- Jiuquan Vocational and Technical University, Jiuquan, China
Background: Clonorchis sinensis is one of the most significant zoonotic food-borne parasites, particularly prevalent in China. The adult form of C. sinensis typically inhabits in the hepatic and biliary tracts of various mammals, including humans, and is considered a major contributor to the incidence of cholangiocarcinoma. The life cycle of C. sinensis is complex, understanding its prevalence among different host species is essential for developing prevention and management measures. Furthermore, the clinical manifestations of clonorchiasis are often overlooked or misdiagnosed. Therefore, the development of early detection methodologies will facilitate timely treatment and reduce associated complications.
Methods: The current review was conducted in accordance with the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses. A comprehensive search for relevant articles was performed across PubMed, Web of Science, Google Scholar, China National Knowledge Infrastructure, Wanfang, and Wipro databases. The selected studies investigated the prevalence of C. sinensis among various host species in China, and developed the corresponding diagnostic approaches.
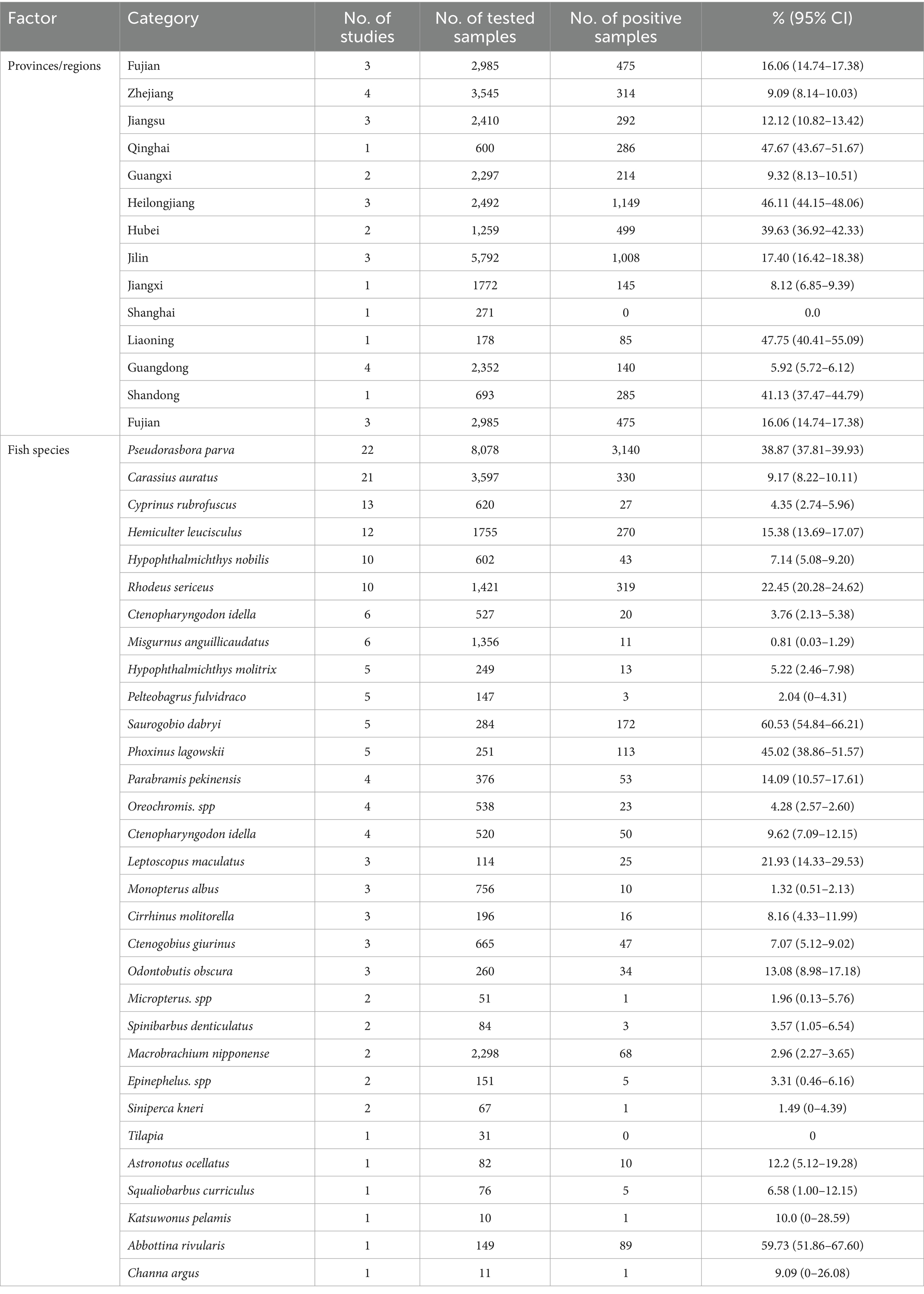
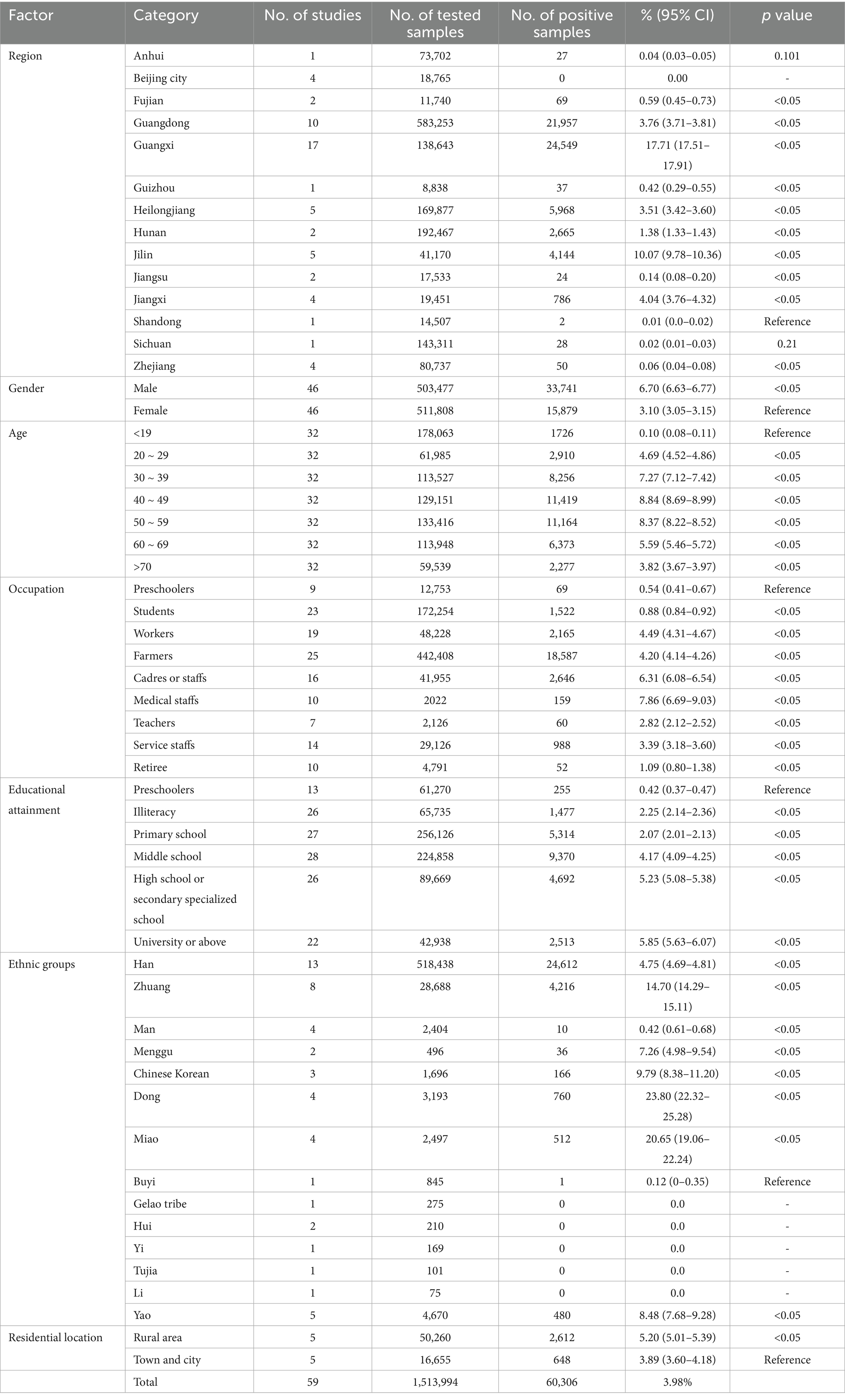
Results: Various diagnostic approaches have been established for detecting C. sinensis. These methods encompass direct identification, molecular techniques, serological assays, and imaging modalities, each of which targets distinct aspects such as morphological characteristics, DNA sequences, specific antibodies, and the visual representation of C. sinensis. The prevalence characteristics of C. sinensis across different host species have been examined in China, with the majority of studies concentrating on the second intermediate hosts, specifically fishes, and the definitive hosts, namely humans. The overall prevalence of C. sinensis among fish species was found to be 18.36% (4,892/26,646, 95% CI 17.90–18.82), with notably high detection rates observed in the provinces of Heilongjiang, Qinghai, Liaoning, and Shandong. In Chinese human population, the average detection rate of this pathogen was recorded at 3.98% (60,306/1,513,994, 95% CI 3.95–4.01), with the highest rates occurring in the provinces of Jiangxi, Hunan, Guangdong, and Heilongjiang Provinces.
Conclusion: This review emphasized on the widespread prevalence of C. sinensis among different host species in China, and summarized the existing diagnosis methods developed in China. These findings will establish a foundational framework for the prevention, control, and potential eradication of clonorchiasis in China.
1 Introduction
Clonorchis sinensis, initially classified as Distoma sinensis in 1875, was first identified in human hosts and is recognized as one of the most significant food-borne parasites, demonstrating widespread prevalence in specific regions of China (1). According to the third national survey on important human parasitic diseases conducted between 2016 and 2021 in China. Approximately 9.46 million (95% BCl, 8.22 million–10.88 million) individuals were estimated to be infected with C. sinensis (2). This disease has been prevalent in nineteen provinces and regions of China, with particularly high infection rates reported in Guangxi, Heilongjiang, Guangdong, and Jilin Provinces (2).
C. sinensis has a multifaceted life cycle characterized by seven developmental stage: eggs, miracidium, sporocyst, redia, cercaria, metacercaria, and adult. Successful completion of its life cycle requires three different types of hosts (Figure 1). In brief, the eggs of C. sinensis, excreted in the feces of the definitive host, are released into aquatic environments and ingested by the first intermediate host, freshwater snail. Within the snail’s gastrointestinal tract, the eggs hatch into micracidia, and subsequently develop into sporocysts, rediae, and cercariae (1). The cercariae are then released into the water and infect the second intermediate hosts, including freshwater fish and freshwater shrimp. Inside these hosts, cercariae encyst in muscle tissue and develop into the metacercariae (3, 4). Definitive mammalian hosts (such as humans, cats, and dogs) acquire infection by consuming the raw or undercooked fish or shrimp containing metacercariae, leading to the development of adult worms in the hepatobiliary system (5). Also, infection with C. sinensis can lead to severe hepatic and gallbladder damage, resulting in conditions such as pyogenic cholangitis, choleithiasis, and even cholangiocarcinoma (6).
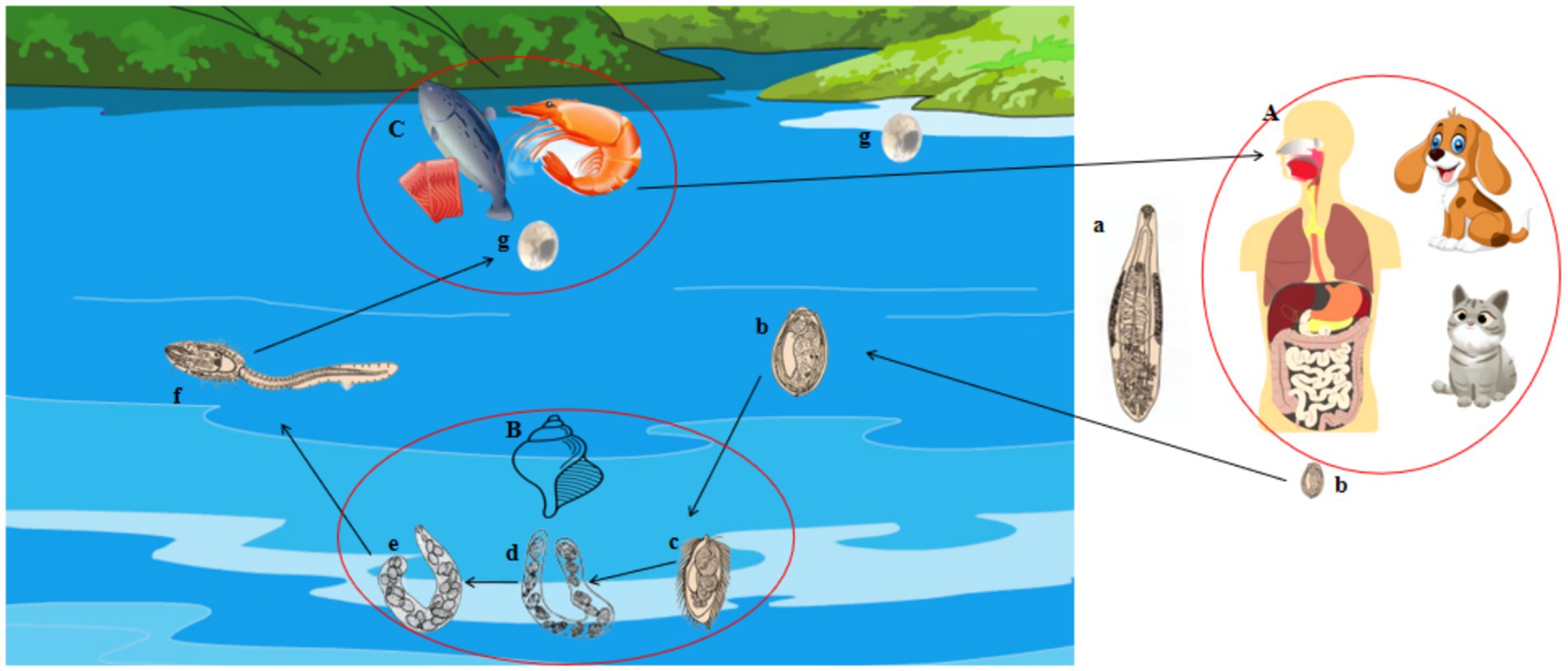
Figure 1. The life cycle of the adult C. sinensis is characterized by several distinct stages. These stages include: (a) the C. sinensis adult; (b) the eggs; (c) sporocysts, (d) sporocysts; (e) rediae; (f) cercariae; (g) metacercariae; (A) the definitive hosts for this organism include human beings, dogs, cats, pigs, and other species; (B) the first intermediate hosts are represented by various species of freshwater snails; (C) the second intermediate hosts consist of freshwater fish and shrimp species.
In recent years, changing dietary preferences have led to the increased consumption of raw or semi-raw fish “yusheng” and “drunken shrimp” in specific regions of China (7, 8). These dietary changes may contribute to a higher incidence of C. sinensis infections among human populations in China (4). Timely diagnosis is essential for detecting the presence of C. sinensis, developing effective prevention strategies, and assessing its prevalence across various host species. This review provides updated information on C. sinensis in China, including its diagnostic methods and prevalence among susceptible hosts, which is critical for the prevention and control of this disease.
2 Methods
2.1 Study protocol
This review was performed in accordance with the guidelines established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (9). The objective was to identify related literature concerning the prevalence of C. sinensis across various host species, as well as the advancement of diagnostic methodologies for the detection of this pathogen within China.
2.2 Searching strategies and information sources
A comprehensive search was conducted across six databases, namely PubMed, Web of Science, Google Scholar, China National Knowledge Infrastructure, Wanfang, and Wipro, to systematically retrieve relevant articles.
In this review, we intend to synthesize the prevalent characteristics of C. sinensis across different host species, with a particular focus on humans and fish, in recent years. Three national surveys have been conducted to investigate the prevalence of significant human parasites, including clonorchiasis, in China during the periods of 1988–1992, 2001–2004, and 2016–2020. Additionally, an analysis of the prevalent characteristics of C. sinensis in China from 2000 to 2016 has been undertaken (10). Consequently, this section is confined to research published from 2010 to April 30, 2025 in both Chinese and English databases. The diagnostic methodologies for the identification of C. sinensis developed in China have been systematically summarized, encompassing literature published from the time of its initial discovery to the present.
2.3 Data synthesis and statistical analysis
Graphs and tables were employed to depict the prevalence of C. sinensis across different host species. The pooled prevalence and its corresponding 95% confidence intervals (95% CI) for C. sinensis were determined using the epidemiological calculator EpiTools software. Potential risk factors influencing its prevalence in humans, including region, gender, age, occupation, education level, ethnic group, and residential location, were examined through Chi-square test in SPSS version 24.0 (SPSS Inc., Chicago, IL, United States). A p-value of less than 0.05 was considered indicative of a statistical difference. In addition, a correlation analysis was conducted using GraphPad. Prism version 9.0 software (GraphPad Software Inc., CA, United States) to assess the concordance between the prevalence of C. sinensis in both fish and human populations.
3 Results
3.1 Current diagnostic methods for detecting Clonorchis sinensis
The prevalence of C. sinensis poses a significant threat to public health in China, thus early diagnosis is essential for mitigating the impact of the disease and reducing treatment costs for affected individuals. In recent years, a range of diagnostic techniques has been developed for detecting C. sinensis in clinical samples, which can be categorized into four primary types: morphological identification that involves the detection of eggs, encysted metacercariae and adult form of C. sinensis; molecular methods that target nucleic acids, serological technologies that identify specific antibodies, and the imaging modalities for the visualization of C. sinensis. This section presents an overview of these diagnostic methods developed in China, their respective characteristics are summarized in Table 1.
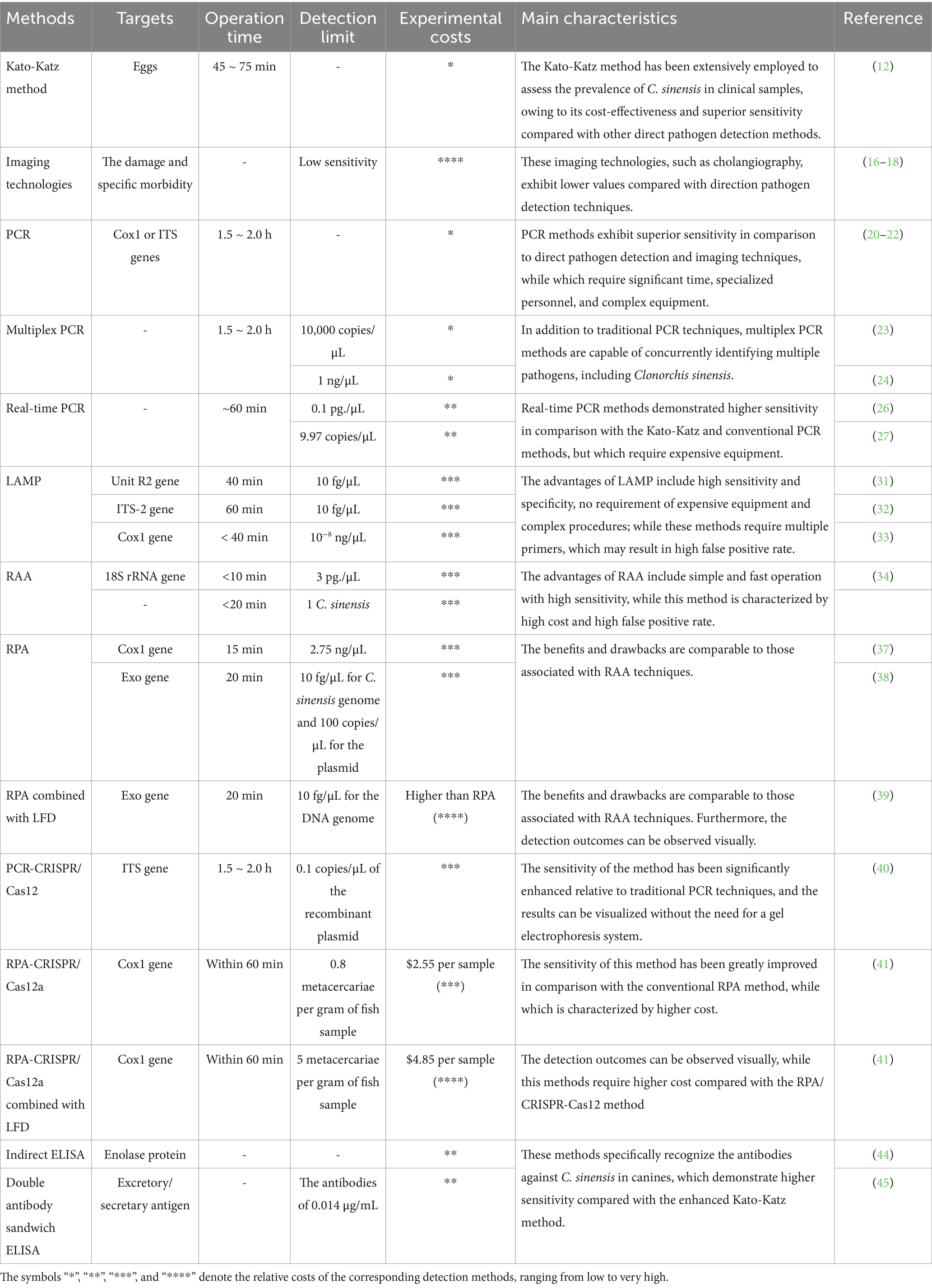
Table 1. The pooled prevalence of C. sinensis across various fish species in different provinces/regions in China.
3.1.1 Traditional detection methods
3.1.1.1 Direct examination
The detection of C. sinensis in fecal samples is considered the “gold standard” for identifying eggs. This diagnostic process incorporates various techniques, including the saturated saltwater flotation method (11), water washing precipitation method (12), and Kato-Katz method (13). Among them, the Kato-Katz method stands is particularly notable for its enhanced sensitivity compared to other diagnostic techniques, which is frequently used to assess the prevalence of C. sinensis in stool samples among humans and other definitive hosts (13–15).
3.1.1.2 Imaging techniques
Imaging techniques, including ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), and cholangiography, have been extensively utilized to detect the damage and specific pathologies associated with particular specific organs and tissues. This application aids in assessing the potential impacts of C. sinensis and other parasitic infections (16).
Among these techniques, cholangiography exhibits a higher sensitivity in detecting the presence of C. sinensis in comparison to other imaging methods (17, 18). The sensitivity and specificity of these imaging technologies are considerably influenced by disease severity, demonstrating lower values when compared to other alternative direct pathogen detection methods. Furthermore, the majority these applications have been primarily focused on the identification of pathogens in human subjects.
3.1.2 Molecular detection approaches
3.1.2.1 Polymerase chain reaction (PCR)
In recent years, conventional PCR techniques have been widely employed for molecular diagnosis of C. sinensis, demonstrating high sensitivity and specificity (19–22). These established PCR methods primarily target conserved genomic regions of C. sinensis, such as the internal transcribed spacer (ITS) and the mitochondrial cytochrome c oxidase subunit 1 (cox1) genes (20). Given the concurrent presence of multiple trematode species in fish, various multiplex PCR approaches have been developed to enable the simultaneous detection of C. sinensis alongside other species (23, 24). For example, Gao et al. developed a multiplex PCR method for the identification of C. sinensis, Metorchis orientalis, and Holostephanus dubinini, with a detection limit of 10,000 copies/μL for all three trematode species (23). Similarly, Li et al. introduced a novel multiplex PCR technique that could concurrently detect the presence of C. sinensis, M. orientalis, Metagonimus yokogawai, Echinochasmus japonicus and Echinostoma hortense, achieving a detection limit of 1 ng/μL for C. sinensis (24).
3.1.2.2 Real-time PCR
Real-time PCR techniques have been widely employed for early diagnosis of various pathogens in clinical samples, primarily owing to their enhanced sensitivity and accuracy in comparison to traditional PCR methodologies (25). Qiao et al. developed a TaqMan-based real-time PCR method for the detection of C. sinensis in gallbladder stone samples, achieving a detection limit of 0.1 pg./μL (26). More recent, a novel TaqMan-based real-time PCR assay has been established for the identification of C. sinensis eggs in fecal samples, which reached a detection limit of 9.97 copies/μL and exhibited superior sensitivity relative to the modified Kato-Katz method (27). In a related vein, Cai et al. introduced an assay that integrates real-time PCR method with high-resolution melting analysis, facilitating the differentiation between C. sinensis and O. viverrini, with a detection limit of 5 eggs per gram, 1 metacercaria, or less than 1 pg. of the purified DNA genome of C. sinensis (28).
3.1.2.3 Loop-mediated isothermal amplification (LAMP)
LAMP is a nucleic acid amplification technique that operates under constant temperature conditions (29). LAMP was initially invented in 2000, it has since been widely applied for the molecular detection of various pathogens (30). In a notable study, Shi et al. introduced a novel LAMP method that specifically targeted the ITS-2 gene of C. sinensis, achieving a detection limit of 10 pg./μL (31). In a similar vein, Sun and colleagues used the Primer Explorer 5.0 software to design three pairs of primers directed at the Unit R2 gene of C. sinensis, thereby establishing an effective LAMP technique (32). This method exhibited a detection limit of 10 fg/μL, demonstrating a sensitivity that was 100 times greater than that of conventional PCR, with results obtainable within a 40-min timeframe (32).
3.1.2.4 Recombinase aided isothermal amplification (RAA)
The RAA technique, akin to the LAMP method, is an isothermal rapid amplification technology used for detecting nucleic acids. This approach is characterized by its ability to maintain constant temperatures, typically between 37°C and 42°C, and offering high specificity, efficiency, and straightforward operational protocols (33). Zhang et al. (34) utilized a conserved region of the 18S rRNA sequence from C. sinensis to design a pair of primers for developing a RAA method, which demonstrating a detection limit of 10 copies/μL of the recombinant plasmid and 3 pg./μL of the C. sinensis genome. Notably, the positive results obtained through this innovative approach could be monitored within 10 min, making it suitable for the detection of C. sinensis in fecal and fish samples (34). Chen et al. (35) used a commercially available RAA detection kit to assess its effectiveness in identifying C. sinensis in freshwater fish specimens. The findings revealed that this kit was capable of specifically detecting a single C. sinensis metacercaria in a 5-gram sample of freshwater fish within 20 min. Furthermore, the detection rate of C. sinensis using the RAA method was found to be significantly superior to that achieved through the direct compression method (35).
3.1.2.5 Recombinase polymerase amplification (RPA)
RPA is a modern isothermal amplification technique that was first introduced in 2006. RPA has gained recognition as a viable alternative to LAMP and RAA methods, due to its numerous advantageous characteristics, such as cost-effectiveness, high sensitivity, rapid processing times, ease of use, and the minimal equipment requirements (36). Zhang et al. (37) employed the conserved region of the cox1 gene from C. sinensis to establish a rapid RPA technique, which can be conducted within 15 min at a constant temperature of 35°C. This innovative method demonstrated a detection limit of 2.75 ng/μL, the results can be visually interpreted without the necessity for specialized equipment. Similarly, Zhang HY developed an RPA method targeting the Exo gene of C. Sinensis, which can be performed at a constant temperature of 39°C and completed within 20 min (38). Moreover, this technique demonstrated high specificity in detecting the nucleic acids of C. sinensis across various developmental stages, with a detection limit of 10 fg/μL for the DNA genome and 100 copies/μL for the plasmid (38).
In a recent investigation, Ma et al. (39) devised a rapid and visually interpretable method for identifying C. Sinensis, utilizing a combination of RPA and lateral flow dipstick technology. The sensitivity of this innovative technique was 10 fg/μL for the DNA genome, with the ability to detect fewer than one purified egg or metacercariae. Furthermore, this technique was performed at 39°C within 20 min, indicating its potential as an alternative tool for the identification of C. sinensis in clinical specimens (39).
3.1.2.6 CRISPR/Cas12 detection system
The clustered regularly interspaced short palindromic repeats (CRISPR) system was identified within the genomes of bacteria and archaea, serving as a “gene weapon” to defend against the intrusion of foreign genetic material (40). Presently, CRISPR/CRISPR-associated protein (Cas) systems are extensively used across diverse domains and are classified into several types based on the genetic variability of Cas gene (29). Among these systems, the CRISPR/Cas12a system has emerged as a promising approach for nucleic acid detection (27). Usually, the sensitivity and convenience of CRISPR/Cas12a system can be significantly improved when combined with the isothermal amplification techniques, such as RPA (41) and LAMP (42).
Xu et al. developed a novel PCR-CRISPR/Cas12 technology for the rapid detection of C. sinensis (43). In this approach, the conserved region of the ITS gene from C. sinensis was amplified using PCR, the resultant PCR products were subsequently introduced into the CRISPR/Cas12a system, enabling the evaluation of detection outcomes based on fluorescence signal intensity (43). The detection limit of this methodology was 0.1\u00B0copies/μL of the recombinant plasmid; however, the operational time was significantly longer compared to other isothermal amplification techniques (40). To mitigate this drawback, an alternative research group proposed a portable detection platform using RPA-CRISPR/Cas12a with dual readout capabilities, specifically targeting the mitochondrial cox1 gene of C. sinensis (41). This innovative method can be completed within 60 min and has a detection limit of approximately 0.8 metacercariae per gram of fish sample, with an estimated cost of 2.55 dollars (41). When this method is combined with a lateral flow assay, the detection limit increases to 5 metacercariae per gram of fish sample, resulting in a total cost of 4.85 dollars (41).
3.1.3 Serological approaches for the detection of Clonorchis sinensis
Currently, there are no commercially available vaccines for C. sinensis infection in China, thus serological detection methods can be applied to distinguish between infections caused by C. sinensis and those uninfected individuals. In recent years, a variety of serological techniques, primarily based on ELISA methodologies, have been developed to identify the specific antibodies against C. sinensis (44, 45). The identification of soluble antigens with high sensitivity and specificity is essential for the enhancement of ELISA methodologies.
Numerous studies have assessed various antigens suitable for the development of ELISA methods. For example, Pan et al. utilized the enolase protein from C. sinensis to create an indirect ELISA, which specifically detected antibodies against C. sinensis in canine subjects (44). Furthermore, the specificity of this ELISA method was markedly superior to that of the enhanced Kato-Katz method, yielding detection rates of 1.13% (4/353) and 0.28% (1/353), respectively (44). Similarly, Zhu et al. used the excretory/secretory antigen (EAS) of C. sinensis to establish a double antibody sandwich ELISA method (45). This technique demonstrated high sensitivity and specificity, enabling the specific identification of antibodies against C. sinensis with a detection limit of 0.014 μg/mL (45).
To improve the detection efficiency and optimize the testing process, Ma et al. developed a point-of-care testing assay for detecting C. sinensis using a Eu-(III) nanoparticles (EuNPs)-tandem repeat sequence 1 (CSTR1) fluorescent probe-based immunoassay (46). In this methodology, EuNPs functioned as signaling probes, which were conjugated with the CSTR1 antigen to selectively binding antibodies specific to C. sinensis. This complex was then analyzed on a test line that emitted a fluorescent signal upon exposure to ultraviolet light (46). Overall, this innovative approach facilitated the specific detection of antibodies against C. sinensis, achieving a detection limit of serum samples corresponding to 24 eggs per gram in fecal examinations, and could be completed within a 10-min timeframe, thereby enhancing the feasibility of point-of-care detection for C. sinensis (46).
3.2 Prevalence of Clonorchis sinensis among various hosts in China
3.2.1 Literature search
As illustrated in the PRISM 2020 flow chart (Figure 2), a comprehensive search across six databases yielded a total of 486 articles examining the prevalence of C. sinensis among different host species in China from 2010 to April 30, 2025. Following the removal of 127 duplicate entries, 359 articles remained. Subsequently, 177 articles were eliminated based on the title and abstract screening, and an additional 50 articles were not available in full text, leading to a total of 132 articles being evaluated for eligibility. However, 26 reports were excluded for several reasons, resulting in the inclusion of 106 studies in this analysis.
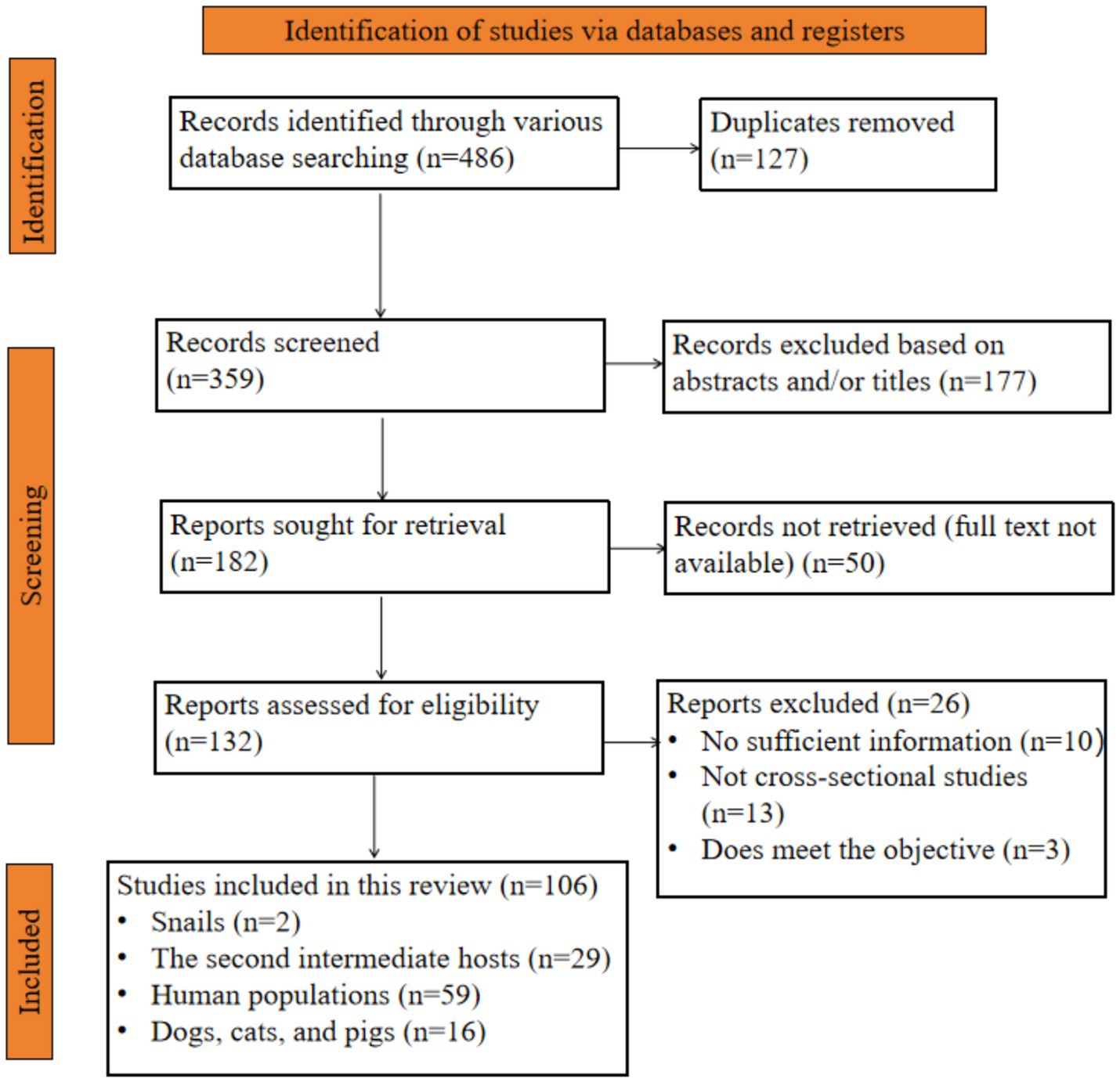
Figure 2. Flow chart of the selected studies that examine the prevalence of C. sinensis across various host species in China.
3.2.2 The prevalence of Clonorchis sinensis among the first intermediate hosts
Snails serve as the primary intermediate host for C. sinensis. Numerous fresh-water snail species are distributed across different regions of China, and more than eight species have been reported to be infected with C. sinensis (1). However, the prevalence of C. sinensis among these snails has often been neglected in recent years. Chen et al. developed a LAMP method to detect C. sinensis in freshwater snails and reported an average detection rate of 19.66% (92/468) (47). The prevalence in Bithynia fuchsianus, Parafossarulus striatulus, and Alocinma longicornis were 33.33% (2/6), 19.46% (87/447), and 20.0% (3/15), respectively (47). In a previous research, 73 (1.15%, 73/630) snails tested positive for C. sinensis eggs in the Zhujiang Delta of Guangdong Province (48). The detection rates among P. striatulus, Parafossarulus sinensis, B. fuchsianus, and A. longicornis were 20.0% (41/204), 11.8% (13/110), 12.3% (12/97), and 8.2% (7/85), respectively (48).
3.2.3 The prevalence of Clonorchis sinensis among the second intermediate hosts
To assess the prevalence of C. sinensis in second intermediate hosts (mainly referred to freshwater fish) in China in recent years, we reviewed 29 representative studies (Supplementary Table 1) conducted from 2010 to to April 30, 2025 covering 13 provinces and cities in China. A total of 26,646 freshwater fish samples were included, among which 4,892 were found to be positive for C. sinensis metacercariae, resulting in an overall infection rate of 18.36% (95% CI 17.90–18.82). As shown in Figure 3, the prevalence of C. sinensis varied widely by region ranging from 0.0% in Shanghai City to 47.75% in Liaoning Province. Notably, four provinces, Heilongjiang, Qinghai, Liaoning, and Shandong, had infection rates exceeding 40%, while five provinces reported ranged between 0.0 to 10.0%.
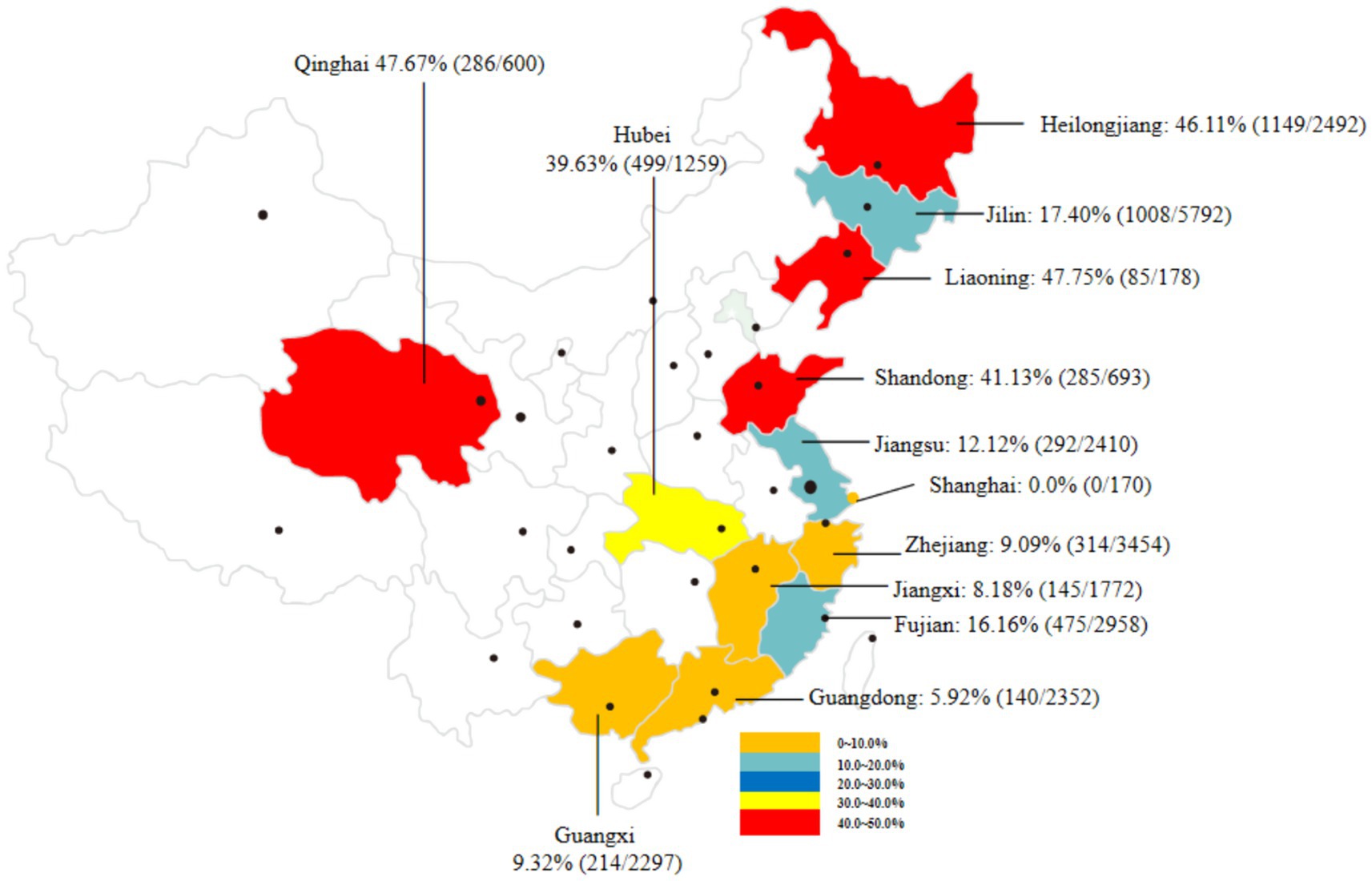
Figure 3. An overview of the prevalence of C. sinensis in various second intermediate hosts from different provinces and regions in China from 2010 to April 30, 2025.
As presented in Table 2, the prevalence of C. sinensis has been investigated in 30 fish species across China. Among these species, Saurogobio dabryi and Abbottina rivularis exhibited the highest detection rates, at 60.53% (172/284) and 59.73% (89/149), respectively. Several freshwater fish species, include Pseudorasbora parva, Carassius auratus, Cyprinus rubrofuscus, Hemiculter leucisculus, Hypophthalmichthys nobilis, and Rhodeus sericeus, have been frequently studied for the prevalence of C. sinensis in China. The average detection rates of these freshwater fishes were 25.68% (4,129/16,073). In addition to freshwater fish, Yu et al. reported an average detection rate of 2.96% (34/1,149) for C. sinensis in samples of Macrobrachium nipponense collected from Zhejiang Province, China (49).
3.2.4 The prevalence of Clonorchis sinensis among the definitive hosts in China
3.2.4.1 The prevalence of Clonorchis sinensis among humans in China
A variety of definitive hosts, including humans, dogs, cats, pigs, and other mammals, are susceptible to C. sinensis infection. This review compiled 59 representative studies (Supplementary Table 2) that investigated the prevalence of C. sinensis among human population in China from 2010 to April 30, 2025. In total, 1,513,994 samples were analyzed across 14 provinces or regions, with 60,306 samples testing positive for either the eggs or specific antibodies against C. sinensis, yielding an overall detection rate of 3.98% (95 CI 3.95–4.01). The detection rates of C. sinensis exhibited considerable variation by province, ranging from 0.0% (0/18,765) to 17.71% (24,549/138,643). The highest prevalence was observed in Guangxi (17.71%, 24,549/138,643) and Jilin (10.07%, 4,144/41,170), both exceeding 10.0%. In the provinces of Jiangxi, Hunan, Guangdong, and Heilongjiang, the prevalence rates ranged from 1.0 to 10.0%, while other provinces, including Beijing, Shandong, Jiangsu, Anhui, Zhejiang, Fujian, and Guizhou, reported prevalence rates below 1.0% (Figure 4). A recent report indicated comparable prevalence characteristics of C. sinensis infection across various provinces, highlighting a notably high crude prevalence in Guangxi, Heilongjiang, Jilin, and Guangdong Provinces (2).
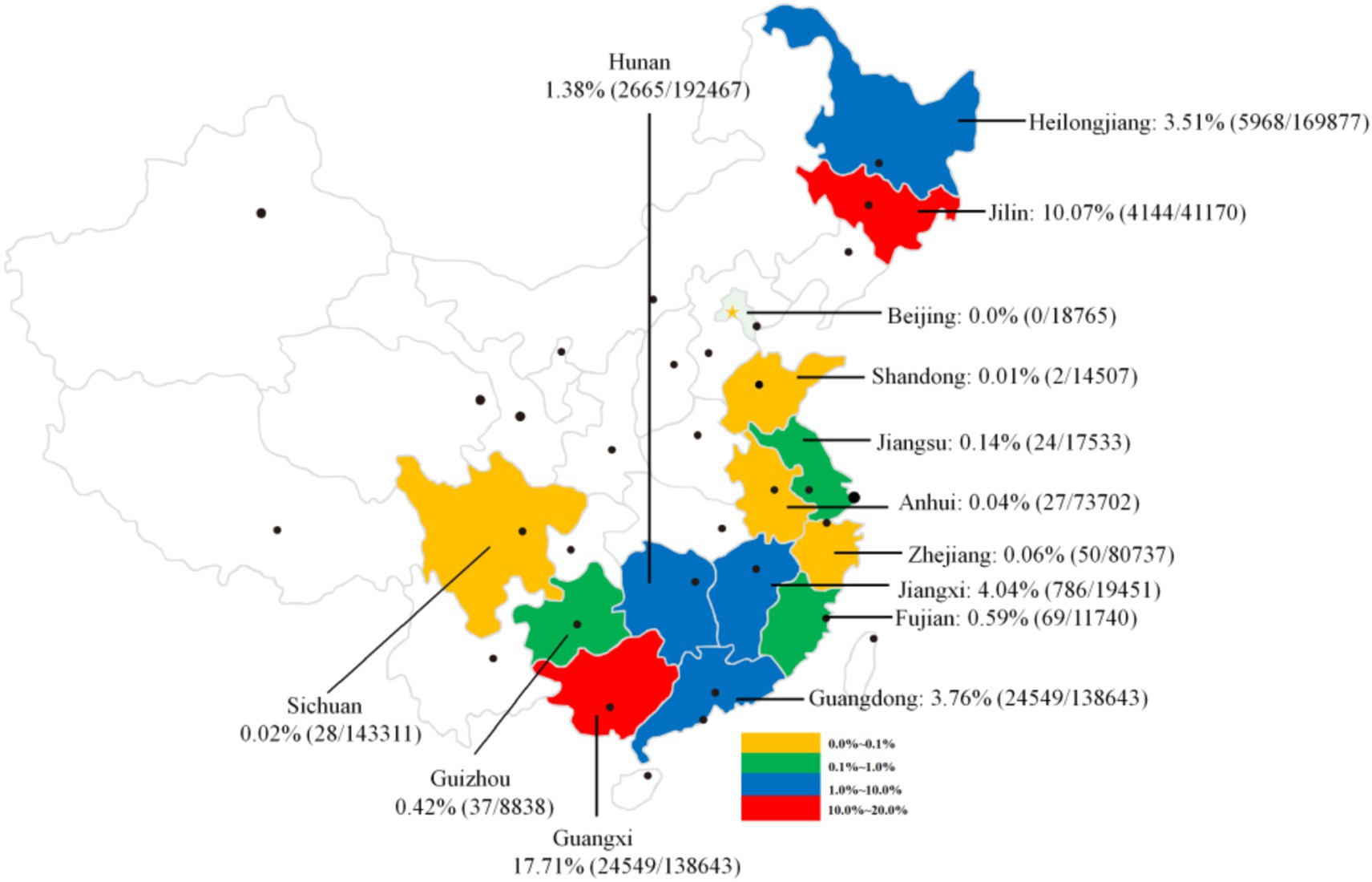
Figure 4. An overview of the prevalence of C. sinensis among human populations across various provinces and regions in China, spanning from 2010 to April 30, 2025.
Next, we conducted an analysis of various potential risk factors associated with the prevalence of C. sinensis, including age, gender, occupation, educational attainment, ethnic groups, and residential location. As demonstrated in Table 3, the data indicated a significant gender disparity in infection rates, with males exhibiting a higher prevalence of C. sinensis infection (6.70%, 33,741/503,477) compared to females (3.10%, 15,879/511,808). Moreover, an analysis of age-related prevalence indicated that the highest detection rate of C. sinensis was observed in individuals aged 30 to 59 years (8.20%, 30,839/376,094), followed by those aged over 60 years (4.99%, 8,650/173,487), while the lowest rate was recorded in individuals under 30 years (1.93%, 4,636/240,048).
The aggregated detection rates of C. sinensis by occupation demonstrated that medical personnel and administrative staff had the highest detection rates, recorded at 7.86% (159/2,022) and 6.31% (2,646/41,955), respectively. The prevalence of C. sinensis among laborers and agricultural workers was 4.49% (2,165/48,228) and 4.20% (18,587/442,408), respectively. However, students and preschool-aged children exhibited the lowest detection rates, recorded at 0.88% (1,522/172,254) and 0.54% (69/12,753), respectively. In terms of educational attainment, the incidence of C. sinensis infection in humans showed a positive correlation with educational levels. Specifically, individuals with a bachelor’s degree or higher had the highest infection rate of infection at 5.85% (2,513/42,038), whereas those with preschool-aged children had the lowest detection rate at 0.42% (255/61,270). Moreover, the detection rate of C. sinensis infection among individuals residing in rural areas (5.20%, 2,612/50,260) was significantly higher than those individuals living in urban settings (3.89%, 648/16,655) in China.
3.2.4.2 The prevalence of Clonorchis sinensis among other definitive animals in China
In recent years, the infection of C. sinensis has been documented not only in humans but also in various animal reservoirs including cats (49, 50), dogs (50), pigs (51), rats (52), and forest musk deer (53). However, research has primarily focused on the prevalence of this pathogen in cats, dogs, and pigs in China (54). In detail, the prevalence of C. sinensis infection among dogs, cats and pigs have been recorded across eight provinces in China (Figure 5), with the average detection rates of 10.25% (171/1,669) for dogs, 14.50% (284/1,958) for cats, and 1.82% (6/329) for pigs.
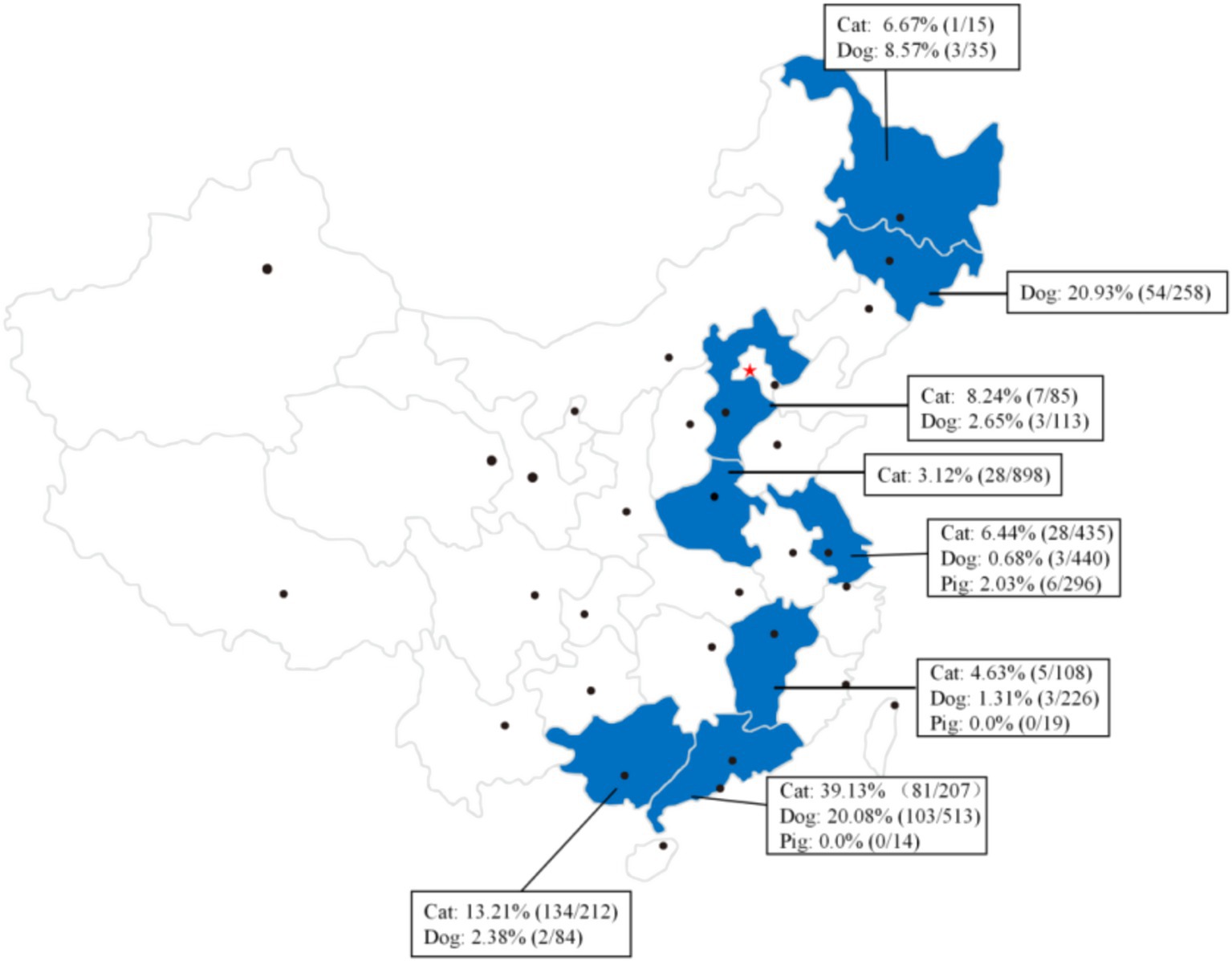
Figure 5. Summary of the prevalence of C. sinensis in canine, feline, and pigs populations across various provinces and regions in China.
4 Perspective and conclusion remarks
Clonorchis sinensis has been acknowledged as a significant risk factor that considerably affects public health in certain regions of China (55, 56). Additionally, this pathogen is identified a major contributor to hepatobiliary diseases, such as cholelithiasis and cholangiocarcinoma (57, 58). The average medical costs for outpatient and inpatient care related to the treatment of C. sinensis infection are 21.72 dollars and 913.80 dollars, respectively (59). This information underscore the considerable economic burden that the widespread occurrence of this parasitic disease places on the public (59). Therefore, it is essential to develop rapid detection approaches and to conduct comprehensive investigations into the prevalence of C. sinensis across various host species, as these initiatives are vital for the prevention and control of the disease.
Given the significant public health concerns linked to C. sinensis infection, a range of detection methodologies have been developed to evaluate the prevalence of this organism across various host species in China. These methodologies can be primarily classified into four distinct categories: traditional parasitological techniques (e.g., Kato-Katz method), imaging modalities, immunological methods, and molecular techniques. Among these, traditional parasitological methods are frequently used to determine the prevalence of C. sinensis infection in different host species. It is noteworthy that the detection rate of C. sinensis using molecular methods, such as PCR, is considerably higher than that achieved with the Kato-Katz method (60). Furthermore, similar to the limitations inherent in the Kato-Katz method, ELISA techniques have low specificity and sensitivity, particularly in instances of mild infection intensity (61).
In light of these challenges, a range of molecular techniques has been developed for detecting C. sinensis, aimed at overcoming the limitations of serological and traditional parasitological methods. However, the implementation of these molecular techniques in clinical studies assessing the prevalence of C. sinensis has been restricted, which may be due to several inherent drawbacks: (1) conventional molecular detection methods, such as PCR and real-time PCR, require complex procedural steps, expensive instrumentation, and considerable time commitments; (2) these innovative visual detection methods, including LAMP (31–33), RPA (37, 38), RAA (34), and the combination of CRISPR/Cas12a with RPA (41), do not demand costly and sophisticated equipment. However, their high sensitivity may lead to an increased occurrence of false-positive results, and the associated high costs of these techniques have further limited their widespread application.
This review presents a comprehensive analysis of C. sinensis epidemiology across various host species in China, focusing on its prevalence in the secondary immediate hosts (freshwater fish species) and the definitive host (human populations). The aggregated detection rates indicate a prevalence of 15.71% (6,325/40,268) in freshwater species and 3.98% (60,306/1,513,994) in human populations. Notably, elevated prevalence rates of C. sinensis were observed among fish in the provinces of Heilongjiang, Liaoning, Shandong, Hubei, Sichuan, and Fujian. In contrast, high human infections were documented in the provinces of Jilin, Guangxi, Heilongjiang, Hunan, and Jiangxi. A weak correlation was identified between the prevalence of C. sinensis in freshwater fish and the human populations, as indicated by an R2 value of 0.0242 (Figure 6), indicating that additional factors beyond fish infection levels may play a significant role in influencing human infection rates.
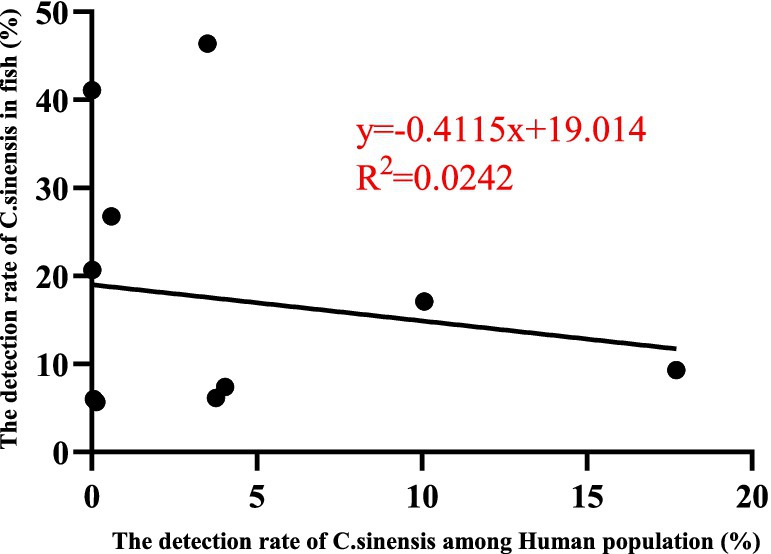
Figure 6. The concordance rate between the detection rates of C. sinensis in various second intermediate hosts and human populations across different provinces and regions (11 points) was analyzed utilizing the GraphPad PRISM version 9.0 software.
The prevalence of C. sinensis across different fish species indicates that certain freshwater fish species exhibit a notably high rate of infection. These species include P. parva, E. ilishaeformis, C. auratus, and A. nobilis, many of which are regarded as optional ingredients in the preparation of “yusheng” in some regions of China. Thus, the consumption of “yusheng” may pose considerable public health risks in these areas where C. sinensis is epidemic (62).
The prevalence of C. sinensis among humans is influenced by several risk factors, including regions, sex, age, occupation, educational attainment, and ethnic groups (Table 3). Specifically, individuals residing in rural areas demonstrating a higher rate of infection in comparison to those living in urban towns and cities in China. Two reasons may contribute to this situation: Firstly, individuals residing in rural areas have a higher risk of contacting C. sinensis, primarily due to dietary practices such as the consumption of raw fish harboring this parasite. Secondly, the availability of medical resources and health education in rural regions is comparatively limited in China. Moreover, the prevalence of C. sinensis infection is elevated among individuals with high education levels. “Yusheng” represents a unique facet of culinary culture with high price in certain regions in China, including Guangxi and Guangdong Provinces (62). Individuals possessing advanced educational qualifications are observed to partake in “Yusheng” more frequently in urban towns and locales, despite having access to substantial medical resources and health education. In summary, these elderly male farmers, migrant workers, and medical personnel with higher levels of education are at an elevated risk of infection in epidemic areas. Consequently, it is imperative to implement targeted interventions aimed at modifying unhealthy dietary practices and enhancing food safety protocols to decrease infection rates within these at-risk populations. Additionally, ongoing monitoring and community engagement initiatives can further mitigate the transmission risks.
In recent years, Chinese government and researchers have made considerable efforts to investigate the epidemiological characteristics of C. sinensis infection across different host species within China. Nonetheless, several limitations in this body of research merit attention: (1) Snails, which are the primary intermediate hosts for C. sinensis, are frequently neglected in epidemiological studies conducted in China; (2) Likewise, domestic cats and dogs, which serve as definitive hosts for C. sinensis infection, are likely to interact with humans and indirectly increase their infection risk, have not been sufficiently studied regarding their prevalence in certain regions in China; (3) The enhanced Kato-Katz method has often been employed to assess the prevalence of C. sinensis across different definitive hosts; however, this method is known for its low sensitivity, which may result in false-negative outcomes in patients and other hosts with mild infections. Given these considerations, future research initiatives should focus on the development of novel diagnostic instruments and the improvement of epidemiological data related to C. sinensis infections in these under-explored host species.
Author contributions
JZ: Writing – review & editing, Formal analysis, Resources, Funding acquisition, Validation, Supervision, Project administration, Writing – original draft, Data curation, Visualization, Methodology, Software, Conceptualization, Investigation. JC: Writing – original draft, Conceptualization. LC: Conceptualization, Writing – original draft. CZ: Writing – original draft, Formal analysis, Conceptualization. LG: Methodology, Writing – original draft. XK: Conceptualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the High Efficiency Production Technology Innovation Team Project for Herbivores Provided by Jiuquan Vocational and Technical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1618633/full#supplementary-material
References
1. Liu, K, Tan, J, Xiao, L, Pan, RT, Yao, XY, Shi, FY, et al. Spatio-temporal disparities of Clonorchis sinensis infection in animal hosts in China: a systematic review and meta-analysis. Infect Dis Poverty. (2023) 12:97. doi: 10.1186/s40249-023-01146-4
2. Qian, MB, Huang, JL, Wang, L, Zhou, CH, Zhu, TJ, Zhu, HH, et al. Clonorchiasis in China: geospatial modeling of the population infected and at risk, based on national surveillance. J Infect. (2025) 91:106528. doi: 10.1016/j.jinf.2025.106528
3. Chen, YD, Zhou, CH, Zhu, HH, Huang, JL, Duan, L, Zhu, TJ, et al. National survey on the current statues of important human parasitic diseases in China in 2015. Chin J Parasitol Parasit Dis. (2020) 38:5–16. doi: 10.12140/j.issn.1000-7423.2020.01.002
4. Li, X, Chen, Y, Huang, G, Sun, X, Mo, G, and Peng, X. Epidemiology and risk factors of Clonorchis sinensis infection in the mountainous areas of Longsheng County, Guangxi: insights from automated machine learning. Parasitol Res. (2025) 124:26. doi: 10.1007/s00436-025-08470-8
5. Wang, W, Huang, X, and Wang, H. Effects of fish-human transmission and different life stages of fish on Clonorchiasis: a novel mathematical model. Math Biosci. (2024) 373:109209. doi: 10.1016/j.mbs.2024.109209
6. Qian, MB, Utzinger, J, Keiser, J, and Zhou, XN. Clonorchiasis. Lancet. (2016) 387:800–10. doi: 10.1016/S0140-6736(15)60313-0
7. Xin, H, Yang, Y, Jiang, Z, Qian, M, Chen, Y, Li, S, et al. An investigation of human Clonorchiasis prevalence in an endemic county in Guangxi Zhuang autonomous region, China, 2016. Food Waterborne Parasitol. (2021) 22:e00109. doi: 10.1016/j.fawpar.2020.e00109
8. Peng, Z, Tan, X, Xiong, P, Ren, Q, Sun, D, and Lin, Z. Case report: intrahepatic cholangiectasis with Clonorchis sinensis infection. Radiol Case Rep. (2024) 19:3258–62. doi: 10.1016/j.radcr.2024.04.085
9. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
10. Lai, YS, Zhou, XN, Pan, ZH, Utzinger, J, and Vounatsou, P. Risk mapping of clonorchiasis in the People's Republic of China: a systematic review and Bayesian geostatistical analysis. PLoS Negl Trop Dis. (2017) 11:e0005239. doi: 10.1371/journal.pntd.0005239
11. Yu, WW, Zu, XF, Zheng, LP, and Xu, JJ. Investigation of epidemic status of Clonorchiasis sinensis in Pucheng country from 2016 to 2021. Parasitosers and Infectious Diseases. (2024) 22:31–4.
12. Qian, MB, Zhuang, SF, Zhu, SQ, Deng, XM, Li, ZX, and Zhou, XN. Improving diagnostic performance of the Kato-Katz method for Clonorchis sinensis infection through multiple samples. Parasit Vectors. (2019) 12:336. doi: 10.1186/s13071-019-3594-5
13. Lin, P, Chen, C, and Zheng, DF. Comparison of four laboratory detection methods of Clonorchiasis. Parasitoses Infect Dis. (2012) 10:233–9.
14. Fang, Y, Wang, J, He, G, Zhang, Q, Xiao, J, Hu, J, et al. Long-term trend analysis of major human helminth infections-Guangdong Province, China, 1988-2021. China CDC Wkly. (2022) 4:912–9. doi: 10.46234/ccdcw2022.188
15. Xu, M, Jiang, Y, Yin, J, Cao, S, Shen, Y, and Cao, J. Risk factors for Clonorchis sinensis infection in residents of Binyang, Guangxi: a cross-sectional and logistic analysis study. Front Public Health. (2021) 9:588325. doi: 10.3389/fpubh.2021.588325
16. Qian, MB, Li, HM, Jiang, ZH, Yang, YC, Lu, MF, Wei, K, et al. Severe hepatobiliary morbidity is associated with Clonorchis sinensis infection: the evidence from a cross-sectional community study. PLoS Negl Trop Dis. (2021) 15:e0009116. doi: 10.1371/journal.pntd.0009116
17. Li, XL, Zhi, FC, Huang, BY, Yang, LC, Xu, TL, Wang, FQ, et al. Comparison study on diagnostic value of ERCP, US and CT on clonorchiasis and clonorchiasis-related cholangiopancreatic diseases. Zhonghua Xiao Hua Za Zhi. (2005) 25:583–5.
18. Tao, LY, Wang, HG, Guo, QM, Liu, SZ, Guo, X, Yang, MY, et al. Peroral cholangioscopy-guided diagnosis and treatment of Clonorchis sinensis liver flukes. Endoscopy. (2024) 56:E498–9. doi: 10.1055/a-2333-9258
19. Tang, YZ, Hao, YL, Sun, QS, Peng, P, Wu, XP, Wang, XL, et al. Identification of Clonorchis sinensis eggs in Jilin and establishment of PCR detection. Hubei Agric Sci. (2013) 52:1601–4. doi: 10.14088/j.cnki.issn0439-8114.2013.07.047
20. Yang, QL, Shen, JQ, Jiang, ZH, Yang, YC, Li, HM, Chen, YD, et al. Identification of Clonorchis sinensis metacercariae based on PCR targeting ribosomal DNA ITS regions and cox1 gene. Chin J Parasit Dis. (2014) 32:217–30.
21. Wang, ZY, Jiang, L, Zhang, YG, He, YY, Ma, XJ, Zhu, Q, et al. Establishment and application of PCR detection technique for Clonorchis sinensis in freshwater fish. J Trop Med. (2018) 18:1435–40.
22. Zhang, X, Sun, B, Tang, Q, Chen, R, and Han, S. Molecular identification and phylogenetic analysis of nuclear rDNA sequences of Clonorchis sinensis isolates from human fecal samples in Heilongjiang Province, China. Front Microbiol. (2019) 10:26. doi: 10.3389/fmicb.2019.00026
23. Gao, JF, Wang, X, Mao, RF, Sun, YY, Wang, CR, and Huang, CQ. Establishment of multiplex PCR for detection of three kinds of trematode metacercariae in freshwater fish. Chin J Prev Vet Med. (2022) 44:157–61. doi: 10.3969/j.issn.1008-0589.202101039
24. Li, B. Survey of helminth infection in freshwater animals and establishment of a multiplex PCR assay to detect five species of zoonotic trematode metacercariae in Heilongjiang Province Heilongjiang Bayi Agricultural University (2024). doi: 10.27122/d.cnki.ghlnu.2024.000089
25. Tan, L, Li, J, Duan, Y, Liu, J, Zheng, S, Liang, X, et al. Current knowledge on the epidemiology and prevention of avian leukosis virus in China. Poult Sci. (2024) 103:104009. doi: 10.1016/j.psj.2024.104009
26. Qiao, T, Zheng, PM, Ma, RH, Luo, XB, and Luo, ZL. Development of a real-time PCR assay for the detection of Clonorchis sinensis DNA in gallbladder bile and stone samples from patients with cholecystolithiasis. Parasitol Res. (2012) 111:1497–503. doi: 10.1007/s00436-012-2986-7
27. Ma, WC, Yang, D, Yu, X, Zhao, XY, and Li, WJ. Taqman MGB real-time PCR method for detection of Clonorchis sinensis eggs in human feces. Acta Parasitol Med Entomol Sin. (2023) 30:143–7. doi: 10.3969/j.issn.1005-0507.2023.03.003
28. Cai, XQ, Yu, HQ, Li, R, Yue, QY, Liu, GH, Bai, JS, et al. Rapid detection and differentiation of Clonorchis sinensis and Opisthorchis viverrini using real-time PCR and high resolution melting analysis. Sci World J. (2014) 2014:893981. doi: 10.1155/2014/893981
29. Feng, Y, He, T, Zhang, B, Yuan, H, and Zhou, Y. Epidemiology and diagnosis technologies of human metapneumovirus in China: a mini review. Virol J. (2024) 21:59. doi: 10.1186/s12985-024-02327-9
30. Yang, L, Sun, Y, Sun, L, Wang, Z, Feng, J, and Liang, Y. Application of loop-mediated isothermal amplification in plant pathogen detection. Phytopathology. (2025) 115:6–13. doi: 10.1094/PHYTO-10-23-0391-KC
31. Shi, W, Huang, KH, Liu, ZL, and Huang, WY. Preliminary establishment of LAMP technology for detecting DNA of Clonorchis sinensis eggs in fecal samples. Heilongjiang Anim Sci Vet Med. (2012) 9:110–3.
32. Sun, XJ, Ma, Q, Tian, T, Zhang, L, Liu, LJ, Yao, J, et al. Establishment and priliminary application of a LAMP assay for the detection of Clonorchis sinensis. Heilongjiang Anim Sci Vet Med. (2024) 13:47–51. doi: 10.13881/j.cnki.hljxmsy.2024.01.0017
33. Li, HM, Qian, MB, Yang, YC, Jiang, ZH, Wei, K, Chen, JX, et al. Performance evaluation of existing immunoassays for Clonorchis sinensis infection in China. Parasit Vectors. (2018) 11:35. doi: 10.1186/s13071-018-2612-3
34. Zhang, Q, Ding, X, Wu, XM, Liu, YH, Liu, JF, Xu, XZ, et al. Establishment and priliminary evaluation of recombinase aided isothermal applification (RAA) assay for specific nucleic acid detection of Clonorchis sinensis. Chin J Schisto Control. (2019) 31:468–73. doi: 10.16250/j.32.1374.2019178
35. Chen, JF, Wang, ZQ, Huang, WM, Wang, J, Chen, LJ, Sun, YL, et al. Priliminary application of recombinase-aided amplification in detection of Clonorchis sinensis metacercariae in freshwater fish. Chin J Schisto Control. (2023) 35:458–63. doi: 10.16250/j.32.1374.2023020
36. Lobato, IM, and O'Sullivan, CK. Recombinase polymerase amplification: basics, applications and recent advances. Trends Analyt Chem. (2018) 98:19–35. doi: 10.1016/j.trac.2017.10.015
37. Zhang, CL, Qiu, YY, Hao, S, Bai, X, Liu, MY, Zhang, LX, et al. Development of RPA assay for the detection of Clonorchis sinensis metacercaria. Chin Vet Sci. (2021) 51:411–55. doi: 10.16656/j.issn.1673-4696.2021.0066
38. Zhang, HY. Establishment and application of RPA visual detection method for Clonorchis sinensis Jilin University (2024). doi: 10.27162/d.cnki.gjlin.2024.004682
39. Ma, X, Bai, X, Li, H, Ding, J, Zhang, H, Qiu, Y, et al. A rapid and visual detection assay for Clonorchis sinensis based on recombinase polymerase amplification and lateral flow dipstick. Parasit Vectors. (2023) 16:165. doi: 10.1186/s13071-023-05774-5
40. Mao, Z, Chen, R, Wang, X, Zhou, Z, Peng, Y, Li, S, et al. CRISPR/Cas12a-based technology: a powerful tool for biosensing in food safety. Trends Food Sci Technol. (2022) 122:211–22. doi: 10.1016/j.tifs.2022.02.030
41. Huang, T, Li, L, Li, J, Li, X, Li, S, Wang, X, et al. Rapid, sensitive, and visual detection of Clonorchis sinensis with an RPA-CRISPR/Cas12a-based dual readout portable platform. Int J Biol Macromol. (2023) 249:125967. doi: 10.1016/j.ijbiomac.2023.125967
42. Lei, L, Liao, F, Tan, L, Duan, D, Zhan, Y, Wang, N, et al. LAMP coupled CRISPR-Cas12a module for rapid, sensitive and visual detection of porcine circovirus 2. Animals (Basel). (2022) 12:2413. doi: 10.3390/ani12182413
43. Xu, Y, Liu, T, Xu, H, Zeng, XJ, Lan, YM, Gong, ZH, et al. Establishment and application of PCR-CRISPR/Cas12a-based detection method for Clonorchis sinensis metacercaria. Chin J Parasitol Dis. (2023) 41:421–7. doi: 10.12140/j.issn.1000-7423.2023.04.004
44. Pan, C, Yang, Y, Chen, XQ, and Du, AF. Development and primary application of an indirect ELISA for detecting Clonorchis sinensis. Chin J Vet Sci. (2015) 35:1792–8. doi: 10.16303/j.cnki.1005-4545.2015.11.13
45. Zhu, MC, Liu, JF, Lu, ZK, and Kong, DL. Establishment of monoclonal antibodies against excretory/secretory antigen of Clonorchis sinensis and development of a quantitative double-antibody sandwich ELISA for ESA. Chin J Zoonoses. (2018) 34:873–7. doi: 10.3969/j.issn.1002-2694.2018.00.154
46. Ma, X, Zhang, H, Fang, Y, Wang, J, Wang, X, Li, C, et al. A point-of-care testing assay for Clonorchiasis using a EuNPs-CsTR1 fluorescent probe-based immunoassay. PLoS Negl Trop Dis. (2024) 18:e0012107. doi: 10.1371/journal.pntd.0012107
47. Chen, Y, Wen, T, Lai, DH, Wen, YZ, Wu, ZD, Yang, TB, et al. Development and evaluation of loop-mediated isothermal amplification (LAMP) for rapid detection of Clonorchis sinensis from its first intermediate hosts, freshwater snails. Parasitology. (2013) 140:1377–83. doi: 10.1017/S0031182013000498
48. Zhang, RL, Gao, ST, Geng, YJ, Huang, DN, Yu, L, Zhang, SX, et al. Epidemiological study on Clonorchis sinensis infection in Shenzhen area of Zhujiang delta in China. Parasitol Res. (2007) 101:179–83. doi: 10.1007/s00436-006-0441-3
49. Yu, XT, Luo, XJ, Chen, PF, Zhang, GM, Chen, ZX, Wang, XH, et al. An investigation on the status of wild freshwater fish and shrimp infected with metacetcaria of Clonorchis sienensis in Jinhua City. Zhejiang Preventive and Medicine. (2015) 7:722–4. doi: 10.19485/j.cnki.issn1007-0931.2015.08.005
50. Lin, RQ, Tang, JD, Zhou, DH, Song, HQ, Huang, SY, Chen, JX, et al. Prevalence of Clonorchis sinensis infection in dogs and cats in subtropical southern China. Parasit Vectors. (2011) 4:180. doi: 10.1186/1756-3305-4-180
51. Xiao, B. Diagnosis and treatment report of a case of Clonorchiasis in pigs. Chin J Anim Husb Vet Med. (2015) 11:80.
52. Liu, L, Zhang, FY, Zhang, J, and Zhang, H. A comparative study of adult Clonorchis sinensis parasitized in experimental mice and human. Lab Anim Sci. (2022) 39:53–6.
53. Cai, CH, Zhang, ZP, Zuo, MY, Guo, LP, Fu, ZH, and Liu, YR. Trematode egg internal transcribed space-2 sequence based analysis for diagnosis of Opisthorchis viverrini and Opisthorchis sinensis infections. Chin J Parasitol Parasit Dis. (2023) 41:427–32. doi: 10.12140/j.issn.1000-7423.2023.04.005
54. Dai, Y, Xu, X, Liu, J, Jin, X, Shen, M, Wang, X, et al. Prevalence of intestinal helminth infections in Jiangsu Province, eastern China; a cross-sectional survey conducted in 2015. BMC Infect Dis. (2019) 19:604. doi: 10.1186/s12879-019-4264-0
55. Yang, QL, Lu, XW, Fang, ZL, Gao, YQ, He, YN, Huang, Y, et al. The association between Clonorchis sinensis seropositivity and hepatocellular carcinoma in an endemic area: a study in Guangxi, China. BMC Infect Dis. (2025) 25:270. doi: 10.1186/s12879-025-10675-2
56. Feng, Y, Yu, K, Chen, H, Zhang, X, Lu, Q, Wang, X, et al. Soil-transmitted helminths, intestinal protozoa and Clonorchis sinensis infections in Southeast China. BMC Infect Dis. (2021) 21:1195. doi: 10.1186/s12879-021-06879-x
57. Qian, M-B, Keiser, J, Utzinger, J, and Zhou, X-N. Clonorchiasis and opisthorchiasis: epidemiology, transmission, clinical features, morbidity, diagnosis, treatment, and control. Clin Microbiol Rev. (2024) 37:e0000923. doi: 10.1128/cmr.00009-23
58. Chu, Y, Shi, D, Wang, N, Ren, L, Liu, N, Hu, F, et al. Clonorchis sinensis legumain promotes migration and invasion of cholangiocarcinoma cells via regulating tumor-related molecules. Parasit Vectors. (2023) 16:71. doi: 10.1186/s13071-023-05694-4
59. Zhong, JF, Wang, M, Luo, L, and Huang, ZJ. Study on the burden of Clonorchiosis in a city of the Pearl River Delta from 2019 to 2023. Modern Preventive Medicine. (2025) 52:424–9.
60. Xu, M, Yin, JH, Cao, SK, Cao, JP, Zhang, XF, and Shen, YJ. Comparison of efficiency of Kato-Katz technique and PCR assay for detecting Clonorchis sinensis infection. Chin J Schisto Control. (2019) 31:165–8. doi: 10.16250/j.32.1374.2018233
61. Li, J, Macdonald, J, and von Stetten, F. Review: a comprehensive summary of a decade development of the recombinase polymerase amplification. Analyst. (2018) 144:31–67. doi: 10.1039/c8an01621f
Keywords: Clonorchis sinensis, prevalence, different hosts, detection approaches, China
Citation: Zhang J, Cao J, Cao L, Zhang C, Gong L and Kang X (2025) Current knowledge on the prevalence and detection techniques of Clonorchis sinensis in China. Front. Vet. Sci. 12:1618633. doi: 10.3389/fvets.2025.1618633
Edited by:
Gustavo Viozzi, National University of Comahue, ArgentinaReviewed by:
Verónica Roxana Flores, National University of Comahue, ArgentinaMartin Miguel Montes, National Council of Scientific and Technical Research (CONICET), Argentina
Copyright © 2025 Zhang, Cao, Cao, Zhang, Gong and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianming Zhang, Z2FsbGFudHpoYW5nQDE2My5jb20=
 Jianming Zhang
Jianming Zhang Jian Cao
Jian Cao