- 1Department of Veterinary Sciences, University of Torino, Torino, Italy
- 2Animal Oncology and Imaging Center AG, Hünenberg, Switzerland
- 3Department of Molecular Biotechnology and Health Sciences, Molecular Biotechnology Center, University of Torino, Torino, Italy
- 4Department of Medical Veterinary Science, University of Parma, Parma, Italy
- 5Clinica Veterinaria Tyrus, Terni, Italy
- 6MyLav La Vallonea, Veterinary Analysis Laboratory srl, Alessano, Italy
The most appropriate approach to regional/sentinel lymph nodes (LN) for staging canine oral malignant melanoma (OMM) is still controversial. This study aims to retrospectively evaluate the prognostic impact of neck dissection modality and LN metastasis in a homogeneous cohort of dogs treated by surgery and adjuvant anti-CSPG4 electrovaccination. Seventy-seven dogs were enrolled and divided into two groups based on the presence (Group A, 24 dogs) or absence (Group B, 53 dogs) of histologically confirmed LN metastasis at the time of surgery. The overall LN metastatic rate was 31%; metastasis was found mostly in the mandibular lymph center (83%). Median survival time (MST) and disease-free interval (DFI) in Group A were 406 and 134 days, respectively. Although shorter, these values were not significantly different from MST and DFI in Group B (534 and 219 days, respectively; p = 0.16 and p = 0.11). Stratifying the cases based on the type of lymphadenectomy performed, no statistical differences were observed between Groups 1 (ipsilateral lymphadenectomy) and 2 (bilateral lymphadenectomy) regarding both MST and DFI. Similarly, no significant differences in MST and DFI were observed among subgroups based on ipsilateral (Group 4) and bilateral (Group 6) removal versus ipsilateral (Group 3) and bilateral (Group 5) non-removal of even the medial retropharyngeal LN. No association was found between LN metastasis and recurrence or distant metastasis. Finally, no association was found between lymphadenectomy pattern and progressive disease. The results recorded in this study, i.e., that ipsilateral mandibular lymphadenectomy may be a reasonable surgical option in OMM, apply for this cohort of dogs only, and the translation of this principle to canine OMMs differently treated needs further investigations. Additionally, further efforts should be addressed to studies on sentinel LN identification for canine OMM staging.
Introduction
Canine oral malignant melanoma (OMM) is an aggressive tumor characterized by rapid growth and high local invasiveness; the reported metastatic rate, from presentation to post-treatment follow-up, is from 30.3 to 74.0% in regional lymph nodes (LN) and from 14 to 92% in lungs and other distant sites (1–6).
The biological behavior of canine OMM can be predicted evaluating the tumor size and volume, clinical stage, presence/absence of bone invasion, and histological and immunohistochemical factors, such as the degree of Ki67 expression, the mitotic count (MC), nuclear atypia (1, 2, 4, 7–11, 58, 59).
The current standard of care for local control of canine OMM consists of wide surgical excision, including 1.5–2 cm of macroscopically normal tissue and adjacent bone if it is part of the excision margin (3, 5, 8, 12, 13). In cases of incomplete excision, non-resectable tumors or if owners refuse surgery, radiotherapy should be considered (14, 15). Electrochemotherapy may be proposed as an alternative, although its efficacy is limited in presence of bone involvement (16, 17).
Despite the achievement of local tumor control, most dogs with OMM die or are euthanized because of distant metastases. Adjuvant chemotherapy, primarily based on carboplatin administration, has not been proved to significantly improve survival, particularly in stage III and IV OMMs, where the median survival time remains less than 1 year (12, 13, 18–22). Metronomic therapy may offer some palliative benefits in dogs with oral cancers, including MM, although further validation is needed (22, 23).
Given the immunogenic features of OMM, immunotherapy has emerged as a promising adjuvant treatment (60). Melanoma-associated antigens have been used to generate vaccines able of evoking an immune response against canine OMM, specifically tyrosinase (24–28, 57) and chondroitin sulfate proteoglycan 4 (CSPG4) (2, 3, 29–31).
Despite a consensus has been reached regarding OMM local control, modalities of neck dissection for clinical staging and their impact on prognosis remain an area of ongoing research and debate (3, 5, 12, 32).
It is well-established that a reliable assessment of the nodal metastatic status should be based on histopathology of the excised nodes, even when they appear clinically and/or cytologically normal (33–37). Given that and considering the propensity of OMM to unpredictably metastasize to homolateral and contralateral cervical nodes, an aggressive surgical approach to those nodes seem to be warranted to accurately assess nodal involvement and therefore correctly define the N parameter of the TNM clinical tumor staging. Consequently, elective neck dissection is often performed to ensure removal of all potentially affected nodes (34, 38). Alternatively, to balance the need for accurate staging with the objective of minimizing the surgical dose and the potential complications associated with an aggressive neck dissection, sentinel LN identification via Computed Tomography (CT) indirect lymphangiography (CTL) alone or in association with intraoperative blue dye or near infrared fluorescence imaging (NIRF) with indocyanine green (ICG), lymphoscintigraphy or contrast-assisted ultrasound (CEUS) has been also investigated (39–44), and some promising results have been reported.
Elective neck dissection and bilateral nodal extirpation and sentinel lymph node biopsy (SLNB) have been proposed, but there are no data if any of those is superior or yields a prognostic benefit for the patients (45, 46).
In this retrospective study, the authors aim to assess the prognostic impact of LN metastases in dogs with OMM treated with surgical excision of the primary tumor and adjuvant anti-CSPG4 electrovaccination. Specifically, the study investigates how different types of lymphadenectomies, ranging from a more selective to a more extensive LN-neck dissection, affect survival time (ST) and disease-free interval (DFI). In addition, the incidence and anatomical distribution of metastatic LNs in the cervical region are analyzed to explore potential associations with clinical outcomes. By focusing on a homogeneous cohort of patients receiving the same therapeutic protocol, the study aims to minimize confounding factors and to better identify the prognostic relevance of nodal involvement and of the extent of neck dissection.
Materials and methods
Patients’ selection and data collection
Client-owned dogs affected by OMM, which were presented at the Veterinary Teaching Hospital of Grugliasco (Turin, Italy) from December 1st, 2014, to December 31st, 2023, were retrospectively considered for this study. Dogs were referred for either surgical treatment or adjuvant anti-CSPG4 DNA electrovaccination or both. Dogs were treated according to the Good Clinical Practice guidelines for animal clinical studies; a specific written consent form was signed by the owners for animals’ enrollment in the electrovaccination study, and for anesthetic, diagnostic and surgical procedures. Both the Ethics Committee of the University of Turin and the Italian Ministry of Health had approved the immunotherapy trials (0004230–20/02/2018-DGSAF-MDS-P and 0015537–28/06/2017-DGSAF-MDS-P).
Inclusion criteria for this study were: (a) complete staging, consisting of either a CT scan or three-view thoracic radiographs and abdominal ultrasound, with no evidence of distant metastases at presentation, (b) no concurrent life-threatening disease (mild to severe renal, hepatic or cardiac diseases or other simultaneous tumors), (c) surgical excision of the primary OMM concurrent with regional lymphadenectomy (ipsilateral or bilateral mandibular and/or medial retropharyngeal LNs), (d) definitive histological diagnosis of OMM, (e) immunohistochemical CSPG4 positivity, (f) histology of the cervical excised LNs and their classification as metastatic or not metastatic, (g) adjuvant anti-CSPG4 electrovaccination. Each dog included in the study had a minimum follow up of 1 year.
For each dog, the collected data included age, gender, weight, breed, tumor size and localization, clinical and at imaging enlargement of cervical LNs (mandibular and medial retropharyngeal), type of surgery performed (wide surgical excision of OMM, including mandibulectomy and maxillectomy or marginal resection) and pattern of regional lymphadenectomy (ipsilateral or bilateral mandibular +/− medial retropharyngeal nodes), complete TNM stage after LN histology and adjuvant treatment performed (anti-CSPG4 DNA electrovaccination; second surgery and/or radiotherapy or electrochemotherapy at local recurrence occurrence; and metronomic therapy at disease’s progression). Only dogs bearing an OMM with a CSPG4 score ≥ 3/8 were suitable for vaccination. Briefly, a modified semi-quantitative scoring system was adopted to evaluate membrane staining in 10 randomly selected high-power fields (400x) within the tumor. A score representing the estimated proportion of positively stained tumor cells was assigned as follows: 0 (none); 1 (<1/100); 2 (1/100–1/10); 3 (1/10–1/3); 4 (1/3–2/3); and 5 (>2/3). An intensity score was also assigned to represent the estimated average staining intensity of positive tumor cells (0, none; 1, weak; 2, intermediate; 3, strong). The proportion and intensity scores were then summed to obtain a total score ranging from 0 to 8. Samples with a total score greater than or equal to 3 were considered positive (47).
For all the canine OMM samples the following histological and immunohistochemical data were recorded: excision margin status (surgical margins were considered complete if the narrowest histologic margin was >2 mm), LN status (metastatic or not metastatic), presence of bone invasion, MC (cut-off of 4/10 high power field - hpf), score of Ki67 expression (using polyclonal Ki67 antibody A-047; DAKO; the Ki67 labeling index was determined by counting the number of positively labeled neoplastic cell nuclei within the area of a 1 mm2 optical grid reticle at 400×. Five grid areas within the highest labeling were counted and averaged to determine the Ki67 labeling index. Areas under regions of ulceration were avoided. Cut-off of 19.5) (2, 9, 48, 61) and CSPG4 immunohistochemical score (47, 62).
Staging, study design and treatment
Before surgery, all dogs underwent a thorough physical examination, blood exam (complete blood count and serum biochemistry) and urinalysis. For both clinical staging and surgical planning, a pre-operative total body CT scan was accomplished; alternatively, according to the owners’ decision, skull radiographs, three views chest radiographs and abdominal ultrasound were carried out.
All the dogs of this cohort underwent a surgical excision of the OMM and regional lymphadenectomy, then dogs were adjuvantly treated with the anti-CSPG4 DNA electrovaccination. Under brief general anesthesia, dogs were vaccinated with plasmids coding for the CSPG4 antigen, as already described (2, 3, 30, 31). The vaccination was started after surgery and repeated after 2 weeks and then monthly for a minimum of 6 and a maximum of 24 immunizations. According to the vaccination protocol, the dogs were re-evaluated monthly for the initial 6 months, and every 3 months thereafter. Examination consisted of clinical assessment, blood work, and CT scans of the head, neck, and thorax. Abdominal ultrasound examinations were conducted every 3 months (29).
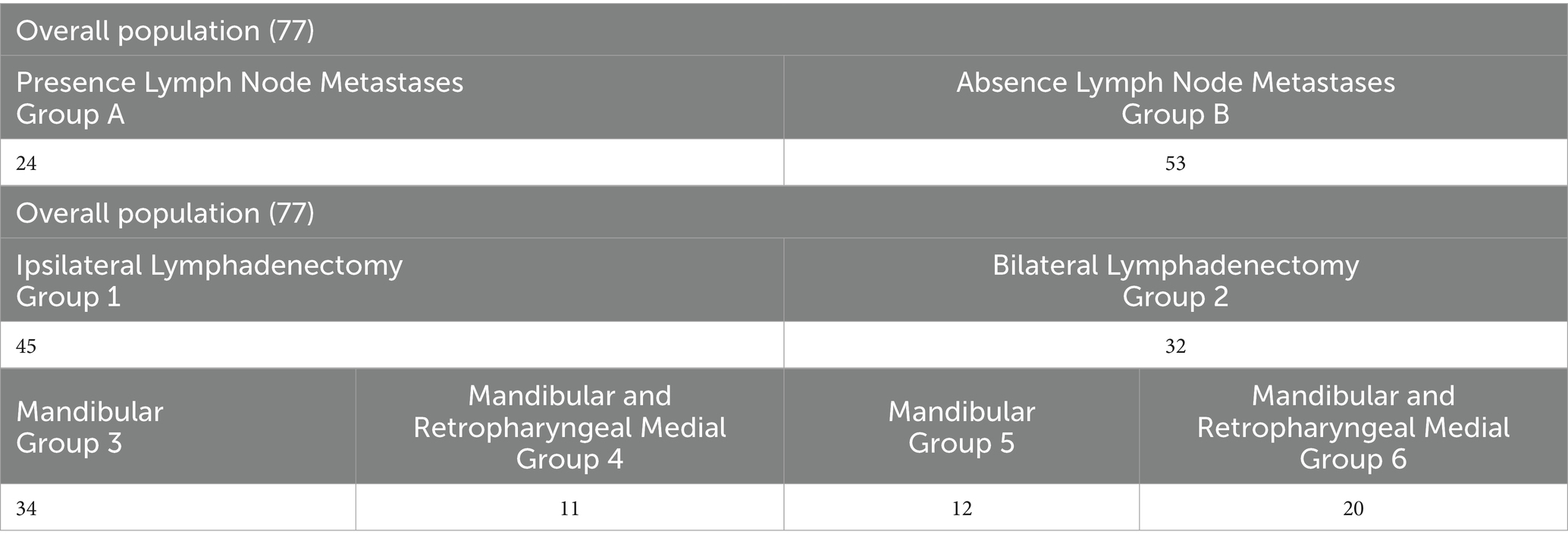
Initially, dogs were stratified based on the presence (A) or absence (B) of regional LN metastasis at presentation. Dogs were then categorized according to the type of LN extirpation performed. Group 1 included dogs that underwent ipsilateral LN dissection, while Group 2 included those that underwent bilateral LN dissection. Group 1 was further subdivided based on the lymph centers removed: only the mandibular LNs (Group 3) or both the mandibular and medial retropharyngeal LNs (Group 4). The same subdivision was applied to Group 2, resulting in Groups 5 and 6, respectively (Table 1). Given the low rate of metastasis in canine oral cancers, parotid LN excision was not considered in this study.
Statistical analysis
The analyses were carried out using GraphPad Prism (version 10.3.1 for Windows, GraphPad Software, San Diego, California1), with statistical significance set at a p < 0.05.
The data were summarized using descriptive statistics and were indicated as median and range. Distribution was checked graphically using the Shapiro–Wilk Test; Mann–Whitney and Kruskal-Wallis tests were used to assess statistical differences among different groups regarding age, weight, stages, MC, Ki67, CSPG4 expression and clinical tumor stage. The disease-free interval (DFI) and the median survival time (MST) were estimated using the Kaplan–Meier method, and differences in DFI and MST among treatment groups were assessed with the log-rank test. A power analysis for the log-rank test was performed using the online MedCalc software2 (see Appendix material). The DFI of dogs was calculated from the day of surgery to the first tumor recurrence or metastasis while the ST was defined as the period from the day of surgery to the patient’s death. Dogs which died from OMM unrelated causes, those lost to follow-up and those still alive at the end of the study were censored. Finally, Fisher’s exact test was used to test a potential association between the different patterns of neck dissection and the probability of local recurrence and/or metastasis and to assess the relationships between LN metastasis and primary tumor size and location within the oral cavity.
Results
Signalment
Seventy-seven client-owned dogs bearing an OMM entirely fulfilling the inclusion criteria were enrolled. Of these dogs, 42/77 (54.5%) were males (27 neutered and 15 intact) and 35/77 (45.5%) were females (33 spayed and 2 intact). The median age at presentation was 11 years (range, 7–15) and the median weight was 22 kg (range, 3–45 kg) (Table 2).
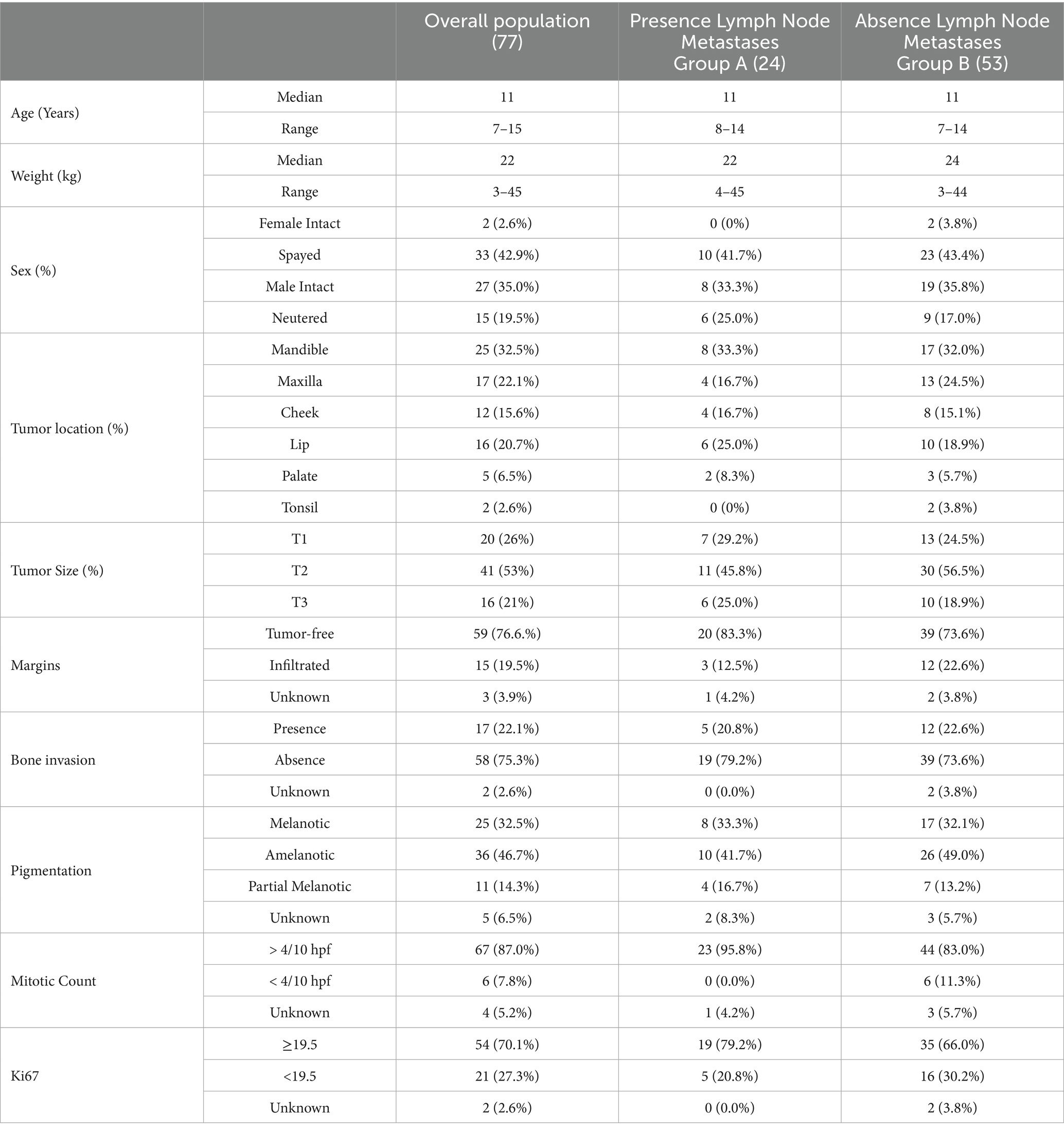
Table 2. Clinical characteristics of the dogs, and histological and immunohistochemical parameters of OMM enrolled in the study.
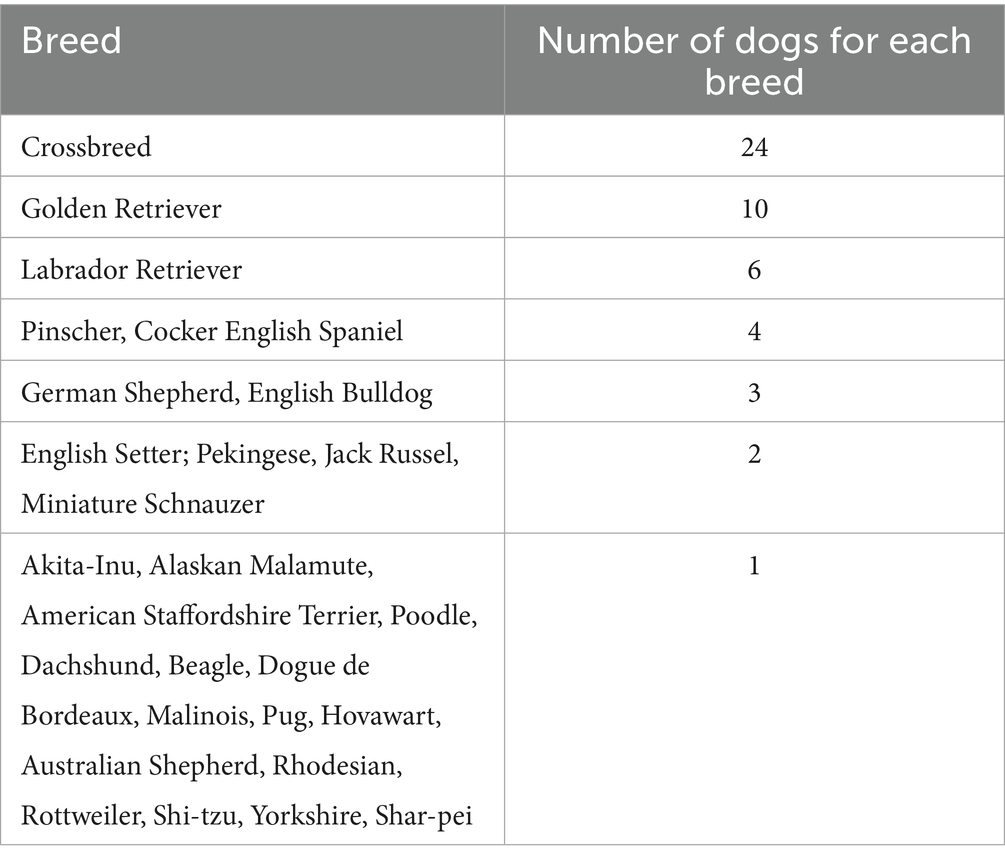
Thirty-one percent (24/77) of this canine population was represented by crossbreed dogs, while the most represented pure breed was Golden Retrievers (10 dogs). The remaining breeds are listed in Table 3.
Clinical and histological characteristics
Overall, 25/77 (32.5%) dogs had an OMM on the gingiva of the lower arcade, 17/77 (22.1%) dogs on the gum of the upper arcade, and 16/77 (20.7%) dogs on the lips. Additionally, 12/77 (15.6%) dogs had an OMM at the level of the cheek, 5/77 (6.5%) dogs on the palatal mucosa, and 2/77 (2.6%) dogs in the tonsil (Table 2).
Mandibulectomy and maxillectomy were performed in 23/77 (30.0%) and 14/77 (18.0%) dogs, respectively. Twenty-seven (27/77, 35.0%) dogs underwent a wide excision (11, 13, 3 OMMs of the cheek, lips, and palatal mucosa, respectively). A marginal excision was performed by the referring veterinarian in 13/77 (17.0%) dogs, followed by a revision surgery in 5 dogs (performed within a range of 32–74 days) and by radiotherapy in 2 further dogs (started within 2 weeks after the marginal excision). In the remaining 6/13 cases, surgical revision was not performed due to the absence of macroscopic residual disease at clinical examination and staging.
Histological status of surgical margins was considered clear in 59/77 (76.6%) cases, infiltrated in 15/77 (19.5%) cases and unknown in the remaining 3/77 (3.9%) cases. Local bone invasion was detected on CT scan and histology in 17/77 (22.1%) dogs. Regarding histological and immunohistochemical prognostic factors, MC was ≥4/10 hpf in 67/77 (87.0%) OMMs, <4/10 hpf in 6/77 (7.8%) OMMs and unknown in 4/77 (5.2%) samples. The Ki67 expression was ≥19.5 in 54/77 (70.0%) OMMs, <19.5 in 21/77 (27.4%) OMMs and not available in 2/77 (2.6%) cases. The CSPG4 expression was ≥ 3 in all the OMM. Twenty-five/77 (32.5%) OMMs were melanotic, 36/77 (46.7%) OMMs were classified as amelanotic, 11/77 (14.3%) as partially melanotic, and in 5/77 (6.5%) cases the grade of pigmentation was not reported (Table 2).
A regional lymphadenectomy was performed in all dogs. Ipsilateral LNs were removed in 45 out of 77 (58.4%) dogs (Group 1) while a bilateral lymphadenectomy was carried out in the remaining 32/77 (41.6%) dogs (Group 2). In the first group, 34/45 (75.6%) dogs underwent only mandibular lymphadenectomy (Group 3) while in 11/45 (24.4%) also the medial retropharyngeal LN was extirpated (Group 4). Considering dogs that underwent bilateral LN excision (Group 2), lymphadenectomy of the mandibular LNs only was performed in 12/32 (37.5%) dogs (Group 5) while both mandibular and medial retropharyngeal LNs extirpation was done in 20/32 (62.5%) dogs (Group 6) (Table 1).
Regional LNs appeared enlarged on both clinical evaluation and CT in 24/77 cases (31.2%). Of these, only 12 (50.0%) were truly metastatic while the other 12 enlarged LNs were histologically normal. Considering the metastatic LNs, the other 12 were normal on both clinical and imaging evaluation. Indirect CTL for sentinel LN was performed only in 8 dogs that underwent a bilateral lymphadenectomy (Group 6). In 7 dogs, CTL identified the ipsilateral mandibular LN as the sentinel node. Among these, 5 LNs were histologically normal without any evidence of metastasis; in the remaining 2 dogs, CTL correctly identified the metastatic LNs. In one case, CTL identified the ipsilateral medial retropharyngeal LN as the sentinel node, but histology revealed metastasis in the contralateral mandibular LN.
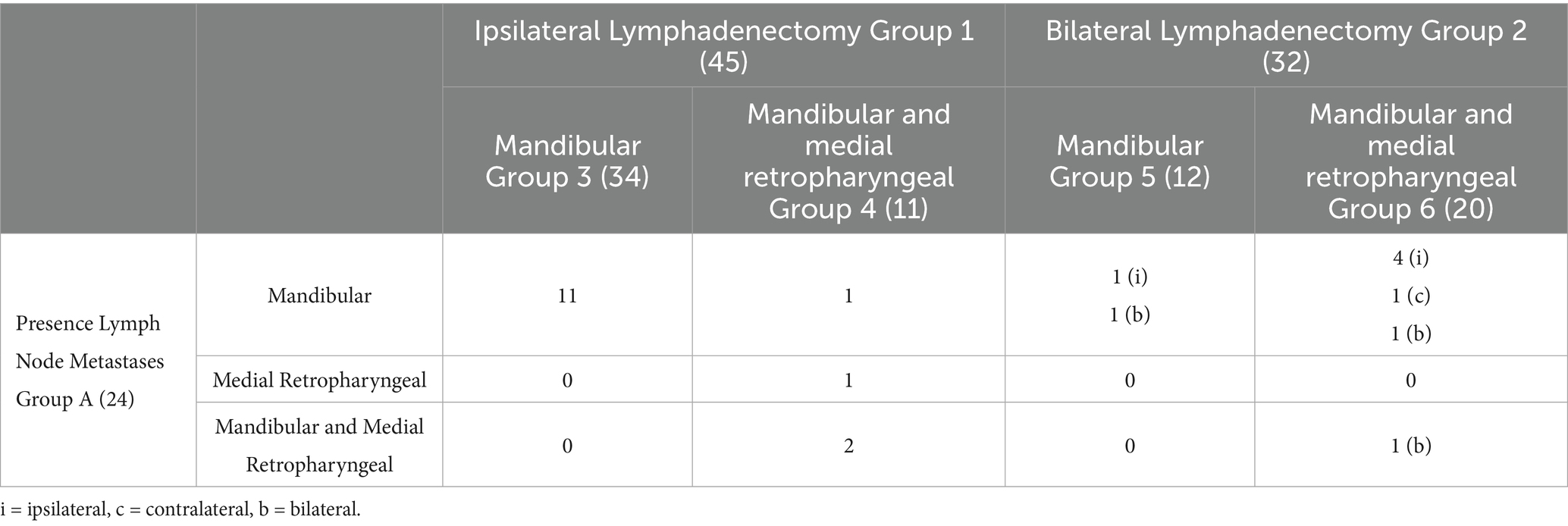
Based on postoperative histology, 24 out of 77 (31.2%, Group A) dogs displayed metastatic LNs. The regional LN metastatic rate was 33.3% (15/45) in Group 1 and 28.1% (9/32) in Group 2. When analyzing metastatic involvement in the subgroups, 11 dogs (32.3%) in Group 3 exhibited mandibular LNs metastasis. In Group 4, metastases were observed in 1 dog at a mandibular LN, in 1 dog at a medial retropharyngeal LN, and in 2 dogs at both the mandibular and medial retropharyngeal LNs. In Group 5, 1 dog presented metastasis at an ipsilateral mandibular LN while another dog had bilateral mandibular LNs involvement. In Group 6, metastatic spread was identified in 4 dogs at the ipsilateral mandibular LNs, in 1 dog at the contralateral mandibular LN, in 1 dog at both bilateral mandibular LNs, and in 1 dog metastases were observed in all the LNs excised (after bilateral mandibular and medial retropharyngeal LN excision) (Table 4).
Final tumor staging was as follows:13 dogs were classified as stage I (17.0%), 30 as stage II (39.0%) and 34 as stage III (44.0%) (Table 5).
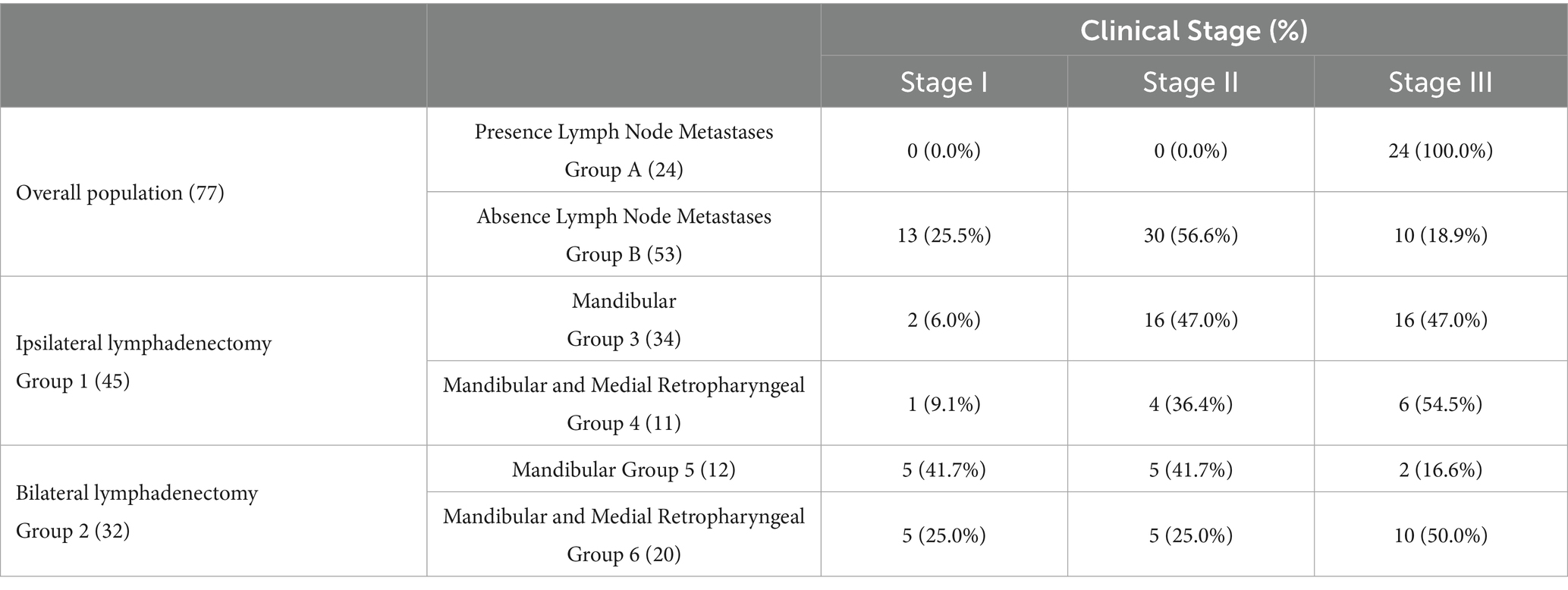
Table 5. Clinical staging, according to TNM classification system (55), of the dogs enrolled in the study.
All dogs received adjuvant anti-CSPG4 DNA vaccination, with a median of 7 vaccinations administered (range, 4–26). In case of progressive disease (lung and/or LN metastasis and/or local recurrence), 18 dogs (23.4%) also received metronomic chemotherapy consisting of a combination of piroxicam, cyclophosphamide and thalidomide.
No statistical differences were observed between the groups when considering MC, Ki67, CSPG4, bone invasion, pigmentation, clinical stage and adjuvant treatments.
Follow up and statistical data
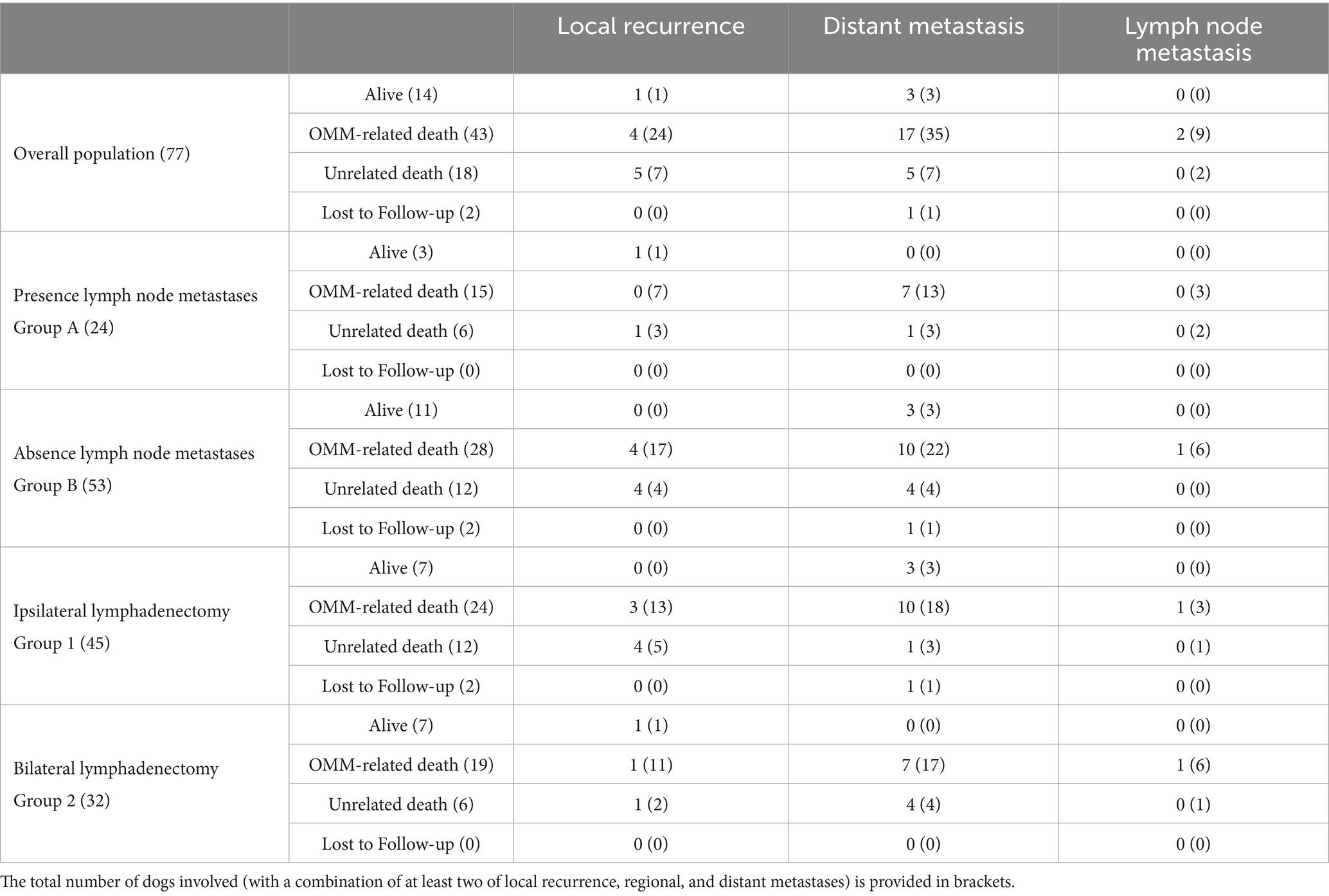
At the end of the study, 14/77 dogs (18.2%) were still alive (range, 367–2,167 days), while 61/77 (79.2%) had died (range, 171–2,252 days), 43 (68.3%) of which for OMM-related causes (range, 171–1,063 days), while 2/77 (2.6%) were lost to follow-up (512 and 962 days, DFI of 241 days for the latter). Sixty dogs out of 77 (78%) developed progressive disease, with 10 dogs (16.7%) experiencing a local recurrence only, 26 (43.3%) distant metastases only, 2 (3.3%) LN metastasis only, while 22 dogs (36.7%) displayed a combination of at least two among local recurrence, regional and distant metastases. Data are summarized in Table 6.
Among the 32/77 dogs (41.5%), which developed local recurrence, this was further managed with radiotherapy, electrochemotherapy and surgery in 3 (3.9%), 5 (6.5%), and 18 (23.4%) dogs, respectively. Electrochemotherapy, following intravenous bleomycin infusion, was applied in an attempt to reduce the size of local recurrence. Additionally, LNs extirpation were performed in 6 dogs (7.8%) to treat the development of new LN metastases. Specifically, 3 dogs had initially undergone bilateral mandibular and medial retropharyngeal LN dissection (Group 6), 1 had received ipsilateral mandibular lymphadenectomy (Group 3), and in 2 dogs, bilateral mandibular LNs had been excised (Group 5).
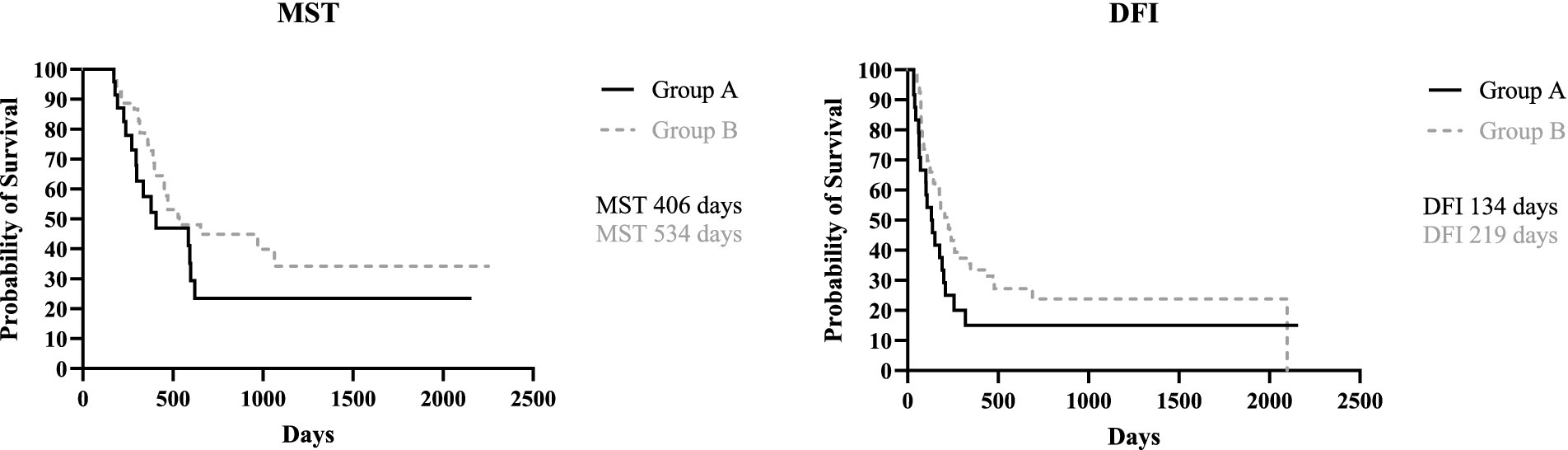
When dividing the study population based on presence/absence of LN metastases, the MST for group A (metastatic) and group B (not metastatic) was, respectively, 406 days (range, 171–2,157 days) and 534 days (range, 171–2,252 days), with no statistical difference between the two groups (p = 0.16). The DFI for group A and B was 134 days (range, 34–2,157 days) and 219 days (range, 36–2098 days), respectively; again, no statistical difference was found (p = 0.11) (Figure 1).
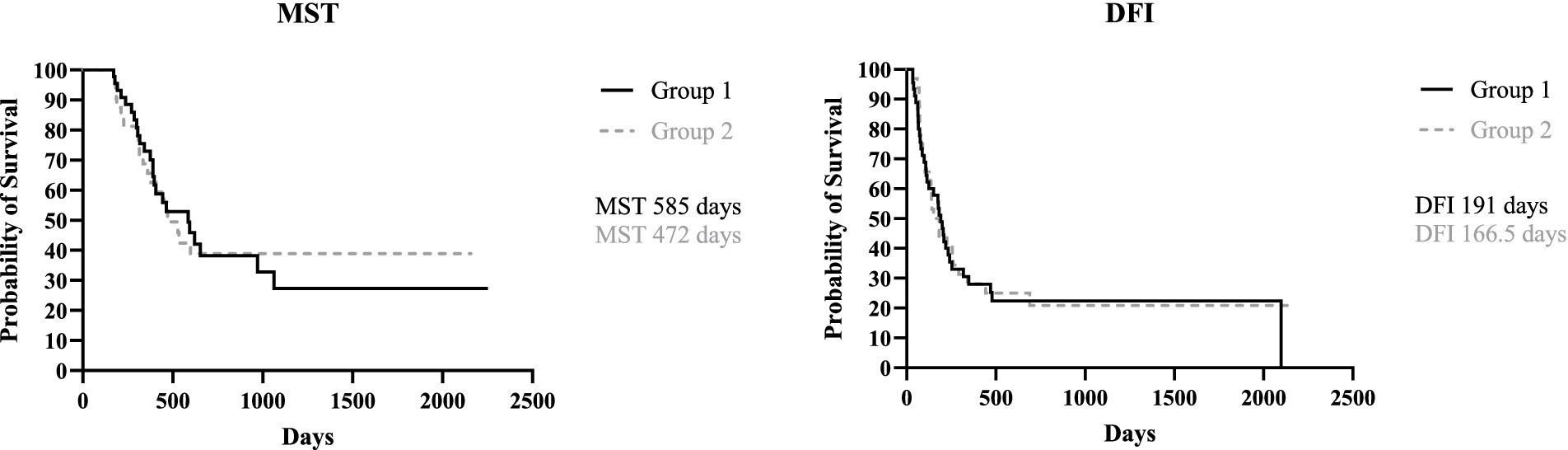
When dividing the canine population based on the type of lymphadenectomy performed, MST for Group 1 (ipsilateral excision) was 585 days (171–2,252 days) while MST for Group 2 (bilateral lymphadenectomy) was 472 days (range, 171–2,157 days); DFI for Group 1 was 191 days (range, 34–2098 days) and 166.5 days (range, 36–2,157 days) for Group 2, with no statistical difference found for both MST and DFI between the two groups (p = 0.98 and p = 0.84, respectively) (Figure 2).
The MST and DFI were then assessed in the subgroups categorized by whether or not the medial retropharyngeal LN had been surgically removed. The MST for Group 3 (ipsilateral mandibular lymphadenectomy) and Group 4 (ipsilateral mandibular + medial retropharyngeal lymphadenectomy) was, respectively, 585 days (range, 171–2,252 days) and 594 days (range, 179–920 days), while the DFI for Group 3 was 195 days (range, 34–2098 days) and 177 days (range, 39–920 days) for Group 4, with no statistically significant difference found between the two groups (p = 0.86 and p = 0.94). When Group 5 (bilateral mandibular lymphadenectomy) and Group 6 (bilateral mandibular + retropharyngeal lymphadenectomy) were compared, MST of Group 5 was not reached (range, 187–2,157 days) and, even if longer, there was no statistical difference with MST of Group 6 that was 430 days (range, 171–1,424 days) (p = 0.06). Similarly, the DFI of Group 5 was 259.5 days (range, 36–2,157 days), longer than that of Group 6 that was 141 days (range, 61–990 days), but no significant difference was found between the two groups (p = 0.43) (Figure 3).
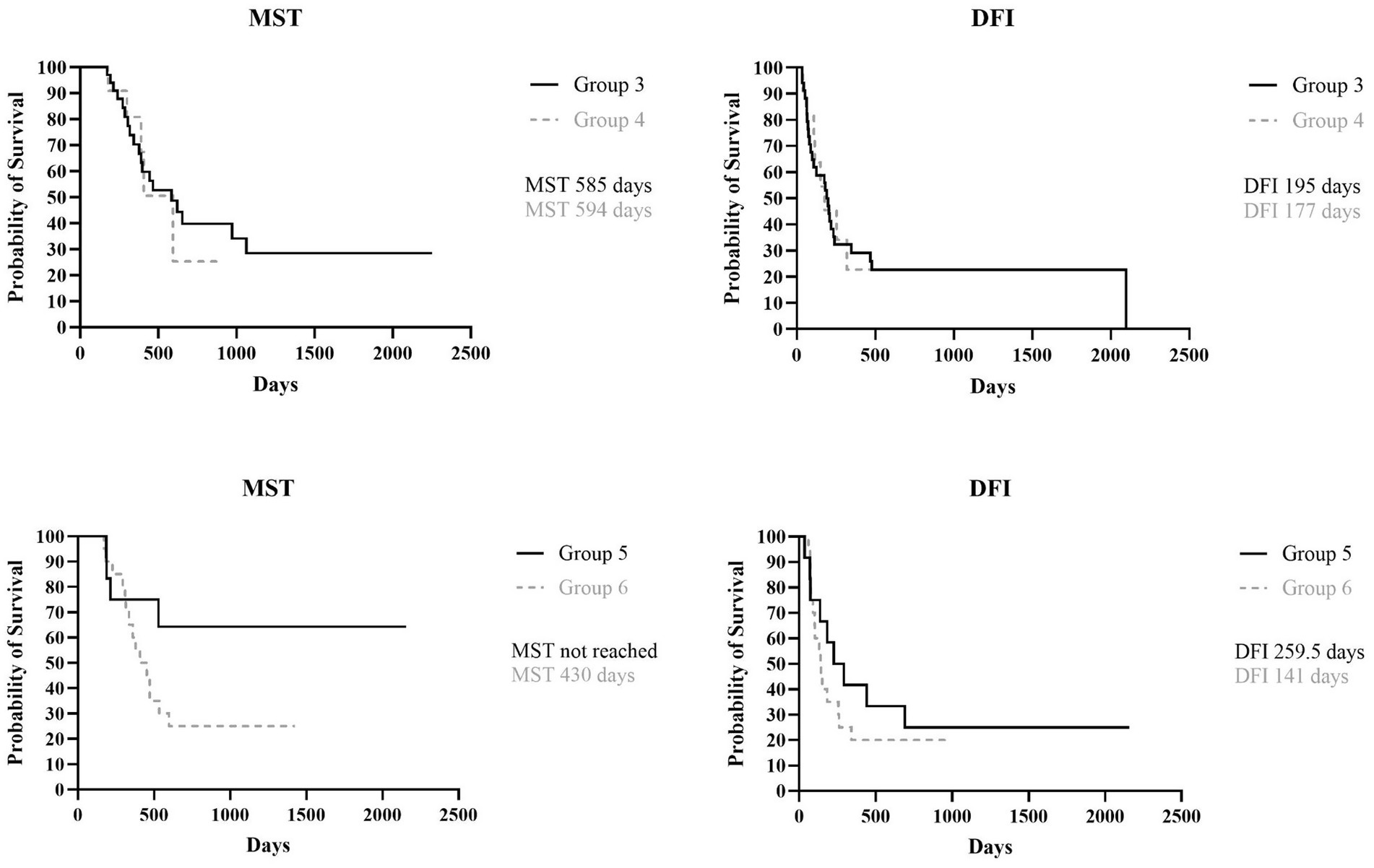
Figure 3. Kaplan Meyer analysis of MST (p = 0.86) and DFI (p = 0.94) of Group 3 and 4, and MST (p = 0.06) and DFI (p = 0.43) of Group 5 and 6.
Furthermore, when Group 3, in which fewer LNs were removed, was compared with Group 6, in which both mandibular and medial retropharyngeal LNs were bilaterally removed, no statistical differences were found for both MST and DFI (p = 0.34 and p = 0.76, respectively).
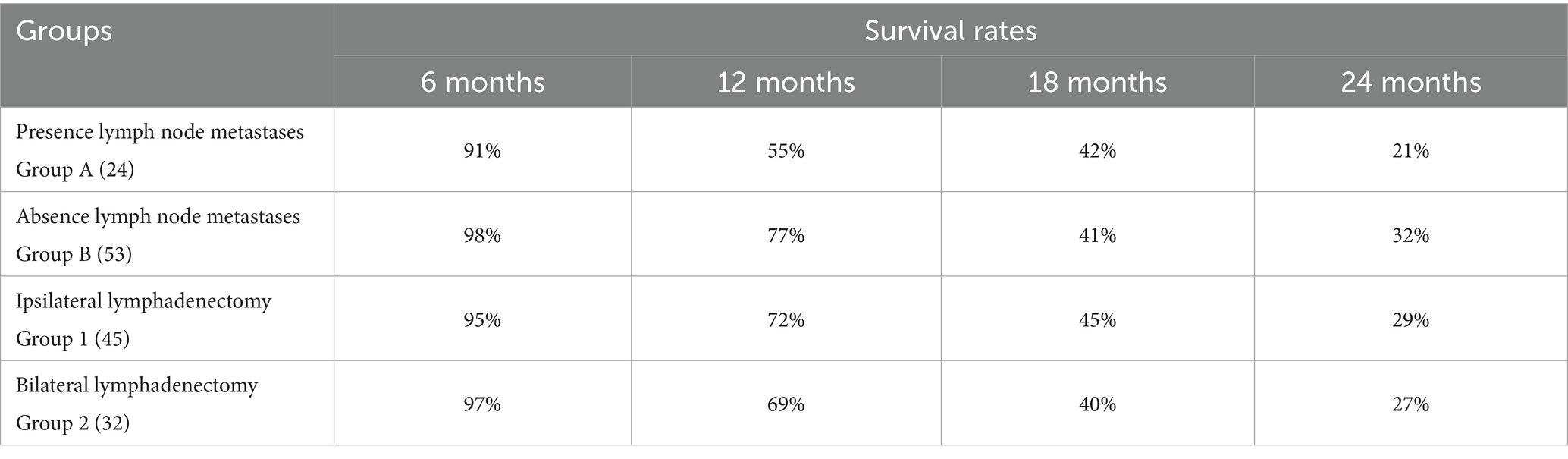
The survival rates at 6, 12, 18 and 24 months for each group are reported in Table 7.
The overall MST for the entire canine population was 529 days (range, 171–2,252 days) while the overall DFI was 183 days (range, 34–2,157 days).
No statistical association was found between LNs metastasis at the time of surgery and the primary tumor dimension (p = 0.67) or its location within the oral cavity (p = 0.91).
Additionally, no association was found between the extent of lymphadenectomy performed and the disease progression (p = 0.99), the occurrence of distant metastases (p = 0.48), the incidence of local recurrence (p = 0.81), and the development of new regional LN metastasis (p = 0.18). Finally, no statistical association was observed between the presence of LN metastasis at diagnosis and the occurrence of recurrence (p = 0.62) or distant metastases (p = 0.46).
Discussion
A consensus has been reached on the need for a multimodal approach to canine OMM; however the appropriate extent of surgical dose for staging the regional/sentinel LNs, as well as the potential therapeutic effect and prognostic impact of elective neck dissection on the disease progression, remain still unclear (5, 32). To fill this gap of knowledge, the present study was conducted to assess the impact of LN metastases on the long-term survival in a specific cohort of dogs with OMM. Furthermore, we aimed at evaluating if elective neck dissection and bilateral nodal extirpation (45) offers a prognostic benefit compared to a targeted – and therefore less invasive – approach to the regional/sentinel LNs.
In this study, the prevalence of histologically confirmed LN metastasis at presentation was 31.2%, consistent with previous literature (1, 4, 38). The mandibular lymph center exhibited the highest incidence of metastasis (20/24 cases, 83.0%), differing from earlier reports in which metastasis was more evenly distributed also to the medial retropharyngeal LN (34, 36).
The histological metastatic status of the excised nodes was not associated with primary tumor size or location, nor with nodal clinical size, thus corroborating the unreliability of LN clinical size to predict their status.
This result once again confirms that histopathology should be considered the most reliable method to assess nodal metastases (37, 38, 41, 43). In this study 12 enlarged LNs were histologically reactive, while 12 metastatic LNs appeared clinically normal on clinical and CT examination, as already reported in other studies (37). However, no cytology was performed on these LNs preoperatively, as their removal was already planned as part of the protocol; so no comparison with post-excisional histology was conducted in this study.
The risk of distant metastases or local recurrence was not associated with the presence of LN metastasis at the time of surgery, indicating that factors other than the nodal status may play a more critical role in disease progression. Nevertheless, it should be noted that the MST of dogs with metastatic LNs was lower than that of dogs without LN metastasis at the time of surgery although this difference was not statistically significant (p = 0.16); a similar trend was observed for DFI. Despite this, the clinically relevant difference in survival between the 2 groups supports the potential importance of lymphadenectomy in the management of dogs with OMM.
The most effective pattern of LN extirpation was assessed by stratifying our canine OMMs population based on the type of lymphadenectomy performed. However, the comparison among these groups did not reveal any significant differences in terms of MST and DFI. Furthermore, when comparing the group of dogs with the fewest LNs removed (ipsilateral mandibular only) with the group with the highest number of LNs excised (bilateral mandibular and medial retropharyngeal), no statistical differences were observed in terms of both MST and DFI. No significant association was found between the type of lymphadenectomy performed and either the risk of local recurrence, distant metastases, or tumor dissemination to other LNs. The lack of statistical significance here may be due to the limited sample size. However, these findings align with the “marker hypothesis” proposed for human melanoma, which suggests that LN metastases reflect a biologically aggressive tumor phenotype with a systemic dissemination potential, rather than being a direct cause of disease progression (49–51). More recently, Faries (51) emphasized that SLN metastases serve primarily as markers of systemic disease rather than as effective barriers to metastatic dissemination, reinforcing the concept that extensive lymphadenectomy may not significantly impact the clinical outcomes (51).
In this context, the lack of prognostic benefit from extensive lymphadenectomy could be explained by the hypothesis that removal of additional nodes beyond the primary metastatic site may not influence the systemic disease progression if tumor dissemination has already occurred.
Therefore, the results of the present study suggest that an extensive lymphadenectomy including bilateral removal of the mandibular and retropharyngeal nodes may not improve outcome and therefore the increased surgical dose and potentially morbidity compared to ipsilateral regional/sentinel lymphadenectomy may not be justified by an oncological benefit. This is also supported by the higher incidence of metastasis found in this series of dogs at the level of the ipsilateral mandibular LNs. On the other hand, the extirpation of the ipsilateral mandibular LNs only may result in undetected nodal metastases.
To reduce the risk of missing nodal metastases–potentially compromising the oncological outcome–while still minimizing the surgical dose, SLN mapping techniques should be preferred over regional lymphadenectomy; these techniques indeed allow for removal of the nodes at highest risk of harboring nodal metastases while reducing the number of excised nodes compared to elective neck dissection (40, 43, 52).
In this study, CTL was performed only in 8 dogs. The SLNs were accurately identified in all but one case. As previously reported, CTL has a reported accuracy of up to 97% for oral tumors in dogs (63). The limited use of indirect CTL or other preoperative/intraoperative sentinel mapping techniques in the present study represents a significant limitation, thus preventing the authors from confirming the efficacy of CTL and assessing its impact on the choice between a radical vs. a more selective lymphadenectomy procedure.
Another important limitation of this study is the absence of a control group of dogs in which lymphadenectomy was not performed, impeding the evaluation of the real role of cervical lymphatic metastasis in prognosis. Nevertheless, it should be outlined that the non-removal of the regional (or better, sentinel) LNs does not represent the current “standard of care” for OMM; in fact, in this scenario, only the overt metastatic LNs would have been diagnosed and excised, thus underestimating the real clinical stage in case of micro metastatic LNs.
A further limitation of this study is the lack of a control group of dogs that underwent surgery and lymphadenectomy without adjunctive vaccine treatment. The choice to include only dogs with OMM treated by surgery and adjuvant anti-CSPG4 electrovaccination was to ensure a homogeneous population of patients for statistical analysis. Nevertheless, this may have introduced a confounding factor when assessing the impact of lymphadenectomy on MST and DFI. The anti-CSPG4 vaccine may have contributed to limiting the metastatic spread, thereby potentially influencing the correct evaluation of the prognostic significance of LN excision on outcome.
Additionally, the potential variability in the number of LNs removed by the surgeons involved in this study could have resulted in an underestimation of LN metastasis in some cases and, consequently, even of the definitive clinical stage. This was not the case for the medial retropharyngeal LN that, being usually a single LN, was never missed if the goal was to remove it, uni-or bilaterally. Nevertheless, missed LNs, if not enlarged, may have occurred intraoperatively for a lateral retropharyngeal LN, present in about 1/3 of dogs only, and the mandibular LNs, present in dogs in a variable number of 2–3 up to 5 on each side (53, 54). Missed LNs may account for the development of subsequent nodal metastases, even in dogs that underwent total bilateral lymphadenectomy or excision of nodes within the same lymph center in cases of mandibular lymphadenectomy. The prognostic impact of a missed LN is at present unknown. Strict monitoring and restaging during the follow-up may permit a quick detection and treatment of new LN metastases.
The results recorded in this study apply only for this specific cohort of dogs and a translation to canine OMMs differently treated requires further investigations. However, based on this study, it can be concluded that (1) a bilateral mandibular and medial retropharyngeal lymphadenectomy, while ensuring the highest detection rate of LN metastasis, was not associated with a significative survival improvement, and (2) an ipsilateral mandibular lymphadenectomy (at least, prudently, for an ipsilateral OMM, unless differently dictated by the preoperative clinical, cytological and imaging findings) may be a reasonable option as it was here associated with a high probability of removing the nodes characterized by the highest risk of metastasis. Nevertheless, apart from the excision of the clearly metastatic LNs already at presentation, authors emphasize the absolute need to implement the procedures aimed at identifying SLN or, ambitiously, those LNs apparently normal but microscopically metastatic. In all circumstances, after the initial wide surgical excision followed by the adjuvant treatment chosen among those the clinician feels more confident with, a strict monitoring and restaging during the follow-up may permit further therapeutic procedures with the ultimate goal of prolonging the survival.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal studies were approved by both the Ethics Committee of the University of Turin and the Italian Ministry of Health (0004230–20/02/2018-DGSAF-MDS-P and 0015537–28/06/2017-DGSAF-MDS-P). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
DG: Methodology, Validation, Formal analysis, Visualization, Data curation, Project administration, Conceptualization, Software, Writing – original draft. MO: Writing – review & editing. EF: Writing – review & editing. GM: Writing – review & editing. LoM: Writing – review & editing. MCa: Writing – review & editing. FR: Writing – review & editing. FC: Writing – review & editing. LT: Writing – review & editing. MCi: Writing – review & editing. AD: Writing – review & editing. SI: Writing – review & editing. EL: Writing – review & editing. LuM: Writing – review & editing. RD: Writing – review & editing. PB: Validation, Conceptualization, Writing – review & editing, Project administration, Supervision. EM: Supervision, Visualization, Project administration, Conceptualization, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
SI was employed by Veterinary Analysis Laboratory srl.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Bergman, PJ, Selmic, LE, and Kent, MS. Melanoma In: DM Vail, DH Thamm, and JM Liptak, editors. The Withrow and Mac Ewen's small animal clinical oncology. 6th ed. St. Louis: Elsevier (2020). 367–81.
2. Camerino, M, Giacobino, D, Manassero, L, Iussich, S, Riccardo, F, Cavallo, F, et al. Prognostic impact of bone invasion in canine oral malignant melanoma treated by surgery and anti-CSPG4 vaccination: a retrospective study on 68 cases (2010–2020). Vet Comp Oncol. (2022) 20:189–97. doi: 10.1111/vco.12761
3. Giacobino, D, Camerino, M, Riccardo, F, Cavallo, F, Tarone, L, Martano, M, et al. Difference in outcome between curative intent vs marginal excision as a first treatment in dogs with oral malignant melanoma and the impact of adjuvant CSPG4-DNA electrovaccination: a retrospective study on 155 cases. Vet Comp Oncol. (2021) 19:651–60. doi: 10.1111/vco.12690
4. Liptak, JM. Oral tumors In: DM Vail, DH Thamm, and JM Liptak, editors. The Withrow and Mac Ewen's small animal clinical oncology. 6th ed. St. Louis, MO: Elsevier (2020). 432–48.
5. Polton, G, Borrego, JF, Clemente-Vicario, F, Clifford, CA, Jagielski, D, Kessler, M, et al. Melanoma of the dog and cat: consensus and guidelines. Front Vet Sci. (2024) 11:1359426. doi: 10.3389/fvets.2024.1359426
6. Ramos-Vara, JA, Beissenherz, ME, Miller, MA, Johnson, GC, Pace, LW, Fard, A, et al. Retrospective study of 338 canine oral melanomas with clinical, histologic, and immunohistochemical review of 129 cases. Vet Pathol. (2000) 37:597–608. doi: 10.1354/vp.37-6-597
7. Kim, WS, Vinayak, A, and Powers, B. Comparative review of malignant melanoma and histologically well-differentiated melanocytic neoplasm in the Oral cavity of dogs. Vet Sci. (2021) 8:261. doi: 10.3390/vetsci8110261
8. Silva, ML, Martinho, I, Rocha, M, Martano, M, Spindler, KP, Buracco, P, et al. Relative tumour volume in canine Oral melanoma staging and prognosis. Vet Comp Oncol. (2024) 22:641–50. doi: 10.1111/vco.13018
9. Smedley, RC, Sebastian, K, and Kiupel, M. Diagnosis and prognosis of canine melanocytic neoplasms. Vet Sci. (2022) 9:175. doi: 10.3390/vetsci9040175
10. Song, E, Lawrence, J, Greene, E, Christie, A, and Goldschmidt, S. Risk stratification scheme based on the TNM staging system for dogs with oral malignant melanoma centered on clinicopathologic presentation. Front Vet Sci. (2024) 11:1472748. doi: 10.3389/fvets.2024.1472748
11. Spangler, WL, and Kass, PH. The histologic and epidemiologic bases for prognostic considerations in canine melanocytic neoplasia. Vet Pathol. (2006) 43:136–49. doi: 10.1354/vp.43-2-136
12. Boston, SE, Lu, X, Culp, WT, Montinaro, V, Romanelli, G, Dudley, RM, et al. Efficacy of systemic adjuvant therapies administered to dogs after excision of oral malignant melanomas: 151 cases (2001–2012). J Am Vet Med Assoc. (2014) 245:401–7. doi: 10.2460/javma.245.4.401
13. Tuohy, JL, Selmic, LE, Worley, DR, Ehrhart, NP, and Withrow, SJ. Outcome following curative-intent surgery for oral melanoma in dogs: 70 cases (1998-2011). J Am Vet Med Assoc. (2014) 245:1266–73. doi: 10.2460/javma.245.11.1266
14. Baja, AJ, Kelsey, KL, Ruslander, DM, Gieger, TL, and Nolan, MW. A retrospective study of 101 dogs with oral melanoma treated with a weekly or biweekly 6 Gy × 6 radiotherapy protocol. Vet Comp Oncol. (2022) 20:623–31. doi: 10.1111/vco.12815
15. Cancedda, S, Rohrer Bley, C, Aresu, L, Dacasto, M, Leone, VF, Pizzoni, S, et al. Efficacy and side effects of radiation therapy in comparison with radiation therapy and temozolomide in the treatment of measurable canine malignant melanoma. Vet Comp Oncol. (2016) 14:e146–15. doi: 10.1111/vru.12308
16. Moretti, G, Dentini, A, Beccati, F, Arcelli, R, Matteo, ID, Giovannini, G, et al. Palliative repeated electroporations of oral tumours in dogs: a case series. Front Vet Sci. (2022) 9:1004811. doi: 10.3389/fvets.2022.1004811
17. Tellado, MN, Maglietti, FH, Michinski, SD, Marshall, GR, and Signori, E. Electrochemotherapy in treatment of canine oral malignant melanoma and factors influencing treatment outcome. Radiol Oncol. (2020) 54:68–78. doi: 10.2478/raon-2020-0014
18. Brockley, LK, Cooper, MA, and Bennett, PF. Malignant melanoma in 63 dogs (2001–2011): the effect of carboplatin chemotherapy on survival. N Z Vet J. (2013) 61:25–31. doi: 10.1080/00480169.2012.699433
19. Dank, G, Rassnick, KM, Sokolovsky, Y, Garrett, LD, Post, GS, Kitchell, BE, et al. Use of adjuvant carboplatin for treatment of dogs with oral malignant melanoma following surgical excision. Vet Comp Oncol. (2014) 12:78–84. doi: 10.1111/j.1476-5829.2012.00338.x
20. Murphy, S, Hayes, AM, Blackwood, L, Maglennon, G, Pattinson, H, and Sparkes, AH. Oral malignant melanoma - the effect of coarse fractionation radiotherapy alone or with adjuvant carboplatin therapy. Vet Comp Oncol. (2005) 3:222–9. doi: 10.1111/j.1476-5810.2005.00082.x
21. Wouda, RM, Hocker, SE, and Higginbotham, ML. Safety evaluation of combination carboplatin and toceranib phosphate (Palladia) in tumour-bearing dogs: a phase I dose finding study. Vet Comp Oncol. (2018) 16:E52–60. doi: 10.1111/vco.12332
22. Xia, Y, Liao, AT, and Lee, J. A retrospective study of chemotherapeutic effect without wide-margin surgery or radiation therapy in dogs with oral malignant melanoma. Can Vet J. (2024) 65:343–50.
23. Marchetti, V, Giorgi, M, Fioravanti, A, Finotello, R, Citi, S, Canu, B, et al. First-line metronomic chemotherapy in a metastatic model of spontaneous canine tumours: a pilot study. Investig New Drugs. (2012):1725–30. doi: 10.1007/s10637-011-9672-y
24. Bergman, PJ, McKnight, J, Novosad, A, Charney, S, Farrelly, J, Craft, D, et al. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clin Cancer Res. (2003) 9:1284–90.
25. Grosenbaugh, DA, Leard, AT, Bergman, PJ, Klein, MK, Meleo, K, Susaneck, S, et al. Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. Am J Vet Res. (2011) 72:1631–8. doi: 10.2460/ajvr.72.12.1631
26. Ottnod, JM, Smedley, RC, Walshaw, R, Hauptman, JG, Kiupel, M, and Obradovich, JE. A retrospective analysis of the efficacy of the Oncept vaccine for the adjunct treatment of canine oral malignant melanoma. Vet Comp Oncol. (2013) 11:219–29. doi: 10.1111/vco.12057
27. Treggiari, E, Grant, JP, and North, SM. A retrospective review of outcome and survival following surgery and adjuvant xenogeneic DNA vaccination in 32 dogs with oral malignant melanoma. J Vet Med Sci. (2016) 78:845–50. doi: 10.1292/jvms.15-0510
28. Verganti, S, Berlato, D, Blackwood, L, Amores-Fuster, I, Polton, GA, Elders, R, et al. Use of Oncept melanoma vaccine in 69 canine oral malignant melanomas in the UK. J Small Anim Pract. (2017) 58:10–6. doi: 10.1111/jsap.12613
29. Camerino, M, Giacobino, D, Tarone, L, Dentini, A, Martano, M, Morello, E, et al. Clinical evaluation of HuDo-CSPG4 DNA electroporation as adjuvant treatment for canine oral malignant melanoma: comparison of two vaccination protocols. Vet Q. (2025) 45:1–16. doi: 10.1080/01652176.2025.2473717
30. Piras, LA, Riccardo, F, Iussich, S, Maniscalco, L, Gattino, F, Martano, M, et al. Prolongation of survival of dogs with oral malignant melanoma treated by en bloc surgical resection and adjuvant CSPG4-antigen. Vet Comp Oncol. (2017) 3:996–1013. doi: 10.1111/vco.12239
31. Riccardo, F, Tarone, L, Camerino, M, Giacobino, D, Iussich, S, Barutello, G, et al. Antigen mimicry as an effective strategy to induce CSPG4-targeted immunity in dogs with oral melanoma: a veterinary trial. J Immunother Cancer. (2022) 10:e004007. doi: 10.1136/jitc-2021-004007
32. Congiusta, M, Lawrence, J, Rendahl, A, and Goldschmidt, S. Variability in recommendations for cervical lymph node pathology for staging of canine oral neoplasia: a survey study. Front Vet Sci. (2020) 7:506. doi: 10.3389/fvets.2020.00506
33. Grimes, JA, Matz, BM, Christopherson, PW, Koehler, JW, Cappelle, KK, Hlusko, KC, et al. Agreement between cytology and histopathology for regional lymph node metastasis in dogs with melanocytic neoplasms. Vet Pathol. (2017) 54:579–87. doi: 10.1177/0300985817698209
34. Grimes, JA, Mestrinho, LA, Berg, J, Cass, S, Oblak, ML, Murphy, S, et al. Histologic evaluation of mandibular and medial retropharyngeal lymph nodes during staging of oral malignant melanoma and squamous cell carcinoma in dogs. J Am Vet Med Assoc. (2019) 254:938–43. doi: 10.2460/javma.254.8.938
35. Herring, ES, Smith, MM, and Robertson, JL. Lymph node staging of Oral and maxillofacial neoplasms in 31 dogs and cats. J Vet Dent. (2002) 19:122–6. doi: 10.1177/089875640201900301
36. Skinner, OT, Boston, SE, and Souza, CHDM. Patterns of lymph node metastasis identified following bilateral mandibular and medial retropharyngeal lymphadenectomy in 31 dogs with malignancies of the head. Vet Comp Oncol. (2017) 15:881–9. doi: 10.1111/vco.12229
37. Williams, LE, and Packer, RA. Association between lymph node size and metastasis in dogs with oral malignant melanoma: 100 cases (1987–2001). J Am Vet Med Assoc. (2003) 222:1234–6. doi: 10.2460/javma.2003.222.1234
38. Skinner, OT, Boston, SE, Giglio, RF, Whitley, EM, Colee, JC, and Porter, EG. Diagnostic accuracy of contrast-enhanced computed tomography for assessment of mandibular and medial retropharyngeal lymph node metastasis in dogs with oral and nasal cancer. Vet Comp Oncol. (2018) 16:562–70. doi: 10.1111/vco.12415
39. Chiti, LE, Stefanello, D, Manfredi, M, Zani, DD, De Zani, D, Boracchi, P, et al. To map or not to map the cN0 neck: Impact of sentinel lymph node biopsy in canine head and neck tumours. Vet Comp Oncol. (2021) 19:661–70. doi: 10.1111/vco.12697
40. Goldschmidt, S, Stewart, N, Ober, C, Bell, C, Wolf-Ringwall, A, Kent, M, et al. Contrast-enhanced and indirect computed tomography lymphangiography accurately identifies the cervical lymphocenter at risk for metastasis in pet dogs with spontaneously occurring oral neoplasia. PLoS One. (2023) 18:e0282500. doi: 10.1371/journal.pone.0282500
41. Grimes, JA, Secrest, SA, Wallace, ML, Laver, T, and Schmiedt, CW. Use of indirect computed tomography lymphangiography to determine metastatic status of sentinel lymph nodes in dogs with a pre-operative diagnosis of melanoma or mast cell tumour. Vet Comp Oncol. (2020) 18:818–24. doi: 10.1111/vco.12592
42. Lurie, DM, Seguin, B, Schneider, PD, Verstraete, FJ, and Wisner, ER. Contrast-assisted ultrasound for sentinel lymph node detection in spontaneously arising canine head and neck tumors. Investig Radiol. (2006) 41:415–21. doi: 10.1097/01.rli.0000201230.29925.95
43. Randall, EK, Jones, MD, Kraft, SL, and Worley, DR. The development of an indirect computed tomography lymphography protocol for sentinel lymph node detection in head and neck cancer and comparison to other sentinel lymph node mapping techniques. Vet Comp Oncol. (2020) 18:634–44. doi: 10.1111/vco.12585
44. Wan, J, Oblak, ML, Ram, AS, McKenna, C, Singh, A, and Nykamp, S. Evaluating the feasibility and efficacy of a dual-modality nanoparticle contrast agent (Nanotrast-CF800) for image-guided sentinel lymph node mapping in the Oral cavity of healthy dogs. Front Vet Sci. (2021) 8:721003. doi: 10.3389/fvets.2021.721003
45. Green, K, and Boston, SE. Bilateral removal of the mandibular and medial retropharyngeal lymph nodes through a single ventral midline incision for staging of head and neck cancers in dogs: a description of surgical technique. Vet Comp Oncol. (2017) 15:208–14. doi: 10.1111/vco.12154
46. Odenweller, PH, Smith, MM, and Taney, KG. Validation of regional lymph node excisional biopsy for staging Oral and maxillofacial malignant neoplasms in 97 dogs and 10 cats (2006-2016). J Vet Dent. (2019) 36:97–103. doi: 10.1177/0898756419869841
47. Mayayo, SL, Prestigio, S, Maniscalco, L, Rosa, GL, Aricò, A, Maria, RD, et al. Chondroitin sulfate proteoglycan-4: a biomarker and a potential immunotherapeutic target for canine malignant melanoma. Vet J. (2011) 190:e26–30. doi: 10.1016/j.tvjl.2011.02.020
48. Bergin, IL, Smedley, RC, Esplin, DG, Spangler, WL, and Kiupel, M. Prognostic evaluation of Ki67 threshold value in canine Oral melanoma. Vet Pathol. (2011) 48:41–53. doi: 10.1177/0300985810388947
49. Di Palma, S, Vitale, C, Sabattini, S, Marconato, L, Riondato, F, Lauzi, S, et al. Prognostic significance of sentinel lymph node biopsy in canine oral malignant melanoma: a retrospective study. Vet Comp Oncol. (2021) 19:412–21.
50. Morton, DL, Thompson, JF, Cochran, AJ, Mozzillo, N, Nieweg, OE, Roses, DF, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. (2014) 370:599–609. doi: 10.1056/NEJMoa1310460
51. Faries, MB. Sentinel lymph nodes in melanoma: necessary as ever for optimal treatment. Clin Exp Metastasis. (2024) 41:369–74. doi: 10.1007/s10585-023-10254-2
52. Menghini, TL, Schwarz, T, Dancer, S, Gray, C, Mac Gillivray, T, Bowlt,, et al. Contrast-enhanced CT predictors of lymph nodal metastasis in dogs with oral melanoma. Vet Radiol Ultrasound. (2023) 64:694–705. doi: 10.1111/vru.13254
53. Bezuidenhout, AJ. The lymphatic system In: HE Evans and A De Lahunta, editors. The Miller’s anatomy of the dog. 4th ed: Elsevier Publishing (2013). 535–62.
54. Liptak, JM, and Boston, SE. Nonselective lymph node dissection and sentinel lymph node mapping and biopsy. Vet Clin North Am Small Anim Pract. (2019) 49:793–807. doi: 10.1016/j.cvsm.2019.04.003
55. Owen, LN. World Health Organization TNM classification of tumors in domestic animals. 1st ed. Geneva: WHO; Veterinary Public Health Unit & WHO Collaborating Center for Comparative Oncology (1980).
56. Faries, MB, Thompson, JF, Cochran, AJ, Andtbacka, RH, Mozzillo, N, Zager, JS, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. (2017) 376:2211–22. doi: 10.1056/NEJMoa1613210
57. Pellin, MA. The use of Oncept melanoma vaccine in veterinary patients: a review of the literature. Vet Sci. (2022) 9:597. doi: 10.3390/vetsci9110597
58. Smedley, RC, Bongiovanni, L, Bacmeister, C, Clifford, CA, Christensen, N, Dreyfus, JM, et al. Diagnosis and histopathologic prognostication of canine melanocytic neoplasms: a consensus of the oncology-pathology working group. Vet Comp Oncol. (2022) 20:739–51. doi: 10.1111/vco.12827
59. Hahn, K. A., Bravo, L., Adams, W. H., and Frazier, D. L. (1994) Naturally occurring tumors in dogs as comparative models for cancer therapy research. In Vivo. 8:133–43.
60. Modiano, J. F., Ritt, M. G., and Wojcieszyn, J. (1999). The molecular basis of canine melanoma:pathogenesis and trends in diagnosis and therapy. J Vet Intern Med. 13:163–74. doi: 10.1892/0891-6640(1999)013<0163
61. Kamstock, D. A., Ehrhart, E. J., Getzy, D. M., Bacon, N. J., Rassnick, K. M., and Moroff, S. D. (2011). Recommended guidelines for submission, trimming, margin evaluation, and reporting of tumor biopsy specimens in veterinary surgical pathology. american college of veterinary pathologists’ oncology committee. Vet Pathol. 48:19–31. doi: 10.1177/0300985810389316
62. Rolih, V., Barutello, G., Iussich, S., De Maria, R., Quaglino, E., and Buracco, P. (2017). CSPG4: a prototype oncoantigen for translational immunotherapy studies. J Transl Med. 15:151. doi: 10.1186/s12967-017-1250-4
63. Goldschmidt, S., Stewart, N., Ober, C., Bell, C., Wolf-Ringwall, A., and Kent, M. (2023). Contrast-enhanced and indirect computed tomography lymphangiography accurately identifies the cervical lymphocenter at risk for metastasis in pet dogs with spontaneously occurring oral neoplasia. PLoS One. 18:e0282500.
Appendix material
A power analysis based on the log-rank test was performed to calculate the number of dogs required in each group to conduct the statistical analyses. An alpha error of 0.05 and a statistical power of 80% were assumed. When the population was stratified according to the presence or absence of lymph node metastases at diagnosis, a 1-year survival rate of 50% was hypothesized based on Authors clinical experience for dogs with lymph node metastases (Group A) and 80% for those without lymph node metastases (Group B). According to Boston et al. (12) the number of patients in Group A was approximately half that of Group B.
Similarly, when the population was stratified based on the type of lymphadenectomy performed, a 1-year survival rate of 50% was hypothesized based on Authors clinical experience for dogs that underwent ipsilateral lymphadenectomy (Group 1), and 80% for those that underwent bilateral lymphadenectomy (Group 2). According to Camerino et al. (29) the number of dogs in Group 1 was approximately 1.3 times higher than in Group 2. We collected a population that mirrors the indications provided by the preliminary statistical analyses. It should be noted that subgroup comparisons were performed with the awareness that the statistical power may be reduced due to the limited number of cases.
Keywords: lymph node, oral malignant melanoma, dog, CSPG4, lymph node dissection
Citation: Giacobino D, Olimpo M, Ferraris EI, Martinelli G, Maniscalco L, Camerino M, Riccardo F, Cavallo F, Tarone L, Cino M, Dentini A, Iussich S, Lardone E, Manassero L, Maria RD, Buracco P and Morello E (2025) Influence of the extent of cervical lymph node dissection and lymph nodes metastases on prognosis in a cohort of dogs with oral malignant melanoma treated by surgical resection and adjuvant anti-CSPG4 electrovaccination: a retrospective study on 77 cases. Front. Vet. Sci. 12:1616419. doi: 10.3389/fvets.2025.1616419
Edited by:
Brigitte Degasperi, Vetmeduni Vienna, AustriaReviewed by:
Majlind Sulce, Agricultural University of Tirana, AlbaniaLavinia Elena Chiti, University of Zurich, Switzerland
Copyright © 2025 Giacobino, Olimpo, Ferraris, Martinelli, Maniscalco, Camerino, Riccardo, Cavallo, Tarone, Cino, Dentini, Iussich, Lardone, Manassero, Maria, Buracco and Morello. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emanuela Morello, ZW1hbnVlbGEubW9yZWxsb0B1bml0by5pdA==
 Davide Giacobino
Davide Giacobino Matteo Olimpo
Matteo Olimpo Erica Ilaria Ferraris1
Erica Ilaria Ferraris1 Greta Martinelli
Greta Martinelli Lorella Maniscalco
Lorella Maniscalco Federica Riccardo
Federica Riccardo Federica Cavallo
Federica Cavallo Lidia Tarone
Lidia Tarone Elena Lardone
Elena Lardone Luca Manassero
Luca Manassero Raffaella De Maria
Raffaella De Maria Paolo Buracco
Paolo Buracco Emanuela Morello
Emanuela Morello