- 1International Joint Research Center of National Animal Immunology, College of Veterinary Medicine, Henan Agricultural University, Zhengzhou, China
- 2Ministry of Education Key Laboratory for Animal Pathogens and Biosafety, Henan Agricultural University, Zhengzhou, China
- 3Ministry of Agriculture and Rural Affairs, Key Laboratory of Quality and Safety Control of Poultry Products, Henan Agricultural University, Zhengzhou, China
- 4College of Veterinary Medicine, Huazhong Agricultural University, Wuhan, China
Hypervirulent Klebsiella pneumoniae (HvKP) is a notorious zoonotic pathogen that poses a significant threat to public health, as it can cause severe infections with high morbidity and mortality among young and healthy individuals. Commonly, a positive string test is primarily used to identify HvKP strains, but which is laborious and time-consuming. A rapid assay to identify HvKP is needed for public health personnel to track the spread of these strains and provide timely efforts for their control. Given this context, rapid SYBR Green I-based singleplex real-time PCR assays were developed for defining HvKP on the basis of the biomarkers iroB, iucA, peg-344 and plasmid-borne rmpA, respectively. These four singleplex PCR assays all displayed a high degree of linearity (R2
1 Introduction
Klebsiella pneumoniae is an increasingly important opportunistic pathogen in zoonoses that is clinically isolated from a wide range of samples, including the gastrointestinal tract, respiratory tract and urinary tract in both animals, humans and the environment, and commonly causes nosocomial infections, including bacteremia, pneumonia and urinary tract infections (1–4). Unlike classical K. pneumoniae (cKP), a distinct pathotype of K. pneumoniae, hypervirulent K. pneumoniae (HvKP) has emerged as a notorious pathogen capable of causing community-acquired and increasingly healthcare-associated infections, including pyogenic liver abscess and other severe organ and life-threatening infections, among healthy individuals of any age. Alarmingly, carbapenem-resistant HvKP, colistin-resistant HvKP and tigecycline-resistant HvKP have emerged, creating a new challenge in combating these threats, as they are hypervirulent, multidrug-resistant and highly transmissible (5–9). Given the increasing emergence and global dissemination of HvKP, rapidly detecting and continuously surveilling such organism is a priority.
Traditional methods, such as colony morphology, Galleria mellonella or mouse lethality assays and serum killing assay, especially the string test, have been widely used for identifying HvKP (10, 11). However, these methods are costly, time-consuming and are not suitable for the rapid detection of HvKP. Moreover, for the widely used string test, studies have shown that not all HvKP isolates are hypermucoviscous, which limits the accuracy of this method to 90% (2, 12). The development of accurate and rapid methods for the identification of HvKP is urgently needed for healthcare providers and public health personnel to track the dissemination of these strains and take timely actions for their control.
With the advantages of accuracy, low cost, rapidity and robustness, PCR-based methods have been widely used for the identification of pathogens (13). Recently, a quintuplex PCR was developed for the detection of HvKP, which targets the plasmid replicon gene, iroN (encodes siderophore salmochelin receptor), iutA (encodes ferric aerobactin receptor), plasmid-borne rmpA and plasmid-borne rmpA2 (regulates capsular polysaccharide biosynthesis), and agarose gel electrophoresis with GelStar nucleic acid gel stain revealed that the amplicons ranged from 160 bp to 683 bp in size (14). Moreover, a nonuplex PCR was established for the detection of HvKP, which targets genes allS (associates with allantoin metabolism), entB (involves in siderophore enterobactin biosynthesis), iutA, kfu (mediates ferric iron uptake), magA (relates with the K1 serotype), mrkD (encodes type 3 fimbrial adhesin), plasmid-borne rmpA, wzi (relates with the K2 serotype) and ybtS (involves in siderophore yersiniabactin biosynthesis), and agarose gel electrophoresis staining with ethidium bromide revealed that the amplicons were in the range of 242 base pairs (bp) to 1,283 bp (15). However, these traditional PCR methods require agarose gels or other post-PCR processing methods, which always suffering from unsatisfactory limit of detection (LOD), poor specificity and contamination.
As a superior alternative, real-time PCR coupled with melting curve analysis has the advantages of avoiding post-PCR operations, reducing contamination risks, and improving the LOD and specificity has been used for the screening of virulence genes in HvKP, such as the singleplex real-time PCRs which target iucA (involves in siderophore aerobactin biosynthesis), peg-344 (metabolite transporter) and rmpA (both plasmid-borne rmpA, rmpA2 and chromosome-borne rmpA), respectively (16). However, for the identification of HvKP, in addition to the above-mentioned genes, other genes, such as iroB (involves in siderophore salmochelin biosynthesis), irp2 (involves in siderophore yersiniabactin biosynthesis), peg-589 (metabolite transport), peg-1631 (metabolite transport), terB (encodes tellurite resistance protein), wzx (K serotype-specific allele) and wzy (K serotype-specific allele), are associated with HvKP, which suggests that more real-time PCRs for these related genes should be developed and used simultaneously to differentiate HvKP from cKP (12, 14, 15, 17). Fortunately, studies have proposed that the virulence-related genes iroB, iucA, peg-344 and plasmid-borne rmpA are biomarkers for the differentiation of HvKP from cKP with diagnostic accuracies greater than 0.96 (12, 18–20). Therefore, in this study, SYBR Green I-based singleplex real-time PCR methods that target biomarkers of HvKP, including iroB, iucA, peg-344 and plasmid-borne rmpA were developed. Moreover, in terms of cost savings and rapidity, duplex detection methods with melting curve analysis were established. These developed assays with low LODs and high specificities provide efficient tools for identifying and tracking the spread of HvKP in animals, humans and the environment.
2 Materials and methods
2.1 Samples
HvKP strain 20 K-368 (ST65, K2; iroB+, iucA+, peg-344+, plasmid-borne rmpA+), and HvKP strain 19 K-29 (iucA+, plasmid-borne rmpA+ and rmpA2+) were isolated and identified as HvKP by the string test (which showed viscous string stretching from the bacterial colony 5 mm) and sequencing analysis. Other K. pneumoniae strains, 19 K-1018, 19 K-1024 and 19 K-1028, were isolated and identified as cKP. Other isolates used in this study, including Gram-negative bacteria, such as Escherichia coli EC23030102 and Salmonella typhimurium SQ1, and Gram-positive bacteria, such as Staphylococcus aureus SA23033004 and Streptococcus suis L915 were collected and stored in our laboratory. The colony counting method was used to determine the bacterial number. Bacterial genomic DNA was rapidly extracted from the isolates via MightyPrep reagent (TaKaRa Bio, Kusatsu, Shiga, Japan).
2.2 Primer design
The genome sequences of the biomarkers iroB, iucA, peg-344 and plasmid-borne rmpA carried by the HvKP strain NTUH-K2044 (accession number AP006726) were retrieved from GenBank (National Library of Medicine, Bethesda, MD, USA). On the basis of the DNA sequences of iroB with 1,116 bp, iucA with 1791 bp, peg-344 with 903 bp and plasmid-borne rmpA with 633 bp, primers were designed via Oligo 7 under default settings (Molecular Biology Insights, Colorado Springs, CO, USA). And primers for these biomarkers in the published literatures were also retrieved. The primer sets were synthesized, purified and confirmed by Sangon Biotech (Shanghai, China).
2.3 Establishment of singleplex and duplex PCR
PCR was performed via a CFX96 real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA, USA). Each singleplex and duplex PCR was performed in triplicate, with a final reaction mixture of 20 μL containing 10 μL of 2 × SYBR Green I master mix (Tolo Biotech, Shanghai, China), 2 μL of the DNA template, an appropriate volume of primers and PCR water. The primers were used at a final concentration of 0.2 to 0.5 μM. A two-step amplification protocol was applied, which preceded the initial denaturation at 95°C for 5 min, followed by 35 cycles of 95°C for 15 s and 58°C for 30 s. Then, a melting curve analysis was used with a ramp speed of 0.1°C/s from 65 to 95°C. The LOD in singleplex and duplex PCR was defined as the lowest amount of HvKP that can produce a melting curve with one or two peaks which distinguished from the background noise. The primers for each target in singleplex real-time PCR were screened on the basis of the LOD and specificity. And the primers used in the duplex PCR melting curve assay were screened via two distinguishable melting peaks appeared or not. In addition, DNA templates extracted from other pathogens were used to evaluate the specificity of the developed singleplex and duplex PCRs.
2.4 Detection of HvKP in clinical specimens
BALB/c female mice, 6–8 weeks old, 16–18 grams in body weight were obtained from Huaxing Experimental Animal Farm (Zhengzhou, China) and quarantined for 5 days before use. HvKP strains 20 K-368 and 19 K-29; cKP strains 19 K-1018, 19 K-1024 and 19 K-1028; and E. coli strain EC23030102, S. typhimurium strain SQ1, S. aureus strain SA23033004, S. suis strain L915 at 103 cfu were used in the intraperitoneal challenge of nine BALB/c mice, respectively. Tail vein blood samples were collected from mice infected with HvKP strains or non-HvKP isolates at the Animal Care Center of Henan Agricultural University, with all operations strictly abiding by the laws and guidelines of China on the care and use of laboratory animals (under experimental license HNND2024030727). A 10 μL sample was mixed with 90 μL of MightyPrep reagent, and incubated at 95°C for 10 min on a block heater. After centrifugation at 12,000 rpm for 2 min, the supernatant was used directly as the DNA template in the developed singleplex and duplex PCRs. Additionally, the string test and sequencing analysis were used as reference methods for these clinical samples.
3 Results
3.1 Development of singleplex real-time PCR for biomarkers
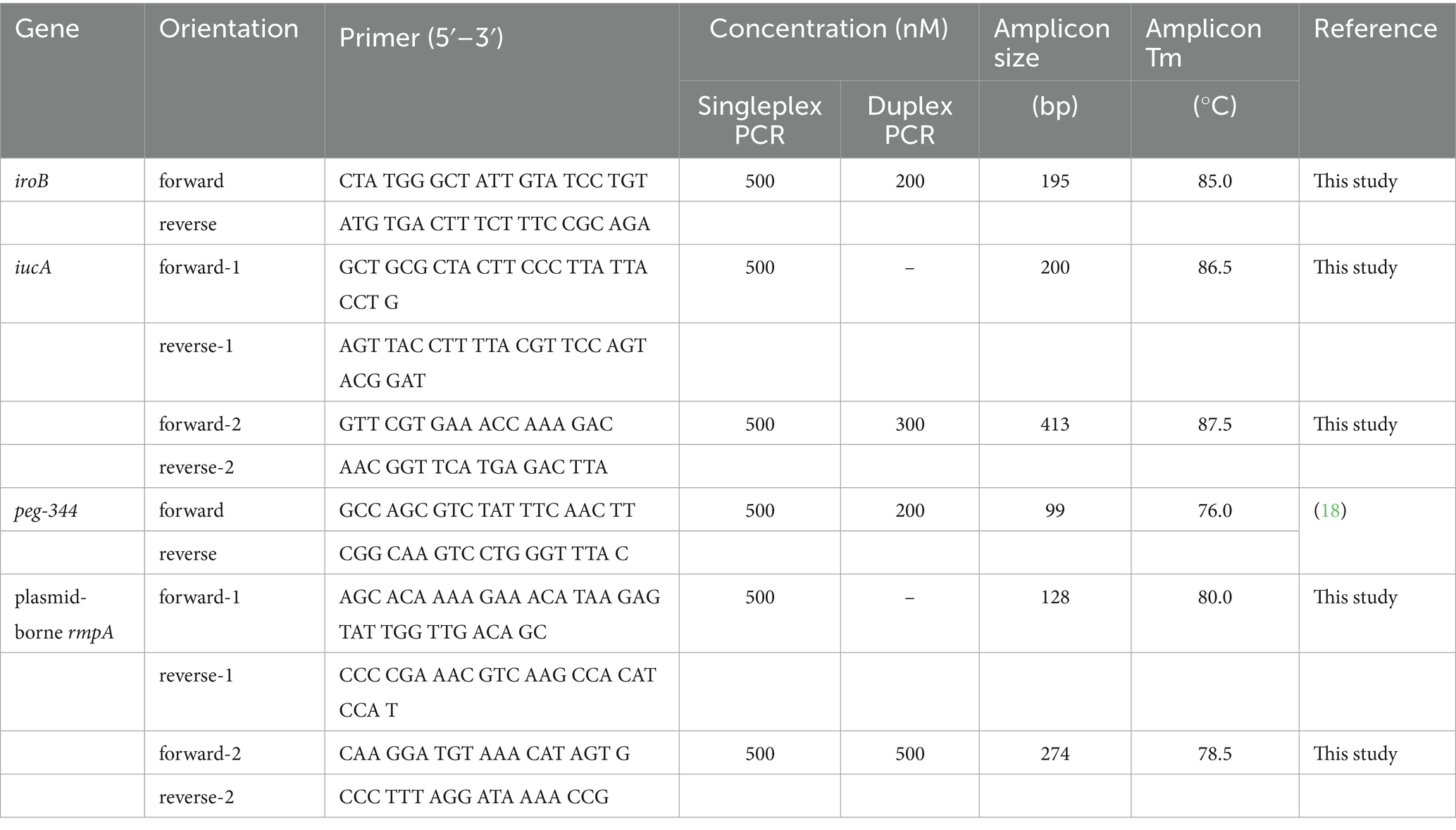
To reduce the testing time, rapid DNA extraction within 12 min was applied in this study, and the extraction yield was confirmed to be comparable to that of the classical mini spin column-based method according to our previous report (21). After initial screening of primer sets on the basis of the detection limit and specificity, primers whose amplicons were in the range of 99 to 413 bp, as shown in Table 1, were selected for use in subsequent real-time PCR assays.
One HvKP strain termed 20 K-368, which harboring biomarker genes iroB, iucA, peg-344 and plasmid-borne rmpA, was used in the development of singleplex real-time PCR. The DNA template was extracted from ten-fold serial dilutions of bacterial cultures at concentrations ranging from 1 102 to 1 109 cfu/mL. The linear range of singleplex real-time PCR was identified as the template concentrations where the corresponding Ct values were fitted into a straight line. As presented in Figure 1, standard curves were fitted with the coefficients of correlation (R2) greater than 0.99, and the linear ranges were from 104 to 109 cfu/mL for iroB, iucA (with primer iucA-1 being used), peg-344 and plasmid-borne rmpA (with primer rmpA-1 being used). The efficiency of the developed singleplex real-time PCR method was 96.5% for iroB, 93.8% for iucA, 101.3% for peg-344 and 82.7% for plasmid-borne rmpA. The LOD was determined to be 103 cfu/mL of HvKP, which was 10 cfu/reaction since 10 μL of bacteria and 90 μL of MightyPrep reagent were mixed for DNA preparation. Melting curve analysis revealed that the average melting temperatures (Tm) of these biomarkers were between 76.0 to 86.5°C (Table 1).
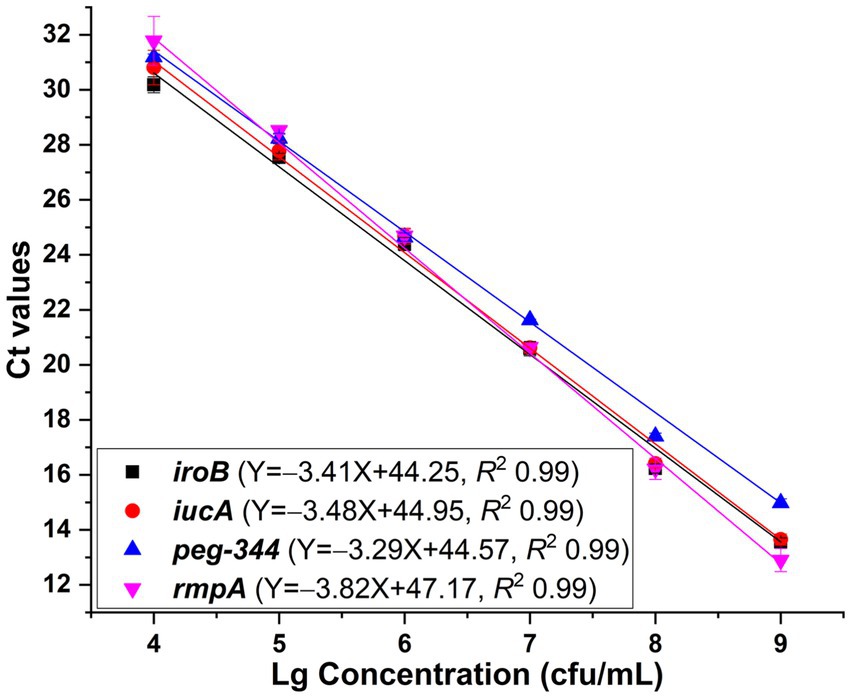
Figure 1. Standard curves generated for iroB, iucA, peg-344 and plasmid-borne rmpA, respectively via the SYBR Green I-based real-time PCR assays. The primers iucA-1 and rmpA-1 were used for the amplification of iucA and plasmid-borne rmpA, respectively. PCRs showed efficiencies of 96.5, 93.8, 101.3 and 82.7% for iroB, iucA, peg-344 and plasmid-borne rmpA, respectively, with all correlation coefficients higher than 0.99.
The reproducibility was evaluated, with coefficients of variation of no more than 1.26% for iroB, no more than 2.04% for iucA, no more than 1.00% for peg-344 and no more than 3.26% for plasmid-borne rmpA, which revealed the good repeatability and reproducibility of the established singleplex real-time PCR assays. Moreover, the specificity of the developed singleplex real-time PCR method was measured by testing 106 cfu/mL 19 K-1018 (cKP), EC23030102 (E. coli), SA23033004 (S. aureus), L915 (S. suis) and SQ1 (S. typhimurium) isolates. As shown in Figure 2, compared with the signal produced by the 20 K-368 (HvKP) strain, no obvious fluorescent signals were recorded for non-HvKP strains during the amplification process, which highlights the high specificity of these assays for the detection of iroB, iucA, peg-344 and plasmid-borne rmpA carried by HvKP.
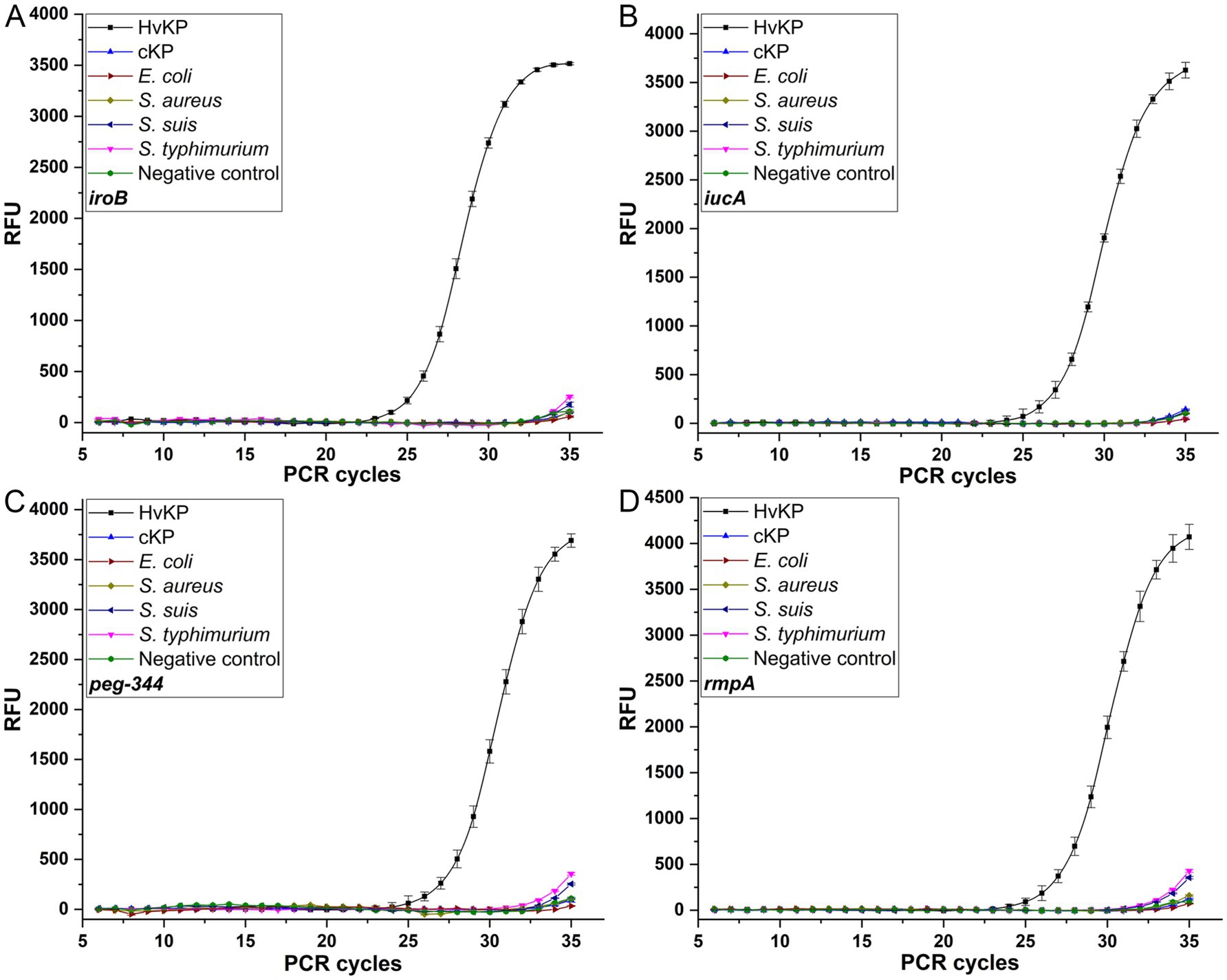
Figure 2. The specificities of the SYBR Green I-based real-time PCR assays for iroB (A), iucA (B), peg-344 (C) and plasmid-borne rmpA (D). An amount of 106 cfu/mL HvKP, cKP, E. coli, S. aureus, S. suis and S. typhimurium were used in this study.
3.2 Development of duplex PCR melting curve assays for biomarkers
In addition to the primers iucA-1 and rmpA-1, singleplex real-time PCR assays were developed with primers iucA-2 and rmpA-2, respectively. Standard curves were fitted with R2 values greater than 0.99 when 104 to 109 cfu/mL HvKP strain 20 K-368 was used (Figure 3). Analysis shown that the PCR efficiencies were 75.3% for iucA and 80.7% for plasmid-borne rmpA, which were lower than that of singleplex real-time PCR when the primers iucA-1 and rmpA-1 were used, respectively.
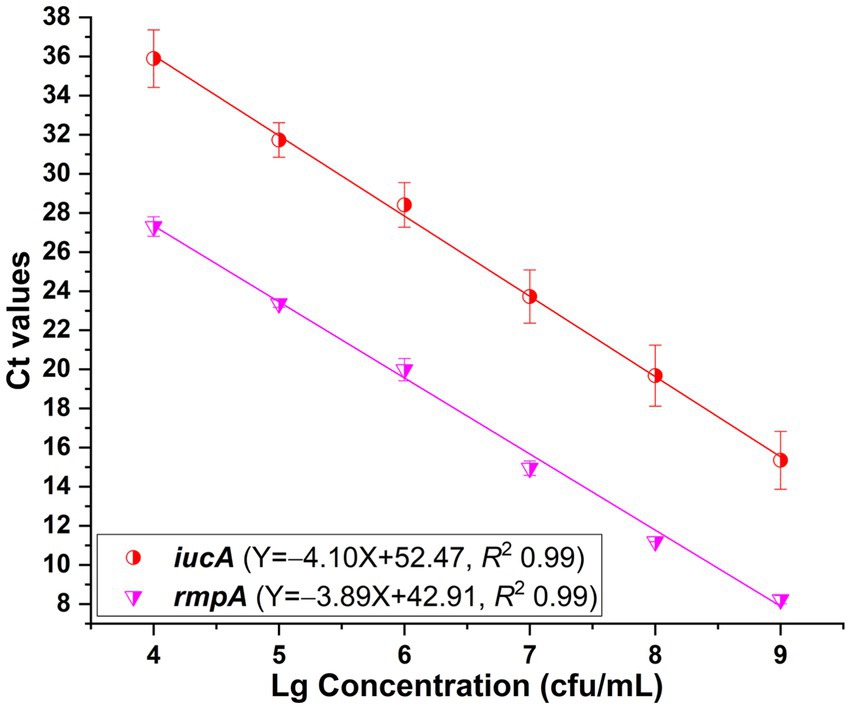
Figure 3. Standard curves plotted for iucA and plasmid-borne rmpA, respectively in the SYBR Green I-based real-time PCR assays. The primers iucA-2 and rmpA-2 were used for the amplification of iucA and plasmid-borne rmpA, respectively. PCRs showed efficiencies of 75.3 and 80.7% for iucA and plasmid-borne rmpA, respectively, with all correlation coefficients higher than 0.99.
As shown in Table 1, the above developed singleplex real-time PCR and melting curve assays revealed two groups of melting peaks with one at approximately 85.0°C for iroB and 86.5–87.5°C for iucA, and the other at approximately 76.0°C for peg-344 and 78.5–80.0°C for plasmid-borne rmpA. The main combination criterion of the primers for duplex PCRs was to ensure that two distinguishable melting peaks could be obtained in the mixed reaction. Therefore, one primer of iroB or iucA in the former group (Tm between 85.0–87.5°C) and the other primer of peg-344 or plasmid-borne rmpA in the latter group (Tm between 76.0–80.0°C) were tried to be mixed in one reaction. After screening, the performance of primer iucA-2 in duplex PCR melting curve assays showed lower detection limits than primer iucA-1 did, as did primer rmpA-2 rather than rmpA-1. In addition, when the primer iroB was mixed with rmpA-1 (or rmpA-2) in one PCR reaction, a melting curve was recorded with only one melting peak, not two different peaks, even when different primer concentrations were attempted, indicating the failure of developing duplex PCR for iroB and plasmid-borne rmpA. Therefore, primers iroB in combination with peg-344, primers iucA-2 in combination with peg-344, and primers iucA-2 in combination with rmpA-2 were selected for the development of duplex PCRs.
To establish the duplex PCR melting curve analysis, the conditions, mainly the primer concentrations were optimized. For singleplex real-time PCR, the concentration was 500 nM for primers iroB, iucA-1, peg-344 and rmpA-1. In duplex PCR for the iroB/peg-344 combination, the concentrations were optimized at 200 nM for primers iroB and peg-344. For the iucA/peg-344 combination, the concentrations were optimized at 300 nM for primer iucA-2 and 200 nM for primer peg-344. And for the iucA/rmpA combination, the optimal primer concentrations were 300 nM for iucA-2 and 500 nM for rmpA-2. However, the mixing of primers iroB and rmpA-2 in duplex PCR was failed to generate a melting curve with two different peaks. The melting curves for the above three duplex PCR assays were shown in Figure 4. Two distinct melting peaks can be seen in a dilution range of 104 to 109 cfu/mL for the iroB (Tm 85.0°C)/peg-344 (Tm 76.0°C) combination and the iucA (Tm 87.5°C)/peg-344 (Tm 76.0°C) combination, and 105 to 109 cfu/mL for the iucA (Tm 87.5°C)/rmpA (Tm 78.5°C) combination. That is, for an unknown sample, if melting peaks appear at 85.0 or 76.0°C in duplex PCR for the iroB/peg-344 combination, it indicates that this sample contains the virulence-related genes iroB or peg-344. The LODs of the duplex PCR melting curve assays for iroB/peg-344, iucA/peg-344 and iucA/rmpA combination were 104, 104 and 105 cfu/mL, respectively, which were 102, 102 and 103 cfu/reaction.
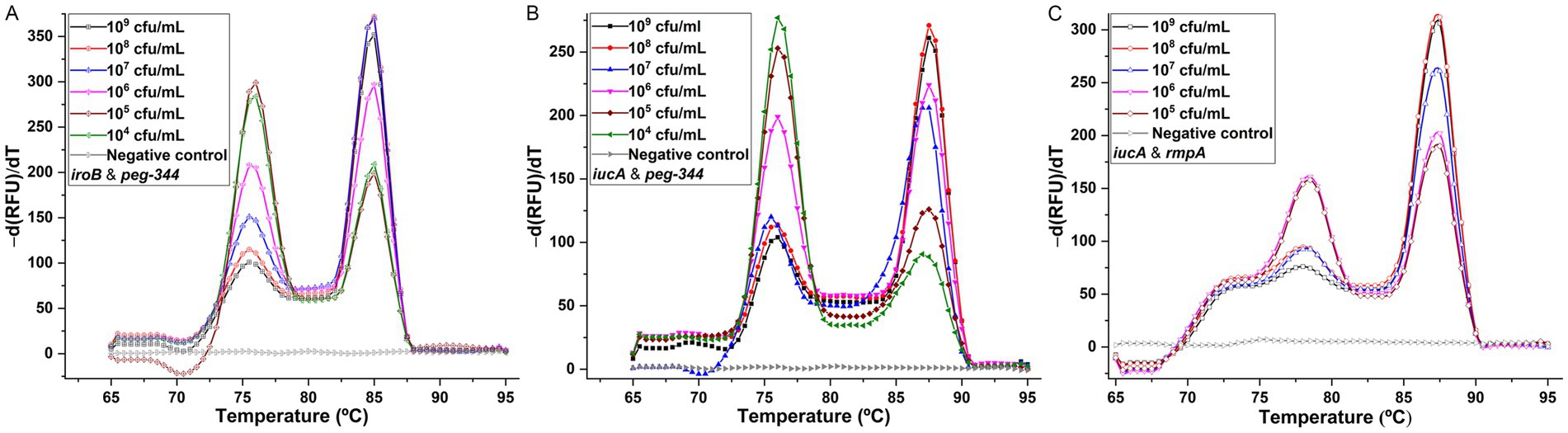
Figure 4. Melting curve analyses for duplex detection of iroB/peg-344 combination (A), iucA/peg-344 combination (B), and iucA/rmpA combination (C). The primers iroB, iucA-2, peg-344 and rmpA-2 were used for SYBR Green I-based amplification and melting analysis of iroB, iucA, peg-344 and plasmid-borne rmpA, respectively. The distinguishable and sharp peaks obtained for iroB (Tm 85.0°C), iucA (Tm 87.5°C), peg-344 (Tm 76.0°C) and plasmid-borne rmpA (Tm 78.5°C) were shown in the duplex PCRs.
The specificities of the developed duplex PCRs were determined by testing for HvKP-positive, and other Gram-positive and Gram-negative pathogens, and the results were shown in Figure 5. Compared with two clearly distinguishable melting peaks generated from the 106 cfu/mL 20 K-368 (HvKP) strain, no obvious melting peaks were recorded from non-HvKP strains, including 106 cfu/mL 19 K-1018 (cKP), EC23030102 (E. coli), SA23033004 (S. aureus), L915 (S. suis) and SQ1 (S. typhimurium) isolates. These results suggest that the developed duplex PCR melting curve analyses are highly specific for the detection and differentiation of iroB, iucA, peg-344 and plasmid-borne rmpA carried by HvKP.
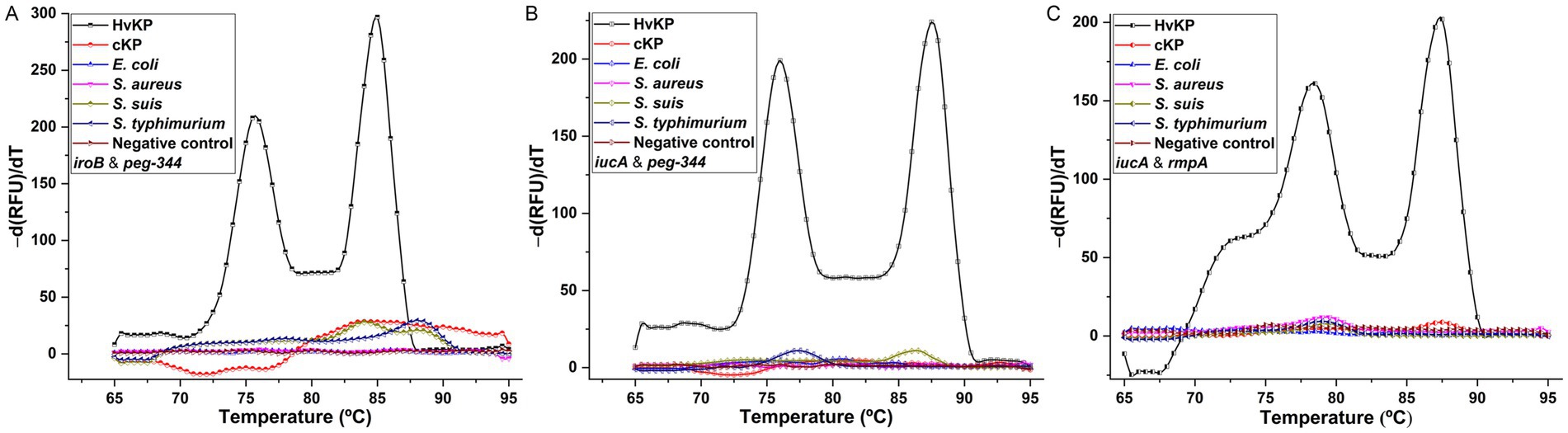
Figure 5. The specificities of melting curve analyses for duplex detection of iroB/peg-344 combination (A), iucA/peg-344 combination (B), and iucA/rmpA combination (C). An amount of 106 cfu/mL HvKP, cKP, E. coli, S. aureus, S. suis and S. typhimurium were used in this study.
3.3 Evaluation of singleplex and duplex PCR in clinical samples
The developed singleplex and duplex PCR assays were used to analyze blood samples from nine infected mice. The results from the developed singleplex and duplex PCR assays revealed that seven out of nine mice were negative for the biomarkers iroB, iucA, peg-344 and plasmid-borne rmpA, which were recorded as non-HvKP infection. The other two samples were recorded as HvKP infection. The results of singleplex and duplex PCRs showed that four melting peaks corresponding to genes iroB, iucA, peg-344, plasmid-borne rmpA were produced in the detection of strain 20 K-368 infection; and two melting peaks corresponding to genes iucA, plasmid-borne rmpA were generated in the detection of strain 19 K-29 infection, which depicting the high specificity of developed PCR methods. The typical clinical symptoms observed in these two mice were poor body condition, a ruffled hair coat, ocular swelling and redness. The above PCR results were confirmed by isolation of bacterial pathogens from blood samples, and identification through sequencing and the string test. These detection results supported the reliability of the established singleplex and duplex PCRs for the rapid detection of HvKP.
4 Discussion
Since the emergence of HvKP in the mid-1980s in Asia, serious and life-threatening infections caused by HvKP have been reported in countries and regions around the world in recent years (22–24). As a notorious zoonotic pathogen, HvKP can be isolated not only from humans but also from livestock, pets, wildlife and the environment (9, 25–28). Notably, the convergent K. pneumoniae clones with multidrug-resistant, hypervirulent and highly transmissible profiles are emerging and are now posing unprecedented threats and challenges to public health (29–31). Therefore, methods to identify and surveil the spread of HvKP are urgently needed.
A culture-based string test of K. pneumoniae on a blood-agar plate has been widely used to define HvKP when the viscous string is longer than 5 mm from the surface of the plate (1, 23). However, the correlation between the string test and clinically defined HvKP ranged from 51 to 98%, suggesting that more accurate methods are needed for the identification of HvKP (1). Other methods, such as Galleria mellonella or mouse lethality tests, qualitative plate siderophore production assay, and serum killing assay have been developed for identifying HvKP (11). However, these methods are labor-intensive, time-consuming, costly and are not suitable for the rapid and simple identification of HvKP.
Molecular-based PCR with the advantages of being timely and cost effective has attracted increasing attention (32–37). However, the challenge is which biomarkers can be used to define HvKP. One study revealed that iroB, iucA, peg-344, plasmid-borne rmpA and plasmid-borne rmpA2 are biomarkers for defining HvKP with accuracies ranging from 95 to 97% (12). Further studies revealed that the presence of four or more of the above-mentioned biomarkers can be used to predict one isolate as HvKP with an accuracy of 84%, and the presence of all five biomarkers was more accurate for the definition of HvKP, with an accuracy of 94% (19, 20). Among these five biomarkers, plasmid-borne rmpA (encoding RmpA) and plasmid-borne rmpA2 (encoding RmpA2), with identities of 84.9% for nucleotides (covering 97%) and 81.3% for proteins (covering 46%), regulate capsular polysaccharide biosynthesis in HvKP. When a total of 1767 sequenced K. pneumoniae isolates were analyzed in our laboratory, 26.0% (459/1767) of the isolates carried plasmid-borne rmpA. Further analysis revealed that 98.3% (451/459) of the isolates coharbored both plasmid-borne rmpA and plasmid-borne rmpA2, which suggests that almost all plasmid-borne rmpA-positive isolates were positive for plasmid-borne rmpA2. To reduce the detection cost, plasmid-borne rmpA (other than both plasmid-borne rmpA and plasmid-borne rmpA2) and other biomarkers iroB, iucA and peg-344 were used as the PCR targets for the identification of HvKP in this study.
Recently, singleplex real-time PCR methods for the detection of iucA, peg-344 and rmpA, respectively have been reported, but their LODs and amplification efficiencies have not been reported (16). Since the primers used for rmpA target the consensus sequences of plasmid-borne rmpA, plasmid-borne rmpA2 and chromosome-borne rmpA, the developed singleplex PCR method can detect the above-mentioned three rmpA genes simultaneously. The other one singleplex real-time PCR method was developed for peg-344 with an amplification efficiency of 97.1% (18). The same primer was used in this study for the detection of peg-344, and an efficiency of 101.3% was obtained, confirming the robustness of the reported singleplex real-time PCR assay for peg-344. To reduce the cost and detection time, duplex PCR methods with the capacity to detect two targets simultaneously were developed in this study. Like in previous studies, melting curve analyses were used for the detection of iroB/peg-344, iucA/peg-344 and iucA/rmpA combinations, respectively (38, 39). The LOD of the duplex PCR melting curve assay for iucA/rmpA was not as low as those of the other two combinations. Further studies are needed to improve the LOD. In the study for triplex detection of Bacillus cereus, Listeria monocytogenes and S. aureus through melting curve analysis, the SYTO9 dye was applied, and an LOD of 3.7 102 cfu/mL was achieved, which implies that superior fluorescent DNA intercalating dyes, such as EvaGreen and SYTO9, with less PCR inhibition other than SYBR Green I can be applied in subsequent studies (39). Recently, a quadruplex real-time PCR was developed for the detection of iroB, iucA, plasmid-borne rmpA and plasmid-borne rmpA2 with molecular beacon probes labeled with the dyes ROX, VIC, FAM and Cy5, respectively (40). An LOD of 1.5 103 cfu/mL was obtained, which is lower than that reported in this study. In the other one quadruplex real-time PCR, in which the targets include iucA and plasmid-borne rmpA/rmpA2 with molecular beacon probes labeled with the dyes ROX and VIC, respectively, an LOD of 20 cfu/reaction was acquired (41). Through these studies, molecular beacon probes based real-time PCR other than melting curve analysis can be employed to improve the detection limit, but the costs increase. Other methods, such as CRISPR/Cas-based diagnostic tools and recombinase-aided amplification assay have also been developed, and their advantages of ease of use, portability and rapidity are now attracting increasing attention (21, 42–49).
In summary, as shown in Table 2, traditional methods, including in vivo models and phenotypic tests, are commonly used for differentiating HvKP strains from cKP. Among these methods, the murine infection model is recognized as the standard. The median lethal dose (LD50) of HvKP is generally between 10–106 cfu after intraperitoneal or subcutaneous challenge, whereas the LD50 of cKP is usually greater than 107 cfu. Other methods, such as quantitative mucoviscosity assay, serum killing assay, siderophore production assessment and string test, are based on the hypermucoviscous phenotype of HvKP. However, not all HvKP strains are hypermucoviscous, which limits the accuracy and specificity (60.0–94.0%) of these methods. Additionally, complex operation, high cost and time consuming are the main disadvantages that limit the usage of these traditional tests. PCR-based tests, especially multiplex PCR, which target the biomarkers iroB, iucA, peg-344, plasmid-borne rmpA and rmpA2 of HvKP, are easy to use, accurate, specific and time-saving. The developed PCR methods can detect as few as 2 cfu of HvKP, and the specificity can reach 100%. In addition, CRISPR/Cas-based detection, loop-mediated isothermal amplification (LAMP) and recombinase-aided amplification (RAA) provide alternative methods for rapid and specific detection of HvKP.
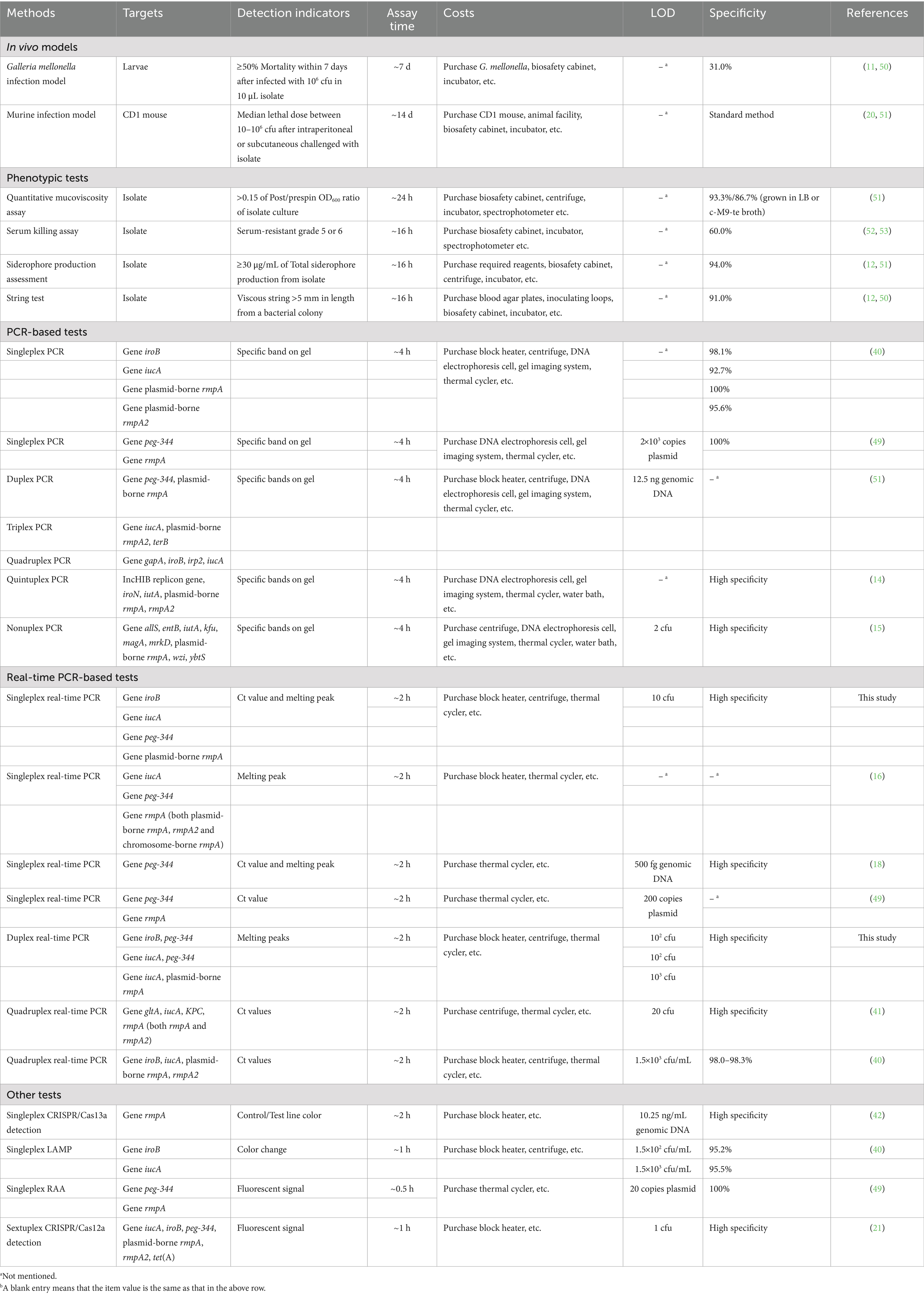
Table 2. Comparison of developed real-time PCRs with several published methods for the identification of HvKP.
In this report, the PCR targets were the HvKP-specific genes iroB, iucA, peg-344 and plasmid-borne rmpA. However, when the DNA sequence of iroB in HvKP (NTUH-K2044 isolate) was used as the query sequence for NCBI BLAST, Enterobacter hormaechei and E. coli, which carry this iroB gene with an identity greater than 88.1% (query coverage 100%), were identified. Similarly, the iucA gene in HvKP (NTUH-K2044 isolate) was found in E. coli, with an identity higher than 88.3% (query coverage higher than 94%). These data suggest that for K. pneumoniae strains, the developed singleplex and duplex PCR can be used to differentiate HvKP from cKP. However, for an unknown isolate, bacterial identification of which one belongs to K. pneumoniae or not is essential. Moreover, more hypervirulent strains of other bacterial species require to be collected and used for further specification test of the developed PCRs.
In conclusion, SYBR Green I-based singleplex real-time PCRs for iroB, iucA, peg-344 and plasmid-borne rmpA, respectively were developed with the LOD of 10 cfu/reaction. And duplex PCR melting curve analyses for iroB/peg-344, iucA/peg-344 and iucA/rmpA combinations were performed with LODs of 102, 102 and 103 cfu/reaction, respectively. These methods were found to be reliable and specific for the identification of HvKP. The highlights are (1) rapid SYBR Green I-based singleplex real-time PCRs for HvKP were developed; (2) new duplex PCR melting curve assays for HvKP were established; (3) the detection limit of the singleplex PCRs was 10 cfu; (4) singleplex and duplex tests provide fast, accurate and specific diagnoses of HvKP infection.
Through the initial evaluation of the developed singleplex and duplex PCRs against HvKP, cKP, E. coli, S. aureus, S. suis and S. typhimurium, a specific response was obtained for HvKP, which highlights the high specificity of these PCR methods. Further studies will focus on the collection of more HvKP, cKP and other hypervirulent bacterial strains to evaluate the accuracy, sensitivity and specificity of developed PCRs and the development of molecular beacon-based multiplex real-time PCRs for HvKP and other pathogens.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Science Ethics Committee of Henan Agricultural University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JW: Data curation, Methodology, Writing – original draft. YW: Data curation, Investigation, Methodology, Software, Writing – review & editing. LZ: Data curation, Methodology, Software, Writing – original draft. MX: Data curation, Validation, Writing – review & editing. YH: Investigation, Validation, Writing – review & editing. SP: Investigation, Writing – review & editing. CX: Writing – review & editing. HY: Funding acquisition, Writing – review & editing. CL: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. X-DD: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Foundation of Henan (No. 242300420152), the National Natural Science Foundation of China (No. 32002342), the Open Fund Project of the National Key Laboratory of Veterinary Public Health and Safety (No. 2025SKLVPHS04), the National Training Program of Innovation and Entrepreneurship for Undergraduates (No. 202210466002) and the National Key Research and Development Project of China (No. 2021YFD1800900).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Choby, JE, Howard-Anderson, J, and Weiss, DS. Hypervirulent Klebsiella pneumoniae – clinical and molecular perspectives. J Intern Med. (2020) 287:283–300. doi: 10.1111/joim.13007
2. Russo, TA, and Marr, CM. Hypervirulent Klebsiella pneumoniae. Clin Microbiol Rev. (2019) 32:e00001-19. doi: 10.1128/CMR.00001-19
3. Wall, K, Macori, G, Koolman, L, Li, F, and Fanning, S. Klebsiella, a hitherto underappreciated zoonotic pathogen of importance to one health: a short review. Zoonoses. (2023) 3:38. doi: 10.15212/ZOONOSES-2023-0016
4. Wyres, KL, and Holt, KE. Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Curr Opin Microbiol. (2018) 45:131–9. doi: 10.1016/j.mib.2018.04.004
5. Pu, D, Zhao, J, Chang, K, Zhuo, X, and Cao, B. "superbugs" with hypervirulence and carbapenem resistance in Klebsiella pneumoniae: the rise of such emerging nosocomial pathogens in China. Sci Bull. (2023) 68:2658–70. doi: 10.1016/j.scib.2023.09.040
6. Liu, X, Wu, Y, Zhu, Y, Jia, P, Li, X, Jia, X, et al. Emergence of colistin-resistant hypervirulent Klebsiella pneumoniae (CoR-HvKp) in China. Emerg Microbes Infect. (2022) 11:648–61. doi: 10.1080/22221751.2022.2036078
7. Yao, H, Qin, S, Chen, S, Shen, J, and Du, X-D. Emergence of carbapenem-resistant hypervirulent Klebsiella pneumoniae. Lancet Infect Dis. (2018) 18:25. doi: 10.1016/S1473-3099(17)30628-X
8. Li, Y, Wang, Z, Dong, H, Wang, M, Qin, S, Chen, S, et al. Emergence of tet(X4)-positive hypervirulent Klebsiella pneumoniae of food origin in China. LWT. (2023) 173:114280. doi: 10.1016/j.lwt.2022.114280
9. Mario, E, Hamza, D, and Abdel-Moein, K. Hypervirulent Klebsiella pneumoniae among diarrheic farm animals: a serious public health concern. Comp Immunol Microbiol Infect Dis. (2023) 102:102077. doi: 10.1016/j.cimid.2023.102077
10. Liao, W, Long, D, Huang, Q, Wei, D, Liu, X, Wan, L, et al. Rapid detection to differentiate hypervirulent Klebsiella pneumoniae (hvKp) from classical K. pneumoniae by identifying peg-344 with loop-mediated isothermal amplication (LAMP). Front Microbiol. (2020) 11:1189. doi: 10.3389/fmicb.2020.01189
11. Zhang, Q-B, Zhu, P, Zhang, S, Rong, Y-J, Huang, Z-A, Sun, L-W, et al. Hypervirulent Klebsiella pneumoniae detection methods: a minireview. Arch Microbiol. (2023) 205:326. doi: 10.1007/s00203-023-03665-y
12. Russo, TA, Olson, R, Fang, C-T, Stoesser, N, Miller, M, MacDonald, U, et al. Identification of biomarkers for differentiation of hypervirulent Klebsiella pneumoniae from classical K. pneumoniae. J Clin Microbiol. (2018) 56:e00776-18. doi: 10.1128/JCM.00776-18
13. Yang, S, and Rothman, RE. PCR-based diagnostics for infectious diseases: uses, limitations, and future applications in acute-care settings. Lancet Infect Dis. (2004) 4:337–48. doi: 10.1016/S1473-3099(04)01044-8
14. Yu, F, Lv, J, Niu, S, Du, H, Tang, Y-W, Pitout, JDD, et al. Multiplex PCR analysis for rapid detection of Klebsiella pneumoniae carbapenem resistant (sequence type 258 [ST258] and ST11) and hypervirulent (ST23, ST65, ST86, and ST375) strains. J Clin Microbiol. (2018) 56:e00731-18. doi: 10.1128/JCM.00731-18
15. Compain, F, Babosan, A, Brisse, S, Genel, N, Audo, J, Ailloud, F, et al. Multiplex PCR for detection of seven virulence factors and K1/K2 capsular serotypes of Klebsiella pneumoniae. J Clin Microbiol. (2014) 52:4377–80. doi: 10.1128/JCM.02316-14
16. Parrott, AM, Shi, J, Aaron, J, Green, DA, Whittier, S, and Wu, F. Detection of multiple hypervirulent Klebsiella pneumoniae strains in a new York City hospital through screening of virulence genes. Clin Microbiol Infect. (2021) 27:583–9. doi: 10.1016/j.cmi.2020.05.012
17. Turton, JF, Perry, C, Elgohari, S, and Hampton, CV. PCR characterization and typing of Klebsiella pneumoniae using capsular type-specific, variable number tandem repeat and virulence gene targets. J Med Microbiol. (2010) 59:541–7. doi: 10.1099/jmm.0.015198-0
18. Bulger, J, MacDonald, U, Olson, R, Beanan, J, and Russo, TA. Metabolite transporter PEG344 is required for full virulence of hypervirulent Klebsiella pneumoniae strain hvKP1 after pulmonary but not subcutaneous challenge. Infect Immun. (2017) 85:e00093-17. doi: 10.1128/IAI.00093-17
19. Russo, TA, Alvarado, CL, Davies, CJ, Drayer, ZJ, Carlino-MacDonald, U, Hutson, A, et al. Differentiation of hypervirulent and classical Klebsiella pneumoniae with acquired drug resistance. MBio. (2024) 15:e02867-23. doi: 10.1128/mbio.02867-23
20. Russo, TA, Lebreton, F, and McGann, PT. A step forward in hypervirulent Klebsiella pneumoniae diagnostics. Emerg Infect Dis. (2025) 31:1–3. doi: 10.3201/eid3101.241516
21. Li, C, Wu, Y, Chen, Y, Xu, C, Yao, H, Yu, W, et al. Violet phosphorene nanosheets coupled with CRISPR/Cas12a in a biosensor with a low background signal for onsite detection of tigecycline-resistant hypervirulent Klebsiella pneumoniae. Sens Actuators B Chem. (2023) 395:134509. doi: 10.1016/j.snb.2023.134509
22. Arcari, G, and Carattoli, A. Global spread and evolutionary convergence of multidrug-resistant and hypervirulent Klebsiella pneumoniae high-risk clones. Pathog Glob Health. (2023) 117:328–41. doi: 10.1080/20477724.2022.2121362
23. Dai, P, and Hu, D. The making of hypervirulent Klebsiella pneumoniae. J Clin Lab Anal. (2022) 36:e24743. doi: 10.1002/jcla.24743
24. Shon, AS, Bajwa, RPS, and Russo, TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. (2013) 4:107–18. doi: 10.4161/viru.22718
25. Anzai, EK, de Souza Junior, JC, Peruchi, AR, Fonseca, JM, Gumpl, EK, Pignatari, ACC, et al. First case report of non-human primates (Alouatta clamitans) with the hypervirulent Klebsiella pneumoniae serotype K1 strain ST 23: a possible emerging wildlife pathogen. J Med Primatol. (2017) 46:337–42. doi: 10.1111/jmp.12296
26. Ma, Q, Zhu, Z, Liu, Y, Wang, J, Pan, Z, Yao, H, et al. Keeping alert to the hypervirulent K1, K2, K3, K5, K54 and K57 strains of Klebsiella pneumoniae within dairy production process. Microbes Infect. (2023) 25:105106. doi: 10.1016/j.micinf.2023.105106
27. Zhang, Z, Lei, L, Zhang, H, Dai, H, Song, Y, Li, L, et al. Molecular investigation of Klebsiella pneumoniae from clinical companion animals in Beijing, China, 2017–2019. Pathogens. (2021) 10:271. doi: 10.3390/pathogens10030271
28. Zou, H, Zhou, Z, Berglund, B, Zheng, B, Meng, M, Zhao, L, et al. Persistent transmission of carbapenem-resistant, hypervirulent Klebsiella pneumoniae between a hospital and urban aquatic environments. Water Res. (2023) 242:120263. doi: 10.1016/j.watres.2023.120263
29. Dong, N, Yang, X, Chan, EW-C, Zhang, R, and Chen, S. Klebsiella species: taxonomy, hypervirulence and multidrug resistance. EBioMedicine. (2022) 79:103998. doi: 10.1016/j.ebiom.2022.103998
30. Liu, C, Dong, N, Chan, EWC, Chen, S, and Zhang, R. Molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae in China, 2016–20. Lancet Infect Dis. (2022) 22:167–8. doi: 10.1016/S1473-3099(22)00009-3
31. Zhao, W, Li, S, Schwarz, S, Li, A, Yao, H, and Du, X-D. Detection of a NDM-5-producing Klebsiella pneumoniae sequence type 340 (CG258) high-risk clone in swine. Vet Microbiol. (2021) 262:109218. doi: 10.1016/j.vetmic.2021.109218
32. Chen, N, Ye, M, Xiao, Y, Li, S, Huang, Y, Li, X, et al. Development of universal and quadruplex real-time RT-PCR assays for simultaneous detection and differentiation of porcine reproductive and respiratory syndrome viruses. Transbound Emerg Dis. (2019) 66:2271–8. doi: 10.1111/tbed.13276
33. Deng, L, He, C, Zhou, Y, Xu, L, and Xiong, H. Ground transport stress affects bacteria in the rumen of beef cattle: a real-time PCR analysis. Anim Sci J. (2017) 88:790–7. doi: 10.1111/asj.12615
34. Guo, Z, Li, K, Qiao, S, Chen, X-X, Deng, R, and Zhang, G. Development and evaluation of duplex TaqMan real-time PCR assay for detection and differentiation of wide-type and MGF505-2R gene-deleted African swine fever viruses. BMC Vet Res. (2020) 16:428. doi: 10.1186/s12917-020-02639-2
35. Lu, S-J, Ma, M-Y, Yan, X-G, Zhao, F-J, Hu, W-Y, Ding, Q-W, et al. Development and application of a low-priced duplex quantitative PCR assay based on SYBR Green I for the simultaneous detection of porcine deltacoronavirus and porcine sapelovirus. Vet Med. (2023) 68:106–15. doi: 10.17221/79/2022-VETMED
36. Yan, Y, Cui, Y, Zhao, S, Jing, J, Shi, K, Jian, F, et al. Development of a duplex PCR assay for detecting Theileria luwenshuni and Anaplasma phagocytophilum in sheep and goats. Exp Appl Acarol. (2021) 85:319–30. doi: 10.1007/s10493-021-00662-y
37. Zheng, LL, Jin, XH, Wei, FS, Wang, CQ, Chen, HY, Wang, YB, et al. Simultaneous detection of porcine pseudorabies virus, porcine parvovirus and porcine circovirus type 2 by multiplex real-time PCR and amplicon melting curve analysis using SYBR green I. Vet Med. (2018) 63:358–66. doi: 10.17221/3/2018-VETMED
38. Barletta, F, Mercado, EH, Lluque, A, Ruiz, J, Cleary, TG, and Ochoa, TJ. Multiplex real-time PCR for detection of Campylobacter, Salmonella, and Shigella. J Clin Microbiol. (2013) 51:2822–9. doi: 10.1128/JCM.01397-13
39. Forghani, F, Wei, S, and Oh, D-H. A rapid multiplex real-time PCR high-resolution melt curve assay for the simultaneous detection of Bacillus cereus, listeria monocytogenes, and Staphylococcus aureus in food. J Food Prot. (2016) 79:810–5. doi: 10.4315/0362-028X.JFP-15-428
40. Cai, Y, Wang, W, Liang, H, Huang, Q, Qin, J, Guo, Z, et al. Sensitive and specific LAMP and multiplex qRT-PCR assays for detection of hypervirulent Klebsiella pneumoniae. Diagn Microbiol Infect Dis. (2025) 111:116684. doi: 10.1016/j.diagmicrobio.2025.116684
41. Xu, Z, Li, B, Jiang, Y, Huang, J, Su, L, Wu, W, et al. Development of a quadruple qRT-PCR assay for simultaneous identification of hypervirulent and carbapenem-resistant Klebsiella pneumoniae. Microbiol Spectr. (2024) 12:e00719-23. doi: 10.1128/spectrum.00719-23
42. Bhattacharjee, G, Gohil, N, Khambhati, K, Gajjar, D, Abusharha, A, and Singh, V. A paper-based assay for detecting hypervirulent Klebsiella pneumoniae using CRISPR-Cas13a system. Microchem J. (2024) 203:110931. doi: 10.1016/j.microc.2024.110931
43. Chang, Y, Deng, Y, Li, T, Wang, J, Wang, T, Tan, F, et al. Visual detection of porcine reproductive and respiratory syndrome virus using CRISPR-Cas13a. Transbound Emerg Dis. (2020) 67:564–71. doi: 10.1111/tbed.13368
44. Wang, Z, Yang, P-P, Zhang, Y-H, Tian, K-Y, Bian, C-Z, and Zhao, J. Development of a reverse transcription recombinase polymerase amplification combined with lateral-flow dipstick assay for avian influenza H9N2 HA gene detection. Transbound Emerg Dis. (2019) 66:546–51. doi: 10.1111/tbed.13063
45. Wang, Y, Yu, F, Zhang, K, Shi, K, Chen, Y, Li, J, et al. End-point RPA-CRISPR/Cas12a-based detection of Enterocytozoon bieneusi nucleic acid: rapid, sensitive and specific. BMC Vet Res. (2024) 20:540. doi: 10.1186/s12917-024-04391-3
46. Wu, Y, Tian, K, Zhang, Y, Guo, H, Li, N, Wang, Z, et al. Rapid and visual detection of Lawsonia intracellularis with an improved recombinase polymerase amplification assay combined with a lateral flow dipstick. BMC Vet Res. (2019) 15:97. doi: 10.1186/s12917-019-1841-9
47. Zhang, K, Sun, Z, Shi, K, Yang, D, Bian, Z, Li, Y, et al. RPA-CRISPR/Cas12a-based detection of Haemophilus parasuis. Animals. (2023) 13:3317. doi: 10.3390/ani13213317
48. Zhao, Y, Dai, J, Zhang, Z, Chen, J, Chen, Y, Hu, C, et al. CRISPR-Cas13a-based lateral flow assay for detection of bovine leukemia virus. Animals. (2024) 14:3262. doi: 10.3390/ani14223262
49. Yan, C, Zhou, Y, Du, S, Du, B, Zhao, H, Feng, Y, et al. Recombinase-aided amplification assay for rapid detection of hypervirulent Klebsiella pneumoniae (hvKp) and characterization of the hvKp pathotype. Microbiol Spectr. (2023) 11:e03984-22. doi: 10.1128/spectrum.03984-22
50. Li, G, Shi, J, Zhao, Y, Xie, Y, Tang, Y, Jiang, X, et al. Identification of hypervirulent Klebsiella pneumoniae isolates using the string test in combination with galleria mellonella infectivity. Eur J Clin Microbiol Infect Dis. (2020) 39:1673–9. doi: 10.1007/s10096-020-03890-z
51. Russo, TA, MacDonald, U, Hassan, S, Camanzo, E, LeBreton, F, Corey, B, et al. An assessment of siderophore production, mucoviscosity, and mouse infection models for defining the virulence spectrum of hypervirulent Klebsiella pneumoniae. mSphere. (2021) 6:e00045-00021. doi: 10.1128/mSphere.00045-21
52. Wang, T-C, Lin, J-C, Chang, J-C, Hiaso, Y-W, Wang, C-H, Chiu, S-K, et al. Virulence among different types of hypervirulent Klebsiella pneumoniae with multi-locus sequence type (MLST)-11, serotype K1 or K2 strains. Gut Pathog. (2021) 13:40. doi: 10.1186/s13099-021-00439-z
Keywords: singleplex real-time PCR, duplex PCR, biomarkers, melting curve analysis, hypervirulent Klebsiella pneumoniae
Citation: Wang J, Wu Y, Zhao L, Xing M, Huang Y, Peng S, Xu C, Yao H, Li C and Du X-D (2025) Singleplex real-time PCR and duplex PCR platform for the rapid detection of hypervirulent Klebsiella pneumoniae. Front. Vet. Sci. 12:1611750. doi: 10.3389/fvets.2025.1611750
Edited by:
Gabriele Rossi, Murdoch University, AustraliaReviewed by:
Ifeanyi Elibe Mba, University of Ibadan, NigeriaElsa Gladys Aguilar Ancori, National University of Saint Anthony the Abbot in Cuzco, Peru
Copyright © 2025 Wang, Wu, Zhao, Xing, Huang, Peng, Xu, Yao, Li and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenglong Li, bXlsaWNoZW5nbG9uZ0AxMjYuY29t
‡ORCID: Chenglong Li, orcid.org/0000-0003-3043-7230
†These authors have contributed equally to this work
 Jiaqi Wang1,2,3†
Jiaqi Wang1,2,3† Chunyan Xu
Chunyan Xu Hong Yao
Hong Yao Chenglong Li
Chenglong Li Xiang-Dang Du
Xiang-Dang Du