- 1Department of Animal Sciences, University of Illinois Urbana-Champaign, Urbana, IL, United States
- 2Roy J. Carver Biotechnology Center, University of Illinois, Urbana, IL, United States
- 3Kerry Group, Beloit, WI, United States
- 4Kerry (Canada), Laval, QC, Canada
- 5Science Made Simple, LLC, Winston Salem, NC, United States
- 6College of Veterinary Medicine, University of Illinois Urbana-Champaign, Urbana, IL, United States
- 7Division of Nutritional Sciences, University of Illinois Urbana-Champaign, Urbana, IL, United States
Abrupt dietary transitions are common in pets, but can lead to digestive disturbances, altered gut microbiota composition, and impaired intestinal integrity. The consumption of live microorganisms may have potential to mitigate these effects by stabilizing the gut microbiota and enhancing intestinal functionality. The current study aimed to evaluate the effects of Bacillus subtilis ATCC PTA-122264 supplementation on fecal characteristics, microbiota composition, and dysbiosis index of dogs undergoing an abrupt dietary change. Twelve healthy adult spayed female beagle dogs (6.0 ± 1.14 yr; 8.7 ± 0.91 kg body weight) were used in a replicated 3 × 3 Latin square design. In each experimental period, dogs were allotted to one of three treatments and fed a high-fiber kibble diet for 28 d: (1) 250 mg/d of maltodextrin (control), (2) 1 × 109 colony-forming units (CFU)/d of B. subtilis, or (3) 5 × 109 CFU/d of B. subtilis. All dogs were then abruptly transitioned to a high-protein, high-fat canned diet and fed for 14 d. Fresh fecal samples were collected before (d 0) and 2, 6, 10, and 14 d after the diet change for fecal scoring, pH, dry matter (DM) content, and microbiota analysis. Data were statistically analyzed to identify differences due to treatment, time, and treatment*time interactions, with p < 0.05 accepted as being significant. Diet change did not impact fecal pH or scores but reduced fecal DM percentage and bacterial alpha diversity measures. Bacterial beta diversity analysis revealed a distinct shift in the microbial community following the diet transition. Diet change reduced (p < 0.05) the abundances of short-chain fatty acid (SCFA)-producing bacteria and increased (p < 0.05) the relative abundance of potentially pathogenic bacteria, resulting in an elevated (p < 0.05) dysbiosis index. B. subtilis supplementation did not attenuate the microbial shifts caused by the diet transition. These findings confirm that an abrupt diet change significantly impacts some stool characteristics and fecal microbiota populations of dogs. Further investigation of Bacillus spp. strains and dosages is required to determine the potential benefits that they may provide during dietary transition.
Introduction
Diet exerts a significant influence on the gastrointestinal (GI) health of dogs, modulating fecal microbial populations and metabolite concentrations. Abrupt dietary change, which is common in dogs, has been associated with digestive disturbances, GI discomfort, and loose stools (1, 2). Furthermore, such dietary changes can adversely affect intestinal integrity, alter gut microbiota composition, and disrupt the production of fermentation end-products (1, 3–5). In general, significant changes in the gut microbiota occur within a few days following a dietary change. In dogs fed different diets, microbiota shifts have been observed as early as 2 d after an abrupt diet change, with stability typically achieved within 6 to 10 d (5).
Probiotic studies with companion animals have demonstrated their ability to mitigate GI disorders triggered by dietary changes. A study evaluating Bacillus subtilis C-3102 in dogs demonstrated that B. subtilis-treated dogs had firmer feces, higher fecal scores, and lower fecal ammonia concentrations than control dogs (6). Dietary supplementation with B. subtilis C-3102 has also been shown to improve fecal quality, increase nutrient digestibility, and positively shift gut health parameters by reducing ammonia concentrations, increasing fecal SCFA concentrations, and increasing fecal Lactobacillus spp. and enterococci (7). Similarly, Saccharomyces cerevisiae CNCM I-5660 supplementation was shown to reduce fecal pH and biogenic amine, ammonia, and aromatic compound concentrations, while increasing fecal butyrate concentrations and lowering the dysbiosis index (DI) of dogs following an abrupt dietary change when compared with controls (3). Collectively, those studies suggest that the consumption of live microorganisms may enhance intestinal functionality in dogs and may provide benefits during dietary transition.
Although probiotics are recognized for their potential health benefits, there is a lack of studies evaluating the specific effects of B. subtilis ATCC PTA-122264 on gut microbiota, dysbiosis index, and fecal characteristics in dogs subjected to abrupt dietary change. In a study recently conducted by our team, B. subtilis ATCC PTA-122264 supplementation reduced the relative abundances of Streptococcus, Escherichia coli, and Blautia in dogs, indicating its potential to modulate the gut microbiota (8). Those findings indicate that B. subtilis may improve stool quality, reduce fecal odor, and positively modulate the gut microbiota of dogs. Given that B. subtilis-based probiotics may have the capacity to positively influence intestinal and microbial function, they may aid during dietary transition. Therefore, the objective of the current study was to assess the fecal characteristics and microbiota of B. subtilis-supplemented dogs undergoing an abrupt dietary change. We hypothesized that dogs supplemented with B. subtilis would have a more stable gut microbiome and greater ability to adapt to a new diet.
Materials and methods
All animal procedures were approved by the University of Illinois Institutional Animal Care and Use Committee prior to experimentation (protocol #22217).
Animals, treatments and diets
A replicated 3 × 3 Latin square design experiment was conducted. Twelve healthy adult spayed female beagle dogs (6.0 ± 1.14 yr old; 8.7 ± 0.91 kg) were used. All dogs were housed in an environmentally controlled facility at the University of Illinois Urbana-Champaign. Dogs always had free access to fresh water. Based on previous feeding records, dogs were fed once daily (8–9 am) to maintain body weight. Dogs were weighed and body condition was assessed (9) once a week prior to feeding.
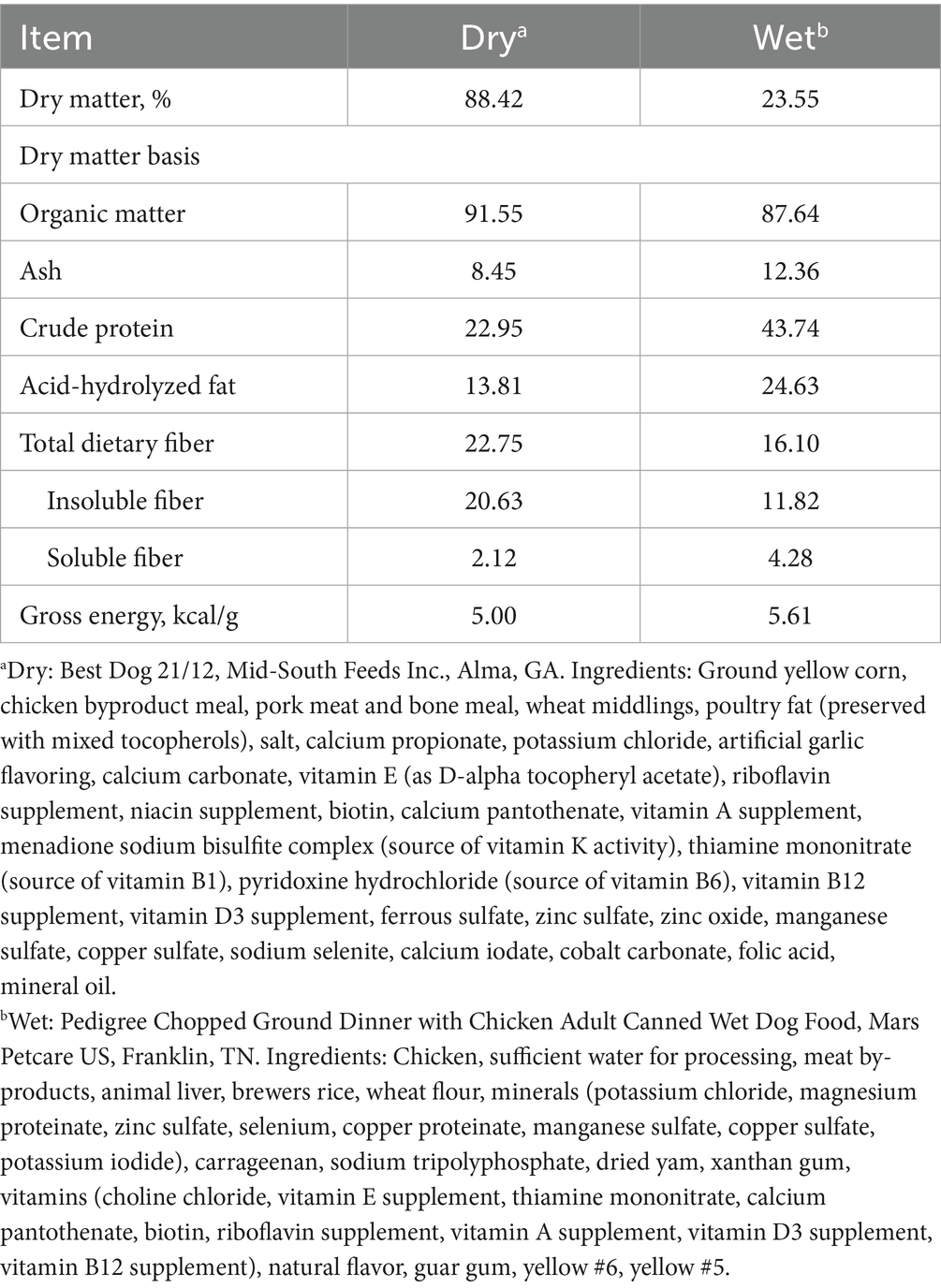
The following treatments were tested: 250 mg/d of maltodextrin (control); 1 × 109 colony-forming units (CFU)/d of B. subtilis ATCC PTA-122264 (Low dose; Kerry, Inc., Beloit, WI); and 5 × 109 CFU/d of B. subtilis ATCC PTA-122264 (High dose). Maltodextrin and B. subtilis treatments were provided prior to each meal with gelatin capsules before each meal. During the first 28 d of each experimental period, dogs were allotted to one of the three treatments and fed a dry extruded kibble commercial diet formulated to meet all Association of American Feed Control Officials nutrient recommendations for adult dogs at maintenance (10) and containing no probiotics or prebiotics and little fermentable fiber (Best Dog 21/12; Mid-South Feeds Inc., Alma, GA). At that time, all dogs were then abruptly changed to a wet canned diet (Pedigree Chopped Ground Dinner with Chicken Adult Canned Wet Dog Food; Mars Petcare US, Franklin, TN) formulated to meet all Association of American Feed Control Officials nutrient recommendations for adult dogs at maintenance (10) and fed for 14 d. Analyzed chemical composition of diets is listed in Table 1.
Fecal collection, scoring, and handling
Fresh (within 15 min of defecation) fecal samples were collected prior to (d 0) and 2, 6, 10, and 14 d after diet change for scoring and the measurement of pH, dry matter (DM) percentage, and microbiota. First, fecal samples were scored using the following scale: 1 = hard, dry pellets, small hard mass; 2 = hard, formed, dry stool; remains firm and soft; 3 = soft, formed, and moist stool, retains shape; 4 = soft, unformed stool, assumes shape of container; and 5 = watery, liquid that can be poured. Fecal pH was measured immediately using an AP10 pH meter (Denver Instrument, Bohemia, NY) equipped with a Beckman Electrode (Beckman Instruments Inc., Fullerton, CA). After pH was measured, an aliquot was collected for DM determination in accordance with AOAC (11) procedures using a 105°C oven. An aliquot of fresh feces was then transferred to sterile cryogenic vials (Nalgene, Rochester, NY), placed on dry ice, and then stored at −80°C until microbiota analysis.
Chemical analyses
Diet and fecal samples were analyzed for DM and ash according to the Association of Official Analytical Chemists (11); (methods 934.01 and 942.05), with organic matter calculated. Crude protein was calculated from Leco (TruMac N, Leco Corporation, St. Joseph, MI) total nitrogen values according to AOAC (11). Total lipid content (acid-hydrolyzed fat) was determined according to the methods of the American Association of Cereal Chemists (12) and Budde (13). Total dietary fiber of diets was determined according to Prosky et al. (14). Gross energy was measured using an oxygen bomb calorimeter (Model 6200, Parr Instruments, Moline, IL).
Fecal DNA extraction and PacBio sequencing of 16S rRNA gene amplicons
Total DNA from fecal samples was extracted using Mo-Bio PowerSoil kits (MO BIO Laboratories, Inc., Carlsbad, CA). Concentrations of extracted DNA were quantified using a Qubit 3.0 Fluorometer (Life Technologies, Grand Island, NY). The quality of extracted DNA was assessed by electrophoresis using agarose gels (E-Gel EX Gel 1%; Invitrogen, Carlsbad, CA). The Roy J. Carver Biotechnology Center at the University of Illinois performed PacBio sequencing. The 16S amplicons were generated with the barcoded full-length 16S primers from PacBio and the 2× Roche KAPA HiFi Hot Start Ready Mix (Roche, Wilmington, MA). Full-length 16S PacBio (Pacific Biology, Menlo Park, CA) primers (forward: AGRGTTYGATYMTGGCTCAG; reverse: RGYTACCTTGTTACGACTT) were added in accordance with the PacBio protocol. The amplicons were pooled and converted to a library with the SMRT Bell Express Template Prep kit 3.0 (Pacific Biology, Menlo Park, CA). The library was sequenced on a SMRT cell 8 M in the PacBio Sequel IIe using the CCS sequencing mode and a 15-h movie time. Analysis of CCS was done using SMRT Link V11.1.0 using the following parameters: minimum passes 3, and minimum rq 0.999; HiFi presets (minimum score of 80; minimum end score of 50, minimum reference (read) span of 0.75); asymmetric (different, minimum number of scoring barcode regions 2).
Microbial data analysis
PacBio-based FASTQ reads were processed using a Nextflow-based workflow and analyzed with DADA2 v1.22 (15) for trimming, denoising, and generating amplicon sequence variants (ASV). Taxonomic classification was performed using the Ribosomal Database Project classifier implemented in DADA2 (36) with the SILVA 138.1 database formatted for PacBio HiFi reads.1 Multiple sequence alignment and phylogenetic analysis were conducted using DECIPHER v2.22 (37) and FastTree v2.1.10 (38). Quality filtering retained sequences with a minimum quality score of 20, and samples were rarefied to 49,463 reads. The taxonomic classifications produced by DADA2, as well as their quantifications, were imported into phyloseq (version 1.44.0) in R (version 4.3.1). The rarefied samples were used for alpha and beta diversity analysis. Principal coordinate analysis was performed using weighted and unweighted unique fraction metric (UniFrac) distances. Analysis of compositions of microbiomes with bias correction (ANCOMBC) was estimated using the ANCOMBC package (version 2.4.0) to determine specific taxa that were statistically responsible for the observed discrimination between treatment and period, with Benjamini–Hochberg adjusted p-value, and q < 0.05 was accepted as statistically significant. Spearman’s rank correlation coefficient (r) carried out using microbiome package (version 1.24.0), with Benjamini–Hochberg adjusted p-value, and q < 0.05 was accepted as statistically significant.
Quantitative PCR and dysbiosis index
DNA was extracted from an aliquot of 100–120 mg fecal sample using a bead-beating method with a MoBio Power soil DNA isolation kit. qPCR assays were used to quantify total bacteria, Blautia, Clostridium (Peptacetobacter) hiranonis, Escherichia coli, Faecalibacterium, Fusobacterium, Streptococcus, and Turicibacter according to (39). Both positive and negative controls were included for all qPCR assays to ensure the accuracy and reliability of the results. The DI was calculated based on the results of the qPCR assays using a previously described algorithm (39).
Statistical analysis
Data were analyzed using the Mixed Models procedure of SAS version 9.4 (SAS Institute, Inc., Cary, NC). Treatment and day were considered fixed effects, while dog was considered a random effect. Data were tested for normality using the UNIVARIATE procedure of SAS. Differences between treatment, day, and treatment*day interactions were determined using repeated measures and a Fisher-protected least significant difference test with a Tukey adjustment to control for experiment-wise error. A probability of p < 0.05 was accepted as being statistically significant. Reported pooled standard errors of the means were determined according to the Mixed Models procedure of SAS.
Results
Fecal scores and pH were not affected by B. subtilis supplementation or dietary change (Table 2). However, the abrupt dietary change reduced (p < 0.0001) fecal DM % and increased (p < 0.0001) fecal dysbiosis index (Figure 1). The dietary change also increased (p < 0.0001) fecal Streptococcus, E. coli, and C. hiranonis abundances and decreased (p < 0.01) fecal Faecalibacterium, Turicibacter, Blautia, and Fusobacterium abundances (Figure 1). Diet-induced changes to fecal characteristics, DI, and bacterial abundances were not impacted by treatment (i.e., B. subtilis supplementation) or treatment*day interactions (Figure 1).
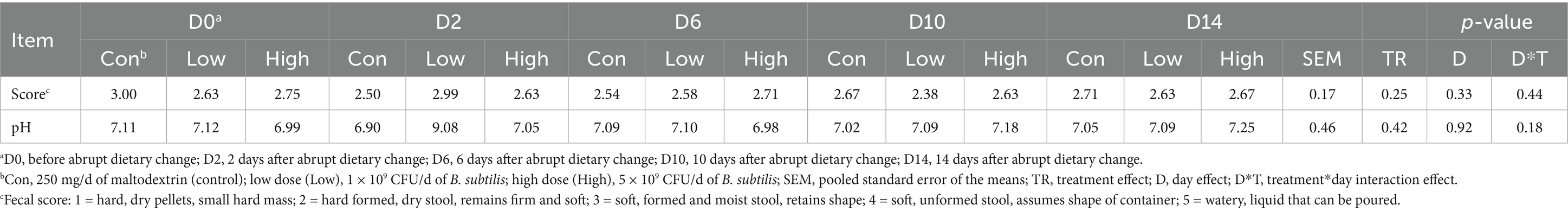
Table 2. Fecal characteristics of dogs supplemented with a B. subtilis probiotic before and after an abrupt diet change.
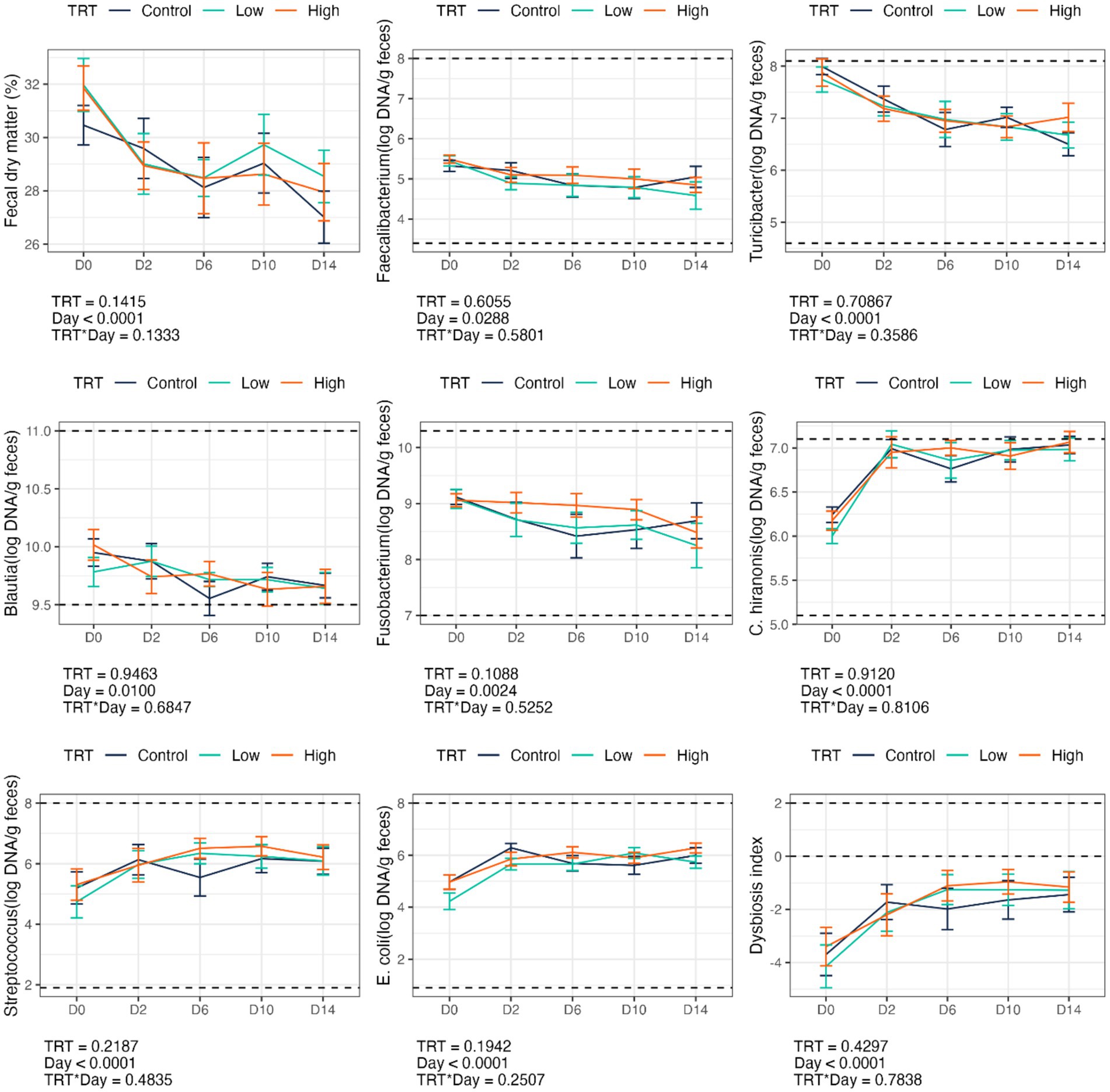
Figure 1. Fecal dry matter, bacterial abundance (log DNA/g feces), and dysbiosis index of dogs supplemented with a B. subtilis probiotic before and after an abrupt diet change. D0, before abrupt dietary change; D2, 2 days after abrupt dietary change; D6, 6 days after abrupt dietary change; D10, 10 days after abrupt dietary change; D14, 14 days after abrupt dietary change.
Fecal bacterial alpha diversity measures were reduced (p < 0.05) with diet change (day effect) but were not affected by B. subtilis supplementation or treatment*day interactions (Figures 2, 3). Three of the four alpha diversity measures were affected, with observed ASV, Fisher Index, and the Shannon Index being reduced (p < 0.005) by diet change. Beta diversity, as assessed by unweighted and weighted UniFrac distances, was also affected by diet change (Figures 4, 5). The principal coordinate analysis plots of weighted and unweighted UniFrac distances show separation of microbial communities, with samples shifting after dietary change. Samples collected prior to dietary change were different (p < 0.05) than samples collected at all other time points. Moreover, samples collected 2 d after dietary change were different (p < 0.05) than those collected 14 d after dietary change.
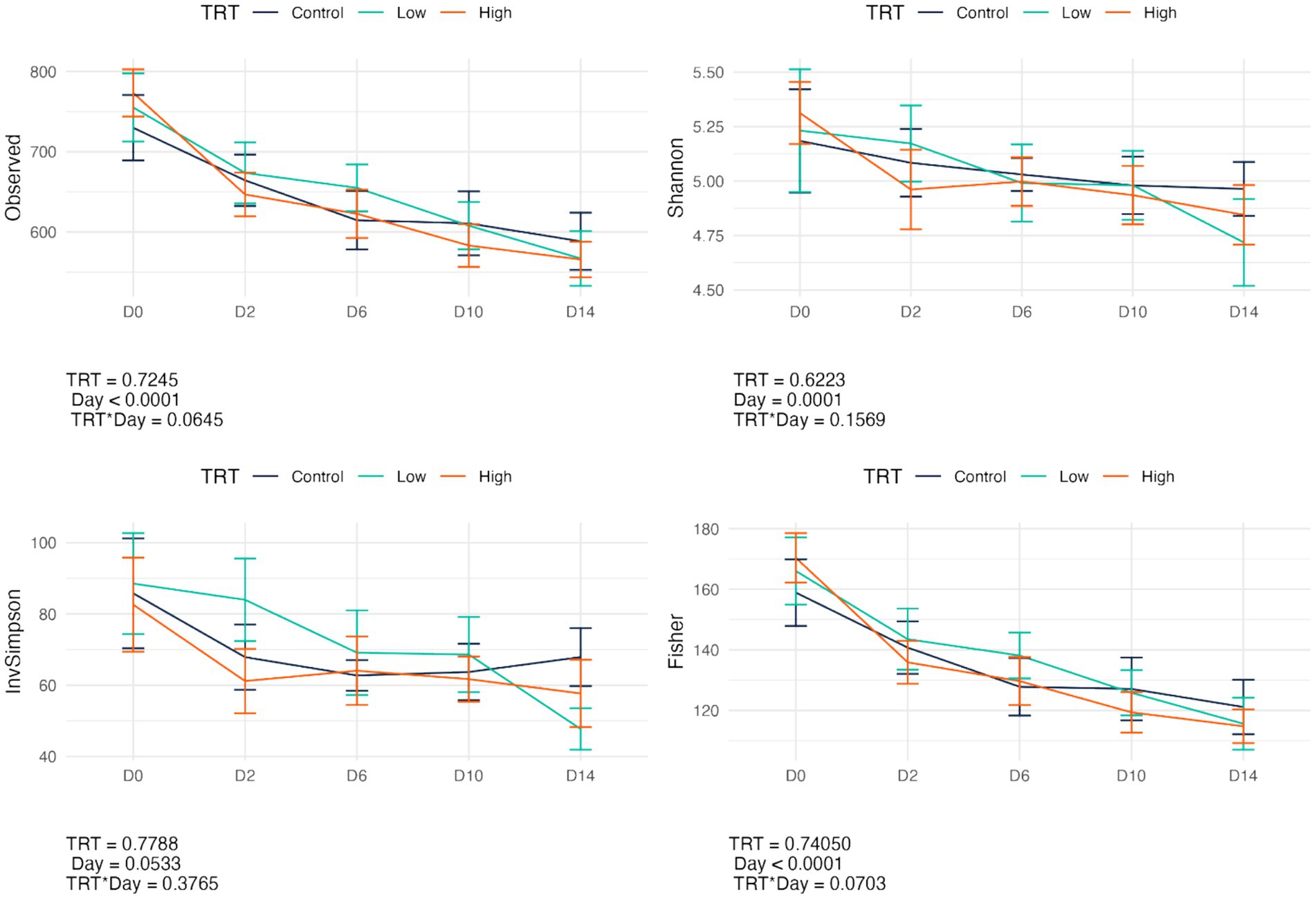
Figure 2. Change in bacterial alpha diversity measures (Observed, Observed ASV; Shannon, Shannon Index; InvSimpson, Inverse Simpson Index; Fisher, Fisher index) of fecal samples from dogs supplemented with B. subtilis before and after an abrupt diet change. D0, before abrupt dietary change; D2, 2 days after abrupt dietary change; D6, 6 days after abrupt dietary change; D10, 10 days after abrupt dietary change; D14, 14 days after abrupt dietary change.
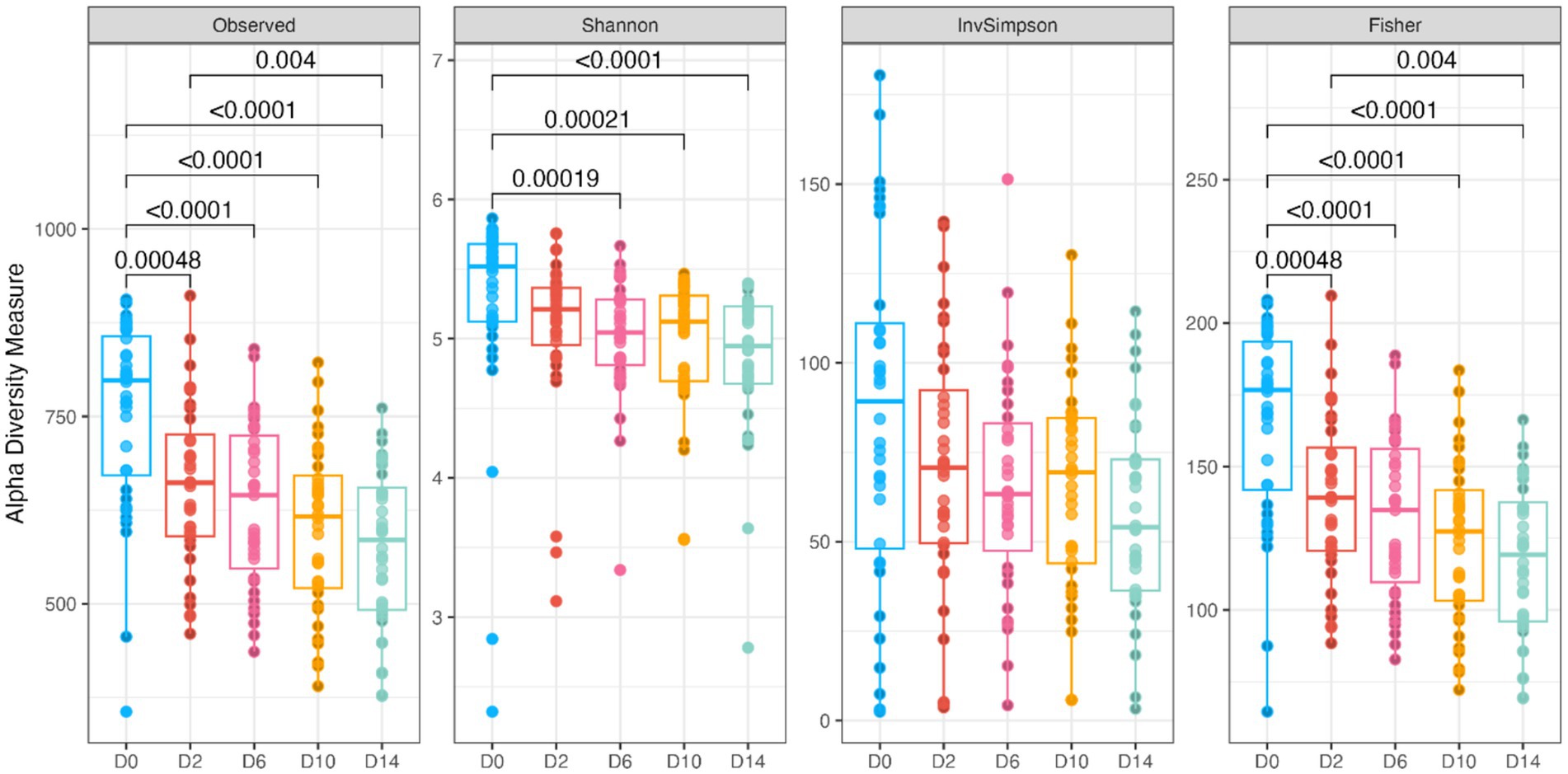
Figure 3. Bacterial alpha diversity measures (Observed, Observed ASV; Shannon, Shannon Index; InvSimpson, Inverse Simpson Index; Fisher, Fisher index) of fecal samples from dogs were affected by diet change. D0, before abrupt dietary change; D2, 2 days after abrupt dietary change; D6, 6 days after abrupt dietary change; D10, 10 days after abrupt dietary change; D14, 14 days after abrupt dietary change.
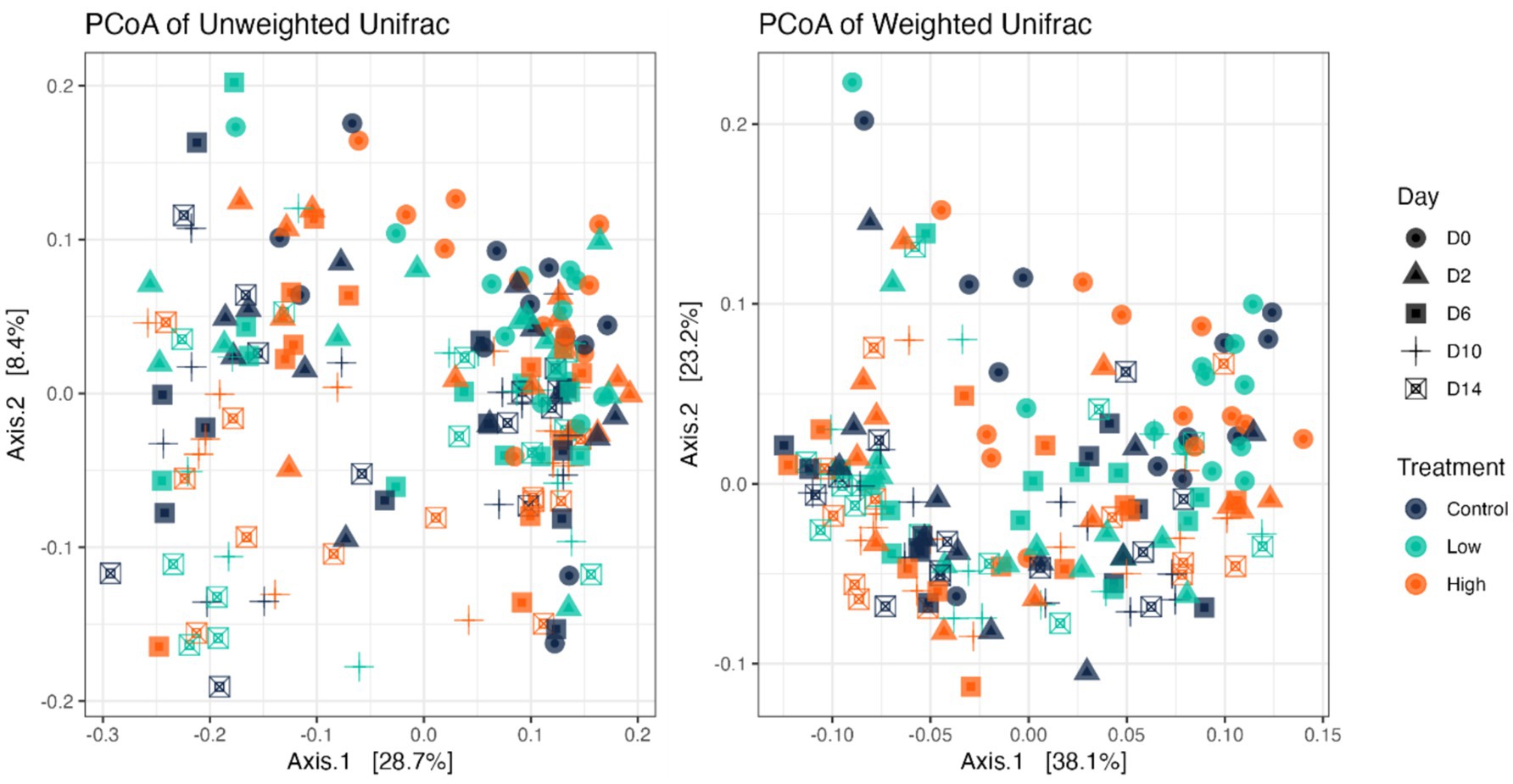
Figure 4. Bacterial beta diversity indices of fecal samples from dogs supplemented with B. subtilis before and after an abrupt diet change, as assessed by unweighted and weighted UniFrac distances. D0, before abrupt dietary change; D2, 2 days after abrupt dietary change; D6, 6 days after abrupt dietary change; D10, 10 days after abrupt dietary change; D14, 14 days after abrupt dietary change.
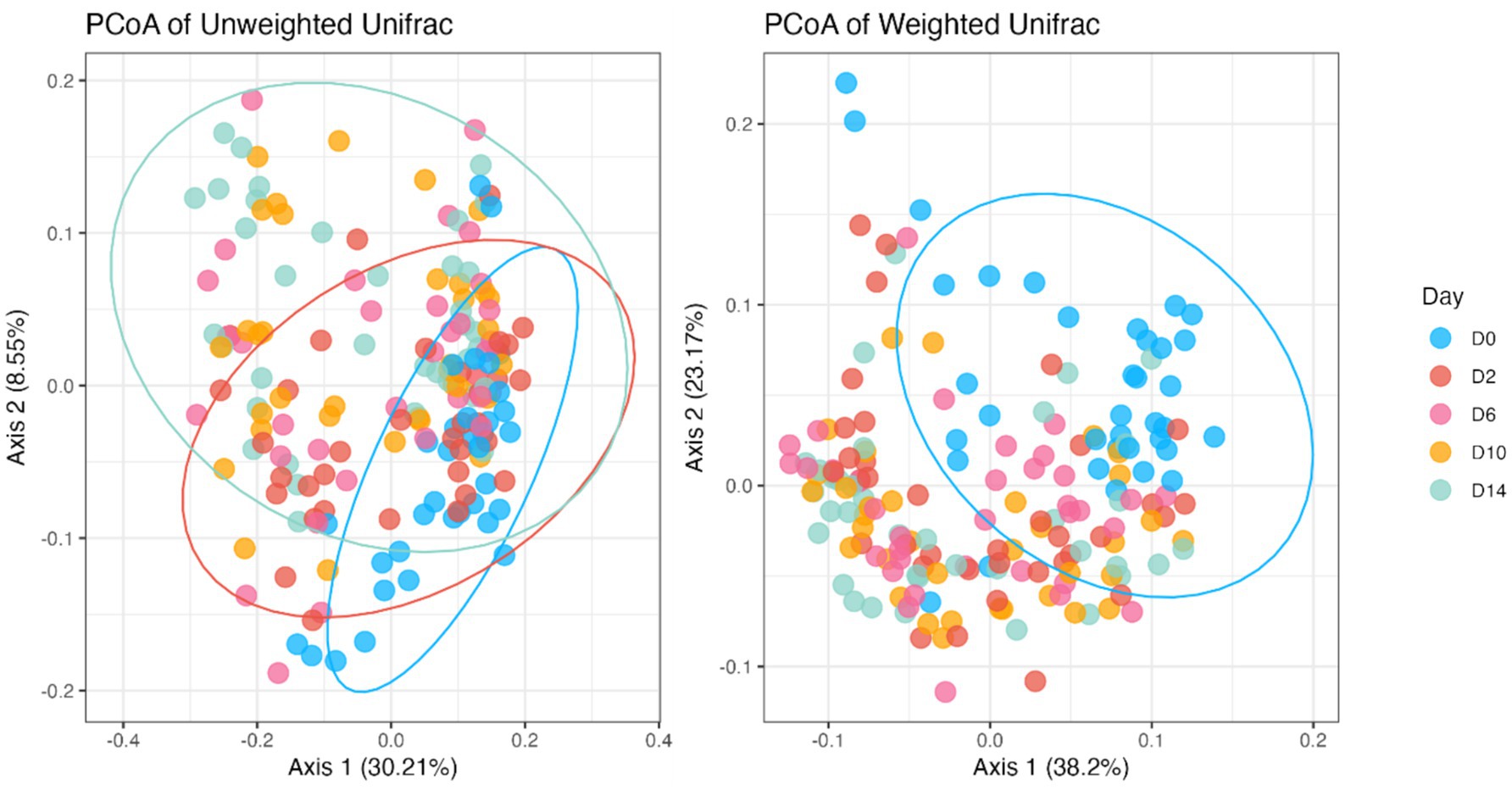
Figure 5. Bacterial beta diversity indices of fecal samples from dogs before and after an abrupt dietary change, as assessed by unweighted and weighted UniFrac distances. D0, before abrupt dietary change; D2, 2 days after abrupt dietary change; D6, 6 days after abrupt dietary change; D10, 10 days after abrupt dietary change; D14, 14 days after abrupt dietary change.
The relative abundances of several bacterial phyla and genera were affected by diet change, but not by B. subtilis supplementation or treatment*day interactions (Figures 6, 7). Using ANCOMBC analysis, the relative abundances of fecal Turicibacter, Prevotella, Lactobacillus, Lachanospiraceae NK4A136 group, Faecalibaculum, Faecalibacterium, Cellulosilyticum, Bifidobacterium, and Anaerofilum were reduced (p < 0.05) with the dietary change. In contrast, the relative abundances of fecal Sutterella, Peptoclostridium, Fusobacterium, Escherichia-Shigella, Collinsella, Ruminococcus gnavus group, and Eubacterium brachy group were increased (p < 0.05) with the dietary change.
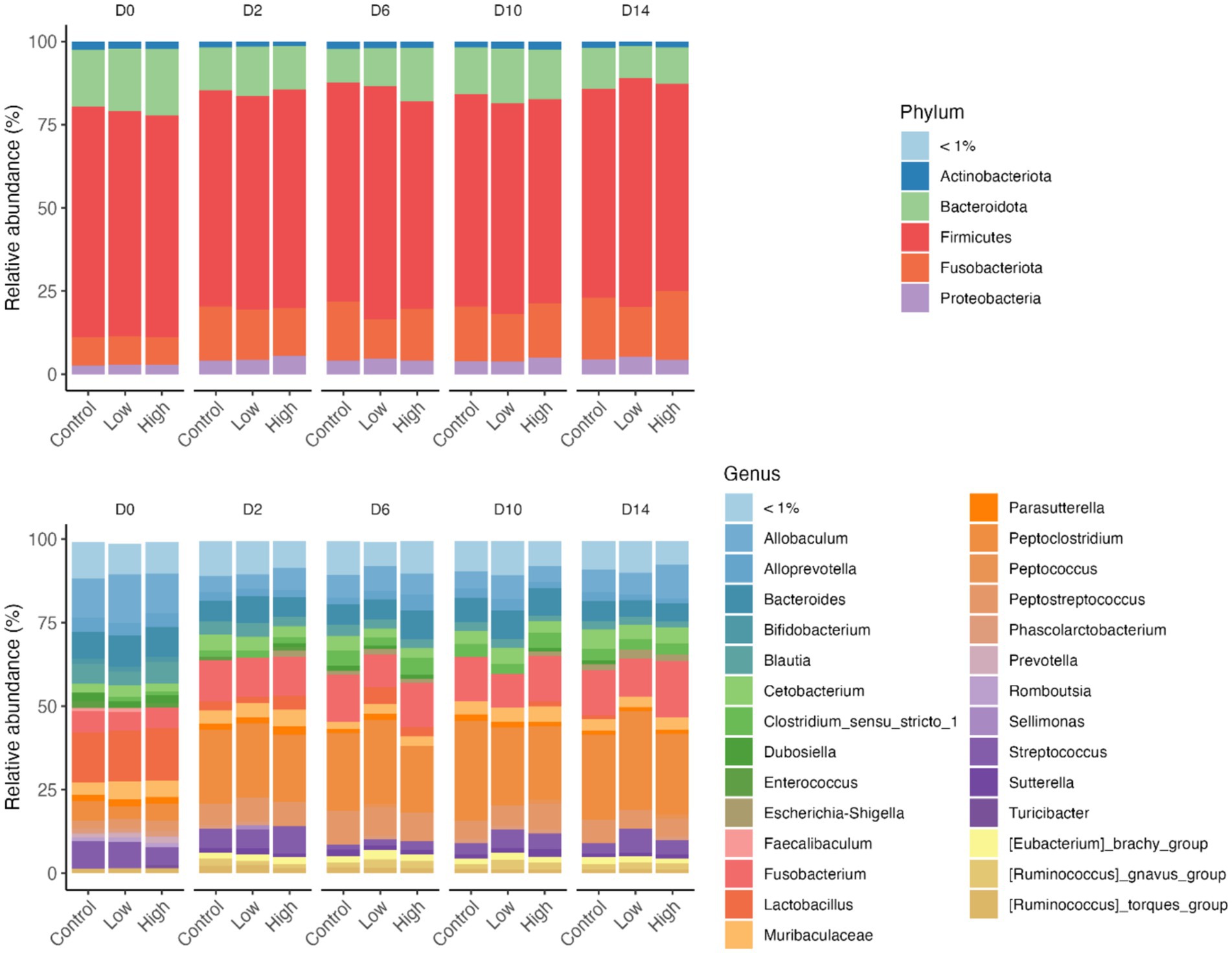
Figure 6. Relative abundance of bacteria phyla and genus of fecal samples from dogs supplemented with B. subtilis before and after an abrupt diet change. Only abundant taxa are shown; phyla and genera in low relative abundances (<1% in all samples) were combined into “<1%.” D0, before abrupt dietary change; D2, 2 days after abrupt dietary change; D6, 6 days after abrupt dietary change; D10, 10 days after abrupt dietary change; D14, 14 days after abrupt dietary change.
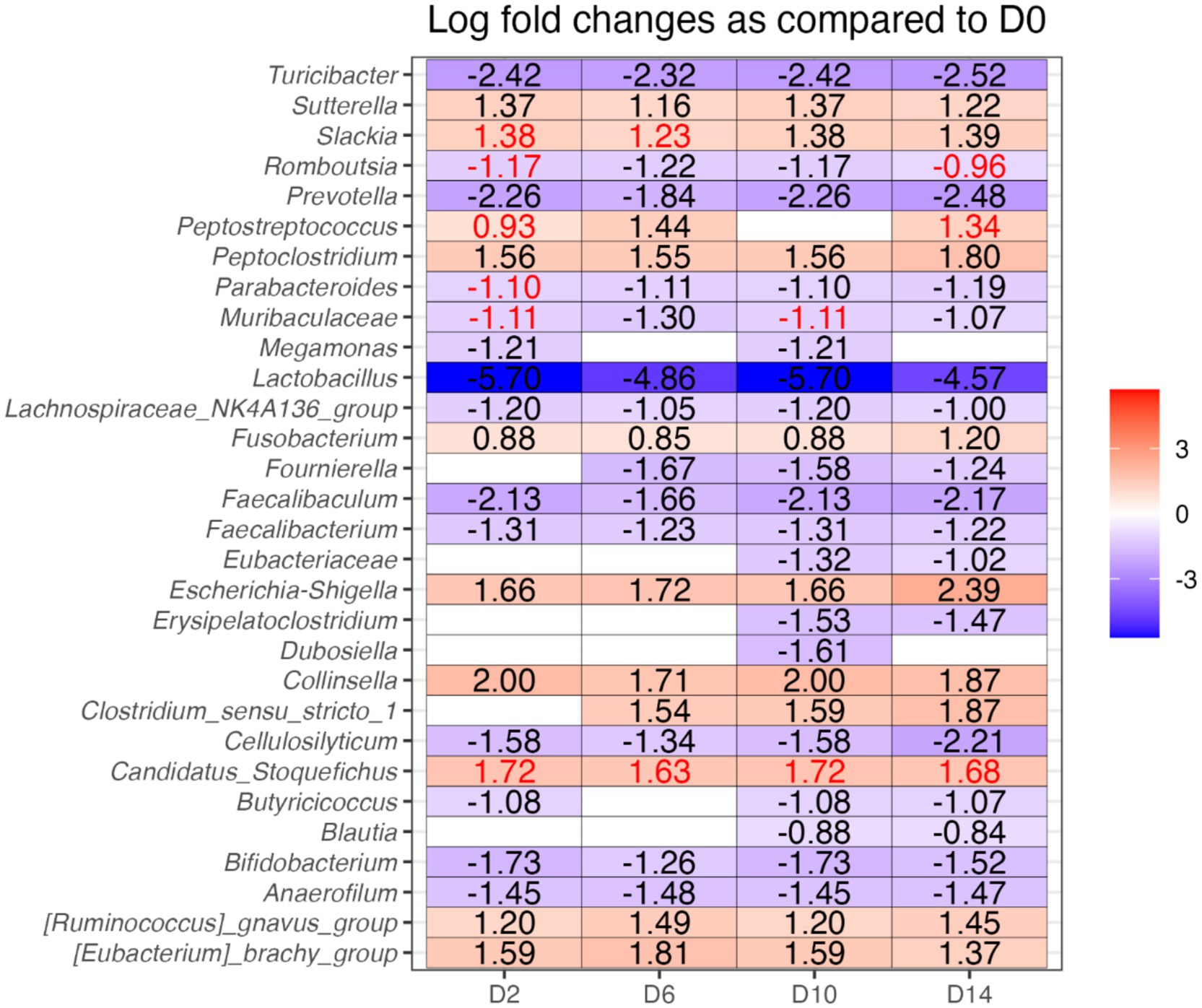
Figure 7. Analysis of composition of microbiomes with bias correction (ANCOMBC), illustrating which bacterial genera were differentially abundant between fecal samples of dogs before and after dietary change (difference greater than 1 and q < 0.05). D0, before abrupt dietary change; D2, 2 days after abrupt dietary change; D6, 6 days after abrupt dietary change; D10, 10 days after abrupt dietary change; D14, 14 days after abrupt dietary change. The values in red text did not pass the sensitivity analysis for pseudo-count addition.
Discussion
Abrupt dietary change is common in dogs and can lead to GI disturbances, including altered fecal consistency and fecal microbiota and metabolite shifts (1–5). While the gut microbiota adapt within a few days following a diet transition, the extent and stability of these changes depend on various factors, including microbiota balance and the presence of beneficial taxa (3, 4). Live microorganisms, such as B. subtilis, have been studied for their role in modulating gut microbiota, enhancing fecal characteristics, and mitigating digestive disturbances (6, 7). However, the specific effects of B. subtilis ATCC PTA-122264 on microbiome stability, dysbiosis index, and fecal characteristics in dogs undergoing abrupt dietary transitions remain unexplored. This study evaluated whether supplementation with B. subtilis ATCC PTA-122264 could promote a more stable gut microbiome and improve fecal characteristics in dogs following an abrupt diet change.
Consistent with previous research, diets with markedly different ingredient compositions, macronutrient profiles, and formats (wet vs. dry) were selected to facilitate shifts in the microbiome. Dogs were moved from a dry extruded kibble diet containing moderate protein and fat concentrations and high dietary fiber concentrations to a canned diet containing higher protein and fat concentrations and lower dietary fiber concentrations. The fiber type was also different with twice the amount of soluble fiber present in the canned diet. Diet transition immediately reduced fecal DM but was stabilized after 6 days. The diet change did not impact fecal scores or pH, however. Previous studies have shown that fecal scores (increased, indicating looser stools) and fecal DM (decreased) shifted and stabilized within 2 days of a dietary change (5), while fecal pH also decreased following the transition and reached stability within the same period (3).
Compared with the control, supplementation with B. subtilis ATCC PTA-122264 did not affect fecal scores, DM, or pH after the dietary change. These results were similar to the supplementation of S. cerevisiae (0.12 g per dog per day) in a previous study, where fecal pH, scores, or DM were similar to control animals following a dietary transition (3). Without the challenge of dietary transition, previous studies have reported that B. subtilis C-3102 supplementation at 1 × 109 CFU/d and 2 × 109 CFU/d led to firmer stools, higher fecal DM, and lower fecal pH than control dogs (7). Another study demonstrated that dietary supplementation with 0.01% B. subtilis C-3102 (1 × 101⁰ CFU/g) resulted in drier feces and higher fecal scores than those observed in control dogs (6). In our previous study testing B. subtilis ATCC PTA-122264 at doses of up to 5 × 109 CFU/day, fecal characteristics were not affected but there was a tendency for reduced apparent total tract digestibility of DM, organic matter, and energy (8). While fecal DI tended to be affected, supplementation resulted in lower abundances of Streptococcus, E. coli, and Blautia in dogs receiving the low treatment (1 × 109 CFU/day), suggesting potential microbiota-modulating effects (8). Those findings highlight strain-dependent differences in B. subtilis effects on fecal characteristics and microbiota modulation, suggesting that the abrupt dietary transition in this study may have presented a greater challenge to GI stability than B. subtilis supplementation alone could mitigate.
Transitioning from the high-fiber kibble diet to the high-protein, high-fat canned diet led to a reduction in SCFA-producing bacteria (e.g., Faecalibacterium, Turicibacter, Blautia, Fusobacterium) and an increase in potentially pathogenic bacteria (e.g., E. coli, C. hiranonis, and Streptococcus), resulting in a higher DI. Similarly, dogs fed a high-protein (44%, DM basis), high-fat (28%, DM basis) bones and raw food diet had a greater abundance of E. coli, Streptococcus, and C. perfringens, as well as a higher DI, than dogs fed commercially available kibble (31% crude protein and 18% fat, DM basis). Additionally, fecal Faecalibacterium abundance was lower in dogs consuming the bones and raw food diet than those fed kibble (16). In a previous study evaluating S. cerevisiae during an abrupt dietary change, dogs in the treatment group demonstrated a lower DI, lower E. coli abundance, and higher Turicibacter abundance than control animals, regardless of the day. Furthermore, transitioning from a low-protein (21%, DM basis), low-fiber (6%, DM basis) diet to a high-protein (28%, DM basis), high-fiber (27%, DM basis) diet improved (reduced) the DI, increased fecal Fusobacterium abundance, and decreased fecal C. hiranonis and Streptococcus abundances (3). Those findings suggest that dietary fiber content plays a significant role in microbiota modulation and that specific probiotic strains may offer benefits during dietary transition.
The ANCOMBC analysis in the current study revealed a reduction in SCFA-producing genera (e.g., Turicibacter, Prevotella, Lactobacillus, Lachanospiraceae NK4A136 group, Faecalibaculum, Faecalibacterium, Cellulosilyticum, Bifidobacterium, Anaerofilum) and an increase in potentially pathogenic bacteria (e.g., Peptoclostridium, Escherichia-Shigella, Ruminococcus gnavus group) following the abrupt dietary change. These findings are consistent with prior research showing that fiber intake in dogs promotes the growth of beneficial SCFA-producing bacteria, such as Turicibacter, Lactobacillus, Lachnospira, Faecalibacterium, Bifidobacterium and decrease Escherichia and Peptoclostridium (17–19). SCFA-producing genera, including Bifidobacterium, Lactobacillus, Faecalibacterium, Anaerofilum, Prevotella, Lachnospiraceae NK4A136 group, and Faecalibaculum utilize dietary fibers as substrates, contributing to GI health (19–25). Additionally, Cellulosilyticum has been identified as a bacterium capable of breaking down both fiber and protein, highlighting its potential role in microbial metabolism (26). High-protein diets often increase fecal Peptoclostridium, E. coli, and Streptococcus, decrease fecal Bifidobacterium and Faecalibacterium, increase concentrations of fecal protein catabolites (e.g., phenols, indoles, and branched-chain fatty acids), and reduce fecal concentrations of SCFA (16, 27–31). Moreover, C. perfringens, E. coli, and Streptococcus are recognized as potential pathogens (32–34), while Ruminococcus gnavus group has been positively associated with parvovirus GI infections in dogs (35).
Abrupt dietary changes in dogs, particularly those involving significant shifts in fiber, protein, and fat content, can lead to notable alterations in GI function, including changes in fecal characteristics and disruptions to the gut microbiota. While the gut microbiota has the capacity to adapt to dietary transitions, the extent of this adaptability can vary based on factors such as the presence of beneficial microbes. In this study, B. subtilis ATCC PTA-122264 supplementation was not able to attenuate the changes to fecal characteristics or microbiota. Other studies have shown positive effects of different probiotic strains, including Bacillus species, under stable conditions, suggesting that further exploration of different doses or strains may reveal more pronounced benefits. Nevertheless, the dietary change in this study led to an increase in potentially pathogenic bacteria and a decrease in beneficial SCFA-producing genera, which is consistent with previous research. This highlights the complexity of the relationship between diet and the gut microbiome and suggests that further investigation into the effects of different Bacillus strains and dietary interventions is necessary to better understand their role in maintaining GI health during abrupt diet changes in dogs.
Data availability statement
The raw sequencing data are available in the NCBI Sequence Read Archive (SRA) under BioProject PRJNA1277247. The data are publicly available.
Ethics statement
The animal study was approved by University of Illinois Institutional Animal Care and Use Committee prior to experimentation. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
PO: Investigation, Writing – review & editing, Formal analysis, Data curation, Writing – original draft. OS: Data curation, Investigation, Writing – review & editing, Formal analysis. YK: Formal analysis, Writing – review & editing. JMi: Formal analysis, Investigation, Writing – review & editing, Data curation. JMe: Writing – review & editing, Resources. EV: Writing – review & editing, Resources. MM: Resources, Conceptualization, Writing – review & editing. MK: Resources, Conceptualization, Writing – review & editing. KS: Project administration, Funding acquisition, Conceptualization, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding for this study was provided by Kerry Group (Beloit, WI).
Conflict of interest
The authors declare that this study received funding from Kerry Group. The funder had the following involvement in the study: assisted with study design and revision of the paper.
JMe, EV, and MM are employees of Kerry Group. MK is a private consultant for Kerry Group. MK was affiliated to Science Made Simple, LLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ANCOMBC, Analysis of compositions of microbiomes with bias correction; AOAC, Association of Official Analytical Chemists; ASV, Amplicon sequence variants; CFU, Colony-forming units; DI, Dysbiosis index; DM, Dry matter; GI, Gastrointestinal; SCFA, Short-chain fatty acids; UniFrac, Unique fraction metric.
Footnotes
References
1. Liao, P, Yang, K, Huang, H, Xin, Z, Jian, S, Wen, C, et al. Abrupt dietary change and gradual dietary transition impact diarrheal symptoms, fecal fermentation characteristics, microbiota, and metabolic profile in healthy puppies. Animals. (2023) 13:1300. doi: 10.3390/ANI13081300
2. Stavisky, J, Radford, AD, Gaskell, R, Dawson, S, German, A, Parsons, B, et al. A case-control study of pathogen and lifestyle risk factors for diarrhoea in dogs. Prev Vet Med. (2011) 99:185–92. doi: 10.1016/J.PREVETMED.2011.02.009
3. Bastos, TS, Souza, CMM, Legendre, H, Richard, N, Pilla, R, Suchodolski, JS, et al. Effect of yeast Saccharomyces cerevisiae as a probiotic on diet digestibility, fermentative metabolites, and composition and functional potential of the fecal microbiota of dogs submitted to an abrupt dietary change. Microorganisms. (2023) 11:506. doi: 10.3390/microorganisms11020506
4. Lin, CY, Carroll, MQ, Miller, MJ, Rabot, R, and Swanson, KS. Supplementation of yeast cell wall fraction tends to improve intestinal health in adult dogs undergoing an abrupt diet transition. Front Vet Sci. (2020) 7:597939. doi: 10.3389/FVETS.2020.597939
5. Lin, C-Y, Jha, AR, Oba, PM, Yotis, SM, Shmalberg, J, Honaker, RW, et al. Longitudinal fecal microbiome and metabolite data demonstrate rapid shifts and subsequent stabilization after an abrupt dietary change in healthy adult dogs. Anim Microbiome. (2022) 4:46. doi: 10.1186/s42523-022-00194-9
6. Félix, AP, Netto, MVT, Murakami, FY, de Brito, CBM, Oliveira, SG, and Maiorka, A. Digestibility and fecal characteristics of dogs fed with Bacillus subtilis in diet. Cienc Rural. (2010) 40:2169–73. doi: 10.1590/S0103-84782010005000166
7. Kahraman, O, Gurbuz, E, Inal, F, Arık, HD, Alatas, MS, Inanc, ZS, et al. Effects of Bacillus subtilis C-3102 addition on nutrient digestibility, faecal characteristics, blood chemistry and faecal Lactobacilli spp., Enterococci spp., and Escherichia coli in healthy dogs. Ital J Anim Sci. (2023) 22:568–77. doi: 10.1080/1828051X.2023.2218871
8. Oba, PM, Swanson, OR, Kang, Y, Mioto, JC, Menton, JF, Vinay, E, et al. Effects of Bacillus subtilis ATCC PTA-122264 on apparent total tract macronutrient digestibility and fecal characteristics, metabolites, and microbiota of healthy adult dogs. J Anim Sci. (2025) 103:skaf038. doi: 10.1093/JAS/SKAF038
9. Laflamme, D . Development and validation of a body condition score system for cats: a clinical tool. Feline Pract. (1997) 25:13–8.
10. AAFCO . Official publication. Champaign, IL: Association of American Feed Control Officials (2023).
11. AOAC . Official methods of analysis of AOAC international. 17th ed. St. Paul: Association of Official Analysis Chemists International (2006).
12. AACC . Approved methods of the American association of cereal chemists. St. Paul: American Association of Cereal Chemists (2000).
13. Budde, EF . The determination of fat in baked biscuit type of dog foods. J AOAC Int. (1952) 35:799–805. doi: 10.1093/jaoac/35.3.799
14. Prosky, L, Asp, N-G, Schweizer, TF, Devries, JW, and Furda, I. Determination of insoluble and soluble dietary fiber in foods and food products: collaborative study. J AOAC Int. (1992) 75:360–7. doi: 10.1093/jaoac/75.2.360
15. Callahan, BJ, McMurdie, PJ, Rosen, MJ, Han, AW, Johnson, AJA, and Holmes, SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. (2016) 13:581–3. doi: 10.1038/nmeth.3869
16. Schmidt, M, Unterer, S, Suchodolski, JS, Honneffer, JB, Guard, BC, Lidbury, JA, et al. The fecal microbiome and metabolome differs between dogs fed bones and raw food (BARF) diets and dogs fed commercial diets. PLoS One. (2018) 13:e0201279. doi: 10.1371/JOURNAL.PONE.0201279
17. Alexander, C, Cross, TWL, Devendran, S, Neumer, F, Theis, S, Ridlon, JM, et al. Effects of prebiotic inulin-type fructans on blood metabolite and hormone concentrations and faecal microbiota and metabolites in overweight dogs. Br J Nutr. (2018) 120:711–20. doi: 10.1017/S0007114518001952
18. Beloshapka, AN, Dowd, SE, Suchodolski, JS, Steiner, JM, Duclos, L, and Swanson, KS. Fecal microbial communities of healthy adult dogs fed raw meat-based diets with or without inulin or yeast cell wall extracts as assessed by 454 pyrosequencing. FEMS Microbiol Ecol. (2013) 84:532–41. doi: 10.1111/1574-6941.12081
19. Panasevich, MR, Kerr, KR, Dilger, RN, Fahey, GC, Guérin-Deremaux, L, Lynch, GL, et al. Modulation of the faecal microbiome of healthy adult dogs by inclusion of potato fibre in the diet. Br J Nutr. (2015) 113:125–33. doi: 10.1017/S0007114514003274
20. Liu, H, Ivarsson, E, Dicksved, J, Lundh, T, and Lindberg, JE. Inclusion of chicory (Cichorium intybus L.) in pigs’ diets affects the intestinal microenvironment and the gut microbiota. Appl Environ Microbiol. (2012) 78:4102–9. doi: 10.1128/AEM.07702-11
21. Myint, H, Iwahashi, Y, Koike, S, and Kobayashi, Y. Effect of soybean husk supplementation on the fecal fermentation metabolites and microbiota of dogs. Anim Sci J. (2017) 88:1730–6. doi: 10.1111/ASJ.12817
22. Qiu, M, Hu, J, Peng, H, Li, B, Xu, J, Song, X, et al. Research note: the gut microbiota varies with dietary fiber levels in broilers. Poult Sci. (2022) 101:101922. doi: 10.1016/J.PSJ.2022.101922
23. Turroni, F, van Sinderen, D, and Ventura, M. Genomics and ecological overview of the genus Bifidobacterium. Int J Food Microbiol. (2011) 149:37–44. doi: 10.1016/J.IJFOODMICRO.2010.12.010
24. Wu, M-R, Chou, T-S, Tzu, T, Hospital, C, Huang, C-Y, and Hsiao, J-K. A potential probiotic-Lachnospiraceae NK4A136 group: evidence from the restoration of the dietary pattern from a high-fat diet. Res Sq. (2020):1–24. doi: 10.21203/RS.3.RS-48913/V1
25. Zhao, Y, Bi, J, Yi, J, Peng, J, and Ma, Q. Dose-dependent effects of apple pectin on alleviating high fat-induced obesity modulated by gut microbiota and SCFAs. Food Sci Human Wellness. (2022) 11:143–54. doi: 10.1016/J.FSHW.2021.07.015
26. Wang, L, Nong, Q, Zhou, Y, Sun, Y, Chen, W, Xie, J, et al. Changes in serum fatty acid composition and metabolome-microbiome responses of Heigai pigs induced by dietary N-6/n-3 polyunsaturated fatty acid ratio. Front Microbiol. (2022) 13:917558. doi: 10.3389/FMICB.2022.917558
27. Bermingham, EN, Maclean, P, Thomas, DG, Cave, NJ, and Young, W. Key bacterial families (Clostridiaceae, Erysipelotrichaceae and Bacteroidaceae) are related to the digestion of protein and energy in dogs. PeerJ. (2017) 5:e3019. doi: 10.7717/peerj.3019
28. Ephraim, E, Cochrane, CY, and Jewell, DE. Varying protein levels influence metabolomics and the gut microbiome in healthy adult dogs. Toxins. (2020) 12:517. doi: 10.3390/TOXINS12080517
29. Herstad, KMV, Gajardo, K, Bakke, AM, Moe, L, Ludvigsen, J, Rudi, K, et al. A diet change from dry food to beef induces reversible changes on the faecal microbiota in healthy, adult client-owned dogs. BMC Vet Res. (2017) 13:147. doi: 10.1186/S12917-017-1073-9
30. Kim, J, An, JU, Kim, W, Lee, S, and Cho, S. Differences in the gut microbiota of dogs (Canis lupus familiaris) fed a natural diet or a commercial feed revealed by the Illumina MiSeq platform. Gut Pathogens. (2017) 9:68. doi: 10.1186/s13099-017-0218-5
31. Sandri, M, Dal Monego, S, Conte, G, Sgorlon, S, and Stefanon, B. Raw meat based diet influences faecal microbiome and end products of fermentation in healthy dogs. BMC Vet Res. (2016) 13:65. doi: 10.1186/s12917-017-0981-z
32. Uzal, FA, Vidal, JE, McClane, BA, and Gurjar, AA. Clostridium perfringens toxins involved in mammalian veterinary diseases. Open Toxicol J. (2013) 2:24. doi: 10.2174/1875414701003010024
33. Vázquez-Baeza, Y, Hyde, ER, Suchodolski, JS, and Knight, R. Dog and human inflammatory bowel disease rely on overlapping yet distinct dysbiosis networks. Nat Microbiol. (2016) 1:16177–5. doi: 10.1038/nmicrobiol.2016.177
34. Weese, JS, Staempfli, HR, Prescott, JF, Kruth, SA, Greenwood, SJ, and Weese, HE. The roles of Clostridium difficile and enterotoxigenic Clostridium perfringens in diarrhea in dogs. J Vet Intern Med. (2001) 15:374–8. doi: 10.1111/J.1939-1676.2001.TB02332.X
35. Wang, B, and Wang, XL. Species diversity of fecal microbial flora in Canis lupus familiaris infected with canine parvovirus. Vet Microbiol. (2019) 237:108390. doi: 10.1016/J.VETMIC.2019.108390
36. Lan, Y, Wang, Q, Cole, JR, and Rosen, GL. Using the RDP classifier to predict taxonomic novelty and reduce the search space for finding novel organisms. PLoS ONE. (2012) 7:e32491. doi: 10.1371/journal.pone.0032491
37. Wright, ES . DECIPHER: Harnessing local sequence context to improve protein multiple sequence alignment. BMC Bioinform. (2015) 16:322. doi: 10.1186/s12859-015-0749
38. Price, MN, Dehal, PS, and Arkin, AP. FastTree: Computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. (2009) 26:1641–1650. doi: 10.1093/molbev/msp077
Keywords: canine microbiota, canine nutrition, dietary transition, probiotic, biotics
Citation: Oba PM, Swanson OR, Kang Y, Mioto JC, Menton JF, Vinay E, Millette M, Kelly MR and Swanson KS (2025) Evaluation of Bacillus subtilis ATCC PTA-122264 on the fecal characteristics and microbiota of healthy adult dogs subjected to an abrupt diet change. Front. Vet. Sci. 12:1617072. doi: 10.3389/fvets.2025.1617072
Edited by:
Jirayu Tanprasertsuk, KatKin, United KingdomReviewed by:
Dennis E. Jewell, Kansas State University, United StatesDavid Atuahene, University of Turin, Italy
Copyright © 2025 Oba, Swanson, Kang, Mioto, Menton, Vinay, Millette, Kelly and Swanson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kelly S. Swanson, a3Nzd2Fuc29AaWxsaW5vaXMuZWR1
 Patrícia M. Oba
Patrícia M. Oba Olivia R. Swanson1
Olivia R. Swanson1
 Julio C. Mioto
Julio C. Mioto Mathieu Millette
Mathieu Millette Kelly S. Swanson
Kelly S. Swanson