- 1College of Animal Science and Technology, Shihezi University, Shihezi, Xinjiang, China
- 2Laboratory and Equipment Management Division, Shihezi University, Shihezi, Xinjiang, China
Introduction: Neonatal calf diarrhea incidence varies seasonally, increasing during climatic fluctuations. This study investigated body weight and size, immune function, intestinal permeability, rectal microbiota, and short-chain fatty acid (SCFA) levels in diarrheal and healthy lactating calves across seasons.
Methods: Ten lactating healthy and ten diarrhea calves were selected in each season, blood and rectal contents samples were collected. Serum immunity, cytokines, and intestinal permeability markers were measured using kits. Rectal microbiota composition was analyzed via 16S rDNA amplicon sequencing, and SCFA profiles of rectal contents were characterized using targeted metabolomics.
Results: Significantly higher levels of interleukin-1β (IL-1β), endotoxin (ET), and diamine oxidase (DAO) and lower levels of interleukin-10 (IL-10), immunoglobulin (IgA and IgG) levels were observed in diarrheic calves compared to those of healthy controls across all four seasons. Acetate and valeric acid concentrations were significantly lower in diarrheic calves in summer, autumn, and winter. In addition, diarrheic calves exhibited significantly reduced alpha diversity compared to than that of healthy controls, as indicated by lower Chao1, Observed_features, and Shannon indices. The relative abundances of Escherichia-Shigella, Fusobacterium, and Peptostreptococcus in the diarrheal calf group were significantly higher, while Faecalibacterium and Bifidobacterium were significantly lower. Clostridium_sensu_stricto_1 content was positively correlated with ET and DAO and negatively correlated with IL-10 and IgA. Escherichia-Shigella was positively correlated with ET, DAO, IL-1β, and TNF-α, but negatively correlated with IL-10 and IgA. Fusobacterium was positively correlated with ET, DAO, and IL-1β.
Discussion: In conclusion, seasonal factors have an influence on the indicators related to diarrhea in calves. Diarrheic lactating calves showed the characteristics of reduced immunity, increased inflammatory response, reduced rectal microbial diversity, and altered microbiota profiles and SCFA content, and these alterations were closely related to the occurrence of diarrhea in calves.
1 Introduction
Calf diarrhea is one of the most common diseases on dairy farms. Due to their immature digestive and nervous systems, calves are highly susceptible to diarrhea (1) and their weak self-regulatory abilities (2, 3). Calf diarrhea induces gut microbiota imbalance, barrier dysfunction, immune dysregulation, and metabolic disturbances (4), and in severe cases, this can lead to the death of calves. Diarrhea accounts for up to 57% of calf deaths (5), severely affecting their subsequent growth, development, and productive performance. This leads to substantial economic and productivity losses, thereby compromising the health and sustainable development of the livestock industry (6, 7).
Calf diarrhea has complex and varied etiologies, comprising bacterial infections (Escherichia coli and Salmonella), viral infections (rotavirus and coronavirus), and parasitic infections (Cryptosporidium), as well as improper feeding management practices (8). Weaning stress also influences diarrhea in calves by decreasing the immune function (9), making diarrhea the most common response to weaning stress (10). The incidence of diarrhea in newborn calves varies across seasons. Calf diarrhea is not confined to a specific season but tends to occur more frequently during periods of climate fluctuation, such as early spring, late summer, and early autumn (11). Additionally, calf diarrhea can occur year-round, with a higher prevalence in winter and early spring (12). These discrepancies may be attributed to regional climatic differences. Gut microbes are critical in maintaining animal health and inhibiting disease progression by influencing gastrointestinal conditions. Short-chain fatty acids (SCFAs), which are indirect nutrients produced by intestinal microbiota, primarily comprise acetate, propionate and butyrate, accounting for approximately 85% of the total SCFAs (13, 14). They are critical in protecting the intestinal mucosal barrier, promoting nutrient absorption, and inhibiting the growth of harmful bacteria (15). In addition, SCFAs can influence the colonization of calf intestinal flora, maintain intestinal barrier integrity, and enhance anti-inflammatory ability, thereby promoting the healthy growth of calves (16). SCFAs have anti-inflammatory properties, and SCFA-producing bacteria can reverse the gut microbiota imbalance and inhibit the secretion of pro-inflammatory cytokines (17). However, the relationship between body weight, immunity, inflammation, intestinal permeability, gut microbiota composition, and SCFA levels in diarrheic suckling calves across different seasons remains unclear.
This study aimed to analyze the body weight, serum immune indices, cytokine levels, and intestinal permeability indices of healthy and diarrheic suckling calves across different seasons. 16S rDNA high-throughput sequencing and targeted metabolomics were employed to compare the composition of the rectal microbiota and SCFA content in healthy and diarrheic suckling calves. Additionally, we investigated the relationships between serum immune markers, inflammatory factors, intestinal permeability indicators, rectal microbiota, SCFAs, and the incidence of calf diarrhea.
2 Materials and methods
2.1 Animals
Fecal samples were collected from Holstein lactating calves with similar body weight and day age (21 ± 3) at Shihezi dairy farm in April (spring), July (summer), October (autumn), and January (winter), respectively. The animal care protocol was approved by the Animal Welfare Committee of Shihezi University (Xinjiang, China) (Ethics No. A2023-313). One day before sample collection, the defecation status of Holstein suckling calves with comparable body weight and age in the farm was observed, and the calf fecal scores were performed according to the method described by Lee et al. (18). Calves with a fecal scores < 3 were categorized as the healthy, while those with a score ≥ 3 were classified as diarrheic. From the healthy and diarrheic calves, 10 individuals were randomly selected to form the healthy group (H) and the diarrheal group (D), respectively.
2.2 Sample collection
Ten healthy and ten diarrhea calves in each season were selected to collecting the blood and rectal contents samples. The blood samples collected from the jugular vein of calves were allowed to stand for 30 min, and the serum was separated by centrifugation at 3,000 rpm for 15 min and stored at −20°C. Fecal samples were collected from the rectum of calves, immediately transferred into enzyme-free and sterile freezing tubes, and rapidly frozen in liquid nitrogen. The samples were subsequently transported to the laboratory and stored in the refrigerator at −80°C.
2.3 Measurement of weight and body measurements
Body weight, height, slant length, and chest circumference were measured according to the Technical Specification for Measuring the Production Performance of Beef Cattle (NY/T 2660-2014).
2.4 Measurement of serum indicators
Serum samples for alanine aminotransferase (ALT), total protein (TP), albumin (ALB), globulin (GLB), menthyltransferase (AST), alkaline phosphatase (ALPU), urea (UN), blood glucose (GLU), triglycerides (TG), total cholesterol (TC), creatine kinase (CK), lactate dehydrogenase (LDH), uric anhydride (UA) were measured using an automatic biochemical analyzer (OLYMPUS AU5800; Olympus Corporation, Tokyo, Japan). Immunoglobulin M (IgM), immunoglobulin A (IgA), immunoglobulin G (IgG), tumor necrosis factor-α (TNF-α), interleukin-10 (IL-10), interleukin-1β (IL-1β), transforming growth factor-β (TGF-β), endotoxin (ET) and diamine oxidase (DAO) were measured using commercial ELISA kits (Shanghai Enzyme-linked Biotechnology Co., Ltd., China).
2.5 16S rDNA high-throughput sequencing and analysis
Total bacterial DNA was extracted from the cecal contents of 10 calves per group using a TIANamp Stool DNA kit (Tiangen, Beijing, China). DNA sequencing was performed on the Illumina MiSeq desktop sequencer (Illumina, CA, USA) by Macrogene Inc. (Majorbio, Shanghai, China), targeting the V3-V4 hypervariable region of the 16S rDNA gene with primer 5′-ACTCCTACGGGAGGCAGCA-3′ and 5′-GGACTACHVGGGTWTCTAAT-3′. High-quality sequences were processed using the QIIME2 software. The sequences were analyzed using UCLUST (version 7.1) and clustered into operational taxonomic units (OTUs) at a similarity level of 97%. Representative sequences were aligned against the Greengenes 13.5 database using PyNAST, and the default parameters were set using QIIME2. Alpha diversity analysis included an abundance-based coverage estimator, Chao1 richness estimate, and Shannon and Simpson diversity indices. Principal coordinate analysis (PCoA) was used to assess the UniFrac distances and sample clustering. Differential bacterial abundances among the groups were analyzed using linear discriminant analysis effect size (LEfSe). Functional prediction of the metabolic pathways in the gut flora was performed using PICRUSt2. Raw sequencing data were deposited in the NCBI Sequence Read Archive under accession number PRJNA1238067.
2.6 Targeted metabolomics of short-chain fatty acids
2.6.1 Preparation of samples
An appropriate amount of calf rectal contents was placed in a 1.5 mL centrifuge tube, followed by the addition of 500 μL of water and 100 mg of glass beads. The sample was homogenized for 1 min and then centrifuged at 13,400×g for 10 min at 4°C to obtain the supernatant (200 μL). Subsequently, 100, 20, and 280 μL of 15% phosphoric acid, 4-methylpentanoic acid (375 μg/mL), and ether, respectively, were added to the supernatant.
2.6.2 GC/MS analysis
2.6.2.1 Gas chromatography conditions
The GC analysis was performed using a Trace 13 1 0 gas chromatograph (Thermo Fisher Scientific, USA). The GC was fitted with a capillary column Agilent HP-INNOWAX (30 m × 0.25 mm ID × 0.25 μm), and helium was used as the carrier gas at 1 mL/min. The injection was made in split mode at 10:1 with an injection volume of 1 μL and an injector temperature of 250°C. The temperature of the ion source and MS transfer line were 300 and 250°C, respectively. The column temperature was programmed to increase from an initial temperature of 90°C, followed by an increase to 120°C at 10°C/min, 150°C at 5°C/min, and finally to 250°C at 25°C/min, which was maintained for 2 min.
2.6.2.2 Mass spectrum conditions
Mass spectrometric detection of metabolites was performed on an ISQ LT (Thermo Fisher Scientific, Waltham, MA, USA) in an electron impact ionization mode. The single-ion monitoring (SIM) mode was used with an electron energy of 70 eV. The calibration curve, linearity, correlation coefficients, limit of detection, and quantitation stability are presented in Supplementary Table S1.
2.7 Statistical analysis
Data were analyzed by two-way ANOVA using SPSS software (version 27.0), p < 0.05 was accepted as statistically significant. The repeated measures model contained fixed effects of treatment, season, and the interaction of treatment and season, and the random effect of calf identity. Significantly different blood markers, rectal microbiota (q < 0.05, relative abundance > 0.5%), and rectal content differential SCFA (q < 0.05) were selected for interaction analysis. Correlations with an absolute Spearman’s correlation coefficient ≥ 0.50 and a q-value <0.05 were considered significant. The Majorbio tools website1 was used to visualize the correlations.
3 Results
3.1 Weight and body size of healthy and diarrheic lactating calves in different seasons
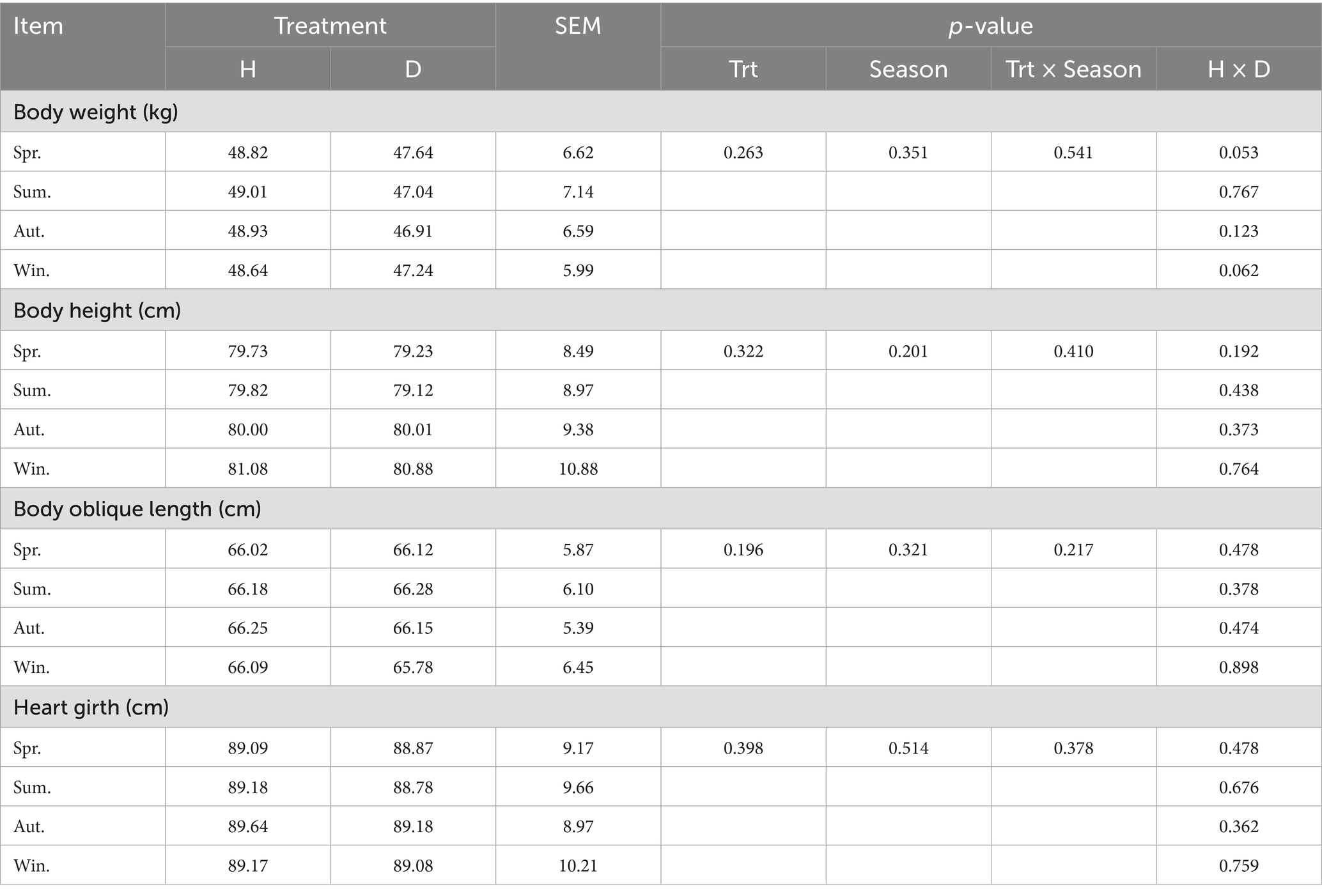
No significant differences were observed between the groups and seasons in body size indices, such as body weight, body height, body slant length, and chest circumference of healthy and diarrhea-lactating calves in different seasons (p > 0.05; Table 1). However, lactating calves with diarrhea tended to have lower body weights (p = 0.0532 and p = 0.0622, respectively) during spring and winter.
3.2 Blood biochemical indices of healthy and diarrheal lactating calves in different seasons
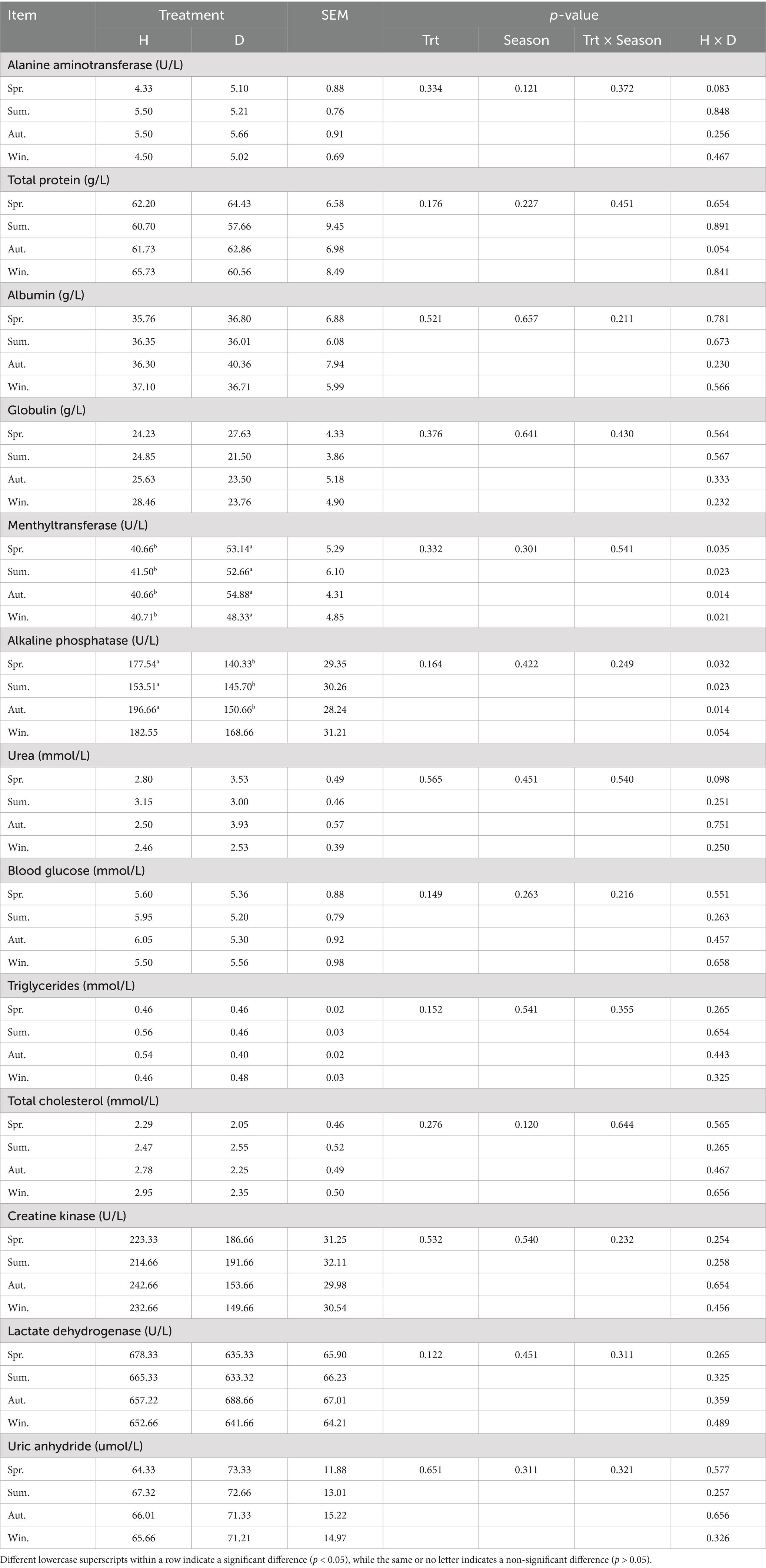
No significant differences were observed in the serum of healthy and diarrheic lactating calves in different seasons for alanine aminotransferase, total protein, albumin, globulin, urea, blood glucose, triglycerides, total cholesterol, creatine kinase, lactate dehydrogenase, and uric anhydride (p ˃ 0.05; Table 2). Serum levels of mentholatum transferase were significantly higher in diarrheic lactating calves than those in healthy calves in all seasons (p < 0.05). However, serum levels of alkaline phosphatase were significantly higher in healthy lactating calves than those in calves with diarrhea in spring, summer, and autumn (p < 0.05), and no significant difference was observed in winter (p > 0.05). None of the blood biochemical indices differed significantly between seasons (p > 0.05).
3.3 Serum immunoglobulin levels in healthy and diarrheic lactating calves in different seasons
Serum IgA and IgG levels in healthy lactating calves were significantly higher than those in diarrheic calves in different seasons (p < 0.05; Table 3), while IgM levels were not significantly different between the two groups of calves (p > 0.05). In addition, none of the abovementioned immunological indices differed significantly between seasons (p > 0.05).
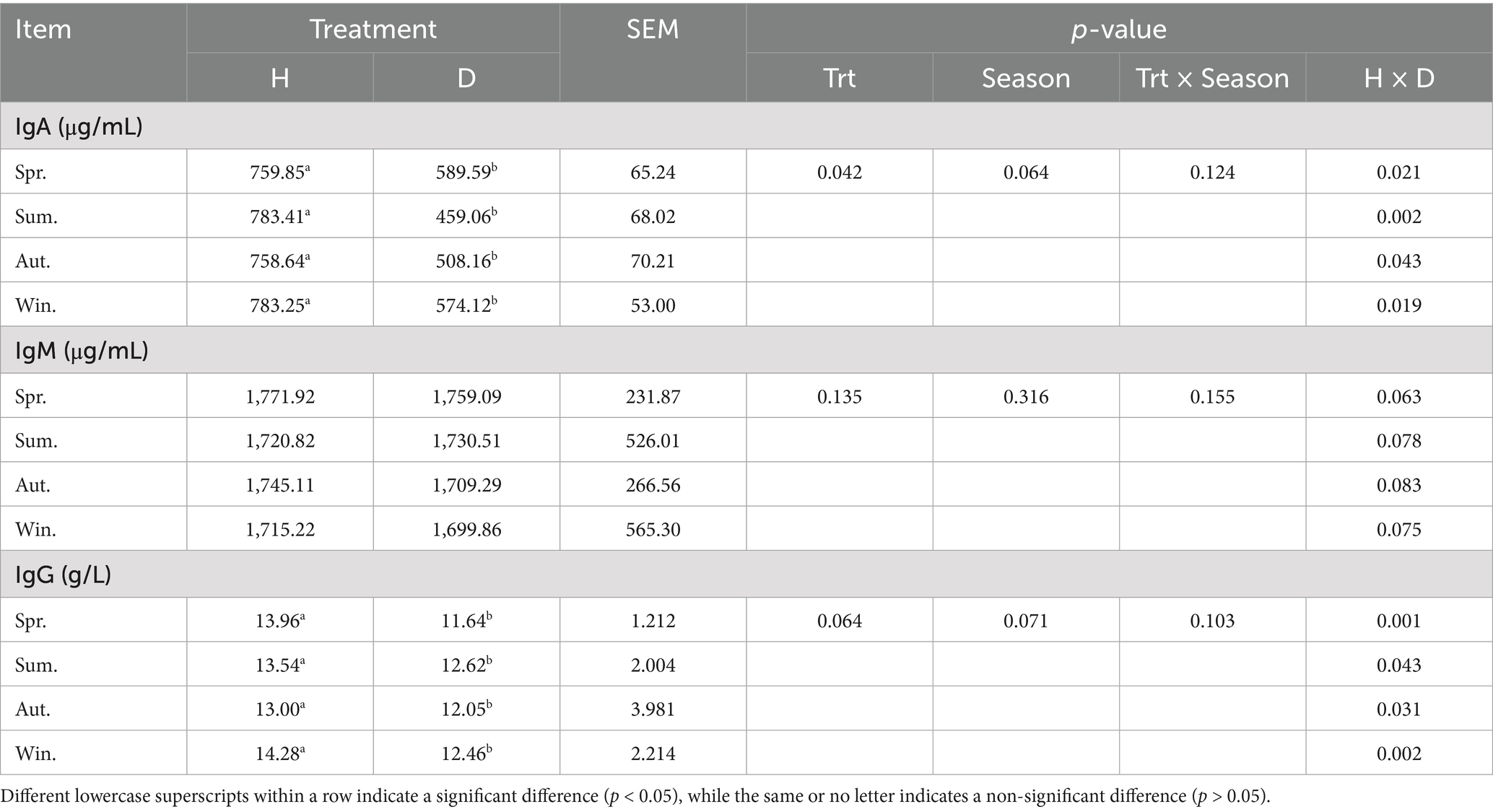
Table 3. Serum immunoglobulin levels in healthy and diarrheic lactating calves in different seasons.
3.4 Serum cytokine levels in healthy and diarrheic lactating calves in different seasons
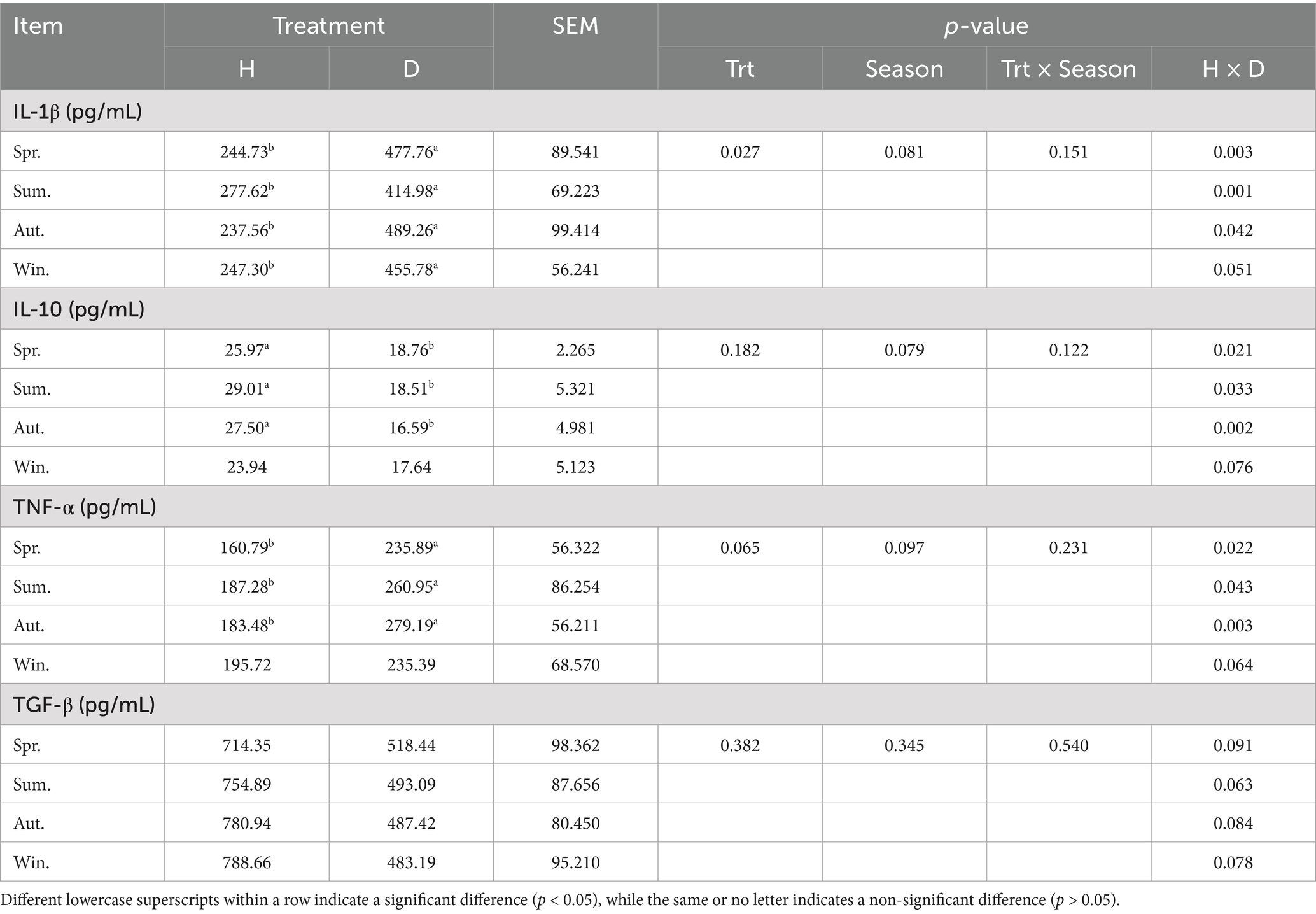
The IL-1β content in diarrheic calves was significantly higher than that of healthy calves in all seasons. Similarly, the TNF-α levels were higher in diarrheic calves during spring, summer, and autumn (p < 0.05; Table 4). The serum levels of IL-10 were significantly higher in healthy calves than those in calves with diarrhea in spring, summer, and autumn (p < 0.05), but the difference was not significant in winter (p > 0.05). In addition, no significant difference was observed in the TGF-β content between healthy and diarrheic calves, as well as in each index between seasons (p > 0.05).
3.5 Indicators of blood-intestinal permeability in healthy and diarrheic lactating calves in different seasons
Serum botulinum toxin (ET) and diamine oxidase (DAO) levels were significantly higher in diarrheic calves in different seasons than in healthy calves (p < 0.05), but the levels of each indicator were not significantly different between seasons (p > 0.05) (Table 5).
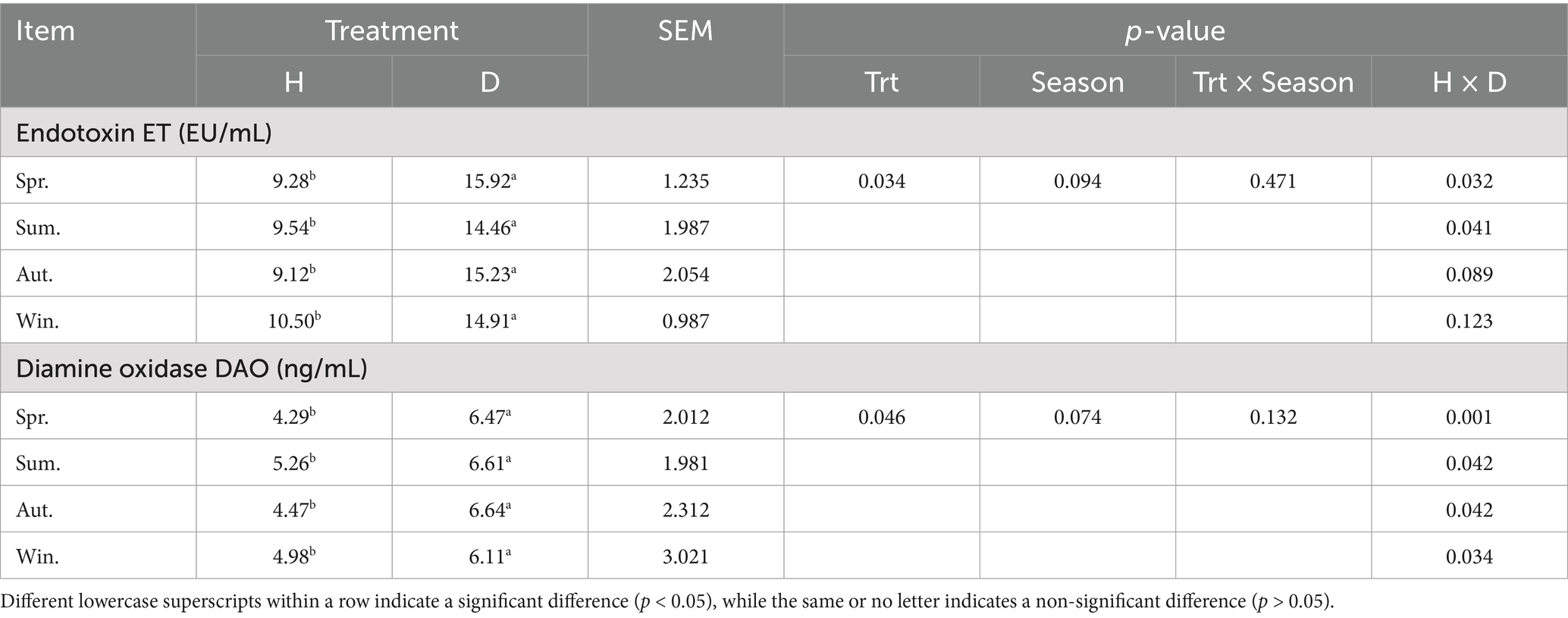
Table 5. Indicators of intestinal permeability of blood in healthy and diarrheic lactating calves in different seasons.
3.6 Multivariate statistical analysis of short-chain fatty acid standards
The relative standard deviation (RSD) values < 10% indicated good methodological stability and reliable data (Supplementary Figure S1A). The TIC profiles exhibited effective separation and well-defined peaks for all seven SCFAs and the internal standard (isocaproic acid), confirming the suitability of this method for analyzing SCFAs (Supplementary Figures S1B,C).
The proportions of the first principal component (PC1) and the second principal component (PC2) in the combined raw information were 83.24 and 9.43%, respectively, indicating a better distinction between healthy and diarrheic calves across different seasons. This suggests differences in SCFAs between the groups (Figure 1).
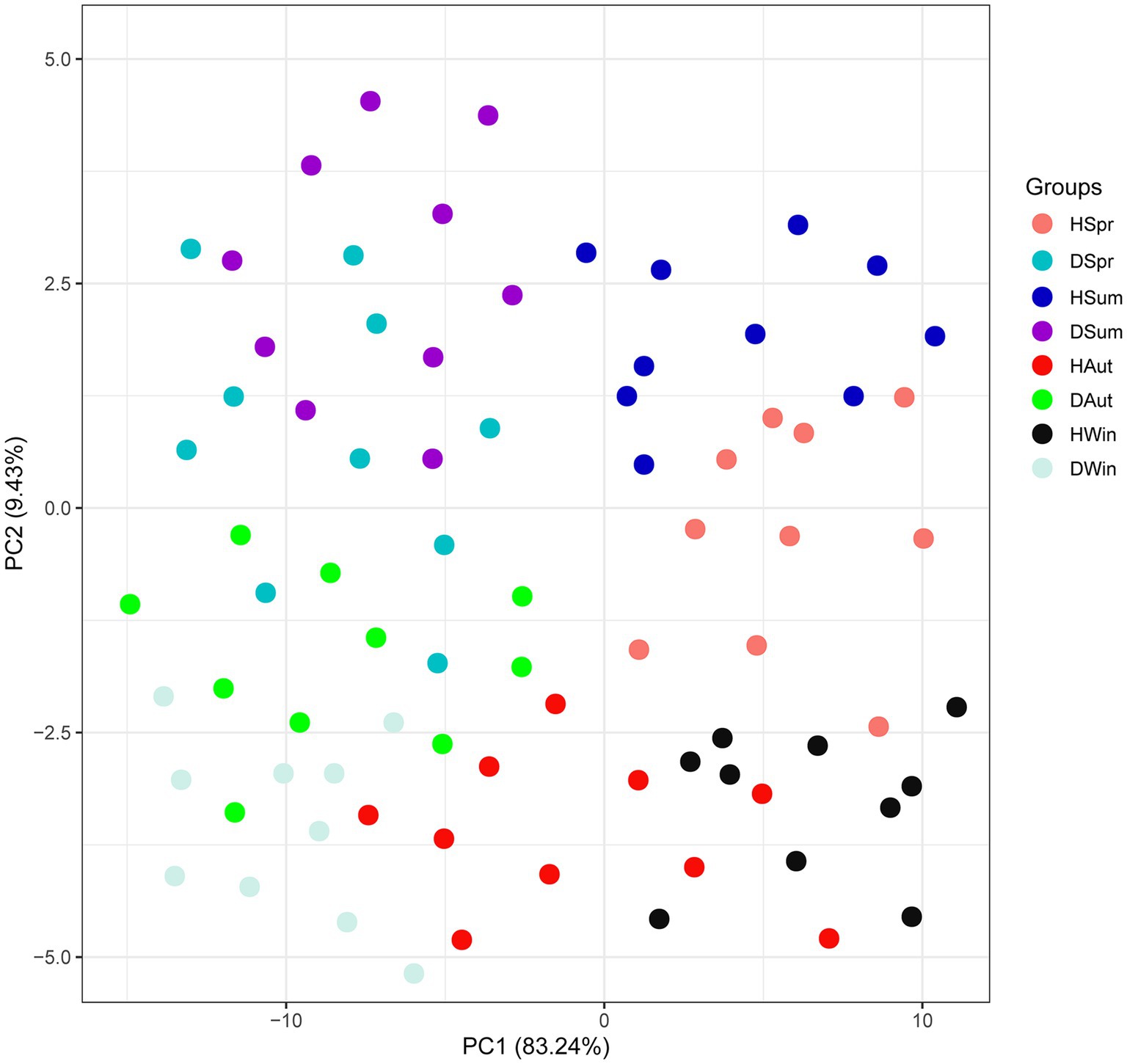
Figure 1. CA scores of rectal short-chain fatty acids in healthy and diarrheic calves in different seasons.
3.7 Comparative analysis of short-chain fatty acids
Table 6 indicates that rectal acetate levels in diarrheic calves were significantly lower than those in healthy calves in summer, autumn, and winter, as well as valeric acid levels in winter (p < 0.05); no significant differences were observed in other SCFAs between the groups (p > 0.05). In addition, the levels of acetate, propionate, valeric, and isovaleric acids differed significantly (p < 0.05) between seasons.
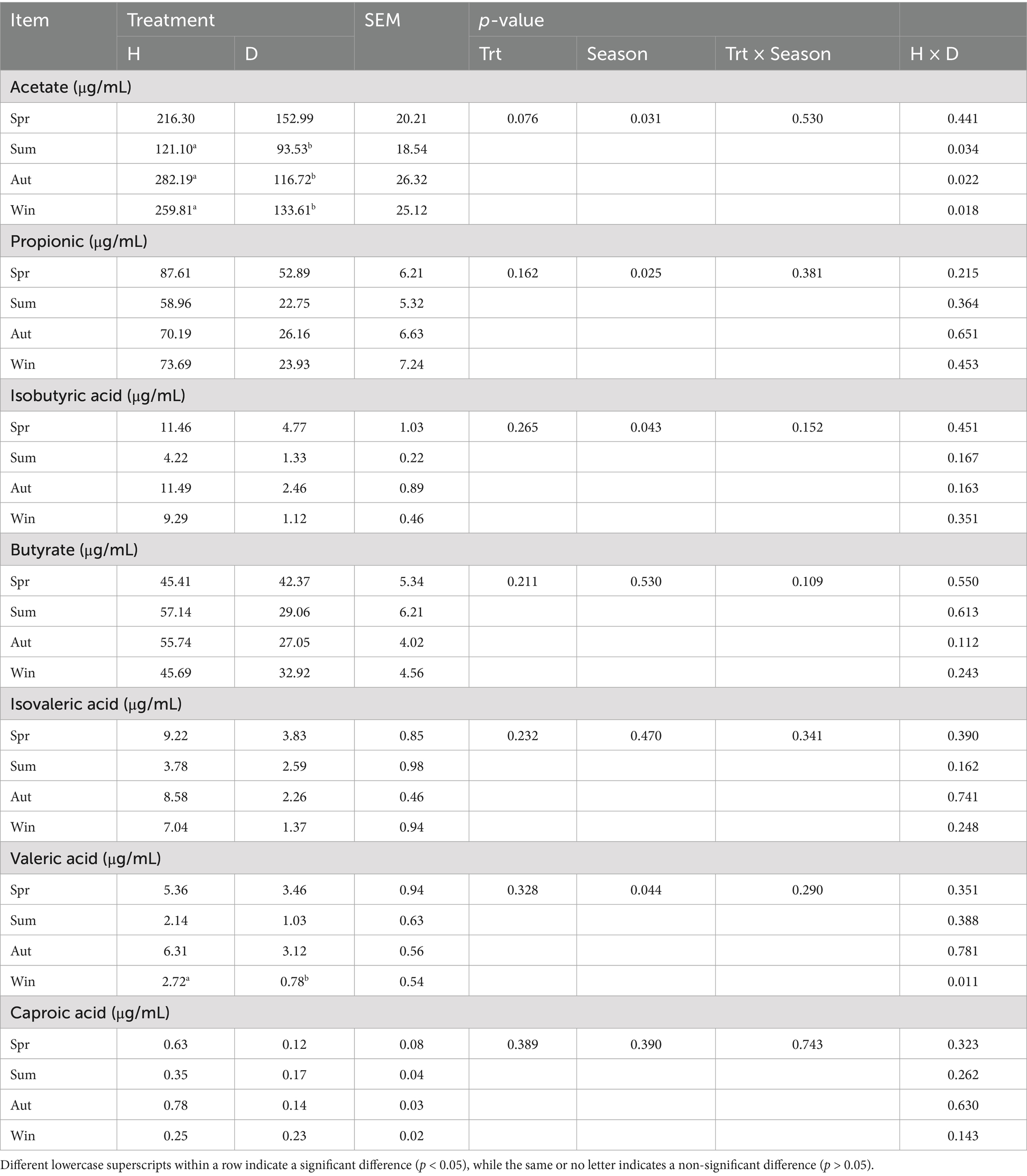
Table 6. Rectal short-chain fatty acid content of healthy and diarrheic calves in different seasons.
3.8 Rectal microbial changes
As shown in the Venn diagram (Figure 2A), 2,736 OTUs were identified, with a total of 36 OTUs in the healthy and diarrheal calf groups in the different seasons. A total of 1,157 and 1,143 OTUs were specific to the healthy and diarrheal calf groups, respectively. The number of OTUs specific to different seasons was 687 in the spring healthy group and 307 in the diarrheal group; 142 in the summer healthy group and 87 in the diarrheal group; 549 in the autumn healthy group and 309 in the diarrheal group; and 179 in the winter healthy group and 440 in the diarrheal group. The diversity rank-abundance curves demonstrated that the healthy calf group exhibited a broader extension along the horizontal axis, indicating higher species richness and even species distribution, with an overall flatter curve profile (Figure 2B). The species accumulation boxplot (Figure 2C) revealed that the boxplot positions gradually stabilized, confirming the adequacy of the sample size in this study.
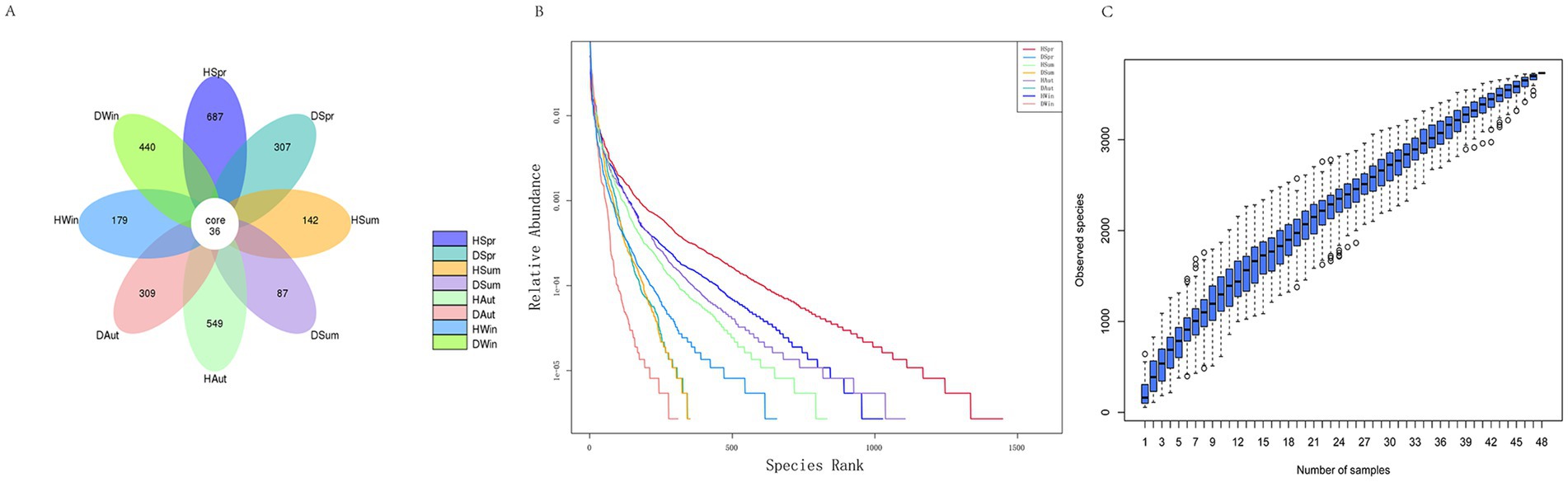
Figure 2. Rectal microbial Venn diagram, diversity rank-abundance clustering curves and species accumulation boxplot diversity in rectal of healthy and diarrhea calves. (A) Venn diagram. (B) Rank-abundance. (C) Species accumulation boxplot.
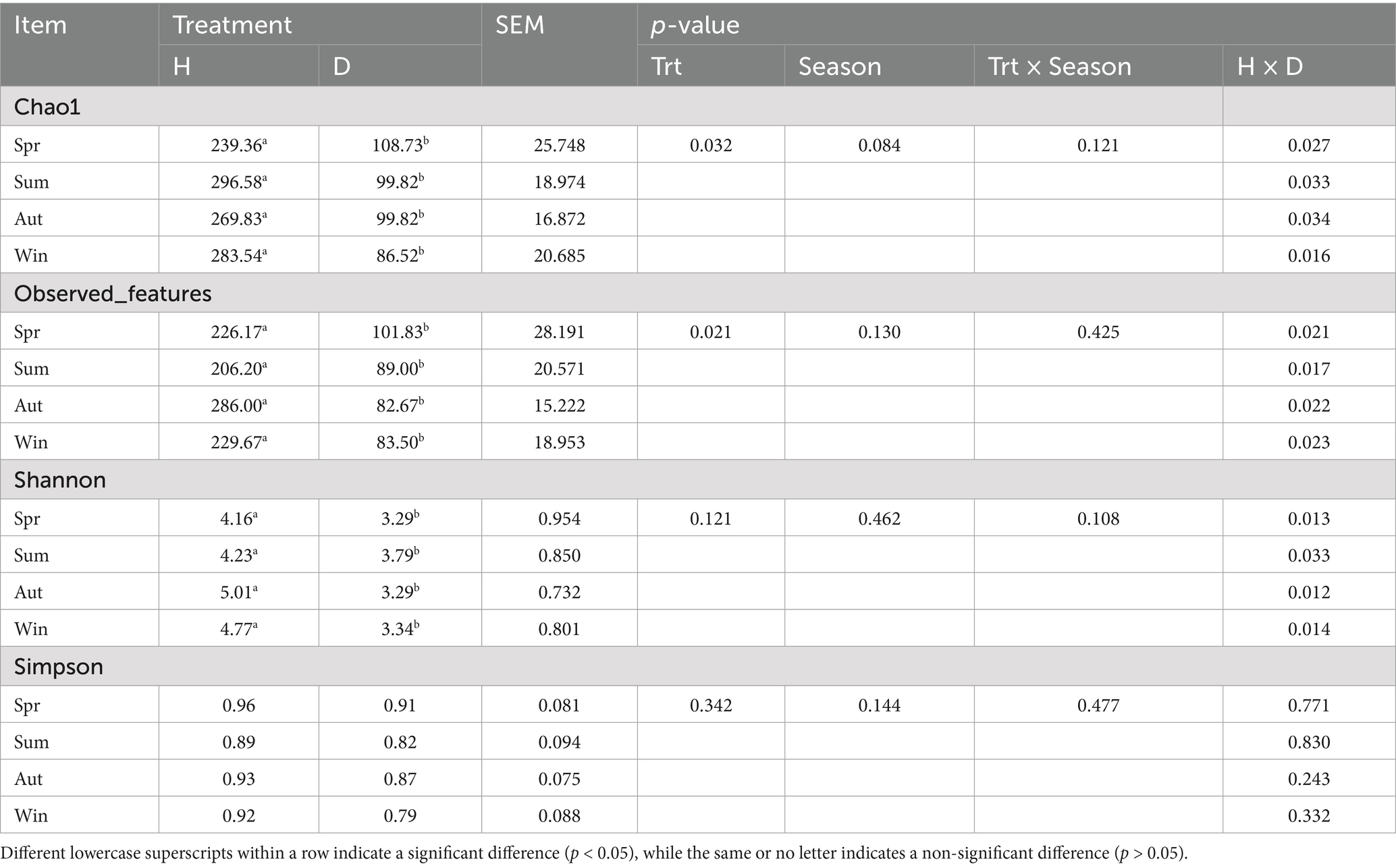
To evaluate the ɑ-diversity of samples, the Chao1, Observed_features, Simpson, and Shannon indices were calculated in this experiment. Diarrheic calves exhibited significantly lower Chao1, Observed_features, and Shannon indices across different seasons than those of healthy calves (p < 0.05), whereas no significant difference was observed in the Simpson index between the two groups (p > 0.05). Additionally, none of the α-diversity indices showed significant seasonal variations (p > 0.05) (Table 7).
The principal coordinates 1 and 2 in the PCOA accounted for 29.41 and 24.22% of the variation, respectively (Figure 3A). In the PCOA plot, samples from healthy calves clustered closely together across different seasons, indicating a high similarity in their microbial composition. In contrast, samples from diarrheic calves exhibited more dispersed distribution patterns, indicating considerable variability in the community structure among diarrheic individuals. The NMDS plot (Figure 3B) revealed that samples from healthy calves were tightly clustered regardless of the season, demonstrating minimal within-group variation and high structural consistency in their microbial communities. Conversely, samples from diarrheic calves exhibited a more scattered distribution, indicating significant heterogeneity in their microbial community structures.
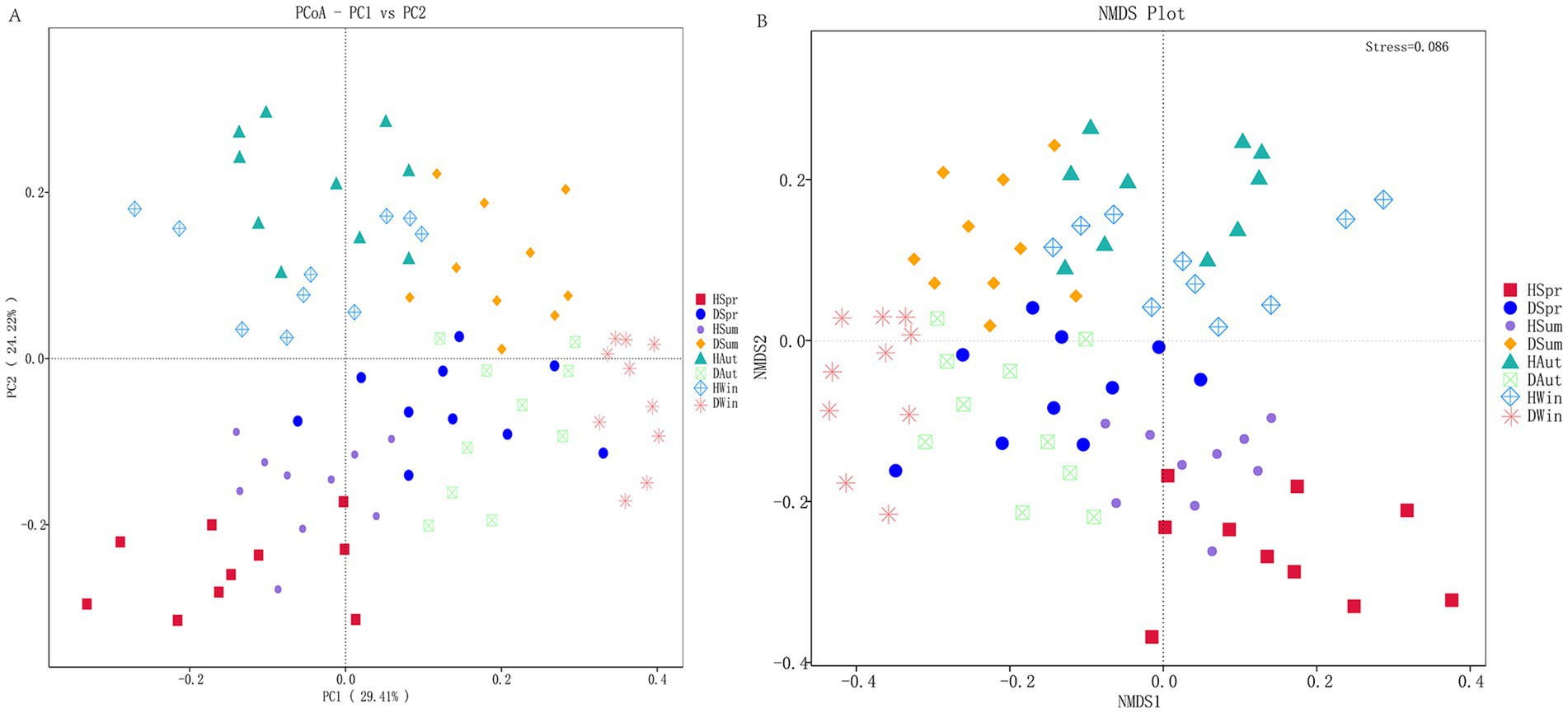
Figure 3. Principal coordinate analysis and non-metric multidimensional scaling of rectal microbes in healthy and diarrheic calves. (A) Principal coordinate analysis. (B) Non-metric multidimensional scaling.
Figures 4A,C show the top 10 phyla in healthy and diarrheic calves, respectively, across different seasons. The analysis results (Figure 4E) showed that Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Fusobacteria were the major phyla in both calf groups. Figures 4B,D show the top 15 genera in the healthy and diarrheal calf groups across different seasons, respectively. Escherichia-Shigella, Bacteroides, and Faecalibacterium were the dominant genera in the healthy and diarrheic calf groups (Figure 4F).
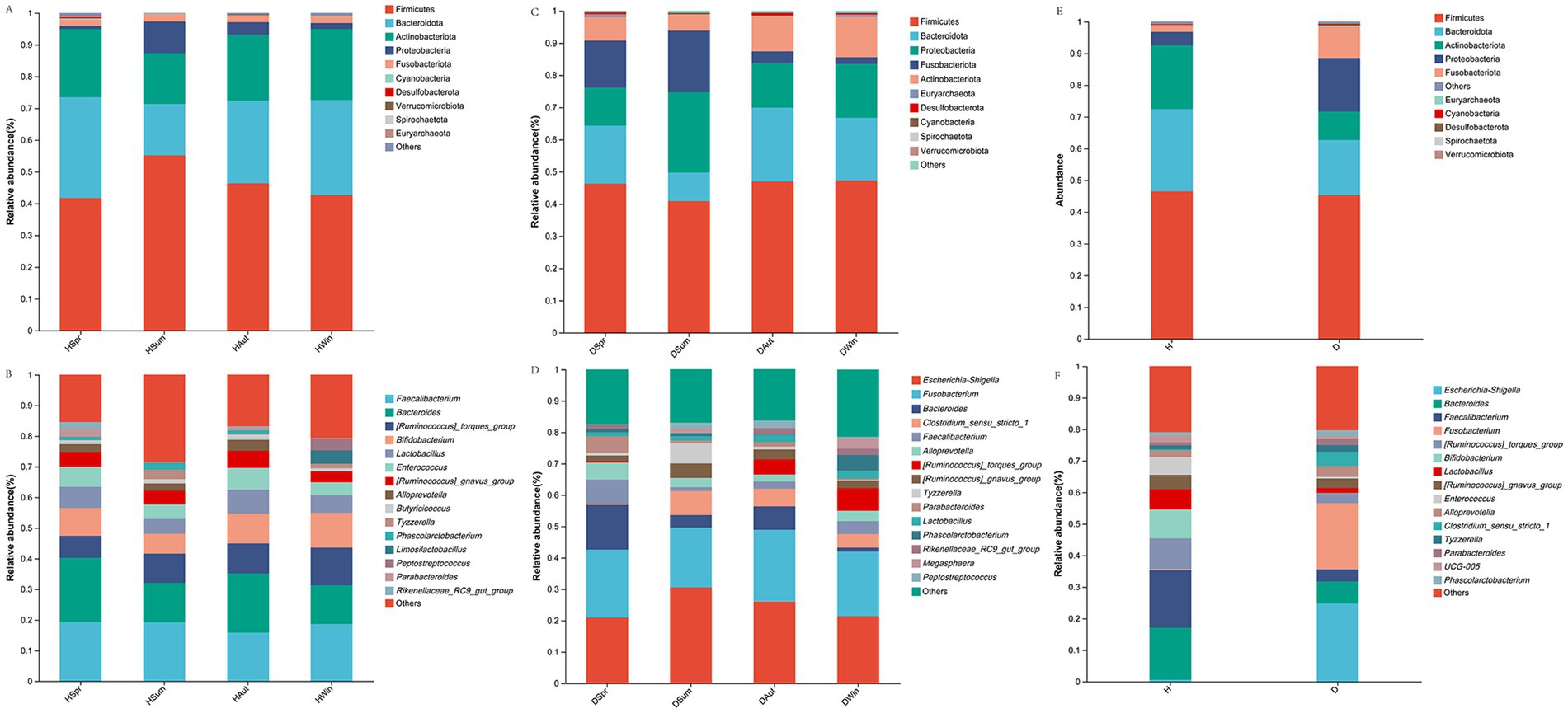
Figure 4. Rectal microbial community composition in healthy and diarrheic calves. (A,C,E) Microbiota phylum level composition. (B,D,F) Microbiota genus level composition.
In healthy calves, UCG_005, Parabacteroides, and Olsenella exhibited higher relative abundances in spring. Butyricoccus and Tyzzerella were more prevalent in the summer. Rikenellaceae_RC9_gut_groupand Stenotrophomonas dominated in autumn, whereas Eubacterium_nodatum_group and Chryseobacterium were enriched in winter (Figure 5A). Conversely, diarrheic calves exhibited distinct seasonal patterns: Alloprevotella was predominant in spring, Olsenella in summer, and Ruminococcus_torques_group with Stenotrophomonas in winter (Figure 5B). LEfSe analysis further confirmed significant differences in the rectal flora composition between the groups (Figure 5C). The diarrheic group demonstrated considerable enrichment of Escherichia-Shigella, Fusobacterium, Peptostreptococcus, and Clostridium_sensu_stricto_1 (p < 0.05). In contrast, healthy calves exhibited significantly higher abundances of Faecalibacterium, Bifidobacterium, Bacteroides, Ruminococcus_torques_group, Enterococcus, and Lactobacillus (p < 0.05).
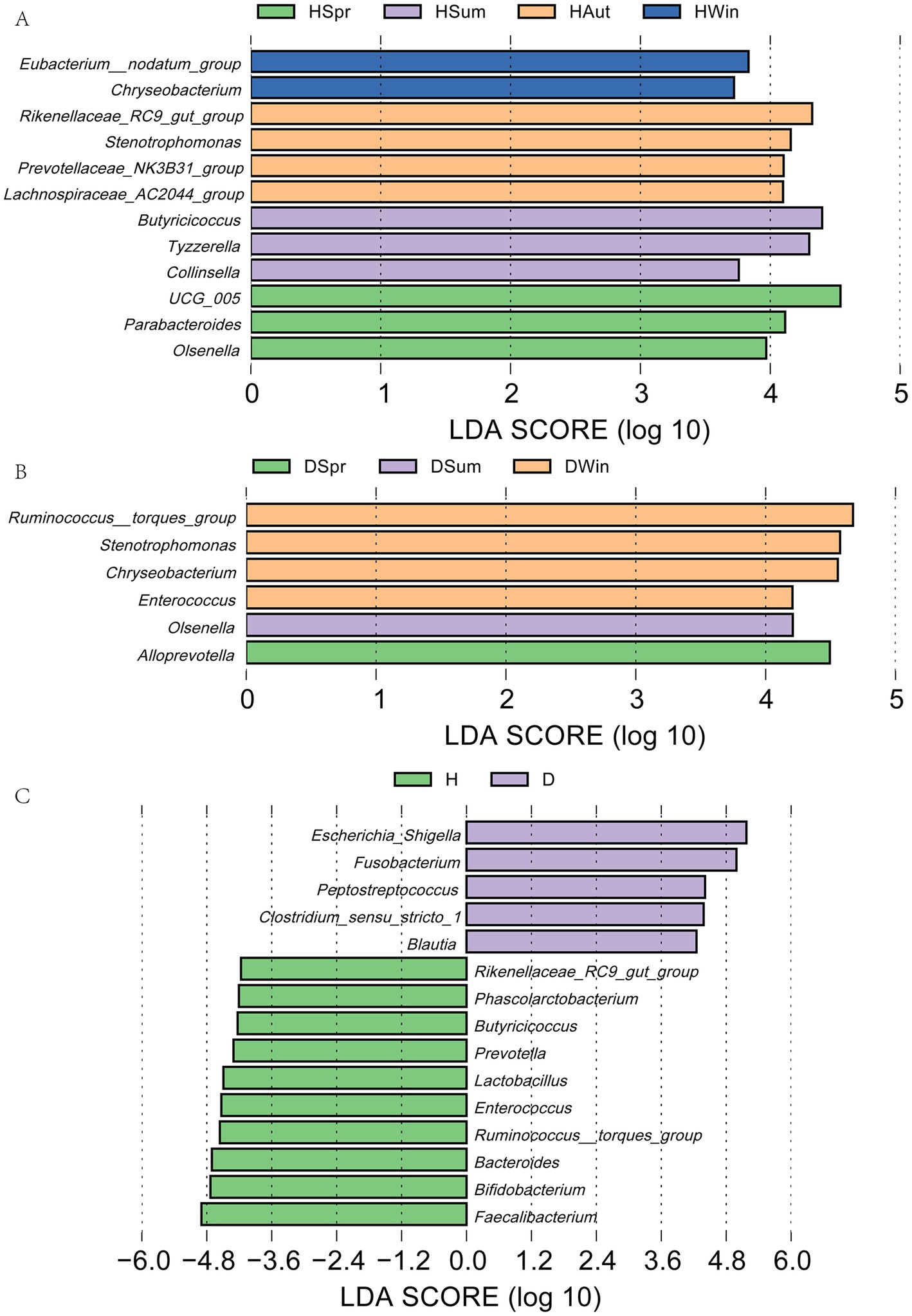
Figure 5. LEfSe analysis of rectal microflora in healthy and diarrheic calves. LDA discriminant plot with vertical coordinates indicating microorganisms with significant differences in multiple groups and horizontal coordinates (LDA scores) indicating species abundance. (A) LDA plots of healthy calves in different seasons; (B) LDA plots of calves with diarrhea in different seasons; (C) LDA plots of healthy and diarrheic calves.
To further investigate the potential functions of the calf rectal microbiota, we performed functional prediction analysis using the PICRUSt2 software based on the KEGG database. At KEGG pathway level 3, the healthy calf group showed upregulation of nine pathways in spring, including glycosaminoglycan degradation, flagellar assembly, and lipopolysaccharide biosynthesis. Summer was characterized by the enrichment of nine pathways, including drug metabolism—other enzymes, phosphotransferase system (PTS), and starch and sucrose metabolism. In autumn and winter, polyketide sugar unit biosynthesis, histidine metabolism, and bacterial secretion systems were upregulated (Figure 6A). In contrast, the diarrheic calf group exhibited the upregulation of four pathways in spring, including glycosaminoglycan degradation and a folate-mediated one-carbon pool. Sixteen pathways were enriched during summer, including the Caulobacter cell cycle, mismatch repair, and peptidoglycan biosynthesis. During winter, 24 pathways were upregulated, including geraniol degradation, phenylalanine metabolism, and cationic antimicrobial peptide (CAMP) resistance (Figure 6B). Twenty-four pathways were upregulated in the diarrheic group, including PTS, lipopolysaccharide biosynthesis, Escherichia coli biofilm formation, nitrotoluene degradation, glutathione metabolism, and fatty acid metabolism. The healthy group exhibited enrichment in 13 pathways, which included N-glycan biosynthesis, biosynthesis of various antibiotics, and oxidative phosphorylation (Figure 6C).
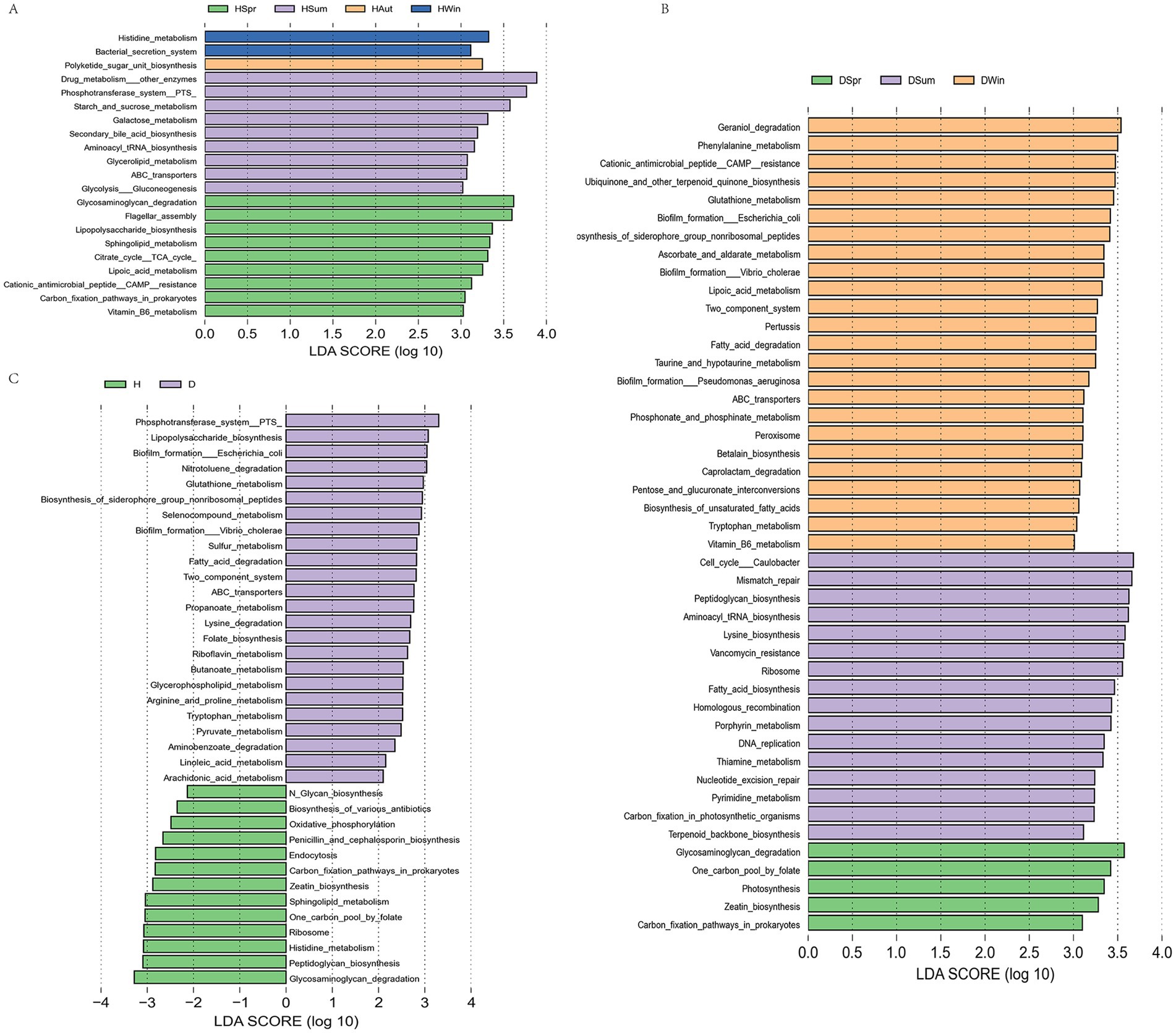
Figure 6. Functional prediction analysis of healthy and diarrheic rectal flora [KEGG pathway level 3 level (C)]. (A) LDA plots of healthy calves in different seasons; (B) LDA plots of calves with diarrhea in different seasons; (C) LDA plots of healthy and diarrheic calves.
3.9 Correlation analysis of blood immunity, inflammation and intestinal permeability indicators, rectal short-chain fatty acids, and rectal flora
The correlation analysis showed that the rectal Clostridium_sensu_stricto_1 content in calves was positively correlated with ET and DAO (p < 0.05) and negatively correlated with IL-10 and IgA (p < 0.05). The relative abundance of Escherichia-Shigella was positively correlated with ET, DAO, IL-1β, and TNF-α (p < 0.05) and negatively correlated with IL-10 and IgA (p < 0.05). Fusobacterium was positively correlated with ET, DAO, and IL-1β (p < 0.05). Parabacteroides levels were positively correlated with the intestinal permeability indicators isobutyric, isovaleric, acetate, propionate, and valeric acids (p < 0.05) and negatively correlated with ET (p < 0.05). Alloprevotella was positively correlated with IgM, valeric acid, and propionate levels (p < 0.05). The relative abundance of Enterococcus was positively correlated with IL-10, IgA, and valeric acid contents (p < 0.05) and negatively correlated with ET and IL-1β (p < 0.05). Faecalibacterium was positively correlated with butyrate, propionate, IL-10, IgA, and IgG (p < 0.05) and negatively correlated with IL-1β, ET, and DAO (p < 0.05). Bifidobacterium was positively correlated with butyrate, propionate, valeric acid, IL-10, and IgA contents (p < 0.05) and negatively correlated with IL-1β, ET, and DAO (p < 0.05). The relative abundance of the [Ruminococcus]_torques_group was positively correlated with butyrate content (p < 0.05) and negatively correlated with IL-1β and TNF-α (p < 0.05). The relative abundance of Bacteroides was positively correlated with propionate, IL-10, and IgA contents (p < 0.05) and negatively correlated with IL-1β, ET, and DAO (p < 0.05). Lactobacillus was positively correlated with propionate, IL-10, and IgG contents (p < 0.05) and negatively correlated with IL-1β, ET, and DAO (p < 0.05). The relative abundance of Phascolarctobacterium was positively correlated with isovaleric acid and IgA contents (p < 0.05) and negatively correlated with the intestinal permeability indices ET and IL-1β (p < 0.05) (Figure 7).
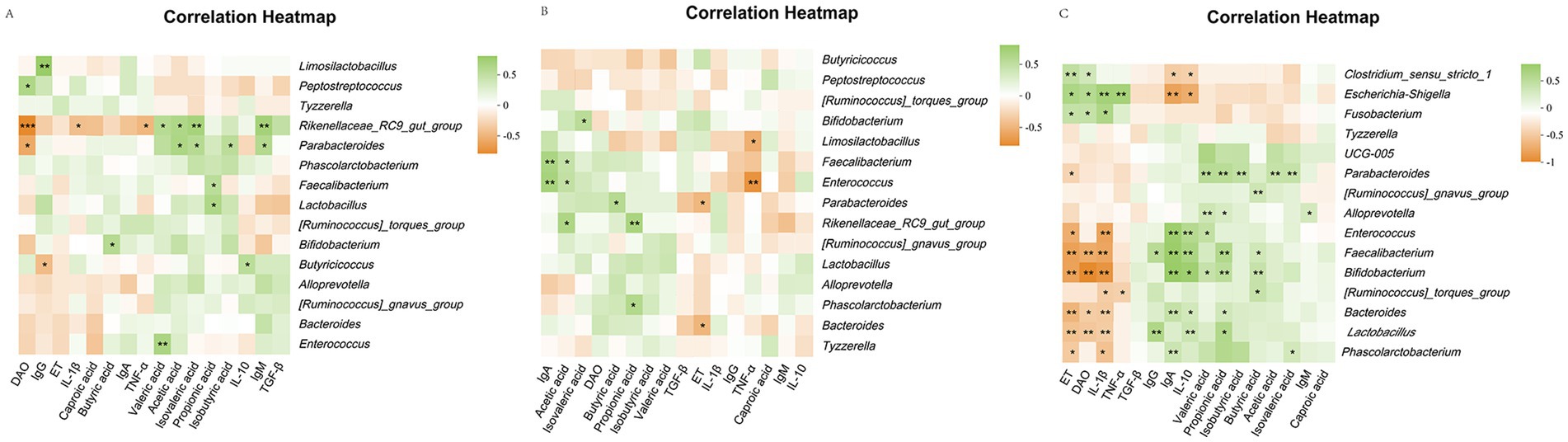
Figure 7. Heatmap of correlation analysis between serum immunity, inflammation and intestinal permeability differential indicators, rectal differential flora and differential short chain fatty acids in calves. (A) Heatmap for correlation analysis between indicators of healthy calves; (B) Heatmap for correlation analysis between indicators of diarrhea calves; (C) Heatmap for correlation analysis between indicators of healthy and diarrhea calves.
4 Discussion
Calf body weight and body measurements are important parameters for assessing overall health. Diarrhea often results in poor nutrient absorption; therefore, body weight measurements can be used as an indirect indicator to assess the nutritional status of calves, consequently providing a basis for adjusting feeding management programs. In the present study, we observed that calves with diarrhea tended to lose weight in both spring and winter. This finding is consistent with previous findings indicating that diarrhea in calves significantly affects digestibility, thus adversely affecting weight gain (19).
Immunoglobulins play a direct role in humoral immunity, and their concentrations partially indicate the immune ability of animals, which is crucial for the host to resist attacks by pathogenic microorganisms. IgG, the predominant antibody in serum (accounting for approximately 75% of total immunoglobulins), is a key indicator of the innate immunity response and the ability of an organism to combat infection (20). IgA, the second most abundant serum immunoglobulin, is a key element of the mucosal defense system. It inhibits microbial adhesion and constitutes the primary barrier against pathogenic invasion. Serum IgG levels indicate immune status, whereas IgM is related to anti-inflammation. Higher IgG and IgM levels typically indicate better immune function (21). The results of this study demonstrated that calves with diarrhea had significantly lower serum IgG and IgA levels than those of healthy calves.
Cytokines play a crucial role in livestock diarrhea by regulating immune responses and influencing intestinal physiological functions. Interleukin-1β (IL-1β), a key pro-inflammatory cytokine, is essential for immune response and pathological inflammation (22). IL-10, secreted by Th2 cells, is a vital regulator of immune homeostasis by controlling the chronic stimulation of the gut microbiota and food antigens (23). IL-10 is a potent anti-inflammatory cytokine that terminates inflammatory responses. Tumor necrosis factor-α (TNF-α) is a pleiotropic cytokine that acts on multiple cell types. It is a master regulator of the inflammatory response and contributes to the pathogenesis of various inflammatory and autoimmune diseases (24). Dysregulated or excessive activation of TNF-α signaling is associated with chronic inflammation, potentially leading to pathological complications such as autoimmune disorders (25). Chen et al. (26) reported that clinically diarrheic piglets showed significantly increased levels of pro-inflammatory cytokines (TNF-α and IL-1β) in jejunal tissues than those of healthy pigs, while the anti-inflammatory cytokine IL-10 exhibited a decreasing trend (though not statistically significant). Consistent with the above findings, the present study found that diarrheic calves had increased serum IL-1β and TNF-α levels but reduced IL-10 concentrations than those of healthy calves. Gut microbiota dysbiosis may influence increased pro-inflammatory cytokines, subsequently suppressing immunoglobulin synthesis, which is a plausible explanation for the decreased immunoglobulin levels observed in diarrheic calves (27). This mechanistic link further explains why the diarrheic calves in our study exhibited significantly lower serum IgG and IgA activities than those of healthy calves.
Impairment of the intestinal barrier function increases disease susceptibility in animals, making the maintenance of gut barrier integrity crucial for animal health. Serum diamine oxidase (DAO) is a key biomarker for evaluating gastrointestinal integrity. As an intracellular enzyme that is highly active in the upper villi of the small intestinal mucosa, DAO protects the intestinal epithelium by regulating the intracellular ion balance to promote cellular repair (28). Endotoxin (ET), a component of Gram-negative bacterial cell walls, can induce cellular inflammation and is widely used to establish inflammatory cell models (29). DAO functions as a marker enzyme in the cytoplasm of the intestinal epithelial cells. When the intestinal barrier function is compromised and mucosal permeability increases, substantial amounts of DAO and ET enter the bloodstream. Consequently, serum DAO activity and ET levels directly indicate the integrity of the intestinal barrier. Serum DAO activity in diarrheic calves is lower than that in control calves (30). In addition, increased intestinal permeability in animals, including calves, results in the entry of ET into circulation, subsequently triggering a systemic inflammatory response and liver injury. ET increases the secretion of pro-inflammatory factors by activating inflammatory signaling pathways and affects liver function through the gut-liver axis (31). This explains the increased serum levels of pro-inflammatory factors in calves with diarrhea in the present study.
SCFAs are the end products of the gut microbial fermentation of indigestible dietary fibers and serve as energy substrates for the host while protecting the intestinal mucosal barrier and suppressing gut inflammation (32). Major SCFAs, including acetate, propionate, butyrate, and isovalerate, play pivotal roles in the regulation of gut microbiota composition, immunity, metabolism, and the improvement of intestinal function (33, 34). Acetate (primarily produced by Lactobacillus) acidifies the intestinal environment to promote microbial balance, while excess acetate upregulates pro-inflammatory cytokines (IL-1β and IL-6), potentially triggering inflammation (35). Propionate can stimulate anti-inflammatory factors and upregulate claudin-1 expression to strengthen intestinal barrier integrity. Butyrate is the primary energy source for colonocytes and is critical for maintaining the gut barrier function (36). The results of this study revealed a significant reduction in rectal acetate and valeric acid levels in calves with diarrhea.
The intestinal flora, characterized by its abundance, diverse species, and complex structure, is a crucial component of the intestinal microecosystem. Interdependence and organizational crosstalk between intestinal flora lead to metabolic dysregulation and inflammation (37, 38).
The genus Faecalibacterium, which belongs to Firmicutes, is a symbiotic bacterium that colonizes the gastrointestinal tract of mammals and is one of the predominant bacterial taxa in the gut microbiota of healthy hosts (39). Faecalibacterium is a primary producer of butyrate and plays a crucial role in intestinal microecology (40). In addition to serving as an important energetic substance, butyrate is critical in immunomodulation, maintenance of the intestinal epithelial mucosal barrier function, and intestinal homeostasis (41, 42). Butyrate can regulate intestinal peristalsis and hormone secretion by activating G protein-coupled receptors, thus maintaining intestinal epithelial barrier function (43).
Clostridium_sensu_stricyto_1 is a highly diverse group comprising multiple species with distinct functional roles in the intestinal ecosystem. Although particular Clostridium species function as beneficial commensals that contribute to gut homeostasis, others exhibit pathogenic potential. Supplementation with Clostridium_sensu_stricyto_1 significantly increases IgA and IgG levels in calf serum (44), decreases DAO and D-lactate levels in piglet serum (45), and induces intestinal IL-10 production (46). Contrary to these findings, the present study found that the relative abundance of Clostridium_sensu_stricyto_1 was significantly higher in the diarrheal calf group than that in the healthy calf group, was positively correlated with ET and DAO, and negatively correlated with IL-10 and IgA. These phenomena may result from complex interactions among multiple factors. Certain pathogenic species within the Clostridium group may produce toxins or induce inflammatory responses, thereby compromising intestinal barrier integrity and disrupting immune homeostasis. However, a combination of factors, including gut barrier dysfunction, exacerbation of the inflammatory response, imbalance in the structure of the intestinal microbial community, developmental stage of the calf immune system, and stress response may also have contributed to this phenomenon.
Escherichia coli, a Gram-negative, parthenogenetic anaerobic bacterium of the Enterobacteriaceae family, has various pathogenic mechanisms, such as adhesion, invasion, and toxin secretion, which contribute to the induction of intestinal infections (47). The genus Shigella also belongs to the Enterobacteriaceae family and is phylogenetically closely related to the Escherichiaceae genus, with some strains sharing higher genetic homology with certain E. coli strains. Shigella is a major pathogen that causes bacillary dysentery, and its pathogenic mechanism involves multiple pathways, including evasion of host immune surveillance and induction of intestinal inflammation (48). Pathogenic E. coli can trigger severe intestinal inflammatory responses, leading to increased intestinal permeability and high levels of inflammatory cytokines (49). Consistent with these findings, we observed that the relative abundance of Escherichia-Shigella in the rectum of diarrheic calves was significantly higher than that in healthy calves. Moreover, it was positively correlated with ET, DAO, IL-1β, and TNF-α while negatively correlated with IL-10 and IgA.
The genus Ruminococcus torques is an important butyrate-producing bacterial group in gut microbiota. Butyrate, a SCFA, is crucial in maintaining intestinal homeostasis and regulating immune responses. Ruminococcus torques exhibit anti-inflammatory properties and inhibit the production of pro-inflammatory cytokines by modulating immune responses. Additionally, the abundance of this genus was positively correlated with butyrate levels (50). Consistent with these results, the present study revealed that the relative abundance of Ruminococcus_torques_group in the rectum of healthy calves was significantly higher than that in calves with diarrhea. Additionally, its relative abundance was positively correlated with butyrate levels and negatively correlated with pro-inflammatory cytokines IL-1β and TNF-α, suggesting its potential beneficial role in maintaining intestinal health in calves.
Fusobacterium is a key component of plaque biofilms and is essential in the development of periodontal diseases. Fusobacterium can activate host cells through surface adhesins and invasins, induce inflammatory responses, and promote the expression of pro-inflammatory cytokines. Additionally, it can disrupt tight junctions in intestinal epithelial cells, increasing intestinal permeability and leading to increased serum levels of ET and DAO (51). Consistent with the above findings, our study observed that the relative abundance of Fusobacterium was significantly higher in diarrheic calves than in healthy calves. Moreover, its relative abundance was positively correlated with ET, DAO, and IL-1β.
Faecalibacterium, a group of strict anaerobes belonging to the phylum Firmicutes, is a vital component of the gut microbiota in humans and animals. Faecalibacterium produces SCFAs such as butyrate and propionate. Butyrate is important in enhancing intestinal barrier integrity by regulating the expression of tight junction proteins in intestinal epithelial cells, thereby reducing intestinal permeability (52). Previous studies have confirmed a positive correlation between Faecalibacterium and the anti-inflammatory cytokine IL-10 as well as immunoglobulin IgA and IgG (53). Our findings further support these observations, demonstrating that the relative abundance of Faecalibacterium in the rectum of healthy calves was significantly higher than that in calves with diarrhea. Moreover, its abundance was positively correlated with butyrate, propionate, IL-10, IgA, and IgG while negatively correlated with IL-1β, ET, and DAO. These results highlight the critical role of Faecalibacterium in maintaining intestinal health and immune homeostasis in calves.
Bifidobacterium can perform various functions that are beneficial for host health. For example, exopolysaccharide (EPS) produced by Bifidobacterium can significantly modulate host immune function by promoting the secretion of the anti-inflammatory cytokine IL-10 while inhibiting the expression of the pro-inflammatory cytokine TNF-α (54). In addition, Bifidobacterium enhances the concentration of SCFAs in the gut, considerably improving the composition and functionality of the gut microbiota and strengthening the integrity of the intestinal barrier (55). Consistent with these findings, the present study revealed that the abundance of Bifidobacterium was significantly higher in healthy calves than in those with diarrhea. Furthermore, its abundance was positively correlated with butyrate, propionate, valerate, IL-10, and IgA while negatively correlated with IL-1β, ET, and DAO.
This study systematically evaluated the differences in body measurements, blood biochemical parameters, serum immune markers, cytokine levels, intestinal permeability, rectal microbiota composition, and SCFA profiles between diarrheic and healthy suckling calves across different seasons. We also preliminarily explored the relationships among blood biochemical indicators, gut microbiota, and SCFAs, providing valuable insights into the effect of diarrhea on calf health. However, this study has certain limitations. First, all the experimental samples were collected from a single farm, which may limit the generalizability and representativeness of the findings. Diarrhea is a multifactorial disease, and its different etiologies may lead to distinct patterns of gut microbial and metabolic disturbances. Therefore, future studies should consider the influence of causative agents. To gain a comprehensive understanding of the mechanisms underlying diarrhea, future studies should employ experimental interventions to validate the causal relationships between gut microbial and metabolic changes and diarrhea. Additionally, integrating multi-omics approaches, such as metagenomics, metabolomics, and transcriptomics, can help comprehensively elucidate the gene expression and metabolic functions of the calf gut microbiota, as well as host immune responses. These advancements can provide a basis for developing more effective strategies for the prevention and treatment of diarrhea.
5 Conclusion
The present study showed that diarrhea in calves significantly affected their serum immunity indexes, inflammatory factor levels and intestinal microecological structure. Diarrheic calves were generally characterized by decreased immune parameters, increased inflammatory factors and increased intestinal permeability. Regarding intestinal flora, diarrheic calves showed reduced rectal flora diversity and altered microbiota profiles, with increased abundance of specific pathogenic bacteria and decreased levels of some beneficial bacteria and short-chain fatty acids. In addition, we observed that seasonal factors may have an effect on some indicators, such as significant differences in alkaline phosphatase, TNF-α and IL-10 levels between healthy and diarrheal groups in spring, summer and autumn, but not in winter, and significantly higher levels of acetate in healthy calves than diarrheal groups in summer, autumn and winter. Additionally, these results provide a rationale for developing novel prevention and treatment strategies targeting microbial modulation to manage diarrheal conditions in calves.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The animal study was approved by Biology Ethics Committee of Shihezi University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
QW: Writing – original draft. QLu: Writing – review & editing. YT: Writing – review & editing. QLi: Writing – review & editing. PG: Writing – review & editing. SP: Writing – review & editing. WZ: Writing – review & editing. CN: Writing – review & editing. JN: Writing – review & editing. XM: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (32260855) and Shihezi University Youth Innovative Talent Cultivation Plan (CXBJ202310).
Acknowledgments
We thank the faculty members of the College of Animal Science and Technology and the members of the Laboratory of Animal Nutrition and Feed Science, especially the Shihezi Xijin Dairy Farm for their support of this experiment and the 16S rDNA high-throughput sequencing analysis performed by Beijing Novozymes Technology Co.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1615310/full#supplementary-material
FIGURE S1 | Stability of short-chain fatty acids in QC samples, mixed sample and sample TIC plots. (A) Stability of short-chain fatty acids in QC samples; (B) mixed-label TIC plot; (C) sample TIC plot.
Footnotes
References
1. Colleen, EC, Peter, SE, Quigley, JD, Hill, TM, Bateman, HG, Suarez-Mena, FX, et al. Effect of milk replacer program on calf performance and digestion of nutrients with age of the dairy calf. J Dairy Sci. (2016) 99, 2740–2747. doi: 10.3168/jds.2015-10372
2. Marina, B, Martín, A, Flavia, F, Celina, GV, Andrés, W, and Viviana, P. Passive immunity to control bovine coronavirus diarrhea in a dairy herd in Argentina. Rev Argent Microbiol. (2018) 50, 23–30. doi: 10.1016/j.ram.2017.03.007
3. Kate, FJ, Natalie, C, Charlotte, CB, and Wathes, DC. Prospective cohort study to assess rates of contagious disease in pre-weaned UK dairy heifers: management practices, passive transfer of immunity and associated calf health. Vet Record Open. (2017) 4:e000226. doi: 10.1136/vetreco-2017-000226
4. Yong, IC, and Karina, JY. An overview of calf diarrhea - infectious etiology, diagnosis, and intervention. J Vet Sci. (2014). doi: 10.4142/jvs.2014.15.1.1
5. Tae Woong, W, Hyun Sik, K, Na-Ri, S, Hojun, S, Min-Soo, K, Joon Yong, K, et al. Calf diarrhea caused by prolonged expansion of autochthonous gut Enterobacteriaceae and their lytic bacteriophages. mSystems. (2021) 15:1–17. doi: 10.1128/msystems.00816-20
6. Jeong-Byoung, C, Hyeon-Cheol, K, Joon Soon, K, Kyoung-Seong, C, Joon-Seok, C, Dong-Hun, Y, et al. The prevalence of causative agents of calf diarrhea in Korean native calves. J Anim Sci Technol. (2021) 63:864–871. doi: 10.5187/jast.2021.e63
7. El-Seedy, FR, Ahmed, HA, Yanni, HA, and El-Rahman, SAAA. Prevalence of Salmonella and E. coli in neonatal diarrheic calves. Beni-Suef Univ J Basic Appl Sci. (2016) 5:45–51. doi: 10.1016/j.bjbas.2015.11.010
8. Edmund, J, Lynna, L, Renaud, DL, Adronie, V, Jennifer, LM, Lisa, G, et al. Neonatal calf diarrhea and gastrointestinal microbiota: etiologic agents and microbiota manipulation for treatment and prevention of diarrhea. Vet Sci. (2024) 11:108–122. doi: 10.3390/vetsci11030108
9. Duff, GC, and Galyean, ML. Board-invited review: recent advances in management of highly stressed, newly received feedlot cattle. J Anim Sci. (2007) 85:823–40. doi: 10.2527/jas.2006-501
10. Hulbert, LE, and Sonia, JM. Stress, immunity, and the management of calves. J Dairy Sci. (2016) 99:3199–3216. doi: 10.3168/jds.2015-10198
11. Matías, C, and Rodney, C. Viral enteritis in cattle: to well known viruses and beyond. Microbiol Res. (2021) 12:663–682. doi: 10.3390/microbiolres12030048
12. R, M, R, T, TT, P, O, ND, F, B, H, H, et al. Mortality, diarrhea and respiratory disease in Danish dairy heifer calves: effect of production system and season. Prev Vet Med. (2018) 155:21–26. doi: 10.1016/j.prevetmed.2018.04.007
13. Bilal Ahmad, P, Mohammed Fahad, A, Arif Tasleem, J, and Irfan, AR. Leaky gut and autoimmunity: an intricate balance in individuals health and the diseased state. Int J Mol Sci. (2020) 21:9770. doi: 10.3390/ijms21249770
14. Anna, LG, Daniel, AP, and Andrew, JH. A short chain fatty acid–centric view of Clostridioides difficile pathogenesis. PLoS Pathog. (2021) 17:e1009959. doi: 10.1371/journal.ppat.1009959
15. L, K, L, F, P, V-D, DS, J, MS, P, and MM, D. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate†. Inflamm Bowel Dis. (2010) 16:1138–48. doi: 10.1002/ibd.21177
16. Elizabeth, RM, Ying Ka, L, and Holm, HU. Short-chain fatty acids: linking diet, the microbiome and immunity. Nat Rev Immunol. (2024) 24:577–595. doi: 10.1038/s41577-024-01014-8
17. Paúl, C, G, D, P-V, B, F, N, F, M, C, H, et al. Effect of Saccharomyces boulardii Cncm I-745 as complementary treatment of Helicobacter pylori infection on gut microbiome. Eur J Clin Microbiol Infect Dis. (2020) 39:1365–1372. doi: 10.1007/s10096-020-03854-3
18. Lee, HJ, Khan, MA, Lee, WS, Y, S-Y, Kim, SB, K, K-S, et al. Influence of equalizing the gross composition of milk replacer to that of whole milk on the performance of Holstein calves. J Anim Sci. (2009) 87:1129–37. doi: 10.2527/jas.2008-1110
19. Carla, F, Richard Van Vleck, P, Erika, G, Marilia Souza, G, Eduardo, M, Thiago, S, et al. Oral Administration of Faecalibacterium Prausnitzii Decreased the incidence of severe diarrhea and related mortality rate and increased weight gain in Preweaned dairy heifers. PLoS One. (2015) 10:e0145485. doi: 10.1371/journal.pone.0145485
20. Hematianlarki, M, and Nimmerjahn, F. Immunomodulatory and anti-inflammatory properties of immunoglobulin G antibodies. Immunol Rev. (2024) 328:372–86. doi: 10.1111/imr.13404
21. Liu, W, Long, X, Wan, K, Yin, M, Yin, Y, Zhang, B, et al. The endogenous factors affecting the detection of serum SARS-CoV-2 IgG/IgM antibodies by ELISA. J Med Virol. (2022) 94:1976–82. doi: 10.1002/jmv.27557
22. Li, X. Il-1β: an inflammatory sentinel that senses invasion signals from viral proteases. Chin Vet Sci. (2024) 54:1278. doi: 10.16656/j.issn.1673-4696.2024.0222
23. Qinmei, L, Yao, C, Baichang, X, Yuhan, W, Feng, L, Lijun, Z, et al. Main active components of Jiawei Gegen Qinlian decoction protects against ulcerative colitis under different dietary environments in a gut microbiota-dependent manner. Pharmacol Res. (2021) 170:105694. doi: 10.1016/j.phrs.2021.105694
25. Dan-In, J, Lee, AH, Hea Kyeong, S, Hyo-Ryeong, S, Jong-Hwi, P, Tae-Bong, K, et al. The role of tumor necrosis factor alpha (Tnf-Α) in autoimmune disease and current Tnf-Α inhibitors in therapeutics. Int J Mol Sci. (2021) 22:2719. doi: 10.3390/ijms22052719
26. Chen, X, Li, Z, Wu, Y, Zhang, C, and Zhang, Y. Changes of renin-angiotensin system (Ras) expression in jejunal tissues of pigs with clinical diarrhea and its relationship with intestinal inflammation. J Anim Husb Vet Sci. (2024) 55:751–8.
27. Ji Youn, Y, Maureen, G, Samia Valéria Ozório, D, Anujit, S, and Daniel, M. Gut microbiota and immune system interactions. Microorganisms. (2020) 8:1587–1609. doi: 10.3390/microorganisms8101587
28. Jon, ST, David, B, Rodney, SM, and William, PV. Intestinal mucosa diamine oxidase activity reflects intestinal involvement in Crohn's disease. Am J Gastroenterol. (1988) 83:756–60. doi: 10.26402/jpp.2019.2.03
29. Keqi, Y, Susan, L, Chuanfei, L, Yafen, S, Zhechuan, M, and Lin, L. Establishment of a lipopolysaccharide-induced inflammation model of human fetal colon cells. Mol Biol Rep. (2023) 50:5557–5564. doi: 10.1007/s11033-023-08465-7
30. Tatsuya, F, Tsukano, K, and Kyuma, S. Serum diamine oxidase activity in Japanese black calves with diarrhea. Sangyo Dobutsu Rinsho Igaku Zasshi. (2021). doi: 10.4190/jjlac.12.59
31. Surinder, SC, Veettiparambil Pandarathil, R, Bagath, M, Veerasamy, S, and Frank, RD. Impacts of heat stress on immune responses and oxidative stress in farm animals and nutritional strategies for amelioration. Int J Biometeorol. (2021) 65:1–14. doi: 10.1007/s00484-021-02083-3
32. Boushra, D, Lukas Van, O, Bram, V, and Kristin, V. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol. (2019) 16:461–78. doi: 10.1038/s41575-019-0157-3
33. Paulina, MK, and Katarzyna, Ś. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients. (2020) 12:1107. doi: 10.3390/nu12041107
34. Yao, Y, Xiaoyu, C, Weidong, F, Yiqing, Y, Miaomiao, Z, and Caihong, Z. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit Rev Food Sci Nutr. (2020) 62:11–12. doi: 10.1080/10408398.2020.1854675
35. José, LM, Pilar, SC, Christiano Eduardo, V, Russel, JR, and Javier, GG. A review of the molecular aspects of melatonin’s anti-inflammatory actions: recent insights and new perspectives. J Pineal Res. (2012) 54:1–14. doi: 10.1111/j.1600-079x.2012.01014.x
36. Bindu, K, Ushā, K, Singh, DK, Gulam Mohammed, H, Kanika, P, Anshul, S, et al. Molecular targets of valeric acid: a bioactive natural product for endocrine, metabolic, and immunological disorders. Endocrine Metab Immune Disord Drug Targets. (2024). doi: 10.2174/0118715303262653231120043819
37. Mohammed, E, Arun, JS, and Jacob, G. Mafld: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. (2020) 158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312
38. Rohit, L, Scott, LF, and Gerald, IS. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. (2021) 184:2537–2564. doi: 10.1016/j.cell.2021.04.015
39. F, C, MAS, T, AGV, T, Van Richard Vleck, P, D, J, Carrillo G, N, et al. Isolation and characterization of Faecalibacterium prausnitzii from calves and piglets. PLoS One. (2014) 9:e116465. doi: 10.1371/journal.pone.0116465
40. Mari, I, Satoru, K, Tomohiro, O, Kentaro, A, Mitsuru, H, Toru, K, et al. In vitro evaluation of Lactiplantibacillus Plantarum Hokkaido strain, effective lactic acid Bacteria for calf diarrhea. Front Vet Sci. (2023) 10:1145445. doi: 10.3389/fvets.2023.1145445
41. Hazel, T, Chien-Hsiung, Y, Lisa, AS, Marcel, RZ, Man Lyang, K, Martha, Z, et al. NLRP1 restricts butyrate producing commensals to exacerbate inflammatory bowel disease. Nat Commun. (2018) 9:3728. doi: 10.1038/s41467-018-06125-0
42. Mafalda, C, Pedro, G, Fernando, M, and Fátima, M. Microbiota-derived butyrate regulates intestinal inflammation: focus on inflammatory bowel disease. Pharmacol Res. (2020) 159:104947. doi: 10.1016/j.phrs.2020.104947
43. Guangxin, C, Xin, R, Bai, L, Yuhang, L, Dewei, H, Bingxu, H, et al. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. EBioMedicine. (2018) 317–325. doi: 10.1016/j.ebiom.2018.03.030
44. Zhu, Y, Li, Z, Yang, Y, Zhan, T, Bu, D, and Ma, L. Early-life Clostridium butyricum supplementation improved rumen development and immune by promoting the maturation of intestinal microbiota. J Agric Food Res. (2024) 18:101517. doi: 10.1016/j.jafr.2024.101517
45. Xiaopeng, T. Probiotic roles of Clostridium butyricum in piglets: considering aspects of intestinal barrier function. Animals. (2024) 14:1069. doi: 10.3390/ani14071069
46. Magdalena, KS, Jeewon, G-S, Nicholas, JJ, Julia, NM, Surabhi, T, Madeleine, N, et al. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes. (2021) 13:21–28. doi: 10.1080/19490976.2021.1907272
47. Matthew, AC, Robyn, JL, Roland, S, Kristie, MK, Marta, W, and Finlay, BB. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev. (2013) 26:822–80. doi: 10.1128/cmr.00022-13
48. El Bethel Lalthavel, H, Sujata, P, and Hemanta Kumar, S. The role of Shigella spp. in propagating bacillary dysentery in humans and the prominence of nanotechnology in disease prevention. Future J Pharm Sci. (2024) 10:97. doi: 10.1186/s43094-024-00676-4
49. David, BH, James, PN, Herbert, LD, Paresh, K, Ashwini, DM, Pablo, CO, et al. Enteroaggregative Escherichia coli is a cause of acute diarrheal illness: a meta-analysis. Clin Infect Dis. (2006) 43:556–63. doi: 10.1086/505869
50. Jeonghyeon, M, Lee, AR, Hee Jung, K, JooYeon, J, Seon-Yeong, L, Jeong Won, C, et al. Faecalibacterium prausnitzii alleviates inflammatory arthritis and regulates IL-17 production, short chain fatty acids, and the intestinal microbial flora in experimental mouse model for rheumatoid arthritis. Arthritis Res Ther. (2023) 25:130. doi: 10.1186/s13075-023-03118-3
51. Martha, AZ-R, Samuel, SM, Heather, B, Hanrui, W, Aitor, B-M, Paolo, M, et al. A distinct Fusobacterium nucleatum clade dominates the colorectal cancer niche. Nature. (2024) 628:424–432. doi: 10.1038/s41586-024-07182-w
52. Ferreira-Halder, CV, AVS, F, and Andrade, SS. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract Res Clin Gastroenterol. (2017) 31:643–648. doi: 10.1016/j.bpg.2017.09.011
53. Rebeca, M, David, R-C, Eugénie, H, Sandrine, A, Sajad, K, Luis, GB-H, et al. Faecalibacterium: A bacterial genus with promising human health applications. FEMS Microbiol Rev. (2023) 47, 1–18. doi: 10.1093/femsre/fuad039
54. R, L, D, S, R-M, P, S, B, and M, A. Bifidobacteria and their molecular communication with the immune system. Front Microbiol. (2017) 8:2345. doi: 10.3389/fmicb.2017.02345
55. Bowen, L, Mengfan, D, Xiaoming, L, Jianxin, Z, Ross, RP, Catherine, S, et al. Bifidobacterium breve Ccfm1078 alleviates collagen-induced arthritis in rats via modulating the gut microbiota and repairing the intestinal barrier damage. J Agric Food Chem. (2022) 70:1–18. doi: 10.1021/acs.jafc.2c04602
Keywords: lactating calves, diarrhea, seasons, intestinal permeability, rectal flora, short-chain fatty acids
Citation: Wang Q, Lu Q, Tang Y, Li Q, Gao P, Pang S, Zhang W, Nie C, Niu J and Ma X (2025) Integrated 16S rDNA-Seq and metabolomics reveal seasonal dynamics of gut microbial–SCFA–immune crosstalk in diarrheic calves. Front. Vet. Sci. 12:1615310. doi: 10.3389/fvets.2025.1615310
Edited by:
Zhiyuan He, Beijing University of Agriculture, ChinaReviewed by:
Yimin Zhuang, China Agricultural University, ChinaDingkun Fan, Chinese Academy of Agricultural Sciences (CAAS), China
Copyright © 2025 Wang, Lu, Tang, Li, Gao, Pang, Zhang, Nie, Niu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junli Niu, bml1anVubGlhYUAxMjYuY29t; Xiaoling Ma, MTE2NTI2MjEwM0BxcS5jb20=
 Qianqian Wang
Qianqian Wang Qicheng Lu1
Qicheng Lu1 Wenju Zhang
Wenju Zhang Junli Niu
Junli Niu Xiaoling Ma
Xiaoling Ma