- 1Department of Veterinary Diagnostic and Production Animal Medicine, College of Veterinary Medicine, Iowa State University, Ames, IA, United States
- 2Genus plc PIC, Hendersonville, TN, United States
Introduction: A risk-based approach to animal selection for sampling enhances pathogen detection by increasing the probability of selecting an animal harboring the pathogen while requiring a smaller sample size. Postmortem tongue fluids (TF) have emerged as a promising risk-based approach, with a PRRSV RNA positivity rate similar to serum, processing fluids, and family oral fluids. Thus, this study assessed the effect of stillborn presence, litter size, and PRRSV RNA detection by RT-qPCR in stillborn TF on the probability of having viremic piglets within the litter.
Methods: Samples from 130 litters were collected within 12 hours after farrowing from two breeding herds. TF and intracardiac blood were collected from stillborns, and tail blood swabs were collected from liveborn littermates within the selected litters. Samples were individually tested for PRRSV RNA detection by RT-qPCR. Litters with ≤ 11 liveborn piglets were defined as small. Generalized linear regression models were used to evaluate the litter size, presence of stillborns, and stillborn PRRSV results on the probability that a litter or at least one liveborn littermate would test PRRSV-positive.
Results: The live piglets’ mean positivity within the litter was 5.0%, while the total born was 4.6%. Litters with at least one stillborn had 12.5 times higher odds of having a PRRSV-positive result, and 4.8 times higher odds of having at least one viremic liveborn piglet. In small litters, the odds of having a PRRSV-positive result increased 12.2 times, whereas the odds of having a viremic liveborn littermate increased 10.8 times. When the stillborn TF was positive, the odds of having a viremic liveborn littermate increased 17.6 times.
Discussion: In conclusion, stillborn TFs were a reliable indicator of PRRSV status among litters. Liveborn piglets from litters with PRRSV-positive stillborn TF or small litters had greater odds of testing PRRSV-positive. Therefore, stillborn TF collection and targeting small litters improve PRRSV detection and support farrowing room biocontainment strategies.
1 Introduction
Surveillance through periodic collection of porcine biological samples is essential for objectively classifying breeding herds undergoing porcine reproductive and respiratory syndrome virus (PRRSV) control and elimination (1). Specifically, as the virus prevalence within a herd declines, identifying PRRSV circulation by reverse transcription polymerase chain reaction (RT-qPCR) testing requires a larger sample size and more sensitive sample types to ensure timely detection (2), which highly encouraged researchers to develop population-based samplings for a wider population screening (3–6). However, PRRSV-positive pigs are often clustered in barns, with non-homogeneous distribution among the population (7, 8). To overcome this challenge, targeted sampling, also known as risk-based sampling, can be adopted (9).
Risk-based sampling involves stratifying the source population based on characteristics associated with the probability of pathogen occurrence (10, 11). Thus, the advantage of risk-based sampling lies in its efficiency (i.e., it maximizes the probability of detection), particularly when the disease occurs at low prevalence rates (9). For instance, in the context of PRRSV, younger parities are more susceptible to the virus than older parities (12), and PRRSV can cause embryonic death in early gestation, clinical manifestation in late gestation, and is characterized by increased abortions and stillborn piglets, fetal death, and early farrowing (13–15).
Population-based samples combined with a risk-based approach have been reported as an effective method to enhance on-farm PRRSV detection with a lower sample size than traditional recommendations. For instance, collecting family oral fluids (FOF), a sample originating from suckling litters before weaning, from young parity litters (≤ 2 parity) or small litter sizes (≤ 11 piglets) can increase the odds of detecting PRRSV by 3.4 and 9.9 times, respectively (7, 16). Similarly, Vilalta et al. (17) reported that collecting processing fluids (PF), a fluid recovered from tissues collected at castration from piglets, from young parity female litters should be prioritized for PRRSV RNA detection by RT-qPCR. Following this concept, tongue fluid (TF) appears to align with the risk-based category, as it targets dead animals (6). Moreover, it has been described as an alternative population-based sample with similar positivity to FOF, PF, and serum samples (18). Also, Dürlinger et al. (19) reported in a longitudinal field study that TF had the highest viral load when examined at a litter level compared to serum, PF, and oral fluids.
Since PRRSV can be transmitted from infected sows to their fetuses during late gestation, vertical transmission represents an important source of PRRSV spread within swine populations (20, 21). However, a significant gap in the current monitoring programs is the lack of a convenient, population-based approach to detect PRRSV circulation within a gestating population. An alternative approach that might be used to indirectly measure PRRSV circulation in the gestating population is by collecting samples from the offspring that were possibly vertically infected. TF collected from stillborn piglets were reported as a well-suited sample for detecting vertical transmission within the herds, with a strong positive correlation and high level of agreement in PRRSV RNA load in TF and serum from stillborn piglets (19, 22). Moreover, collecting TF from stillborn pigs was demonstrated to have a higher PCR positivity and lower cycle threshold (Ct) value than serum from liveborn littermate pigs and PF from the same litter (23). However, less is known about how PRRSV-RNA positive results in stillborn piglets reflect the PRRSV status of their liveborn littermates, suggesting an important information gap, as it potentially contributes to the further virus spreading across farrowing room populations.
Therefore, based on PRRSV dynamics, such as increased stillborn rates and decreased liveborn piglets per sow, this study evaluated PRRSV monitoring programs’ effectiveness in using TF from stillborn piglets and how results reflected liveborn littermates’ PRRSV status in commercial breeding herds. Secondly, this study assessed the probability of a PRRSV RNA-positive result in litters with at least one stillborn compared to those without and the association of litter size on PRRSV-RNA detection within the litter.
2 Materials and methods
2.1 Study design
A cross-sectional field study was conducted to estimate the association of the presence of stillborn piglets, litter size, and stillborn TF results on the probability of having viremic piglets in the litter. From two PRRSV-unstable breeding herds (2), samples were collected from liveborn and stillborn piglets from 130 litters within 12 h after farrowing to assess PRRSV status at birth. TF and intracardiac blood (IB) were collected from the stillborn piglets, and tail blood swabs (BS) were collected from the liveborn littermates. Samples were individually tested for PRRSV RNA by RT-qPCR by the investigator.
Generalized linear regression models were used to evaluate the following: whether the presence of stillborn piglets had an effect on detecting PRRSV in the litter and liveborn piglets; if PRRSV RT-qPCR results from stillborn TF and IB were indicators of PRRSV status in liveborn piglets; and whether litter size impacted the probability that at least one live littermate tested PRRSV-RNA positive. This study was approved by the Institutional Animal Care and Use Committee (IACUC) of Iowa State University, IA, USA, under protocol IACUC-22-101 “Field surveillance for swine pathogens” and IBC-24-096 “Field surveillance of swine pathogens using post-mortem tissues”.
2.2 Inclusion criteria for breeding herd selection
To obtain a satisfactory proportion of PRRSV RNA-positive samples from piglets, two PRRSV-positive unstable breeding herds (Herds A and B) in the Midwestern USA undergoing PRRSV elimination were selected based on weekly PRRSV RNA detection in PF from the suckling pig population. Both herds broke with PRRSV-2 RFLP 1–8-4 Lineage 1H (24) and immediately underwent live virus inoculation (LVI) protocols after PRRSV-2 wild-type detection. Herd A was a 2,500-sow farm with a four-week batch farrowing system that received LVI 120 days before the first sampling day; Herd B was a 7,000-sow farm with a weekly batch farrowing system that received LVI 107 days before the first sampling day.
2.3 Sample collection and processing
Over a consecutive period of 5 days in Herd A and 9 days in Herd B, within 12 h of farrowing, a total of 130 litters from both herds were sampled (26 litters from Herd A and 104 from Herd B): 66 litters without stillborn piglets and 64 with stillborn piglets, totaling 1,723 liveborn and 105 stillborn sampled piglets. Both herds had been actively assisted farrowing by farm personnel. Throughout the study, the same trained investigator performed the stillborn piglet sampling, while two performed the liveborn piglet sampling. All liveborn and stillborn piglets within each litter were sampled. Stillborn piglets were defined as those found dead at birth, often presenting a brown-greenish colour, discoloured skin, or retention within the placenta, with intact thimbles covering their feet. Mummified piglets, defined as fetuses that died in utero and exhibited signs of decomposition such as dehydration and sunken eyes, were not sampled.
Tongue tips were collected from all stillborn piglets within the sampled litters. Briefly, three centimetres of each stillborn tongue tip was collected using sterile disposable scalpel blades (Size 20, Securos Surgical, Fiskdale, MA, USA), individually placed in 50 mL centrifuge tubes (Thermo Fisher Scientific, Pittsburgh, PA, USA) containing 1 mL of phosphate buffered saline (PBS) (PBS 1x, RPI Research Products International, Mt. Prospect, IL, USA), followed by a freezing process under-20°C for 24h. Gloves and scalpel blades were discarded after each stillborn sampling. The samples were thawed at 4°C for 6 h, the fluid was extracted, placed into 5 mL tubes (Thermo Fisher Scientific, Pittsburgh, PA, USA), and frozen at-20°C until laboratory diagnostic testing.
Intracardiac blood (IB) from stillborn piglets were collected directly from the piglet’s heart using a single-use 5 mL syringe (MonojectTM, Cardinal Health, Waukegan, IL, USA) and a single-use 20G x 1” needle (MonojectTM, Covidien, Mansfield, MA, USA) and kept at 4°C until laboratory shipment. In the laboratory, IB samples were centrifuged and sera were transferred into 5 mL tubes (Thermo Fisher Scientific, Pittsburgh, PA, USA) before diagnostic testing.
From the liveborn piglets, tail blood swabs (BS) were collected using cotton-tipped swabs (Puritan Medical Products Company, LLC, Guilford, ME, USA) and disposable scalpel blades (Size 20, Securos Surgical, Fiskdale, MA, USA) from the piglets’ tails after the tail docking process, and swabs were then placed into 5 mL tubes (Thermo Fisher Scientific, Pittsburgh, PA, USA) containing 1 mL of PBS (PBS 1x, RPI Research Products International, Mt. Prospect, IL, USA). Scalpel blades were discarded after each piglet sampling. BS samples were frozen at-20°C before diagnostic testing.
2.3.1 PRRSV RNA extraction and PRRSV RT-qPCR
All samples were individually tested using RT-qPCR in the research facilities of a National Animal Health Laboratory Network-accredited veterinary diagnostic laboratory at Iowa State University. Briefly, nucleic acids were extracted from the samples using the RealPCR*DNA/RNA Magnetic Bead Kit (IDEXX Laboratories, Inc., Westbrook, ME, USA), following the manufacturer’s instructions and automated extraction equipment (Kingfisher Flex System Magnetic Beads Processor, Thermo-Fisher Scientific, Waltham, MA, USA) (25). A positive amplification control (IDEXX Laboratories, Inc., Westbrook, ME, USA), a negative amplification control (nuclease-free water), a positive PRRSV-2 extraction control sample, and a PRRSV-2 negative extraction control sample were included for each RT-qPCR plate (25). According to the manufacturer’s recommendations, samples with Ct values < 40 were considered PRRSV-positive.
2.4 Statistical analysis
Data analyses were conducted in R program software (version 4.1.2). The Cohen Kappa agreement test was performed between TF and IB PRRSV RT-qPCR testing results at an animal level. Multivariate generalized linear regression models were used to estimate the effects of stillborn presence, stillborn PRRSV-RNA result (IB and TF), and litter size on the probability that a litter or at least one liveborn littermate would test PRRSV-positive (five analyses in total). For these analyses, the variables were classified as categorical: the presence of at least one stillborn piglet within the litter (presence, absence), at least one PRRSV-positive stillborn piglet (yes, no), at least one TF PRRSV-positive within the litter (yes, no), at least one IB PRRSV-positive within the litter (yes, no), and litter size (small, large). Litters with ≤ 11 liveborn piglets were defined as small litters based on the first quantile (the lowest 25%) for liveborn piglets registered in both herds during the collection period, whereas litters with ≥ 12 were defined as large. The “Herd” identifier was initially tested as a random effect in a generalized linear mixed regression. The model variance–covariance resulted in 0, and ‘Herd’ was excluded since there was no evidence of hierarchical structure. Model assumptions were assessed using the Wald test, deviance analysis, and Goodness-of-Fit test. Non-parametric analysis was conducted using Kruskal-Wallis and Dunn tests to assess the Ct values differences between the sample types. A p-value < 0.05 was used to determine the statistical significance of all analyses.
3 Results
A total of 1,723 liveborn piglets and 105 stillborn piglets were individually sampled from 130 litters from two herds: 26 litters from Herd A and 104 from Herd B (Table 1). Results from Herds A and B were simultaneously analyzed. Regarding the litter’s parity: in Herd A, 14 were parity 1 sows, seven were parity 2, four were parity 3, and one parity 4; in Herd B, all 104 litters came from parity 1 sows.
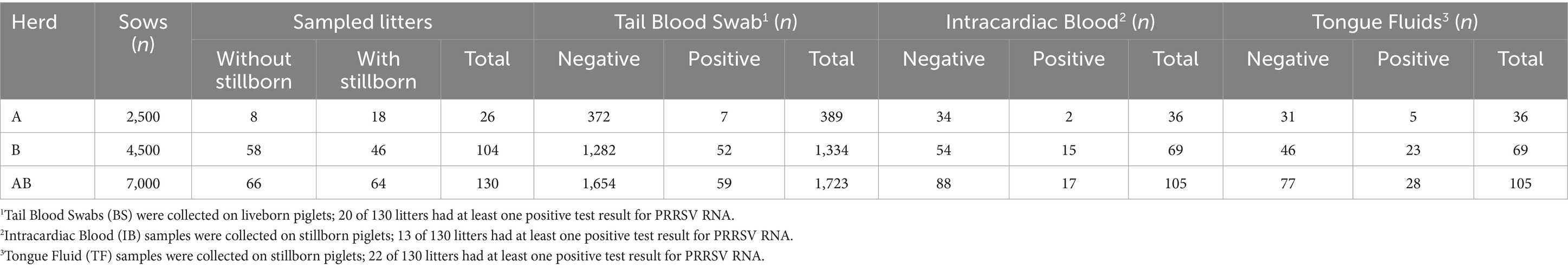
Table 1. Descriptive information for herds, litters, and piglets sampled for tail blood swabs, intracardiac blood, and tongue fluids.
Considering all sample types (TF, IB, and BS), the percentage of PRRSV-positive litters based on PRRSV RNA testing was 22.3% (29 of 130 litters), of which 15.4% (20 of 130 litters) were BS PRRSV RNA-positive, 10% (13 of 130 litters) were IB PRRSV RNA-positive, and 16.9% (22 of 130 litters) were TF PRRSV RNA-positive. At an animal-level, considering both herds, 3.4% (59 of 1,723 liveborn piglets) were BS PRRSV-RNA positive, 16.2% (17 of 105 stillborn piglets) were IB PRRSV-RNA positive, and 26.7% (28 of 105 stillborn piglets) were TF PRRSV-RNA positive (Table 1). The mean positivity of liveborn piglets within the litter was 5%, varying from 0 to 85.7%, while the total born (stillborn and liveborn piglets combined) was 4.6% (0 to 76.4%).
Regarding sample types’ Ct values, BS had a median of 35.5 (interquartile range [IQR]: 29.2–37.7), IB a median of 22.4 (IQR: 20.7–26.0), and TF a median of 29.2 (IQR: 25.2–35.7) (Figure 1). IB exhibited the lowest median Ct value, which was significantly different from the median Ct values of the compared sample types (p-value < 0.05).
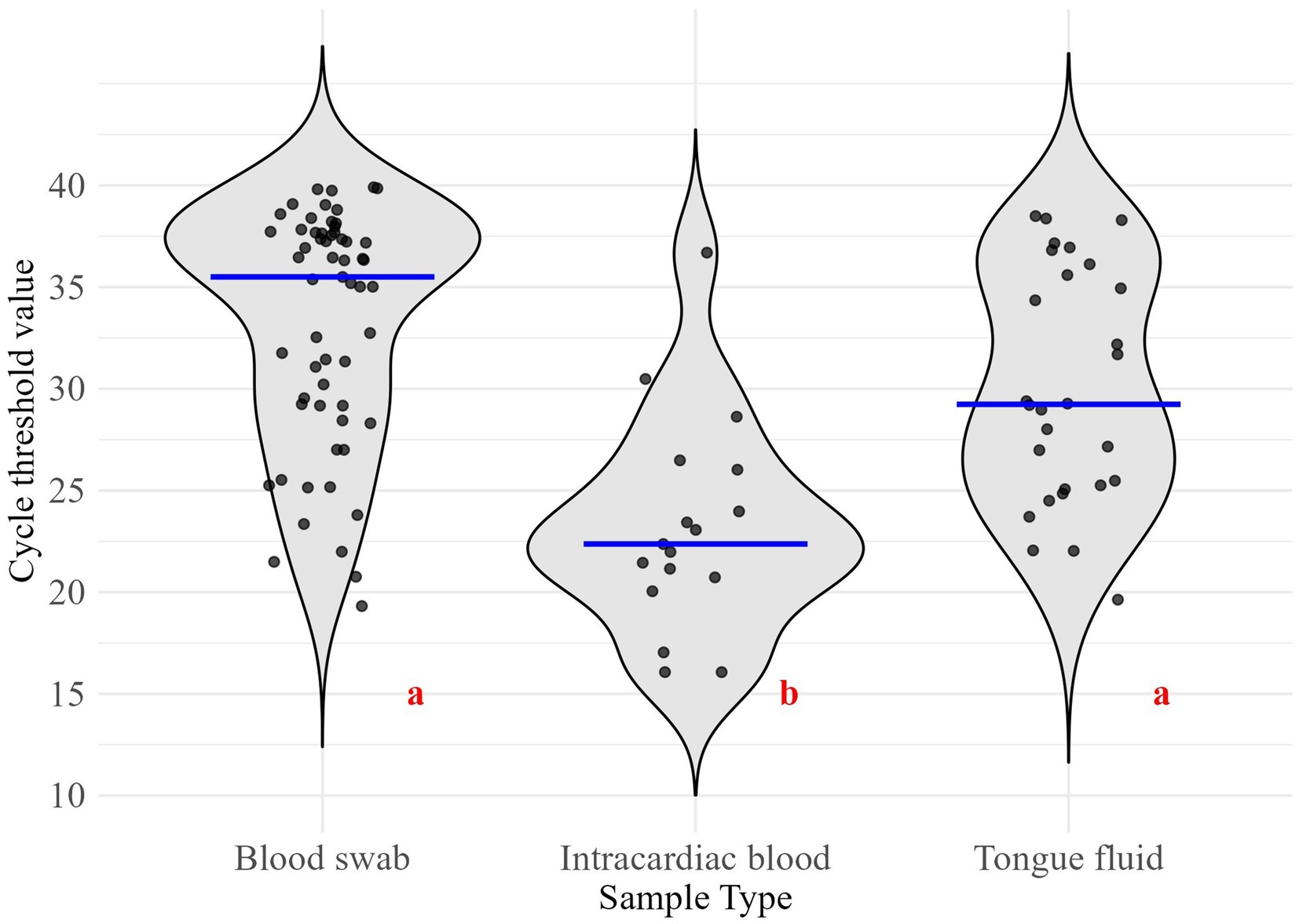
Figure 1. Distribution of cycle threshold values for the collected sample types. Different red letters indicate significant differences (α = 0.05). Blue lines represent group medians. Sample sizes for PRRSV-positive results: Blood swab (n = 59), Intracardiac blood (n = 17), Tongue fluid (n = 28).
At the animal level, TF and IB had a substantial agreement (Kappa = 0.694, p-value < 0.001). Results of the five multivariate logistic regression models are available in Table 2, and their predicted probabilities of PRRSV-RNA detection in litters or liveborn piglets by the assessed predictor variables (litter size, presence of stillborn piglet, and stillborn piglet PRRSV result [TF, IB, or both]) are available in Table 3.
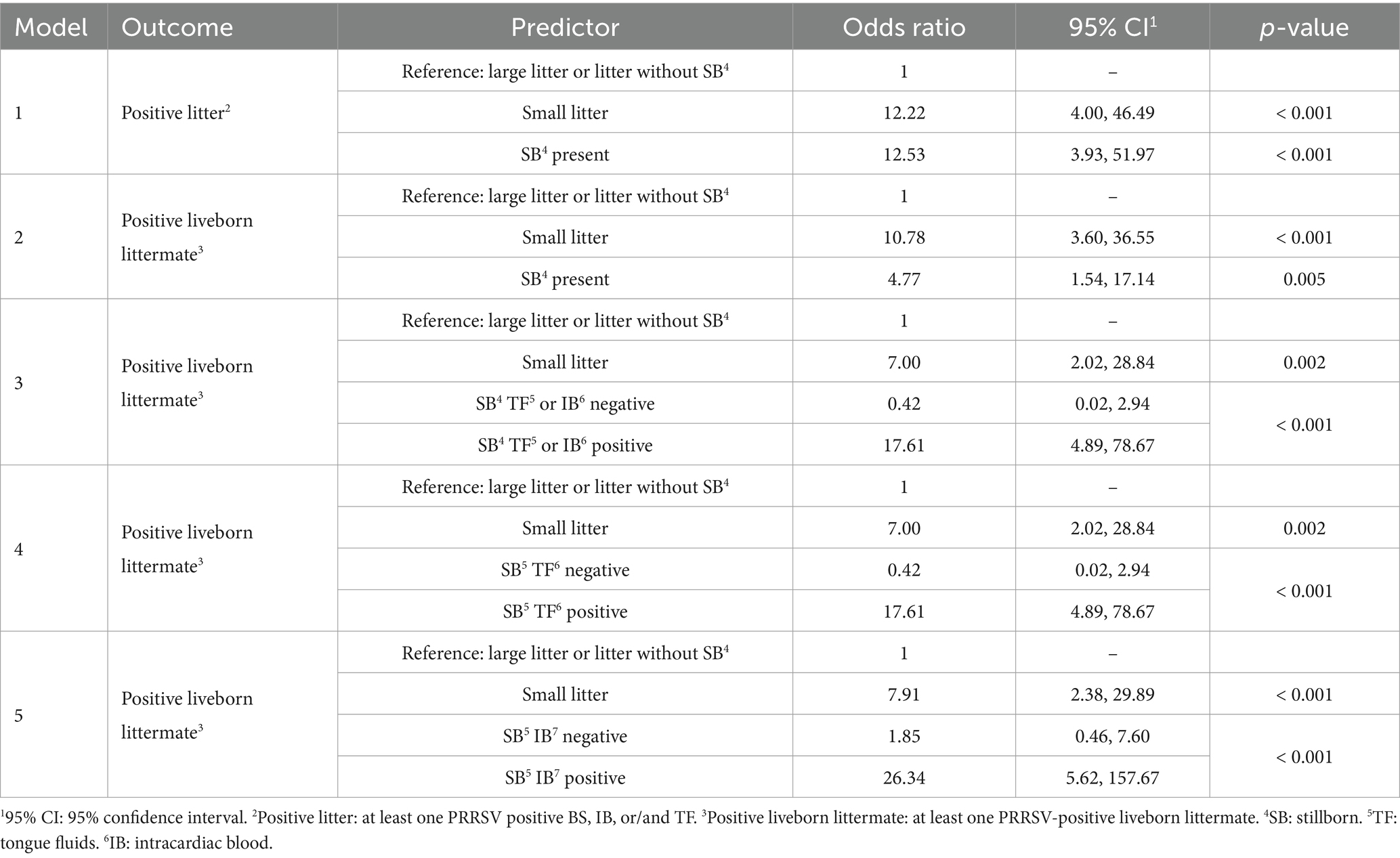
Table 2. Multivariable logistic regression analyses of predictors for PRRSV-RNA detection in litters or liveborn littermates.
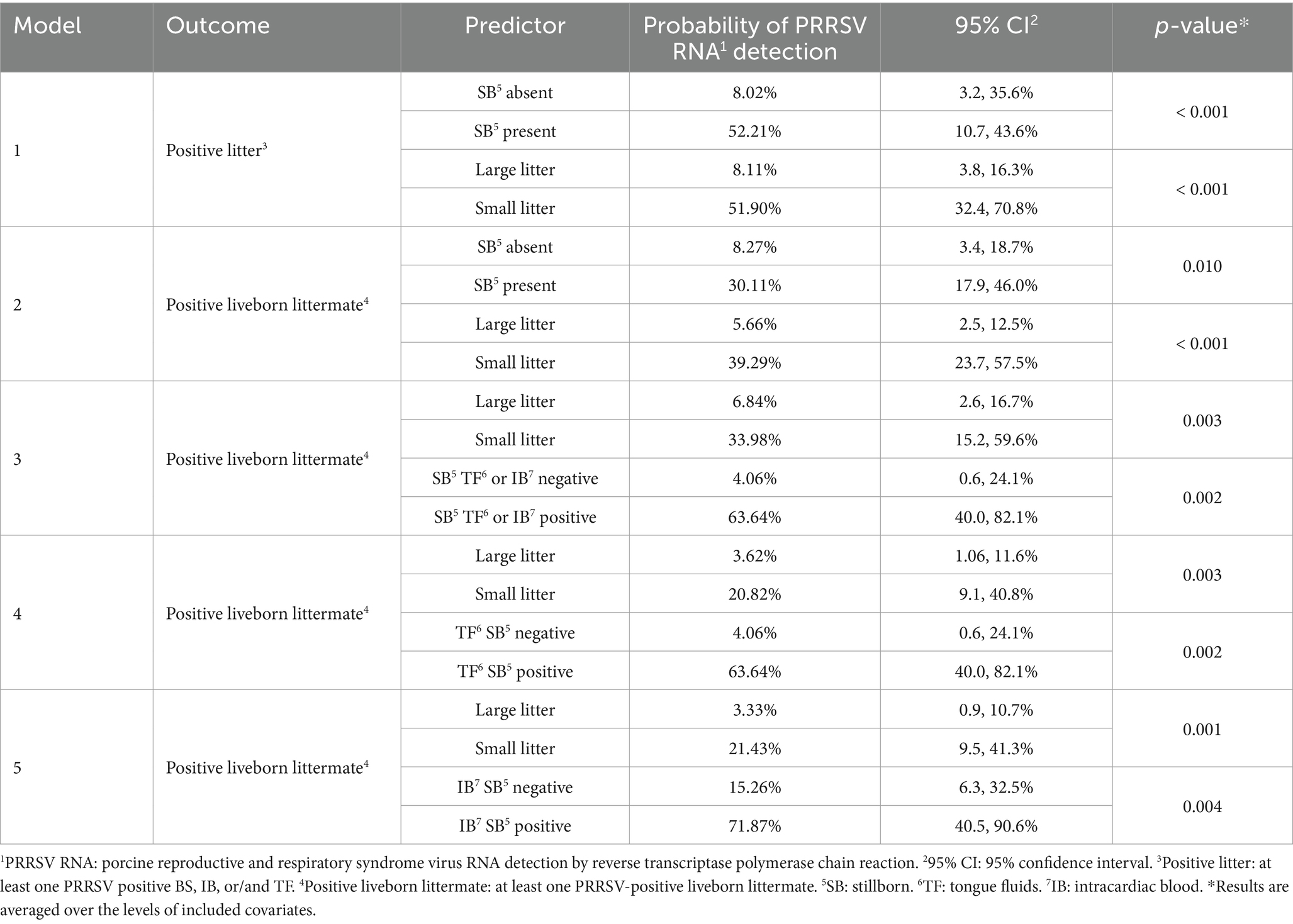
Table 3. Predicted probabilities of PRRSV-RNA detection in litters or liveborn piglets by the assessed predictors.
In model 1, small litters had 12.2 times higher odds (95% CI: 4.00 – 46.49, p-value < 0.001) of having a PRRSV-positive result compared to large litters, holding the stillborn variable constant, with a 51.9% probability of detecting PRRSV-positive piglets in small litters compared to 8.1% in large litters. Litters with at least one stillborn piglet had 12.5 times higher odds (95% CI: 3.93, 51.97, p-value < 0.001) of having a PRRSV-positive piglet, holding litter size constant, with a 52.2% probability of detecting a PRRSV-positive piglet when at least one stillborn was present, compared to 8.0% when no stillborns were present. No significant interaction between litter size and stillborn presence was found (p-value = 0.076). In model 2, small litters had 10.8 times higher odds (95% CI: 3.60 – 36.55, p-value < 0.001) of having a viremic liveborn littermate compared to large litters, holding the stillborn variable constant. Litters with at least one stillborn piglet had 4.8 times higher odds (95% CI: 1.54, 17.14, p-value = 0.005) of having a viremic liveborn littermate, compared to litters without stillborn piglets, holding litter size constant. Further probabilities to detect PRRSV-positive liveborn in the litter are described in Table 3.
Stillborn PRRSV RNA results were included as predictor variables in models 3, 4, and 5, along with litter size. Model 3 included both TF and IB results, model 4 included the TF only, and model 5 IB only. Litters with PRRSV-positive stillborn piglets – detected either by combined TF or IB, or TF only – had 17.6 times higher odds (95% CI: 4.89, 78.67, p-value < 0.001) of having a viremic liveborn littermate compared to those without stillborn piglets, holding litter size constant. Small litters had 7.0 times higher odds (95% CI: 2.02, 28.84, p-value = 0.002) of having a viremic liveborn littermate compared to large litters, holding the stillborn PRRSV result constant. Further probabilities to detect PRRSV-positive liveborn in the litter are described in Table 3.
4 Discussion
The US swine industry continuously updates its PRRSV surveillance programs, incorporating standardized systems for PRRSV classification and sample type recommendations (1, 2). Surveillance and classification systems are highly reliant on diagnostic testing and play an important role in facilitating communication between veterinarians and swine producers. They also support the implementation of regional and national efforts toward PRRSV control and elimination. Over the years, multiple population-based sample types, such as PF, oral fluids, FOF, and TF, were developed to improve the monitoring programs and to overcome labor and cost issues (3–6). As a result, the AASV PRRSV classification committee incorporated both individual and population-based samples in its latest recommendations for a broader population coverage and provided additional evidence to increase confidence in detecting PRRSV-positive pigs (2). Similarly, risk-based approaches have been reported to be associated with an increased probability of detecting viral infections (16, 17). Thus, using a risk-based approach, the current study provided important insights into detecting PRRSV RNA across multiple sample types from liveborn and stillborn piglets.
PRRSV is known to cause negative reproductive impacts. When it infects the gestating population, it can cause an increase in abortions, fetal death, and stillborn piglet rates (26, 27). When horizontally transmitted, PRRSV can cause viremia as early as 12 h post-exposure (28–30). In this study, litters with at least one stillborn piglet had 12.5 times higher odds of testing PRRSV RNA-positive (based on IB, TF, or BS) within 12 h of farrowing than those without stillborns. Additionally, the odds of having a viremic liveborn littermate were 4.8 times higher in such litters, and when the stillborn tested TF PRRSV RNA-positive, the odds of finding a viremic liveborn littermate increased dramatically by 17.6 times. Thus, stillborn piglet occurrence can be an indicator of the potential presence of PRRSV in the litter, which can support decisions during PRRSV outbreak management programs. For instance, once the virus prevalence of the herd decreases, e.g., AASV Status 1B to II (2), implementing measures to prevent further spread must be considered, such as handling mortalities at the end of the day, adhering to McREBEL practices, and limiting cross-fostering protocols, especially in litters where stillborn piglets are found (31, 32).
Litter size and the number of pigs born alive are key performance indicators in pork production, as they directly impact the number of pigs weaned per sow. However, PRRSV negatively affects litter size (33). Litters with 11 or fewer liveborn piglets per sow demonstrated 12.2 times higher odds of yielding a PRRSV RNA-positive result (based on IB, TF, or BS) than larger litters, and 10.8 times higher for detecting at least one PRRSV RNA-positive liveborn piglet. For instance, in a scenario where a breeding herd is under a PRRSV elimination process with herd closure following the AASV PRRSV elimination guidelines, a target sampling focusing on small litters can be adopted to improve the monitoring program, similar to those reported for FOF and PF (16, 17).
Both herds had TF-positive results in 22 litters: five out of 26 litters in Herd A and 17 out of 104 litters in Herd B. Since TF is typically collected as an aggregated sample from multiple animals, if they had been collected as an aggregate sample in this study, i.e., all tongue tips in one bag, both herds would likely have yielded PRRSV RNA-positive results, effortlessly assessing the population’s PRRSV status. According to a previous study (34), PRRSV RNA detection in BS is comparable to serum from the jugular vein, with the advantage of being practical and less time-consuming. When PRRSV prevalence is as low as 4%, sampling 89 liveborn piglets with BS samples is needed to detect at least one PRRSV RNA-positive result. Based on BS PRRSV RNA results in this study from liveborn piglets, the liveborn piglet positivity was 3.42% (59 positives out of 1,723 sampled piglets). Thus, collecting tongue tips from multiple piglets into a single aggregated TF sample – such as from 30 to 100 individuals (6) – offers not only accurate PRRSV detection but also serve as a more time-and cost-effective alternative to individually sampling and testing 89 liveborn piglets at a prevalence of 4%. However, further studies are needed to estimate the probability of testing PRRSV-positive using RT-qPCR in an aggregated TF sample, particularly when only one tongue tip is PRRSV RNA-positive among an aggregate of negative tongue tip samples.
In this study, two PRRSV high-prevalence herds were selected to evaluate the effects of stillborn TF results and litter size comparison for PRRSV-RNA detection by RT-qPCR, aiming to maximize PRRSV-RNA positive results. This study is a proof of concept, and external validity is limited to herds sharing similar characteristics and PRRSV infection stage as the study herd. Due to internal farm management practices, most of the sampled litters were from young parities (e.g., first parity), which did not allow the assessment of the parity effect. Nevertheless, based on the disease’s ecology, stillborn piglets would still be expected in lower-prevalence herds, as virus-harboring dams continue to produce stillborn piglets, especially in the lower parity population (35).
In conclusion, under the conditions of this study, sampling litters with stillborn piglets or small litter sizes provided a higher probability of detecting PRRSV RNA-positive in the farrowing room’s population. Moreover, TF samples from stillborn piglets were a reliable indicator of the PRRSV status of their liveborn littermates, indicating that TF can be an effective risk-based approach to assess PRRSV circulation within liveborn piglets. Therefore, veterinarians and pig producers are encouraged to collect TF, targeting stillborn piglets or small litters to increase the likelihood of detecting PRRSV. This risk-based sampling strategy within the first hours post-farrowing enhances the effectiveness of PRRSV monitoring programs in breeding herds that support timely interventions, such as McREBEL and cross-fostering (31, 32). Lastly, while TFs were individually collected in this study to test the hypothesis, collecting them as an aggregated sample, similar to PF, is recommended (6), as this approach allows for a larger number of screened animals.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal studies were approved by Institutional Animal Care and Use Committee (IACUC) of Iowa State University, IA, USA, under protocol IACUC-22-101 “Field surveillance for swine pathogens.” The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
IM: Writing – review & editing, Writing – original draft, Investigation, Supervision, Data curation, Methodology, Software, Conceptualization, Resources, Funding acquisition, Visualization, Project administration, Formal analysis, Validation. PL: Conceptualization, Resources, Investigation, Project administration, Funding acquisition, Methodology, Writing – review & editing. JX: Project administration, Investigation, Writing – review & editing, Methodology. TP: Resources, Project administration, Writing – review & editing, Supervision, Conceptualization, Investigation, Methodology. AS: Validation, Writing – review & editing, Methodology, Formal analysis, Software. OO: Writing – review & editing, Methodology, Conceptualization, Investigation, Formal analysis, Software, Funding acquisition. LP: Supervision, Methodology, Investigation, Writing – review & editing. PG: Methodology, Writing – review & editing, Supervision, Investigation. GT: Methodology, Supervision, Writing – review & editing, Investigation. GS: Formal analysis, Writing – review & editing, Funding acquisition, Investigation, Methodology, Supervision, Software. DL: Investigation, Validation, Project administration, Supervision, Conceptualization, Funding acquisition, Writing – review & editing, Software, Methodology, Formal analysis, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The Funding was provided by The Swine Health Information Center (project #23–076).
Acknowledgments
The authors acknowledge Dr. Chris Sievers from Swine Vet Center (St Peter, MN) for providing the facilities and animals.
Conflict of interest
LP was employed by Genus plc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Holtkamp, DJ, Polson, DD, Torremorell, M, Classen, DM, Becton, L, Henry, S, et al. Terminology for classifying swine herds by porcine reproductive and respiratory syndrome virus status. J Swine Health Produc. (2011) 19:44–56. doi: 10.54846/jshap/669
2. Holtkamp, DJ, Torremorell, M, Corzo, CA, Linhares, D, Almeida, MN, Yeske, P, et al. Proposed modifications to porcine reproductive and respiratory syndrome virus herd classification. Amer Assoc Swine Vet. (2021) 29:261–70. doi: 10.54846/jshap/1218
3. Rotolo, ML, Sun, Y, Wang, C, Giménez-Lirola, L, Baum, DH, Gauger, PC, et al. Sampling guidelines for oral fluid-based surveys of group-housed animals. Vet Microbiol. (2017) 209:20–9. doi: 10.1016/j.vetmic.2017.02.004
4. Lopez, W., and Linhares, D., (2017). Processing fluids, blood serum, and tail blood swabs to detect PRRSV RNA and PCV2 DNA by PCR-based assays. In James McKean Swine Disease Conference.
5. Almeida, MN, Rotto, H, Schneider, P, Robb, C, Zimmerman, JJ, Holtkamp, DJ, et al. Collecting oral fluid samples from due-to-wean litters. Prev Vet Med. (2020) 174:104810. doi: 10.1016/j.prevetmed.2019.104810
6. Baliellas, J, Novell, E, Enric-Tarancón, V, Vilalta, C, and Fraile, L. Porcine reproductive and respiratory syndrome surveillance in breeding herds and nurseries using tongue tips from dead animals. Vet Sci. (2021) 8:259. doi: 10.3390/vetsci8110259
7. Almeida, MN, Zhang, M, Lopez, WAL, Vilalta, C, Sanhueza, J, Corzo, CA, et al. A comparison of three sampling approaches for detecting PRRSV in suckling piglets. Prev Vet Med. (2021) 194:105427. doi: 10.1016/j.prevetmed.2021.105427
8. Osemeke, OH, de Freitas Costa, E, Weide, V, Jayaraman, S, Silva, GS, and Linhares, DC. In-silico characterization of the relationship between the porcine reproductive and respiratory syndrome virus prevalence at the piglet and litter levels in a farrowing room. Porcine Health Manage. (2023) 9:14. doi: 10.1186/s40813-023-00309-x
9. Dohoo, I.R., Martin, W., and Stryhn, H.E., (2003). Veterinary epidemiologic research. Charlottetown (PE): Atlantic Veterinary College.
10. Salman, MD. Surveillance and monitoring systems for animal health programs and disease surveys In: MD Salman, editor. Animal disease surveillance and survey systems: Methods and applications (2003). 3–13.
11. Stärk, KD, Regula, G, Hernandez, J, Knopf, L, Fuchs, K, Morris, RS, et al. Concepts for risk-based surveillance in the field of veterinary medicine and veterinary public health: review of current approaches. BMC Health Serv Res. (2006) 6:1–8. doi: 10.1186/1472-6963-6-20
12. Lewis, CRG, Torremorell, M, and Bishop, SC. Effects of porcine reproductive and respiratory syndrome virus infection on the performance of commercial sows and gilts of different parities and genetic lines. J Swine Health Produc. (2009) 17:140–7. doi: 10.54846/jshap/582
13. Terpstra, C, Wensvoort, G, and Pol, JMA. Experimental reproduction of porcine epidemic abortion and respiratory syndrome (mystery swine disease) by infection with Lelystad vims: Koch’s postulates fulfilled. Vet Q. (1991) 13:131–6. doi: 10.1080/01652176.1991.9694297
14. Mengeling, WL, Lager, KM, and Vorwald, AC. Temporal characterization of transplacental infection of porcine fetuses with porcine reproductive and respiratory syndrome virus. Am J Vet Res. (1994) 55:1391–8. doi: 10.2460/ajvr.1994.55.10.1391
15. Prieto, C, Suarez, P, Simarro, I, Garcia, C, Fernandez, A, and Castro, JM. Transplacental infection following exposure of gilts to porcine reproductive and respiratory syndrome virus at the onset of gestation. Vet Microbiol. (1997) 57:301–11. doi: 10.1016/S0378-1135(97)00112-0
16. Almeida, MN, Zhang, M, Zimmerman, JJ, Holtkamp, DJ, and Linhares, DCL. Finding PRRSV in sow herds: family oral fluids vs. serum samples from due-to-wean pigs. Prev Vet Med. (2021) 193:105397. doi: 10.1016/j.prevetmed.2021.105397
17. Vilalta, C, Sanhueza, J, Alvarez, J, Murray, D, Torremorell, M, Corzo, C, et al. Use of processing fluids and serum samples to characterize porcine reproductive and respiratory syndrome virus dynamics in 3 day-old pigs. Vet Microbiol. (2018) 225:149–56. doi: 10.1016/j.vetmic.2018.09.006
18. Machado, IF, Magalhães, ES, Poeta Silva, APS, Moraes, DC, Cezar, G, Mil-Homens, MP, et al. Porcine reproductive and respiratory syndrome virus RNA detection in tongue tips from dead animals. Front Vet Sci. (2022) 9:993442. doi: 10.3389/fvets.2022.993442
19. Dürlinger, S, Kreutzmann, H, Unterweger, C, Martin, V, Hamar, F, Knecht, C, et al. Detection of PRRSV-1 in tongue fluids under experimental and field conditions and comparison of different sampling material for PRRSV sow herd monitoring. Porcine Health Manage. (2024) 10:18. doi: 10.1186/s40813-024-00370-0
20. Harding, JC, Ladinig, A, Novakovic, P, Detmer, SE, Wilkinson, JM, Yang, T, et al. Novel insights into host responses and reproductive pathophysiology of porcine reproductive and respiratory syndrome caused by PRRSV-2. Vet Microbiol. (2017) 209:114–23. doi: 10.1016/j.vetmic.2017.02.019
21. Rudy, K, Jeon, D, Smith, AA, Harding, JCS, and Pasternak, JA. PRRSV-2 viral load in critical non-lymphoid tissues is associated with late gestation fetal compromise. Front Microbiol. (2024) 15:1352315. doi: 10.3389/fmicb.2024.1352315
22. Machado, I., Li, P., Poeta Silva, AP, Magalhaes, ES, Osemeke, OH, Jayaraman, S., Moraes, DCA, Mil-Homens, M., Cezar, GA, Petznick, T., Silva, GS, and Linhares, D. (2024). Evaluation of PRRSV vertical transmission using stillborn tongue tip fluids sampling. In 55th AASV annual meeting, research topics. Nashville, Tennessee. March 2024
23. Jansen, T., Waddell, J., Linhares, D., Machado, I., and LeFevre, C. (2023). Comparison of tongue tips, serum, and processing fluids for PRRSV monitoring in neonates. In 54th AASV annual meeting, Sunday concurrent session #1: Student seminar. Aurora, Denver. March 2023
24. Yim-Im, W, Anderson, TK, Paploski, IA, VanderWaal, K, Gauger, P, Krueger, K, et al. Refining PRRSV-2 genetic classification based on global ORF5 sequences and investigation of their geographic distributions and temporal changes. Microbiol Spec. (2023) 11:e0291623–3. doi: 10.1128/spectrum.02916-23
25. Machado, IF, Osemeke, OH, Doolittle, K, Moura, CA, Galina Pantoja, L, Trevisan, G, et al. Effect of time and temperature on the detection of PRRSV RNA and endogenous internal sample control in porcine tongue fluids. Vet Sci. (2025) 12:59. doi: 10.3390/vetsci12010059
26. Dea, S, Bilodeau, R, Athanassious, R, Sauvageau, R, and Martineau, GP. Swine reproductive and respiratory syndrome in Quebec: isolation of an enveloped virus serologically-related to Lelystad virus. Can Vet J. (1992) 33:801–8.
27. Lager, KM, and Halbur, PG. Gross and microscopic lesions in porcine fetuses infected with porcine reproductive and respiratory syndrome virus. J Vet Diagn Invest. (1996) 8:275–82. doi: 10.1177/104063879600800301
28. Van der Linden, IFA, Voermans, JJM, Van der Linde-Bril, EM, Bianchi, ATJ, and Steverink, PJGM. Virological kinetics and immunological responses to a porcine reproductive and respiratory syndrome virus infection of pigs at different ages. Vaccine. (2003) 21:1952–7. doi: 10.1016/S0264-410X(02)00822-8
29. Klinge, KL, Vaughn, EM, Roof, MB, Bautista, EM, and Murtaugh, MP. Age-dependent resistance to porcine reproductive and respiratory syndrome virus replication in swine. Virol J. (2009) 6:1–11. doi: 10.1186/1743-422X-6-177
30. Lunney, JK, Fang, Y, Ladinig, A, Chen, N, Li, Y, Rowland, B, et al. Porcine reproductive and respiratory syndrome virus (PRRSV): pathogenesis and interaction with the immune system. Ann Rev Animal Biosci. (2016) 4:129–54. doi: 10.1146/annurev-animal-022114-111025
31. McCaw, MB. Effect of reducing crossfostering at birth on piglet mortality and performance during an acute outbreak of porcine reproductive and respiratory syndrome. J Swine Health Produc. (2000) 8:15–21.
32. Cano, JP, Dee, SA, Murtaugh, MP, Rovira, A, and Morrison, RB. Infection dynamics and clinical manifestations following experimental inoculation of gilts at 90 days of gestation with a low dose of porcine reproductive and respiratory syndrome virus. Can J Vet Res. (2009) 73:303–7.
33. Baysinger, AK, Dewey, CE, Straw, BE, Brumm, MC, Schmitz, J, Doster, A, et al. The effect of PRRSV on reproductive parameters in swine herds. J Swine Health Produc. (1997) 5:173–6.
34. Osemeke, OH, Cezar, GA, Paiva, RC, Moraes, DC, Machado, IF, Magalhaes, ES, et al. A cross-sectional assessment of PRRSV nucleic acid detection by RT-qPCR in serum, ear-vein blood swabs, nasal swabs, and oral swabs from weaning-age pigs under field conditions. Front Vet Sci. (2023) 10:1200376. doi: 10.3389/fvets.2023.1200376
Keywords: tongue fluids, risk-based, PRRSV, monitoring, targeted sampling, swine
Citation: Machado IF, Li P, Xiao J, Petznick T, Silva APP, Osemeke OH, Pantoja LG, Gauger P, Trevisan G, Silva GS and Linhares DCL (2025) Evaluating stillborn and litter size as indicators of PRRSV detection in live piglets and the use of stillborn tongue fluids as risk-based samples for PRRSV monitoring. Front. Vet. Sci. 12:1600064. doi: 10.3389/fvets.2025.1600064
Edited by:
Victor Manuel Petrone-García, Universidad Nacional Autonoma de Mexico, MexicoReviewed by:
Annalisa Scollo, University of Turin, ItalyCaihong Bi, Brigham and Women’s Hospital, Harvard Medical School, United States
Copyright © 2025 Machado, Li, Xiao, Petznick, Silva, Osemeke, Pantoja, Gauger, Trevisan, Silva and Linhares. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel C. L. Linhares, bGluaGFyZXNAaWFzdGF0ZS5lZHU=
 Isadora F. Machado
Isadora F. Machado Peng Li
Peng Li Jinnan Xiao1
Jinnan Xiao1 Ana Paula P. Silva
Ana Paula P. Silva Onyekachukwu H. Osemeke
Onyekachukwu H. Osemeke Lucina Galina Pantoja
Lucina Galina Pantoja Phillip Gauger
Phillip Gauger Giovani Trevisan
Giovani Trevisan Gustavo S. Silva
Gustavo S. Silva Daniel C. L. Linhares
Daniel C. L. Linhares