- 1Heilongjiang Provincial Key Laboratory of Prevention and Control of Bovine Diseases, College of Animal Science and Veterinary Medicine, Heilongjiang Bayi Agricultural University, Daqing, China
- 2Heilongjiang Mudanjiang Agricultural Reclamation Jin’ao Dairy Farming Specialized Cooperative, Mudanjiang, China
- 3Heilongjiang Bayi Agricultural University Hospital, Daqing, China
Ketosis is a prevalent metabolic disease in dairy cows, characterized by adverse effects on both animal health and production performance. Propylene glycol (PG), recognized for its glucogenic properties, is widely utilized in the therapeutic management of ketosis. This study evaluated the efficacy of two PG-based treatment protocols in mitigating ketosis and enhancing the metabolic health of Holstein cows. Ninety cows were randomly allocated into three groups (n = 30 each): control (Group C, no PG), original PG protocol (Group O, 500 mL PG orally drenched once daily on days 0, 1, 2, 7, 8, and 9 post-calving), and novel PG protocol (Group N, 500 mL PG orally drenched once daily on days 0, 7, and 14 post-calving). Data were collected for body condition score, milk yield, metabolic biomarkers, and the incidence of ketosis from 14 (±3) days prepartum to 50 days postpartum. The results demonstrated that the novel PG protocol, compared with the control group, significantly enhanced energy metabolism by modulating glucose, insulin, and leptin levels while reducing β-hydroxybutyric acid and non-esterified fatty acid concentrations (p < 0.05). Additionally, the novel PG protocol effectively decreased the incidence of ketosis (from 33.3% in Group C to 6.7% in Group N at 14 days postpartum), alleviated liver injury, and mitigated oxidative stress in dairy cows (p < 0.05). These findings underscore the potential of the novel PG protocol to improve metabolic health and reduce the risk of ketosis during the critical transition period in dairy cows. This offers a promising strategy for managing this condition in modern dairy production systems.
1 Introduction
The transition period in dairy cows, defined as the 3 weeks before calving to the 3 weeks after calving (1), is a critical phase marked by significant physiological changes associated with pregnancy, parturition, and lactation. These changes result in a rapid increase in energy demands, yet dry matter intake (DMI) often fails to meet these physiological requirements, leading to a state of negative energy balance (NEB) (2, 3). To compensate for this energy deficit, dairy cows mobilize substantial body fat reserves, which increases circulating concentrations of non-esterified fatty acids (NEFA) (4). Excessive NEFA is channeled into the ketone body synthesis pathway, producing high levels of β-hydroxybutyrate (BHBA), acetoacetate, and acetone, thereby contributing to the onset and progression of ketosis in dairy cows (5). Globally, the prevalence of ketosis in high-producing dairy cows is estimated to be approximately 15%, with an increasing trend in incidence (6, 7).
Studies have shown that ketosis not only causes a significant decline in milk yield but also adversely affects milk quality (8). Furthermore, ketosis severely impairs reproductive performance, as evidenced by reduced conception rates and prolonged intervals from calving to first insemination (9). Additionally, ketosis predisposes dairy cows to various secondary disorders, including displaced abomasum, mastitis, and metritis (10, 11). The combined impact of these factors leads to increased treatment costs, elevated culling rates, and substantial economic losses for the dairy industry (12). In this context, propylene glycol (PG) has emerged as a promising intervention strategy. Its potential as a preventive and therapeutic solution for managing ketosis warrants further investigation to improve the health and productivity of dairy cows during the transition period.
Propylene glycol is widely utilized as both a therapeutic and prophylactic agent for mitigating ketosis in dairy cows (13, 14). Its mode of action primarily involves increasing serum glucose levels and reducing circulating ketone body concentrations (15, 16). Recent studies have demonstrated that PG supplementation can effectively lower the incidence of both clinical and subclinical ketosis, improve milk yield, and enhance overall health during the transition period (17, 18). Despite these benefits, the long-term use of PG raises certain challenges. Prolonged reliance on PG may reduce feed palatability, while excessive administration can lead to rumen acidosis and other metabolic disturbances (19). Consequently, research has predominantly focused on optimizing PG administration, either as a dosing agent or as a feed additive, to minimize the incidence of ketosis (15, 20, 21). Despite these efforts, ketosis continues to pose a significant challenge in modern dairy management systems, highlighting the pressing need for innovative and more effective strategies to further reduce its prevalence and impact.
Extensive research has shown that oral administration of PG can significantly lower plasma BHBA concentrations, elevate serum glucose levels, and improve both the health status and milk yield of dairy cows (22, 23). Emerging evidence suggests that PG alleviates oxidative stress and enhances immunity in ketotic cows by modulating amino acid and lipid metabolism (24). Despite these benefits, current treatment protocols have notable limitations, particularly in addressing the effects of different PG regimens on energy metabolism and oxidative stress in dairy cows, which remain insufficiently explored. This study introduces a novel health management protocol based on PG. It was hypothesized that, compared to the original PG regimen, the new PG regimen would demonstrate improved efficacy in alleviating oxidative stress, enhancing liver function, and improving energy metabolism in dairy cows. These findings offer valuable theoretical insights and present innovative strategies for advancing health management practices in modern dairy farming systems.
2 Materials and methods
2.1 Ethics
This study was carried out in strict accordance with the guidelines for the care and use of animals of Heilongjiang Bayi Agricultural University. All animal experimental procedures were approved by the Animal Welfare and Research Ethics Committee of Heilongjiang Bayi Agricultural University (Daqing, China) (protocol code DWKJXY2023057; approval date: August 1st, 2023) and the study complied with the ARRIVE guidelines.
2.2 Animals and diets
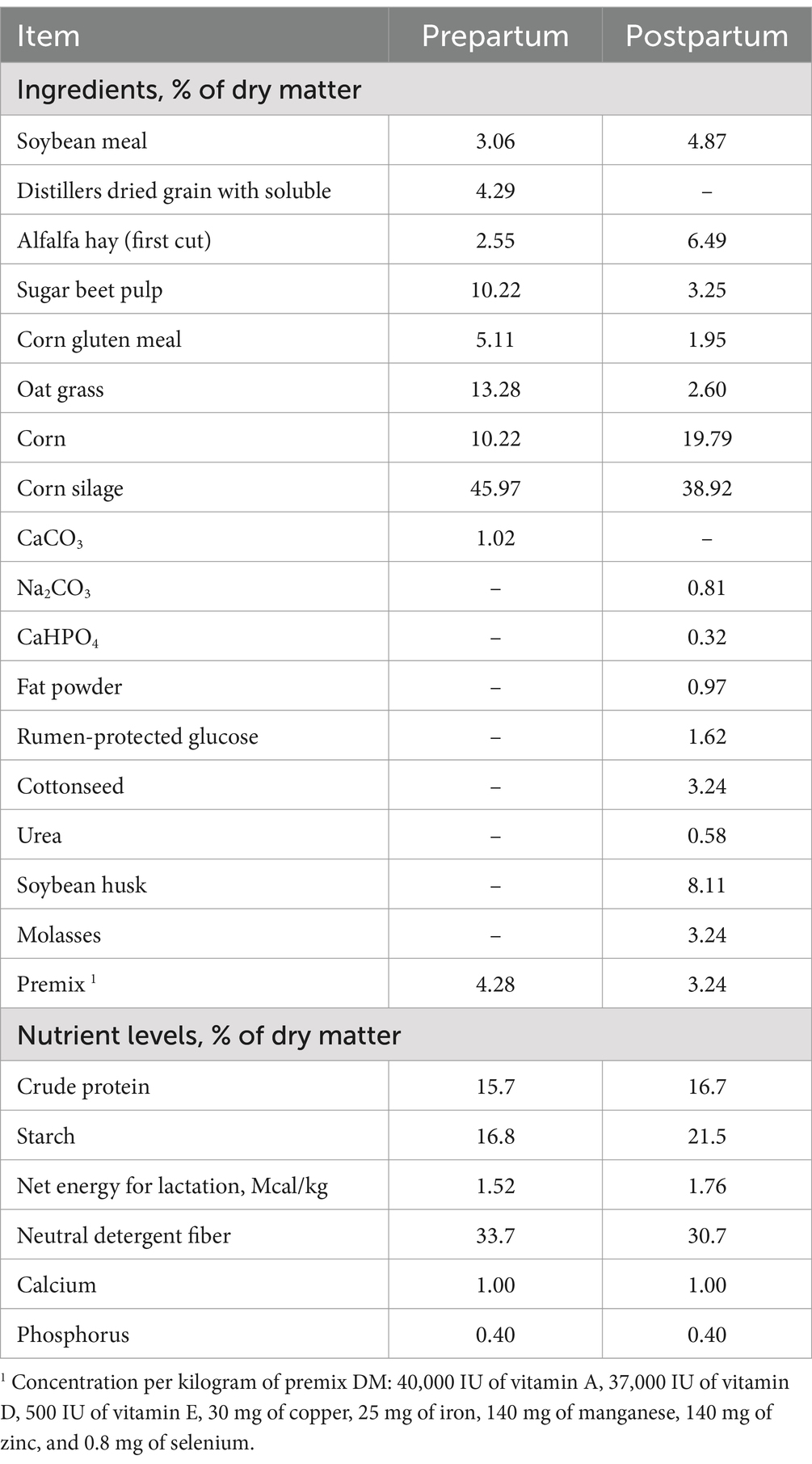
The experiment was conducted using Holstein cows on a large intensive cattle farm in the central region of Heilongjiang Province. This experiment was performed using a completely randomized design. Ninety multiparous Holstein cows of similar age, parity, body condition score (BCS) and milk yield (40.33 ± 2.22 months of age, 2.37 ± 0.10 of parity, 3.06 ± 0.09 of BCS, 9383.29 ± 111.26 kg of 305 d milk yield, mean ± SEM) were randomly selected 21 (±3) days before their expected calving date as experimental subjects. From 14 (±3) days prepartum, the cows were randomly allocated into three groups: the control group (Group C, n = 30; 41.69 ± 4.21 months of age, 2.40 ± 0.16 of parity, 3.00 ± 0.15 of BCS, 9407.43 ± 209.86 kg of 305 d milk yield), the original protocol group (Group O, n = 30; 47.96 ± 3.81 months of age, 2.40 ± 0.22 of parity, 3.05 ± 0.18 of BCS, 9123.40 ± 173.39 kg of 305 d milk yield), and the novel protocol group (Group N, n = 30; 41.36 ± 3.7 months of age, 2.30 ± 0.15 of parity, 3.13 ± 0.14 of BCS, 8910.53 ± 184.34 kg of 305 d milk yield). All cows were provided with a scientifically formulated total mixed ration based on NRC (2001) standards (25), with the composition and nutritional levels of the basal diet detailed in Table 1.
2.3 Experimental design and management
The experimental dairy cows were housed in three isolated tie-stall barns, each containing 30 cows from a homogeneous group. These barns were physically segregated from the primary housing facilities utilized by other cattle herds. Feeding was conducted three times daily (07:00, 14:00, and 21:00) with ad libitum access to fresh water. The feed intake of each barn was recorded for 3 consecutive days per week from 2 weeks prepartum to 7 weeks postpartum. The DMI of each group was calculated by analyzing the dry matter content in the feed. The average of the 3 daily values recorded each week was used as the weekly DMI average for each group. Following calving, the cows were milked three times per day (06:00, 13:00, and 22:00) until 50 days postpartum, and individual milk yields were recorded. Cows in Group C did not receive PG. Those in Group O were administered 500 mL of PG on the day of calving, at 1 and 2 days postpartum, and at 7, 8, and 9 days postpartum, totaling 3,000 mL. Cows in Group N received 500 mL of PG on the day of calving and at 7 and 14 days postpartum, totaling 1,500 mL. Cows treated with PG were given orally drenched.
2.4 Data collection
Specific software (Afifarm, Afimilk, Kibbutz Afikim, 1,514,800, Israel) was utilized to record data on age, parity, milk yield, and ketosis incidence in the experimental cows. Body condition score was assessed by two trained veterinarians using a standardized 5-point scale (26). Clinical ketosis was defined as a serum BHBA concentration ≥ 3.0 mmol/L, while subclinical ketosis was diagnosed when serum BHBA exceeded 1.2 mmol/L (27). In this study, cows with serum BHBA concentrations ≥1.20 mmol/L were classified as having ketosis based on the diagnostic criteria.
2.5 Sample collection
The official experimental period spanned from 14 (±3) days prepartum to 50 days postpartum. Blood samples (10 mL) were collected from the coccygeal vein prior to morning feeding on days −14 (±3), 0, + 7, + 14, + 21, + 28, and + 50 relative to calving. Samples were placed into centrifuge tubes left at room temperature for 30 min to allow for full clotting and centrifuged and at 3500 × g for 10 min. The resulting serum was aliquoted and stored at −80°C for subsequent analysis.
2.6 Serum detection
Serum concentrations of calcium, phosphorus, magnesium, potassium, aspartate aminotransferase (AST), albumin (ALB), total cholesterol (TC), and glucose were determined using commercial biochemical reagent kits (Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China) and analyzed with a fully automated biochemical analyzer (Mindray BS-830S). Serum total antioxidant capacity (T-AOC), malondialdehyde (MDA), glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) activities, and NEFA levels were measured using a microplate reader (Multiskan FC, Thermo Fisher Scientific Co., Ltd., Shanghai, China) and commercial colorimetric reagent kits (Nanjing Jiancheng Bioengineering Institute Co., Ltd., Nanjing, China). Additionally, serum levels of leptin and insulin were quantified using bovine-specific enzyme-linked immunosorbent assay (ELISA) kits (Shanghai Lengton Bioscience Co., Ltd., Shanghai, China), while serum total bilirubin (TBIL) and BHBA levels were measured using bovine-specific ELISA kits (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China). The intra-assay and inter-assay coefficients of variation for all assays were maintained below 10%.
2.7 Statistical analysis
IBM SPSS Statistics 26.0 software (SPSS Inc., Chicago, IL, USA) was used to perform the biostatistical analysis of the experimental data. Baseline characteristics including age, parity, BCS, and 305 d milk yield, as well as DMI were compared across the three groups (C, O, and N) using one-way analysis of variance (ANOVA) with Tukey’s post-hoc test. A Chi-square test was employed to assess the risk of subclinical ketosis in the test cows, and the odds ratio (OR) along with the 95% confidence interval (CI) were calculated. Serum data were analyzed using univariate ANOVA, as in previous studies that did not conduct repeated measures analysis (28–30). Statistical significance was defined as p < 0.05, and results are presented as means ± SEM.
3 Results
3.1 Effects of different PG protocols on DMI, Milk yield, and BCS in dairy cows
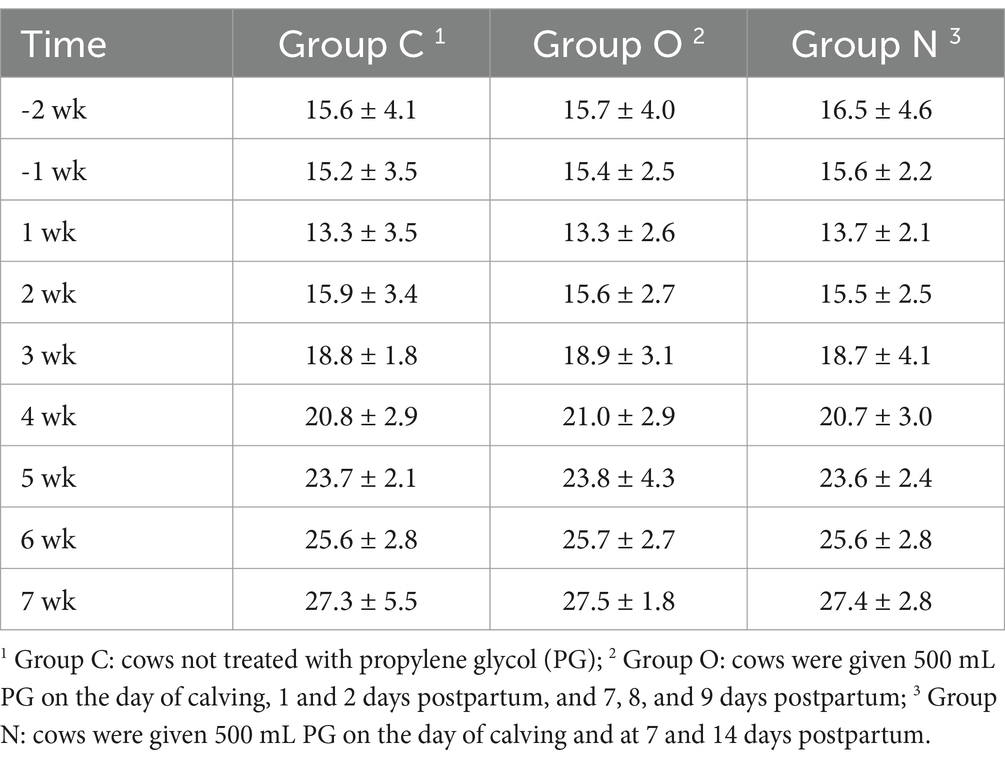
There was no significant difference in DMI among the three groups of cows from 2 weeks prepartum to 7 weeks postpartum (p > 0.05) (Table 2). On day 7 postpartum, the milk yield of cows in Group O was higher than that of cows in the other two groups (p < 0.05). At 14, 21, 28, and 50 days postpartum, the milk yield of cows in Group N increased rapidly, surpassing that of both Group C and Group O (p < 0.05). On days 14, 21, and 50 postpartum, cows in Group O exhibited higher milk yield than those in Group C (p < 0.05) (Figure 1a). From days 7 to 21 postpartum, the BCS of cows in Group O was higher than that of cows in the other two groups (p < 0.05). From days 14 to 21 postpartum, cows in Group N had higher BCS than those in Group C (p < 0.05). On day 28 postpartum, the BCS of cows in Group N had increased, surpassing that of cows in both Group C and Group O (p < 0.05). No significant differences in BCS were observed among the groups on day 50 postpartum (Figure 1b).
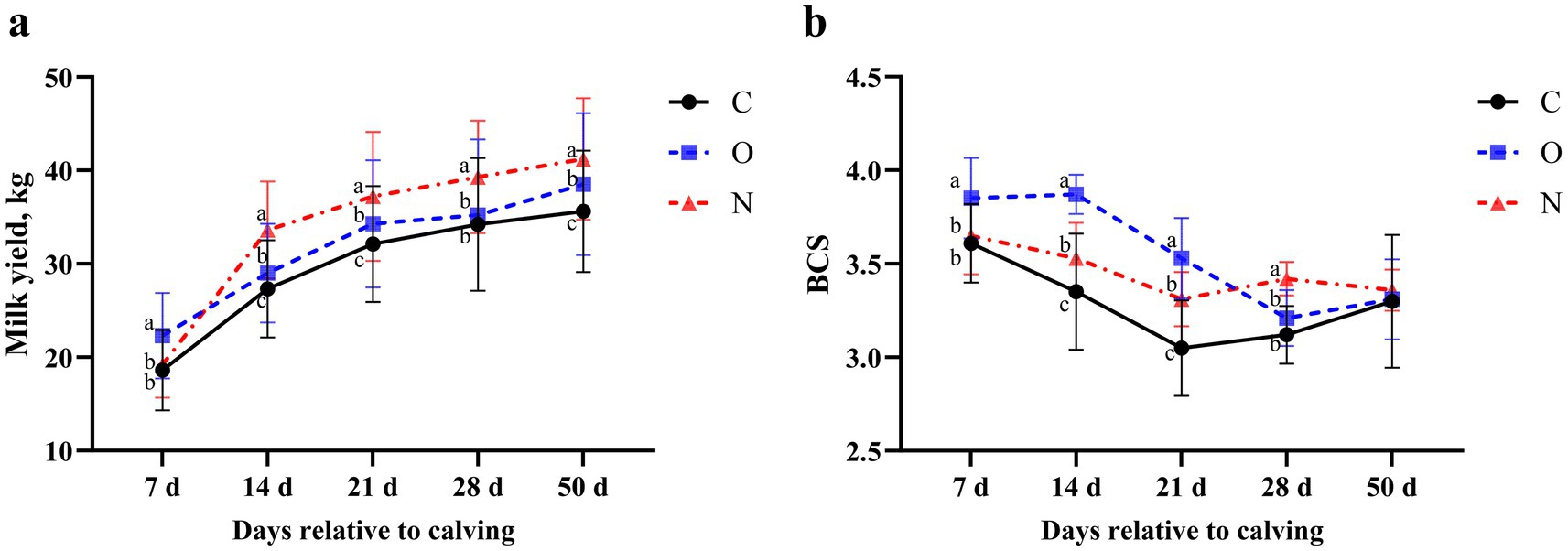
Figure 1. Effects of different propylene glycol protocols on (a) milk yield and (b) body condition score (BCS) in dairy cows. C = control group (●), O = original protocol group (■), N = novel protocol group (▲). Significant differences (p < 0.05) are indicated by different lowercase letters.
3.2 Incidence and risk analysis of ketosis in dairy cows
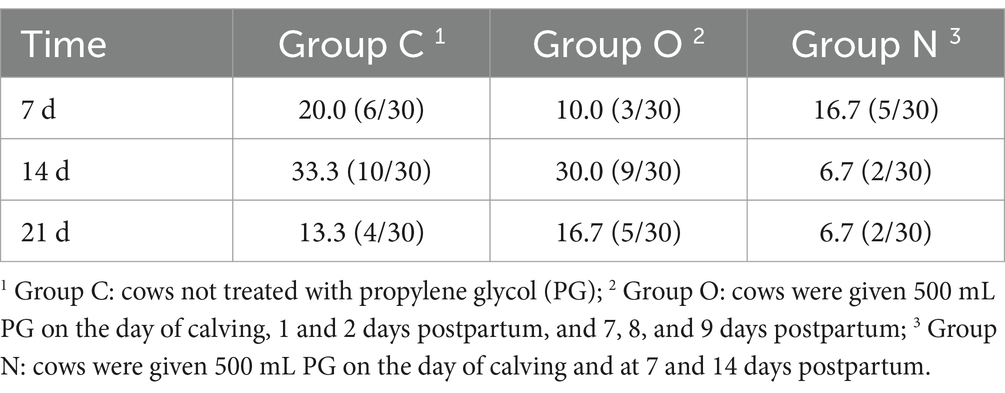
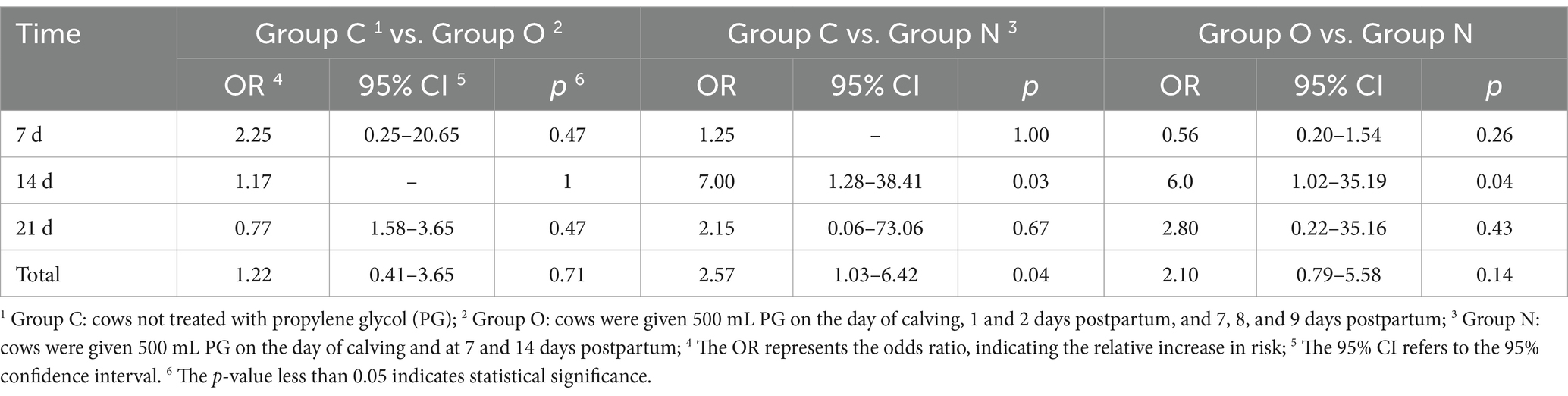
On day 7 postpartum, cows in Group C exhibited the highest incidence of ketosis, followed by those in Group N, while cows in Group O had the lowest incidence. At 14 and 21 days postpartum, cows in Group N demonstrated lower incidence of ketosis compared to the other two groups (Table 3). Additionally, the risk of ketosis in Group N decreased by 6-fold compared to Group O (p < 0.05), the risk of ketosis in Group N decreased by 7-fold compared to Group C (p < 0.05). Overall, these results demonstrate that the risk of ketosis in Group N was 2.57 times lower than that in Group C (p < 0.05) (Table 4).
3.3 Effects of different PG protocols on serum energy metabolites in dairy cows
No differences were observed in serum levels of BHBA, glucose, NEFA, and TC across the groups from 14 (± 3) days prepartum to the day of calving, and at 50 days postpartum. On day 14 postpartum, serum BHBA levels in cows from Group N were lower than those in Groups C and O (p < 0.05). At 21 days postpartum, serum BHBA levels in cows from Group N were lower than those in Group C (p < 0.05). Similarly, at 28 days postpartum, serum BHBA levels in cows from Group N were lower than those in Group C (p < 0.05) (Figure 2a). At 7 days postpartum, serum glucose levels in cows from Group O were higher than those in Groups C and N (p < 0.05). From days 14 to 21 postpartum, serum glucose levels in cows from Groups O and N were higher than those in Group C (p < 0.05) (Figure 2b). From days 7 to 21 postpartum, serum NEFA levels in cows from Groups O and N were lower than those in Group C (p < 0.05), with Group N showing lower levels than Group O at the same time points. At day 28 postpartum, serum NEFA levels in cows from Group N were lower than those in Groups C and O (p < 0.05) (Figure 2c). On day 7 postpartum, serum TC levels in cows from Group N were higher than those in the other two groups (p < 0.05). From days 14 to 28 postpartum, serum TC levels in cows from Groups C and N were lower than those in Group O (p < 0.05) (Figure 2d).
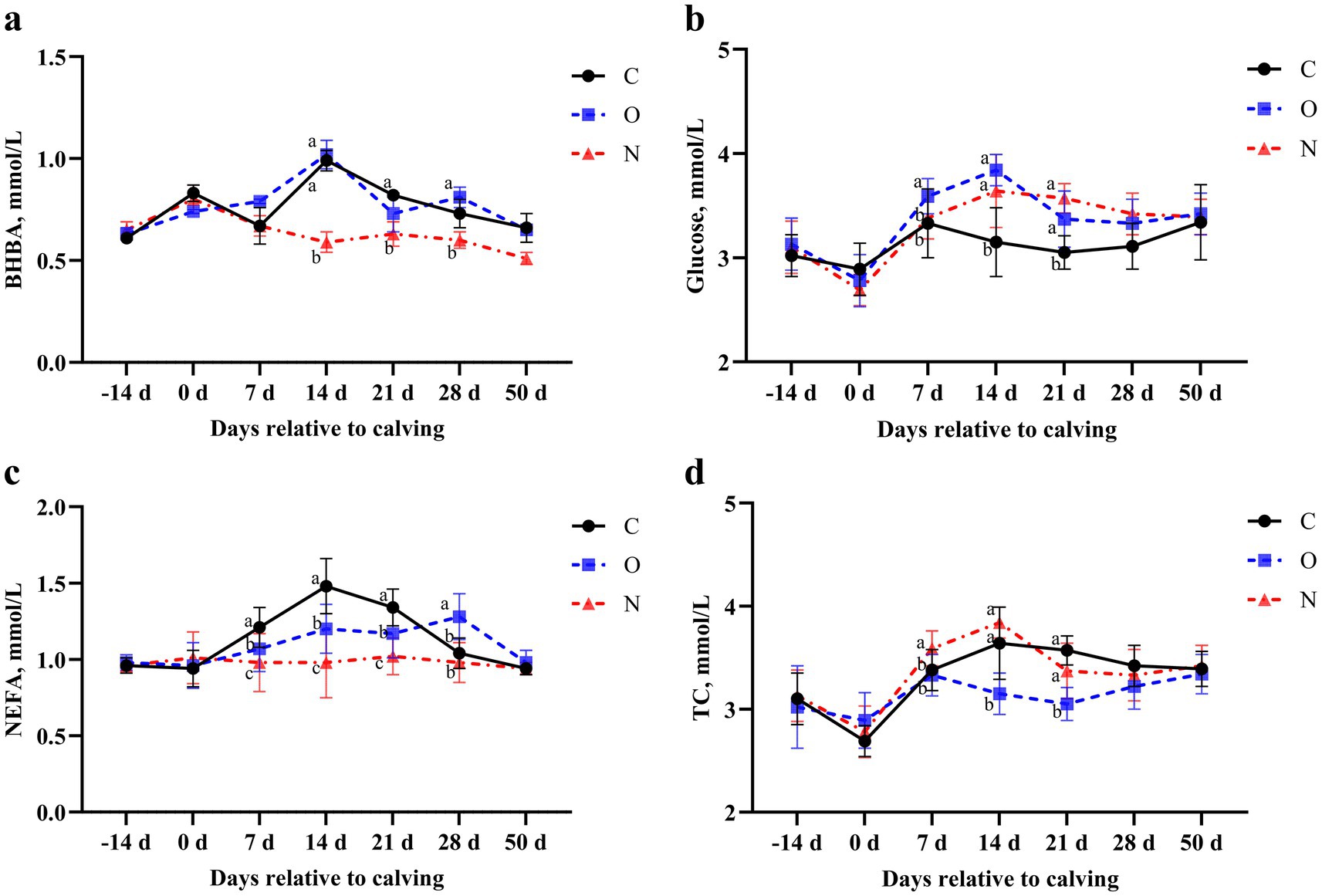
Figure 2. Effects of different propylene glycol protocols on serum (a) BHBA, (b) Glucose, (c) NEFA, and (d) TC levels in dairy cows. BHBA = β-hydroxybutyrate, NEFA = non-esterified fatty acids, TC = total cholesterol, C = control group (●), O = original protocol group (■), N = novel protocol group (▲). Significant differences (p < 0.05) are indicated by different lowercase letters.
3.4 Effects of different PG protocols on serum hormone levels in dairy cows
No differences were observed in serum insulin and leptin levels between Group C and Group O. Notably, serum insulin levels in cows from Group N were higher than those in Group C on day 7 postpartum (p < 0.05). From days 14 to 28 postpartum, serum insulin levels in cows from Group N were higher than those in Groups O and C (p < 0.05) (Figure 3a). At day 7 postpartum, serum leptin levels in cows from Group O were higher than those in Groups C and N (p < 0.05), and this difference persisted until day 28 postpartum (Figure 3b).
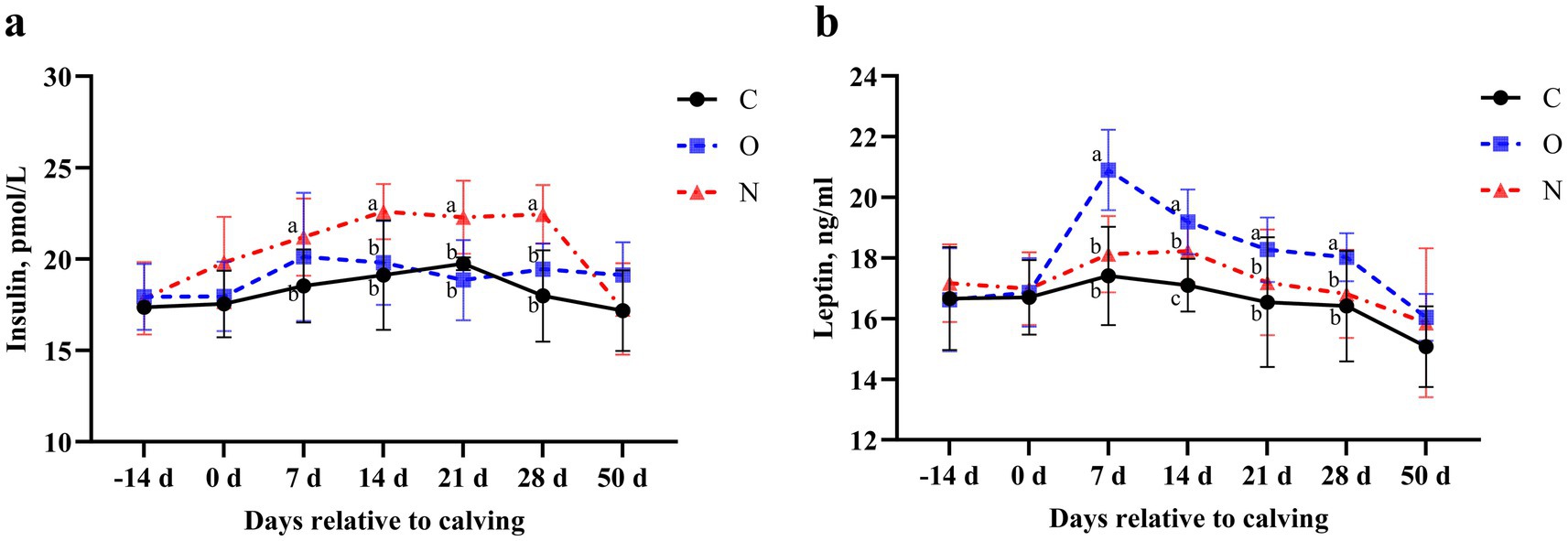
Figure 3. Effects of different propylene glycol protocols on serum (a) insulin and (b) leptin levels in dairy cows. C = control group (●), O = original protocol group (■), N = novel protocol group (▲). Significant differences (p < 0.05) are indicated by different lowercase letters.
3.5 Effects of different PG protocols on serum liver function indicators in dairy cows
In terms of liver function, no differences were observed in serum levels of AST and ALB among the three groups from 14 days prepartum to the day of calving, and from days 28 to 50 postpartum. On days 7 and 14 postpartum, serum AST levels in cows from Groups O and N were lower than those in Group C (p < 0.05). On day 21 postpartum, serum AST levels in Group C remained higher than those in Group N (p < 0.05) (Figure 4a). At 7 days postpartum, serum ALB levels in cows from Group N were higher than those in the other two groups (p < 0.05). On day 14 postpartum, serum ALB levels in cows from Groups O and N were higher than those in Group C (p < 0.05). At day 21 postpartum, serum ALB levels in cows from Group O were higher than those in Group C (p < 0.05) (Figure 4b). Notably, no differences in serum TBIL levels were observed among the three groups during the lactation period (Figure 4c).
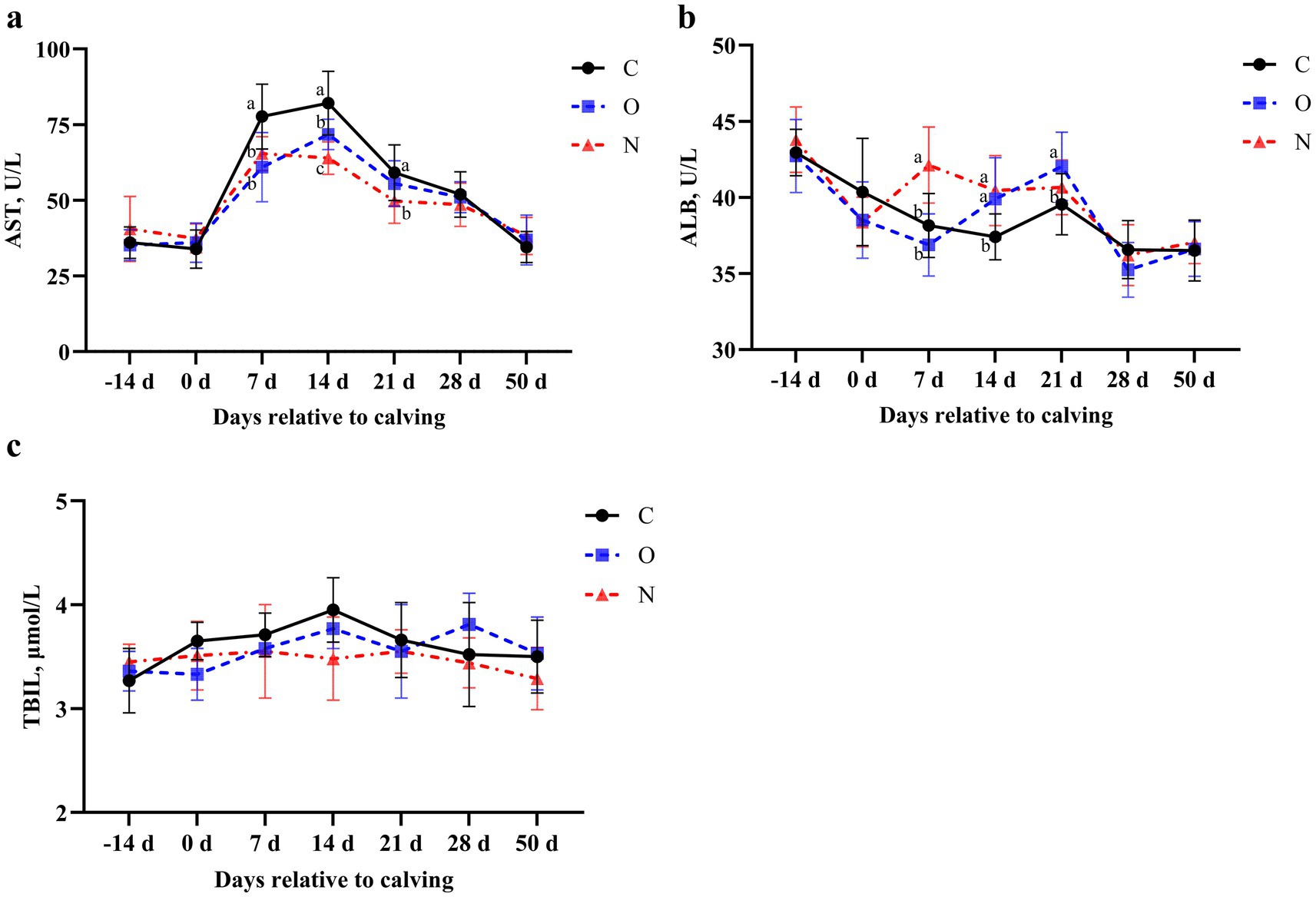
Figure 4. Effects of different propylene glycol protocols on serum (a) AST, (b) ALB, and (c) TBIL levels in dairy cows. AST = aspartate aminotransferase, ALB = albumin, TBIL = total bilirubin, C = control group (●), O = original protocol group (■), N = novel protocol group (▲). Significant differences (p < 0.05) are indicated by different lowercase letters.
3.6 Effects of different PG protocols on serum mineral status in dairy cows
At 7 days postpartum, cows in Group O had higher serum calcium levels compared to those in Group C (p < 0.05). No differences in serum calcium levels were observed among the three groups at other time points (Figure 5a). Throughout the study period, no differences in serum phosphorus or magnesium levels were observed among the three groups (p > 0.05) (Figures 5b,c). Cows in Group N had higher serum potassium levels than those in the other groups at days 21 and 28 postpartum (p < 0.05) (Figure 5d).
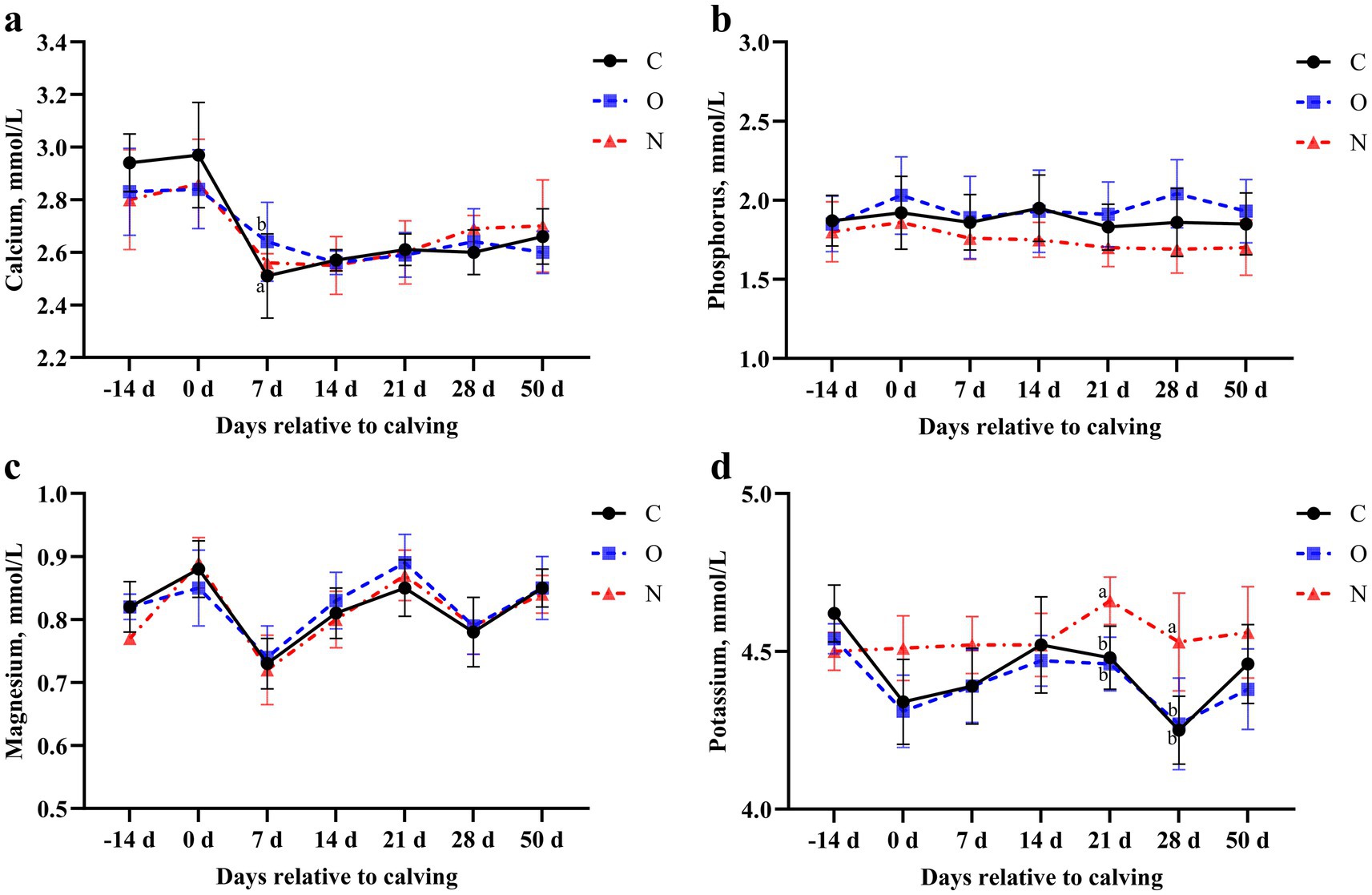
Figure 5. Effects of different propylene glycol protocols on serum (a) calcium, (b) phosphorus, (c) magnesium, and (d) potassium levels in dairy cows. C = control group (●), O = original protocol group (■), N = novel protocol group (▲). Significant differences (p < 0.05) are indicated by different lowercase letters.
3.7 Effects of different PG protocols on serum oxidative stress biomarkers in dairy cows
From days 7 to 21 postpartum, serum MDA levels in cows from Groups O and N were lower than those in Group C (p < 0.05). At 21 days postpartum, serum MDA levels in cows from Group N were also lower than those in Group O (p < 0.05) (Figure 6a). At 7 days postpartum, serum T-AOC levels in cows from Groups O and N were higher than those in Group C (p < 0.05). At 14 days postpartum, serum T-AOC levels in cows from Group N were higher than those in Groups C and O (p < 0.05) (Figure 6b). At 7 days postpartum, serum GSH-Px levels in cows from Group N were increased and higher than those in Group C. Additionally, at 14 days postpartum, serum GSH-Px levels in Group N were higher than those in both Groups C and O (p < 0.05) (Figure 6c). From days 7 to 14 postpartum, serum SOD levels in cows from Group N were higher than those in Groups C and O. At 21 days postpartum, serum SOD levels in Group N remained higher than those in Group C (p < 0.05) (Figure 6d).
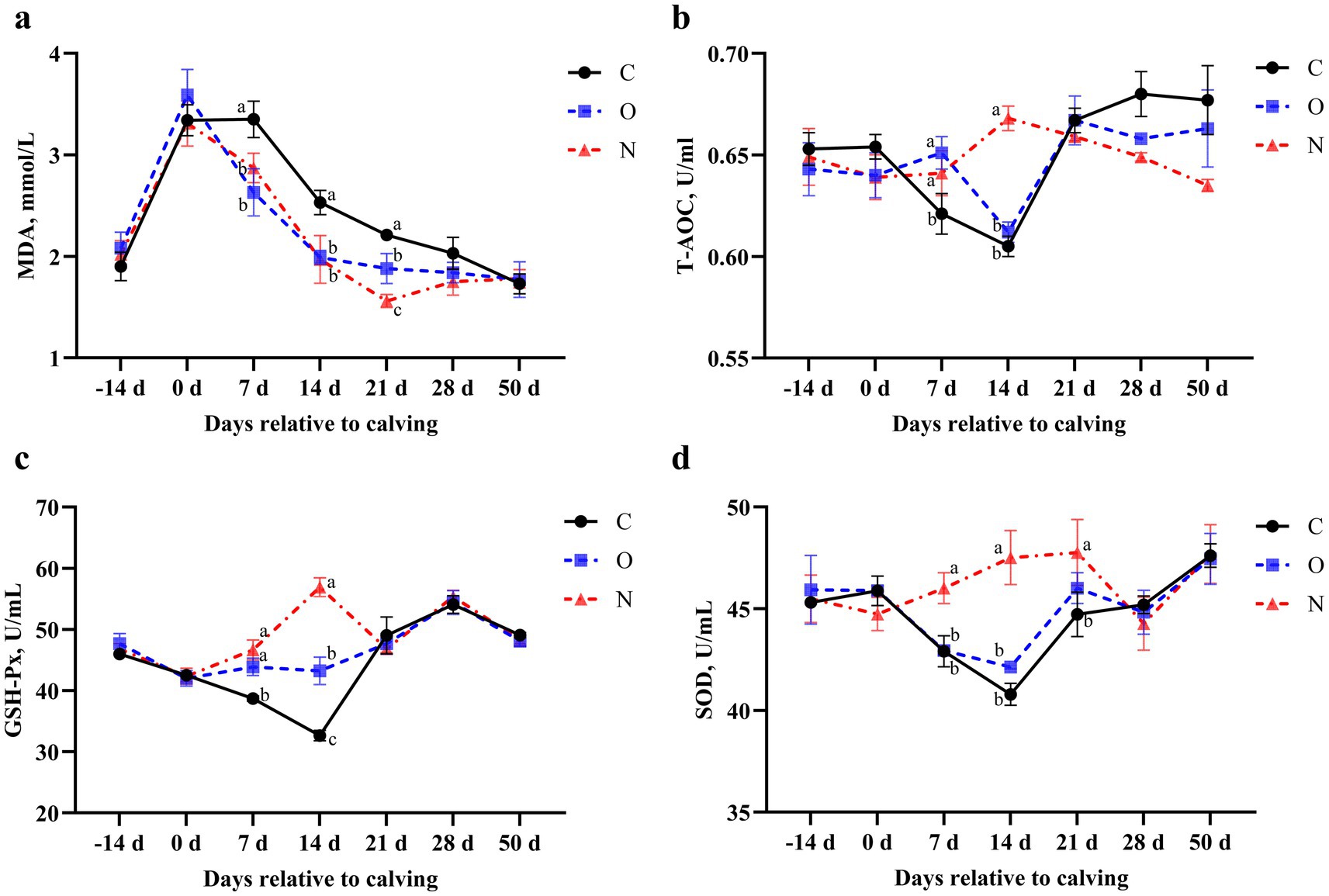
Figure 6. Effects of different propylene glycol protocols on serum (a) MDA, (b) T-AOC, (c) GSH-Px, and (d) SOD levels in dairy cows. MDA = malondialdehyde, T-AOC = total antioxidant capacity, GSH-Px = glutathione peroxidase, SOD = superoxide dismutase, C = control group (●), O = original protocol group (■), N = novel protocol group (▲). Significant differences (p < 0.05) are indicated by different lowercase letters.
4 Discussion
Energy metabolism and oxidative stress are pivotal in dairy cows’ ketosis onset and progression. Postpartum, NEB triggers lipolysis and ketone body production, impacting health, liver function, and oxidative stress. Understanding metabolic interventions’ impact is vital for enhancing cow health and performance. This study aims to evaluate the effects of a novel PG protocol on ketosis, energy metabolism, liver function, and oxidative stress in dairy cows, providing a scientific foundation for optimizing health management and enhancing production outcomes.
High-yielding cows experience an increased demand for glucose during early lactation, leading to a hypoglycemic state, which subsequently results in elevated levels of BHBA (31). This phenomenon is primarily due to the mobilization of fatty acids, which suppresses gluconeogenesis, thereby decreasing serum glucose levels and increasing BHBA levels (32). When energy intake fails to meet energy requirements, cows enter a state of NEB. This increased fat mobilization associated with NEB leads to elevated concentrations of plasma NEFA (33), resulting in large amounts of NEFA accumulating in the liver, where they are oxidized to produce ketone bodies, consequently triggering ketosis (5). Cholesterol plays important physiological roles in the body, including the formation of cell membranes and hormone synthesis (34, 35). The increase in BHBA levels in ketosis cows disrupts cholesterol metabolism and leads to a decrease in TC levels (36, 37). Studies by Nguyen and Singh et al. have demonstrated that PG supplementation elevates serum glucose levels while reducing NEFA and BHBA levels, thereby alleviating the symptoms of ketosis (38, 39). Our results are consistent with these findings, showing that cows in Group N had the lowest incidence of ketosis, while cows in Groups C and O exhibited higher BHBA and NEFA levels and lower TC levels. Notably, the lack of reduction in BHBA and NEFA levels in Group O cows after PG supplementation may be attributed to the potential production of toxic compounds due to excessive PG intake, possibly resulting in adverse toxic effects (19). On days 7 and 14 postpartum, the serum BHBA and glucose levels in cows from Groups O and N did not exhibit the expected inverse relationship due to PG supplementation during this period, which influenced glucose and ketone dynamics. These results suggest that the PG health protocol in Group N was more effective than that in Group O.
Research indicates that during ketosis, downstream insulin signaling in adipose tissue is disrupted, leading to a decreased secretion of insulin by the body (40–43). Insulin plays a critical role in promoting glucose uptake and utilization, as well as facilitating the synthesis of fats and proteins (44). When insulin levels are low, fat mobilization increases, the rate of fatty acid oxidation rises, and the formation of ketone bodies is promoted (45). Consistent with these findings, the present study demonstrated that insulin levels in cows from Groups C and O were lower than those in Group N. Additionally, studies have shown that higher body fat reserves are associated with increased leptin secretion (46), which suppresses appetite and reduces feed intake in cows (47, 48). For postpartum cows, this suppression of appetite often results in insufficient DMI to meet energy demands, causing NEB and subsequently leading to ketosis (19). In our study, postpartum cows in Group O exhibited higher leptin levels than those in Groups N and C. Cows in Group C had the lowest leptin levels, yet the highest incidence of ketosis, which may be attributed to their lower BCS and greater loss of BCS throughout the experimental period. These findings suggest that the novel PG protocol not only effectively increases insulin levels but also reduces leptin secretion in cows.
Liver damage compromises cell membrane integrity, resulting in the leakage of intracellular components, such as AST, into the bloodstream. Consequently, elevated AST levels are commonly regarded as biomarkers of liver cell damage (49, 50). In this study, serum AST levels in cows from all three experimental groups increased as postpartum days progressed. This trend may be associated with enhanced fat mobilization and the accumulation of NEFA in the liver, leading to fatty infiltration (51). Albumin is primarily synthesized by hepatocytes, and a reduction in ALB levels reflects decreased protein synthesis or an insufficient protein supply in the liver (52, 53). In this study, cows in Groups C and O exhibited compromised liver function, as indicated by higher AST levels and lower ALB levels, compared to cows in Group N. These findings suggest that the novel PG protocol employed in Group N effectively mitigated liver damage associated with ketosis.
Oxidative stress is a critical factor contributing to the onset and progression of ketosis, leading to metabolic disturbances and health complications in dairy cows. Malondialdehyde, a byproduct of lipid peroxidation, is generated when oxidative stress induces the peroxidation of lipids within cell membranes. Elevated serum MDA levels are indicative of exacerbated oxidative stress (54, 55). Cows with ketosis often experience a NEB, which accelerates lipolysis. This imbalance between pro-oxidant and antioxidant processes results in oxidative stress and subsequent cellular damage. The T-AOC, SOD, and GSH-Px are essential components of the antioxidant defense system in dairy cows. Reduced activity of these enzymes reflects compromised antioxidant defenses during ketosis (56, 57). Tan et al. (24) reported that oxidative stress in ketotic cows was alleviated through oral administration of PG. Our findings align with these observations. Serum MDA levels in cows from Groups N and O were lower than those in Group C. Moreover, MDA levels in Group N were lower than in Group O, suggesting milder oxidative stress in Group N. Additionally, serum T-AOC, SOD, and GSH-Px levels were higher in Group N compared to Groups C and O, indicating enhanced antioxidant capacity in Group N cows. These results suggest that the novel PG protocol employed in Group N effectively strengthened antioxidant defenses, thereby mitigating oxidative stress.
5 Conclusion
In conclusion, the novel PG protocol demonstrates substantial efficacy in mitigating oxidative stress, enhancing liver function, and improving energy metabolism in dairy cows. Cows in Group N exhibited superior energy metabolism, as evidenced by the modulation of glucose, insulin, and leptin levels, compared to those in Groups C and O. This metabolic improvement was accompanied by a reduction in serum BHBA and NEFA concentrations and improved liver function. Furthermore, the elevated levels of antioxidant markers, including T-AOC, GSH-Px, and SOD, indicate enhanced antioxidant defense mechanisms in Group N cows. While most existing studies have predominantly focused on the short-term effects of PG supplementation, future research should prioritize investigating its long-term impacts, particularly its sustained efficacy across the entire lactation period in dairy cows.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Animal Welfare and Research Ethics Committee of Heilongjiang Bayi Agricultural University (Daqing, China). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YS: Funding acquisition, Writing – review & editing. XJ: Writing – original draft. YH: Formal Analysis, Writing – original draft. RS: Visualization, Writing – original draft. YB: Project administration, Writing – original draft. GS: Data curation, Writing – review & editing. WR: Methodology, Writing – review & editing. CX: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by National Key R&D Program of China (grant number 2023YFD1801100), Heilongjiang Province’s ‘Unveiling the List and Appointing the Leader’ Key Science and Technology Project (grant number 2023ZXJ02B03), New Era Heilongjiang Outstanding Master’s and Doctoral Dissertation Program (grant number LJYXL2023-073), China Postdoctoral Science Foundation (grant number 2024MD753940), and Heilongjiang Postdoctoral Financial Assistance (grant number LBH-Z23243). The APC was funded by Key Project of Natural Science Foundation of Heilongjiang Province of China (grant number ZD2021C006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Drackley, JK. ADSA foundation scholar award. Biology of dairy cows during the transition period: the final frontier? J Dairy Sci. (1999) 82:2259–73. doi: 10.3168/jds.S0022-0302(99)75474-3
2. Imhasly, S, Bieli, C, Naegeli, H, Nyström, L, Ruetten, M, and Gerspach, C. Blood plasma lipidome profile of dairy cows during the transition period. BMC Vet Res. (2015) 11:252. doi: 10.1186/s12917-015-0565-8
3. Zebeli, Q, Ghareeb, K, Humer, E, Metzler-Zebeli, BU, and Besenfelder, U. Nutrition, rumen health and inflammation in the transition period and their role on overall health and fertility in dairy cows. Res Vet Sci. (2015) 103:126–36. doi: 10.1016/j.rvsc.2015.09.020
4. Contreras, GA, and Sordillo, LM. Lipid mobilization and inflammatory responses during the transition period of dairy cows. Comp Immunol Microbiol Infect Dis. (2011) 34:281–9. doi: 10.1016/j.cimid.2011.01.004
5. White, HM. The role of TCA cycle anaplerosis in ketosis and fatty liver in periparturient dairy cows. Animals. (2015) 5:793–802. doi: 10.3390/ani5030384
6. Bellato, A, Tondo, A, Dellepiane, L, Dondo, A, Mannelli, A, and Bergagna, S. Estimates of dairy herd health indicators of mastitis, ketosis, inter-calving interval, and fresh cow replacement in the Piedmont region. Italy Prev Vet Med. (2023) 212:105834. doi: 10.1016/j.prevetmed.2022.105834
7. Duffield, T. Subclinical ketosis in lactating dairy cattle. Vet Clin North Am Food Anim Pract. (2000) 16:231–53. doi: 10.1016/S0749-0720(15)30103-1
8. Gantner, V, Bobić, T, and Potočnik, K. Prevalence of metabolic disorders and effect on subsequent daily milk quantity and quality in Holstein cows. Arch Anim Breed. (2016) 59:381–6. doi: 10.5194/aab-59-381-2016
9. Walsh, RB, Walton, JS, Kelton, DF, LeBlanc, SJ, Leslie, KE, and Duffield, TF. The effect of subclinical ketosis in early lactation on reproductive performance of postpartum dairy cows. J Dairy Sci. (2007) 90:2788–96. doi: 10.3168/jds.2006-560
10. Benedet, A, Manuelian, CL, Zidi, A, Penasa, M, and De Marchi, M. Invited review: β-hydroxybutyrate concentration in blood and milk and its associations with cow performance. Animal. (2019) 13:1676–89. doi: 10.1017/S175173111900034X
11. Berge, AC, and Vertenten, G. A field study to determine the prevalence, dairy herd management systems, and fresh cow clinical conditions associated with ketosis in western European dairy herds. J Dairy Sci. (2014) 97:2145–54. doi: 10.3168/jds.2013-7163
12. Duffield, TF, Lissemore, KD, McBride, BW, and Leslie, KE. Impact of hyperketonemia in early lactation dairy cows on health and production. J Dairy Sci. (2009) 92:571–80. doi: 10.3168/jds.2008-1507
13. Mikuła, R, Pruszyńska-Oszmałek, E, Kołodziejski, PA, and Nowak, W. Propylene glycol and maize grain supplementation improve fertility parameters in dairy cows. Animals. (2020) 10:2147. doi: 10.3390/ani10112147
14. Gohary, K, Overton, MW, Von Massow, M, LeBlanc, SJ, Lissemore, KD, and Duffield, TF. Economic value of ionophores and propylene glycol to prevent disease and treat ketosis in Canada. Can Vet J. (2016) 57:733–40.
15. Kristensen, NB, and Raun, BM. Ruminal and intermediary metabolism of propylene glycol in lactating Holstein cows. J Dairy Sci. (2007) 90:4707–17. doi: 10.3168/jds.2007-0295
16. Piantoni, P, and Allen, MS. Evaluation of propylene glycol and glycerol infusions as treatments for ketosis in dairy cows. J Dairy Sci. (2015) 98:5429–39. doi: 10.3168/jds.2015-9476
17. Jeong, JK, Choi, IS, Moon, SH, Lee, SC, Kang, HG, Jung, YH, et al. Effect of two treatment protocols for ketosis on the resolution, postpartum health, milk yield, and reproductive outcomes of dairy cows. Theriogenology. (2018) 106:53–9. doi: 10.1016/j.theriogenology.2017.09.030
18. Lomander, H, Frössling, J, Ingvartsen, KL, Gustafsson, H, and Svensson, C. Supplemental feeding with glycerol or propylene glycol of dairy cows in early lactation-effects on metabolic status, body condition, and milk yield. J Dairy Sci. (2012) 95:2397–408. doi: 10.3168/jds.2011-4535
19. Zhang, F, Nan, X, Wang, H, Zhao, Y, Guo, Y, and Xiong, B. Effects of propylene glycol on negative energy balance of postpartum dairy cows. Animals. (2020) 10:1526. doi: 10.3390/ani10091526
20. Butler, ST, Pelton, SH, and Butler, WR. Energy balance, metabolic status, and the first postpartum ovarian follicle wave in cows administered propylene glycol. J Dairy Sci. (2006) 89:2938–51. doi: 10.3168/jds.S0022-0302(06)72566-8
21. Nielsen, NI, and Ingvartsen, KL. Propylene glycol for dairy cows: a review of the metabolism of propylene glycol and its effects on physiological parameters, feed intake, milk production, and risk of ketosis. Anim Feed Sci Technol. (2004) 115:191–213. doi: 10.1016/j.anifeedsci.2004.03.008
22. Hubner, AM, Canisso, IF, Peixoto, PM, Coelho, WM Jr, Ribeiro, L, Aldridge, BM, et al. A randomized controlled trial examining the effects of treatment with propylene glycol and injectable cyanocobalamin on naturally occurring disease, milk production, and reproductive outcomes of dairy cows diagnosed with concurrent hyperketonemia and hypoglycemia. J Dairy Sci. (2022) 105:9070–83. doi: 10.3168/jds.2021-21328
23. McArt, JA, Nydam, DV, Ospina, PA, and Oetzel, GR. A field trial on the effect of propylene glycol on milk yield and resolution of ketosis in fresh cows diagnosed with subclinical ketosis. J Dairy Sci. (2011) 94:6011–20. doi: 10.3168/jds.2011-4463
24. Tan, J, Zhao, H, Li, L, Wang, Y, Pan, Y, Fang, L, et al. Propylene glycol alleviates oxidative stress and enhances immunity in ketotic cows through modulating amino acid and lipid metabolism. Antioxidants. (2024) 13:1146. doi: 10.3390/antiox13091146
25. NRC (National Research Council). Nutrient requirements of dairy cattle. 7th ed. Washington, DC, USA: National Academy Press (2001).
26. Edmonson, AJ, Lean, IJ, Weaver, LD, Farver, T, and Webster, G. A body condition scoring chart for Holstein dairy cows. J Dairy Sci. (1989) 72:68–78. doi: 10.3168/jds.S0022-0302(89)79081-0
27. Macmillan, K, López Helguera, I, Behrouzi, A, Gobikrushanth, M, Hoff, B, and Colazo, MG. Accuracy of a cow-side test for the diagnosis of hyperketonemia and hypoglycemia in lactating dairy cows. Res Vet Sci. (2017) 115:327–31. doi: 10.1016/j.rvsc.2017.06.019
28. Liu, L, Shen, T, Yang, W, Yu, H, Gao, S, Huang, B, et al. Ketotic cows display a different serum nonesterified fatty acid composition. J Dairy Res. (2020) 87:52–5. doi: 10.1017/S002202991900092X
29. Walter, LL, Gärtner, T, Gernand, E, Wehrend, A, and Donat, K. Effects of parity and stage of lactation on trend and variability of metabolic markers in dairy cows. Animals. (2022) 12:1008. doi: 10.3390/ani12081008
30. Zhao, C, Bai, Y, Fu, S, Wu, L, Xia, C, and Xu, C. Metabolic alterations in dairy cows with subclinical ketosis after treatment with carboxymethyl chitosan-loaded, reduced glutathione nanoparticles. J Vet Intern Med. (2020) 34:2787–99. doi: 10.1111/jvim.15894
31. Wankhade, PR, Manimaran, A, Kumaresan, A, Jeyakumar, S, Ramesha, KP, Sejian, V, et al. Metabolic and immunological changes in transition dairy cows: a review. Vet World. (2017) 10:1367–77. doi: 10.14202/vetworld.2017.1367-1377
32. Shin, EK, Jeong, JK, Choi, IS, Kang, HG, Hur, TY, Jung, YH, et al. Relationships among ketosis, serum metabolites, body condition, and reproductive outcomes in dairy cows. Theriogenology. (2015) 84:252–60. doi: 10.1016/j.theriogenology.2015.03.014
33. Gross, JJ, Schwarz, FJ, Eder, K, van Dorland, HA, and Bruckmaier, RM. Liver fat content and lipid metabolism in dairy cows during early lactation and during a mid-lactation feed restriction. J Dairy Sci. (2013) 96:5008–17. doi: 10.3168/jds.2012-6245
34. Fedorov, NS, Malomouzh, AI, and Petrov, AM. Effects of membrane cholesterol-targeting chemicals on skeletal muscle contractions evoked by direct and indirect stimulation. J Muscle Res Cell Motil. (2024) 45:221–31. doi: 10.1007/s10974-024-09675-7
35. Hossain, MS, Barua, A, Tanim, MAH, Hasan, MS, Islam, MJ, Hossain, MR, et al. Ganoderma applanatum mushroom provides new insights into the management of diabetes mellitus, hyperlipidemia, and hepatic degeneration: a comprehensive analysis. Food Sci Nutr. (2021) 9:4364–74. doi: 10.1002/fsn3.2407
36. Gross, JJ, Schwinn, AC, Müller, E, Münger, A, Dohme-Meier, F, and Bruckmaier, RM. Plasma cholesterol levels and short-term adaptations of metabolism and milk production during feed restriction in early lactating dairy cows on pasture. J Anim Physiol Anim Nutr. (2021) 105:1024–33. doi: 10.1111/jpn.13531
37. Zhou, S, Chen, M, Meng, M, Ma, N, Xie, W, Shen, X, et al. Subclinical ketosis leads to lipid metabolism disorder by downregulating the expression of acetyl-coenzyme a acetyltransferase 2 in dairy cows. J Dairy Sci. (2023) 106:9892–909. doi: 10.3168/jds.2023-23602
38. Nguyen, HT, and Diep, TT. Efficacy of propylene glycol on prevention and treatment of ketosis for dairy cows in lactation stage. J Agric Dev. (2020) 19:28–35. doi: 10.52997/jad.4.02.2020
39. Singh, G, Randhawa, SS, Uppal, SK, Randhawa, CS, and Chand, N. Evaluation of efficacy of propylene glycol in the treatment of subclinical ketosis and its effect on plasma concentration of various metabolic parameters. J Anim Res. (2017) 7:691–7. doi: 10.5958/2277-940X.2017.00106.1
40. Nassef, E, Abouzeid, TK, and Shokry, M. Impact of dietary propylene glycol supplementation on some biochemical parameters, productive and reproductive performance of lactating dairy cows. AJVS. (2019) 62:47–54. doi: 10.5455/ajvs.51226
41. Zhang, F, Li, D, Wu, Q, Sun, J, Guan, W, Hou, Y, et al. Prepartum body conditions affect insulin signaling pathways in postpartum adipose tissues in transition dairy cows. J Anim Sci Biotechnol. (2019) 10:38. doi: 10.1186/s40104-019-0347-4
42. Elmeligy, E, Oikawa, S, Mousa, SA, Bayoumi, SA, Hafez, A, Mohamed, RH, et al. Role of insulin, insulin sensitivity, and abomasal functions monitors in evaluation of the therapeutic regimen in ketotic dairy cattle using combination therapy with referring to milk yield rates. Open Vet J. (2021) 11:228–37. doi: 10.5455/OVJ.2021.v11.i2.7
43. Xia, C, Wang, Z, Xu, C, and Zhang, HY. Concentrations of plasma metabolites, hormones, and mRNA abundance of adipose leptin and hormone-sensitive lipase in ketotic and nonketotic dairy cows. J Vet Intern Med. (2012) 26:415–7. doi: 10.1111/j.1939-1676.2011.00863.x
44. Fleming, A, Schenkel, FS, Chen, J, Malchiodi, F, Bonfatti, V, Ali, RA, et al. Prediction of milk fatty acid content with mid-infrared spectroscopy in Canadian dairy cattle using differently distributed model development sets. J Dairy Sci. (2017) 100:5073–81. doi: 10.3168/jds.2016-12102
45. Langin, D. Adipose tissue lipolysis revisited (again!): lactate involvement in insulin antilipolytic action. Cell Metab. (2010) 11:242–3. doi: 10.1016/j.cmet.2010.03.003
46. Ahima, RS, Prabakaran, D, Mantzoros, C, Qu, D, Lowell, B, Maratos-Flier, E, et al. Role of leptin in the neuroendocrine response to fasting. Nature. (1996) 382:250–2. doi: 10.1038/382250a0
47. Holtenius, K, Agenäs, S, Delavaud, C, and Chilliard, Y. Effects of feeding intensity during the dry period. 2. Metabolic and hormonal responses. J Dairy Sci. (2003) 86:883–91. doi: 10.3168/jds.S0022-0302(03)73671-6
48. Młynek, K, Strączek, I, and Głowińska, B. The occurrence of a negative energy balance in Holstein-Friesian and Simmental cows and its association with the time of resumption of reproductive activity. Metabolites. (2022) 12:448. doi: 10.3390/metabo12050448
49. Lv, XH, Yang, JL, and Deng, K. Letter to the editor: COVID-19-related liver injury: the interpretation for aspartate aminotransferase needs to be cautious. Hepatology. (2021) 73:874. doi: 10.1002/hep.31509
50. Kew, MC. Serum aminotransferase concentration as evidence of hepatocellular damage. Lancet. (2000) 355:591–2. doi: 10.1016/S0140-6736(99)00219-6
51. Fan, X, Xu, J, Hu, Y, Wang, K, Zhao, Y, Cai, J, et al. Effect of high NEFA concentration on lipid metabolism disorders in hepatocytes based on lipidomics. Front Pharmacol. (2024) 15:1372296. doi: 10.3389/fphar.2024.1372296
52. Spinella, R, Sawhney, R, and Jalan, R. Albumin in chronic liver disease: structure, functions and therapeutic implications. Hepatol Int. (2016) 10:124–32. doi: 10.1007/s12072-015-9665-6
53. Amouzandeh, M, Sundström, A, Wahlin, S, Wernerman, J, Rooyackers, O, and Norberg, Å. Albumin and fibrinogen synthesis rates in advanced chronic liver disease. Am J Physiol Gastrointest Liver Physiol. (2023) 325:G391–7. doi: 10.1152/ajpgi.00072.2023
54. Ayala, A, Muñoz, MF, and Argüelles, S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med Cell Longev. (2014) 2014:360438. doi: 10.1155/2014/360438
55. Cordiano, R, Di Gioacchino, M, Mangifesta, R, Panzera, C, Gangemi, S, and Minciullo, PL. Malondialdehyde as a potential oxidative stress marker for allergy-oriented diseases: an update. Molecules. (2023) 28:5979. doi: 10.3390/molecules28165979
56. Zal, F, Miladpour, B, Taheri, R, Heidari, I, and Mostafavi-Pour, Z. Estrogen and/or progesterone effects on HepG2 human cell lines; oxidant or antioxidant? JAMSAT. (2015) 1:35–41. doi: 10.18869/nrip.jamsat.1.1.35
Keywords: dairy cow, ketosis, propylene glycol, oxidative stress, energy metabolism
Citation: Song Y, Jiang X, Hao Y, Sun R, Bai Y, Shao G, Ren W and Xia C (2025) Effectiveness of a novel propylene glycol protocol in reducing ketosis in transition dairy cows. Front. Vet. Sci. 12:1609300. doi: 10.3389/fvets.2025.1609300
Edited by:
Jae-Cheol Jang, Institute of Agricultural and Life Science, Republic of KoreaCopyright © 2025 Song, Jiang, Hao, Sun, Bai, Shao, Ren and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wanxia Ren, d2FueGlhbWFudGlhbkB5ZWFoLm5ldA==; Cheng Xia, eGN3bHh5ZjIwMTRAMTYzLmNvbQ==
 Yuxi Song
Yuxi Song Xuejie Jiang1
Xuejie Jiang1 Yunlong Bai
Yunlong Bai Cheng Xia
Cheng Xia