- 1Institute of Animal Rearing Technologies, Faculty of Animal Sciences, Lithuanian University of Health Sciences, Kaunas, Lithuania
- 2Department of Food Safety and Quality, Faculty of Veterinary Medicine, Lithuanian University of Health Sciences, Kaunas, Lithuania
- 3Department of Anatomy and Physiology, Faculty of Veterinary Medicine, Lithuanian University of Health Sciences, Kaunas, Lithuania
- 4Institute of Microbiology and Virology, Faculty of Veterinary Medicine, Lithuanian University of Health Sciences, Kaunas, Lithuania
- 5Centre for Veterinary Systems Transformation and Sustainability, Clinical Department for Farm Animals and Food System Science, University of Veterinary Medicine, Vienna, Austria
Recently, fermented feed materials (FFM) have gained attention for their potential to improve overall performance in piglets. In this study, the effect of supplementing FFM to the diet of Topigs Norsvin Yorkshire piglets (weaning) on growth performance and health parameters was investigated. The whole experiment was divided into two phases: suckling (days 7 to 25) and weaning (days 25 to 69). During the suckling phase, 36 piglets (divided into three groups of 12 piglets/group) were assigned to three groups to differently ‘program’ their gut: (1) control (C) group, receiving a full-fledged commercial pre-starter feed, and (2) the Pp and (3) Pa groups, which received 25 mL of fermented milk permeate prepared either with Pediococcus pentosaceus LUHS183 and Pediococcus acidilactici LUHS29, respectively. In weaning, the pigs received two diets: C group received a non-fermented basal diet; Pp and Pa—same Lactiplantibacillus plantarum LUHS122, Lactobacillus casei LUHS210, Latilactobacillus curvatus LUHS51, and Lacticaseibacillus paracasei LUHS244 FFM. Results showed that weaned pigs of the Pp and Pa groups had higher body weight on day 69 compared to C group. Feed conversion ratio was similar in all three groups. On day 69, the highest concentration of immunoglobulins IgG was found in Pa group compared to other groups, while plasma alanine aminotransferase (ALT) levels were lower in treated groups compared to the C group. Diet did not influence ALT, aspartate aminotransferase (AST), faecal pH or dry matter content. On day 69, the faeces of the Pp and Pa groups exhibited higher texture hardness compared to the control (C) group. Additionally, the lactic acid bacteria (LAB) count differed significantly between the Pa and control groups. The C group had high abundances of beneficial lactobacilli and Prevotellaceae but the lowest bacterial diversity compared to the Pp and Pa groups. On day 69, faeces of treated groups had greater variability in individual volatile compounds (VCs) compared to the C group. Significant correlations between VC and faecal microbiological parameters were observed. In conclusion, the findings from this study show that with pediococci (LUHS183 and LUHS29), and lactobacilli FFM supports gut microbial diversification and homeostasis, potentially leading to improved BW gain.
1 Introduction
The gut microbiome has long been recognized for its crucial role in animal health and overall well-being. In pigs, the early establishment and maintenance of a beneficial gut microbiota are essential, as the first microbial colonizers play a key role in shaping the long-term microbial community, which ultimately influences the health and growth performance of pigs as they mature (1). It has been reported that the maternal gut microbiota of sows plays a key role in regulating fetal metabolism and immune function throughout pregnancy (2). Given the importance of these early gut colonizers, it is vital to comprehend the factors (diet, antimicrobials, probiotics, and prebiotics, among others) that influence the development of the piglet microbiome at weaning. Each of these elements can affect the piglet’s gut microbiome at weaning, influencing microbial diversity, structure, and succession within the gut. The gut microbiota offers the pig numerous benefits, including improved energy harvesting, production of volatile fatty acids, synthesis of vitamin K, cellulose fermentation, and enhanced resistance to pathogenic bacteria (3–5). As this field of study continues to evolve, it is clear that new roles and interactions between the gut microbiome and animal growth performance are still to be discovered. Additionally, further research is needed to investigate how supplementing FFM could impact piglet growth and health, particularly during the weaning period and in later stages of life. Weaning induces physiological changes in the structure and function of the intestine (6). Moreover, the gut microbiota of young piglets undergoes rapid ecological succession in response to various factors during the weaning period. One of the key factors is the abrupt change in diet from simple to more complex nutrient sources, which can affect the absorption capacity of the small intestine and likely influence growth and feed efficiency. During the weaning period piglets are exposed to thousands of new bacterial species, which may play significant roles in establishing an adult-like microbiota later in life. Additionally, establishing a beneficial microbiota during weaning is crucial, as piglets still have an immature immune system and rely on sow’s milk to prevent the colonization and overgrowth of opportunistic pathogens.
A study by Badaras et al. (7) reported that supplementing Topigs Norsvin Yorkshire newborn piglets with milk permeate fermented by lactic acid bacteria (LAB) strains Pediococcus pentosaceus LUHS183 (MPPp) and P. acidilactici LUHS29 (MPPa), which possess antimicrobial properties, could lead to beneficial changes in piglet growth performance and health parameters (7). The study concluded that feeding piglets with MPPa was beneficial for promoting weight gain, as well as fostering the growth of specific bacterial species and contributing to a distinct volatile compound (VC) profile in their faeces. While piglets fed with MPPp showed the highest IgM concentration in blood plasma. Both experimental groups demonstrated increased Lactobacillus counts in the faeces. The novelty of this study lies in the use of a novel combination of Lactiplantibacillus plantarum LUHS122, Lactobacillus casei LUHS210 Latilactobacillus curvatus LUHS51, and Lacticaseibacillus paracasei LUHS244 strains, which possess antimicrobial properties (8), for the preparation of fermented feed material (FFM), and in testing its influence on piglets, previously fed fermented milk permeate during the suckling period (7), throughout the weaning period.
The whole experiment was divided into two phases: suckling and weaning. During the suckling phase (from day 7 to 25 of life), 36 Topigs Norsvin Yorkshire piglets (divided into three groups of 12 piglets/group) were assigned to (1) the control (C) group, receiving a full-fledged commercial pre-starter feed (FFCP), and (2) the Pp and (3) Pa groups, receiving 25 mL of fermented milk permeate with Pediococcus pentosaceus LUHS183 and Pediococcus acidilactici LUHS29, respectively. During the suckling phase, the piglets had access to the sow’s milk till day 25 (7). The experiment began at the weaning phase (from the 25th day of life) and lasted for 43 days. After, we hypothesize that continuing feeding of piglets (from suckling to weaning and in the later stages) with FFM may be beneficial for enhancing their growth performance and health parameters. Therefore, in this study, the effect of supplementing FFM to the diet of piglets weaning on growth performance and health parameters including body weight (BW), feed conversion ratio (FCR), plasma immunoglobulins (IgA, IgM, IgG), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) concentrations, faecal microbiological parameters, pH, dry matter (DM), metataxonomic and the faecal volatile compound (VC) profiles were investigated.
2 Materials and methods
2.1 Fermented feed preparation
The Lb. plantarum LUHS122, Lb. casei LUHS210, Lb. curvatus LUHS51, and Lb. paracasei LUHS244 strains were obtained from the Lithuanian University of Health Sciences collection (Kaunas, Lithuania). Our previous studies have shown that the aforementioned strains inhibit various pathogenic and opportunistic microorganisms (8). Characteristics of the LAB strains used are given in Supplementary File 1. The LAB strains were stored at −80°C in a Microbank system (Pro-Lab Diagnostics, UK) and separately propagated in de Man-Rogosa-Sharpe (MRS) broth (CM 0359, Oxoid Ltd., Hampshire, UK) at 30 ± 3°C for 48 h before their use for feed fermentation.
The fermented feed part (composition: crude protein—20.08%, crude fiber—7.34%, crude oil and fats—6.23%, lysine—1.01%, methionine—0.40%, tryptophan—0.28%, threonine—0.87%, Ca—0.33%, total P—0.64%, and Na—0.02%, NaCL—0.11%, Mg—0.27%, K—0.76%, S—0.2%, starch—31.95%, sugar—5.9%), water, and LAB strains (equal parts of each strain by volume) suspension (3% from dry matter of feed mass, v/m), containing 8.9 log10 CFU/mL, was fermented at 30 ± 2°C for 24 h. The final moisture content of the feed was 60 g/100 g. The moisture content was determined by drying the samples at 103 ± 2°C to a constant weight. Whole fermented feed mass (100%) was divided into two parts (15 and 85%, by mass): 85% of the fermented feed was used for piglet feeding, and 15% was used as a starter for additional feed fermentation cycles. The principal scheme of the experiment of feed fermentation is shown in Figure 1. To ensure the stability of the quality of each batch, a WEDA (Dammann & Westerkamp GmbH, Germany) feeding system was used for animal feeding. The system is equipped with integrated industrial pH meters and thermometers. An alarm is triggered if the temperature or pH reaches the specified limit values.
2.2 Animals’ housing and experimental design
All animal procedures were conducted according to the EU Directive (9) of the European Parliament and of Council from 22 September 2010 on the protection of animals used for scientific purposes and Requirements for the Keeping, Maintenance and Use of Animals Intended for Science and Education Purposes, approved by the order of the Lithuanian Director of the State Food and Veterinary Service (10).
The study was conducted at a pig farm in the Klaipeda district (Kantvainų vill., Lithuania) and at the Institute of Animal Rearing Technologies, Lithuanian University of Health Sciences (Kaunas, Lithuania). The farm participates in the Lithuanian Agricultural and Rural Development 2023–2027 of the intervention measure “Animal welfare” of the Strategic Plan, the activity “Support for 20%” “Ensuring a larger housing area for fattening pigs” implementation rules were prepared during the implementation of the 2021 December 2 Regulation (EU) 2021/2115 of the European Parliament and of the Council (11). Piglets are also raised with unclipped tails and unground.
The principal scheme of the whole experiment is shown in Figure 2.
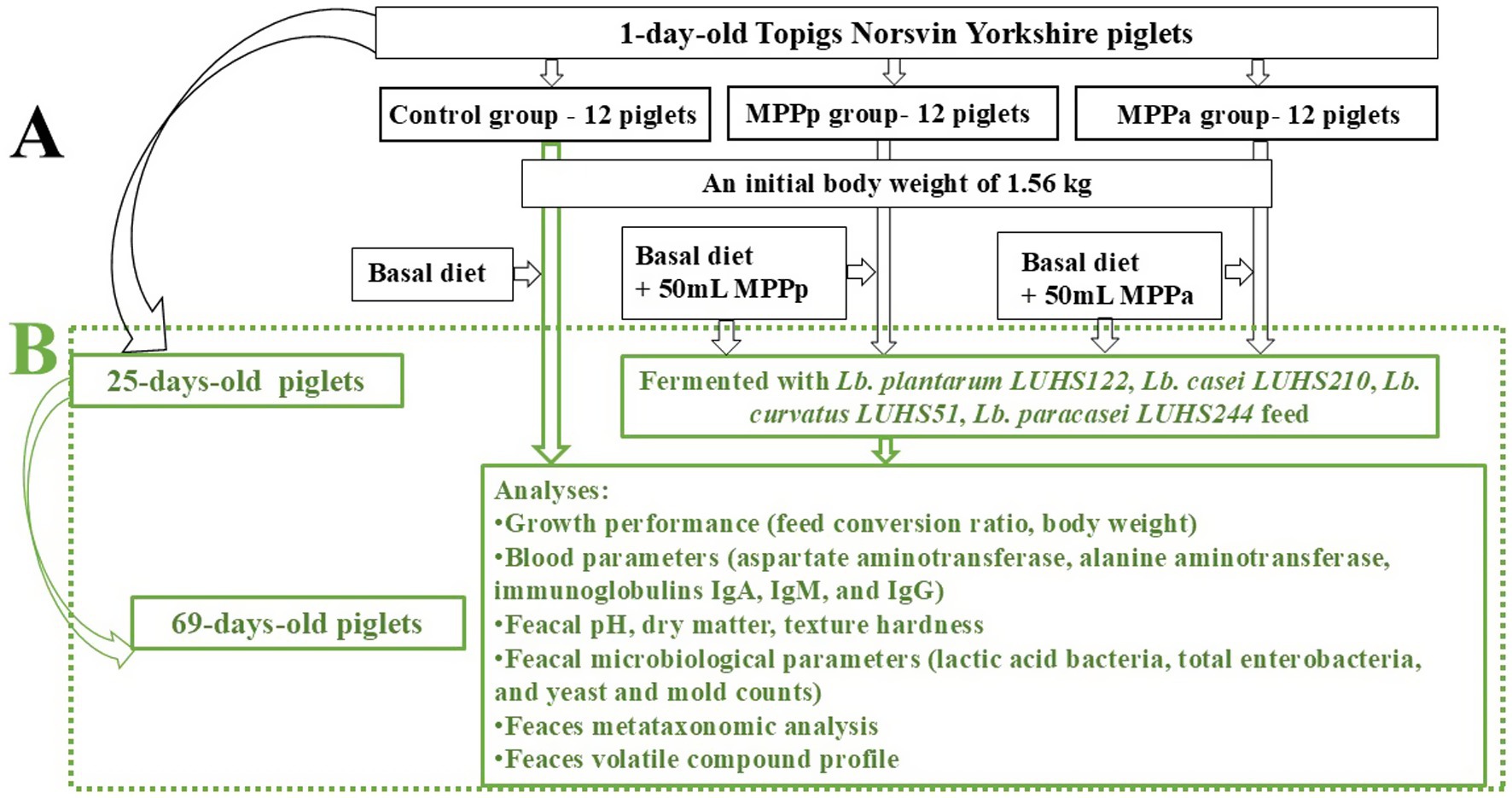
Figure 2. The principal scheme of the experiment (Part A: C, control group—fed with basal diet; MPPp, treated piglets group fed with basal diet and with 25 mL/day fermented with Pediococcus pentosaceus LUHS183 milk permeate; MPPa, treated piglets group fed with basal diet and with 25 mL/day fermented with Pediococcus acidilactici LUHS29 (2); Part B: continuous experiment: two dietary treatments for three piglet groups were compared (C, control group—fed with basal non-fermented diet, previously received full-fledged combined pre starter feed for piglets PANTO®; for treated groups, which additionally to traditional diet from the 7th day of life received fermented with Pp and Pa milk permeate (groups Pp and Pa, respectively) during the continuous experiment additionally received fermented with Lb. plantarum LUHS122, Lb. casei LUHS210, Lb. curvatus LUHS51, and Lb. paracasei LUHS244 feed material)).
The whole experiment was divided into two phases: suckling (days 7 to 25) and weaning (days 25 to 69). During the suckling phase (from day 7 to 25 of life), 36 Topigs Norsvin Yorkshire piglets (divided into three groups of 12 piglets/group) were assigned to (1) the control (C) group, receiving a full-fledged commercial pre-starter feed (FFCP), and (2) the Pp and (3) Pa groups, receiving 25 mL of fermented milk permeate with P. pentosaceus LUHS183 and P. acidilactici LUHS29, respectively. During the suckling phase, the piglets had access to the sow’s milk till day 25. Results of the suckling phase are reported by Badaras et al. (7).
The experiment began at the weaning phase (from the 25th day of life) and lasted for 43 days. At weaning, the body weight of the piglets was 6.95 ± 0.07 kg in the control (C), Pp and Pa groups. The diet of piglets (from 25th till 69th days) was composed of crude protein—19.09%, crude fiber—3.01%, crude fats—5.98%, av. lysine—1.55%, av. methionine—0.67%, av. tryptophan—0.25%, av. threonine—0.98%, Ca—0.86% and total P—0.62%. The weaned pigs were kept in a section with two climate zones. The first had a heated concrete floor (32°C) with a roof over it, while the second had plastic piglet floors and optimally ventilated air and temperature for the active period. The barn also housed other piglets from the same weaning week. It contained 24 pens—12 on the right and 12 on the left—separated by a central drive path. The feeding system was designed to deliver two different feed formulations through a single feeding tube within the same barn: fermented feed to the left side and commercial feed to the right side. Both experimental groups were fed from the same trough, while the control group was fed from a separate trough to allow accurate calculation of feed consumption. Drinking water and compound liquid feed were available ad libitum throughout the trial. Antimicrobial treatment was not applied.
The piglets were distributed into three groups, and blood plasma samples from 6 animals per group were collected. Weaning, two dietary treatments were compared for three groups: (i) non fermented basal diet for piglets previously fed with full-fledged combined pre starter feed for piglets PANTO® (control group) and (ii) fermented with Lb. plantarum LUHS122, Lb. casei LUHS210, Lb. curvatus LUHS51 and Lb. paracasei LUHS244 strains combination for piglets previously fed with fermented with Pp and Pa milk permeate (groups Pp and Pa, respectively) (Figure 2). Fermented feed comprised 450 g/kg of total feed. The basal feed was formulated according to the nutritional requirements prescribed in the Nutrient Requirements of Swine (12). The feed composition and nutritional values are shown in Table 1. Dietary components were selected according to the AOAC recommendations (13).
2.3 Evaluation of piglets’ growth performance
Both the experimental and control groups were weighed at age of 25, 26, 34, 41, 48, 55, 62, and 69 days of life using an electronic scale (model: IT1000, SysTec GmbH, Bergheim, Germany). To weigh only the selected piglets after driving them out of the barn, the necessary ones were separated in the corridor towards the scale, trying to cause the piglets as little stress as possible. All three study groups had individually numbered ear tags of different colors to facilitate their identification within the group. Feed conversion ratio (FCR) was calculated from feed intake (87% dry matter) and body weight (BW) gain recorded on the same days using a WEDA (Dammann & Westerkamp GmbH, Germany) automated feeding system. The feed components were mixed in a mixer and fed to pigs according to a feeding curve. The feed flow meter calculated the amount of feed added to each trough during feeding.
2.4 Blood plasma analysis
Blood samples were taken on days 25 (starting point) and 69 (end of FFM supplementation), before morning feeding. Piglets were fixed with a nose twister and blood was collected from the jugular vein into vacuum blood tubes (BD Vacutainer, Plymouth, UK) with a 18Gx1 ½″ needle number. The parameters included immunoglobulins IgA, IgM, IgG, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were analysed with an automatic biochemistry analyzer in the accredited laboratory “Anteja” (Klaipeda, Lithuania). Blood samples were collected by a licensed veterinarian (Sarunas Badaras veterinary practice license Nr. 1610) working on the farm.
2.5 Evaluation of faecal parameters
2.5.1 Evaluation of faecal pH, dry matter, and texture hardness
For the evaluation of faecal pH, dry matter, texture hardness, and microbiological analysis, faecal samples were individually collected from 12 piglets in each group before (on day 25) and after (on day 69) the experiment. The samples were stored in vials at +4°C in a transport medium (Faecal Enteric Plus, Oxoid, Basingstoke, UK) and analysed on the same day.
2.5.2 Microbiological analysis of faecal samples
For the evaluation of viable LAB count, 10 g of the faecal sample were homogenized with 90 mL of saline (9 g/L NaCl solution). Serial dilutions of 10−4 to 10−8 with saline were used for sample preparation. Sterile De Man, Rogosa and Sharpe (MRS) agar (CM0361, Oxoid) of 5 mm thickness was used for bacterial growth on Petri dishes. The dishes were separately seeded with the sample suspension using surface sowing and were incubated under anaerobic conditions at 30°C for 72 h. All results were expressed in log10 CFU/g (colony forming units per g of sample). Violet Red Bile Glucose (VRBG) agar (Oxoid Ltd., Basingstoke, United Kingdom) was used to analyze the total enterobacteria count (TEC) and Dichloran Rose Bengal Chloramphenicol (DRBC) agar (Liofilchem, Milan, Italy) was used to analyze yeast/mold (Y/M) count in faecal samples.
2.6 Metataxonomic analysis of the bacterial composition in the faecal samples of piglets’
On day 25 (starting point) and on day 69 (end of feed supplementation) faeces from all piglets in all the groups were collected using sterile cotton swabs. Pooled samples representing each of the group were made by mixing equal amount (0.5 g) of faeces taken individually, for targeted sequencing of 16S rRNA. Pooled samples were stored in were stored in DNA/RNA Shield (1:10 dilution; R1100-250, Zymo Research, USA) at −70°C until DNA extraction. ZymoBIOMICS®-96 MagBead DNA Kit (Zymo Research, Irvine, CA) was used to extract DNA using an automated platform according to manufacturers instructions. Bacterial 16S ribosomal RNA gene targeted sequencing was performed using the Quick-16S™ NGS Library Prep Kit (Zymo Research, Irvine, CA). The bacterial 16S custom-designed by Zymo Research primers amplified the V3-V4 region of the 16S rRNA gene. The sequencing library was prepared using real-time PCR. The final PCR products were quantified with qPCR fluorescence readings and pooled together based on equal molarity. The final pooled library was cleaned with the Select-a-Size DNA Clean & Concentrator™ (Zymo Research, Irvine, CA), then quantified with TapeStation®(Agilent Technologies, Santa Clara, CA) and Qubit® (Thermo Fisher Scientific, Waltham, WA). The final library was sequenced on Illumina® Nextseq™ with a P1 reagent kit (600 cycles). The sequencing was performed with 30% PhiX spike-in. Unique amplicon sequence variants were inferred from raw reads using the DADA2 pipeline (14). Potential sequencing errors and chimeric sequences were also removed with the Dada2 pipeline. Taxonomy assignment was performed using Uclust from Qiime v.1.9.1 with the Zymo Research Database, a 16S database that is internally designed and curated, as reference. Composition visualization and alpha-diversity analyse were performed with Qiime v.1.9.1 (15). The number of genome copies per microliter DNA sample was calculated by dividing the gene copy number by an assumed number of gene copies per genome.
2.7 Analysis of the faecal volatile compound profile
Faeces were prepared for gas chromatography (GC) analysis by using solid-phase microextraction (SPME). An SPME device with Stableflex (TM) fibre, coated with a 50-μm DVB-PDMS-Carboxen™ layer (Supelco, USA), was used for sample preparation. For gas chromatography–mass spectrometry (GC–MS), a GCMS-QP2010 (Shimadzu, Japan) was used. The gas chromatograph was equipped with an AOC-5000 Plus Shimadzu autosampler, upgraded with an SPME analysis kit. Analysis was performed according to the procedure described by Vadopalas et al. (16).
2.8 Statistical analysis
In order to compare the differences in parameter means between the different groups (C, Pp, Pa), a paired samples t-test was used (for growth performance (n = 12 for each group); for blood plasma parameters at the beginning and at the end of the experiment (n = 6 for each group); for the pH, dry matter, texture, VC profile, and microbiological parameters of faecal samples before (on day 25) and after (at day 69) the experiment (n = 12 for each group)). For metataxonomic analysis of metataxonomic analysis of the bacterial composition in the faecal samples, at the beginning of experiment, samples were collected from 12 piglets from each group and a single pooled sample was prepared for microbiome profiling, after the experiment, faeces from 12 piglets from the C, Pp, and Pa groups were collected and thereafter, three pooled samples, representing each of the groups, were prepared and analysed. The p values of factor (diet) influence were determined by multivariate tests of between-subjects effects. Baseline measurements were used as covariates to account for the experimental conditions. The mean values were compared using Duncan’s multiple range post-hoc test with the significance level defined at p ≤ 0.05. In the tables, the results are presented as mean values with pooled standard errors. Also, Pearson’s correlations between characteristics were calculated, strength of the correlations was interpreted according to Evans (17). Correlations were considered significant when p ≤ 0.05. Differences in bacterial genera/species between the groups were assessed using the Z-test calculator for two population proportions (18). The number of reads of each genera/species from the total number of reads in samples was counted. Relative abundance of the genera/species in samples then were compared between the groups. Two-tailed hypothesis was counted. For the quality control of taxonomical identification, standard (D6300, Zymo Research, Murphy Ave. Irvine, CA, USA) of mixed known bacterial cultures were sequenced together with sample sequencing. Statistical comparisons were considered significant when p ≤ 0.05. Heatmap visualisation and analysis were conducted using the R statistical programming software package “ComplexHeatmap” (version 2.14.0). Partial least squares discriminant analysis (PLS-DA) and variable importance of projection (VIP) analyses were conducted using the “ropls” package (version 1.30.0). Volcano plots were generated with the “EnhancedVolcano” package (version 1.16.0). Missing values in samples (not detected in chromatogram) were filled using half minimum method. Pearson’s correlation analysis and visualizations were carried out using “psych” (version 2.4.12) and “corrplot” (version 0.95) packages.
3 Results
3.1 Piglets’ growth performance
Piglets’ body weights (kg) at age of 25, 26, 34, 41, 48, 55, 62, and 69 days of life are shown in Figure 3. On days 25 and 26, the piglets fed with Pa fermented milk permeate during the suckling phase had the highest body weight compared to the piglets fed the C and Pp fermented milk permeate (p < 0.05). On day 25, their body weight was, on average, 9.65% higher than that of the control group and the group previously fed with Pp fermented milk permeate. On day 26, their weight was, on average, 8.97% higher than that of the control group and the Pp group, respectively. However, on day 34, Pp piglets started to gain weight, and by day 41, both the Pa and Pp groups had, on average, 13.7% higher body weight than the control group. Similar trends were observed until the end of the experiment, except on day 55, when the Pa piglets’ body weight was lower compared to the Pp group.
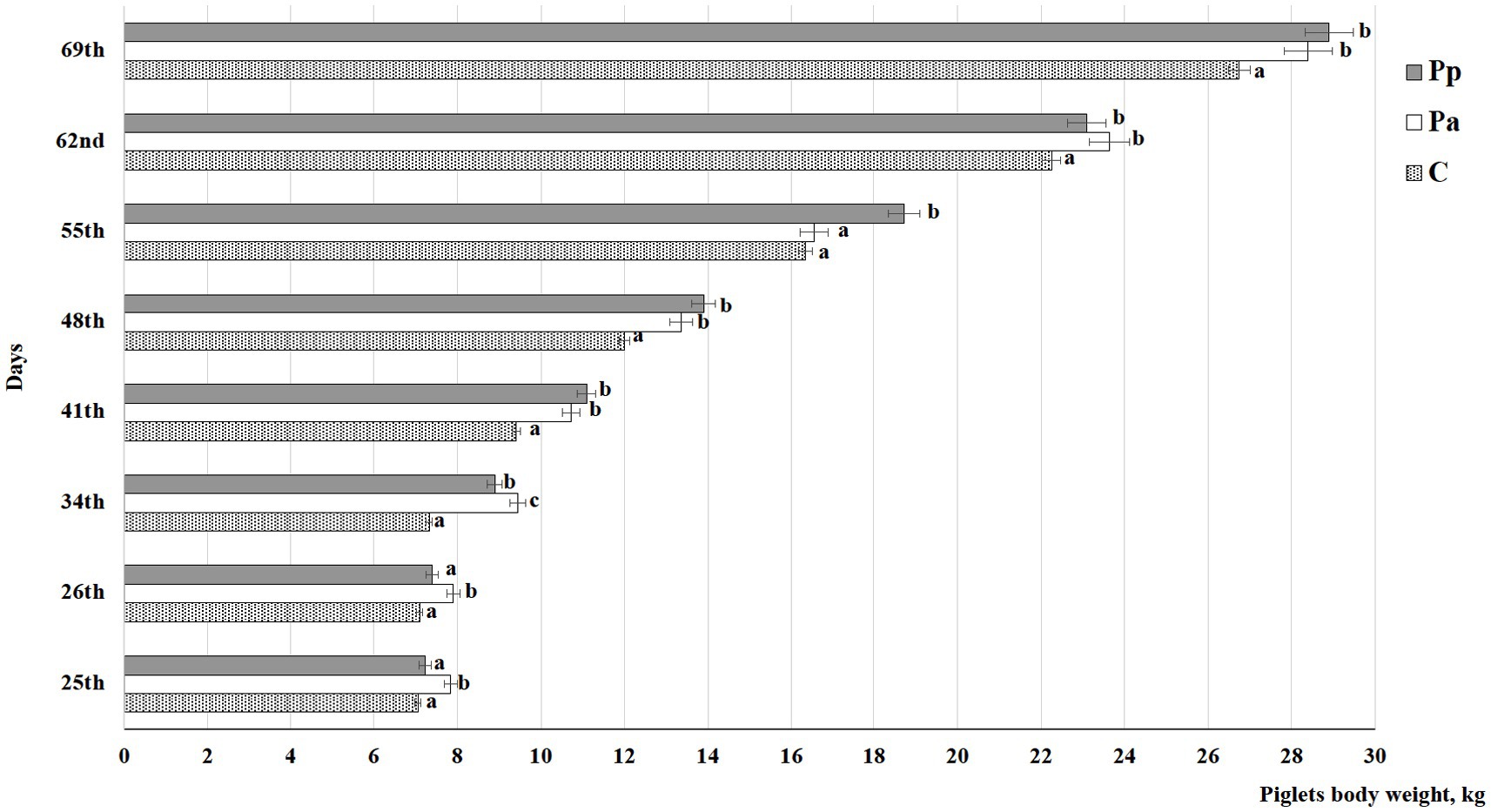
Figure 3. Piglets’ body weight, kg (C, control group—fed with basal non-fermented diet, previously received full-fledged combined pre starter feed for piglets PANTO®; Pp and Pa groups—previously, additionally to traditional diet from the 7th day of life received fermented with Pp and Pa milk permeate (groups Pp and Pa, respectively) and during the continuous experiment additionally received fermented with Lb. plantarum LUHS122, Lb. casei LUHS210, Lb. curvatus LUHS51, and Lb. paracasei LUHS244 feed material); a,cDifferent letters indicate differences among different piglet groups on the same day (p ≤ 0.05). The data are presented as the mean ± standard deviation (n = 12/group).
The feed conversion ratio (FCR) calculated on day 69 is shown in Figure 4. Significant differences in the FCR between the C, Pp, and Pa groups were not established.
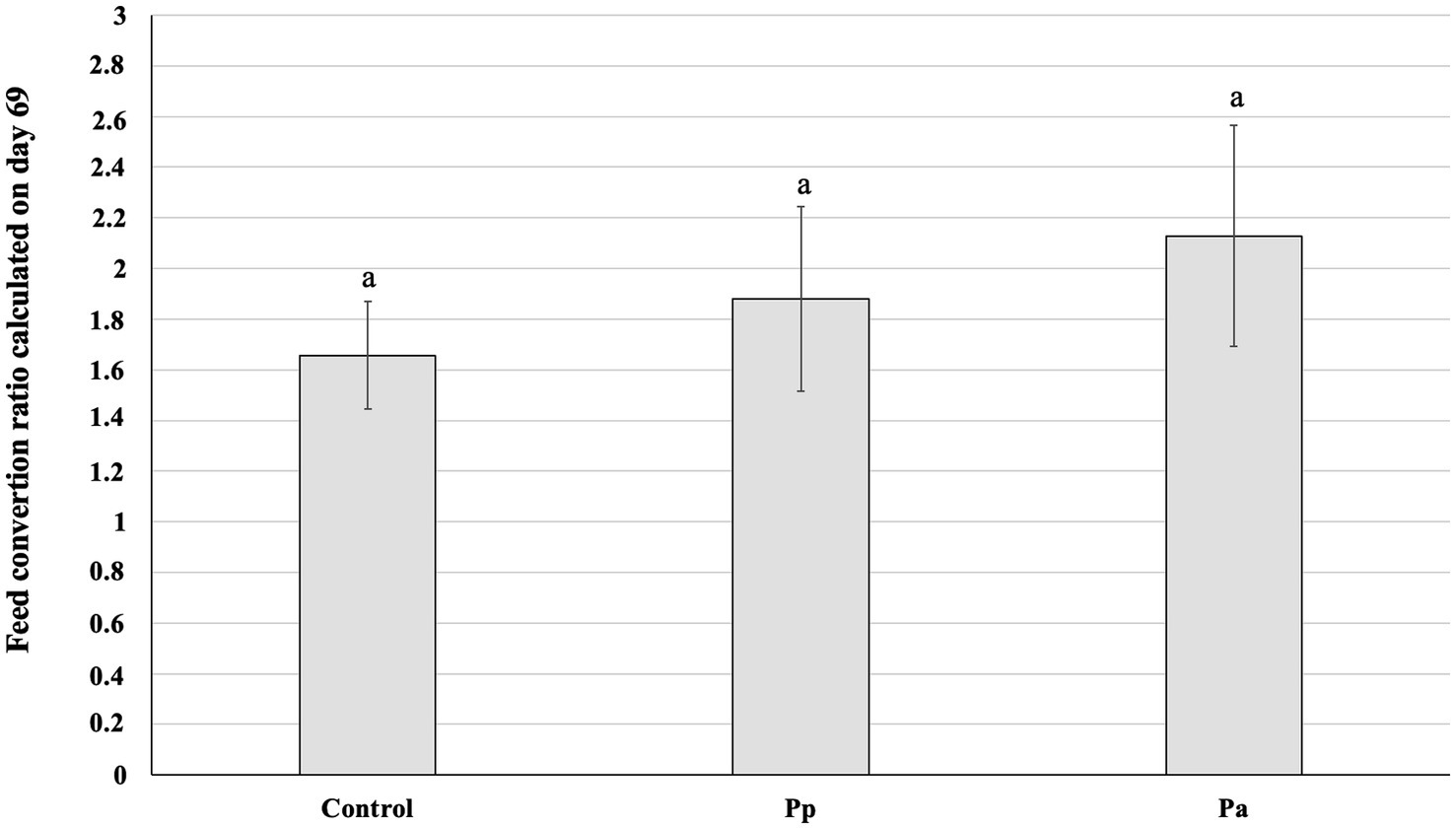
Figure 4. Feed conversion ratio calculated on day 69 (Control, control group—fed with basal non-fermented diet, previously received full-fledged combined pre starter feed for piglets PANTO®; Pp and Pa groups—previously, additionally to traditional diet from the 7th day of life received fermented with Pp and Pa milk permeate (groups Pp and Pa, respectively) and during the continuous experiment additionally received fermented with Lb. plantarum LUHS122, Lb. casei LUHS210, Lb. curvatus LUHS51, and Lb. paracasei LUHS244 feed material). The data are presented as the mean ± standard deviation (n = 12/group). a Different letters indicate differences among different piglet groups (p ≤ 0.05).
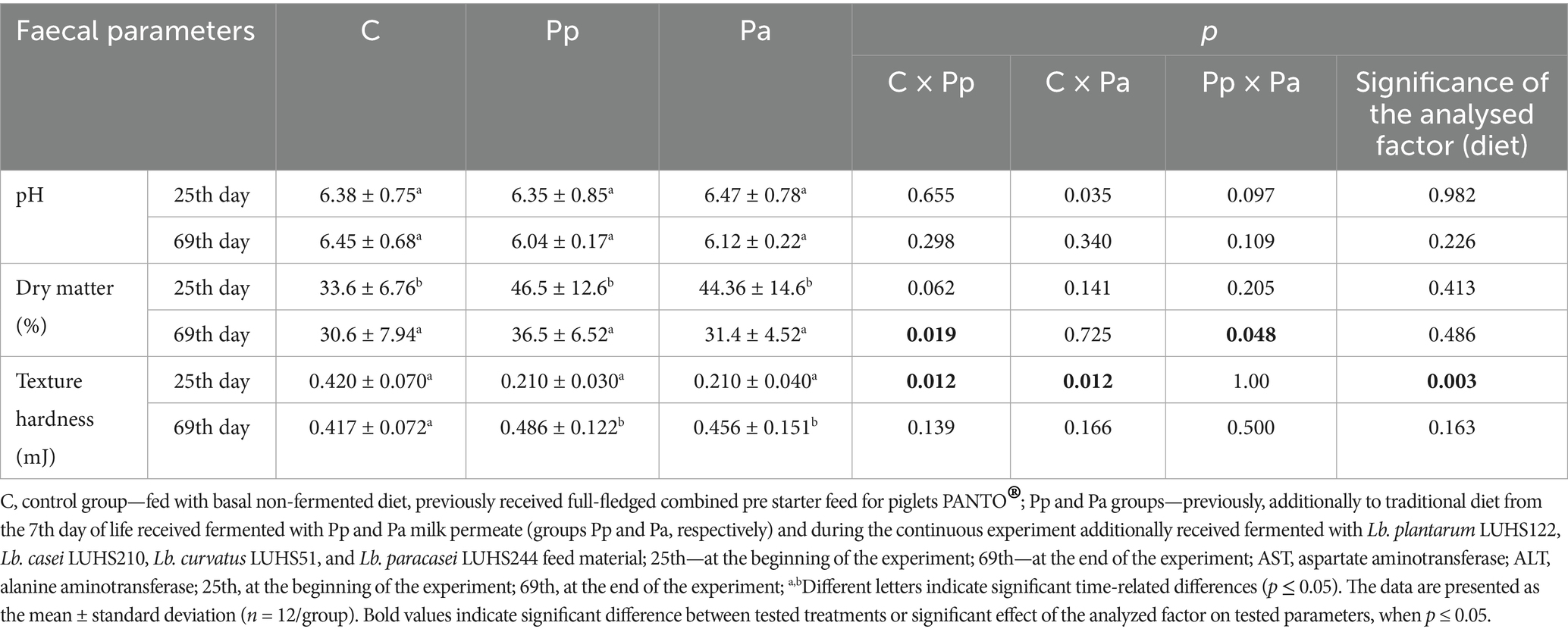
3.2 Piglets’ blood plasma parameters
The blood plasma parameters of piglets on 25 and 69 days old are shown in Table 2. In all groups, at both ages, the IgA concentration in piglet blood plasma was <0.33 g/L. Although no significant differences were found between the piglet groups in IgM concentration in blood plasma, diet at 25 days was a significant factor for IgM levels (p = 0.003). Additionally, when comparing IgM concentration in piglet blood plasma on 25 and 69 days, higher IgM concentrations were found in the C and Pa groups on 69 days. Higher concentration of IgG was found in the blood plasma of Pa piglets on day 69, in comparison with the C group. However, no significant differences in IgG concentrations were found between the control and Pp groups, and diet was not a significant factor for IgG concentration. When comparing ALT concentration in piglet blood plasma, ALT levels on 69 days were lower in both treated groups (Pp and Pa) compared to the C group (p < 0.05). However, diet was not found to be a significant factor affecting ALT concentration. AST concentration was higher in the C and Pa groups versus the Pp group after 69 days of the experiment, but not on day 25. However, diet was not found to be a significant factor for AST levels in piglet blood plasma.
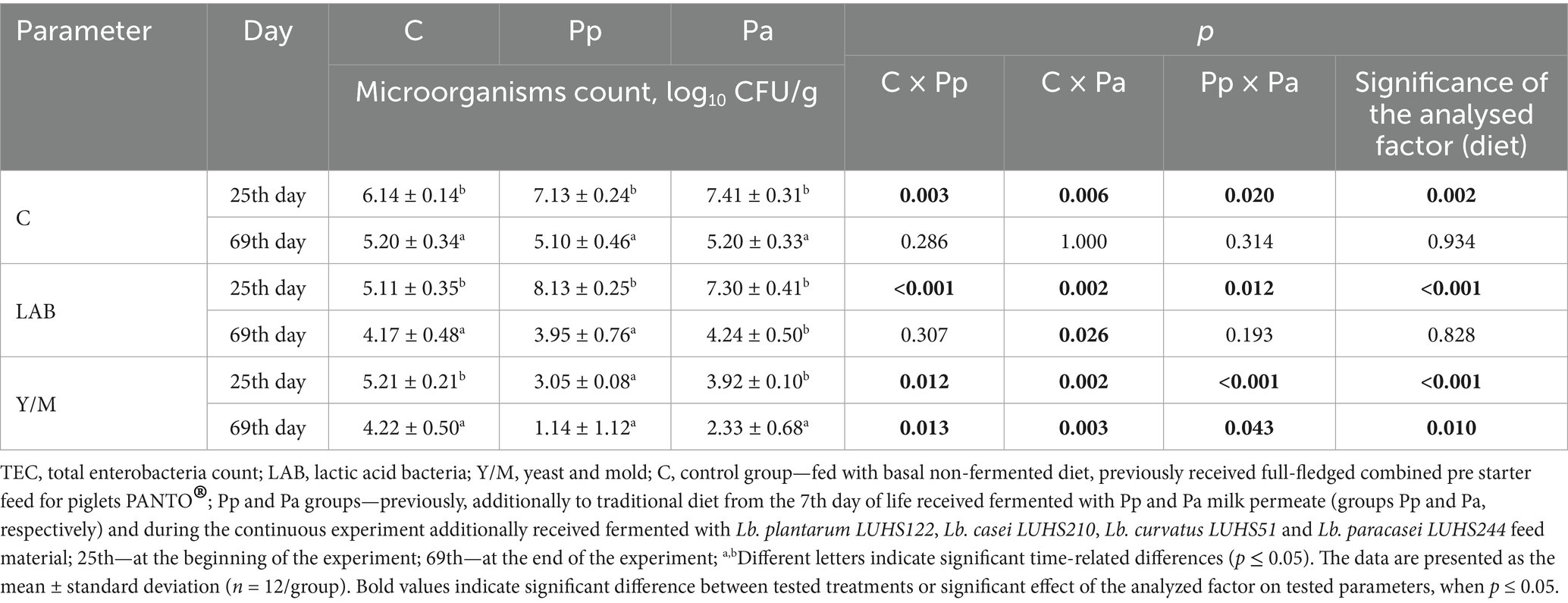
3.3 Piglets’ faecal pH, dry matter, and texture hardness
The pH, dry matter, and texture hardness of piglets’ faeces are shown in Table 3. No significant differences in faecal pH were found between groups. However, on day 69, the highest faecal dry matter content was observed in the Pp piglets. Diet was not a significant factor for faeces pH or dry matter content. At the end of the experiment, both treated groups showed higher faecal texture hardness compared to the control group. However, on day 69, diet was not a significant factor for faecal texture hardness.
3.4 Microbiological parameters of piglets’ faeces
Microbiological parameters of piglet faecal samples are shown in Table 4. In all cases, a significantly higher total enterobacteria count (TEC) was observed in piglet faeces at day 25 of life. On day 69, no significant differences in TEC were found between the different groups. On day 69, the significantly higher LAB count was observed in the Pa group, compared to the control group (p = 0.026). However, no significant differences in LAB counts were found between the Pp and C group as well as between Pp and Pa groups. Additionally, the control group exhibited the highest yeast and mold counts (at the beginning and at the end of the experiment). In comparison, the yeast and mold count in the Pp and Pa groups were lower, with the Pp group showing the lowest count. At the end of the experiment, diet was a significant factor in the yeast and mold counts in piglet faeces.
3.5 Metataxonomic composition of faecal microbiota
Table 5 demonstrates faecal bacterial composition in pigs’ faeces at a genus level on the 25th day.
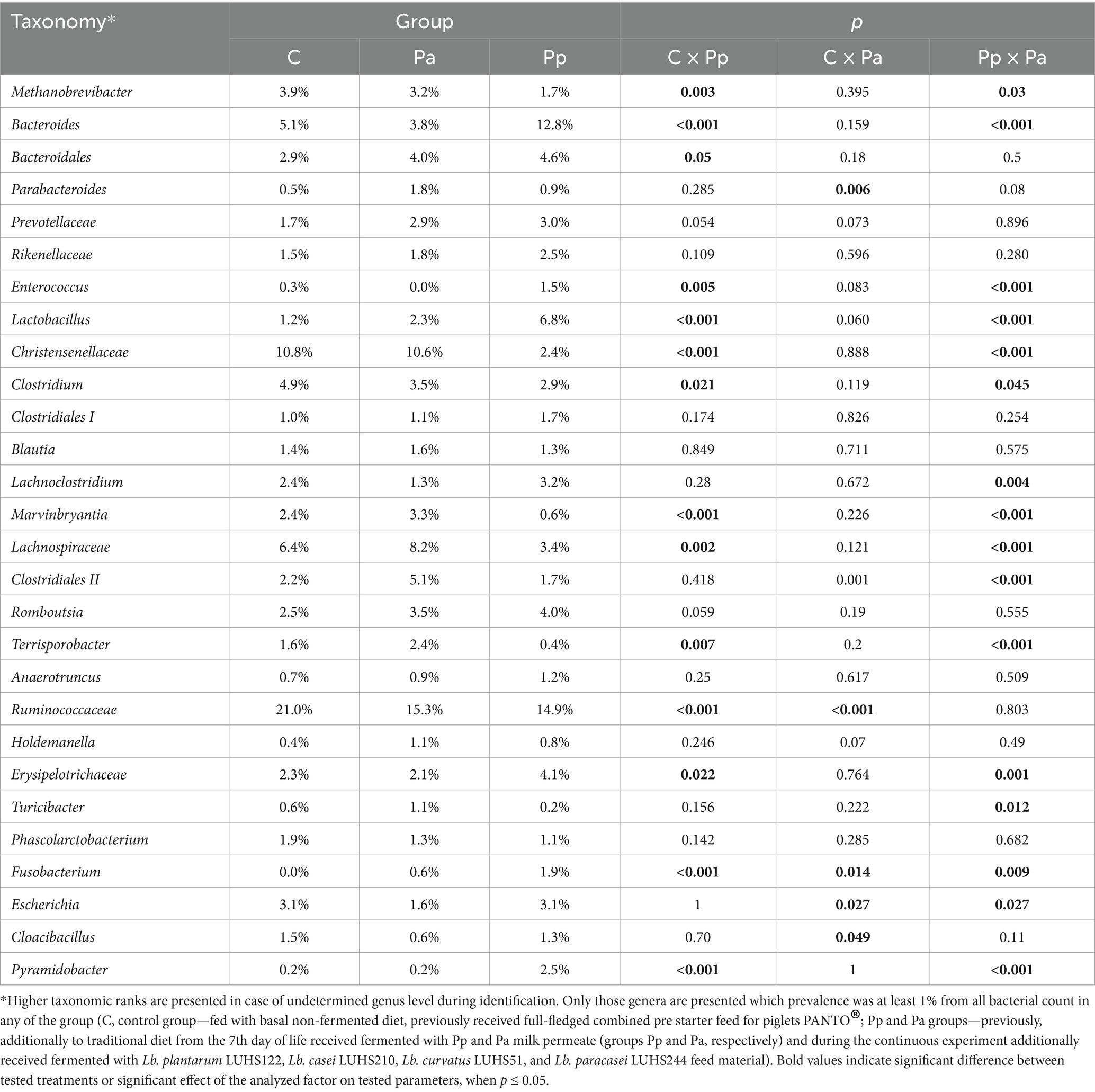
Table 5. Differences in relative abundances (% of all reads) of bacterial genera in pigs’ faeces on the 25th day.
On day 25, in all the groups of pigs, the most prevalent bacteria belonged to an unclassified genus within the family Ruminococcaceae with a relative abundance of 14.9 to 21.0% of all sequences. The second most prevalent family in the C and Pa groups was Christensenellaceae, with a relative abundance of 10.8 and 10.6%, respectively. In the Pp group, the second most prevalent genus was Bacteroides, with a relative abundance of 12.8%. Bacteria from this genus were also highly abundant in the other two groups. The most prevalent bacteria detected at species level on the 25th day are presented in Figure 5.
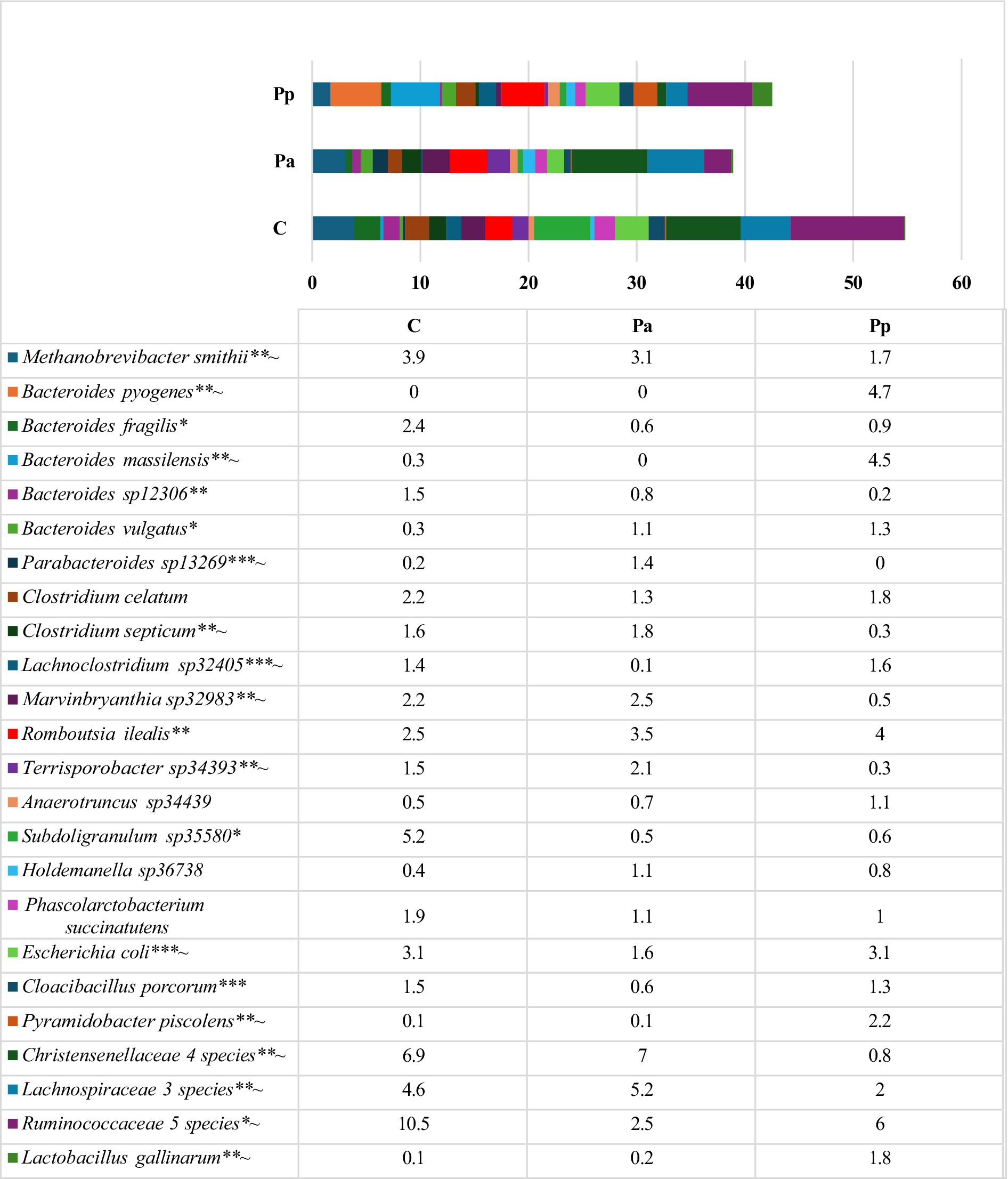
Figure 5. Differences in relative abundances (% of all reads) of bacterial amplicon sequence variants (ASV) in the pigs’ faeces on the 25th day (Only these ASV are presented which prevalence was ≥ 1% from all bacterial count in any of the animal group; C, control group—fed with basal non-fermented diet, previously received full-fledged combined pre starter feed for piglets PANTO®; Pp and Pa groups—previously, additionally to traditional diet from the 7th day of life received fermented with Pp and Pa milk permeate (groups Pp and Pa, respectively) and during the continuous experiment additionally received fermented with Lb. plantarum LUHS122, Lb. casei LUHS210, Lb. curvatus LUHS51, and Lb. paracasei LUHS244 feed material). *Statistically significant results (p ≤ 0.05) between the control and experimental groups; **Statistically significant results (p ≤ 0.05) between the control and Pp group; ***Statistically significant results (p ≤ 0.05) between the control and Pa group; ~Statistically significant results (p ≤ 0.05) between Pa and Pp groups.
Data from amplicon sequencing showed a very complex bacterial community in pigs of all groups, without clearly dominant species however, the overall composition has some differences in each animal group. In the C group the most prevalent species were Methanobrevibacter smithii, Escherichia coli, Bacteroides fragilis, Romboutsia ilealis, Clostridium celatum, Marvinbryanthia sp32983 and different species from Rumonococcaceae, Christensenellaceae and Lachnospiraceae. Besides from those species, in group Pa the most abundant species were Romboutsia ilealis, Methanobrevibacter smithii, Marvinbryanthia sp32983 and Terrisporobacter sp34393, whereas in Pp group—Bacteroides pyogenes, Bacteroides massilensis, Romboutsia ilealis, Escherichia coli and Pyramidobacter piscolens. This group also comprised the highest relative abundance of Lactobacillus gallinarum (1.8%) and other Lactobacillus spp.
On day 69, the most prevalent genera/families in faeces of the C group were Lactobacillus (19.8%), Prevotellaceae (11.0%), Ruminococcaceae (10.1%) and Lachnospiraceae (7.4%) which made almost half (48.3%) of all the bacterial load in pigs’ faeces. In Pp group, the most prevalent genera/families were Ruminococcaceae (14.9%), Prevotellaceae (10.1%), Lactobacillus (5.7%) and Lachnospiraceae (5,5%), i.e., the same bacterial genera as in the control group. In Pa group the most prevalent genera/families were Ruminococcaceae (17.4%), Lachnospiraceae (10.2%), Prevotellaceae (7.7%), Lactobacillus (4.4%) and Bacteroidales (5.0%). Bacteroidales also were abundant in other two groups as well. Bacterial composition on the 69th day at the genus level in all the animal groups is presented in Table 6.
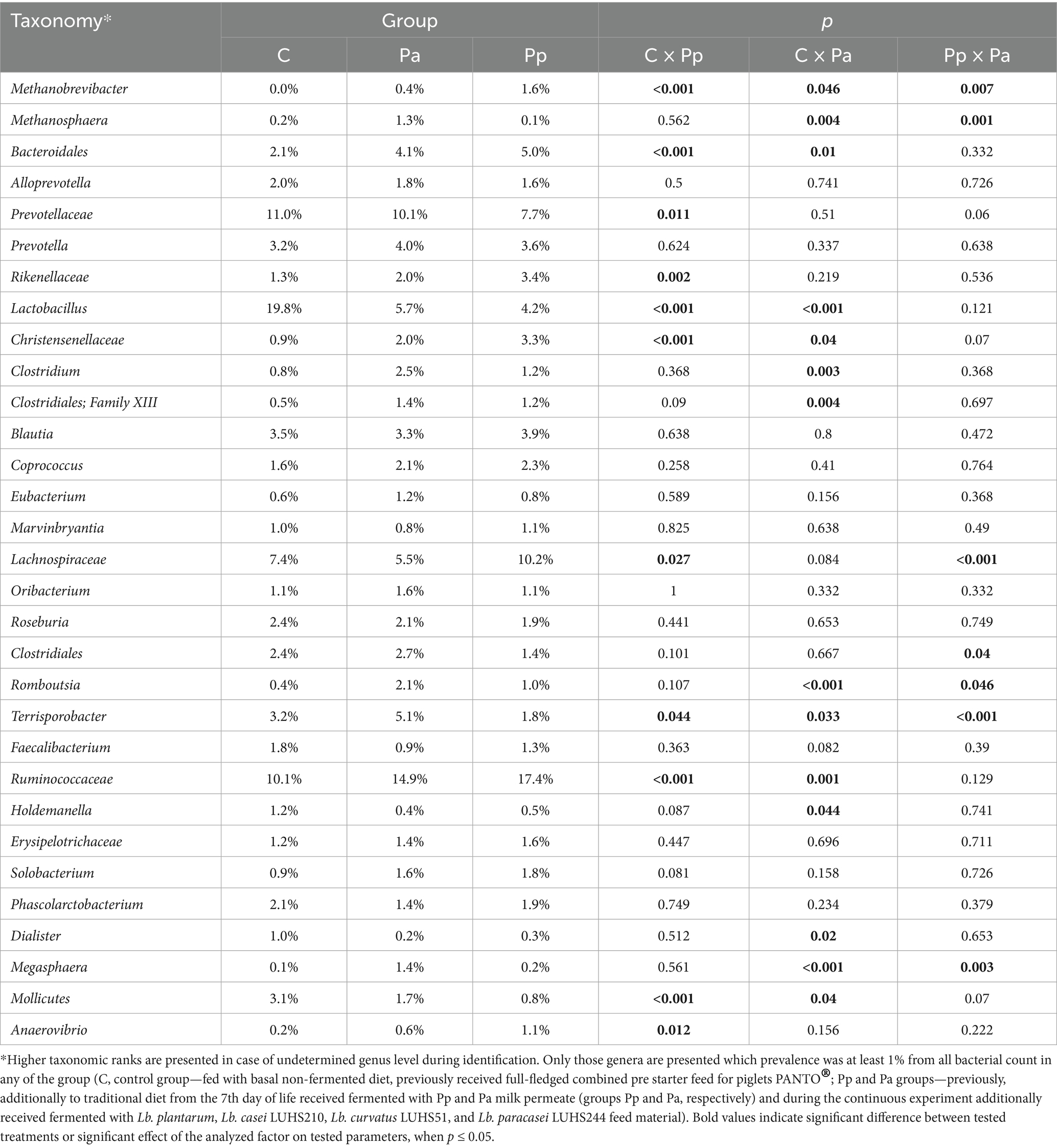
Table 6. Differences in relative abundances (% of all reads) of bacterial genera in pigs’ faeces on day 69.
On the species level (Figure 6) the most prevalent bacteria in the control group were different species of Lactobacillus, from which only minority of them were identified up to the species level (L. pontis, L. amylovorus, L. panis species) which has the highest prevalence among all other bacteria (18.2%). The other most prevalent species in this group were different species of Prevotellaceae (10.6%), Terrisporobacter (2.8%) and Clostridiales (2.3%). In Pp group the most prevalent bacteria were species from Prevotellaceae (9.8%), Lactobacillus (4.5%), Terrisporobacter (4.6%), and Bacteroidales (2.9%). In group Pa, the most prevalent bacteria were Prevotellaceae (7.4%), Lactobacillus (3.4%) and Bacteroidales (3.4%).
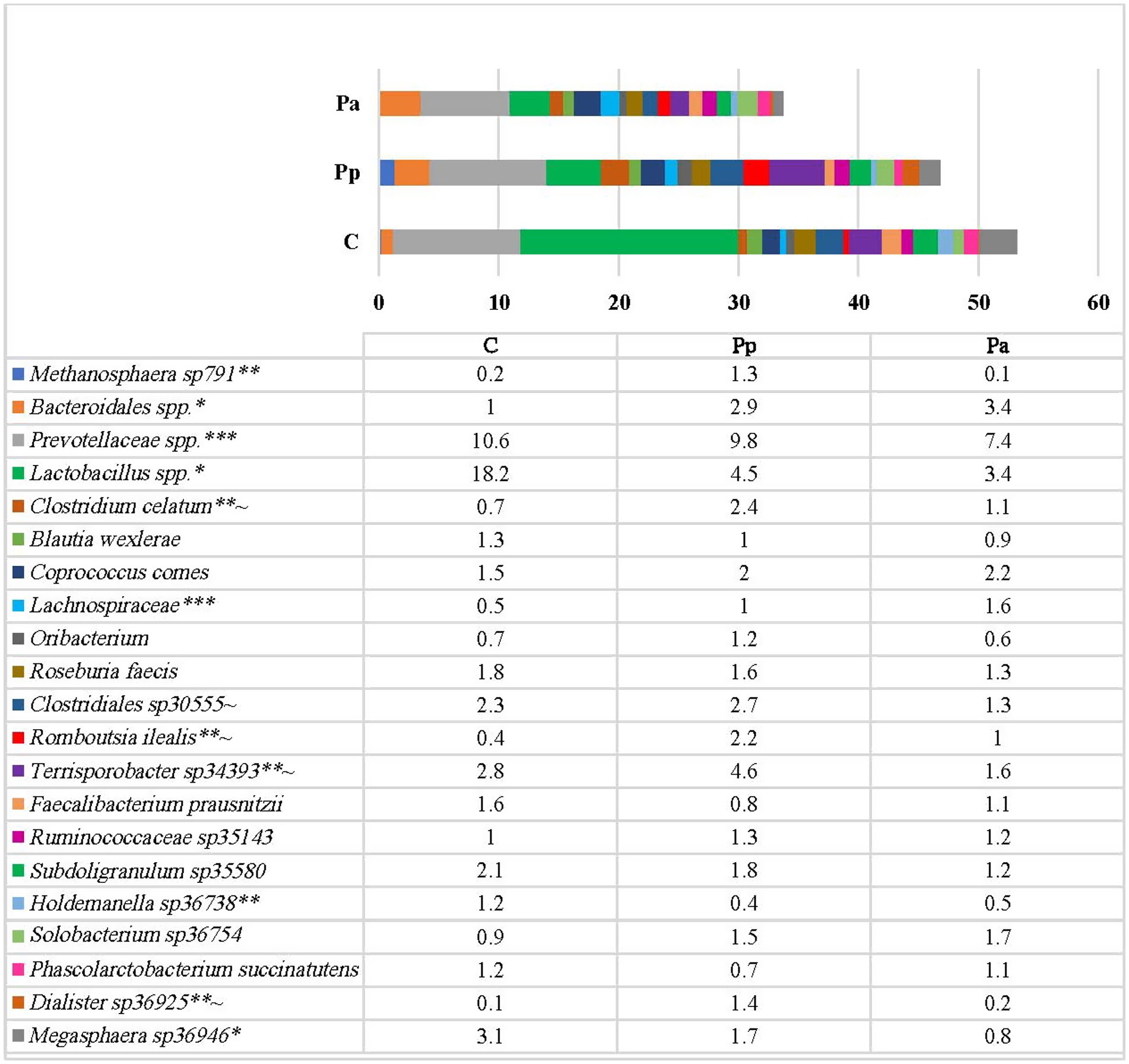
Figure 6. Differences in relative abundances (% of all reads) of bacterial amplicon sequence variants (ASV). Only these species are presented which prevalence was ≥ 1% from all bacterial count in any of the animal group (C, control group—fed with basal non-fermented diet, previously received full-fledged combined pre starter feed for piglets PANTO®; Pp and Pa groups—previously, additionally to traditional diet from the 7th day of life received fermented with Pp and Pa milk permeate (groups Pp and Pa, respectively) and during the continuous experiment additionally received fermented with Lb. plantarum LUHS122, Lb. casei LUHS210, Lb. curvatus LUHS51, and Lb. paracasei LUHS244 feed material). *Statistically significant results (p ≤ 0.05) between the control and experimental groups; **Statistically significant results (p ≤ 0.05) between the control and Pp group; ***Statistically significant results (p ≤ 0.05) between the control and Pa group; ~Statistically significant results (p ≤ 0.05) between Pa and Pp groups.
Figure 7 demonstrates alpha diversity (richness of the species) among all animal groups on days 25 and 69.
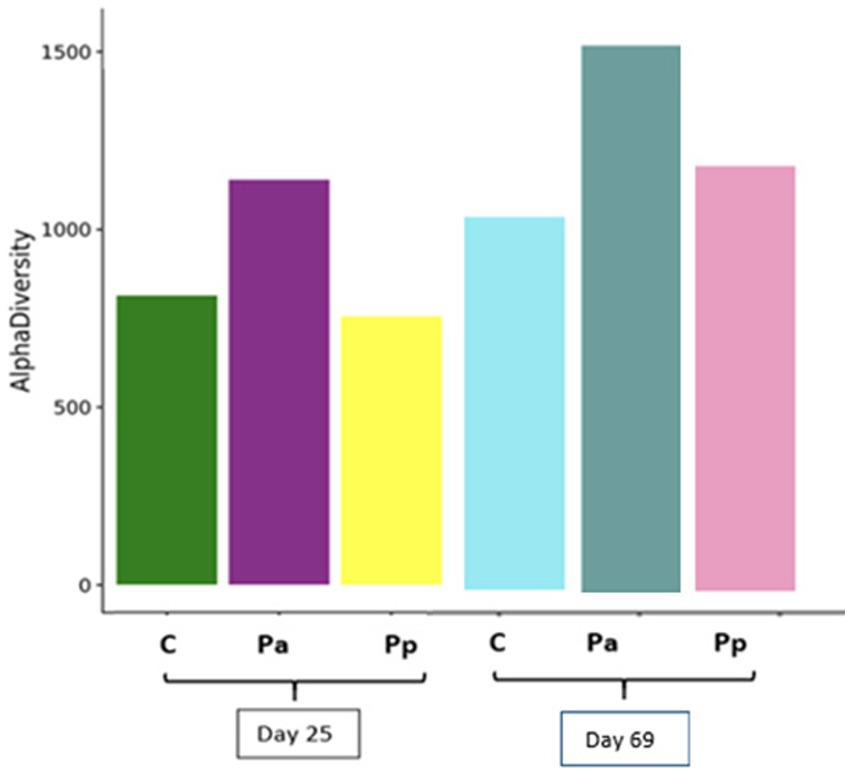
Figure 7. Species richness of the bacterial communities in the faeces of pigs on day 25 and 69 (C, control group—fed with basal non-fermented diet, previously received full-fledged combined pre starter feed for piglets PANTO®; Pp and Pa groups—previously, additionally to traditional diet from the 7th day of life received fermented with Pp and Pa milk permeate (groups Pp and Pa, respectively) and during the continuous experiment additionally received fermented with Lb. plantarum LUHS122, Lb. casei LUHS210, Lb. curvatus LUHS51, and Lb. paracasei LUHS244 feed material).
It was determined that species variety increased with the pig age. The higher richness of species diversity was detected at the end of the feeding trial in comparison with day 25. Although the C group has highest numbers of some beneficial bacteria (Lactobacillus and Prevotellaceae), it also has the lowest bacterial richness in comparison with experimental groups of pigs.
3.6 Volatile compound profiles of piglets’ faeces
Volatile compound (VC) profiles of piglets’ faeces are shown in Figure 8. When comparing the faecal VC profiles on day 69 between C and treated groups, butyl 2-methylbutanoate, 3-methylbutanoic acid butyl ester, non-(2E)-enal, methyl octyl ketone, benzeneacetic acid methyl ester, ethyl hydrocinnamate, decyl methyl ketone, geranyl acetone, Z-2-dodecenol, cyclododecanol, (2E)-2-tetradecenal, hexadecene epoxide, (Z)-7-hexadecenal, nonadecane, hexadecanoic acid methyl ester, hexadecanoic acid, eicosane, and octadecanal were detected only in the faeces of the Pa and Pp groups but not in those of the C group. On the other hand, 1,3-di-tert-butylbenzene, 11-dodecen-1-yl acetate, pentadecanol, and cis-9-hexadecenal were found exclusively in the faeces to the C group.
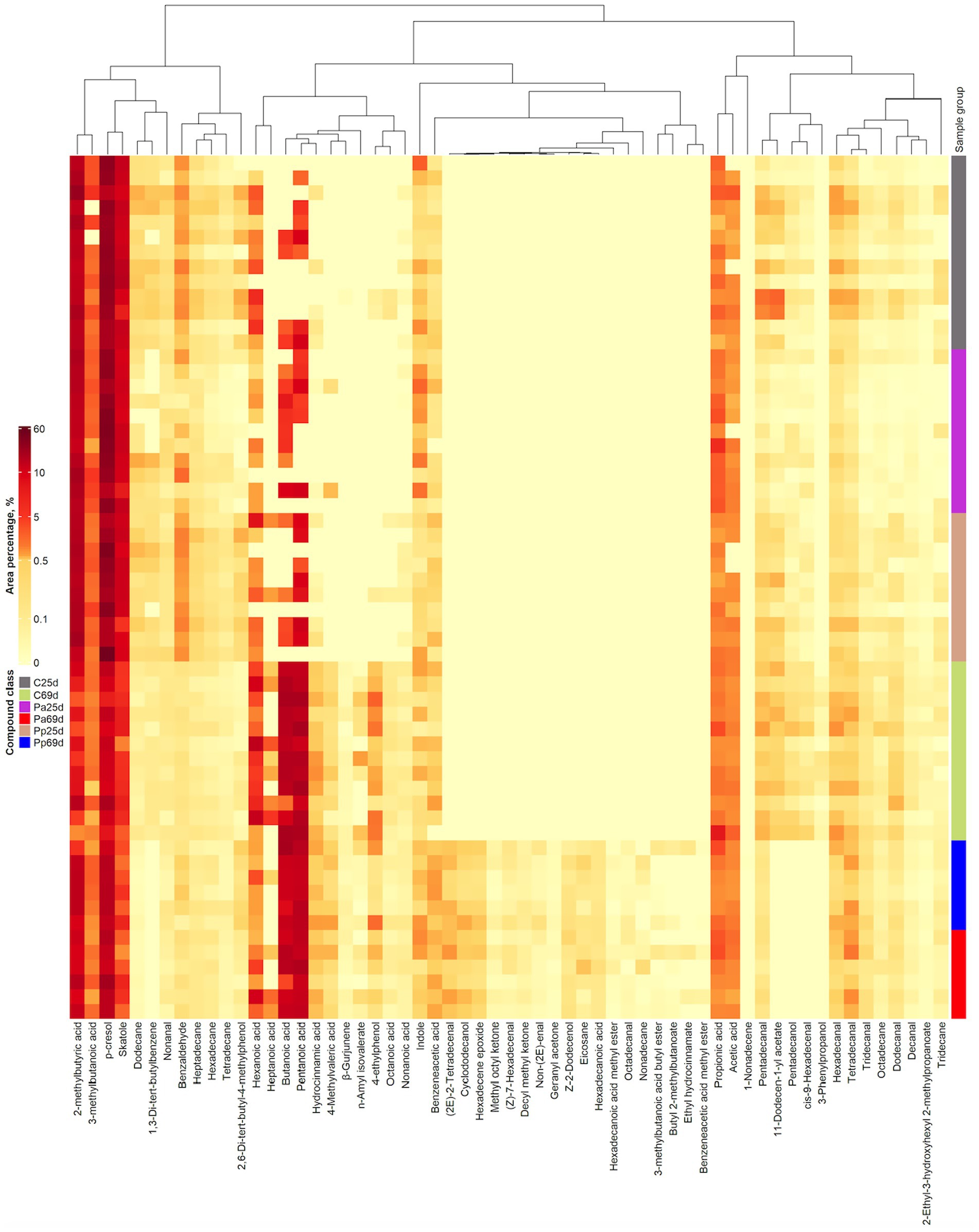
Figure 8. Volatile compound profiles of piglets’ faeces (C, control group—fed with basal non-fermented diet, previously received full-fledged combined pre starter feed for piglets PANTO®; Pp and Pa groups—previously, additionally to traditional diet from the 7th day of life received fermented with Pp and Pa milk permeate (groups Pp and Pa, respectively) and during the continuous experiment additionally received fermented with Lb. plantarum LUHS122, Lb. casei LUHS210, Lb. curvatus LUHS51, and Lb. paracasei LUHS244 feed material; 25th—at the beginning of the experiment; 69th—at the end of the experiment).
PLS-DA analysis (Figure 9A) clearly separated the C69d (C group on day 69) sample group from the other sample groups. Moreover, the Pp25 (day 25) and Pa25 (day 25) groups were clustered separately. The Pp and Pa group samples on day 69 are overlapping and exhibit much greater scattering. This higher scattering suggests higher variability in the individual VCs. In contrast, the C sample group showed the opposite trend, suggesting a much simpler VC profile in the faecal samples.
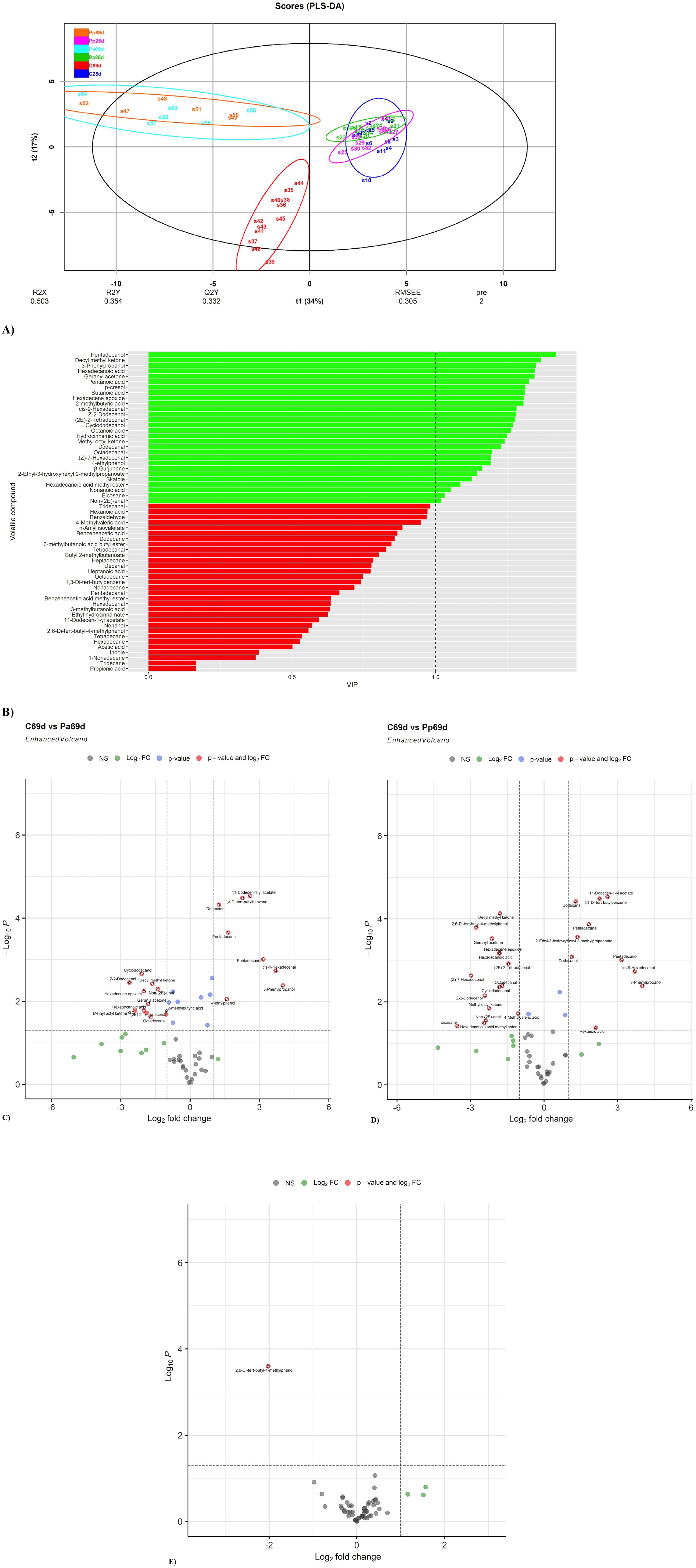
Figure 9. (A) PLS-DA plot of the detected VCs in piglet faecal sample groups (C, control group—fed with basal non-fermented diet, previously received full-fledged combined pre starter feed for piglets PANTO®; Pp and Pa groups—previously, additionally to traditional diet from the 7th day of life received fermented with Pp and Pa milk permeate (groups Pp and Pa, respectively) and during the continuous experiment additionally received fermented with Lb. plantarum LUHS122, Lb. casei LUHS210, Lb. curvatus LUHS51, and Lb. paracasei LUHS244 feed material; 25th—at the beginning of the experiment; 69th—at the end of the experiment). (B) VIP score bar plot of the detected VCs in piglet faecal sample groups (Green bars denote VCs, where VIP ≥ 1, while red bars denote VCs, where VIP < 1). (C) Volatile compound profiles of piglets’ faeces (volatile compound distribution in the control piglets’ group on day 69 vs. Pp group on day 69). (D) Volatile compound profiles of piglets’ faeces (volatile compound distribution in the control piglets’ group on day 69 vs. Pa group on day 69). (E) Volatile compound profiles of piglets’ faeces (volatile compound distribution in the Pa piglets’ group on day 69 vs. Pa group on day 69).
The VIP score analysis (Figure 9B) identified 19 analytes (VIP ≥ 1) as the most significant contributors to the variation between the sample groups. The top five VCs contributing most to group separation were pentadecanol, decyl methyl ketone, 3-phenylpropanol, hexadecanoic acid, and geranyl acetone. Other significant VCs included various acids (such as pentanoic, butanoic, 2-methylbutyric, octanoic, hydrocinnamic, nonanoic), aldehydes (such as cis-9-hexadecenal, (2E)-2-tetradecenal, dodecanal, octadecanal, (Z)-7-hexadecenal, non-(2E)-enal), phenolic compounds (p-cresol, 4-ethylphenol), alcohols (Z-2-dodecenol, cyclododecanol), esters (2-ethyl-3-hydroxyhexyl 2-methylpropanoate, hexadecanoic acid methyl ester), indoles (skatole), ketones (methyl octyl ketone), sesquiterpenes (β-gurjunene), alkanes, and their derivates (eicosane, hexadecene epoxide).
On day 69, the faecal VC profile of the C piglet group showed lower quantities of the following compounds, or they were not detected at all: cyclododecanol, Z-2-dodecenol, decyl methyl ketone, hexadecene epoxide, non-(2E)-enal, geranyl acetone, hexadecanoic acid, methyl octyl ketone, (2E)-2-tetradecenal, octadecanal, and 2-methylbutyric acid compared to the Pa group (Figure 9C). In contrast, the quantities of 11-dodecen-1-yl acetate, 1,3-di-tert-butylbenzene, dodecane, pentadecanal, pentadecanol, cis-9-hexadecenal, 3-phenylpropanol, and 4-ethylphenol were higher in the C group faeces compared to the Pa group.
When comparing the Pp and C groups’ faeces on day 69, the faecal VC profile of the C piglet group showed lower quantities of the following compounds, or they were not detected at all compared to the Pp group: geranyl acetone, hexadecanoic acid, decyl methyl ketone, 2,6-di-tert-butyl-4-methylphenol, (2E)-2-tetradecenal, Z-2-dodecenol, (Z)-7-hexadecenal, hexadecene epoxide, methyl octyl ketone, hexadecanoic acid methyl ester, octadecanal, cyclododecanol, eicosane, 2-methylbutyric acid (Figure 9D). In contrast, the quantities of 1,3-di-tert-butylbenzene, dodecane, 11-dodecen-1-yl acetate, pentadecanal, 2-ethyl-3-hydroxyhexyl 2-methylpropanoate, pentadecanol, cis-9-hexadecenal, dodecanal, 3-phenylpropanol, and hexanoic acid were higher in the C group’s faeces.
When comparing Pa and Pp groups’ faeces on day 69, at the end of the experiment, the faecal VC profile of the Pa piglet group showed lower quantities of the 2,6-Di-tert-butyl-4-methylphenol (Figure 9E).
4 Discussion
There is growing interest in using fermented feed for pigs. This study results showed that both treated groups (Pa and Pp) on day 69 showed higher body weights, in comparison with C group. However, significant differences in the FCR between the C, Pp, and Pa groups were not established. In the swine industry, the growth performance and health of weaned piglets are crucial for both animal productivity and economic profitability. Therefore, exploring healthier nutritional strategies to enhance the growth performance of weaned pigs is essential. Lactic acid bacteria with advantageous metabolic pathways for piglet fermented feed is essential for boosting nutrition, promoting gut health, increasing feed efficiency, and maintaining pigs’ general wellbeing. These bacteria may differ in the individual characteristics (tolerance to the acid environment, carbohydrate metabolism, etc.), the production of antimicrobial metabolites, vitamins and short-chain fatty acids as well as adaptation to the gut environment. Fermented feed is commonly inoculated with Pediococcus spp. and L. plantarum alone or in combination, because they produce significant amounts of lactic acid and reduce the feed’s pH (19). It was reported that P. acidilactici strain 72 N showed the greatest capacity to modify the quantity of lactobacilli and Enterobacteriaceae while also demonstrating the greatest ability to enhance intestinal structure in pigs (20). P. acidilactici synthesizes pediocin which is active against Gram-positive bacteria (21). It was found that P. acidilactici FT28 improved intestinal health and alleviated diarrheal symptoms in pigs by increasing the LAB population (22). By preserving the integrity of the gut epithelium, P. pentosaceus can reduce inflammation and regulate immunity (23). Lactobacillus is the main genera found in pigs’ digestive tracts and may improve gut metabolic capabilities (digestibility of nutrients and energy, energy content, and nitrogen retention), enhance weaning piglet immune systems, and preserve gut microbiota equilibrium (inhibit Enterobacteriaceae proliferation and Escherichia coli-induced intestinal permeability) (24). It was reported that in the digestive tracts of newborn piglets, Lactobacillus reuteri I5007 reduces harmful microorganisms and enhances the growth of a good bacteria (24). Lactobacillus strains MB 3123 (L. johnsonii), MB 3182 (L. salivarius group), and MB 3083 (L. plantarum) demonstrated high antimicrobial activity and organic acid production (19).
It has been reported that fermented feed helps alleviate stress in young pigs after weaning (25) and can improve the growth performance (26–28). It has also been reported that supplementing with fermented feed boosted the average daily gain of piglets between 28 and 42 days of age (29, 30). Including better digestibility (i.e., fermented) ingredients in piglet diets is crucial due to their immature digestive and immune systems (25). After fermentation, the macromolecular substances in feed are broken down into smaller molecules, making them easier for piglets to absorb and improving feed utilization (31). Fermenting feed, especially during the early growth stage of pigs, offers health benefits (32), suppress pathogen proliferation by lowering gastrointestinal pH (33), and improving gut microecology (26, 34). Additionally, solid fermented feed enhances palatability, which can increase feed intake in animals (35). Hu et al. (36) reported that a solid-state fermented diet increased the apparent total tract nutrient digestibility. The improved crude protein digestibility may result from the microbial degradation of antinutritional factors, such as cell wall components and phytates, as well as the breakdown of large protein molecules into smaller ones (36). Dietary supplementation with fermented feed has been shown to enhance nutrient digestion, promote feed intake, and ultimately improve the growth performance of weaned pigs (32). This complex effect of fermented feed material can explain the piglet weight results obtained in our experiment. However, the results may be related to the strain used. It has been reported that supplementation with 5% fermented soybean meal was found to improve FCR without affecting the blood profile (37). Supplementation of Bacillus subtilis to diets has been reported to significantly increase average daily gain (ADG) and reduce FCR in piglets, consistent with previous findings (38, 39). Another study showed that there was no significant difference in initial and final BWs between the P. pentosaceus CACC616 and control diet groups (23). However, none of the groups showed clinical symptoms, such as diarrhoea or mortality. The CACC616 group exhibited a lower FCR than the control group. The authors concluded that although P. pentosaceus CACC616 treatment led to increased average daily feed intake (ADFI) and improved FCR in weaned piglets after 26 days, the mechanisms through which supplementation with CACC616-based functional probiotics improves weaned piglet growth remain unclear. However, another studies have shown that diets supplemented with P. pentosaceus notably enhanced the growth performance of pigs while reducing the incidence of faecal noxious gases and diarrhoea (40, 41).
In our study analysis of blood parameters revealed no significant differences in IgA and IgM concentrations between the piglet groups. However, the highest IgG concentration was observed in the blood plasma of Pa piglets on day 69, although diet did not significantly affect IgG levels. Research by Grela et al. (42) suggests that fermented products enhance immunity in animals through both probiotic and prebiotic effects. It was found that piglets receiving Bacillus subtilis had significantly higher levels of IgA and IgG compared to those in the control and antibiotic-treated groups (43), and these outcomes align with earlier studies (44). It has been reported that P. pentosaceus can modulate immune function and reduce gut inflammation by maintaining the integrity of the gut epithelium (45, 46). Another blood plasma characteristics: ALT and AST are markers of hepatocellular damage. The liver, being the main organ for metabolism and detoxification, plays a crucial role in eliminating toxins, detoxifying blood, and removing unwanted drug metabolites that accumulate in other tissues (47, 48). Due to its distinct anatomical position, the liver is often exposed to circulating antigens, endotoxins, and microorganisms (49). In our study ALT levels were lower in both treated groups at the end of experiment, compared to the control group. However, diet was not found to significantly influence ALT or AST concentrations.
Also, no significant differences in faecal pH were observed between the groups when analyzing faecal indicators. However, on day 69, the Pp piglets had the highest faecal dry matter content, and both treated groups showed greater faecal texture hardness compared to the control group. Despite these findings, diet was not determined to be a significant factor for these parameters. It is also worth mentioning that no cases of diarrhea were reported in any of the groups by the end of the experiment. Microbiological analysis of piglet faecal samples revealed that all groups had higher TEC on day 69 compared to day 25. Enterobacteriaceae are part of the natural microflora in the guts of humans and animals. While they can lead to enteric diseases, many of these bacteria exist as commensals, with only a few species recognized as strict pathogens (50). On day 69, the highest LAB count was observed in the control and Pa groups compared to the Pp group. However, no significant differences in LAB counts were found between the Pp and Pa groups. Early intervention aimed at the gut microbiota could offer potential benefits in improving intestinal microbial balance (51–54). LAB are essential in preventing diseases by maintaining gut health (55, 56). Lactobacillus, specifically, are frequently used as probiotics after weaning because they are commensal bacteria that support gut immune function, help maintain the balance of gut microbiota and mitigate inflammation (57–60). In general, the microorganisms used in fermentation produce organic acids that significantly lower the feed’s pH (to 3.5–4.5), helping to inhibit the growth of pathogens in animals’ digestive tracts. Another benefit of using fermented feed is the presence of metabolites from microorganisms and LAB, and when these reach the animals’ intestines, they improve the intestinal microbiome, leading to more efficient energy utilization. However, despite that some LAB inhibits mold growth in vitro (61, 62), survival of individual species is thus often contingent on the niche created by metabolic activities of the others and yeast metabolites are benefit the LAB (63). By producing antimicrobial compounds (bacteriocins) and creating an acidic environment (lactic acid production), LAB prevents the growth or adhesion of pathogenic bacteria (64). By vying for binding sites on the intestinal epithelium or for nutrients in the intestine, LAB can suppress harmful bacteria (65). It was reported that L. casei reduced the quantity of E. coli that colonizes the gnotobiotic piglets’ jejunal mucosa (66). LAB have the ability to increase mucus secretion, which shields the intestinal lining and fosters a wholesome intestinal environment (64). By aiding in the breakdown of complex proteins and carbohydrates, LAB increases the availability of nutrients for absorption. Certain LAB strains exhibit immunomodulatory characteristics and the capacity to scavenge free radicals, which seems to be strain-dependent (64). In the small intestine of neonatal piglets, Enterococcus faecium EF1 produced a potent anti-inflammatory response (67).
Faecal microbiome composition showed that at the start point, i.e., the 25th day, the microbial composition in faeces was similar in all groups, with the dominance of Ruminococcaceae, Christensenellaceae, Bacteroides, and a moderate amount of Lactobacillus. Only Pp group has higher number of lactobacilli due to previous addition (0–25 days) of milk permeate to the feed of this group. The above-mentioned bacteria are known as normal predominant bacteria at this period of pigs’ life (1, 68). Lambo et al. (69) reported that using combinations and synergistic effect of different microorganisms (e.g., Lactobacillus, Pediococcus, Streptococcus, Enterococcus, Lactococcus, Bifidobacteria, etc.) can achieve greater positive effects on animal health and productivity than using a single microorganism. Wang et al. (70) observed that the synergistic effects of Lactobacillus fermentum and Pediococcus acidilactici improved intestinal health, more effectively reduced serum inflammatory factors, increased short-chain fatty acid production, and inhibited the proliferation of pathogenic microorganisms. Bacterial variety at this age showed a complex bacterial community without clearly dominant species but with clear increasing of alpha diversity during the growth of piglets. Such data is in coincidence with data obtained by other authors on the increasing bacterial variety in pigs’ gut from birth to the following growth stages (71, 72).
At the end of the experiment, on day 69, the predominant bacteria, including Ruminococcaceae, Lachnospiraceae, Prevotellaceae, Lactobacillus, and Bacteroidales, were observed in all animal groups; these species made about half amount from all faecal microbiomes. The above-mentioned bacteria are known as a core microbiota of pigs (73). The main difference between the groups at the end of experiment was observed in the prevalence of Lactobacillus, which surprisingly was significantly higher in the control group fed without fermented feed. Early-life seeding, stemming from maternal microbiota transmission and environmental exposure, establishes the foundational Lactobacillus populations that are crucial for neonatal gut health and disease resistance (1, 74, 75). Subsequently, in weaned piglets, nutrition, particularly the composition of dietary carbohydrates, and fermented feed material, become primary drivers of Lactobacillus abundance and diversity. The reason of such increase of Lactobacillus in this animal group remain unknown. The data on the dynamics of Lactobacillus after the weaning period in different studies are contradictory. Some research indicates that Lactobacillus populations remain consistent with pre-weaning levels (76, 77), while other authors report a significant increase in faecal samples during the post-weaning period (78). In our study, the variety of Lactobacillus species detected in faecal content of pigs included Limosilactobacillus mucosae, Lactobacillus johnsonii, Limosilactobacillus reuteri, Limosilactobacillus vaginalis, Lactobacillus delbrueckii and Lactobacillus gallinarum. However, most of the reads remained unidentified up to the species level. Despite the higher numbers of Lactobacillus in the control group, the alpha diversity in this group was lowest in comparison with groups fed with fermented feed. It is known that healthy microbial community often demonstrates high taxonomic diversity, high microbial gene richness, and stable core microbiota (79). As body weight was higher in the pigs fed with fermented feed, it may be assumed that a single bacterial taxon, even if it is known as beneficial, is unable to provide better clinical status, higher immunoglobulin level or better zootechnical parameters. It also should be noted that the faecal microbiome does not provide clear insight into microbial composition in separate parts of the gastrointestinal tract, therefore microbial indices in faeces should not be treated as the key parameter within this study.
It has been reported that faecal VCs may serve as biomarkers of gastrointestinal functionality, as changes in microbial and cellular metabolism can lead to variations in the VC profile of faeces (80). The faecal samples from the Pp and Pa groups on day 69 showed significantly greater variability in individual VCs compared to the control group. It could be seen from the Figure 10 that there are significant correlations between separate VC and faeces microbiological parameters. Out of the 59 VCs identified in piglet faeces, significant correlations were found between 43 VCs and LAB count, between 47 VCs and TEC, and between 34 VCs and yeast and mold count. In our previous studies, similar tendencies were established (7). On day 69, butyl 2-methylbutanoate, 3-methylbutanoic acid butyl ester, non-(2E)-enal, methyl octyl ketone, benzeneacetic acid methyl ester, ethyl hydrocinnamate, decyl methyl ketone, geranyl acetone, Z-2-dodecenol, cyclododecanol, (2E)-2-tetradecenal, hexadecene epoxide, (Z)-7-hexadecenal, nonadecane, hexadecanoic acid methyl ester, hexadecanoic acid, eicosane, and octadecanal were only detected in treated groups (Pa and Pp) faeces. Additionally, both treated groups showed higher body weights at the end of the experiment. Butyl 2-methylbutanoate is a fatty acid ester, which is a carboxylic ester derivative of a fatty acid. The synthesis of this ester may be mediated by a bacterial esterase, such as the one present in E. coli (81) or by enzyme(s) derived from the intestinal mucosa (82). There is a paucity of literature in this area, but reports suggest that the intestinal mucosa may be a major site for the synthesis of fatty acid ethyl esters (83). Although esters naturally occur in many feedstuffs, they are expected to be primarily hydrolyzed by the piglet’s digestive system. Geranyl acetone is a monoterpene ketone, where an (E)-geranyl group is bonded to one of the alpha-methyl groups of acetones. It is a component of essential oils from various plants. Methylketones can be produced by many bacterial species and by fungi, which convert the respective alkanoic acids, however, some compounds in this group may also have dietary origins (84). Aliphatic aldehydes can result from bacterial activity or autooxidation of unsaturated fatty acids, so their upregulation may be associated with a high-fat diet (85). Alkanes of various chain lengths can originate from plants and be excreted in manure or faeces due to limited absorption in the intestine. Alternatively, like aldehydes, they can also be produced through lipid peroxidation (86, 87).
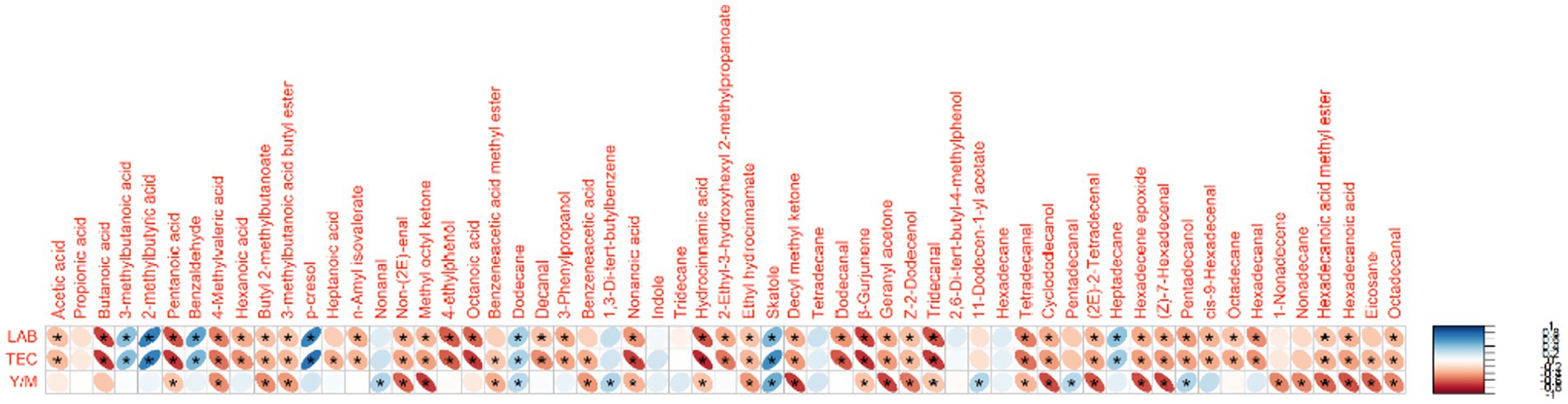
Figure 10. Correlations between faeces microbiological parameters and volatile compounds (LAB, lactic acid bacteria; TEC, total enterobacteria count; Y/M, yeast and mold). *Denotes statistically significant correlations (p ≤ 0.05).
Finally, greater variability in individual VCs compared to the control group could be associated with the greater variability of bacterial species in the treated group’s faeces, as well as with deeper nutrient degradation.
5 Conclusion
This study showed that continuing to feed piglets (from suckling to weaning and into the later stages) with FFM may be beneficial for enhancing their growth performance and health parameters. Both treated groups (Pa and Pp) on day 69 showed higher body weights compared to the control group. Additionally, the control and Pa groups on day 69 had higher blood plasma IgM concentrations and lower ALT levels. Both treated groups exhibited higher faecal texture hardness compared to the control group. The faeces from the treated groups showed greater bacterial diversity. Furthermore, samples from the Pp and Pa groups on day 69 showed significantly greater variability in individual faecal VCs compared to the control group, with significant correlations between individual VCs and faecal microbiological parameters. Finally, the greater bacterial diversity and variability in individual VCs in the treated groups could be linked to enhanced nutrient degradation and absorption as well as higher body weights of the piglets.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s. Sequences were deposited in the NCBI database under accession number PRJNA1205751.
Ethics statement
The study was conducted in accordance with the EU and local legislation as well as institutional requirements (Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes; Requirements for the Keeping, Maintenance, and Use of Animals Intended for Science and Education Purposes, coordinated release by the order of the Lithuanian Director of the State Food and Veterinary Service). According to the State Food and Veterinary Service of the Republic Lithuania, no bioethics permissions are required for routine veterinary procedures. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
SB: Writing – original draft, Investigation, Formal analysis, Validation, Data curation, Methodology. VS: Writing – original draft, Software, Investigation, Methodology. ErnM: Investigation, Writing – original draft, Methodology, Software, Visualization, Formal analysis, Validation. MR: Formal analysis, Visualization, Investigation, Validation, Writing – original draft, Writing – review & editing, Methodology, Software. DK: Formal analysis, Validation, Writing – original draft, Investigation, Methodology, Software. EriM: Writing – original draft, Visualization, Investigation, Formal analysis. JD: Writing – original draft, Formal analysis, Investigation, Validation. AD: Validation, Formal analysis, Investigation, Writing – original draft. LV: Investigation, Writing – original draft, Formal analysis, Methodology. BM-Z: Writing – review & editing. EB: Project administration, Conceptualization, Writing – original draft, Supervision, Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1616209/full#supplementary-material
SUPPLEMENTARY TABLE S1 | Carbohydrate metabolism, gas production, tolerance to temperature (10, 30, 37, and 45°C) and low pH conditions (pH 2.5 for 2 h) of the Lb. plantarum LUHS122, Lb. casei LUHS210, Lb. curvatus LUHS51, and Lb. paracasei LUHS244 strains.
SUPPLEMENTARY TABLE S2 | Inhibition zones of the tested lactic acid bacteria (LAB) strains against pathogenic opportunistic microorganisms.
SUPPLEMENTARY TABLE S3 | Antimicrobial activities of the tested lactic acid bacteria (LAB) strains against pathogenic opportunistic microorganisms in liquid medium (+ indicates pathogen growth; − indicates that pathogen growth was not observed).
SUPPLEMENTARY TABLE S2.1 | Volatile compound profiles of piglets’ faeces.
References
1. Guevarra, RB, Hong, SH, Cho, JH, Kim, B-R, Shin, J, Lee, JH, et al. The dynamics of the piglet gut microbiome during the weaning transition in association with health and nutrition. J Anim Sci Biotechnol. (2018) 9:54. doi: 10.1186/s40104-018-0269-6
2. Chen, Z, Yang, H, Fu, H, Wu, L, Liu, M, Jiang, H, et al. Gut bacterial species in late trimester of pregnant sows influence the occurrence of stillborn piglet through pro-inflammation response. Front Immunol. (2023) 13:1101130. doi: 10.3389/fimmu.2022.1101130
3. Kim, HB, and Isaacson, RE. The pig gut microbial diversity: understanding the pig gut microbial ecology through the next generation high throughput sequencing. Vet Microbiol. (2015) 177:242–51. doi: 10.1016/j.vetmic.2015.03.014
4. Stokes, CR. The development and role of microbial-host interactions in gut mucosal immune development. J Anim Sci Biotechnol. (2017) 8:12. doi: 10.1186/s40104-016-0138-0
5. Yang, H, Huang, X, Fang, S, He, M, Zhao, Y, Wu, Z, et al. Unraveling the fecal microbiota and metagenomic functional capacity associated with feed efficiency in pigs. Front Microbiol. (2017) 8:1555. doi: 10.3389/fmicb.2017.01555
6. Pluske, JR. Feed-and feed additives-related aspects of gut health and development in weanling pigs. J Anim Sci Biotechnol. (2013) 4:1–7. doi: 10.1186/2049-1891-4-1
7. Badaras, S, Starkute, V, Mockus, E, Ruzauskas, M, Klupsaite, D, Mozuriene, E, et al. Influence of fermented milk permeate containing antimicrobial Lactobacillus and galactooligosaccharides on growth performance and health parameters in neonatal piglets. Front Vet Sci. (2025) 12:1501117. doi: 10.3389/fvets.2025.1501117
8. Bartkiene, E, Lele, V, Ruzauskas, M, Domig, KJ, Starkute, V, Zavistanaviciute, P, et al. Lactic acid bacteria isolation from spontaneous sourdough and their characterization including antimicrobial and antifungal properties evaluation. Microorganisms. (2020) 8:64. doi: 10.3390/microorganisms8010064
9. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010. On the protection of animals used for scientific purposes text with EEA relevance. Eur Parliam Counc (2010) 47.
10. B1-866 Dėl Mokslo ir mokymo tikslais naudojamų gyvūnų laikymo, priežiūros ir naudojimo reikalavimų patvi. (2012) Available online at: https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/TAIS.437081?positionInSearchResults=0&searchModelUUID=64a1f51f-6356-4b60-a21e-3a67ac3b1107 (Accessed March 26, 2025)
11. Official Journal of the European Union. Regulation (EU) 2021/2115 of the European Parliament and of the Council of 2 December 2021. (2021). Available online at: https://eur-lex.europa.eu/homepage.html (Accessed May 12, 2025).
12. National Research Council; Division on Earth and Life Studies; Board on Agriculture and Natural Resources; Committee on Nutrient Requirements of Swine. Nutrient Requirements of Swine: Eleventh Revised Edition. Washington, DC: National Academies Press (2012). 420 p.
13. AOAC. Official methods of analysis of Association of Official Agricultural Chemists. Rockford, MD, USA: AOAC (2019).
14. Callahan, BJ, McMurdie, PJ, Rosen, MJ, Han, AW, Johnson, AJA, and Holmes, SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. (2016) 13:581–3. doi: 10.1038/nmeth.3869
15. Caporaso, JG, Kuczynski, J, Stombaugh, J, Bittinger, K, Bushman, FD, Costello, EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. (2010) 7:335–6. doi: 10.1038/nmeth.f.303
16. Vadopalas, L, Zokaityte, E, Zavistanaviciute, P, Gruzauskas, R, Starkute, V, Mockus, E, et al. Supplement based on fermented Milk permeate for feeding newborn calves: influence on blood, growth performance, and faecal parameters, including microbiota, volatile compounds, and fatty and organic acid profiles. Animals. (2021) 11:2544. doi: 10.3390/ani11092544
17. Evans, JD. Straightforward statistics for the behavioral sciences. Belmont, CA, US: Thomson Brooks/Cole Publishing Co. (1996) xxii, 600 p.
18. Social Science Statistics. Available online at: https://www.socscistatistics.com/ (2019) (Accessed May 11, 2025)
19. Missotten, JAM, Goris, J, Michiels, J, Van Coillie, E, Herman, L, De Smet, S, et al. Screening of isolated lactic acid bacteria as potential beneficial strains for fermented liquid pig feed production. Anim Feed Sci Technol. (2009) 150:122–38. doi: 10.1016/j.anifeedsci.2008.08.002
20. Pupa, P, Apiwatsiri, P, Sirichokchatchawan, W, Pirarat, N, Maison, T, Koontanatechanon, A, et al. Use of Lactobacillus plantarum (strains 22F and 25F) and Pediococcus acidilactici (strain 72N) as replacements for antibiotic-growth promotants in pigs. Sci Rep. (2021) 11:12028. doi: 10.1038/s41598-021-91427-5
21. Morales-Partera, Á, Cardoso Toset, F, Luque, I, Maldonado, A, Tarradas, C, and Gómez-Laguna, J. Supplementing feed with Pediococcus acidilactici reduces Campylobacter load in finishing pigs. Vet Rec. (2020) 187:e45–5. doi: 10.1136/vr.105591
22. Dowarah, R, Verma, AK, Agarwal, N, Patel, BHM, and Singh, P. Effect of swine based probiotic on performance, diarrhoea scores, intestinal microbiota and gut health of grower-finisher crossbred pigs. Livest Sci. (2017) 195:74–9. doi: 10.1016/j.livsci.2016.11.006
23. Park, S, Song, J, Park, MA, Jang, H-J, Son, S, Kim, D-H, et al. Assessing the probiotic effects of Pediococcus pentosaceus CACC616 in weaned piglets. Microorganisms. (2023) 11:2890. doi: 10.3390/microorganisms11122890
24. Wang, Q, Sun, Q, Qi, R, Wang, J, Qiu, X, Liu, Z, et al. Effects of Lactobacillus plantarum on the intestinal morphology, intestinal barrier function and microbiota composition of suckling piglets. J Anim Physiol Anim Nutr. (2019) 103:1908–18. doi: 10.1111/jpn.13198
25. Płacheta, B, Motyl, I, Berłowska, J, and Mroczyńska-Florczak, M. The use of fermented plant biomass in pigs feeding. Sustainability. (2022) 14:14595. doi: 10.3390/su142114595
26. Yuan, L, Chang, J, Yin, Q, Lu, M, Di, Y, Wang, P, et al. Fermented soybean meal improves the growth performance, nutrient digestibility, and microbial flora in piglets. Anim Nutr Zhongguo Xu Mu Shou Yi Xue Hui. (2017) 3:19–24. doi: 10.1016/j.aninu.2016.11.003
27. Zhang, Y, Shi, C, Wang, C, Lu, Z, Wang, F, Feng, J, et al. Effect of soybean meal fermented with Bacillus subtilis BS12 on growth performance and small intestinal immune status of piglets. Food Agric Immunol. (2018) 29:133–46. doi: 10.1080/09540105.2017.1360258
28. Zhu, J, Gao, M, Zhang, R, Sun, Z, Wang, C, Yang, F, et al. Effects of soybean meal fermented by L. plantarum, B. Subtilis and S. Cerevisieae on growth, immune function and intestinal morphology in weaned piglets. Microb Cell Factories. (2017) 16:191. doi: 10.1186/s12934-017-0809-3
29. Kim, YG, Lohakare, JD, Yun, JH, Heo, S, and Chae, BJ. Effect of feeding levels of microbial fermented soy protein on the growth performance, nutrient digestibility and intestinal morphology in weaned piglets. Asian Australas J Anim Sci. (2007) 20:399–404. doi: 10.5713/ajas.2007.399
30. Yun, HM, Nyachoti, CM, and Kim, IH. Effect of dietary supplementation of fermented garlic by Leuconostoc mesenteroides KCCM35046, on growth performance, blood constituents, nutrient digestibility, fecal microflora, and fecal scores in sows and their piglets. Can J Anim Sci. (2019) 99:349–56. doi: 10.1139/cjas-2017-0203
31. Liu, S, Xiao, H, Xiong, Y, Chen, J, Wu, Q, Wen, X, et al. Effects of fermented feed on the growth performance, intestinal function, and microbiota of piglets weaned at different age. Front Vet Sci. (2022) 9:841762. doi: 10.3389/fvets.2022.841762
32. Lu, Y, Zhang, R, Lei, H, Hang, Y, Xue, H, Cai, X, et al. Supplementation with fermented feedstuff enhances orexin expression and secretion associated with increased feed intake and weight gain in weaned pigs. Animals. (2022) 12:1329. doi: 10.3390/ani12101329
33. Scholten, RHJ, van der Peet-Schwering, CMC, Verstegen, MWA, den Hartog, LA, Schrama, JW, and Vesseur, PC. Fermented co-products and fermented compound diets for pigs: a review. Anim Feed Sci Technol. (1999) 82:1–19. doi: 10.1016/S0377-8401(99)00096-6
34. Sugiharto, S, Lauridsen, C, and Jensen, BB. Gastrointestinal ecosystem and immunological responses in E. coli challenged pigs after weaning fed liquid diets containing whey permeate fermented with different lactic acid bacteria. Anim Feed Sci Technol. (2015) 207:278–82. doi: 10.1016/j.anifeedsci.2015.06.019
35. Meinlschmidt, P, Ueberham, E, Lehmann, J, Schweiggert-Weisz, U, and Eisner, P. Immunoreactivity, sensory and physicochemical properties of fermented soy protein isolate. Food Chem. (2016) 205:229–38. doi: 10.1016/j.foodchem.2016.03.016
36. Hu, P, Wang, L, Hu, Z, Jiang, L, Hu, H, Rao, Z, et al. Effects of multi-Bacteria solid-state fermented diets with different crude Fiber levels on growth performance, nutrient digestibility, and microbial flora of finishing pigs. Animals. (2021) 11:3079. doi: 10.3390/ani11113079
37. Ding, Z, Chang, KH, and Kim, I. Effects of fermented soybean meal on growth performance, nutrients digestibility, blood profile and fecal microflora in weaning pigs. Korean J Agric Sci. (2020) 47:1–10. doi: 10.7744/kjoas.20190062
38. Kyriakis, SC, Tsiloyiannis, VK, Vlemmas, J, Sarris, K, Tsinas, AC, Alexopoulos, C, et al. The effect of probiotic LSP 122 on the control of post-weaning diarrhoea syndrome of piglets. Res Vet Sci. (1999) 67:223–8. doi: 10.1053/rvsc.1999.0308
39. Jørgensen, JN, Laguna, JS, Millán, C, Casabuena, O, and Gracia, MI. Effects of a Bacillus-based probiotic and dietary energy content on the performance and nutrient digestibility of wean to finish pigs. Anim Feed Sci Technol. (2016) 221:54–61. doi: 10.1016/j.anifeedsci.2016.08.008
40. Upadhaya, SD, and Kim, IH. Maintenance of gut microbiome stability for optimum intestinal health in pigs – a review. J Anim Sci Biotechnol. (2022) 13:140. doi: 10.1186/s40104-022-00790-4
41. Song, D, Lee, J, Kim, K, Oh, H, An, J, Chang, S, et al. Effects of dietary supplementation of Pediococcus pentosaceus strains from kimchi in weaned piglet challenged with Escherichia coli and Salmonella enterica. J Anim Sci Technol. (2023) 65:611–26. doi: 10.5187/jast.2023.e31
42. Grela, ER, Czech, A, Kiesz, M, Wlazło, Ł, and Nowakowicz-Dębek, B. A fermented rapeseed meal additive: effects on production performance, nutrient digestibility, colostrum immunoglobulin content and microbial flora in sows. Anim Nutr. (2019) 5:373–9. doi: 10.1016/j.aninu.2019.05.004
43. Li, H-H, Jiang, X-R, and Qiao, J-Y. Effect of dietary Bacillus subtilis on growth performance and serum biochemical and immune indexes in weaned piglets. J Appl Anim Res. (2021) 49:83–8. doi: 10.1080/09712119.2021.1877717
44. Lee, SH, Ingale, SL, Kim, JS, Kim, KH, Lokhande, A, Kim, EK, et al. Effects of dietary supplementation with Bacillus subtilis LS 1–2 fermentation biomass on growth performance, nutrient digestibility, cecal microbiota and intestinal morphology of weanling pig. Anim Feed Sci Technol. (2014) 188:102–10. doi: 10.1016/j.anifeedsci.2013.12.001
45. Wang, P, Chen, S, Liao, C, Jia, Y, Li, J, Shang, K, et al. Probiotic properties of chicken-derived highly adherent lactic acid bacteria and inhibition of enteropathogenic bacteria in caco-2 cells. Microorganisms. (2022) 10:2515. doi: 10.3390/microorganisms10122515
46. Lee, J, Lee, K-S, Lee, J, Lee, K-S, and Park, S-Y. Weissella koreensis and Pediococcus pentosaceus bacterial ghosts induce inflammatory responses as immunostimulants. Biochem Biophys Res Commun. (2023) 676:213–9. doi: 10.1016/j.bbrc.2023.07.049
47. Dhainaut, JF, Marin, N, Mignon, A, and Vinsonneau, C. Hepatic response to sepsis: interaction between coagulation and inflammatory processes. Crit Care Med. (2001) 29:S42–7. doi: 10.1097/00003246-200107001-00016
48. Zhang, Y, Xue, W, Zhang, W, Yuan, Y, Zhu, X, Wang, Q, et al. Histone methyltransferase G9a protects against acute liver injury through GSTP1. Cell Death Differ. (2020) 27:1243–58. doi: 10.1038/s41418-019-0412-8
49. Compare, D, Coccoli, P, Rocco, A, Nardone, OM, De Maria, S, Cartenì, M, et al. Gut--liver axis: the impact of gut microbiota on non alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. (2012) 22:471–6. doi: 10.1016/j.numecd.2012.02.007
50. Zhao, D, Wu, T, Yi, D, Wang, L, Li, P, Zhang, J, et al. Dietary supplementation with Lactobacillus casei alleviates lipopolysaccharide-induced liver injury in a porcine model. Int J Mol Sci. (2017) 18:2535. doi: 10.3390/ijms18122535
51. Al Nabhani, Z, and Eberl, G. GAPs in early life facilitate immune tolerance. Sci Immunol. (2017) 2:eaar 2465. doi: 10.1126/sciimmunol.aar2465
52. El Aidy, S, Derrien, M, Merrifield, CA, Levenez, F, Doré, J, Boekschoten, MV, et al. Gut bacteria-host metabolic interplay during conventionalisation of the mouse germfree colon. ISME J. (2013) 7:743–55. doi: 10.1038/ismej.2012.142
53. El Aidy, S, Hooiveld, G, Tremaroli, V, Bäckhed, F, and Kleerebezem, M. The gut microbiota and mucosal homeostasis: colonized at birth or at adulthood, does it matter? Gut Microbes. (2013) 4:118–24. doi: 10.4161/gmic.23362
54. Gensollen, T, Iyer, SS, Kasper, DL, and Blumberg, RS. How colonization by microbiota in early life shapes the immune system. Science. (2016) 352:539–44. doi: 10.1126/science.aad9378
55. Konstantinov, SR, Awati, AA, Williams, BA, Miller, BG, Jones, P, Stokes, CR, et al. Post-natal development of the porcine microbiota composition and activities. Environ Microbiol. (2006) 8:1191–9. doi: 10.1111/j.1462-2920.2006.01009.x
56. Choi, Y-J, Lim, H-J, and Shin, H-S. Immunomodulatory effects of seven viable and sonicated Lactobacillus spp. and anti-bacterial activities of L. rhamnosus and L helvetilus. Microbiol Soc Korea. (2019) 55:392–9. doi: 10.7845/kjm.2019.9123
57. Daly, K, Darby, AC, Hall, N, Nau, A, Bravo, D, and Shirazi-Beechey, SP. Dietary supplementation with lactose or artificial sweetener enhances swine gut Lactobacillus population abundance. Br J Nutr. (2014) 111:S30–5. doi: 10.1017/S0007114513002274
58. Jiao, J, Wu, J, Zhou, C, Tang, S, Wang, M, and Tan, Z. Composition of ileal bacterial community in grazing goats varies across non-rumination, transition and rumination stages of life. Front Microbiol. (2016) 7:1364. doi: 10.3389/fmicb.2016.01364
59. Shang, Q, Shan, X, Cai, C, Hao, J, Li, G, and Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of lactobacillus and ruminococcaceae. Food Funct. (2016) 7:3224–32. doi: 10.1039/C6FO00309E
60. Vujkovic-Cvijin, I, Swainson, LA, Chu, SN, Ortiz, AM, Santee, CA, Petriello, A, et al. Gut-resident Lactobacillus abundance associates with IDO1 inhibition and Th17 dynamics in SIV-infected macaques. Cell Rep. (2015) 13:1589–97. doi: 10.1016/j.celrep.2015.10.026
61. Bartkiene, E, Bartkevics, V, Lele, V, Pugajeva, I, Zavistanaviciute, P, Mickiene, R, et al. A concept of mould spoilage prevention and acrylamide reduction in wheat bread: application of lactobacilli in combination with a cranberry coating. Food Control. (2018) 91:284–93. doi: 10.1016/j.foodcont.2018.04.019
62. Juodeikiene, G, Bartkiene, E, Cernauskas, D, Cizeikiene, D, Zadeike, D, Lele, V, et al. Antifungal activity of lactic acid bacteria and their application for Fusarium mycotoxin reduction in malting wheat grains. LWT. (2018) 89:307–14. doi: 10.1016/j.lwt.2017.10.061
63. Ponomarova, O, Gabrielli, N, Sévin, DC, Mülleder, M, Zirngibl, K, Bulyha, K, et al. Yeast creates a niche for symbiotic lactic acid Bacteria through nitrogen overflow. Cell Syst. (2017) 5:345–357.e6. doi: 10.1016/j.cels.2017.09.002
64. Yang, F, Hou, C, Zeng, X, and Qiao, S. The use of lactic acid Bacteria as a probiotic in swine diets. Pathogens. (2015) 4:34–45. doi: 10.3390/pathogens4010034
65. Malago, JJ, and JFJG, K. Probiotic-pathogen interactions and enteric cytoprotection In: JJ Malago, K JFJG, and R Marinsek-Logar, editors. Probiotic bacteria and enteric infections: cytoprotection by probiotic bacteria. Dordrecht: Springer Netherlands (2011). 289–311.
66. Bomba, A, Nemcová, R, Gancarcíková, S, Herich, R, and Kastel, R. Potentiation of the effectiveness of Lactobacillus casei in the prevention of E. coli induced diarrhea in conventional and gnotobiotic pigs. Adv Exp Med Biol. (1999) 473:185–90. doi: 10.1007/978-1-4615-4143-1_18
67. Huang, YC, Li, YL, Huang, Q, Cui, ZW, Yu, DY, Rajput, IR, et al. Effect of orally administered Enterococcus faecium EF1 on intestinal cytokines and chemokines production of suckling piglets. Pak Vet J Pak (2012): 81–84 32. Available online at: https://agris.fao.org/search/en/providers/122650/records/647364fc53aa8c89630c6708 (Accessed May 22, 2025)
68. Wang, C, Wei, S, Chen, N, Xiang, Y, Wang, Y, and Jin, M. Characteristics of gut microbiota in pigs with different breeds, growth periods and genders. Microb Biotechnol. (2022) 15:793–804. doi: 10.1111/1751-7915.13755
69. Lambo, MT, Chang, X, and Liu, D. The recent trend in the use of multistrain probiotics in livestock production: An overview. Animals. (2021) 11:2805. doi: 10.3390/ani11102805
70. Wang, S, Yao, B, Gao, H, Zang, J, Tao, S, Zhang, S, et al. Combined supplementation of Lactobacillus fermentum and Pediococcus acidilactici promoted growth performance, alleviated inflammation, and modulated intestinal microbiota in weaned pigs. BMC Vet Res. (2019) 15:239. doi: 10.1186/s12917-019-1991-9
71. Saladrigas-García, M, Durán, M, D’Angelo, M, Coma, J, Pérez, JF, and Martín-Orúe, SM. An insight into the commercial piglet’s microbial gut colonization: from birth towards weaning. Anim Microbiome. (2022) 4:68. doi: 10.1186/s42523-022-00221-9
72. Karasova, D, Crhanova, M, Babak, V, Jerabek, M, Brzobohaty, L, Matesova, Z, et al. Development of piglet gut microbiota at the time of weaning influences development of postweaning diarrhea – a field study. Res Vet Sci. (2021) 135:59–65. doi: 10.1016/j.rvsc.2020.12.022
73. Holman, DB, Brunelle, BW, Trachsel, J, and Allen, HK. Meta-analysis to define a core microbiota in the swine gut. mSystems. (2017) 2:e00004. doi: 10.1128/msystems.00004-17
74. Guevarra, RB, Lee, JH, Lee, SH, Seok, M-J, Kim, DW, Kang, BN, et al. Piglet gut microbial shifts early in life: causes and effects. J Anim Sci Biotechnol. (2019) 10:1. doi: 10.1186/s40104-018-0308-3
75. Quan, J, Xu, C, Ruan, D, Ye, Y, Qiu, Y, Wu, J, et al. Composition, function, and timing: exploring the early-life gut microbiota in piglets for probiotic interventions. J Anim Sci Biotechnol. (2023) 14:143–20. doi: 10.1186/s40104-023-00943-z
76. Motta, V, Luise, D, Bosi, P, and Trevisi, P. Faecal microbiota shift during weaning transition in piglets and evaluation of AO blood types as shaping factor for the bacterial community profile. PloS One. (2019) 14:e0217001. doi: 10.1371/journal.pone.0217001
77. Arsenault, M, Lillie, B, Nadeem, K, Khafipour, E, and Farzan, A. Progression of swine fecal microbiota during early stages of life and its association with performance: a longitudinal study. BMC Microbiol. (2024) 24:182. doi: 10.1186/s12866-024-03336-y
78. Ortiz Sanjuán, JM, Manzanilla, EG, Cabrera-Rubio, R, Crispie, F, Cotter, PD, Garrido, JJ, et al. Fine-tuning of post-weaning pig microbiome structure and functionality by in-feed zinc oxide and antibiotics use. Front Cell Infect Microbiol. (2024) 14:1354449. doi: 10.3389/fcimb.2024.1354449
79. Fan, Y, and Pedersen, O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. (2021) 19:55–71. doi: 10.1038/s41579-020-0433-9
80. Pérez-Calvo, E, Wicaksono, AN, Canet, E, Daulton, E, Ens, W, Hoeller, U, et al. The measurement of volatile organic compounds in faeces of piglets as a tool to assess gastrointestinal functionality. Biosyst Eng. (2019) 184:122–9. doi: 10.1016/j.biosystemseng.2019.06.005
81. Elgaali, H, Hamilton-Kemp, TR, Newman, MC, Collins, RW, Yu, K, and Archbold, DD. Comparison of long-chain alcohols and other volatile compounds emitted from food-borne and related gram positive and gram negative bacteria. J Basic Microbiol. (2002) 42:373–80. doi: 10.1002/1521-4028(200212)42:6<373::AID-JOBM373>3.0.CO;2-4
82. Diczfalusy, MA, Björkhem, I, Einarsson, C, Hillebrant, CG, and Alexson, SE. Characterization of enzymes involved in formation of ethyl esters of long-chain fatty acids in humans. J Lipid Res. (2001) 42:1025–32. doi: 10.1016/S0022-2275(20)31590-X
83. WHO Library Cataloguing in Publication Data. International Programme on chemical safety, Environmental Health Criteria, Methylene chloride (EHC 164). 2nd Edition. WHO. (1996). Available online at: https://www.inchem.org/documents/ehc/ehc/ehc164.htm (Accessed March 25, 2025)
84. Garner, CE, Smith, S, de Lacy, CB, White, P, Spencer, R, Probert, CSJ, et al. Volatile organic compounds from feces and their potential for diagnosis of gastrointestinal disease. FASEB J. (2007) 21:1675–88. doi: 10.1096/fj.06-6927com
85. Uchikawa, M, Kato, M, Nagata, A, Sanada, S, Yoshikawa, Y, Tsunematsu, Y, et al. Elevated levels of proinflammatory volatile metabolites in feces of high fat diet fed KK-ay mice. Sci Rep. (2020) 10:5681. doi: 10.1038/s41598-020-62541-7
86. Koch, F, Kowalczyk, J, Mielke, H, Schenkel, H, Bachmann, M, Zeyner, A, et al. Preference and possible consumption of provided enrichment and bedding materials and disinfectant powder by growing pigs. Porcine Health Manag. (2022) 8:1–13. doi: 10.1186/s40813-021-00243-w
Keywords: faeces bacterial composition, immunoglobulins, faeces volatile compounds, antimicrobials, metataxonomic
Citation: Badaras S, Starkute V, Mockus E, Ruzauskas M, Klupsaite D, Mozuriene E, Dailidaviciene J, Dauksiene A, Vadopalas L, Metzler-Zebeli BU and Bartkiene E (2025) Modeling the weaning diet of piglets with fermented feed material: effects on growth performance and health parameters. Front. Vet. Sci. 12:1616209. doi: 10.3389/fvets.2025.1616209
Edited by:
Fulin Yang, Fujian Agriculture and Forestry University, ChinaReviewed by:
Shea Beasley, Sheaps Oy, FinlandSandra Traverso, Santa Catarina State University, Brazil
Copyright © 2025 Badaras, Starkute, Mockus, Ruzauskas, Klupsaite, Mozuriene, Dailidaviciene, Dauksiene, Vadopalas, Metzler-Zebeli and Bartkiene. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena Bartkiene, ZWxlbmEuYmFydGtpZW5lQGxzbXUubHQ=
 Sarunas Badaras1
Sarunas Badaras1 Vytaute Starkute
Vytaute Starkute Modestas Ruzauskas
Modestas Ruzauskas Dovile Klupsaite
Dovile Klupsaite Agila Dauksiene
Agila Dauksiene Barbara U. Metzler-Zebeli
Barbara U. Metzler-Zebeli Elena Bartkiene
Elena Bartkiene