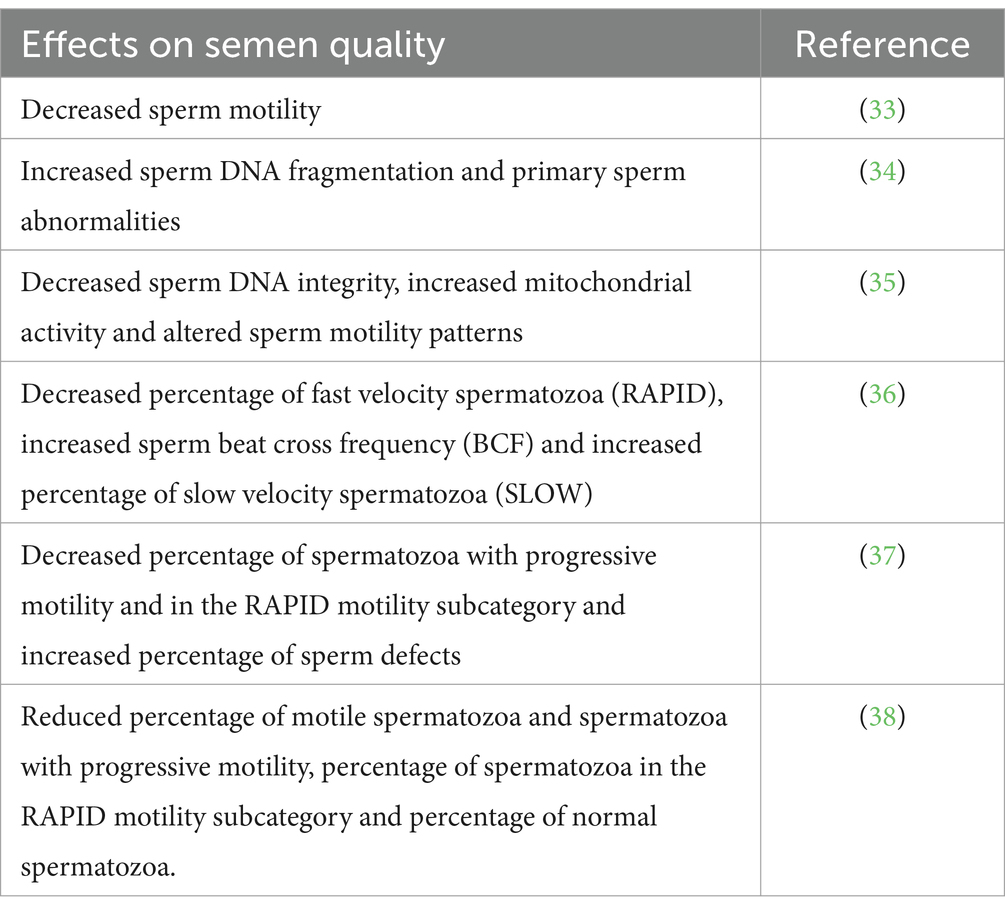
BPH does not necessarily lead to infertility, but this condition is one of the leading causes of infertility in male dogs (10, 15–17). This is usually associated with a decrease in semen quality. There are relatively few studies on the effect of BPH on semen quality in dogs (Table 1).
Fila and Berglavaz (33) reported significant decrease in sperm motility in dogs with BPH. No significant changes in standard semen parameters were found, but a significant increase in sperm DNA defragmentation and an increased percentage of spermatozoa with primary defects formed during spermatogenesis were found in dogs with BPH compared to healthy dogs. The most common morphological defect observed was the Dag defect (circular, coiled tails) (34). Flores et al. (35) reported that BPH decreases sperm DNA integrity, increases mitochondrial activity and alters sperm motility patterns. In a study by Angrimani et al. (36), dogs with BPH showed a lower percentage of fast velocity spermatozoa (RAPID), a higher sperm beat cross frequency (BCF) and a higher percentage of slow velocity spermatozoa (SLOW) than in healthy dogs. A lower percentage of spermatozoa with progressive motility and in the RAPID motility subcategory and a higher percentage of sperm defects were observed in dogs with BPH compared to healthy dogs (37). The mean percentage of motile spermatozoa and spermatozoa with progressive motility, the percentage of spermatozoa in the RAPID motility subcategory and the percentage of normal spermatozoa were significantly lower in dogs with BPH than in healthy dogs (38). The changes described above in spermatozoa of dogs with BPH reduce their fertilizing ability and impair dog fertility.
BPH is most common in older dogs. It is generally accepted that aging has a negative effect on semen quality (37, 39). However, the decrease in semen quality in dogs with BPH is significantly greater than in healthy dogs of similar age (37, 38).
The mechanisms underlying the effects of BPH on semen quality are not fully understood. Hormonal imbalance, oxidative stress, biochemical changes in prostatic fluid and haematospermia have been discussed as possible causes of reduced semen quality in dogs with BPH (Figure 1).

Figure 1. Possible mechanisms for the effect of BPH on semen quality. Reduced testosterone levels in dogs with BPH can impair spermatogenesis. A chronic inflammatory response in prostate induced by age depended hormonal changes results in the generation of large amounts of ROS. Oxidative stress in BPH dogs can cause sperm cell damage. BPH can lead to changes in the biochemical composition of the seminal plasma, resulting in reduced semen quality. The frequent presence of blood in semen of BPH dogs can adversely affect semen quality.
BPH is linked to hormonal changes associated with aging. Testosterone plays an essential role in maintaining spermatogenesis (40). Lower testosterone levels have been found in dogs with BPH than in healthy dogs (32, 36, 41).
BPH is associated with oxidative stress and the accumulation of ROS can induce sperm cell damage, resulting in decreased sperm motility, velocity and morphological integrity (27, 35). Dearakhshandeh et al. (42) reported a decrease in antioxidant enzyme activities in the blood serum of dogs induced for BPH by testosterone and estradiol. Lower total serum antioxidant activity was observed in dogs with BPH compared to healthy dogs (25). A decrease in total antioxidant capacity and an increase in protein oxidation in prostatic fluid and spermatozoa were observed in dogs with BPH (43). Dogs with BPH had a higher proportion of spermatozoa producing nitric oxide, a subclass of ROS, than control dogs (38).
Prostatic fluid accounts for approximately 90% of the seminal fluid volume and provides transport and support for sperm (1). Normal prostatic fluid is clear and slightly acidic (pH 6.15–6.5) and contains various electrolytes, minerals, cholesterol, bicarbonate, fructose, lactic acid and proteins (19, 44–46). Canine prostate specific esterase accounts for more than 90% of prostate fluid proteins (44). BPH can cause changes in the biochemical composition of the seminal plasma, resulting in reduced semen quality. In dogs with BPH, a higher pH of the prostate fluid, reduced zinc and copper concentrations, and increased cholesterol concentrations have been observed (34). Ferré-Dolcet et al. (1) reported low concentrations of zinc in the prostatic fluid of dogs with BPH. In a study by Aquino-Cortez et al. (44), concentrations of glucose, cholesterol and triglycerides in prostatic fluid were higher in dogs with BPH than in healthy dogs.
Haematospermia is common in dogs with BPH. The hyperplastic gland has increased vascularity, which results in vascular leakage or hemorrhage into the gland and the blood is present in prostatic fluid and ejaculate (23). The effect of the presence of blood in semen on fertility in dogs is controversial. It is generally accepted that haematospermia may reduce sperm longevity and is associated with infertility (20, 47). However, dogs with some blood in the ejaculate may be fertile (15, 16). Rijsselaere et al. (48) showed that blood admixtures of up to 10% had no negative effect on the functional characteristics of chilled canine spermatozoa. However, blood admixtures of 2% or more in an ejaculate negatively affected semen parameters after freezing and thawing.
BPH predisposes to mainly chronic, rarely acute, bacterial infections of the prostate (3, 9, 24). Chronic bacterial prostatitis often occurs at the same time as BPH and has an additive negative effect on semen quality and fertility (10, 15, 24). It results mainly from an ascending infection from the urinary tract, and less frequently from haematogenous spread. The symptoms of chronic prostatitis are similar to those of BPH. Chronic prostatitis is often an accidental finding when infertility or recurrent cystitis are investigated (6). Diagnosis requires bacteriological and/or cytological examination of prostate fluid or prostate tissue obtained by ultrasound-guided biopsy or fine needle aspiration. Urine culture is also a reliable diagnostic tool (6, 49). The most common isolate in bacterial prostatitis is Escherichia (E.) coli. In addition to E. coli, other opportunistic bacteria ascending from the urethra such as Staphylococcus sp., Pseudomonas sp., Klebsiella sp., Proteus sp., Enterobacter sp., Pasteurella sp., Hemophilus sp., Streptococcus sp. and Ureaplasma sp. can cause bacterial prostatitis (49).
Chronic prostatitis has a high rate of recurrence and may progress to acute prostatitis or prostatic abscessation. Acute prostatitis can cause severe symptoms such as fever, apathy, abdominal pain, vomiting, dysuria, obstipation and unwilligness to breed. The prostate is painfull. A frequent symptom in dogs is purulent-bloody preputial discharge (4, 23, 49). In case the of an abscess the prostate is greatly enlarged and fluctuation is typical (10). The diagnosis is generally made with ultrasonography of the prostate, in combination with cytological and bacteriological examination of prostatic fluid (3, 6).
4 Treatment of BPH to restore fertility
There are many different methods of treating BPH in dogs. In non-breeding dogs, surgical or pharmacological castration is the treatment of choice. In breeding dogs, treatment with pharmacological agents that inhibit the production or activity of androgens is preferred in order to preserve fertility (9, 10, 24). The antiandrogen approved for the treatment of BPH in dogs is osaterone acetate (OA), which reduces the uptake of DHT in the prostate, decreases 5α-reductase activity and suppresses DHT receptor expression in prostate tissue (48). OA is available in four tablet strengths suitable for dogs weighing 3 kg to 60 kg, given orally at a dose of 0.25–0.5 mg/kg, once a day, for 7 days. Treatment with OA for 7 days rapidly reduces clinical signs and prostate volume in dogs with BPH (32, 50–52). The clinical response persists for approximately 5 months after treatment but, in some dogs, relapse of clinical signs may occur earlier (32, 51). OA had no effect on libido, but resulted in a transient decrease in seminal plasma volume, percentage of motile and progressive spermatozoa, and an increase in sperm concentration (32). Dogs treated with OA remain fertile and can still be used for mating (32, 53).
Another drug that may be considered for the treatment of BPH in breeding dogs is the 5α-reductase inhibitor finasteride, which reduces the conversion of testosterone to DHT. Treatment with finasteride at a dose of 0.2 mg/kg for 3–4 months reduced prostate size (54–56). Serum DHT concentration decreased without affecting testosterone concentration (36, 56, 57). During treatment, ejaculate volume decreased, resulting in an increase in sperm concentration (54, 55, 56). Finasteride has no effect on sperm DNA integrity (58). Fertility is not affected by treatment with finasteride (54, 55). However, finasteride is not approved in EU for use in dogs.
In the case of combined BPH and prostatitis, simultaneous treatment of both conditions is required. Treatment should also include antimicrobial agents with a good ability to penetrate prostate tissue, such as fluoroquinolones, erythromycins, clindamycin and chloramphenicol for 4–6 weeks (10, 24, 49). Prostatic abscesses require drainage and omentalization in addition to antimicrobial therapy (6, 23).
Oxidative stress plays a role in the pathogenesis of BPH and infertility. It may be increased by antioxidant deficiency. In our previous study, suboptimal Se and vitamin E status was found in male dogs with reduced fertility (59). Therefore, the supportive use of antioxidants in the treatment of BPH seems justified. Several studies have reported positive effects of selenium and vitamin E (59–61) and polyphenol (62, 63) supplementation on oxidative status and semen quality in dogs. Further studies on the usefulness of antioxidants in the supportive treatment of BPH are needed.
5 Conclusion
BPH is one of the major causes of infertility in male dogs. It is associated with reduced semen quality. Hormonal imbalance, oxidative stress, biochemical changes in prostatic fluid and haematospermia may contribute to this decline. Chronic bacterial prostatitis often co-occurs with BPH and has an additive negative effect on semen quality and fertility. In breeding dogs with infertility following BPH, treatment with the anti-androgen osaterone acetate or the 5α-reductase inhibitor finasteride is recommended to restore fertility. In the case of combined BPH and prostatitis, treatment should also include antimicrobial agents with a good ability to penetrate prostate tissue. The adjunctive use of antioxidants in the treatment of BPH seems justified.
Author contributions
AD-W: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. SZ: Conceptualization, Writing – original draft, Writing – review & editing. AR: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funded by the Minister of Science under the Regional Initiative of Excellence Program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ferré-Dolcet, L, Frigotto, L, Contiero, B, Bedin, S, and Romagnoli, S. Prostatic fluid composition and semen quality in dogs with benign prostatic hyperplasia undergoing treatment with osaterone acetate. Reprod Domest Anim. (2022) 57:72–9. doi: 10.1111/rda.14030
2. Sun, F, Baez-Diaz, C, and Sanches-Margallo, FM. Canine prostate models in preclinical studies of minimally invasive interventions: part II, benign prostatic hyperplasia models. Transl Androl Urol. (2017) 6:547–55. doi: 10.21037/tau.2017.03.62
3. Smith, J. Canine prostatic disease: a review of anatomy, pathology, diagnosis, and treatment. Theriogenology. (2008) 70:375–83. doi: 10.1016/j.theriogenology.2008.04.039
4. Leis-Filho, AF, and Fonseca-Alves, C. Anatomy, histology, and physiology of the canine prostate gland. London: IntechOpen (2019).
5. Ryman-Tubb, T, Lothion-Roy, JH, Metzler, VM, Harris, AE, Robinson, BD, Rizvanov, AA, et al. Comparative pathology of dog and human prostate cancer. Vet Med Sci. (2022) 8:110–20. doi: 10.1002/vms3.642
6. Lévy, X, Niżański, W, von Heimendahl, A, and Mimouni, P. Diagnosis of common prostatic conditions in dogs: an update. Reprod Domest Anim. (2014) 49:50–7. doi: 10.1111/rda.12296
7. Polisca, A, Troisi, A, Fontaine, E, Menchetti, L, and Fontbonne, A. A retrospective study of canine prostatic diseases from 2002 to 2009 at the Alfort veterinary college in France. Theriogenology. (2016) 85:835–40. doi: 10.1016/j.theriogenology.2015.10.030
8. Johnston, SD, Kamolpatana, K, Root-Kustritz, MV, and Johnston, GR. Prostatic disorders in the dog. Anim Reprod Sci. (2020) 60-61:405–15. doi: 10.1016/s0378-4320(00)00101-9
9. Cunto, M, Ballotta, G, and Zambelli, D. Benign prostatic hyperplasia in the dog. Anim Reprod Sci. (2022) 247:107096. doi: 10.1016/j.anireprosci.2022.107096
11. DeKlerk, DP, Coffey, DS, Ewing, LL, McDermott, IR, Reiner, WG, Robinson, CH, et al. Comparison of spontaneous and experimentally induced canine prostatic hyperplasia. J Clin Invest. (1979) 64:842–9.
12. Berry, SJ, Strandberg, JD, Saunders, WJ, and Coffey, DS. Development of canine benign prostatic hyperplasia with age. Prostate. (1986) 9:363–73. doi: 10.1002/pros.2990090406
13. Wieszczeczyński, M, Krakowski, L, Opielak, G, Krakowska, I, Furmaga, J, Brodzki, P, et al. MicroRNA and vascular endothelial growth factor (VEGF) as new useful markers in the diagnosis of benign prostatic hyperplasia in dogs. Theriogenology. (2021) 171:113–8. doi: 10.1016/j.theriogenology.2021.05.017
14. Laurusevičius, T, Šiugždaitė, J, Juodžiukynienė, N, Kerzienė, S, Anskienė, L, Jackutė, V, et al. Comparative evaluation of methods for subclinical benign prostatic hyperplasia in intact breeding male dogs. Animals (Basel). (2024) 14:1204. doi: 10.3390/ani14081204
15. Fontbonne, A. Infertility in male dogs: recent advances. Rev Bras Reprod Anim. (2011) 35:266–73.
16. Romagnoli, S. Two common causes of infertility in the male dog In: M Svoboda, editor. Proceedings of 31st world small animal veterinary association, October 11–14. Prague: Czech Republic (2006). 687–90.
17. Memon, MA. Common causes of male dog infertility. Theriogenology. (2007) 68:322–8. doi: 10.1016/j.theriogenology.2007.04.025
18. Domosławska, A, and Zduńczyk, S. Clinical and spermatological findings in male dogs with acquired infertility: a retrospective analysis. Andrologia. (2020) 52:e13802. doi: 10.1111/and.13802
19. Barsanti, JA, and Finco, DR. Canine prostatic diseases. Vet Clin North Am Small Anim Pract. (1986) 16:587–99. doi: 10.1016/s0195-5616(86)50063-2
20. Gobello, C, and Corrada, Y. Noninfectious prostatic diseases in dogs. Compend Contin Educ Vet. (2002) 2:99–107.
21. Meikle, AW, Collier, ES, Stringham, JD, Fang, SM, and Taylor, GN. Elevated intranuclear dihydrotestosterone in prostatic hyperplasia of aging dogs. J Steroid Biochem. (1981) 14:331–5. doi: 10.1016/0022-4731(81)90150-3
22. Tunn, S, Hochstrate, H, Habenicht, UF, and Krieg, M. 5 alpha-reductase activity in epithelium and stroma of prostates from intact and castrated dogs treated with androstenedione, the aromatase inhibitor 1-methyl-1,4-androstadiene-3,17-dione, and cyproterone acetate. Prostate. (1988) 12:243–53. doi: 10.1002/pros.2990120307
23. Lopate, CH. Clinical approach to conditions of the male In: G England and A Von Heimendahl, editors. BSAVA manual of canine and feline reproduction and neonatology. 2nd ed. Gloucester, UK: BSAVA (2010). 191–211.
24. Niżański, W, Levy, X, Ochota, M, and Pasikowska, J. Pharmacological treatment for common prostatic conditions in dogs - benign prostatic hyperplasia and prostatitis: an update. Reprod Domest Anim. (2014) 49:8–15. doi: 10.1111/rda.12297
25. Domosławska, A, Zduńczyk, S, Kankofer, M, and Bielecka, A. Oxidative stress biomarkers in dogs with benign prostatic hyperplasia. Ir Vet J. (2022) 75:21. doi: 10.1186/s13620-022-00228-3
26. Domosławska-Wyderska, A, Zduńczyk, S, and Rafalska, A. Potential role of oxidative stress in pathogenesis of benign prostatic hyperplasia in male dogs. Reprod Domest Anim. (2024) 59:e14580. doi: 10.1111/rda.14580
27. Agarwal, A, Virk, G, Ong, C, and Du Plessis, SS. Effect of oxidative stress on male reproduction. World J Mens Health. (2014) 32:1–17. doi: 10.5534/wjmh.2014.32.1.1
28. Mahapokai, W, van den Ingh, TS, van Mil, F, van Garderen, E, Schalken, JA, Mol, JA, et al. Immune response in hormonally-induced prostatic hyperplasia in the dog. Vet Immunol Immunopathol. (2001) 78:297–303. doi: 10.1016/s0165-2427(01)00236-7
29. Palmieri, C, Hood, G, Fonseca-Alves, CE, Laufer-Amorim, R, and Allavena, R. An immunohistochemical study of T and B lymphocyte density in prostatic hyperplasia and prostate carcinoma in dogs. Res Vet Sci. (2019) 122:189–92. doi: 10.1016/j.rvsc.2018.11.022
30. Vital, P, Castro, P, and Ittmann, M. Oxidative stress promotes benign prostatic hyperplasia. Prostate. (2016) 76:58–67. doi: 10.1002/pros.23100
31. Roumeguère, T, Sfeir, J, El Rassy, E, Albisinni, S, Van Antwerpen, P, Boudjeltia, KZ, et al. Oxidative stress and prostatic diseases. Mol Clin Oncol. (2017) 7:723–8. doi: 10.3892/mco.2017.1413
32. Niżański, W, Ochota, M, Fontaine, C, and Pasikowska, J. Comparison of clinical effectiveness of Deslorelin acetate and Osaterone acetate in dogs with benign prostatic hyperplasia. Animals (Basel). (2020) 10:1936. doi: 10.3390/ani10101936
33. Fila, D, and Berglavaz, A. Benign prostatic hyperplasia in aged dogs affects semen gross and individuality motility. Proceedings of 7th International Symposium On Canine and Feline Reproduction and 15th Congress of the European Veterinary Society for Small Animal Reproduction, July 26–29, Whistler, Canada. (2012).
34. Krakowski, L, Wąchocka, A, Brodzki, P, Wrona, Z, Piech, T, and Wawron, W. Sperm quality and selected biochemical parameters of seminal fluid in dogs with benign prostatic hyperplasia. Anim Reprod Sci. (2015) 160:120–5. doi: 10.1016/j.anireprosci.2015.07.014
35. Flores, RB, Angrimani, DSR, Rui, BR, Brito, MM, Abreu, RA, and Vannucchi, CI. The influence of benign prostatic hyperplasia on sperm morphological features and sperm DNA integrity in dogs. Reprod Domest Anim. (2017) 52:310–5. doi: 10.1111/rda.12817
36. Angrimani, DSR, Brito, MM, Rui, BR, Nichi, M, and Vannucchi, CI. Reproductive and endocrinological effects of benign prostatic hyperplasia and finasteride therapy in dogs. Sci Rep. (2020) 10:14834. doi: 10.1038/s41598-020-71691-7
37. Niżański, W, Eberhardt, M, Ochota, M, Fontaine, C, Levy, X, and Pasikowska, JA. Comparative study of the effects of Osaterone acetate and Deslorelin acetate on sperm kinematics and Morpho-functional parameters in dogs. Animals (Basel). (2022) 12:1548. doi: 10.3390/ani12121548
38. Domosławska-Wyderska, A, Orzołek, A, Zduńczyk, S, and Rafalska, A. Nitric oxide production by spermatozoa and sperm characteristics in dogs with benign prostatic hyperplasia. Pol J Vet Sci. (2023) 26:621–8. doi: 10.24425/pjvs.2023.148281
39. Rijselaere, T, Maes, D, Hoflack, G, de Kruif, A, and Van Soom, A. Effect of body weight, age and breeding history on canine sperm quality parameters measured by the Hamilton-Thorne analyser. Reprod Domest Anim. (2007) 42:143–8. doi: 10.1111/j.1439-0531.2006.00743.x
40. Ramaswamy, S, and Weinbauer, GF. Endocrine control of spermatogenesis: role of FSH and LH/testosterone. Spermatogenesis. (2015) 4:e996025. doi: 10.1080/21565562.2014.996025
41. Cochran, RC, Ewing, LL, and Niswender, GD. Serum levels of follicle stimulating hormone, luteinizing hormone, prolactin, testosterone, 5 alpha-dihydrotestosterone, 5 alpha-androstane-3 alpha, 17 beta-diol, 5 alpha-androstane-3 beta, 17 beta-diol, and 17 beta-estradiol from male beagles with spontaneous or induced benign prostatic hyperplasia. Investig Urol. (1981) 19:142–7.
42. Dearakhshandeh, N, Mogheiseh, A, Nazifi, S, Ahrari Khafi, MS, Abbaszadeh Hasiri, M, and Golchin-Rad, K. Changes in the oxidative stress factors and inflammatory proteins following the treatment of BPH induced dogs with an anti-proliferative agent called tadalafil. J Vet Pharmacol Ther. (2019) 42:665–72. doi: 10.1111/jvp.12805
43. Domosławska, A, Zduńczyk, S, Bielecka, A, and Kankofer, M. The effect of benign prostatic hyperplasia on total antioxidant capacity and protein peroxidation in canine prostatic fluid and spermatozoa. Pol J Vet Sci. (2023) 26:667–73. doi: 10.24425/pjvs.2023.148286
44. Aquino-Cortez, A, Pinheiro, BQ, Lima, DBC, Silva, HVR, Mota-Filho, AC, Martins, JAM, et al. Proteomic characterization of canine seminal plasma. Theriogenology. (2017) 95:178–86. doi: 10.1016/j.theriogenology.2017.03.016
45. Umbach, AK, Failing, K, Goericke-Pesch, S, and Wehrend, A. Concentrations of minerals in the canine prostatic fluid. Reprod Domest Anim. (2019) 54:1064–8. doi: 10.1111/rda.13467
46. Chapdelaine, P, Dubé, JY, Frenette, G, and Tremblay, RR. Identification of arginine esterase as the major androgen-dependent protein secreted by dog prostate and preliminary molecular characterization in seminal plasma. J Androl. (1984) 5:206–2010. doi: 10.1002/j.1939-4640.1984.tb02395
47. England, GC, and Allen, WE. Factors affecting the viability of canine spermatozoa. II. Effects of seminal plasma and blood. Theriogenology. (1992) 37:373–81. doi: 10.1016/0093-691X(92)90195-W
48. Rijsselaere, T, Van Soom, A, Maes, D, Verberckmoes, S, and de Kruif, A. Effect of blood admixture on in vitro survival of chilled and frozen-thawed canine spermatozoa. Theriogenology. (2004) 61:1589–602. doi: 10.1016/j.theriogenology.2003.09.008
49. Kutzler, M. Reproductive microbiome alterations: canine prostatitis. Clin Theriogenol. (2019) 11:649–58. doi: 10.58292/ct.v11.9467
50. Takezawa, Y, Fukabori, Y, Yamanaka, H, Mieda, M, Honma, S, Kushitani, M, et al. Effects of the new steroidal antiandrogen TZP-4238 on hormone-induced canine prostatic hyperplasia. Prostate. (1992) 21:315–29. doi: 10.1002/pros.2990210408
51. Albouy, M, Sanquer, A, Maynard, L, and Eun, HM. Efficacies of osaterone and delmadinone in the treatment of benign prostatic hyperplasia in dogs. Vet Rec. (2008) 163:179–83. doi: 10.1136/vr.163.6.179
52. Socha, P, Zduńczyk, S, Tobolski, D, and Janowski, T. The effects of osaterone acetate on clinical signs and prostate volume in dogs with benign prostatic hyperplasia. Pol J Vet Sci. (2018) 21:797–566. doi: 10.24425/pjvs.2018.125601
53. Tsutsui, T, Hori, T, Shimizu, M, Tatsuzawa, C, and Kawakami, E. Effect of osaterone acetate administration on prostatic regression rate, peripheral blood hormone levels and semen quality in dogs with benign prostatic hypertrophy. J Vet Med Sci. (2001) 63:453–6. doi: 10.1292/jvms.63.453
54. Iguer-Ouada, M, and Verstegen, JP. Effect of finasteride (Proscar MSD) on seminal composition, prostate function and fertility in male dogs. J Reprod Fertil. (1997) 51:139–49.
55. Sirinarumitr, K, Johnston, SD, Kustritz, MV, Johnston, GR, Sarkar, DK, and Memon, MA. Effects of finasteride on size of the prostate gland and semen quality in dogs with benign prostatic hypertrophy. J Am Vet Med Assoc. (2001) 218:1275–80. doi: 10.2460/javma.2001.218.1275
56. Strzeżek, R, Janowski, T, and Barański, W. Effect of finasteride on the functioning of the prostate gland and semen quality (in polish). Med Weter. (2004) 60:211–4.
57. Kamolpatana, K, Johnston, SD, Hardy, S, and Castner, S. Effect of finasteride on serum concentrations of dihydrotestosterone and testosterone in three clinically normal sexually intact adult male dogs. Am J Vet Res. (1998) 59:762–4. doi: 10.2460/ajvr.1998.59.06.762
58. Angrimani, DSR, Bicudo, LC, Llamas Luceño, N, Rui, BR, Silva, MF, Losano, JDA, et al. Does finasteride treatment for benign prostatic hyperplasia influence sperm DNA integrity in dogs? Basic Clin Androl. (2020) 30:9. doi: 10.1186/s12610-020-00108-2
59. Domosławska, A, Zduńczyk, S, Franczyk, M, Kankofer, M, and Janowski, T. Selenium and vitamin E supplementation enhances the antioxidant status of spermatozoa and improves semen quality in male dogs with lowered fertility. Andrologia. (2018) 50:e13023. doi: 10.1111/and.13023
60. Kawakami, E, Kobayashi, M, Hori, T, and Kaneda, T. Therapeutic effects of vitamin E in 4 dogs with poor semen quality and low superoxide dismutase activity in seminal plasma. J Vet Med Sci. (2015) 77:1711–4. doi: 10.1292/jvms.15-0294
61. Alonge, S, Melandri, M, Leoci, R, Lacalandra, GM, Caira, M, and Aiudi, GG. The effect of dietary supplementation of vitamin E, selenium, zinc, folic acid, and N-3 polyunsaturated fatty acids on sperm motility and membrane properties in dogs. Animals (Basel). (2019) 9:34. doi: 10.3390/ani9020034
62. Tufarelli, V, Rizzo, A, Lacalandra, GM, Guaricci, AC, Laudadio, V, and Valentini, L. Effects of the supplementation with an high-polyphenols extra-virgin olive oil on kinetic sperm features and seminal plasma oxidative status in healthy dogs. Reprod Domest Anim. (2018) 53:582–7. doi: 10.1111/rda.13145
Keywords: dog, benign prostatic hyperplasia, fertility, semen quality, prostate
Citation: Domosławska-Wyderska A, Zduńczyk S and Rafalska A (2025) Negative impact of benign prostatic hyperplasia on fertility in dogs–a mini-review. Front. Vet. Sci. 12:1582705. doi: 10.3389/fvets.2025.1582705
Edited by:
Amal M. Aboelmaaty, National Research Centre (Egypt), EgyptReviewed by:
Ellen Leonel, Université Catholique de Louvain, BelgiumLeszek Krakowski, University of Life Sciences in Lublin, Poland
Copyright © 2025 Domosławska-Wyderska, Zduńczyk and Rafalska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Domosławska-Wyderska, YW5uYS5kb21vc2xhd3NrYUBnbWFpbC5jb20=
 Anna Domosławska-Wyderska
Anna Domosławska-Wyderska Sławomir Zduńczyk
Sławomir Zduńczyk Agata Rafalska
Agata Rafalska