- 1Department of Fish Nutrition, Faculty of Aquatic and Fisheries Sciences, Kafrelsheikh University, Kafrelsheikh, Egypt
- 2College of Fisheries and Life Science, Shanghai Ocean University, Shanghai, China
- 3Department of Aquaculture, Faculty of Aquatic and Fisheries Sciences, Kafrelsheikh University, Kafrelsheikh, Egypt
- 4Department of Fish Nutrition and Feed Technology, Central Laboratory for Aquaculture Research, Agricultural Research Center, Abbassa, Abo-Hammad, Egypt
- 5Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafrelsheikh, Egypt
- 6Department of Fish Production, Faculty of Agriculture, Al-Azhar University, Cairo, Egypt
- 7Department of Pharmaceutical Sciences, College of Pharmacy, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
- 8College of Veterinary Medicine, University of Al Dhaid, Sharjah, United Arab Emirates
- 9Department of Animal Medicine, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafrelsheikh, Egypt
- 10Department of Physiology, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafrelsheikh, Egypt
- 11Department of Integrative Agriculture, College of Agriculture and Veterinary Medicine, United Arab Emirates University, Abu Dhabi, United Arab Emirates
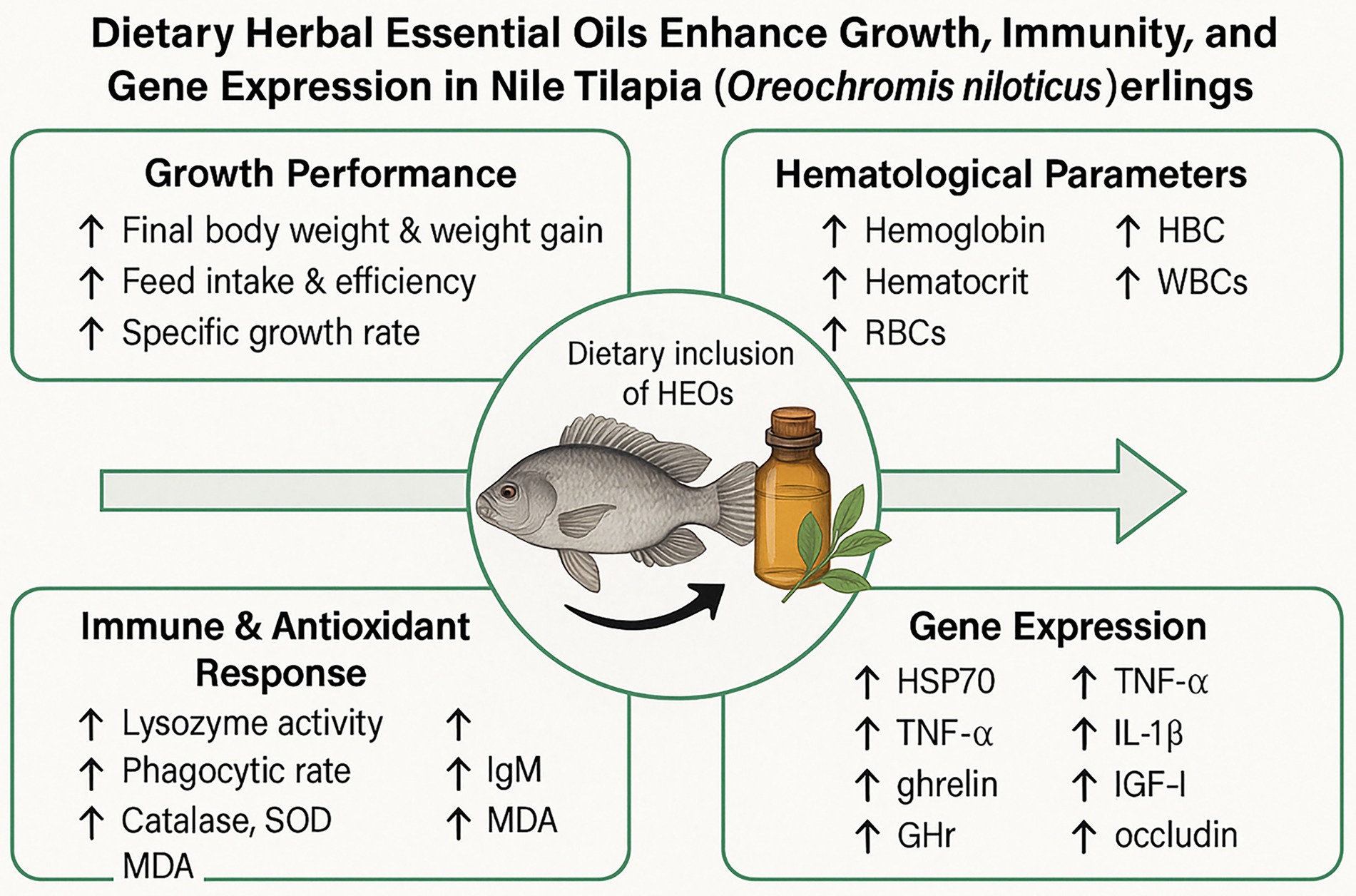
Introduction: The increasing global demand for sustainable aquaculture practices has prompted the search for natural and effective alternatives to synthetic feed additives. Herbal essential oils (HEOs) have emerged as promising candidates due to their bioactive properties that support growth, health, and immunity in fish.
Methods: This study evaluated the effects of dietary supplementation with blended HEOs—comprising carvacrol, oregano oil, 1,8-cineole, thymol, α-pinene, β-pinene, limonene, and propylene glycol—on growth performance, hematological indices, antioxidant status, immune response, intestinal morphology, and gene expression in Nile tilapia (Oreochromis niloticus) fingerlings. Over a 72-day trial, fish were fed diets with 0 (control), 30, 60, 120, and 240 mL/kg of HEOs.
Results and discussion: The 30 and 60 mL/kg groups showed significantly improved final body weight, weight gain, specific growth rate, and feed conversion ratio (p < 0.05). Hematological parameters increased, while serum cholesterol and triglyceride levels decreased. Enhanced lysozyme activity, phagocytic rate, IgM concentration, and antioxidant enzymes (SOD and CAT) were observed in the 30 and 60 mL/kg groups. Additionally, these doses significantly upregulated the expression of growth- and immunity-related genes (GHr, IGF-I, IL-1β, TNF-α, ZO-1, and occludin) while downregulating HSP70, indicating improved stress resilience. Histological analysis revealed increased villi height and surface area in the intestine, suggesting better nutrient absorption. These findings demonstrate that dietary supplementation with 30–60 mL/kg of HEOs can enhance physiological and immunological health, offering a natural strategy to improve Nile tilapia aquaculture productivity.
1 Introduction
Aquaculture is a rapidly expanding endeavor throughout all continents, rising at an approximate pace of 7% annually, and constitutes over half of the fish consumed by humans (1). Aquaculture farms need to optimize feed ingredients to improve the digestibility of the immune system, decrease FCR microbiota, and reduce fish production costs (2). Freshwater fish farming accounts for over two-thirds of global aquaculture output (3). Nile tilapia is the second most cultivated fish globally, following carp, and accounted for 8.3% of global fish production in 2018 (4).
Sufficient nutrition is essential for sustaining healthy, disease-free fish in diverse aquaculture settings. Natural immunostimulants, growth enhancers, and medicinal herbs are now considered safe and effective alternatives to chemotherapy and antibiotics in aquaculture settings (5). Essential oils (EOs) extracted from plants have been utilized in aquaculture research because they enhance animal health, growth, and welfare (6). Furthermore, incorporating plant-based foods into fish feed is highly intriguing in aquaculture; hence, plant-based extracts should be considered to enhance growth performance and health (probiotics) (7). It has been observed that phenolic chemicals found in essential oils (EOs), including thymol, carvacrol, p-cymene, and ɞ-terpinene, can inhibit both gram-negative and positive pathogens (8). Essential oils include antimicrobial, antiviral, and antifungal characteristics; as a result, they gained popularity as natural feed additives (9). Research on feed additives for farmed fish, including phytogenics, essential oils, prebiotics, and probiotics, has been ongoing for quite some time, and the results have been promising in terms of safety, cost-effectiveness, and environmental friendliness (10).
Essential oils’ many beneficial effects, including their ability to fight cancer, alleviate pain, promote growth, and protect the liver, have increased their use in recent years (11). Immunostimulants derived from medicinal plants, such as spices, seaweeds, and herbs, have a long history of use. They are eco-friendly, cheap, easy to make, and deliver an immune-system-safe substitute for antibiotics and immunoprophylaxis. The active chemicals in these plants possess antibacterial, growth-promoting, immune-enhancing, and stress-relieving characteristics in fish (12, 13).
Better digestion is possible with the help of essential oils since they increase the absorption of broken-down molecules and stimulate enzymes. Antioxidant and antibacterial properties are demonstrated by Hendam et al. (14). Due to their benefits for digestion, gut microbial ecology, growth, and welfare, EOs may 1 day supplement animal diets instead of antibiotics (15). Eos and other natural dietary supplements with bioactive compounds can boost fish’s feed efficiency and growth performance by enhancing digestive secretion (16, 17). Juvenile tilapia fed a diet with OAB had better intestinal health and a higher survival rate (18). Considering that the purpose of this study was to examine the impact on immune response, feed utilization, growth performance, biochemical and hematological variables, and genes related to immunity by supplementing the diet of fingerling Nile tilapia, O. niloticus, with a mixture of herbal essential oils (HEOs).
2 Materials and methods
2.1 Experimental design and fish diets
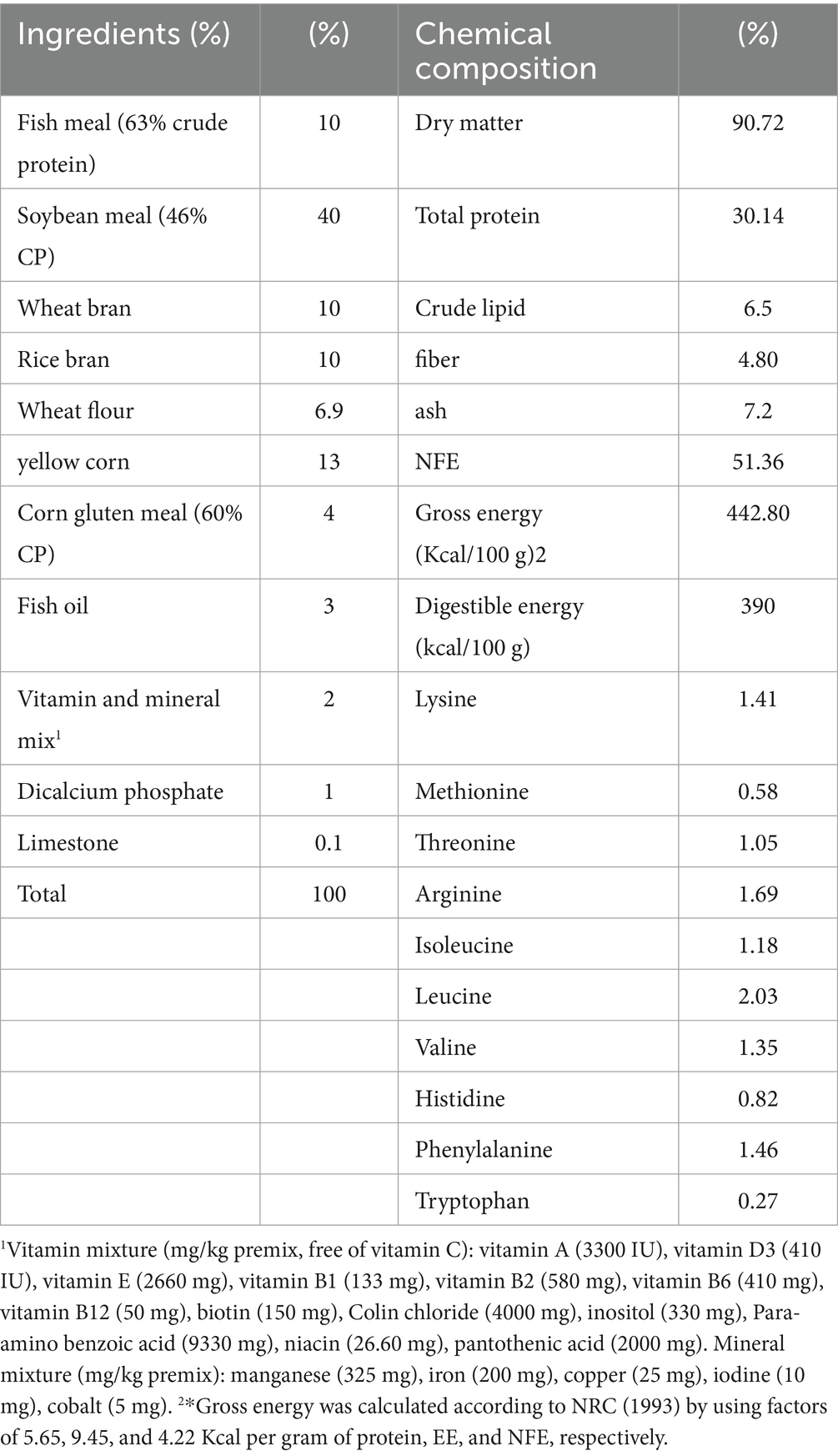
Five experimental diets were created to meet the specific dietary needs of fingerlings of Nile tilapia, all of which were isonitrogenous and had the same amount of calories. Five groups were assigned different diets: Group 1 (G1, HEOs 0 mL/kg) received no chemicals, and Groups 2 (G2, HEOs 30 mL/kg) and 3 (G3, HEOs 60 mL/kg) received 30 and 60 mL of herbal essential oils (HEOs) per kilogram of feed, respectively. Supplementation levels were increased for Groups 4 (G4, HEOs 120 mL/kg) and 5 (G5, HEOs 240 mL/kg) to 120- and 240-ml HEOs/kg of diet, respectively—Supplementary Table S1 - GC–MS Composition of HEO Blend.
The experimental diets were formulated to be isonitrogenous and isolipidic, with a target of 32% crude protein and 7% lipid. All ingredients were dried to constant weight and mixed on a dry matter (DM) basis. Proximate composition was determined according to AoOA Chemists and AoOA Chemists (19) methods: crude protein by the Kjeldahl method (Method 2001.11), crude lipid by ether extraction (Method 920.39), ash by incineration at 550°C (Method 942.05), and moisture by oven drying at 105°C (Method 930.15).
The basic elements of the diet include fish meal (the main source of animal protein), wheat bran, rice bran, wheat flour, soybean meal, corn gluten, yellow maize, di-calcium phosphate, fish oil, limestone, and a premixed vitamin and mineral blend. Five equal quantities of the materials were measured for each treatment after thorough mixing Table 1.
The liquid herbal essential oils (TRI VIR, Tri Pharma Company, Egypt) were added to each treatment diet in a specific dosage. These oils included carvacrol (45 g/L), oregano oil (45 g/L), 1,8-cineole (16 g/L), thymol (39.2 g/L), α-pinene (4.6 g/L), β-pinene (2.6 g/L), limonene (3 g/L), and propylene glycol (150 g/L). Each batch was mixed with fish oil after the HEOs were added, and then 400 cc of water per kilogram of feed was added to make a homogenous dough. To make pellets that are just right for fingerlings, this dough was run through laboratory pelleting equipment fitted with a 2 mm die. The pellets were left to dry at room temperature for a full day before being sealed in plastic bags and kept at 4°C until needed. We used industry-standard methods to examine the diets’ chemical makeup (20).
2.2 Experimental setup
Nile tilapia were collected from a freshwater farm with no prior health issues or mortality. Fish were sedated onsite using MS-222 (40 mg/L, Syndel, Canada), then transported in labeled plastic bags containing one-third clean water and two-thirds pure oxygen. Upon arrival at the Animal Health Research Institute, Agriculture Research Center, Kafrelsheikh, Egypt, fish underwent a 10-min iodine bath (20 ppm Betadine®, 5% povidone-iodine, Nile Company for Pharmaceuticals) (21).
At first, the Nile tilapia fingerlings were kept in a 1,000-liter fiberglass tank at the Sakha Aquaculture Research unit after being acquired from a private fish farm in Kafrelsheikh, Egypt. Fifteen days before the trial’s start, the fish were given a commercial meal with 30 % crude protein to help them adjust to the lab environment. After acclimatization, 300 fingerlings (27.20 ± 0.06 g) were accidentally allocated to 30 glass aquaria (each with a capacity of 60 liters), with 10 fish stocked per tank. Three aquaria were designated for each dietary treatment, and all tanks were continuously aerated using electric air pumps. The photoperiod was maintained at 12 h light and 12 h dark using programmable LED lighting. Initial biomass density was approximately 4.5 kg/m3 (10 fish averaging 27.2 g per 60-L tank). Dissolved oxygen was measured daily at 9:00 a.m. and 5:00 p.m. using a DO meter (YSI Professional Plus), and ranged from 6.5 to 7.0 mg/L across all tanks. Tank positions were randomized at the beginning of the experiment using a computer-generated random number sequence and re-randomized weekly to eliminate potential positional or environmental bias (light, airflow). Dechlorinated water was used throughout the study. Waste was removed daily by siphoning, with 50% of the water in each tank replaced daily, and a full water change was carried out once weekly following cleaning. Fish were fed the experimental diets twice daily—at 8:00 a.m. and 2:00 p.m.—for 72 days. Feeding was based on 3% of the fish’s body weight and was adjusted biweekly according to growth. Water quality parameters were regularly monitored, with the following average values recorded: temperature at 28.53 ± 0.01°C, dissolved oxygen at 6.73 ± 0.14 mg/L, pH at 7.62 ± 0.1, and total ammonia nitrogen at 0.38 ± 0.02 mg/L.
2.3 Growth and feed efficiency
The duration of the feeding trial was 72 days. Each fish in each tank was weighed before and after the experiment to see how much they had grown. The fish were fasted for 24 h before the final sampling to reduce stress. The following equations were used to assess the efficiency of feed use and growth performance:
1. Final Weight Gain (%) = ((Final Mean Body Weight–Initial Mean Body Weight)/Initial Mean Body Weight) × 100
2. Weight Gain (%) = 100 × (Final Average Body Weight− Initial Average Body Weight)/Initial Average Body Weight
3. Daily Weight Gain (g/fish/day) = Total Weight Gain(g)/Duration of Experiment (days)
4. Specific Growth Rate (SGR; %/day) = {ln (Final Body Weight) − ln (Initial Body Weight)} × 100/Number of Days, where ln is the natural logarithm.
5. Feed Conversion Ratio (FCR) = Dry Feed Intake(g)/Wet Weight Gain(g)
6. Protein Efficiency Ratio (PER) = Weigh Gain(g)/Protein Intake(g)
7. Survival Rate (%) = (Final Number of Fish/Initial Number of Fish) × 100
2.4 Sampling
Prior to sampling, fish were fasted for 24 h to eliminate postprandial effects on hematological and biochemical parameters. Blood was collected from the caudal vein using 1 mL heparinized syringes under anesthetic conditions.
Fish blood was drawn from the caudal vertebral vein at the end of the experiment using sterilized hypodermic syringes and an anesthetic. Blood samples were separated into two tubes. Hematological tests were performed in the first tube using EDTA as an anticoagulant. The second tube without an anticoagulant was left at room temperature for 3 h to allow clot formation. After centrifuging the clotted blood at 3000 RPM for 15 min, serum was extracted. The serum was stored at −20°C until biochemical analysis.
2.4.1 Determination of haemato-immunological variables
Hb, WBC, and (RBC) counts were all measured following an established protocol (22). The differential leukocytic counts were performed using Giemsa-stained blood smears. The microhematocrit technique is used to determine the packed cell volume (PCV).
2.4.2 Determination of blood biochemical variables, antioxidative status, and immunity
Serum total protein and albumin concentrations were measured using commercially available colorimetric assay kits, following the manufacturer’s protocols (23, 24).
By subtracting albumin values from total protein measurements, globulin levels were estimated. The RA-50 semi-automated chemistry analyzer (Bayer) was used to measure several biochemical parameters in the blood, such as amylase and lipase enzyme activity (AST and ALT), immunoglobulin M (IgM), creatinine, urea, uric acid, total cholesterol, and triglycerides. The biochemical assays were carried out using diagnostic kits provided by Spinreact Co., Spain, following the manufacturer-specified protocols.
A turbidimetric method was used to assess the activity of serum lysozyme (25), produced by studying the gram-positive bacteria Micrococcus lysodeikticus (Sigma, United States). We used the following approaches to evaluate leukocyte phagocytes (26). The phagocytosis assay smear was used to quantify the amount of leukocytes that absorbed bacteria as a ratio to the total leukocyte count. The phagocytic index and phagocytic activity were evaluated according to the methods described by Kawahara et al. (27).
Serum (MDA) concentrations were assessed with the thiobarbituric acid assay. The samples were calorimetrically cleared utilizing a commercial kit (LPO 586 Kit, OXIS International Inc., Portland, United States) as outlined by Ohkawa et al. (28). The serum (SOD) measurement follows McCord and Fridovich (29), and (CAT) following Aebi (30) using (Biodiagnostic Co., Giza, Egypt).
2.5 Gene expression
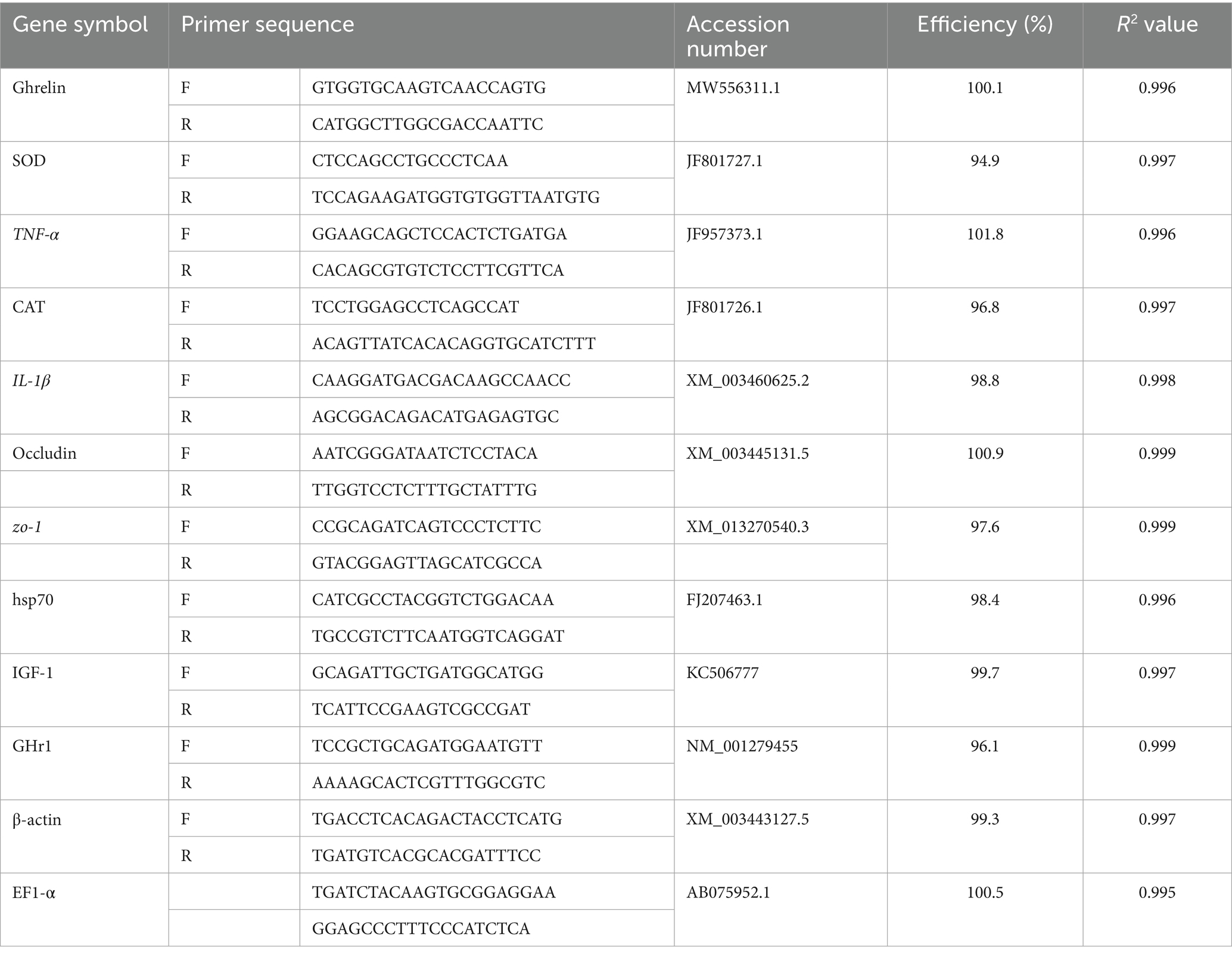
The Quantitative gene expression analysis was done using real-time reverse transcription PCR (RT-PCR). The genes EF1-α and β-actin served as internal controls for data normalization (primer sequences are provided in Table 2). Total RNA was isolated from tissue samples using Trizol reagent (iNtRON Biotechnology), following the manufacturer-supplied protocol. The integrity of RNA was confirmed through electrophoresis on a 2% agarose gel, and concentrations were determined using a Nanodrop spectrophotometer (Quawell, United States).
To synthesize cDNA, 2 μg of high-quality total RNA from each sample was reverse transcribed using a Bioline cDNA synthesis kit (United Kingdom), adhering to the manufacturer’s recommendations. PCR amplification was conducted using the SensiFast SYBR Lo-Rox kit (Bioline) in a 20 μL total volume. Each reaction included 2 μL of cDNA template, 10 μL of SYBR Green master mix, and 0.5 μM of each primer.
The thermal cycling program included an initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 min. Amplification data and Ct values were collected automatically using the Rotor-Gene Q system (Qiagen, Valencia, CA, United States). The relative quantification of target gene expression was calculated using the 2^−ΔΔCt method (31). To validate the stability of the two reference genes (EF1-α and β-actin), expression data were analyzed using **geNorm** and **NormFinder** software tools. The geNorm analysis revealed M-values of 0.41 (EF1-α) and 0.44 (β-actin), both below the accepted threshold of 1.5, indicating stable expression under our experimental conditions. NormFinder also ranked these genes as the two most stable among the four candidates tested (including GAPDH and 18S rRNA; data not shown), with a combined stability value = 0.28. Primer efficiency was calculated from standard curves generated by serial 10-fold dilutions of pooled cDNA. All primer pairs exhibited efficiencies between **95 and 104%** and R2 values > 0.99.
2.6 Histological and morphometric analysis of intestinal villi
Five randomly selected fish from each treatment (HEOs 0, 30, 60, 120, and 240 mL/kg) were histopathologically examined following deep anesthesia with 40% ethyl alcohol. Tissue samples were taken from the front, middle, and posterior intestines. The materials were dehydrated in 70–100% ethyl alcohol after a one- to two-day fixation in 10% formaldehyde. After dehydration, samples were paraffin waxed and xylene washed. According to histopathological and morphometric analysis techniques, Hematoxylin and eosin staining were performed on 4–5 μm sections (32, 33). Image analysis software (NIH, Bethesda, MD) evaluated intestinal villi length, breadth, and crypt depth. Each set of five intestinal cross-sections had 10 randomly selected villi and crypts, which were examined using one-way ANOVA (SPSS version 22, SPSS Inc., IL, United States). A p-value under 0.05 was significant. Although five fish per treatment were histologically examined, we confirm that three non-overlapping intestinal sections per fish were evaluated to increase statistical robustness. This results in 15 observations per treatment group for morphometric analysis.
2.7 Statistical model
All statistical analyses were conducted using SPSS version 22 (IBM, United States). The experimental unit for all analyses was the tank (n = 3 per treatment), and tank-level means were used to avoid pseudoreplication.
Data were first checked for normality using the Shapiro–Wilk test and for homogeneity of variances using Levene’s test. When assumptions were met, one-way analysis of variance (ANOVA) was used to detect differences among treatments, followed by means were compared using the Tukey–Kramer post hoc test to account for unequal sample sizes. The level of significance was set at α = 0.05.
In addition, a linear mixed model (LMM) was fitted using the `PROC MIXED` procedure to account for random tank effects. The model equation was:
Where Y_ij is the observed response in tank j of treatment i, μ is the overall mean, T_i is the fixed effect of treatment, and e_ij is the residual error (random). A variance components (VC) covariance structure was used. Model fit was assessed using Akaike Information Criterion (AIC) and residual plots. Residuals were confirmed to be normally distributed with homoscedasticity. For key response variables (e.g., SGR, FCR, GHr, IGF-I), both one-way ANOVA and LMM approaches yielded consistent results.
2.8 Sample size and power consideration
A total of 15 tanks (3 per treatment group) were used in the study. Post hoc power analysis was performed using G*Power version 3.1.9.7 to assess whether this design had sufficient sensitivity to detect significant treatment effects. Using a one-way ANOVA model with five groups, α = 0.05, and n = 3 replicates per group, the analysis showed that effect sizes of f = 0.40–0.50 (based on observed SGR and gene expression differences) yielded power levels >0.80. This indicates the sample size was sufficient for detecting large and biologically relevant treatment effects.
3 Results
3.1 Efficiency in growth, feed consumption, and mortality rate
At the end of the 72-day feeding experiment, significant improvements in growth performance metrics were noted in Nile tilapia (p ≤ 0.05), as outlined in Table 3. The initial body weight (IBW) of fish across all treatment groups showed no statistical differences (p =0.654), indicating that all groups began the trial under equivalent conditions.
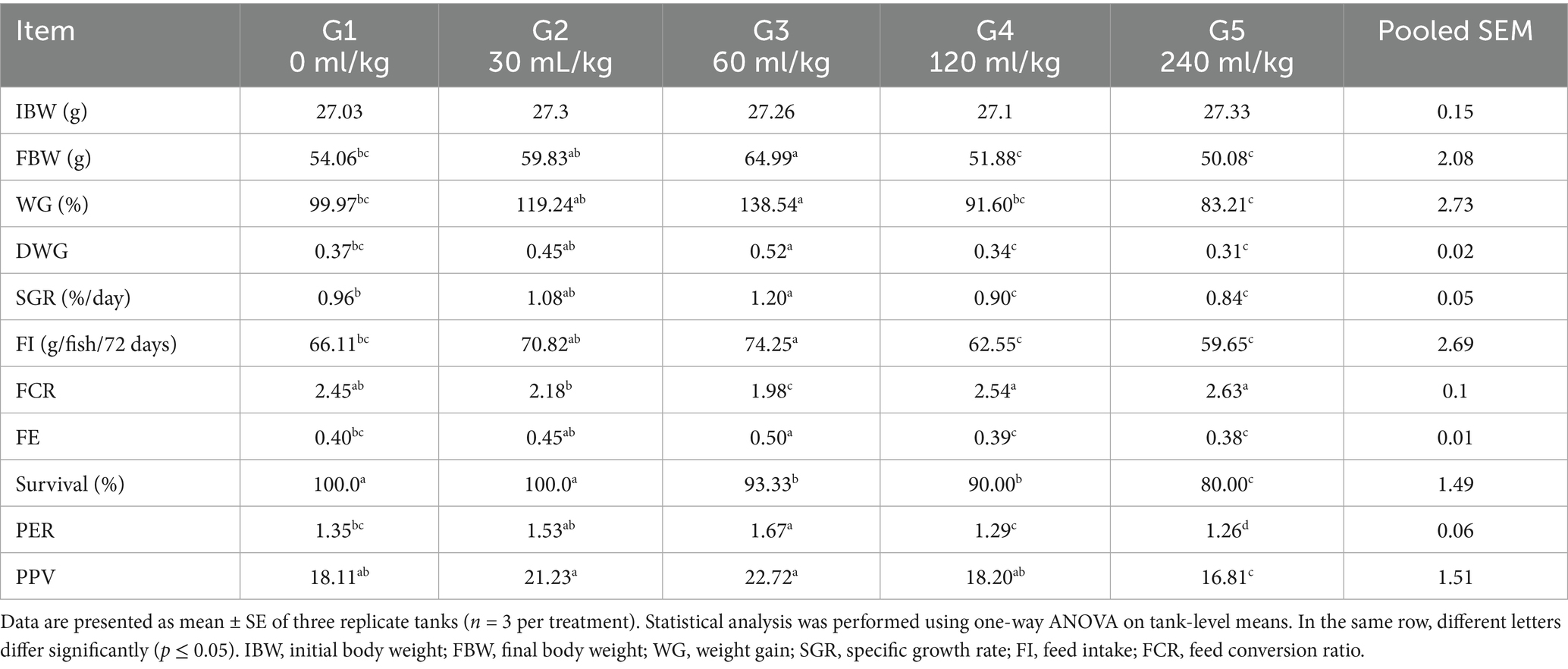
Table 3. Effect of HEOs combination (ml/kg) on the growth performance of fingerlings Nile tilapia (O. niloticus).
Tilapia-fed diets enriched with 30 or 60 mL/kg of herbal essential oils (HEOs) achieved the best growth responses, recording significantly higher values for final body weight (FBW), weight gain percentage (WG%), daily weight gain (DWG), feed intake (FI), feed efficiency (FE), and specific growth rate (SGR) compared to the other groups (p =0.0241).
On the other hand, the poorest growth outcomes were observed in the control group, and fish fed higher HEO inclusion levels (120 and 240 mL/kg). Feed conversion ratio (FCR) was significantly improved (i.e., reduced) in the 30 and 60 mL/kg HEO groups, whereas the control and high-inclusion groups did not show significant changes in FCR. Furthermore, both protein efficiency ratio (PER) and protein productive value (PPV) were substantially higher in the 30 and 60 mL/kg treatment groups than in the control or higher-dose groups (p =0.0145). Survival rates were also significantly better in the control group and those fed 30 and 60 mL/kg of HEOs, showing a noticeable decline in the groups receiving 120 and 240 mL/kg (p =0.0214).
3.2 Haemato-biochemical parameters
Introducing HEOs substantially impacted blood hematological and biochemical parameters (p =0.0324). Table 4 shows that, associated with the control group, the Nile tilapia fish given a diet with 30, 60, 120, and 240 mL HEOs/kg−1 diet had increased levels of hemoglobin, platelet-count value (p =0.014), (RBCs), and (WBCs). Nevertheless, the current study did not find any significant impacts of utilizing a combination of HEOs on the blood biochemical features of Nile tilapia (p =0.0214; Table 5). Treatment groups also had reduced levels of triglycerides and cholesterol. As a result, they consumed less HEO in their diet compared to the control group and other groups, using a combination of 30 and 60 mL/kg−1 HEO (Table 5).
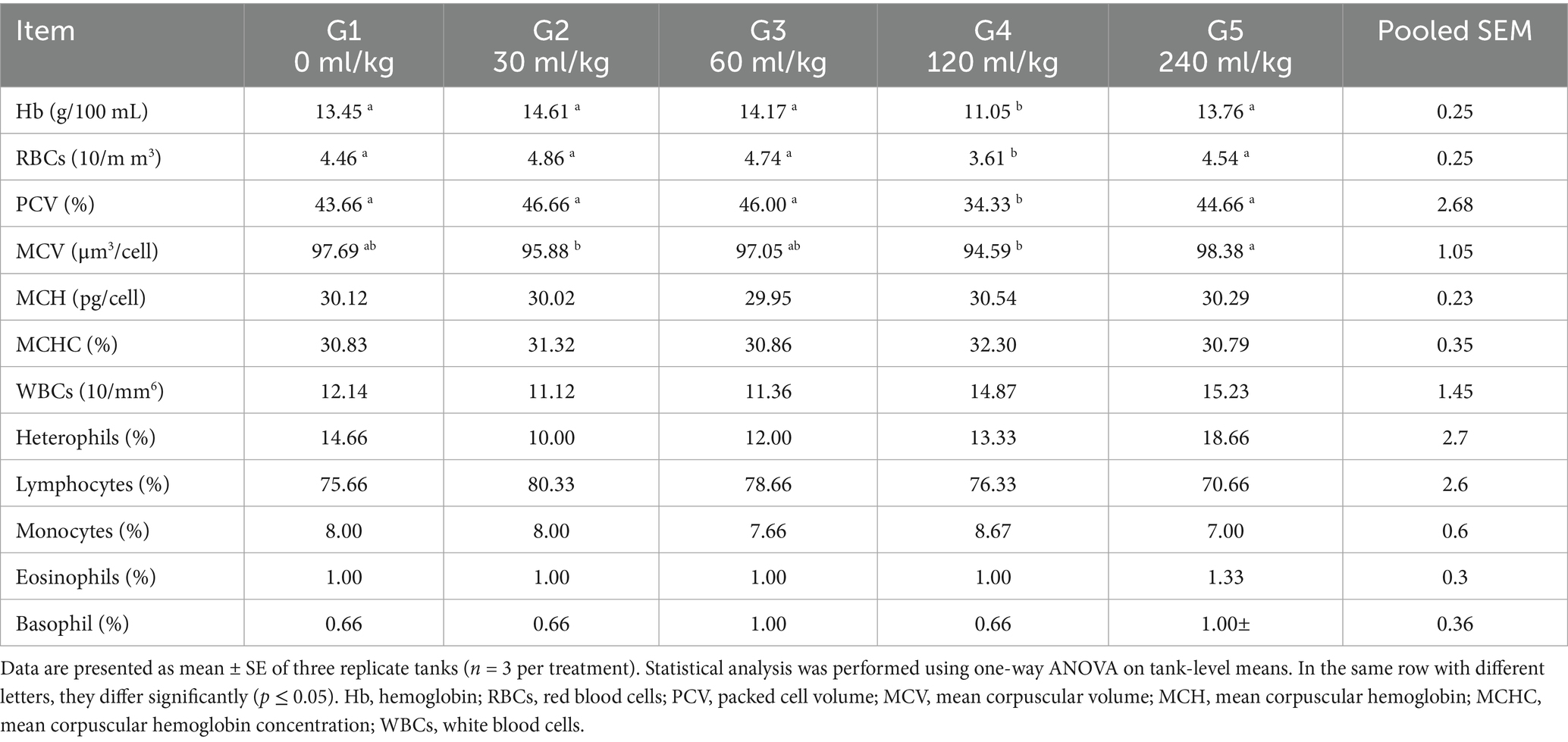
Table 4. Effect of HEOs combination (ml/kg) on the hematological traits of fingerlings Nile tilapia (O. niloticus).
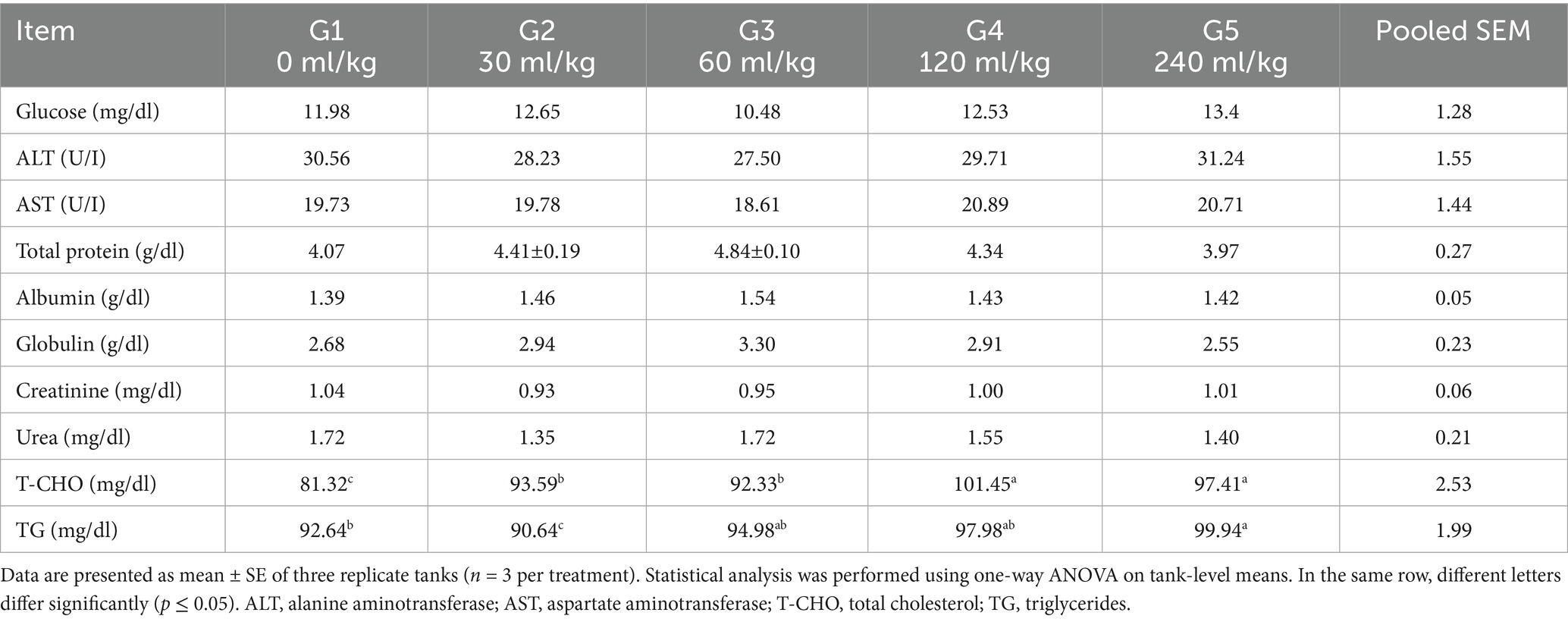
Table 5. Effect of HEOs combination (ml/kg) on blood biochemical traits of fingerlings Nile tilapia (O. niloticus).
3.3 Immune parameters
There were notable group variations in the fish immune response markers; those groups that consumed higher concentrations of HEO had an improved immune response (Table 6). In fingerlings, lysozyme and phagocytic activity showed a greater value. Unlike the other groups, Nile tilapia O. niloticus fed a diet containing 30 and 60 mL HEOs/kg−1 and fared relatively well. Several experimental meals had similar phagocytic index values (p =0.756). In the fish-fed diet with HEOs, IgM levels were substantially higher than in the control and fish-fed 60 mL HEOs/kg−1 diet (p < 0.05; Table 6).
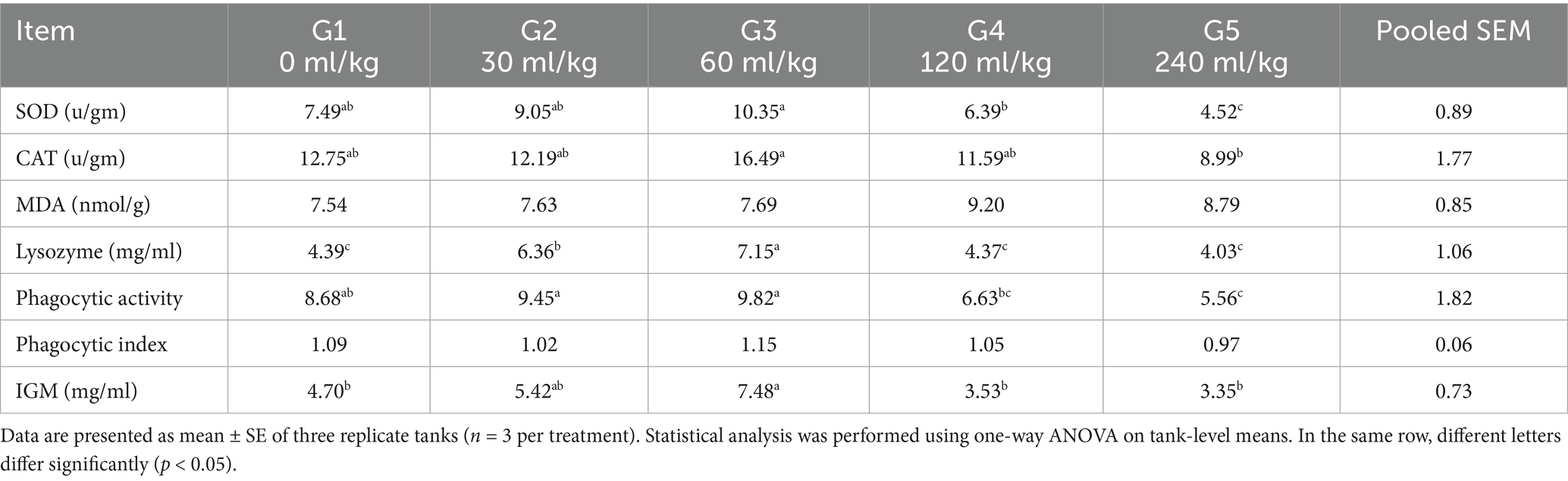
Table 6. Effect of HEOs combination (ml/kg) on Immune and antioxidative responses of fingerlings Nile tilapia O. niloticus.
3.4 Antioxidant enzymes and gene expression in Oreochromis niloticus
Feeding Nile tilapia fingerlings with herbal essential oils (HEOs) significantly affected antioxidant enzyme activity (Table 6). Both (SOD) and (CAT) activities were substantially elevated (p ≤ 0.05) in fish-fed diets containing HEOs associated with the control group. The MDA levels showed no significant differences across all treatment groups (p =0.6547).
Regarding gene expression, the relative mRNA levels of HSP70 were substantially reduced in fish fed 30 and 60 mL/kg of HEOs (p =0.0145). In contrast, fish receiving 120 and 240 mL/kg diets exhibited a significant upregulation of HSP70 expression (Figure 1).
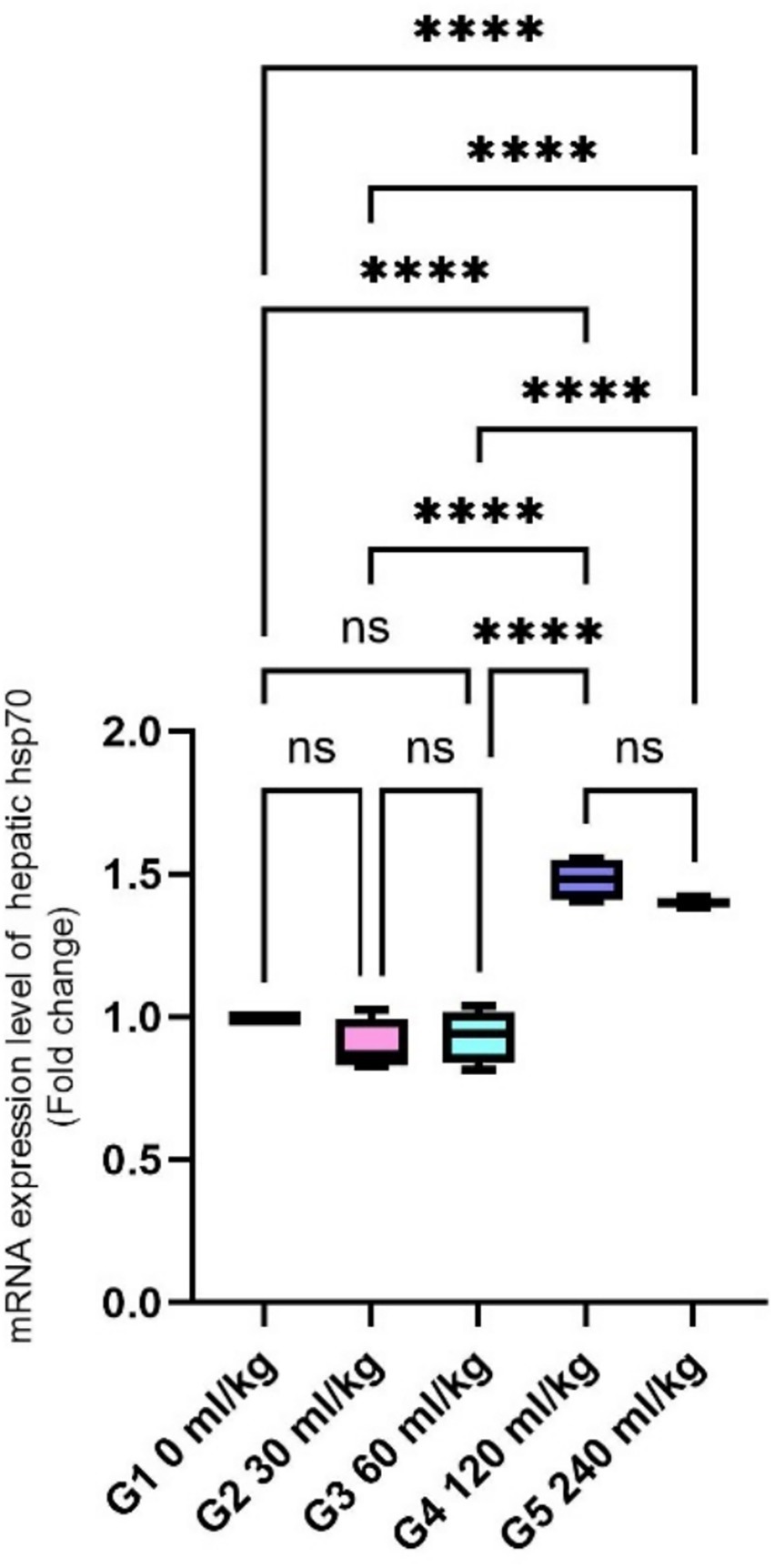
Figure 1. Effect of HEOs supplementation on the expression levels of HSP70 in fingerlings of Nile tilapia O. niloticus. HSP70: heat shock protein. Data are mean ± SE; values with different superscripts in the same column differ (p < 0.05).
Fish fed 30 and 60 mL/kg HEOs showed lower IL-1β and TNF-α levels than other dietary groups (p =0.014; Figures 2A,B), indicating reduced inflammatory activity.
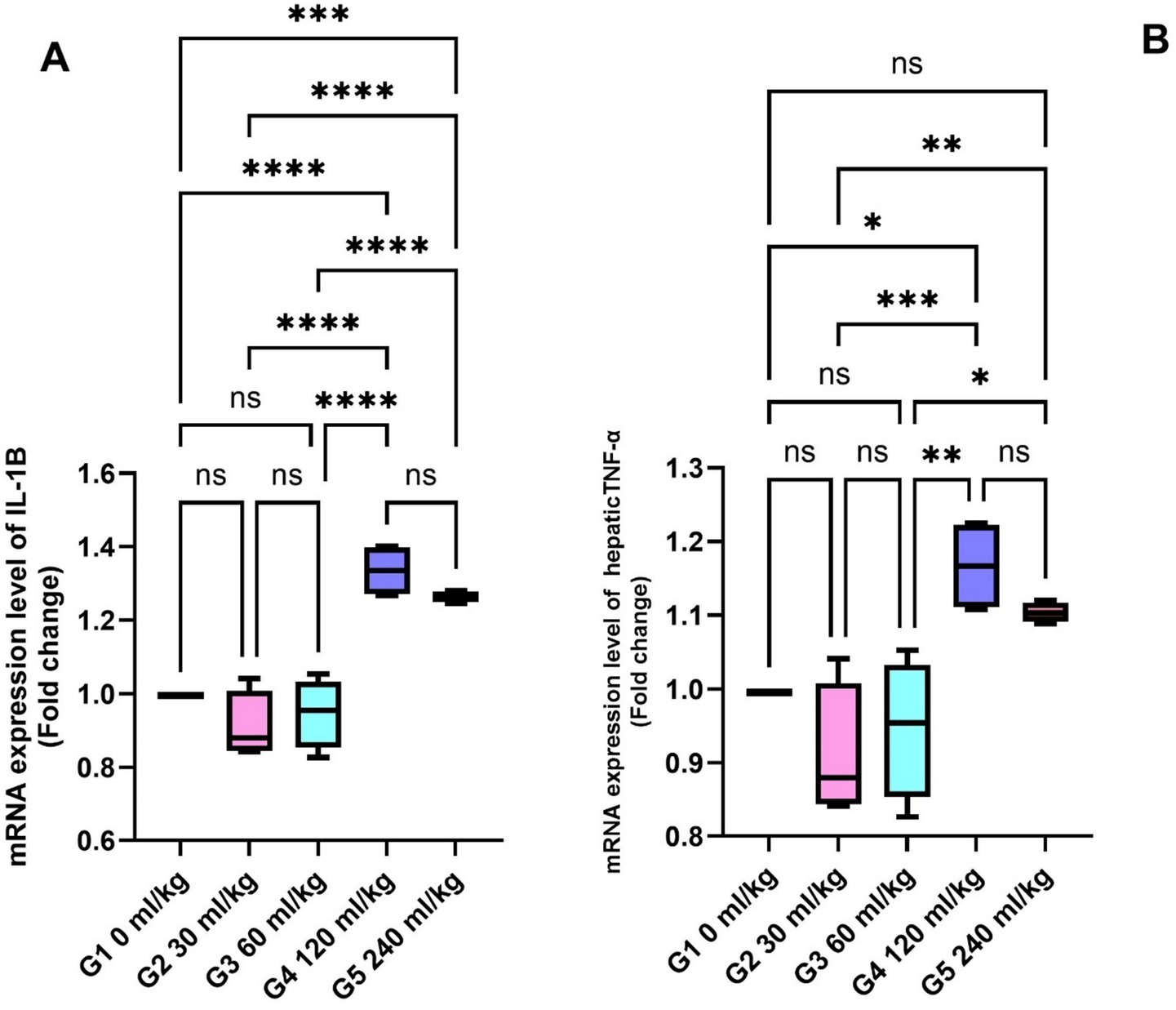
Figure 2. Effect of HEOs supplementation on the expression levels of (A) IL-1β, and (B) TNF-α in fingerlings Nile tilapia O. niloticus. IL-1β: interleukin1β and tumor necrosis factor alpha (TNF-α). Data are mean ± SE; values with different superscripts in the same column differ (p < 0.05).
The expression of (GHr) in liver tissue was significantly upregulated in fish supplemented with 30 and 60 mL/kg of HEOs (Figure 3A), while ghrelin—a hormone associated with metabolism and appetite regulation—was also significantly more expressed in the stomachs of fish from these groups (p =0.017; Figure 3B).
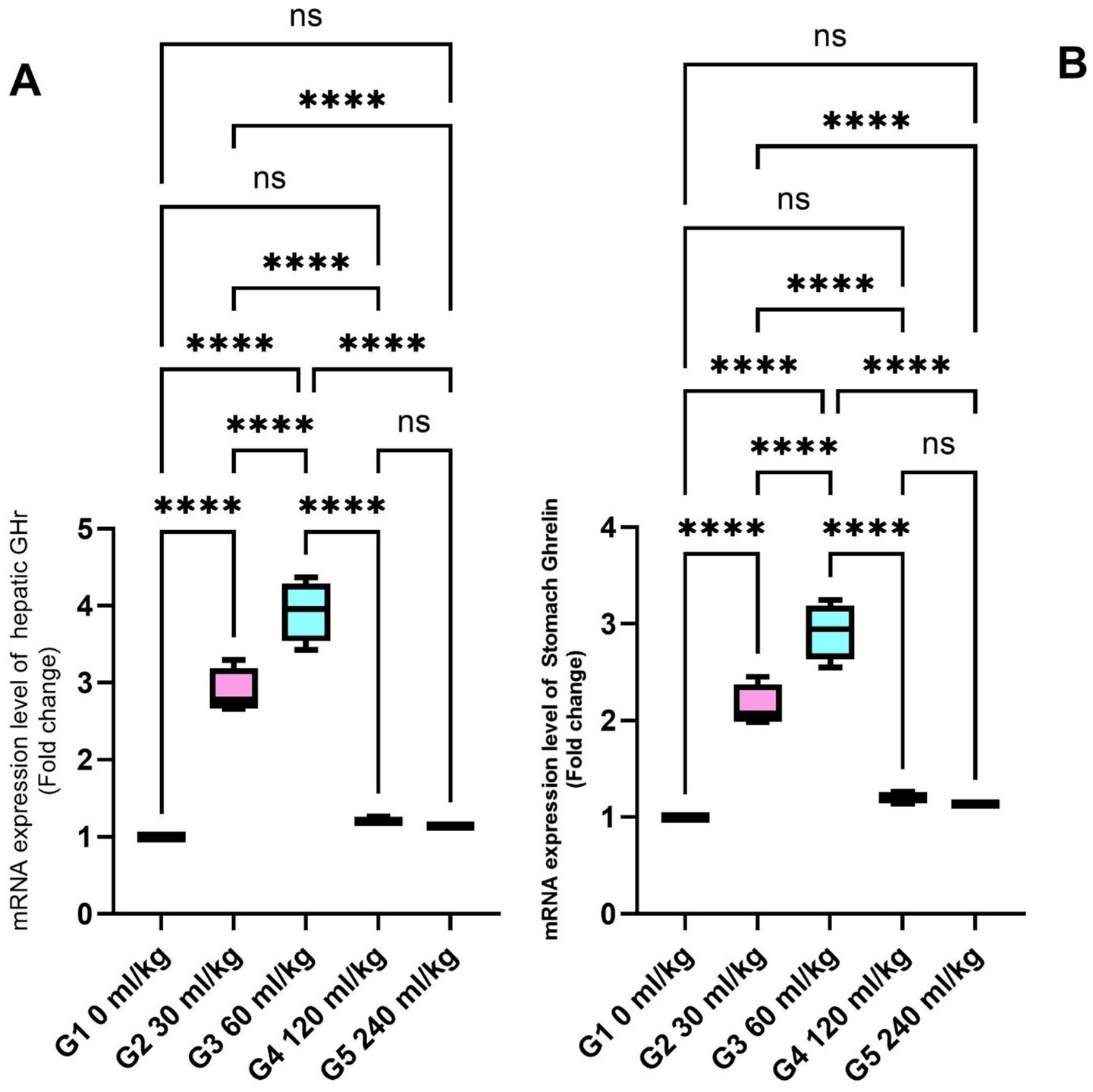
Figure 3. Effect of HEOs supplementation on the expression levels of (A) GH and (B) Ghrelin in fingerlings Nile tilapia O. niloticus. GH is a growth hormone, and Ghrelin is a peptide hormone. Data are mean ± SE; values with different superscripts in the same column differ (p < 0.05).
Additionally, IGF-I gene expression in the liver was significantly higher in fish-fed diets containing 30 and 60 mL/kg of HEOs than in the other groups (p =0.025; Figure 4), suggesting enhanced growth signaling.
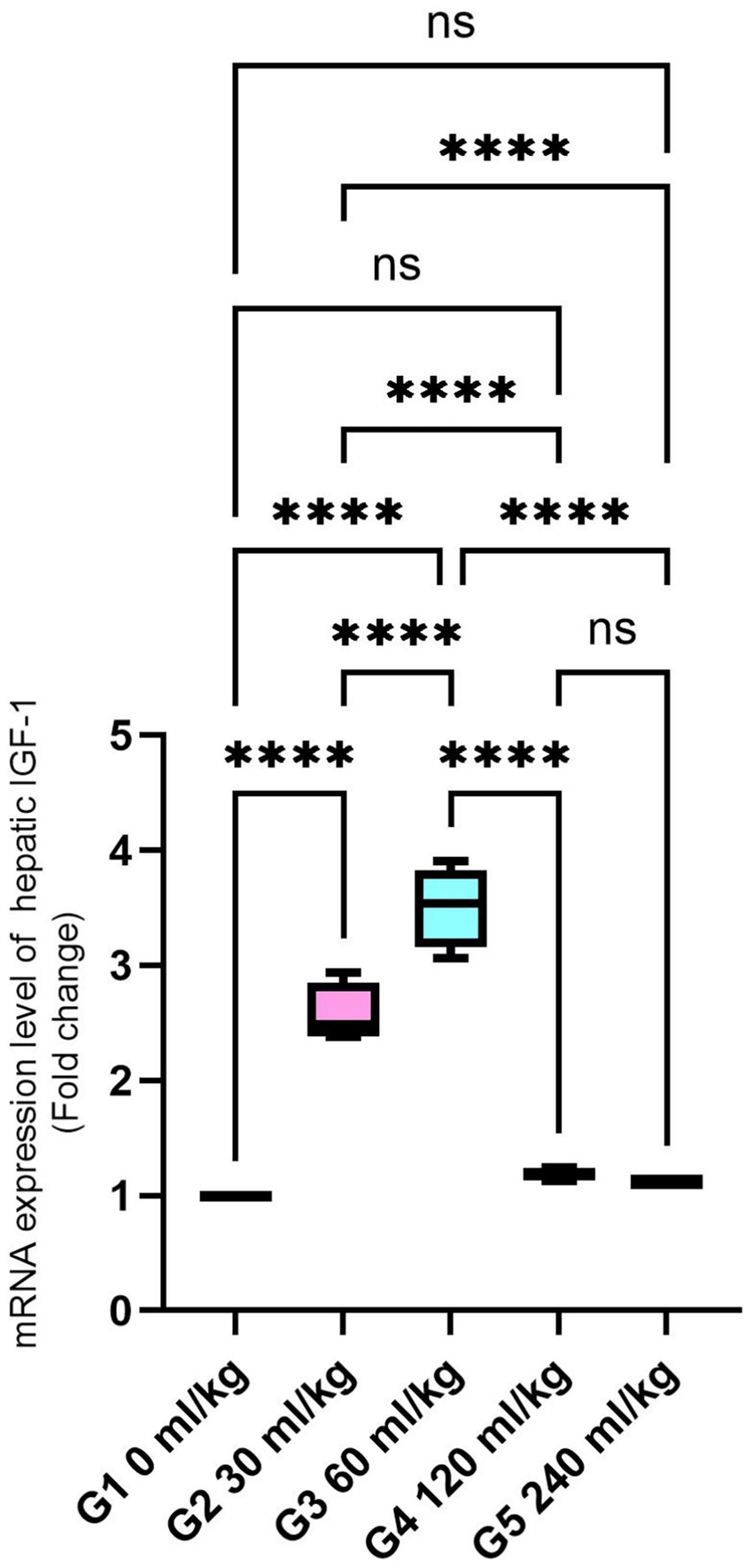
Figure 4. Effect of HEOs supplementation on the expression levels of IGF-I in fingerlings of Nile tilapia O. niloticus. Insulin-like growth factor 1 (IGF-I) Data are mean ± SE; values with different superscripts in the same column are different (p < 0.05).
SOD and CAT were also significantly increased in the liver tissues of fish fed 30 and 60 mL/kg of HEOs (p ≤ 0.05; Figures 5A,B), consistent with the enzyme activity results. Zonula occludin-1 (ZO-1) and occludin showed significant increases in the groups receiving 30 and 60 mL/kg of HEOs, with the greatest expression detected in the 60 mL/kg group (p =0.036; Figures 6A,B), indicating improved gut barrier integrity.
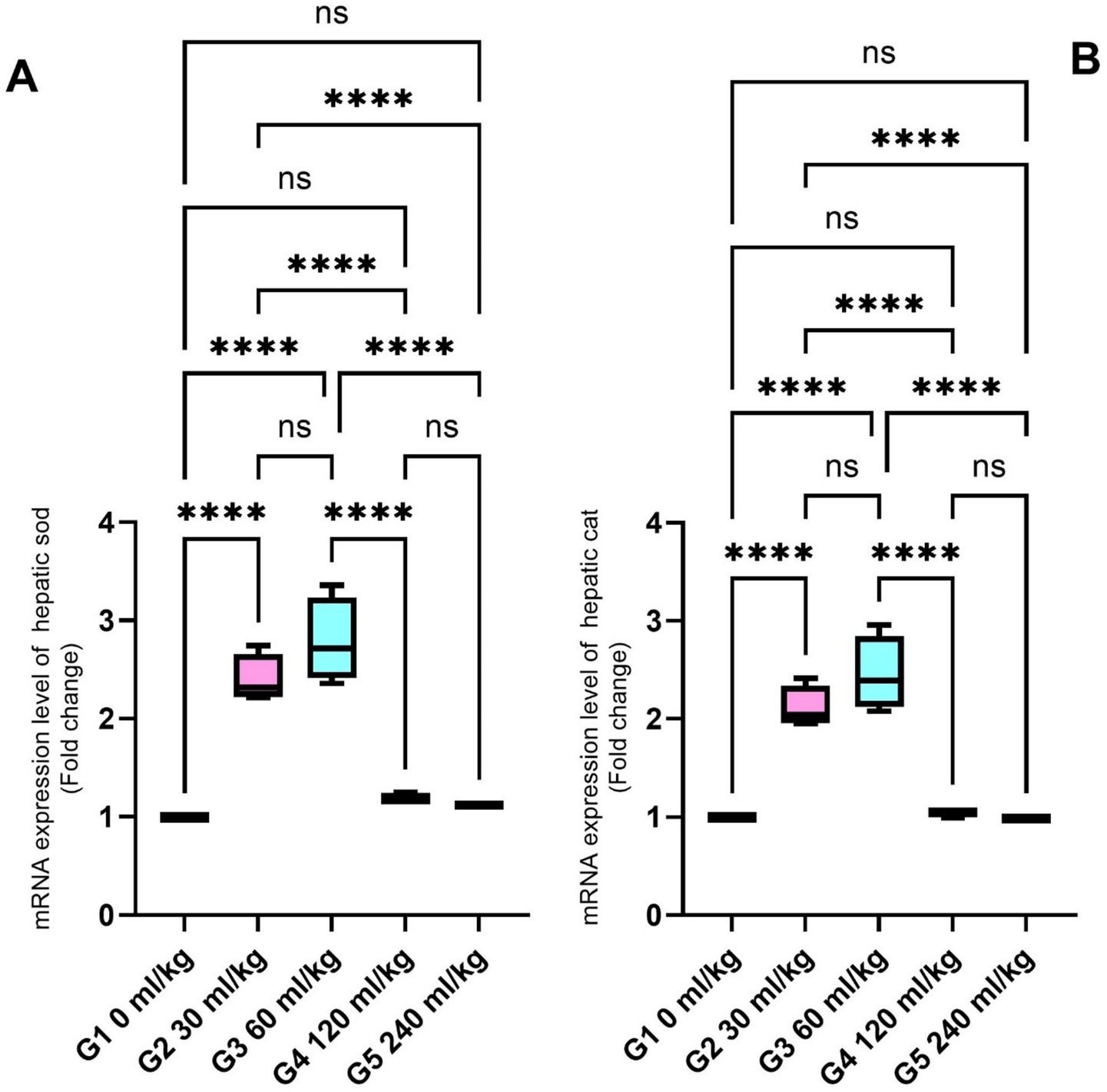
Figure 5. Effect of HEOs supplementation on the expression levels of (A) SOD and (B) CAT in fingerlings of Nile tilapia O. niloticus. Superoxide dismutase (SOD) and catalase (CAT). Data are mean ± SE; values with different superscripts in the same column differ (p < 0.05).
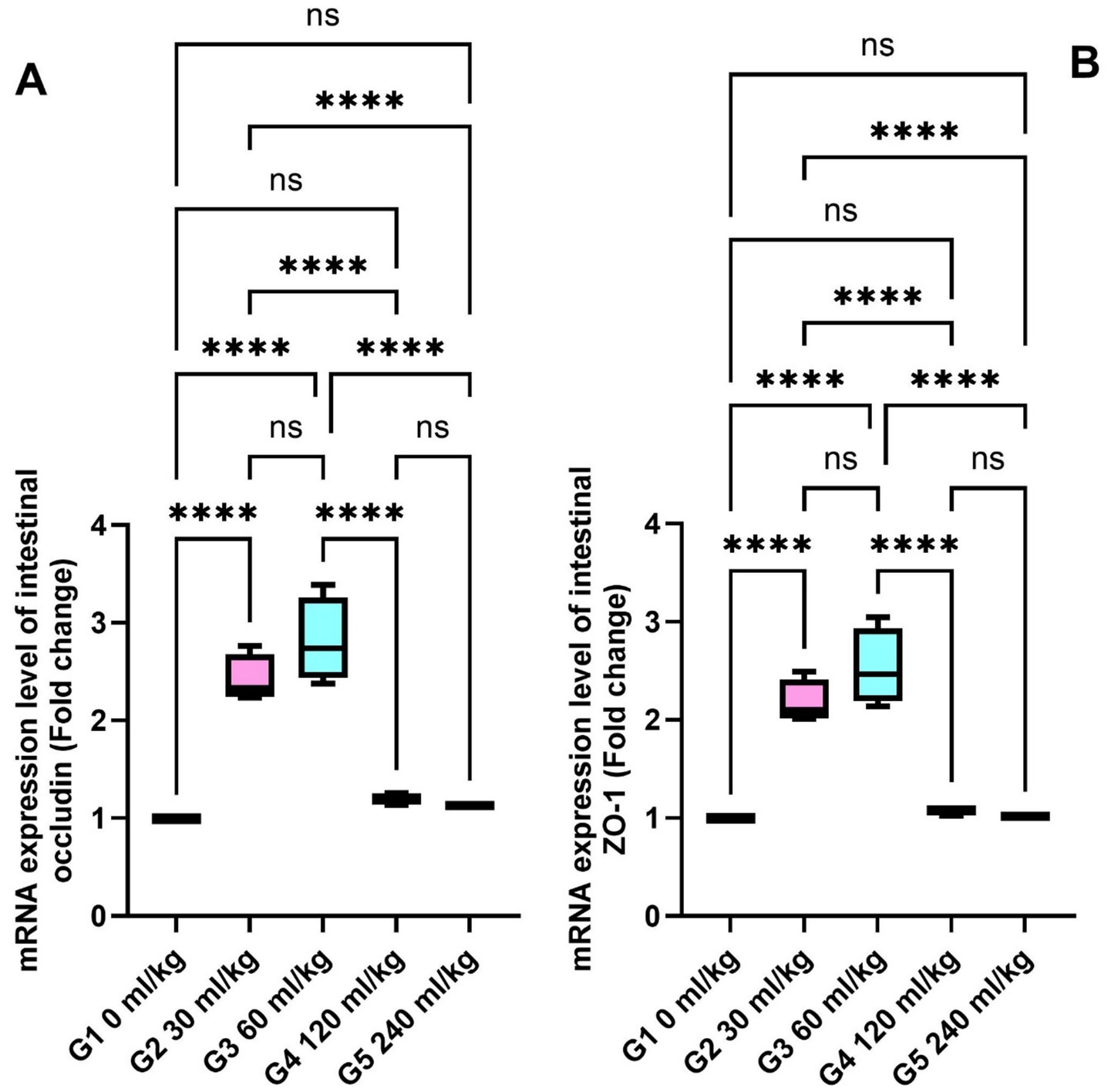
Figure 6. Effect of HEOs supplementation on the expression levels of (A) ZO-1 and (B) occludin in fingerlings Nile tilapia O.niloticus. Zonula occludens-1 (ZO-1) and occludin. Data are mean ± SE; values with different superscripts in the same column differ (p < 0.05).
3.5 Histopathological findings
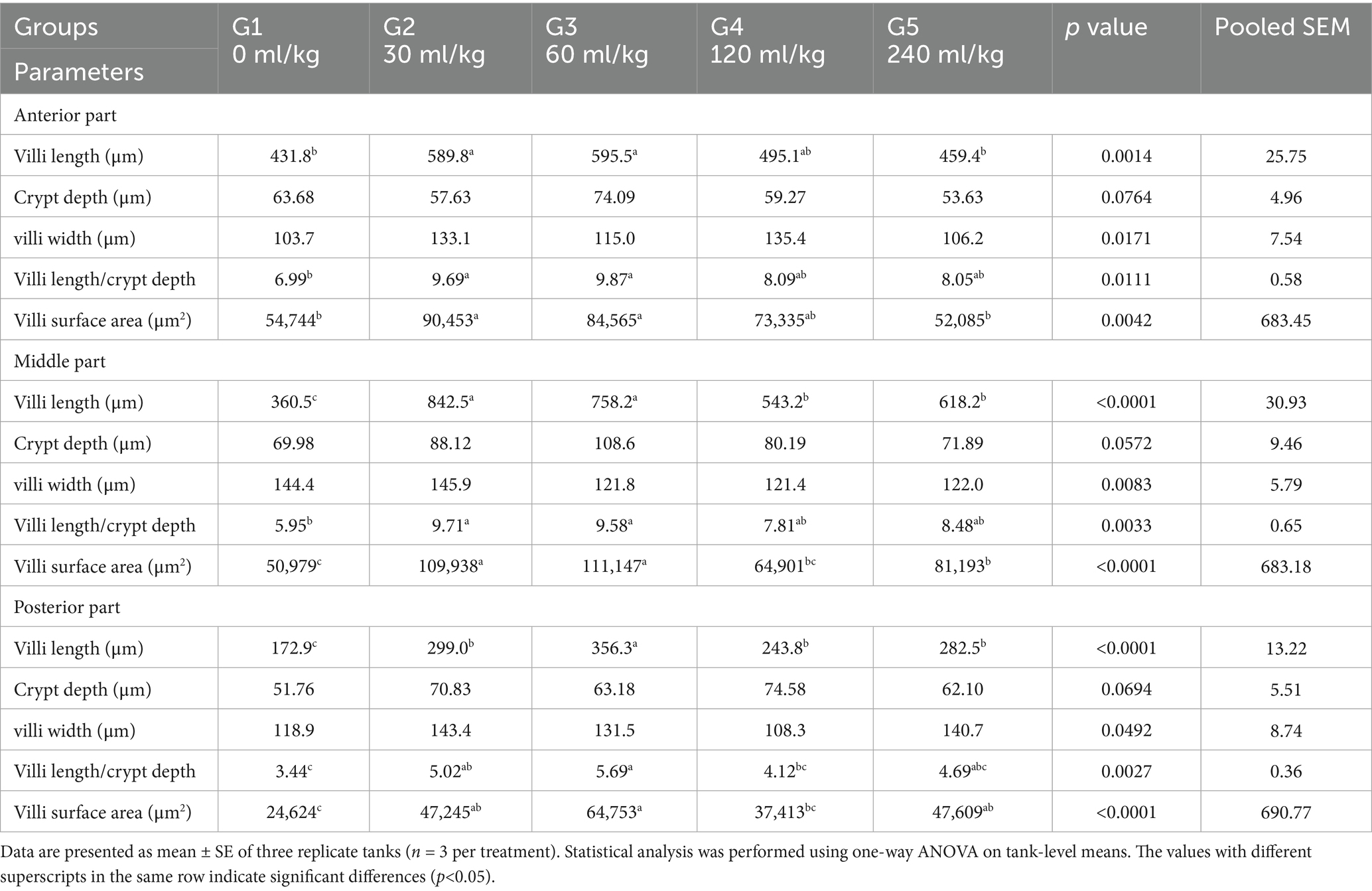
Many intestinal villi are bordered by simple columnar epithelium containing goblet cells, and the intestinal wall seems intact in all three types. Their lamina muscularis and tunica serosa are external coverings for a core of loose connective tissue. Compared to other groups, the intestinal villi in the 30 mL/kg and 60 mL/kg HEOs groups exhibit high branching. Villi length, surface area, and villi length/crypt depth were all significantly increased in the 30 mL/kg and 60 mL/kg HEO groups compared to the 0 mL/kg, 120 mL/kg, and 240 mL/kg groups in the morphometric analysis of data acquired from the measurement of intestinal villi. In Table 7, you can see a summary of the results of the morphological analysis in Figure 7 and Supplementary Table S2.
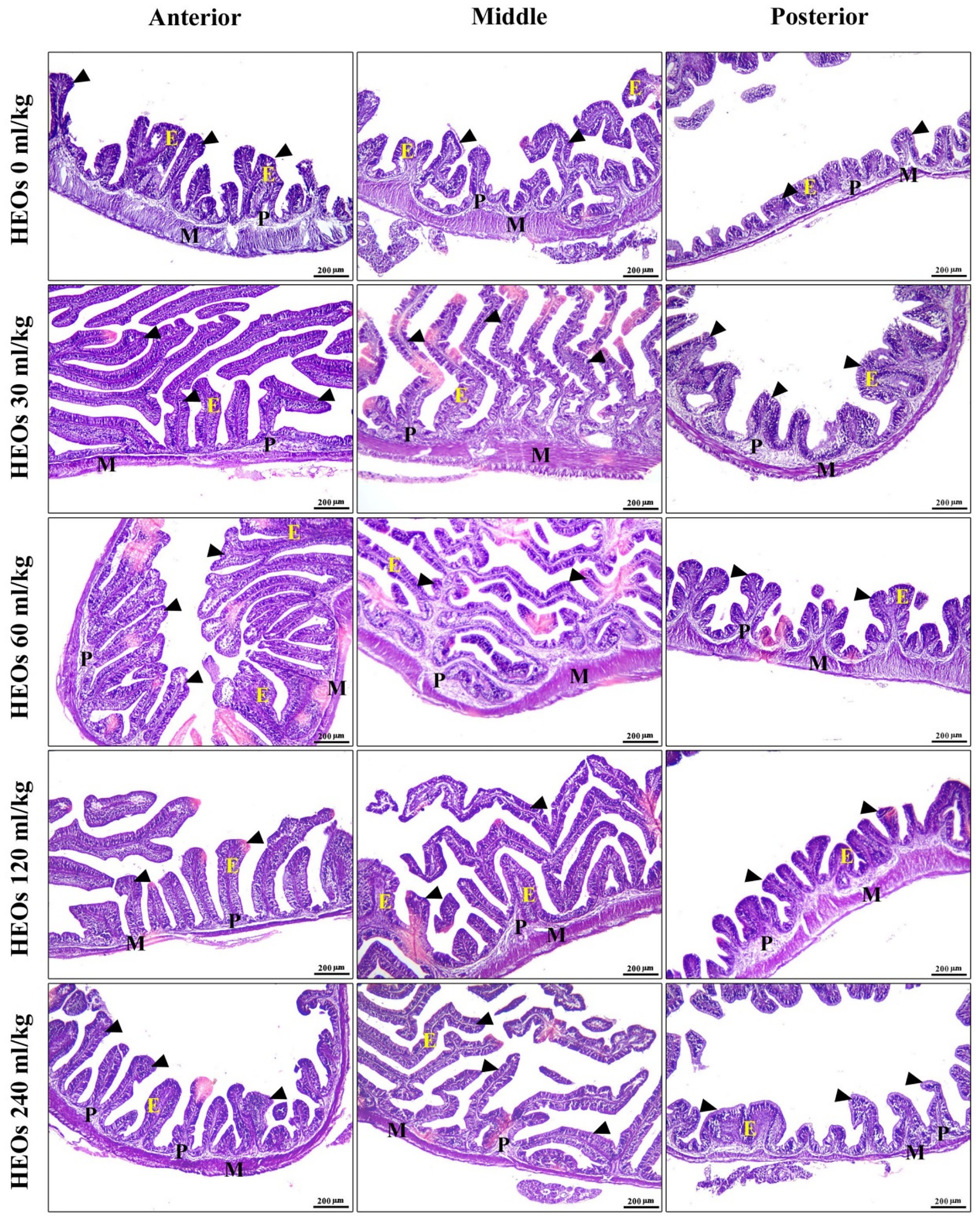
Figure 7. photomicrograph of H&E-stained panel of anterior, middle, and posterior parts of intestine of HEOs 0, 30, 60, 120, and 240 mL/kg groups showing intestinal villi (arrowheads) lined by simple columnar epithelium of lamina epithelialis (E), lamina propria (P), and lamina muscularis (M).
3.6 Dose–response modeling
To determine the optimal inclusion level of HEOs, broken-line and quadratic regression analyses were performed for specific growth rate (SGR), feed conversion ratio (FCR), and expression of GHr and IGF-I. The broken-line model estimated the optimal HEO inclusion level at 52.6 mL/kg diet for SGR and 55.4 mL/kg for FCR. Beyond this threshold, no further performance improvements were observed, and higher levels were associated with reduced gains.
These results are illustrated in Figure 8 and support our conclusion that the optimal HEO dose lies between 50 and 60 mL/kg.
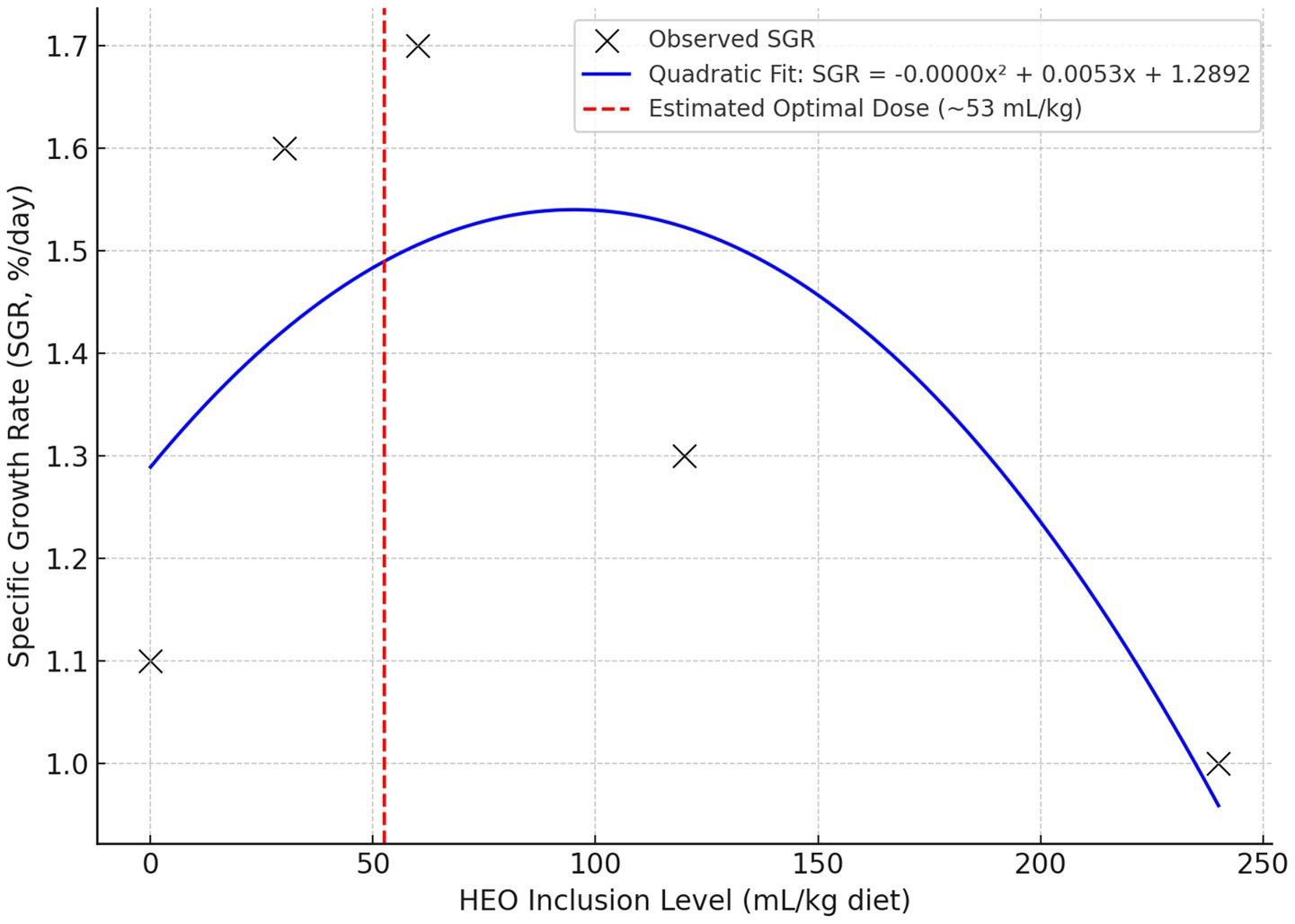
Figure 8. The quadratic relationship between HEO dose and specific growth rate (SGR), estimating the optimal dose near 53 mL/kg.
4 Discussion
This study demonstrated that supplementing Nile tilapia diets with 30 and 60 mL/kg of blended herbal essential oils (HEOs) significantly improved growth performance metrics, including final body weight (FBW), specific growth rate (SGR), and feed conversion ratio (FCR). These enhancements can be mechanistically linked to the bioactive components of the HEO blend—particularly carvacrol, thymol, and 1,8-cineole—which are known to stimulate appetite, enhance digestive enzyme secretion, and improve intestinal morphology. Carvacrol and thymol, for example, modulate gut microbiota and reduce pathogenic load, promoting better nutrient absorption and feed utilization. These functional properties may explain the improved protein efficiency ratio (PER) and feed efficiency observed in our study. Additionally, 1,8-cineole has been shown to exert anti-inflammatory effects in the gut, which may reduce energy diversion toward immune responses and favor growth. By enhancing both digestive function and gut integrity, the HEO blend supported more efficient nutrient metabolism and somatic growth, consistent with findings reported in Nile tilapia and other aquaculture species (34, 35).
Supplementation with 30 and 60 mL/kg of HEOs significantly increased hemoglobin (Hb), red blood cells (RBCs), and white blood cells (WBCs), reflecting improved oxygen transport and immunocompetence. These hematological responses are likely driven by the stimulatory effects of thymol and carvacrol on hematopoiesis and leukocyte activation. Additionally, the observed reduction in serum cholesterol and triglycerides may be attributed to the hypolipidemic effects of carvacrol and limonene, which modulate lipid metabolism through inhibition of key hepatic enzymes. Importantly, liver and kidney markers (ALT, AST, urea, and creatinine) remained stable, indicating no adverse systemic effects. These results suggest that HEOs not only enhance blood health but also help maintain metabolic balance under physiological conditions, aligning with findings from El-Bab et al. (9).
Although moderate inclusion levels of HEOs (30–60 mL kg−1) significantly improved growth and immune performance, higher doses (≥120 mL kg−1) resulted in depressed growth, lower feed intake, and slightly reduced survival. Several factors may explain this dose-dependent response. First, the high concentrations of volatile compounds such as carvacrol and thymol may reduce feed palatability due to strong odor or flavor, leading to reduced voluntary intake, as previously observed in rainbow trout fed high oregano oil levels (36). Second, while essential oils can act as antioxidants at low levels, they may exert pro-oxidant or cytotoxic effects at high concentrations, thereby increasing metabolic stress or damaging the intestinal epithelium (37). This is supported by Reverter et al. (38), who reported biphasic effects of plant-derived bioactives in aquafeeds. Third, the cumulative intake of the carrier solvent, propylene glycol—especially at 240 mL/kg HEO (~36 g/kg diet)—may have exerted subclinical toxicity or impaired digestive enzyme activity, although this was not directly measured. Together, these findings highlight the importance of determining optimal inclusion levels. Based on our regression modeling and observed trends, we recommend an upper inclusion limit of 60 mL kg−1, beyond which HEOs may exert counterproductive effects.
The immune-enhancing effects observed in fish fed 30 and 60 mL/kg HEOs—evident through elevated lysozyme activity, phagocytic rate, and IgM levels—suggest stimulation of both innate and adaptive immune pathways (34). These effects are likely mediated by carvacrol and thymol, which have been shown to activate macrophages, promote phagocytosis, and modulate cytokine release. Carvacrol, in particular, enhances the expression of pattern recognition receptors and increases microbial clearance. Furthermore, the significant upregulation of antioxidant enzymes (SOD and CAT) in these groups indicates improved oxidative defense, potentially driven by the phenolic structure of thymol and limonene, which facilitates neutralization of reactive oxygen species (ROS). The lack of significant changes in MDA levels supports the notion that oxidative stress was well controlled across treatments. These findings demonstrate that moderate doses of HEOs promote immune readiness and redox homeostasis, contributing to improved disease resilience (9, 34).
The Studies of functional feed additives on aquatic species generally use the transcription of growth, immunological, and related genes to discover the genetic mechanism of action (39). In Nile Tilapia fed 0.4 and 0.5% OVLE, Hsp70 was downregulated, which is important for fish health (40). According to the study, HSP70 expression was dramatically reduced in fish fed 30- and 60-ml HEOs/kg−1 food. This research supports prior findings (34). Fish fed 1 g/kg of oregano essential oil showed a considerable reduction, with the lowest level.
Glycoprotein indicators such as IL-1β, IL-8, IL-10, and IgM regulate fish immune response, represent innate immunity, and alter viral disease response (41, 42). Consistent with Giri et al. (43), IL-1β, a pro-inflammatory cytokine, activates lymphocytes and enhances the release of other cytokines like TNF-ɑ. Our investigation found that fish fed 30- and 60-ml HEOs/kg−1 had considerably lower IL-1β and TNF-α mRNA levels than other experimental groups. Similarly El-Bab et al. (9), results indicate that fish given 0.2 and 0.4% OVLE had higher levels of IL-1β, whereas those fed 0.4 and 0.5% OVLE had higher levels of IL-8. It supported these findings (44). Who examined how thyme and Nigella sativa affect these Nile tilapia genes through food. Additionally, NS dietary additives dramatically increased IL-1β, IL-8, and IgM expression (44). Abd El-Hamid et al. (45) increased CNE levels led to greater IL-1β, IL-8, TNFα, and IL-10 gene expression compared to the control group. Zebrafish fed encapsulated cinnamaldehyde demonstrated increased IL-1β, TNFα, and interferon-gamma expression levels (46).
Occludin and ZO-1 help tighten junctions. Occludin is the major structural and functional component of tight junction proteins that preserves junction integrity (47). As a tight junction-associated adaptor protein, ZO-1 links occludin/claudin and actin cytoskeleton to maintain tight junction stability and function (47). The study found that fish fed 30- and 60-ml HEOs/kg−1 diets had considerably higher ZO-1 and occludin expression levels than other groups. The 60 mL diet had the highest mRNA level. According to an earlier study, Oxazolone inhibited ZO-1 and occludin transcription in zebrafish (48). The GH/IGF axis controls somatic growth. One transmembrane receptor regulates the (GHRs) in teleost fish, which have two paralogous versions, GHR-I and GHR-II, with complementary roles (49). Higher fish weights are related to larger IGF-I mRNA expression in fish of the same species, age, and rearing conditions under optimal feeding regimens (35). In our investigation, fish fed 30- and 60-ml HEOs/kg−1 exhibited significantly greater GHr and IGF-I mRNA levels in hepatic tilapia compared to other groups. Our findings contradict recent research showing higher IGF-I expression in (O. mossambicus) (50). Previous Nile tilapia research found a positive association between liver IGF-1 protein, mRNA levels, body weight increase, and SGR (51). Polyphenols can increase hunger and food consumption by improving the digestive enzymes, DNA, RNA, GH, IGF-1 creation, and ghrelin (GHRL) expression. Additionally, improving the microbiota in the intestines can improve metabolic functions and growth, contributing to general health and development (35).
The dose–response pattern was nonlinear, with performance improvements plateauing between 30 and 60 mL/kg, followed by a decline at higher doses. This was confirmed using broken-line and quadratic regression models, which estimated an optimal HEO dose of ~53–56 mL/kg for growth and gene expression endpoints. The decline in performance at higher doses may be due to bioactive compound overload or excessive carrier (propylene glycol) intake.
One limitation of the current study is the relatively low number of replicates (three tanks per treatment), which may limit the detection of subtle effects. However, post hoc power analysis indicated sufficient power (>80%) to detect medium-to-large effects observed in growth performance and gene expression. Future studies with increased replication and factorial designs are encouraged to confirm and refine these findings.
5 Conclusion
The present study demonstrates that dietary inclusion of herbal essential oils (HEOs) at 30–60 mL kg−1 diet significantly improves growth performance, feed utilization, antioxidant defense, immune response, and intestinal morphology in Nile tilapia. These benefits are associated with upregulation of growth- and immunity-related genes and improved intestinal histoarchitecture. However, higher inclusion levels (≥120 mL kg−1) were detrimental, suggesting a threshold beyond which HEOs may exert adverse effects. Therefore, 30–60 mL kg−1 appears to be the optimal inclusion range under current conditions. Future research should validate these findings under commercial aquaculture conditions, considering different life stages and feeding regimes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
All animals involved in the experiment were handled with appropriate care, and the National Institutes of Health carried out all procedures and guidelines for the care and use of laboratory animals. The study protocol received ethical approval from the Faculty of Veterinary Medicine Ethics Committee, Kafr El-Sheikh University, Egypt (Approval No. KFS/VET-IACUC/1005/2023)—all methods adhered to relevant ethical standards, including compliance with the ARRIVE guidelines (https://arriveguidelines.org). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
MK: Visualization, Conceptualization, Formal analysis, Writing – original draft, Validation, Supervision. SS: Investigation, Writing – original draft, Funding acquisition, Data curation, Conceptualization, Formal analysis. MHA: Writing – original draft, Resources, Writing – review & editing, Investigation, Project administration, Methodology. AA: Formal analysis, Resources, Funding acquisition, Project administration, Writing – review & editing, Data curation, Conceptualization. FF: Writing – review & editing, Writing – original draft, Funding acquisition, Data curation, Conceptualization. MFA: Software, Writing – review & editing, Writing – original draft, Methodology. BA: Conceptualization, Data curation, Writing – review & editing, Formal analysis, Writing – original draft. MA: Data curation, Funding acquisition, Software, Writing – original draft, Formal analysis, Resources. MS: Writing – original draft, Formal analysis, Writing – review & editing, Data curation, Conceptualization. AE: Funding acquisition, Formal analysis, Writing – review & editing, Data curation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R73), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Acknowledgments
We appreciate the resources provided by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R73), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1620632/full#supplementary-material
References
1. Rocha, CP, Cabral, HN, Marques, JC, and Gonçalves, AM. A global overview of aquaculture food production with a focus on the activity’s development in transitional systems—the case study of a south European country (Portugal). J Mar Sci Eng. (2022) 10:417. doi: 10.3390/jmse10030417
2. Rohani, MF, Islam, SM, Hossain, MK, Ferdous, Z, Siddik, MA, Nuruzzaman, M, et al. Probiotics, prebiotics and synbiotics improved the functionality of aquafeed: upgrading growth, reproduction, immunity and disease resistance in fish. Fish Shellfish Immunol. (2022) 120:569–89. doi: 10.1016/j.fsi.2021.12.037
3. Boyd, CE, McNevin, AA, and Davis, RP. The contribution of fisheries and aquaculture to the global protein supply. Food Secur. (2022) 14:805–27. doi: 10.1007/s12571-021-01246-9
4. Miao, W, and Wang, W. Trends of aquaculture production and trade: carp, tilapia, and shrimp. Asian Fish Sci. (2020) 33:1–10. doi: 10.33997/j.afs.2020.33.S1.001
5. Ashry, AM, Habiba, MM, Abdel-wahab, A, Younis, EM, Davies, SJ, Elnakeeb, MA, et al. Dietary effect of powdered herbal seeds on zootechnical performance, hemato-biochemical indices, immunological status, and intestinal microbiota of European sea bass (Dicentrarchus labrax). Aquac Rep. (2024) 36:102074. doi: 10.1016/j.aqrep.2024.102074
6. Mariappan, B, Kaliyamurthi, V, and Binesh, A. Medicinal plants or plant derived compounds used in aquaculture In: B Mariappan, editor. Recent advances in aquaculture microbial technology. Amsterdam, Netherlands: Elsevier (2023). 153–207.
7. Bhanja, A, Payr, P, and Mandal, B. Phytobiotics: response to aquaculture as substitute of antibiotics and other chemical additives. South Asian Journal of Experimental Biology. (2023) 13:341–55. doi: 10.38150/sajeb.13(5).p341-355
8. Zejli, H, Fitat, A, Lefrioui, Y, Siddique, F, Bourhia, M, Bousseraf, FZ, et al. Phytochemical analysis and biological activities of essential oils extracted from Origanum grossii and Thymus pallidus: in vitro and in silico analysis. Sci Rep. (2023) 13:20021. doi: 10.1038/s41598-023-47215-4
9. El-Bab, AFF, Amer, AA, El-Nawsany, MM, Ibrahim, IH, Gouda, AH, El-Bahlol, AA, et al. Oregano leaf extract dietary administration modulates performance, redox status, intestinal health, and expression of some related genes of Nile tilapia (Oreochromis niloticus L.). Ann Anim Sci. (2024) 24:179–90. doi: 10.2478/aoas-2023-0068
10. Zidan, EM, Goma, AA, Tohamy, HG, Shukry, M, and Naiel, MA. Impact of feeding Artemia franciscana enriched with various oil resources on growth, blood biochemical and behavioral indices, and survival of Oreochromis niloticus. Ann Anim Sci. (2024) 24:1251–62. doi: 10.2478/aoas-2024-0045
11. Omar, AA, Gado, MS, Kandel, HE, Farrag, FA, and Shukry, M. Probiotic efficacy in aquaculture: the role of Technospore® (Bacillus coagulans) in improving Nile Tilapia (Oreochromis niloticus) performance and disease resistance: a study on gut health, immunological response, and gene expression. Probiotics Antimicrob Proteins. (2024) 1:1–18. doi: 10.1007/s12602-024-10279-3
12. Abdel-Latif, HM, Soliman, AA, Gewaily, MS, Amer, AA, Shukry, M, Khalil, RH, et al. Dietary effects of Saccharomyces cerevisiae and Allium sativum on growth, antioxidant status, hepatic and intestinal histoarchitecture, expression of growth-and immune-related genes, and resistance of Oreochromis niloticus to Aeromonas sobria. Fish Shellfish Immunol. (2024) 148:109493. doi: 10.1016/j.fsi.2024.109493
13. Tawfeek, WS, Kassab, AS, Al-Sokary, ET, Abass, ME, and Sherif, AH. Chlorella vulgaris algae ameliorates chlorpyrifos toxicity in Nile tilapia with special reference to antioxidant enzymes and Streptococcus agalactiae infection. Mol Biol Rep. (2024) 51:616. doi: 10.1007/s11033-024-09535-0
14. Hendam, BM, Baromh, MZ, Khafaga, AF, Shukry, M, El-Son, MA, and Abdel-Latif, HM. Effects of dietary baobab, Adansonia digitata on growth, haemato-immunological status, antioxidant biomarkers, intestinal histomorphometry, gene expression responses, and disease resistance in Nile tilapia, Oreochromis niloticus. Aquaculture. (2024) 581:740473. doi: 10.1016/j.aquaculture.2023.740473
15. Naiel, MA, Ismael, NE, Negm, SS, Ayyat, MS, and Al-Sagheer, AA. Rosemary leaf powder–supplemented diet enhances performance, antioxidant properties, immune status, and resistance against bacterial diseases in Nile Tilapia (Oreochromis niloticus). Aquaculture. (2020) 526:735370. doi: 10.1016/j.aquaculture.2020.735370
16. Abdo, SE, El-Nahas, AF, Abdellatif, RE, Mohamed, R, Helal, MA, Azzam, MM, et al. Combined dietary Spirulina platensis and Citrus limon essential oil enhances the growth, immunity, antioxidant capacity and intestinal health of Nile Tilapia. Vet Sci. (2024) 11:474. doi: 10.3390/vetsci11100474
17. Sherif, AH, Khalil, RH, Talaat, TS, Baromh, MZ, and Elnagar, MA. Dietary nanocomposite of vitamin C and vitamin E enhanced the performance of Nile tilapia. Sci Rep. (2024) 14:15648. doi: 10.1038/s41598-024-65507-1
18. Addam, KGS, Pereira, SA, Jesus, GFA, Cardoso, L, Syracuse, N, Lopes, GR, et al. Dietary organic acids blend alone or in combination with an essential oil on the survival, growth, gut/liver structure and de hemato-immunological in Nile tilapia Oreochromis niloticus. Aquac Res. (2019) 50:2960–71. doi: 10.1111/are.14250
19. Chemists AoOA, Chemists AoOA. Official methods of analysis of the Association of Official Analytical Chemists, vol. 2. Rockville, MD: Association of Official Analytical Chemists (1920).
20. Cunniff, P. Official methods of analysis of AOAC international. 16th ed. Rockville, MD: Association of Official Analytical Chemists (1997).
21. Okasha, LA, Abdellatif, JI, Abd-Elmegeed, OH, and Sherif, AH. Overview on the role of dietary Spirulina platensis on immune responses against Edwardsiellosis among Oreochromis Niloticu s fish farms. BMC Vet Res. (2024) 20:290. doi: 10.1186/s12917-024-04131-7
22. Houston, A. Blood and circulation In: CB Schreck and PB Moyle, editors. Methods for fish biology. Bethesda, MD: American Fisheries Society (1990). 415–88.
23. Doumas, BT, Bayse, DD, Carter, RJ, Peters, T Jr, and Schaffer, R. A candidate reference method for determination of total protein in serum. I. Development and validation. Clin Chem. (1981) 27:1642–50. doi: 10.1093/clinchem/27.10.1642
24. Farag, EA, Baromh, MZ, El-Kalamwi, N, and Sherif, AH. Vitamin E nanoparticles enhance performance and immune status of Nile tilapia. BMC Vet Res. (2024) 20:561. doi: 10.1186/s12917-024-04398-w
25. Ellis, SG, Vandormael, MG, Cowley, MJ, DiSciascio, G, Deligonul, U, Topol, EJ, et al. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Multivessel angioplasty prognosis study group. Circulation. (1990) 82:1193–202. doi: 10.1161/01.CIR.82.4.1193
26. Cai, Y, Luo, Q, Sun, M, and Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. (2004) 74:2157–84. doi: 10.1016/j.lfs.2003.09.047
27. Kawahara, E, Ueda, T, and Nomura, S. In vitro phagocytic activity of white-spotted char blood cells after injection with Aeromonas salmonicida extracellular products. Fish Pathol. (1991) 26:213–4. doi: 10.3147/jsfp.26.213
28. Ohkawa, H, Ohishi, N, and Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. (1979) 95:351–8. doi: 10.1016/0003-2697(79)90738-3
29. McCord, JM, and Fridovich, I. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J Biol Chem. (1969) 244:6049–55.
30. Aebi, H. Catalase in vitro In: H Aebi, editor. Methods Enzymol, vol. 105. Amsterdam, Netherlands: Elsevier (1984). 121–6.
31. Livak, KJ, and Schmittgen, TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. (2001) 25:402–8. doi: 10.1006/meth.2001.1262
32. Moustafa, EM, Dawood, MA, Assar, DH, Omar, AA, Elbialy, ZI, Farrag, FA, et al. Modulatory effects of fenugreek seeds powder on the histopathology, oxidative status, and immune related gene expression in Nile tilapia (Oreochromis niloticus) infected with Aeromonas hydrophila. Aquaculture. (2020) 515:734589. doi: 10.1016/j.aquaculture.2019.734589
33. Fadel, A, Mahmoud, MA, Abdelsalam, M, Eissa, E-SH, and Sherif, AH. Aeromonas veronii infection in cultured Oreochromis niloticus: prevalence, molecular and histopathological characterization correlated to water physicochemical characteristics, with the protective autochthonous probiotic. Aquac Int. (2025) 33:298. doi: 10.1007/s10499-025-01960-7
34. Magouz, FI, Amer, AA, Faisal, A, Sewilam, H, Aboelenin, SM, and Dawood, MA. The effects of dietary oregano essential oil on the growth performance, intestinal health, immune, and antioxidative responses of Nile tilapia under acute heat stress. Aquaculture. (2022) 548:737632. doi: 10.1016/j.aquaculture.2021.737632
35. Aanyu, M, Betancor, MB, and Monroig, O. Effects of dietary limonene and thymol on the growth and nutritional physiology of Nile tilapia (Oreochromis niloticus). Aquaculture. (2018) 488:217–26. doi: 10.1016/j.aquaculture.2018.01.036
36. Ju, J. Essential oils as antimicrobial agents in food preservation. Boca Raton, FL: CRC Press (2023).
37. Hoseinifar, SH, Shakouri, M, Yousefi, S, Van Doan, H, Shafiei, S, Yousefi, M, et al. Humoral and skin mucosal immune parameters, intestinal immune related genes expression and antioxidant defense in rainbow trout (Oncorhynchus mykiss) fed olive (Olea europea L.) waste. Fish Shellfish Immunol. (2020) 100:171–8. doi: 10.1016/j.fsi.2020.02.067
38. Reverter, M, Bontemps, N, Lecchini, D, Banaigs, B, and Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: current status and future perspectives. Aquaculture. (2014) 433:50–61. doi: 10.1016/j.aquaculture.2014.05.048
39. Diab, AM, El-Rahman, FA, Khalfallah, MM, Salah, AS, Farrag, F, Darwish, SI, et al. Evaluating the impact of dietary and water-based probiotics on Tilapia health and resistance to Aeromonas hydrophila. Probiotics Antimicrob Proteins. (2024) 1:1–17. doi: 10.1007/s12602-024-10415-z
40. Fath El-Bab, AF, El-Ratel, IT, Abdel-Warith, AWA, Younis, EM, Davies, SJ, and El-Raghi, AA. Investigating the impact of nanoemulsion of curcumin-loaded olive oil on growth performance, feed utilization, immunological responses, and redox status of Litopenaeus vannamei shrimp with emphasis on economic efficiency of supplementation. J Anim Physiol Anim Nutr (Berl). (2024) 108:1877–89. doi: 10.1111/jpn.14027
41. Acar, Ü, Kesbiç, OS, Yılmaz, S, İnanan, BE, Zemheri-Navruz, F, Terzi, F, et al. Effects of essential oil derived from the bitter orange (Citrus aurantium) on growth performance, histology and gene expression levels in common carp juveniles (Cyprinus carpio). Animals. (2021) 11:1431. doi: 10.3390/ani11051431
42. Sherif, AH, Khalil, RH, Tanekhy, M, Sabry, NM, Harfoush, MA, and Elnagar, MA. Lactobacillus plantarum ameliorates the immunological impacts of titanium dioxide nanoparticles (rutile) in Oreochromis niloticus. Aquac Res. (2022) 53:3736–47. doi: 10.1111/are.15877
43. Giri, SS, Sen, SS, Chi, C, Kim, HJ, Yun, S, Park, SC, et al. Effect of guava leaves on the growth performance and cytokine gene expression of Labeo rohita and its susceptibility to Aeromonas hydrophila infection. Fish Shellfish Immunol. (2015) 46:217–24. doi: 10.1016/j.fsi.2015.05.051
44. Valladão, GMR, Gallani, SU, Kotzent, S, Assane, IM, and Pilarski, F. Effects of dietary thyme essential oil on hemato-immunological indices, intestinal morphology, and microbiota of Nile tilapia. Aquac Int. (2019) 27:399–411. doi: 10.1007/s10499-018-0332-5
45. Abd El-Hamid, MI, Ibrahim, SM, Eldemery, F, El-Mandrawy, SA, Metwally, AS, Khalifa, E, et al. Dietary cinnamaldehyde nanoemulsion boosts growth and transcriptomes of antioxidant and immune related genes to fight Streptococcus agalactiae infection in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. (2021) 113:96–105. doi: 10.1016/j.fsi.2021.03.021
46. Faikoh, EN, Hong, Y-H, and Hu, S-Y. Liposome-encapsulated cinnamaldehyde enhances zebrafish (Danio rerio) immunity and survival when challenged with Vibrio vulnificus and Streptococcus agalactiae. Fish Shellfish Immunol. (2014) 38:15–24. doi: 10.1016/j.fsi.2014.02.024
47. Tan, X, Sun, Z, Zhou, C, Huang, Z, Tan, L, Xun, P, et al. Effects of dietary dandelion extract on intestinal morphology, antioxidant status, immune function and physical barrier function of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol. (2018) 73:197–206. doi: 10.1016/j.fsi.2017.12.020
48. Meng, R, Wu, S, Chen, J, Cao, J, Li, L, Feng, C, et al. Alleviating effects of essential oil from Artemisia vulgaris on enteritis in zebrafish via modulating oxidative stress and inflammatory response. Fish Shellfish Immunol. (2022) 131:323–41. doi: 10.1016/j.fsi.2022.10.010
49. Fuentes, EN, Valdés, JA, Molina, A, and Björnsson, BT. Regulation of skeletal muscle growth in fish by the growth hormone–insulin-like growth factor system. Gen Comp Endocrinol. (2013) 192:136–48. doi: 10.1016/j.ygcen.2013.06.009
50. Midhun, SJ, Arun, D, Edatt, L, Sruthi, M, Thushara, V, Oommen, OV, et al. Modulation of digestive enzymes, GH, IGF-1 and IGF-2 genes in the teleost, Tilapia (Oreochromis mossambicus) by dietary curcumin. Aquac Int. (2016) 24:1277–86. doi: 10.1007/s10499-016-9984-1
51. Chaklader, MR, Ahmed, HA, Khafaga, AF, Shukry, M, Selema, TAA, and Abdel-Latif, HM. Silybum marianum promotes growth, hepatic antioxidative activity, and splenic immunity but does not influence the intestinal barrier function of Nile tilapia, Oreochromis niloticus. Aquaculture. (2024) 583:740554. doi: 10.1016/j.aquaculture.2024.740554
Keywords: Nile tilapia, herbal essential oils, growth performance, immune-related genes, antioxidant enzymes, intestinal morphology
Citation: Khalafalaa M, Shehab SM, Aboraya MH, Amer AA, Farrag F, Abdelghany MF, Alotaibi BS, Abdelmegeid M, Shukry M and Elolimy AA (2025) Herbal essential oils improve growth, antioxidant response, and gene expression in Nile Tilapia fingerlings. Front. Vet. Sci. 12:1620632. doi: 10.3389/fvets.2025.1620632
Edited by:
Awad A. Shehata, Helmholtz Association of German Research Centers (HZ), GermanyReviewed by:
Hosny El-Adawy, Friedrich Loeffler Institut, GermanyNariman Essmat, Faculty of Medicine, Semmelweis University, Hungary
Ahmed Sherif, Animal Health Research Institute (Egypt), Egypt
Lorenzo Danilo Granados Rivera, National Institute of Forestry and Agricultural Research (INIFAP), Mexico
Copyright © 2025 Khalafalaa, Shehab, Aboraya, Amer, Farrag, Abdelghany, Alotaibi, Abdelmegeid, Shukry and Elolimy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed A. Elolimy, ZWxvbGlteUB1YWV1LmFjLmFl
 Malik Khalafalaa1
Malik Khalafalaa1 Mustafa Shukry
Mustafa Shukry