- 1Department of Poultry Diseases, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
- 2Department of Poultry Diseases, Animal Health Research Institute, Giza, Egypt
- 3Department of Zoology, Faculty of Science, Fayoum University, Fayoum, Egypt
- 4Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
- 5Department of Biological Sciences, College of Science, University of Jeddah, Jeddah, Saudi Arabia
- 6Department of Biology, College of Science, Qassim University, Buraydah, Saudi Arabia
- 7Department of Biology, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
- 8Department of Biology, College of Science, King Khalid University, Abha, Saudi Arabia
- 9Department of Biology, College of Science, Jazan University, Jazan, Saudi Arabia
- 10Department of Biology, College of Science, United Arab Emirates University, Al Ain, United Arab Emirates
- 11Department of Parasitology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
Introduction: Haemoproteus columbae is a common haemosporidian worldwide blood parasite affecting domestic pigeons (Columba livia). Therefore, this study aimed to detect the incidence of H. columbae infection in domestic pigeons with morpho-molecular identification.
Methods: In the current study, blood samples were collected from 125 domestic pigeons between 2023 and 2024 and analyzed using both microscopic and molecular techniques. H. columbae positive birds underwent postmortem (PM) and histopathological examinations, as well as cytokine immunological reaction assessments.
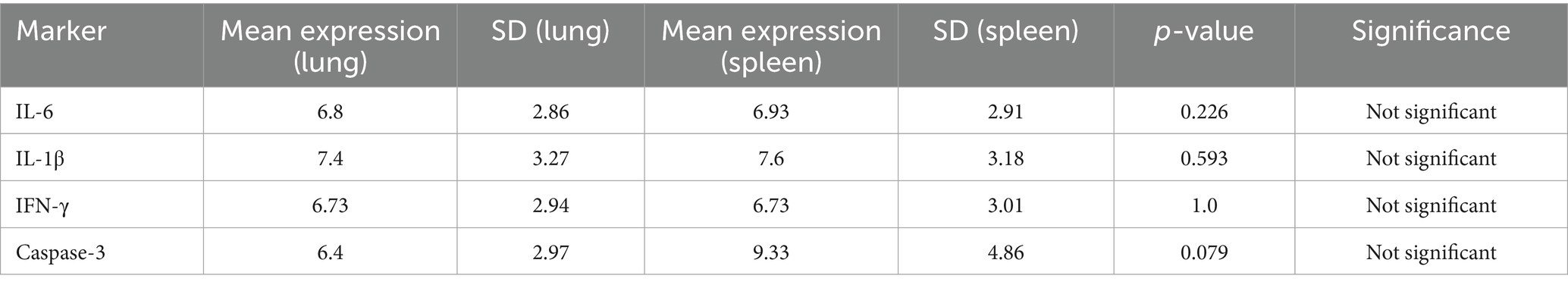
Results: It was found that around 8% (10/125) of pigeons were positive for H. columbae infection, and their morphological characteristics were reported. H. columbae induces observable macroscopic and microscopic alterations in the infected tissues, which increases the cytokine immunological reaction in the infected birds. The infected birds suffered from severe histopathological changes in most haemopoietic and parenchymatous organs. The transcript levels of inflammatory markers such as IL-6, IFN-γ, and IL-1β were significantly upregulated in H. columbae-infected birds. Additionally, the H. columbae samples’ mRNA level of the apoptotic Cas-3 indicated apoptotic activity.
Discussion: Hematic parasites can pose a serious health threat to pigeons as they invade red blood cells and internal organs, leading to anemia, weakness, weight loss, and even death in severe cases. Epidemiological studies and surveys are essential for monitoring these hematologic parasites. Furthermore, additional research is recommended to evaluate the efficacy of various herbal extracts in comparison to the most frequently used drugs for managing this issue in affected pigeons.
Introduction
Parasitic infections in poultry present a substantial economic burden due to their impact on bird health and productivity (1–3). Among these, avian haemosporidian parasites, particularly those from the genera Plasmodium and Haemoproteus (Apicomplexa: Haemosporidia), are widespread pathogens affecting both domestic and wild bird populations (4, 5).
One of the most studied avian haemosporidians is Haemoproteus columbae, a protozoan parasite primarily infecting pigeon (Columba livia) (6, 7). H. columbae follows a heteroxenous life cycle, with asexual replication occurring in erythrocytes and tissues of the avian host, and sexual reproduction taking place in blood-sucking dipterans, specifically louse flies (Pseudolynchia canariensis) (8, 9). Sporogony in the vector takes approximately 6.5–10 days (9).
Haemosporidians have traditionally been grouped into four genera: Plasmodium, Haemoproteus, Leucocytozoon, and Fallisia (10). The genus Haemoproteus is further divided into subgenera: Haemoproteus (transmitted by Hippoboscidae) and Parahaemoproteus (transmitted by Ceratopogonidae) based on vector specificity (11, 12).
Although often considered non-pathogenic in their natural avian hosts, H. columbae can cause significant pathology in immunologically naïve or stressed birds, especially in captivity. The parasite has been implicated in a disease commonly referred to as “pigeon malaria,” which can be fatal in young birds (13). Gametocytes characteristically appear in erythrocytes as crescent or horseshoe-shaped forms (13). Acute infections are often linked to pre-erythrocytic tissue stages, such as the rupture of megalomeronts in the liver and muscle, which can lead to systemic damage and mortality (14, 15).
Despite the global presence of avian haemosporidians, detailed studies focusing on the immune response and pathological alterations associated with natural H. columbae infections in pigeons remain scarce. In particular, there is a paucity of data integrating morphological, histopathological, and molecular findings with cytokine profiling to understand the host-pathogen interactions and potential consequences of infection.
Recently, it has been posited that pigeon haemoproteosis caused by H. columbae is predominantly found in tropical and subtropical regions. Therefore, the objective of this study is to identify the causative agents of mortality in pigeons, ascertain the incidence of H. columbae through morphological identification, and evaluate the tissue responses to this infection, including histopathological alterations and cytokine responses in various tissues of the infected pigeons.
Materials and methods
Ethical approval and consent to participate
The work was carried out according to the IACUC guidelines and was approved by the Ethical Committee, Faculty of Veterinary Medicine, Cairo University, Cairo, Egypt, with code “Vet CU 8032022407.”
Collection of pigeons
For this study, 125 domestic pigeons of different ages (4 months to 4 years old) and mixed sex were collected from poultry markets and different poultry clinics. From the suspected birds with general signs of loss of weight, ruffled feathers, and general depression, blood samples were ethically collected from the wing vein.
In addition, apparently healthy birds confirmed to be negative for blood and gastrointestinal parasites via parasitological examination were kept as negative control birds.
Parasitological examination
Blood samples were ethically collected from the wing vein to prepare blood smears. For every blood sample, two to three thin blood films were prepared on sterile glass slides, allowed to air-dry, and subsequently fixed in 100% methanol for 10 min. Giemsa’s solution (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) was used to stain blood films.
The slides were examined under a 40× and 100× magnification (16) using Olympus BH-2 (Olympus Optical Co. Ltd., Tokyo, Japan) light microscope equipped with a digital camera and software (Jenoptik ProgRes Camera, C12plus, Frankfurt, Germany). The infection intensity was calculated using the number of infected erythrocytes (presence of male or female gametocytes) per 10 microscopic fields (17, 18).
The morphometric properties of the infected erythroparasites were microscopically assessed, and the morphological characteristics key established by Valkiūnas and Iezhova (19) was used to identify H. columbae.
Transcript levels analysis of the infected spleen and lung with H. columbae
Lung and spleen tissues were aseptically collected from 10 H. columbae-infected pigeons and 10 uninfected controls. A tissue sample of 100 mg from each organ was homogenized using a FastPrep-24 system (MP Biomedicals, Irvine, California, United States) with two 30-s cycles at 6 m/s and then placed in Lysing Matrix D tubes. Total RNA was extracted using Ambion RNA isolation kits (Thermo Fisher Scientific Inc., Waltham, Massachusetts, United States), and its concentration and purity were assessed with a NanoDrop spectrophotometer (Thermo Fisher Scientific).
Subsequently, 500 ng of RNA per sample was treated with DNase I (Thermo Fisher Scientific) and converted to cDNA using the High-Capacity cDNA Archive Kit (Thermo Fisher Scientific). Quantitative real-time PCR was conducted using the Applied Biosystems 7500 platform with SYBR Green-based chemistry (Wiz Pure™ qPCR Master Mix, Wizbiosolutions Inc., Seongnam-si, South Korea) and ROX as the reference dye.
The 10 μL reaction mixture included 0.2 μL of 50 × ROX dye, 0.5 μL of each primer (10 pmol/μL), and nuclease-free water. Thermal cycling involved an initial denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 58°C for 1 min. Gene expression levels of IL-6, IL-1β, IFN-γ, and caspase-3 were quantified with β-actin, which was used as a housekeeping gene for normalization.
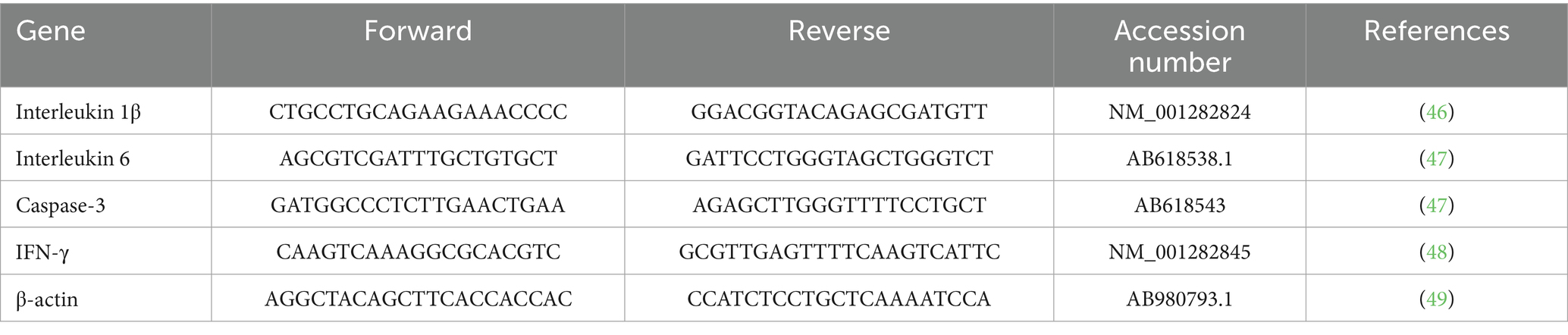
The relative fold changes were determined using the 2−ΔΔCt method. Each sample was analyzed in triplicate to ensure data reliability and accuracy. Detailed primer sequences and references are provided in Table 1.
Histopathological examination
The tissue specimens from the heart, lungs, liver, and spleen of the infected pigeon were fixed in 10% buffered neutral formalin and processed routinely and stained with hematoxylin and eosin (20).
The stained tissue sections were viewed, and the consistency of meronts was carefully examined using Olympus BH-2 microscope. The lesions and meronts were photographed using an Olympus DP27 camera linked to software (cellSens dimensions, version 1.13).
Statistical analysis
Python (pandas, scipy, and seaborn) and GraphPad Prism were used for all statistical analyses. First, the Shapiro–Wilk and Levene’s tests were used to check the data for normality and homogeneity of variance, respectively. Contingency tables comparing infectivity status across categorical groups were subjected to a chi-square test of independence to assess the impact of age, sex, and location on infection prevalence.
Transcript levels of IL-6, IL-1β, IFN-γ, and caspase-3 were measured using the 2−ΔΔCt method and normalized to β-actin for gene expression analysis. Independent samples t-tests were used to evaluate differences in gene expression between tissue types (lung versus spleen), with significance defined at P<0.05. Bar charts with mean values ± standard deviation (SD) and standard error bars were used to show gene expression data, and the plots were annotated with P values.
Results
Clinical signs and postmortem (PM) examination
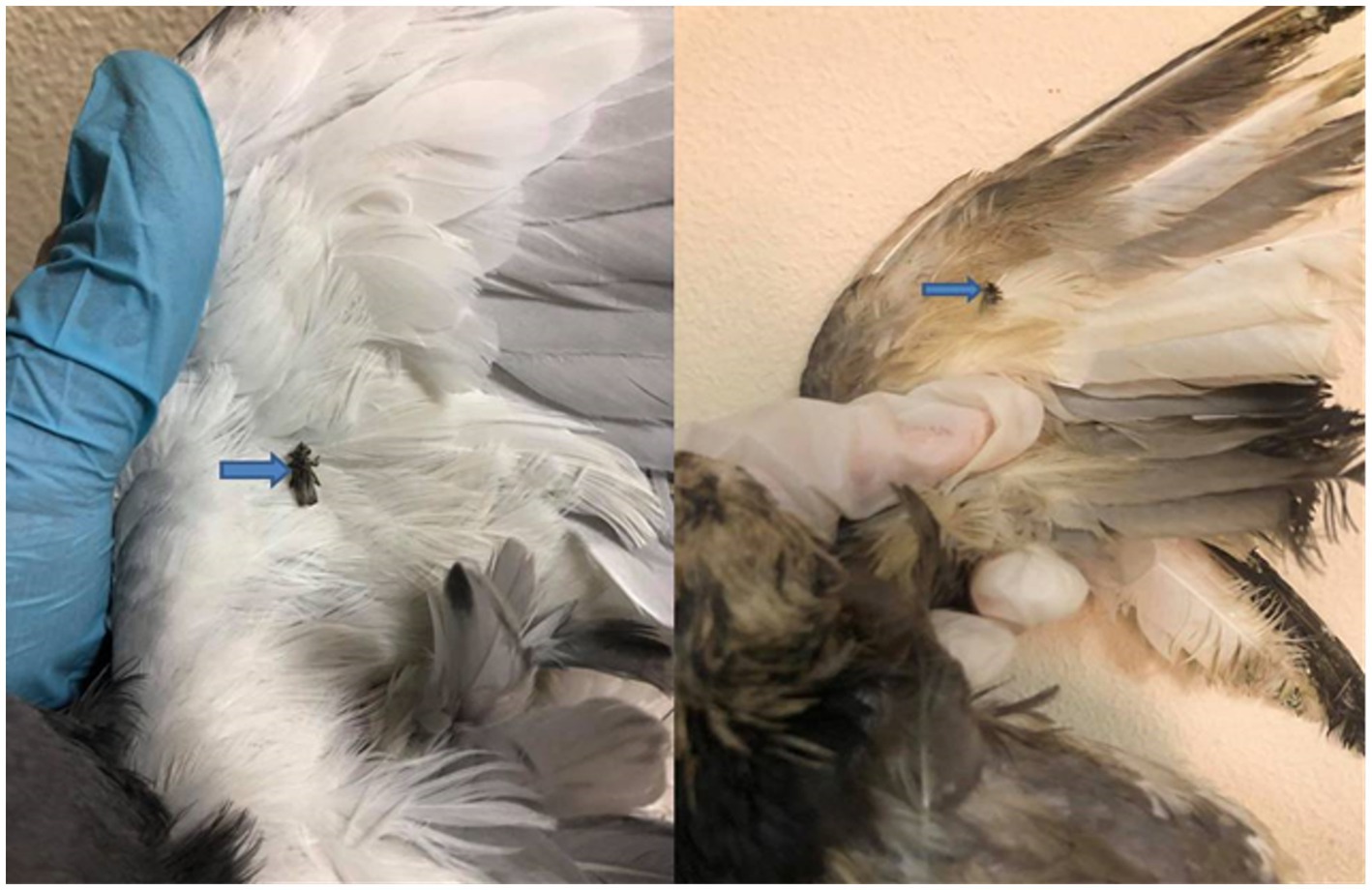
Hippoboscid flies were identified when examining pigeon bodies. These flies were collected from nine infected birds, and upon closer inspection, they were identified as P. canariensis, a pigeon dipteran parasite (Figure 1).
The examined birds exhibited general signs of depression, emaciation, anemic appearance, ruffled feathers, and some birds showed nervous and/or respiratory manifestations. The PM lesions had significant splenic congestion, with hemorrhages seen in the thigh and breast muscles, alongside cardiomegaly and enlargement, as well as congestion in the majority of parenchymatous organs. Pneumonia was also reported in some cases (Figure 2).

Figure 2. Postmortem lesion of freshly dead pigeons showing (A) congested lung (white arrow). (B) Focal pneumonia (white arrow). (C) Congested spleen (yellow star) and enlarged heart (red ring). (D) Pale and degenerated liver (white arrow) and dark, congested heart (red ring).
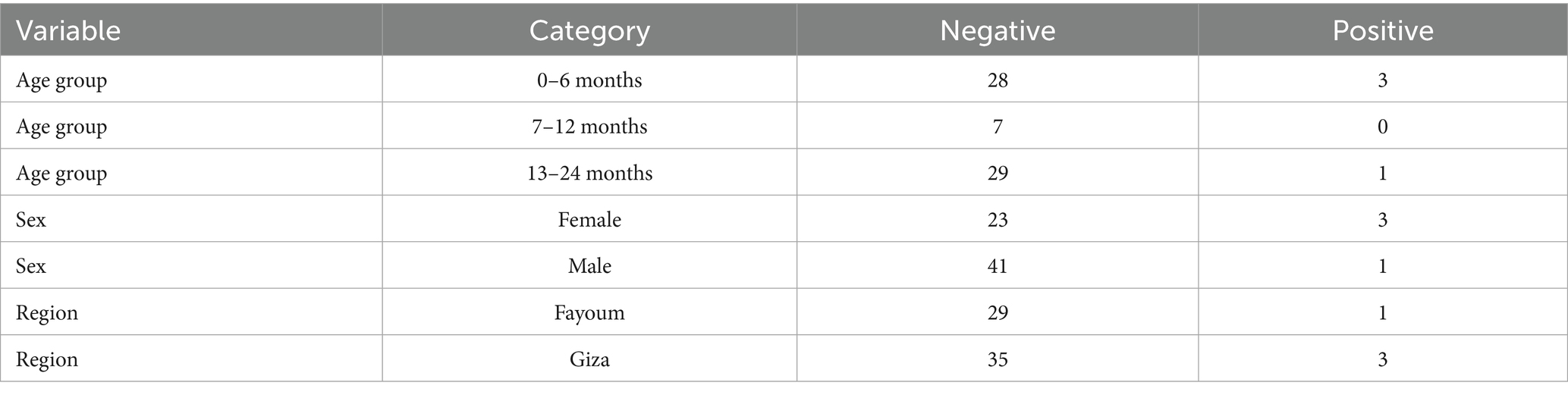
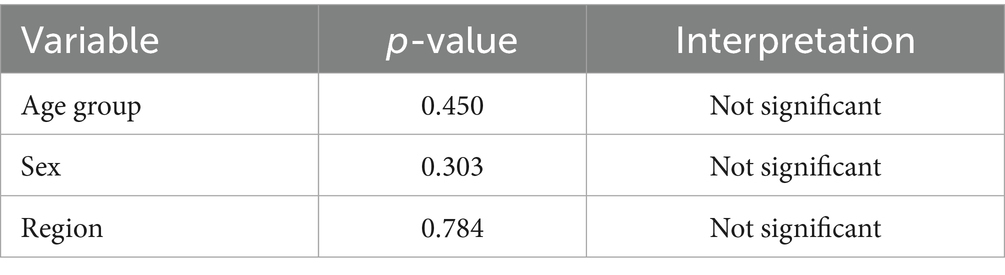
None of the variables—age, sex, or locality (region)—showed a statistically significant association with infectivity status (all P values were >0.05). This indicates that differences in infection rates across these groups may be due to random variation rather than meaningful demographic or geographic trends (Tables 2, 3).
Parasitological examination
Examining every positive case revealed that the infected erythrocytes of the pigeons contained 1–2 gametocytes per 10 microscopic fields, with an incidence of 8% infected pigeons (10/125). The gametocytes encircled the host cell nucleus, either male or female. The male or microgametocyte had a light red cytoplasm with granules at two poles, while the macrogametocyte had a dark cytoplasm with diffused granules (Figure 3).
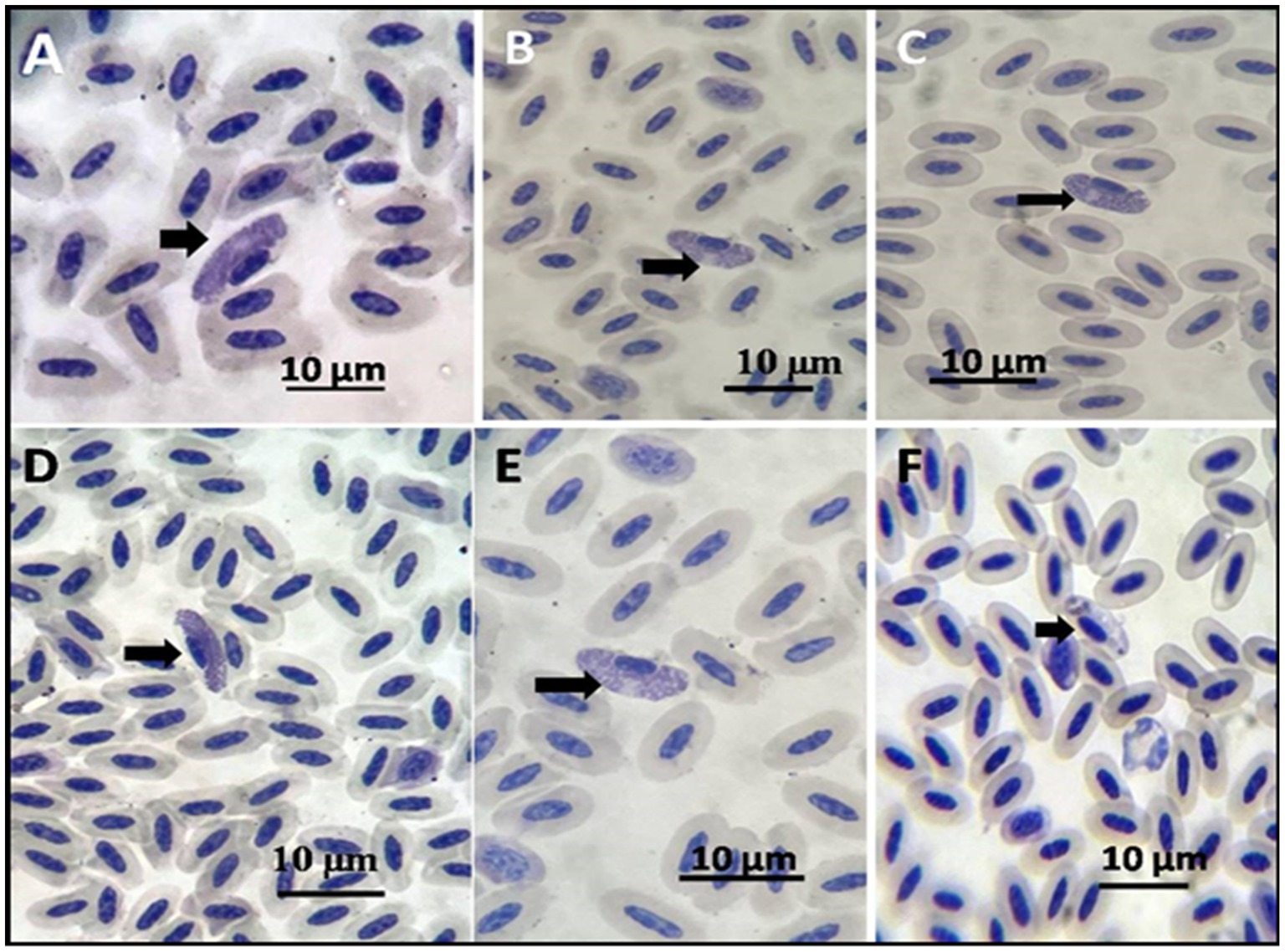
Figure 3. Pigeon-stained thin blood film with Giemsa stain showing (A–E) macrogametocytes shown by arrows. (F) Microgametocyte.
Transcript levels of the cytokines in the infected spleen and lung of the pigeons
Gene expression analysis showed upregulation of IL-6, IL-1β, and IFN-γ in infected tissues. Mean values were higher in lungs than spleens, though not statistically significant (IL-6 P = 0.226; IL-1β P = 0.593; IFN-γ, P = 1.000).
Caspase-3 was also elevated, suggesting apoptosis (Figures 4A–D). Cytokine expression shows the mean ± SD of each marker (IL-6, IL-1β, IFN-γ, caspase-3) in lung and spleen tissues, with P values and significance interpretation (Table 4).
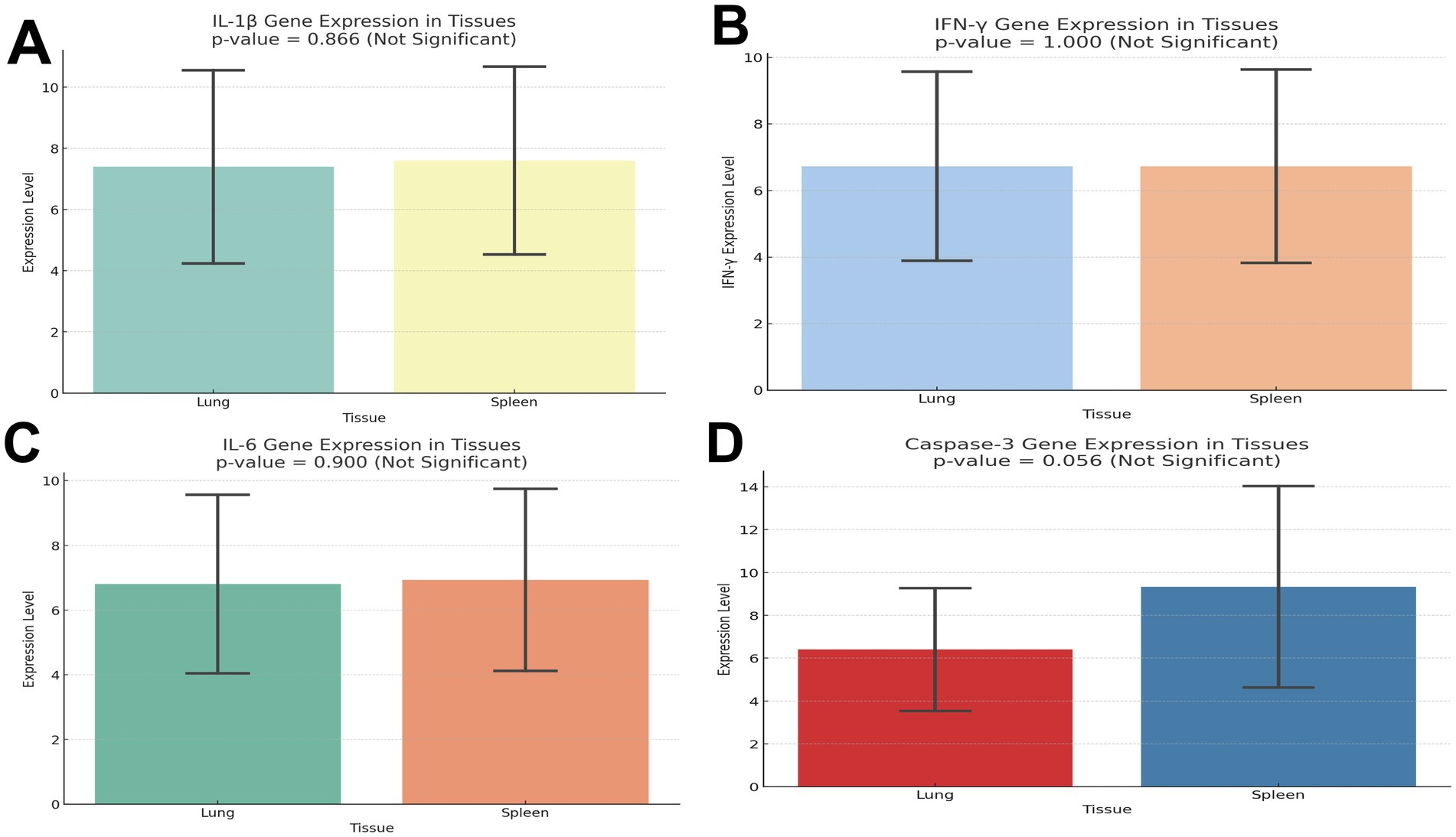
Figure 4. (A) Transcript levels of interleukin 1β in lung and spleen of the infected and control pigeon. (B) Transcript levels of IFN-γ in lung and spleen of the infected and control pigeon. (C) Transcript levels of IL-6 in lung and spleen of the infected and control pigeon. (D) Transcript levels of caspase-3 in lung and spleen of the infected and control pigeon.
Histopathological findings
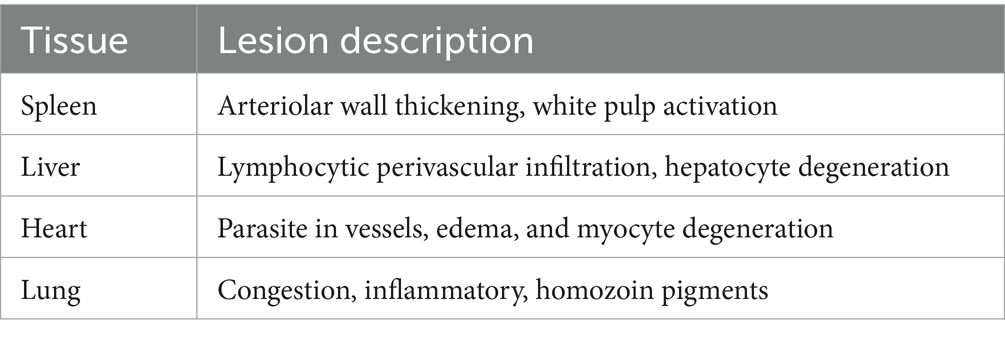
The lesions in the infected pigeon were very clear in internal organs, including the heart, lungs, liver, and spleen (Table 5). The lesions were distinct in the blood vessels of such organs and were associated with degenerative and inflammatory reactions. In the heart, the infected blood cells within the blood vessels appeared abnormally elongated in shape. The cells contained granular, elongated basophilic stages of Haemoproteus in the cytoplasm, representing the developing stages of this parasite (Figure 5A).

Figure 5. Photomicrograph of the heart of Columba livia infected with Haemoproteus columbae and stained with hematoxylin and eosin showing (A) infected blood cells (bc) with abnormal elongated shape with granular basophilic cytoplasm; these cells contain the stages of hemoproteus parasite (p). (B) The infected cells are observed between the cardiac muscles (cm) with edema and hyalinosis of the muscle fibers. (C) Higher magnification of the previous image showing the infected blood cells (bc) containing hemoproteus stages within the blood capillary together with eosinophils (es). (D) Blood vessel (bvs) in the cardiac muscle showing swelling of the endothelium and thickening of its wall.
The infected blood cells were also noticed in the deep blood capillaries, between the cardiac muscles. In such cases, the cardiac muscles showed edema and hyalinosis, along with a few eosinophil infiltrations (Figures 5B,C). The blood vessel itself showed swelling of the endothelium and thickening of its wall (Figure 5D).
In the lung tissue, some parts of the lung tissue showed congestion of both large blood vessels and perialveolar capillaries and contained apparently normal nucleated red blood cells without stages of the Haemoproteus (Figure 6A). Other parts of the examined lung tissue showed infected blood cells containing the stages of Haemoproteus (Figure 6B). The infected cells in such cases were surrounded by numerous hemozoin pigments (Figure 6C). Mononuclear inflammatory cells infiltration, mainly lymphocytes and heterophils, was not uncommon (Figure 6D).
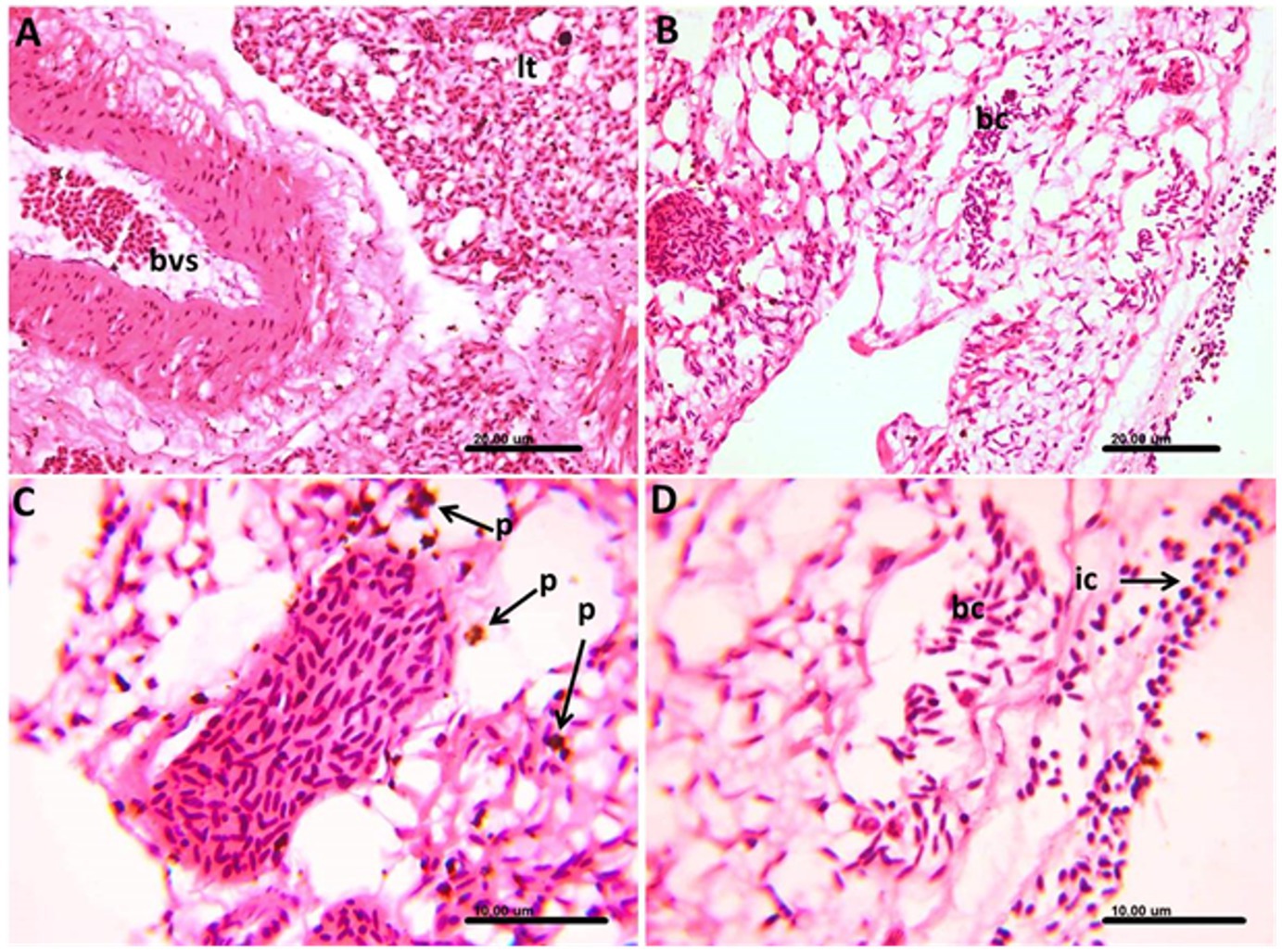
Figure 6. Photomicrograph of the lung of Columba livia infected with Haemoproteus columbae, and stained with hematoxylin and eosin showing (A) apparently normal nucleated red blood cells within the blood vessels (bvs) and congestion of the perialveolar capillaries of the lung tissue (lt). (B) Lung tissue showing infected blood cells (bc) containing the stages of hemoproteus parasite. (C) Infected blood cells surrounded by numerous hemozoin pigments (p). (D) Infected blood cells (bc) in the lung tissue surrounded by mononuclear inflammatory cells (ic) infiltration mainly lymphocytes and heterophils.
In the spleen, marked thickening in the wall of follicular arterioles was common, and the white pulp showed activation of the hemopoietic tissue together with hemozoin pigment deposition (Figures 7A,B).
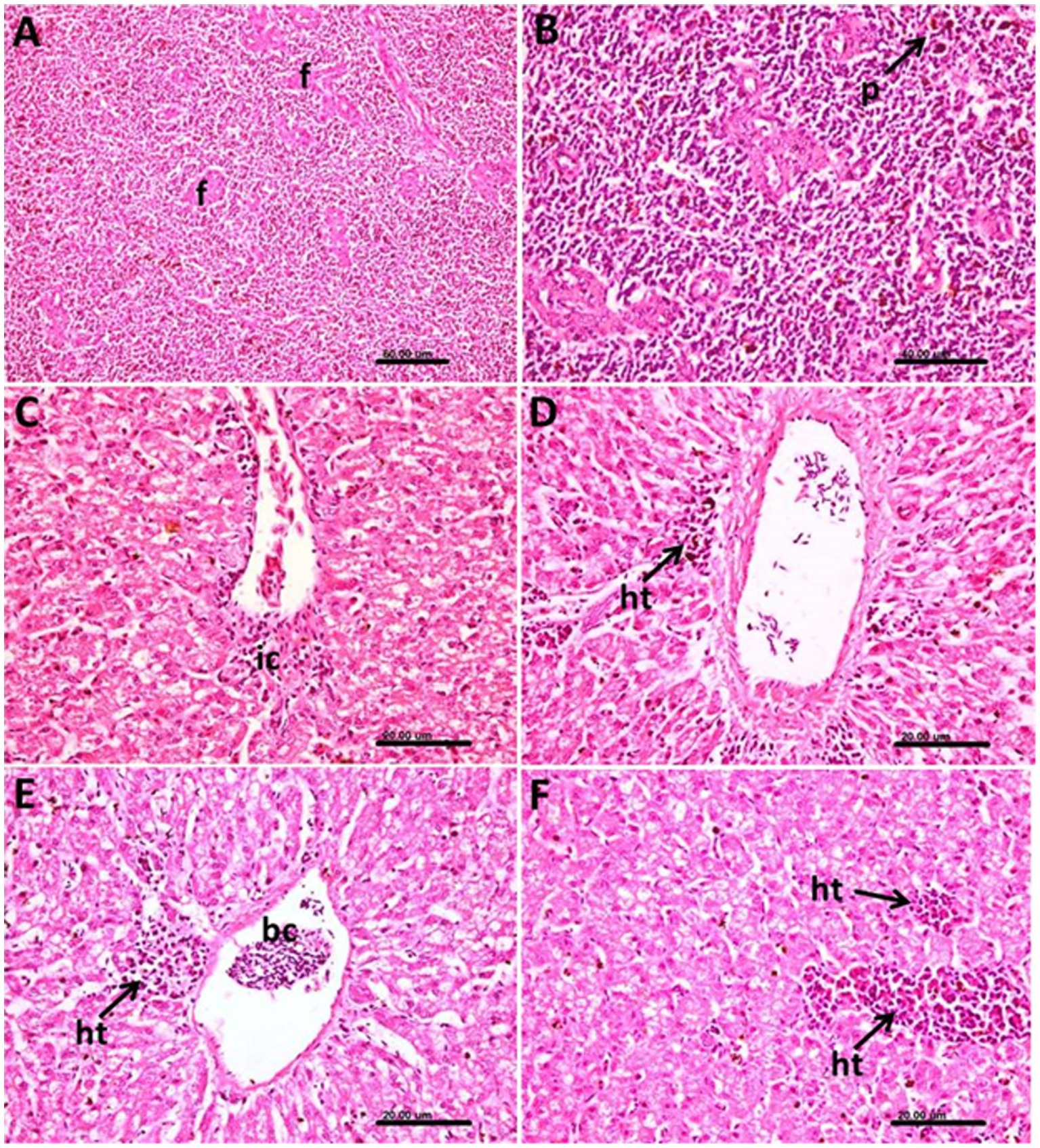
Figure 7. Photomicrograph of the spleen and liver of Columba livia infected with Haemoproteus columbae and stained with hematoxylin and eosin showing (A) thickening of the wall of the follicular arterioles (f) of the spleen. (B) Hemozoin pigments (p) in the splenic tissue. (C) Hepatic tissue showing mononuclear inflammatory cells (ic) infiltration mainly lymphocytes around the central vein. (D) Liver showing infected blood cells in the central vein and heterophils (ht) aggregation in the hepatic tissue. (E) Focal aggregation of heterophils (ht) and lymphocytes around the central vein containing infected blood cells (bc) and vacuolar degeneration of the hepatocytes. (F) Multiple foci of heterophils (ht) and lymphocytes aggregation in the hepatic tissues.
In liver, the pathological changes were prominent where perivascular mononuclear inflammatory cells infiltration mainly lymphocytes around the central veins were common (Figure 7C), and the infected blood cells within the central vein were also noticed (Figure 7D). In such cases, a heterophils aggregation was also observed.
The heterophils and lymphocytes were sometimes noticed as a focal aggregation around the central vein containing infected blood cells (Figure 7E) or as multiple foci aggregated extravascular in the hepatic tissue (Figure 7F). The hepatocytes in such cases showed vacuolar degeneration.
Discussion
Haemoproteus infections are common in wild birds; however, they are not usually deadly (21, 22). Nonetheless, Hartley (23) reported deaths in Australian wild currawongs (Strepera graculind). The study’s high infection was largely caused by the louse fly, P. canariensis, a known vector of H. columbae (24, 25). This was probably the outcome of repeated re-infection with H. columbae in captivity. In Saudi Arabia, domestic pigeons were found to harbor H. columbae (26).
However, in the current study, a macroscopic and histological investigation showed a correlation between the tissue stages of H. columbae megaloschizonts and the cardiac muscle lesions. The widespread necrotic lesions in the legs, wings, and breast muscles resembled lesions caused by nutritional myopathy resulting from a selenium or vitamin E deficiency. Comparable results have been recorded in many studies by Simpson (27), Atkinson et al. (28), Gardiner et al. (29), and Atkinson et al. (30).
According to Cepeda et al. (31), the pigeon used in this investigation contained thin-walled aseptate elongated, spherical, or irregularly shaped schizonts within the lumina of blood arteries in the lung, liver, kidney, and proventriculus. According to Kucera et al. (32), these schizonts contained merozoites, cytomeres, or other forms. It has been documented that birds infected during the prepatent period perish have severe hemoproteosis as a result of the rupture of megalomeronts in the muscles and liver and the subsequent pathogenic changes as suggested by Youssefi et al. (33).
Natural Haemoproteus infection and subsequent consequences analysis have demonstrated that haemoproteosis can result in a remarkable pressure on the hosts’ survival, behavior, reproductive efficiency and community framework (34, 35). In feral domestic pigeons living, the amount of H. columbae parasitemia and body mass are negatively correlated. In the present investigation, these could lead to body mass loss and other adverse health outcomes that in consistent with Youssefi et al. (33), and Nebel et al. (36).
A significant disease was brought on by the pigeon’s increased gametocyte to erythrocyte ratio. The infected erythrocyte counts inside the microscopic field in the positive instances, which demonstrated greater affection of 1–2 erythrocytes, also indicative of milder infection in the birds, supported the findings of Mushi et al. (37), and Gicik and Arslan (38).
It is well accepted that the pathogenicity of Haemoproteus spp. in their natural avian hosts is essentially negligible, particularly in comparison to the pathology of the avian hosts of the genera Plasmodium and Leucocytozoon. Inadvertent hosts, like captive avian species in zoos and aviaries, have been shown to get clinical and lethal Haemoproteus infections (39, 40). In this regard, schizonts were detected in the current study at the lungs’ vascular periphery, indicating development among endothelial cells. Despite the study’s lack of finding any correlation between schizonts and endothelial cells, it was not always possible to pinpoint the precise kind and developmental stage of the structures inside the megaschizonts (37).
The host’s defenses against infection by foreign microorganisms depend on a functioning immune response. Intense reactions could be harmful to the host. In our study, the transcript levels of inflammatory markers such as IL-6, IFN-γ, and IL-1β were found to be significantly upregulated in H. columbae infected birds. Cytokines play an important role and are considered as an effective element of the avian immunity (41–43).
They are usually considered in case of parasitic infections, especially intracellular parasites (44). On the other hand, and in contrary to endoerythrocytic parasites, Mohammed (24) and Rennenberg et al. (45) have paid attention to the effect of cysteine protease inhibitor, which is capable of blocking cell death (apoptosis) of host hepatocytes in the case of exoerythrocytic Plasmodium infection. However, the endoerythrocytic nature of Hemoproteus infection could explain the increased apoptotic activity marker (Cas-3) in our study.
IFN-γ is a vital Th1 cytokine that promotes antiviral immunity and activates macrophages. There was no tissue-specific immune activation, as indicated by the equal expression in the spleen and lung. This could suggest a homeostatic state in which both organs’ baseline levels of IFN-γ are maintained. Additionally, the lack of differential expression suggests that there was no active inflammation or infection in the tissues collected at the time of assessment.
Fever induction and early innate immune responses are significantly influenced by IL-1β. Its comparatively consistent expression in different tissues points to widespread surveillance activity in the spleen and lung, which may be connected to the lymphoid and mucosal compartments’ mutual requirement for cytokine responsiveness. A multipurpose cytokine, IL-6 plays a role in immunological responses and inflammation. Its similar expression in the two tissues would indicate a baseline level of IL-6 activity across the body, most likely in a non-inflammatory or steady-state state. This implies that, in the circumstances examined, neither tissue exhibits preferential IL-6 upregulation.
The spleen of infected pigeons had higher transcriptional activity (mean = 9.33 ± 4.86) than the lung (mean = 6.40 ± 2.97), according to the gene expression study of caspase-3, a crucial apoptotic marker. The difference did not achieve statistical significance (P = 0.079), despite the fact that this trend suggests increased apoptotic signaling in splenic tissue. These findings imply that although there is an increase in caspase-3 expression, which might be due to tissue-specific immune responses or parasite-induced apoptosis, the ability to detect a statistically significant difference may have been diminished by the variability among biological replicates or the small sample size. However, the observed rise is consistent with the histologically documented tissue damage and inflammatory profile, hence corroborating the significance of apoptosis in the pathophysiology of H. columbae infection.
Although H. columbae typically causes subclinical infections, this study revealed histological lesions in vital organs and mild immune activation. Cytokine transcription was elevated but not statistically significant, possibly due to the small sample size (n = 5 per group). These results mirror findings in accidental hosts where immune dysregulation and pathology are more severe.
Our qPCR data align with prior studies on avian cytokine response during parasitic infections (41–43). The lack of statistical significance may reflect limitations in power and inter-individual variability. Histopathologically, the presence of parasites in endothelial and parenchymal tissues suggests developmental schizogony stages. These lesions, along with cytokine upregulation and caspase-3 activity, underscore the potential for immune-mediated tissue damage.
The circular basophilic globular aggregates resemble cytomeres, as reported by Gardiner et al. (29) and Msoffe et al. (39). There are a few incomplete septa that resemble the outer walls, which suggest that the megaloschizont’s compartmentalization was caused by ingrowth from the walls. The morphology of both doves’ merozoites and the descriptions of merozoites from other Haemoproteus species showed a favorable correlation (28, 30).
Conclusion
In the current study, H. columbae was detected in domesticated farmed pigeons with an incidence of 8%. However, H. columbae causes a low mortality rate, and the infected birds suffered from severe pathological changes in most hematopoietic and parenchymatous organs. The transcript levels of inflammatory markers such as IL-6, IFN-γ, and IL-1β were found to be significantly upregulated in H. columbae infected birds. Additionally, the mRNA level of the apoptotic Cas-3 in the H. columbae samples indicated apoptotic activity. Further studies are recommended to study the effect of different herbal extracts in comparison to the most commonly used drugs in controlling such a problem in infected pigeons.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was carried out according to the IACUC guidelines and approved by the Ethical Committee, Faculty of Veterinary Medicine, Cairo University, Cairo, Egypt with code “Vet CU 8032022407.” The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
HS: Conceptualization, Writing – review & editing, Writing – original draft, Investigation. AI: Writing – original draft, Writing – review & editing, Formal analysis. SB: Investigation, Formal analysis, Writing – original draft, Data curation. MAM: Writing – review & editing, Methodology. HA: Writing – review & editing, Methodology, Writing – original draft. MAlm: Writing – original draft, Writing – review & editing, Methodology, Formal analysis. LA: Writing – review & editing, Writing – original draft, Funding acquisition, Project administration. MAlq: Writing – review & editing, Formal analysis, Writing – original draft. SA: Project administration, Writing – review & editing, Writing – original draft, Resources, Formal analysis. KE-T: Writing – original draft, Formal analysis, Conceptualization, Funding acquisition, Resources, Writing – review & editing. MAt: Writing – original draft, Writing – review & editing, Methodology.
Funding
The author(s) declare that financial support was received for the research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R457), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. Deanship of Research and Graduate Studies at King Khalid University funded this work through Large Research Project under grant number RGP2/581/46. This research was funded by the UAEU Program for Advanced Research, Grant Number 12S169 to KE-T.
Acknowledgments
The authors gratefully acknowledge Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2025R457), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Large Research Project under Grant Number RGP2/581/46.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Saleh, S, Elseadawy, R, Elmorsy, MA, Essam, A, Abbas, I, and El-Alfy, ES. Intestinal parasites of domestic pigeons (Columba livia domestica) in Egypt: update on the prevalence and species diversity. J Parasit Dis. (2025) 49:130–41. doi: 10.1007/s12639-024-01728-5
2. Abd El-Salama, AM, Dyab, AK, Arafa, MI, Nasr, AA, and Abd-Elrahman, SM. Protozoal and rickettsial infections among clinically ill pigeons in Assiut, Egypt: prevalence, biodiversity, and potential public risk. Assiut Vet Med J. (2025) 71:672–83. doi: 10.21608/avmj.2025.336497.1471
3. Ramadan, RM, Salem, MA, Mohamed, HI, Orabi, A, El-Bahy, MM, and Taha, NM. Dermanyssus gallinae as a pathogen vector: phylogenetic analysis and associated health risks in pigeons. Vet Parasitol Reg Stud Reports. (2025) 57:101198. doi: 10.1016/j.vprsr.2025.101198
4. Zerek, A, Ceylan, O, Erdem, I, Simsek, FN, Úngari, LP, Sevik, M, et al. Molecular detection of two new Haemoproteus mitochondrial cytb lineages in Ciconiform and Charadriiform migratory birds in Turkey. Pak Vet J. (2024) 44:2074–7764. doi: 10.29261/pakvetj/2024.150
5. Santos, R, Lourenço, R, da Fonseca, IP, Louro, M, Barros, S, Casero, M, et al. Molecular survey of hemosporidian parasites in owls in mainland Portugal. J Wildl Dis. (2025) 61:434–47. doi: 10.7589/JWD-D-24-00063
6. Martinsen, ES, Perkins, SL, and Schall, JJ. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Mol Phylogenet Evol. (2008) 47:261–73. doi: 10.1016/j.ympev.2007.11.012
7. Bensch, S, Hellgren, O, and Pérez-Tris, J. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour. (2009) 9:1353–8. doi: 10.1111/j.1755-0998.2009.02692.x
8. Farmer, JN. Gizzard lesions associated with Haemoproteus sacharovi infections of pigeons. Proc Iowa Acad Sci. (1964) 71:537–42.
9. Valkiunas, G. Avian malaria parasites and other Haemosporidia. Boca Raton, FL: CRC Press (2005). 946 doi: 10.1201/9780203643792
10. Ahmed, FE, and Mohammed, AHH. Schizogony in Haemoproteus columbae Kruse. J Protozool. (1977) 24:389–93. doi: 10.1111/j.1550-7408.1977.tb04757.x
11. Valkiunas, G, and Ashford, RW. Natural host range is not a valid taxonomic character. Trends Parasitol. (2002) 18:528–9. doi: 10.1016/s1471-4922(02)02429-7
12. Santiago-Alarcon, D, Outlaw, DC, Ricklefs, RE, and Parker, PG. Phylogenetic relationships of haemosporidian parasites in New World Columbiformes, with emphasis on the endemic Galapagos dove. Int J Parasitol. (2010) 40:463–70. doi: 10.1016/j.ijpara.2009.10.003
13. Gamboa-Suárez, BA, Lotta-Arévalo, IA, Sarmiento-Salazar, F, and Matta, NE. Finding a needle in a haystack: DNA Haemoproteus columbae enrichment using percoll density gradient and flow cytometry. Vet Parasitol. (2024) 328:110170. doi: 10.1016/j.vetpar.2024.110170
14. Ferrell, ST, Snowden, K, Marlar, AB, Garner, M, and Lung, NP. Fatal hemoprotozoal infections in multiple avian species in a zoological park. J Zoo Wildl Med. (2007) 38:309–16. doi: 10.1638/1042-7260(2007)038[0309,FHIIMA]2.0.CO;2
15. Donovan, TA, Schrenzel, M, Tucker, TA, Pessier, AP, and Stalis, IH. Hepatic hemorrhage, hemocoelom, and sudden death due to Haemoproteus infection in passerine birds: eleven cases. J Vet Diagn Invest. (2008) 20:304–13. doi: 10.1177/104063870802000307
16. Adriano, EA, and Cordeiro, NS. Prevalence and intensity of Haemoproteus columbae in three species of wild doves from Brazil. Mem Inst Oswaldo Cruz. (2001) 96:175–8. doi: 10.1590/S0074-02762001000200007
17. Godfrey, RD Jr, Fedynich, AM, and Pence, DB. Quantification of hematozoa in blood smears. J Wildl Dis. (1987) 23:558–65. doi: 10.7589/0090-3558-23.4.558
18. Samani, AD, Kheirabadi, KP, and Samani, AD. Prevalence and rate of parasitemia of Haemoproteus columbae in Columba livia domestica in southwest of Iran. Iran J Parasitol. (2013) 8:641–4.
19. Valkiūnas, G, and Iezhova, TA. Keys to the avian Haemoproteus parasites (Haemosporida, Haemoproteidae). Malar J. (2022) 21:269. doi: 10.1186/s12936-022-04235-1
20. Suvarna, SK, Layton, C, and Bancroft, JD Bancroft’s theory and practice of histological techniques. Eighth Edition, Elsevier, Amsterdam, Netherlands (2018). 584 p.
21. Scaglione, FE, Pregel, P, Cannizzo, FT, Pérez-Rodríguez, AD, Ferroglio, E, and Bollo, E. Prevalence of new and known species of haemoparasites in feral pigeons in Northwest Italy. Malar J. (2015) 14:99–5. doi: 10.1186/s12936-015-0617-3
22. Nebel, C, Harl, J, Pajot, A, Weissenböck, H, Amar, A, and Sumasgutner, P. High prevalence and genetic diversity of Haemoproteus columbae (Haemosporida: Haemoproteidae) in feral pigeons Columba livia in Cape Town, South Africa. Parasitol Res. (2020) 119:447–63. doi: 10.1007/s00436-019-06558-6
23. Hartley, WJ. Some lethal avian protozoan diseases of native birds in eastern Australia. J S Afr Vet Assoc. (1992) 63:90.
24. Mohammed, AHH. Studies on the schizogony of Haemoproteus columbae Kruse, 1880. Proc Egypt Acad Sci. (1967) 19:37–64.
25. Bennett, GF, and Peirce, MA. The haemoproteid parasites of the pigeons and doves (family Columbidae). J Nat Hist. (1990) 24:311–25. doi: 10.1080/00222939000770231
26. Metwally, DM, Al-Talhi, RA, Barakat, IA, and ElKhadragy, MF. Effects of eugenol on Haemoproteus columbae in domestic pigeons (Columba livia domestica) from Riyadh, Saudi Arabia. Biosci Rep. (2019) 39:BSR20190409. doi: 10.1042/BSR20190409
27. Simpson, VR. Leucocytozoon-like infection in parakeets, budgerigars and a common buzzard. Vet Rec. (1991) 129:30–2. doi: 10.1136/vr.129.2.30
28. Atkinson, CT, Greiner, EC, and Forrester, DJ. Pre-erythrocytic development and associated host responses to Haemoproteus meleagridis (Haemosporina: Haemoproteidae) in experimentally infected domestic turkeys. J Protozool. (1986) 33:375–81. doi: 10.1111/j.1550-7408.1986.tb05626.x
29. Gardiner, CH, Jenkins, HJ, and Mahoney, KS. Myositis and death in bobwhites, Colinus virginianus (L.), due to hemorrhagic cysts of a haemosporozoan of undetermined taxonomic status. J Wildl Dis. (1984) 20:308–18. doi: 10.7589/0090-3558-20.4.308
30. Atkinson, CT, and Forrester, DJ. Myopathy associated with megaloschizonts of Haemoproteus meleagridis in a wild Turkey from Florida. J Wildl Dis. (1987) 23:495–8. doi: 10.7589/0090-3558-23.3.495
31. Cepeda, AS, Lotta-Arévalo, IA, Pinto-Osorio, DF, Macías-Zacipa, J, Valkiūnas, G, Barato, P, et al. Experimental characterization of the complete life cycle of Haemoproteus columbae, with a description of a natural host-parasite system used to study this infection. Int J Parasitol. (2019) 49:975–84. doi: 10.1016/j.ijpara.2019.07.003
32. Kucera, ZJ, Marjankova, K, Rachac, V, and Vitovec, J. Haemosporidiosis as a fatal disease in muscovy ducks (Cairina moschata) in South Bohemia. Folia Parasitol. (1982) 29:193–200.
33. Youssefi, MR, Sadeghian, AG, and Esfandiari, B. Prevalence of Haemoproteus columbae infection in Columba livia in north of Iran. World J Zool. (2010) 5:275–7.
34. Valkiūnas, G, Žičkus, T, Shapoval, AP, and Iezhova, TA. Effect of Haemoproteus belopolskyi (Haemosporida: Haemoproteidae) on body mass of the blackcap Sylvia atricapilla. J Parasitol. (2006) 92:1123–5. doi: 10.1645/GE-3564-RN.1
35. Møller, AP, and Nielsen, JT. Malaria and risk of predation: a comparative study of birds. Ecology. (2007) 88:871–81. doi: 10.1890/06-0747
36. Nebel, C, Sumasgutner, P, Pajot, A, and Amar, A. Response time of an avian prey to a simulated hawk attack is slower in darker conditions, but is independent of hawk colour morph. R Soc Open Sci. (2019) 6:190677. doi: 10.1098/rsos.190677
37. Mushi, EZ, Binta, MG, Chabo, RG, Ndebele, R, and Panzirah, R. Parasites of domestic pigeons (Columba livia domestica) in Sebele, Gaborone, Botswana. J S Afr Vet Assoc. (2000) 71:249–50. doi: 10.4102/jsava.v71i4.726
38. Gicik, Y, and Arslan, MÖ. Blood parasites of wild pigeons in Ankara district. Turk J Vet Anim Sci. (2001) 25:169–72.
39. Msoffe, PLM, Muhairwa, AP, Chiwanga, GH, and Kassuku, AA. A study of ecto- and endo-parasites of domestic pigeons in Morogoro Municipality, Tanzania. Afr J Agric Res. (2010) 5:264–7. doi: 10.5897/AJAR09.451
40. Rosyadi, I, Salasia, SIO, Argamjav, B, and Sato, H. Impact of subclinical Haemoproteus columbae infection on farmed domestic pigeons from Central Java (Yogyakarta), Indonesia, with special reference to changes in the hemogram. Pathogens. (2021) 10:440. doi: 10.3390/pathogens10040440
41. Al-Khalaifah, H, and Al-Nasser, A. Cytokines as effective elements of the avian immune system. J Microbiol Genet. (2018) 3:JMGE-119. doi: 10.29011/2574-7371.00019
42. Cui, W, Liu, L, Du, X, Yang, Q, Zhou, C, and Lei, Y. Rutaecarpine regulates the expression of pro-inflammatory cytokines to induce protective effects in the murine model of acute reflux esophagitis. Pak Vet J. (2023) 43:545–52. doi: 10.29261/pakvetj/2023.083
43. Kaya, DA, Güzel, Ö, Sezer, D, Sevim, G, Matur, E, Ergen, E, et al. Effect of propofol induction on antioxidant defense system, cytokines, and CD4+ and CD8+ T cells in cats. Kafkas Univ Vet Fak Derg. (2024) 30:603–10. doi: 10.9775/kvfd.2024.31756
44. Yu, H, Zou, W, Mi, C, Wang, Q, Dai, G, Zhang, T, et al. Research note: expression of T cell-related cytokines in chicken cecal and spleen tissues following Eimeria tenella infection in vivo. Poult Sci. (2021) 100:101161. doi: 10.1016/j.psj.2021.101161
45. Rennenberg, A, Lehmann, C, Heitmann, A, Witt, T, Hansen, G, Nagarajan, K, et al. Exoerythrocytic plasmodium parasites secrete a cysteine protease inhibitor involved in sporozoite invasion and capable of blocking cell death of host hepatocytes. PLoS Pathog. (2010) 6:e1000825. doi: 10.1371/journal.ppat.1000825
46. Wu, YF, Liu, HJ, Chiou, SH, and Lee, LH. Sequence and phylogenetic analysis of interleukin (IL)-1β-encoding genes of five avian species and structural and functional homology among these IL-1β proteins. Vet Immunol Immunopathol. (2007) 116:37–46. doi: 10.1016/j.vetimm.2006.12.010
47. Hayashi, T, Hiromoto, Y, Chaichoune, K, Patchimasiri, T, Chakritbudsabong, W, Prayoonwong, N, et al. Host cytokine responses of pigeons infected with highly pathogenic Thai avian influenza viruses of subtype H5N1 isolated from wild birds. PLoS One. (2011) 6:e23103. doi: 10.1371/journal.pone.0023103
48. Fringuelli, E, Urbanelli, L, Tharuni, O, Proietti, PC, Bietta, A, Davidson, I, et al. Cloning and expression of pigeon IFN-γ gene. Res Vet Sci. (2010) 89:367–72. doi: 10.1016/j.rvsc.2010.03.021
Keywords: blood parasites, cytochrome b gene, cytokine, gene expression, Haemoproteus columbae
Citation: Morphological characterization, histopathological alteration, and cytokine response of different tissues of Columba livia naturally infected with Haemoproteus columbae. Front. Vet. Sci. 12:1610416. doi: 10.3389/fvets.2025.1610416
Edited by:
Jinhong Zhao, Wannan Medical College, ChinaReviewed by:
Qingxia Wu, Tibet Agricultural and Animal Husbandry University, ChinaMeng Qingling, Shihezi University, China
Copyright © 2025 Salem, Ibrahim, Barsoum, Mahmoud, Albohiri, Almayouf, Almutairi, Alqahtani, Areshi, El-Tarabily and Attia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khaled A. El-Tarabily, a3RhcmFiaWx5QHVhZXUuYWMuYWU=
 Heba M. Salem
Heba M. Salem Amira M. Ibrahim2
Amira M. Ibrahim2 Khaled A. El-Tarabily
Khaled A. El-Tarabily