- 1National Reference Laboratory of Veterinary Drug Residues, College of Veterinary Medicine, South China Agricultural University, Guangzhou, China
- 2Guangdong Provincial Key Laboratory of Tea Plant Resources Innovation and Utilization, Tea Research Institute, Guangdong Academy of Agricultural Sciences, Guangzhou, China
Introduction: Transport stress (TS) is unavoidable for livestock and poultry in modern agriculture, which not only affects animal welfare, but also to the quality of meat products. Tea polysaccharide (TPS), one of the major bioactive components of tea leaves, has been shown to have anti-stress effects. However, the antagonistic effect of TPS on TS-induced intestinal damage in chicks is unclear.
Methods: The free radical scavenging and reducing abilities of TPS were determined by chemical methods. RAW264.7 macrophages were stimulated by H2O2 to construct cell oxidative damage model, and the effects of TPS on cell viability and antioxidant enzyme activity were determined. A chick transport stress model was established using the shaker to investigate the effect of TPS on intestinal damage in chick caused by transport stress.
Results: In vitro test results showed that TPS had good free radical scavenging ability and reducing ability. Furthermore, TPS could significantly alleviate cell oxidative damage induced by H2O2. In vivo test results showed that the TS-induced intestinal pathological damage and oxidative damage were markedly alleviated by TPS. Furthermore, TS resulted in the elevated expression of heat shock factors, disrupted expression of heat shock proteins, and similarly disrupted expression of aquaporins in chicks. Surprisingly, chicks treated with TPS showed a significant decrease in the expression of heat shock factors and significant alleviation of the dysregulated expression of heat shock proteins and aquaporins. Analysis of the intestinal flora showed that TS resulted in reduced intestinal flora diversity and altered flora structure in chicks, whereas the intestinal flora was normalized after TPS intervention.
Discussion: TPS has good antioxidant activity and significantly ameliorate TS-induced intestinal damage in chicks, suggesting that TPS could be developed and utilized as a feed additive.
1 Introduction
With the increasing scale and intensity of the livestock and poultry industry, the necessity for seed introduction and export becomes paramount. Consequently, the use of vehicles for off-site transportation has become an indispensable aspect of the breeding industry. During the transportation process, animals might be exposed to a range of stressors, including fluctuations in temperature, fasting, water deprivation, noise, and other factors (1). Transport stress (TS) often has adverse effects, including physical damage to the body, performance degradation, and even death (2). The immature nature of the newborn chick’s body renders it susceptible to stressors encountered during transportation. It has been demonstrated that TS not only disrupts the internal environmental homeostasis of chicks (3), but also causes varying degrees of damage to vital organs, such as the heart, liver, and brain (4–6).
The intestine is the primary organ that responds to stress, which is prone to mucosal damage, slow peristalsis, and other functional disorders when experiencing stress (7, 8). Furthermore, stress can result in a reduction in the diversity and abundance of the intestinal flora, thus affecting the normal development of intestinal metabolism and digestive absorption (9). In addition, TS was observed to cause oxidative damage to jejunal tissue in pigs (10) and a significant increase in small intestinal cell apoptosis in goats (11). However, there is a lack of information on the effects of TS on the intestine of newly hatched chicks.
Tea is an agricultural product derived from the fresh leaves and buds of the tea tree. Tea contains a variety of bioactive components, including polyphenols, theanines, alkaloids, and polysaccharides (12). Tea polysaccharide (TPS), the primary active component of tea, is a high-molecular-weight polymer with a complex structure. Studies have demonstrated that TPS exhibits a range of biological activities, including antioxidant, anti-inflammatory, anti-tumor, and hypoglycemic effects (13–16). Yu extracted a novel acidic polysaccharide, HTP-1, from Hawk tea. The administration of HTP-1 was observed to not only attenuate jejunal injury and improve immune organ indicators in mice, but also increase the abundance of beneficial bacteria (17). Given its multiple biological activities, TPS has significant potential as a natural product in the development of functional feeds. Jin Xuan TPS is an acidic heteropolysaccharide, which was demonstrated to ameliorate metabolic disorders and bolster immune function in immunosuppressed mice (18). However, there is a paucity of research examining the antagonistic effects of TPS on TS.
In this study, we investigated the antioxidant activity of Jin Xuan TPS and evaluated its antagonistic effect on TS-induced intestinal damage in chicks in terms of pathological damage, oxidative damage, heat shock response (HSR), aquaporins (AQPs) and intestinal flora. The aim is to provide a reference basis for the development and utilization of TPS as a feed additive.
2 Materials and methods
2.1 Materials
TPS was extracted in the pre-laboratory stage. The crude polysaccharide was extracted from fresh Jin Xuan tea leaves using ethanol precipitation, followed by deproteinization (Sevage method) and degreasing (petroleum ether). Initial purification was performed using AB-8 macroporous resin and dialysis. Further purification was achieved via DEAE anion-exchange chromatography and Sephacryl S-400 HR gel filtration. The final product was desalted by dialysis and lyophilized to obtain purified TPS. The Mw (Weight-average molecular weight) of TPS is 478.75 kDa with a purity of 68.89%, which consisted mainly of Ara (Arabinose), Gal (Galactose), Gal-UA (Galacturonic acid), Glc (Glucose), Rha (Rhamnose), Man (Mannose), Glc-UA (Glucuronic acid), Xyl (Xylose), and Fuc (Fucose) with the molar ratios of 4.86:5.36:0.21:1.00:1.60:0.49:0.59:0.30:0.13 (19).
2.2 Antioxidant activity
TPS and ascorbic acid (VC) were prepared as 5, 4, 3, 2, 1 and 0.5 mg/mL aqueous solutions, respectively. After weighing 23.64 mg DPPH (2,2-diphenyl-1-picrylhydrazyl) dissolved in a small amount of methanol, it was transferred to a 500 mL volumetric flask, and 50% ethanol was added to fix the volume to obtain 120 μmol/L DPPH solution, which was stored under protection from light. The reaction was carried out by adding 0.1 mL of sample solution and 1.9 mL of DPPH solution. The absorbance change was detected at 525 nm after standing for 20 min at room temperature. The clearance was calculated according to the following formula:
Where A0 is the absorbance of the blank (50% ethanol instead of the sample), A is the absorbance of the sample, and B is the absorbance of the control (50% ethanol instead of DPPH solution).
The ABTS [2,2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate)] free radical scavenging ability, hydroxyl free radical inhibiting ability and iron ion reducing ability of TPS were determined by kit following the supplier’s guidelines (Nanjing Jiancheng Bioengineering Institute Co., Ltd., Nanjing, China).
2.3 Effects of TPS on RAW264.7 macrophages under oxidative stress
2.3.1 Cell culture
RAW264.7 macrophages is purchased from Wuhan Punosai Life Technology Co., LTD. RAW264.7 macrophages were cultured in DMEM (Dulbecco’s modified eagle medium) at 37°C with 5% CO2. When the cell confluence reached 85%, it was digested from the plate and washed three times with PBS for subsequent experiments.
2.3.2 Establishment of oxidative damage model of RAW264.7 macrophages
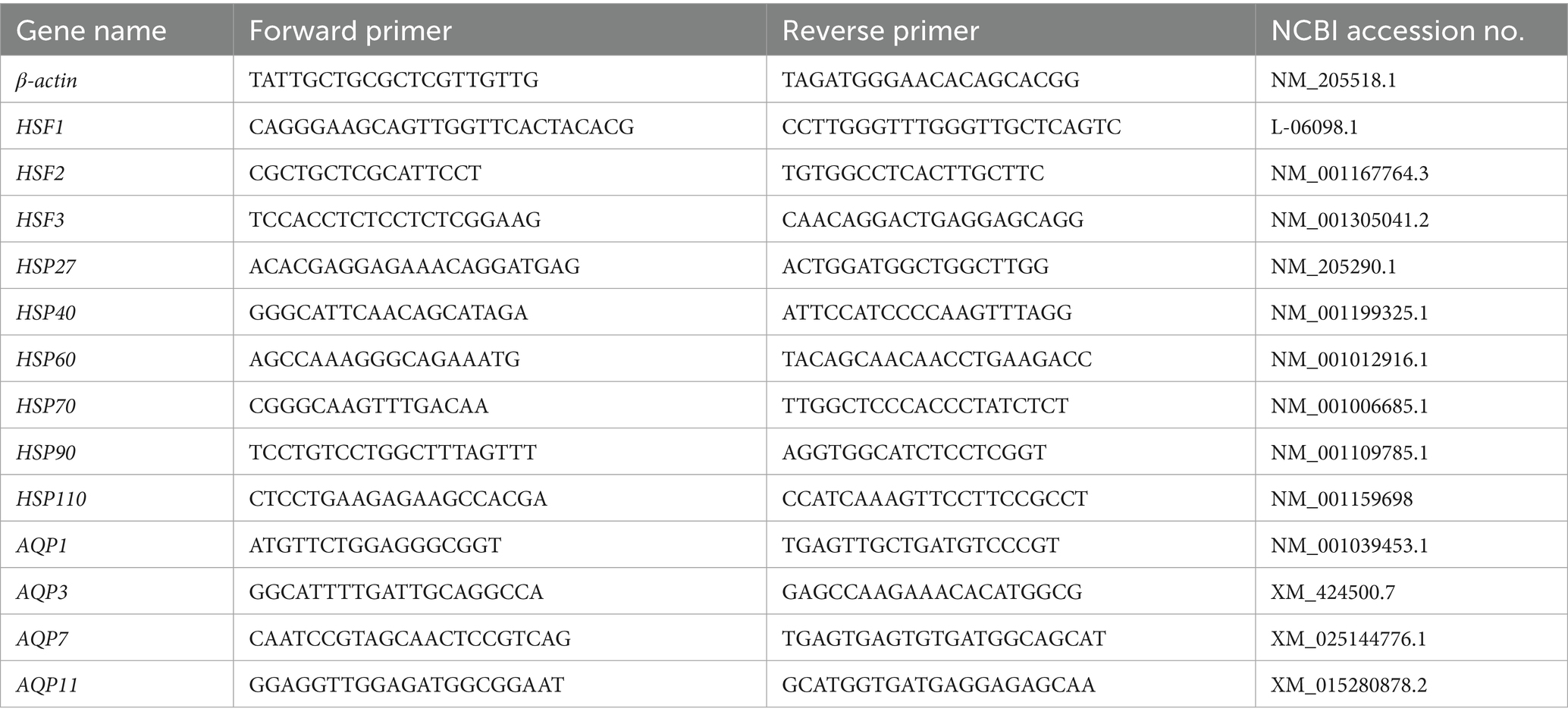
After the cells were inoculated in 96-well plate for 24 h, added H2O2 solutions with different concentrations (final concentrations of 100, 200, 300, 300, 400, 500, 600, and 700 μmol/L) and incubated for another 24 h, respectively. Then, the medium was removed and fresh medium containing 10% CCK-8 solution was added into the plate for 4 h to measure the cell viability.
2.3.3 Cell viability analysis
After the cells were inoculated in 96-well plate for 24 h, added new medium containing TPS solution (final concentration was 4 μg/mL) and incubated for another 24 h. Subsequently, new medium containing H2O2 (finally concentration was 300 μmol/L) replaced the solution for 24 h. Then, the medium was removed and fresh medium that containing 10% CCK-8 solution was added into the plate for 4 h to evaluate the cell viability.
2.3.4 Antioxidant enzyme activity assay
After the cells were inoculated in 96-well plate for 24 h, added new medium containing TPS solution (final concentration was 4 μg/mL) and incubated for another 24 h. Subsequently, new medium containing H2O2 (finally concentration was 300 μmol/L) replaced the solution for 24 h. Then, the medium was removed, washed 3 times with PBS, and the cells were collected. The activity of the total antioxidant capacity (T-AOC) and total superoxide dismutase (T-SOD) in the cells was determined by ELISA (MSD BioTech Co., Ltd. Huhan, China). Glutathione peroxidase (GPx) activities in cells were detected using assay kits following the supplier’s guideline (Beyotime Biotechnology Co., Ltd., Shanghai, China).
2.4 Effect of TPS on TS-induced intestinal damage in chicks
2.4.1 Ethics statement
Every procedure and protocol involving animals was approved by the Experimental Animal Ethics Committee of South China Agricultural University on July 12, 2023 (approval ID: 2023A019).
2.4.2 Animal and trial design
We developed a simulated transport model for newborn chicks based on the experiments of Dadgar and Chen (20, 21). The study utilized a total of 40 one-day-old chicks (Ephedra chicken, Guangdong Wen’s Dahuanong Biotechnology Co., Ltd., Yun-fu, China). The chicks were randomly divided into five groups: a control group (Con), a simulated transport stress group (TS), a TS plus low dose TPS group (L-TPS), a TS plus medium-dose TPS group (M-TPS), and a TS plus high-dose TPS group (H-TPS), with each group comprising eight chicks. We designed the administration dose of TPS based on the experiments of Zhou and Lan (18, 22). One hour before the commencement of the transportation process, the chicks in Con and TS were administered 0.3 mL of deionized water, while the chicks in the L-TPS, M-TPS, and H-TPS received TPS at doses of 200, 400, and 600 mg/kg body weight (b.w.) respectively. The chicks in Con were maintained at 28°C for 5 h within a warm box, while the remaining chicks were subjected to a simulated transportation process on a shaker with an oscillation frequency of 60–80 r/min at 32°C. During the experimental period, the chicks in each group were not provided with food or water. At the conclusion of simulated transport, all chicks were euthanized with carbon dioxide, and jejunal tissues were promptly collected and preserved in liquid nitrogen for subsequent experimentation. A portion of the jejunal tissue was stored in a 4% paraformaldehyde solution. The cecum feces were collected and stored in a − 80°C refrigerator for subsequent analysis.
2.4.3 Histopathological observation
Adequately fixed jejunal tissues were dehydrated through a concentration gradient of ethanol, followed by embedding, sectioning, and hematoxylin and eosin (H&E) staining. After staining, the histopathological changes in the jejunum were observed under a microscope. Villi length and crypt depth were measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.4.4 Antioxidant enzyme activity assay
T-AOC, T-SOD and GPx activities, and the malondialdehyde (MDA) content in chick jejunum were detected using their respective assay kits following the supplier’s guidelines (Beyotime Biotechnology Co., Ltd., Shanghai, China).
2.4.5 Total RNA extraction and quantitative real-time reverse transcription PCR
Jejunal tissue (50 mg) was obtained and processed using the RNAiso Plus reagent (Takara Inc., Dalian, China) in accordance with the manufacturer’s instructions. The resulting total RNA was quantified using an ultra-micro spectrophotometer. Then, 1 μg of total RNA was reverse transcribed into cDNA using an RT reagent Kit (Takara Inc., Dalian, China). The resultant cDNA was then amplified using SYBR qPCR Master Mix (Vazyme Biotech Co. Ltd., Nanjing, China) on an ABI Quant Studio 5 Flex PCR instrument (Applied Biosystems, Foster City, CA, USA) for the qPCR reactions. The primers utilized in the procedure are detailed in Table 1, with β-actin serving as the internal reference gene. The 2-ΔΔCT method was employed to determine the relative mRNA expression levels.
2.4.6 Determination of the intestinal flora
A high-throughput sequencing analysis of the 16S rDNA was conducted on the cecum contents of three randomly selected chick. Genomic DNA was extracted from cecum feces using a DNA extraction kit (Omega Biotek Guangzhou Ltd., Guangzhou, China) and the extracted genomic DNA was detected using 1% agarose gel electrophoresis. The V3-V4 region of 16S rDNA was amplified using Fastpfu DNA Polymerase (TransGen Biotech Co., Ltd., Beijing, China) with the following primer sequences: 338F (ACTCCTAC-GGGGAGGCAGCAG) and 806R (GGACTACHVGGGTWT CTAAT). Purification of the DNA was performed using a DNA Gel Extraction Kit (Heyi Biotech Co., Ltd., Beijing, China). Libraries were then constructed and sequenced on the Illumina NovaSeq PE250 platform (San Diego, CA, USA). Finally, the sequencing reads and operational taxonomic units (OTUs) were analyzed. The impact of TPS on the intestinal flora of TS-treated chicks was evaluated in terms of alpha diversity, beta diversity, and flora structure.
2.5 Statistical analysis
The data were analyzed using SPSS 21.0 (IBM Corp., Armonk, NY, USA), with one-way ANOVA, and the statistical difference was compared using the Tukey test. A p value less than 0.05 was considered statistically significant, and the results are expressed as the mean ± standard deviation. Subsequently, the data were plotted using GraphPad Prism 9.5 (GraphPad Inc., San Diego, CA, USA).
3 Results
3.1 Antioxidant activity of TPS
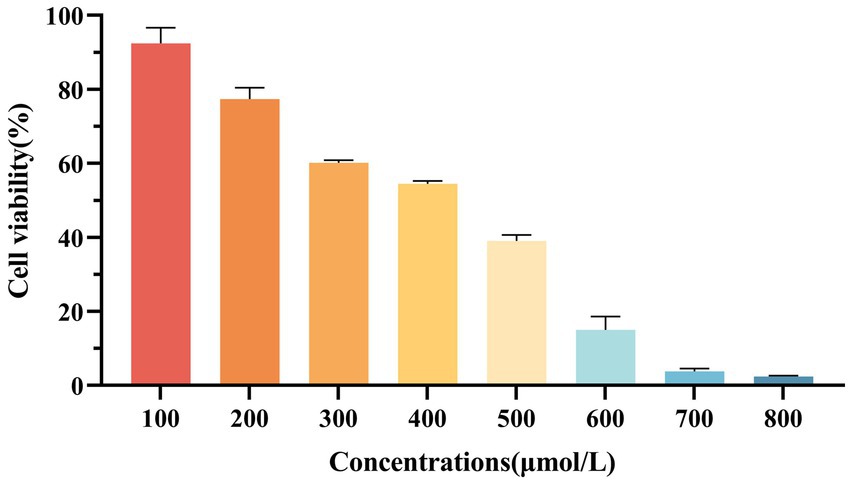
As shown in Figure 1A, the scavenging capacity of TPS for ABTS radicals increased with increasing concentration, reaching a maximum of 1.87 mmol/L at 4 mg/mL. As shown in Figure 1B, the scavenging capacity of TPS for DPPH radicals increased with increasing concentration, and its scavenging rate of DPPH radicals reached a maximum of 91.42% at 3 mg/mL, which was comparable to the effect of the same concentration of VC. As shown in Figure 1C, the hydroxyl radical inhibition ability of TPS increased with increasing concentration, reaching the maximum of 58.74 U/mL at 3 mg/mL. As shown in Figure 1D, the iron ion reducing ability of TPS was always positively correlated with its concentration in the concentration range of 0.5–5 mg/mL.
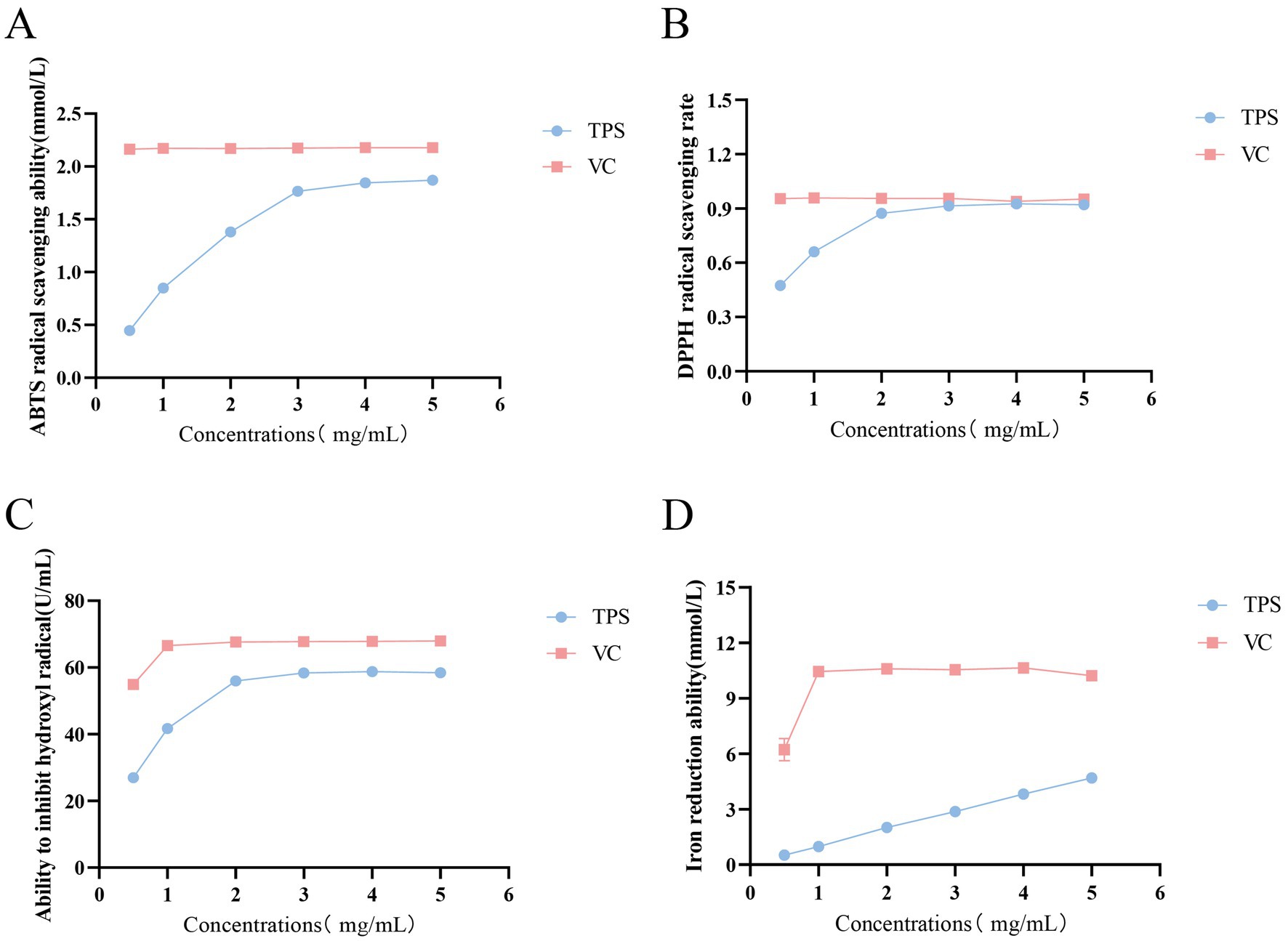
Figure 1. Antioxidant activity of TPS. (A) ABTS free radical scavenging ability. (B) DPPH free radical scavenging ability. (C) Hydroxyl free radical inhibiting ability. (D) Iron ion reducing ability.
3.2 Effects of TPS on RAW264.7 macrophages under oxidative stress
3.2.1 Establishment of oxidative damage model of cells
As shown in Figure 2, when H2O2 concentration was 300 μmol/L, the cell viability was (60.18 ± 0.70) %, indicating that oxidative stress occurred in cells but no irreversible damage occurred. Therefore, 300 μmol/L H2O2 was selected for subsequent tests.
3.2.2 Effect of TPS on the viability of cells induced by H2O2
The effect of TPS on cell viability induced by H2O2-induced oxidative damage is shown in Figure 3. Compared with the control group, the cell viability decreased significantly after H2O2 stimulation (p < 0.05), which was (55.67 ± 0.02) %. Compared with H2O2 group, the cell viability after adding TPS was significantly increased (p < 0.05), which was (102.76 ± 0.02) %. Furthermore, there was no significant difference compared with the control group (p > 0.05). The results showed that TPS could significantly alleviate the apoptosis induced by H2O2.
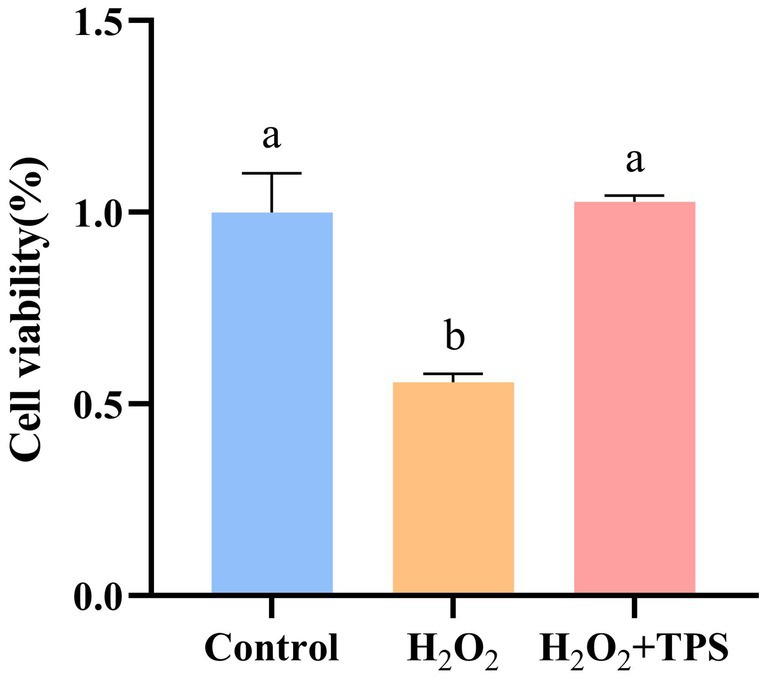
Figure 3. Effect of TPS on the viability of RAW264.7 macrophages induced by H2O2. Distinct letters indicate significant differences by one way analysis of variance (ANOVA) (p < 0.05).
3.2.3 Effect of TPS on antioxidant enzyme activity in cells induced by H2O2
As shown in Figure 4. Compared with the control group, T-SOD and T-AOC activities in the cells of the H2O2 group were significantly reduced (p < 0.05), and there was a tendency for GPx activity to decrease (p > 0.05). This indicated that H2O2 induced oxidative damage in RAW264.7 macrophages. T-SOD, T-AOC, and GPx activities were significantly increased in the H2O2 + TPS group compared to the H2O2 group (p < 0.05). In addition, the T-SOD and T-AOC activities in the TPS group were not significantly different from those in the control group (p > 0.05), and the GPx activity in the TPS group was significantly higher than that in the control group (p < 0.05). The results indicated that TPS significantly alleviated H2O2-induced oxidative damage in cells.
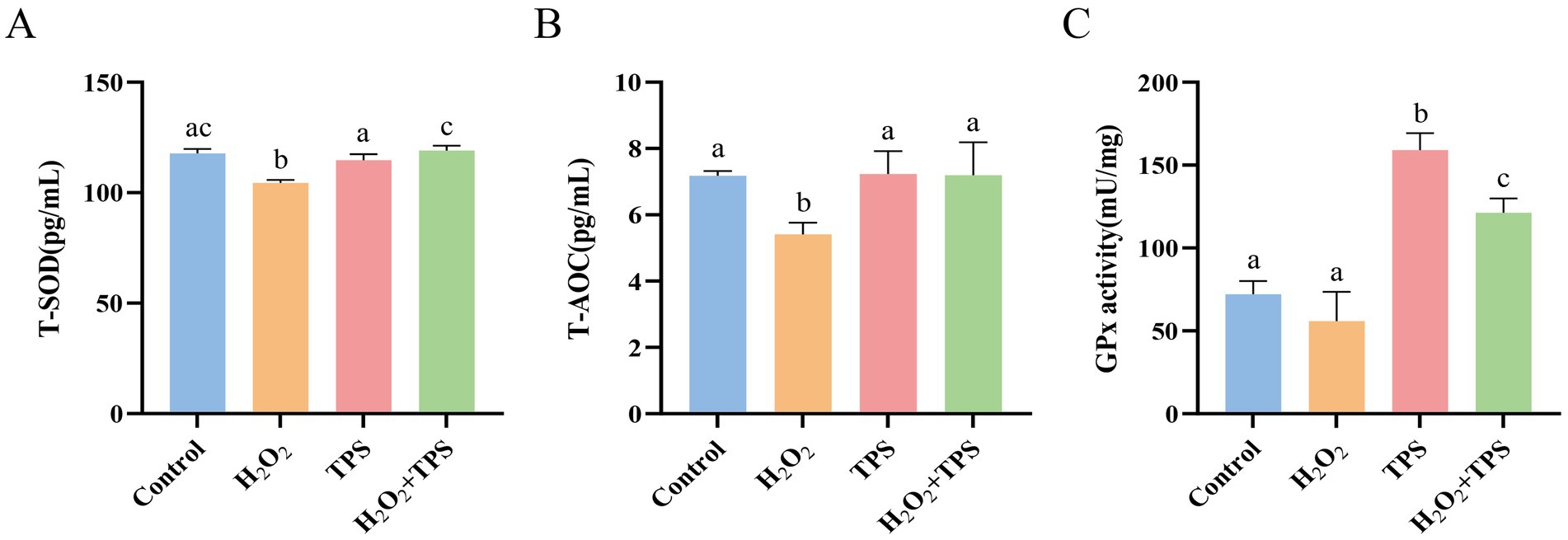
Figure 4. Effect of TPS on the activity of (A) T-SOD, (B) T-AOC and (C) GPx in of RAW264.7 macrophages induced by H2O2. Distinct letters indicate significant differences by one way analysis of variance (ANOVA) (p < 0.05).
3.3 Effect of TPS on TS-induced intestinal damage in chicks
3.3.1 Effect of TPS on TS-induced intestinal pathological damage in chicks
As shown in Figures 5A–E, the jejunal tissues in the Con were clear and intact in all layers, with long intestinal villi and no obvious lesions. In the TS, some epithelial cells of the intestinal villi were detached and there was infiltration of inflammatory cells in the lamina propria. In the L-TPS and M-TPS, the intestinal villi were intact with well-defined cup cells, and there was minimal infiltration of inflammatory cells in the lamina propria. In the H-TPS, the intestinal villi were highly intact with a slight reduction in cup cells. As shown in Figures 5F–H, compared with the Con, the TS had significantly lower intestinal villus height (p < 0.05), significantly higher jejunal crypt depth (p < 0.05), and significantly lower villus height/crypt depth ratio (p < 0.05). Compared with the TS, the M-TPS and H-TPS showed a significant increase in villus height (p < 0.05), a significant decrease in crypt depth (p < 0.05), and a significant increase in the villus height/crypt depth ratio (p < 0.05).
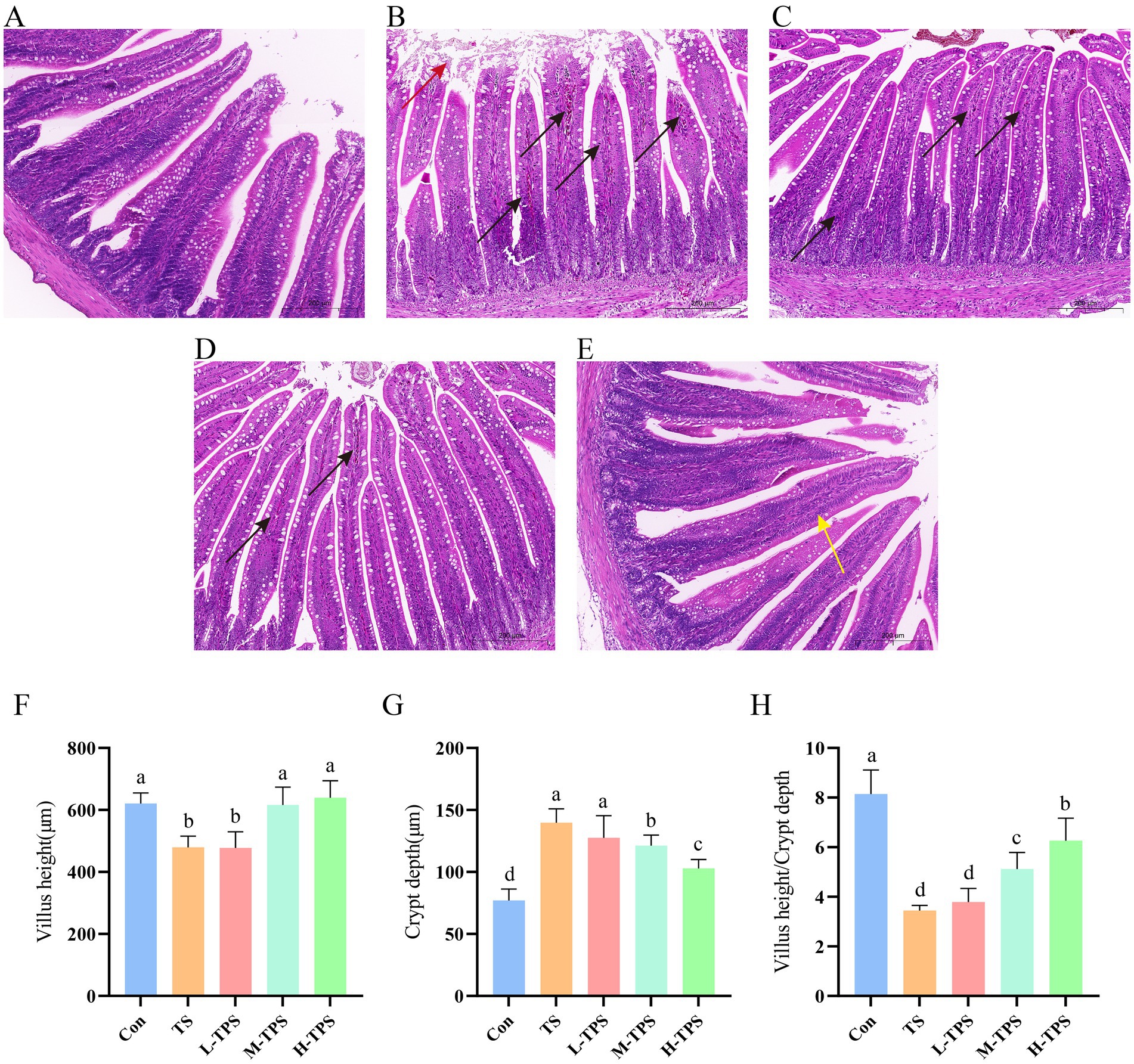
Figure 5. Effect of TPS on TS-induced intestinal pathological damage in chicks. (A–E) Hematoxylin and eosin (H&E) staining of jejunum tissue sections of (A) Con, (B) TS, (C) L-TPS, (D) M-TPS and (E) H-TPS. The red arrows indicate epithelial cell detachment. The black arrows indicate inflammatory cell infiltration. The yellow arrows indicate a decrease in cup cells. (F–H) Quantification of the (F) villi height, (G) crypt depth, and (H) villi height/crypt depth ratio in the jejunum. Distinct letters indicate significant differences by one way analysis of variance (ANOVA) (p < 0.05).
3.3.2 Effect of TPS on TS-induced intestinal oxidative damage in chicks
As shown in Figure 6, compared with the Con, T-AOC, and T-SOD and GPx activities in the TS decreased significantly (p < 0.05), and the MDA content increased significantly (p < 0.05). In comparison to the TS, the L-TPS, M-TPS, and H-TPS exhibited a notable elevation in T-SOD activity (p < 0.05). Additionally, the M-TPS and H-TPS demonstrated a significant enhancement in the T-AOC (p < 0.05). The GPx activity was significantly increased in the L-TPS, M-TPS, and H-TPS (p < 0.05). Meanwhile, the MDA content was significantly decreased in the L-TPS, M-TPS, and H-TPS relative to the TS (p < 0.05).
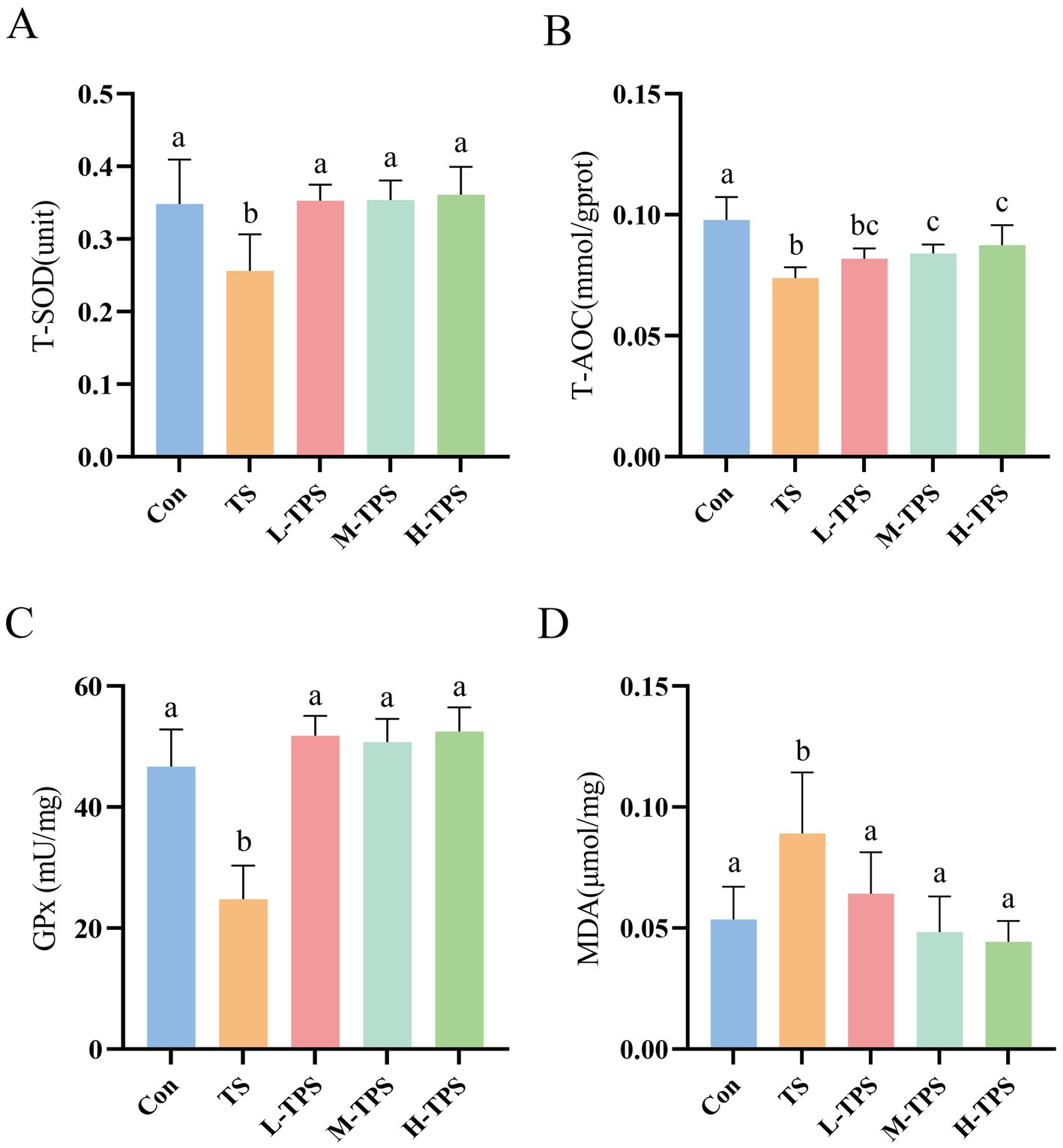
Figure 6. Effect of TPS on TS-induced intestinal oxidative damage in chicks. The contents or activities of (A) T-SOD, (B) T-AOC, (C) GPx and (D) MDA. Distinct letters indicate significant differences by one way analysis of variance (ANOVA) (p < 0.05).
3.3.3 Effect of TPS on TS-induced heat shock response in chick intestine
As shown in Figure 7, significant increases in the mRNA expression levels of HSF1, HSF2, and HSF3 were observed in the TS compared with the Con (p < 0.05). In comparison with the TS, the L-TPS, M-TPS, and H-TPS all showed a notable reduction in the mRNA expression levels of HSF1, HSF2, and HSF3 (p < 0.05).
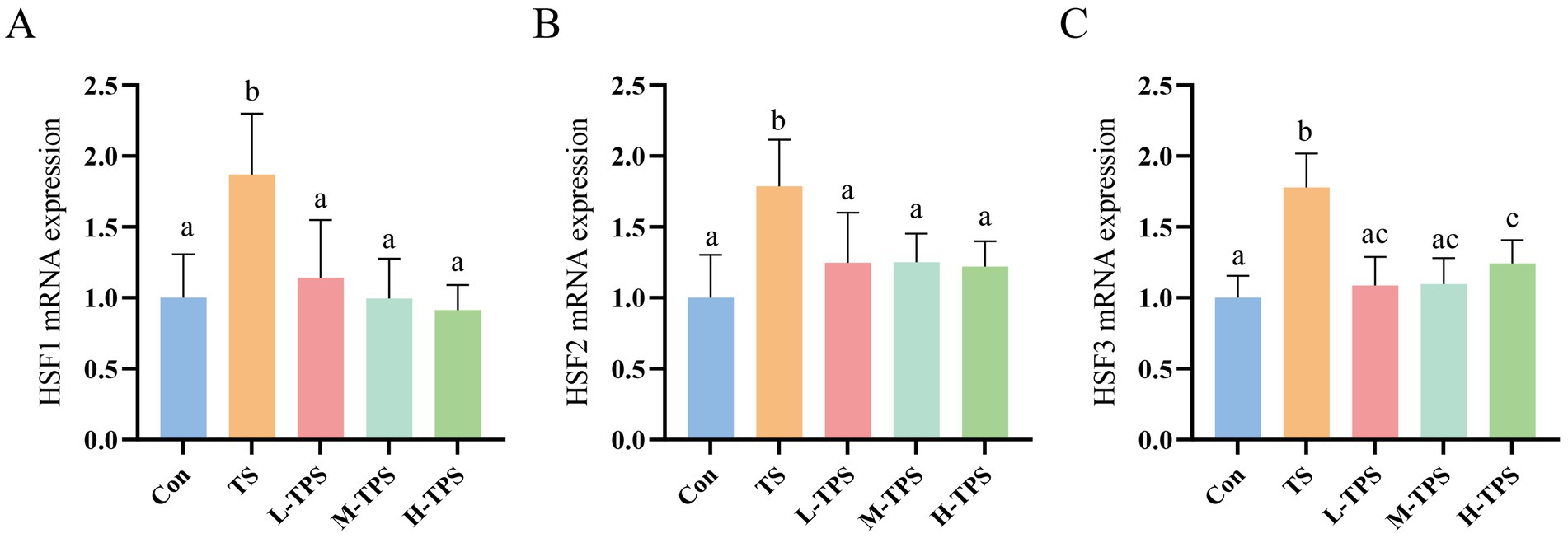
Figure 7. Effect of TPS on TS-induced mRNA expression levels of heat shock factors (HSFs) in the intestine of chicks. The mRNA expression levels of (A) HSF1, (B) HSF2 and (C) HSF3. Distinct letters indicate significant differences by one way analysis of variance (ANOVA) (p < 0.05).
As shown in Figures 8A–F, compared with the Con, the mRNA expression levels of HSP27, HSP60, and HSP70 in the L-TPS, M-TPS, and H-TPS were significantly elevated (p < 0.05), whereas the mRNA expression levels of HSP40, HSP90, and HSP110 were significantly reduced (p < 0.05). In comparison to the TS, the L-TPS, M-TPS, and H-TPS showed a notable reduction in the mRNA expression levels of HSP27, HSP60, and HSP70 (p < 0.05). The HSP40 mRNA expression level in the H-TPS was significantly increased (p < 0.05). No significant alterations were observed in the mRNA expression levels of HSP90 in the L-TPS, M-TPS and H-TPS (p > 0.05). Furthermore, the mRNA expression levels of HSP110 in the M-TPS and H-TPS were notably increased compared with the TS (p < 0.05).
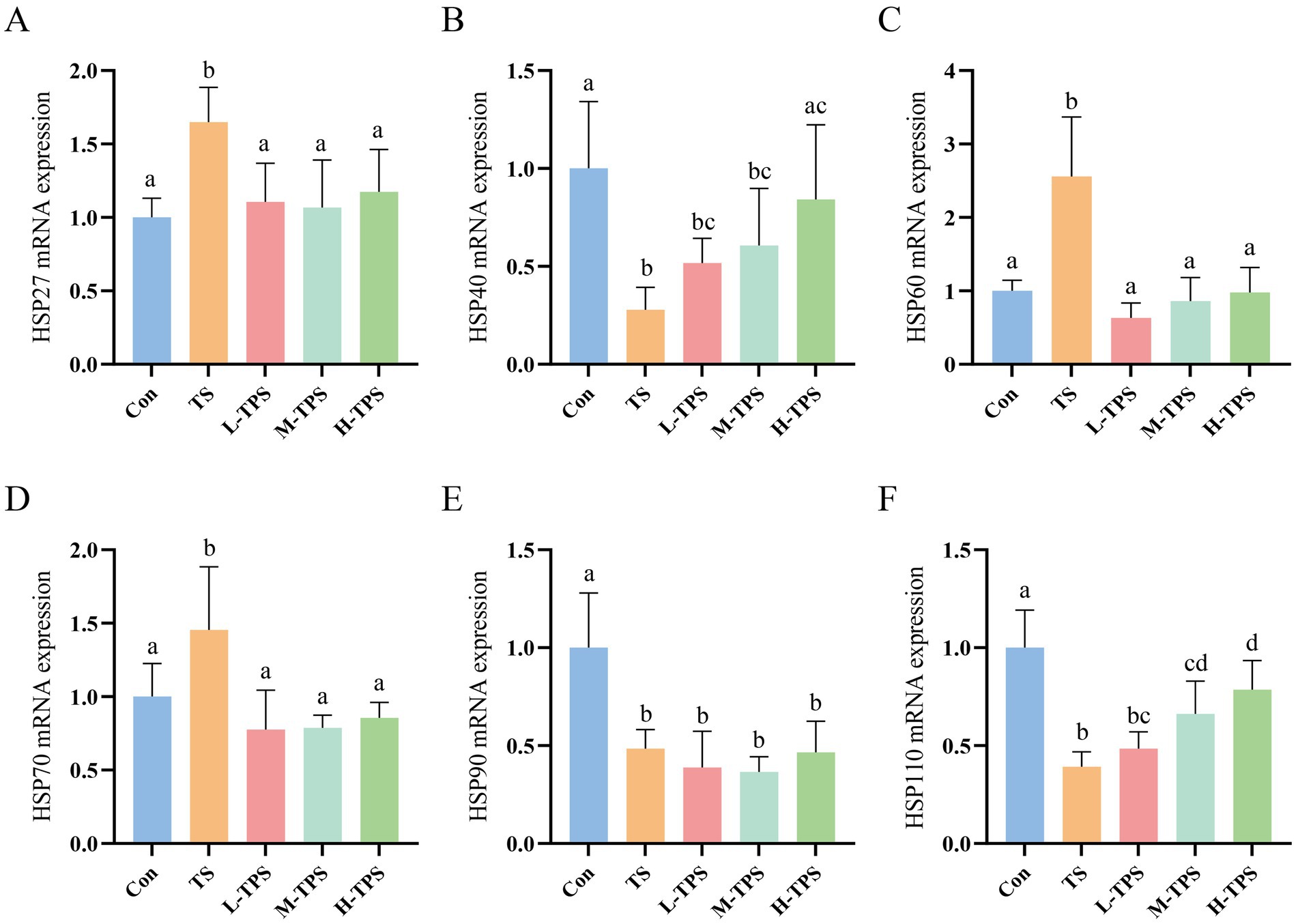
Figure 8. Effect of TPS on TS-induced mRNA expression levels of heat shock proteins (HSPs) in the intestine of chicks. (A–F) The mRNA expression levels of (A) HSP27, (B) HSP40, (C) HSP60, (D) HSP70, (E) HSP90, and (F) HSP110. Distinct letters indicate significant differences by one way analysis of variance (ANOVA) (p < 0.05).
3.3.4 Effect of TPS on aquaporin in TS-induced chick intestines
As shown in Figures 9A–D, compared with the Con, TS caused a significant increase in the mRNA expression levels of AQP1, AQP3, and AQP7 (p < 0.05), and a significant decrease in the mRNA expression of AQP11 (p < 0.05). In comparison to the TS, the AQP1 mRNA expression levels in the M-TPS and H-TPS were notably reduced (p < 0.05); the AQP3 and AQP7 mRNA expression levels in the L-TPS, M-TPS, and H-TPS were significantly decreased (p < 0.05); and the AQP11 mRNA expression levels in the L-TPS, M-TPS, and H-TPS were highly significantly increased (p < 0.05).
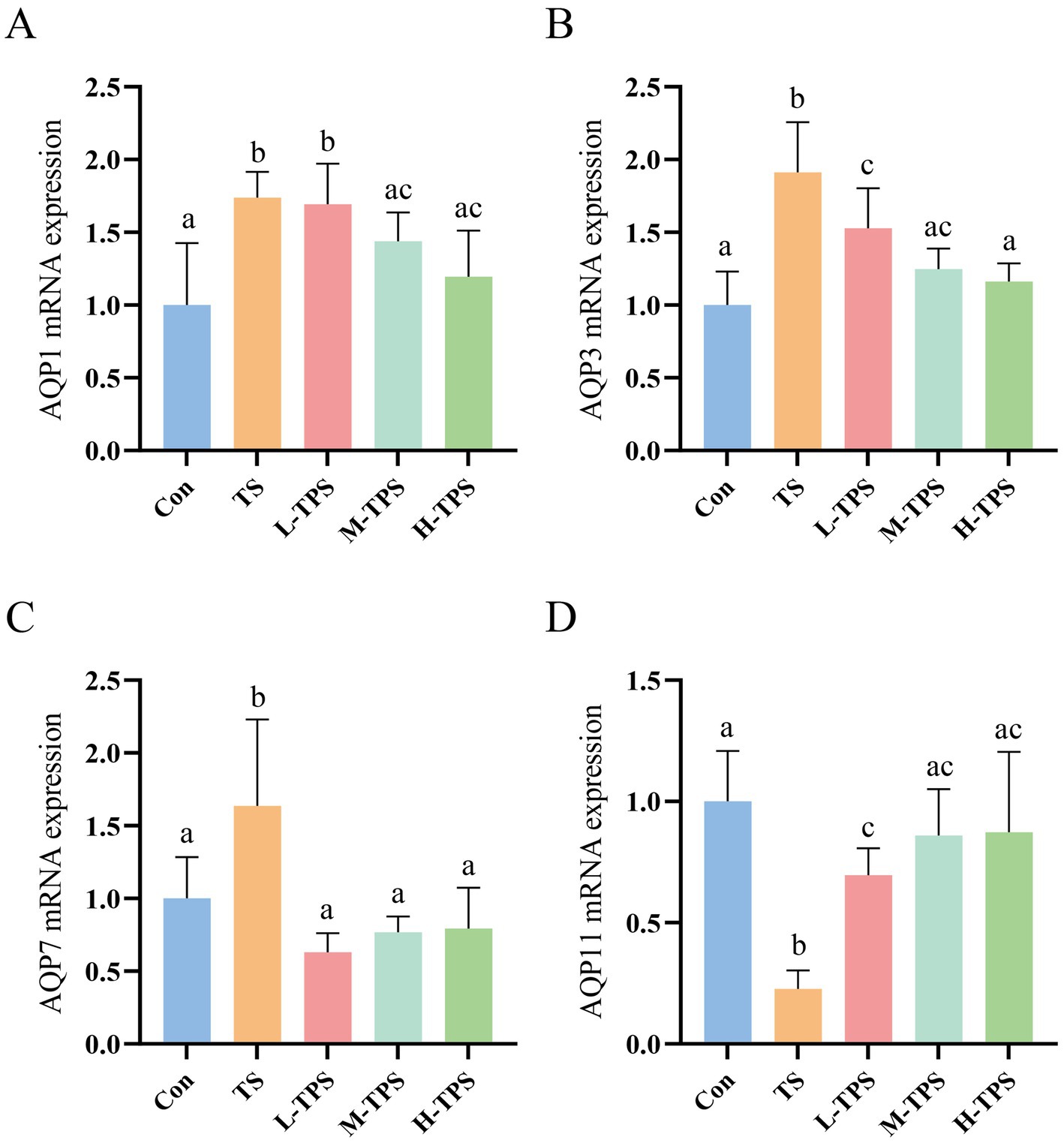
Figure 9. Effect of TPS on TS-induced mRNA expression levels of aquaporins (AQPs) in the intestine of chicks. The mRNA expression levels of (A) AQP1, (B) AQP3, (C) AQP7 and (D) AQP11. Distinct letters indicate significant differences by one way analysis of variance (ANOVA) (p < 0.05).
3.3.5 Effect of TPS on intestinal flora in TS-induced chicks
As illustrated in Figure 10A, in comparison to the Con, the intestinal flora in the TS showed in a notable reduction in the Ace and Chao indexes (p < 0.05); and a declining trend was observed in the Shannon and Invsimpson indexes (p > 0.05). In comparison to the TS, the Shannon, Ace, and Chao indexes were significantly elevated in the M-TPS (p < 0.05). Additionally, the Invsimpson and Ace indexes were significantly elevated in the H-TPS (p < 0.05).
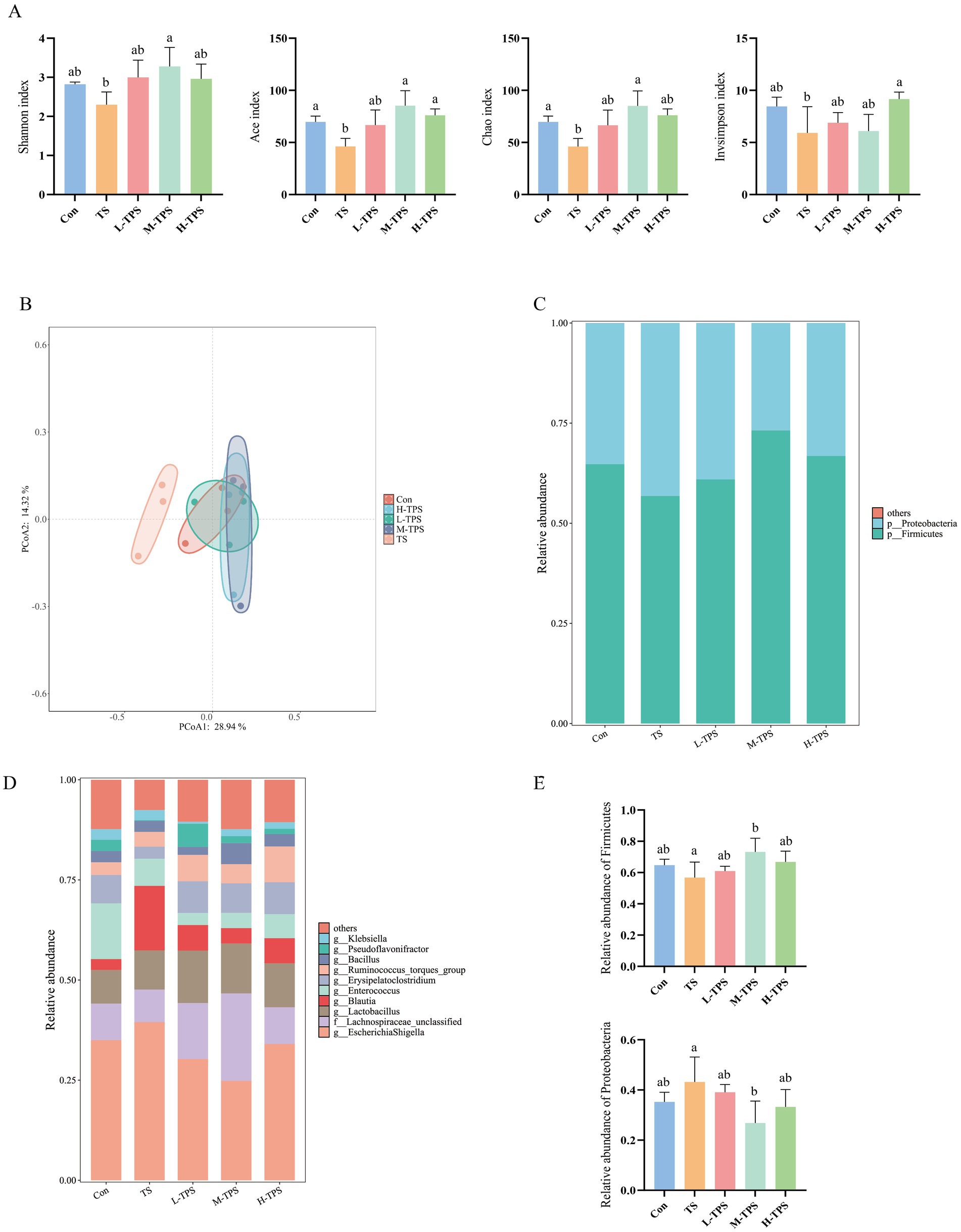
Figure 10. Effect of TPS on the intestinal flora in TS-induced chicks. (A) Alpha diversity indexes. Distinct letters indicate significant differences by one way analysis of variance (ANOVA) (p < 0.05). (B) Principal coordinate analysis (PCoA) plot. (C) Stacked map of species distribution at the phylum level. (D) Stacked map of species distribution at the genus level. (E) Turkey HSD test of relative abundance at the phylum level.
In the principal coordinate analysis (PCoA, Figure 10B), PCoA1 and PCoA2 contributed 28.94 and 14.32% to the variation in the samples, respectively. The PCoA plot revealed notable discrepancies in the distances between the TS and the other groups (Con, L-TPS, M-TPS, and H-TPS). The intestinal flora in the TPS treated groups (L-TPS, M-TPS, and H-TPS) exhibited a closer proximity to the Con.
As illustrated in Figure 10C, at the phylum level, the chick cecum flora was predominantly composed of Firmicutes and Proteobacteria. In comparison to the Con, the TS exhibited a tendency to increase in the relative abundance of Proteobacteria and a tendency to decrease in the relative abundance of Firmicutes(p > 0.05). Compared with the TS, the M-TPS had a significantly higher relative abundance of Firmicutes and a significantly lower relative abundance of the Proteobacteria (p < 0.05). As illustrated in Figure 10D, at the genus level, the predominant intestinal flora observed in each group were Escherichia Shigella, Lachnospiraceae_unclassified, Lactobacillus, Blautia, Enterococcus and Erysipelatoclostridium. The relative abundance of Escherichia Shigella and Blautia exhibited a tendency to increase in the TS compared with the Con (p < 0.05). Compared with the TS, the relative abundances of Escherichia Shigella and Blautia a tendency to decrease in the TPS treated groups (p < 0.05). Notably, the relative abundance of Lactobacillus exhibited a tendency to increase in the TPS treated groups compared with the Con (p < 0.05).
4 Discussion
DPPH, ABTS and hydroxyl radicals are frequently utilized to evaluate antioxidant activity in vitro. DPPH radicals, a highly stable radical, have been extensively employed to assess the free radical scavenging capacity of compounds or extracts (23). ABTS, a water-soluble radical initiator, can be oxidized to green ABTS radicals, and this method has been widely adopted to assess the total antioxidant capacity of compounds (24). Hydroxyl radicals, the most reactive oxygen radicals, are known to be highly invasive and toxic to cells (25). In this study, the DPPH radical and hydroxyl radical scavenging capacity of TPS increased with increasing concentration, demonstrating a positive correlation. The DPPH radical scavenging capacity of TPS reached a maximum of 91.42% at 3 mg/mL, which was comparable to that of VC at the same concentration. The scavenging capacity of TPS for the ABTS radicals reached a maximum at 4 mg/mL, which was 1.87 mmol/ L. The reducing capacity of polysaccharides is also an important indicator for assessing their antioxidant capacity. In this study, the ferric ion reducing ability of TPS always showed a positive correlation with the concentration in the range of 0.5–5 mg/mL. The results indicated that TPS has good antioxidant activity.
RAW264.7 macrophages are frequently chosen as model cells to evaluate the antioxidant activity of polysaccharides (26). It has been reported that H2O2 stimulation can cause oxidative stress, resulting in damage of intracellular biomolecules, such as nucleic acids, membrane lipids, and proteins (27). Therefore, we chose H2O2 to construct the cell oxidative damage model. In the present study, TPS significantly alleviated H2O2-induced apoptosis in RAW264.7 macrophages. Further studies revealed that TPS significantly increased the activities of T-SOD, T-AOC, and GPx in the cells (p < 0.05). Liu et al. investigated the protective effect of Tien Shan green tea polysaccharide (TSPS) against oxidative damage in cells, finding that TSPS protected HepG2 and LO2 cells from H2O2-induced apoptosis. They also found that TSPS regulated the level of ROS and increased the activities of SOD and CAT in cells (28), which was similar to the results of the present study. A multitude of studies have demonstrated that plant polysaccharides can mitigate the damage caused by stress by modulating the antioxidant capacity of the body (29–31). Consequently, it is hypothesized that TPS with potent antioxidant activity may exert an anti-stress effect.
The transportation of animals is not only a matter of animal welfare, but also has implications for economic losses. However, because of the necessity for production and biosecurity, chicks are inevitably subjected to road transportation, which results in their exposure to TS. Various plant polysaccharides have been shown to maintain intestinal health by improving intestinal function (32, 33). In this study, shedding of intestinal villi epithelial cells occurred after 5 h of transport, while the integrity of intestinal villi was improved under TPS intervention. The villus height/crypt depth ratio is an important indicator of the digestive and absorptive function of an organism. In this study, compared with the Con, TS significantly reduced the villus height and the villus height/crypt depth ratio. However, compared with the TS, the M-TPS and H-TPS had significantly higher villus height and a larger villus height/crypt depth ratio, similar to the findings of Zou (34). The results suggested that TPS can ameliorate TS-induced intestinal damage in chicks by improving intestinal villus height and the villus height/crypt depth ratio.
The process of oxidative damage is closely related to stress. Studies have demonstrated that a variety of antioxidant-related signaling pathways, including the Nrf2 (35), PI3K/AKT (36) and NF-kB pathways (21), are activated during periods of stress. The antioxidant system of an organism consists of enzymatic and non-enzymatic systems: T-AOC reflects the cumulative effect of all antioxidants in the non-enzymatic system; the activities of GPx and T-SOD reflect the status of the enzymatic system; and the content of MDA reflects the degree of lipid oxidation of the cells. In this study, the T-AOC, T-SOD and GPx levels were significantly decreased, and the content of MDA was significantly increased in the TS, suggesting that oxidative damage occurred in the jejunum. Surprisingly, T-SOD, T-AOC, GPx, and MDA levels were restored to normal levels after TPS intervention. Liu found that Tien Shan green TPS could protect cells from H2O2 damage by activating the Nrf2 signaling pathway (28). Therefore, we speculated that TPS might alleviate TS-induced oxidative damage by activating the Nrf2 pathway in intestinal tissues, which requires further investigation.
HSR is one of the most conserved pathways in organisms when confronted with stress and unfavorable conditions. It is a classical cellular response to maintain the accurate folding of proteins and to prevent the aggregation of erroneous proteins from interfering with the cell’s normal physiological functions. In eukaryotes, HSR is regulated by heat shock factors (including HSF1-4) (37). HSF1 plays a crucial role in antagonizing environmental perturbations and proteotoxic stresses (38). In this study, TS induced a significant increase in HSF1 mRNA expression, which regulated the expression of heat shock proteins (HSPs). In recent years, HSF2 has been shown to co-regulate the expression of HSPs with HSF1 (39), and thus the expression of HSF1 and HSF2 tended to occur the same direction after TS in the chick jejunum. HSF3 is essential for the HSR in avian. It has been demonstrated that the HSF3 has a higher threshold and increased expression during severe heat shock (40). In this study, the expression of HSF3 was significantly elevated in the TS, indicating that the TS applied to the chicks in this experiment reached the threshold for HSF3 activation. The notable decline in HSF3 expression following TPS intervention indicated that TPS significantly mitigated the adverse effects of TS on the chicks.
HSPs are the main executors of the HSR. However, excessive stress can cause HSPs to lose their protective effects (41). Sun found that the expression of HSPs in chick hearts was elevated in the early stage of TS. However, most of the HSPs were reduced as TS was intensified (4). Therefore, the expression levels of HSPs might be related to the degree of stress. In this study, the mRNA expression levels of HSP27, HSP60, and HSP70 increased, and those of HSP40, HSP90, and HSP110 decreased in response to TS. The results indicated that transportation for 5 h induced a severe stress response in the chick intestines, which was also evidenced by the expression of HSF3. Compared with the TS, the mRNA expression levels of HSP27, HSP60, HSP70 were significantly decreased in TPS treated groups, and the mRNA expression levels of HSP40 and HSP110 were significantly increased. The results indicated that TPS could restore the HSR in the jejunum of chicks that was impaired by TS.
AQPs are widely distributed in a wide range of tissues. They maintain organismal homeostasis mainly by regulating the intra- and extracellular transport of water molecules and small solutes (42). AQPs are functionally classified into three distinct groups: Firstly, AQP1, 2, 4, and 5, which primarily transport water molecules; secondly, a group of aquaglyceroporins, including AQP3, 7, 9, and 10, which transport water, glycerol, and other small solutes; and thirdly, a group including AQP6, 8, 11, and 12, which have a variety of specific cellular localizations and functions (43, 44). In this study, the mRNA expression levels of AQP1, AQP3, and AQP7 were significantly increased, while the expression of AQP11 mRNA was significantly decreased in the intestines of chicks exposed to TS. Wang found that AQPs mRNA levels were significantly increased in the intestines of pyrexic rats, which is consistent with our findings (45). In comparison to the TS, the mRNA expression levels of AQP1, AQP3, and AQP7 were significantly decreased, while the expression level of AQP11 mRNA was significantly increased in the TPS treated groups. These findings suggested that TPS can antagonize the TS-induced intestinal homeostatic imbalance and might serve as a potential therapeutic agent to treat TS-induced intestinal homeostatic imbalance.
The intestinal flora plays a pivotal role in the growth, development, immune function, and metabolism of animals (46). Consequently, an increasing number of studies investigating the function of plant polysaccharides are considering the intestinal flora as a crucial target. Polysaccharides have been shown to enhance intestinal health by inhibiting the proliferation of detrimental bacteria, promoting the growth of beneficial bacteria, and strengthening the intestinal mucosal barrier (47). In this study, 16S rDNA high-throughput sequencing was employed to investigate the impact of TPS on the intestinal flora of TS-treated chicks. The findings indicated a decline in the alpha diversity of the intestinal flora of chicks subjected to TS, accompanied by alterations in beta diversity. However, after TPS intervention, alpha diversity was significantly higher and beta diversity was normalized. At the phylum level, the dominant intestinal flora of chicks was identified as Firmicutes and Proteobacteria. A reduction in the abundance of Firmicutes and an increase in Proteobacteria were observed in the TS, indicating that TS disrupted the intestinal flora structure of the chicks, which is in line with the findings of Zhao (48). However, the abundance of Firmicutes increased and the abundance of Proteobacteria decreased following TPS intervention. At the genus level, the administration of TS resulted in an increase in the relative abundance of Escherichia Shigella and Blautia in the chick. It has been found that stress can increase the level of inflammation, which in turn disrupts the structure of the intestinal flora, resulting in a decrease in the relative abundance of Firmicutes and an increase in the relative abundance of Blautia and Ruminococcaceae in the intestines of mice (49), which is similar to our findings. Therefore, we hypothesized that TS might cause an inflammatory response in the intestine of chicks, which in turn leads to disruption of the intestinal flora. The relative abundance of Escherichia Shigella and Blautia was reduced after TPS intervention. In addition, the relative abundance of Lactobacillus, a beneficial bacterium, increased in the TPS treated groups. The experimental results showed that TS could disrupt the intestinal flora of chicks, whereas TPS could maintain intestinal health by regulating the structure and composition of the intestinal flora.
5 Conclusion
In conclusion, the present study demonstrated that TPS extracted from Jinxuan tea has good antioxidant activity and can effectively alleviate H2O2-induced cellular oxidative damage. A transport stress model was established, and it was found that TS induces oxidative damage, disrupts the HSR and the expression of AQPs in the chick intestine, while also influencing the structure and composition of the intestinal flora. However, TPS was demonstrated to mitigate the oxidative damage, increase the intestinal flora abundance, and modulate the HSR and AQPs expression, thereby mitigating intestinal damage. The present study increases our understanding of intestinal damage in chicks caused by TS and provides a basis for the development and utilization of TPS as an anti-stress feed additive.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Laboratory Animal Center of South China Agricultural University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
XS: Conceptualization, Methodology, Formal analysis, Writing – original draft, Software. QZ: Conceptualization, Writing – review & editing, Formal analysis. JG: Software, Writing – review & editing, Methodology, Formal analysis. SL: Methodology, Writing – review & editing. FZ: Writing – review & editing. CL: Writing – review & editing. BF: Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was financed by the Yunfu City Science and Technology Innovation Project [grant number S2023090103], National Key Research and Development Program of China [grant number 2023YFD1800901], and the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program [grant number 2019BT02N054].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Carlisle, AJ, Mitchell, MA, Hunter, RR, Duggan, JA, and Randall, JM. Physiological responses of broiler chickens to the vibrations experienced during road transportation. Br Poult Sci. (1998) 39:48–49. doi: 10.1080/00071669888340
2. Yerpes, M, Llonch, P, and Manteca, X. Effect of environmental conditions during transport on chick weight loss and mortality. Poult Sci. (2021) 100:129–37. doi: 10.1016/j.psj.2020.10.003
3. Li, ZY, Lin, J, Sun, F, Li, H, Xia, J, Li, XN, et al. Transport stress induces weight loss and heart injury in chicks: disruption of ionic homeostasis via modulating ion transporting ATPases. Oncotarget. (2017) 8:24142–53. doi: 10.18632/oncotarget.15903
4. Sun, F, Zuo, YZ, Ge, J, Xia, J, Li, XN, Lin, J, et al. Transport stress induces heart damage in newly hatched chicks via blocking the cytoprotective heat shock response and augmenting nitric oxide production. Poult Sci. (2018) 97:2638–46. doi: 10.3382/ps/pey146
5. Ge, J, Li, H, Sun, F, Li, XN, Lin, J, Xia, J, et al. Transport stress-induced cerebrum oxidative stress is not mitigated by activating the Nrf2 antioxidant defense response in newly hatched chicks. J Anim Sci. (2017) 95:2871–8. doi: 10.2527/jas.2017.1559
6. Liu, J, Liu, D, Wu, X, Pan, C, Wang, S, and Ma, L. TMT quantitative proteomics analysis reveals the effects of transport stress on iron metabolism in the liver of chicken. Animals (Basel). (2021) 12:52. doi: 10.3390/ani12010052
7. Johnson, JS, Aardsma, MA, Duttlinger, AW, and Kpodo, KR. Early life thermal stress: impact on future thermotolerance, stress response, behavior, and intestinal morphology in piglets exposed to a heat stress challenge during simulated transport. J Anim Sci. (2018) 96:1640–53. doi: 10.1093/jas/sky107
8. Wang, SX, and Wu, WC. Effects of psychological stress on small intestinal motility and bacteria and mucosa in mice. World J Gastroenterol. (2005) 11:2016–21. doi: 10.3748/wjg.v11.i13.2016
9. Bailey, MT, Dowd, SE, Parry, NM, Galley, JD, Schauer, DB, and Lyte, M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect Immun. (2010) 78:1509–19. doi: 10.1128/IAI.00862-09
10. He, Y, Sang, Z, Zhuo, Y, Wang, X, Guo, Z, He, L, et al. Transport stress induces pig jejunum tissue oxidative damage and results in autophagy/mitophagy activation. J Anim Physiol Anim Nutr. (2019) 103:1521–9. doi: 10.1111/jpn.13161
11. Peng, R, Gao, F, Hu, Y, Li, K, Liu, B, Zheng, W, et al. Effects of transport stress on the oxidative index, apoptosis and autophagy in the small intestine of caprine. BMC Vet Res. (2023) 19:117. doi: 10.1186/s12917-023-03670-9
12. Li, H, Guo, H, Luo, Q, Wu, DT, Zou, L, Liu, Y, et al. Current extraction, purification, and identification techniques of tea polyphenols: an updated review. Crit Rev Food Sci Nutr. (2023) 63:3912–30. doi: 10.1080/10408398.2021.1995843
13. Zhang, Z, Wang, X, Li, J, Wang, G, and Mao, G. Extraction and free radical scavenging activity of polysaccharide from ‘Anji Baicha’ (Camellia sinensis (l.) O. Kuntze). Int J Biol Macromol. (2016) 84:161–5. doi: 10.1016/j.ijbiomac.2015.12.016
14. Liu, F, Wang, R, Chen, Y, Geng, R, Gao, H, Wang, F, et al. Structural characterization of a pectic polysaccharide from laoshan green tea and its inhibitory effects on the production of NO, TNF-α and IL-6. Nat Prod Res. (2023) 37:1797–805. doi: 10.1080/14786419.2022.2121831
15. Liu, M, Gong, Z, Liu, H, Wang, J, Wang, D, Yang, Y, et al. Structural characterization and anti-tumor activity in vitro of a water-soluble polysaccharide from dark brick tea. Int J Biol Macromol. (2022) 205:615–25. doi: 10.1016/j.ijbiomac.2022.02.089
16. Lee, YE, Yoo, SH, Chung, JO, Park, MY, Hong, YD, Park, SH, et al. Hypoglycemic effect of soluble polysaccharide and catechins from green tea on inhibiting intestinal transport of glucose. J Sci Food Agric. (2020) 100:3979–86. doi: 10.1002/jsfa.10442
17. Yu, B, Zhang, D, Wu, Y, Tao, W, Luorong, Q, Luo, J, et al. A new polysaccharide from hawk tea: structural characterization and immunomodulatory activity associated with regulating gut microbiota. Food Chem. (2023) 418:135917. doi: 10.1016/j.foodchem.2023.135917
18. Zhou, Q, Gao, J, Sun, X, Du, J, Wu, Z, Liang, D, et al. Immunomodulatory mechanisms of tea leaf polysaccharide in mice with cyclophosphamide-induced immunosuppression based on gut flora and metabolomics. Food Secur. (2024) 13:2994. doi: 10.3390/foods13182994
19. Zhou, Q, Gao, J, Sun, X, Liang, Y, Ye, M, Liang, D, et al. In vitro characterization of polysaccharides from fresh tea leaves in simulated gastrointestinal digestion and gut microbiome fermentation. Food Secur. (2024) 13:1561. doi: 10.3390/foods13101561
20. Dadgar, S, Crowe, TG, Classen, HL, Watts, JM, and Shand, PJ. Broiler chicken thigh and breast muscle responses to cold stress during simulated transport before slaughter. Poult Sci. (2012) 91:1454–64. doi: 10.3382/ps.2011-01520
21. Chen, J, Xu, WY, Gu, Y, Tang, YX, Xu, XW, Li, XN, et al. Inhibition of mtDNA-PRRs pathway-mediated sterile inflammation by astragalus polysaccharide protects against transport stress-induced cardiac injury in chicks. Poult Sci. (2024) 103:103638. doi: 10.1016/j.psj.2024.103638
22. Lan, R, Li, Y, Chang, Q, and Zhao, Z. Dietary chitosan oligosaccharides alleviate heat stress-induced intestinal oxidative stress and inflammatory response in yellow-feather broilers. Poult Sci. (2020) 99:6745–52. doi: 10.1016/j.psj.2020.09.050
23. Yuan, S, Duan, Z, Lu, Y, Ma, X, and Wang, S. Optimization of decolorization process in agar production from gracilaria lemaneiformis and evaluation of antioxidant activities of the extract rich in natural pigments. 3 Biotech. (2018) 8:8. doi: 10.1007/s13205-017-1037-6
24. Ooi, DJ, Chan, KW, Sarega, N, Alitheen, NB, Ithnin, H, and Ismail, M. Bioprospecting the curculigoside-cinnamic acid-rich fraction from molineria latifolia rhizome as a potential antioxidant therapeutic agent. Molecules. (2016) 21:682. doi: 10.3390/molecules21060682
25. Ren, X, He, L, Wang, Y, and Cheng, J. Optimization extraction, preliminary characterization and antioxidant activities of polysaccharides from semen juglandis. Molecules. (2016) 21:1335. doi: 10.3390/molecules21101335
26. Wang, Z, Xie, J, Kan, L, Wang, J, Shen, M, Li, W, et al. Sulfated polysaccharides from Cyclocarya paliurus reduce H2O2-induced oxidative stress in RAW264.7 cells. Int J Biol Macromol. (2015) 80:410–7. doi: 10.1016/j.ijbiomac.2015.06.031
27. Polidoro, L, Properzi, G, Marampon, F, Gravina, GL, Festuccia, C, Di Cesare, E, et al. Vitamin d protects human endothelial cells from h(2)o(2) oxidant injury through the mek/erk-sirt1 axis activation. J Cardiovasc Transl Res. (2013) 6:221–31. doi: 10.1007/s12265-012-9436-x
28. Liu, J, Lin, J, Huang, Z, Zheng, Q, Lin, F, and Wu, L. Chemical characterization of tianshan green tea polysaccharides and its protective effects on cell oxidative injury. J Food Biochem. (2022) 46:e14000. doi: 10.1111/jfbc.14000
29. Yang, Y, Yu, L, Zhu, T, Xu, S, He, J, Mao, N, et al. Neuroprotective effects of rehmannia glutinosa polysaccharide on chronic constant light (CCL)-induced oxidative stress and autophagic cell death via the AKT/mTOR pathway in mouse hippocampus and HT-22 cells. Int J Biol Macromol. (2024) 261:129813. doi: 10.1016/j.ijbiomac.2024.129813
30. Du, K, Wang, L, Wang, Z, Xiao, H, Hou, J, Hu, L, et al. Angelica sinensis polysaccharide antagonizes 5-fluorouracil-induced spleen injury and dysfunction by suppressing oxidative stress and apoptosis. Biomed Pharmacother. (2023) 162:114602. doi: 10.1016/j.biopha.2023.114602
31. Shen, F, Song, Z, Xie, P, Li, L, Wang, B, Peng, D, et al. Polygonatum sibiricum polysaccharide prevents depression-like behaviors by reducing oxidative stress, inflammation, and cellular and synaptic damage. J Ethnopharmacol. (2021) 275:114164. doi: 10.1016/j.jep.2021.114164
32. Zhou, R, He, D, Xie, J, Zhou, Q, Zeng, H, Li, H, et al. The synergistic effects of polysaccharides and ginsenosides from american ginseng (panax quinquefolius l.) ameliorating cyclophosphamide-induced intestinal immune disorders and gut barrier dysfunctions based on microbiome-metabolomics analysis. Front Immunol. (2021) 12:665901. doi: 10.3389/fimmu.2021.665901
33. Ma, G, Kimatu, BM, Zhao, L, Yang, W, Pei, F, and Hu, Q. Impacts of dietary pleurotus eryngii polysaccharide on nutrient digestion, metabolism, and immune response of the small intestine and colon-an iTRAQ-based proteomic analysis. Proteomics. (2018) 18:e1700443. doi: 10.1002/pmic.201700443
34. Zou, Y, Wei, HK, Xiang, QH, Wang, J, Zhou, YF, and Peng, J. Protective effect of quercetin on pig intestinal integrity after transport stress is associated with regulation oxidative status and inflammation. J Vet Med Sci. (2016) 78:1487–94. doi: 10.1292/jvms.16-0090
35. Li, Z, Li, Y, Zhou, X, Dai, P, and Li, C. Autophagy involved in the activation of the Nrf2-antioxidant system in testes of heat-exposed mice. J Therm Biol. (2018) 71:142–52. doi: 10.1016/j.jtherbio.2017.11.006
36. Liu, Y, Cai, H, Guo, X, Aierken, A, Hua, J, Ma, B, et al. Melatonin alleviates heat stress-induced testicular damage in dairy goats by inhibiting the PI3k/AKT signaling pathway. Stress Biol. (2022) 2:47. doi: 10.1007/s44154-022-00068-9
37. Goel, A. Heat stress management in poultry. J Anim Physiol Anim Nutr (Berl). (2021) 105:1136–45. doi: 10.1111/jpn.13496
38. Shen, Q, Wang, R, Liu, X, Song, P, Zheng, M, Ren, X, et al. HSF1 stimulates glutamine transport by super-enhancer-driven lncRNA LINC00857 in colorectal cancer. Cancers (Basel). (2022) 14:3855. doi: 10.3390/cancers14163855
39. Ostling, P, Bjork, JK, Roos-Mattjus, P, Mezger, V, and Sistonen, L. Heat shock factor 2 (HSF2) contributes to inducible expression of hsp genes through interplay with HSF1. J Biol Chem. (2007) 282:7077–86. doi: 10.1074/jbc.M607556200
40. Tanabe, M, Nakai, A, Kawazoe, Y, and Nagata, K. Different thresholds in the responses of two heat shock transcription factors, HSF1 and HSF3. J Biol Chem. (1997) 272:15389–95. doi: 10.1074/jbc.272.24.15389
41. Benjamin, IJ, and Mcmillan, DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. (1998) 83:117–32. doi: 10.1161/01.res.83.2.117
42. King, LS, Kozono, D, and Agre, P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. (2004) 5:687–98. doi: 10.1038/nrm1469
43. Takata, K, Matsuzaki, T, and Tajika, Y. Aquaporins: water channel proteins of the cell membrane. Prog Histochem Cytochem. (2004) 39:1–83. doi: 10.1016/j.proghi.2004.03.001
44. Agre, P, King, LS, Yasui, M, Guggino, WB, Ottersen, OP, Fujiyoshi, Y, et al. Aquaporin water channels-from atomic structure to clinical medicine. J Physiol. (2002) 542:3–16. doi: 10.1113/jphysiol.2002.020818
45. Wang, YH, Liu, TT, Kung, WM, Chen, CC, Wen, YT, Lin, IC, et al. Expression of aquaporins in intestine after heat stroke. Int J Clin Exp Pathol. (2015) 8:8742–53.
46. Valdes, AM, Walter, J, Segal, E, and Spector, TD. Role of the gut microbiota in nutrition and health. BMJ. (2018) 361:k2179–44. doi: 10.1136/bmj.k2179
47. Ye, M, Yu, J, Shi, X, Zhu, J, Gao, X, and Liu, W. Polysaccharides catabolism by the human gut bacterium -bacteroides thetaiotaomicron: advances and perspectives. Crit Rev Food Sci Nutr. (2021) 61:3569–88. doi: 10.1080/10408398.2020.1803198
48. Zhao, H, Li, Y, Lv, P, Huang, J, Tai, R, Jin, X, et al. Salmonella phages affect the intestinal barrier in chicks by altering the composition of early intestinal flora: association with time of phage use. Front Microbiol. (2022) 13:947640. doi: 10.3389/fmicb.2022.947640
Keywords: tea polysaccharide, antioxidant activity, transport stress, intestinal injury, intestinal flora
Citation: Sun X, Zhou Q, Gao J, Liu S, Zhao F, Ling C and Fang B (2025) Antioxidant activity of Jinxuan tea polysaccharide and its protective effect on intestinal injury induced by transport stress in chicks. Front. Vet. Sci. 12:1610218. doi: 10.3389/fvets.2025.1610218
Edited by:
Awad A. Shehata, Helmholtz Association of German Research Centers (HZ), GermanyReviewed by:
Guangliang Shi, Northeast Agricultural University, ChinaAdnan Alrubaye, University of Arkansas, United States
Copyright © 2025 Sun, Zhou, Gao, Liu, Zhao, Ling and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Binghu Fang, ZmFuZ2JoQHNjYXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Xueyan Sun
Xueyan Sun Qiaoyi Zhou
Qiaoyi Zhou Jinjing Gao1
Jinjing Gao1 Binghu Fang
Binghu Fang