- 1College of Life Science and Resources and Environment, Yichun University, Yichun, China
- 2Yichun University Research Center for Traditional Chinese Veterinary Medicine and Animal Embryo Engineering Technology, Yichun, China
- 3Engineering Technology Research Center of Jiangxi Universities and Colleges for Selenium Agriculture, Yichun, China
Introduction: Road transportation exposes goats to thermal, mechanical, and microbial stressors that can compromise their welfare by triggering pulmonary apoptosis and autophagy processes associated with tissue damage and immunosuppression.
Methods: To explore potential biomarkers for transport-related welfare assessment, this study analyzed lung tissues from nine Ganxi goats (n = 9; 0 h control, 2 h/6 h transport groups) through an integrated experimental approach: TUNEL assays quantified apoptosis rates, immunohistochemistry mapped protein localization, Western blotting analyzed protein expression levels, and qPCR profiled gene expression of apoptotic regulators (Bax, Bcl-2) alongside autophagy-related markers (LC3B, p62, PINK1, Parkin).
Results: Results indicated time-dependent cellular stress patterns, where the 2 h group displayed elevated apoptosis rates, while the 6 h group exhibited upregulated Parkin expression (p < 0.05) and altered regulation of apoptotic [Bcl-2-associated X-protein (Bax)/B-cell lymphoma-2 (Bcl-2)] and autophagy-related genes (Microtubule-associated protein 1 light chain 3B (LC3B), p62, PTEN-induced putative kinase 1 (PINK1)/Parkin). Protein localization analyses revealed compartment-specific responses, with Bcl-2/Bax primarily in bronchial epithelia and LC3B/PINK1/Parkin in alveolar cells, suggesting spatially distinct stress adaptation mechanisms. Observed molecular changes coincided with histological evidence of pulmonary alterations, implying a potential interplay between apoptosis and autophagy in transport-induced cellular stress. The identification of time-sensitive molecular shifts (e.g., transient apoptosis elevation at 2 h, and progressive Parkin activation at 6 h) could inform hypotheses for monitoring transport-associated physiological responses.
Discussion: These findings highlight the need for further investigation into transport duration effects, with shorter intervals (e.g., ≤2 h) warranting evaluation for acute stress mitigation, and prolonged transport (e.g., >6 h) requiring characterization of cumulative autophagic impacts. The mechanistic insights can contribute to developing science-informed strategies for assessing transport stress, aligning animal welfare research with objectives to enhance sustainable livestock management practices.
1 Introduction
Transportation stress, driven by compounded oxidative, thermal, and physiological challenges, compromises animal welfare through multi-organ dysfunction and systemic physiological disturbances (1–3). Transport duration critically impacts animal welfare, as prolonged exposure disrupts homeostasis through thermoregulatory failure, antioxidant depletion, and immunosuppression, all of which are biomarkers of welfare compromise (4). Unmitigated stress not only elevates morbidity and mortality risks but can also exacerbate disease transmission susceptibility and meat quality deterioration (e.g., DFD/PSE defects), reflecting both ethical and economic implications for livestock systems (5–7). These welfare compromises extend beyond animal suffering, threatening public health security and sustainable food production, thereby underscoring the urgency for science-driven interventions to align transport practices with welfare-centric husbandry standards.
As a vital component of the respiratory system, the lungs primarily facilitate gas exchange while additionally contributing to drug metabolism and immune regulation (8, 9). Transportation stress induces pathological alterations in the caprine respiratory system, particularly affecting the structural integrity of pulmonary tissues, trachea, and bronchial structures (10). Extended transportation periods have been associated with the development of severe respiratory pathologies, including fibrinous pleuropneumonia and bovine respiratory disease complex (BRDC) (11–13). Emerging evidence suggest that bacterial endotoxins can induce pathological apoptosis in pulmonary epithelial cells through prolonged low-concentration exposure (14, 15). B-cell lymphoma-2 (BCL2)-associated X (Bax)-mediated apoptosis has been demonstrated to accelerate murine mortality and promote the pathogenesis of pulmonary disorders, including emphysema (16). Moderate apoptosis plays a dual physiological role, protecting against pulmonary infections and oncogenesis while preventing the accumulation of dysfunctional cells that may deplete metabolic resources and promote carcinogenesis or autoimmune disorders. This regulated cell death process facilitates recycling of cellular material and energy homeostasis (17–20). Apoptosis is primarily regulated through two distinct pathways: the receptor-mediated pathway and the mitochondrial pathway. The latter is controlled by the B-cell lymphoma-2 (Bcl-2) protein family, wherein Bcl-2 proteins inhibit apoptosis by antagonizing the pro-apoptotic function of Bax proteins (21–23). Cellular autophagy is a conserved catabolic process involving the sequestration of damaged organelles and long-lived proteins within autophagosomes for subsequent lysosomal degradation. Based on distinct cargo delivery mechanisms, autophagy is classified into three primary forms: macroautophagy, microautophagy, and chaperone-mediated autophagy (24, 25). Autophagy serves as a crucial cellular recycling mechanism, maintaining metabolic homeostasis and mitigating stress-induced damage. Notably, in mesenchymal progenitor cells, autophagy has a cytoprotective effect against bone marrow failure following severe intermittent stress (26–28). Recent genetic studies have established autophagy as a critical pathway in the pathogenesis of various human diseases, including neurodegenerative disorders, malignancies, inflammatory conditions, and autoimmune pathologies (29, 30). Excessive autophagy induces myocardial mitochondrial dysfunction and disrupts cellular homeostasis, contributing to both acute and chronic cardiac pathologies (31, 32). Core autophagy regulators, including Microtubule-associated protein 1 light chain 3B (LC3B), Sequestosome-1 (SQSTM1/p62), PTEN-induced putative kinase 1 (PINK1), and Parkin, mediate autophagic flux through interconnected signaling networks. Extensive research has revealed significant crosstalk between apoptotic and autophagic pathways, with their dynamic equilibrium maintaining metabolic homeostasis and modulating immune regulation (33–35). This study investigates the impact of transportation stress on apoptotic and autophagic pathways in caprine pulmonary cells by analyzing key regulatory proteins and genes, and provides mechanistic insights for developing preventive and therapeutic strategies against transportation-induced lung injury.
2 Materials and methods
2.1 Animals and experimental design
Nine healthy Ganxi male goats (Capra hircus) of similar body weight (13.89 ± 2.96 kg) and age (1 year) were selected for this study from Jiangxi Mulei Agriculture and Forestry Development Co., Ltd. (Ganxi Goat Breeding Farm). The selected goats were randomly divided into a control group, a 2 h transportation group, and a 6 h transportation group, with three goats in each group. The transportation groups were subjected to standard road conditions (4.2 × 2.2 × 1.8 m compartment, 35–45 km/h, and 28–32°C) with fasting protocols. The control group was not treated. After transportation, the animals were immediately euthanized using intravenous sodium pentobarbital (90 mg/kg), without rest periods. No transportation-related incidents occurred. Death was confirmed through cardiac auscultation. Euthanasia procedures were followed based on the Chinese Association for Laboratory Animal Sciences guidelines. All protocols were approved by Yichun University’s Animal Ethics Committee (License: JXSTUDKY2019009). Tissue samples were either fixed in 4% paraformaldehyde or snap-frozen in liquid nitrogen and stored at −80°C.
2.2 Preparation of paraffin sections
Paraffin-embedded lung tissue sections were prepared as detailed below. The samples were fixed in 4% paraformaldehyde and rinsed under 24 h running water. This was followed by dehydration using a graded ethanol series (70, 80, 90, 95, and 100%), clearing in xylene, and embedding in paraffin wax. Solidified blocks were sectioned at 5 μm thickness using a rotary microtome (RM2235, Leica Biosystems, Buffalo Grove, IL, United States). The sections were stored at 4°C for subsequent analysis.
2.3 TUNEL assay
Paraffin sections were routinely dewaxed, rehydrated, and immersed in distilled water and PBS (for 5 min each). TUNEL staining was performed using a TUNEL kit (MK1015, Wuhan Boster Biological Technology, Ltd., Wuhan, China). The sections were then incubated with Proteinase K (20 μg/mL in PBS, pH = 7.4) at 37°C for 20 min, followed by five PBS washes (5 min/wash). Test group samples were treated with 50 μL TUNEL reaction mixture, while negative controls were only labeled with 50 μL fluorescein-dUTP. The sections were incubated in a humidified dark chamber (37°C, 60 min) and washed three times in PBS (5 min/wash). Subsequently, 50 μL horseradish peroxidase (POD)-conjugated solution was applied under identical conditions (37°C, 30 min). After three PBS washes, color development was initiated with 50 μL DAB substrate (10 min, room temperature). TUNEL-positive apoptotic cells were identified by yellow/brownish-yellow nuclear staining with characteristic morphological changes (chromatin condensation, margination, fragmentation, or lysis), contrasting with blue-stained normal nuclei. Apoptosis rates were determined by counting positive cells in 10 randomly selected fields per section, viewed under 400 × magnification. The formula used for calculation is: (apoptosis-positive cells/total cells) × 100%.
2.4 Immunohistochemistry
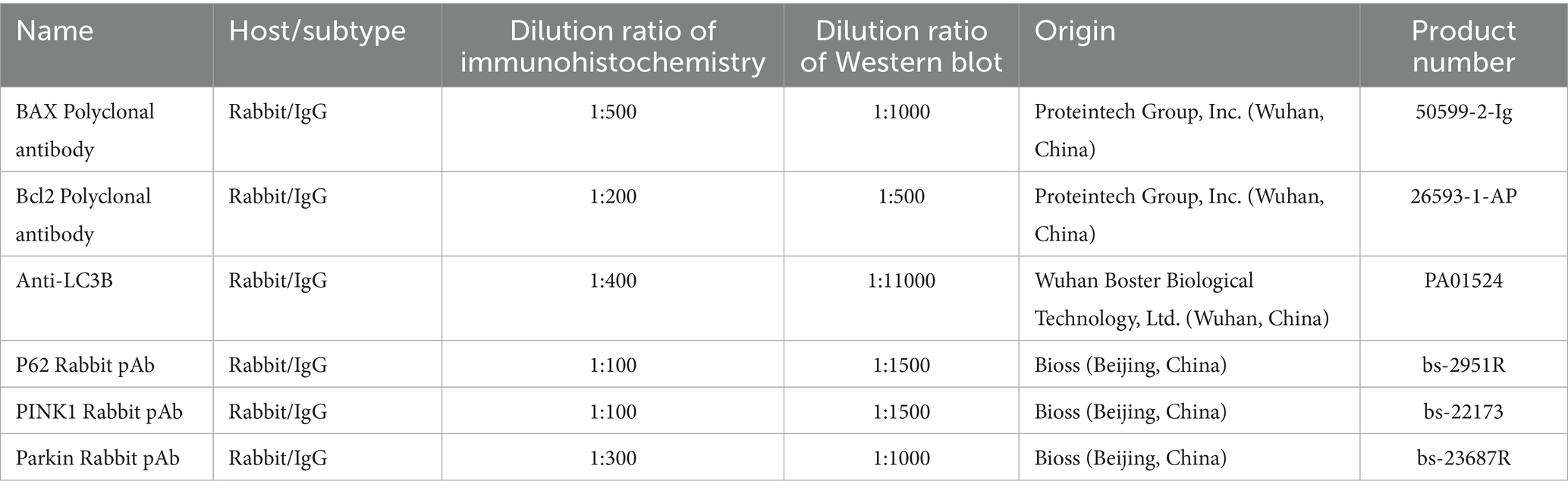
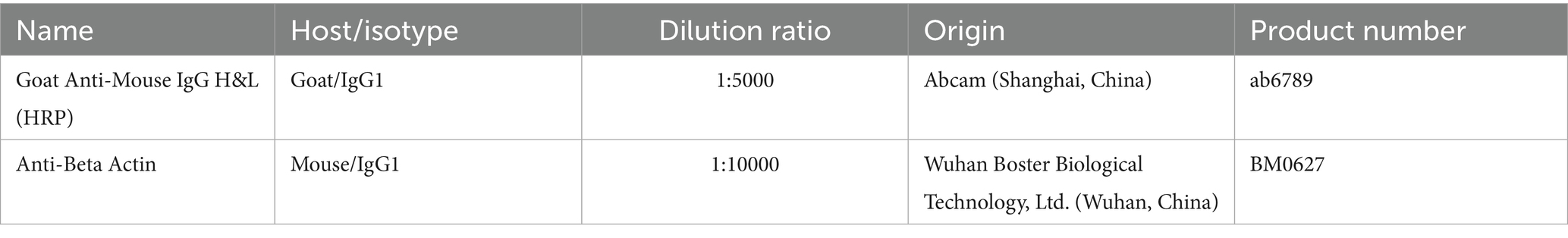
Paraffin sections were processed using a Rabbit Two-Step Assay Kit (PV-9001, Beijing Zhongshan Jinqiao Co., Ltd., Beijing, China) following the manufacturer’s instructions. The sections were treated thrice with xylene (for 10 min each), dehydrated with a gradient of alcohol, rinsed for 1 min with distilled water, and then immersed in PBS buffer. Antigen retrieval was performed using 10 mM sodium citrate buffer (pH 6.0) with microwave heating, followed by washing with PBS three times (3 min/wash). Endogenous peroxidase activity was blocked with 3% H2O2 (37°C, 10 min). Primary antibodies were applied overnight at 4°C (negative controls received PBS only). Following overnight incubation, the sections were treated with enhancement solution (37°C, 20 min), washed with PBS, and incubated with enzyme-conjugated anti-rabbit IgG polymer (37°C, 20 min). After additional PBS washes, color development was performed using the DAB kit (ZLI-9018, Beijing Zhongshan Jinqiao Co., Ltd., Beijing, China) for 7 min at room temperature under light-protected conditions. The sections were sequentially rinsed with tap and distilled water, counterstained with hematoxylin (8 s), differentiated in acid alcohol (1 s × 3), dehydrated, cleared, and mounted. Immunoreactivity was graded as: strong (brown), moderate (yellow), weak (light yellow), or negative (no staining). Scale bars (20 μm) were uniformly positioned in the lower-right corner of all histological images (Figures 1A–F, 2A–L) without obstructing positively stained regions. The primary and secondary antibodies used in this section are shown in Tables 1, 2, respectively.
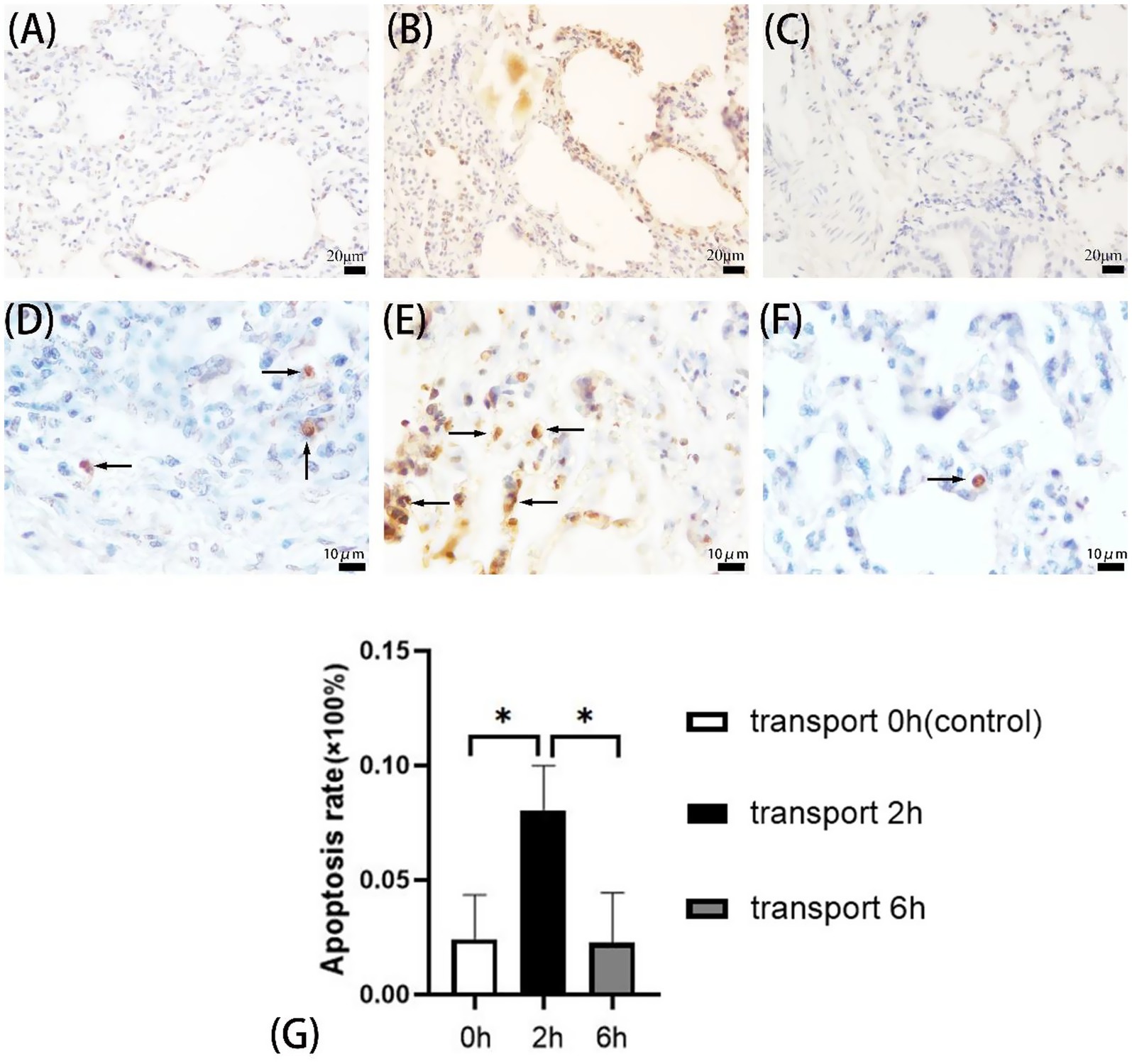
Figure 1. Apoptosis in goat lungs (TUNEL assay). (A–C) 400× microscope field of view, (D–F) 1,000× microscope field of view, (A,D) control group, (B,E) 2 h transport group, (C,F) 6 h transport group; the arrows show the TUNEL reaction positive cells, specifically manifested as yellow/brownish-yellow nuclear staining with characteristic morphological changes (chromatin condensation, margination, fragmentation, or lysis), contrasting with blue-stained normal nuclei. (G) Apoptosis rate in goat lung cells, the heights of the white, black, and gray columns in the bar chart correspond to the apoptosis rates of lung cells in goats in the control group, 2-h transportation group and 6-h transportation group, respectively. *Indicates significant difference (P < 0.05).
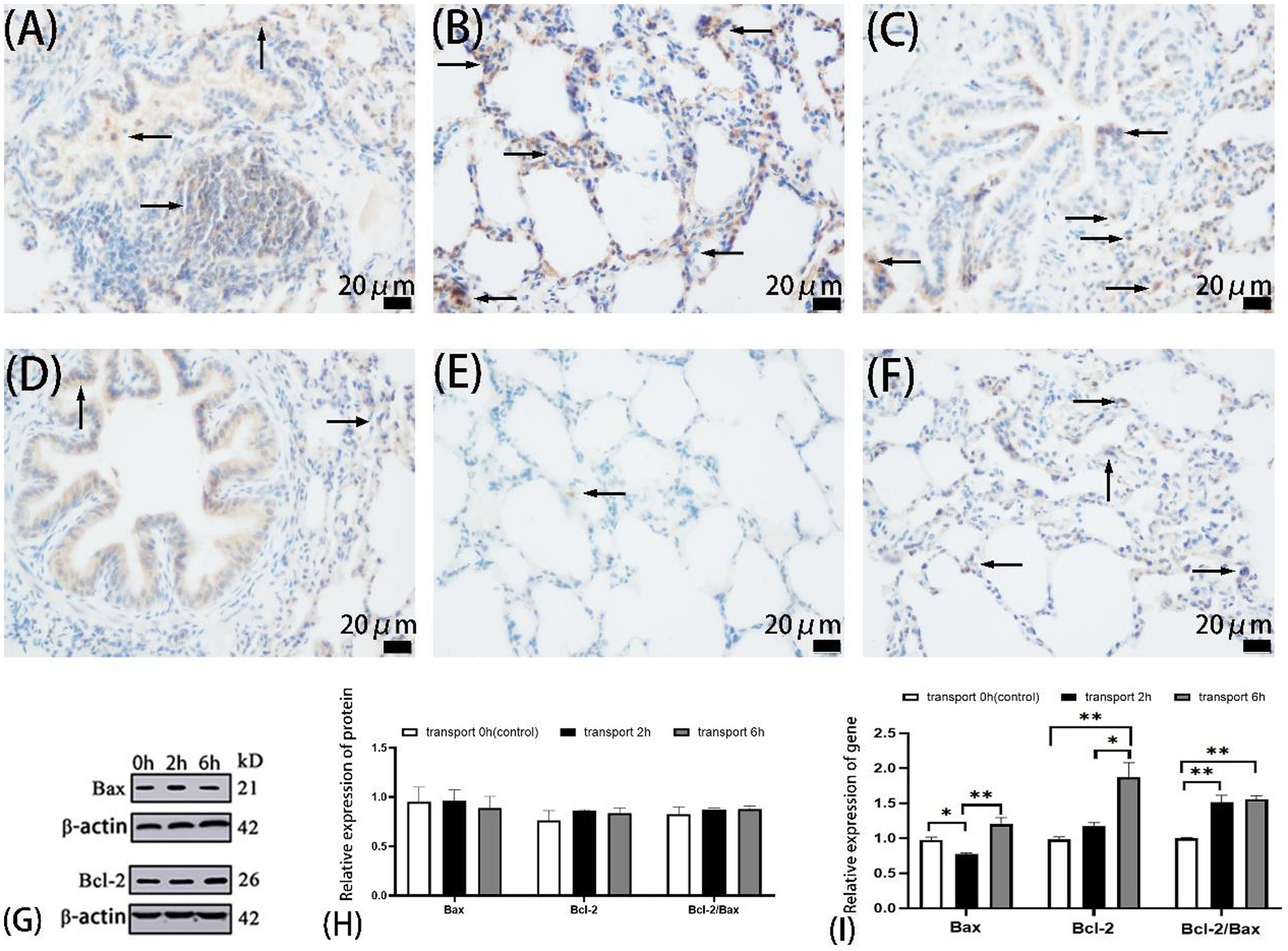
Figure 2. Apoptosis in goat lungs (immunohistochemistry, western blot and qRT-PCR). (A–C) The distribution of Bax protein. (D–F) The distribution of Bcl-2 protein. (A,D) Control groups, (B,E) 2 h transport groups, (C,F) 6 h transport groups; the positive expression regions are shown by arrows (immunohistochemistry 400×), immunoreactivity was graded as: strong (brown), moderate (yellow), weak (light yellow), or negative (no staining). (G) Protein blots of Bax and Bcl-2, the number at the right end of Protein Blots represents the molecular weight of the corresponding protein, while the upper 0 h, 2 h, and 6 h correspond to the Western blot results of the control group, 2-h transportation group and 6-h transportation group, respectively; (H) Expression of apoptosis-related proteins in goat lungs; (I) Expression of apoptosis-related genes in goat lung, the heights of the white, black, and gray bars in bars (H,I) correspond to the relative expression levels of proteins and genes in the lungs of goats in the control group, 2-h transportation group, and a 6-h transportation group, respectively. *Significant difference (P < 0.05), **highly significant difference (P < 0.01). Data are presented as the means ± SD and the internal reference gene is β-actin.
2.5 Western blot analysis
Frozen lung tissue samples (50 mg) were homogenized in 1 mL lysis buffer on ice using a tissue homogenizer. Then, 500 μL of the homogenate was transferred to a 1.5 mL microcentrifuge tube, mixed with 1 mL extraction reagent, and incubated at 4°C for 10 min. After centrifugation at 10,000 × g (4°C, 10 min), the supernatant and lower phase were discarded to retain the intermediate protein membrane. The pellet was air-dried at room temperature with the tube caps open. The dried precipitate was resuspended in an appropriate volume of 2% SDS solution, boiled at 95°C for 10 min, and equilibrated at room temperature for 30 min. The protein supernatant was collected by centrifugation at 12,000 × g for 5 min. Protein concentrations were determined using a BCA assay kit (P1250, Applygen Technologies Inc., Beijing, China), and equal amounts were added to protein loading buffer and were determined using a BCA assay kit (AR0146, Boster Biological Technology, Wuhan, China) following the manufacturer’s instructions, then mixed with protein loading buffer for later use. Samples were mixed with 4 × buffer, denatured (95°C, 5 min), and separated by SDS-PAGE (10% separating gel, 5% stacking gel) for 2 h. Proteins were transferred to 0.45 μm PVDF membranes (IPVH00010, Beijing Qiangxin Biorepublic Co., Ltd., Beijing, China) for 75 min. The membranes were blocked with 5% skim milk for 2 h, and washed with TBST (5 min × 3). Subsequently, the protein membrane was incubated overnight with the primary antibody, with gentle, continuous flipping during the process. After three TBST washes (for 10 min each), the membranes were incubated with secondary antibodies (diluted in 2% skim milk; see Table 2) for 2 h at room temperature and then washed thrice with TBST. Protein bands were detected using an ECL kit (P10100, Suzhou Xinsaimei Biotechnology Co., Ltd., Suzhou, China) using a 1:1 mixed A/B solution and imaged with a chemiluminescence system (Amersham Imager 600, GE Healthcare, United States). Quantitative analysis was performed using Image Pro Plus 6.0 (Media Cybernetics, United States). Relative expression levels were normalized to β-actin. All the experiments were independently repeated three times. Protein markers, molecular weights, and experimental conditions were annotated for clarity. As seen in Figure 3M, separator lines were inserted between samples to distinguish different experimental conditions and improve visual presentation, without obscuring protein expression patterns.
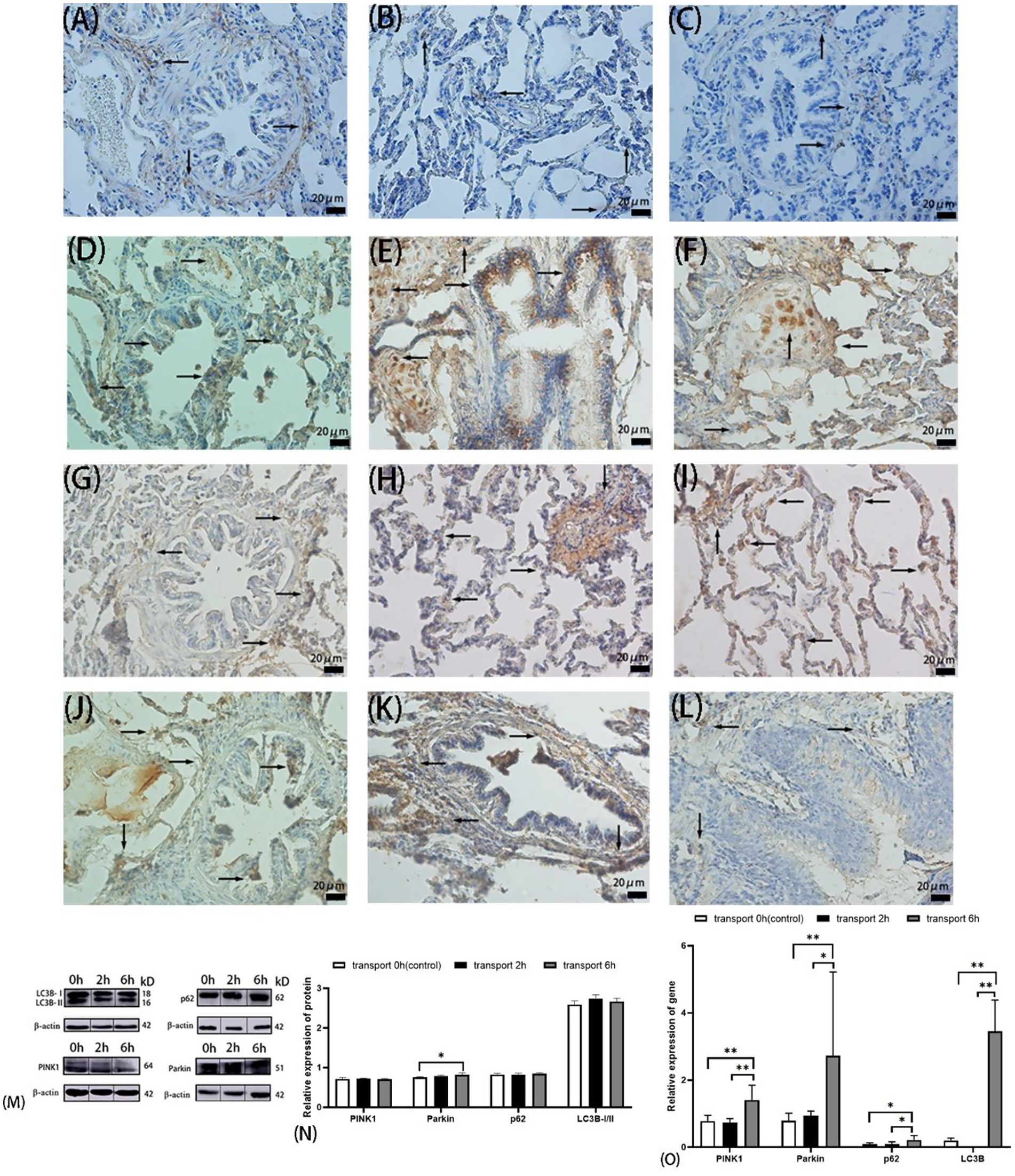
Figure 3. Autophagy in goat lungs (immunohistochemistry, western blot, and qRT PCR). (A–C) The distribution of LC3B protein. (D–F) The distribution of p62 protein. (G–I) The distribution of PINK1 protein. (J–L) The distribution of Parkin protein; (A,D,G,J) control groups; (B,E,H,K) 2 h transport groups; (C,F,I,L) 6 h transport groups; positive expression is shown by the arrows in the figure, immunoreactivity was graded as: strong (brown), moderate (yellow), weak (light yellow), or negative (no staining). (M) Protein bands of PINK1, Parkin, p62, LC3B-I, and LC3B-II, the number at the right end of Protein Blots represents the molecular weight of the corresponding protein, while the upper 0 h, 2 h, and 6 h correspond to the Western blot results of the control group, 2-h transportation group, and a 6-h transportation group, respectively; (N) Expression of PINK1, Parkin, p62, and LC3B-I/II protein; (O) Expression of PINK1, Parkin, p62, and LC3B gene, the heights of the white, black, and gray bars in bars (N,O) correspond to the relative expression levels of proteins and genes in the lungs of goats in the control group, 2-h transportation group and 6-h transportation group, respectively. *Significant difference (P < 0.05), **highly significant difference (P < 0.01). Data are presented as the means ± SD and the internal reference gene is β-actin.
2.6 Real-time fluorescence quantitative PCR
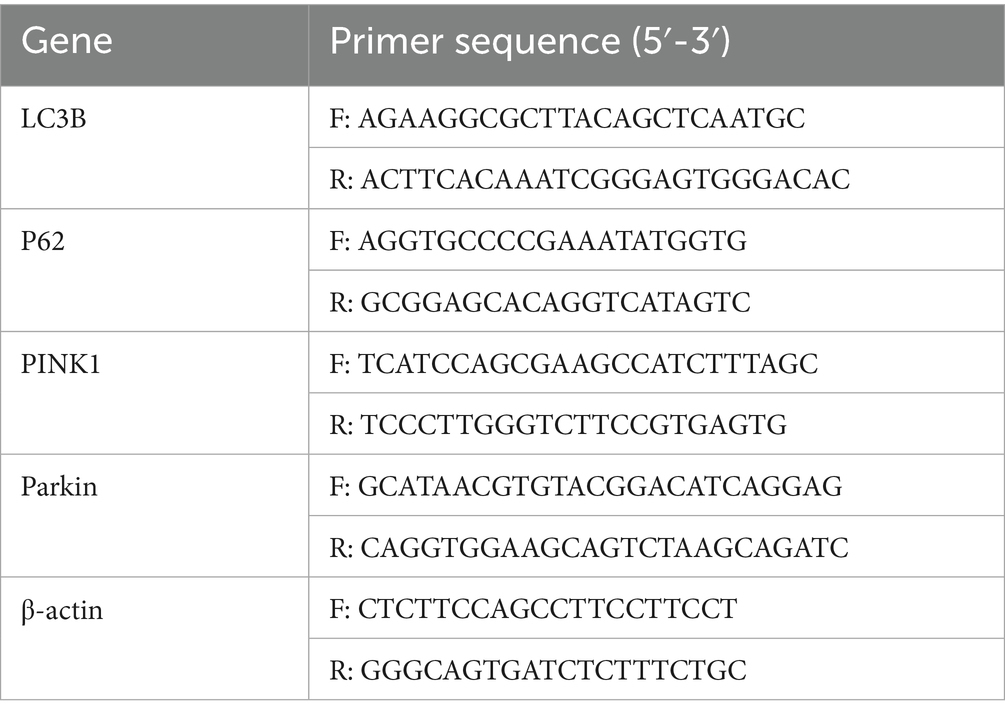
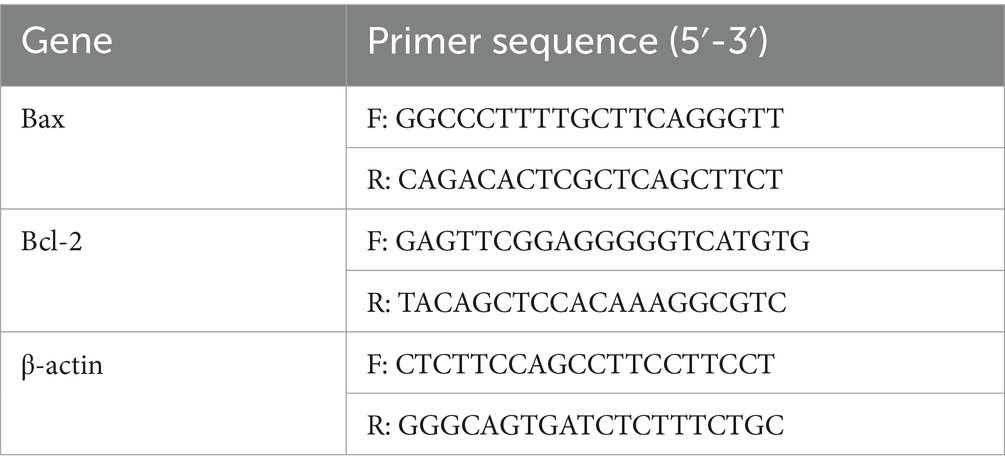
Total RNA was extracted using an HP Total RNA Kit (R6812, United States Omega Bio-Tek (Guangzhou), Guangzhou, China) and quantified spectrophotometrically. Reverse transcription was performed using HiScript II QRT SuperMix (10 μL reaction volume), with cDNA diluted in 20 μL ddH2O for subsequent qPCR analysis. Gene-specific primers were designed using Premier 6.0 software based on caprine reference sequences: Bcl-2 (NM001314213.1), Bax (XM018062750.1), and β-actin (NM001314342.1). Primers were designed using Primer Premier 6.0 (Premier Biosoft, Canada) software and sent to Guangzhou Huada Gene Medical Laboratory Co., Ltd. for synthesis. The primer sequences used in the experiment are shown in Tables 3, 4. qPCR was performed using ChamQ SYBR qPCR Master Mix (Q311-02, Vazyme Biotech Co., Ltd., Nanjing, China) in 10 μL reactions, containing 5 μL 2 × SYBR Primix Ex Taq II, 1 μL each of 4 μmol/L primers, 2.8 μL cDNA, and 0.2 μL 50 × ROX Reference Dye. Reactions were conducted on an ABI 7500 system with the following protocol: 95°C for 3 min; 40 cycles at 95°C for 10 s and at 68°C for 30 s. Relative expression was calculated using the 2-ΔΔCt method.
2.7 Statistical analysis
Statistical analysis was performed using SPSS 26.0 to compare protein and gene expression across transport durations. Parametric data from different groups were compared using one-way ANOVA, followed by the least significant difference (LSD) test. Significance levels were defined as: *p < 0.05, **p < 0.01. Data are presented as mean ± standard deviation (SD). Graphical representations were generated using GraphPad Prism 8.0.
3 Results
3.1 Effects of transportation stress on apoptosis and expression of Bax and Bcl-2 in goat lung cells
3.1.1 Apoptosis in goat lung cells
TUNEL assays revealed pulmonary apoptosis in both control and transported goats (Figures 1A–F). The 2 h transport group exhibited significantly higher apoptosis rates compared to both 6 h transport and control groups (p < 0.05), while no significant difference was observed between the control and 6 h groups (p > 0.05) (Figure 1G).
3.1.2 Localization of apoptosis-related proteins Bax and Bcl-2 in the lungs
Immunohistochemical analysis revealed similar Bax localization patterns across groups, predominantly in alveolar epithelial cytoplasm, bronchiolar epithelium, and select alveolar septal cells (Figures 2A–F). Bax expression was predominantly weak-to-moderate in the control and 6 h groups, while strong-to-moderate in the 2 h group. Bcl-2 exhibited similar cellular distribution but differential expression intensity: weak-to-moderate in the control and 6 h groups, contrasting with minimal expression in the 2 h group.
3.1.3 Expression of Bax and Bcl-2 proteins in goat lungs
Western blot analysis revealed no significant differences (p > 0.05) in Bcl-2, Bax expression, or Bcl-2/Bax ratios among the control, 2 h, and 6 h transport groups (Figure 2H).
3.1.4 Bax and Bcl-2 gene expression in goat lungs
qPCR analysis (Figure 2I) demonstrated significantly reduced Bax expression in the 2 h group compared to the control (p < 0.05) and 6 h groups (p < 0.01). Bcl-2 expression was significantly elevated in the 6 h group in comparison to the control and 2 h groups (p < 0.01), with no difference between the 2 h and control groups (p > 0.05). The Bcl-2/Bax ratio was significantly increased in both the transport groups compared to the controls (p < 0.01).
3.2 Effects of transportation stress on autophagy and expression of LC3B, p62, PINK1, and Parkin in goat lung cells
3.2.1 Expression and localization of autophagy-related proteins LC3B, p62, PINK1, and Parkin in goat lungs
Immunohistochemical analysis revealed moderate to strong cytoplasmic expression of all four autophagy-related proteins in alveolar epithelial cells (Figures 3A–L). LC3B, p62, and PINK1 exhibited similar localization patterns across the control and transported groups. LC3B was predominantly expressed in the cytoplasm of alveolar, bronchiolar, and arteriolar epithelial cells, with notably stronger brownish staining in the 2 h transport group compared to the control and 6 h transport groups. p62 showed no positive expression in the alveolar septa or terminal bronchiolar epithelial cells but displayed extensive, strong brown staining in the hyaline cartilage of small bronchiolar outer membranes (Figures 3E,F). The autophagy-related proteins PINK1 and Parkin were localized in both the nucleus and cytoplasm. Parkin expression in the 2 h and 6 h transport groups was widely distributed in the lungs, exhibiting light yellow or yellow staining, except in the alveolar septa. In contrast, Parkin expression in the control group and PINK1 expression across all groups were nearly absent in the bronchial regions, also referred to as the conductive portion of the lungs (Figures 3G–L).
3.2.2 Expression of LC3B, p62, PINK1, and Parkin proteins in goat lungs
Western blot analysis of autophagy-related proteins (Figure 3N) revealed no significant differences in the relative expression of p62, LC3B, or the LC3B-I/LC3B-II ratio among the control, 2 h transport, and 6 h transport groups (p > 0.05). However, Parkin expression was significantly upregulated in the 6 h transport group compared to the control (p < 0.05).
3.2.3 Expression of LC3B, p62, PINK1, and Parkin genes in goat lungs
qPCR analysis (Figure 3O) revealed that LC3B gene expression was significantly higher in the control group compared to the 2 h and 6 h transport groups (p < 0.01). In contrast, p62, PINK1, and Parkin gene expressions were significantly upregulated in the 6 h transport group (p < 0.05), with PINK1 and Parkin showing highly significant increases compared to the control group (p < 0.01). No significant differences were observed in p62, PINK1, or Parkin gene expressions between the control and 2 h transport groups (p > 0.05).
4 Discussion
Transportation exposes animals to compounded environmental stressors, including thermal discomfort, mechanical vibrations, and toxin exposure, which compromise their welfare by driving oxidative and thermal stress responses, as evidenced by elevated markers (Malondialdehyde, Reactive oxygen species, and DNA/RNA oxidative stress damage products) and upregulated heat shock proteins (HSP27, 70, 90) in goats (36–39). These disruptions exhibit pulmonary tropism, with respiratory dysfunction directly compromising welfare through impaired gas exchange and barrier function. Crucially, transport-induced immunosuppression and pulmonary damage facilitate pathogenic invasion, increasing susceptibility to severe respiratory pathologies, such as Bartonella pneumonia and acute respiratory distress syndrome (ARDS)—conditions directly linked to animal suffering and productivity losses (13, 40–42). Structural alterations in bronchial tissues and oxidative modulation of apoptotic (Bcl-2/Bax) and autophagic mediators (LC3B, PINK1, and Parkin) further underscore the molecular basis of transport-related welfare compromises (10, 43). The interplay of physical injury, inflammation, and stress-mediated cell death pathways highlights systemic welfare threats, with preclinical evidence suggesting apoptosis attenuation via Bcl-2/Bax modulation as a potential welfare-protective strategy (44, 45). By mapping these molecular cascades to organ-level dysfunction, this study identifies biomarkers (e.g., Heat shock proteins and oxidative markers) that could inform welfare-centric transport protocols, balancing ethical husbandry with sustainable livestock management.
Our findings demonstrate that transportation stress induces pulmonary apoptosis in goats, with TUNEL assays revealing significantly elevated apoptosis rates following 2 h of transport. Interestingly, the 6 h transport group exhibited apoptosis rates comparable to the controls, indicating a net reduction in apoptotic cells between 2 h and 6 h of transport. The observed apoptotic cell reduction suggests an intrinsic anti-stress mechanism that simultaneously inhibits apoptosis and facilitates apoptotic cell clearance in caprine pulmonary tissues during prolonged transport. Existing evidence demonstrates extensive crosstalk between stress-induced apoptotic and autophagic pathways. Autophagy and apoptosis share common inducers and exhibit bidirectional regulation, where autophagy prevents apoptosis by eliminating damaged mitochondria and associated death signals, while mitochondrial release of cytochrome c during autophagy can activate caspase-mediated apoptosis. Furthermore, PINK1 and Parkin modulate apoptotic signaling through Bax and p53 regulation. Apoptotic regulators, including p53 and Bcl-2 family proteins, modulate mitochondrial membrane stability, thereby influencing mitophagy. Additional crosstalk mediators encompass ERLIN and Beclin1, among others (33, 46, 47). The apoptotic–autophagic crosstalk is critically regulated by calcium-mediated signaling pathways (35, 48). qPCR analysis revealed significant upregulation of the Bcl-2/Bax ratio following 2 h transport, while LC3B, p62, PINK1, and Parkin expression increased after 6 h transport. These findings indicate time-dependent autophagosome formation and ubiquitin-mediated mitophagy activation in response to transportation stress. The concurrent upregulation of anti-apoptotic Bcl-2 and autophagy-related genes with prolonged transport duration demonstrates functional crosstalk between apoptotic and autophagic pathways in the studied cellular system. This regulatory mechanism likely represents stress-induced apoptosis triggering mitophagy, thereby establishing a cytoprotective feedback loop in caprine pulmonary cells. Apoptotic cell clearance involves a coordinated mechanism combining classical phagocytosis, autophagic processes, and alveolar macrophage-mediated efferocytosis within the pulmonary mucosal and interstitial compartments. Crucially, autophagy maintains cellular ATP homeostasis, thereby facilitating efficient apoptotic cell clearance through these coordinated mechanisms (33, 49, 50). TUNEL assays indicated rapid apoptosis induction within 0–2 h of transport, coinciding with stable autophagy-related gene expression. This temporal pattern suggests that transport-induced DNA damage or death receptor activation triggers immediate apoptosis, potentially suppressing concurrent autophagic responses during this amplification phase. However, the temporal relationship between stress-induced apoptosis and autophagy requires further investigation (35). Notably, baseline apoptosis was observed in non-transported controls, indicating constitutive expression of apoptotic regulators Bax and Bcl-2 in caprine pulmonary epithelium. Their balanced interaction maintains epithelial cell population homeostasis.
Immunohistochemical analysis revealed comparable Bax and Bcl-2 expression patterns across control and transport groups, with predominant localization in alveolar epithelium, bronchiolar epithelium, and select alveolar septa. Most cells in the controls and 6 h transport groups exhibited weak-to-moderate Bax/Bcl-2 expression. In contrast, the 2 h group showed strong-to-moderate Bax expression with minimal Bcl-2 detection, correlating with elevated apoptosis rates through altered Bcl-2/Bax expression dynamics. All four autophagy markers demonstrated positive alveolar epithelial expression, with LC3B, p62, and PINK1 exhibiting consistent cellular localization patterns across control and transport groups. LC3B exhibited predominant cytoplasmic localization in alveolar, bronchiolar, and arteriolar epithelial cells, while p62 demonstrated intense immunoreactivity in bronchiolar hyaline cartilage. PINK1 and Parkin demonstrated nuclear and cytoplasmic localization. Parkin exhibited moderate pulmonary expression (excluding alveolar septa) in transport groups, while both Parkin in controls and PINK1 showed minimal bronchiolar expression across all airway levels (Figures 2G,J). Immunohistochemical analysis revealed transport stress-induced apoptosis and autophagy in specific pulmonary regions. Previous studies have demonstrated similar stress-mediated apoptosis in caprine immune organs (intestinal epithelium, spleen, and lymph nodes), resulting in immunosuppression and subsequent pulmonary macrophage depletion (16, 49, 50). Transport stress induces cardiac enzyme accumulation and mitochondrial dysfunction, promoting excessive mitophagy. Notably, mitochondrial complex I in pulmonary epithelium is crucial for lung development, indicating that integrated mitochondrial stress responses determine epithelial cell fate (51, 52). In summary, transport-induced apoptosis compromises pulmonary integrity through dual mechanisms: direct pathogen-mediated alveolar-capillary barrier disruption and indirect structural–functional impairment via immunosuppression, collectively impairing respiratory and immune functions.
Western blot analysis revealed comparable expression levels of Bcl-2, Bax, LC3B, PINK1, and p62 across all experimental groups. Parkin expression was significantly elevated in the 6 h transport group compared to the controls. The discordance between gene and protein expression patterns of Bax and Bcl-2 in transport groups suggests a temporal lag, where rapid transcriptional activation precedes protein translation completion. Similarly, the observed gene-protein expression discrepancy in autophagy markers (LC3B, p62, PINK1, and Parkin) likely reflects differential kinetics between transcriptional activation and protein synthesis. Notably, similar gene–protein expression discrepancies have been observed in caprine spleen, lymph nodes, and intestinal tissues following transport stress, suggesting a systemic temporal dissociation between transcriptional and translational regulation of apoptotic markers. This phenomenon reflects delayed protein synthesis kinetics relative to transcriptional activation (7). However, tissue-specific variations in gene-protein expression correlations likely reflect molecular and cellular context-dependent regulatory mechanisms.
Predominant Bax expression during initial transport phases was revealed by qPCR analysis, indicating early apoptotic activation. Following 2 h and 6 h transport, pulmonary Bcl-2/Bax ratios significantly increased compared to the controls, demonstrating time-dependent predominance of anti-apoptotic signaling. Notably, autophagy-related genes (LC3B, p62, PINK1, and Parkin) exhibited significant upregulation following 6 h transport, coinciding with reduced apoptotic activity. These observations indicate a potential mechanism whereby stress-induced apoptosis can initiate ubiquitin-dependent mitophagy, which can subsequently reduce apoptotic risk through clearance of mitochondria containing death signals, potentially offering cytoprotection in caprine pulmonary cells. Previous studies have demonstrated context-dependent protective roles of autophagy against cell death. For instance, reticulum-mediated autophagy mitigates PM2.5-induced endothelial apoptosis, while cadmium-exposed trophoblasts exhibit p62-dependent caspase-9 degradation through autophagy activation. Notably, cadmium-induced autophagy appears apoptosis-dependent, with autophagy-related gene 4B (Atg4B)-Bcl-2 interactions potentially modulating apoptotic-autophagic crosstalk via Bcl-2-Beclin1 dissociation (46, 53, 54). Additional stress-responsive mechanisms may contribute to cellular protection, including the nuclear factor-erythroid 2-related factor 2 pathway-mediated regulation of oxidative stress markers (Reactive oxygen species, Heat shock proteins, Nitric oxide), which collectively exert antioxidant and anti-apoptotic effects (29, 55). The elevated apoptosis rate in the 2 h transport group, contrasting with gene expression patterns, may reflect delayed clearance of stress-induced apoptotic cells by pulmonary scavenging mechanisms. This temporal pattern, coupled with early Bax downregulation in the 2 h group, suggests rapid and intense apoptotic activation during initial transport phases in caprine pulmonary cells.
The interplay between autophagy and apoptosis critically influences pulmonary health, with direct implications for animal welfare under transport stress. In fibrotic lung diseases (idiopathic pulmonary fibrosis, cystic fibrosis, and silicosis), autophagy modulates disease progression through fibroblast apoptosis regulation and epithelial cell senescence, while dysregulated macrophage autophagy exacerbates fibrosis mechanisms, underscoring the delicate balance required to maintain respiratory welfare (44, 56, 57). Similarly, preterm neonates with bronchopulmonary dysplasia exhibit oxidative stress-driven programmed cell death pathways (Notch4/SIRT1/P53/Bax, RIPK3/NF-κB) that impair alveolar development, paralleling transport stress scenarios where pulmonary structural integrity is compromised (58, 59). The mitochondrial PINK1/Parkin pathway’s role in attenuating inflammation and apoptosis further highlights potential therapeutic targets to mitigate welfare risks (46, 60). Critically, prolonged transport stress disrupts the apoptosis–autophagy equilibrium, with excessive apoptosis threatening pulmonary homeostasis and respiratory function, which is a welfare concern linked to impaired physiological resilience. Conversely, stress-induced autophagy may serve as an adaptive mechanism to counterbalance apoptotic cell death (61–63). This study demonstrates that transport stress induces apoptosis and autophagy in lung cells. Significant alterations in the expression of apoptosis-related genes (Bax and Bcl-2) and autophagy-related genes (LC3B, p62, PINK1, and Parkin) were observed in goat lungs following varying transport durations, highlighting their roles in transport stress-mediated apoptosis and autophagy. These findings emphasize the need to optimize transport durations and conditions to preserve pulmonary autophagy–apoptosis crosstalk, thereby safeguarding respiratory welfare in livestock systems.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by Animal Care and Use Committee of Yichun University, Yichun, China (License number: JXSTUDKY2019009). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YZ: Conceptualization, Data curation, Methodology, Visualization, Writing – original draft, Writing – review & editing. YH: Conceptualization, Formal analysis, Methodology, Writing – review & editing. YJ: Conceptualization, Writing – review & editing. TY: Conceptualization, Data curation, Investigation, Methodology, Resources, Writing – review & editing. YY: Conceptualization, Investigation, Methodology, Project administration, Writing – review & editing. BL: Conceptualization, Data curation, Funding acquisition, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. WZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Software, Supervision, Validation, Visualization, Writing – review & editing. SD: Formal analysis, Methodology, Writing – review & editing. XY: Data curation, Supervision, Validation, Writing – review & editing. LZ: Investigation, Methodology, Project administration, Validation, Writing – review & editing. WH: Funding acquisition, Investigation, Methodology, Software, Supervision, Writing – review & editing. MF: Data curation, Formal analysis, Methodology, Resources, Supervision, Validation, Writing – review & editing. WY: Funding acquisition, Investigation, Methodology, Writing – review & editing. WX: Methodology, Project administration, Software, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Science and Technology Project of the Education Department of Jiangxi Province, Grant/Award Number: GJJ201622/GJJ211630, and Jiangxi Modern Agricultural Scientific Research Collaborative Innovation Project, Grant/Award Number: JXXTCX201702.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. The author(s) declare that generative artificial intelligence (AI) tools were used during the preparation of this manuscript. Specifically, DeepSeek was employed to assist in reviewing grammar, syntax, spelling, and sentence structure. After utilizing this tool, the author(s) rigorously revised the content, verified its accuracy, and accept full responsibility for the integrity and originality of the final published work.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pascual-Alonso, M, Miranda-de la Lama, GC, Aguayo-Ulloa, L, Villarroel, M, Mitchell, M, and María, GA. Thermophysiological, haematological, biochemical and behavioural stress responses of sheep transported on road. J Anim Physiol Anim Nutr (Berl). (2017) 101:541–51. doi: 10.1111/jpn.12455
2. Zappaterra, M, Faucitano, L, and Nanni Costa, L. Road transport: a review of its effects on the welfare of piglets. Animals (Basel). (2023) 13:1604. doi: 10.3390/ani13101604
3. Alcalde, MJ, Suárez, MD, Rodero, E, Álvarez, R, Sáez, MI, and Martínez, TF. Effects of farm management practices and transport duration on stress response and meat quality traits of suckling goat kids. Animal. (2017) 11:1626–35. doi: 10.1017/S1751731116002858
4. Jacobs, L, Delezie, E, Duchateau, L, Goethals, K, Ampe, B, Buyse, J, et al. Impact of transportation duration on stress responses in day-old chicks from young and old breeders. Res Vet Sci. (2017) 112:172–6. doi: 10.1016/j.rvsc.2017.04.015
5. Schwartzkopf-Genswein, KS, Faucitano, L, Dadgar, S, Shand, P, González, LA, and Crowe, TG. Road transport of cattle, swine and poultry in North America and its impact on animal welfare, carcass and meat quality: a review. Meat Sci. (2012) 92:227–43. doi: 10.1016/j.meatsci.2012.04.010
6. Earley, B, Drennan, M, and O’Riordan, EG. The effect of road transport in comparison to a novel environment on the physiological, metabolic and behavioural responses of bulls. Res Vet Sci. (2013) 95:811–8. doi: 10.1016/j.rvsc.2013.04.027
7. Peng, R, Gao, F, Hu, Y, Li, K, Liu, B, Zheng, W, et al. Effects of transport stress on the oxidative index, apoptosis and autophagy in the small intestine of caprine. BMC Vet Res. (2023) 19:117. doi: 10.1186/s12917-023-03670-9
8. Kamiya, M, Carter, H, Espindola, MS, Doyle, TJ, Lee, JS, Merriam, LT, et al. Immune mechanisms in fibrotic interstitial lung disease. Cell. (2024) 187:3506–30. doi: 10.1016/j.cell.2024.05.015
9. Shaikh, SB, Goracci, C, Tjitropranoto, A, and Rahman, I. Impact of aging on immune function in the pathogenesis of pulmonary diseases: potential for therapeutic targets. Expert Rev Respir Med. (2023) 17:351–64. doi: 10.1080/17476348.2023.2205127
10. Zheng, W, Liu, B, Hu, W, and Cui, Y. Effects of transport stress on pathological injury and main heat shock protein expression in the respiratory system of goats. J Anim Physiol Anim Nutr (Berl). (2021) 105:1–13. doi: 10.1111/jpn.13430
11. Akalu, M, Vemulapati, B, Abayneh, T, Degefa, T, Deresse, G, and Gelaye, E. Serotyping, antibiogram, and detection of bacterial pathogens associated with bovine respiratory disease in selected areas of Ethiopia. Ir Vet J. (2022) 75:3. doi: 10.1186/s13620-022-00210-z
12. Cool, K, Gaudreault, NN, Trujillo, JD, Morozov, I, McDowell, CD, Bold, D, et al. Experimental co-infection of calves with SARS-CoV-2 Delta and omicron variants of concern. Emerg Microbes Infect. (2024) 13:2281356. doi: 10.1080/22221751.2023.2281356
13. Lawrence, PK, and Dassanayake, RP. Ovis aries CR4 is involved in Mannheimia haemolytica leukotoxin-induced cytotoxicity. Vet Immunol Immunopathol. (2010) 135:266–74. doi: 10.1016/j.vetimm.2009.12.007
14. Zhang, Y, Li, J, Qiu, Z, Huang, L, Yang, S, Li, J, et al. Insights into the mechanism of action of pterostilbene against influenza a virus-induced acute lung injury. Phytomedicine. (2024) 129:155534. doi: 10.1016/j.phymed.2024.155534
15. Cheng, KT, Xiong, S, Ye, Z, Hong, Z, Di, A, Tsang, KM, et al. Caspase-11-mediated endothelial pyroptosis underlies endotoxemia-induced lung injury. J Clin Invest. (2017) 127:4124–35. doi: 10.1172/JCI94495
16. Matsuyama, S, Palmer, J, Bates, A, Poventud-Fuentes, I, Wong, K, Ngo, J, et al. Bax-induced apoptosis shortens the life span of DNA repair defect Ku70-knockout mice by inducing emphysema. Exp Biol Med (Maywood). (2016) 241:1265–71. doi: 10.1177/1535370216654587
17. Tian, X, Wang, M, Ying, X, Dong, N, Li, M, Feng, J, et al. Co-exposure to arsenic and fluoride to explore the interactive effect on oxidative stress and autophagy in myocardial tissue and cell. Ecotoxicol Environ Saf. (2023) 253:114647. doi: 10.1016/j.ecoenv.2023.114647
18. Singh, R, Letai, A, and Sarosiek, K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. (2019) 20:175–93. doi: 10.1038/s41580-018-0089-8
19. O’Reilly, LA, and Strasser, A. Apoptosis and autoimmune disease. Inflamm Res. (1999) 48:5–21. doi: 10.1007/s000110050369
20. Pritchard, DM, and Watson, AJ. Apoptosis and gastrointestinal pharmacology. Pharmacol Ther. (1996) 72:149–69. doi: 10.1016/s0163-7258(96)00102-7
21. Pattingre, S, and Levine, B. Bcl-2 inhibition of autophagy: a new route to cancer? Cancer Res. (2006) 66:2885–8. doi: 10.1158/0008-5472.CAN-05-4412
22. Sasaki, H, Hirose, T, Oura, T, Otsuka, R, Rosales, I, Ma, D, et al. Selective Bcl-2 inhibition promotes hematopoietic chimerism and allograft tolerance without myelosuppression in nonhuman primates. Sci Transl Med. (2023) 15:eadd5318. doi: 10.1126/scitranslmed.add5318
23. Youle, RJ, and Strasser, A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. (2008) 9:47–59. doi: 10.1038/nrm2308
24. Levine, B, and Kroemer, G. Biological functions of autophagy genes: a disease perspective. Cell. (2019) 176:11–42. doi: 10.1016/j.cell.2018.09.048
25. Mizushima, N, and Komatsu, M. Autophagy: renovation of cells and tissues. Cell. (2011) 147:728–41. doi: 10.1016/j.cell.2011.10.026
26. Naldurtiker, A, Batchu, P, Kouakou, B, Terrill, TH, McCommon, GW, and Kannan, G. Differential gene expression analysis using RNA-seq in the blood of goats exposed to transportation stress. Sci Rep. (2023) 13:1984. doi: 10.1038/s41598-023-29224-5
27. Levine, B, and Kroemer, G. Autophagy in the pathogenesis of disease. Cell. (2008) 132:27–42. doi: 10.1016/j.cell.2007.12.018
28. Feng, Y, Chen, Y, Wu, X, Chen, J, Zhou, Q, Liu, B, et al. Interplay of energy metabolism and autophagy. Autophagy. (2024) 20:4–14. doi: 10.1080/15548627.2023.2247300
29. Mizushima, N, and Levine, B. Autophagy in human diseases. N Engl J Med. (2020) 383:1564–76. doi: 10.1056/NEJMra2022774
30. Awan, MUF, and Deng, Y. Role of autophagy and its significance in cellular homeostasis. Appl Microbiol Biotechnol. (2014) 98:5319–28. doi: 10.1007/s00253-014-5721-8
31. Onorati, AV, Dyczynski, M, Ojha, R, and Amaravadi, RK. Targeting autophagy in cancer. Cancer. (2018) 124:3307–18. doi: 10.1002/cncr.31335
32. Chi, R-F, Li, L, Wang, A-L, Yang, H, Xi, J, Zhu, Z-F, et al. Enhanced oxidative stress mediates pathological autophagy and necroptosis in cardiac myocytes in pressure overload induced heart failure in rats. Clin Exp Pharmacol Physiol. (2022) 49:60–9. doi: 10.1111/1440-1681.13583
33. Maiuri, MC, Zalckvar, E, Kimchi, A, and Kroemer, G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. (2007) 8:741–52. doi: 10.1038/nrm2239
34. Chen, R, Zou, J, Zhong, X, Li, J, Kang, R, and Tang, D. HMGB1 in the interplay between autophagy and apoptosis in cancer. Cancer Lett. (2024) 581:216494. doi: 10.1016/j.canlet.2023.216494
35. Sorice, M. Crosstalk of autophagy and apoptosis. Cells. (2022) 11:1479. doi: 10.3390/cells11091479
36. Buczyńska, A, Sidorkiewicz, I, Kościuszko, M, Adamska, A, Siewko, K, Dzięcioł, J, et al. Clinical significance of oxidative stress markers as angioinvasion and metastasis indicators in papillary thyroid cancer. Sci Rep. (2023) 13:13711. doi: 10.1038/s41598-023-40898-9
37. Wang, R, Fortier, TM, Chai, F, Miao, G, Shen, JL, Restrepo, LJ, et al. PINK1, Keap1, and Rtnl1 regulate selective clearance of endoplasmic reticulum during development. Cell. (2023) 186:e18:4172–88. doi: 10.1016/j.cell.2023.08.008
38. Xu, H-L, Li, H, Bao, R-K, Tang, Y-X, Elsherbeni, AIA, Gharib, HBA, et al. Transport stress induced cardiac NO-NOS disorder is mitigated by activating Nrf2/HO-1/NQO1 antioxidant defense response in newly hatched chicks. Front Vet Sci. (2022) 9:938826. doi: 10.3389/fvets.2022.938826
39. Refaey, MM, and Li, D. Transport stress changes blood biochemistry, antioxidant defense system, and hepatic HSPs mRNA expressions of channel catfish Ictalurus punctatus. Front Physiol. (2018) 9:1628. doi: 10.3389/fphys.2018.01628
40. Christenson, SA, Smith, BM, Bafadhel, M, and Putcha, N. Chronic obstructive pulmonary disease. Lancet. (2022) 399:2227–42. doi: 10.1016/S0140-6736(22)00470-6
41. Lucas, R, Hadizamani, Y, Gonzales, J, Gorshkov, B, Bodmer, T, Berthiaume, Y, et al. Impact of bacterial toxins in the lungs. Toxins (Basel). (2020) 12:223. doi: 10.3390/toxins12040223
42. Pauwels, RA, and Rabe, KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet. (2004) 364:613–20. doi: 10.1016/S0140-6736(04)16855-4
43. Hu, W, Fang, M, Yang, Y, Ye, T, Liu, B, and Zheng, W. Detection of heat shock protein 27, 70, 90 expressions in primary parenchymatous organs of goats after transport stress by real-time PCR and ELISA. Vet Med Sci. (2020) 6:788–95. doi: 10.1002/vms3.327
44. Ornatowski, W, Lu, Q, Yegambaram, M, Garcia, AE, Zemskov, EA, Maltepe, E, et al. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol. (2020) 36:101679. doi: 10.1016/j.redox.2020.101679
45. Muruganandah, V, and Kupz, A. Immune responses to bacterial lung infections and their implications for vaccination. Int Immunol. (2022) 34:231–48. doi: 10.1093/intimm/dxab109
46. Li, Z, Li, Q, Lv, W, Jiang, L, Geng, C, Yao, X, et al. The interaction of Atg4B and Bcl-2 plays an important role in cd-induced crosstalk between apoptosis and autophagy through disassociation of Bcl-2-Beclin1 in A549 cells. Free Radic Biol Med. (2019) 130:576–91. doi: 10.1016/j.freeradbiomed.2018.11.020
47. Debnath, J, Gammoh, N, and Ryan, KM. Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol. (2023) 24:560–75. doi: 10.1038/s41580-023-00585-z
48. Gordy, C, and He, YW. The crosstalk between autophagy and apoptosis: where does this lead? Protein Cell. (2012) 3:17–27. doi: 10.1007/s13238-011-1127-x
49. Pellegrini, FR, De Martino, S, Fianco, G, Ventura, I, Valente, D, Fiore, M, et al. Blockage of autophagosome-lysosome fusion through SNAP29 O-GlcNAcylation promotes apoptosis via ROS production. Autophagy. (2023) 19:2078–93. doi: 10.1080/15548627.2023.2170962
50. Zheng, DJ, Abou Taka, M, and Heit, B. Role of apoptotic cell clearance in pneumonia and inflammatory lung disease. Pathogens. (2021) 10:134. doi: 10.3390/pathogens10020134
51. Han, S, Lee, M, Shin, Y, Giovanni, R, Chakrabarty, RP, Herrerias, MM, et al. Mitochondrial integrated stress response controls lung epithelial cell fate. Nature. (2023) 620:890–7. doi: 10.1038/s41586-023-06423-8
52. Jones, AJ, Blaza, JN, Varghese, F, and Hirst, J. Respiratory complex I in Bos taurus and Paracoccus denitrificans pumps four protons across the membrane for every NADH oxidized. J Biol Chem. (2017) 292:4987–95. doi: 10.1074/jbc.M116.771899
53. Wang, Y, and Tang, M. PM2.5 induces autophagy and apoptosis through endoplasmic reticulum stress in human endothelial cells. Sci Total Environ. (2020) 710:136397. doi: 10.1016/j.scitotenv.2019.136397
54. Zhu, H-L, Xu, X-F, Shi, X-T, Feng, Y-J, Xiong, Y-W, Nan, Y, et al. Activation of autophagy inhibits cadmium-triggered apoptosis in human placental trophoblasts and mouse placenta. Environ Pollut. (2019) 254:112991. doi: 10.1016/j.envpol.2019.112991
55. Hotchkiss, RS, Schmieg, RE, Swanson, PE, Freeman, BD, Tinsley, KW, Cobb, JP, et al. Rapid onset of intestinal epithelial and lymphocyte apoptotic cell death in patients with trauma and shock. Crit Care Med. (2000) 28:3207–17. doi: 10.1097/00003246-200009000-00016
56. Guan, R, Yuan, L, Li, J, Wang, J, Li, Z, Cai, Z, et al. Bone morphogenetic protein 4 inhibits pulmonary fibrosis by modulating cellular senescence and mitophagy in lung fibroblasts. Eur Respir J. (2022) 60:2102307. doi: 10.1183/13993003.02307-2021
57. Lee, YY, Han, JI, Lee, KE, Cho, S, and Suh, EC. Neuroprotective effect of dexmedetomidine on autophagy in mice administered intracerebroventricular injections of Aβ25-35. Front Pharmacol. (2023) 14:1184776. doi: 10.3389/fphar.2023.1184776
58. Liu, C, Fu, C, Sun, Y, You, Y, Wang, T, Zhang, Y, et al. Itaconic acid regulation of TFEB-mediated autophagy flux alleviates hyperoxia-induced bronchopulmonary dysplasia. Redox Biol. (2024) 72:103115. doi: 10.1016/j.redox.2024.103115
59. Deng, X, Bao, Z, Yang, X, Mei, Y, Zhou, Q, Chen, A, et al. Molecular mechanisms of cell death in bronchopulmonary dysplasia. Apoptosis. (2023) 28:39–54. doi: 10.1007/s10495-022-01791-4
60. Lv, W, Sui, L, Yan, X, Xie, H, Jiang, L, Geng, C, et al. ROS-dependent Atg4 upregulation mediated autophagy plays an important role in cd-induced proliferation and invasion in A549 cells. Chem Biol Interact. (2018) 279:136–44. doi: 10.1016/j.cbi.2017.11.013
61. Cao, Y, Chen, X, Pan, F, Wang, M, Zhuang, H, Chen, J, et al. Xinmaikang-mediated mitophagy attenuates atherosclerosis via the PINK1/Parkin signaling pathway. Phytomedicine. (2023) 119:154955. doi: 10.1016/j.phymed.2023.154955
62. Zhao, H, Wang, Y, Qiu, T, Liu, W, and Yao, P. Autophagy, an important therapeutic target for pulmonary fibrosis diseases. Clin Chim Acta. (2020) 502:139–47. doi: 10.1016/j.cca.2019.12.016
Keywords: transport stress, apoptosis, autophagy, Bcl-2/Bax, PINK1/Parkin
Citation: Zhuo Y, Hu Y, Jin Y, Ye T, Yang Y, Liu B, Zheng W, Ding S, Yang X, Zheng L, Hu W, Fang M, Yi W and Xing W (2025) Effect of transport stress on apoptosis and autophagy in goat lung cells. Front. Vet. Sci. 12:1585008. doi: 10.3389/fvets.2025.1585008
Edited by:
Honghong He, Gansu Agricultural University, ChinaReviewed by:
Carol Geralyn Chitko-McKown, U.S. Meat Animal Research Center, United StatesYangyang Pan, Gansu Agricultural University, China
Copyright © 2025 Zhuo, Hu, Jin, Ye, Yang, Liu, Zheng, Ding, Yang, Zheng, Hu, Fang, Yi and Xing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ben Liu, bGl1YmVucmVzQDE2My5jb20=; Wenya Zheng, emhlbmd3ZW55YXJlc0AxNjMuY29t
 Yu Zhuo
Yu Zhuo Yunhai Hu1
Yunhai Hu1 Lucheng Zheng
Lucheng Zheng Manxin Fang
Manxin Fang