- 1Department of Animal Medicine, Production and Health, University of Padua, Legnaro, Italy
- 2Department of Reproduction and Artificial Insemination, Faculty of Veterinary Medicine, Ankara University, Ankara, Türkiye
Oviduct represents the original place of fertilisation and early embryo development in all the domestic animals. In past time it has been considered a mere channel but new reproductive biotechnology approaches suggested the need of structurally and functionally efficient oviductal environment for in vitro embryo production. Recreating the oviductal microenvironment in IVP systems represents a paradigm shift in reproductive biotechnology. By incorporating reproductive fluids and utilising advanced 3D culture models could be reduced adverse IVP outcomes, and bring assisted reproduction closer to its natural counterpart.
The oviduct: more than a mere channel
In vitro embryo production (IVP) technologies often fail to fully replicate the highly dynamic and selective environment of the oviduct, leading to suboptimal outcomes such as reduced embryo quality and developmental anomalies. Can mimicking the oviductal microenvironment resolve these limitations and improve success rates? To answer this question, it is essential to first understand the complex physiological and anatomical characteristics of the oviduct that support natural fertilization and early embryonic development. The oviduct, also known as the salpinx or Fallopian tube in humans, is a highly specialized reproductive organ essential for fertilization and early embryo development. Previously considered a simple conduit for gamete transport, it is now recognized for its crucial roles in sperm selection, capacitation, fertilization, and early embryonic support (1–3).
Anatomically, the oviduct consists of distinct regions. The uterine-tubal junction (UTJ) acts as a selective barrier, allowing capacitated spermatozoa with specific surface markers, such as members of ADAMs (a disintegrin and metalloprotease) protein family, particularly ADAM3 to pass through, thereby ensuring optimal fertilization to occur (4–7). The isthmus serves as a sperm reservoir and a site for capacitation. The ampulla is the primary site of fertilization, where gamete fusion occurs. The infundibulum captures the ovulated oocyte via fimbriae and directs it into the oviduct. These regions exhibit specialized structures that optimize reproductive processes. The isthmus’s narrow, convoluted structure facilitates sperm storage, while the ampulla’s wider lumen and folded mucosa create an ideal environment for fertilization and early embryonic development (1, 2). These region-specific adaptations raise the question: what if such in vivo conditions could be replicated in vitro to improve embryo quality?
This mini-review aims to summarize the key structural and functional features of the oviduct, highlight the limitations and pathological consequences of current IVP protocols, and discuss emerging strategies that aim to mimic in vivo conditions through the integration of reproductive fluids (RFs), extracellular vesicles (EVs), and three dimensional (3D) culture systems. The reviewed literature includes studies conducted on humans, livestock species (primarily bovine and porcine), and laboratory animals (notably mice and rabbits). The relevance and limitations of animal models in representing human reproductive processes are also briefly addressed.
Oviductal cells and their functions
The oviductal epithelium consists of ciliated cells and secretory cells. Ciliated cells play a pivotal role in gamete transport by generating directional flow through rhythmic ciliary beating. This motility is tightly regulated by hormonal cues: estrogen significantly enhances ciliary beat frequency and promotes ciliogenesis, while progesterone downregulates this activity during the luteal phase (8). In parallel, estrogen also stimulates the proliferation and activity of secretory cells, increasing the synthesis and release of oviductal fluid (OF), which provides biochemical support for sperm capacitation, fertilization, and early embryo development. The composition of OF dynamically changes in response to ovarian signals, mainly steroids such as 17 beta-estradiol (E2) and progesterone (P4), and uterine signals; in fact the vascular supply of the oviduct, via ovarian and uterine arteries, ensures communication between oviduct, ovary, and uterus through exchanging of metabolites, hormones and signaling molecules, modulating the reproductive microenvironment (9, 10).
Oviductal fluid: a crucial medium for gametes and embryo
Oviductal fluid is a complex biochemical medium containing nutrients, enzymes, hormones, and signaling molecules (3, 11). Its movement is governed by the activity of the secretory cells, predominantly in the isthmus, ciliary beating, and oviductal peristalsis. These movements are not only mechanical but also under precise hormonal regulation. The release of oviductal fluid is hormonally regulated estrogen enhances the activity of secretory and ciliated cells, promoting fluid production and ciliary beating, while progesterone generally downregulates secretion during the luteal phase. Estrogen and progesterone influence oviductal peristalsis and fluid dynamics, which in turn facilitate gamete interaction and synchronize embryo transport with uterine implantation window (4, 12–16). Prior to fertilization, OF undergoes key modifications that facilitate gamete interaction and fertilization. Changes in viscosity regulate sperm motility, while the release of chemotactic agents guides sperm toward the oocyte. Viscosity greatly influences the chemotaxis of sperm in the oviduct by altering their motility and facilitating navigation through viscous fluids. Sperm exhibit increased linear and progressive movements in high-viscous fluid compared to the low viscosity environments. The interplay between change of viscosity, flow (rheotaxis) and temperature (thermotaxis) seem to enhance the energetic efficiency sperm motility (3). Thermotaxis, which relies on temperature gradients, aids sperm migration to the ampulla (9). Polyspermy is regulated through zona pellucida modifications (cortical/zona reaction) and the release of repellent-like molecules (i.e., osteopontin) immediately after fertilization by the oviduct. Emerging studies highlight the impact of OF on epigenetic modulation, potentially mitigating IVP-associated anomalies (1, 17). Addressing these challenges requires media formulations that are dynamic and customized to each developmental stage, possibly using bioengineered substrates and time-controlled factor release systems.
Negative outcomes of IVP: the need for more natural conditions
Despite technological advancements, IVP techniques such as ovum pick-up (OPU) and intracytoplasmic sperm injection (ICSI) still struggle to match natural fertilization success rates (18–21). Common negative outcomes include large offspring syndrome (LOS), which is characterized by macrosomia, dystocia, prolonged gestation, and congenital abnormalities and is due to perturbation of the environment the embryo has to face with (18, 22, 23). One of the main known environmental disruptors is serum, accelerating embryo development and increasing the number of blastocysts produced but reducing their quality (23–25). Metabolic dysregulation is another concern, leading to altered glucose metabolism, increased systolic blood pressure, and higher adiposity (26, 27). Epigenetic alterations are also observed, causing deviations in gene expression that affect long-term health (22, 28). Bovine LOS, analogous to Beckwith-Wiedemann syndrome in humans, underscores the importance of optimizing IVP conditions to minimize epigenetic disruptions (17, 28). During the IVP there is the need of a continuous equilibrium, if developmental speed is prioritized the embryo’s quality will be affected (Figure 1). In future research, reducing reliance on serum-based media and developing dynamic, stage-specific alternatives may improve outcomes.
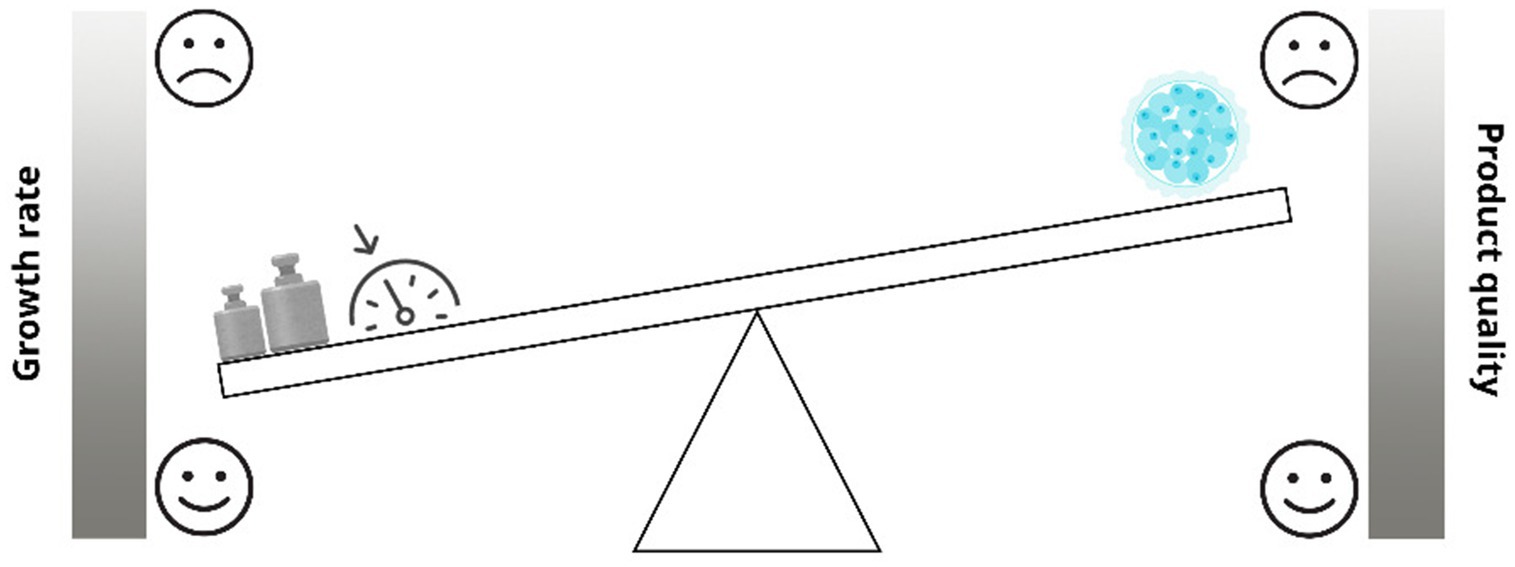
Figure 1. Impact of accelerated in vitro conditions on embryo quality: a developmental trade-off. As shown, during the IVP there is the need of a continuous equilibrium, if developmental speed is prioritized the embryo’s quality will be affected.
Mimicking in vivo conditions: the role of reproductive fluids and organoids
To enhance in vitro production (IVP) outcomes, researchers are exploring the addition of reproductive fluids to IVP media to improve embryo viability and reduce aberrant growth patterns (29). Mimicking natural conditions in in vitro cultured systems is a key factor to obtain high-quality embryos. This can be achieved by supplementing the culture media with different nutritive factors (such as proteins) or by co-culturing embryos with oviductal epithelial cells, which has demonstrated beneficial effects on embryo development (30).
To better resemble the natural environment of the embryo, reproductive fluids (RFs), such as oviductal fluid (OF) and uterine fluid (UF), have been added to in vitro systems. At low concentrations, RFs support embryo development and improve quality: OF has been shown to enhance embryo cryotolerance and upregulate the expression of development-related genes, while UF exhibits antioxidant properties (24, 31, 32). However, higher concentrations may exert dose-dependent adverse effects.
RF supplementation aims to reproduce the complex maternal-conceptus communication that occurs in vivo, which involves multiple “omics” pathways, paracrine/autocrine signals, and stage-specific molecular cues. These interactions are dynamically influenced by the presence of gametes and embryos and evolve throughout development (33–35). Therefore, the culture medium must be tailored to match the changing needs of the developing embryo.
Recent attention has also focused on extracellular vesicles (EVs) found within RFs. EVs differ between oviductal regions and act as key messengers in embryo–maternal communication. Studies suggest that supplementing in vitro culture systems with OF-derived EVs can enhance embryo quality and/or development by influencing, among other, gene expression and lipid metabolism (25, 36).
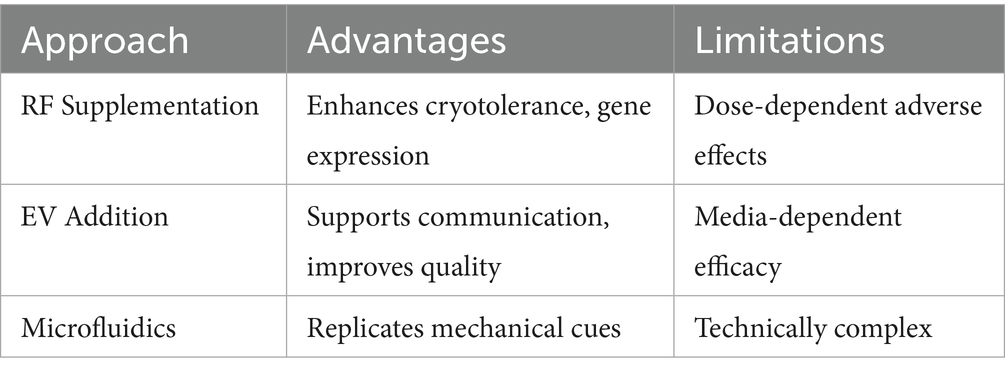
Additionally, mimicking natural mechanical stimulation in the oviduct is gaining interest. Innovative culture systems that simulate physical forces using tilting platforms, microfluidics, vibration systems, or soft substrate materials have shown promise in replicating the biomechanical environment of the oviduct (35).
A summary of these approaches is presented in Table 1.
Advancements in 3D culture systems
Unlike 2D cultures, which rapidly lose cellular differentiation and function, 3D cultures maintain essential characteristics such as polarity, ciliary activity, and secretory function resembling in vivo conditions (9). Embryonic development is enhanced with improved blastocyst formation rates and a more favorable inner cell mass to trophectoderm ratio (37). Organoids are composed of key components, including adult stem cells (ASCs) or induced pluripotent stem cells (iPSCs), an extracellular matrix (ECM) that provides structural support, and growth factors that maintain cellular differentiation and function (38). Future directions in this field include 3D bioprinting to recreate oviductal microstructures and the use of microfluidic systems to simulate dynamic hormonal and gamete interactions (39). For instance, Belda-Perez et al. (37) demonstrated that 3D-printed culture materials can improve the performance and biocompatibility of in vitro embryo development systems.
Challenges and limitations
Current models cannot fully mimic complex parameters such as sperm selection by uterine-tubal junction (UTJ), time-dependent EV changes, or dynamic hormonal fluctuations. Ethical and biosafety implications are underdeveloped: potential risks include immunological reactions and regulatory concerns related to using biological fluids or stem-cell derived structures in vitro.
Translational models
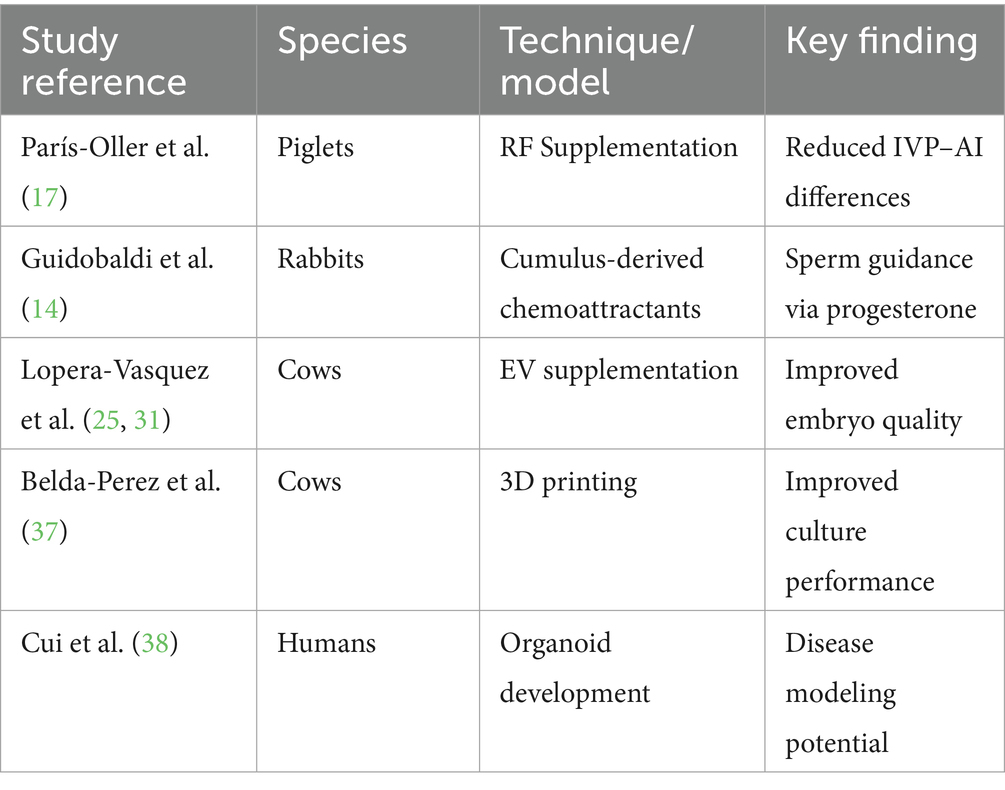
A table summarizing the current advances per species (see Table 2) has been provided to highlight translational opportunities. Ethical frameworks and cost–benefit analyses will be essential to transition these innovations from laboratory settings to routine clinical or production applications.
Integration with artificial intelligence (AI)
Many types of AI models have been proposed for the optimization of in vitro embryo productions. Despite the undoubtable value of the algorithms created to reduce the subjectivity of embryo assessment, shortening the training curve for new embryologists, and improving consistency across practitioners not yet shared AI models are available. To validate the performance of an AI model in a clinical context and to reveal any improvements over current practices are necessary randomized controlled trials (40–42).
Conclusion
Recreating the oviductal microenvironment in in vitro production (IVP) systems represents a major advancement in reproductive biotechnology. However, calling it a “paradigm shift” requires further substantiation—perhaps in reference to changing how future embryo technologies are conceptualized and regulated. To bring assisted reproduction closer to its natural counterpart, future research should prioritize the standardization of reproductive fluid supplementation (25), scalable organoid systems (38), and the validation of microfluidic technologies under field conditions (17, 37).
To date, the integration of biophysical cues, molecular signals, and cellular architecture in IVP remains fragmented. Coordinated multi-disciplinary efforts are needed to create fully biomimetic systems that reflect the temporal and spatial complexity of the oviductal environment.
In addition, creating species-specific protocols and cross-comparison models using data from bovine, porcine, rabbit, and human studies will be critical to fine-tuning these technologies.
Author contributions
ED: Investigation, Writing – review & editing, Writing – original draft. KT: Investigation, Supervision, Writing – review & editing, Writing – original draft. CS: Methodology, Writing – original draft, Supervision, Conceptualization, Writing – review & editing, Project administration.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Coy, P, García-Vázquez, FA, Visconti, PE, and Avilés, M. Roles of the oviduct in mammalian fertilization. Reproduction. (2012) 144:649–60. doi: 10.1530/REP-12-0279
2. Varner, DD. Odyssey of the spermatozoon. Asian J Androl. (2015) 17:522–8. doi: 10.4103/1008-682X.153544
3. Giojalas, LC, and Guidobaldi, HA. Getting to and away from the egg, an interplay between several sperm transport mechanisms and a complex oviduct physiology. Mol Cell Endocrinol. (2020) 518:110954. doi: 10.1016/j.mce.2020.110954
4. Nishimura, H, Kim, E, Nakanishi, T, and Baba, T. Possible function of the ADAM1a/ADAM2 fertilin complex in the appearance of ADAM3 on the sperm surface. J Biol Chem. (2004) 279:34957–62. doi: 10.1074/jbc.M314249200
5. Yamaguchi, R, Yamagata, K, Ikawa, M, Moss, SB, and Okabe, M. Aberrant distribution of ADAM3 in sperm from both angiotensin-converting enzyme (ace)- and calmegin (Clgn)-deficient mice. Biol Reprod. (2006) 75:760–6. doi: 10.1095/biolreprod.106.052977
6. Tokuhiro, K, Ikawa, M, Benham, AM, and Okabe, M. Protein disulfide isomerase homolog PDILT is required for quality control of sperm membrane protein ADAM3 and male fertility. Proc Natl Acad Sci USA. (2012) 109:3850–5. doi: 10.1073/pnas.1117963109
7. Mahé, C, Zlotkowska, AM, Reynaud, K, Tsikis, G, Mermillod, P, Druart, X, et al. Sperm migration, selection, survival, and fertilizing ability in the mammalian oviduct. Biol Reprod. (2021) 105:317–31. doi: 10.1093/biolre/ioab105
8. Hunter, RHF. Components of oviduct physiology in eutherian mammals. Biol Rev. (2012) 87:244–55. doi: 10.1111/j.1469-185X.2011.00196.x
9. Ferraz, MAMM, Henning, HHW, Stout, TAE, Vos, PLAM, and Gadella, BM. Designing 3-dimensional in vitro oviduct culture systems to study mammalian fertilization and embryo production. Ann Biomed Eng. (2017) 45:1731–44. doi: 10.1007/s10439-016-1760-x
10. Lamya, J, Liereb, P, Pianosb, A, Aprahamianb, F, Mermilloda, P, and Saint-Dizier, M. Steroid hormones in bovine oviductal fluid during the estrous cycle. Theriogenology. (2016) 86:1409–20. doi: 10.1016/j.theriogenology.2016.04.086
11. Apichela, SA, Argañaraz, ME, Zampini, R, Vencato, J, Miceli, DC, and Stelletta, C. Biochemical composition and protein profile of alpaca (Vicugna pacos) oviductal fluid. Anim Reprod Sci. (2015) 154:79–85. doi: 10.1016/j.anireprosci.2014.12.013
12. Li, S, and Winuthayanon, W. Oviduct: roles in fertilization and early embryo development. J Endocrinol. (2017) 232:R1–R26. doi: 10.1530/JOE-16-0302
13. Hino, T, and Yanagimachi, R. Active peristaltic movements and fluid production of the mouse oviduct: their roles in fluid and sperm transport and fertilization. Biol Reprod. (2019) 101:40–9. doi: 10.1093/biolre/ioz061
14. Guidobaldi, HA, Teves, ME, Uñates, DR, Anastasía, A, and Giojalas, LC. Progesterone from the cumulus cells is the sperm chemoattractant secreted by the rabbit oocyte cumulus complex. PLoS One. (2008) 3:e3040. doi: 10.1371/journal.pone.0003040
15. Zervomanolakis, I, Ott, HW, Hadziomerovic, D, Mattle, V, Seeber, BE, Virgolini, I, et al. Physiology of upward transport in the human female genital tract. Ann N Y Acad Sci. (2007) 1101:1–20. doi: 10.1196/annals.1389.032
16. Wijayagunawardane, MPB, Miyamoto, IA, Cerbito, WA, Acosta, ITJ, Takagi, M, and Sate, K. Local distributions of oviductal estradiol, progesterone, prostaglandins, oxytocin and endothelin-I in the cyclic cow. Theriogenology. (1998) 49:607–18. doi: 10.1016/s0093-691x(98)00011-9
17. París-Oller, E, Soriano-Úbeda, C, Belda-Pérez, R, Sarriás-Gil, L, Lopes, JS, Canha-Gouveia, A, et al. Reproductive fluids, added to the culture media, contribute to minimizing phenotypical differences between in vitro-derived and artificial insemination-derived piglets. J Dev Orig Health Dis. (2022) 13:593–605. doi: 10.1017/S2040174421000702
18. Lazzari, G, Wrenzycki, C, Herrmann, D, Duchi, R, Kruip, T, Niemann, H, et al. Cellular and molecular deviations in bovine in vitro-produced embryos are related to the large offspring syndrome. Biol Reprod. (2002) 67:767–75. doi: 10.1095/biolreprod.102.004481
19. Nava-Trujillo, H, and Rivera, RM. Review: large offspring syndrome in ruminants: current status and prediction during pregnancy. Animal. (2023) 17:100740. doi: 10.1016/j.animal.2023.100740
20. Li, Y, Boadu, F, Highsmith, MR, Hagen, DE, Cheng, J, and Rivera, RM. Allele-specific aberration of imprinted domain chromosome architecture associates with large offspring syndrome. iScience. (2022) 25:104269. doi: 10.1016/j.isci.2022.104269
21. Li, Y, Sena Lopes, J, Coy-Fuster, P, and Rivera, RM. Spontaneous and ART-induced large offspring syndrome: similarities and differences in DNA methylome. Epigenetics. (2022) 17:1477–96. doi: 10.1080/15592294.2022.2067938
22. Chen, Z, Robbins, KM, Wells, KD, and Rivera, RM. Large offspring syndrome a bovine model for the human loss-of-imprinting overgrowth syndrome Beckwith-Wiedemann. Epigenetics. (2013) 8:591–601. doi: 10.4161/epi.24655
23. Young, LE, Sinclair, KD, and Wilmut, I. Large offspring syndrome in cattle and sheep. Rev Reprod. (1998) 3:155–63. doi: 10.1530/ror.0.0030155
24. Hamdi, M, Lopera-Vasquez, R, Maillo, V, Sanchez-Calabuig, MJ, Núnez, C, Gutierrez-Adan, A, et al. Bovine oviductal and uterine fluid support in vitro embryo development. Reprod Fertil Dev. (2018) 30:935–45. doi: 10.1071/RD17286
25. Lopera-Vasquez, R, Hamdi, M, Maillo, V, Gutierrez-Adan, A, Bermejo-Alvarez, P, Angel Ramirez, M, et al. Effect of bovine oviductal extracellular vesicles on embryo development and quality in vitro. Reproduction. (2017) 153:461–70. doi: 10.1530/REP-16-0384
26. Chen, M, Wu, L, Zhao, J, Wu, F, Davies, MJ, Wittert, GA, et al. Altered glucose metabolism in mouse and humans conceived by IVF. Diabetes. (2014) 63:3189–98. doi: 10.2337/db14-0103
27. Cánovas, S, Heras, S, Romero-Aguirregomezcorta, J, Quintero-Moreno, AA, Gadea, J, Coy, P, et al. Metabolic profile and glycemic response in fully-grown sows born using assisted reproductive technologies. Theriogenology. (2024) 230:314–21. doi: 10.1016/j.theriogenology.2024.10.002
28. Brioude, F, Kalish, JM, Mussa, A, Foster, AC, Bliek, J, Ferrero, GB, et al. Clinical and molecular diagnosis, screening and management of Beckwith-Wiedemann syndrome: an international consensus statement. Nat Rev Endocrinol. (2018) 14:229–49. doi: 10.1038/nrendo.2017.166
29. Coy, P, Canovas, S, Mondejar, I, Saavedra, MD, Romar, R, Grullón, L, et al. Oviduct-specific glycoprotein and heparin modulate sperm–zona pellucida interaction during fertilization and contribute to the control of polyspermy. Proc Natl Acad Sci USA. (2008) 105:15809–14. doi: 10.1073/pnas.0804422105
30. Leese, HJ, Baumann, CG, Brison, DR, McEvoy, TG, and Sturmey, RG. Metabolism of the viable mammalian embryo: quietness revisited. Mol Hum Reprod. (2008) 14:667–72. doi: 10.1093/molehr/gan065
31. Lopera-Vasquez, R, Hamdi, M, Maillo, V, Lloreda, V, Coy, P, Gutierrez-Adan, A, et al. Effect of bovine oviductal fluid on development and quality of bovine embryos produced in vitro. Reprod Fertil Dev. (2017) 29:621–9. doi: 10.1071/RD15238
32. Mermillod, P, Dalbiès-Tran, R, Uzbekova, S, Thélie, A, Traverso, J-M, Perreau, C, et al. Factors affecting oocyte quality: who is driving the follicle? Reprod Domest Anim. (2008) 43:393–400. doi: 10.1111/j.1439-0531.2008.01190.x
33. Almiñana, C. Snooping on a private conversation between the oviduct and gametes/embryos. Anim Reprod. (2015) 12:366–74.
34. Saint-Dizier, M, Mahé, C, Reynaud, K, Tsikis, G, Mermillod, P, and Druart, X. Sperm interactions with the female reproductive tract: a key for successful fertilization in mammals. Mol Cell Endocrinol. (2020) 516:110956. doi: 10.1016/j.mce.2020.110956
35. Ferraz, MAMM, Rho, HS, Hemerich, D, Henning, HHW, van Tol, HTA, Hölker, M, et al. An oviduct-on-a-chip provides an enhanced in vitro environment for zygote genome reprogramming. Nat Commun. (2018) 9:4934. doi: 10.1038/s41467-018-07119-8
36. Almiñana, C, Corbin, E, Tsikis, G, Alcântara-Neto, AS, Labas, V, Reynaud, K, et al. Oviduct extracellular vesicles protein content and their role during oviduct–embryo cross-talk. Reproduction. (2017) 154:153–68. doi: 10.1530/REP-17-0054
37. Belda-Perez, R, Heras, S, Cimini, C, Romero-Aguirregomezcorta, J, Valbonetti, L, Colosimo, A, et al. Advancing bovine in vitro fertilization through 3D printing: the effect of the 3D printed materials. Front Bioeng Biotechnol. (2023) 11:1260886. doi: 10.3389/fbioe.2023.1260886
38. Cui, Y, Zhao, H, Wu, S, and Li, X. Human female reproductive system organoids: applications in developmental biology, disease modelling, and drug discovery. Stem Cell Rev Rep. (2020) 16:1173–84. doi: 10.1007/s12015-020-10039-0
39. de Figueiredo Pessôa, LV, Bressan, FF, and Freude, KK. Induced pluripotent stem cells throughout the animal kingdom: availability and applications. World J Stem Cells. (2019) 11:491–505. doi: 10.4252/wjsc.v11.i8.491
40. Kragh, MF, and Karstoft, H. Embryo selection with artificial intelligence: how to evaluate and compare methods? J Assist Reprod Genet. (2021) 38:1675–89. doi: 10.1007/s10815-021-02254-6
41. Bormann, CL, Thirumalaraju, P, Kanakasabapathy, MK, Kandula, H, Souter, I, Dimitriadis, I, et al. Consistency and objectivity of automated embryo assessments using deep neural networks. Fertil Steril. (2020) 113:781–7. doi: 10.1016/j.fertnstert.2019.12.004
Keywords: oviductal fluid, in vitro production IVP, large offspring syndrome, embryos, extracellular vesicles, 3D cell culture
Citation: Dalle Palle E, Tekin K and Stelletta C (2025) Nature does it better: mimicking in vivo conditions to resolve in vitro production side effects. Front. Vet. Sci. 12:1617740. doi: 10.3389/fvets.2025.1617740
Edited by:
Rosa Maria Garcia-Garcia, Complutense University of Madrid, SpainReviewed by:
Regiane R. Santos, Schothorst Feed Research, NetherlandsKarina Cañón-Beltrán, Complutense University of Madrid, Spain
Copyright © 2025 Dalle Palle, Tekin and Stelletta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enrico Dalle Palle, ZW5yaWNvLmRhbGxlcGFsbGVAcGhkLnVuaXBkLml0
 Enrico Dalle Palle
Enrico Dalle Palle Koray Tekin
Koray Tekin Calogero Stelletta
Calogero Stelletta