- 1Department of Animal Production, Faculty of Agriculture, Zagazig University, Zagazig, Egypt
- 2Department of Biology, College of Science, Imam Mohammad Ibn Saud Islamic University (IMSIU), Riyadh, Saudi Arabia
- 3Department of Animal Nutrition and Clinical Nutrition, Faculty of Veterinary Medicine, New Valley University, El-Kharga, Egypt
- 4Department of Integrative Agriculture, College of Agriculture and Veterinary Medicine, United Arab Emirates University, Al Ain, Abu Dhabi, United Arab Emirates
- 5Department of Physiology, Biochemistry, and Pharmacology, College of Veterinary Medicine, University of Kirkuk, Kirkuk, Iraq
Introduction: This research assessed the influence of probiotics in low-energy diets on the performance and health status of rabbits during the growing phase. The growth parameters, carcass metrics, serum immunological state, lipid profile, and hepatic and renal functioning of rabbits have been analysed.
Methods: One hundred male New Zealand White rabbits, aged 5 weeks, were split into five groups at random. The rabbits within each group were allocated into 10 replicates, comprising 2 rabbits each. The initial group (T1) received a standard energy diet (10.85 MJ/kg), the (T2) group was provided with a low energy diet (10.25 MJ/kg), whereas the T3, T4, and T5 groups were administered a low energy diet mixed with Bifidobacterium (1 ml/kg diet), Spirulina extract (2 ml/kg diet), and yeast extract (2 ml/kg diet), respectively.
Results and Discussion: The results showed a significant increase in growth performance with the treatment of biological feed additives, and the group treated with spirulina extract increased final body weight and weight gain (5-13 wk), while the group treated with bifidobacterium improved feed intake and feed conversion ratio (5–13 wk). Carcass traits were not significantly affected by probiotic supplementation (P > 0.05). Moreover, haematological parameters showed no significant changes (P > 0.05) with probiotic supplementation, except for red blood cells (RBCs), white blood cells (WBCs), platelets (PLT), and basophils (BAS), which showed a significant variation (P < 0.05). Liver and kidney function tests showed a significant increase (P < 0.05) with probiotic treatments. Furthermore, thyroid hormones such as T3 and T4 were significantly enhanced by supplementation of probiotics (P < 0.0001). Immunoglobulins (IgA and IgG) were significantly enhanced by supplementation of probiotics when compared to low-energy diet group. Conclusively, probiotics in low-energy diet significantly enhanced rabbit growth, and serum immunity while improving lipid profiles and supporting liver and kidney functions. This supplementation strategy promoted both performance and overall health during the fattening period.
Introduction
Researchers have been seeking to identify harmless and effective feed additives to boost feed efficiency, production, and economic viability without compromising animal health to address the challenges posed by feed shortages that impede animal output. Probiotics are a medication that may lessen the risk of contamination in birds (1). A variety of probiotics are employed in avian nutrition, like Lactobacillus and Bifidobacteria (2, 3), Lactobacillus strains (4, 5), and Saccharomyces cerevisiae (6). Probiotics have demonstrated the ability to enhance feed conversion ratios and promote weight gain (7, 8), decrease mortality rates (9), mitigate disease infections (10), and stimulate the immune system (11).
Spirulina is a widely utilized natural feed additive that functions as a probiotic and comprises bioactive elements. It is safe and cost-effective as a nutritional supplement for people as well as livestock. The SP is utilized as a protein source for human and animal consumption, comprising 60–70% crude protein, all necessary amino acids, and highly digestible minerals (12). It also comprises approximately 1.3–15% important fatty acids (FAs), including myristic, palmitic, oleic, and gamma-linolenic acids, which offer numerous health benefits. Additionally, it serves as a substantial supply of pro-vitamin A and antioxidant qualities (redox status). Recent studies have demonstrated that including microalgae in the diets of rabbits (13), chicks (14, 15), quails (16, 17), various fish species (18, 19), and ruminants (20, 21) enhance feed intake, nutritional digestibility, and overall productive performance.
Yeast, such as Saccharomyces cervisiae, has served as an animal feed additive for years. The supplementation of yeast to livestock diets has been revealed to enhance the feed nutritional value and the animal’s productivity (22). Additionally, yeast and mannan oligosaccharides, as well as fructo-oligosaccharides sourced from the cell wall of yeast, have revealed the capacity to suppress enteric pathogens and alter immune responses (23, 24). It was hypothesized that adding biological feed additives to the low-energy diet of fattening rabbits could improve their productive performance and physiological and health status. The current work targeted growing rabbits to study the influence of biological feed additives in the low-energy diet on numerous parameters, including productive parameters, lipid profile, renal function, hepatic function, immunoglobulin levels, and antioxidant status.
Materials and methods
Tested probiotics
Saccharomyces cervisiae extract: An extract of Saccharomyces cervisiae was prepared utilizing a method delineated by Abdel-Rahim et al. (25), which facilitated the efficient growth and proliferation of yeast cells (dry yeast at 100 g/L) under optimal aerobic and nutritional conditions conducive to the synthesis of novel valuable substances such as amino acids, carbohydrates, sugars, proteins, fatty acids, hormones, etc. Subsequently, these substances were liberated from the yeast cells in readily accessible forms through 2 cycles the 1st is freezing followed by thawing to disrupt the yeast cells and release their contents. Spirulina platensis extract: The spirulina isolate was obtained from Soda Lake in Wadi el Natrun, Behera governorate, Egypt. S. platensis extract was prepared according to Alghamdi et al. (26). Bifidobacterium: Bifidobacterium (1 × 107 CFU) was obtained from Agricultural Microbiology, Agriculture Faculty, Zagazig University, Egypt.
Experimental design, animals and diets
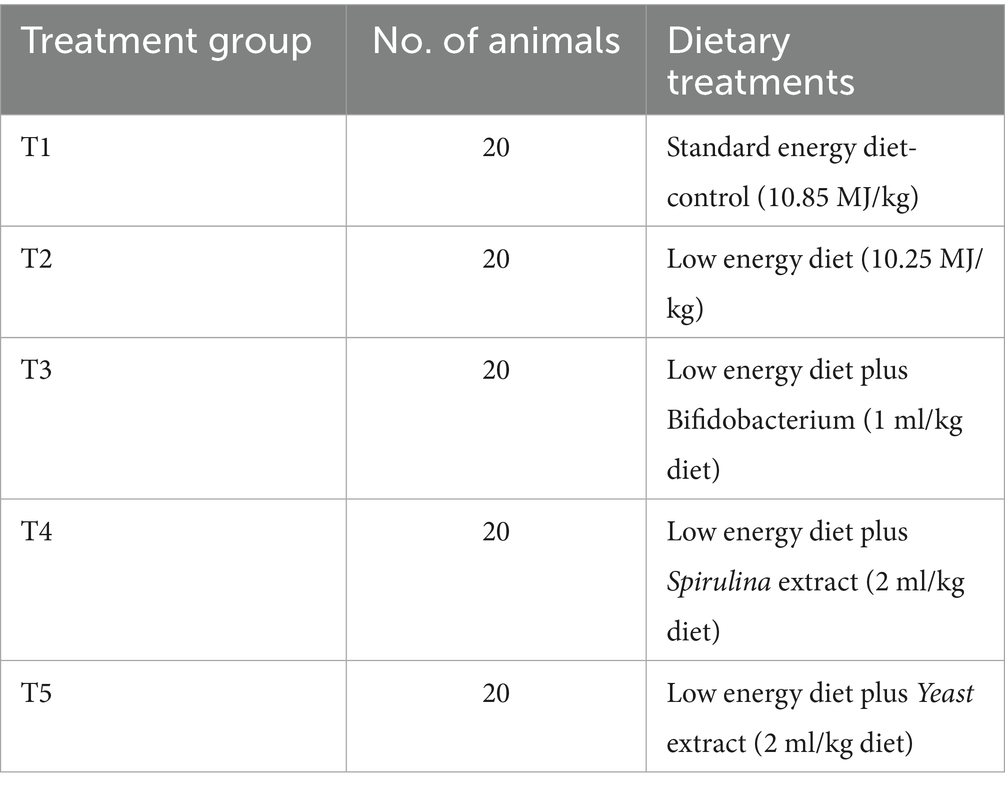
Five different experimental treatments were used in a completely randomized design involving 100 Japanese quail male New Zealand White rabbits, aged 5 weeks. Each treatment comprised ten replications, with each replicate consisting of two rabbits. The experimental design is shown in Table 1.
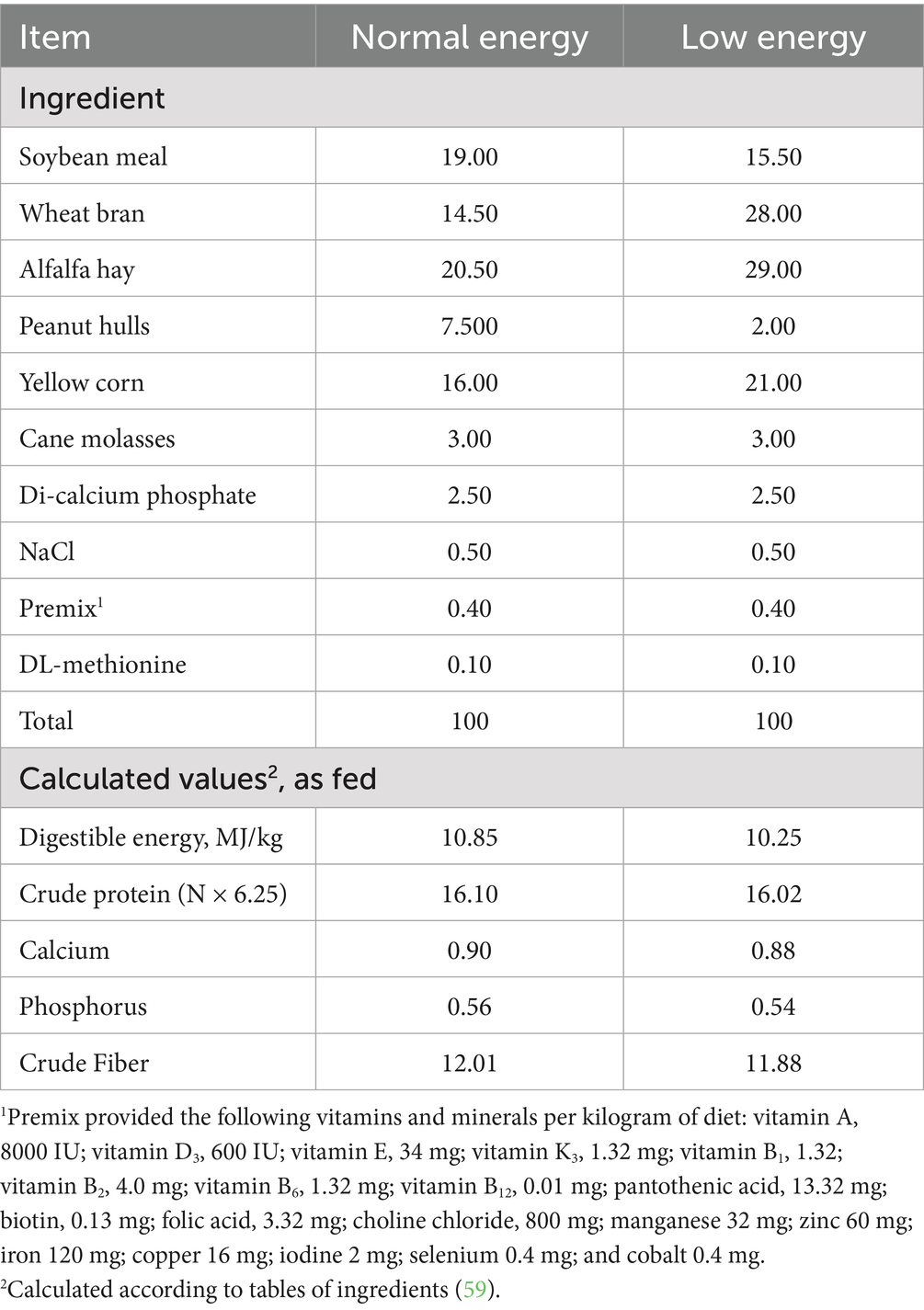
The doses of biological feed additives have been selected according to Mancini and Paci (27). Energy intake on the low-energy diet was reduced by 5.50% compared to the standard energy diet (control group). The cage was supplied with an auto nipple waterer and manual galvanized metal feeders, and its dimensions were 35 × 40 × 60 cm to ensure a constant supply of fresh and clean water and feed. Rabbits were reared under the same management, environmental, and hygienic conditions. Before the trial began, the rabbits were allotted 1 week for acclimatization. The basal feeds were fully pelleted and prepared to meet the necessary nutrient requirements for fattening rabbits, as per NRC (28) (Table 2). The chemical analysis and formulation of the basal feeds administered to rabbits were detailed in the ethics statement, which complies with the rules established by the Ethics Committee of the Egyptian Research for the Utilisation and Welfare of Laboratory Animals at Zagazig University (ZU-IACUC/2/F/313/2023).
Growth performance measurements
The research was extended for 8 weeks from 5 to 13 weeks. The rabbits were weighed separately at 5, 7, 9, 11, and 13 weeks of age (29). At the conclusion of each cycle, the feed intake (FI) of the fattening rabbits was recorded. Consequently, body weight gains (BWG), feed intake (FI), and feed conversion ratio (FCR) were computed (30).
Carcass traits
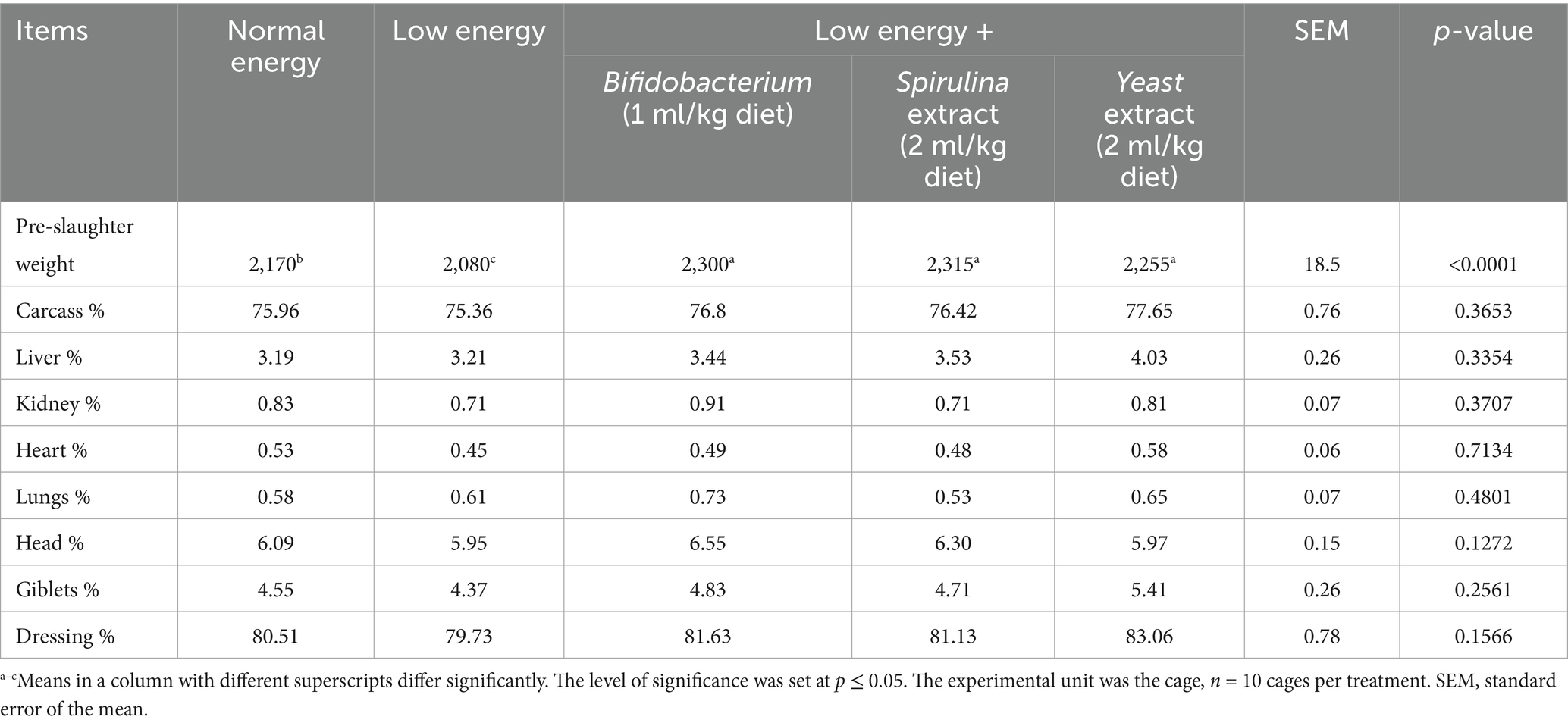
At 13 weeks of age, 5 rabbits from each trial group were randomly taken, weighed, and slaughtered to evaluate the carcass characteristics. The carcass, liver, kidney, heart, lungs, spleen, and abdominal fat weights were documented and expressed as a % of the pre-slaughter weight. The dressing percentage and giblets were measured.
Blood sampling and analysis
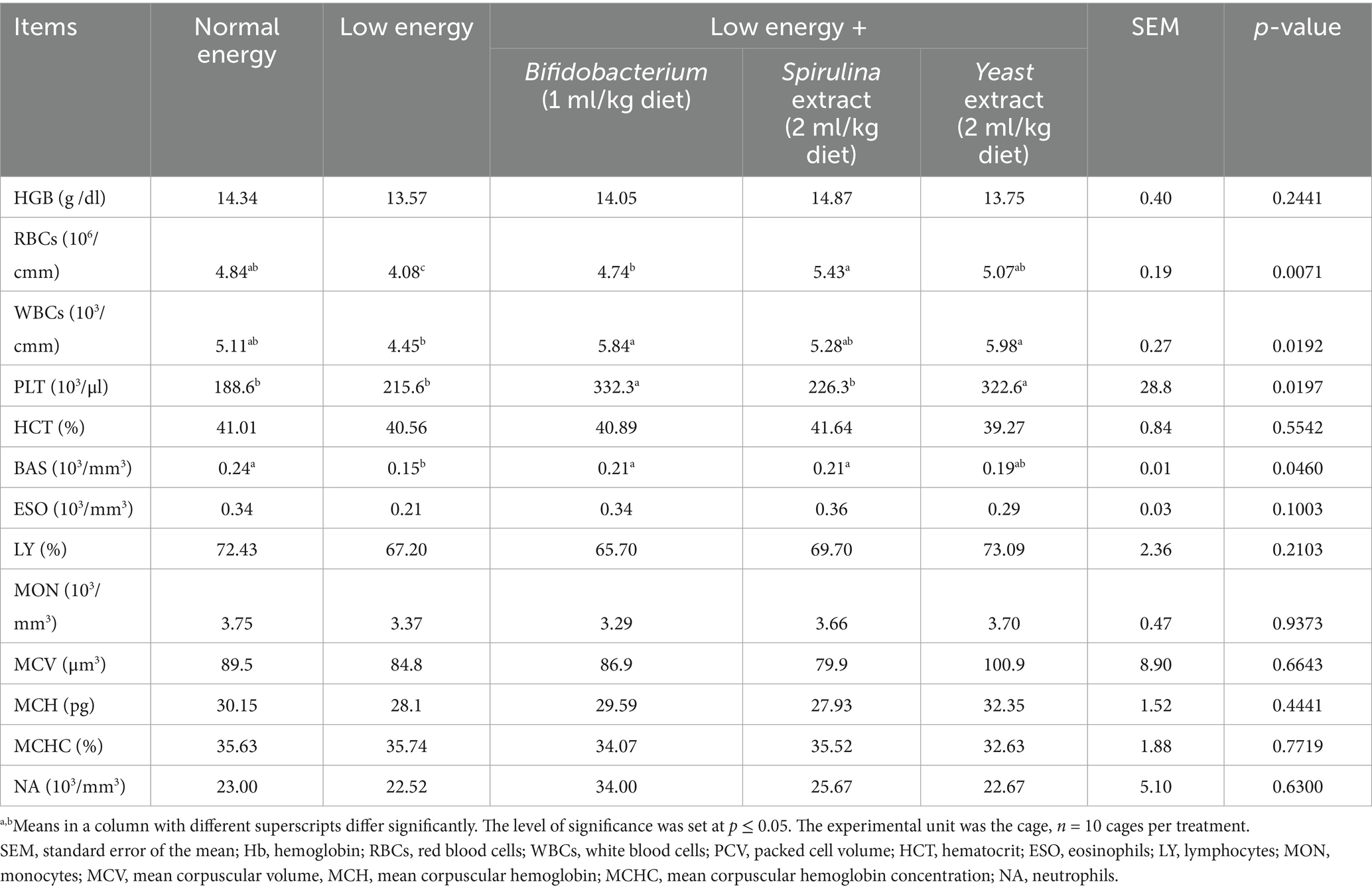
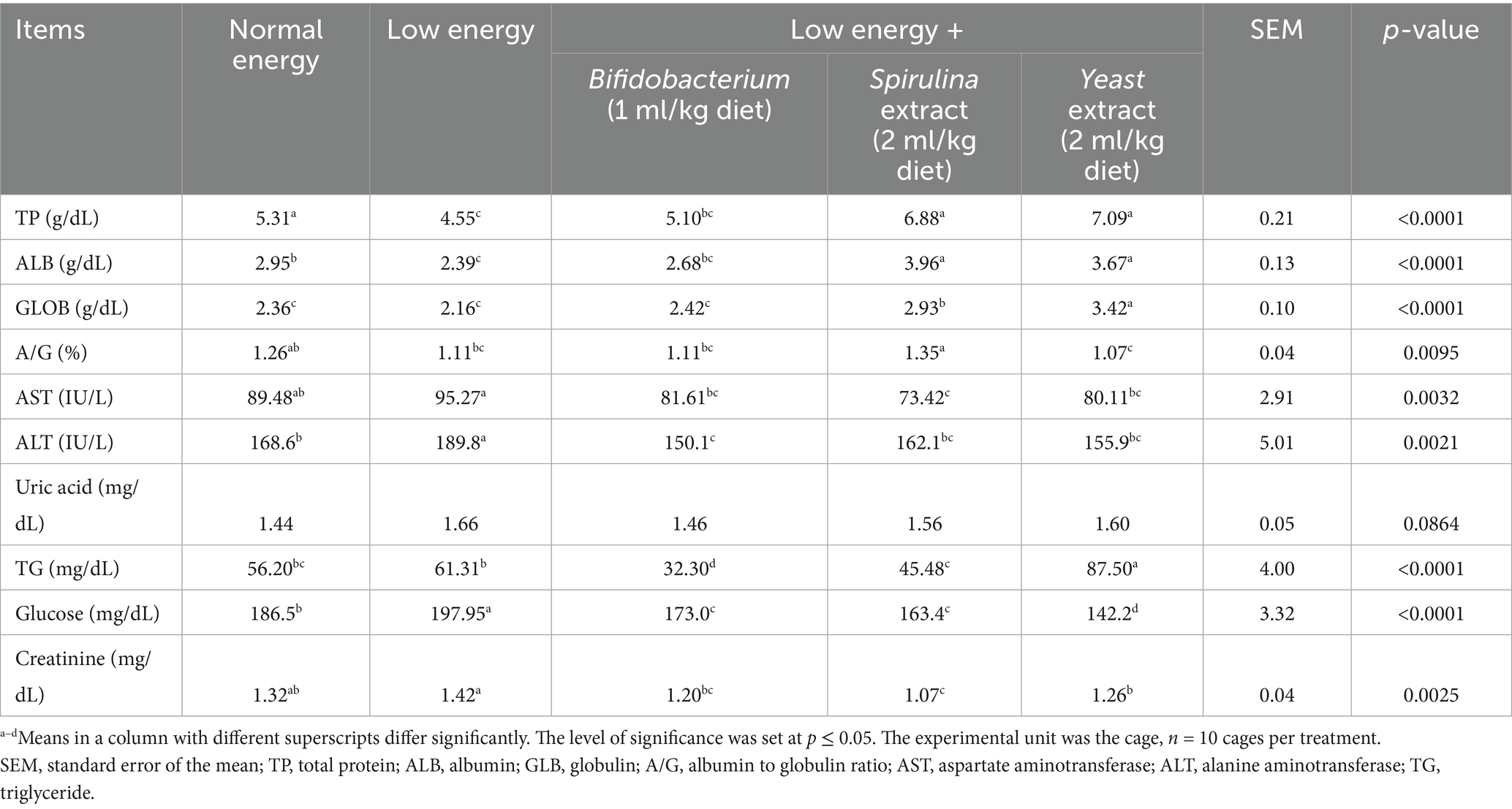
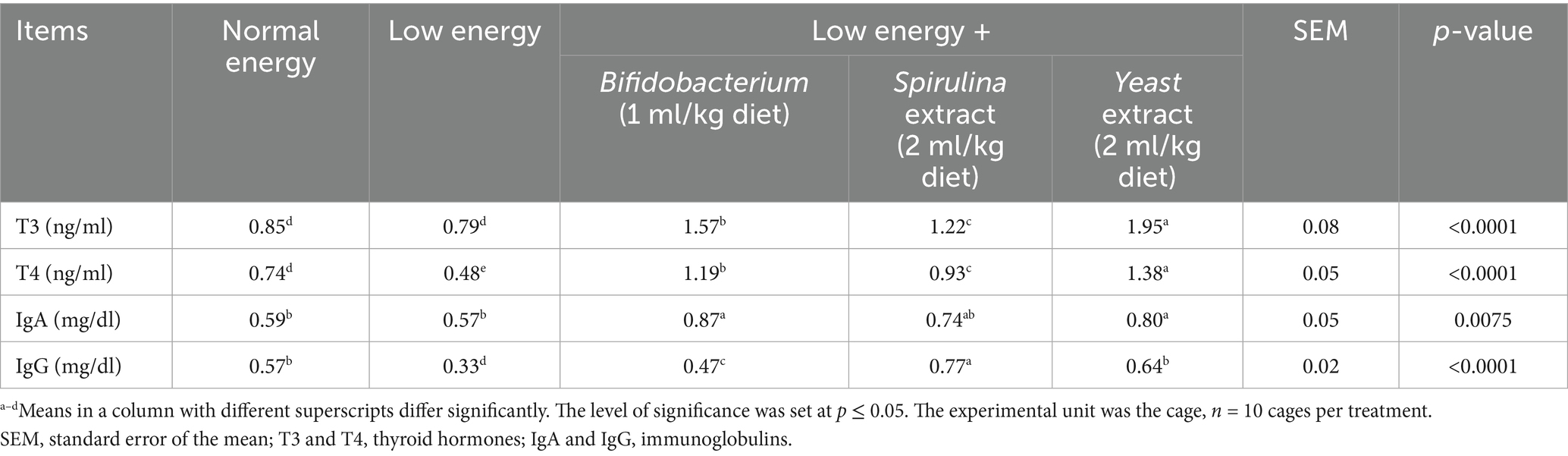
At 13 weeks of age, 5 rabbits from each group were used for blood sampling. Two blood samples per animal were collected from rabbits during slaughter. The initial sample was collected in a sterile tube containing an anticoagulant (heparin) for the assessment of hematological parameters in whole blood, as per Brooks et al. (31), utilizing an automated hematology analyzer (Hospitex Hema Screen 18, Sesto Fiorentino, Italy). The 2nd sample was collected, permitted to coagulate, and subsequently centrifuged for 15 min at 1507 g to get serum samples, which were then kept at −20°C until biochemical analysis. The hematological parameters assessed included hemoglobin (Hb, g/dL), red blood cells (RBCs, 106/mm3), white blood cells (WBCs, 106/mm3), platelets, packed cell volume (PCV, %), hematocrit (HCT), eosinophils (ESO), lymphocytes (LY), monocytes (MON), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and sodium (Na). The serum concentrations of albumin (g/dL), total protein (TP g/dL), globulin (GLB g/dL), uric acid (mg/dL), creatinine (mg/dL), and the concentrations of aspartate aminotransferase (AST), and alanine aminotransferase (ALT) (IU/L) enzymes and triglyceride (TG) were quantified utilizing commercial kits (Diamond Diagnostic Company, Giza, Egypt), in accordance with the manufacturer’s guidelines. The serum globulin was determined by subtracting albumin from total protein (globulin = TP - albumin). Thyroid hormones (T3, T4) and immunoglobulins (IgA, IgG mg/mL) were quantified utilizing commercial ELISA kits in accordance with the makers’ directions.
Statistical analysis
The one-way ANOVA test was employed to examine the data with the subsequent statistical model: Yij = Gi + μ + eij.
In this context, Yij denotes the observation, Gi represents the treatment effect (T1:T5), μ indicates the general mean, and eij refers to the experimental error. Mean differences were assessed using Tukey’s test. The level of p < 0.05 was established as the limit for statistical significance. Statistical analyses were done utilizing SPSS (32).
Results
Performance parameters
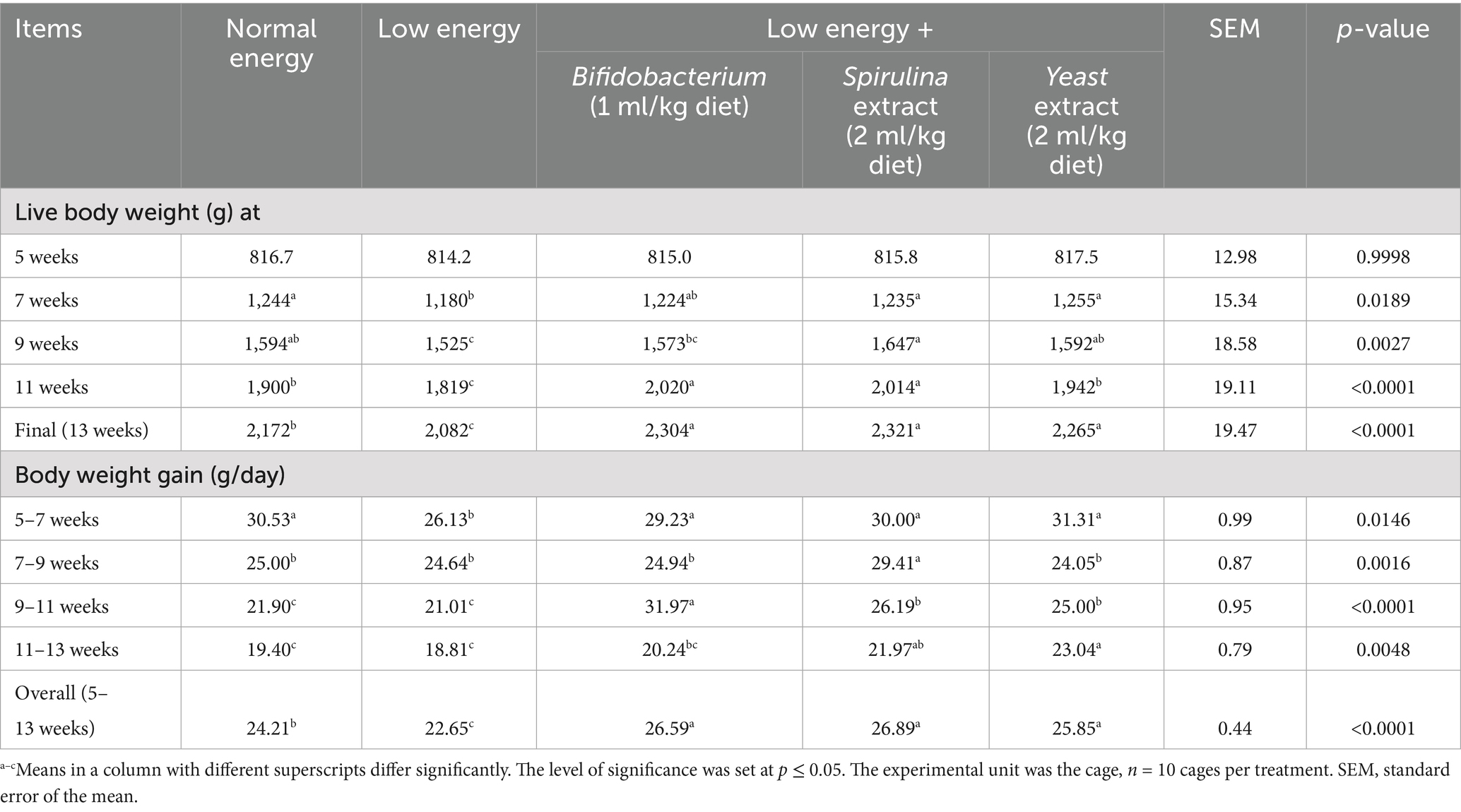
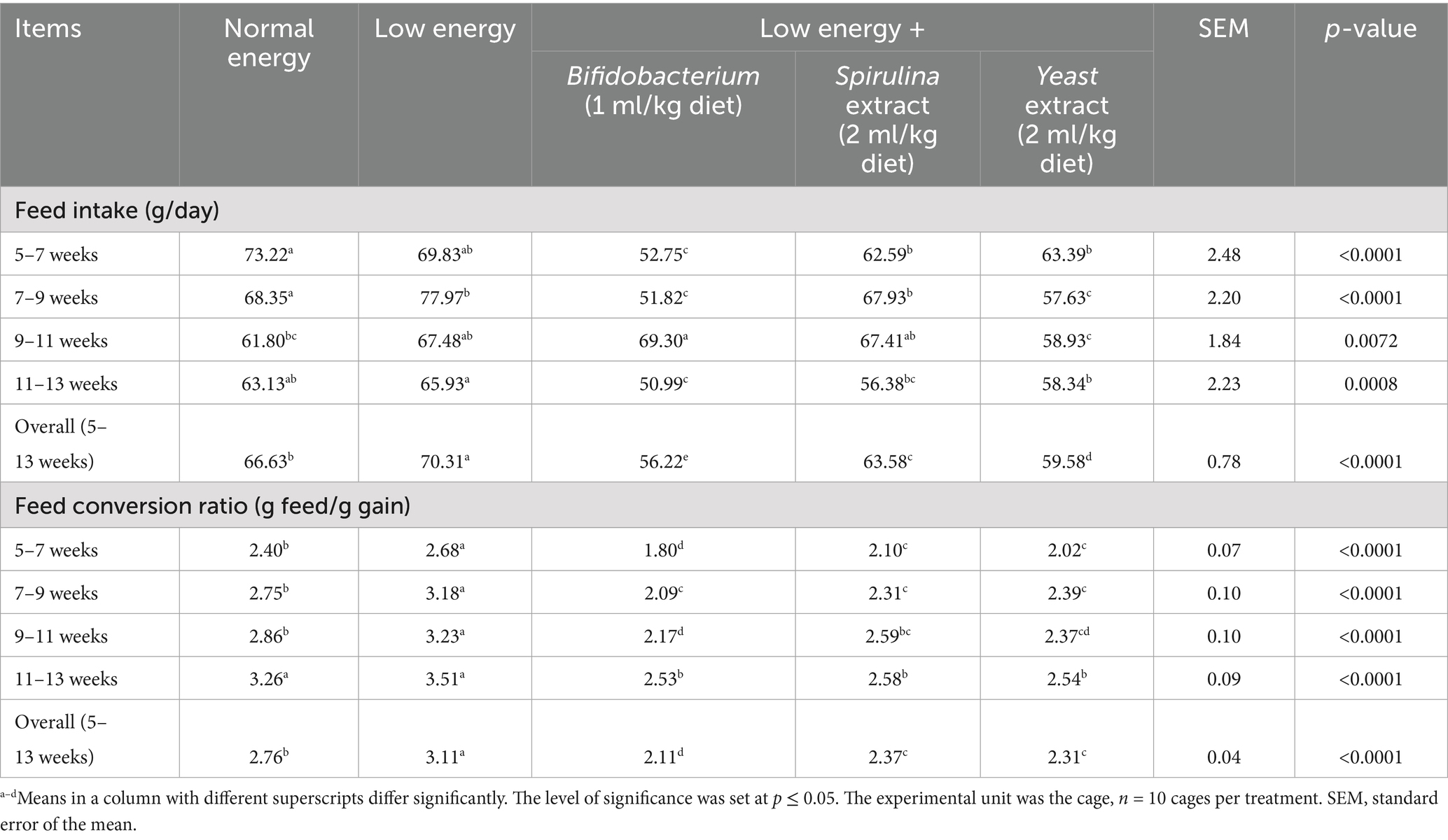
The influence of nutritional supplements with probiotics on the growth parameters of growing rabbits is displayed in Table 3. The outcomes displayed a substantial increase (p < 0.05) in final live body weight and weight gain with probiotic supplementation in low-energy diets, and the group supplemented with Spirulina extract displayed the augmented final body weight and body weight gain (2321.67 and 26.89 g) at 13 week and overall (5–13 weeks). Moreover, the feed utilization of fattening rabbits supplied a low-energy diet and treated with biological feed additives revealed a substantial reduction (p < 0.05) in feed intake with the supplementation. The group supplemented with Bifidobacterium (1 ml/kg diet) displayed a decreased feed intake (56.22 g); in contrast, the group fed low-energy feeds without any supplementation displayed an increased feed intake (70.31 g) along with a trial period of 5–13 weeks. Furthermore, the feed conversion ratio substantially improved (p < 0.05) with biological feed additives administration, and the group supplemented with Bifidobacterium (1 ml/kg diet) displayed improved feed conversion ratio (2.11); in contrast, the group fed low-energy diets without supplementation displayed the worst feed conversion ratio (3.11) along with a trial period (5–13) week as shown in Table 4.
Carcass criteria
Table 5 displays the carcass traits of fattening rabbits treated with a low-energy diet and probiotics. The data revealed a nonsignificant variation (p > 0.05) in carcass criteria with the administration of biological feed additives, especially in the carcass, liver, kidney, heart, lung, head, giblets, and dressing percentage.
Blood hematology
The hematological parameters of growing rabbits treated with biological feed additives are illustrated in Table 6. The finding presented a non-substantial increase in Hb (p = 0.2441) with the administration of probiotics. The group treated with Spirulina extract (2 ml/kg diet) displayed the highest results (14.87), followed by Bifidobacterium (1 ml/kg diet) (14.05), while the energy-fed group demonstrated the lowest results (13.57). Moreover, the findings displayed a substantial variation (p < 0.05) in RBCs, WBCs, PLT, and BAS. The group treated with Spirulina extract (2 ml/kg diet) displayed the highest RBCs levels (5.43), while the group treated with Yeast extract (2 ml/kg diet) displayed increased WBCs levels (5.98). In contrast, the group treated with a low-energy diet revealed decreased RBCs and WBCs levels (4.08 and 4.45). The other hematological parameters, such as HCT, ESO, LY, MON, MCV, MCH, MCHC and NA, presented a non-significant variation (p > 0.05) with supplementation with probiotics.
Serum biochemistry
Dietary supplementation of growing rabbits with probiotics significantly affected (p < 0.05) liver and kidney function tests, as illustrated in Table 7. The results presented a significant increase in TP and GLOB (p < 0.0001, p < 0.0001) in the group supplemented with Yeast extract (2 ml/kg diet) (7.09 and 3.42 g/dL) in comparison to the low energy supplemented group (4.55 and 2.16 g/dL). Furthermore, the Spirulina extract (2 ml/kg diet) treated group displayed a substantial increase (p < 0.0001, p = 0.0095) in ALB and A/G % (3.96 and 1.35 g/dL) in comparison to the low energy supplemented group (2.39, 1.11 g/dL). Additionally, AST and ALT significantly decreased (p = 0.0032, p = 0.0021) with the supplementation of biological feed additives. Spirulina extract (2 ml/kg diet) treated group revealed the AST decreased levels (73.42 IU/L), whereas the Bifidobacterium (1 ml/kg diet) treated group revealed the ALT decreased levels (150.12 IU/L). Uric acid serum levels displayed a non-significant decrease (p = 0.0864) with treatment and the Bifidobacterium (1 ml/kg diet) treated group revealed the decreased levels (1.46 mg/dL). In contrast, creatinine levels displayed a significant (p = 0.0025) reduction with treatment, and the Spirulina extract (2 ml/kg diet) treated group revealed the reduced levels (1.07 mg/dL) relative to the low energy and normal energy treated groups.
Growth hormones and immunity
Dietary treatment of growing rabbits with probiotics meaningfully increased growth hormone (T3 and T4) levels and improved immunity (IgA and IgG) (p < 0.0001), as shown in Table 8. Yeast extract (2 ml/kg diet) treated group substantially (p < 0.0001, p < 0.0001) revealed the boosted T3 and T4 levels (1.95 and 1.38). Furthermore, the Bifidobacterium (1 ml/kg diet) treated group expressively (p = 0.0075) displayed improved IgA levels (0.87), whereas the Spirulina extract (2 ml/kg diet) treated group significantly (p < 0.0001) displayed improved IgG levels (0.77) relative to low and normal energy treated groups.
Discussion
This study examined the positive effects of dietary probiotic supplementation, such as Bifidobacterium, Spirulina extract, and Yeast extract, in the diets of growing rabbits. It demonstrated that food supplementation with yeast yielded beneficial impacts on growth efficacy and health condition. The results established the former findings of several other researchers (33, 34). Ezema and Eze (35) recommended that an additional concentration of 0.12 g of yeast per kg of feed may promote weight gain in the rabbits. The improved growth parameters in rabbits attributed to yeast-containing feed supplements may be due to improved digestion and absorption of feed ingredients (36). It is hypothesized that certain advantages in the growth efficiency of rabbits may result from the beneficial effects of yeast on gastrointestinal health, namely through the enhancement of villus height. Zhang et al. (37) proposed that these results could elucidate the growth-enhancing impact of yeast cell wall constituents on gastrointestinal tract shape. Priya and Babu (38) proved that the incorporation of Saccharomyces cervisiae into diets will alter feed digestion, hence improving growth performance. Soliman et al. (39) noted that rabbits consuming a yeast-treated diet achieved markedly greater market weight, increased weight gain, and showed optimal feed conversion efficiency. Conversely, Foad (40) determined that the supplementation of yeast had no impact on body weight (BW), body weight gain (BWG), and feed conversion ratio (FCR) in rabbits.
There is some agreement between the present results and those published by Attia et al. (33), who noted nonsignificant variations in the carcass characteristics of rabbits attributable to dietary yeast administration or mannan oligosaccharides. Shehata et al. (36) reported that the dressing percentage of growing New Zealand White rabbits improved when supplemented with Saccharomyces cervisiae. Our findings contradict those of Khanna et al. (34), who stated that the average weights of rabbit carcasses’ fore and hind parts were considerably advanced in yeast-supplemented groups.
The hematology profile offers important findings on the general health of animals. The administration of diverse biological feed additives did not affect blood health, except hemoglobin (Hb), red blood cell (RBC), and white blood cell (WBC) count. Rabbits consuming diets with biological feed additives such as Bifidobacterium, Spirulina extract, or Yeast extract exhibited elevated hemoglobin levels relative to those on a low-energy diet, suggesting that these substances help mitigate anaemia caused by a low-energy diet in rabbits. Özsoy and Yalçin (41) and Belhassen et al. (42) found that adding active yeast to the feed does not impact the blood parameters of growing rabbits. Attia et al. (33) found similar findings and concluded that dietary supplementation of zinc-bacitracin and mannan oligosaccharides did not substantially modify the hematological characteristics of growing rabbits.
The current finding displayed a significant enhancement (p < 0.05) in hepatic and renal function in growing rabbits as an increase in total protein, albumin, and globulin and a decrease in ALT, AST, uric acid, and creatinine with biological feed additive treatments, either Bifidobacterium or Spirulina extract, or Yeast extract. These findings align with the outcome of (43), who reported that the elevation in Spirulina concentrations in the T3 and T4 groups in rabbits culminated in considerable (p < 0.05) higher serum albumin and substantially minor plasma globulin, urea, creatinine, and ALT concentrations than those in the 2nd and 1st groups. In addition, SP supplementation led to increased levels of TP and ALB, ascribed to its elevated levels of protein, complete amino acids, vitamins, minerals, lipids, and other components (44). However, the decrease in serum GLB level with increasing SP supplementation may be due to the growth inhibition of harmful intestinal bacteria, which reduces inflammatory secretions and globulin synthesis in the liver and other tissues (45). Moreover, the favorable effects of SP addition on serum creatinine and urea levels are related to its functional role as a good influence on feed utilization and kidney activities. Similarly, Ribeiro et al. (46) observed comparable results with rabbits fed SP alone or with yeast diets. The results of AST and ALT are supported by previous research that revealed that SP might work as a preventive agent against liver dysfunctions (47) possibly due to its high contents of different vital nutrients that have numerous positive effects on health. In contrast, some studies found no significant variations in ALT and AST with the addition of zinc or selenium-enriched Spirulina (48) to the growing rabbit diets. However, Hassanein et al. (49) stated that Spirulina and Chlorella vulgaris supplementation in rabbit diets did not affect blood parameters. Additionally, Spirulina can activate the immune system due to its rich content of vital organic components, like all amino acids, omega-3 PUFAs, and essential minerals and vitamins for regulating body functions (50, 51). It contains natural active substances that have the potential to function as potent antioxidants, anti-inflammatories, and antiviral agents (52).
The treatment significantly influenced thyroid function, as evidenced by T3 and T4 concentrations in the rabbit’s blood serum. Supplemented rabbits exhibited elevated levels of T3 and T4 when administered Bifidobacterium, Spirulina extract, or Yeast extract. These findings suggest that the treatments substantially impacted the metabolic hormones of the thyroid gland, indicating typical thyroid action in the supplemented groups. This aligns with the findings of Ansorge et al. (53), who proposed that Propolis exerts an anabolic effect akin to the increase in T3 and T4 observed in groups receiving biological feed additives. Moreover, the present finding demonstrated a substantial enhancement (p < 0.05) in immunity parameters (IgA and IgG) in growing rabbits with biological feed additive treatments, either Bifidobacterium or Spirulina extract or Yeast extract. These outcomes align with (54), who described that the administration of SP simulated cytokines, antibodies, and the mobilization of T and B cells. In addition, supplementing SP has been shown to improve most of the immunological parameters of stressed doe rabbits more than any other supplements, either vitamin E or their combination with SP or the control ones El-Ratel et al. (13). However, Mahmoud et al. (55) noted that substituting soybean meal with SP at 20, 40, and 60% in growing rabbit diets exhibited no substantial impact on the immunological parameters. Furthermore, nourishing doe rabbits SP diets improved their productive and reproductive performance and economic efficiency. Our results coordinate with those of Hassanein et al. (49) and (43). A factual demonstration that, under the impact of dietary probiotic supplementation, the healthier gut environment of animals (with its better-balanced microbiota and strengthened mucosa) can function more efficiently was provided by Park et al. (56), who found feed digestion to be enhanced in animals fed probiotic-supplemented diets for 35 days. Besides the exertion of a positive effect on gut health, dietary supplementation of probiotic has been reported to enhance animals’ general health (57, 58).
Conclusion
The study demonstrates that biological feed additives in low-energy diets effectively boost the well-being and growth performance of rabbits throughout the fattening stage. The significant improvement in final body weight, feed intake, liver and kidney functions, and immunological markers highlights the potential of these additives to optimize rabbit production. Overall, the findings support the use of biological feed additives as a beneficial strategy for improving rabbit welfare and productivity in low-energy feeding systems.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal studies were approved by the Ethics Committee of the Egyptian Research for the Utilisation and Welfare of Laboratory Animals at Zagazig University (ZU-IACUC/2/F/313/2023). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
MS: Writing – original draft, Writing – review & editing, Investigation, Methodology. AhA: Writing – review & editing, Writing – original draft. UAM: Writing – review & editing, Writing – original draft, Conceptualization, Supervision. SB: Supervision, Writing – original draft, Writing – review & editing. BK: Supervision, Writing – review & editing, Writing – original draft. AS: Writing – review & editing, Writing – original draft. AbA: Writing – original draft, Writing review & editing. HR: Writing – original draft, Writing – review & editing. AE: Writing – review & editing, Writing – original draft, Funding acquisition. KA: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2501).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rashid, S, Alsayeqh, AF, Akhtar, T, Abbas, RZ, and Ashraf, R. Probiotics: alternative of antibiotics in poultry production. Int J Vet Sci. (2023) 12:45–53. doi: 10.47278/journal.ijvs/2022.1752023
2. Salah, AS, Ahmed-Farid, OA, and El-Tarabany, MS. Carcass yields, muscle amino acid and fatty acid profiles, and antioxidant indices of broilers supplemented with synbiotic and/or organic acids. J Anim Physiol Anim Nutr. (2019) 103:41–52. doi: 10.1111/jpn.12994
4. Lan, PTN, Le, TB, and Benno, Y. Impact of two probiotic Lactobacillus strains feeding on fecal lactobacilli and weight gains in chicken. J Gen Appl Microbiol. (2003) 49:29–36. doi: 10.2323/jgam.49.29
5. Mehmood, A, Nawaz, M, Rabbani, M, and Mushtaq, MH. Probiotic effect of Limosilactobacillus fermentum on growth performance and competitive exclusion of Salmonella gallinarum in poultry. Pak Vet J. (2023) 43:659–64. doi: 10.29261/pakvetj/2023.103
6. Lila, ZA, Mohammed, N, Yasui, T, Kurokawa, Y, Kanda, S, and Itabashi, H. Effects of a twin strain of Saccharomyces cerevisiae live cells on mixed ruminal microorganism fermentation in vitro. J Anim Sci. (2004) 82:1847–54. doi: 10.2527/2004.8261847x
7. Alagawany, M, Elewa, MS, Abou-Kassem, DE, Ismail, TA, Salah, AS, Madkour, M, et al. Effect of parsley (Petroselinum crispum) oil as feed additive on broiler performance, carcass, liver and kidney functions, antioxidant, lipid profile, and immunity. Anim Sci J. (2024) 95:e13981. doi: 10.1111/asj.13981
8. Salah, AS, El-Tarabany, MS, and Ali, MA. Impact of dietary supplementation with a synbiotic, organic acids or their combination on growth performance, carcass traits, economic efficiency, jejunum histomorphometry and some blood indices of broiler chickens. Anim Prod Sci. (2018) 59:1318–26. doi: 10.1071/AN18156
9. Jin, LZ, Ho, YW, Abdullah, N, and Jalaludin, S. Probiotics in poultry: modes of action. World Poult Sci J. (1997) 53:351–68. doi: 10.1079/WPS19970028
10. Yu, H, Rahman, A, Sadique, MA, Batool, T, Imtiaz, B, Zaman, MA, et al. Impact of Bacillus subtilis probiotic on growth performance, bone health, intestinal morphology, and cecal microbiota in cobb broiler chicks. Pak Vet J. (2024) 44:1243–8. doi: 10.29261/pakvetj/2024.254
11. Raza, A, Abbas, RZ, Karadağoğlu, Ö, Raheem, A, Khan, AMA, Khalil, MZ, et al. Role of probiotics in increasing meat and egg production in poultry: a review. Kafkas Univ Vet Fak Derg. (2024) 30:753–60. doi: 10.9775/kvfd.2024.32861
12. Hassan, MAE, Shehabeldin, AM, Omar, MEA, Khalil, WA, Swelum, AA, Lu, Y, et al. Effect of Spirulina nanoparticles or selenium coated Spirulina nanoparticles supplemented to freezing extender on bull sperm freezability. Pak Vet J. (2023) 43:739–47. doi: 10.29261/pakvetj/2023.111
13. El-Ratel, IT. Reproductive performance, oxidative status and blood metabolites of doe rabbits administrated with Spirulina alga. Egypt Poult Sci J. (2017) 37:1153–72.
14. Salah, AS, El-Tarabany, MS, Mostafa, M, Zaki, RS, Azzam, MM, El Euony, OI, et al. Impact of dietary Spirulina on performance, antioxidant status, carcass traits and pathological alteration in broilers exposed to ochratoxin a stress. Front Vet Sci. (2025) 11:1532353. doi: 10.3389/fvets.2024.1532353
15. Salah, AS, Lestingi, A, El-Tarabany, MS, Mostafa, M, Zaki, RS, Azzam, MM, et al. Effect of Spirulina supplementation on growth, immunity, antioxidant status and pathomorphological perspectives in broilers exposed to dietary aflatoxin B1. J Appl Poult Res. (2025) 34:100519. doi: 10.1016/j.japr.2025.100519
16. Abd Elzaher, HA, Ibrahim, ZA, Ahmed, SA, Salah, AS, Osman, A, Swelum, AA, et al. Growth, carcass criteria, and blood biochemical parameters of growing quails fed Arthrospira platensis as a feed additive. Poult Sci. (2023) 102:103205. doi: 10.1016/j.psj.2023.103205
17. Mohamed, SM, Alagawany, M, El-Kholy, MS, El-Mekkawy, MM, Salah, AS, Attia, YA, et al. Effect of dietary microalgae on growth performance and health in meat-type quails. Poult Sci. (2025) 104:104709. doi: 10.1016/j.psj.2024.104709
18. Abozaid, H, Elnady, A, Aboelhassan, DM, Mansour, H, Abedo, A, Ghaly, IS, et al. Impact of Spirulina platensis as a dietary supplement on growth performance, blood biochemical parameters, and expression of growth-related genes in Nile tilapia (Oreochromis niloticus). Egypt J Vet Sci. (2023) 54:1265–77. doi: 10.21608/ejvs.2023.250660.1678
19. Ahmed, BS. Nutritional effects of dietary Spirulina (Arthrospora platensis) on morphological performance, hematological profile, biochemical parameters of common carp (Cyprinus carpio L.). Egypt J Vet Sci. (2023) 54:515–24. doi: 10.21608/ejvs.2023.191557.1441
20. Gaafar, HM, Riad, WA, Elsadany, AEGY, El-Reidy, KF, and Abu El-Hamd, MA. Effect of Spirulina (Arthrospira platensis) on productive and reproductive performance of Friesian cows. Egypt J Agric Res. (2017) 95:893–911. doi: 10.21608/ejar.2017.148904
21. Hassanein, HA, Youssef, HF, Mahrous, AA, Hafez, YH, Phillip, YL, and Gaballah, MM. Effect feeding of supplemented ration with algae on milk yield and its composition for Damascus goats. Egypt J Nutr Feeds. (2015) 18:65–75. doi: 10.21608/ejnf.2015.104671
22. Elghandour, MM, Abu Hafsa, SH, Cone, JW, Salem, AZ, Anele, UY, and Alcala-Canto, Y. Prospect of yeast probiotic inclusion enhances livestock feeds utilization and performance: an overview. Biomass Convers Biorefinery. (2024) 14:2923–35. doi: 10.1007/s13399-022-02562-6
23. Anwar, MI, Tahir, MA, Awais, MM, Raza, A, Akhtar, M, Muhammad, F, et al. Purification, characterization and protective effects of indigenous yeast derived β-glucans against salmonellosis in broilers. Pak Vet J. (2023) 43:297–302. doi: 10.29261/pakvetj/2023.013
24. Li, GQ, Wang, XZ, Liu, Y, Yang, YZ, Wang, C, Gong, SM, et al. The effect of Bacillus subtilis and fructooligosaccharide as antibiotic substituent on goose performance parameters, serum biochemical indicators and intestinal morphology. Kafkas Univ Vet Fak Derg. (2023) 29:247–54. doi: 10.9775/kvfd.2023.29134
25. Abdel-Rahim, EA, Shallan, MA, and El-Scheik, AM. Biochemical studies on production of new thermophilic yeast alkaline proteases applied for the purposes of laundry detergents industry. J Agric Sci Mansoura Univ. (1988) 21:1971–85.
26. Alghamdi, MA, Reda, FM, Mahmoud, HK, Bahshwan, SM, Salem, HM, Alhazmi, WA, et al. The potential of Spirulina platensis to substitute antibiotics in Japanese quail diets: impacts on growth, carcass traits, antioxidant status, blood biochemical parameters, and cecal microorganisms. Poult Sci. (2024) 103:103350. doi: 10.1016/j.psj.2023.103350
27. Mancini, S, and Paci, G. Probiotics in rabbit farming: growth performance, health status, and meat quality. Animals (Basel). (2021) 11, 11:3388. doi: 10.3390/ani11123388
28. NRC. Nutrient requirements of rabbits. 2nd Revised ed. Washington, DC. USA: National Academy of Sciences (1977).
29. Khalifa, WH, Sallam, MG, Kamel, NN, Samy, A, Yassein, SA, El-Mallah, GM, et al. Using in-ovo injection of olive pulp extract and vitamin C to improve hatchability, post hatch growth performance, carcass traits and some biochemical blood analysis in broiler chickens. Int J Vet Sci. (2023) 12:353–9. doi: 10.47278/journal.ijvs/2022.200
30. Susalam, MK, Harnentis,, Marlida, Y, Jamsari,, and Ardani, LR. The effect of probiotics consortium isolated from fermented fish (Budu) on broiler performances and meat quality. Int J Vet Sci. (2024) 13:100–7. doi: 10.47278/journal.ijvs/2023.066
31. Brooks, M. B., Harr, K. E., Seelig, D. M., Wardrop, K. J., and Weiss, D. J., editors. (2022). Schalm's veterinary hematology. Wiley Online Library.
33. Attia, YA, Hamed, RS, El-Hamid, A, Al-Harthi, MA, Shahba, HA, and Bovera, F. Performance, blood profile, carcass and meat traits and tissue morphology in growing rabbits fed mannanoligosaccharides and zinc-bacitracin continuously or intermittently. Anim Sci Paper Rep. (2015) 33:85–101.
34. Khanna, S, Gulati, HK, Verma, AK, Sihag, SS, Sharma, DP, and Kapoor, PK. Effect of yeast supplementation and alternative housing systems on performance of rabbits. Haryana Vet. (2014) 53:23–27.
35. Ezema, C, and Eze, DC. Determination of the effect of probiotic (Saccharomyces cerevisiae) on growth performance and hematological parameters of rabbits. Comp Clin Pathol. (2012) 21:73–6. doi: 10.1007/s00580-010-1066-6
36. Shehata, SA, Mahrose, KM, and Ismail, EI. Effect of amino yeast addition on growth performance, digestion, carcass traits and economic efficiency of growing rabbit. Egypt J Nutr Feed. (2012) 15:75–80.
37. Zhang, AW, Lee, BD, Lee, SK, Lee, KW, An, GH, Song, KB, et al. Effects of yeast (Saccharomyces cerevisiae) cell components on growth performance, meat quality, and ileal mucosa development of broiler chicks. Poult Sci. (2005) 84:1015–21. doi: 10.1093/ps/84.7.1015
38. Priya, B. S., and Babu, S. S. (2013). Effect of different levels of supplemental probiotics (Saccharomyces cerevisiae) on performance, haematology, biochemistry, microbiology, histopathology, storage stability and carcass yield of broiler chicken. International Journal of Pharmaceutical & Biological Archive, 4. Available at: http://ijpba.info/index.php/ijpba/article/view/1008
39. Soliman, A. Z. M., El-Kady, R. I., El-Shahat, A. A., and Sedik, M. Z. Effect of some commercial growth promoters on the growth performance and caecum microbiology of growing New Zealand white rabbits. EJRS. (2000) 10:239–252.
40. Foad, KAEE. Evaluation of baker's yeast (Saccharomyces cerevisiae) supplementation on the feeding value of hydroponic barley sprouts for growing rabbits. Egypt Poult Sci J. (2017) 37:833–54. doi: 10.21608/epsj.2017.7735
41. Özsoy, B, and Yalçın, S. The effects of dietary supplementation of yeast culture on performance, blood parameters and immune system in broiler turkeys. Ankara Üniv Vet Fak Derg. (2011) 58:117–22. doi: 10.1501/Vetfak_0000002460
42. Belhassen, T, Bonai, A, Gerencsér, Z, Matics, ZS, Tuboly, T, Bergaoui, R, et al. Effect of diet supplementation with live yeast Saccharomyces cerevisiae on growth performance, caecal ecosystem and health of growing rabbits. World Rabbit Sci. (2016) 24:191–200. doi: 10.4995/wrs.2016.3991
43. Abdou, AA, Raafat, SM, Younan, EG, Farag, EM, Abd El-Wahab, MW, Shahin, FG, et al. Effect of Spirulina algae levels supplements on the productive and reproductive performance of doe rabbits. Egypt J Vet Sci. (2024) 55:2095–106. doi: 10.21608/ejvs.2024.267807.1826
44. Farag, MR, Abd EL-Aziz, RM, Ali, HA, and Ahmed, SA. Evaluating the ameliorative efficacy of Spirulina platensis on spermatogenesis and steroidogenesis in cadmium-intoxicated rats. Environ Sci Pollut Res Int. (2016) 23:2454–66. doi: 10.1007/s11356-015-5314-9
45. Bhowmik, D, Dubey, J, and Mehra, S. Probiotic efficiency of Spirulina platensis-stimulating growth of lactic acid bacteria. World J Dairy Food Sci. (2009) 4:160–3.
46. Ribeiro, DM, Martins, CF, Kuleš, J, Horvatić, A, Guillemin, N, Freire, JP, et al. Influence of dietary Spirulina inclusion and lysozyme supplementation on the longissimus lumborum muscle proteome of newly weaned piglets. J Proteome. (2021) 244:104274. doi: 10.1016/j.jprot.2021.104274
47. Abdel-Daim, MM, Abushouk, AI, Alkhalf, MI, Toraih, EA, Fawzy, MS, Ijaz, H, et al. Antagonistic effects of Spirulina platensis on diazinon-induced hemato-biochemical alterations and oxidative stress in rats. Environ Sci Pollut Res Int. (2018) 25:27463–70. doi: 10.1007/s11356-018-2761-0
48. El-Ratel, IT, Elbasuny, ME, El-Nagar, HA, Abdel-Khalek, AKE, El-Raghi, AA, El Basuini, MF, et al. The synergistic impact of Spirulina and selenium nanoparticles mitigates the adverse effects of heat stress on the physiology of rabbits bucks. PLoS One. (2023) 18:e0287644. doi: 10.1371/journal.pone.0287644
49. Hassanein, H, Arafa, MM, Abo Warda, MA, and Abd–Elall, A. Effect of using Spirulina platensis and Chlorella vulgaris as feed additives on growing rabbit performance. Egypt J Rabbit Sci. (2014) 24:413–31. doi: 10.21608/EJRS.2014.47489
50. Michalak, I, Mironiuk, M, Godlewska, K, Trynda, J, and Marycz, K. Arthrospira (Spirulina) platensis: an effective biosorbent for nutrients. Process Biochem. (2020) 88:129–37. doi: 10.1016/j.procbio.2019.10.004
51. Soni, RA, Sudhakar, K, and Rana, RS. Spirulina–from growth to nutritional product: a review. Trends Food Sci Technol. (2017) 69:157–71. doi: 10.1016/j.tifs.2017.09.010
52. Gabr, GA, El-Sayed, SM, and Hikal, MS. Antioxidant activities of phycocyanin: a bioactive compound from Spirulina platensis. J Pharm Res Int. (2020) 32:73–85. doi: 10.9734/jpri/2020/v32i230407
53. Ansorge, S, Reinhold, D, and Lendeckel, U. Propolis and some of its constituents down-regulate DNA synthesis and inflammatory cytokine production but induce TGF-β1 production of human immune cells. Zeitschrift für Naturforschung C. (2003) 58:580–9. doi: 10.1515/znc-2003-7-823
54. Gad, AS, Khadrawy, YA, El-Nekeety, AA, Mohamed, SR, Hassan, NS, and Abdel-Wahhab, MA. Antioxidant activity and hepatoprotective effects of whey protein and Spirulina in rats. Nutrition. (2011) 27:582–9. doi: 10.1016/j.nut.2010.04.002
55. Mahmoud, AE, Naguib, MM, Higazy, AM, Sultan, YY, and Marrez, DA. Effect of substitution soybean by blue green alga Spirulina platensis on performance and meat quality of growing rabbits. Am J Food Technol. (2017) 12:51–9. doi: 10.3923/ajft.2017.51.59
56. Park, JH, Lee, SI, and Kim, IH. Effect of dietary Spirulina (Arthrospira) platensis on the growth performance, antioxidant enzyme activity, nutrient digestibility, cecal microflora, excreta noxious gas emission, and breast meat quality of broiler chickens. Poult Sci. (2018) 97:2451–9. doi: 10.3382/ps/pey093
57. Bondar, A, Horodincu, L, Solcan, G, and Solcan, C. Use of Spirulina platensis and Curcuma longa as nutraceuticals in poultry. Agriculture. (2023) 13:1553. doi: 10.3390/agriculture13081553
58. Lestingi, A, Alagawany, M, Di Cerbo, A, Crescenzo, G, and Zizzadoro, C. Spirulina (Arthrospira platensis) used as functional feed supplement or alternative protein source: a review of the effects of different dietary inclusion levels on production performance, health status, and meat quality of broiler chickens. Life. (2024) 14:1537. doi: 10.3390/life14121537
Keywords: feed additives, low energy diet, production, blood metabolites, Bifidobacterium , Yeast extract, Spirulina , rabbit
Citation: Shaheen MS, Allam AA, Abdel Monem UM, Bassiony SM, Khalil BA, Salah AS, Alawam AS, Rudayni HA, Elolimy AA and Abass KS (2025) Bifidobacterium, Spirulina, and Yeast extracts in low-energy diets for rabbits: effects on performance, hematology, lipid metabolism, hepatorenal function, immunity and hormones. Front. Vet. Sci. 12:1615203. doi: 10.3389/fvets.2025.1615203
Edited by:
Aisha Khatoon, University of Agriculture, PakistanReviewed by:
Dongjin Chen, Fujian Academy of Agriculture Sciences, ChinaZain Ul Abidin, Veterinary Research Institute, Pakistan
Copyright © 2025 Shaheen, Allam, Abdel Monem, Bassiony, Khalil, Salah, Alawam, Rudayni, Elolimy and Abass. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed A. Elolimy, ZWxvbGlteUB1YWV1LmFjLmFl
 Mohamed S. Shaheen1
Mohamed S. Shaheen1 Ahmed A. Allam
Ahmed A. Allam Ayman S. Salah
Ayman S. Salah Hassan A. Rudayni
Hassan A. Rudayni Ahmed A. Elolimy
Ahmed A. Elolimy Kasim Sakran Abass
Kasim Sakran Abass