- 1Department of Veterinary Pathology, Faculty of Veterinary Medicine, Kasetsart University, Nakhon Pathom, Thailand
- 2Faculty of Veterinary Medicine, Kamphaeng Saen Veterinary Diagnostic Center, Kasetsart University, Nakhon Pathom, Thailand
- 3Department of Veterinary Public Health, Faculty of Veterinary Medicine, Kasetsart University, Nakhon Pathom, Thailand
Introduction: A retrospective study of non-typhoidal Salmonella isolation from poultry and pig farms in Nakhon Pathom and Suphan Buri provinces was conducted from 2008 to 2015. The aim of study was to examine the prevalence of antimicrobial resistance and class I integrons related to gene resistance of Salmonella in livestock and its environment.
Methods: A total of 636 Salmonella isolates was collected from livestock and environmental samples. The isolates included 1.42% S. Typhimurium, 4.40% S. Enteritidis, and 1.26% S. Virchow; however, neither S. Infantis nor S. Hadar were found. All Salmonella isolates was tested for antimicrobial susceptibility and minimum inhibitory concentrations (CLSI Vet03-S2 2014, NCCLS standard).
Results: The top three drug resistances were to cephalexin, gentamicin, and amoxicillin. S. Typhimurium showed resistance rates of 100%, 100%, and 22.22% to these antibiotics, respectively; S. Enteritidis showed resistance rates of 100%, 100%, and 90.91%; and S. Virchow revealed resistance at the rates of 50%, 50%, 12.50%, respectively. The conserved segment integrase 1 and gene cassette were found by polymerase chain reaction (PCR) in all serotypes. The resistance gene of aadb, IntI1, aac(6′)-la, aac(6′)-lb, blaPSE-1, CmlA, Sul, dfrA1, A10 and A12 were not detected from S. Typhimurium and fewer resistance genes were detected when compared to the other two subtypes.
Discussion: These findings could be used to set up the prevention and control strategies for addressing future antimicrobial resistance of Salmonella, which remains a major food safety concern.
Introduction
Salmonella spp. is a Gram-negative, non-spore-forming bacterium in the Enterobacteriaceae family. It is facultative anaerobe, digests glucose, generates hydrogen sulfide, but cannot ferment lactose. Salmonella is a mesophilic bacterium and grows well between 35–42°C, however, the lowest temperature could be growth at 5.2°C and it could be survived within a pH range of 4.5–9.0 (1). Salmonella spp. is a significant bacterium in veterinary public health because it causes Salmonellosis, a serious gastrointestinal disease that affects populations worldwide, including Thailand and South Asia (2, 3). It causes of foodborne illness worldwide and global public health impact. In case that the multidrug resistance (MDR) happened in Salmonella, it has been exacerbating with the limitation of disease treatment options (4). As indicated in the “Thailand Disease Outbreak Laws 2015,” Salmonella is categorized as an outbreak livestock disease affecting both humans and animals. The most common serovar causing human salmonellosis in Thailand was Salmonella Enterica Weltevleden, the data from 1993 until 2002 (5). Non-host specific serovars such as Salmonella Enteritidis (S. enteritidis), S. Typhimurium, S. Hadar, S. infantis, and S. Virchow cause non-typhoidal Salmonella (NTS) diseases in animals, including cows, buffaloes, pigs, ducks, chickens and Tilapia fish (6). Infected animals may be asymptomatic carriers, transmitting the disease through food products to consumers. Infected patients may experience gastrointestinal tract infections and, in some cases, bacteremia, particularly in children, senior adults, or immunocompromised individuals, alongside antimicrobial resistance (7). Currently, antibiotic resistance in meats and animal products is an escalating problem impacting human health due to the extensive use of antibiotics in livestock for disease control, prevention, treatment, and growth promoters. Reports indicate antimicrobial resistance in swine farms to antibiotics such as Ampicillin, Tetracycline, Streptomycin, Sulfamethoxazole plus Trimethoprim, and Chloramphenicol especially for tetA of tetracycline resistance isolates (8), and similar resistance patterns have been observed in poultry farms (9, 10). Salmonella isolates from Tilapia showed resistance rates of 5.5% for Ceftazidime, Chloramphenicol, Meropenem, Nitrofurantoin and Streptomycin and 22.2% to Penicillin-G in the fish sample (6). There was also reported of MDR from 3 of Salmonella spp. and 2 of E coli isolated from this study.
The antimicrobial resistance of Salmonella spp. is increasing, leading to multidrug resistance. Resistance genes can be transferred to other bacteria via important resistance mechanisms, notably through class 1 integrons, which contain multiple resistance genes in the form of gene cassettes. These integrons, housing over 100 antimicrobial resistance genes (11), result in multidrug resistance patterns in Salmonella. Additionally, mobile genetic elements facilitate the transfer of resistance gene cassettes between similar and different bacterial species. The use of antimicrobials in livestock can thus lead to outbreaks of multidrug-resistant genes. As Thailand exports significant quantities of livestock products, stringent disease control and prevention measures, particularly against Salmonella spp., are necessary (7, 12). The purpose of this study is to conduct a retrospective analysis of antimicrobial resistance patterns of Salmonella spp. and their functions on class 1 integrons from samples collected from livestock. This study will provide valuable data to mitigate the economic and public health impact of Salmonella outbreaks in Thailand.
Materials and methods
Sample collection
The retrospective study utilized Salmonella isolation data collected from livestock and their environments in the Nakhon Pathom and Suphan Buri provinces in the western region of Thailand from 2008 to 2015. Samples were collected from six swine farms and seven poultry farms (six duck farms and one broiler farm), with the total of 630 isolated samples. Bacterial isolation was performed using cloacal swabs, soil, and water samples collected from the farms. Among these, 86 samples were from cleaning water before and after use, 39 samples from piglets’ floors, 24 samples from piglets, 18 soil samples, and 4 samples from drinking water collected before and after treatment. Additionally, 544 cloacal swabs were collected from poultry farms, including 523 samples from duck farms and 21 samples from the broiler farm.
Bacterial isolation and identification
Salmonella was isolated using the standard methods outlined in ISO 6579:2002, which identifies non-host-specific serovars such as S. Typhimurium, S. enteritidis, S. Virchow, S. infantis and S. Hadar. The collected samples were initially placed in lactose broth and incubated at 37°C for 18–24 h. They were then transferred to Tetrathionate broth and Rappaport Vassiliadis medium (RVS), followed by plating on Xylose-lysine Deoxycholate agar (XLD) and Brilliant Green Phenol Red Lactose Sucrose agar (BPLS) and incubated at 37°C for 18–24 h. Subsequently, isolated colonies of specific Salmonella were selected and tested on Triple Sugar Iron agar (TSI), subjected to the Urease test and L-lysine decarboxylase test at 37°C for 18–24 h, and then examined with a Slide Agglutination test using poly OH antigen for Salmonella spp. serovars identification. The purified Salmonella colonies were stored in 1.5 mL of skim milk at −20°C until further analysis.
Antibiotic sensitivity testing
The antibiotic sensitivity testing for Salmonella spp. was conducted by determining the Minimum inhibitory concentration (MIC) using a VITEK II machine, operated according to the CLSI Vet03-S2 2014 NCCLS standard (The National Committee for Clinical Laboratory Standards). This testing included 16 antibiotics: Amikacin, Amoxicillin/Clavulanic Acid, Ampicillin, Cefalexin, Cefovecin, Cefpodoxime, Ceftiofur, Chloramphenicol, Enrofloxacin, Gentamicin, Imipenem, Marbofloxacin, Nitrofurantoin, Piperacillin, Polymyxin B, Rifampicin, Tetracycline, Tobramycin, and Trimethoprim/Sulfamethoxazole. The frequency comparison was tested using Fisher’s exact test with the statistically significant difference at (p < 0.05) by GraphPad Prism Statistical Software, version 5.01, 2007 (GraphPad Software, Institute., USA.).
The study of genotypic resistance gene of Salmonella spp.
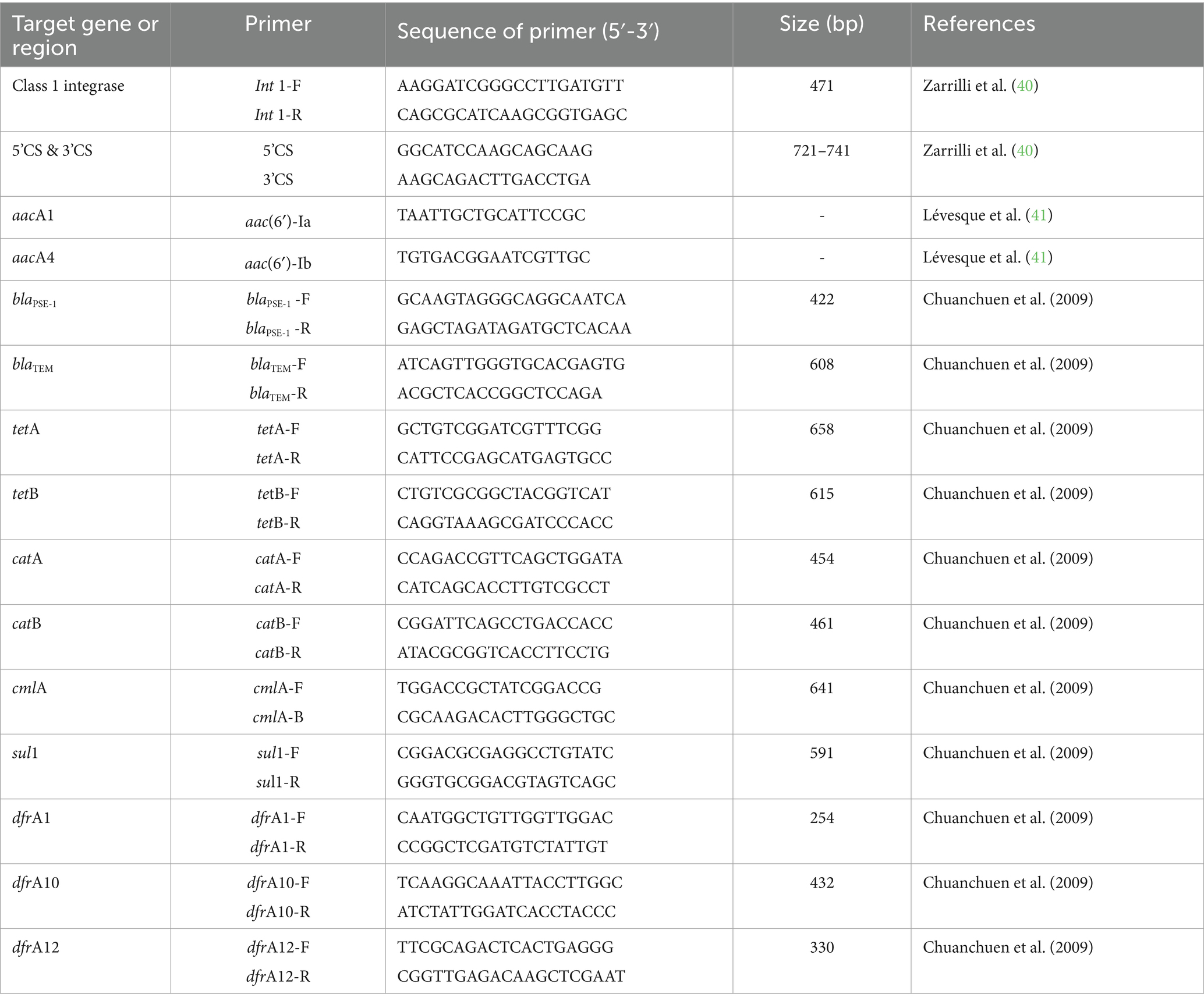
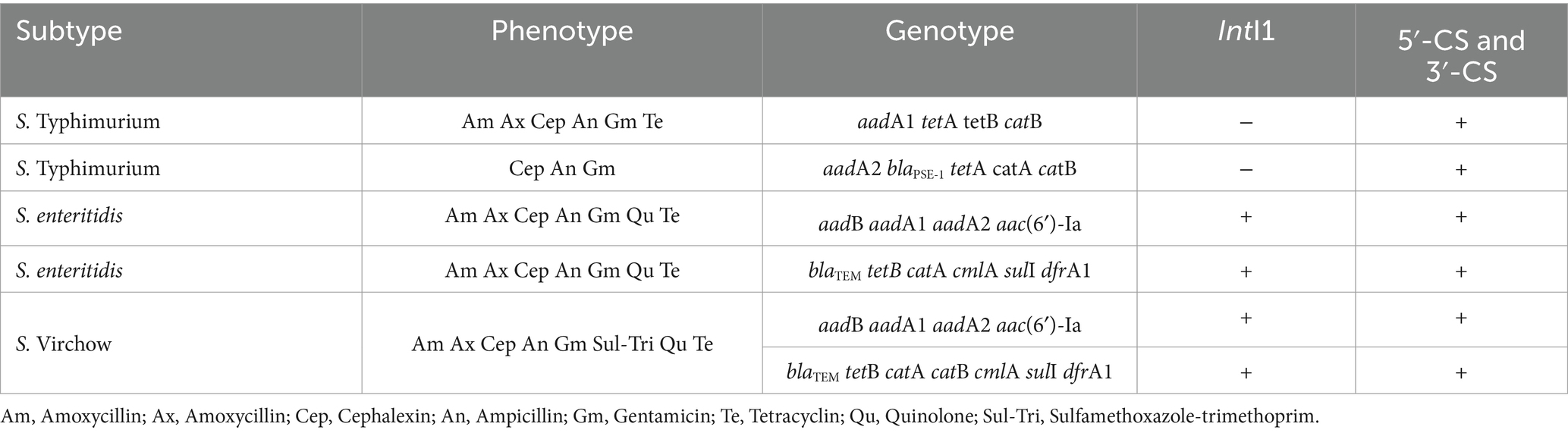
The study of class I Integron by the integrase gene, IntI1, and gene cassettes located at the conserved segments of 5’CS and 3’CS was conducted using Polymerase Chain Reaction (PCR), referenced in Table 1. The Ampicillin-resistance genes are Blapsc1 (n = 5) and BlaTEM (n = 5); Chloramphenicol-resistance genes are catA (n = 5), catB (n = 5), and cmlA (n = 5); Tetracycline-resistance genes are tetA (n = 5) and tetB (n = 5); Trimethoprim-resistance genes are dfrA1 (n = 5), dfrA10 (n = 5), and dfrA12 (n = 5); and the Sulfamethoxazole-resistance gene is sul1 (n = 5).
The DNA template was prepared by selecting a pure colony on McConkey Agar, incubated at 37°C for 18–24 h. The colony was then transferred to 50 μL of TE buffer and boiled at 100°C for 5 min. The DNA fraction was then centrifuged at 11,000 rpm for 1 min, and the supernatant was collected for PCR testing. The PCR solution was prepared using 100 μL of Eppendorf® Master Mix (Eppendorf, Hamburg, Germany) containing 75 μL of sterile distilled water, 10 μL of 10x Tag buffer (100 mM Tris–HCL, pH 8.3), 50 μL MgCl2, 3 μL of 10 mM dNTPs, 1 μL of F-R primer (10 pmol/μL), 5 μL of DNA template, and Tag DNA polymerase (Apsalagon 5 U/μL). The first step of initial denaturation was set at 94°C for 5 min for 1 cycle, followed by DNA amplification in 3 steps for a total of 30 cycles: denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s. The final step was an extension at 72°C for 5 min, 1 cycle. The PCR product (2 μL) was mixed with an equal volume of gel red (Biotium, California) and loaded for electrophoresis in 1% agarose gel (Axygen Biosciences, USA) at 100 V for 30 min. A ladder was used as the marker (Solis Biodyne, Estonia), and the results were interpreted on a UV-trans illuminator (Gel DocTM EZ Imager).
Results
Sample collection
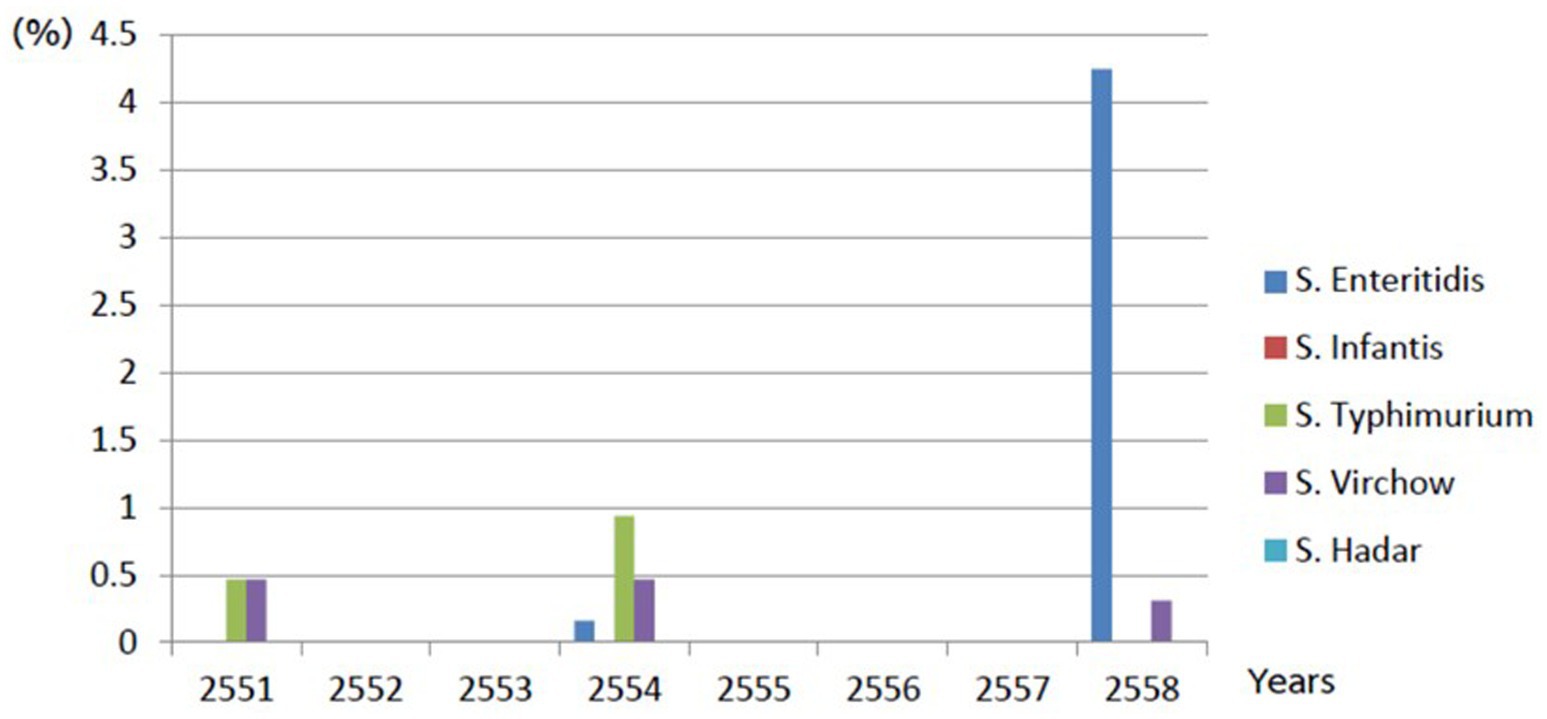
This retrospective study gathered Salmonella spp. from livestock and their environments during 2008–2015. Among 636 samples, 86 samples were collected from swine farms, 39 from cleaning water within the farm, 24 from nursery piglets’ floor swabs, 18 from the soil,4 from drinking water (before and after chlorine treatment), 529 from duck farms and 21 samples were from the broiler farms. However, no Salmonella of non-host-specific serovars was detected during 2009–2010 and 2012–2014, as shown in Figure 1.
Serovar identification
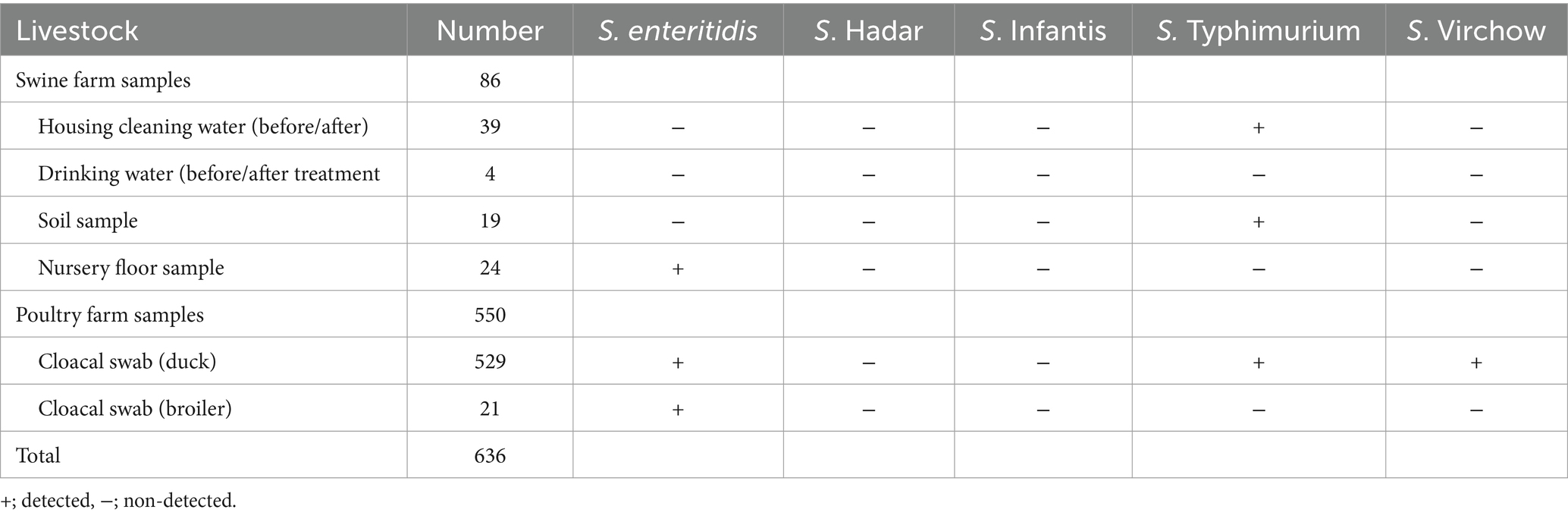
Among 45 non-host-specific Salmonella isolates, 9 were identified as S. Typhimurium, 28 as S. enteritidis, and 8 as S. Virchow. None isolates of S. infantis or S. Hadar were detected. In detail, it was indicated that S. Typhimurium was detected in 1.42% (9/636 samples: 3 environmental and 6 cloacal swabs), S. enteritidis in 4.40% (28/636 samples: 4 environmental and 24 cloacal swabs), and S. Virchow in 1.26% (8/636 samples: 8 cloacal swabs), as shown in Table 2.
Drug resistance testing
Drug sensitivity testing was performed using the VITEK II system according to CLSI Vet03-S2 2014 standards. S. Typhimurium showed resistance rates of 22.22% towards Ampicillin, Amoxicillin, Piperacillin, and Tetracycline; 44.44% to Nitrofurantoin; and 100% to Cephalexin, Amikacin, Gentamicin, and Tobramycin. S. Virchow exhibited resistance rates of 12.50% towards Ampicillin, Amoxicillin, Amoxicillin-clavulanic acid, Imipenem, Sulfamethoxazole-trimethoprim, Chloramphenicol, Marbofloxacin, and Tetracycline; 25% for Piperacillin; 37.5% for Enrofloxacin and Nitrofurantoin; and 50% for Cefpodoxime, Amikacin, and Tobramycin. Lastly, S. enteritidis demonstrated resistance rates of 18.18% for Amoxicillin-clavulanic acid, Cefpodoxime, Imipenem, Enrofloxacin, Marbofloxacin, and Tetracycline; 54.55% for Nitrofurantoin; 90.91% for Ampicillin, Amoxicillin, and Piperacillin; and 100% for Cephalexin, Amikacin, Gentamicin, and Tobramycin and shown in Figure 2.
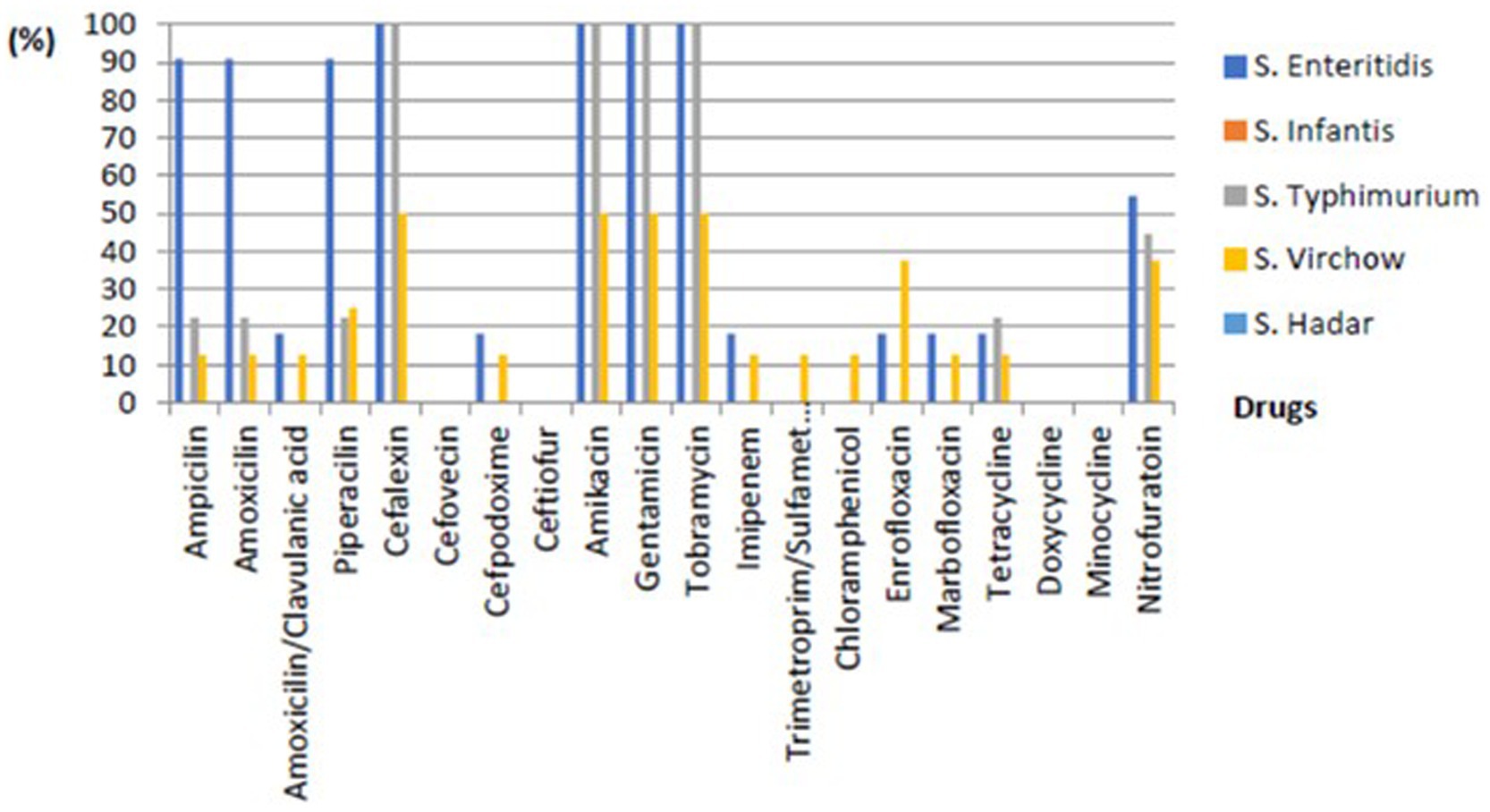
Figure 2. The percentage of phenotypic resistance of non-typhoidal Salmonella of each subtype from environmental and livestock samples.
Study of drug resistance gene cassette in integron by PCR
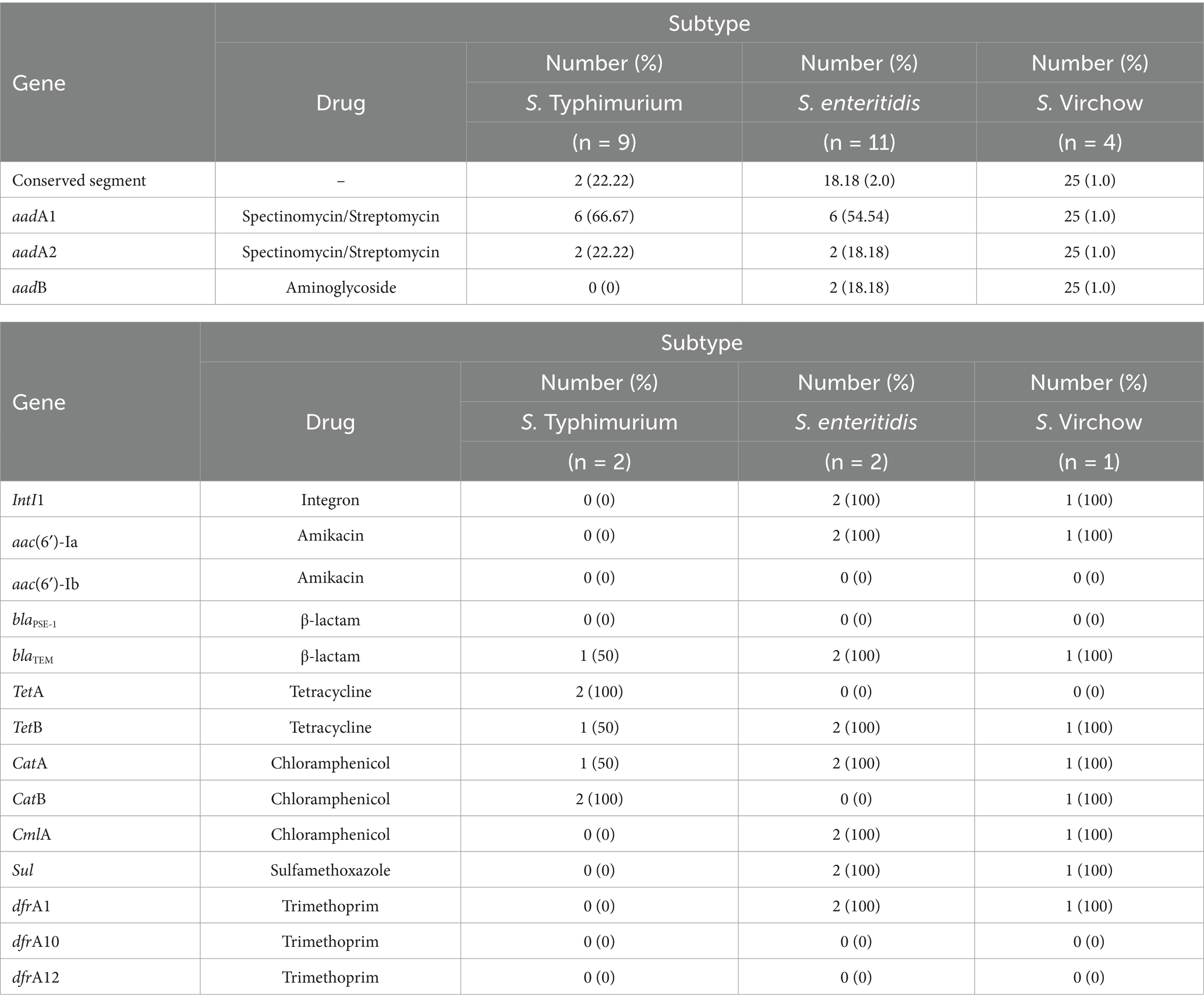
Gene cassette detection following MIC testing revealed resistance rates of 22.22% for S. Typhimurium, 18.18% for S. enteritidis, and 25% for S. Virchow, with an overall detection rate of 20.83%. Among the conserved segments found, the integrase gene was detected in 60% of cases, with 100% detection in both S. enteritidis and S. Virchow, but not detected at all in S. Typhimurium, as shown in Table 3 and Figure 3. According to Falagas and Karageorgopoulos (13), the concept of multidrug resistance indicates that bacteria resist at least three antibiotics. In this study, the aadA1 resistance gene, associated with Spectinomycin and Streptomycin resistance, was detected in 54.17% of isolates, with detection rates of 66.67% in S. Typhimurium, 8.33% in S. enteritidis, and 4.17% in S. Virchow. The aadA2 gene showed a resistance rate of 21.83%, with 22.22, 18.18, and 25%, respectively. The aadB gene against gentamicin was detected in 18.56% of cases, with 12.5, 18.18, and 25% detection in the same order. Using specific primers for the integron gene cassette on Salmonella conserved segments, they found of 100% IntI1 detected for S. enteritidis and S. Virchow but not for S. Typhimurium. Whereas, the study of aac(6′)-Ia resistance gene for Amikacin was detected in 100% of both S. enteritidis and S. Virchow, but none detected for the aac(6′)-Ib gene in these subtypes. The blaPSE-1 gene from the β-lactam group was not detected in all isolated Salmonella, but the blaTEM gene was detected in 80% of cases, with 50, 100, and 100% detection for S. Typhimurium, S. enteritidis, and S. Virchow, respectively. The tetA gene for Tetracycline resistance was detected in 40% of cases, with 100% detection only for S. Typhimurium. The tetB gene showed an 80% detection rate, divided into 50, 100, and 100% in the same order. The catA gene for chloramphenicol resistance was found in 80% of cases (50, 100, 100%), the catB gene in 60% of cases (100, 100, 60%), and the cmlA gene in 60% of cases (100% detection for S. enteritidis and S. Virchow). Lastly, the sulI-drfA1 resistance gene towards Sulfamethoxazole-trimethoprim was detected in 60% of cases (100% detection for S. enteritidis and S. Virchow), while the dfrA10 and dfrA12 genes were not detected in isolated Salmonella spp. as shown in Table 4.
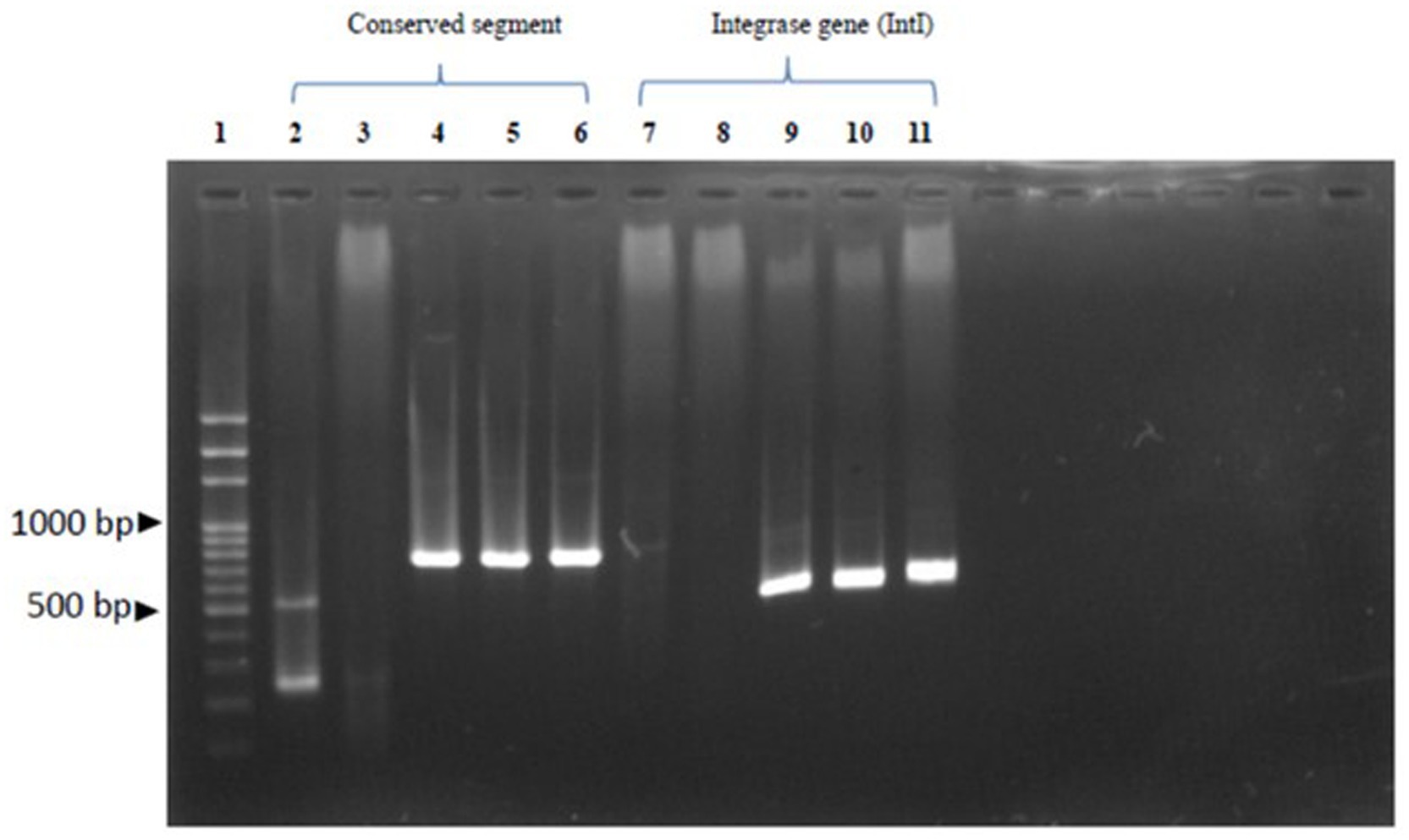
Figure 3. Demonstrates of the antibiotic resistance gene: Row 1 reveals 100 bp DNA Ladder Ready to load (Solis Biodyne, Tartu Estonia.); Row 2-6 reveals conserved segment (5’CS-3’CS) which row 2 and 3 were S. Typhimurium, row 4 and 5 were S. Enteritidis and row 6 was S. Virchow. Row 7-11 revealed of intergron class I by integrase gene (IntI) which row 7 and 8 were S. Typhimurium, row 9 and 10 were S. Enteritidis and row 11 was S. Virchow, respectively.
Discussion
According to former reports between 2003 to 2006 in Thailand, 80% of S. Typhimurium isolated from environmental samples showed antibiotic resistance, especially in the pig farm environments (14). In this study, Non-Typhoidal Salmonella spp. (NTS) collected from pig farm environments were detected at the rate of 33.33% (3 out of 9 samples), they all were collected from cleaning water and contaminated soil samples. In northern Thailand, S. Typhimurium and S. enteritidis were found in 18.44 and 1.78% of samples, respectively. However, 28% isolation rate from retail chicken meat was also reported (10, 15). S. enteritidis is commonly found on pig farm floors, which similar to the others finding of 3.12% in pig farm environments and 6.6% from chicken feces sample (15, 16). Interestingly, this finding was different from the current studied of Salmonella Enterica isolated from animal feedstuffs in year 2017, they reported that the most serotypes found were S. Rissen, S. Mbandaka and S. Livingstone, respectively (17).
There was also a report of finding 66% of S. Typhimurium in pigs and farm environments in Spain, which is a significant problem for both S. Typhimurium and S. enteritidis in America. Additionally, S. enteritidis and S. Typhimurium have been isolated from broiler farms and their environments in Thailand (12), which correlates well with the 7.81% detection rate for both serotypes in broiler farms in Algeria (18) and the presence of S. Virchow in broiler farms in China (19).
Antibiotic resistance study
This study demonstrated antibiotic resistance in NTS, specifically S. Typhimurium, S. enteritidis, and S. Virchow. Resistance to one or two antibiotics was observed in 37.5% of cases, while 62.5% exhibited MDR. Among the MDR isolates, resistance to more than three drugs were found in 38.4% (5/13) of duck samples, 83% (5/6) of pig samples, and 100% (5/5) of broiler samples. They were resistant to beta-lactam, Cephalosporins, and Aminoglycoside antibiotics.
Comparing these findings with previous reports on Salmonella spp. isolated from broiler and pig feces from 2003 to 2005 in central Thailand, it was found that the bacteria were 100% sensitive to Gentamicin and Ciprofloxacin (9). However, another study reported bacterial resistance in broilers, especially S. enteritidis and S. Virchow, with 100% resistance to Nalidixic acid and only 16.7% resistance to Kanamycin. Studies in Vietnam and Thailand demonstrated of antibiotic resistance rates of 28 and 59%, respectively, particularly for ampicillin and tetracycline (20). During 2004–2007, there were reports of high antibiotic resistance for Nalidixic acid, Ciprofloxacin, and Ampicillin (21), with an increasing trend in resistance to Cephalosporins and Aminoglycosides. In addition, the study of S. enterica isolated from animal feedstuffs in Thailand, of which they found MDR in those isolates, therefore, the commercial feeds and raw material involved people should be monitored (17).
Resistance gene detection
The MIC testing for the three serotypes revealed resistance to Aminoglycosides, with the detection of aadA1, aadA2, and aadB genes. This finding is consistent with reports of the aadA1 gene in Salmonella spp. isolated from pigs and chickens (22, 23), as well as similar findings in pig and chicken farms in Thailand (24). In this study, S. Typhimurium isolated from swine environments showed resistance to beta-lactam, Cephalosporin, Aminoglycoside, and Tetracycline antibiotics. The resistance genes aadA1, tetA, tetB, and catB were well-correlated with findings abroad and in Chloramphenicol resistance (12, 23).
There have been reports of the detection of aadA2, blaPSE-1, tetA, catA, and catB in gene cassettes from S. Typhimurium, indicating MDR (25). The detection of multiple resistance genes in S. Typhimurium suggests the potential for future MDR development. This study found that S. enteritidis was resistant to Aminoglycosides, Cephalosporins, beta-lactams, Quinolones, and Tetracycline. The detected genes included aadB, aadA1, aadA2, aac(6′)-la, blaTEM, tetB, catA, cmlA, sulI, and dfrA1, with genotypic patterns similar to S. enteritidis isolated from broilers in Romania (22), indicating MDR. Moreover, S. Virchow in this study showed resistance to Aminoglycosides, Cephalosporins, beta-lactams, Quinolones, Tetracycline, and Sulfamethoxazole/trimethoprim. The phenotypic pattern included aadB, aadA1, aac(6′)-la, blaTEM, tetB, catA, catB, cmlA, sulI, and dfrA1, which were consistent with gene cassettes drfA1,2-ortf-aadA2 in S. Typhimurium showing MDR (26). The gene patterns detected in S. Virchow were similar to those in S. enteritidis, S. Typhimurium, and indicating potential MDR (27).
Class I integron and MDR
Chuanchuen et al. (12) and Partridge et al. (28) reported that MDR in Salmonella spp. is caused by abundant gene cassettes in class I integron (IntI), which can be mobile genetic elements (MGEs) in gram-negative bacteria (25, 29). Since MGEs composed of various elements such as integrons, transposons and plasmids which were considered to responsible for the Horizontal gene transfer (HGT). HGT is crucial for the propagation of increased of antibiotic resistance (30, 31). The gene cassettes in S. enteritidis isolated from chickens in China indicated MDR, with a resistance rate of 65% by MIC testing (CLSI Vet03-S2 2014 NCCLS standard) and a 33.3% detection rate for gene cassettes. Two different gene cassette sizes were detected, distinguishing S. Typhimurium from S. enteritidis and S. Virchow, which had identical cassette sizes. The gene cassette size difference indicates structural differences in the genes (FEMS Microbial Rev., 2009). In this study, IntI gene cassettes were detected in 60% (3/5) of S. Typhimurium and 100% (2/2) and 100% (1/1) for S. enteritidis and S. Virchow, respectively, whereas the IntI may induce MDR in S. enteritidis and S. Virchow in broilers. Since, class 1 integrons is one of the major integrons classes, which normally associated with the HGT of antibiotic resistance and it could be existed in environmental sample with sequence diversity (32, 33). The antimicrobial resistance in broiler farms was higher than in the native chickens, attributed to high use of antibiotics in the broiler industry. Therefore, careful recognition of antibiotic use in farms is necessary attributed to the increasing trend of MDR in Salmonella spp. especially in developed and developing countries (34). Even after the cessation of antibiotic use, the residues and persistence of the resistance genes in environment may increase the likelihood of MDR in Salmonella which could be entering the food chains (35, 36).
There was report indicated the similar MDR serotype of Salmonella spp. and E. coli could be detected in farm workers, infected pigs and their environment in Thailand (37, 38). The presence of MDR circulation in the food web poses significant to public health and medical concerns, since the incidents of antibiotic resistance in NTS have been reported in America (39), Thailand and Vietnam (20). Once an outbreak occurs, prevention and control measures become challenging and serious concern for global public health.
Conclusion
This study identified an increasing trend in antibiotic resistance, particularly MDR, among Salmonella isolates from Nakhon Pathom and Suphan Buri provinces, especially for the three serotypes of examined. The observed resistance patterns are likely linked to the continued use of antibiotics in livestock production systems. Despite laws regulating the antibiotics and control strategies enforcing of reasonable use, the long-term misuse of antibiotics in livestock still persists. This misuse leads to environmental contamination and the spread of resistance genes into the bacteria, particularly Salmonella as known as a major foodborne pathogen in food safety.
Therefore, prevention and control strategies are essential to delaying antibiotic resistance in bacteria. The determination of class I integron genes and gene cassettes in Salmonella spp. serves as a valuable tool for understanding resistance of the bacteria and monitoring MDR in the livestock. This study framework can be applied for further explore the relationship between IntI and resistance gene cassettes of Salmonella isolates from livestock and their environments. These findings are critical for developing the predictive tools and effective MDR prevention and control strategies for livestock production industry which become one of a major industry in Thailand.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The protocol was approved by Faculty of Veterinary Medicine, Kasetsart University, which was a routine collection protocol in the farm handled by farm veterinarian. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
PL: Data curation, Formal analysis, Validation, Visualization, Writing – review & editing. SP: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – review & editing. PT: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Grant in aids from the Faculty of Veterinary Medicine, Kasetsart University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Wang, Y, Ge, H, Wei, X, and Zhao, X. Research progress on antibiotic resistance of Salmonella. Food Quality and Safety. (2022) 6:fyac035. doi: 10.1093/fqsafe/fyac035
3. Talukder, H, Alam, R, Debnath, K, Sharma, B, Ahmed, J, and Roy, S. Prevalence and antimicrobial resistance profile of Salmonella isolated from human, animal and environment samples in South Asia: a 10-year Meta-analysis. J Epidemiol Glob Health. (2023) 13:637–52. doi: 10.1007/s44197-023-00160-x
4. Ayuti, SR, Khairullah, AR, Al-Arif, MA, Lamid, M, Warsito, SH, Moses, IB, et al. Tackling salmonellosis: a comprehensive exploration of risks factors, impacts, and solutions. Open Vet J. (2024) 14:1313–29. doi: 10.5455/OVJ.2024.v14.i6.1
5. Bangtrakulnonth, A, Pornreongwong, S, Pulsrikarn, C, Sawanpanyalert, P, Hendriksen, RS, Wong, DMLF, et al. Salmonella Serovars from humans and other sources in Thailand, 1993-2002. Emerg Infect Dis. (2004) 10:131–6. doi: 10.3201/eid1001.02-0781
6. Mumbo, MT, Nyaboga, EN, Kinyua, JK, Muge, EK, Mathenge, SGK, Rotich, H, et al. Antimicrobial resistance profiles of Salmonella spp. and Escherichia coli isolated from fresh Nile tilapia (Oreochromis niloticus) fish marketed for human consumption. BMC Microbiol. (2023) 23:306. doi: 10.1186/s12866-023-03049-8
7. Chaisakdanukul, Y, Praikanahok, N, and Chaturapahu, U. (2012). Surveillance of salmonellosis in livestock products. Bureau of Animal Disease Control and Prevention. Bangkok. Access date January 27, 2020.
8. Hendriksen, RS, Bangtrakulnonth, A, Pulsrikarn, C, Pornreongwong, S, Hasman, H, Song, SW, et al. Antimicrobial resistance and molecular epidemiology of Salmonella Rissen from animals, food products, and patients in Thailand and Denmark. Foodborne Pathog Dis. (2008) 5:605–19. doi: 10.1089/fpd.2007.0075
9. Pattanasopon, P, Narongsak, W, and Charoenpoj, S. Prevalence Serovars and antibiotic sensitivity of Salmonella spp. isolated from broiler and pig farms in central region. Veterinary J. (2007) 58:49–53.
10. Tadee, P, Kumpapong, K, Sinthuya, D, Yamsakul, P, Chokesajjawatee, N, Nuanualsuwan, S, et al. Distribution, quantitative load and characterization of Salmonella associated with swine farms in upper-northern Thailand. J Vet Sci. (2014) 15:327–34. doi: 10.4142/jvs.2014.15.2.327
11. Fluit, AC, and Schmitz, FJ. Resistance integrons and super-integrons. Clin Microbiol Infect. (2004) 10:272–88. doi: 10.1111/j.1198-743X.2004.00858.x
12. Chuanchuen, R, Pathanasophon, P, Khemtong, S, Wannaprasat, W, and Padungtod, P. Susceptibilities to antimicrobials and disinfectants in Salmonella isolates obtained from poultry and swine in Thailand. J Vet Med Sci. (2008) 70:595–601. doi: 10.1292/jvms.70.595
13. Falagas, ME, and Karageorgopoulos, DE. Pandrug resistance (PDR), extensive drug resistance (XDR), and multidrug resistance (MDR) among gram-negative bacilli: need for international harmonization in terminology. Clin Infect Dis. (2008) 46:1121–2. doi: 10.1086/528867
14. Hendriksen, RS, Bangtrakulnonth, A, Pulsrikarn, C, Pornruangwong, S, Noppornphan, G, Emborg, HD, et al. Risk factors and epidemiology of the ten Most common Salmonella Serovars from patients in Thailand: 2002-2007. Foodborne Pathog Dis. (2009) 6:8. doi: 10.1089/fpd.2008.0245
15. Tadee, P, Patchanee, P, Pascoe, B, Sheppard, SK, Meunsene, D, Buawiratlert, T, et al. Occurrence and sequence type of antimicrobial resistant Salmonella spp. circulating in antibiotic-free organic pig farms of northern-Thailand. J Vet Med. (2021) 51:14.
16. Boonmar, S, Bangtrakulnonth, A, Pornrunangwong, S, Marnrim, N, Kaneko, K, and Ogawa, M. Salmonella in broiler chickens in Thailand with special reference to contamination of retail meat with Salmonella enteritidis. J Vet Med Sci. (1998) 60:1233–6. doi: 10.1292/jvms.60.1233
17. Sanguankiat, A, Pinniam, N, and Tulayakul, P. Surveillance of antimicrobial resistance, phenotypic, and genotypic patterns of Salmonella enterica isolated from animal feedstuffs: annual study. Vet world. (2023) 16:939–45. doi: 10.14202/vetworld.2023.939-945
18. Mezali, L, and Hamdi, TM. Prevalence and antimicrobial resistance of Salmonella isolated from meat and meat products in Algiers (Algeria). Foodborne Pathog Dis. (2012) 9:522–9. doi: 10.1089/fpd.2011.1032
19. Lu, Y, Zhao, H, Sun, J, Liu, Y, Zhou, X, Beier, CB, et al. Characterization of multidrug-resistant Salmonella enterica Serovars Indiana and Enteritidis from chickens in eastern China. PLoS One. (2014) 9:e96050. doi: 10.1371/journal.pone.0096050
20. Padungtod, P, Tribuddharat, C, and Chuanchuen, R. Widespread presence of dfra12 and its association with drfa12-aada2 cassette in Salmonella enterica isolates from swine. Southeast Asian J Trop Med Public Health. (2011) 42:1471–6.
21. Utrarachkij, F, Nakajima, C, Siripanichgon, K, Changkaew, K, Thongpanich, Y, Ornraungwong, S, et al. Genetic diversity and antimicrobial resistance pattern of Salmonella enterica serovar Enteritidis clinical isolates in Thailand. J Infect Chemother. (2016) 22:209–15. doi: 10.1016/j.jiac.2015.12.011
22. Dan, SD, Tabaran, A, Mihaiu, L, and Mihaiu, M. Antibiotic susceptibility and prevalence of foodborne pathogens in poultry meat in Romania. J Infect Developing Countries. (2015) 9:035–41. doi: 10.3855/jidc.4958
23. Voss-Rech, D, Ziech, RE, Vaz, CSL, Coldebella, A, Kuchiishi, SS, Balzan, C, et al. Association between antimicrobial resistance and biofilm forming ability of Salmonella enterica serotypes from commercial broiler farms in Brazil. British Poult Sci. (2023) 64:224–30. doi: 10.1080/00071668.2022.2136511
24. Chuanchuen, R, and Padungtod, P. Antimicrobial resistance genes in Salmonella enterica isolates from poultry and swine in Thailand. J Vet Med Sci. (2009) 71:1349–55. doi: 10.1292/jvms.001349
25. Molla, B, Miko, A, Pries, K, Hildebrandt, G, Kleer, J, Schroeter, A, et al. Class 1 integrons and resistance gene cassettes among multidrug resistant Salmonella serovars isolated from slaughter animals and foods of animal origin in Ethiopia. Acta Trop. (2007) 103:142–9. doi: 10.1016/j.actatropica.2007.05.018
26. Jin, Y, and Liang, JM. Prevalence of integrons in antibiotic-resistant Salmonella spp. in Hong Kong. Japanese J Infect Dis. (2009) 62:432–9. doi: 10.7883/yoken.JJID.2009.432
27. Bacci, C, Lanzoni, E, Vismarra, A, Alpigiani, I, Nuvoloni, R, Bonardi, S, et al. Antibiotic resistance and resistance genes in Salmonella enterica isolated from pork meat and pig carcasses in northern Italy. Large Anim Rev. (2014) 20:201–7.
28. Partridge, SR, Tsafnat, G, Coiera, E, and Iredell, JR. Gene cassettes and cassette arrays in mobile resist in mobile resistance integrons. FEMS Microbiol Rev. (2009) 33:757–84. doi: 10.1111/j.1574-6976.2009.00175.x
29. Bennett, PM. Integrons and gene cassettes: a genetic construction kit for bacteria. J Antimicrob Chemother. (1999) 43:1–4. doi: 10.1093/jac/43.1.1
30. Ochman, H, Lawrence, JG, and Groisman, EA. Lateral gene transfer and the nature of bacterial innovation. Nature. (2000) 405:299–304. doi: 10.1038/35012500
31. von Wintersdorff, CJH, Penders, J, van Niekerk, JM, Mills, ND, Majumder, S, van Alphen, LB, et al. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front Microbiol. (2016) 7:173. doi: 10.3389/fmicb.2016.00173
32. Boucher, Y, Labbate, M, Koenig, JE, and Stokes, HW. Integrons: mobilizable platforms that promote genetic diversity in bacteria. Trends Microbiol. (2007) 15:301–9. doi: 10.1016/j.tim.2007.05.004
33. Gillings, MR, Krishnan, S, Worden, PJ, and Hardwick, SA. Recovery of diverse genes for class 1 integrons-integrases from environmental DNA samples. FEMS Microbiol Lett. (2008b) 287:56–62. doi: 10.1111/j.1574-6968.2008.01291.x
34. Ashtiani, M, Monajemzadeh, M, and Kashi, L. Trends in antimicrobial resistance of fecal Shigella and Salmonella isolates in Tehran, Iran. Indian J Pathol Microbiol. (2009) 52:55. doi: 10.4103/0377-4929.44964
35. Hsu, CY, Hsu, BM, Ji, WT, Chen, JS, Hsu, TK, Ji, DD, et al. Antibiotic resistance pattern and gene expression of non-typhoid Salmonella in riversheds. Environ Sci Pollut Res. (2015) 22:7843–50. doi: 10.1007/s11356-014-4033-y
36. Tulayakul, P, Boonsoongnern, A, Kasemsuwan, S, Wiriyarampa, S, Pankumnoed, P, Tippayaluck, S, et al. Comparative study of heavy metal and pathogenic bacterial contamination in sludge and manure in biogas and non-biogas swine farm. J Environ Sci. (2011) 23:991–7. doi: 10.1016/S1001-0742(10)60484-6
37. Dawangpa, A, Lertwatcharasarakul, P, Ramasoota, P, Boonsoongnern, A, Ratanavanichrojn, N, Sanguankiat, A, et al. Genotypic and phenotypic situation of antimicrobial drug resistance of Escherichia coli in water and manure between biogas and non-biogas swine farms in Central Thailand. management Environ Manage. (2021) 279:111659. doi: 10.1016/j.jenvman.2020.111659
38. Sinwat, N, Angkittitrakul, S, Coulson, KF, Pilapil, FMIR, Meunsene, D, and Chuanchuen, R. High prevalence and molecular characteristics of multidrug-resistant Salmonella in pigs, pork and humans in Thailand and Laos provinces. J Med Microbiol. (2016) 65:1182–93. doi: 10.1099/jmm.0.000339
39. Gu, W, Medalla, F, and Hoekstra, RM. Bayesian hierarchical model of ceftriaxone resistance proportions among Salmonella serotype Heidelberg infections. Spat Spatiotemporal Epidemiol. (2018) 24:19–26. doi: 10.1016/j.sste.2017.10.003
40. Zarrilli, R, Crispino, M, Bagattini, M, Barretta, E, Di Popolo, A, Triassi, M, et al. Molecular epidemiology of sequential outbreaks of Acinetobacter baumannii in an intensive care unit shows the emergence of carbapenem resistance. J Clin Microbiol. (2004) 42:946–53. doi: 10.1128/JCM.42.3.946-953.2004
Keywords: Salmonella , antimicrobial resistance, multidrug resistance, environment, livestock
Citation: Lertwatcharasarakul P, Phatthanakunanan S and Tulayakul P (2025) Retrospective analysis of antimicrobial resistance of Salmonella spp. isolated from livestock and its environment in Thailand. Front. Vet. Sci. 12:1584940. doi: 10.3389/fvets.2025.1584940
Edited by:
Zhigang Qiu, Tianjin Institute of Environmental and Operational Medicine, ChinaReviewed by:
Chengshi Ding, Zaozhuang University, ChinaChelea Matchawe, Institute of Medical Research and Studies of Medicinal Plants (IMPM), Cameroon
Copyright © 2025 Lertwatcharasarakul, Phatthanakunanan and Tulayakul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Phitsanu Tulayakul, ZnZldHBudEBrdS5hYy50aA==
 Preeda Lertwatcharasarakul1
Preeda Lertwatcharasarakul1 Phitsanu Tulayakul
Phitsanu Tulayakul