- 1Key Laboratory of Clinical Diagnosis and Treatment Techniques for Animal Disease, Ministry of Agriculture, Inner Mongolia Agricultural University, Hohhot, China
- 2Laboratory of Veterinary Clinical Pharmacology, College of Veterinary Medicine, Inner Mongolia Agricultural University, Hohhot, China
- 3Inner Mongolia Bayannaoer City Municipal Center for Disease Control and Prevention, Linhe, China
Introduction: Bovine endometritis is a common postpartum uterine infection that significantly impacts the health and production performance of dairy cows, leading to economic losses for farms. Bovine endometritis is closely associated with pathogenic microorganisms, disturbances in uterine microecology, and localized inflammatory damage. Escherichia coli (E. coli) is the primary pathogenic bacterium responsible for bovine endometritis. Prostaglandin D2 (PGD2) is abundant in the uterine environment. However, its role in E. coli-induced endometritis remains largely unknown. We used bovine bone marrow-derived macrophages (BMDMs) and bovine endometrial tissue to investigate the specific genes and molecular mechanisms involved in E. coli-induced bovine endometritis.
Methods and results: Transcriptomic data show that E. coli infection significantly upregulated 2,141 genes and downregulated 2,381 genes in bovine BMDMs. E. coli activates various molecular functions in bovine BMDMs, with the most closely related being the inflammatory response, in which Prostaglandin-Endoperoxide Synthase 2 (PTGS2) plays a crucial role. Additionally, ELISA analysis revealed that E. coli infection significantly promoted the secretion of PGD2 in BMDMs. In the early stage of infection, ELISA results showed that exogenous PGD2 significantly promoted the secretion of TNF-α, IL-1β, and IL-6 in BMDMs and endometrial tissues, suggesting its role in enhancing the inflammatory response during early infection. Further q-PCR and immunofluorescence analyses demonstrated that PGD2 markedly upregulated the expression of damage-associated molecules, including high mobility group box 1 (HMGB-1) and hyaluronic acid-binding protein 2 (HABP-2). In addition, immunofluorescence and MTT assay results indicated that PGD2 enhanced the intracellular survival of E. coli in macrophages. H&E staining showed that PGD2 exacerbated pathological damage in bovine endometrial tissues. Contrastingly, at later stages, PGD2 suppresses the expression of inflammatory mediators, decreases E. coli survival, and alleviates tissue damage.
Discussion: These results not only deepen our understanding of the multifaceted role of exogenous PGD2 in uterine pathophysiology but also provide potential therapeutic implications for the treatment of bovine endometritis.
1 Introduction
Endometritis is a common bacterial infection affecting the reproductive system of cattle globally. It severely impacts bovine the reproductive efficiency, hinders animal welfare, and can delay or prevent successful pregnancies (1). Escherichia coli (E. coli) is the primary pathogen associated with this condition, with bacterial colonization occurring in the uterus of 80–100% of cows within 2 weeks postpartum (2). The reported incidence of E. coli-induced endometritis during this period is 49.2% (3). Postpartum, the uterus is highly vulnerable to infection by E. coli, leading to increased inflammation triggered by microbe- and damage-associated molecular patterns (DAMPs) (4). E. coli infection also contributes to cell death, tissue damage, and necrosis (5), posing a significant threat to the health and recovery of postpartum cows, while reducing the economic efficiency of dairy farming (6). Consequently, strategies to protect the uterus from E. coli infection are of growing research interest.
At present, the primary clinical approaches for managing bovine endometritis involve the use of antibiotics and non-steroidal anti-inflammatory drugs (NSAIDs) (7, 8). However, long-term or inappropriate antibiotic use can lead to the development of antibiotic-resistant bacterial strains. This resistance not only makes the treatment of subsequent infections more difficult but may also significantly reduce the effectiveness of antibiotics, thereby posing a serious challenge to herd health management (9–11). In addition, the issue of antibiotic residues in meat and dairy products cannot be overlooked. Antibiotic residues may trigger allergic reactions in humans and contribute to the development of antibiotic resistance, thereby weakening the body's immune defense mechanisms and posing a potential threat to human health (12–14). NSAIDs are another commonly used treatment for bovine endometritis, primarily acting by reducing inflammation, alleviating pain, and improving clinical symptoms (15–17). However, NSAIDs inhibit the synthesis of all prostaglandins, which can interfere with the normal tissue repair processes in the uterus and delay the healing of the endometrial lining (18, 19). Consequently, it is crucial to identify novel therapeutic targets that can provide effective treatment while reducing the risk of adverse effects.
The imbalance between infection and self-defense mechanisms results in postnatal reproductive diseases, including puerperal inflammation, clinical endometritis, subclinical endometritis, etc. (20). Endometritis affects over 45% of cows within 3 weeks postpartum, with 15–20% developing clinical endometritis and 30% developing subclinical endometritis (6). Upon E. coli invasion, it adheres to and colonizes the mucosal surface of the endometrium, particularly in areas with tissue damage (21). Endometrial epithelial and mesenchymal cells detect DAMPs via innate immune receptors, triggering the release of cytokines, chemokines, and prostaglandins that amplify the inflammatory response (22). These mediators recruit and activate neutrophils and macrophages, which aid in eliminating pathogens and resolving tissue damage (23). Macrophages play a key role in immune responses, facilitating phagocytosis, clearing infections, and regulating inflammation to promote tissue repair and restore homeostasis (24). Monocytes and macrophages in the bone marrow and peripheral blood primarily originate from hematopoietic stem cells in the bone marrow (25). As primary immune cells, they retain the key physiological functions of tissue-resident macrophages. Moreover, under specific induction conditions, bone marrow-derived macrophages (BMDMs) can be reliably polarized into either M1 or M2 phenotypes (26), making them an ideal in vitro model for studying macrophage-mediated inflammatory responses during E. coli infection in dairy cows. Based on these advantages, BMDMs were selected as the experimental model in this study. Cytokines, chemokines and prostaglandins (PGs) are recognized as key regulators of E. coli-induced endometritis (27). However, the mechanism by which PGs influence this process in bovine endometritis remains unclear.
PGs, members of the eicosanoid family of lipid compounds, play critical roles in regulating inflammation and immune responses. The major PGs include Prostaglandin D2 (PGD2), E2 (PGE2), H2 (PGH2), and I2 (PGI2). PGD2 is synthesized via the catalytic activities of cyclooxygenases and PGD2 synthases in mast cells, macrophages, and other cellular sources (28). PGD2 has been implicated in microbial infections, immunomodulation, inflammation during cancer progression, and renal injury (29). PGD2 mediates its biological effects through two G protein-coupled receptors, DP1 and DP2 (28). However, the pathophysiological role of PGD2 remains controversial. Some studies have highlighted its pro-inflammatory properties, showing that it enhances immune cell chemotaxis and accumulation at inflammation sites, exacerbating conditions such as inflammatory bowel disease, rhinitis, and asthma (30–32). Conversely, other research highlights its anti-inflammatory functions, demonstrating that PGD2 signaling suppresses inflammasome hyperactivation, providing protection against Helicobacter pylori-induced gastritis, acute lung inflammation, and brain inflammation (33–35). Intriguingly, PGD2 exhibits anti-inflammatory effects in colitis, but paradoxically promotes carcinogenesis during its resolution phase (30). Recent studies have also provided insight into PGD2′s dual role in inflammatory conditions. For instance, our research group demonstrated that exogenous PGD2 enhances E. coli induced inflammatory responses in mouse macrophages (36). Additionally, the PGD2-DP1 pathway may exert protective effects against endometritis in dairy cows (37). Hence, the paradoxical role of PGD2 has attracted attention; however, its role in bovine endometritis remains largely unexplored.
Therefore, this study aims to explore novel therapeutic targets, with the goal of providing new strategies for the treatment of bacterial bovine endometritis. To this end, this study employed transcriptomics to select inflammation-related genes associated with E. coli infection in BMDMs. Using bovine BMDMs and endometrial tissue as models, we conducted a comprehensive and systematic investigation of the molecular mechanisms through which PGD2 modulates E. coli-induced bovine endometritis.
2 Materials and methods
2.1 Ethical statement
All animal experiments adhered to the regulations stipulated in the Administration of Affairs Concerning Experimental Animals in China and received approval from the Animal Welfare and Research Ethics Committee of Inner Mongolia Agricultural University (Approval ID: NND2021013).
2.2 Bacterial strains
The E. coli O157:H7 strain used in this study was purchased from Bena Culture Collection (BNCC186579, Beijing, China). A 1 ml suspension of E. coli O157:H7 strain (at a concentration of 1 × 107 CFU) preserved in the laboratory was inoculated into 100 ml of Luria-Bertani (LB) broth (Oxoid, Basingstoke, LTD, UK). Incubate the culture at 37°C with shaking at 200 rpm for 12 h, or until the OD600 of the culture reaches 0.9. The bacterial suspension was serially diluted and plated onto LB agar. After incubation at 37°C for 18 h, colonies were counted, and the concentration was quantified as CFU/ml.
2.3 Infection of BMDMs and treatment in vitro
In this study, bovine rib samples were obtained from healthy adult Holstein cows at the Beiya Slaughterhouse in Hohhot, Inner Mongolia, China. All animals had passed veterinary health inspections and quarantine assessments, and were confirmed to be free of major diseases. Slaughter was conducted solely for commercial food production purposes. After slaughter at the abattoir, cow rib bones were collected immediately post-mortem, placed on ice, and transported to the laboratory for further processing. All bone marrow samples were processed within 1 hour of animal death to preserve cell viability and ensure reproducibility of the results. Bone marrow was extracted from cow ribs by flushing with phosphate-buffered saline (PBS; Hyclone, Logan, UT, USA). The collected cells were centrifuged at 2,900 g for 8 min, and the supernatant was discarded. The cells were subsequently resuspended in erythrocyte lysis buffer and incubated for 5 min to facilitate lysis. The cells were then centrifuged at 1,300 g for 8 min and subsequently cultured in RPMI 1,640 medium supplemented with 20% fetal bovine serum (Hyclone, Logan, UT, USA) and 20 ng/ml M-CSF (Kingfisher Biotech Inc., USA) at 37°C in a 5% CO2 atmosphere. After 7 days, unattached cells were removed, and adherent cells were used for the experiments. To induce M1 macrophages, cells (2 × 106 per well) were treated with 1 μg/ml of lipopolysaccharide for 24 h, followed by an 8-h resting period. M1 BMDMs were confirmed by immunofluorescence (Supplementary Figure 1).
2.4 Experimental animals and treatment
The tissues used in this study were obtained from animals slaughtered at the Beiya Slaughterhouse in Hohhot, Inner Mongolia, China. Prior to slaughter and tissue collection, all animals underwent health screening, including tests for common reproductive diseases. To minimize the potential impact of underlying infections on the study results, only animals that met these health standards were selected. The experiment selected 30 healthy Holstein dairy cows aged 15–18 months, and fresh uterine horn samples were collected (~400 kg). All samples were collected from sexually mature cows in the proestrus phase of the estrous cycle. Endometrial tissue culture was established following the method of Li (5). After rinsing the uterine horns 3 times with PBS containing 100 IU/ml penicillin, 100 IU/ml streptomycin, and 2.5 mg/ml amphotericin B, the uterine horns were incubated at 4°C for 1 h. Under aseptic conditions, the uterine horns of cows were longitudinally incised, and small pieces of endometrial tissue measuring 2 × 2 mm were excised. These explants were then randomly allocated to six-well plates for culture.
2.5 Experimental infection and treatment in vitro
BMDMs were randomly divided into four groups: Control, PGD2-treated, E. coli-infected, and PGD2 + E. coli co-treatment groups. In the PGD2-treated and co-treatment groups, cells were pretreated with PGD2 at a final concentration of 1 × 10−6 M (1 × 10−6 M; Cayman Chemical Company, Ann Arbor, MI, USA) for 24 h prior to infection (see Supplementary Figure 2 for concentration selection). Subsequently, E. coli was added at a multiplicity of infection (MOI) of 5:1 for further experimentation. After 1 h of infection with 100 μg/ml tobramycin to remove extracellular bacteria, the medium was changed to continue the culture (38).
The bovine endometrial tissues were randomly assigned to the following experimental groups: Control, PGD2 (1 × 10−5 M), PGD2 (4 × 10−9 M), E. coli, PGD2 (1 × 10−5 M) + E. coli, and PGD2 (4 × 10−9 M) + E. coli. Prior to infection, tissues in the PGD2 treatment groups were pretreated with the corresponding concentration of PGD2 for 24 h. Subsequently, infection was performed using E. coli at a concentration of 1 × 106 CFU/ml. Each group was set up in three independent wells, with tissue samples obtained from different individual cows to enhance biological reproducibility. In addition, each well was analyzed in triplicate, and the average value was used for subsequent data analysis.
2.6 RNA isolation, library preparation and transcriptome analysis
BMDMs were treated with PGD2 (1 × 10−6 M) for 24 h, followed by infection with E. coli at a MOI of 5:1 for 4 h. Total RNA was isolated from the cells using TRIzol reagent. The RNA concentration and integrity were then evaluated using a NanoDrop spectrophotometer (Thermo Scientific, USA) to confirm that the RNA remained intact. The libraries were constructed following the manufacturer's instructions, after which transcriptome sequencing was performed and the data analyzed by OE Biotech Co (Shanghai, China). Gene counts for each sample were normalized using DESeq2 software, with expression levels estimated based on the baseMean value. The fold changes were calculated, and significance was assessed using a negative binomial distribution test (NB test). The differentially expressed genes (DEGs) selected met the criteria of |log2 Fold Change| > 1.5 and a significance P-value < 0.05. Significantly enriched genes and related pathways were identified through GO and KEGG enrichment analysis and their main biological functions were explored (with a significance threshold of P < 0.05). Finally, further enrichment analysis of GO and KEGG pathways was conducted using the OECloud tool (https://cloud.oebiotech.com/task/).
2.7 q-PCR
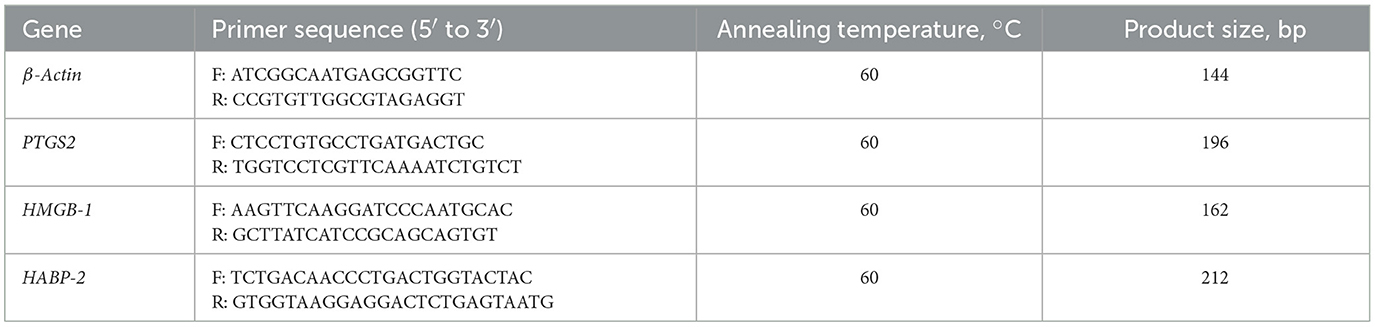
Cells were collected at 2, 4, and 6 h post-E. coli infection. Total RNA was extracted using the Axygen RNA Kit (Axygen Scientific, USA), and RNA was reverse-transcribed into cDNA using the PrimeScript RT kit (Vazyme, Nanjing, China). The mRNA levels of various genes including Prostaglandin-Endoperoxide Synthase 2 (PTGS2), HMGB-1, and HABP-2 were quantified using the SYBR Green Master Mix Kit (Roche Applied Science, Mannheim, Germany). Gene expression was normalized according to actin mRNA levels, and data were analyzed using the 2−ΔΔCt method (39). All primers information is in Table 1.
2.8 Molecular docking analysis
We performed molecular docking analysis of the drug PGD2 with the key target protein PTGDR. After removing all associated ligands, the three-dimensional structures of PGD2 and PTGDR were obtained from PubChem and the Protein Data Bank (PDB), respectively. AutoDock v1.5.7 was then used for ligand preparation, including the removal of water molecules, processing of nonpolar hydrogen atoms, and identification of the active binding site. Docking conformations were calculated using AutoDock Vina, and the optimal docking model was selected. Finally, the key binding amino acids and optimal binding conformation were visualized using the PyMOL molecular graphics system v2.0 in the Python environment (40).
2.9 Enzyme-linked immunosorbent assay
The concentrations of PGD2, TNF-α, IL-1β, IL-6, IL-8 and IL-10 in the supernatants of BMDMs and endometrial tissue were measured using bovine PGD2 (Cayman Chemical Company, Ann Arbor, MI, USA), TNF-α and IL-6 (R&D Systems, Minneapolis, MN, USA), and IL-1β, IL-8, and IL-10 (Kingfisher Biotech, St. Paul, MN, USA) ELISA kits according to the manufacturer's instructions.
2.10 Western blot analysis
Total protein was extracted from treated cells using the M-PER mammalian protein extraction reagent (Thermo Scientific, Waltham, MA, USA), and protein concentrations were determined using the BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA). A total of 10 μg of protein from each sample was separated using a 12% SDS-PAGE gel, and the proteins were subsequently transferred to a polyvinylidene fluoride (PVDF) membrane for Western blot analysis. Subsequently, the membrane was blocked with StartingBlock™ (TBS) blocking buffer (Thermo Fisher, MA, USA) at room temperature for 1 h, followed by incubation with primary antibodies at 4°C for 14 h. The primary antibodies employed were antiphospho-ERK, anti-ERK, antiphospho-p38, anti-p38, antiphospho-NF-κB p65, anti-NF-κB p65 (Cell Signaling Technology, 1:1,000 dilution), and anti-GAPDH (1:10,000) monoclonal antibodies. Immunoreactive bands were visualized via chemiluminescence using horseradish peroxidase-conjugated secondary anti-rabbit and anti-mouse antibodies along with chemiluminescent substrate (Thermo Scientific). The band density on the blots was quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.11 Cell viability assay
Cell viability and intracellular E. coli survival were assessed via the MTT assay. BMDMs were seeded in 96-well plates at a density of 1 × 104 cells per well, with 180 μl of culture medium added to each well, and cultured at 37°C in 5% CO2. After 24 h of PGD2 treatment, cells were infected with E. coli at an MOI of 5:1 for 2.5 and 6 h. Cell viability was evaluated using the MTT assay following the guidelines provided by the manufacturer (Solarbio, Beijing, China).
2.12 Phagocytosis and bacterial killing of E. coli by BMDMs
To investigate the impact of PGD2 on phagocytosis and bacterial killing, BMDMs were cultured at a density of 2 × 106 cells per 35 mm glass-bottom Petri dish. After treatment, cells were labeled with 8 μm 11′-dioctadecyl-3,33′3′-tetramethylindocarbocyanine perchlorate (DiI, Thermo Scientific). After labeling E. coli with Hoechst 33258 dye for 30 mins, BMDMs were infected for 30 mins, 2.5 h, and 6 h at 37°C. The cells were then fixed with 4% paraformaldehyde and imaged using a confocal microscope (LSM 800; Carl Zeiss, Oberkochen, Germany) at × 400 magnification.
2.13 Immunofluorescence and histological analysis
Immunofluorescence analysis of endometrial tissues from dairy cows was performed using established methods (41). After frozen sections, they were incubated overnight at 4°C using primary antibodies (1:100 dilution) to HMGB-1 and HABP-2. After blocking, tissue sections were incubated with donkey anti-rabbit IgG-Alexa Fluor 647 fluorescent secondary antibody (1:1000 dilution; Abcam) for 1 h in the dark. Imaging and quantification of fluorescence intensity were conducted using confocal microscopy (LSM 800, Zeiss, Oberkochen, Germany) at × 400 magnification.
Sections were made by dehydration through an alcohol gradient (70%, 80%, 90%, 100%) and paraffin embedding.These sections were then stained with hematoxylin and eosin (H&E) and imaged using an Axio Scan Z1 slide scanner (Zeiss, Thornwood, NY, USA). All images were captured under identical conditions.
2.14 Statistical analysis
Data were analyzed using GraphPad Prism 8 (GraphPad Software, CA, USA), and results are presented as mean ± standard deviation (SD). Statistical significance was evaluated using one-way analysis of variance (ANOVA) with Tukey's multiple comparisons test or two-way ANOVA with Bonferroni's post-hoc test, depending on the experimental design. Differences were considered statistically significant when the P value was < 0.05 (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001).
3 Results
3.1 Altered gene levels in E. coli-infected BMDMs
Transcriptome sequencing was performed to identify potential target genes associated with E. coli infection in BMDMs. The principal component analysis (PCA) identified distinct differences among various sample groups based on transcriptome data, with PCA1 and PCA2 accounting for 83.77 % and 12.49 % of the total variation, respectively (Figure 1A). Transcriptomic analysis of E. coli-infected BMDMs identified 4,522 significantly dysregulated genes, including 2,141 upregulated and 2,381 downregulated genes, based on a q-value < 0.05 and |log2 fold change (FC)| > 1 (Figures 1A, B). Gene Ontology (GO) analysis revealed that the most significant differential gene expression was observed in the inflammatory response (Figure 1C). The key genes involved were IL17A, IL17F, TNFAIP6, and PTGS2 (Figure 1D). Among these, we focused on PTGS2, which showed a significant increase in mRNA expression at 2, 4, and 6 h post-infection (Figure 1E, P < 0.01). In E. coli-infected macrophages, the secretion levels of PGD2 were elevated at 6 and 24 h (Figure 1F, P < 0.0001). These results indicate that PGD2 plays a critical role in the response of BMDMs to E. coli infection.
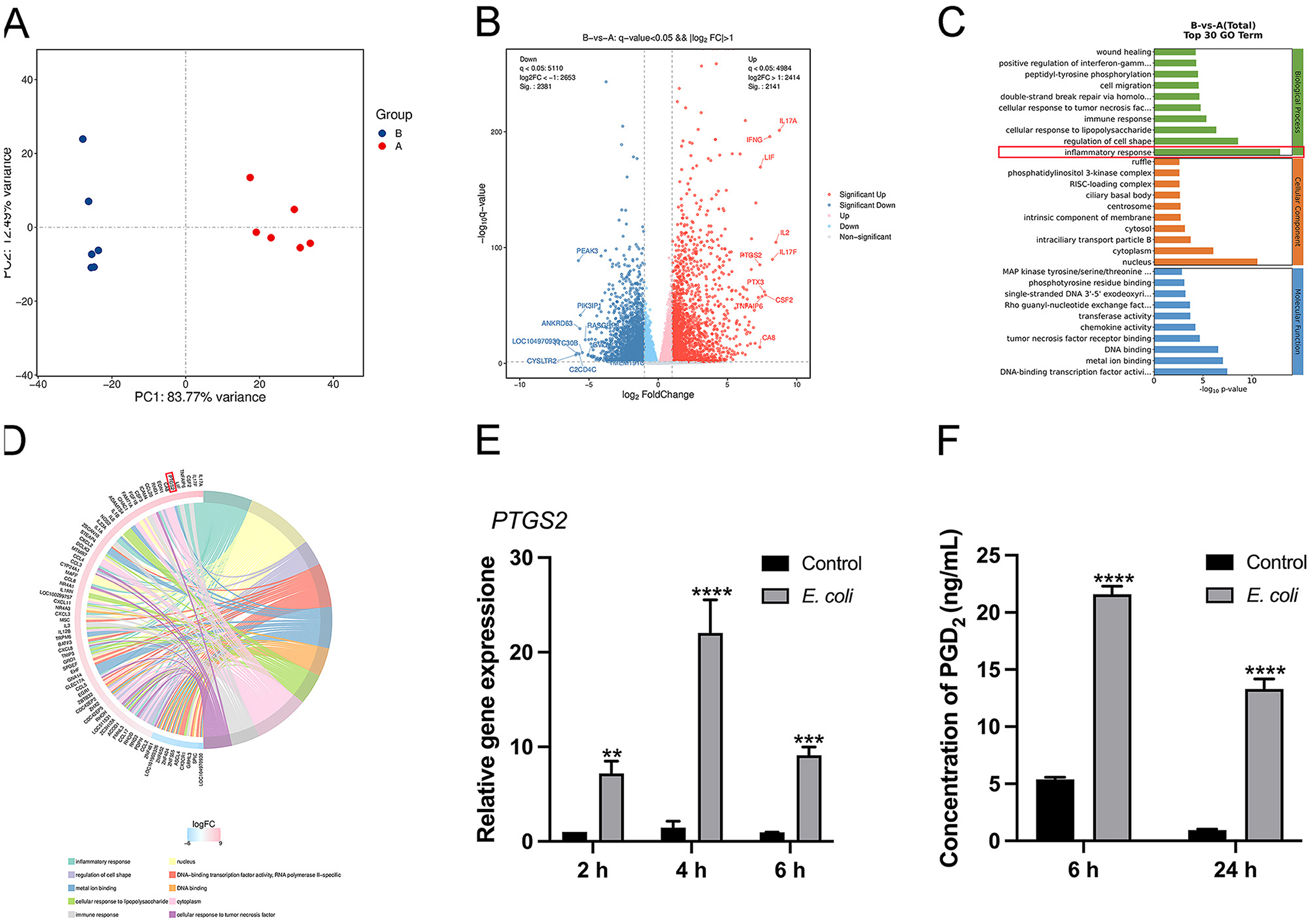
Figure 1. Escherichia coli induces PTGS2 and PGD2 expression in BMDMs of dairy cows. (A) PCA score plots. A: control, B: E. coli. (B–D) The GO enrichment analysis and KEGG enrichment analysis results of genes in E. coli-induced BMDMs. (E) The mRNA expression of PTGS2. (F) Secretion of PGD2. Results were expressed as the mean ± SD of multiple independent experiments and analyzed by two-way ANOVA with Bonferroni's post-test (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
3.2 The effect of the drug PGD2 on BMDMs
Molecular docking analysis of the interaction between PGD2 and the PTGDR-associated protein (PDB ID: 7M8W) revealed that PGD2 forms a stable binding with the PTGDR-associated protein, with a binding energy of −7.9 kcal/mol (Figure 2A). In addition, MTT assay results showed no significant difference in cell viability between PGD2-treated BMDMs for 24 h and the control group, indicating that PGD2 did not significantly impact macrophage viability (Figure 2B). These results indicate that PGD2 binds effectively to PTGDR and does not exert a significant effect on macrophage viability.
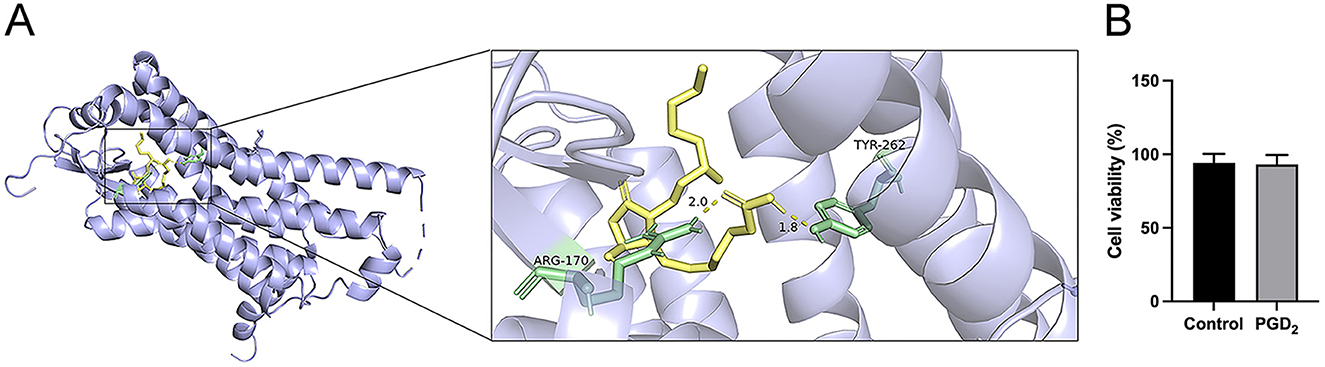
Figure 2. Effect of the drug PGD2 on the activity of BMDMs. (A) The molecular docking results. Amino acids are in pale green, with hydrogen bond distances shown as yellow dashed lines. (B) The effect of PGD2 on BMDM viability.
3.3 Effects of exogenous PGD2 on cytokine and chemokine production and inflammatory pathways during E. coli infection in BMDMs
Based on KEGG enrichment analysis, differentially expressed genes were significantly enriched in multiple inflammation-related signaling pathways, such as TNF signaling pathway, NF-κB pathway, cytokine-cytokine receptor interaction, and MAPK signaling pathway (Figure 3A). Therefore, we measured the secretion of cytokines and chemokine using ELISA and analyzed the activation of NF-κB and MAPK signaling pathways by Western blot. The ELISA results showed that PGD2 pretreatment significantly increased the secretion of TNF-α, IL-1β, IL-6, IL-8, and IL-10 in BMDMs at 6 h post-E. coli infection. However, at 12 and 24 h post-infection, PGD2 significantly downregulated the secretion of TNF-α, IL-1β, and IL-8 compared to the E. coli infection group, while the secretion of IL-6 and IL-10 remained elevated (Figures 3B–F, P < 0.01).
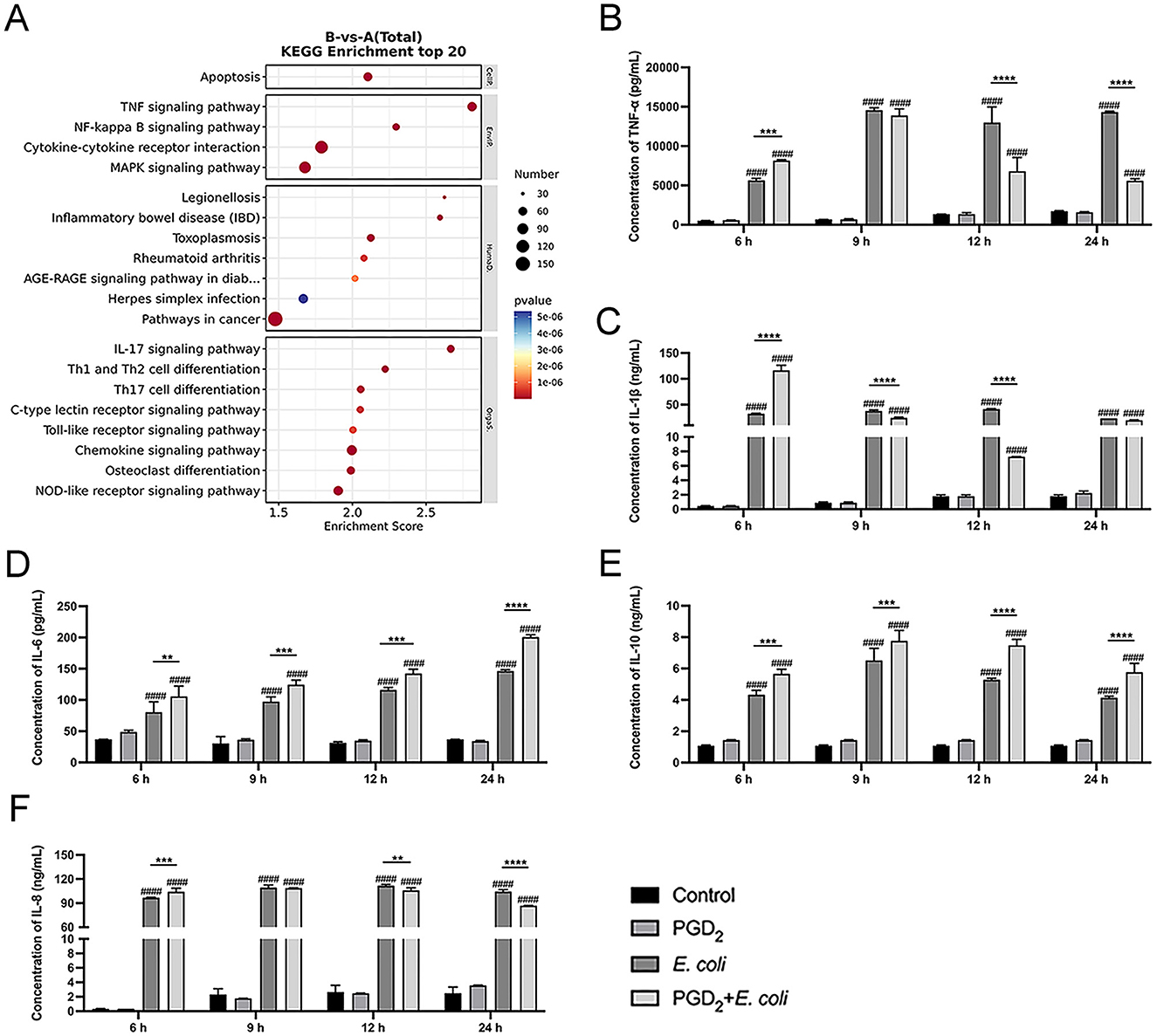
Figure 3. Effect of PGD2 on Escherichia coli induced cytokine secretion in BMDMs. (A) Top 20 KEGG pathway enrichment of upregulated genes in E. coli-infected BMDMs. (B–F) Secretion of TNF-α, IL-1β, IL-6, IL-10 and, IL-8. Results are expressed as mean ± SD of three independent experiments and were analyzed using two-way ANOVA with Bonferroni's post-hoc test. #P < 0.05, ##P < 0.01, ###P < 0.001 and ####P < 0.0001 compared to control group. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 indicate statistically significant differences between two experimental groups.
In addition, PGD2 treatment significantly enhanced the phosphorylation levels of MAPK (ERK, P38) and NF-κB (P65) in E. coli-infected BMDMs at 15 and 30 min (Figure 4, P < 0.01). However, at 60 min post-infection, PGD2 attenuated the phosphorylation levels of MAPK in BMDMs (Figure 4, P < 0.05). These findings suggest that PGD2 may influence the activation of macrophage MAPK and NF-κB signaling pathways during E. coli infection, thereby affecting the secretion of pro-inflammatory cytokines and chemokines.
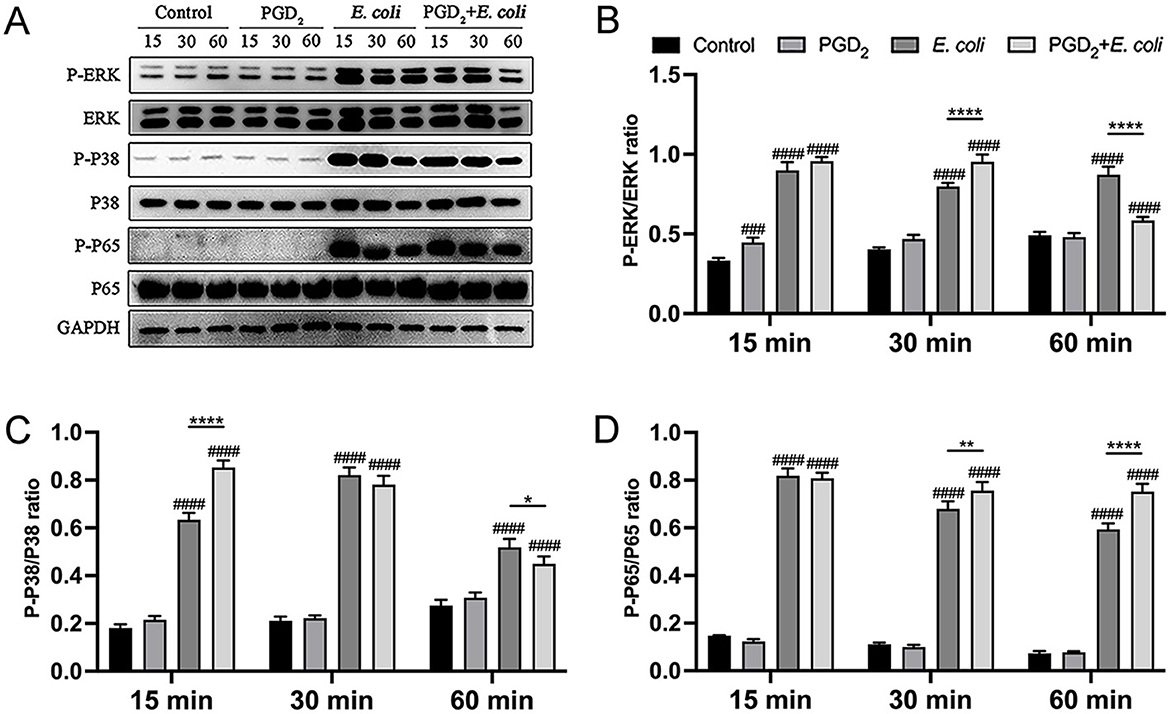
Figure 4. Effect of PGD2 on the activation of BMDMs signaling pathway induced in Escherichia coli. Phosphorylation of ERK, p38, and p65 was assessed by western blotting at 15, 30, and 60 min post-infection, with GAPDH as the loading control. Grayscale values were quantified using ImageJ software. Results are expressed as mean ± SD of three independent experiments and were analyzed using two-way ANOVA with Bonferroni's post-hoc test. #P < 0.05, ##P < 0.01, ###P < 0.001 and ####P < 0.0001 compared to control group. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 indicate statistically significant differences between two experimental groups.
3.4 Impact of exogenous PGD2 on BMDMs phagocytosis and intracellular killing
The effect of PGD2 on the ability of BMDMs to phagocytose and kill E. coli was assessed using Dil-labeled BMDMs and Hoechst-stained E. coli. After 0.5 h post-infection, no significant differences were observed, suggesting that PGD2 does not affect the phagocytic ability of BMDMs (Figures 5A, C). After 2.5 h of infection, the fluorescence intensity of bacteria in the PGD2 was higher in the treatment group than the E. coli infection group, indicating that PGD2 reduced the killing ability of BMDMs. However, after 6 h of infection, the fluorescence intensity of bacteria in the PGD2 treatment group was lower, suggesting that PGD2 enhanced the killing ability of BMDMs against E. coli (Figures 5B, D, P < 0.05). The effect of PGD2 on E. coli survival within macrophages was evaluated through the MTT assay. Consistent with these observations, PGD2 demonstrated a significant increase in the survival of internalized E. coli in macrophages (Figure 5E, P < 0.05). Together, these results suggest that PGD2 decreased intracellular killing in macrophages.
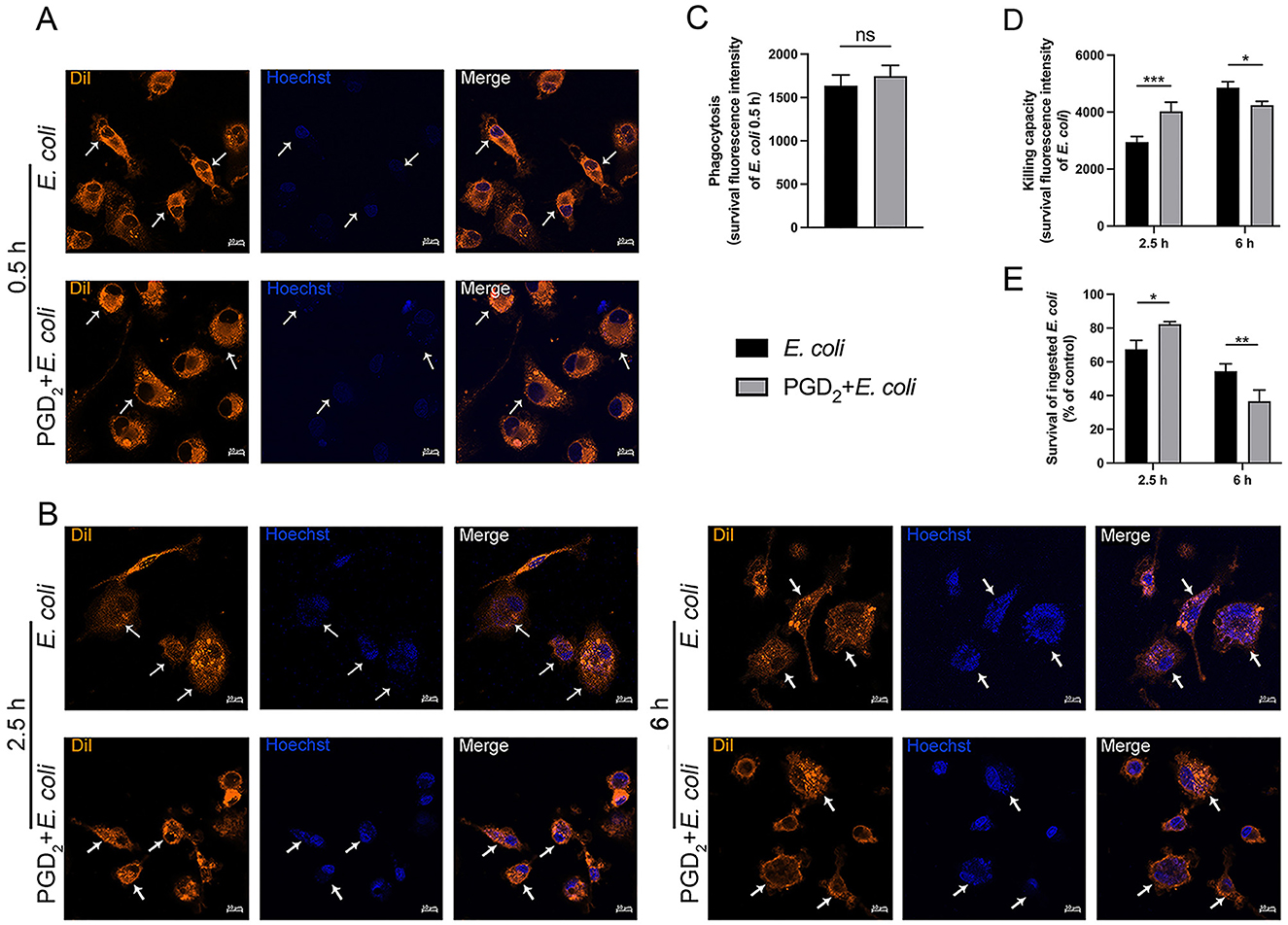
Figure 5. Effect of exogenous PGD2 on BMDMs phagocytosis and intracellular killing of Escherichia coli. (A, B) Phagocytosis and intracellular killing of Hoechst 33258-labeled E. coli (blue) within DiI-labeled BMDMs (orange) were analyzed via microscopy (×400, scale label = 10 μm). (C) The effect of PGD2 on E. coli phagocytosis by BMDMs. (D) The impact of PGD2 on E. coli killing by BMDMs. (E) The bacterial killing capacity of macrophages was quantified by a tetrazolium dye reduction assay. Results were expressed as the mean ± SD of multiple independent experiments and analyzed by two-way ANOVA with Bonferroni's post-test. *P < 0.05, **P < 0.01, ***P < 0.001. ns, not significant.
3.5 Effects of exogenous PGD2 on the expression of DAMPs in BMDMs and endometrial tissues of dairy cows infected with E. coli
To determine the effect of PGD2 on the damage caused by E. coli infection in BMDMs and bovine endometrial tissues, we measured the expression of DAMPs (HMGB-1, HABP-2) using qPCR and immunofluorescence. Compared to the E. coli infection group, PGD2 significantly increased HMGB-1 and HABP-2 mRNA expression levels in BMDMs (Figure 6A, P < 0.05). Immunofluorescence staining of E. coli-infected endometrial explants showed that PGD2 treatment led to an increased expression of HMGB-1 and HABP-2 at 9 h post-infection (Figures 6B–D, P < 0.0001). However, this expression was decreased at 24 h (Figures 6B–6D, P < 0.0001). In conclusion, our data demonstrate that PGD2 modulates the release of HMGB-1 and HABP-2 in E. coli-infected endometritis in dairy cows, exhibiting distinct roles at different times.
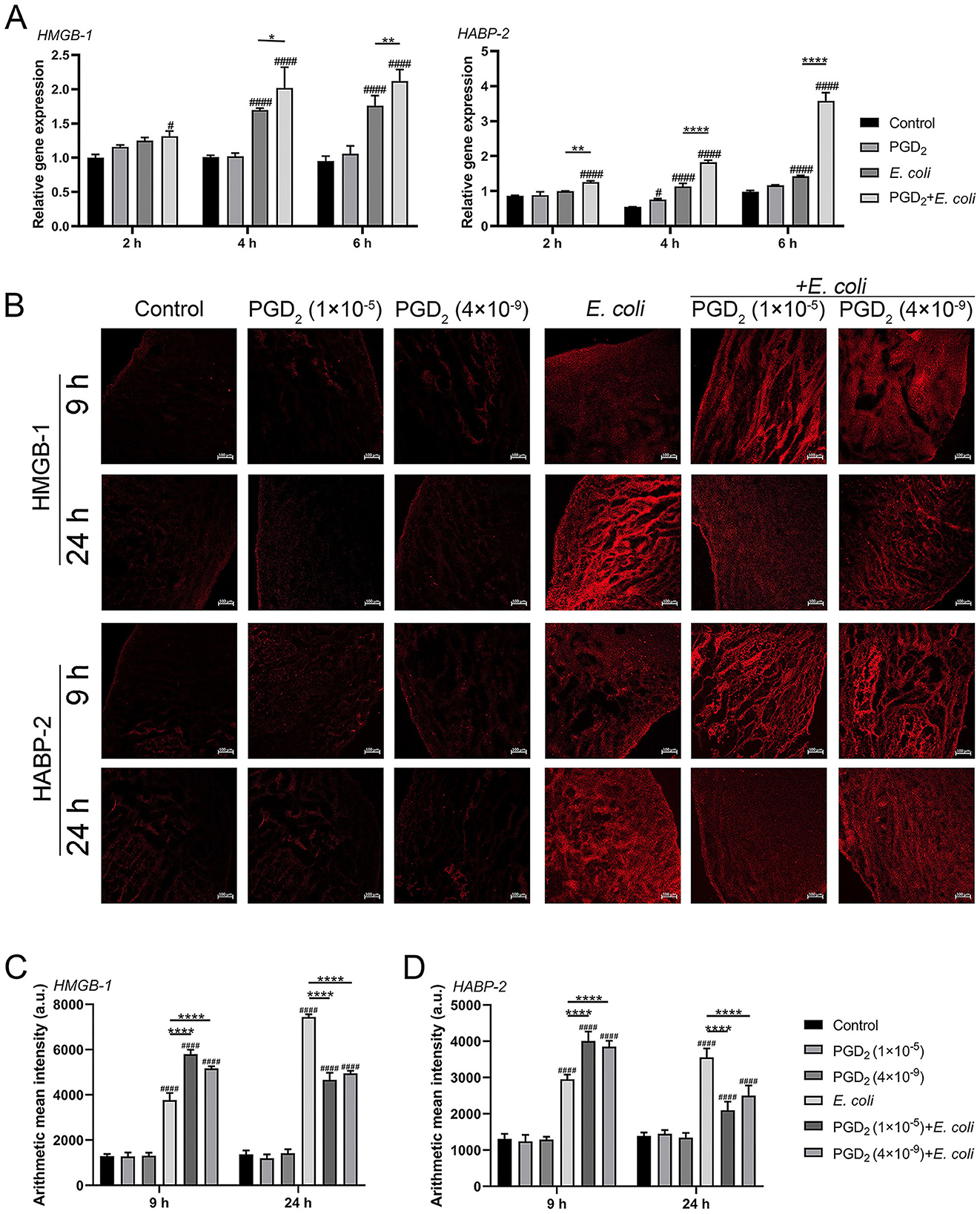
Figure 6. Effect of exogenous PGD2 on Escherichia coli-induced expression of HMGB-1 and HABP-2 in bovine BMDMs and endometrial tissues. (A) HMGB-1 and HABP-2 mRNA expression in BMDMs. (B–D) HMGB-1 and HABP-2 expression in E. coli-infected dairy cow endometrial tissue was assessed using ZEN software by immunofluorescence (Zeiss, ×100 magnification, scale label = 100 μm). Results are expressed as mean ± SD of three independent experiments and were analyzed using two-way ANOVA with Bonferroni's post-hoc test. #P < 0.05, ##P < 0.01, ###P < 0.001 and ####P < 0.0001 compared to control group. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 indicate statistically significant differences between two experimental groups.
3.6 Effect of exogenous PGD2 on histomorphometry of endometrium in E. coli infected cows
To gain more insight into the effects of PGD2 on uterine injury, we assessed histologic changes in the endometrium of dairy cows. Hematoxylin and Eosin (H&E) staining results demonstrate that both the control and PGD2-treated groups maintained intact endometrial structures, characterized by tightly arranged epithelial cells and well-defined glands and blood vessels. At 9 h post-infection, E. coli-infected tissues exhibited complete shedding of endometrial epithelial cells although glandular epithelial cells remained largely unaffected. PGD2-treated groups (1 × 10−5 M and 4 × 10−9 M) also showed endometrial epithelial cells shedding and loosening of glandular epithelial cells, some of which exhibited partial disintegration (Figure 7A). At 24 h post-infection, PGD2 treatment reduced endometrial damage, with the E. coli-infected group showing severe epithelial and glandular cell loss and necrosis, whereas the PGD2-treated groups retained relatively intact glandular and vascular structures (Figure 7B). Additionally, measurements of endometrial epithelial thickness and gland count (42) revealed no significant differences in epithelial thickness between the PGD2-treated and E. coli-infected groups at both 9 and 24 h. Notably, at 9 h post-infection, the PGD2-treated group showed a reduction in gland count compared to the E. coli-infected group, a trend that reversed by 24 h (Figures 7C, D, P < 0.05). These findings provide further evidence that PGD2 may play a dual role in E. coli-induced endometritis, aggravating tissue damage during the early phase of infection while contributing to tissue protection at later stages.

Figure 7. PGD2 attenuates Escherichia coli-induced endometrial tissue damage in dairy cows. (A, B) Micrographs of H&E-stained endometrial tissue sections. (C) Thickness of endometrial epithelium. (D) Number of endometrial glands. The black dotted line represents the endometrial epithelium, green arrows represent glands, and blue arrows represent the vessels. Scale label = 200 μm and 50 μm. Results are expressed as mean ± SD of three independent experiments and were analyzed using two-way ANOVA with Bonferroni's post-hoc test. #P < 0.05, ##P < 0.01, ###P < 0.001 and ####P < 0.0001 compared to control group. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 indicate statistically significant differences between two experimental groups.
3.7 Regulation of inflammatory mediators by exogenous PGD2 in E. coli-infected endometrial tissues of cows
We assessed cytokine and chemokine secretion in E. coli-infected endometrial tissue. After 9 h, PGD2 treatment significantly increased TNF-α, IL-1β, IL-6, and IL-8 levels compared to the E. coli infection group, while IL-10 levels decreased. However, at 24 h post-infection, the expression levels of TNF-α, IL-1β, and IL-6 were markedly reduced, while the secretion of the chemokine IL-8 continued to increase, and the anti-inflammatory cytokine IL-10 was significantly upregulated (Figure 8, P < 0.05). These findings suggest that PGD2 exerts a time-dependent immunoregulatory effect during E. coli infection, promoting inflammatory responses at the early stage while potentially exerting anti-inflammatory effects at the later stage.
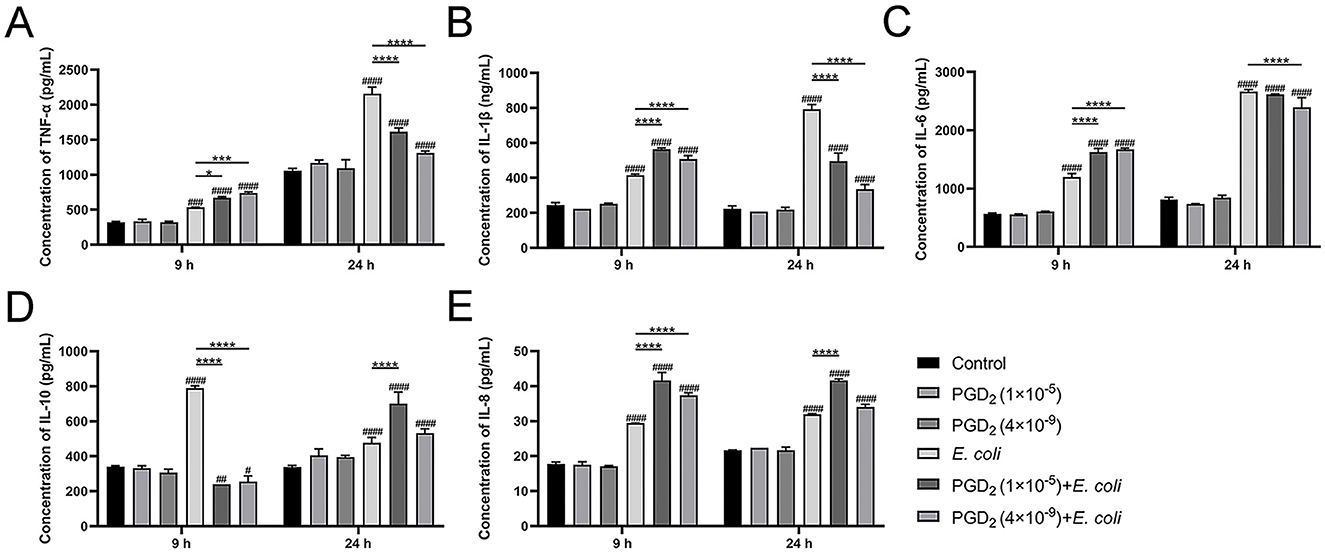
Figure 8. PGD2 modulates the level of Escherichia coli-induced inflammation in endometrial tissue of dairy cows. (A) TNF-α. (B) IL-1β. (C) IL-6. (D) IL-10. (E) IL-8. Results are expressed as mean ± SD of three independent experiments and were analyzed using two-way ANOVA with Bonferroni's post-hoc test. #P < 0.05, ##P < 0.01, ###P < 0.001 and ####P < 0.0001 compared to control group. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 indicate statistically significant differences between two experimental groups.
4 Discussion
This study explored the novel role and underlying molecular mechanisms of PGD2 in dairy cow endometritis. We applied PGD2 to E. coli-induced BMDMs and endometrial tissues from dairy cows, demonstrating its dual pro- and anti-inflammatory effects, depending on the stage of inflammation. Notably, the clinical features and prognosis of endometritis correlated with the duration of PGD2 action, irrespective of its concentration. Initially, we confirmed PGD2 expression in E. coli-induced BMDMs and then assessed its effects in a model of endometritis. Our results revealed that PGD2 amplifies the inflammatory response in the early stages of infection but mitigates it as the infection progresses. We measured the levels of various inflammatory mediators and tissue damage at the early (9 h) and late (24 h) stages of infection, finding consistency with cellular results. We hypothesized that PGD2 modulates the expression of inflammatory mediators and the killing capacity of BMDMs through different receptors at various infection stages, affecting endometrial tissue damage. However, further studies are required to fully elucidate these regulatory mechanisms.
Transcriptomic analysis revealed that the most significant changes in gene expression following E. coli infection in BMDMs were associated with the “inflammatory response,” with a notable increase in cytokines and PTGS2 expression. In this study, we observed increased PGD2 secretion following E. coli infection in BMDMs. Similarly, inflammation induced by Staphylococcus aureus led to elevated PGD2 levels in mouse peritoneal macrophages (43). In other contexts, excessive PGD2 promotes eosinophilia and elevates Th2 cytokine levels, exacerbating allergic lung inflammation in mice (44). These findings suggest that PGD2 plays a crucial role in regulating inflammation and immune responses. Our KEGG enrichment analysis of the top 20 E. coli-altered signaling pathways revealed strong associations with inflammation, particularly involving the TNF, NF-κB, cytokine-cytokine receptor interaction, and MAPK pathways. Bacterial infections typically activate inflammatory responses that protect the host by eliminating harmful stimuli and promoting tissue repair (45). However, the specific effects of PGD2 on the activation of NF-κB and MAPK signaling pathways, as well as its regulation of cytokine secretion during E. coli-induced endometritis in dairy cows remains unclear.
We observed that 24 h of PGD2 pretreatment followed by 6 h of E. coli infection increased pro-inflammatory cytokines (TNF-α, IL-1β, and IL-8) secretion in BMDMs. This response was accompanied by enhanced phosphorylation of ERK, p38, and p65 within 15–30 min. However, cytokine secretion and phosphorylation levels decreased at later stages of infection. These findings suggest that PGD2 initially promotes, but later suppresses inflammation, showing both pro- and anti-inflammatory effects, depending on infection duration. Similar dual roles have also been observed in other models in which PGD2 exacerbated allergic inflammation but protected against liver damage (46). Another study showed that PGD2 suppresses inflammation by inhibiting NF-κB kinase, leading to reduced secretion of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) and iNOS expression in macrophages (47). Further, PGD2-DP1 signaling plays a protective role in Helicobacter pylori-induced gastritis (33) and enhances antiviral immunity against respiratory syncytial virus infection (48). We hypothesized that these opposing effects are mediated by the differential activation of DP1 and DP2 receptors, which have distinct influences on cyclic AMP production, inositol phosphate conversion, and intracellular Ca2+ mobilization (49). Additionally, PGD2 upregulated both IL-6 and IL-10, regardless of the infection stage. IL-6 is a pleiotropic cytokine involved in both pro- and anti-inflammatory processes (50), whereas IL-10 inhibits pro-inflammatory cytokine production and promotes tissue repair (51, 52). Our results suggest that increased IL-10 levels in early E. coli infection may act as a compensatory response to innate immune overactivation, whereas its upregulation in later stages reflects PGD′2s anti-inflammatory action.
Macrophages are crucial for host defenses, playing key roles in phagocytosis and bacterial clearance (53). Our findings showed that PGD2 initially reduced the killing capacity of BMDMs during early E. coli infection but enhanced it in later stages. This suggests that PGD2 modulates the immune function by regulating the killing capacity of BMDMs, potentially mitigating E. coli-induced endometrial tissue injury in dairy cows. Previous evidence has linked heightened inflammatory responses and bacterial burden to lung injury (54), supporting our findings on s role in immune regulation.
HMGB-1 and HABP-2 are critical tissue damage biomarkers associated with inflammatory responses (45). HMGB-1, released by damaged cells, acts as a “necrosis marker,” enabling the immune system to identify tissue damage, initiate repair responses, and facilitate lymphocyte maturation (55). When secreted, HMGB-1 binds to various immune receptors and induces inflammation by activating NF-κB signaling, leading to the release of cytokines and recruitment of leukocytes (56). During inflammation, damaged cells release numerous endogenous molecules called DAMPs, such as heat shock proteins and low-molecular-weight hyaluronic acid (57). Notably, hyaluronic acid levels consistently increase with the severity of liver injury (58). HABP-2, a hyaluronic acid-binding protein associated with the endometrial tissue in cows, plays a vital role in endometrial function (5). The upregulation of HMGB-1 and HABP-2 by exogenous PGD2 in E. coli-induced BMDMs suggests that PGD2 enhances cellular damage and inflammatory responses. We investigated the biological impact of exogenous PGD2 on the intrauterine pathophysiology of dairy cows using a model of E. coli-induced endometritis. Previous findings indicate that high and low concentrations of PGD2 mediate opposing effects on cell growth, with high concentrations promoting growth and low concentrations inhibiting it (59). Therefore, we selected high (drug-level) and low (physiological-level) PGD2 concentrations to treat endometrial tissues from E. coli-infected cows and assessed their roles in dairy cow endometritis. Our results show that at 9 h post-E. coli infection, both concentrations of PGD2 significantly increased the expression of HMGB-1 and HABP-2, indicating that PGD2 exacerbates tissue damage and sustains inflammation. However, after 24 h, PGD2 significantly downregulated the expression of DAMPs in the infected bovine endometrial tissues, suggesting a reduction in inflammation and alleviation of tissue damage. Morphological observations yielded consistent results, showing that PGD2 exacerbated tissue damage at 9 h and alleviated it at 24 h post-E. coli infection. These results suggest that PGD2 may exhibit a pro-inflammatory effect during the initial phases of endometrial infection in E. coli-infected cows, followed by an anti-inflammatory effect in the later stages of infection, regardless of the PGD2 concentration. This phenomenon may result from PGD2 activating different receptors at different stages of endometritis in dairy cows. Supporting this hypothesis, maternal inflammation has been shown to exacerbate inflammatory responses, oxidative stress, and neuronal apoptosis through the activation of the COX-2-PGD2-DP2 pathway, thereby increasing the susceptibility of the offspring to brain injury (60). Systemic knockout of the DP2 receptor in mice moderately attenuates inflammation-induced kidney injury (61). Similarly, mice modestly attenuate inflammation-induced kidney injury, demonstrating D2′s broader role in mediating pro-inflammatory damage. In contrast to the detrimental effects mediated by DP2, the PGD2-DP1 signaling pathway offers protective effects in different contexts. For instance, DP1 signaling protects against aluminum overload-induced neuronal damage in primary cultured rat hippocampal cells (62), and PGD2 shields neurons from glutamate toxicity or ischemia-reperfusion injury via DP1 receptor activation (63). The PGD2-DP1 pathway is also protective in conditions such as acute lung injury and bovine endometritis (37, 64). Therefore, PGD2 cannot be strictly classified as pro-inflammatory or anti-inflammatory, and its effects depend on the disease stage, cell type, and specific synthase and receptor interactions.
Previous research has demonstrated that inflammatory cytokines TNF-α or IL-1 can stimulate monocyte-macrophages to release HMGB-1, which, in turn, stimulates the secretion of inflammatory cytokines and induces the chemotaxis of neutrophils (65). In this study, assessment of cytokine secretion revealed that exogenous PGD2 increased the production of pro-inflammatory cytokines and chemokines while diminishing the secretion of anti-inflammatory cytokines in tissues during the early stages of infection. Conversely, diametrically opposite outcomes were observed during the later stages of infection. This highlights the multifaceted roles of PGD2 throughout the course of infection. The activation of this cytokine and chemokine cascade ultimately triggers an inflammatory response, leading to organ damage if left unchecked (54). Based on these findings, we hypothesized that PGD2 initially binds to the DP2 receptor in E. coli-infected tissues and cells, thereby increasing inflammatory responses and exacerbating tissue damage. Subsequently, PGD2 may bind to the DP1 receptor, exerting an anti-inflammatory effect that mitigates tissue damage by downregulating pro-inflammatory mediators and upregulating IL-10 secretion during E. coli infection.
By thoroughly exploring the dual role of PGD2 and its mechanism in endometritis in dairy cows, we can provide a theoretical basis for the development of safer and more effective treatments. PGD2 is expected to serve as a new therapeutic target to improve uterine health in dairy cows, thereby safeguarding the quality and safety of dairy products.
5 Conclusions
This study highlights the key role of PGD2 in the response to E. coli-induced bovine endometritis. Our findings demonstrate the different roles of exogenous PGD2 throughout the infection process and its molecular mechanisms (Figure 9): in the early stages, PGD2 enhances the inflammatory response, while later, it increases the bactericidal capacity of BMDMs against E. coli by reducing pro-inflammatory cytokines and chemokines, promoting anti-inflammatory factor production, and decreasing DAMPs expression, ultimately alleviating endometrial damage. Importantly, these results not only advance our understanding of the immunomodulatory role of PGD2 in the context of uterine inflammation but also have broad implications for improving the reproductive health of dairy cows. By elucidating a potential non-antibiotic pathway for controlling inflammation and enhancing host defense, this study provides a promising foundation for the development of novel therapeutic strategies aimed at reducing antibiotic dependence. Such strategies are critical for promoting animal welfare, improving herd fertility, and ensuring the long-term sustainability and productivity of the dairy industry.
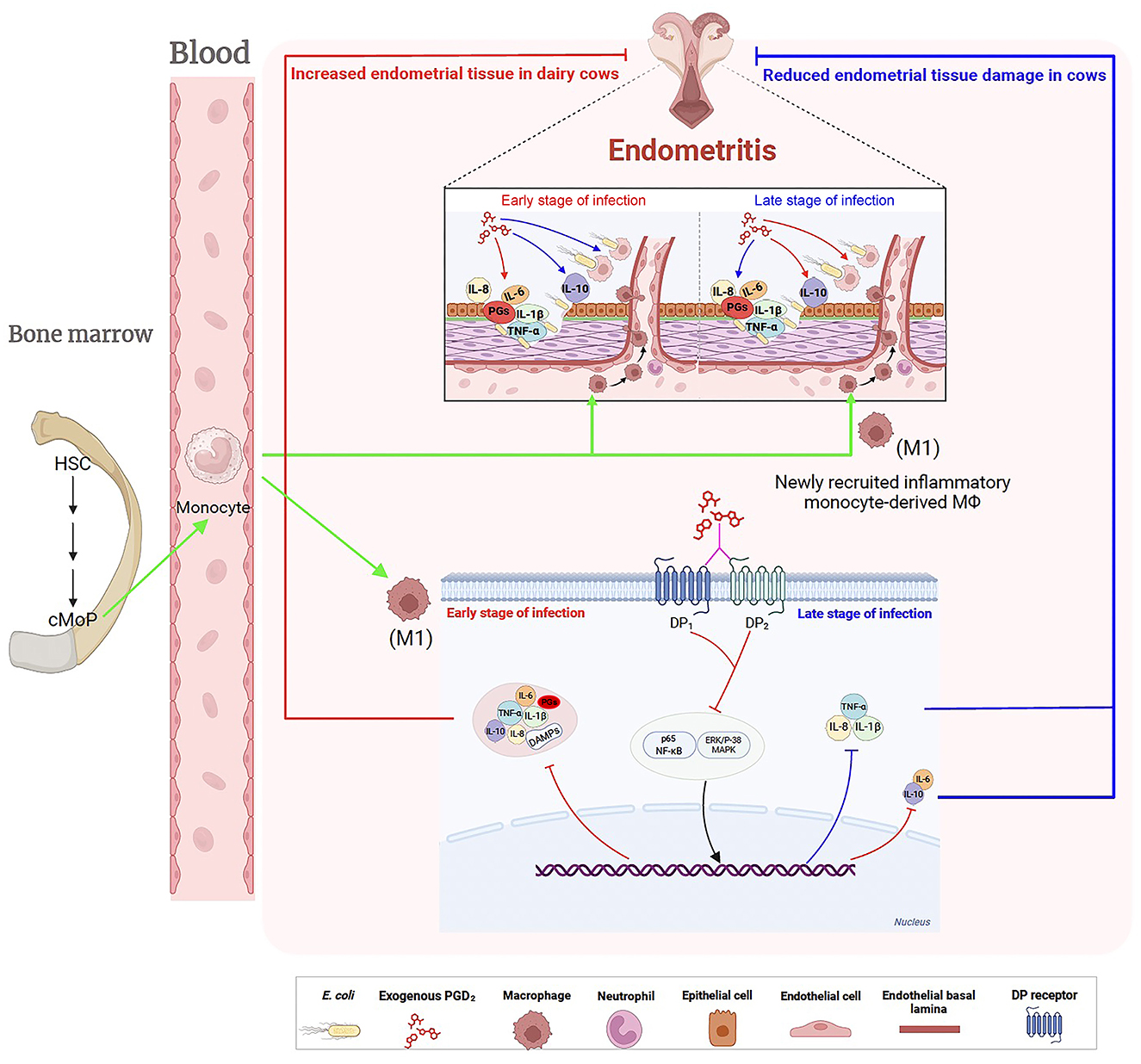
Figure 9. Regulatory effects of exogenous PGD2 on Escherichia coli-induced endometritis in dairy cows. Postpartum, E. coli invades the uterus, triggering the production of inflammatory mediators, including prostaglandins. This response recruits and activates hematopoietic cells, particularly macrophages, which differentiate into M1 macrophages to eliminate the invading E. coli. PGD2 binds to two distinct receptors, exacerbating endometrial tissue damage in the early stages of E. coli-induced endometritis in dairy cows by enhancing signaling pathways, increasing pro-inflammatory cytokine production, and reducing the killing capacity of BMDMs. However, in the later stages of infection, PGD2 exhibits a protective role. Green arrows indicate activation, migration, the red lines indicate up-regulation, the blue lines indicate down-regulation, pink lines (?) indicate recognition or interaction. HSC, hematopoietic stem cell; cMoP, common monocyte progenitor; DAMPs, damage-associated molecular patterns.
Data availability statement
The datasets generated and/or analyzed during the current study are publicly available in the Gene Expression Omnibus (GEO) repository at NCBI under accession number GSE275904, which can be accessed at the following link: https://www.ncbi.nlm.nih.gov.
Ethics statement
The animal study was approved by Animal Welfare and Research Ethics Committee of Inner Mongolia Agricultural University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
XY: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. SZ: Conceptualization, Project administration, Supervision, Writing – review & editing. BL: Conceptualization, Investigation, Project administration, Supervision, Visualization, Writing – review & editing. LG: Formal analysis, Investigation, Methodology, Software, Writing – review & editing. PG: Methodology, Software, Validation, Visualization, Writing – review & editing. JW: Methodology, Validation, Visualization, Writing – review & editing. YZ: Data curation, Investigation, Writing – review & editing. WM: Funding acquisition, Resources, Supervision, Writing – review & editing. JC: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project received funding from the National Natural Science Foundation of China (32160851), Inner Mongolia Autonomous Region “Young Talents in Science and Technology in Higher Education Institutions” Program of China (NJYT22041), Inner Mongolia Autonomous Region Science and Technology Major Project of China (2021ZD0013) and Special Research Program for First-Class Disciplines, Department of Education of Inner Mongolia Autonomous Region of China (YLXKZX-NND-012) for research, technological development.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1618203/full#supplementary-material
References
1. Ma X, Yin B, Guo S, Umar T, Liu J, Wu Z, et al. Enhanced expression of miR-34a enhances Escherichia coli lipopolysaccharide-mediated endometritis by targeting LGR4 to activate the NF-κB pathway. Oxid Med Cell Longev. (2021) 2021:1744754. doi: 10.1155/2021/1744754
2. Wang L, Yao D, Deepak RNVK, Liu H, Xiao Q, Fan H, et al. Structures of the human PGD(2) receptor CRTH2 reveal novel mechanisms for ligand recognition. Mol Cell. (2018) 72:48–59.e4. doi: 10.1016/j.molcel.2018.08.009
3. Xinyue WU, Kitahara G, Suenaga T, Naramoto K, Sekiguchi S, Goto Y et al. Association of intrauterine presence of Lactobacillus spp. with inflammation and pathogenic bacteria in the uterus in postpartum dairy cows. J Reprod Dev. (2021) 67:340–4. doi: 10.1262/jrd.2021-023
4. Kelly P, Meade KG, O'Farrelly C. Non-canonical inflammasome-mediated IL-1β production by primary endometrial epithelial and stromal fibroblast cells is NLRP3 and caspase-4 dependent. Front Immunol. (2019) 10:102. doi: 10.3389/fimmu.2019.00102
5. Li T, Mao W, Liu B, Gao R, Zhang S, Wu J, et al. LP induced/mediated PGE2 synthesis through activation of the ERK/NF-κB pathway contributes to inflammatory damage triggered by Escherichia coli-infection in bovine endometrial tissue. Vet Microbiol. (2019) 232:96–104. doi: 10.1016/j.vetmic.2019.03.005
6. Ma X, Li Y, Shen W, Oladejo AO, Yang J, Jiang W, et al. LPS mediates bovine endometrial epithelial cell pyroptosis directly through both NLRP3 classical and non-classical inflammasome pathways. Front Immunol. (2021) 12:676088. doi: 10.3389/fimmu.2021.676088
7. Jeremejeva J, Toomas O, Waldmann A, Kask K. Treatment of dairy cows with PGF2α or NSAID, in combination with antibiotics, in cases of postpartum uterine inflammation. Acta Vet Scand. (2012) 54:45. doi: 10.1186/1751-0147-54-45
8. Hussain AM. Bovine endometritis: current and future alternative therapy. Zentralblatt Fur Veterinarmedizin. (1991) 38:641–51. doi: 10.1111/j.1439-0442.1991.tb01060.x
9. Hoelzer K, Wong N, Thomas J, Talkington K, Jungman E, Coukell A. Antimicrobial drug use in food-producing animals and associated human health risks: what, and how strong, is the evidence? BMC Vet Res. (2017) 13:211. doi: 10.1186/s12917-017-1131-3
10. Patel SJ, Wellington M, Shah RM, Ferreira MJ. Antibiotic stewardship in food-producing animals: challenges, progress, and opportunities. Clin Ther. (2020) 42:1649–58. doi: 10.1016/j.clinthera.2020.07.004
11. Espadamala A, Pereira R, Pallarés P, Lago A, Silva-Del-Río N. Metritis diagnosis and treatment practices in 45 dairy farms in California. J Dairy Sci. (2018) 101:9608–16. doi: 10.3168/jds.2017-14296
12. Arsène MMJ, Davares AKL, Viktorovna PI, Andreevna SL, Sarra S, Khelifi I, et al. The public health issue of antibiotic residues in food and feed: causes, consequences, and potential solutions. Vet World. (2022) 15:662–71. doi: 10.14202/vetworld.2022.662-671
13. Niu C, Cheng C, Liu Y, Huang S, Fu Y, Li P. Transcriptome profiling analysis of bovine vaginal epithelial cell response to an isolated lactobacillus strain. mSystems. (2019) 4:e00268–19. doi: 10.1128/mSystems.00268-19
14. Piras C, Hale OJ, Reynolds CK, Jones AKB, Taylor N, Morris M, et al. LAP-MALDI MS coupled with machine learning: an ambient mass spectrometry approach for high-throughput diagnostics. Chem Sci. (2022) 13:1746–58. doi: 10.1039/D1SC05171G
15. LeBlanc SJ, Duffield TF, Leslie KE, Bateman KG, Keefe GP, Walton JS, et al. The effect of treatment of clinical endometritis on reproductive performance in dairy cows. J Dairy Sci. (2002) 85:2237–49. doi: 10.3168/jds.S0022-0302(02)74303-8
16. Hwang U, Belland LK, Handel DA, Yadav K, Heard K, Rivera-Reyes L, et al. Is all pain is treated equally? A multicenter evaluation of acute pain care by age. Pain. (2014) 155:2568–74. doi: 10.1016/j.pain.2014.09.017
17. Hamza M, Dionne RA. Mechanisms of non-opioid analgesics beyond cyclooxygenase enzyme inhibition. Curr Mol Pharmacol. (2009) 2:1–14. doi: 10.2174/1874467210902010001
18. Chan WY, Berezin I, Daniel EE. Effects of inhibition of prostaglandin synthesis on uterine oxytocin receptor concentration and myometrial gap junction density in parturient rats'. Biol Reprod. (1988) 39:1117–28. doi: 10.1095/biolreprod39.5.1117
19. Engelking LE, Gobikrushanth M, Oba M, Ambrose DJ. Effects of dietary butyrate supplementation and oral nonsteroidal antiinflammatory drug administration on uterine inflammation and interval to first ovulation in postpartum dairy cows. JDS Commun. (2022) 3:362–7. doi: 10.3168/jdsc.2022-0207
20. Ciliberti MG, Francavilla M, Intini S, Albenzio M, Marino R, Santillo A, et al. Phytosterols from Dunaliella tertiolecta reduce cell proliferation in sheep fed flaxseed during post partum. Mar Drugs. (2017) 15:216. doi: 10.3390/md15070216
21. Fu S, Guo J, Li R, Qiu Y, Ye C, Liu Y, et al. Transcriptional profiling of host cell responses to virulent Haemophilus parasuis: new insights into pathogenesis. Int J Mol Sci. (2018) 19:1320. doi: 10.3390/ijms19051320
22. Belville C, Ponelle-Chachuat F, Rouzaire M, Gross C, Pereira B, Gallot D, et al. Physiological TLR4 regulation in human fetal membranes as an explicative mechanism of a pathological preterm case. Elife. (2022) 11:e71521. doi: 10.7554/eLife.71521
23. Teles AM, Pontes LPP, Guimarães SJA, Butarelli AL, Silva GX. do Nascimento FRF, et al. Marine-derived Penicillium purpurogenum reduces tumor size and ameliorates inflammation in an erlich mice model. Mar Drugs. (2020) 18:541. doi: 10.3390/md18110541
24. Fernando MR, Giembycz MA, McKay DM. Bidirectional crosstalk via IL-6, PGE2 and PGD2 between murine myofibroblasts and alternatively activated macrophages enhances anti-inflammatory phenotype in both cells. Br J Pharmacol. (2016) 173:899–912. doi: 10.1111/bph.13409
25. Lin W, Li Q, Zhang D, Zhang X, Qi X, Wang Q, et al. Mapping the immune microenvironment for mandibular alveolar bone homeostasis at single-cell resolution. Bone Res. (2021) 9:17. doi: 10.1038/s41413-021-00141-5
26. Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. (2014) 5:514. doi: 10.3389/fimmu.2014.00514
27. Cao L, Huang F, Massey IY, Wen C, Zheng S, Xu S, et al. Effects of microcystin-LR on the microstructure and inflammation-related factors of jejunum in mice. Toxins. (2019) 11:482. doi: 10.3390/toxins11090482
28. Oguma T, Asano K, Ishizaka A. Role of prostaglandin D(2) and its receptors in the pathophysiology of asthma. Allergol Int. (2008) 57:307–12. doi: 10.2332/allergolint.08-RAI-0033
29. Dave M, Amin AR. Yin-Yang regulation of prostaglandins and nitric oxide by PGD2 in human arthritis: reversal by celecoxib. Immunol Lett. (2013) 152:47–54. doi: 10.1016/j.imlet.2013.04.002
30. Sturm EM, Radnai B, Jandl K, Stančić A, Parzmair GP, Högenauer C, et al. Opposing roles of prostaglandin D2 receptors in ulcerative colitis. J Immunol. (2014) 193:827–39. doi: 10.4049/jimmunol.1303484
31. Ratner P, Andrews CP, Hampel FC, Martin B, Mohar DE, Bourrelly D, et al. Efficacy and safety of setipiprant in seasonal allergic rhinitis: results from Phase 2 and Phase 3 randomized, double-blind, placebo- and active-referenced studies. Allergy Asthma Clin Immunol. (2017) 13:18. doi: 10.1186/s13223-017-0183-z
32. Xiao L, Ornatowska M, Zhao G, Cao H, Yu R, Deng J, et al. Lipopolysaccharide-induced expression of microsomal prostaglandin E synthase-1 mediates late-phase PGE2 production in bone marrow derived macrophages. PLoS One. (2012) 7:e50244. doi: 10.1371/journal.pone.0050244
33. Kim HJ, Kang TW, Haam K, Kim M, Kim SK, Kim SY, et al. Whole genome MBD-seq and RRBS analyses reveal that hypermethylation of gastrointestinal hormone receptors is associated with gastric carcinogenesis. Exp Mol Med. (2018) 50:1–14. doi: 10.1038/s12276-018-0179-x
34. Sadam H, Pihlak A, Kivil A, Pihelgas S, Jaago M, Adler P, et al. Prostaglandin D2 receptor DP1 antibodies predict vaccine-induced and spontaneous narcolepsy type 1: large-scale study of antibody profiling. EBioMedicine. (2018) 29:47–59. doi: 10.1016/j.ebiom.2018.01.043
35. Murata T, Aritake K, Matsumoto S, Kamauchi S, Nakagawa T, Hori M, et al. Prostagladin D2 is a mast cell-derived antiangiogenic factor in lung carcinoma. Proc Natl Acad Sci U S A. (2011) 108:19802–7. doi: 10.1073/pnas.1110011108
36. Gong Z, Mao W, Jin F, Zhang S, Zhao J, Ren P, et al. Prostaglandin D(2) regulates Escherichia coli-induced inflammatory responses through TLR2, TLR4, and NLRP3 in macrophages. Prostaglandins Other Lipid Mediat. (2023) 169:106772. doi: 10.1016/j.prostaglandins.2023.106772
37. Wu J, Bai F, Mao W, Liu B, Yang X, Zhang J, et al. Anti-inflammatory effects of the prostaglandin D(2)/prostaglandin DP1 receptor and lipocalin-type prostaglandin D(2) synthase/prostaglandin D(2) pathways in bacteria-induced bovine endometrial tissue. Vet Res. (2022) 53:98. doi: 10.1186/s13567-022-01100-6
38. Yang X, Yin Y, Yan X, Yu Z, Liu Y, Cao J. Flagellin attenuates experimental sepsis in a macrophage-dependent manner. Crit Care. (2019) 23:106. doi: 10.1186/s13054-019-2408-7
39. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. (2001) 25:402–8. doi: 10.1006/meth.2001.1262
40. Guo DD, Huang HY, Liu HE, Liu K, Luo XJ. Orientin reduces the effects of repeated procedural neonatal pain in adulthood: network pharmacology analysis, molecular docking analysis, and experimental validation. Pain Res Manag. (2023) 20:14. doi: 10.1155/2023/8893932
41. Li T, Liu B, Mao W, Gao R, Wu J, Deng Y, et al. Prostaglandin E2 promotes nitric oxide synthase 2, platelet-activating factor receptor, and matrix metalloproteinase-2 expression in Escherichia coli-challenged ex vivo endometrial explants via the prostaglandin E2 receptor 4/protein kinase a signaling pathway. Theriogenology. (2019) 134:65–73. doi: 10.1016/j.theriogenology.2019.04.028
42. Xu X, Xing Q, Liu R, Dong L, Yu Z, Wang Y, et al. Therapeutic effects and repair mechanism of HGF gene-transfected mesenchymal stem cells on injured endometrium. Stem Cells Int. (2022) 2022:5744538. doi: 10.1155/2022/5744538
43. Bao H, Gong Z, Zhao J, Ren P, Yu Z, Su N, et al. Prostaglandin D(2) is involved in the regulation of inflammatory response in Staphylococcus aureus-infected mice macrophages. Int Immunopharmacol. (2024) 129:111526. doi: 10.1016/j.intimp.2024.111526
44. Kataoka N, Satoh T, Hirai A, Saeki K, Yokozeki H. Indomethacin inhibits eosinophil migration to prostaglandin D2: therapeutic potential of CRTH2 desensitization for eosinophilic pustular folliculitis. Immunology. (2013) 140:78–86. doi: 10.1111/imm.12112
45. Shen Y, Gong Z, Zhang S, Cao J, Mao W, Fu Y, et al. Braun lipoprotein protects against escherichia coli-induced inflammatory responses and lethality in mice. Microbiol Spectr. (2023) 11:e0354122–e0354122. doi: 10.1128/spectrum.03541-22
46. Krzystek-Korpacka M, Fleszar MG, Fortuna P, Gostomska-Pampuch K, Lewandowski Ł, Piasecki T, et al. Modulation of prostanoids profile and counter-regulation of SDF-1α/CXCR4 and VIP/VPAC2 expression by sitagliptin in non-diabetic rat model of hepatic ischemia-reperfusion injury. Int J Mol Sci. (2021) 22:13155. doi: 10.3390/ijms222313155
47. Lim TKY, Anderson KM, Hari P, Di Falco M, Reihsen TE, Wilcox GL, et al. Evidence for a role of nerve injury in painful intervertebral disc degeneration: a cross-sectional proteomic analysis of human cerebrospinal fluid. J Pain. (2017) 18:1253–69. doi: 10.1016/j.jpain.2017.06.002
48. Ullah MA, Rittchen S, Li J, Hasnain SZ, Phipps S. DP1 prostanoid receptor activation increases the severity of an acute lower respiratory viral infection in mice via TNF-α-induced immunopathology. Mucosal Immunol. (2021) 14:963–72. doi: 10.1038/s41385-021-00405-7
49. Woodward DF, Jones RL, Narumiya S. International union of basic and clinical pharmacology. LXXXIII: classification of prostanoid receptors, updating 15 years of progress. Pharmacol Rev. (2011) 63:471–538. doi: 10.1124/pr.110.003517
50. Gong J, Wu ZY Qi H, Chen L, Li HB Li B, et al. Maresin 1 mitigates LPS-induced acute lung injury in mice. Br J Pharmacol. (2014) 171:3539–50. doi: 10.1111/bph.12714
51. Yu HS, Park MK, Kang SA, Cho KS, Mun SJ, Roh HJ. Culture supernatant of adipose stem cells can ameliorate allergic airway inflammation via recruitment of CD4(+)CD25(+)Foxp3 T cells. Stem Cell Res Ther. (2017) 8:8. doi: 10.1186/s13287-016-0462-5
52. Pincus SH, Bhaskaran M, Brey RN. 3rd, Didier PJ, Doyle-Meyers LA, Roy CJ. Clinical and pathological findings associated with aerosol exposure of macaques to ricin toxin. Toxins. (2015) 7:2121–33. doi: 10.3390/toxins7062121
53. Chin WY, He CY, Chow TW Yu QY, Lai LC, Miaw SC, et al. Adenylate kinase 4 promotes inflammatory gene expression via Hif1alpha and AMPK in macrophages. Front Immunol. (2021) 12:630318. doi: 10.3389/fimmu.2021.630318
54. Gong Z, Zhang S, Gu B, Cao J, Mao W, Yao Y, et al. Codonopsis pilosula polysaccharides attenuate Escherichia coli-induced acute lung injury in mice. Food Funct. (2022) 13:7999–8011. doi: 10.1039/D2FO01221A
55. Arumugam T, Ramachandran V, Gomez SB, Schmidt AM, Logsdon CD. S100P-derived RAGE antagonistic peptide reduces tumor growth and metastasis. Clin Cancer Res. (2012) 18:4356–64. doi: 10.1158/1078-0432.CCR-12-0221
56. Papadaki M, Rinotas V, Violitzi F, Thireou T, Panayotou G, Samiotaki M, et al. New insights for RANKL as a proinflammatory modulator in modeled inflammatory arthritis. Front Immunol. (2019) 10:97. doi: 10.3389/fimmu.2019.00097
57. Liu X, Chen W, Zhu G, Yang H, Li W, Luo M, et al. Single-cell RNA sequencing identifies an Il1rn(+)/Trem1(+) macrophage subpopulation as a cellular target for mitigating the progression of thoracic aortic aneurysm and dissection. Cell Discov. (2022) 8:11. doi: 10.1038/s41421-021-00362-2
58. Gudowska M, Gruszewska E, Panasiuk A, Cylwik B, Flisiak R, Swiderska M, et al. Hyaluronic acid concentration in liver diseases. Clin Exp Med. (2016) 16:523–8. doi: 10.1007/s10238-015-0388-8
59. Hu S, Lu T, Shang J, Cai Y, Ding M, Zhou X, et al. PGD2 displays distinct effects in diffuse large B-cell lymphoma depending on different concentrations. Cell Death Discov. (2023) 9:39. doi: 10.1038/s41420-023-01311-6
60. Li Y, Luo W, Zhang J, Luo Y, Han W, Wang H, et al. Maternal inflammation exaggerates offspring susceptibility to cerebral ischemia-reperfusion injury via the COX-2/PGD2/DP2 pathway activation. Oxid Med Cell Longev. (2022) 2022:1571705. doi: 10.1155/2022/1571705
61. Montgomery TA, Xu L, Mason S, Chinnadurai A, Lee CG, Elias JA, et al. Breast regression protein-39/chitinase 3-like 1 promotes renal fibrosis after kidney injury via activation of myofibroblasts. J Am Soc Nephrol. (2017) 28:3218–26. doi: 10.1681/ASN.2017010110
62. Ma J, Yang Q, Wei Y, Yang Y, Ji C, Hu X, et al. Effect of the PGD2-DP signaling pathway on primary cultured rat hippocampal neuron injury caused by aluminum overload. Sci Rep. (2016) 6:24646. doi: 10.1038/srep24646
63. Liu H, Li W, Rose ME, Pascoe JL, Miller TM, Ahmad M, et al. Prostaglandin D2 toxicity in primary neurons is mediated through its bioactive cyclopentenone metabolites. Neurotoxicology. (2013) 39:35–44. doi: 10.1016/j.neuro.2013.08.001
64. Gupta A, Kalantar-Zadeh K, Reddy ST. Ramatroban as a novel immunotherapy for COVID-19. J Mol Genet Med. (2020) 14:457. doi: 10.37421/jmgm.2020.14.457
Keywords: endometritis, Escherichia coli, prostaglandin D2, bone marrow-derived, endometrial tissue
Citation: Yang X, Zhang S, Liu B, Guo L, Gong P, Wu J, Zhao Y, Mao W and Cao J (2025) Exogenous prostaglandin D2 as a modulator in bovine endometritis: implications for reducing antibiotic use in dairy cattle. Front. Vet. Sci. 12:1618203. doi: 10.3389/fvets.2025.1618203
Received: 25 April 2025; Accepted: 14 July 2025;
Published: 12 August 2025.
Edited by:
Guangbin Zhou, Sichuan Agricultural University, ChinaReviewed by:
Jiakang He, Guangxi University, ChinaWenqiang Sun, Sichuan Agricultural University, China
Copyright © 2025 Yang, Zhang, Liu, Guo, Gong, Wu, Zhao, Mao and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinshan Cao, amluc2hhbmNhb0BpbWF1LmVkdS5jbg==; Wei Mao, bWFvd2VpMjAxNEBpbWF1LmVkdS5jbg==
 Xiaolin Yang
Xiaolin Yang Shuangyi Zhang
Shuangyi Zhang Bo Liu
Bo Liu Lili Guo1,2
Lili Guo1,2 Wei Mao
Wei Mao