- 1Laboratory of Infectious Diseases, Joint Faculty of Veterinary Medicine, Kagoshima University, Kagoshima, Japan
- 2Transboundary Animal Diseases Center, Joint Faculty of Veterinary Medicine, Kagoshima University, Kagoshima, Japan
- 3Biotechnology Department, Animal Health Research Institute, Agricultural Research Center, Giza, Egypt
- 4Division of Infectious Diseases, Animal Medicine Department, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt
- 5Faculty of Medicine, Mansoura University, Mansoura, Egypt
- 6Laboratory of Veterinary Histopathology, Joint Faculty of Veterinary Medicine, Kagoshima University, Kagoshima, Japan
- 7Laboratory of Animal Microbiology, Graduate School of Agricultural Science, Tohoku University, Sendai, Japan
Piroplasmosis is a tick-borne disease that can affect livestock, particularly cattle. Its causative pathogens are intracellular apicomplexan parasites belonging to the order Piroplasmida. We recently identified one such emergent pathogen (Colpodella spp.) in ticks infesting camel in Egypt. Accordingly, we aimed to ascertain the presence of hemoprotozoan parasites in ticks infesting cattle. We removed ticks from household cattle during veterinary examinations, and submitted them for morphological examination and PCR analyses for species identification. Ticks and hemoprotozoan species obtained from tick samples were also evaluated using BLAST analysis, followed by confirmatory phylogenetic analyses. The collected ticks were identified as belonging to three species: Hyalomma dromedarii, Hyalomma marginatum, and Rhipicephalus annulatus. Phylogenetic analysis based on the 16S rRNA gene revealed that these ticks were clustered with those of the relevant species previously documented in Egypt. Molecular analysis targeting the 18S rRNA gene revealed Colpodella spp., the second such report in Egypt and the first in R. annulatus ticks infesting cattle. The Colpodella minimum infection rate (MIR) was 2.3% (per sample of pooled ticks from a single bovine host). Furthermore, Babesia bovis, Theileria. annulata, and Theileria orientalis were detected with MIRs of 3.5%, 4.7%, and 0.39%, respectively. In the phylogenetic analysis, each detected pathogen clustered with its corresponding species. Specifically, the Colpodella spp. were grouped with Colpodella spp. previously detected in Rhipicephalus microplus, Rhipicephalus haemaphysaloides ticks, and humans in China (accession numbers MH208620, MH208621, and GQ411073), and H. dromedarii ticks infesting camel in southern Egypt (accession numbers LC775361 and LC775361). We confirmed the detection of B. bovis and T. annulata through PCR assays with specific primers targeting the spherical body protein-4 gene and the major merozoite surface antigen gene, respectively. The detection of Colpodella spp. in ticks infesting cattle highlights the need for ongoing surveillance of this parasites. Both cattle and camels may serve as sentinel species, emphasizing the importance of monitoring these livestock for emerging parasites.
1 Introduction
Piroplasmosis is a disease caused by intracellular apicomplexan parasites in the order Piroplasmida, mainly Theileria or Babesia spp. (1). The primary vectors are ixodid ticks, which are known to pose a significant threat to human and animal health as hematophagous ectoparasites that transmit pathogens when they feed on a host's blood (2). Beyond the threat to health, ticks and the protozoan pathogens they transmit are a major cause of economic loss in the agricultural sector, as tick-infested livestock are exposed to the pathogens that cause piroplasmosis. Infected livestock may show classic tick-borne disease symptoms such as weight loss and progressive anemia, and adverse effects on the udder, skin, and hide, and may die or have to be slaughtered (3, 4).
Piroplasmosis has been detected in various provinces of Egypt, suggesting that large parts of the country are prone to outbreaks of tick-borne diseases (5–9). Recent reports have highlighted newly emergent tick-borne protozoa in Egypt; specifically, Babesia naoakii was detected in camels (10), and a Colpodella spp. was detected in Hyalomma dromedarii ticks collected from camels (11). These findings demonstrate the importance of monitoring the distribution of tick species and tick-borne pathogens in livestock across Egypt. Although camels are commonly used in Egyptian agriculture, cattle are the predominant livestock species across the whole country; thus, there is a need to extend monitoring for the pathogens recently identified in ticks infesting camel to cattle.
Of the newly detected protozoan parasites in Egypt mentioned above, Colpodella spp. was the most recently reported, and is particularly worthy of attention as the potential epidemiological implications for human and animal health are not yet well understood. Colpodella spp. are a group of small predatory flagellates (12). They are predominantly free-living microorganisms that typically feed on algae or protozoa, and have only occasionally been found in vertebrates and arthropod vectors (13, 14). The first human case of Colpodella spp. infection was reported in China in 2012. In that case, cell morphology exams (Giemsa staining) revealed multiple, ring-like forms infecting erythrocytes, and the detection of a Colpodella spp. was formally confirmed through molecular analysis (15). Additionally, a Colpodella spp. has been identified in Rhipicephalus microplus ticks infesting cattle in Mozambique, Africa (16). In 2018, Colpodella spp. was found in ticks, and in a human patient exhibiting neurological symptoms, at the same location as that of the first human case in China (Qinghai Province). Notably, Colpodella tetrahymenae and Colpodella spp. were obtained in that report, and four sequences were identified (accession numbers MH012044-MH012047) (17). Recently, in China, Colpodella spp. have been reported in horses (18), and in Amur tigers (Panthera tigris altaica) and the ticks attached to them (19). Our previous report on Colpodella spp. was made in camels in the Luxor and Aswan regions of Upper Egypt. The spread of this previously little-reported pathogen from East Asia to North Africa is a cause for concern, especially considering the severity of the symptoms documented in the human case.
In Egypt, the prevailing climatic conditions provide a highly favorable habitat for numerous tick species, and the control measures against the potential threat to public health and the livestock-based economy remain insufficient (20). Thus, there is a pressing need to correct the paucity of epidemiological data on tick species and the protozoan parasites they transmit, especially in cattle populations, which represent the mainstay of Egyptian agriculture. Thus, the primary goal of this research was to identify tick species infesting cattle and any protozoa present in these ticks, in three governorates in Upper Egypt (including an area where Colpodella spp. had previously been detected) using PCR-sequencing assays, to provide crucial information for developing effective strategies to combat tick-borne diseases.
2 Materials and methods
2.1 Study area and design
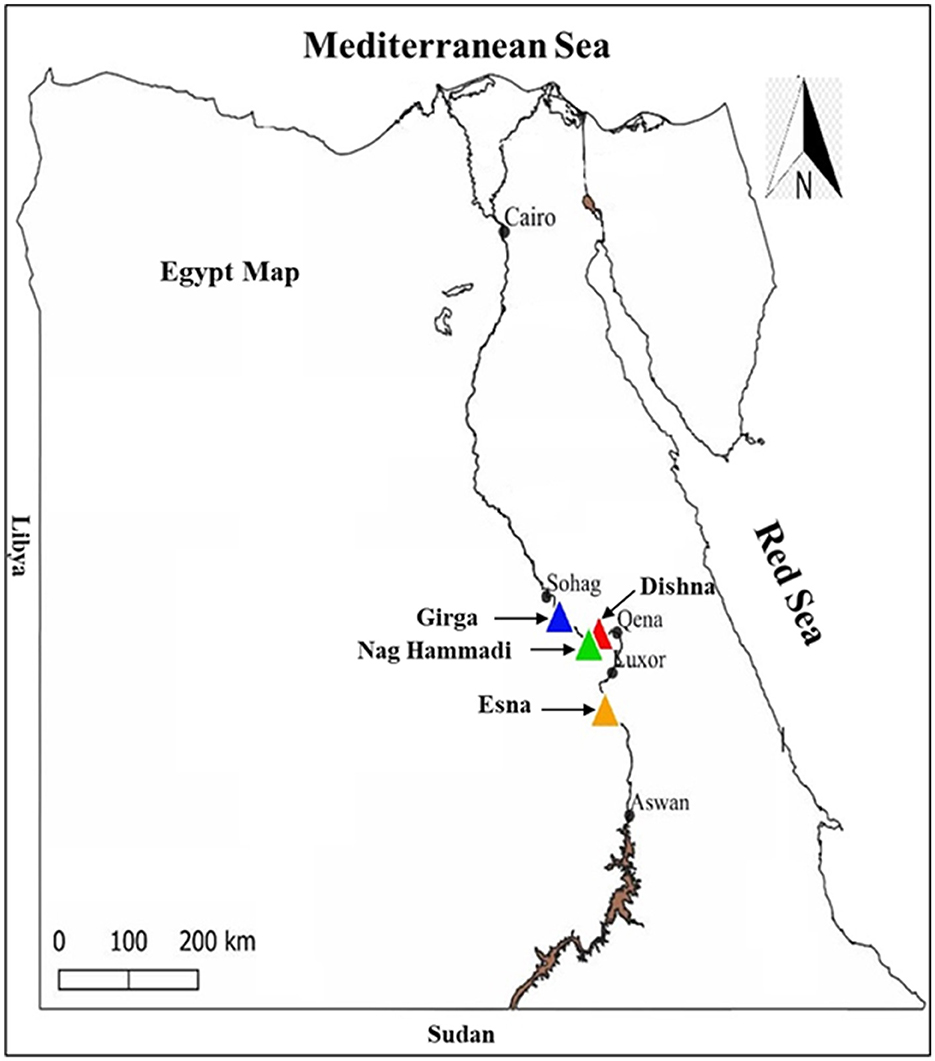
This cross-sectional study was conducted in four districts across southern Egypt: Deshna (26°7′18.595"N, 32°28′17.511"E) and Naja' Hammadi (26°2′55.6"N, 32°14′25.12"E) in the Qena governorate, Girga (26°20′13.96"N, 31°53′34.6"E) in the Sohag governorate, and Esna in the Luxor governorate (25°17′24.3"N, 32°33′23"E). These areas were selected based on their high livestock densities and documented history of tick infestations and tick-borne diseases, making them epidemiologically relevant for studying tick-pathogen dynamics. The hot and arid climate of southern Egypt, characterized by minimal annual rainfall and elevated temperatures, provides favorable conditions for tick survival and pathogen transmission. The geographical positions of the study areas within southern Egypt are shown in Figure 1.
The current study was conducted to investigate the presence and molecular diversity of tick species and associated piroplasmids in southern Egypt. The aim was to provide baseline data on tick infestation patterns and tick-borne protozoa with a focus on identifying emerging or underreported tick-borne pathogens. Collected ticks were morphologically identified and pooled according to species, developmental stage, and geographic origin for molecular analysis targeting piroplasmids and related protozoan parasites. All laboratory procedures, including DNA extraction, PCR amplification, and sequencing, were performed using standardized protocols to ensure reproducibility and accuracy.
2.2 Tick collection and tick morphological identification
A total of 110 cattle were selected for tick collection during routine veterinary inspections conducted between January and July 2019. The sample size was determined based on accessibility and logistical constraints, with the aim of generating preliminary baseline data on tick species composition and associated pathogens in the study area. We attempted to collect a uniform number (2, 3) of ticks from each bovine host. All ticks were removed manually from the medial aspect of the thighs, udder, dewlap, or axilla, taking care to minimize any discomfort to the animal. Sampling was not randomized or stratified due to logistical constraints and the observational nature of the study; however, efforts were made to include animals from multiple locations within each location to attain variability in tick populations. The owner of each cattle provided informed consent for the collection of ticks from their cattle, and the use of the samples in this study. The number of collected ticks was recorded for each cattle at each location. After collection, the ticks were preserved in tubes containing 70% ethyl alcohol. Each tick was subjected to a cleaning process before species identification by microscopy. The procedure involved placing the tick in a fine mesh and subjecting it to a gentle stream of tap water to eliminate surface dirt and debris. Subsequently, the ticks were immersed in 70% ethanol for 2 min and then rinsed twice in sterile Milli-Q water. Ticks were cleaned thoroughly to enhance the visibility of their taxonomic characteristics. Tick species were identified using standard identification keys (21–24).
2.3 Tick processing and DNA isolation
Ticks underwent an initial cleansing process with 70% ethanol and were washed with sterile Milli-Q water before air-drying. Each tick was then placed in a 2-ml tube also containing a stainless-steel bead for subsequent crushing, following overnight freezing at −80°C. The crushing procedure utilized an Automill crusher (Tokken. Inc, Japan) for three cycles, each lasting 30 s at 2,000 rpm. Following crushing, 200 μl of 1 M Tris-HCl (pH = 7.5) was added to each sample tube, and the tube was well shaken for 15 min, to ensure thorough mixing of the contents. After removing the stainless-steel beads, the tubes were centrifuged at 14,000 g for 5 min at 4°C. A 200-μl amount of tick homogenate was then carefully collected for DNA isolation.
The tick samples collected from each animal were pooled according to species, life cycle stage, and sampling area before DNA extraction. One hundred seventy-seven pools were generated based on sample size (1– 3 ticks/pool). The DNA isolation process employed a fully automated nucleic acid extractor, (magLEAD 6gc, Precision System Science Co., Ltd., Chiba, Japan), employing MagDEA® Dx SV (Precision System Science Co., Ltd., Chiba, Japan), and was conducted following the manufacturer's instructions. This method was chosen over manual extraction due to its higher efficiency, reduced contamination risk, and improved reproducibility, ensuring standardized DNA yields. The DNA concentration was determined using a NanodropTM 2,000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The DNA samples were stored at −30°C for subsequent analysis.
2.4 Molecular characterization of ticks
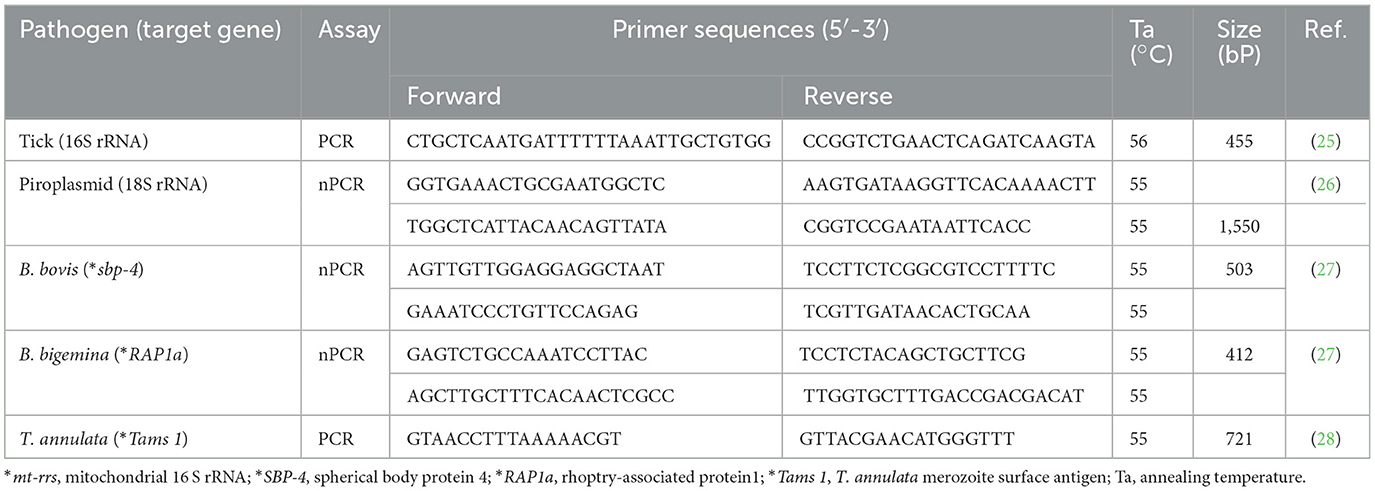
Samples were then subjected to a conventional PCR assay to confirm the existence of tick genomic DNA and as assure the absence of PCR inhibitors. In this assay, we targeted a specific partial fragment of the 16S rRNA gene unique to tick species (25) for amplification. The PCR mixture was prepared at a total volume of 10 μl, and comprised 5 μl of 2 × Gflex PCR buffer (containing Mg2+, dNTP plus; Takara Bio Inc., Kusatsu, Shiga, Japan), two 0.5 μl primer solutions (one each for the forward and reverse primers) with a concentration of 10 μm, 0.5 μl of Tks GflexTM DNA polymerase (1.25 U/μl; Takara Bio Inc.), 3.2 μl of Milli-Q water, and 0.5 μl of the extracted DNA template, ranging in concentration from 10 to 30 ng/μl. The sequences and annealing temperatures of the used primers are stated in Table 1. Nuclease-free water was utilized as a negative control and template for contamination monitoring. A confirmed NA sample for Haemaphysalis longicornis that had been stored in our laboratory and subject to confirmatory sequencing was utilized as the positive control, and exhibited a DNA concentration of 10 ng/μl.
2.5 Molecular detection of piroplasms
A nested PCR assay was used to detect the 1,550-bp fragment of the 18S rRNA gene specific to piroplasms (26). Moreover, we employed nested PCR assays to pinpoint gene fragments specific to pathogens: the 503-bp fragment of the spherical body protein-4 (SBP-4) gene for B. bovis (27), and the 412-bp fragment of the rhoptry-associated protein 1 (RAP1a) gene for Babesia bigemina (27). Additionally, a conventional PCR assay was conducted to detect the 721-bp fragment of the major merozoite surface antigen (Tams1) gene, which is specific to T. annulata (28). The PCR mixture was prepared at a total volume of 10 μl. It included 5 μl of 2 × Gflex PCR buffer (Mg2+, dNTP plus) (Takara Bio Inc.), two 0.5 μl primer solutions (one each for the forward and reverse primers) at a concentration of 10 μm, 0.5 μl of Tks GflexTM DNA polymerase (1.25 U/μl) (Takara Bio Inc.), 3.2 μl of Milli-Q water, and 0.5 μl of the extracted DNA template with a concentration ranging from 10 to 30 ng/μl. In the nested PCR assays, 0.5 μl of the first PCR product (diluted 25-fold) was used as the template in the second PCR reaction. The sequences and annealing temperatures of the used primers are provided in Table 1. All PCR assays included strict measures for contamination monitoring, and employed nuclease-free water as the negative control. Additionally, confirmed DNA samples for each pathogen (Piroplasma spp., B. bovis, B. bigemina, and T. annulata) that had been obtained from Egyptian cattle blood and stored in our laboratory were used as positive controls. These positive control samples had undergone confirmatory sequencing, and they demonstrated DNA concentrations ranging from 10 to 15 ng/μl.
The PCR products obtained from the assays above were subjected to electrophoresis on a 1.5% agarose gel in 1 × Tris-acetate-EDTA (TAE) buffer. Gel electrophoresis was performed using a Mupid electrophoresis device (Mupid Co., Ltd., Tokyo, Japan). After electrophoresis, the DNA bands were visualized using a gel documentation system with a UV device (WUV-M20; ATTO Co., Ltd., Tokyo, Japan). Bands were visualized after staining the gel with ethidium bromide at a 5 μg/ml concentration in 1 × TAE buffer.
2.6 Sequencing and phylogenetic analysis
The positive amplicons obtained from each gene detection were subjected to purification from the agarose gel, employing the NucleoSpin Gel and PCR Clean-up kit (Macherey-Nagel, Leicestershire, Duren, Germany), in accordance with the manufacturer's instructions. Subsequently, the purified DNA samples were sent to a commercial laboratory (Eurofins NSC Japan KK, Kanagawa, Japan) for sequence analysis using a 3730 × 1 DNA Analyzer (Thermo Fisher Scientific, Waltham, Massachusetts, USA). The bi-directional sequencing results were first evaluated using SnapGene Viewer software (GSL Biotech, LLC., Boston, USA) (https://www.snapgene.com/). The forward and reverse sequences were aligned and combined using MEGA 11 software to produce complete sequences for subsequent analysis.
To validate the identification of the tick-borne protozoa, the obtained sequences were compared with reference sequences in the GenBank database utilizing the BLASTn tool. Obtained sequences were aligned with reference sequences from the GenBank database, using the ClustalW algorithm in MEGA 11 software. After alignment, the sequences were trimmed, and a model test was conducted in MEGA 11 to identify the most suitable evolutionary model for the data. Subsequently, phylogenetic trees were constructed using the Maximum Likelihood method, with the Tamura 3-parameter model applied for identifying tick species, B. bovis SBP4 gene, and T. annulata Tams-1 gene. For the detection of Protozoal spp. 18S rRNA genes, the Tamura-Nei model was used as the evolutionary model. Tree reliability was evaluated by bootstrap analysis with 1,000 replicates (29).
2.7 Statistical analysis
The minimum infection rate (MIR) was calculated as the number of positive pools divided by the total number of the tested ticks within each geographic location and in relation to the examined corresponding tick spp., multiplied by 100. This metric was used to estimate the lower bound of infection at the individual tick level, particularly because samples were analyzed in pools. As prevalence could not be directly determined from pooled testing due to the inability to identify the exact number of infected individuals, MIR was selected as the primary measure of infection frequency in this study.
3 Results
3.1 Morphological and molecular tick identification
We collected 258 ticks from 110 cattle across the study locations. In initial morphological characterization. Specifically, in the Qena governorate, 79 ticks were retrieved from 36 cattle. Broken down by location, we collected 35 ticks from 17 cattle at Deshna (R. annulatus: 23 adult females, 11 nymphs; H. dromedarii: one adult female), 44 ticks from 19 cattle at Naja' Hammadi (R. annulatus: 35 adult females, 8 nymphs; H. dromedari: one adult male). At the Girga location in the Sohag governorate, we collected 82 ticks from 34 cattle (R. annulatus: 54 adult females, one adult male; H. dromedarii: 12 adult males, 15 adult females). At the Esna location in the Luxor governate, we collected 96 ticks from 40 cattle (R. annulatus: 68 adult females, 23 nymphs; H. dromedarii: one adult male; H. marginatum: four adult females). For each species, and at each geographical location, adult females accounted for the largest proportion of collected ticks. Representative examples displaying the morphological characteristics of each collected species are shown in Figures 2, 3.
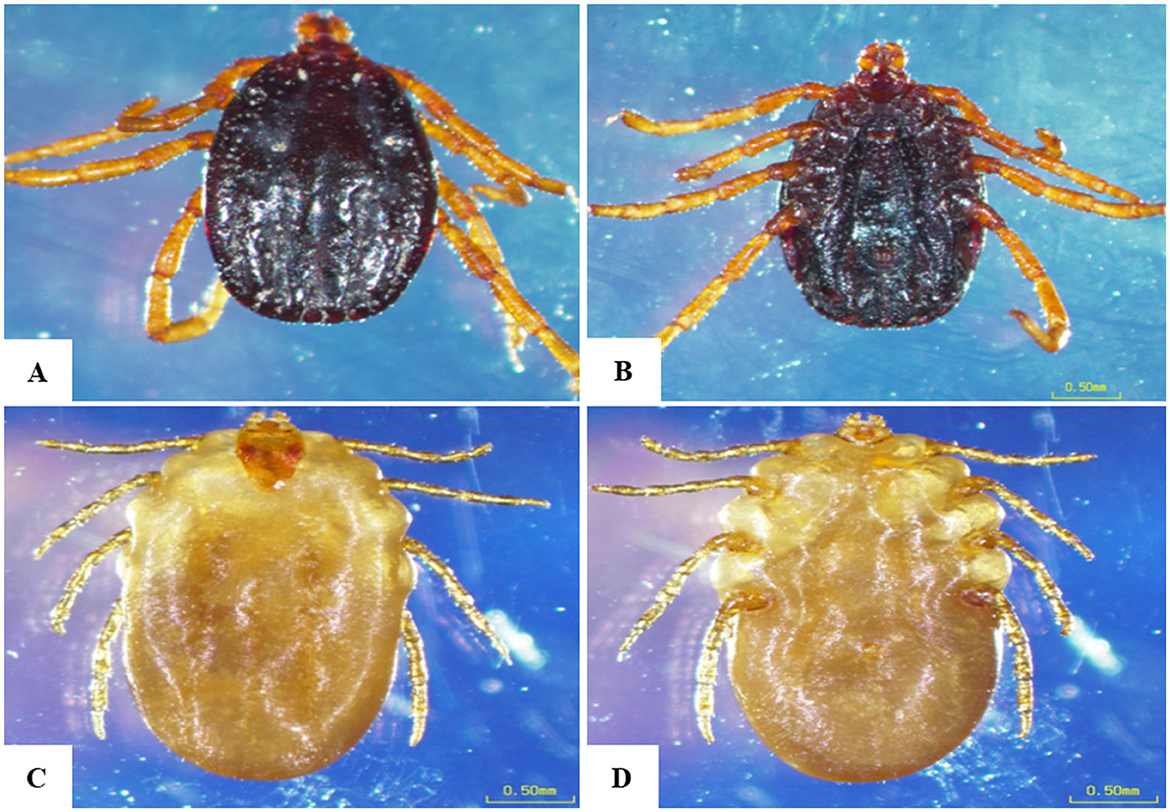
Figure 2. Morphological characteristics of Rhipicephalus annulatus obtained in the present study. (A) Male, dorsal surface, (B) male, ventral surface, (C) female, dorsal surface, (D) female, ventral surface; scale bar: 0.50 mm.
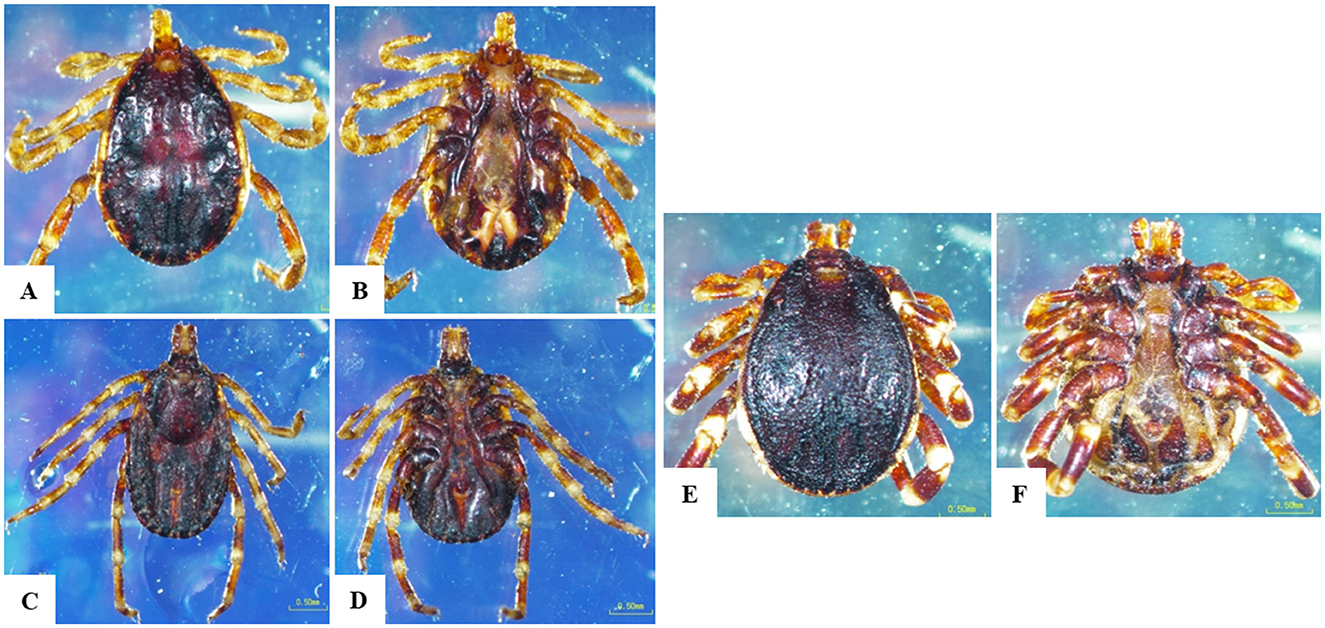
Figure 3. Morphological characteristics of Hyalomma species obtained in the current study. (A) H. dromedarii male, dorsal surface, (B) H. dromedarii male, ventral surface, (C) H. dromedarii female, dorsal surface, (D) H. dromedarii female, ventral surface, (E) H. marginatum male, dorsal surface, (F) H. marginatum male, ventral surface; scale bar: 0.50 mm.
We then targeted 177 pooled tick samples for DNA extraction and PCR analysis. Each pooled sample exhibited the expected 455-bp band of the 16S rRNA gene specific to tick species, thus confirming successful DNA extraction. Twenty PCR products representative for each morphologically identified tick species were then subjected to sequencing and phylogenetic analysis as shown in Figure 4. The H. dromedarii sequences identified in this study exhibited 99.8% similarity with H. dromedarii sequences (accession numbers MG757400 and MN960580) previously obtained from Egypt and Tunisia, respectively. Meanwhile, the H. marginatum sequences we obtained displayed a 99.3% similarity with H. marginatum sequences from Tunisia and Turkey (accession numbers OQ269610 and OQ975265, respectively). Additionally, the R. annulatus sequences we identified exhibited a 99.6% similarity with an R. annulatus sequence from Egypt (accession number: KY945491). The obtained sequences for tick species identification were deposited in the GenBank database with accession numbers PP937570, PP937571, and PP937573 for R. annulatus, PP937568 for H. marginatum, and PP937569, PP937572, and PP937574 for H. dromedarii.
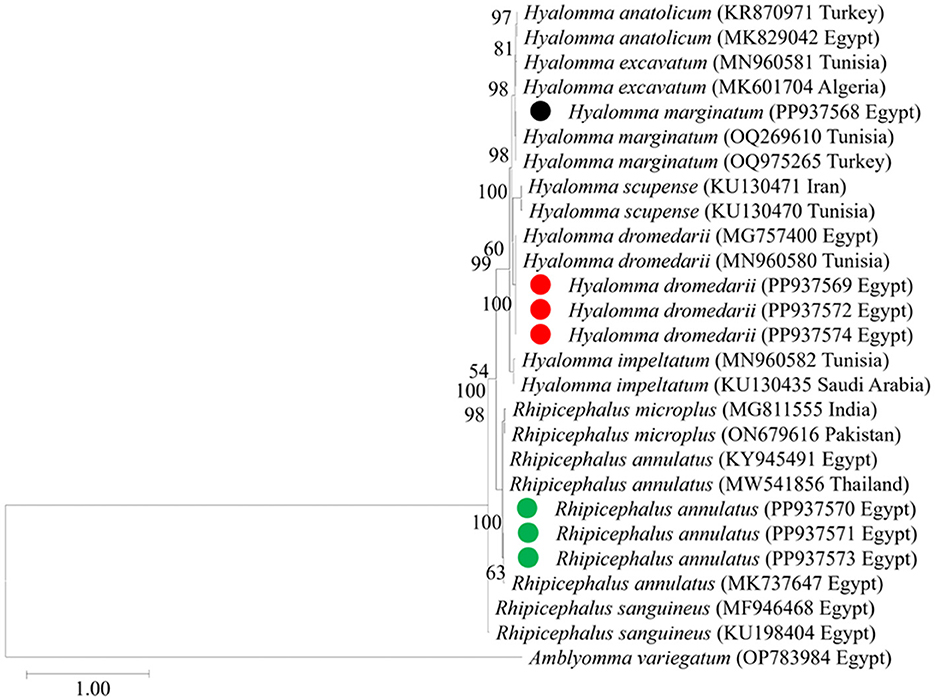
Figure 4. The phylogenetic analysis of tick spp. identified in this study based on mt-rrs (16S rRNA) gene sequences. The phylogenetic tree was constructed using the Maximum Likelihood method with a Tamura 3-parameter model, employing MEGA version 11 software. The numbers displayed at the nodes of the tree indicate the percentage occurrence of each clade based on 1,000 bootstrap replications of the data. Amblyomma variegatum (OP783984) was used as the out group. The sequences obtained in this study are circled.
3.2 Detection of tick-borne protozoa
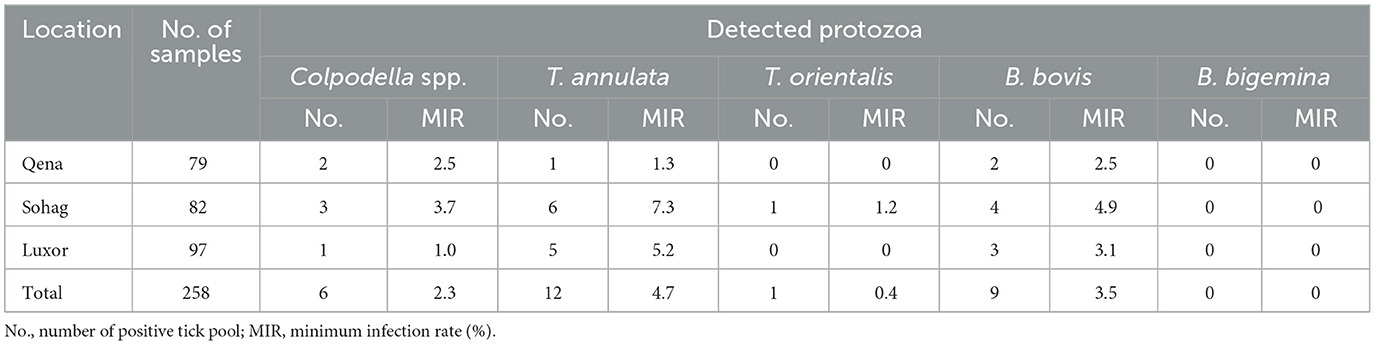
In this study, 177 pooled tick DNA samples were examined using nested PCR to detect the 18S rRNA gene, which is indicative of piroplasmid species. Seventeen of these DNA samples tested positive, representing a MIR of 9.6%, with each positive finding representing a single bovine host. These 17 positive samples then underwent further PCR testing to determine the MIR of specific piroplasmid pathogens (B. bovis, B. bigemina, and T. annulata) employing primers specific for each species. A nested PCR assay targeting the SBP-4 gene of B. bovis revealed the presence of this piroplasmid pathogen in 9/17 pooled samples. Contrastingly, B. bigemina, was not identified in any sample in an assay targeting its RAP1a gene. A conventional PCR assay targeting the Tams1 gene of T. annulata revealed this pathogen was present in 12/17 pooled samples. The MIR for these tick-borne protozoans are displayed together with the absolute numbers of samples in which they were detected, in Table 2.
In Hyalomma ticks, MIR was 5.9% for T. annulata, 2.9% for B. bovis, and 0% (undetected) for T. orientalis and Colpodella spp. In R. annulatus ticks, the MIR was 4.4% for T. annulata, 3.3% for B. bovis, 0.5% for T. orientalis, and 2.7% for Colpodella spp. The MIRs for all pathogens in different tick species by sampling location are presented in Table 3. In addition, multiple pathogens were detected within single tick pools. Table 4 provides detailed information on the co-infection rates for the detected protozoa, categorized by the tick collection sites and the specific tick species.
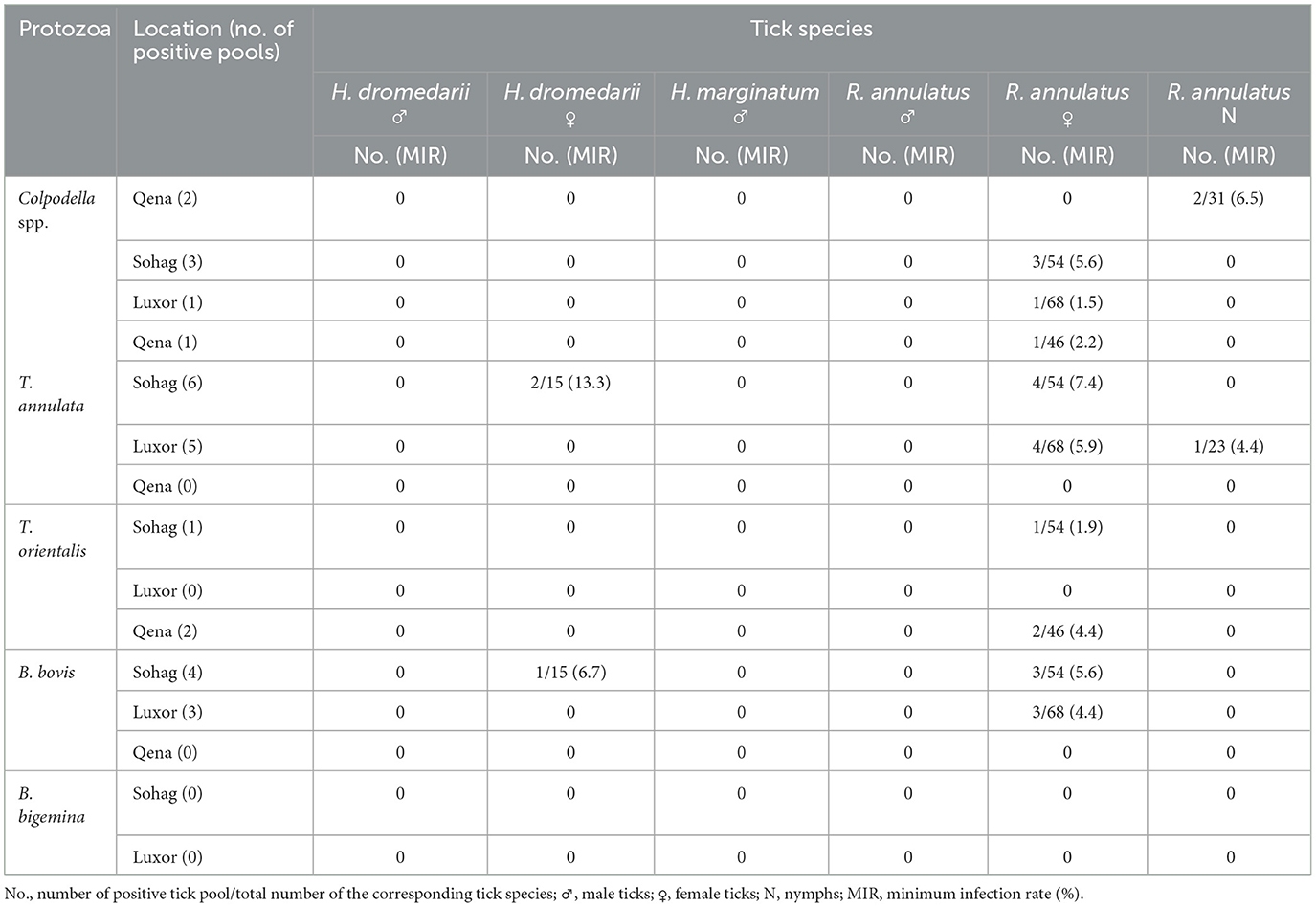
Table 3. Minimum infection rates of the detected protozoa in tick pools by PCR-sequencing assay according to tick species in relation to sampling location.
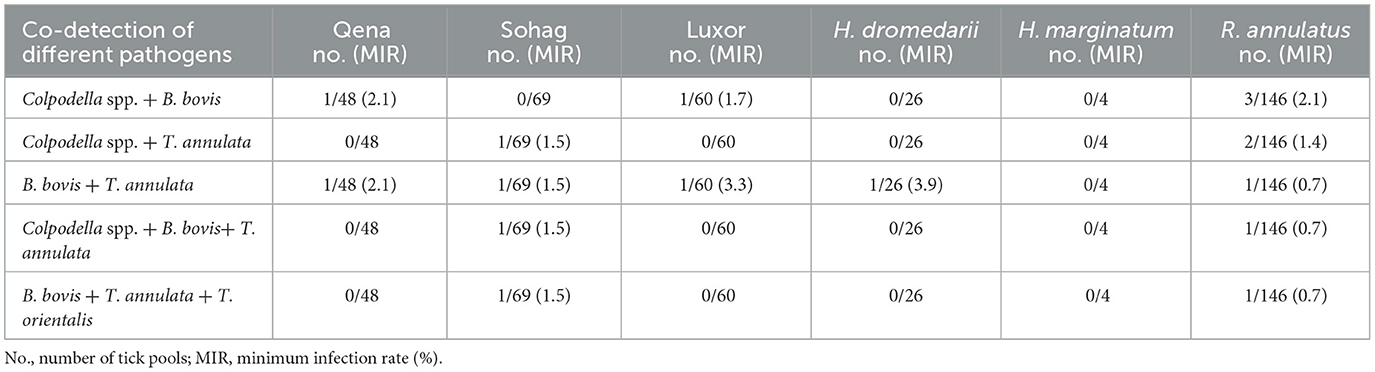
Table 4. Co-infection rates of the detected protozoa in overall tick pools according to tick collection sites and tick specie.
3.3 Sequencing and phylogenetic analysis
We then submitted the 17 pooled piroplasm (18S rRNA gene)-positive samples described above for direct sequencing. Among the obtained sequences, the numbers exhibiting high similarity with existing sequences in the GenBank database were six for Colpodella spp., six for T. annulata, four for B. bovis, and one for T. orientalis. The obtained sequences for the 18S rRNA gene were submitted to the GenBank database with accession numbers PP937594, PP937595 and PP937596 for Colpodella spp., PP937591 for T. orientalis, PP937589 and PP937590 for T. annulata, and PP937592 and PP937593 for B. bovis. The phylogenetic tree depicting the evolutionary relationship between the detected protozoa and other apicomplexans is shown in Figure 5. Positive PCR products obtained with B. bovis or T. annulata-specific primers were selected for sequencing. For B. bovis (n = 1), the obtained sequence showed approximately 99% similarity with B. bovis sequences previously reported in South Africa (accession number KF626637) and Egypt (accession number LC775376). Furthermore, the obtained sequences for T. annulata (n = 2) showed 98.6% identity with T. annulata sequences previously reported in Tunisia (accession number AF214904) and China (accession numbers MH538103 and MF116148). We thus confirmed the presence of B. bovis and T. annulata in the samples analyzed. The obtained sequences were deposited in the GenBank database with accession numbers PP941969 (for B. bovis SBP-4 gene), or PP941967 or PP941968 (for T. annulata Tams1 gene). The phylogenetic trees for B. bovis and T. annulata, which illustrate the relationships and evol.utionary connections between different strains of B. bovis and T. annulata, are shown in Figures 6, 7, respectively.
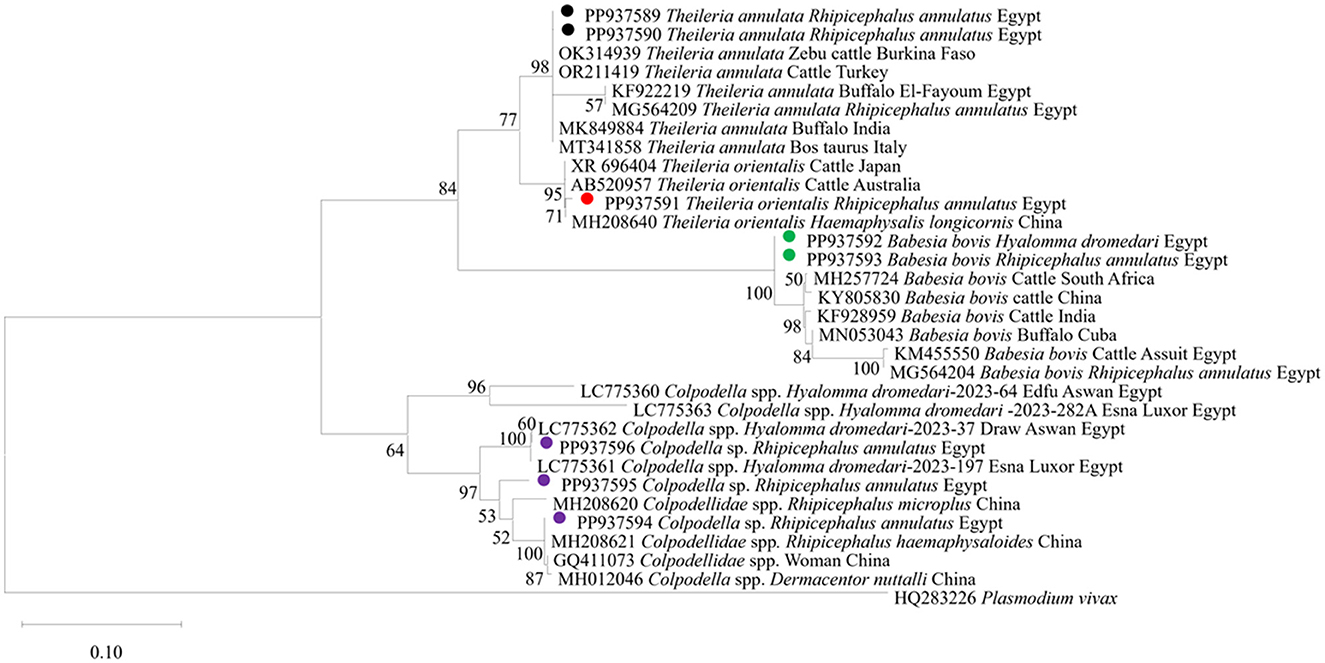
Figure 5. The phylogenetic analysis showing the detected protozoa based on 18S rRNA gene sequences. The phylogenetic tree was constructed using the Maximum Likelihood method with Tamura-Nei model, employing MEGA version 11 software. The numbers displayed at the nodes of the tree indicate the percentage occurrence of each clade based on 1,000 bootstrap replications of the data. Plasmodium vivax (HQ283226) was used as out group. The sequences obtained in this study are circled.
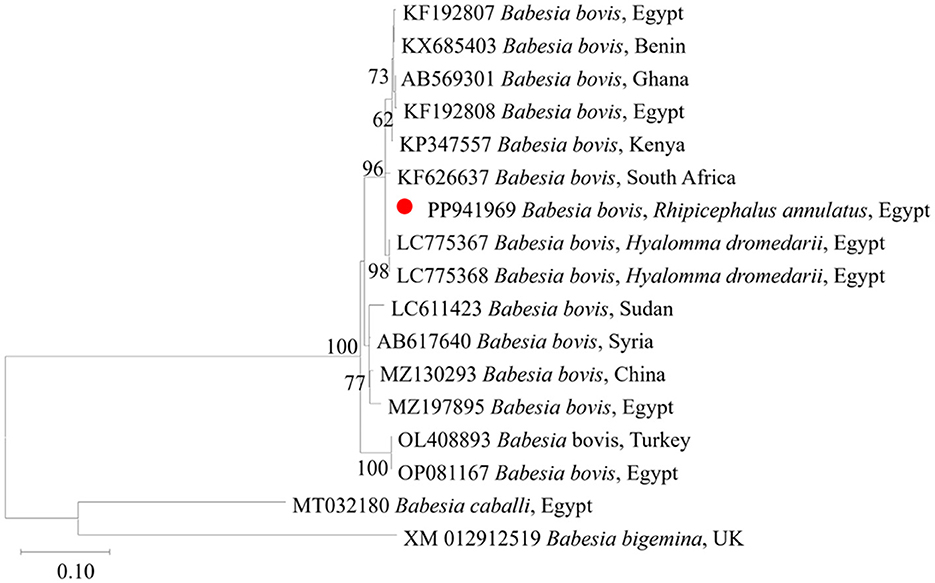
Figure 6. Phylogenetic analysis of B. bovis identified in this study based on SBP4 gene sequences. The phylogenetic tree was constructed using the Maximum Likelihood method with the Tamura 3-parameter model, employing MEGA version 11 software. The numbers displayed at the nodes of the tree indicate the percentage occurrence of each clade based on 1000 bootstrap replications of the data. Babesia bigemina (XM012912519) was used as out group. The sequence obtained in this study is circled in red.
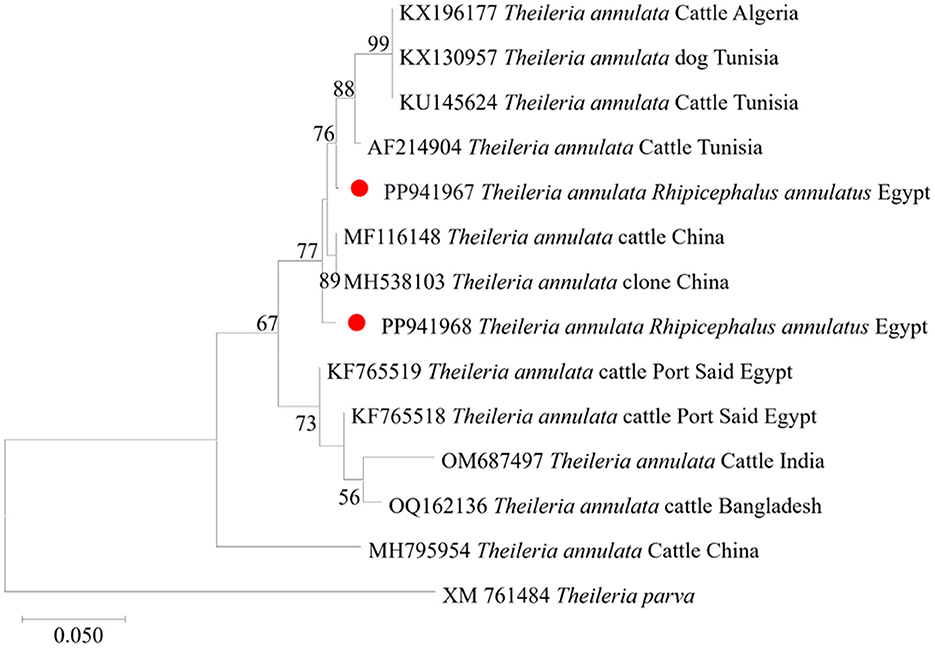
Figure 7. Phylogenetic analysis of T. annulata identified in this study based on Tams 1 gene sequences. The phylogenetic tree was constructed using the Maximum Likelihood method with the Tamura 3-parameter model, employing MEGA version 11 software. The numbers displayed at the nodes of the tree indicate the percentage occurrence of each clade based on 1000 bootstrap replications of the data. Theileria parva (XM761484) was used as out group. The sequences obtained in this study are circled in red.
4 Discussion
Here, we present a comprehensive molecular investigation of ixodid tick infestation in cattle in Upper Egypt, coupled with a detailed molecular characterization of the hemoprotozoan parasites identified in ticks infesting cattle in this region. To the best of our knowledge, this study provides the most recent epidemiological data on ticks and associated tick-borne pathogens in the cattle population in southern Egypt, including crucial data on protozoan parasites (specifically, Colpodella spp.) that have only recently been in identified Egypt, in ticks infesting another livestock species (camels).
All collected ticks were classified as H. dromedarii, H. marginatum, or R. annulatus based on morphological criteria (Figure 3). Because morphological identification can be challenging when dealing with engorged ticks (21, 30), we employed a molecular technique (31) targeting the protozoan 16S rRNA gene, to validate tick species identification (2, 31–34). In our phylogenetic analyses, sequences for morphologically identified tick species formed distinct clusters, validating our identification of H. dromedarii, H. marginatum, and R. annulatus (Figure 4).
The current study provides further molecular evidence of Colpodella spp. within ixodid ticks in Upper Egypt, following our recent identification of this parasites in H. dromedarii ticks infesting camel, in the first such report in Egypt (11). Here, we identified Colpodella spp. in ticks infesting cattle, specifically R. annulatus, at an MIR of 2.7%. We successfully amplified 1,550-bp DNA fragments of the 18S rRNA gene associated with Colpodella spp., showing sequence identities between 95% and 100% with Colpodella spp. entries in the GenBank database (Figure 4). Our phylogenetic analysis placed the Colpodella spp. sequences in a sister group to the apicomplexan clade, encompassing microorganisms such as Babesia spp. and Theileria spp. The phylogenetic tree (Figure 5) demonstrated that the Colpodella spp. sequences obtained from H. dromedarii ticks previously recorded in Aswan and Luxor governorates of Egypt (LC775360, LC775361, LC775362, and LC775363) clustered tightly together, forming a well-supported clade with high bootstrap values (96%−100%), indicating a close genetic relationship among these isolates. This monophyletic grouping may reflect a conserved lineage of Colpodella spp. specifically associated with H. dromedarii in southern Egypt. In contrast, the sequences PP937595 and PP937596 derived from Rhipicephalus annulatus ticks, in this study in different locations in southern Egypt, showed a separate clustering pattern, positioned closer to Colpodella sequences from Chinese Rhipicephalus species (e.g., MH208620, MH208621), suggesting potential host- or region-specific genetic divergence. These results imply that Colpodella spp. may exhibit host-specific genotypes and that their genetic diversity is influenced by tick species and possibly ecological factors. The phylogenetic proximity of Egyptian and Asian isolates further raises the possibility of a broader transboundary distribution or ancient lineage conservation, warranting further investigation into the evolutionary dynamics and zoonotic potential of these protozoans.
Our findings provide further evidence on the presence of Colpodella spp. in ixodid ticks across Upper Egypt, although this presence does not necessarily indicate biological transmission from ticks to cattle. The cattle infested with Colpodella-carrying ticks in this study were all clinically healthy at the time of tick collection. Our findings suggest that cattle, like camels, could serve as a valuable sentinel species for detecting emerging tick-borne diseases, such as Colpodella spp. infections. A critical concern related to Colpodella spp. is its zoonotic potential, as documented in reports from China (15, 17). Further studies must address the transmission mechanism, pathogenicity, and zoonotic potential of this emerging pathogen. Importantly, research is needed to develop protective measures for individuals who are in frequent proximity to animals susceptible to tick infestations. Protozoan parasites in the genus Colpodella represent an emerging health threat that appears to have a wider geographical reach than previously thought, and they may induce a disease with severe symptoms in humans (based on the small number of cases reported so far).
The MIR for piroplasma microorganisms we detected in pooled cattle-infested ticks (one pool collected from one bovine host) in this study was 9.6%, which is lower than the rates reported in tick-infested cattle populations in other regions of Egypt. Specifically, previous studies have reported rates of 52.5% in cattle in El-Wady El-Gadid governorate (35), 17% in household cattle across Beni-Suef, Qalyubia, El-Wady El-Gadid, Qena, and Behera governorates (36), and 20% in cattle in Qena and Sohag governorates (37).
Among the individual piroplasma species, we found B. bovis at an MIR of around 3.5% in our pooled tick samples, specifically 3.3% in both H. dromedarii and R. annulatus ticks. As with the findings for piroplasma microorganisms overall, higher infection rates for these pathogens have been reported in cattle; specifically, 55% in Giza governorate (38), 4% in Beheira and Faiyum governorates (5), 52.4% in Assiut governorate (39), 9% in Qena and Sohag governorates (40), 3.2% in Behera and Menofia governorates (41), and 5.3% in Qena and Sohag governorates (37). We did not detect B. bigemina in any sample in our study, although this pathogen has previously been reported in Egyptian cattle populations at infection rates ranging from 1.1% to 66% (5, 36, 37, 40, 42, 43).
In the present study, T. annulata was detected in our samples with an MIR of 4.7%; specifically, 6.7% and 4.5% in H. dromedarii and R. annulatus ticks, respectively. Our results are consistent with findings in previous studies Dahl on this pathogen in Egypt. Notably, previous studies have recorded infection rates in cattle of 9.6% in Menoufia, Behera, Giza, and Sohag governates (42), 63.6% in El-Wady El-Gadid governate (19), 22% in Beni-Suef, Faiyum, and El-Wady El-Gadid governates (43), 15.9% in Beni-Suef, Qalyubia, El-Wady El-Gadid, Qena, and Behera governorates (36), 18.1% in Faiyum, Assiut, and Kharja governates (9), 9.6% in Behera and Menofia governorates (41), and 10.7% in Qena and Sohag governorates (37). We detected T. orientalis in one pooled R. annulatus tick sample collected from one bovine host in the Sohag governorate (representing an MIR of 0.5%). This finding aligns with previous research, including the reports by (41), who found a rate of 0.68% in the Behera and Menofia governorates, and (44), who found an infection rate of 8.8% in northern Egypt. The presence of T. orientalis in R. annulatus ticks suggests a potential route of infection for this piroplasmid microorganism. We speculate that there are regional variations in the spread of this pathogen across Egypt, but this requires further investigation. Multiple pathogens were identified in both pooled tick samples and individual ticks. However, it is crucial to note that detecting various pathogens in pooled samples does not necessarily indicate that individual ticks were co-infected; such findings could result from several mono-infected ticks being present in a single pool. Our results highlight the benefits of molecular techniques, which offer the advantage of detecting a wide range of pathogens in ticks (6, 20).
This study has a number of limitations. As a cross-sectional study, its design did not allow for investigation of seasonal fluctuations in tick population densities, the impact of animal movement on tick dispersal, or the associated challenges in correlating infection and infestation risk factors. Since the study was conducted in a specific region, Upper Egypt, we cannot draw any conclusions on the tick-related health threats in other regions across the country. To comprehensively evaluate the current health risk posed by ticks and tick-borne pathogens in Egypt, longitudinal research spanning a wider geographic area and invol.ving a greater number of samples, including samples collected from ticks infesting humans, is imperative. Furthermore, there is a lack of specific primers for detecting Colpodella spp. using genes other than the 18S rRNA, and this represents a significant limitation for this research. Addressing this constraint requires future studies to integrate whole genome sequencing of Colpodella spp., facilitating the development of appropriate specific primers.
5 Conclusion
In conclusion, we elucidated pathogenic piroplasms carried by ixodid ticks infesting cattle in southern Egypt. Specifically, we detected Colpodella spp. in R. annulatus ticks retrieved from infested cattle, following our previous detection of this pathogen in H. dromedarii ticks infesting camel in a similar study area. T. orientalis was detected in R. annulatus ticks, suggesting this tick species may act as a vector for transmitting the pathogen to cattle in Egypt. Although we identified piroplasms in ticks, we cannot confirm their biological transmission to cattle in this study, and further surveillance and control measures to mitigate the risk of tick-borne diseases in animal and human populations are crucial. Here, we provide important evidence on the MIR of ticks and tick-pathogens that may affect cattle in Upper Egypt.
Data availability statement
All data supporting the findings of this study are presented in the article. The sequences obtained for tick species identification have been deposited in the GenBank database under accession numbers PP937568-PP937574. The 18S rRNA gene sequences are also available in GenBank, with accession numbers PP937594, PP937595, and PP937596 for Colpodella spp., PP937591 for T. orientalis, PP937589 and PP937590 for T. annulata, and PP937592 and PP937593 for B. bovis. Additionally, the SBP-4 gene for B. bovis is deposited under PP941969, while the Tams1 gene for T. annulata is listed under PP941967 and PP941968.
Ethics statement
This study was approved by the Ethical Research Committee of the Faculty of Veterinary Medicine, South Valley University, Qena, Egypt (Approval No.19/11.08/2021). All procedures in this study accorded with Egyptian national animal welfare regulations and the research regulations and bylaws of South Valley University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
AS: Investigation, Validation, Visualization, Formal analysis, Conceptualization, Data curation, Writing – review & editing, Methodology, Funding acquisition, Writing – original draft. HM: Conceptualization, Formal analysis, Writing – review & editing. MA: Writing – review & editing, Conceptualization, Validation, Formal analysis. SM: Writing – review & editing, Conceptualization, Formal analysis, Validation. TH: Validation, Conceptualization, Writing – review & editing. KT-K: Conceptualization, Validation, Writing – review & editing. TT: Project administration, Formal analysis, Data curation, Conceptualization, Funding acquisition, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by JSPPS KAKENHI Grant Numbers JP20KK0154 and JP22H02522, and JSPPS Bilateral Program Grant Number JPJSBP120206002, JPJSBP120219936, and JPJSBP120239937. Ahmed M. Soliman was funded by a full scholarship (PD60) support from the Ministry of Higher Education and Scientific Research of the Arab Republic of Egypt as a Ph.D. student (Animal Health Research Institute, Agricultural Research Center). Hassan Y. A. H. Mahmoud received financial support from the Arab Republic of Egypt, Ministry of Higher Education and Scientific Research (Faculty of Veterinary Medicine, South Valley University), in the form of a post-doctoral researcher.
Acknowledgments
We thank Henry Smith of the Joint Faculty of Veterinary Medicine, Kagoshima University, Japan, for his assistance with the English edition of this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Selim A, Khater H. Seroprevalence and risk factors associated with Equine piroplasmosis in North Egypt. Comp Immunol Microbiol Infect Dis. (2020) 73:101549. doi: 10.1016/j.cimid.2020.101549
2. de la Fuente J, Estrada-Pena A, Venzal JM, Kocan KM, Sonenshine DE. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front Biosci. (2008) 13:6938–46. doi: 10.2741/3200
3. Jonsson NN. The productivity effects of cattle tick (Boophilus microplus) infestation on cattle, with particular reference to Bos indicus cattle and their crosses. Vet Parasitol. (2006) 137:1–10. doi: 10.1016/j.vetpar.2006.01.010
4. Walker AR, Bouattou A, Camicas JL. Ticks of Domestic Animals in Africa: A Guide to Identification of Species. Edinburgh: Bioscience Reports (2003).
5. Ibrahim HM, Adjou Moumouni PF, Mohammed-Geba K, Sheir SK, Hashem ISY, Cao S, et al. Molecular and serological prevalence of Babesia bigemina and Babesia bovis in cattle and water buffaloes under small-scale dairy farming in Beheira and Faiyum Provinces, Egypt. Vet Parasitol. (2013) 198:187–92. doi: 10.1016/j.vetpar.2013.08.028
6. El-Ashker M, Hotzel M, Gwida M, El-Beskawy M, Silaghi C, Tomaso H. Molecular biological identification of Babesia, Theileria, and Anaplasma species in cattle in Egypt using PCR assays, gene sequence analysis and a novel DNA microarray. Vet Parasitol. (2015) 207:329–34. doi: 10.1016/j.vetpar.2014.12.025
7. Mahmoud MS, El-Ezz NT, Abdel-Shafy S, Nassar SA, El Namaky AH, Khalil WK. Assessment of Theileria equi and Babesia caballi infections in equine populations in Egypt by molecular, serological and hematological approaches. Parasit Vectors. (2016) 9:260. doi: 10.1186/s13071-016-1539-9
8. Abo El Fadl EA, El-Ashker M, Suganuma K, Kayano M. Discriminant analysis for the prediction and classification of tick-borne infections in some dairy cattle herds at Dakahlia Governorate, Egypt. JJVR. (2017) 65:127–33. doi: 10.14943/jjvr.65.3.127
9. AL-Hosary AM, Răileanu C, Tauchmann O, Fischer S, Nijhof AM, Silaghi C. Tick species identification and molecular detection of tick-borne pathogens in blood and ticks collected from cattle in Egypt. Tick Tick-born Dis. (2021) 12:101676. doi: 10.1016/j.ttbdis.2021.101676
10. Salman D, Sivakumar T, Otgonsuren D, Mahmoud ME, Elmahallawy EK, Khalphallah A, et al. Molecular survey of Babesia, Theileria, Trypanosoma, and Anaplasma infections in camels (Camelus dromedarius) in Egypt. Parasitol Int. (2022) 90:102618. doi: 10.1016/j.parint.2022.102618
11. Soliman AM, Mahmoud HYA, Hifumi T, Tanaka T. Discovery of Colpodella spp. in ticks (Hyalomma dromedarii) infesting camels in southern Egypt. Ticks Tick-Borne Dis. (2024) 5:102352. doi: 10.1016/j.ttbdis.2024.102352
12. Kuvardina ON, Leander BS, Aleshin VV, Myl'Nikov AP, Keeling PJ, Simdyanov TG. The phylogeny of colpodellids (Alveolata) using small subunit rRNA gene sequences suggests they are the free-living sister group to apicomplexans. J Eukaryot Microbiol. (2002) 49:498–504. doi: 10.1111/j.1550-7408.2002.tb00235.x
13. Leander BS, Keeling PJ. Morphostasis in alveolate evolution. Trends Ecol Evol. (2003) 18:395–402. doi: 10.1016/S0169-5347(03)00152-6
14. Sam-Yellowe TY, Addepalli K, Yadavalli R, Peterson JW. New trichrome stains identify cysts of Colpodella spp. (Apicomplexa) and Bodo caudatus. Int J Microbiol. (2020) 23:303–11. doi: 10.1007/s10123-019-00104-1
15. Yuan CL, Keeling PJ, Krause PJ, Horak A, Bent S, Rollend L, et al. Colpodella spp-like parasite infection in woman, China. Emerg Infect Dis. (2012) 18:125–7. doi: 10.3201/eid1801.110716
16. Matsimbe AM, Magaia V, Sanches GS, Neves L, Noormahomed E, Antunes S, et al. Molecular detection of pathogens in ticks infesting cattle in Nampula province, Mozambique. Exp Appl Acarol. (2017) 3:91–102. doi: 10.1007/s10493-017-0155-5
17. Jiang JF, Jiang RR, Chang QC, Zheng YC, Jiang BG, Sun Y, et al. Potential novel tick-borne Colpodella species parasite infection in patient with neurological symptoms. PLoS Negl Trop Dis. (2018) 12:e0006546. doi: 10.1371/journal.pntd.0006546
18. Xu M, Hu Y, Qiu H, Wang J, Jiang J. Colpodella sp. (Phylum Apicomplexa) identified in horses shed light on its potential transmission and zoonotic pathogenicity. Front Microbiol. (2022) 13:857752. doi: 10.3389/fmicb.2022.857752
19. Chiu HC, Sun X, Bao Y, Fu W, Lin K, Chen T, et al. Molecular identification of Colpodella spp. of South China tiger Panthera tigris amoyensis (Hilzheimer) in the Meihua Mountains, Fujian, China. Folia Parasitol. (2022) 69:28. doi: 10.14411/fp.2022.019
20. AL-Hosary A, Ahmed L, Ahmed J, Nijhof A, Clausen PH. Epidemiological study on tropical theileriosis (Theileria annulata infection) in the Egyptian Oases with special reference to the molecular characterization of Theileria spp. Ticks Tick Borne Dis. (2018) 9:1489–93. doi: 10.1016/j.ttbdis.2018.07.008
21. Estrada-Peña A, Bouattour A, Camicas JL, Walker AR. Ticks of Domestic Animals in the Mediterranean Region. Zaragoza: University of Zaragoza (2004).
22. Apanaskevich DA, Schuster AL, Horak IG. The genus Hyalomma: VII redescription of all parasitic stages of H (Euhyalomma) dromedarii and H (E) schulzei (Acari: Ixodidae). J Med Entomol. (2008) 45:817–31. doi: 10.1093/jmedent/45.5.817
23. Kwak ML. The introduction and subsequent extinction of the camel tick Hyalomma (Euhyalomma) dromedarii (Acari, Ixodidae) in Australia, with a review of the introduction of foreign ticks to Australia. Exp Appl Acarol. (2018) 74:329–33. doi: 10.1007/s10493-018-0218-2
24. Okely M, Bakkes DK, Chitimia-Dobler L. Morphological abnormalities in Hyalomma dromedarii and Hyalomma rufipes (Acari: Ixodidae) collected from dromedary camels (Camelus dromedarius) in Aswan, Egypt. Exp Appl Acarol. (2022) 88:225–41. doi: 10.1007/s10493-022-00747-2
25. Black WC, Piesman J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rRNA sequences. Proc Natl Acad Sci USA. (1994) 91:10034–8. doi: 10.1073/pnas.91.21.10034
26. Galay R, Manalo A, Dolores SL, Aguilar I, Sandalo-De Ramos KA, Cruz K, Divina B, Andoh M, Masatani T, Tanaka T. Molecular detection of tick-borne pathogens in canine population and Rhipicephalus sanguineus (sensu lato) ticks from southern Metro Manila and Laguna, Philippines. Parasit Vectors. (2018) 11:643. doi: 10.1186/s13071-018-3192-y
27. Terkawi MA, Huyen NX, Shinuo C, Inpankaew T, Maklon K, Aboulaila M, et al. Molecular and serological prevalence of Babesia bovis and Babesia bigemina in water buffaloes in the northeast region of Thailand. Vet Parasitol. (2011) 178:201–7. doi: 10.1016/j.vetpar.2011.01.041
28. d'Oliveira C, van der Weide M, Habela MA, Jacquiet P, Jongejan F. Detection of Theileria annulata in blood samples of carrier cattle by PCR. J Clin Microbiol. (1995) 33:2665–2669. doi: 10.1128/jcm.33.10.2665-2669.1995
29. Kumar S, Stecher G, Li M, Knyaz C, Tamura K, MEGA X. Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. (2018) 35:1547–9. doi: 10.1093/molbev/msy096
30. AL-Hosary AAT. Comparison between conventional and molecular methods for diagnosis of bovine babesiosis (Babesia bovis infection) in tick infested cattle in Upper Egypt. J Parasit Dis. (2017) 41:243–6. doi: 10.1007/s12639-016-0785-2
31. Paternina LE, Verbel-Vergara D, Bejarano EE. Comparison of 16S and COX1 genes mitochondrial regions and their usefulness for genetic analysis of ticks (Acari: Ixodidae). Biomedica. (2016) 36:295–302. doi: 10.7705/biomedica.v36i2.3116
32. Lv J, Wu S, Zhang Y, Chen Y, Feng C, Yuan X, et al. Assessment of four DNA fragments (COI, 16S rDNA, ITS2, 12S rDNA) for species identification of the Ixodida (Acari: Ixodida). Parasit Vectors. (2014) 7:93. doi: 10.1186/1756-3305-7-93
33. Abdullah HHA, El-Molla A, Salib FA, Allam NAT, Ghazy AA, Abdel-Shafy S. Morphological and molecular identification of the brown dog tick Rhipicephalus sanguineus and the camel tick Hyalomma dromedarii (Acari: Ixodidae) vectors of rickettsioses in Egypt. Vet World. (2016) 9:1087–101. doi: 10.14202/vetworld.2016.1087-1101
34. Boyer PH, Almeras L, Plantard O, Grillon A, Talagrand-Reboul E, McCoy K, et al. Identification of closely related Ixodes species by protein profiling with MALDI-TOF mass spectrometry. PLoS ONE. (2019) 14:e0223735. doi: 10.1371/journal.pone.0223735
35. Nasreldin N, Ewida R, Hamdon H, Elnaker Y. Molecular diagnosis and biochemical studies of tick-borne diseases (anaplasmosis and babesiosis) in Aberdeen Angus cattle in New Valley, Egypt. Vet World. (2020) 13:1884–91. doi: 10.14202/vetworld.2020.1884-1891
36. Abdullah HHAM, Amanzougaghene N, Dahmana H, Louni M, Raoult D, Mediannikov O. Multiple vector-borne pathogens of domestic animals in Egypt. PLoS Negl Trop Dis. (2021) 15:e0009767. doi: 10.1371/journal.pntd.0009767
37. Mahmoud HYAH, Rady AA, Tanaka T. Molecular detection and characterization of Theileria annulata, Babesia bovis, and Babesia bigemina infecting cattle and buffalo in southern Egypt. Parasite Epidemiol Control. (2024) 1:e00340. doi: 10.1016/j.parepi.2024.e00340
38. Adham F. Abd-el-Samie E, Gabre R, El-Hussein H. Detection of tick blood parasites in Egypt using PCR assay, Babesia bovis and Babesia bigemina. Parasitol Res. (2009) 105:721–30. doi: 10.1007/s00436-009-1443-8
39. Al-Hosary AAT. Loop-mediated isothermal amplification (LAMP) assay for diagnosis of bovine babesiosis (Babesia bovis infection) in Egypt. J Adv Vet Res. (2017) 7:71–4.
40. Fereig F, Mohamed S, Mahmoud H, AbouLaila M, Guswanto A, Nguyen T, et al. Seroprevalence of Babesia bovis, bigemina, Trypanosoma evansi, and Anaplasma marginale antibodies in cattle in southern Egypt. Ticks Tick Borne Dis. (2017) 8:125–31. doi: 10.1016/j.ttbdis.2016.10.008
41. Elsify A, Sivakumar T, Nayel M, Salama A, Elkhtam A, Rizk M, et al. An epidemiological survey of bovine Babesia and Theileria parasites in cattle, buffaloes, and sheep in Egypt. Parasit Int. (2015) 64:79–85. doi: 10.1016/j.parint.2014.10.002
42. Rizk M, Salama A, El-Sayed S, Elsify A, El-Ashkar M, Ibrahim H, et al. Animal level risk factors associated with Babesia and Theileria infections in cattle in Egypt. Acta Parasitol. (2017) 62:796–804. doi: 10.1515/ap-2017-0096
43. El-Dakhly KM, Arafa WM, Soliman S, Abdel-Fatah OR, Wahba AA, Esteve-Gasent MD, et al. Molecular detection, phylogenetic analysis, and genetic diversity of Theileria annulata, Babesia bigemina, and Anaplasma marginale in cattle in three districts of Egypt. Acta Parasitol. (2020) 65:620–7. doi: 10.2478/s11686-020-00189-z
Keywords: Colpodella spp., Babesia bovis, Theileria spp., Hyalomma dromedarii, Hyalomma marginatum, Rhipicephalus annulatus, cattle, Egypt
Citation: Soliman AM, Mahmoud HYAH, Amer MM, Mohamed S, Hifumi T, Tsukiyama-Kohara K and Tanaka T (2025) First detection of Colpodella spp. in Rhipicephalus annulatus and molecular characterization of piroplasmids in southern Egypt. Front. Vet. Sci. 12:1617204. doi: 10.3389/fvets.2025.1617204
Received: 24 April 2025; Accepted: 21 May 2025;
Published: 18 June 2025.
Edited by:
Nicola Pugliese, University of Bari Aldo Moro, ItalyReviewed by:
Ahmed Kamal Dyab, Assiut University, EgyptArchile Paguem, University of Buea, Cameroon
Copyright © 2025 Soliman, Mahmoud, Amer, Mohamed, Hifumi, Tsukiyama-Kohara and Tanaka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tetsuya Tanaka, dGV0c3V5YS50YW5ha2EuYTNAdG9ob2t1LmFjLmpw
 Ahmed M. Soliman
Ahmed M. Soliman Hassan Y. A. H. Mahmoud
Hassan Y. A. H. Mahmoud Moaz M. Amer
Moaz M. Amer Samah Mohamed
Samah Mohamed Tatsuro Hifumi6
Tatsuro Hifumi6 Kyoko Tsukiyama-Kohara
Kyoko Tsukiyama-Kohara Tetsuya Tanaka
Tetsuya Tanaka